Abstract
Objective
This study aimed to demonstrate noninferiority using telehealth in treating obesity with phentermine in patients with BMI ≥ 27 kg/m2 with comorbidities or BMI ≥ 30 compared with the standard in‐person approach over a 90‐day period.
Methods
A 12‐week, randomized, prospective, single‐center, open label trial compared the use of virtual visits versus in‐person visits for the treatment of obesity using phentermine. The primary end point was percentage mean change in body weight from baseline to 12 weeks. A noninferiority approach assuming a 3% noninferiority region was used to assess effect size differences.
Results
The weight loss in the virtual visit arm was noninferior to the in‐person arm at all time points. At 12 weeks, the mean change in weight was −6.5% among the virtual group and −7.7% among the in‐person group. In addition, 65% of virtual patients and 71% of in‐person patients demonstrated a weight reduction of at least 5%. There was no difference in medication tolerance, adherence, and compliance.
Conclusions
These results indicate that the virtual obesity pharmacotherapy visits in adults aged 18 to 65 years prescribed phentermine are effective and noninferior in achieving meaningful weight loss after 12 weeks. Future clinical trials are needed to better assess the effectiveness of televisits for obesity pharmacotherapy.
Study Importance.
What is already known?
Phentermine is a commonly used medication for treatment of obesity with well‐established weight‐loss outcomes. Phentermine is affordable and well tolerated.
Standard obesity care, specifically for pharmacotherapy, is in‐person (face‐to‐face) visits. During the COVID‐19 pandemic, there was an increased shift from in‐person to virtual visits.
What does this study add?
The use of virtual visits for prescription of 12‐week phentermine in adults aged 18 to 65 years showed similar results (noninferior) for weight loss when compared with standard in‐person visits.
Even though the study was not designed to assess safety, there were no significant differences in side effects (e.g., increased heart rate and blood pressure) or tolerability to phentermine between groups.
How might these results change the direction of research or the focus of clinical practice?
The use of virtual visits for obesity pharmacotherapy, specifically phentermine, should be considered as a valuable method to manage obesity in current and future clinical practices.
This trial demonstrates that patients are comfortable with the use of virtual visits for treatment of obesity and that virtual visits produce similar weight‐loss results.
INTRODUCTION
Obesity is a chronic disease and a major health care problem in the United States affecting more than 40% of the population, often increasing the risk of other comorbidities such as hypertension, dyslipidemia, and type 2 diabetes mellitus, and it is also associated with increased risk of all‐cause mortality and cardiovascular death [1]. Modest weight loss of 5% has been associated with improvement in obesity related comorbidities and quality of life [2]. The cornerstone for treatment of obesity is lifestyle modification, including changes in diet and physical activity [3]. Metabolic adaptation and compensatory physiological responses of increased appetite and decreased satiety can contribute to the difficulty of maintaining weight loss. Antiobesity medications (AOMs) are often required as adjuvant therapy for obesity in combination with lifestyle interventions [4, 5, 6]. Unfortunately, AOMs remain underused, providing treatment to only a minority of patients, and the prescribing of these agents is often met with barriers (e.g., lack of insurance coverage). Phentermine is an affordable, commonly prescribed medication to treat obesity; however, phentermine is confronted with challenges as it is Food and Drug Administration approved only for short‐term use (90 days), and some states, such as Ohio, require monthly in‐person visits [7].
Telemedicine has rapidly emerged as an effective platform to deliver medical and obesity care, allowing providers to overcome more traditional barriers of access for patients with severe obesity and other comorbidities [8], most recently during the COVID‐19 pandemic [9]. Although self‐monitoring apps and telehealth were used before the coronavirus pandemic to deliver obesity care, most focused on behavioral interventions and sought to bolster patient attrition [10, 11, 12, 13]. At the start of the COVID‐19 pandemic, the federal government and the state of Ohio relaxed standards for prescribing controlled substances, allowing providers to provide patients with up to three 30‐day prescriptions of phentermine over 90 consecutive days (and other scheduled AOMs) via virtual visits. This trial was conducted to demonstrate the effectiveness and safety of obesity treatment with phentermine via virtual visits. The primary overall objective of this study was to demonstrate noninferiority of using telehealth in treating obesity with phentermine in patients with body mass index (BMI) ≥ 27 kg/m2 with comorbidities or with BMI ≥ 30 compared with the standard in‐person approach over a 90‐day course of treatment.
METHODS
Study design and participants
This was a 12‐week, randomized, prospective, single‐center, open label trial comparing the use of virtual visits versus standard in‐person visits for the treatment of obesity with phentermine. The study was conducted at the Cleveland Clinic's Endocrinology and Metabolism Institute and at the Bariatric and Metabolic Institute in Cleveland, Ohio, from December 2020 to August 2021. The protocol and amendments were approved by the institutional review board of Cleveland Clinic. All participants provided written informed consent prior to participation. No one received compensation or was offered any incentive for participating in this study.
Eligible participants included adults who were aged 18 to 65 years, who had obesity (BMI ≥ 30) or overweight (BMI ≥ 27), who had one or more comorbidities (hypertension, diabetes, sleep apnea, fatty liver disease, polycystic ovarian syndrome, dyslipidemia, congestive heart failure, osteoarthritis), who were English speaking, and who had a smartphone and the ability and willingness to join an online virtual visit platform. Key exclusion criteria included a contraindication to phentermine (history of cardiovascular disease [e.g., coronary artery disease, stroke, history of arrhythmias, congestive heart failure, uncontrolled hypertension], hyperthyroidism, glaucoma, agitated states, history of drug abuse, known hypersensitivity, or idiosyncrasy to the sympathomimetic amines), pregnancy or intention to become pregnant, breast‐feeding or of child‐bearing age without adequate contraceptive methods, participation in another clinical trial within 30 days before screening, uncontrolled hypertension, history of arrhythmias, treatment with any medication with the primary intention of weight loss within 180 days before screening, and a previous history of bariatric surgery or use of minimally invasive weight‐loss devices. Patients who were already scheduled for an in‐person weight‐management appointment were prescreened and, if eligible, were contacted ahead of their visit to explain the study. If interested, the consent was sent for their review and then signed inperson on visit 1.
Randomization and interventions
Eligible participants were randomized (1:1) via centralized allocation to either an in‐person weight‐management program (standard of care) or a virtual weight‐management program (intervention). A single randomization list was created and uploaded to the study database. As patients were enrolled, randomization was performed within the database using this list. All patients, independently of the randomization group, were seen in person on visit 1 by one of our seven obesity‐medicine providers in a 1:1 consultation. A visit with the registered dietitian and exercise physiologist (in‐person or virtual) was offered. At visit 1, patients were prescribed phentermine (37.5‐mg tablet) and instructed to take half a tablet per day for 2 weeks and, if tolerating, to increase to a full tablet until the end of the trial. A Mediterranean or ketogenic diet was offered to patients (but was not mandatory to follow). Weight and vital signs were monitored at each visit; all patients randomized to virtual visits received a remote scale and a remote blood pressure cuff (linked to Cleveland Clinic's electronic health record EPIC, My Practice). Participants had a total of three 1:1 follow‐up encounters every 28 ± 7 days. Modifiable lifestyle factors were reviewed at each visit, including nutrition, physical activity, circadian rhythm and sleep, stress, and psychosocial issues. Other educational topics related to nutrition plan, emotional eating, weight set point, hunger/fullness, food preparation, healthy sleeping habits, and/or behavioral modification were discussed. If the patient opted to see the nutrition and exercise physiologist, a personalized nutrition and exercise program was offered. In addition, if felt relevant by the provider, patients were also referred to a mental health specialist and/or sleep clinic. The effects, and side effects, of phentermine were reviewed at each visit. Vital signs including heart rate and blood pressure were monitored remotely via Bluetooth for patients randomized to virtual visits. Phentermine was e‐prescribed to participants' pharmacies and coverage varied.
End points
The primary end point was mean percentage change in body weight from baseline (visit 1) to 12 weeks (visit 4). All variables were assessed at baseline and at 4, 8, and 12 weeks (±7 days). The initial weight measurement for the in‐person participants was obtained at the in‐person baseline visit. The initial weight measurement for the virtual management participants was obtained using the remote scale within 24 hours of the baseline visit, first thing in the morning. Subsequent weight measurements were obtained on a designated calibrated scale or remote scale at 4, 8, and 12 weeks for the in‐person and virtual patients, respectively. Secondary end points included average weight loss in pounds, BMI change from baseline to week 12, percentage of patients who lost >5% body weight (at week 4, 8, and 12), adherence to weight‐management program (numbers of missed visits), medication compliance, and medication tolerance, assessed verbally by the physician at each visit. Medication compliance was divided into the following categories: patient taking full dose; not taking full dose owing to medical reasons; and not taking full dose owing to other reasons (nonadherence). Medication tolerance was defined in four categories: taking full dose without side effects; full dose with side effects; decreased dose owing to side effects; and discontinued because of side effects. For participants who did not attend the last visit, an attempt was made to obtain a weight measure within the visit window by the following hierarchy: 1) research coordinator visit with in‐person weight check (in‐person group) or retrieved from remote scale reading from EPIC (virtual group); 2) most recent weight recorded in the electronic medical record (if within the study visit window); or 3) participant self‐reported.
Statistical analysis
Sample size calculations were performed on percentage weight change at 12 weeks. In one of the phentermine/topiramate trials [14], at 12 weeks, patients taking phentermine demonstrated a mean change in weight of approximately 5%. The standard deviation (SD) was not explicitly stated, but, based on the confidence intervals (CI) reported, power calculations were performed assuming SD of 4.5%. A total of 29 patients per group was required to achieve 80% power to detect noninferiority assuming a 3% noninferiority region. After accounting for an anticipated dropout rate of 15%, 35 patients were recruited in each group to detect noninferiority effect size differences in the primary end point.
Categorical variables were described using frequencies and percentages, whereas continuous variables were described using means and SD. Standardized differences were calculated for demographic and medical history variables to measure the effect size between the two groups. Primary outcomes were assessed using intention‐to‐treat analysis (the full set of all randomized patients regardless of adherence to randomized treatment). To evaluate noninferiority and superiority of virtual visits relative to in‐person visits on percentage weight loss, linear mixed effect models were used. Baseline weight was included initially, but it did not improve model fit and was dropped. A 3% noninferiority region was assumed, and a one‐sided test was performed to evaluate this hypothesis. If this test was significant, a superiority test was performed. To compare groups on 5% weight loss, visit specific log‐binomial models were fit to estimate relative risks between groups at each visit. Relative risks reflect the ratio of the observed event rates of the virtual group relative to the in‐person group. Noninferiority was tested against a lower bound of 0.8, and then superiority tests followed if noninferiority tests were significant. Odds ratios were also calculated from mixed effect logistic regression models. Mixed effect models include patient as a random effect and group, time, and their interaction as predictors. Findings from mixed effect models remained valid under the assumption that data from patients with missed visits were missing at random, so no additional imputation of missing weight values was performed. This assumption was checked graphically. Visit specific models included only group as a predictor, with patient as a repeated effect [15]. Results are presented as means or relative risks with 95% CI with p values for noninferiority and, if appropriate, superiority. For both mean change and 5% weight loss, two sensitivity analyses were performed: first a per‐protocol analysis that included only patients who did not discontinue medication throughout the study and second a sensitivity analysis adjusting for baseline weight in the analysis of all patients. Comparisons of visit and medication compliance, medication tolerance, medication adherence, and side effects were evaluated using Pearson χ2 tests, Fisher exact tests for nominal factors, and Wilcoxon rank sum tests. Secondary end point analysis of compliance and safety measures was performed among those completing each visit. Analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, North Carolina). A significance level of 0.05 was assumed for all tests.
RESULTS
Study participants
A total of 70 patients were enrolled from December 2020 to June 2021. The last patient visit occurred in August 2021. Participants were predominately (90%) women with a mean (SD) age of 42.2 (11.4) years and baseline weight of 250.7 (54.6) lb. The mean BMI was 41.2 (8.7). Table 1 provides a summary of baseline characteristics of the groups. Based on the standardized difference it appears that those with in‐person visits included a larger number of African American patients and appeared to have a higher prevalence of osteoarthritis and gastroesophageal reflux disease, along with fewer cases of depression/anxiety. Overall, the most frequent comorbidities were hypertension (27.1%) and dyslipidemia (25.7%), followed by depression/anxiety (21.4%) and osteoarthritis (20%). Overall, 87% of patients qualified based on BMI ≥ 30.
TABLE 1.
Baseline characteristics
| Overall (N = 70) | Virtual visits (n = 35) | In‐person encounters (n = 35) | ||||
|---|---|---|---|---|---|---|
| n | Statistics | n | Statistics | n | Statistics | |
| Age (y), mean ± SD | 70 | 42.2 ± 11.4 | 35 | 42.7 ± 8.9 | 35 | 41.6 ± 13.5 |
| Gender, n (%) | 70 | 35 | 35 | |||
| Female | 63 (90.0) | 32 (91.4) | 31 (88.6) | |||
| Male | 7 (10.0) | 3 (8.6) | 4 (11.4) | |||
| Ethnicity, n (%) | 62 | 30 | 32 | |||
| Hispanic or Latino | 3 (4.8) | 3 (10.0) | 0 (0.00) | |||
| Not Hispanic or Latino | 59 (95.2) | 27 (90.0) | 32 (100.0) | |||
| Race, n (%) | 68 | 33 | 35 | |||
| Black or African American | 31 (45.6) | 12 (36.4) | 19 (54.3) | |||
| White | 33 (48.5) | 19 (57.6) | 14 (40.0) | |||
| More than one race | 4 (5.9) | 2 (6.1) | 2 (5.7) | |||
| Comorbidities | 70 | 35 | 35 | |||
| Hypertension, n (%) | 70 | 19 (27.1) | 35 | 8 (22.9) | 35 | 11 (31.4) |
| Dyslipidemia, n (%) | 70 | 18 (25.7) | 35 | 8 (22.9) | 35 | 10 (28.6) |
| NAFLD, n (%) | 70 | 2 (2.9) | 35 | 0 (0.00) | 35 | 2 (5.7) |
| CKD, n (%) | 70 | 0 (0.00) | 35 | 0 (0.00) | 35 | 0 (0.00) |
| OSA, n (%) | 70 | 7 (10.0) | 35 | 2 (5.7) | 35 | 5 (14.3) |
| Osteoarthritis, n (%) | 70 | 14 (20.0) | 35 | 4 (11.4) | 35 | 10 (28.6) |
| Metabolic syndrome, a n (%) | 70 | 11 (15.7) | 35 | 4 (11.4) | 35 | 7 (20.0) |
| Depression/anxiety, n (%) | 70 | 15 (21.4) | 35 | 10 (28.6) | 35 | 5 (14.3) |
| Urinary incontinence, n (%) | 70 | 1 (1.4) | 35 | 0 (0.00) | 35 | 1 (2.9) |
| GERD, n (%) | 70 | 12 (17.1) | 35 | 3 (8.6) | 35 | 9 (25.7) |
| Diabetes mellitus, n (%) | 70 | 3 (4.3) | 35 | 1 (2.9) | 35 | 2 (5.7) |
| PCOS, n (%) | 63 | 5 (7.9) | 32 | 1 (3.1) | 31 | 4 (12.9) |
| Male hypogonadism, n (%) | 7 | 0 (0.00) | 3 | 0 (0.00) | 4 | 0 (0.00) |
Note: Statistics presented as n (column %).
Abbreviations: CKD, chronic kidney disease; GERD, gastroesophageal reflux disease; NAFLD, nonalcoholic fatty liver disease; OSA, obstructive sleep apnea; PCOS, polycystic ovarian syndrome.
Three or more of the following: fasting glucose ≥ 100 mg/dL or treatment for diabetes or prediabetes; HDL‐c < 50 mg/dL in men or <40 mg/dL in women or treatment for low HDL‐c; triglycerides ≥ 150 mg/dL or drug treatment for elevated triglycerides; abdominal obesity, waist ≥ 102 cm in men or ≥88 cm in women; blood pressure ≥ 130/85 mm Hg or drug treatment for hypertension.
Three patients dropped out of the study (one patient became pregnant, one had sleeve gastrectomy outside the United States, and one patient started taking topiramate for weight loss prescribed by another physician), and three patients withdrew consent (two patients owing to high insurance cost, and one patient did not like the randomization group assigned).
Final weight measures were obtained in 59 of 67 eligible patients (88%): 34 patients (100%) in the virtual group and 25 patients (76%) in the in‐person group. Overall, 34 patients had their weights measured by remote scale, 24 on‐site, 1 by electronic medical record (in‐person group), and none by self‐report. None of the patients reached by telephone provided a self‐report weight within the study window (Figure 1).
FIGURE 1.
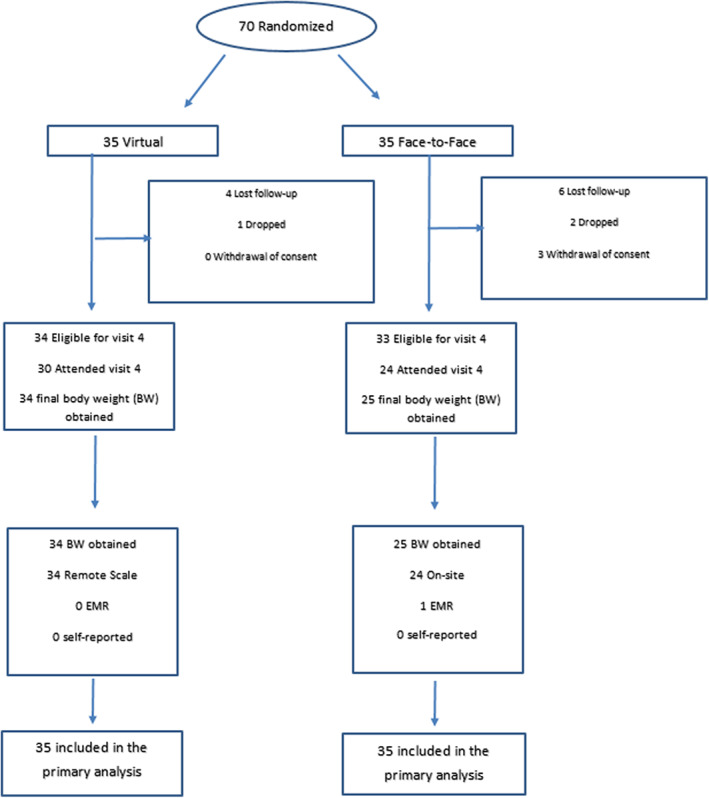
Trial scheme [Color figure can be viewed at wileyonlinelibrary.com]
Primary end point
Table 2 contains the results for the comparison of weight loss between groups. Significant reductions in weight were demonstrated at all study visit time points. The weight loss in the virtual visit group was noninferior to the in‐person group at all time points but was not superior to the in‐person group at any of the time points. Estimated change in mean weight at 4 weeks was −4.3% among the virtual group and −3.4% among the in‐person group, with a mean difference of 0.9 (95% CI: −2.5 to 0.7). The estimated change in weight was −6.0% among the virtual group and −5.1% among the in‐person group, with a mean difference of 1.0 (95% CI: −2.6 to 0.7) at 8 weeks. At 12 weeks, the mean change in weight was −6.6% among the virtual group and −7.7% among the in‐person group, with a mean difference of 1.1 (95% CI: −0.6 to 2.7), indicating noninferiority but not superiority (Figure 2). Sensitivity analysis in the per‐protocol population results was similar to the primary analysis.
TABLE 2.
Primary outcome of weight change (%) and secondary outcome of 5% weight change
| Time | Virtual change (95% CI) | In‐person change (95% CI) | Mean difference (95% CI) | Noninferiority (3%) | Superiority | Odds ratio (95% CI) | Noninferiority (20%) |
|---|---|---|---|---|---|---|---|
| Primary analysis of weight change (%) | |||||||
| 12 weeks | −6.6 (−7.7 to −5.5) | −7.7 (−8.9 to −6.4) | 1.1 (−0.6 to 2.7) | 0.011 | 0.90 | ||
| Secondary analysis of weight change (%) | |||||||
| 4 weeks | −4.3 (−5.4 to −3.2) | −3.4 (−4.6 to −2.2) | −0.9 (−2.5 to 0.7) | <0.001 | 0.14 | ||
| 8 weeks | −6.0 (−7.2 to −4.9) | −5.1 (−6.3 to −3.9) | −1.0 (−2.6 to 0.7) | <0.001 | 0.13 | ||
| Primary analysis of 5% weight change | |||||||
| 4 weeks | 29.4 (17.5 to 49.5) | 15.5 (6.2 to 38.8) | 1.9 (0.7 to 5.4) | 0.054 | |||
| 8 weeks | 57.2 (42.4 to 77.3) | 55.6 (39.8 to 77.8) | 1.0 (0.7 to 1.6) | 0.14 | |||
| 12 weeks | 64.7 (50.5 to 82.9) | 70.5 (55.1 to 90.1) | 0.9 (0.6 to 1.3) | 0.22 | |||
FIGURE 2.
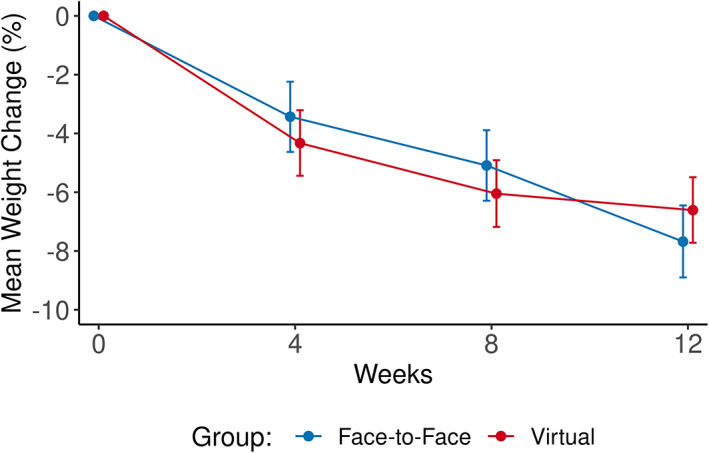
Mean percentage weight change from baseline in body weight over time by group (intention‐to‐treat analysis). There is no statistical difference between groups [Color figure can be viewed at wileyonlinelibrary.com]
Secondary end points
Table 2 and Figure 3 contain the results for the comparisons of 5% weight loss. At 12 weeks, 65% of virtual patients and 71% of in‐person patients demonstrated a weight reduction of at least 5%. The relative risk was 0.92 (95% CI: 0.65‐1.30). Using a noninferiority boundary of 0.8, we could not conclude noninferiority for this time point or any of the other time points, so superiority testing was not performed. Figure 3 shows the proportion of patients attaining at least 5%, 10%, and 15% of weight loss and Figure 4 (and Supporting Information Figures [Link], [Link]) show visual representations of the changes in weight from the analysis tables. Only 29 patients (41%) went to see the dietitian and/or exercise physiologist. There was no difference between study groups.
FIGURE 3.
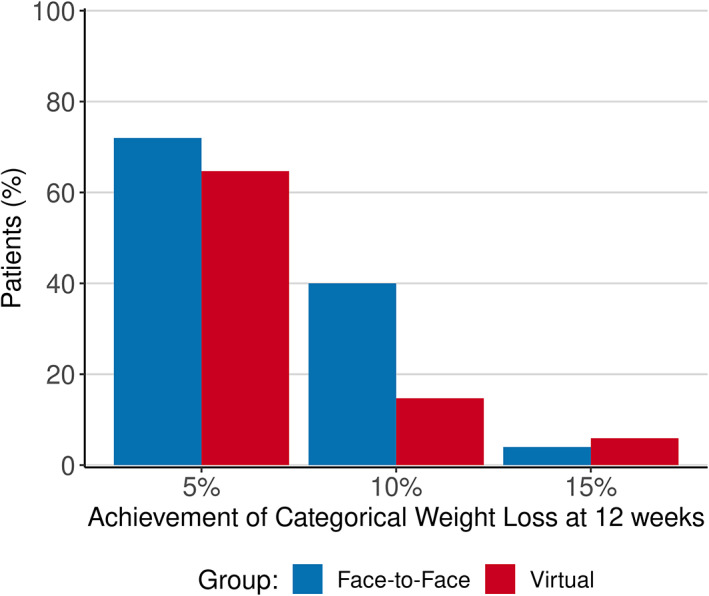
Proportions of patients achieving body weight reductions of at least 5%, 10%, and 15% from baseline to week 12 by group (intention‐to‐treat). There is no statistical difference between groups [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
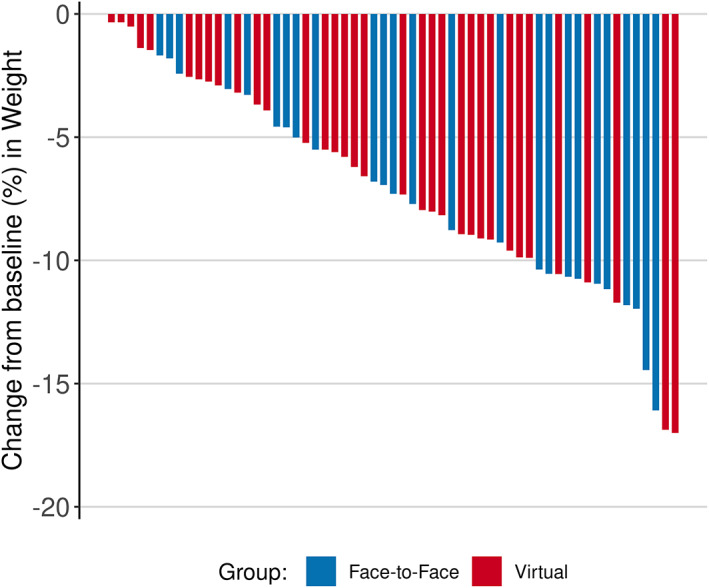
Waterfall plot—All patients. Individual weight loss among all patients from baseline to week 12 [Color figure can be viewed at wileyonlinelibrary.com]
Table 3 contains the results for the comparisons of visit compliance and adherence between study groups. Although there was some evidence that the virtual group had better overall visit compliance (percentage of patients who attended all visits was 82.9% in the virtual group vs. 62.9% in the in‐person encounter), this did not reach statistical significance (p = 0.053).
TABLE 3.
Adherence and compliance
| Factor | Virtual visits (n = 35) | In‐person encounters (n = 35) | p value | ||
|---|---|---|---|---|---|
| n | Statistics | n | Statistics | ||
| Medication compliance | 30 | 24 | 0.58 a | ||
| Full dose | 24 (80.0) | 18 (75.0) | |||
| Not full dose (medical reasons) | 4 (13.3) | 2 (8.3) | |||
| Not full dose (nonadherence) | 2 (6.7) | 4 (16.7) | |||
| Medication tolerance: 4 levels | 28 | 20 | 0.84 a | ||
| Full dose: no side effects | 17 (60.7) | 11 (55.0) | |||
| Full dose: side effects | 7 (25.0) | 7 (35.0) | |||
| Decreased dose: side effects | 2 (7.1) | 1 (5.0) | |||
| Discontinued dose: side effects | 2 (7.1) | 1 (5.0) | |||
| Full visit compliance, n (%) | 35 | 29 (82.9) | 35 | 22 (62.9) | 0.060 b |
| Number of follow‐up visits made, n (%) | 35 | 35 | 0.053 a | ||
| 0 | 1 (2.9) | 4 (11.4) | |||
| 1 | 2 (5.7) | 5 (14.3) | |||
| 2 | 3 (8.6) | 4 (11.4) | |||
| 3 | 29 (82.9) | 22 (62.9) | |||
Note: Statistics presented as n (column %).
Wilcoxon rank sum test.
Pearson χ2 test.
None of the medication measures (medication tolerance, adherence, and compliance to full or lower dose) significantly differed between groups. Medication compliance to full dose was 80% in the virtual group and 75% in the in‐person group (not statistically significantly different between groups). Among completers, nonadherence was documented in two patients (6.7%) in the virtual group and four patients in the in‐person group. All of them were due to being unable to fill the prescription within the window allowed by the state of Ohio (28 ± 7 days).
Safety
Table 3 demonstrates tolerability of full dose of phentermine completers: 85.7% in the virtual group and 90% in the in‐person group. Only three patients (two in the virtual group and one in in‐person group) required a dose decreased related to side effects (hypertension in two patients and insomnia in one patient). Also, among the completers, a total of six patients discontinued phentermine. Three of those patients stopped phentermine because of side effects: one in the in‐person group because of insomnia, anxiety, and constipation; and two patients in the virtual group, one because of palpitations and tachycardia (resting tachycardia was confirmed with the remote blood pressure cuff) and the other patient because of dizziness and vomiting. One patient discontinued the medication as instructed by another physician in the context of hospitalization not related to the study. Two patients discontinued use because of nonadherence, as stated previously.
No serious adverse events during the study were reported. Table 4 demonstrates the observed side‐effect profile of phentermine, which did not differ between groups at any time points. The most common side effect was dry mouth followed by palpitations and constipation. Supporting Information Table S1 shows average blood pressure changes and heart rate changes.
TABLE 4.
Side effects
| Factor | Overall (N = 70) | Virtual visits (n = 35) | In‐person encounters (n = 35) | p value | |||
|---|---|---|---|---|---|---|---|
| n | Statistics | n | Statistics | n | Statistics | ||
| Any side effects of phentermine | 65 | 24 (36.9) | 34 | 12 (35.3) | 31 | 12 (38.7) | 0.78 a |
| Dry mouth | 65 | 11 (16.9) | 34 | 3 (8.8) | 31 | 8 (25.8) | 0.068 a |
| Palpitation | 65 | 4 (6.2) | 34 | 3 (8.8) | 31 | 1 (3.2) | 0.61 b |
| Constipation | 65 | 5 (7.7) | 34 | 2 (5.9) | 31 | 3 (9.7) | 0.66 b |
| Diarrhea | 65 | 0 (0.00) | 34 | 0 (0.00) | 31 | 0 (0.00) | |
| Insomnia | 65 | 4 (6.2) | 34 | 1 (2.9) | 31 | 3 (9.7) | 0.34 b |
| Headache | 65 | 3 (4.6) | 34 | 1 (2.9) | 31 | 2 (6.5) | 0.60 b |
| High blood pressure | 65 | 5 (7.7) | 34 | 5 (14.7) | 31 | 0 (0.00) | 0.054 b |
| Anxiety | 65 | 3 (4.6) | 34 | 1 (2.9) | 31 | 2 (6.5) | 0.60 b |
| Other c | 65 | 6 (9.2) | 34 | 3 (8.8) | 31 | 3 (9.7) | 0.99 b |
Note: Statistics presented as n (column %).
Pearson χ2 test.
Fisher exact test.
Other side effects included the following: bad taste in mouth, smell disturbances, dizziness, nausea, vomiting, mood disturbances, and tachycardia.
DISCUSSION
In this single‐center, randomized clinical trial, adults with age between 18 and 65 years with obesity/overweight with comorbidities who were randomized to virtual visits demonstrated similar weight‐loss results (noninferior but not superior) to in‐person visits at 12 weeks while receiving phentermine. The study was not statistically powered to show differences on the secondary outcomes (e.g., categorical weight loss). There was no difference with respect to medication compliance or tolerance between groups. Patients randomized to the virtual group were more likely to attend study visits even though the results were not statistically significant. These results demonstrate that a virtual visit intervention for management of obesity can produce meaningful results compared with the gold standard of in‐person visits.
This study addressed an important clinical issue in the field of obesity, specifically, the use of virtual visits (using remote weight, blood pressure, and pulse measurements) to deliver effective obesity care using AOMs, particularly one that is a controlled substance, as part of a comprehensive obesity therapeutic program. We were able to demonstrate a clinically significant weight loss in both groups, with an average weight loss of 7.2% (6.6% with virtual visits vs. 7.7% with the in‐person encounters) and with the majority of patients able to reach at least 5% weight reduction. These results are consistent with a pooled analysis of short‐term phentermine trials in which patients on phentermine lost an average of 7.92 lb more weight than placebo [16].
Our study demonstrated that the use of virtual visits is convenient for patients on phentermine, when compared with in‐person visits. Certainly, we understand there are additional benefits to virtual visits, including improved access to medical care [17] and reduced loss of work time, commute time, and frequency of in‐person visits. In addition, virtual visits can be more convenient for patients who can see their provider from home, workplace, or even from their automobile. This study demonstrates that obesity care, including the use of AOMs like phentermine, can be conveniently and effectively delivered through virtual visits. This can not only break down barriers that may be contributing to the underuse of AOMs [18], but can also address direct or indirect barriers of other restrictive regulations seen in states like Ohio, which mandate patients to be seen every 28 days in order for phentermine to be refilled. In general, phentermine is the most commonly prescribed AOM and it remains a safe, tolerable, and cost‐effective medication [19, 20, 21]. Younger patients with obesity who have a low cardiovascular disease risk are the ideal candidates to manage virtually. Patients' use of technology and, perhaps, their medical literacy to describe potential side effects should be taken into consideration when selecting ideal patients.
Similar to clinical practice experiences with AOMs like phentermine, our study showed that patients tolerated phentermine at different doses. Patients who needed to de‐escalate the dose of phentermine owing to side effects tolerated the lower dose. We believe the use of remote monitoring (body weight scale and blood pressure/pulse monitoring) in our virtual visit group facilitated early identification and management of medication side effects. This may explain why all of the cases of high blood pressure as a side effect of phentermine were reported in the virtual visit group compared with none in the in‐person group. However, it may also suggest that cases of high blood pressure in the in‐person group may have been missed owing to the reduced frequency of blood pressure monitoring (i.e., at the medical visit). Therefore, early detection of side effects may be another advantage of remote monitoring in patients taking AOMs like phentermine regardless of the use of virtual visits. In addition, remote monitoring provided the opportunity to consistently and accurately track body weight (i.e., using the same scale and measurement technique [i.e., with or with clothing]).
The emergency COVID‐19 pandemic has forever changed the landscape of health care, enhancing the use of telemedicine and remote monitoring of care. As an increase of environmental stressors and other factors led to weight gain among Americans [22], the management of obesity became an important part of medical care. Telehealth has the potential to soon become the new standard of care [23, 24], and studies like ours demonstrate that obesity care, including a combination of behavioral and pharmacological interventions, can be effectively delivered. Although the number of the patients in our study was relatively small, the study was appropriately powered to demonstrate noninferior weight loss with virtual versus in‐person care. It is important to understand that this was a short‐term randomized controlled trial that is unable to address long‐term outcomes or side effects of phentermine. Close monitoring of patients on phentermine is recommended given that it is classified as a controlled substance and that it has a potential for side effects. At the present time, we are not aware of other studies demonstrating the effective use of phentermine (or other AOMs) in the treatment of obesity via virtual visits. We believe this study will add to our understanding of virtual visits and provide insight to the future of obesity care.
This study has several strengths, including the ability of conducting it during the challenges of the COVID‐19 pandemic. Data from remote scales and blood pressure cuffs were easily incorporated into medical charts and they provided valuable objective information to patients and providers. Some of the limitations of this study include generalizability as this is a single institution with a relatively small number of patients. We believe the patient demographics were similar to the American population that seeks treatment of their obesity and that our data were appropriately statistically powered despite the small number of patients. Even though the initial visit for all patients before randomization was an in‐person visit, we believe the management of obesity through remote monitoring and virtual visits was uniquely different from the in‐person group. That said, this approach may be best described as a hybrid approach with telehealth follow‐up visits. The heavy predominance of female sex has been consistently observed with the majority of weight‐loss clinical trials. This report does not provide long‐term data regarding weight loss and compliance; however, in many US states, phentermine is approved to be used only up to 3 months, so assessing chronic outcomes is not possible at this point in time. There was also a higher percentage of patients for whom final weight was unable to be obtained than anticipated in the in‐person group, as most of those patients when called were not able to provide a self‐report weight owing to lack of a home scale. Some patients were not able to renew their phentermine prescription because they were out of the window for renewal (Ohio law allows a patient to renew phentermine only every 28 ± 7 days). The majority of patients in the trial decided not to see a nutrition team member and/or exercise physiologist owing to costs and lack of insurance coverage. Even though we discussed nutrition and exercise topics during the visits, we were not able to provide a full multidisciplinary approach to all patients.
CONCLUSION
This study addresses a very important issue in the management of obesity: the use of virtual visits to facilitate treatment of obesity with phentermine. The results of this trial indicate that the use of virtual visits in adults aged 18 to 65 years with obesity or BMI ≥ 27 and comorbidities and with no contraindication to phentermine is noninferior when compared with in‐person care in achieving meaningful weight loss with the use of phentermine. This trial was not designed to address the safety of phentermine in the short or long term. Additional trials focused on the administration of obesity care and prescribing of AOMs, particularly controlled substances, over both the short and long term are necessary in order to optimize the quality of care to those with overweight or obesity and to determine the best modality by which that care is delivered.
FUNDING INFORMATION
This research was financially supported from internal Cleveland Clinic accounts dedicated to investigator‐initiated research as well as an internally awarded research grant.
CONFLICT OF INTEREST
W. Scott Butsch reports being a health care consultant for the Clinical Advisory Board for Rhythm Pharmaceuticals and the Obesity Educational Advisory Board for Novo Nordisk, A/S. Kevin M. Pantalone in the past 12 months reports receiving research support from Bayer, Novo Nordisk A/S, Merck, and Twinhealth, consulting honoraria from AstraZeneca, Bayer, Corcept Therapeutics, Diasome, Eli Lilly & Co., Merck, Novo Nordisk A/S, and Sanofi, and speaker honoraria from AstraZeneca, Corcept Therapeutics, Merck, and Novo Nordisk A/S. The other authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT04614545.
Supporting information
Figure S1 Waterfall plot—Face‐to‐face patients. Individual weight loss among all patients from baseline to week 12.
Figure S2 Waterfall plot—Virtual patients. Individual weight loss among all patients from baseline to week 12.
Table S1 Summary of blood pressure and heart rate changes.
Griebeler ML, Butsch WS, Rodriguez P, et al. The use of virtual visits for obesity pharmacotherapy in patients with overweight or obesity compared with in‐person encounters. Obesity (Silver Spring). 2022;30(11):2194‐2203. doi: 10.1002/oby.23548
Funding information Internal Cleveland Clinic Grant for Investigator Initiated Research
DATA AVAILABILITY STATEMENT
Deidentified study data representing reported findings may be available on reasonable request from the corresponding author.
REFERENCES
- 1. Flegal KM, Kruszon‐Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen MD, Ryan DH, Apovian CM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102‐S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84:951‐958. [DOI] [PubMed] [Google Scholar]
- 5. Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220‐233. [DOI] [PubMed] [Google Scholar]
- 6. LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity‐related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:1172‐1191. [DOI] [PubMed] [Google Scholar]
- 7. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 8. Brown JD, Hales S, Evans TE, et al. Description, utilisation and results from a telehealth primary care weight management intervention for adults with obesity in South Carolina. J Telemed Telecare. 2020;26:28‐35. [DOI] [PubMed] [Google Scholar]
- 9. Baum A, Kaboli PJ, Schwartz MD. Reduced in‐person and increased telehealth outpatient visits during the COVID‐19 pandemic. Ann Intern Med. 2021;174:129‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasselli JR, Juray S, Trasino SE. Success and failures of telehealth during COVID‐19 should inform digital applications to combat obesity. Obes Sci Pract. 2021;8:254‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wadden TA, Neiberg RH, Wing RR, et al. The Look AHEAD Research Group. Four‐year weight losses in the look AHEAD study: factors associated with long‐term success. Obesity (Silver Spring). 2011;19:1987‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duenas S, Arnoriaga M, Brizuela A, et al. Information and communication technology in obesity. Integr Obes Diabetes. 2017;3. doi: 10.15761/IOD.1000184 [DOI] [Google Scholar]
- 13. Jiandani D, Wharton S, Rotondi MA, Ardern CI, Kuk JL. Predictors of early attrition and successful weight loss in patients attending an obesity management program. BMC Obes. 2016;3:14. doi: 10.1186/s40608-016-0098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allison DB, Gadde KM, Garvey WT, et al. Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bell ML, Fairclough DL. Practical and statistical issues in missing data for longitudinal patient‐reported outcomes. Stat Methods Med Res. 2014;23:440‐459. [DOI] [PubMed] [Google Scholar]
- 16. Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Ann Rheum Dis. 2007;66:433‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ross KM, Hong YR, Krukowski RA, Miller DR, Lemas DJ, Cardel MI. Acceptability of research and health care visits during the COVID‐19 pandemic: cross‐sectional survey study. JMIR Form Res. 2021;5:e27185. doi: 10.2196/27185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Claridy MD, Czepiel KS, Bajaj SS, Stanford FC. Treatment of obesity: pharmacotherapy trends of office‐based visits in the United States from 2011 to 2016. Mayo Clin Proc. 2021;96:2991‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pantalone KM, Smolarz BG, Ramasamy A, et al. Effectiveness of combining antiobesity medication with an employer‐based weight management program for treatment of obesity: a randomized clinical trial. JAMA Netw Open. 2021;4:e2116595. doi: 10.1001/jamanetworkopen.2021.16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shibuya K, Ali KF, Ji X, et al. The benefit of shot‐term weight loss with anti‐obesity medications in real‐world clinical practice. Endocr Pract. 2019;25:1022‐1028. [DOI] [PubMed] [Google Scholar]
- 21. Lee M, Lauren BN, Zhan T, et al. The cost‐effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract. 2020;6:162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Razzoli M, Pearson C, Crow S, Bartolomucci A. Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76:154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burguera B, Pantalone KM, Griebeler ML, et al. The need and benefit of implementing telemedicine in clinical practice. Endocr Pract. 2020;26:794‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griebeler ML, Pantalone KM, Gambino R, et al. The importance of implementing inpatient virtual coverage in an endocrinology practice: lessons learned thus far from the COVID‐19 pandemic. Clin Diabetes Endocrinol. 2021;7:5. doi: 10.1186/s40842-021-00118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Waterfall plot—Face‐to‐face patients. Individual weight loss among all patients from baseline to week 12.
Figure S2 Waterfall plot—Virtual patients. Individual weight loss among all patients from baseline to week 12.
Table S1 Summary of blood pressure and heart rate changes.
Data Availability Statement
Deidentified study data representing reported findings may be available on reasonable request from the corresponding author.


