Abstract
Aims
Sewage treatment effluent with pharmaceutical residues is discharged into surface waters, raising societal concerns. The aim of this paper is to describe the Dutch chain approach on medicinal residues in water that has been implemented by the Dutch government. We show how stakeholders from both the health and water sectors have got actively involved. Within this chain approach, source measures as well as end‐of‐pipe measures are identified and, where feasible and effective, implemented.
Methods
Descriptive paper on the Dutch chain approach.
Results
Getting the water and health care sectors to talk with each other instead of about each other was the key accomplishment. Comprehension of each other's viewpoints, terminology, policy goals and span of control, was pivotal in setting shared goals, creating perspective about possible measures and actually taking (joint) action. Together, stakeholders agreed to act within their own possibilities, without pointing at others, and to focus on pragmatic measures. In this article, we provide examples of measures taken, pilot projects performed, and of measures that were not implemented. Besides this, we discuss the most important barriers encountered during this process and how they were overcome.
Conclusion
The issue of pharmaceuticals in the environment is a wicked problem, which makes it necessary to work together with many stakeholders on possible solutions, avoiding paralysis by complexity. Most importantly, stakeholders need to invest in mutual understanding, keep an open communication, and feel invited to bring in solutions for their part of the chain.
Keywords: pharmaceuticals, policy formulation, stakeholder approach, sustainability, water quality
What is already known about this subject
Pharmaceuticals enter the water system after excretion and (incomplete) sewage treatment and may affect environmental organisms.
The Dutch chain approach was implemented as a cross‐sectoral approach to deal with this issue, together with stakeholders from the water and healthcare sector.
What this study adds
The issue of pharmaceuticals in water is a so‐called wicked problem with many trade‐offs.
There is no single best solution.
Approach to get the whole value chain involved: communicate openly, take all stakeholders seriously, invite them to bring in solutions for their part of the chain.
1. INTRODUCTION
1.1. General
When patients use pharmaceuticals, residues of pharmaceuticals and their metabolites end up in the sewerage system via the toilet or wastewater. Pharmaceutical residues are generally not fully removed in sewage treatment plants (STPs) and are discharged into surface waters. They have been frequently detected in surface waters worldwide. 1 , 2 Even at low concentrations their presence potentially impacts aquatic ecosystems and drinking water resources. Following chronic exposure, environmental risks to wildlife are due to subtle effects that can impact individual fitness and population health. Examples include histopathological changes to tissues, feminisation of male fish and behavioural changes in both fish and aquatic invertebrates. 3 , 4 , 5 , 6 , 7 Besides this, the presence of pharmaceutical residues in sewage sludge used for fertilization and treated wastewater used for irrigation may cause uptake of these residues by crops. 8 The environment is vitally important to human health, as reflected in the One Health approach, 9 which underpins the UN Sustainable Development Goals of Life under Water, Life on Land, and Clean Water and Sanitation. The current paper provides insight in the Dutch national strategy to address the issue of pharmaceuticals in the environment, which succeeded in getting a wide variety of stakeholders on board.
The presence of pharmaceutical residues in surface water, groundwater and (sources of) drinking water is raising societal concerns world‐wide. Within the EU Water Framework Directive (EU Directive 2000/60/EC), some pharmaceuticals were flagged as being potentially priority substances for water quality. 10 The European Commission commissioned a report on the scale of the problem in 2013, 11 and has published a strategy on pharmaceuticals in the environment in 2019. 12 Within the United Nations Environment Programme pharmaceuticals have been adopted as an emerging policy issue in the SAICM (Strategic Approach to International Chemicals Management) context. 13 More recently, a publication by the World Bank suggested pharmaceutical residues to be “a prescription for disaster” for water supplies 14 and an Organisation for Economic Co‐operation and Development publication called for policy actions to prevent and remedy these emerging concerns. 6
In the Netherlands, a number of pharmaceuticals in surface water exceed environmental risk limits and effects are shown in and near STP discharges, 15 , 16 , 17 causing societal concerns and a demand for measures by a variety of stakeholders and the Dutch parliament. Therefore, the Dutch government, together with many stakeholders from the health and water sectors, has developed a so‐called chain approach to reduce the emission of pharmaceuticals into surface waters. Within this chain approach, the actors in the chain worked together to identify measures throughout the whole chain and, where feasible and effective, worked on their implementation. In this paper, the history of the chain approach and the process leading to the implementation of measures is described.
2. THE DUTCH CHAIN APPROACH: PHARMACEUTICAL RESIDUES OUT OF WATER
2.1. History of the topic before 2016
In the Netherlands, the issue of pharmaceuticals in the aquatic environment (including antibiotic resistance) was identified in the 1980s, with a focus on wastewater management. 18 In the 1990s the topic of pharmaceuticals in water received renewed attention. 19 , 20 After that, in 2001, the Dutch Health Council (Gezondheidsraad) asked for more attention to be paid to the subject of pharmaceuticals in water. 21 After a study showed feminization of fish close to a STP in Eindhoven, 15 a Dutch report was published with the title “Prevention is better than the cure”. 22 This led to the start of a working group with members from the ministries of environment, health, and a number of governmental institutes. This working group established an action programme and some research projects which identified emission routes of pharmaceuticals. In the following years, measures were identified but never implemented, due to a lack of urgency and reluctancy of stakeholders that would have to bear the costs.
In parallel, in the European water quality arena the topic of pharmaceuticals was not a priority issue. Nutrients, industrial chemicals and plant protection products were seen as more important. In 2011, this changed: during a screening of thousands of substances to determine new priority substances within the Water Framework Directive, 3 pharmaceutical substances ended high up on the list (diclofenac, 17‐β‐oestradiol, 17‐α‐ethinyloestradiol). This led to discussion within the water sector: if indeed these substances were to be put on the priority substances list, water authorities would be obliged to reduce the concentrations in surface waters. This would mean expensive additional treatment at STPs. As a result of the negotiations that followed, specifically for these pharmaceuticals, a watch list was created in 2015, 23 including the above pharmaceuticals and the antibiotics erythromycin, clarithromycin and azithromycin. Substances on the watch list have to be monitored by all EU Member States, but without an Environmental Quality Standard and without obligations regarding measures. However, not only was the watch list created, but at the same time it was also stated that the European Commission should come with a strategy on how to reduce emissions of pharmaceuticals (Article 8c of the Priority Substances Directive [2008/105/EC5 as amended by Directive 2013/39/EU6]).
The success of the lobby keeping pharmaceuticals from the list of priority substances, was less positive for the Dutch drinking water companies, who feared the possible costs of extra treatment for their own sector. In November 2014, the drinking water sector together with the regional water authorities (responsible for sewage treatment) sent a letter to the minister responsible for water affairs, urging her to take action at the source, implying the health care sector. Around the same time, the Dutch parliament organised a round table meeting, urging action. However, specific source control measures for the substances on the watchlist were not possible: environmental legislation and marketing authorisation legislation are not coupled and thus a risk observed in the environment does not affect marketing authorisation nor does it imply mandatory risk mitigation measures. 24 Moreover, for these specific substances source control measures were not feasible, due to ethical and medical constraints (birth control pill) and antibiotic resistance‐based treatment guidance (antibiotics). It became clear that pharmaceuticals other than those on the watchlist pose a risk to the aquatic ecosystem, with risks unknown for many more. 16 Thus, policy was directed towards a national general approach to decrease the total load of pharmaceutical residues entering the water cycle and not only towards those on the watchlist.
At both a national and European level, the pharmaceutical sector gradually became more pro‐active and presented a proposal to better streamline the regulatory environmental risk assessment. 25 Led by their European branch organisations, the Dutch pharmaceutical industry organizations also became more pro‐active in the national approach.
As a result of these processes, the Dutch Ministry of Infrastructure and Water Management (IenW) took the lead, and in the beginning of 2016 the chain approach on pharmaceuticals out of water was started.
2.2. Need for action
Before taking action combined with a need for (financial) resources, the water sector asked for objective facts on the scale of the issue. Therefore, IenW let the National Institute for Public Health and the Environment report on the facts and the implications for evidence‐based policy making. In this report, the available monitoring data for the Dutch waters were compared to concentrations that were considered safe for aquatic systems, the predicted no‐effect concentrations 16 (updated in 2020 17 ). It showed that the presence of pharmaceuticals was widespread and that for some pharmaceuticals risks for the water system were apparent. It also made clear that for many pharmaceuticals, data were lacking. From the 2000 active ingredients on the Dutch market, only around 80 were monitored on a regular or project basis. This report set the scene about the need for action. Despite the identified lack of knowledge for most pharmaceuticals, the explicit choice was made not to wait until more research was performed on the topic, since the data available already showed risks. More research and monitoring would only underpin the necessity of action and due to the large amount of pharmaceutical substances, uncertainties will always exist. 16 , 17 Thus, it was decided that time and resources should be used for the development of measures.
2.3. The chain approach: First steps
The first step towards tackling the problem was to get the various sectors involved to work together. To this end, IenW worked together with the Ministry of Health, Welfare and Sport, the Ministry of Agriculture, Nature and Food Quality, and regional authorities, as well as a broad spectrum of stakeholders representing the healthcare, pharmaceutical and water sectors. All stakeholders together identified the essential steps in the chain from pharmaceutical development and production, via prescription and use, to waste and water treatment. This process was aided by a professional artist who drew the chain during the interactive session with the stakeholders (Figure 1). This drawing showed the interdependency of the various stakeholders: although the health and water sectors are not natural partners, within this chain approach they need to work together to solve the issue.
FIGURE 1.
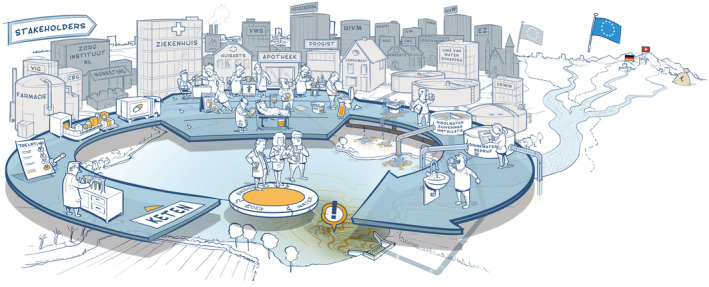
Pharmaceutical chain and stakeholders: from development to drinking water. Source: www.medicijnresten.org
It quickly became clear that this issue met all the characteristics of a wicked problem. 26 Wicked problems are characterised by scientific uncertainties, multiple stakeholders with different values and interests, institutional complexity, and lack an easy solution. Because of this, it was not possible to define policy measures without collaboration of all stakeholders.
As stakeholders from the water sector and the health care sector do not automatically work together and use a completely different terminology, the picture (Figure 1) also helped in overcoming differences in language use. An example is the term emerging substances, which is a term from the water sector that is not at all close to the mental picture a doctor will have with these words. Besides this, misperceptions played a role: where the participants from the health sector thought flushing pharmaceutical leftovers is an environmentally sound way of acting, since they would be removed in the STP (which is not the case), the participants from the water sector thought that doctors were too lax with their prescriptions of medicines (which is not the case). Another misperception was that the problem would be solved if the pharmaceutical industry would start designing more degradable pharmaceuticals. These misperceptions were not helpful in the discussions nor in finding sound measures. A lot of effort was spent on identifying misperceptions, determining the necessary knowledge base to counteract these misperceptions, and to actively communicate to all stakeholders about this.
After this first part of the process, where the actors got to know each other, some discussion meetings were held to establish the following collective principles 27 :
Pharmaceuticals remain available to patients who need them.
The chain approach acts in a pragmatic way, geared to solving problems (not to take measures for the sake of appearances).
The cost of measures taken by the parties must be socially acceptable.
Parties should start acting, without waiting until another party acts first.
The first principle was particularly important in building trust with the healthcare sector, as they were concerned that life‐saving pharmaceuticals might be banned. The second principle has since taken precedence: ascertain whether a so‐called problem really poses risks to the (aquatic) environment, and determine whether a so‐called solution has significant impact on the emissions. If this is not the case, then no resources should be spent on this. It became clear that, in line with the wicked‐problem theory, 26 it was necessary to break down the large issue of pharmaceuticals in the environment into smaller pieces, for which solutions were feasible. However, sometimes these smaller pieces became wicked problems in themselves, due to the complexity of the health care sector.
Based on these 4 principles, all actors discussed and evaluated measures in all phases of the chain, clustered in themes (Figure 2). At the end of 2016, the principles of the chain approach were laid down in an agreement and undersigned by the various institutions involved.
FIGURE 2.
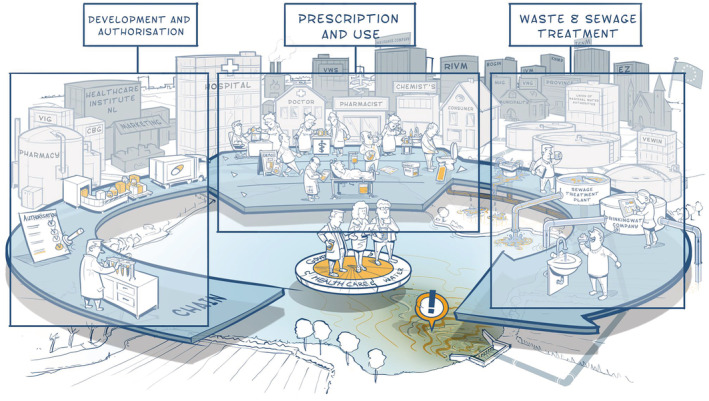
The chain approach: clustering of measures
As the chain approach grew, more and more stakeholders became part of it. This also meant that the complexity of solutions increased as they required more collaboration between different stakeholders. Adding stakeholders means more meetings and more people with whom to communicate and achieve synergy, which takes a great deal of effort. Most measures were designed and implemented via a bottom‐up approach, but, for some stakeholders, possibilities to cooperate via this bottom‐up approach were limited, especially when people work in a traditional bureaucracy with strong hierarchy which limits participation and team‐based (bottom‐up) approaches. Especially in this phase of the approach, it was very important to invest in good interpersonal relationships and to focus on what stakeholders could do themselves.
2.4. The chain approach: Implementation
After this first phase, in 2017 national and regional stakeholders constructively worked together to identify measures that met the above criteria. The measures were purposely designed to be focussed and not aimed at solving the complete issue. This helped to prevent stakeholders not acting because they felt this wicked problem is too large to solve. Focussed measures also helped to show stakeholders what they can do themselves, without the need to point to others that should act.
Thus, for every possible measure, it was evaluated whether it actually solves the problem (directly or indirectly), whether it does not affect patient's health and whether the measures cost in terms of resources (time and/or materials) or finances are acceptable. Because of this, some measures were not implemented (see below for examples). Sometimes, it was first necessary to assess the extent of the specific problem to be solved (e.g., cytostatic emissions). In this phase of identifying suitable measures, it became clear that source measures can be useful, but the majority of the problem will be solved by improving sewage treatment. This is because most pharmaceutical residues enter the water system after normal use by patients, and our society will always need pharmaceuticals in order to provide good health care.
As the knowledge base grew, it was clear that a substance‐by‐substance approach would not be feasible, as there would always be too many pharmaceuticals for which no knowledge on environmental risks were available, and that risks could not be attributed to just 1 (group of) pharmaceuticals. Thus, the chain approach needed to focus on measures for (therapeutic) groups of pharmaceuticals or all pharmaceuticals. Further research just focussed on the feasibility of measures for specific groups of pharmaceuticals (like cytostatics, painkillers, psychoactive substances, or x‐ray contrast media) and the group as a whole (improving sewage treatment).
In 2018, an implementation programme was sent to the Dutch parliament for approval. 27 In this implementation programme, measures are described along the whole chain. A selection of these measures is described in the following sections.
2.5. Examples of measures
Some measures turned out easy to implement, such as a campaign for general practitioners (GPs) and pharmacists to reduce the amount of fluid leftovers that are being flushed into the sink. Others seemed relatively easy to implement at first sight, but in practice turned out to be a wicked problem in themselves. In addition, several measures seemed sensible at first sight, but appeared to be unfeasible or unnecessary. Not all measures identified could be implemented on a national scale, especially regarding legislative measures that are to be solved on a European level. The examples of measures discussed below use the clustering of Figure 2.
2.5.1. Development and authorisation
Development of better biodegradable active ingredients
Often, developing better biodegradable pharmaceuticals is seen as a solution (e.g., 11 , 12 ). However, increasing degradability may also mean that stability of the substance, during distribution as well as in the patient, is affected. 28 Currently, within the IMI‐Horizon 2020 project PREMIER (www.imi-premier.eu), the feasibility of green pharmacy is explored, including GREENER criteria for discovery and development, 29 and possible solutions within product development (personalized medication, other delivery methods).
Availability of information
To assess environmental quality, it is important to have good environmental information of a substance available. For pharmaceuticals, this information is currently often not available to stakeholders from the water sector. For active substances that were marketed after 2006 an environmental risk assessment dossier is provided to the national authorities (nationally authorised products) or the European Medicines Agency (centrally authorised procedures). When a certain trigger value, based on the use of the substance, is met, this dossier contains experimental studies. However, the outcome of this risk assessment and the underlying data are often not publicly available or very hard to find. 11 Dutch water managers have often voiced their need for this data. As this can only be solved in the European context, the Netherlands have been lobbying in the European arena and with industry to increase the availability of this information. Currently, the European Commission has stated in their Strategic Approach 12 that there should be a Union‐wide database. The pharmaceutical industry, together with universities and public partners such as the European Medicines Agency and National Institute for Public Health and the Environment (RIVM), is working on developing a database to meet these requirements within the IMI‐PREMIER project (www.imi-premier.eu).
2.5.2. Prescription and use
Source measures to control emissions of cytostatics
Cytostatics are important—but highly toxic—medicines to treat cancer patients. Several protocols exist to prevent patients contaminating their direct environment. However, via urine, cytostatic residues end up in wastewater: reason for the health care sector to demand measures. Following principle 2 (is this a problem?) the need for measures was investigated. Moermond et al. 30 showed that for most cytostatics in the Dutch situation, their residues do not pose a risk to the environment. They are sufficiently metabolised in the human body and removed in wastewater treatment plants. It was concluded that no specific source measures are necessary, provided that leftovers are disposed of correctly.
Identification of pharmaceuticals to replace pharmaceuticals that impact the environment
Often, health care professionals ask whether they can substitute pharmaceuticals that impact the environment by other pharmaceuticals. Van der Grinten et al. 31 found that professionals are willing to consider substituting treatments, under the condition that the patient's treatment stays qualitatively the same. This means the substitute must at least be equally effective and safe. In practice, for many pharmaceuticals this has not yet proven possible, as the environmental benefits must be substantiated. A system in which environmental information on risk and hazard (e.g. persistent bioaccumulative and toxic – properties) can be weighed against each other and patient safety/efficacy, does not exist. Even if such a system would be developed, it would be hampered by the lack of public data for most pharmaceuticals. 31
Recently, the Dutch College of General Practitioners has started a pilot project to take environmental considerations into account in their guidelines. An example of this is replacing gas inhalators with powder inhalators to decrease greenhouse gas emissions 32 and a revised guideline on pain treatment where it is stated that nonsteroidal anti‐inflammatory drugs have a higher impact on the environment than paracetamol. 33 Besides this, environmental considerations could steer doctors and patients into nonmedical treatment.
Training of healthcare professionals
At present, medical universities lack education modules on environmental aspects, but the demand is growing. For ongoing professional education, a module was created for GPs and pharmacists. Pharmacotherapy audit meetings (PTAM; FTOs in Dutch) are used for their professional education. These PTAMs consist of local groups of 8–12 GPs and 1–3 pharmacists. The groups meet around 6 times a year for 1–2 hours to discuss new developments, (medication) guidelines, and their own medication prescribing and delivery policy. Sometimes they follow education modules, which provide them with education points that are needed to keep their registration. In 2018, the IVM (Dutch Institute for Rational Use of Medicine) together with the professional organizations of GPs and pharmacists, developed a module on pharmaceuticals in the environment, which has already been used by many PTAMs after its implementation in 2019. Experts from the water sector usually join these meetings.
Collection of x‐ray contrast media using urine bags
It is estimated that at least 30 t of radiographic contrast media are flushed into the sewage system every year. 34 This is regarded as undesirable as these agents are given in high doses, are mobile and easily pass the sewage treatment to enter the aquatic environment as well as the drinking water treatment systems. Although these contrast media are not very ecotoxic, they are undesirable in the environment as they are not easily degraded. A first pilot project in 2015 and a larger pilot project in 6 Dutch hospitals in 2020 and 2021 showed that hospital personnel and patients are very willing to use urine bags to collect contrast media. 35 , 36 The further implementation of the use of these urine bags is currently under discussion.
2.5.3. Waste
Leftover medication
A specific set of education materials is available for healthcare professionals, to aid them in disposing of fluid leftovers in the correct way (see Figure 3 for an example of a storyboard that can be put above the sink).
FIGURE 3.
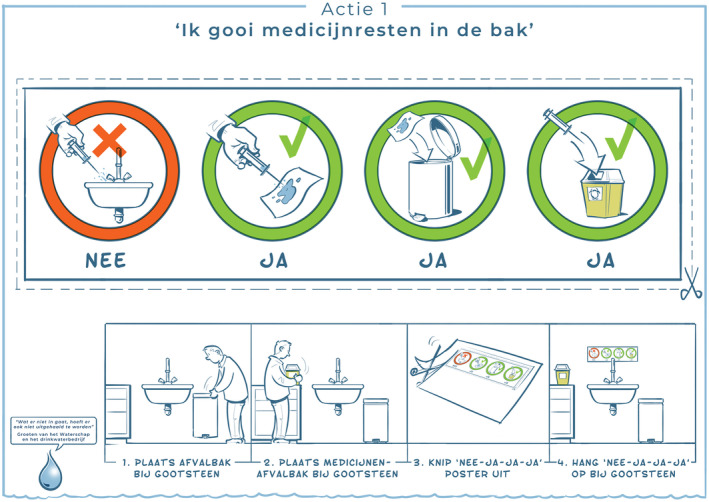
Storyboard for healthcare professionals to show them how to dispose of fluid leftovers. The upper part can be placed above the sink as a reminder. Source: https://www.stowa.nl/kennisimpuls
Collection of left‐over pharmaceuticals
A small proportion of pharmaceutical residues enters the water system after being disposed of in the sink or toilet. It is recommended that pharmaceutical waste is be collected (e.g., by pharmacists) and burnt at high temperatures in order to prevent emissions to the environment. In a response to questions from the partners in the chain approach, more and more municipalities facilitate the collection of left‐over pharmaceuticals at the pharmacists (75% in 2016 to 90% in 2018). 27 In 2020 and 2022, a national collection week was organized including communication to patients.
Upgrading STPs
A programme to speed up improvements at STPs, funded by the national government and regional authorities, started in 2020. This programme includes research projects on new technologies and optimisation of existing technologies, full‐scale implementation of additional treatment modules at existing STPs and impact studies. Information and experiences, also from projects abroad (mainly Germany and Switzerland) are shared in a community of practice. National and regional authorities closely work together with consultancies and knowledge centres.
The STPs have been prioritised for full‐scale improvement with the help of a hotspot analysis, to identify locations where the receiving water was most influenced by STP effluent. This resulted in 80–100 hotspot STPs (depending on the criteria used) out of the 314 Dutch STPs. 37 The programme led to standardised monitoring of pharmaceutical residues at the STP, both in the way samples are taken as well as in the way the samples are analysed. Bioassay methods to determine toxicity of STP effluent were developed, as it is not feasible to analytically determine all pharmaceutical residues.
3. DISCUSSION
3.1. First barriers: Common goals and language
The issue of pharmaceuticals in the environment is very complex, with many stakeholders that act at different parts of the chain (Figure 2) without knowing each other or being aware of the implications of their actions on other stakeholders. Decisions made in 1 part of the chain (e.g., marketing of a certain pharmaceutical) can influence a completely different part of the chain (e.g., purification of drinking water), without the connection being visible to all actors. This issue meets all criteria of a wicked problem. 26
To involve all stakeholders to have them actually working together, it was important to deal with the following barriers:
Stakeholders from different sectors use entirely different terminology, with different meanings for the same terms (e.g., emerging chemicals, alternative medication, green pharmacy).
Freeze response to complexity: Stakeholders did not have an overview or thought the problem was too big to solve, which paralyzed them, thinking that no measure they could take individually would contribute to a solution.
Freeze response to choices: Stakeholders from different sectors criticized each other as these others should solve it or take the first step. The water sector criticized the pharmaceutical industry, or doctors who should not prescribe so much (with Dutch expenditure on medicines already well below the EU average 38 ). The health care sector, however, argued that—since STPs exist to treat sewage—the STPs just should remove pharmaceuticals.
Lack of knowledge on the extent of environmental impacts of pharmaceuticals. With about 2000 active substances authorised for the Dutch market, a risk assessment based on actual water monitoring data was only possible for a couple of dozen of these. This showed risks for a handful of substances. Stakeholders needed to accept that they would never know the real extent of the problem—performing monitoring and ecotoxicity studies for only a dozen more active substances would take years, and then still for the majority there would be no knowledge on actual risks.
These barriers had to be taken away in the first stages of the process. The time spent on getting all actors together to formulate a common goal and speak each other's languages was needed to reach agreement on the measures that need to be taken. Involving all stakeholders in drawing the visual representation of the processes in the chain was a large step towards understanding the consequences of what each stakeholder does, and the interdependencies of the measures taken in each part of the chain. Making the chain visual helped to overcome language (terminology) barriers.
Once the sectors got to know each other's viewpoints, mutual understanding increased. For example, water stakeholders recognized that medicines will always be used and stakeholders from the health care sector realized that it would be impossible to remove all pharmaceutical residues from wastewater.
3.2. Next steps: Dealing with misperceptions, increasing group size, designing measures
During the next phase of the chain approach, actual measures had to be designed by the stakeholders. Again, the lack of knowledge appeared to be an issue for some parties, so parties had to keep investing in disseminating the known facts and communicating openly with these stakeholders about uncertainties. Besides this, the available knowledge was used to help design measures or to decide not to act: e.g. for cytostatics, actual use information showed that specific measures would not be needed (see above). Some misperceptions proved persistent, however, partly because these were voiced in media (newspapers, news shows). For instance, that the design of greener drugs would immediately solve the problem has been a misperception that has the potential to negatively interfere in collaborations between the health care sector and the water sector. Most of these misperceptions could be counteracted with fact‐checking and communication. However, some of them keep re‐occurring, especially those that attracted media attention, and continuous effort is needed to communicate about these misperceptions.
The focus was primarily on solutions that matter in the short to middle term, with win–win solutions for >1 stakeholder. The motto was to solve problems, and not wait for others to act. Measures had to actually contribute to solving the issue, no regret measures that did not contribute only consume a lot of (personal) energy and resources and thus had to be prevented.
In time, the chain approach grew and included more stakeholders and increased complexity. Especially in this phase of the approach it was very important to invest in good interpersonal relationships and to focus on what stakeholders could do themselves—even small steps matter. By breaking the wicked problem down in smaller pieces, it became manageable. This way, it became possible to acknowledge all stakeholders for their individual work. Care should be taken that “the issue being too complex” or “we are not sure about risks for all individual active substances” is not used as a tactic to delay taking measures. Again, open communication about these issues was the key.
Although establishing the chain approach was quite an intensive process, the large group of stakeholders involved led to a process that generated its own energy. During the pilot on collecting x‐ray contrast media, patients themselves started asking for urine bags, which increased enthusiasm among hospital personnel. And in the water sector, a boost was given to the development of new treatment methods, not only to remove pharmaceutical residues, but also a range of other ‘chemicals of emerging concern’. The most important factor in the whole process was to keep an open communication, take all stakeholders seriously, and to invite them to bring in solutions for their part of the chain.
COMPETING INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Caroline Moermond and Marc de Rooy both conceived, wrote, reviewed and edited the paper.
ACKNOWLEDGEMENTS
The authors acknowledge all institutions and individuals who have contributed to the chain approach and an anonymous reviewer for help with the manuscript. The work of Caroline Moermond was funded by the ministry of Infrastructure and Water Management.
Moermond CTA, de Rooy M. The Dutch chain approach on pharmaceuticals in water: Stakeholders acting together to reduce the environmental impact of pharmaceuticals. Br J Clin Pharmacol. 2022;88(12):5074‐5082. doi: 10.1111/bcp.15509
Funding information Ministry of Infrastructure and Water Management
DATA AVAILABILITY STATEMENT
All data are available in the paper or the cited references.
REFERENCES
- 1. Wilkinson JL, Boxall ABA, Kolpin DW, et al. Pharmaceutical pollution of the world's rivers. Proc Natl Acad Sci. 2022;119(8):e2113947119. doi: 10.1073/pnas.2113947119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aus der Beek T, Weber F‐A, Bergmann A, et al. Pharmaceuticals in the environment‐Global occurrences and perspectives. Environ Toxicol Chem. 2016;35(4):823‐835. doi: 10.1002/etc.3339 [DOI] [PubMed] [Google Scholar]
- 3. Miller TH, Bury NR, Owen SF, MacRae JI, Barron LP. A review of the pharmaceutical exposome in aquatic fauna. Environ Pollut. 2018;239:129‐146. doi: 10.1016/j.envpol.2018.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kidd KA, Blanchfield PJ, Mills KH, et al. Collapse of a fish population after exposure to a synthetic estrogen. PNAS. 2007;104(21):8897‐8901. doi: 10.1073/pnas.0609568104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyler CA, Goodhead RM. Impacts of hormone disrupting chemicals on wildlife. In: Maclean N, ed. Silent Summer: the State of Wildlife in Britain and Ireland. UK: Cambridge University Press; 2010:125‐140. doi: 10.1017/CBO9780511778230.011 [DOI] [Google Scholar]
- 6. OECD . Pharmaceutical residues in freshwater: hazards and policy responses. OECD Studies on Water: OECD Publishing, Paris; 2019. doi: 10.1787/c936f42d-en [DOI] [Google Scholar]
- 7. WHO . Chemical mixtures in source water and drinking‐water. Geneva, Switzerland: World Health Organisation; 2017. [Google Scholar]
- 8. Miller EL, Nason SL, Karthikeyan KG, Pedersen JA. Root uptake of pharmaceuticals and personal care product ingredients. Environ Sci Technol. 2016;19(50):525‐541. doi: 10.1021/acs.est.5b01546 [DOI] [PubMed] [Google Scholar]
- 9. https://www.cdc.gov/onehealth/index.html
- 10.Priority substances ‐ Water ‐ Environment ‐ European Commission (https://ec.europa.eu/environment/water/water‐dangersub/pri_substances.htm)
- 11. Bio Intelligence Service . Study on the environmental risks of medicinal products. Final report prepared for Executive Agency for Health and Consumers, European Commission. Bio Intelligence Service, Brussels, Belgium. 2013.
- 12. European Commission . European Union strategic approach to pharmaceuticals in the environment. In: Communication from the Commission to the European parliament, the council and the European economic and social committee. European Commission, Brussels: Belgium; 2019. [Google Scholar]
- 13. UNEP , 2015. 11. UN Environment Programma – Strategic Approach to International Chemicals Management. Pharmaceutical pollutants | SAICM Knowledge [Google Scholar]
- 14. Damania R, Desbureaux S, Rodella A‐S, Russ J, Zaveri E. Quality unknown. The World Bank, Washington, USA: The invisible water crisis; 2019. [Google Scholar]
- 15. Vethaak AD, Rijs GBJ, Schrap SM, Ruiter H, Gerritsen A, Lahr J. Estrogens and xeno‐estrogens in the aquatic environment of the Netherlands. Occurrence, potency and biological effects. RIZA/RIKZ report 2002/001. RIZA/RIKZ, Lelystad, the Netherlands. 2002.
- 16. Moermond CTA, Smit CE, van Leerdam RC, van der Aa NGFM, Montforts MHMM. Geneesmiddelen en Waterkwaliteit. RIVM report 2016‐0111. RIVM, Bilthoven, the Netherlands. 2016.
- 17. Moermond C, Montforts M, Roex E, Venhuis B. Medicijnresten en waterkwaliteit: een update. RIVM report 2020‐0088. RIVM, Bilthoven, the Netherlands. 2000.
- 18. CUWVO . Afvalwaterproblematiek van ziekenhuizen. RIZA, Lelystad, the Netherlands. 1986. https://www.helpdeskwater.nl/publish/pages/130510/ciw41986‐09afvalwaterproblematiek_ziekenhuizen_1986.pdf
- 19. van Vlaardingen PLA, Montforts MHMM. Geneesmiddelen in het milieu. Twee verkennende studies samengevat. RIVM, the Netherlands. 1999.
- 20. Derksen JGM, De Poorter LRM. Geneesmiddelen in oppervlaktewater. Aanwezigheid en risico's. Amsterdam, the Netherlands: RIWA; 1997. [Google Scholar]
- 21. Health Council of the Netherlands . Environmental risks of medicines. Publication no. 2001/17. Health Council, The Hague, the Netherlands. 2001.
- 22. Rijs GBJ, Laane RWPM, De Maagd G‐J. Voorkomen is beter dan genezen. Een beleidsanalyse over ‘geneesmiddelen en watermilieu’. RIZA/RIKZ rapport 2003.037/2003.048. RIZA, Lelystad, the Netherlands. 2003.
- 23.Chemicals ‐ Water pollution ‐ Environment ‐ European Commission (https://ec.europa.eu/environment/water/water‐dangersub/index.htm)
- 24. Keessen A, Freriks A, van Rijkswick M. The clash of the titans: the relation between the European water and medicines legislation. Common Market Law Rev. 2010;47(Issue 5):1429‐1454. doi: 10.54648/COLA2010060 [DOI] [Google Scholar]
- 25.eps‐core‐V9.cdr (https://www.efpia.eu/media/25628/eps‐a‐holistic‐environmental‐risk‐management‐program.pdf)
- 26. Rittel HWJ, Webber MM. Dilemmas in a general theory of planning. Pol Sci. 1973;4(2):155‐169. doi: 10.1007/BF01405730 [DOI] [Google Scholar]
- 27. De Rooy M. Reducing pharmaceutical residues in water: a chain approach. Supplement to parliamentary letter (in Dutch; Full English translation available through the author). Ministry of Infrastructure and the Environment, The Hague, the Netherlands. 2018. https://www.rijksoverheid.nl/documenten/rapporten/2018/06/21/bijlage‐1‐uitvoeringsprogramma‐ketenaanpak‐medicijnresten
- 28. Moermond C, Venhuis B. Green Pharmacy en beter afbreekbare medicijnen: wat is er mogelijk? H2O online, april 2019. 2019. https://www.h2owaternetwerk.nl/images/2019/April/H2O-Online_190424_Greenpharmacy.pdf
- 29. Moermond CTA, Puhlmann N, Brown AR, et al. GREENER pharmaceuticals for more sustainable healthcare. Environ Sci Technol Lett. 2022. doi: 10.1021/acs.estlett.2c00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moermond C, Venhuis B, van Elk M, Oostlander A, van Vlaardingen P, Marinkovic M, van Dijk J. Cytostatics in Dutch surface water: Use, presence and risks to the aquatic environment. RIVM report 2018‐0067. RIVM, Bilthoven, the Netherlands. 2018.
- 31. Van der Grinten E, van der Maaden T, van Vlaardingen PLA, Venhuis BJ, Moermond CTA. Milieuafwegingen in de geneesmiddelvoorziening. (Environmental considerations in the medicinal product chain). RIVM report 2016–0207. RIVM, Bilthoven, the Netherlands. 2017.
- 32.Nieuw hulpmiddel bij keuze inhalator astma en COPD | NHG. https://www.nhg.org/actueel/nieuws/nieuw‐hulpmiddel‐bij‐keuze‐inhalator‐astma‐en‐copd
- 33. Pijn|NHG‐Richtlijnen. https://richtlijnen.nhg.org/standaarden/pijn#volledige-tekst-richtlijnen-diagnostiek
- 34. Evenblij H, Moll S, Schuman E, Kujawa‐Roeleveld K. Inventarisatie röntgencontrastmiddelen. Amersfoort, The Netherlands: Royal HaskoningDHV; 2016. [Google Scholar]
- 35. Diels J, Muis J, Verhoeff A, Van Vliet B, Hendriksen A, Wijn G. Grip op medicijnresten in ons water. Waterschap Groot Salland, Zwolle, The Netherlands: Eindrapportage; 2015. [Google Scholar]
- 36. Hoogenboom J, Bergema K, van Vlie BJM, et al. Brede Proef Plaszakken – Eindrapportage. Rossum, The Netherlands: Van Waarde; 2021. [Google Scholar]
- 37. Vissers M, Vergouwen L, Marc Vissers WS. Landelijke hotspotanalyse geneesmiddelen RWZI's. Amersfoort, the Netherlands: STOWA; 2017. [Google Scholar]
- 38. OECD . Health at a Glance: Europe. OECD Publishing, Paris. Section on Pharmaceutical expenditure: Pharmaceutical expenditure | Health at a Glance: Europe 2020: State of Health in the EU Cycle | OECD iLibrary (oecd‐ilibrary.org). 2021. https://www.oecd‐ilibrary.org/sites/82129230‐en/1/3/2/3/7/index.html?itemId=/content/publication/82129230‐en&_csp_=e7f5d56a7f4dd03271a59acda6e2be1b&itemIGO=oecd&itemContentType=book
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the paper or the cited references.


