Abstract
The major outer membrane protein (MOMP) of Chlamydia trachomatis serovariants is known to be an immunodominant surface antigen. Moreover, it is known that the C. trachomatis MOMP elicits antibodies that recognize both linear and conformational antigenic determinants. In contrast, it has been reported that the MOMP of Chlamydia pneumoniae is not surface exposed and is immunorecessive. We hypothesized that the discrepancies between C. trachomatis and C. pneumoniae MOMP exposure on intact chlamydiae and immunogenic properties might be because the focus of the host's immune response is directed to conformational epitopes of the C. pneumoniae MOMP. We therefore conducted studies aimed at defining the surface exposure of MOMP and the conformational dominance of MOMP antibodies. We present here a description of C. pneumoniae species-specific monoclonal antibody (MAb), GZD1E8, which recognizes a conformational epitope on the surface of C. pneumoniae. This MAb is potent in the neutralization of C. pneumoniae infectivity in vitro. Another previously described C. pneumoniae species-specific monoclonal antibody, RR-402, displayed very similar characteristics. However, the antigenic determinant recognized by RR-402 has yet to be identified. We show by immunoprecipitation of C. pneumoniae with GZD1E8 and RR-402 MAbs and by mass spectrometry analysis of immunoprecipitated proteins that both antibodies GZD1E8 and RR-402 recognize the MOMP of C. pneumoniae and that this protein is localized on the surface of the organism. We also show that human sera from C. pneumoniae-positive donors consistently recognize the MOMP by immunoprecipitation, indicating that the MOMP of C. pneumoniae is an immunogenic protein. These findings have potential implications for both C. pneumoniae vaccine and diagnostic assay development.
Chlamydia pneumoniae is a human respiratory pathogen. It is the third most common cause of community-acquired pneumonia, being responsible for approximately 10% of all community-acquired pneumonia cases and 5% of bronchitis and sinusitis cases (15). Most importantly, C. pneumoniae infection has been associated with a number of chronic diseases, such as asthma, sarcoidosis, otitis media, erythema nodosum, Reiter's syndrome (reviewed in reference 21), and atherosclerosis (reviewed in reference 9).
Laboratory diagnosis of C. pneumoniae infection is based on isolation of the agent, serology, and/or detection of DNA by PCR. Isolation of organisms from clinical specimens has proven to be very difficult, and PCR-based detection is met with technical and standardization problems that currently prevent its routine use in diagnostics (reviewed in reference 4). Serology, primarily the detection of serum antibodies using the micro-immunofluorescence (micro-IF) test, has proven to be the most specific and sensitive test for the diagnosis of C. pneumoniae (15, 34). Despite its utility, the micro-IF test is inapplicable for use in a standard laboratory setting. The test is labor intensive, it requires intact purified organisms as antigen and specialized fluorescent microscopy equipment, and the reading of immunofluorescent results is subjective. The C. pneumoniae species-specific antigen(s) on the surface of intact organisms that is detected by the micro-IF test is unknown. This antigen is, however, a logical and potentially very important component in the development of much-needed nonsubjective serological tests for the diagnosis of C. pneumoniae infection.
Despite extensive studies of the antigenic composition of C. pneumoniae, an immunodominant C. pneumoniae-specific antigen has yet to be identified and characterized. Immunoblot analysis of the anti-C. pneumoniae antibody response in acute and convalescent human sera, as well as hyperimmune mouse and rabbit sera, has identified numerous immunogenic proteins varying in mass from 15 to 99 kDa; however, none of these antigens has been shown to be both C. pneumoniae species specific and consistently recognized by either acute or convalescent sera (10, 11, 14, 16, 19, 20). One explanation proposed for this finding is that in nature, C. pneumoniae isolates differ antigenically and the response to different polypeptides observed by immunoblotting is a reflection of these antigenic differences (3, 17, 33). This explanation is not consistent with micro-IF findings, however, since two prototype strains, AR-39 and TW-183, are routinely used as antigen in the assay to detect C. pneumoniae-specific antibodies in the sera of individuals representing diverse populations. An alternative possibility is that there exists a common species-specific surface antigen that is conformational in nature and whose antigenicity is destroyed by exposure to heat and sodium dodecyl sulfate (SDS), both of which are used in immunoblotting procedures.
Interestingly, the C. pneumoniae species-specific monoclonal antibody (MAb) RR-402 described by Puolakkainen et al. reacts with the surface of C. pneumoniae organisms (12, 28). It is possible that the antigen and epitope recognized by this antibody is similar or identical to the immunodominant surface antigen that is detected in human sera by the micro-IF assay. It has therefore been of considerable interest to identify the C. pneumoniae antigen recognized by RR-402. Immunoblot assays using MAb RR-402 have not yielded a reproducible immunoreactive antigen. Several investigators have employed immunoprecipitation of intrinsically radiolabeled C. pneumoniae proteins using MAb RR-402 to identify a reactive antigen, with differing results (14, 28). Puolakkainen et al. were unable to identify a specific reactivity since the treatment applied in their immunoprecipitation protocol included steps that would have denatured the reactive antigen (28). Essig et al. reported that MAb RR-402, as well as acute human sera, immunoprecipitated an approximately 40-kDa polypeptide. These investigators hypothesized that the antibody reacted with the organism's major outer membrane protein (MOMP); however, evidence for this conclusion was based solely on the relative migration of the precipitated protein (14).
In this study, we address the significance of conformational determinants in eliciting antibody responses specific to the MOMP of C. pneumoniae. We describe a C. pneumoniae species-specific murine MAb GZD1E8 that recognizes a surface-localized antigen. We demonstrate by immunoprecipitation and mass spectrometry (MS) analysis that MAb GZD1E8, as well as MAb RR-402, recognize a conformation-dependent epitope of C. pneumoniae MOMP. We also demonstrate by immunoprecipitation and MS analysis that human sera positive for C. pneumoniae antibody by the micro-IF test consistently recognize a conformational determinant of MOMP. We believe these findings help clarify existing controversy in the literature about the surface exposure and immunogenicity of C. pneumoniae MOMP. In addition, our findings should help in defining surrogate antigens corresponding to MOMP conformational epitopes that could prove useful in the development of new serodiagnostic tests for C. pneumoniae infection.
MATERIALS AND METHODS
Cell culture and organisms.
The following chlamydial organisms were used: C. trachomatis serovars A, B, Ba, C, D, E, F, G, H, I, J, K, L1, L2, and L3; mouse pneumonitis (MoPn); C. psittaci meningopneumonitis/Cal 10 (Mn) and guinea pig inclusion conjunctivitis (GPIC). Growth and purification of C. trachomatis and C. psittaci were performed as previously described (6). C. pneumoniae strain AR-39 (CCL 2.1) was purchased from the American Type Culture Collection (Manassas, Va.) and propagated in HeLa 229 cells. Confluent monolayers of HeLa 229 cells in 24-well plates containing coverslips were infected with each chlamydial serovar at a multiplicity of infection of 0.5. Infected cells were incubated in RPMI 1640 medium (Gibco BRL, Rockville, Md.) supplemented with 10% fetal calf serum plus 10 μg of gentamicin/ml and, for some serovars, with 2 μg of cycloheximide/ml at 37°C in an atmosphere of 5% CO2 and humidified air (35). When mature inclusions were formed (36 to 72 h), monolayers were fixed with acetone and stained by indirect immunofluorescence using either MAb GZD1E8 or EVIH1 and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin serum (Zymed, San Francisco, Calif.).
Antibodies.
MAb GZD1E8, isotype immunoglobulin G1 (IgG1), was generated against C. pneumoniae strain AR-39 by G. Zhong (data not shown). The C. pneumoniae species-specific MAb RR-402, isotype IgG3, was purchased from the Department of Pathobiology, University of Washington, Seattle. The anti-chlamydial genus-specific Hsp60 MAb A57-B9, isotype IgG1, was previously described by Yuan et al. (36). The anti-chlamydial genus-specific lipopolysaccharide (LPS) MAb EVIH1, isotype IgG2a, was generated in our laboratory. The anti-rickettsial MAb 8-13A4A10, isotype IgG2a, was generated and described by Anacker et al. (1). Genus-specific anti-MOMP polyclonal antiserum was generated in our laboratory against linear epitopes of C. psittaci Mn MOMP.
Human sera.
Sera were collected from donors seropositive for C. pneumoniae by the micro-IF test with antibody titers of 1:128 and higher, as well as from donors seronegative for C. pneumoniae by micro-IF.
Microscopy.
For transmission electron microscopy, C. pneumoniae-infected HeLa 229 cells were grown on Thermanox coverslips (Nunc Inc., Naperville, Ill.) and fixed with periodate-lysine-paraformaldehyde fixative (5) for 2 h at room temperature. The coverslips were then permeabilized with phosphate-buffered saline (PBS) containing 0.01% saponin and incubated for 1 h at room temperature with GZD1E8, EVIH1, or 8.13A4A10 MAb. The cultures were rinsed twice with PBS and then incubated for 1 h with horseradish peroxidase-conjugated F(ab′)2 sheep anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) in PBS containing 0.01% saponin. The coverslips were rinsed in PBS and fixed with 1.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.4, plus 5% sucrose for 1 h. They were then rinsed three times with 50 mM Tris-HCl, pH 7.4, containing 7.5% sucrose prior to development with Immunopure Metal Enhanced DAB substrate (Pierce Chemical Co., Rockford, Ill.). Application of high sucrose concentration in the buffers allows optimal conditions for antibody staining of the organisms. However, the high hypertonicity may affect the typical structure of C. pneumoniae elementary bodies (EBs) and reticulate bodies (RBs). After incubation in the DAB substrate, cells were rinsed three times with 50 mM Tris-HCl, pH 7.4, containing 7.5% sucrose and fixed in 4% paraformaldehyde–2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, at 4°C for 2 h. Cells were postfixed in 1.0% OsO4–0.8% K3Fe(CN)6 for 15 min, washed with 0.1 M sodium cacodylate buffer, dehydrated in a graded ethanol series, and embedded in Spurr's resin. Thin sections were cut with an RMC MT-7000 ultramicrotome (Ventana, Tucson, Ariz.), stained with 1% uranyl acetate and Reynold's lead citrate, and observed at 80 kV on an H-7500 transmission electron microscope (Hitachi, Tokyo, Japan). Images were obtained with AMT digital camera.
In vitro neutralization assay.
The complement-dependent neutralization assay described by Caldwell and Perry (7) and Peterson et al. (27) was used with the following modifications. A 0.1-ml volume of C. pneumoniae EBs containing 2 × 105 inclusion-forming units (IFU) was added to sucrose-phosphate-glutamic acid buffer containing 5% normal or heat-inactivated (56°C, 30 min) guinea pig sera (BioWhittaker, Walkersville, Md.) and serial 10-fold dilutions of the MAbs GZD1E8, A57-B9, or 8-13A4A10 in a final volume of 0.5 ml. The mixtures were incubated at 37°C for 45 min, and 0.2 ml per glass coverslip was inoculated onto confluent monolayers of HeLa cells by centrifugation at 900 × g for 1 h (22). The inoculum was removed, monolayers were washed, and the cells were supplemented with RPMI 1640 media containing 2 μg of cycloheximide/ml. The plates were incubated at 37°C in 5% CO2 for 72 h. The monolayers were fixed with methanol and stained by indirect immunofluorescence using a rabbit polyclonal antisera raised in our laboratory against C. pneumoniae AR-39 and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin serum (Zymed). The number of mature IFU per milliliter was counted. The neutralization assay was done in triplicate.
Immunoblot analysis.
Gradient-purified C. pneumoniae EBs (108 IFU) were solubilized in Laemmli sample buffer (23) and heated at 100°C for 5 min, and soluble material was electrophoresed on an SDS–12.5% polyacrylamide gel electrophoresis (PAGE) gel. After separation, proteins were transferred to Immobilon-P membrane (Millipore Corp., Bedford, Mass.) in phosphate buffer (25 mM NaPO4). C. pneumoniae MOMP was detected by probing with either RR-402, GZD1E8, or genus-specific anti-MOMP antibodies followed by alkaline phosphatase-conjugated goat anti-mouse or anti-rabbit IgG (Sigma, St. Louis, Mo.). Polypeptide bands were visualized by development with nitroblue tetrazolium–5-bromo-4-chloro-3-indolyphosphate (NBT-BCIP; Gibco BRL). Equal amounts of chlamydial protein and identical conditions were applied in all three immunoblotting reactions.
Intrinsic radiolabeling and immunoprecipitation.
HeLa 229 cells grown in six-well plates were infected with C. pneumoniae and incubated for 45 h. Infected and uninfected monolayers were washed two times with RPMI 1640 lacking methionine (Met) and cysteine (Cys) (ICN, Costa Mesa, Calif.) and were incubated in this medium for 3 h at 37°C. Cells were then labeled with 100 μCi of EXPRES 35S-labeled protein labeling mix (DuPont-New England Nuclear, Wilmington, Del.)/ml in RPMI 1640 medium lacking Met and Cys with cycloheximide (2 μg/ml) for an additional 20 h at 37°C. Radiolabeled cells were washed once with Hanks balanced salt solution and lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and protease inhibitors for 30 min at 4°C. The cell lysates were centrifuged at 12,000 × g for 2 min to remove insoluble material. Soluble lysates were precleared by incubation in a 50% slurry of protein A-Sepharose (Sigma) for 1 h at room temperature. The protein A-Sepharose slurry was then pelleted by centrifugation, and the supernatants were recovered. Precleared lysates were incubated for 1 h at room temperature with either GZD1E8, RR-402, 8-13A4A10, or human sera. A 50% slurry of protein A-Sepharose was added to the mixtures for an additional 1 h at room temperature, and the protein A-bound immunocomplexes were pelleted by centrifugation (30, 39). The pelleted material was washed three times in lysis buffer, resuspended in Laemmli sample buffer (23), heated at 100°C for 5 min, and centrifuged, and the soluble lysate material was electrophoresed on SDS–12.5% PAGE gels. Fixed and dried gels were visualized autoradiographically.
Protein identification.
Lysates of C. pneumoniae EBs (109 IFU) were prepared in lysis buffer and immunoprecipitated with MAb GZD1E8, MAb RR-402, or human sera as described above for the immunoprecipitation of radiolabeled organisms. Immunoprecipitated proteins were electrophoresed on SDS–12.5% PAGE gels and visualized with either Coomassie brilliant blue R250 or silver stain. A single protein band migrating at ∼40 kDa was excised from the gel and processed for analysis by mass spectrometry (MS) as follows. Briefly, the gel slice was destained twice, equilibrated with 50% acetonitrile in 25 mM ammonium bicarbonate (pH 8.0), washed once with 100% acetonitrile, and dried completely in a speed-vac under low heat and high vacuum. The dried gel slice was rehydrated with 25 μl of porcine trypsin (Promega, Madison, Wis.) at 20 μg/ml in 25 mM ammonium bicarbonate (pH 8.0) and incubated overnight at 37°C. The resulting peptides were then extracted from the gel by twice incubating the gel with 50 μl of 50% acetonitrile in 5% trifluoroacetic acid (TFA) for 1 h at room temperature, followed by a single incubation for 30 min in 100% acetonitrile. The extractions were combined and dried in a speed-vac. The peptides were rehydrated with 2 μl of 0.1% TFA. An MS sample plate was spotted with 0.5 μl of matrix consisting of 20 mg of alpha-cyano-4-hydroxy-cinnamic acid (Aldrich, Milwaukee, Wis.)/ml dissolved in a solution of 10-mg/ml nitrocellulose in 50% acetone–50% isopropyl alcohol. A 0.5-μl volume of the peptide solution was then spotted over the matrix, air dried, and washed twice with cold 0.1% TFA. The sample plate was loaded into a Voyager-DE STR mass spectrometer (PerSeptive Biosystems, Framington, Mass.) for collection of matrix-assisted laser desorption ionization–time of flight (MS) spectra. The instrument was operated at 20 kV using delayed extraction in positive/reflector mode, with 250 scans averaged per spectrum (26). The resulting peak list was submitted for a search of the National Center for Biotechnology Information (NCBI) database using the MS-Fit section from the Protein Prospector MS analysis package developed at the University of California, San Francisco.
RESULTS
MAb GZD1E8 is C. pneumoniae specific.
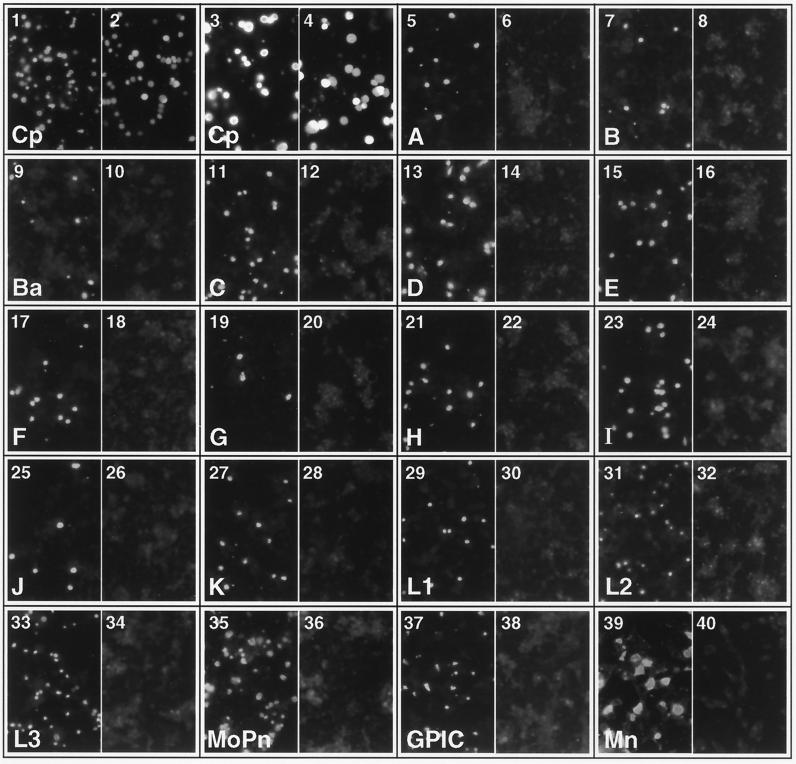
HeLa cells infected with C. pneumoniae AR-39, C. trachomatis (serovars A, B, Ba, C, D, E, F, G, H, I, J, K, L1, L2, L3, and MoPn), and C. psittaci strains Mn (Cal 10) and GPIC were stained by immunofluorescence assay (IFA) using MAb GZD1E8 (Fig. 1) to define the specificity of the antibody. Chlamydiae-infected cells were also stained by IFA using the anti-LPS genus-specific MAb EVIH1 as a positive control for immunoreactivity. Staining of chlamydial inclusions with the anti-LPS MAb is shown in the odd-number photomicrographs in Fig. 1. As shown, all chlamydial strains exhibited strong immunoreactivity following staining with the anti-LPS MAb. In contrast, when chlamydiae-infected cells were stained with the MAb GZD1E8 generated against C. pneumoniae (even-number panels), only C. pneumoniae inclusions were immunoreactive (plates 2 and 4). MAb GZD1E8 was also reactive with inclusions of C. pneumoniae strain TW-183 (data not shown). These findings demonstrate that MAb GZD1E8 is C. pneumoniae specific. Thus, MAb GZD1E8, as defined by its reactivity with chlamydial organisms, has a specificity identical to that of the C. pneumoniae species-specific MAb RR-402 described by Puolakkainen et al. (28).
FIG. 1.
MAb GZD1E8 is C. pneumoniae species specific. HeLa cells were infected with different chlamydial strains or serovars, and inclusions were stained by immunofluorescence with either MAb GZD1E8 or EVIH1. Shown are stainings of the following: C. pneumoniae AR-39 (1, 2, 3, 4,); C. trachomatis serovars A (5, 6), B (7, 8), Ba (9, 10), C (11, 12), D (13, 14), E (15, 16), F (17, 18), G (19, 20), H (21, 22), I (23, 24), J (25, 26), K (27, 28), L1 (29, 30), L2 (31, 32), L3 (33, 34), and MoPn (35, 36); and C. psittaci GPIC (37, 38) and Mn (39, 40). All odd-number panels are immunofluorescent stainings with anti-chlamydial LPS MAb EVIH1. All even-number panels are stainings with MAb GZD1E8. Only C. pneumoniae inclusions showed positive IFA staining with Mab GZD1E8 (2, 4). Magnifications, ×180 (1, 2, 5 to 40) and ×360 (3 and 4).
Epitope recognized by MAb GZD1E8 is surface exposed.
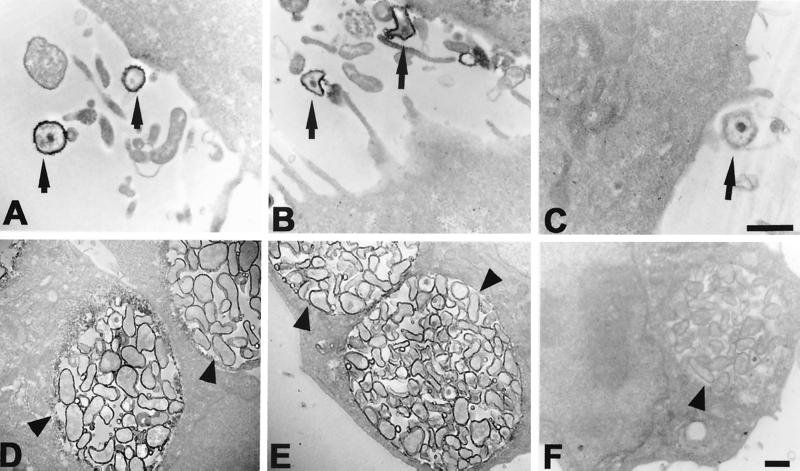
To localize the C. pneumoniae antigen recognized by the GZD1E8 MAb, HeLa cells were infected with C. pneumoniae, stained with GZD1E8 antibody, and processed for transmission electron microscopy (Fig. 2). Staining of C. pneumoniae EBs obtained 2 h postinfection (Fig. 2A) and C. pneumoniae RBs within an inclusion obtained 36 h postinfection (Fig. 2D) with the GZD1E8 MAb indicated surface localization of the antigenic determinant. MAbs EVIH1 and 8-13A4A10 were used as positive and negative controls, respectively. The genus-specific EVIH1 MAb that recognizes an antigenically conserved epitope located on chlamydial LPS strongly stained the outer membrane (OM) of both C. pneumoniae EBs and RBs (Fig. 2B and E). The anti-rickettsial negative control MAb 8-13A4A10 did not react with C. pneumoniae (Fig. 2C and F). These results demonstrate that the species-specific epitope recognized by MAb GZD1E8 is localized to the chlamydial OM and suggests that it is surface exposed.
FIG. 2.
Transmission electron microscopy of C. pneumoniae EBs (A) and RBs (D) stained with the GZD1E8 MAb indicate surface localization of the antigenic determinant. Surface staining of C. pneumoniae EBs (B) and RBs (E) is also shown with an anti-chlamydial LPS MAb, EVIH1. No staining of C. pneumoniae EBs (C) and RBs (F) was observed with anti-rickettsial MAb 8-13A4A10. Arrowheads indicate inclusions and arrows indicate EBs of C. pneumoniae. Bars = 0.5 μm.
MAb GZD1E8 neutralizes C. pneumoniae infectivity.
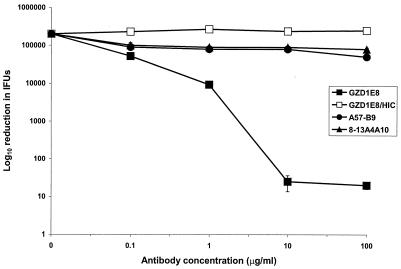
To more definitively characterize the surface exposure of the epitope, we conducted in vitro neutralization assays with MAb GZD1E8. This assay uses viable chlamydial organisms; therefore, specific neutralization of infectivity by antibody clearly defines the antigenic determinant as being surface exposed on native organisms. We found MAb GZD1E8 to be a potent neutralizing antibody (Fig. 3). A 10,000-fold reduction in C. pneumoniae infectivity for HeLa cells was found using concentrations of 10 and 100 μg/ml of MAb GZD1E8. This neutralization was complement dependent and specific since no significant neutralization was observed using two negative controls, the chlamydial anti-HSP60 MAb A57-B9 or anti-rickettsial MAb 8-13A4A10. Similar findings have been reported using the C. pneumoniae-specific MAb RR-402 (28). Together, these results clearly demonstrate that the epitope recognized by MAb GZD1E8 is surface exposed on viable C. pneumoniae EBs.
FIG. 3.
Neutralization of C. pneumoniae infectivity with MAb GZD1E8. In the presence of complement, MAb GZD1E8 resulted in a 10,000-fold reduction of IFU at concentrations of 10 and 100 μg/ml. Incubation of C. pneumoniae with the GZD1E8 MAb in the presence of heat-inactivated guinea pig sera (HIC) did not result in a reduction of IFU. This neutralization assay was additionally performed with anti-chlamydial Hsp60 MAb A57-B9 and anti-rickettsial MAb 8-13A4A10. Most of the standard error bars are contained within the symbols.
MAbs GZD1E8 and RR-402 recognize MOMP.
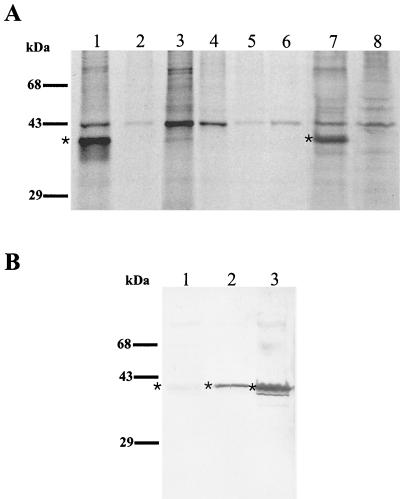
We used immunoprecipitations of intrinsically radiolabeled C. pneumoniae proteins to identify the antigen(s) recognized by the GZD1E8 and RR-402 MAbs. Moreover, the immunoprecipitation assay used here allows detection of both linear and conformational protein antigenic determinants (39). Assays were performed using lysates of [35S]-cysteine- and [35S]methionine-labeled C. pneumoniae-infected and uninfected HeLa cells; therefore, antigens subjected to the immunoassay are representative of EBs, RBs, and non-organism-associated secreted protein antigens. The results of a radioimmunoprecipitation assay using MAbs GZD1E8 and RR-402 are shown in Fig. 4. Both MAbs GZD1E8 and RR-402 were found to specifically immunoprecipitate a polypeptide with a molecular mass of approximately 40 kDa (Fig. 4A, lanes 1 and 7, respectively). The immunoprecipitated 40-kDa polypeptide was specific to C. pneumoniae since the GZD1E8 MAb did not precipitate an antigen with a similar mass from 35S-labeled lysates prepared from C. psittaci GPIC- and C. trachomatis L2-infected cells (lanes 5 and 6, respectively). The antibody specificity for both MAbs was further demonstrated by immunoprecipitation of 35S-labeled C. pneumoniae-infected and uninfected HeLa cells with the anti-rickettsial MAb, 8-13A4A10, which did not immunoprecipitate the 40-kDa antigen (Fig. 4A, lanes 3 and 4). The 40-kDa immunoprecipitated polypeptide corresponds to the predicted mass of the C. pneumoniae MOMP; however, this alone is insufficient evidence to conclude that the MOMP is the protein recognized by each of the species-specific MAbs.
FIG. 4.
(A) Immunoprecipitation of intrinsically 35S-labeled C. pneumoniae antigen by C. pneumoniae MAbs. Autoradiographs show immunoprecipitation results of 35S-labeled C. pneumoniae-infected (lane 1) and uninfected (lane 2) HeLa cells with the GZD1E8 MAb; 35S-labeled C. pneumoniae-infected (lane 3) and uninfected (lane 4) HeLa cells immunoprecipitated with anti-rickettsial MAb 8-13A4A10; 35S-labeled C. psittaci GPIC-infected (lane 5) and C. trachomatis L2-infected (lane 6) HeLa cells immunoprecipitated with MAb GZD1E8; and 35S-labeled C. pneumoniae-infected (lane 7) and uninfected (lane 8) HeLa cells immunoprecipitated with MAb RR-402. ∗, the ∼40-kDa protein in C. pneumoniae-infected HeLa cells detected with MAbs GZD1E8 and RR-402 (lanes 1 and 7). (B) Detection of a ∼40-kDa polypeptide with MAbs GZD1E8 and RR-402 by immunoblotting. The 40-kDa polypeptide was not detected with the RR-402 MAb (lane 1) and was weakly detected with the GZD1E8 MAb (lane 2). A very strong reaction was detected with chlamydial, genus-specific, anti-MOMP antibody (lane 3). ∗, the ∼40-kDa protein.
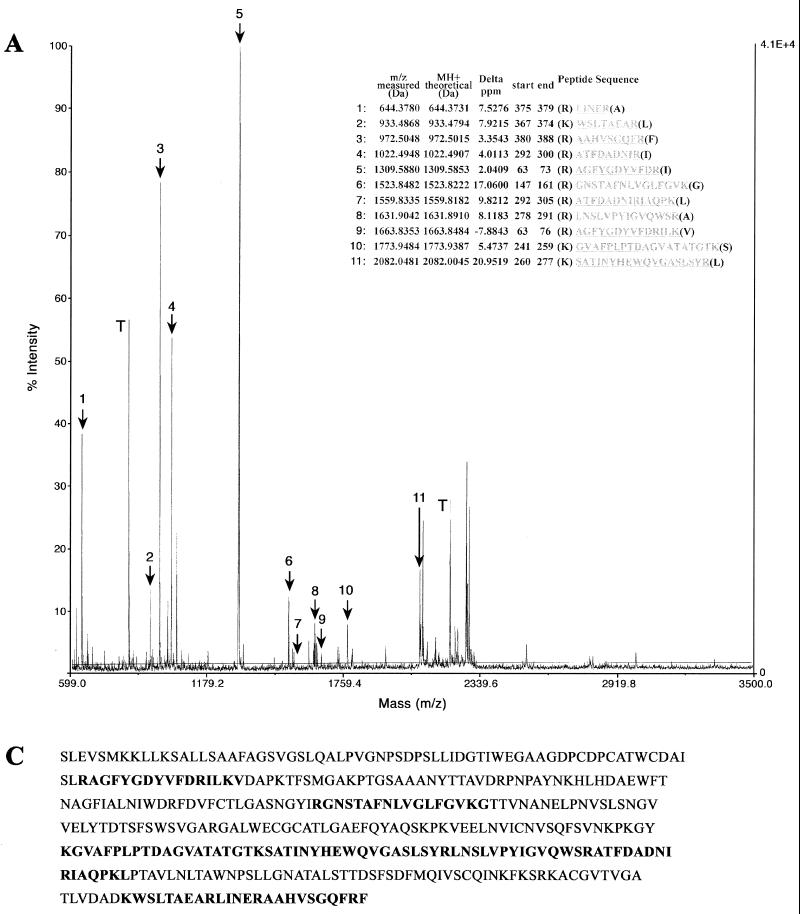
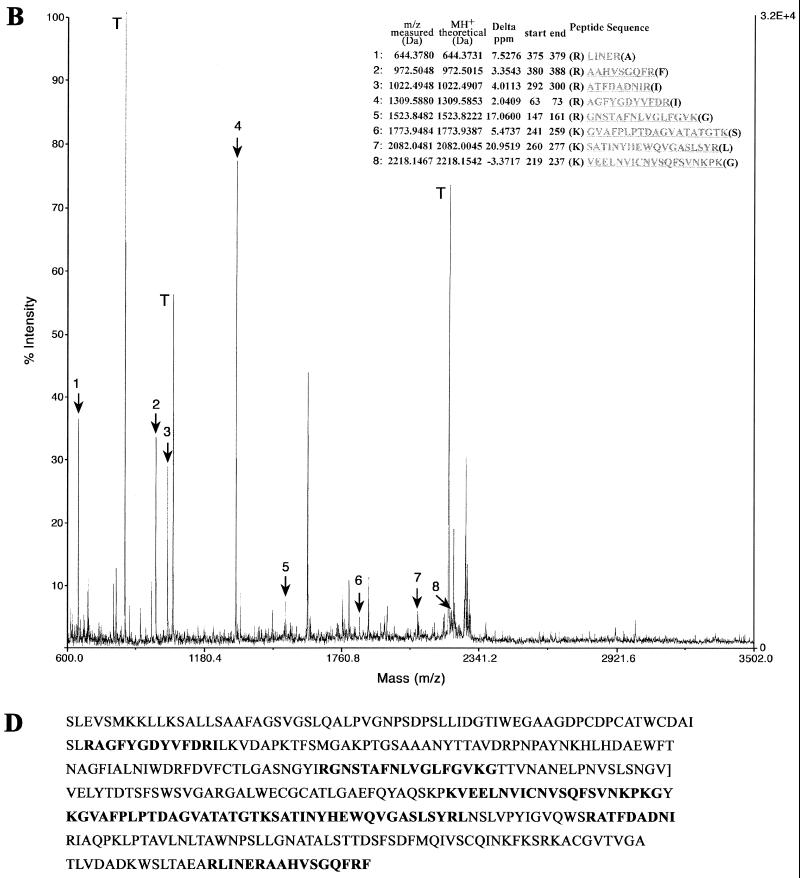
To conclusively identify the 40-kDa antigen recognized by MAbs GZD1E8 and RR-402, we have directly analyzed the immunoprecipitated polypeptide by MS. To obtain sufficient quantities of antigen for sequence analysis, immunoprecipitation assays were done using lysates prepared from gradient-purified (109 IFU) C. pneumoniae EBs. Coomassie brilliant blue- or silver-stained bands were excised, destained, dehydrated, and subjected to trypsinolysis, and the resulting fragmented peptides were analyzed by MS. The resulting peptide list was submitted to a search of the NCBI database using the MS-Fit section of the Protein Prospector MS analysis package. The parameters of the search were set to allow for one missed cleavage site with a mass accuracy of 25 ppm. The search results clearly identified the 40-kDa antigen precipitated by MAbs GZD1E8 and RR-402 as the 41.6-kDa MOMP of C. pneumoniae (Fig. 5).
FIG. 5.
MS-Fit search of peptide monoisotopic mass spectra identified MOMP of C. pneumoniae. Proteins obtained by immunoprecipitation of purified C. pneumoniae EBs with the GZD1E8 (A) and RR-402 (B) MAbs were processed by MS. Masses indicated that fragments 1 to 11 (A) and 1 to 8 (B) matched known MOMP sequence fragments. Trypsin (T) autodigestion peptides were used for internal calibration of the spectrum prior to peptide mass assignment for MS-Fit searching of the NCBI database. The MOMP sequence aligned with the matching peptide masses identified with MAbs GZD1E8 (C) and RR-402 (D).
MAbs GZD1E8 and RR-402 reacted either very weakly (GZD1E8) or were nonreactive (RR-402) with the MOMP by immunoblotting (Fig. 4B), indicating that MAbs GZD1E8 and RR-402 do not recognize an identical epitope. Taken together, these findings strongly support the conclusion that the species-specific MAbs GZD1E8 and RR-402 recognize C. pneumoniae MOMP epitopes in their native conformation.
C. pneumoniae micro-IF-positive human sera immunoprecipitate MOMP.
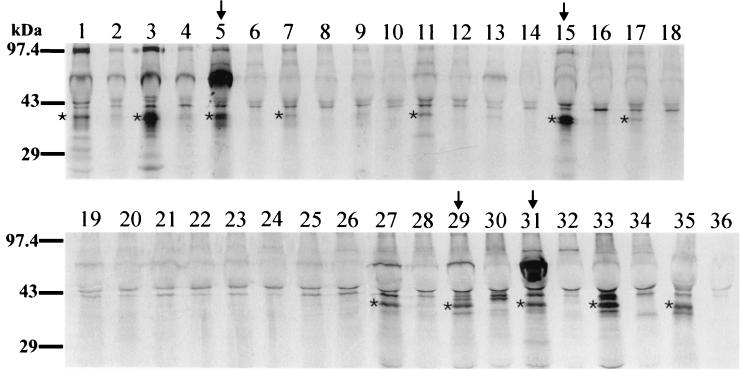
Immunoprecipitation of 35S-labeled C. pneumoniae-infected and uninfected HeLa cells with sera collected from individuals with C. pneumoniae-specific antibody titers of 1:128 or greater was detected by micro-IF. All C. pneumoniae-positive sera (Fig. 6, lanes 1, 3, 5, 15, 27, 29, 31, 33, and 35) immunoprecipitated a ∼40-kDa polypeptide. In some samples, the polypeptide was observed to migrate as a doublet. Several strongly positive samples detected in immunoprecipitation with radiolabeled C. pneumoniae cells were used for MS analysis (Fig. 6, lanes 5, 15, 29, and 31). MS analysis of some of the single 40-kDa bands, as well as some of the double bands obtained by immunoprecipitation with unlabeled C. pneumoniae EBs, identified the 41.6-kDa MOMP of C. pneumoniae (data not shown). Immunoprecipitation of 35S-labeled C. pneumoniae-infected and uninfected HeLa cells with micro-IF-seronegative sera showed very weak reactivity with a 40-kDa polypeptide with some sera; however, the majority of control sera did not immunoprecipitate a 40-kDa polypeptide (Fig. 6).
FIG. 6.
Immunoprecipitation of intrinsically 35S-labeled C. pneumoniae antigen by sera from C. pneumoniae-infected and uninfected individuals. Autoradiographs show immunoprecipitations of 35S-labeled C. pneumoniae-infected and uninfected HeLa cells with human sera from seropositive and seronegative donors. Odd-number lanes contained C. pneumoniae-infected HeLa cells; even-number lanes contained uninfected HeLa cells. Positive samples by immunoprecipitation are shown in lanes 1, 3, 5, 15, 27, 29, 31, 33, and 35, which correspond to seropositive samples, as detected by micro-IF. Micro-IF-seronegative sera were either weakly positive by immunoprecipitation (lanes 7, 11, and 17) or negative (lanes 9, 13, 19, 21, 23, and 25). Arrows indicate the sera used in immunoprecipitation assays with unlabeled C. pneumoniae EBs for protein sequencing. ∗, a ∼40-kDa protein in C. pneumoniae-infected HeLa cells detected with seropositive samples and with a few seronegative samples.
Sera that specifically immunoprecipitated MOMP were analyzed by Western blotting and were found to be nonreactive or weakly reactive with MOMP (data not shown), indicating that the predominant antibody response generated following infection with C. pneumoniae is directed to conformationally dependent antigenic determinants.
DISCUSSION
The ompA gene of C. trachomatis encodes the MOMP (31, 32). The ompA's are highly conserved genes among species of Chlamydia (29). The protein makes up approximately 60% of the organism's OM mass (6) and exists as a large oligomeric structure on the parasite's surface stabilized by disulfide bonds (25). The C. trachomatis MOMP is immunodominant and represents the primary serotyping antigen of C. trachomatis isolates (8, 37, 40, 41). MAbs specific to the MOMP recognize both linear and conformational epitopes (39). Linear epitopes map to four regions of sequence variation, termed variable domains, that are interdispersed within larger constant segments of the molecule (2, 32). MOMP conformational epitopes have not been mapped but are believed to be composed of amino acid residues that are distal to one another in the protein's primary sequence, perhaps within the surface-accessible variable domains, that are closely juxtaposed in the protein's tertiary structure (38). In contrast, studies of the C. pneumoniae antigenic structure have produced a portrayal of the MOMP surface exposure and immunogenicity that are strikingly different than the MOMP of C. trachomatis. In fact, the current thinking about C. pneumoniae MOMP is that it is not surface accessible, it is immunorecessive, and it is not the predominant serotyping antigen of the organism (10, 11, 13). This paradox in MOMP properties among chlamydial species seems rather dubious because of the striking sequential and structural similarities between the ompA genes of C. trachomatis and C. pneumoniae (31). This homology argues, at least hypothetically, for a common rather than a diverse functional and antigenic relationship between the MOMPs of these two chlamydial species. We therefore undertook studies to investigate the immunogenic and topological characteristics of the C. pneumoniae MOMP in an attempt to unravel and perhaps portray a more accurate description of these important properties of the protein.
The majority of research focused on defining immunogenic and serotyping antigens of C. pneumoniae has been based primarily on Western blotting. These reports produced descriptions of numerous immunoreactive antigens of various mass; however, none of the antigens described were consistently recognized by hyperimmune or convalescent sera that exhibited species-specific antigenic properties. Puolakkainen et al. described a C. pneumoniae species-specific MAb, RR-402, that reacted with the surface of C. pneumoniae and neutralized infectivity in vitro (28). Because the species-specific antigen would be potentially useful in diagnostics and vaccine development, considerable effort has gone into identifying its molecular nature. The same investigators attempted to immunoprecipitate the antigen from solubilized, radiolabeled C. pneumoniae cells, without success. However, purified metabolically labeled EBs were extracted first with 2% Triton X-100 and then sequentially with 0.2 and 0.5% SDS (28), a treatment that could have destroyed the antigen and/or antibody.
More recently, Essig et al. described the immunoprecipitation of a 40-kDa polypeptide from lysates of intrinsically 35S-labeled C. pneumoniae with MAb RR-402. In fact, these investigators proposed that the immunoreactive protein was MOMP; however, this conclusion was based solely on the rate of migration in SDS-polyacrylamide gels. The same investigators also reported the immunoprecipitation of a 40-kDa polypeptide with an acute patient's sera, but again, the criteria used to identify the polypeptide as MOMP were based on the relative migration in gels (14).
In this report, we describe and characterize the C. pneumoniae-reactive MAb GZD1E8. We show that this MAb is species specific (Fig. 1), is reactive with the OM of both C. pneumoniae EBs and RBs (Fig. 2), and is a potent neutralizing antibody (Fig. 3). Thus, by these criteria, the antigen recognized by MAb GZD1E8 can be characterized as C. pneumoniae species specific and exposed on the native surface of intact organisms. Moreover, MAb GZD1E8 has properties that are very similar to those of MAb RR-402 described by Puolakkainen et al., who described the epitope recognized by this antibody as surface exposed and species specific (28). We next performed immunoprecipitation with both of these MAbs by using lysates of intrinsically radiolabeled chlamydial organisms obtained from infected HeLa 229 cells. Our findings clearly show that both GZD1E8 and RR-402 specifically precipitate a 40-kDa antigen present only in lysates prepared from C. pneumoniae-infected cells (Fig. 4). Most importantly, we sequenced the 40-kDa polypeptide immunoprecipitated by both MAbs and definitively showed that the protein was the 41.6-kDa MOMP of C. pneumoniae (Fig. 5). These findings provide unambiguous and indisputable evidence that MAb GZD1E8 and MAb RR-402 are specific to C. pneumoniae MOMP. We believe that the epitope(s) recognized by MAbs GZD1E8 and RR-402 are conformational, which may explain in part why the antigen's identification by Western blot analysis, a method known to destroy protein conformation, has remained elusive. We and others (14) were successful in identifying the MOMP by immunoprecipitation because we utilized a mixture of detergents that enabled partial solubilization of the MOMP without significant denaturation, thereby maintaining critical conformation-dependent antigenic determinants. This was not the situation in the studies by Puolakkainen et al., who utilized only SDS in the final lysate buffer (28).
We have also shown that sera from donors with C. pneumoniae-specific antibodies detected by micro-IF immunoprecipitate the MOMP (Fig. 6), which was again confirmed by direct MS analysis of the protein excised from SDS gels. These sera reacted poorly or not at all with the MOMP by Western blotting (data not shown). These findings suggest that the MOMP is an immunogenic protein recognized during C. pneumoniae infection and indicate that the primary antibody response is directed against conformational MOMP epitopes.
We believe our data will have important implications for the development of new diagnostic tests for C. pneumoniae infection and perhaps in the development of an efficacious vaccine against the intracellular parasite. It should now be possible to identify the species-specific antigenic determinant(s) of C. pneumoniae MOMP using phage display methodologies, since this approach has been useful in the identification of conformational epitopes for the proteins of hepatitis B virus and human immunodeficiency virus, in addition to conformation-dependent domains of several ligand receptor molecules (18, 24, 42). Once C. pneumoniae species-specific epitopes are identified, it will be possible to produce synthetic surrogate antigens that mimic these conformational immunogenic determinants that can be used to develop sensitive user-friendly diagnostic assays for the detection of serum antibodies and as possible synthetic antigens for the generation of protective neutralizing antibodies.
ACKNOWLEDGMENTS
We thank T. Hackstadt, M. Scidmore, R. Carabeo, D. Clifton, and K. Fields for critical review of the manuscript.
REFERENCES
- 1.Anacker R L, Mann R E, Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987;25:167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehr W, Zhang Y X, Joseph T, Su H, Nano F E, Everett K D, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black C M, Johnson J E, Farshy C E, Brown T M, Berdal B P. Antigenic variation among strains of Chlamydia pneumoniae. J Clin Microbiol. 1991;29:1312–1316. doi: 10.1128/jcm.29.7.1312-1316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boman J, Gaydos C A, Quinn T C. Molecular diagnosis of Chlamydia pneumoniae infection. J Clin Microbiol. 1999;37:3791–3799. doi: 10.1128/jcm.37.12.3791-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown W J, Farquhar M G. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 1989;31:553–569. doi: 10.1016/s0091-679x(08)61626-x. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell H D, Perry L J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982;38:745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell H D, Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982;35:1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell L A, Kuo C-C, Grayston J T. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–579. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell L A, Kuo C C, Grayston J T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990;58:93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell L A, Kuo C C, Wang S P, Grayston J T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990;28:1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi E Y, Kuo C-C, Grayston J T. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987;169:3757–3763. doi: 10.1128/jb.169.8.3757-3763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christiansen G, Boesen T, Hjerno K, Daugaard L, Mygind P, Madsen A S, Knudsen K, Falk E, Birkelund S. Molecular biology of Chlamydia pneumoniae surface proteins and their role in immunopathogenicity. Am Heart J. 1999;138(Pt. 2):S491–S495. doi: 10.1016/s0002-8703(99)70283-8. [DOI] [PubMed] [Google Scholar]
- 14.Essig A, Simnacher U, Susa M, Marre R. Analysis of the humoral immune response to Chlamydia pneumoniae by immunoblotting and immunoprecipitation. Clin Diagn Lab Immunol. 1999;6:819–825. doi: 10.1128/cdli.6.6.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grayston J T. Infections caused by Chlamydia pneumoniae strain TWAR. Clin Infect Dis. 1992;15:757–761. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- 16.Iijima Y, Miyashita N, Kishimoto T, Kanamoto Y, Soejima R, Matsumoto A. Characterization of Chlamydia pneumoniae species-specific proteins immunodominant in humans. J Clin Microbiol. 1994;32:583–588. doi: 10.1128/jcm.32.3.583-588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jantos C A, Heck S, Roggendorf R, Sen-Gupta M, Hegemann J H. Antigenic and molecular analyses of different Chlamydia pneumoniae strains. J Clin Microbiol. 1997;35:620–623. doi: 10.1128/jcm.35.3.620-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen A, Jensen T H, Kjems J. HIV-1 rev nuclear export signal binding peptides isolated by phage display. J Mol Biol. 1998;283:245–254. doi: 10.1006/jmbi.1998.2085. [DOI] [PubMed] [Google Scholar]
- 19.Kanamoto Y, Iijima Y, Miyashita N, Matsumoto A, Sakano T. Antigenic characterization of Chlamydia pneumoniae isolated in Hiroshima, Japan. Microbiol Immunol. 1993;37:495–498. doi: 10.1111/j.1348-0421.1993.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen K, Madsen A S, Mygind P, Christiansen G, Birkelund S. Identification of two novel genes encoding 97- to 99-kilodalton outer membrane proteins of Chlamydia pneumoniae. Infect Immun. 1999;67:375–383. doi: 10.1128/iai.67.1.375-383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae. Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo C-C, Grayston J T. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988;26:812–815. doi: 10.1128/jcm.26.5.812-815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Motti C, Nuzzo M, Meola A, Galfre G, Felici F, Cortese R, Nicosia A, Monaci P. Recognition by human sera and immunogenicity of HBsAg mimotopes selected from an M13 phage display library. Gene. 1994;146:191–198. doi: 10.1016/0378-1119(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 25.Newhall W J, Jones R B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983;154:998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker K C, Garrels J I, Hines W, Butler E M, McKee A H, Patterson D, Martin S. Identification of yeast proteins from two-dimensional gels: working out spot cross-contamination. Electrophoresis. 1998;19:1920–1932. doi: 10.1002/elps.1150191110. [DOI] [PubMed] [Google Scholar]
- 27.Peterson E M, Zhong G M, Carlson E, de la Maza L M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puolakkainen M, Parker J, Kuo C C, Grayston J T, Campbell L A. Further characterization of Chlamydia pneumoniae specific monoclonal antibodies. Microbiol Immunol. 1995;39:551–554. doi: 10.1111/j.1348-0421.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 29.Read T D, Brunham R C, Shen C, Gill S R, Heidelberg J F, White O, Hickey E K, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg S L, Eisen J, Fraser C M. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scidmore M A, Fischer E R, Hackstadt T. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol. 1996;134:363–374. doi: 10.1083/jcb.134.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 32.Stephens R S, Sanchez-Pescador R, Wagar E A, Inouye C, Urdea M S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagels G, Rasmussen S, Timms P. Comparison of Chlamydia pneumoniae isolates by Western blot (immunoblot) analysis and DNA sequencing of the omp 2 gene. J Clin Microbiol. 1994;32:2820–2823. doi: 10.1128/jcm.32.11.2820-2823.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S. The microimmunofluorescence test for Chlamydia pneumoniae infection: technique and interpretation. J Infect Dis. 2000;181(Suppl. 3):S421–S425. doi: 10.1086/315622. [DOI] [PubMed] [Google Scholar]
- 35.Wolf K, Fischer E, Hackstadt T. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect Immun. 2000;68:2379–2385. doi: 10.1128/iai.68.4.2379-2385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Lyng K, Zhang Y X, Rockey D D, Morrison R P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan Y, Zhang Y-X, Watkins N G, Caldwell H D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y X, Morrison S G, Caldwell H D, Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989;57:1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y X, Stewart S, Joseph T, Taylor H R, Caldwell H D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 40.Zhang Y X, Stewart S J, Caldwell H D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y X, Watkins N G, Stewart S, Caldwell H D. The low-molecular-mass, cysteine-rich outer membrane protein of Chlamydia trachomatis possesses both biovar- and species-specific epitopes. Infect Immun. 1987;55:2570–2573. doi: 10.1128/iai.55.11.2570-2573.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong G, Smith G P, Berry J, Brunham R C. Conformational mimicry of a chlamydial neutralization epitope on filamentous phage. J Biol Chem. 1994;269:24183–24188. [PubMed] [Google Scholar]