ABSTRACT
Fracture liaison services (FLS) are considered to be the most effective organizational approach for secondary fracture prevention. In this study, we evaluated whether FLS care was associated with reduced subsequent fracture and mortality risk over 3 years of follow‐up. In total, 8682 consecutive patients aged 50–90 years with a recent fracture were included. Before FLS introduction, regular fracture treatment procedures were followed (pre‐FLS). After FLS introduction, patients were invited to the FLS and FLS attenders were assessed for osteoporosis, prevalent vertebral fractures, metabolic bone disorders, medication use, and fall risk, and treatment for fracture prevention was initiated according to Dutch guidelines. All fractures were radiographically confirmed and categorized into major/hip (pelvis, proximal humerus or tibia, vertebral, multiple rib, distal femur) and non‐major/non‐hip (all other fractures). Mortality risk was examined using age and sex adjusted Cox proportional hazard models. For subsequent fracture risk, Cox proportional hazard models were adjusted for age, sex, and competing mortality risk (subdistribution hazard [SHR] approach). The pre‐FLS group consisted of 2530 patients (72% women), of whom 1188 (46.9%) had major/hip index fractures, the post‐FLS group consisted of 6152 patients (69% women), of whom 2973 (48.3%) had major/hip index fractures. In patients with a non‐major/non‐hip fracture there was no difference in subsequent non‐major/non‐hip fracture risk or mortality between pre‐FLS and post‐FLS. In patients with a major/hip index fracture, mortality risk was lower post‐FLS (hazard ratio [HR] 0.84; 95% confidence interval [CI], 0.73–0.96) and subsequent major/hip fracture risk was lower in the first 360 days after index fracture post‐FLS compared to pre‐FLS (SHR 0.67; 95% CI, 0.52–0.87). In conclusion, FLS care was associated with a lower mortality risk in the first 3 years and a lower subsequent major/hip fracture risk in the first year in patients with a major/hip index fracture but not in patients with a non‐major/non‐hip fracture. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: AGING, FRACTURE PREVENTION, FRACTURE RISK ASSESSMENT, HEALTH SERVICES RESEARCH, OSTEOPOROSIS
Introduction
Patients with a recent fracture have an increased risk of subsequent fractures and mortality.( 1 , 2 , 3 ) Subsequent fracture risk changes over time and is the highest immediately after an initial fracture.( 4 ) Besides the increased subsequent fracture risk, mortality risk is also increased during the first 5 years after a fracture, even after a non‐hip fracture.( 2 ) Despite the proven effectiveness of anti‐osteoporosis treatment in reducing subsequent fractures, only a minority of patients with a recent fracture receive appropriate fracture risk evaluation and treatment.( 5 ) Therefore, fracture liaison services (FLS) have been developed and implemented to identify, evaluate, and treat patients with an increased risk of subsequent fractures, namely those with a recent fracture. The overall aim of the FLS is to increase the number of patients receiving appropriate fracture risk evaluation and treatment to reduce subsequent fracture risk.( 6 , 7 , 8 )
The effectiveness of FLS in terms of subsequent fracture reduction and reduction of mortality has been summarized in several reviews,( 9 , 10 , 11 ) suggesting variable impacts on mortality and subsequent fracture risk. In a recently published meta‐analysis, Li and colleagues( 12 ) concluded that FLS care was associated with a lower probability of subsequent fractures (odds ratio [OR] 0.70; 95% confidence interval [CI], 0.52–0.93) in the overall comparison, as well as in the post‐FLS versus pre‐FLS comparison (OR 0.62; 95% CI, 0.42–0.91). With respect to the outcome mortality, they concluded that FLS care was not associated with reduced mortality in the overall comparison (OR 0.73; 95% CI, 0.40%–1.09%), whereas in the post‐FLS versus pre‐FLS studies mortality risk was reduced by 35% (OR 0.65; 95% CI, 0.44%–0.95%). The systematic review by Li and colleagues( 12 ) was based on a limited number of heterogeneous studies, limited lengths of follow‐up, mixed groups (ie, before and after the introduction of an FLS in the same hospital [post‐FLS versus pre‐FLS] or between hospitals with and without FLS) and most studies did not apply a competing mortality risk analysis when analyzing subsequent fracture risk.
Our aim was to evaluate whether FLS care was associated with a reduced subsequent fracture and mortality risk within 3 years after a major/hip or non‐major/non‐hip index fracture.
Patients and Methods
Study design and population
This study was designed as a retrospective cohort study and conducted among all consecutive patients aged 50 to 90 years presenting with an index fracture at the Emergency Department (ED) of VieCuri Medical Center (Venlo, the Netherlands) from January 2005 until December 2013. Only patients with radiographically confirmed fractures living in the referral area of this hospital were included. The study was approved by the institutional review board of VieCuri Medical Center (CEM 14‐011).
Outline of the FLS
The FLS was initiated at the end of 2007 at the outpatient clinic at the department of Internal Medicine in close collaboration with the departments of trauma surgery and orthopedic surgery of VieCuri Medical Center. Our staff consisted of a fulltime nurse and two endocrinologists. Patients visiting the ED between January 2005 until December 2007 received regular fracture treatment by trauma surgeons or orthopedic trauma surgeons and were grouped into the “pre‐FLS” group.
The “post‐FLS” group consisted of patients who visited the ED between January 2008 and December 2013. In this period, a trained nurse systematically selected all patients with a clinical fracture based on diagnostic codes on a monthly basis. Patients were invited to the FLS if they were aged 50–90 years, had a radiographically confirmed fracture, and lived in the referral area of VieCuri Medical Center. Patients were not invited to the FLS if they had a fracture of the skull, fractures due to failure of a prosthesis, osteomyelitis, metastasis, an active malignancy, or Paget's disease. If patients were admitted to the hospital, ie, because of hip fracture, then screening and invitation was repeated during the next month's screening.
All patients received an invitation letter. If the patient did not respond to the invitation letter, a reminder letter was sent the next month. All patients who responded positively and visited the FLS completed a detailed questionnaire on demographics, calcium and vitamin D intake (including supplements), comorbidities, medication use, and clinical risk factors for falls and fractures according to the Dutch national guideline.( 13 ) Further, in all patients bone mineral density (BMD) was assessed by dual energy X‐ray absorptiometry (DXA) (Hologic QDR 4500; Hologic, Bedford, MA, USA) at the lumbar spine, total hip, and femoral neck, and categorized according to the World Health Organization (WHO) guideline as normal BMD (T‐score ≥ −1.0), osteopenia (T‐score < −1.0 and > −2.5), and osteoporosis (T‐score ≤ −2.5). In addition, a standard blood sample was collected and analyzed to diagnose underlying contributors to secondary osteoporosis and metabolic bone disorders as previously reported by Bours and colleagues.( 14 ) From 2011 onward, after the implementation of the Dutch national guideline on osteoporosis and fracture prevention, vertebral fracture assessment (VFA) was performed at the same time as the BMD measurements.( 13 )
An appointment at the FLS consisted of a consultation with a specialized nurse and an endocrinologist. Based on the medical history, comorbidities, BMD and VFA results, calcium intake, and serum 25(OH)D levels, patients were counseled on lifestyle, including nutrition, exercise, alcohol, smoking, and fall risks, and treatment was initiated with anti‐osteoporosis medication (AOM) and calcium and vitamin D supplements according to the national guideline for treatment of osteoporosis and fracture prevention.( 13 ) If contributors for secondary osteoporosis and metabolic bone disorders were diagnosed, treatment was initiated according to the specific guidelines for those disorders.
Data collection and outcome measures
For all patients, data were collected retrospectively by yearly anonymized exports of the electronic patient records. The following baseline data were collected: gender, age, index fracture location, and date. All index and subsequent fractures were grouped into hip and major fractures (pelvis, proximal humerus or tibia, vertebral, multiple rib, distal femur) and non‐major/non‐hip according to Center and colleagues.( 15 ) Patients were followed from their index fracture date until death, first subsequent fracture (same groupings as index fractures; ie, major/hip or non‐major/non‐hip) or end of follow‐up, whichever came first. All patients were followed for a maximum of 3 years. The data regarding the outcome of subsequent fractures were obtained by diagnostic codes and additional verification of the radiology reports; only radiographically confirmed fractures were included in the analyses. Subsequent fractures due to failure of a prosthesis, osteomyelitis, malignancy, and Paget's diseases, and fractures of the skull were excluded. In case a fracture in the post‐FLS period was a subsequent fracture from the pre‐FLS period, this was counted as index fracture for the post‐FLS period as well. Data regarding the outcome mortality were obtained by the national death registration database, providing only the date of death. For this study, data of patients who emigrated were excluded. Data on the yearly FLS attendance rate and the proportion of patients that received a prescription for AOM were retrieved on a group level.
Statistical analyses
Data were analyzed using Cox proportional hazard models with mortality or subsequent fracture as event. The proportional hazards assumption was tested using time‐dependent Cox regression analyses with interaction with time tested for each baseline variable separately. In case of violation, the analyses were separated in two time intervals and the −2LogLikelihood were compared between models with different cut‐off points to identify the best cut‐off (ie, the model with the lowest −2LogLikelihood). All analyses were performed with adjustments for age (decades) and gender. To adjust for the competing risk of mortality, the subdistribution hazard approach (SHR) by Fine and Gray( 16 , 17 ) was applied for the analyses with subsequent fracture as outcome. Explorative subgroup analyses with mortality and subsequent fractures as outcomes were performed for gender, age decades and index fracture type. Sensitivity analyses for both mortality and subsequent fractures were performed with classification of index fracture location as major osteoporotic fractures (MOFs; including wrist, humerus, spine, hip fractures) and non‐MOFs (all other fracture types) according to the International Osteoporosis Foundation (IOF) classification.( 18 ) Further, as a consequence of the formal tracking of individuals, their invitation and attendance to the FLS introduced a median lag time of 125 days between index fracture and FLS visit. Therefore, sensitivity analyses were performed for mortality and subsequent fractures with follow‐up initiated at day 126 to minimize immortal time bias( 19 ) for the total post‐FLS group, as well for FLS attenders and non‐attenders separately. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 8682 consecutive patients aged 50–90 years with a clinical index fracture was included. The pre‐FLS group consisted of 2530 patients with a recent fracture (1832 [72.4%] women) with a mean ± standard deviation (SD) age of 68.2 ± 11.7 years of whom 365 (14.4%) had a hip fracture, 704 (27.8%) a major fracture, and 1460 (57.7%) a non‐major/non‐hip fracture (Table 1). Of all patients in the pre‐FLS group, 131 sustained a subsequent fracture in the post‐FLS period. The post‐FLS group consisted of 6152 patients (4244 [69.0%] women), with a mean age of 68.2 ± 11.0 years. In this group, 763 (14.1%) patients had a hip fracture, 1944 (31.7%) a major fracture, and 3445 (54.2%) a non‐major/non‐hip fracture (Table 1). In the post‐FLS group, 53% attended the FLS, of whom 40% had an indication for treatment with AOM. The median follow‐up for both mortality and subsequent fractures was 1095 days.
Table 1.
Baseline Characteristics of All Patients With a Major or Hip Index Fracture and Non‐Major/Non‐Hip Index Fracture Before (Pre‐FLS) and After (Post‐FLS) the Introduction of the FLS
| Major/hip index fracture | Non‐major/non‐hip index fracture | |||
|---|---|---|---|---|
| Characteristic | Pre‐FLS (n = 1188) | Post‐FLS (n = 2973) | Pre‐FLS (n = 1557) | Post‐FLS (n = 3607) |
| Female (%) | 72.8 | 68.4 | 71.3 | 68.8 |
| Age (years), mean ± SD | 72.6 ± 10.4 | 72.2 ± 11.0 | 65.9 ± 10.4 | 65.9 ± 10.4 |
| 50–59 (%) | 15.8 | 17.4 | 34.2 | 33.3 |
| 60–69 (%) | 18.0 | 21.4 | 27.7 | 30.7 |
| 70–79 (%) | 33.7 | 28.8 | 24.9 | 22.5 |
| 80–90 (%) | 32.5 | 32.5 | 13.2 | 13.5 |
Mortality
In patients presenting with a major/hip index fracture, the cumulative mortality during the 3‐year follow‐up period was significantly lower in the post‐FLS group (n = 668; 22.5%) compared to the pre‐FLS group (n = 308; 25.9%; p = 0.019), whereas in patients presenting with a non‐major/non‐hip index fracture mortality pre‐FLS and post‐FLS was comparable, 9.3% pre‐FLS versus 8.0% post‐FLS respectively (p = 0.122).
In patients with a major/hip index fracture, the adjusted mortality risk was significantly lower in the post‐FLS group (HR 0.84; 95% CI, 0.73–0.96). In patients with a non‐major/non‐hip index fracture, there was no difference in mortality risk between pre‐FLS and post‐FLS (Table 2).
Table 2.
Multivariable Cox Regression Model for Mortality Risk During 3 Years of Follow‐Up in Patients With a Major/Hip Index Fracture and Patients With a Non‐Major/Non‐Hip Index Fracture
| Major/hip index fracture | Non‐major/non‐hip index fracture | |||
|---|---|---|---|---|
| Parameter | Number of deaths | Hazard ratio (95% CI) | Number of deaths | Hazard ratio (95% CI) |
| Pre‐FLS | 308 | Reference | 145 | Reference |
| Post‐FLS | 668 | 0.84 (0.73–0.96) | 290 | 0.86 (0.70–1.04) |
| Men | 345 | Reference | 135 | Reference |
| Women | 631 | 0.56 (0.49–0.64) | 300 | 0.61 (0.50–0.75) |
| Age at fracture (years) | ||||
| 50–59 | 23 | Reference | 38 | Reference |
| 60–69 | 81 | 3.20 (2.01–5.09) | 56 | 1.81 (1.20–2.74) |
| 70–79 | 261 | 7.61 (4.97–11.66) | 122 | 5.44 (3.76–7.86) |
| 80 and older | 611 | 20.90 (13.76–31.73) | 219 | 19.91 (14.03–28.26) |
Major/hip: fractures of hip, pelvis, proximal humerus or tibia, vertebral, multiple rib, distal femur. Non‐major/non‐hip: all others.
In subgroup analyses, mortality risk post‐FLS was significantly lower in patients with a major index fracture, in patients aged 80+ years and in women (Fig. 1). In men and the age group 70–79 years, the analyses were separated in two time intervals due to violation of the proportional hazard assumption. In men, mortality risk was significantly lower in the first 330 days after a major/hip index fracture in the post‐FLS group and in patients aged 70–79 mortality risk was significantly lower in the first 480 days after a major/hip index fracture (Fig. 1).
Fig. 1.
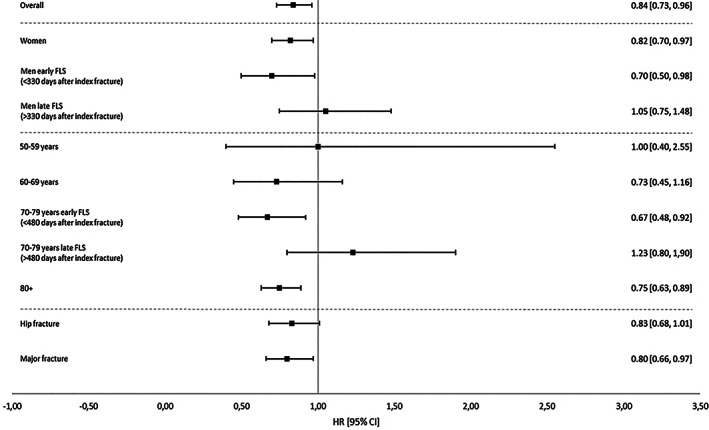
Mortality risk after a major or hip index fracture during 3 years of follow‐up, starting from date of index fracture.
Subsequent fractures
The cumulative incidence of a subsequent major/hip fracture after major/hip index fracture during the 3‐year follow‐up period was comparable between pre‐FLS and post‐FLS, 6.0% and 5.6%, respectively (p = 0.616). Further, the cumulative incidence of subsequent non‐major/non‐hip fracture after non‐major/non‐hip index fracture was 3.3% pre‐FLS and 3.2% post‐FLS (p = 0.852).
The risk of a subsequent major/hip fracture after a major/hip index fracture was significantly lower in the first 360 days after index fracture post‐FLS compared to pre‐FLS, taking the competing risk of death into account (SHR 0.67; 95% CI, 0.52–0.87), but there was no difference in the second period (SHR 1.29; 95% CI, 0.97–1.73) (Table 3). There was no difference in the risk of subsequent non‐major/non‐hip fracture risk after a non‐major/non‐hip index fracture between pre‐FLS and post‐FLS.
Table 3.
Multivariable Cox Regression Model for Subsequent Major/Hip Fracture Risk After Major/Hip Index Fracture, and Subsequent Non‐Major/Non‐Hip Fracture After Non‐Major/Non‐Hip Index Fracture for 3 Years Follow‐Up, Starting From Date of Index Fracture
| Major/hip index fracture | Non‐major/non‐hip index fracture | |||||
|---|---|---|---|---|---|---|
| Parameter | Number of events | Hazard ratio (95% CI) | SHR (95% CI) | Number of events | Hazard ratio (95% CI) | SHR (95% CI) |
| Pre‐FLS | 153 | Reference | Reference | 83 | Reference | Reference |
| Post‐FLS | 199 | 1.03 (0.80–1.33) | 1.04 (0.81–1.35) | |||
| Post‐FLS ≤ 360 days | 153 | 0.66 (0.51–0.85) | 0.67 (0.52–0.87) | |||
| Post‐FLS > 360 days | 192 | 1.25 (0.94–1.68) | 1.29 (0.97–1.73) | |||
| Men | 112 | Reference | Reference | 62 | Reference | Reference |
| Women | 386 | 1.26 (1.02–1.56) | 1.41 (1.14–1.74) | 220 | 1.49 (1.12–1.99) | 1.52 (1.14–2.02) |
| Age at fracture (years) | ||||||
| 50–59 | 52 | Reference | Reference | 81 | Reference | |
| 60–69 | 73 | 1.19 (0.83–1.70) | 1.14 (0.80–1.63) | 86 | 1.14 (0.84–1.55) | 1.13 (0.84–1.53) |
| 70–79 | 160 | 1.93 (1.41–2.64) | 1.71 (1.26–2.34) | 66 | 1.13 (0.81–1.57) | 1.09 (0.79–1.51) |
| 80 and older | 213 | 2.91 (2.15–3.96) | 2.12 (1.57–2.87) | 49 | 1.65 (1.15–2.37) | 1.39 (0.97–2.00) |
Major/hip: fractures of hip, pelvis, proximal humerus or tibia, vertebral, multiple rib, distal femur. Non‐major/non‐hip: all others.
SHR = subdistribution hazard ratio.
In subgroup analyses, women and patients with a major index fracture had a significantly lower risk of subsequent major/hip fractures within the first 360 and 210 days after index fracture, respectively (Fig. 2).
Fig. 2.
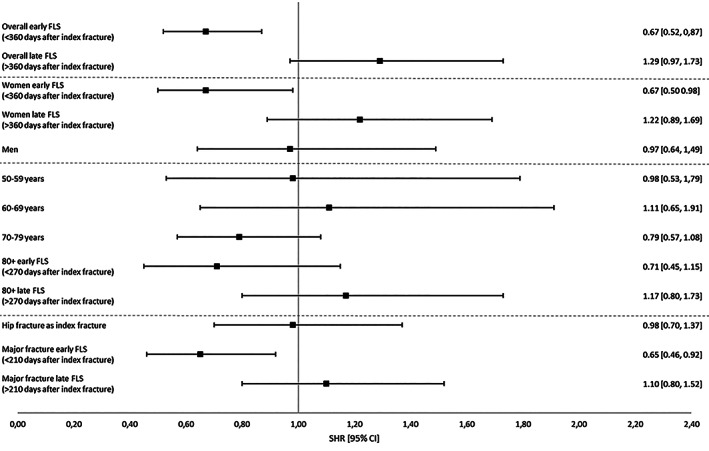
Subsequent major or hip fracture risk after a major or hip index fracture during 3 years of follow‐up, starting from date of index fracture.
Sensitivity analyses in patients with MOF
The adjusted mortality risk in patients with an index MOF (wrist, humerus, spine, hip fracture) was significantly lower in the post‐FLS group (HR 0.79; 95% CI, 0.70–0.91). There was no difference in subsequent MOF fracture risk after an index MOF fracture between pre‐FLS and post‐FLS.
In subgroup analyses, mortality risk was lower in women post‐FLS (Fig. SS1). In men, mortality risk was lower in the first 330 days after an index MOF fracture (HR 0.63; 95% CI, 0.46–0.86), but there was no difference in the second period. Mortality risk was lower in post‐FLS patients aged 70–79 years, in patients aged 80 years, and in patients with an index clinical vertebral fracture.
In subgroup analyses, there was no difference in subsequent MOF fracture risk after an index MOF fracture between pre‐FLS and post‐FLS (Fig. S2).
Sensitivity analyses with follow‐up initiated at 126 days after fracture
In the analyses where day 126 after the index fracture was used as the first day of follow‐up, the adjusted mortality risk was significantly lower in the post‐FLS group in patients with a major/hip index fracture (HR 0.79; 95% CI, 0.67–0.93) and in patients with an index MOF (HR 0.75; 95% CI, 0.64–0.88). There was no difference in subsequent major/hip fracture risk after a major/hip index fracture, or subsequent MOF risk after an index MOF between pre‐FLS and post‐FLS (HR 1.00 [95% CI, 0.79–1.27]; HR 0.93 [95% CI, 0.55–1.14], respectively).
In FLS attenders with a major/hip index fracture, the adjusted mortality risk was significantly lower (HR 0.43; 95% CI, 0.34–0.56) compared to the pre‐FLS group, whereas the adjusted mortality risk was not different in FLS non‐attenders (HR 1.05; 95% CI, 0.88–1.25). The subsequent major/hip risk after a major/index fracture, both in attenders and non‐attenders, was not significantly different as compared to the pre‐FLS group (SHR 0.80 [95% CI, 0.60–1.07] in attenders; SHR 1.18 [95% CI, 0.93–1.53] in non‐attenders, respectively).
In accordance with the main analyses, the major/hip subsequent fracture risk after a major/hip index fracture was lower in the FLS attenders in the first 360 days after index fracture compared to the pre‐FLS group (SHR 0.62; 95% CI, 0.40–0.95), whereas in non‐attenders there was no difference (SHR 0.99; 95% CI, 0.70–1.38). In the late post‐FLS period (from 360 days onward), subsequent major/hip fracture risk was not different in FLS attenders (SHR 1.00; 95% CI, 0.67–1.50), but higher in non‐attenders (SHR 1.42; 95% CI, 1.00–1.99) presenting with a major/hip index fracture.
Discussion
In the present study, we found that the adjusted mortality risk in patients with a major/hip index fracture was 16% lower in the post‐FLS group as compared to the pre‐FLS group. Further, subsequent major/hip fracture after a major/hip index fracture was 33% lower in the first 360 days after index fracture post‐FLS compared to pre‐FLS, taking the competing risk of death into account. However, in patients with a non‐major/non‐hip index fracture, there was no difference in mortality or subsequent fracture risk between post‐FLS and pre‐FLS.
Studies on the effectiveness of the implementation of an FLS in terms of subsequent fracture risk reduction and mortality are heterogeneous, with respect to the length of follow‐up (most often 2 years or less), the design of the study (ie, post‐FLS versus pre‐FLS comparison, or comparison of hospitals with and without FLS), the included study population (age, index fracture types), and the classifications of groups of fractures, and most previous studies did not apply a competing mortality risk analysis when analyzing subsequent fracture risk.( 12 )
The finding of a 33% lower 3‐year mortality risk in our study is in line with the recent published meta‐analysis of Li and colleagues,( 12 ) showing a 35% lower probability of mortality post‐FLS compared to pre‐FLS. Because five out of the six studies included in the meta‐analysis had a follow‐up duration of 2 years or less, and the only study with a median pre‐post FLS follow‐up period >2 years in that meta‐analysis had a post‐FLS follow‐up period of 1.5–1.7 year,( 20 ) our study is the first that indicates a longer‐term mortality reduction 3 years after implementation of FLS care.
We found a 33% lower subsequent major/hip fracture risk post FLS, in the first year after a major/hip index fracture, taking the competing risk of death into account. Due to violation of the proportional hazards assumption, we did not analyse the risk of subsequent fractures during the complete follow‐up period of 3 years, rather the analyses were separated into two time intervals. However the finding of a lower subsequent fracture risk in the first period followed by a nonsignificant difference in the second period suggests that FLS care is associated with a longer‐term subsequent fracture risk reduction. Regarding the risk of subsequent fractures, there are four published FLS studies that used the competing risk analysis method described by Fine and Gray.( 16 , 20 , 21 , 22 , 23 ) Hawley and colleagues( 21 ) reported a lower mortality risk, but no difference in the risk of subsequent hip fractures in the first year after an index hip fracture, after implementation of orthogeriatric and nurse‐led FLS models. By using the subdistribution hazard approach by Fine and Gray,( 16 ) patients who died before sustaining a subsequent fracture (event of interest) are not censored, but these patients retain in the risk set for sustaining a subsequent fracture. If the competing risk of mortality is ignored, the incidence of subsequent fractures is overestimated. By taking the competing risk of death into account, a true estimate of the subsequent fracture risk is presented. Axelsson and colleagues( 20 ) reported an SHR of 0.73 (95% CI, 0.66–0.82) for subsequent MOFs after an index MOF, including pelvis fractures as MOFs, with a median FLS follow‐up of 1.7 years, which is in line with our study. Nakayama and colleagues( 22 ) reported an SHR of 0.67 (95% CI, 0.47–0.95) for subsequent fractures over 3 years when comparing an FLS hospital with no‐FLS hospital. Compared to our study, the study of Nakayama and colleagues( 22 ) did not compare a pre‐post FLS period but showed a comparison of a FLS hospital versus a non‐FLS hospital, had a smaller sample size, and the 3‐year incidence of fractures (11% post‐FLS and 6% pre‐FLS) was substantially higher than in our study. Furthermore, in that study there was no violation of the proportional hazard assumption. Davidson and colleagues( 23 ) reported an SHR of 0.58 (95% CI, 0.35–0.95) for subsequent fractures over 3 years when comparing the effectiveness of a nurse‐led FLS versus pre‐FLS in patients with a minimal trauma fracture (MTF). MTFs were defined fractures from femur, tibia, fibula, ankle, pelvis, humerus, and wrist resulting from a standing height or less. The 3‐year incidence of fractures in this small study of 140 patients aged 45 years and older was markedly higher as compared to our study (10.5% post‐FLS versus 19.1% pre‐FLS).( 23 ) Overall, the findings of our study and the four other studies on subsequent fracture risk, indicate that FLS care is associated with a lower risk of subsequent fractures in the first 2 years after FLS implementation, when taking the competing risk of death into account. Studies reporting longer‐term benefits in subsequent fracture risk reduction, especially when taking competing mortality risk into account, are currently lacking in FLS literature.
We found no difference in mortality and non‐major/non‐hip subsequent fracture risk in patients presenting with a non‐major/non‐hip fracture. Only Huntjens and colleagues,( 24 ) Nakayama and colleagues,( 22 ) and Shin and colleagues( 25 ) evaluated the outcomes of FLS‐care in patients with a non‐major/non‐hip index fracture and distal radius fractures, respectively. Huntjens and colleagues( 24 ) reported that subsequent fracture and mortality risk in patients with a minor fracture between a non‐FLS and FLS hospital was not reduced, but the competing risk of death was not taken into account. In line with our study, Nakayama and colleagues( 22 ) reported that the reduction of minor refractures was not as pronounced as the reduction in major refractures between the FLS‐hospital and non‐FLS hospital, but due to small the number of events, the authors did not perform a separate subgroup analysis for patients with a minor fracture. Shin and colleagues( 25 ) evaluated the effect of osteoporosis care after a distal radius fracture and reported a risk reduction of 65% for subsequent fractures, but the competing risk of death was not taken into account.
The early benefits in terms of subsequent fracture risk reduction, in the first year after index fracture combined with the lower 3‐year mortality risk in this study can only partially be explained by the use of AOM because only 40% of the FLS attenders were treated with AOM. It is likely that improvements of fracture‐related procedures in combination with the integrated approach after implementation of FLS care resulted in favorable outcomes in the post‐FLS period. FLS attenders were extensively evaluated, not only by BMD measurement and VFA, but also for the presence of underlying metabolic bone disorders (including calcium and vitamin D deficiencies). Furthermore, next to initiation of AOM, comorbidities were treated, medication was reviewed and optimized, and patients were followed up to a year postfracture.
The outcome of FLS care as presented in this study could potentially be further improved by reaching higher FLS attendance rates, because only 53% of patients with a fracture visited our FLS. Furthermore, at the time of FLS care in this study, alendronic acid was the first choice AOM according to the Dutch guideline, zoledronic acid was hardly used, denosumab was not available in the first years of the post‐FLS period (introduced in the Netherlands in 2011), and teriparatide treatment could only be prescribed in patients who had a third fracture during treatment with an oral bisphosphonate.( 13 ) Although oral bisphosphonates have proven their effect in fracture risk reduction in clinical trials in patients with an increased fracture risk (ie, osteoporosis), real‐life persistence with this type of medication is often poor, which might in turn dilute the fracture risk reduction. Klop and colleagues( 26 ) evaluated persistence with bisphosphonates in newly treated fracture patients in the Netherlands and concluded that persistence was 75% 1 year after treatment initiation and only 45% 5 years after initiation, respectively. More recently, the treatment options for fracture prevention have been enlarged with teriparatide and romosozumab, bone forming agents, which have early and superior fracture risk reductions as compared to oral bisphosphonates. Therefore, it has to be advocated that future studies evaluating the FLS should include all treatment options for osteoporosis including treatment persistence rates as well. Further, these future studies should have a longer follow‐up period (ie, 5 years) and, as advised by Li and colleagues,( 12 ) the competing risk of mortality should be taken into account while exploring the FLS effect on subsequent fracture risk.
This study has strengths and limitations. A strength of this study is that we were able to evaluate all patients with radiographically confirmed index fracture and subsequent fractures. Further, we included all consecutive patients, including those who did not attend the FLS. Although this approach might result in a dilution of the FLS effect, especially in subsequent fracture risk, we consider this as the proper method to evaluate the real‐life outcome of FLS care and to minimize selection bias. Our analyses were performed including the competing risk of mortality. Ignoring this competing risk could introduce bias in the estimation of fracture risk. An important limitation of this study is that we were not able to identify the FLS attenders on an individual level, in whom treatment was initiated either with AOM or underlying causes and we have no data on treatment persistence during follow‐up.
In conclusion, FLS care was associated with a lower mortality risk in the first 3 years and a lower subsequent major/hip fracture risk in the first year in patients with a major/hip index fracture but not in patients with a non‐major/non‐hip fracture. Although the early benefits suggests that the multidisciplinary approach at the FLS could improve the outcomes of patients with a recent fracture, there is still a window of opportunity by increasing FLS attendance and treatment rates, the use of anabolic medication, and long‐term follow‐up with attention to treatment adherence.
AUTHOR CONTRIBUTIONS
Lisanne Vranken: Data curation; writing – original draft; writing – review and editing. Irma de Bruin: Writing – original draft; writing – review and editing. Annemariek Driessen: Formal analysis; methodology; writing – original draft; writing – review and editing. Piet Geusens: Conceptualization; writing – review and editing. John A. Eisman: Conceptualization; writing – review and editing. Jacqueline R Center: Conceptualization; writing – review and editing. Robert van der Velde: Writing – review and editing. Heinrich Janzing: Writing – review and editing. Sjoerd Kaarsemaker: Writing – review and editing. Joop van den Bergh: Conceptualization; methodology; supervision; writing – original draft; writing – review and editing. Caroline Wyers: Conceptualization; data curation; formal analysis; project administration; writing – original draft; writing – review and editing.
Conflicts of Interest
IJAdB: received personal fees from Sanofi and Amgen, outside the submitted work. JAE: has consulted for and/or received research funding from Amgen, deCode, Merck Sharp and Dohme, Sanofi‐Aventis and Theramax outside the submitted work. JRC: advisory boards for Amgen; research funding from Amgen, outside the submitted work. RYvdV: received funding from Novo Nordisk an Lilly, outside the submitted work. JPWvdB: consultant for Amgen and UCB, research funding from Amgen and UCB, outside the submitted work. AHMD, LV, PPMG, JHMD, HMJJ, SK, CEW: no conflicts of interest.
Supporting information
Fig. S1 Mortality risk after a MOF index fracture during 3 years of follow‐up, starting from date of index fracture.
Fig. S2 Subsequent fracture risk after a MOF index fracture during 3 years of follow‐up, starting from date of index fracture.
Acknowledgments
This study was funded by the research foundation of VieCuri Medical Center Noord‐Limburg, The Netherlands and Stichting de Wijerhorst.
DATA AVAILABILITY STATEMENT
Research data are not shared due to privacy or ethical restrictions.
References
- 1. Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2015;30(4):637‐646. [DOI] [PubMed] [Google Scholar]
- 2. Tran T, Bliuc D, van Geel T, et al. Population‐wide impact of non‐hip non‐vertebral fractures on mortality. J Bone Miner Res. 2017;32(9):1802‐1810. [DOI] [PubMed] [Google Scholar]
- 3. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2010;15(4):721‐739. [DOI] [PubMed] [Google Scholar]
- 4. Laurs ‐ van Geel TACM, Center JR, Geusens PP, Dinant G‐J, Eisman JA. Clinical fractures cluster in time after initial fracture. Maturitas. 2010;67(4):339‐342. [DOI] [PubMed] [Google Scholar]
- 5. Borgstrom F, Karlsson L, Ortsater G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta‐analysis. Osteoporos Int. 2012;24(2):393‐406. [DOI] [PubMed] [Google Scholar]
- 7. Lems WF, Dreinhöfer KE, Bischoff‐Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis. 2017;76(5):802‐810. [DOI] [PubMed] [Google Scholar]
- 8. Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039‐2046. [DOI] [PubMed] [Google Scholar]
- 9. Briot K. Fracture liaison services. Curr Opin Rheumatol. 2017;29(4):416‐421. [DOI] [PubMed] [Google Scholar]
- 10. de Bruin IJA, Wyers CE, van den Bergh JPW, Geusens PPMM. Fracture liaison services: do they reduce fracture rates? Ther Adv Musculoskelet Dis. 2017;9(7):157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu CH, Tu ST, Chang YF, et al. Fracture liaison services improve outcomes of patients with osteoporosis‐related fractures: a systematic literature review and meta‐analysis. Bone. 2018;111:92‐100. [DOI] [PubMed] [Google Scholar]
- 12. Li N, Hiligsmann M, Boonen A, et al. The impact of fracture liaison services on subsequent fractures and mortality: a systematic literature review and meta‐analysis. Osteoporos Int. 2021;32(8):1517‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Working group CBO . Guideline Osteoporosis and Fracture prevention, third edition [Dutch]. Utrecht: CBO; 2011. [Google Scholar]
- 14. Bours SPG, van den Bergh JPW, van Geel TACM, Geusens PPMM. Secondary osteoporosis and metabolic bone disease in patients 50 years and older with osteoporosis or with a recent clinical fracture: a clinical perspective. Curr Opin Rheumatol. 2014;26(4):430‐439. [DOI] [PubMed] [Google Scholar]
- 15. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low‐trauma fracture in men and women. JAMA. 2007;297(4):387. [DOI] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496‐509. [Google Scholar]
- 17. Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670‐2677. [DOI] [PubMed] [Google Scholar]
- 18. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16(3):241‐249. [DOI] [PubMed] [Google Scholar]
- 20. Axelsson KF, Johansson H, Lundh D, Möller M, Lorentzon M. Association between recurrent fracture risk and implementation of fracture liaison services in four Swedish hospitals: a cohort study. J Bone Miner Res. 2020;35(7):1216‐1223. [DOI] [PubMed] [Google Scholar]
- 21. Hawley S, Javaid MK, Prieto‐Alhambra D, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population‐based longitudinal study. Age Ageing. 2016;45(2):236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakayama A, Major G, Holliday E, Attia J, Bogduk N. Evidence of effectiveness of a fracture liaison service to reduce the re‐fracture rate. Osteoporos Int. 2016;27(3):873‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidson E, Seal A, Doyle Z, Fielding K, McGirr J. Prevention of osteoporotic refractures in regional Australia. Aust J Rural Health. 2017;25(6):362‐368. [DOI] [PubMed] [Google Scholar]
- 24. Huntjens KM, van Geel TACM, van den Bergh JP, et al. Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am. 2014;96(4):e29. [DOI] [PubMed] [Google Scholar]
- 25. Shin YH, Hong WK, Kim J, Gong HS. Osteoporosis care after distal radius fracture reduces subsequent hip or spine fractures: a 4‐year longitudinal study. Osteoporos Int. 2020;31(8):1471‐1476. [DOI] [PubMed] [Google Scholar]
- 26. Klop C, Welsing PMJ, Elders PJM, et al. Long‐term persistence with anti‐osteoporosis drugs after fracture. Osteoporosis Int. 2015;26(6):1831‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Mortality risk after a MOF index fracture during 3 years of follow‐up, starting from date of index fracture.
Fig. S2 Subsequent fracture risk after a MOF index fracture during 3 years of follow‐up, starting from date of index fracture.
Data Availability Statement
Research data are not shared due to privacy or ethical restrictions.


