Abstract
Objective
In patients with status epilepticus (SE), the clinical significance of ictal changes on magnetic resonance imaging (MRI) is insufficiently understood. We here studied whether the presence of ictal MRI changes was associated with neurological deterioration at discharge.
Methods
The retrospective cohort comprised all identifiable patients treated at Odense University Hospital in the period 2008–2017. All amenable MRIs were systemically screened for ictal changes. Patient demographics, electroencephalography, seizure characteristics, treatment, and SE duration were assessed. Neurological status was estimated before and after SE. The predefined endpoint was the association of neurological deterioration and ictal MRI changes.
Results
Of 261 eligible patients, 101 received at least one MRI during SE or within 7 days after cessation; 43.6% (44/101) had SE due to non‐ or less brain‐damaging etiologies. Patients who received MRI had a longer duration of SE, less frequently had a history of epilepsy, and were more likely to have SE due to unknown causes. Basic characteristics (including electroencephalographic features defined by the Salzburg criteria) did not differ between patients with (n = 20) and without (n = 81) ictal MRI changes. Timing of MRI was important; postictal changes were rare within the first 24 h and hardly seen >5 days after cessation of SE. Ictal MRI changes were associated with a higher risk of neurological deterioration at discharge irrespective of etiology. Furthermore, they were associated with a longer duration of SE and higher long‐term mortality that reached statistical significance in patients with non‐ or less brain‐damaging etiologies.
Significance
In this retrospective cohort, ictal changes on MRI were associated with a higher risk of neurological deterioration at discharge and, possibly, with a longer duration of SE and poorer survival.
Keywords: duration, DWI, ictal MRI changes, imaging, long‐term outcome, status epilepticus
Key Points.
Ictal MRI changes are associated with neurological deterioration
The chance of detecting ictal MRI changes decreases rapidly after cessation of SE
Ictal MRI changes were associated with the duration of SE
Ictal MRI changes may be associated with long‐term survival
1. INTRODUCTION
Ictal magnetic resonance imaging (MRI) changes after status epilepticus (SE) or single seizures are a well‐known phenomenon and are also referred to as peri‐ictal or postictal MRI changes. 1 , 2 , 3 Increased signal of the cerebral cortex and subcortical structures on T2 and diffusion‐weighted images (DWI) are the most common manifestations; however, thalamic and basal ganglia affection are also well described. 4 , 5 In most patients, ictal changes on MRI match the epileptic focus of ongoing seizure activity on electroencephalography (EEG). However, transient MRI changes compatible with ictal changes have also been described in the nonaffected hemisphere. 4 , 5 , 6 , 7 , 8 Notably, especially lateralized periodic discharges appear to be associated with ictal MRI changes. 7 , 9 Several longitudinal case series proved the transient nature of these changes in the majority of patients, with an 80% remission rate after 1–6 weeks. 10 Patients with incomplete remission on control MRI typically showed local atrophy or changes described as gliosis or cortical necrosis, whereas DWI changes disappeared. 5 , 6 , 8 , 10 , 11 , 12 Case studies reporting biopsy results from the affected cortical regions described local edema combined with histopathological signs of hypoxia of the affected neuronal layers, 5 , 13 , 14 , 15 which is in line with results from animal experiments indicating mitochondrial dysfunction, calcium accumulation, and subsequent necrosis. 16
Despite recent advances in diagnostics, the clinical significance of these changes remains obscure. The majority of published studies focused on the description of radiological changes, and cohorts with >20 patients and systematic clinical long‐term follow‐up have not yet been reported. 4 , 7 , 10 , 11 Published cohorts reported null associations between in‐hospital mortality and ictal MRI changes 6 , 7 , 9 ; data on long‐term survival of patients has not been published so far. Acquired and persistent neurological deficits likely linked to the ictal MRI changes have been described in multiple case reports. 6 , 17 , 18 A selection bias is, however, difficult to exclude, and a common critique was the difficulty of rejecting diffuse or focal anoxia as an alternative explanation for the MRI changes. 19 Thus, available data supporting the concept of SE‐induced neuronal damage are controversial, and etiology is usually considered the major determinant of outcome. 20 , 21 A recent study comprising 25 patients with SE and ictal MRI changes reported a higher rate of fatal etiologies among patients with ictal changes and a higher rate of patients with increased disability quantified using the modified Rankin Scale at discharge. 22 However, the data did not allow conclusions on the association between ictal changes and clinical deterioration. We therefore aimed at clarifying the association between ictal MRI changes and neurological deterioration in a large cohort of extensively characterized patients with SE and available long‐term follow‐up.
2. MATERIALS AND METHODS
2.1. Patients
Patient identification and selection are shown in Figure 1, and the cohort has previously been reported in another study. 23 Data reporting complies with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. 24 In brief, between January 1, 2008 and December 31, 2017, patients were retrospectively identified at the Odense University Hospital (OUH), from referrals for acute EEG or based on the diagnosis of SE at discharge (n = 261 patients). All patients were treated at OUH and had either clinical or EEG‐verified SE diagnosed using the International League Against Epilepsy (ILAE) or Salzburg criteria. 25 , 26 The study was approved by the Danish Health Authorities (3‐3013‐26 611) and the Region of Southern Denmark (data handling: 18‐58 576).
FIGURE 1.

STROBE (Strengthening the Reporting of Observational Studies in Epidemiology)‐compliant flowchart for patient screening and identification. DWI, diffusion‐weighted imaging; EEG, electroencephalography; FLAIR, fluid‐attenuated inversion recovery; ICD, International Classification of Diseases; MRI, magnetic resonance imaging; SE, status epilepticus.
All patients were screened for MRIs performed at the Department of Radiology during or within 7 days after cessation of SE; all MRIs were assessed retrospectively. Preictal MRIs and MRIs without fluid‐attenuated inversion recovery (FLAIR) and DWI sequences were excluded from the study.
Clinical characterization was based on the complete patients' electronic medical files and performed by L.E.R. and O.M.; if consensus could not be reached, C.P.B. provided the final assessment. To secure a quasiblinded design, clinical characterization was performed as the first step; analysis of MRI and EEG was performed later and independently.
2.2. MRI evaluation
MRIs were systematically screened for ictal changes by a first MRI examiner (S.M.D.) who was blinded to the original radiological report and all clinical details (e.g., seizure type, etiology, medication, and duration of SE) apart from the SE diagnosis. During the study period, patients were scanned with an Achieva dStream 3.0T (Philips Medical Systems), and occasionally with an Ingenia 3.0T (Philips Medical Systems). The type of scanner, was, however, not assessed for individual patients. Possible ictal changes on MRI were defined as an increased signal of the cerebral cortex and subcortical structures on T2/FLAIR and DWI sequences. All detected ictal changes were registered. Results from the screening were reviewed and compared with the original radiological report by a second rater (C.D.C.). In patients with disagreements between the screening results and the radiological reports, an expert neuroradiologist (N.N.) reassessed MRIs, aware of the SE diagnosis but blinded to all other details. Altogether, 49 MRIs were reassessed. Figure 1 gives the steps of the screening process.
2.3. Outcomes
Data collection was based on electronic medical records as previously described by Roberg et al. 23 and allowed for a complete assessment of demographics, seizure semiology, SE severity scores, and etiology of SE. Survival data were available for all patients due to the linkage of the electronic medical files with the Danish Civil Registration System. For all patients, National Institutes of Health Stroke Scale (NIHSS) scores were estimated by L.E.R. and O.M. (supervised by C.P.B.) before SE and at discharge/first control after discharge; an increase in NIHSS was defined as neurological deterioration, and an increase in NIHSS of >5 was defined as “significant neurological deficit.” 27 Estimation of NIHSS before admission was based on description of the patient by their relatives and previous medical notes, if available in case of, for example, previous stroke. Estimation of NIHSS at discharge was based on the neurological examination at discharge, the detailed examinations by the physiotherapist used for rehabilitation assessment, and—if available—a neurological examination at first clinical visit after discharge. A score of 0 was given if data were not available. Patients who died in hospital received an NIHSS score of 42 at discharge.
Semiology and etiology were classified based on the ILAE recommendations. 26 , 28 Furthermore, we individually assessed the etiology of each patient and classified it as likely non‐ or less brain‐damaging or not. 23 The group of non‐ or less brain‐damaging etiologies was defined by the lack of acute changes (e.g., stroke, bleeding, tumor) on cerebral imaging and no clinical indication for infection/autoimmune encephalitis. The length of SE was estimated based on the information provided by paramedics, the emergency department, and the notes of the treating neurologist.
All available EEGs were analyzed systematically according to the Salzburg criteria 25 by L.E.R., O.M., and T.K., who were blinded to clinical data apart from the SE diagnosis. Details of the analysis were published previously in Monsson et al. 29
2.4. Statistics
Data were stored using REDCap provided by the Region of Southern Denmark. 30 The primary predefined endpoint of the study was the association of neurological deterioration with the presence of ictal MRI changes in all patients with SE; all other analyses were exploratory. The statistical tests used are given in the tables/figures, and statistical significance was assumed for alpha error > .05. Due to statistical planning, correction for multiple comparisons was omitted for the primary endpoint but performed for all exploratory analyses. For the exploratory binary logistic regression analysis, 2‐year overall survival was used as endpoint. Due to the low number of patients, Epidemiology‐Based Mortality Score in Status Epilepticus (EMSE), with the components etiology, comorbidity, age, and EEG, was pragmatically chosen as surrogate marker for disease severity. Linear association of EMSE with SE severity was postulated and not tested. IBM SPSS (v29) was used for statistical analyses.
3. RESULTS
3.1. Characteristics of the cohort
From the previously described cohort of 261 patients, 23 101 had at least one MRI during SE or within 7 days after cessation of SE. Characteristics of patients with and without MRI and with and without ictal MRI changes are given in Table 1. Patients receiving MRI in temporal proximity to SE had a longer duration of SE and a higher rate of treatment in intensive care. Furthermore, the underlying etiologies differed, with more unknown etiologies and fewer patients with previous history of epilepsy in the MRI group. Survival and the rate of neurological deterioration at discharge did not differ between patients who received MRI and patients who did not (Table 1).
TABLE 1.
Patient demographics
| Characteristic | All patients | Patients with MRI, all etiologies | All etiologies, MRI vs. no MRI, p a | Patients with MRI, non‐ or less brain‐damaging etiologies | |||
|---|---|---|---|---|---|---|---|
| Patients, n | 261 | 101 | 44 | ||||
| Sex, n (%) | |||||||
| Female | 132 (50.6%) | 48 (47.5%) | n.s. | 19 (43.2%) | |||
| Male | 129 (49.4%) | 53 (52.5%) | 25 (56.8%) | ||||
| Age, mean years (IQR) | 67.2 (58.0–78.0) | 65.5 (56.0–75.0) | n.s. b | 66.2 (55.0–75.5) | |||
| SE duration, mean h (IQR) | 132.8 (23.2–157.1) | 193.5 (47.1–210.3) | <.001 b , c , d | 235.6 (67.2–281.8) | |||
| GTC seizures, n (%) | 1.5 (.0–2.0) | 1.7 (.0–2.0) | n.s. | 1.6 (.0–2.5) | |||
| Refractory status, n (%) | |||||||
| No | 84 (32.2%) | 29 (28.7%) | n.s. | 11 (25.0%) | |||
| Yes | 177 (67.8%) | 72 (71.3%) | 33 (75.0%) | ||||
| Treatment in ICU, n (%) | |||||||
| No | 180 (69.0%) | 60 (59.4%) | .008 c , d | 23 (52.3%) | |||
| Yes | 81 (31.0%) | 41 (40.6%) | 21 (47.7%) | ||||
| Etiology per ILAE, n (%) | |||||||
| Acute symptomatic | 102 (39.1%) | 39 (38.6%) | .02 d | 0 (.0%) | |||
| Remote symptomatic | 80 (30.7%) | 24 (23.8%) | 27 (61.4%) | ||||
| Progressive central nervous system disorder | 44 (16.9%) | 21 (20.8%) | 0 (.0%) | ||||
| SE in defined electroclinical syndromes | 8 (3.1%) | 1 (1.0%) | 1 (2.3%) | ||||
| Unknown | 27 (10.3%) | 16 (15.8%) | 16 (36.4%) | ||||
| History of epilepsy per STESS, n (%) | |||||||
| No | 146 (55.9%) | 71 (70.3%) | <.001 c , d | 25 (56.8%) | |||
| Yes | 115 (44.1%) | 30 (29.7%) | 19 (43.2%) | ||||
| Level of consciousness per STESS, n (%) | |||||||
| Alert to somnolent or confused/GCS > 12 [0] | 120 (46.0%) | 48 (47.5%) | n.s. | 17 (38.6%) | |||
| Stuporous to comatose/GCS < 13 [1] | 141 (54.0%) | 53 (52.5%) | 27 (61.4%) | ||||
| Change in NIHSS grouped, n (%) | |||||||
| <5‐point NIHSS increase | 151 (70.2%) | 56 (66.7%) | n.s. | 26 (66.7%) | |||
| 5–10‐point NIHSS increase | 37 (17.2%) | 16 (19.0%) | 7 (17.9%) | ||||
| >10‐point NIHSS increase | 27 (12.6%) | 12 (14.3%) | 6 (15.4%) | ||||
| Survival status at 3 months, n (%) | |||||||
| Alive at 3 months | 186 (71.3%) | 74 (73.3%) | n.s. | 35 (79.5%) | |||
| Dead at 3 months | 75 (28.7%) | 27 (26.7%) | 9 (20.5%) | ||||
| Survival status at 2 years, n (%) | |||||||
| Alive at 2 years | 138 (52.9%) | 50 (49.5%) | n.s. | 25 (56.8%) | |||
| Dead at 2 years | 123 (47.1%) | 51 (50.5%) | 19 (43.2%) | ||||
Abbreviations: GCS, Glasgow Coma Scale; GTC, generalized tonic–clonic; ICU, intensive care unit; ILAE, International League Against Epilepsy; IQR, interquartile range; MRI, magnetic resonance imaging; n.s., not significant; NIHSS, National Institutes of Health Stroke Scale; SE, status epilepticus; STESS, Status Epilepticus Severity Score.
Chi‐squared test.
Mann–Whitney U test; crude p‐values are given.
Significant associations after controlling for multiple testing using Bonferroni–Holm correction.
Statistically significant.
3.2. Association of ictal MRI changes and neurological deterioration at first follow‐up/discharge
Basic demographics, prognostic factors, treatment, and etiology were similar between patients with and without ictal MRI changes (Table 2). Although the vast majority of patients with ictal MRI changes had definite or possible nonconvulsive SE, neither the proportion of nonconvulsive SE (Table 2) nor the different components of the Salzburg criteria differed consistently (Table S1). In contrast, the association of ictal MRI changes and neurological deterioration at first follow‐up/discharge differed significantly between patients with and without ictal MRI changes. As compared to patients with MRIs without ictal changes (mean increase of NIHSS = .8, interquartile range [IQR] = .0–20.0), ictal MRI changes were associated with a higher increase in NIHSS scores at discharge (mean increase in NIHSS = 4.9, IQR = .0–1.5.0; p = .006, Kruskal–Wallis test) as compared to admission (Figure 2A). Mortality was higher in the presence of ictal MRI changes, but the difference was not statistically significant in a Kaplan–Meier analysis (Figure 2B).
TABLE 2.
Characteristics of patients with and without ictal MRI changes
| Characteristic | Non‐ or less brain‐damaging etiologies | p a | All etiologies | p a | ||
|---|---|---|---|---|---|---|
| No ictal MRI changes | Ictal MRI changes | No ictal MRI changes | Ictal MRI changes | |||
| All patients, n (%) | 35 (100%) | 9 (100%) | 81 (100%) | 20 (100%) | ||
| Sex, n (%) | ||||||
| Female | 16 (45.7%) | 3 (33.3%) | n.s. | 38 (46.9%) | 10 (50.0%) | n.s. |
| Male | 19 (54.3%) | 6 (66.7%) | 43 (53.1%) | 10 (50.0%) | ||
| Age at admission, mean years (IQR) | 66.5 (54.0–77.0) | 65.2 (56.0–74.0) | n.s. b | 65.4 (56.0–75.0) | 65.8 (57.5–74.5) | n.s. b |
| Time between cessation and discharge, mean days (IQR) | 14.4 (3.0–19.9) | 11.5 (2.0–21.0) | n.s. | 16.0 (3.0–21.0) | 13.5 (2.0–22.0) | n.s. |
| Refractory status, n (%) | ||||||
| No | 9 (25.7%) | 2 (22.2%) | n.s. | 27 (33.3%) | 2 (10.0%) | .04 c |
| Yes | 26 (74.3%) | 7 (77.8%) | 54 (66.7%) | 18 (90.0%) | ||
| Treatment in ICU, n (%) | ||||||
| No | 20 (57.1%) | 3 (33.3%) | n.s. | 49 (60.5%) | 11 (55.0%) | n.s. |
| Yes | 15 (42.9%) | 6 (66.7%) | 32 (39.5%) | 9 (45.0%) | ||
| Etiology per ILAE, n (%) | ||||||
| Acute symptomatic | 0 (.0%) | 0 (.0%) | n.s. | 32 (39.5%) | 7 (35.0%) | n.s. |
| Remote symptomatic | 21 (60.0%) | 6 (66.7%) | 18 (22.2%) | 6 (30.0%) | ||
| Progressive disorders | 0 (.0%) | 0 (.0%) | 17 (21.0%) | 4 (20.0%) | ||
| Electroclinical syndromes | 1 (2.9%) | 0 (.0%) | 1 (1.2%) | 0 (.0%) | ||
| Unknown | 13 (37.1%) | 3 (33.3%) | 13 (16.0%) | 3 (15.0%) | ||
| History of epilepsy, n (%) | ||||||
| No | 20 (57.1%) | 5 (55.6%) | n.s. | 57 (70.4%) | 14 (70.0%) | n.s. |
| Yes | 15 (42.9%) | 4 (44.4%) | 24 (29.6%) | 6 (30.0%) | ||
| Level of consciousness, n (%) | ||||||
| GCS > 12 | 15 (42.9%) | 2 (22.2%) | n.s. | 39 (48.1%) | 9 (45.0%) | n.s. |
| GCS < 13 | 20 (57.1%) | 7 (77.8%) | 42 (51.9%) | 11 (55.0%) | ||
| NCSE per Salzburg criteria, n (%) | ||||||
| Definite | 19 (55.9%) | 6 (66.7%) | n.s. | 49 (62.8%) | 15 (75.0%) | n.s. |
| Possible | 7 (20.6%) | 2 (22.2%) | 15 (19.2%) | 4 (20.0%) | ||
| No NCSE | 8 (23.5%) | 1 (11.2%) | 14 (17.9%) | 1 (5.0%) | ||
Abbreviations: GCS, Glasgow Coma Scale; ICU, intensive care unit; ILAE, International League Against Epilepsy; IQR, interquartile range; MRI, magnetic resonance imaging; n.s., not significant; NCSE, nonconvulsive SE; SE, status epilepticus.
Chi‐squared test.
Mann–Whitney U test; crude p‐values are given. All p‐values given become nonsignificant after controlling for multiple testing using Bonferroni–Holm correction.
Statistically significant.
FIGURE 2.

Neurological deterioration and survival. (A) Neurological deterioration in patients with status epilepticus (SE) with and without ictal magnetic resonance imaging (MRI) changes (p = .07, chi‐squared test; the group with National Institutes of Health Stroke Scale [NIHSS] score > 10 includes patients who died in‐hospital). (B) Survival of patients with and without ictal MRI changes (log‐rank test). (C) Neurological deterioration in patients with SE due to non‐ or less brain‐damaging etiologies depending on the presence or absence of ictal MRI changes (p = .005, chi‐squared test; the group with NIHSS > 10 includes patients who died in‐hospital). (D) Survival of patients with SE due to non‐ or less brain‐damaging etiologies and the presence or absence of ictal MRI changes (log‐rank test).
3.3. Ictal MRI changes in patients with non‐ or less brain‐damaging etiologies
An exploratory analysis of the subgroup of patients with non‐ or less brain‐damaging etiologies (n = 44) confirmed the higher increase in NIHSS at discharge (Figure 2C) in patients with ictal MRI changes. Despite the small sample size, survival differences between patients with and without ictal MRI changes reached statistical significance in this subgroup, which was defined by the lack of all kinds of acute MRI alterations apart from ictal MRI changes (Figure 2C). Given the association of disease severity, duration, and outcome, we performed exploratory logistic regression analyses. Using ictal MRI changes and EMSE (as postulated near‐linear surrogate marker for disease severity) as covariables, 2‐year overall survival appeared mainly dependent on etiology in patients with all etiologies (EMSE: p < .001, exp[b] = 1.03, 95% confidence interval [CI] for exp[b] = 1.01–1.04; ictal MRI changes: p = .17, exp[b] = .48, 95% CI for exp[b] = .16–1.39). In contrast, in patients with non‐ or less brain‐damaging etiologies, ictal MRI changes but not EMSE were significantly associated with survival after 2 years (EMSE: p = .32, exp[b] = 1.02, 95% CI for exp[b] = .99–1.05; ictal imaging changes: p = .04, exp[b] = .16, 95% CI for exp[b] = .03–.90).
3.4. Congruency of ictal MRI changes, ictal EEG focus, and neurological deterioration
Ictal MRI changes showed high congruency with ictal activity on EEG. In all but one patient, seizure activity—defined according to the Salzburg criteria—was detectable in the brain areas with ictal MRI changes (18/19, 94.7%). In four patients, ictal activity was documented also in brain regions without detectable ictal MRI changes (Table 3). With a certain amount of reservation due to the unknown lateralization of the affected brains, the pattern of neurological deficits at discharge were congruent with the ictal MRI changes in the majority of patients who survived the acute phase and developed neurological deficits (Table 3). All four patients with thalamic involvement either died or had severe neurological sequelae.
TABLE 3.
Overview of patients with ictal MRI changes
| Patient | Etiology | MRI | EEG | New significant neurological deficits, NIHSS increase ≥ 5 | ||
|---|---|---|---|---|---|---|
| Laterality | Localization | Laterality | Localization | |||
| 1 | Poststroke | Left | Hippocampal | Left | Temporal | None |
| 2 | HSV | Right | Temporal/insular | Right | Temporoparietal | None |
| 3 | Astrocytoma | Right | Hemisphere | Right | Frontotemporal | None |
| 4 | Dementia | Right | Parietal | Bilateral | Temporal | None |
| 5 | Cryptogenic | Bilateral | Temporal | Bilateral | Frontoparietal | Died before discharge |
| 6 | Poststroke | Right | Frontal | n.a. | n.a. | Orientation, fixed gaze, dysarthria, paresis right arm |
| 7 | Microbleeds | Right | Parietal | Right | Parieto‐occipital | Bilateral weakness, unresponsiveness |
| 8 | Poststroke | Left | Hippocampal | Bilateral | Bilateral | Aphasia, vegetative state |
| 9 | Poststroke | Right | Hemisphere | Right | Temporal | Aphasia, severe dysarthria |
| 10 | Cryptogenic | Right | Temporal | Right | Temporal | Aphasia |
| 11 | AIE | Bilateral | Temporal | Bilateral | Temporal | Aphasia, left‐sided hemiparesis |
| 12 | HSV | Left | Temporal/insular | Left | Temporal | None |
| 13 | Cryptogenic | Left | Temporal/insular | Left | Temporal | Died before discharge |
| 14 | Lymphoma | Bilateral | Parietal | Bilateral | Left frontal and bilateral parieto‐occipital | Ataxia, dysarthria, bilateral weakness |
| 15 | Metastasis | Left | Hemisphere | Left | Temporal | Died before discharge |
| 16 | Alcohol | Right | Hemisphere | Right | Parieto‐occipital | Aphasia, right‐sided paresis |
| 17 | Poststroke | Left | Hemisphere | Left | Frontoparietal | None |
| 18 | Acute stroke | Left | Temporoparieto‐occipital | Bilateral | Frontotemporal | Died before discharge |
| 19 | Cryptogenic | Left | Temporal | Bilateral | Parieto‐occipital | Died before discharge |
| 20 | AIE | Right | Temporal | Right | Temporal | None |
Abbreviations: AIE, autoimmune encephalopathy; EEG, electroencephalograph; HSV, herpes simplex virus; MRI, magnetic resonance imaging; n.a., not available; NIHSS, National Institutes of Health Stroke Scale.
3.5. Temporal aspects of the ictal MRI changes
In two additional exploratory analyses, we studied the association of ictal MRI changes with the duration of SE and timing of the MRI scan. A higher rate of ictal MRI changes was associated with a longer duration of SE (Figure 3A,B). Ictal changes were most common in patients with an SE duration of >1 day (Figure 3C). The rate of ictal MRI changes appeared highest among patients with ongoing SE of long duration, and prevalence declined approximately 1–5 days after cessation (Figure 3C,D).
FIGURE 3.
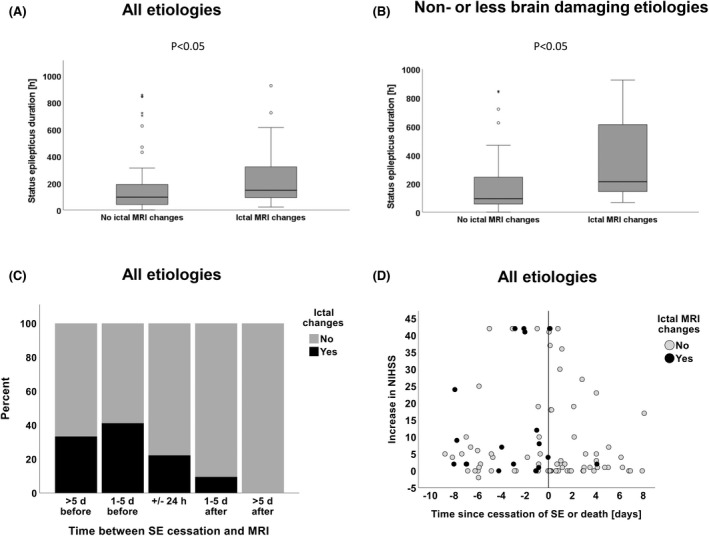
Duration and time of magnetic resonance imaging (MRI) under status epilepticus (SE). (A) Association of ictal MRI changes and the duration of SE in patients with SE of all etiologies and (B) in patients with SE due to non‐brain‐damaging etiologies (Whitney–Mann test). (C) Timing of MRI relative to the cessation of SE and the presence or absence of ictal MRI changes. (D) Association of timing of MRI, change of National Institutes of Health Stroke Scale (NIHSS) score, and ictal MRI changes. *Indicates individual patients (outlayers).
4. DISCUSSION
In this retrospective cohort, ictal MRI changes were associated with neurological deterioration in patients with SE. We found this association in all patients, including a separate group of patients with non‐ or less brain‐damaging etiologies. The latter finding makes an erroneous interpretation of, for example, diffuse or focal anoxia as ictal MRI changes unlikely. Despite the low number of patients, the associations reached statistical significance, indicating a strong and possibly causal association between SE, ictal MRI changes, and neurological outcome, which would be in keeping with the cumulative data from case reports and smaller case series. 12 , 13 , 17 , 18 However, given the lack of a suitable control population (e.g., matched patients with similar SE duration), the small sample size, and the moderate anatomical association between neurological deficits and ictal MRI changes, the causal relationship remains speculative. Although plausible, results from the exploratory logistic regression analysis remain preliminary due to the low number of patients and certainly require confirmation in additional cohorts.
We confirmed that the duration of SE is strongly associated with the rate of ictal MRI changes, which has been reported previously. 7 , 10 , 22 , 31 Furthermore, we found a clear temporal relationship between ictal MRI changes and cessation of SE. The rate of ictal changes among all MRIs was lowest if taken shortly after debut or after cessation of SE. This finding also argues against the claim that other diseases (e.g., stroke or anoxia) may mimic ictal changes. 19 , 32 The association of ictal changes with overall survival reached statistical significance in the subgroup of patients with non‐ or less brain‐damaging etiologies despite a very low number of patients. Again, this indicates a strong biological link between long‐term survival and ictal changes, which is likely explained by the neurological deterioration.
Our cohort is, like all previously published studies, limited by the low number of patients with ictal changes. The rate of ictal changes and the definitions used are in line with previous studies and a recent systematic review. 1 With 20 patients with ictal MRI changes, the number of patients in our study is on the higher end of published studies. 7 , 11 , 22 Systematic and rapid MRI >24 h after seizure onset would likely have increased the proportion of “positive” MRIs. 3 , 10 It is tempting to speculate how and whether the inclusion of early MRI would have changed the results. As expected, the cohort receiving MRIs was substantially biased toward patients with unknown etiologies and a longer duration of SE, and against patients with an epilepsy diagnosis. However, we found no significant differences in basic characteristics among patients with and without peri‐ictal changes. This supports the interpretation that the poorer outcome of patients with ictal MRI changes is essentially due to SE‐related factors and not due to selection bias; we could not confirm the association with more fatal etiologies described by Requena et al. 22 in our cohort.
Only very few patients had an MRI on follow‐up, which is an additional limitation. Although plausible, we could not study the association between atrophy after SE and the presence of neurological deterioration. Especially the natural course of persistent neurological deficits directly or likely linked to the affected regions would have been interesting. Furthermore, systematic MRI at follow‐up would have helped to unequivocally differentiate between ictal MRI changes and inflammatory changes in patients with viral encephalitis.
The quantification of neurological deterioration after SE is difficult. We chose the established NIHSS in this and related studies 23 , 29 because it appeared to be the “least bad established score” available and covers mainly supratentorial functions. 27 We found a modified Rankin Scale and Barthel index, indicating that retrospective assessment of the neurological status using NIHSS provides meaningful results. 23 However, NIHSS has obvious shortcomings, such as the insufficient coverage of temporal lobe functions that are often affected after SE and the exaggeration of a critical illness neuropathy giving scores in all four extremities. Furthermore, NIHSS is a purely descriptive score; not all neurological deficits assessed using NIHSS are likely to be due to the ictal MRI changes but may reflect side effects to treatment (e.g., ataxia) and immobilization (e.g., nonlateralized weakness). As a consequence, a direct link between the NIHSS scores and the ictal MRI changes is difficult. Given that NIHSS was the “least bad score,” the development of a dedicated score for SE‐induced deficits would be warranted.
In summary, we here show that ictal MRI changes are associated with neurological deterioration and, possibly, with increased long‐term mortality and longer duration of SE.
AUTHOR CONTRIBUTIONS
Camilla Dyremose Cornwall: Analysis of MRI and data, writing of the manuscript, approval of the manuscript. Svein Magne Dahl: Analysis of MRI, final approval of the manuscript. Nina Nguyen: Review and final assessment of MRI data, approval of the manuscript. Lars Egil Roberg, Olav Monsson: Analysis of clinical data (including EEG data), approval of the manuscript. Thomas Krøigård: Supervision of the study, assessment of EEG data, approval of the manuscript. Christoph Patrick Beier: Design of the study, supervision, funding, data analysis, writing, and approval of the manuscript.
CONFLICT OF INTEREST
C.P.B. has received honoraria from UCB, Eisai, and Arvelle. T.K. has received honoraria from UCB. The other authors do not report possible conflicts of interest.
Supporting information
TABLE S1
ACKNOWLEDGMENTS
The study was supported by a scholarship from the University of Southern Denmark to C.D.C., L.E.R., and O.M.
Cornwall CD, Dahl SM, Nguyen N, Roberg LE, Monsson O, Krøigård T, et al. Association of ictal imaging changes in status epilepticus and neurological deterioration. Epilepsia. 2022;63:2970–2980. 10.1111/epi.17404
Camilla Dyremose Cornwall and Svein Magne Dahl contributed equally to this work.
REFERENCES
- 1. Williams JA, Bede P, Doherty CP. An exploration of the spectrum of peri‐ictal MRI change; a comprehensive literature review. Seizure. 2017;50:19–32. [DOI] [PubMed] [Google Scholar]
- 2. Kramer R, Lüders H, Lesser R, Weinstein M, Dinner D, Morris H, et al. Transient focal abnormalities of neuroimaging studies during focal status epilepticus. Epilepsia. 1987;28:528–32. [DOI] [PubMed] [Google Scholar]
- 3. Hübers A, Thoma K, Schocke M, Fauser S, Ludolph AC, Kassubek J, et al. Acute DWI reductions in patients after single epileptic seizures—more common than assumed. Front Neurol. 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatzikonstantinou A, Gass A, Förster A, Hennerici MG, Szabo K. Features of acute DWI abnormalities related to status epilepticus. Epilepsy Res. 2011;97:45–51. [DOI] [PubMed] [Google Scholar]
- 5. Raghavendra S, Ashalatha R, Krishnamoorthy T, Kesavadas C, Thomas S, Radhakrishnan K. Reversible periictal MRI abnormalities: clinical correlates and long‐term outcome in 12 patients. Epilepsy Res. 2007;73:129–36. [DOI] [PubMed] [Google Scholar]
- 6. Canas N, Breia P, Soares P, Saraiva P, Calado S, Jordão C, et al. The electroclinical‐imagiological spectrum and long‐term outcome of transient periictal MRI abnormalities. Epilepsy Res. 2010a;91:240–52. [DOI] [PubMed] [Google Scholar]
- 7. Giovannini G, Kuchukhidze G, McCoy MR, Meletti S, Trinka E. Neuroimaging alterations related to status epilepticus in an adult population: definition of MRI findings and clinical‐EEG correlation. Epilepsia. 2018;59:120–7. [DOI] [PubMed] [Google Scholar]
- 8. Xiang T, Li G, Liang Y, Zhou J. A wide spectrum of variably periictal MRI abnormalities induced by a single or a cluster of seizures. J Neurol Sci. 2014;343:167–72. [DOI] [PubMed] [Google Scholar]
- 9. Rennebaum F, Kassubek J, Pinkhardt E, Hübers A, Ludolph AC, Schocke M, et al. Status epilepticus: clinical characteristics and EEG patterns associated with and without MRI diffusion restriction in 69 patients. Epilepsy Res. 2016;120:55–64. [DOI] [PubMed] [Google Scholar]
- 10. Jabeen SA, Cherukuri P, Mridula R, Harshavardhana KR, Gaddamanugu P, Sarva S, et al. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin Neurol Neurosurg. 2017;155:70–4. [DOI] [PubMed] [Google Scholar]
- 11. Cianfoni A, Caulo M, Cerase A, Della Marca G, Falcone C, Di Lella GM, et al. Seizure‐induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013;82:1964–72. [DOI] [PubMed] [Google Scholar]
- 12. Meierkord H, Wieshmann U, Niehaus L, Lehmann R. Structural consequences of status epilepticus demonstrated with serial magnetic resonance imaging. Acta Neurol Scand. 1997;96:127–32. [DOI] [PubMed] [Google Scholar]
- 13. Arman F, Kaya D, Dincer A, Sav A, Necmettin Pamir M. Serial EEG and MRI changes in status epilepticus‐induced excitotoxic neuronal necrosis. Epileptic Disord. 2011;13:446–51. [DOI] [PubMed] [Google Scholar]
- 14. Milligan TA, Zamani A, Bromfield E. Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure. 2009;18:104–8. [DOI] [PubMed] [Google Scholar]
- 15. Nixon J, Bateman D, Moss T. An MRI and neuropathological study of a case of fatal status epilepticus. Seizure. 2001;10:588–91. [DOI] [PubMed] [Google Scholar]
- 16. Walker MC. Pathophysiology of status epilepticus. Neurosci Lett. 2018;667:84–91. [DOI] [PubMed] [Google Scholar]
- 17. Canas N, Soares P, Calado S, Pestana R, Ribeiro C, Vale J. Pathophysiology and long‐term outcome of reversible tumor‐like lesions induced by presenting status epilepticus. J Neuroimaging. 2010b;20:169–74. [DOI] [PubMed] [Google Scholar]
- 18. Donaire A, Carreno M, Gomez B, Fossas P, Bargalló N, Agudo R, et al. Cortical laminar necrosis related to prolonged focal status epilepticus. J Neurol Neurosurg Psychiatry. 2006;77:104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grillo E. Postictal MRI abnormalities and seizure‐induced brain injury: notions to be challenged. Epilepsy Behav. 2015;44:195–9. [DOI] [PubMed] [Google Scholar]
- 20. Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16:323–31. [DOI] [PubMed] [Google Scholar]
- 21. Drislane FW, Blum AS, Lopez MR, Gautam S, Schomer DL. Duration of refractory status epilepticus and outcome: loss of prognostic utility after several hours. Epilepsia. 2009;50:1566–71. [DOI] [PubMed] [Google Scholar]
- 22. Requena M, Sarria‐Estrada S, Santamarina E, Quintana M, Sueiras M, Rovira A, et al. Peri‐ictal magnetic resonance imaging in status epilepticus: temporal relationship and prognostic value in 60 patients. Seizure. 2019;71:289–94. [DOI] [PubMed] [Google Scholar]
- 23. Roberg LE, Monsson O, Kristensen SB, Dahl SM, Ulvin LB, Heuser K, et al. Prediction of long‐term survival after status epilepticus using the ACD score. JAMA Neurol. 2022;79(6):604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 25. Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non‐convulsive status epilepticus—approach to clinical application. Epilepsy Behav. 2015;49:158–63. [DOI] [PubMed] [Google Scholar]
- 26. Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. [DOI] [PubMed] [Google Scholar]
- 27. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. [DOI] [PubMed] [Google Scholar]
- 28. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–6. [DOI] [PubMed] [Google Scholar]
- 29. Monsson OS, Roberg LE, Gesche J, Beier CP, Krøigård T. Salzburg consensus criteria are associated with long‐term outcome after non‐convulsive status epilepticus. Seizure 2022;99:28–35. [DOI] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goyal MK, Sinha S, Ravishankar S, Shivshankar JJ. Peri‐ictal signal changes in seven patients with status epilepticus: interesting MRI observations. Neuroradiology. 2009;51:151–61. [DOI] [PubMed] [Google Scholar]
- 32. Wiest R, Beisteiner R. Recent developments in imaging of epilepsy. Curr Opin Neurol. 2019;32:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1


