Abstract
Background
Respiratory failure is a major cause of morbidity and mortality in patients with Spinal Muscular Atrophy (SMA). Lack of endurance, or “fatigability,” is an important symptom of SMA. In addition to respiratory muscle weakness, respiratory function in SMA may be affected by Respiratory Muscle Fatigability (RMF).
Aim
The purpose of this study was to explore RMF in patients with SMA.
Methods
We assessed a Respiratory Endurance Test (RET) in 19 children (median age [years]: 11) and 36 adults (median age [years]: 34) with SMA types 2 and 3. Participants were instructed to breath against an inspiratory threshold load at either 20%, 35%, 45%, 55%, or 70% of their individual maximal inspiratory mouth pressure (PImax). RMF was defined as the inability to complete 60 consecutive breaths. Respiratory fatigability response was determined by change in maximal inspiratory mouth pressure (ΔPImax) and perceived fatigue (∆perceived fatigue).
Results
The probability of RMF during the RET increased by 59%−69% over 60 breaths with every 10% increase in inspiratory threshold load (%PImax). Fatigability response was characterized by a large variability in ΔPImax (−21% to +16%) and a small increase in perceived fatigue (p = 0.041, range 0 to +3).
Conclusion and Key Findings
Patients with SMA demonstrate a dose‐dependent increase in RMF without severe increase in exercise‐induced muscle weakness or perceived fatigue. Inspiratory muscle loading in patients with SMA seems feasible and its potential to stabilize or improve respiratory function in patients with SMA needs to be determined in further research.
Keywords: fatigue, Respiratory Endurance Test, respiratory muscle strength, SMA
1. INTRODUCTION
Spinal muscular atrophy (SMA) is a severe neuromuscular disease caused by a homozygous deletion of the survival motor neuron‐1 gene, 1 , 2 , 3 which leads to cellular survival motor neuron (SMN) protein deficiency. SMA has an incidence of about 1 in 6000−12,000 live births. 4 It is characterized by a wide range of disease severity and is classified into four types based on age at onset and highest acquired motor milestone. 2 , 3 , 5 , 6 , 7 , 8 Childhood‐onset SMA types 2, 3a and 3b are characterized by delayed gross motor development and progressive loss of motor function and muscle strength. 3 , 9 , 10 , 11 SMA type 2 has its onset between 6 and 18 months, patients acquire the ability to sit, but not to stand or walk. SMA type 3a has its onset between 18 months and 3 years, the ambulation is usually lost in adolescence or early adulthood, whereas patients with SMA type 3b, onset after age of 3 years, lose their ability to walk at a median age of 40 years. 9 , 11
In the last few years, SMN‐augmenting genetic therapies have been introduced, including SMN‐gene therapy and therapies that modify SMN2‐splicing. 12 Efficacy studies have demonstrated, on average, favorable responses in motor function, survival and muscle strength, but the respiratory outcomes vary, with most studies showing no significant improvement in lung function parameters in patients with SMA types 2 and 3. 13 , 14 , 15 , 16
Respiratory failure is the most important cause of morbidity and mortality in patients with SMA. 11 , 17 In SMA, it is primarily due to a decrease in respiratory muscle strength (i.e., muscle weakness), resulting in an impaired cough and poor clearance of lower airway secretions. 11 , 17 The respiratory muscle weakness is most pronounced in the intercostal muscles, while the diaphragm remains relatively spared. 3
In addition to respiratory muscle weakness, respiratory dysfunction in SMA may be caused by a lack of endurance of respiratory muscles, also known as increased Respiratory Muscle Fatigability (RMF). 18 , 19 Fatigability is defined as the inability to continue a task at the same intensity, resulting in a decline in one or more aspects of physical performance, such as peak force and power. 6 , 20 , 21 In a previous study we showed that 85% of patients with SMA demonstrated increased fatigability of leg‐, arm and hand function. 7 , 20 Fatigability of the respiratory muscles has not yet been studied in patients with SMA. More insight into respiratory muscle weakness and especially RMF in SMA will facilitate clinical management, with the aim of reducing respiratory failure in patients with SMA.
In this study we explored the feasibility of inspiratory muscle loading, the dose‐response relationship between inspiratory load and RMF and the fatigability response to RMF in patients with SMA types 2−3.
2. METHODS
This observational study is part of a large cross‐sectional study on fatigability in SMA. 6 , 7 To ensure an accurate and complete report of our work, we followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. 22
2.1. Ethical considerations
The Medical Ethical Committee of the University Medical Center Utrecht approved the research protocol (NL48715.041.14). The study was conducted according to the principles of the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act (WMO). The criteria of the Dutch Association of Pediatrics concerning research involving children were strictly applied. All participants and all parents or legal guardians of adolescents below the age of 18 years signed informed consent.
2.2. Participants
We recruited patients with SMA from the Dutch national SMA registry. 8 Patients aged 8−60 years with a genetically confirmed diagnosis of SMA type 2, 3a, or 3b and the ability to follow test instructions were considered for inclusion. Patients with concomitant medical problems that might have intervened with the outcomes of the testing were excluded. Patients were also excluded if they had: a history of neuromuscular diseases or the use of medication that affects neuromuscular junction function, medical conditions incompatible with exercise, or the inability to perform any of the endurance tests. Strenuous physical activities the day before each visit to the hospital were discouraged.
2.3. Study procedures
We explored RMF with a Respiratory Endurance Test (RET) executed at a percentage of the patients' maximal inspiratory mouth pressure (PImax). RMF was defined as task failure (TF) on a RET and expressed in number of breaths until exhaustion. 6 , 21 , 23 , 24 Fatigability response was determined by change in maximal inspiratory mouth pressure (ΔPImax) and change in perceived fatigue (∆perceived fatigue) before and after the RET. 24 , 25
Participants were included between March 2015 and March 2018. The measurements were performed at three different visits within approximately 6 weeks. We obtained demographics and clinical characteristics from the Dutch SMA registry. 8 At the first visit, pulmonary function, including Forced Vital Capacity (FVC) and Forced Expiratory Volume in the 1st second (FEV1), was assessed using spirometry (Geratherm) in accordance with the European Respiratory Society/American Thoracic Society recommendations. 26 Global lung function reference equations published in 2012 were used. 27 The motor function was assessed with the Hammersmith Functional Motor Scale Expanded (HFMSE). We determined the individual threshold load of the RET (20%, 35%, or 55% of PImax) and the participant practiced the RET during 10 breaths. Participants performed the RET at visits two (RET1) and three (RET2), with a minimum of 2 weeks between the visits. The first 15 participants performed RET1 and RET2 at a 20% threshold load, then the next participants who completed RET1 (60 breaths) performed RET2 at a higher threshold load (35%, 45%, or 70% of PImax). Participants who only partially completed RET1, performed RET2 at the same inspiratory threshold load as RET1 (Figure 1).
Figure 1.
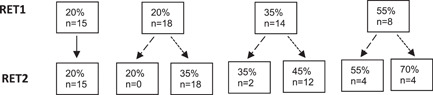
Study procedure: inspiratory threshold load (% of maximal inspiratory mouth pressure [PImax]) of Respiratory Endurance Test (RET) 1 and RET2.
 The first 15 participants performed RET1 and RET2 at 20%, regardless of whether they completed RET1 (60 breaths) or not.
The first 15 participants performed RET1 and RET2 at 20%, regardless of whether they completed RET1 (60 breaths) or not.
 Participants did not complete 60 breaths at RET1 so they performed RET2 at the same threshold load.
Participants did not complete 60 breaths at RET1 so they performed RET2 at the same threshold load.
 Participants did complete 60 breaths at RET1 so they performed RET2 at a higher threshold load. n, number of participants.
Participants did complete 60 breaths at RET1 so they performed RET2 at a higher threshold load. n, number of participants.
2.4. RET
2.4.1. POWERbreathe
The RET was conducted with the POWERbreathe K5, POWERbreathe International Ltd. The POWERbreathe provides immediate feedback on the test execution and applies a patient‐friendly gradual increase in resistance up to the threshold load. The POWERbreathe K series device has an electronically tapered flow resistive loading valve. The resistance is constantly monitored and tapered to match the reducing strength throughout the breath, thus allowing greater flow and maximum volume, resulting in a more fulfilling and effective breath, maximum flow and volume, plus more work per breath compared to traditional threshold pressure devices. 28
2.4.2. Determination of the inspiratory threshold load
At visit one, we determined the individual maximal inspiratory mouth pressure (PImax in centimeter H2O) with the POWERbreathe K5, according to standard procedures. 24 , 29 , 30 , 31 Participants generated a maximal inspiratory effort from residual volume while breathing through the mouthpiece with the nose occluded. The test was repeated at least five times with 30 s rest in between. An additional repeat was required if the last measurement was the highest. We noted the maximum value of three maneuvers that varied by less than 10% 23 and used that value to calculate the individual inspiratory threshold loads of RET1 and RET2.
2.4.3. Execution of the RET
At visits two (RET1) and three (RET2), participants performed the RET with the POWERbreathe K5 against an inspiratory threshold load of their PImax (which was determined at visit one). 23 , 24 They were instructed to inhale repetitively at approximately 50% of their FVC against the set threshold load until too tired or breathless to continue. 24 Sitting position and encouragement were standardized. RMF was defined as the inability to continue for 60 consecutive breaths (i.e., TF) and the number of breaths until TF was recorded.
2.4.4. Quality of the RET
After each RET, all breaths were checked for quality of performance. An inspiration was performed correctly if a participant had overcome at least 50% of the peak inspiratory load. If the participant failed to do this three consecutive times, all subsequent inhalations were subtracted from the total number of breaths, resulting in a net number of breaths which were used for further quantitative analysis. 24
2.5. Respiratory fatigability response
Respiratory fatigability response was expressed as ∆PImax and ∆perceived fatigue. We compared the response in participants with RMF with the response in participants without RMF. We analyzed ∆PImax and ∆perceived fatigue for RET1 as well as RET2.
2.5.1. Change in maximal inspiratory mouth pressure (∆PImax)
∆PImax in centimeter H2O was calculated as the difference between PImax measured immediately after the RET and PImax measured before the RET. We assessed PImax following standard procedures. 23 , 24 PImax was measured at residual volume with the Micro‐Respiratory Pressure Monitor, Viasys. Participants generated maximal inspiratory efforts while breathing through the mouthpiece. We noted the highest value of three maneuvers that varied by less than 10% 23 for analysis.
2.5.2. Change in percieved fatigue ∆perceived fatigue
∆perceived fatigue was calculated as the difference between perceived fatigue measured immediately after the RET and perceived fatigue measured before the RET. Perceived fatigue was evaluated with the OMNI Scale of Perceived Exertion (i.e., OMNI Scale), a valid and reliable instrument for measuring perceived fatigue in children and adults. 32 , 33 The OMNI Scale consists of 11 numbered categories, 0–10, and verbal cues, from “not tired at all” to “very, very tired.” 32 Participants scored their perceived fatigue on an 11‐point Likert scale.
2.6. Sample size calculation
The sample size was not calculated prospectively because of the novelty of the RET in this population and unpredictable effect size. Sample size was determined by the number of eligible patients willing to participate.
2.7. Statistical analysis
We defined RMF as TF on a RET, executed at a predetermined percentage of the patients' PImax, and expressed in number of breaths until exhaustion. To explore at which percentage of PImax the chance of RMF was greatest, we compared threshold loads of 20%, 35%, and 45% of PImax for RET1, and for RET2, threshold loads of 20%, 35%, 45%, 55%, and 70% of PImax.
We checked the data for normality and outliers with histograms and Q−Q plots. The continuous variables were presented as median and interquartile range (M [IQR]). Categorical variables were presented in absolute numbers and percentages (n [%]). We tested differences between groups with the χ 2 test or the Fisher's exact test for nominal data. For interval/ratio data, the Mann−Whitney U test was used for differences between two groups and the Kruskal−Wallis test for differences between more than two groups.
Kaplan−Meier curves were generated for RET1 and RET2, where the event was defined as TF on the RET. The null hypothesis, that is, there is no difference in the probability of TF during 60 breaths between threshold loads, was tested with the log‐rank test. To specify the dose‐response relation between %PImax and TF, the Hazard ratio (HR) was computed with Cox Regression with threshold load in %PImax as a continuous variable.
All statistical analyses were performed using SPSS for Windows (version 25.0; SPSS Inc). A p < 0.05 was considered significant.
3. RESULTS
3.1. Participant characteristics
We included a total of 19 children (median age 11 years [IQR 6 years]; 7 girls) and 36 adults (median age 34 years [IQR 16 years]; 20 women) with SMA. Twenty‐nine participants had SMA type 2, 11 type 3a, and 15 type 3b. The median standardized FEV1 was 67.5% (IQR 62.5%). The median standardized FVC was 69% (IQR 62.75%) and the median standardized PImax was 78% (IQR 51%).
3.2. RETs characteristics
All 55 participants performed the RET twice, resulting in 110 RETs. There were no drop‐outs and no adverse events. POWERbreathe data were missing from eight RETS (RET1: n = 3, RET2: n = 5), and so quality of performance could not be checked; they were, therefore, excluded from analysis, resulting in 52 RETs for RET1 and 50 RETs for RET2.
3.3. Characteristics per threshold load protocol
First, we analyzed the data per inspiratory threshold load protocol (Table 1 for RET1 and Table 2 for RET2). We compared gender, age, SMA subtype, motor function (HFMSE), and lung function (FEV1 and FVC) between the different threshold loads. For RET1, there were three different threshold loads, namely 20%, 35%, and 55% of patients' PImax. Age was the only statistically significant different characteristic in RET1 (p = 0.018). For RET2, there were five different threshold loads, namely 20%, 35%, 45%, 55%, and 70% of patients' PImax. There were no statistically significant differences in the patients' characteristics between the different threshold loads of RET2.
Table 1.
Characteristics per threshold load protocol for RET1
| Threshold load | 20% of PImax n = 31 (60%) | 35% of PImax n = 13 (25%) | 55% of PImax n = 8 (15%) |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 13 (42%) | 7 (54%) | 6 (75%) |
| Age (year), M (IQR) | 26 (22)* | 11 (22)* | 38 (24)* |
| SMA subtype, n (%) | |||
| Type 2 | 15 (48%) | 8 (62%) | 5 (63%) |
| Type 3a | 7 (23%) | 1 (8%) | 2 (25%) |
| Type 3b | 9 (29%) | 4 (31%) | 1 (13%) |
| HFMSE, M (IQR) | 5 (49) | 9 (54) (n = 11) | 4 (34) (n = 7) |
| FEV1 (% of predicted), M (IQR) | 78 (66.5) (n = 29) | 58.5 (54.5) (n = 12) | 70 (72.5) |
| FVC (% of predicted), M (IQR) | 76 (63.75) (n = 30) | 59 (60) | 75 (74) |
Note: If data were missing, n was expressed by (n = …). The missing data were completely at random.
Abbreviations: FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; M (IQR), median and interquartile range; n (%), number of RETs and percentage; PImax, maximal inspiratory mouth pressure; RET, Respiratory Endurance Test; SMA, Spinal Muscular Atrophy.
Significant differences p < 0.05.
Table 2.
Characteristics per threshold load protocol for RET2
| Threshold load | 20% of PImax n = 14 (28%) | 35% of PImax n = 17 (34%) | 45% of PImax n = 11 (22%) | 55% of PImax n = 4 (8%) | 70% of PImax n = 4 (8%) |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Female | 4 (29%) | 8 (47%) | 7 (64%) | 3 (75%) | 3 (75%) |
| Age (year), M (IQR) | 24.5 (26) | 26 (19) | 19 (26) | 47 (34) | 34.5 (16) |
| SMA subtype, n (%) | |||||
| Type 2 | 8 (57%) | 8 (47%) | 7 (64%) | 2 (50%) | 3 (75%) |
| Type 3a | 4 (29%) | 3 (18%) | 0 (0%) | 1 (25%) | 1 (25%) |
| Type 3b | 2 (14%) | 6 (35%) | 4 (36%) | 1 (25%) | 0 (0%) |
| HFMSE, M (IQR) | 4.5 (39) | 7 (57) | 21 (56) (n = 9) | 9 (28) | 4 (n = 3) |
| FEV1 (% of predicted), M (IQR) | 75 (68.25) | 74 (65) (n = 16) | 56 (58.75) (n = 10) | 91.5 (64.25) | 49 (60.25) |
| FVC (% of predicted), M (IQR) | 76 (67.75) | 79 (64.75) (n = 16) | 59 (68) | 97.5 (70.25) | 52 (57.75) |
Note: If data were missing, n was expressed by (n = …). The missing data were completely at random.
Abbreviations: FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; M (IQR), median and interquartile range; n (%), number of RETs and percentage; PImax, maximal inspiratory mouth pressure; RET, Respiratory Endurance Test; SMA, Spinal Muscular Atrophy.
3.4. TF during RET1
TF during RET1 occurred in 75% (n = 6) of the RETs which were executed at 55% of patients' PImax and in 31% (n = 4) and 19% (n = 3) of the RETs which were executed at 35% and 20% of patients' PImax, respectively. With every 10% increase in threshold load (in percentage of PImax), the probability of TF during RET1 increases by 69% (HR 1.69, 95% confidence interval [CI] 1.21−2.36, p = 0.002).
Figure 2A depicts the Kaplan−Meier curves for the different inspiratory threshold loads of RET1. We found that the probability of TF, during RET1 at any point in time, was significantly different between the different threshold loads (p = 0.002). The median number of breaths until TF was 60 at the threshold loads 20% and 35% of PImax. The median number of breaths until TF at the threshold load 55% of PImax was 31 (95% CI 25−37).
Figure 2.
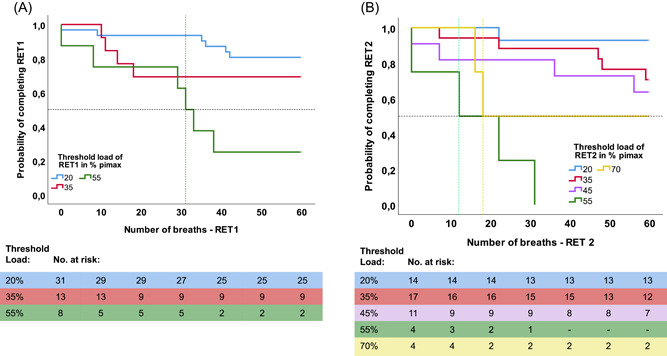
(A) Kaplan−Meier curves for each threshold load of RET1. (B) Kaplan−Meier curves for each threshold load of RET2. RET, Respiratory Endurance Test. Participants who completed the RET are censored at 60 breaths. The intersection between the horizontal and vertical dashed lines depicts the median time (breath) to TF at the threshold load of 55% of RET1 (green, left curve), 55% of RET 2 (green, right curve) and 70% (yellow, right curve). The tables shows the number of participants at risk of TF per threshold load per time point (0−60 breaths). [Color figure can be viewed at wileyonlinelibrary.com]
3.5. TF during RET2
TF during RET2 was observed in 50% (n = 2) of the RETs which were executed at 70% of patients' PImax and in 100% (n = 4), 36% (n = 4), 29% (n = 5) and 7% (n = 1) of the RETs which were executed at 55%, 45%, 35%, and 20% of patients' PImax, respectively. With every 10% increase in threshold load (in percentage of PImax), the probability of TF during RET2 increases by 59% (HR 1.59, 95% CI 1.18−2.15, p = 0.003).
Figure 2B depicts the Kaplan−Meier curves for the different inspiratory threshold loads of RET2. We found that the probability of TF, during RET2 at any point in time, was significantly different between the different threshold loads (p < 0.001). The median number of breaths until TF was 60 at the threshold loads 20%, 35%, and 45% of PImax. The median number of breaths until TF at the threshold load 55% of PImax was 12 (95% CI 0−34), and the median number of breaths until TF at the threshold load 70% of PImax was 18 (95% CI?).
3.6. Respiratory fatigability response for RET2
Second, we analyzed the data per subgroup RMF (patients with TF) and no RMF (patients with no TF) to determine the respiratory fatigability response (Table 3). We compared age, SMA subtype, motor function (HFMSE), lung function (FEV1 and FVC) and inspiratory muscle strength (PImax) between the two subgroups (RMF and no RMF). In addition, we compared respiratory fatigability responses (∆PImax and Δperceived fatigue). The baseline characteristics and respiratory fatigability response in RET1 were similar to those in RET2 (Appendix A).
Table 3.
Characteristics and respiratory fatigability response per subgroup for RET2
| RMF (TF) n = 16 (32%) | No RMF (no TF) n = 34 (68%) | |
|---|---|---|
| Characteristics | ||
| Age (year), M (IQR) | 29 (32) | 25.5 (21) |
| SMA subtype, n (%) | ||
| Type 2 | 10 (63%) | 18 (53%) |
| Type 3a | 3 (19%) | 6 (18%) |
| Type 3b | 3 (19%) | 10 (29%) |
| HFMSE, M (IQR) | 5 (38) (n = 14) | 7 (49) (n = 33) |
| FEV1% of predicted, M (IQR) | 70 (65.25) (n = 14) | 67.5 (63.75) |
| FVC % of predicted, M (IQR) | 67 (66) (n = 15) | 68.5 (65.25) |
| PImax % of predicted1, M (IQR) | 69 (56) | 87 (46) (n = 33) |
| Respiratory fatigability response | ||
| ∆PImax (cm H2O), M (IQR) | −0.5 (5) | 1 (8) (n = 33) |
| ∆PImax (%), M (IQR) | −0.64% (7.47%) | 1.38% (8.02%) (n = 33) |
| ∆perceived fatigue, M (IQR) | 0 (1) (n = 15) | 0 (2) (n = 33) |
| +∆perceived fatigue (increase), n (%) | 5 (33%) | 17 (52%) |
| No differences in perceived fatigue, n (%) | 10 (67%) | 16 (49%) |
Note: If data were missing, n was expressed by (n = …). The missing data were completely at random. 1Reference values of Neder et al. 34 were used for age 20−60 years and those of Hulzebos et al. 35 for age 8−19 years.
Abbreviation: Δ, post RET−pre RET; cm H2O, centimeter of water, FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; M (IQR), median and interquartile range; n (%), number of RETs and percentage; PImax, maximal inspiratory mouth pressure; RET, Respiratory Endurance Test; RMF, Respiratory Muscle Fatigability; SMA, Spinal Muscular Atrophy; TF, task failure.
3.6.1. Baseline characteristics per subgroup for RET2
We compared baseline characteristics of the participants with and without RMF on RET2 (Table 3). There were no statistically significant differences between the characteristics of the two groups for RET2. Notable is the lower median baseline values of PImax % of predicted in the group with RMF compared to the participants without RMF. Fifty‐six percent of the participants with RMF could be classified as inspiratory weak (PImax: <70 cm H20 for women and <80 cm H20 for men 23 ) as opposed to just 33% of the participants without RMF (p = 0.215).
3.6.2. Respiratory fatigability response: ∆PImax per subgroup for RET2
We found no statistically significant differences in ∆PImax between the two subgroups (Table 3). The median change in PImax in the group with RMF for RET2 was −0.5 cm H2O (p = 0.22), with a large variability in individual response. Eight participants with RMF (50%) showed a decrease in ΔPImax, ranging from −1% to −21%. Five participants with RMF (31%) showed an increase in ΔPImax ranging from +2% to +16%. Twelve participants (36%) without RMF showed a decrease in ΔPImax, ranging from −1% to −10%, compared to an increase in 19 participants (58%) in this group, ranging from +1% to +32%.
To gain some more insigth we divided PImax and age in quartiles, so we could see if the youngest/weakest patients showed the most increase in ΔPImax. We found no statistic significant differences in age groups (p = 0.243). We did found statistic significant differences between the PImax groups (p = 0.048), however this was not a clinically relevant difference. The median ∆PImax was 1 cm H2O in the weakest group, −0.5 cm H2O in the Q2 group, −2 cm H2O in the Q3 group and 1 cm H2O in the strongest group.
3.6.3. Respiratory fatigability response: ∆perceived fatigue for RET2
Both groups, participants with and without RMF, demonstrated a small significant increase in ∆perceived fatigue after RET2 (RMF: p = 0.041, no RMF: p < 0.001) (Table 3). We found a similar response in Δperceived fatigue ranging from 0 to +3 in both groups. We found no statistically significant differences in ∆perceived fatigue between the two subgroups.
4. DISCUSSION
In this study we explored RMF in patients with SMA. Patients with SMA demonstrate a dose‐dependent increase in RMF with a large individual variation in fatigability response.
The probability of experiencing RMF in our study was the highest at an inspiratory threshold load of 55% of their individual PImax, which is similar to the probability in healthy individuals 25 and 20% higher than in patients with duchenne muscular dystrophy [DMD]. 24 In a recent study on fatigability of arm and leg muscles, patients with SMA showed increased fatigability compared to patients with DMD, despite similar levels of muscle weakness. 7 , 20 , 36 Therefore, we expected patients with SMA to show RMF at a similar or even lower inspiratory threshold load compared to patients with DMD. 24
The diaphragm acts as the primary inspiratory muscle and accounts for 70% of the inspired air volume during regular breathing. 37 In patients with SMA, the diaphragm is relatively spared, while in DMD, it is one of the most severely affected respiratory muscles. 10 , 38 , 39 This might explain why patients with DMD experience RMF at a lower inspiratory threshold load than patients with SMA.
A decrease in ΔPImax of 9.3%–50% after a RET as a response to RMF was reported by Janssens et al. in healthy individuals, 25 while Matecki et al. 24 reported a mean decrease of 22% in ΔPImax in children with DMD. In contrast, we found no statistically significant decrease in PImax after the RET in patients with SMA. Interestingly, we observed large interindividual differences in ∆PImax, ranging from a decrease of 21% to an increase of 16% in PImax as a response to RMF. We suggest that the observed increase in inspiratory muscle strength after the RET in some patients might be due to a learning effect, similar to what was previously observed during endurance testing of the upper and lower extremities. 7 Intramuscular coordination generally improves after repeated performance of a new motor task. We expected that the learning effect would be greatest in the youngest or weakest patients, but sub‐analysis showed no association between age, strength (PImax), and ∆PImax. The variability in age and subtypes of SMA and the fast recovery of fatigability in patients with SMA may also have contributed to a blunted response in PImax. 6 , 7 To provide insight into the underlying mechanism of RMF during a RET, and to better understand variable responses in ∆PImax, we suggest the use of surface electromyography (sEMG) in future studies. 40
There was a small but significant increase in perceived exertion in both patients with and without RMF. However, these results should be interpreted with some caution. The OMNI scale, used to measure perceived exertion, has only been validated in children and adults during motor activities. 32 , 33 Unfortunately, no validated scale was available for perceived exertion of the respiratory muscles in SMA or other neuromuscular diseases. Further research is needed to determine the validity of the OMNI scale to detect perceived exertion in neuromuscular diseases. To objectify RMF and validate the OMNI scale, we suggest to compare scores to objective outcome measures such as sEMG. 40 Until then, other parameters indicative of exercise intensity will have to be included to monitor the response on respiratory muscle loading such as the experienced intensity of the training and perceived dyspnea measured with a Borg scale. 41 In addition to subjective measurements, objective measurements, for example breathing frequency, use of accessory inspiratory muscles and retractions can be used as indicators of increased work of breathing.
The results of our study show that RMF in patients with SMA is observed at a similar percentage of the individual PImax compared to healthy individuals. The probability of RMF in patients with SMA seems to be highest at a threshold load of 55% of PImax; in healthy individuals, RMF is induced at a threshold load of 60% of PImax. 25 Inspiratory loading appears feasible in patients with SMA and they are capable of breathing against a high inspiratory threshold load, which provides opportunities for respiratory training. This study shows that respiratory muscle endurance seems adequate in patients with SMA, but the low baseline levels of PImax suggest a therapeutic window for respiratory muscle strength training. Furthermore, compared to core and limb muscles relevant for motor function tests, respiratory muscles may be less responsive to SMN‐augmenting therapies. Respiratory muscle training could, therefore, be a complementary therapy to the treatment strategies that have now become increasingly available for patients with SMA. 13 , 14
Due to a lack of knowledge of the feasibility of inspiratory muscle loading in patients with SMA, we decided to use a dose escalation method starting at a low intensity. After 15 patients we noticed that almost all patients completed the RET, and we decided to gradually increase the intensity of the RET. This resulted in relatively small numbers of participants in each subgroup which requires the results to be interpreted with some caution. Our findings do confirm that fatigability in the respiratory muscles seems less prominent than fatigability of the muscles in arms and legs. 7 , 20 Further research in a larger group of patients with SMA is necessary to validate the RMF threshold and further explore the variability in fatigability response. Also, further research is needed to investigate the feasibility and effectiveness of respiratory muscle training in patients with SMA.
5. CONCLUSION
Patients with SMA types 2 and 3 demonstrate a dose‐dependent increase in RMF without severe increase in exercise‐induced muscle weakness or perceived fatigue. Inspiratory muscle loading in patients with SMA seems feasible and its potential to stabilize or improve respiratory function in patients with SMA needs to be determined in further research.
AUTHOR CONTRIBUTIONS
Study concept and design: Kim Kant‐Smits, Erik H. J. Hulzebos, Janke F. de Groot, W. Ludo van der Pol, and Bart Bartels. Acquisition, analysis, or interpretation of data: Kim Kant‐Smits, Erik H. J. Hulzebos, Laura E. Habets, Fay‐Lynn Asselman, Esther S. Veldhoen, Ruben P. A. van Eijk, Janke F. de Groot, W. Ludo van der Pol and, Bart Bartels. Statistical analysis: Kim Kant‐Smits, Ruben P. A. van Eijk,. Drafting of the manuscript: Kim Kant‐Smits, Erik H. J. Hulzebos, and Bart Bartels. Critical revision of the manuscript for important intellectual content: Kim Kant‐Smits, Erik H. J. Hulzebos, Laura E. Habets, Fay‐Lynn Asselman, Esther S. Veldhoen, Ruben P. A. van Eijk, Janke F. de Groot, W. Ludo van der Pol, and Bart Bartels. Study supervision: Erik H. J. Hulzebos, Janke F. de Groot, W. Ludo van der Pol, Bart Bartels. All authors have read and approved the manuscript.
CONFLICTS OF INTEREST
Bart Bartels obtained research grants from Prinses Beatrix Spierfonds and Stichting Spieren voor Spieren, both non‐profit foundations. His employer receives fees for SMA‐related consultancy activities. W. Ludo van der Pol. obtained research grants from Prinses Beatrix Spierfonds and Stichting Spieren voor Spieren, both non‐profit foundations. His employer receives fees for SMA‐related consultancy activities. The remaining authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank all patients who participated in this study. This study was funded by Prinses Beatrix Spierfonds, Stichting Spieren voor Spieren and de Vriendenloterij. The funding parties were not involved in the design of the study, collection, analysis and interpretation of the data, nor in writing the manuscript.
APPENDIX A.
A1. RESPIRATORY FATIGABILITY RESPONSE FOR RESPIRATORY ENDURANCE TEST 1 (RET1)
For RET1 we analyzed the data per subgroup Respiratory Muscle Fatigability (RMF) (patients with task failure [TF]) and no RMF (patients with no TF) to determine the respiratory fatigability response (Table 4). We compared age, Spinal Muscular Atrophy (SMA) subtype, motor function (Hammersmith Functional Motor Scale Expanded [HFMSE]), lung function (Forced Expiratory Volume in the 1 s and Forced Vital Capacity) and inspiratory muscle strength (maximal inspiratory mouth pressure [PImax]) between the two subgroups (RMF and no RMF). In addition, we compared fatigability responses (change in maximal inspiratory mouth pressure [∆PImax] and change in perceived fatigue [Δperceived fatigue])
Table 4.
Characteristics and respiratory fatigability response per subgroup for RET1
| RMF (TF) n = 16 (31%) | No RMF (no TF) n = 36 (69%) | |
|---|---|---|
| Characteristics | ||
| Age (year), M (IQR) | 21.5 (35) | 26 (22) |
| SMA subtype, n (%) | ||
| Type 2 | 10 (63%) | 18 (50%) |
| Type 3a | 4 (25%) | 6 (17%) |
| Type 3b | 2 (13%) | 12 (33%) |
| HFMSE, M (IQR) | 4 (13) (n = 15) | 8.5 (54) (n = 34) |
| FEV1% of predicted, M (IQR) | 56.5 (71.75) (n = 14) | 78 (62) (n = 35) |
| FVC % of predicted, M (IQR) | 60 (72) (n = 15) | 76 (60.75) |
| PImax % of predicted1, M (IQR) | 60 (47) | 85.5 (50) |
| Respiratory fatigability response | ||
| ∆PImax (cm H2O), M (IQR) | 1.5 (14.75) | 0 (7.75) |
| ∆PImax (%), M (IQR) | 3.17% (17.01%) | 0% (10.27%) |
| ∆perceived fatigue, M (IQR) | 0.5 (2) (n = 14) | 0 (2) (n = 33) |
| −∆ perceived fatigue (decrease), n (%) | 1 (7%) | 3 (9%) |
| +∆perceived fatigue (increase), n (%) | 7 (50%) | 16 (49%) |
| No differences in perceived fatigue, n (%) | 6 (43%) | 14 (42%) |
Note: If data were missing, n was expressed by (n = …). The missing data were completely at random. 1Reference values of Neder et al. 34 were used for age 20‐60 yrs and those of Hulzebos et al. 35 for age 8−19 years.
Abbreviations: Δ, post RET−pre RET, cm H2O, centimeter of water; FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; M (IQR), median and interquartile range; n (%), number of RETs and percentage; PImax, maximal inspiratory mouth pressure; RET, Respiratory Endurance Test; RMF, Respiratory Muscle Fatigability; SMA, Spinal Muscular Atrophy; TF, task failure.
A1.1. Baseline characteristics per subgroup for RET1
We compared baseline characteristics of the participants with and without RMF on RET1 (Table 4). There were no statistically significant differences between the characteristics of the two groups for RET1. Notable is the lower mean baseline values of PImax % of predicted in the group with RMF compared to the participants without RMF. Sixty‐three percent of the participants with RMF could be classified as inspiratory weak (PImax % of predicted: <70 cm H20 for women and <80 cm H20 for men 23 ) as opposed to just 36% of the participants without RMF (p = 0.129).
A1.2. Respiratory fatigability response: change in inspiratory mouth pressure (∆PImax) per subgroup for RET1
We found no statistically significant differences in ∆PImax between the two subgroups (Table 4). The median change in PImax in the group with RMF for RET1 was 1.5 cm H2O (p = 0.35) with a large variability in individual response. Six participants with RMF (38%) showed a decrease in ΔPImax, ranging from ‐1% to −24%. Nine participants with RMF (56%) showed an increase in ΔPImax ranging from +3% to +29%. Sixteen participants (44%) without RMF showed a decrease in ΔPImax, ranging from −1% to −18%, compared to an increase in 17 participants (47%) in this group, ranging from +1% to +36%.
A1.3. Respiratory fatigability response: ∆perceived fatigue for RET1
Both groups, participants with and without RMF, demonstrated a small significant increase in ∆perceived fatigue after RET1 (RMF: p = 0.024, no RMF: p = 0.005) (Table 4). We found a similar response in Δperceived fatigue in both groups. In the group with RMF, Δperceived fatigue ranged from −1 to +4; in the group without RMF Δperceived fatigue ranged from –3 to +8. We found no statistically significant differences in ∆perceived fatigue between the two subgroups.
Kant‐Smits K, Hulzebos EHJ, Habets LE, et al. Respiratory muscle fatigability in patients with spinal muscular atrophy. Pediatric Pulmonology. 2022;57:3050‐3059. 10.1002/ppul.26133
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Wadman R, Vrancken A, van den Berg L, van der Pol W. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Am Acad Neurol. 2012;79:2050‐2055. [DOI] [PubMed] [Google Scholar]
- 2. Finkel RS, Sejersen T, Mercuri E, ENMC SMA Workshop Study G. 218th ENMC international workshop: revisiting the consensus on standards of care in SMA. Neuromuscul Disord. 2017;27(6):596‐605. [DOI] [PubMed] [Google Scholar]
- 3. Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120‐2133. [DOI] [PubMed] [Google Scholar]
- 4. Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q‐linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mongiovi P, Dilek N, Garland C, et al. Patient reported impact of symptoms in spinal muscular atrophy (PRISM‐SMA). Neurology. 2018;91(13):E1206‐E1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartels B, Habets LE, Stam M, et al. Assessment of fatigability in patients with spinal muscular atrophy: development and content validity of a set of endurance tests. BMC Neurol. 2019;19:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartels B, De Groot JF, Habets LE, et al. Fatigability in spinal muscular atrophy: validity and reliability of endurance shuttle tests. Orphanet J Rare Dis. 2020;15(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wadman RI, Wijngaarde CA, Stam M, et al. Muscle strength and motor function throughout life in a cross‐sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Eur J Neurol. 2018;25(3):512‐518. [DOI] [PubMed] [Google Scholar]
- 9. Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11(5):443‐452. [DOI] [PubMed] [Google Scholar]
- 10. Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103‐115. [DOI] [PubMed] [Google Scholar]
- 11. Wijngaarde CA, Veldhoen ES, Van Eijk RPA, et al. Natural history of lung function in spinal muscular atrophy. Orphanet J Rare Dis. 2020;15(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723‐1732. [DOI] [PubMed] [Google Scholar]
- 13. Paul GR, Gushue C, Kotha K, Shell R. The respiratory impact of novel therapies for spinal muscular atrophy. Pediatr Pulmonol . 2020; 1‐8. [DOI] [PubMed]
- 14. Heitschmidt L, Pichlmaier L, Eckerland M, et al. Nusinersen does not improve lung function in a cohort of children with spinal muscular atrophy—a single‐center retrospective study. Eur J Paediatr Neurol. 2021;31:88‐91. [DOI] [PubMed] [Google Scholar]
- 15. Audic F, De La Banda MGG, Bernoux D, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real‐life observational study. Orphanet J Rare Dis. 2020;15(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavie M, Diamant N, Cahal M, et al. Nusinersen for spinal muscular atrophy type 1: real‐world respiratory experience. Pediatr Pulmonol. 2020;56:(June) 1‐8. [DOI] [PubMed] [Google Scholar]
- 17. Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027‐1049. [DOI] [PubMed] [Google Scholar]
- 18. Vendrusculo F, Heinzmann‐filho P, Piva T, Marostica P, Donadio M. Inspiratory muscle strength and endurance in children and adolescents with cystic fibrosis. Respir Care. 2016;61(2):184‐191. [DOI] [PubMed] [Google Scholar]
- 19. Fauroux B. Respiratory muscle testing in children. Paediatr Respir Rev. 2003;4:243‐249. [DOI] [PubMed] [Google Scholar]
- 20. Stam M, Wadman RI, Bartels B, et al. A continuous repetitive task to detect fatigability in spinal muscular atrophy. Orphanet J Rare Dis. 2018;13:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kluger B, Krupp L, Enoka R. Fatigue and fatigability in neurologic illnesses. Am Acad Neurol. 2013;80:409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandenbroucke JP, Von Elm E, Altman DG, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐578. [DOI] [PubMed] [Google Scholar]
- 23. Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53:53. [DOI] [PubMed] [Google Scholar]
- 24. Matecki S, Topin N, Hayot M, et al. A standardized method for the evaluation of respiratory muscle endurance in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11:171‐177. [DOI] [PubMed] [Google Scholar]
- 25. Janssens L, Brumagne S, Mcconnell AK, Raymaekers J, Goossens N, Gayan‐ramirez G. The assessment of inspiratory muscle fatigue in healthy individuals: a systematic review. Respir Med. 2013;107(3):331‐346. [DOI] [PubMed] [Google Scholar]
- 26. Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 27. Quanjer PH, Stanojevic S, Cole TJ, et al. Multı‐ethnıc reference values for spırometry for the 3‐95 year age range: the global lung functıon 2012 equatıons: report of the global lung function initiative (GLI), ERS task force to establish improved lung function reference values. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The worlds first intelligent digital breathing trainer [Internet] . Maximise lung volume & boost performance in 4 weeks. Accessed May 7, 2020. https://www.powerbreathe.com/product-category/breathing-trainers/k-series/
- 29. Langer D, Jacome C, Charususin N, et al. Measurement validity of an electronic inspiratory loading device during a loaded breathing task in patients with COPD. Respir Med. 2013;107(4):633‐635. [DOI] [PubMed] [Google Scholar]
- 30. Valkenet K, Trappenburg JC, Gosselink R, et al. Preoperative inspiratory muscle training to prevent postoperative pulmonary complications in patients undergoing esophageal resection (PREPARE study): study protocol for a randomized controlled trial. Trials. 2014;15(144):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gibson GJ, Whitelaw W, Siafakas N, et al. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518‐624. [DOI] [PubMed] [Google Scholar]
- 32. Bulthuis M, van Empelen R, Heimeriks I, Dronkers J. Het gebruik van een belastingschaal bij kinderen tussen de 8 en 12 jaar voor het meten van subjectief ervaren belasting tijdens training. Ned tijdschrijft voor Kinderfysiotherapie. 2010;22(67):24‐31. [Google Scholar]
- 33. Robertson RJ, Goss FL, Boer NF, et al. Children's OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sport Exerc. 2000;32(2):452‐458. [DOI] [PubMed] [Google Scholar]
- 34. Neder JA, Andreoni S, Lerario MC, et al. Reference values for lung function tests. II. maximal respiratory pressures and voluntary ventilation. Brazilian J Med Biol Res. 1999;32:719‐727. [DOI] [PubMed] [Google Scholar]
- 35. Hulzebos E, Takken T, Reijneveld EA, Mulder MG, Bongers BC. Reference values for respiratory muscle strength in children and adolescents. Respiration. 2018;95:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montes J, Blumenschine M, Dunaway S, et al. Weakness and fatigue in diverse neuromuscular diseases. J Child Neurol. 2013;28(10):1277‐1283. [DOI] [PubMed] [Google Scholar]
- 37. Fayssoil A, Behin A, Ogna A, et al. Diaphragm: pathophysiology and ultrasound imaging in neuromuscular disorders. J Neuromuscul Dis. 2018;5(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Neuromuscul Dis muscle. 2017;30:529‐537. [DOI] [PubMed] [Google Scholar]
- 39. Silva I, Pedrosa R, IG A, et al. Respiratory muscle training in children and adults with neuromuscular disease (Review). Cochrane Database Syst Rev. 2019;2019(9):CD011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Habets LE, Bartels B, de Groot JF, et al. Motor unit reserve capacity in spinal muscular atrophy during fatiguing endurance performance. Clin Neurophysiol. 2021;132(3):800‐807. [DOI] [PubMed] [Google Scholar]
- 41. Just N, Bautin N, Danel‐Brunaud V, Debroucker V, Matran R, Perez T. The Borg dyspnoea score: a relevant clinical marker of inspiratory muscle weakness in amyotrophic lateral sclerosis. Eur Respir J. 2010;35(2):353‐360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


