Abstract
Neurofilament proteins (Nf) have been validated and established as a reliable body fluid biomarker for neurodegenerative pathology. This review covers seven Nf isoforms, Nf light (NfL), two splicing variants of Nf medium (NfM), two splicing variants of Nf heavy (NfH), ‐internexin (INA) and peripherin (PRPH). The genetic and epigenetic aspects of Nf are discussed as relevant for neurodegenerative diseases and oncology. The comprehensive list of mutations for all Nf isoforms covers Amyotrophic Lateral Sclerosis, Charcot–Marie Tooth disease, Spinal muscular atrophy, Parkinson Disease and Lewy Body Dementia. Next, emphasis is given to the expanding field of post‐translational modifications (PTM) of the Nf amino acid residues. Protein structural aspects are reviewed alongside PTMs causing neurodegenerative pathology and human autoimmunity. Molecular visualisations of NF PTMs, assembly and stoichiometry make use of Alphafold2 modelling. The implications for Nf function on the cellular level and axonal transport are discussed. Neurofilament aggregate formation and proteolytic breakdown are reviewed as relevant for biomarker tests and disease. Likewise, Nf stoichiometry is reviewed with regard to in vitro experiments and as a compensatory mechanism in neurodegeneration. The review of Nf across a spectrum of 87 diseases from all parts of medicine is followed by a critical appraisal of 33 meta‐analyses on Nf body fluid levels. The review concludes with considerations for clinical trial design and an outlook for future research.
Keywords: biomarker, diagnosis, neurofilament, prognosis, treatment trial
Neurofilament proteins (Nf) are a validated bodyfluid biomarker for neurodegeneration. Nf are obligate, heteropolymers of different molecular sizes. Molecular assembly is hierarchical. Mutations and post‐translational modifications are extensive, relevant to pathology and immunotest development. The mature Nf proteins contain intrinsically unstructured polyampholytic regions relevant for protein–protein interactions. Contemporary state‐of‐the art laboratory tests quantify small, stable, soluble, proteolytic Nf breakdown products. Review and meta‐analyses of the clinical spectrum illustrate the relationship with neurodegeneration. Interpretation of increased human body fluid Nf concentration conveys information on the following: (i) diagnosis; (ii) prognosis; (iii) monitoring of progression and (iv) treatment trial endpoints.
Abbreviations
- AD
Alzheimer disease
- ADP
adenosine diphosphate
- AE
acridinium ester
- AF
atrial fibrillation
- AGEs
advanced glycation end products
- AIDS
acquired immunodeficiency syndrome
- Ala
alanine
- ALS
amyotrophic lateral sclerosis
- AMD
age‐related macular degeneration
- AQP4
aquaporin 4
- Arg
arginine
- Asn
asparagine
- Asp
aspartate
- ATTRm
hereditary transthyretin amyloidosis
- BBB
blood–brain barrier
- BMI
body mass index
- BPAN
beta‐propeller protein‐associated neurodegeneration
- Ca
calcium
- CADASIL
cerebral autosomal dominant arteriopathy
- CAG
cytosine‐adenine‐guanine
- CBD
corticobasal degeneration
- CHD
congenital heart disease
- CI
confidence interval
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CIN
critical illness neuropathy
- CIPN
chemotherapy induced peripheral neuropathy
- CIS
clinically isolated syndrome
- Cit
citrullin
- CJD
Creutzfeldt–Jakob Disease
- CLN
Batten disease gene
- CMT
Charcot Marie Tooth disease
- CNS
central nervous system
- COVID‐19
coronavirus disease 2019
- CPB
cardiopulmonary bypass
- CSF
cerebrospinal fluid
- CTE
chronic traumatic encephalopathy
- CV
coefficient of variation
- Cys
cysteine
- Da
dalton
- DAT
dopamine transporter
- DLB
diffuse lewy body dementia
- DNA
deoxyribonucleic acid
- ELISA
enzyme‐linked immunosorbent assay
- FTLD
frontotemporal lobar dementia
- GAN
giant axonal neuropathy
- GBS
Guillain–Barré Syndrome
- Glu
glutamine
- Gly
glycine
- GM
gangliosidosis
- HD
Huntington's disease
- HE
herpes encephalitis
- HIV
human immunodeficiency virus infection
- HMS
hypogravitatational motor syndrome
- Hyl
hydroxylysine
- Hyp
hydroxyproline
- ICH
intracranial haemorrhage
- IF
immunomagnetic reduction technology
- Ile
isoleucine
- INA
internexin alpha
- IRT
immunomagnetic reduction technology
- LBD
Lewy body disease
- Leu
leucine
- Lys
lysine
- MAG
myelin‐associated glycoprotein
- MCI
minimal cognitive impairment
- MDMA
3,4‐methylenedioxymethamphetamine
- Met
methionine
- MMN
multifocal motor neuropathy
- MOGAD
myelin oligodendrocyte glycoprotein antibody disease
- MS
multiple sclerosis
- MSA
multisystem atrophy
- Mw
molecular weight
- Nf
neurofilament
- NfH
neurofilament heavy chain
- NfL
neurofilament light chain
- NfM
neurofilament medium chain
- nfvPPA
nonfluent and agrammatic variant primary progressive aphasia
- NMOSD
neuromyelitis optica spectrum disease
- ON
optic neuritis
- OSAP
obstructive sleep apnoea syndrome
- P
phosphorus
- PAD
peptidyl arginine deiminases
- PD
Parkinson disease
- pI
isoelectric point
- PML
progressive multifocal leukoencephalopathy
- PNS
peripheral nervous system
- PPA
primary progressive aphasia
- Pro
proline
- PRPH
peripherin
- PSP
progressive supranuclear palsy
- PTM
post‐translational modifications
- RD
retinal detachment
- RNA
ribonucleic acid
- ROM
ratio of means
- ROS
reactive oxygen species
- SAH
subarachnoid haemorrhage
- SAS
sleep apnoea syndrome
- SCA
spinocerebellar ataxia
- SDS
sodium dodecyl sulphate
- Ser
serine
- SIMOA
single‐molecule arrays
- SMA
spinal muscular atrophy
- SUMO
small ubiquitin‐related modifier
- SVD
small vessel disease
- svPPA
semantic variant Ppa
- TBI
traumatic brain injury
- Thr
threonine
- Tyr
tyrosine
- Val
valine
- WNV
West Nile virus
1. INTRODUCTION
The Lady Estelle Wolfson Lectureship in Translational Medicine is awarded annually by the Royal College of Physicians in London to research with demonstrable patient benefit. The 2022 Lectureship was awarded to ‘Neurofilaments’ in acknowledgment of the successful efforts to deliver a reliable biomarker for neurodegeneration. As a consequence of worldwide, collaborative efforts, patients have now access to novel treatment strategies, the efficacy of which was elegantly demonstrated by implementing neurofilaments (Nf) as clinical trial outcome measures. The use of Nf permits to gain much quicker information on the progression of neurodegeneration compared to clinical metrics.
There is consensus that Nf have delivered the first biomarker for neurodegeneration which has, as a laboratory test, broken through the clinical specialty barrier. Industrial scale testing capabilities of Nf from blood samples have become possible by focusing on highly stable and soluble Nf polypeptides. A key development was the move from cerebrospinal fluid (CSF) to blood samples. The first reports on blood Nf concentrations appeared in 2004 (Khalil et al., 2018; Petzold, 2005b). Since, the field has been taken forward by a network approach between basic science, industry, laboratory and clinical science. Analytical sensitivity has been improved by employing Single‐Molecule Arrays (SIMOA) (Kuhle et al., 2016), Acridinium Ester (AE) technology (Center of Disease Control, 2022) and Immunomagnetic Reduction Technology (IRT) (Liu, Lin, et al., 2020). Novel chemical sensing platforms are at the brink of providing point‐of‐care tests (Chen, Tong, & Yang, 2021; Kim, Lee, & Park, 2020). Validation studies covered the performance of the test (Miller et al., 2016; Petzold, Altintas, et al., 2010) and the clinical relevance (Zetterberg & Blennow, 2018). The validation phase was rapidly followed by the successful implementation of Nf as a clinical trial endpoint (Hauser et al., 2020; Tabrizi et al., 2019).
This review will start with the genetics of the Nf proteins. This review expands from the strong focus on NfL in (Khalil et al., 2018) to include all seven known Nf isoforms. The reason for this will be motivated in the genetic section of this review which includes confirmed splicing variants. The section also includes an in‐depth update on known Nf mutations and related diseases. It will be learned that one important aspect of neurodegeneration is related to Nf aggregation. The susceptibility for Nf aggregates can further be increased by post‐translational modifications (PTM), notably phosphorylation, which will be reviewed in detail. Because of the poor solubility of Nf aggregates they do not make for a good body fluid biomarker. Therefore, the review continues with a discussion of described proteolytic breakdown products of Nf isoforms. This includes relevant relationships with disease. Finally, the clinical disease spectrum benefiting from Nf as a biomarker will be reviewed per medical specialty. The wealth of data on Nf has enabled meta‐analyses which will be reviewed in a separate section of the review for two reasons. First, they are likely to increase in number, are frequently cited and influence the interpretation of results. Second, there are meta‐analyses‐specific methodological points to be reviewed (Forgrave et al., 2019). The implications of these findings for clinical trials will be summarised.
2. METHODS
Google Scholar and PubMed were searched. This review refers to human Nf isoforms in general. This review includes Nf structural modelling using Alphafold2 through ColabFold (release version 2022/4/29) (Mirdita et al., 2021). Post‐translational modifications (PTM) were introduced to the Alphafold2 model with PyTMs (Warnecke et al., 2014). PBD files were processed in PyMOL (version 2.5.0) (Schrödinger, LLC, 2015) for creation of images. All scripts and models are available as Supplementary Data.
3. NEUROFILAMENTS: BUILDING BLOCKS FOR NEURONS AND AXONS
Nf are the key building blocks of the cytoskeleton for neurons and axons. Nf belong to the large family of intermediate filaments (IF) which have a diameter of 10 nm. This diameter is intermediate, hence the name, between microfilaments (7 nm) and microtubules (25 nm).
To truly admire the mechanistic achievement of Nf for cellular architecture one must put the size of neurons and axons into relation. For didactic reasons simplified, the neuronal cell bodies (10 μm size) are connected by axons over 1 m length (for the sciatic nerve). This is a factor of 100,000 difference. If St. Paul's cathedral (height 111.25 m) would represent a neuron, then the lengths of the axon would be 111,250 km long and 11 m wide. There would be no town on earth far enough away from London to, within this analogy, represent the next connecting neuron. The distance from London to Paris is 456 km, to New York 5570 km and to Sydney 17,000 km. The imaginary axon, originating from St. Paul's cathedral in London, would only stop about one‐third on the way to the moon. Such a structure would be impossible to build for men. And yet the function of Nf is not only to keep this structure stable, but also helps with the housekeeping.
4. FROM DNA TO PTM
All Nf genes arose through gene duplication from an ancestral IF gene 800 million years ago (Lasek et al., 1985). Chromosome 8 encodes for Nf light (NfL) and Nf medium (NfM), chromosome 22 encodes for Nf heavy (NfH), chromosome 10 for internexin‐α (INA) and peripherin (PRPH) on chromosome 12. The genetic nomenclature has developed over the years with many synonyms, not all correct, used in the literature which may be found confusing. Therefore, an overview is given on the terms currently used (EMBL‐EBI, 2022; NIH, 2022). The structures of the Nf isoforms and splicing variants are shown in (Figure 1).
FIGURE 1.
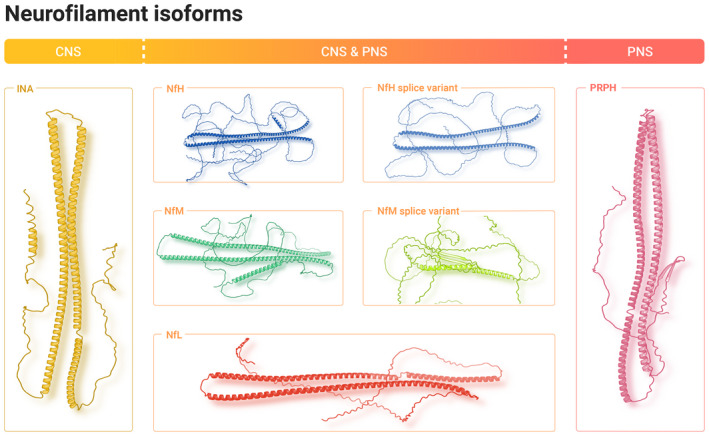
The seven human neurofilament isoforms. Structure models of the Nf isoforms are shown as a protein cartoon. The α‐helical domains are structured and contain a highly conserved rod domain common to all Nf isoforms. Expression is restricted to the CNS for INA (in yellow) and to the PNS for PRPH (in magenda). The remaining isoforms NfH (in blue), NfM (in green) and NfL (in red) are not specific for one single nervous system. NfL, NfM and NfH are expressed both in the CNS and PNS. Currently available immunoassays quantify NfL, NfH or NfM.
4.1. Nf identifiers and aliases
The order of the Nf isoforms is descending, according to the molecular weight (Table 1).
TABLE 1.
The genetic background of Nf isoforms explains changes in reported weight due to alternative splicing for NfH and NfM
| NfH | NfM | NfL | INA | PRPH | |
|---|---|---|---|---|---|
| Chromosome | 22 | 8 | 8 | 10 | 12 |
| Gene location | 29,480,218–29,491,390 | 24,913,761–24,919,093 | 24,950,955–24,956,612 | 49,295,147–49,298,686 | 49,295,147–49,298,686 |
| Length 1 a | 1020 | 916 | 543 | 499 | 470 |
| Length 2 b | 924 | 540 | − | − | − |
| Weight 1 (Mw) c | 112477.56734 ± 7.23404 | 102470.81258 ± 6.54664 | 61400.80804 ± 3.95853 | 55389.99135 ± 3.54851 | 53650.26292 ± 3.46054 |
| Weight 2 (kDa) d | 105.6 | 102.5 | 61.5 | 55.4 | 53.7 |
| Weight 3 (kDa) e | 190–210 | 150 | 68 | 66 | 57 |
| Charge f | −11 | −64 | −49 | −14 | −15 |
| Phosphorylation | +++ g | ++ | + | + | + |
| O‐glycosylation | ++ | ++ | + | − | − |
| Genetic risk for | ALS/SMA, CMT | ALS, PD | ALS, CMT | PD, LBD | ALS |
Note: The weight increases following translation due to post‐translational modifications, the most important of which is phosphorylation. A number of mutations (see main text) have been associated with an increased risk for disease which is either autosomal dominant, autosomal recessive or considered a genetic susceptibility factor. Da, Dalton; Mw, molecular weight; pI, basal isoelectric point.
Full protein length.
Shorter lengths following alternative splicing.
Calculated from DNA sequence.
Reported from processed DNA sequence (EMBL‐EBI, 2022).
Migration in SDS gel which differs from the calculated weights because of post‐translational modification (PTM).
Calculated from amino acid sequence.
NfH is the most extensively phosphorylated protein of the human body.
4.1.1. NfH
The gene ID for NfH is 4744, MIM 162230 (NIH, 2022) and P12036 (EMBL‐EBI, 2022). The location is on Chromosome 22 (29480218.0.29491390), NC. Aliases used are Neurofilament heavy polypeptide, NfH, NEFH, NfHHUMAN, CMT2CC, KIAA0845, NFH200 kDa neurofilament protein, Neurofilament triplet H protein.
4.1.2. NfM
The gene ID for NfM is 4741, MIM 162250 (NIH, 2022) and P07197 (EMBL‐EBI, 2022). The location is on Chromosome 8 (24913761.0.24919093), NC. Aliases used are Neurofilament medium polypeptide, NfM, NEFM, NFMHUMAN, NEF3, NF‐M, 160 kDa neurofilament protein, Neurofilament triplet M protein, Neurofilament 3.
4.1.3. NfL
The gene ID for NfL is 4747, MIM 162280 (NIH, 2022) and P07196 (EMBL‐EBI, 2022). The location is on Chromosome 8 (24950955.0.24956612), NC. Aliases used in the contemporary literature are as follows: Neurofilament light polypeptide, 68 kDa neurofilament protein, Neurofilament triplet L protein, NfL, NEFL, NFLHUMAN, CMT1F, CMT2E, CMTDIG, NF‐L, NF68, PPP1R110.
4.1.4. INA
The gene ID for INA is 9118, MIM 605338 (NIH, 2022) and Q16352 (EMBL‐EBI, 2022). The location is on Chromosome 10 (103277138.0.103290346), NC. Aliases used are Alpha‐internexin, ‐internexin, Alpha‐Inx, INA, AINXHUMAN, FLJ18662, FLJ57501, NEF5, NF‐66, NF66, TXBP‐1, tax‐binding protein, neuronal intermediate filament protein alpha, 66 kDa neurofilament protein, Neurofilament‐66, Neurofilament 5.
4.1.5. PRPH
The gene ID for peripherin is 5630, MIM 170710, PRPH and P41219 (EMBL‐EBI, 2022). The location is on Chromosome 12 (49295147.49298686), NC. Aliases used are Peripherin, NEF4, PERIHUMAN and Neurofilament 4.
4.2. Nf mutations
Pathologically relevant mutations of the Nf genes have been described mainly for ALS (NFH) and CMT (NFL). These mutations reviewed comprehensively here, expand substantially on previous reviews by the inclusion of synonymous variants which have been suggested to be also association with an increased genetic risk for disease and inclusion of protective variants (Table 1).
ALS: NfL (E7K, Q93Q, I261I, I351V R241R, R421X, Y242Y,Y443Y, Y470S, G527del, GAG deletion 528E, Ter531G [Lin, et al., 2021]), NFM (S7W,F35Y, A144T, R295S, S279R, R311L, G407S, A475T, P499P, V718A, V755L, T831T, V858I [Lin, et al., 2021]), NfH (G35G, A90V, Q117X, R148P, E152D, E152D, A152V, Q171H, D187N, A203P, G249S, S285R, A314V, T338I, R346H, R352S, A380T, A380T, E463K, E491K, P505L, P5125, A528delGCT, V578V, P615L, T642M, P655deIAGA, E658 K665del, P663deITGAGAAGGCCAAGTCCCC, P663delTGAA744deIGCC, V670E, A672E, E673E, A674A, A708ins84bp, S787R, 2368–2370delAAG, K794K, E805A, P848S, K867N, E868K, T905I, E918G ([Lin, et al., 2021]), PRPH (A141T (Leung et al., 2006)). An intronic NfH (TTTA) variant (rs140814097) halved the risk for spinal onset ALS (Theunissen et al., 2022).
CMT: NfL (P8L, P8Q, P8R, T21Afs*83, P22R, P22S, P22T, E90K, L94P, N98.S, N98T, E140*, A149V, E186*, E210, Y265C, L268P, L268R, Y265C, L268P, L268R, L311P, L312P, C322N326del, Q332P, L333P, Q334P, L336P, I384F, Y389C, E396K, G397L, R421*, F439I, P440L, Y443N, K467N (Butinar et al., 2008; Horga et al., 2017; Kim et al., 2022)). This list seems long, but overall only 27/798 (9%) of patients with CMT in a large Japanese cohort were due to a mutation in NFL (Higuchi & Takashima, 2022). NH (P739S, L1003A, A1004G, P1007A, P1008A, L1010G, L1015G, L1020G, L1020I (Ando et al., 2022; Pipis et al., 2021; Yan et al., 2020)). Only eight out of 2494 (0.003%) of patients with CMT in a large UK cohort were due to a mutation in the NfH gene making this an extremely rare condition (Pipis et al., 2021). All the NfH mutations in CMT affect the tail domain of the protein.
SMA: NfH (P1007A in one single case (Ando et al., 2022))
PD: NfM (S336G (Lavedan et al., 2002)). It was suggested that this might represent a PD susceptibility factor similar to NFM (P725G and deletion of valine in position 829) (Han et al., 2005; Krüger et al., 2003). INA (E46K) (Zarranz et al., 2003).
LBD: INA (E46K) (Zarranz et al., 2003).
4.3. PTMs of Nf
The transcription of Nf (Nf DNA Nf mRNA) and translation (Nf mRNA Nf amino acid sequence) is followed by PTMs (Figure 2). The most extensive PTM is Nf phosphorylation (Grant & Pant, 2000) (Table 1). The function of PTMs is to biochemically modify the ‘naked’ amino acid sequence (MacTaggart & Kashina, 2021). It has been anticipated that all reactive residues, at least at the protein surface, are subjected to PTMs of which only a fraction are currently known (MacTaggart & Kashina, 2021). PTMs regulate intermediate filament function (Snider & Omary, 2014). This entails the modification of axonal growth and myelination, synapse plasticity, neuronal differentiation, axonal diameter and transport, reaction to damage which includes autoimmunity. PTMs are also relevant to stability and protein interactions (Zecha et al., 2022). The list of known IF PTMs has increased from previous reviews (Khalil et al., 2018; Petzold, 2005b; Sihag et al., 2007).
FIGURE 2.
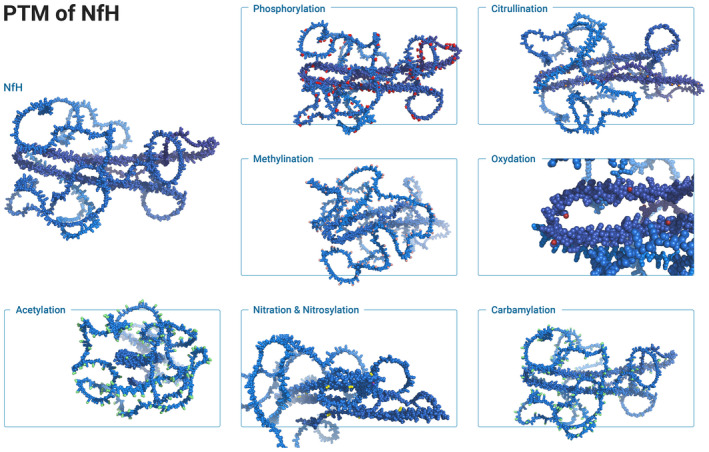
Post‐translational modifications of neurofilaments. The structure of NfH is represented as spheres in blue. Residues modified by PTMs are coloured. The most abundant PTM is phosphorylation (red) at Ser, Thr and Tyr. There is extensive phosphorylation of the unstructured NfH tail domain. In contrast, citrullination (orange) is more extensive in the α‐helical domains. The 3D orientation of NfH is changed through this image which permits a better perception of the predominant effect of acetylation (green) and methylenation (salmon) to the unstructured domains. Data on oxygenation (dark red) are sparce and here shown in magnification for the residues where the two large α‐helices meet (far left). Likewise, nitration and nitrosylation are concentrated to the opposite end (far right) of the same α‐helices. Finally, carbamylation (green) is more expensive in the intrinsically unstructured domains. PTMs for the other Nf isoforms follow a similar pattern, but are less extensive than for NfH.
4.3.1. Nf phosphorylation & dephosphorylation
NfH is the most extensively phosphorylated protein of the human body. Phosphorylation of Nf isoforms occurs at three amino acids: ThrThr(P), SerSer(P) and TyrTyr(P) (see red dots in Figure 2) and is catalysed by kinases (Grant & Pant, 2000). Nf isoform phosphorylation affects protein charge (more negative) and molecular weight (heavier, see also Table 1). Phosphorylation is largely enzymatic and most abundant in the C‐terminal tail domain. The C‐terminal tail domain of NfH contains 42 KSP (Lys‐Ser‐Pro) repeats. Consequently, phosphorylation of the C‐terminal tail domain is, on the whole, regulated by proline‐directed kinases (Grant & Pant, 2000). The N‐terminal domain of the Nf isoforms is phosphorylated by a range of non‐proline‐directed enzymes: cyclin‐dependent kinase‐5, glycogen synthase kinase‐3 and extracellular signal‐regulated kinases (Grant & Pant, 2000; Petzold, 2005b).
Phosphorylation causes charge‐repulsion (Jones & Safinya, 2008). Charge‐repulsion modifies the axonal diameter. Increased Nf phosphorylation results in an increase in axonal diameter. Resistance to proteases also increases with phosphorylation (Goldstein et al., 1987), as does immunogenicity (Carden et al., 1985; Cloos & Christgau, 2004). Phosphorylated Nf isoforms remain immunogenic for millennia (Petzold et al., 2020). Phosphorylation affects Nf solubility and dynamics within the cytoskeleton which helps reorganisation of the Intermediate filament network (MacTaggart & Kashina, 2021). Nf phosphorylation increases with myelination during postnatal development (Shaw & Weber, 1982). Myelin‐associated glycoprotein (MAG) modifies phosphorylation of NfH and NfM (Dashiell et al., 2002). Abnormal Nf phosphorylation is observed in ALS and AD. The data for human NfH, NfM and NfL phosphorylation were recently summarised (MacTaggart & Kashina, 2021) and have been further updated (PhosphoSitePlus) and expanded here to include INA and PRPH (Hornbeck et al., 2015).
NfH at amino acid residues: Ser54, Ser59, Ser61, Ser63, Tyr111, Tyr146, Ser337, Tyr385, Ser431, Thr501, Ser503, Ser511, Ser518, Ser526, Ser532, Ser540, Ser546, Ser552, Ser560, Ser566, Ser574, Ser580, Ser594, Ser600, Ser606, Ser614, Ser620, Ser628, Ser634, Ser640, Ser648, Ser654, Lys657, Ser660, Ser668, Ser674, Ser682, Ser688, Ser696, Ser702, Ser710, Ser716, Ser724, Ser730, Thr738, Ser744, Ser752, Ser758, Ser769, Ser782, Ser793, Ser801, Ser807, Ser828, Thr911, Ser948
NfM at amino acid residues: Ser23, Ser28, Ser30, Ser33, Ser37, Ser44, Ser55, Ser73, Ser226, Ser333, Tyr384, Tyr401, Ser467, Ser511, Ser545, Ser553, Ser558, Ser559, Ser615, Ser620, Ser628, Ser633, Ser641, Ser646, Ser654, Ser659, Ser667, Ser670, Ser672, Ser680, Ser685, Ser736, Thr748, Thr750, Ser783, Ser788, Ser821, Ser837, Tyr872
NfL at amino acid residues: Ser3, Tyr14, Thr21, Ser56, Ser103, Thr154, Ser215, Ser221, Tyr372, Tyr389, Ser472, Thr520
INA at amino acid residues: Ser23, Ser30, Ser58, Ser78, Tyr272, Tyr287, Ser335, Tyr379, Tyr396, Thr442, Ser445, Thr463, Ser464, Ser496
PRPH at amino acid residues: Ser13, Tyr17, Ser28, Ser50, Ser59, Ser62, Ser75, Tyr287, Ser405, Ser413, Thr421
4.3.2. Nf citrullination
Citrullination (synonymous: peptidylarginine deimination or just deamination) alters peptidyl‐Arginine, but not free Arginine, to peptidyl‐Citrulline (ArgCit). This is achieved through Ca2+‐dependent hydrolysis of ketamine to keton (Mondal & Thompson, 2021). The reaction is catalysed by a group of peptidyl arginine deiminases (PADs). Citrullination reduces charge (−1 per deimination), weight (+0.98 mW), susceptibility to proteolysis, structure, function, neurodegeneration and autoimmunity (Briot et al., 2020). Given the amount of data on citrullination of human MBP (Cao et al., 1998; Srour et al., 2022; Wang, Chen, et al., 2022; Yu & Proost, 2022), which has only 29 Arg residues, it is surprising how little is known regarding the citrullination of human NfH (44 Arg), NfM (37 Arg), NfL (35 Arg), INA (45 Arg), PRPH (48 Arg). Citrullination in Figure 2 was modelled in orange spheres. Analytical limitations for experimental study of citrullination (Verheul et al., 2018) have been overcome with high‐resolution mass spectrometry (Yu & Proost, 2022).
4.3.3. Nf glycosylation
Glycosylation reversibly adds carbohydrates. Glycosylation has been demonstrated for NfL, NfM and NfH (Dong et al., 1993). In humans, there is O‐ and N‐glycosylation. N‐glycosylation targets Asn. O‐glycosylation (O‐GlcNAcylation) occurs at the hydroxyl‐group of Thr, Ser, Hyl and Hyp: AspAsp‐glycan, SerSer‐glycan. Glycosylation is central to a range of immune processes. Altered protein glycosylation may trigger an autoimmunity (Cloos & Christgau, 2004). Impaired glycosylation has been related to neurodegeneration (Yuzwa et al., 2012). Reduced O‐GlcNAc glycosylation of NfM is linked to AD and ALS (Deng et al., 2007; Dong et al., 1996). Glycosylation of NfH increased with glucose deprivation (Cheung & Hart, 2008).
4.3.4. Nf glycation
Glycation describes the non‐enzymatic addition of a sugar aldehyde or ketone to an amino acid residue (Kikuchi, 2003). This is the main difference to glycosylation which requires enzymes, but the terms are not always strictly differentiated in the literature. Glycation is rapid for surface Lys residues, particularly if adjacent to His. The late stage of glycation leads to formation of advanced glycation end products (AGEs) which are cytotoxic. Many AGEs are unstable and some are immunogenic (Chou et al., 1998; Cloos & Christgau, 2004; Virella et al., 2003). There is a likely synergistic role of glycation and oxidative stress regarding neurotoxicity in AD and ALS (Kikuchi, 2003).
4.3.5. Nf acetylation
Acetylation of Lys and Arg (both basic) neutralises the side chain resulting in a larger residue with reduced polarity (green coloured in Figure 2) (Lacoursiere et al., 2022). There are at least 40 lysine acetyltransferases (Donev, 2021) and 20 deacetylases (Ho et al., 2020). Acetylation in human Nf has been demonstrated for (MacTaggart & Kashina, 2021):
NfH at amino acid residues: Lys659, Lys663, Lys907, Lys908
NfM at amino acid residues: Lys298, Lys403, Lys653, Lys693, Lys698
NfL at amino acid residues: Lys362, Lys379, Lys391
INA at amino acid residues: Lys290, Lys398, Lys483, Lys498
PRPH at amino acid residues: Lys288, Lys398
4.3.6. Nf deamidation
Deamidation has been described as a molecular clock because it is closely related to the physiological half life of a protein (Ying & Li, 2022). The lifetime of Nf isoforms was calculated to be in the range of 1–2 years (Lee & Cleveland, 1996). Spontaneous deamination mostly affects AsnAsp and is very slow for GlnGlu. Enzymatic deamidation is catalysed by tissue transglutaminase in a Ca2+‐dependent manner. With high‐resolution mass spectrometry it has become easier to quantify deamidation (mass shift of 0.984 Da) (Ying & Li, 2022). Deamidation has been described in AD, PD among other neurodegenerative conditions (Briot et al., 2020). Therefore, it is interesting to note that yet so little is known about deamidation of Nf isoforms (see supplementary material in (Trimpin et al., 2004)). Deamination is interrelated with isomerisation and racemisation.
4.3.7. Nf isomerisation
Isomerisation describes the conversion of for example AspisoAsp or GLyisoGly. Isomerisation almost always affects susceptibility to proteolysis. Protein isomerisation has been implicated in ALS pathogenesis (Parakh et al., 2013).
4.3.8. Nf racemisation
AspD‐Asp (D‐isoAsp), GlxDGlu (D‐isoGlu). Occasionally also other residues such as Ala, Ser, Thr may racemise. Susceptibility to proteolysis is always affected. There are yet no systematic data on racemisation and isomerisation on human Nf isoforms.
4.3.9. Nf oxidation
Oxidative stress causes damage to proteins which is relevant in neurodegenerative disease (Lee et al., 2021). Even in absence of pathology, there will be oxidative stress to any given protein over time. One major target of oxidation is the thiol side‐chain of Cys (dark red spheres in Figure 2) (Lee et al., 2021). Given the longevity of Nf isoforms, it is not surprising that oxidation has been described (Trimpin et al., 2004). Oxidising compounds such as free radicals are commonly known as reactive oxygen species (ROS) and the major cellular sources are mitochondria. It has been proposed that NfH protects other axonal proteins by accumulating the oxidative damage (Couillard‐Despres et al., 1998).
4.3.10. Nf S‐nitrosylation
Similar to oxidation, S‐nitrosylation (S‐nitrosation) also affects the thiol side‐chain of Cys. A good pre‐analytical quality control pipeline is relevant to avoid artefacts (Wang, Zhou, et al., 2022). Modelling of nitrosylation and nitration is shown in yellow colour in Figure 2.
4.3.11. Nf methylation
The methylation of Lys and Arg residues is catalysed by methyltransferases and reversed by demethylases. Both amino acids can be mono‐ and dimethylated, with trimethylation also being possible for Lys. Methylation of Lys is illustrated in Figure 2 (salmon colour), but there are no experimental data yet confirming that this happens in vivo. Contemporary experimental data provide evidence for methylation of Arg in Nf isoforms (Ho et al., 2020; MacTaggart & Kashina, 2021):
NfH at amino acid residues: Arg164, Arg412, Arg389
NfM at amino acid residues: Arg21, Arg26, Arg42, Arg54, Lys102, Arg111. Arg382
NfL at amino acid residues: Arg23, Arg30, Arg37
INA at amino acid residues: Arg24, Arg39, Arg104, Arg377
PRPH at amino acid residues: Arg72, Lys98
4.3.12. N‐terminal Nf modifications
In humans, the N‐terminus is frequently N‐acetyl ‘blocked’ and common residues are as follows: Ala, Ser, Met, Gly or Thr. Enzymatic removal of these residues is possible. N‐terminal acetylation has been shown for all Nf isoforms (Trimpin et al., 2004).
4.3.13. C‐terminal Nf modifications
Amidation of the C‐terminus is common. Gly is a frequent donor for the amide. Other mechanisms are methylation and isoprenylation for GPI anchors and ADP‐dependent ribosylation of C‐terminal Lys (Dutour‐Provenzano & Etienne‐Manneville, 2021; Srour et al., 2022; Ying & Li, 2022).
4.3.14. Nf ubiquitination
Ubiquitination largely affects lysine residues (Lacoursiere et al., 2022). This is catalysed by three enzymes, E1 (activates), E2 (conjugates) and E3 (ligates) ubiquitin (8.5 kDa) onto the Lys within Nf. Ubiquitination for Nf isoforms has been demonstrated for (MacTaggart & Kashina, 2021):
NfH at amino acid residues: Lys31, Lys336, Lys531, Lys565, Lys659, Lys895
NfM at amino acid residues: Lys118, Lys259, Lys263, Lys271, Lys445, Lys451, Lys871, Lys875
NfL at amino acid residues: Lys15, Lys157, Lys271, Lys370
INA at amino acid residues: Lys95, Lys290
PRPH at amino acid residues: Lys288, Lys290, Lys398, Lys402
4.3.15. Nf SUMOylation
The Lys residues of the Nf isoforms are a target for the covalent addition of a small ubiquitin‐related modifier (SUMO). This is catalysed by SUMO‐specific ligases and reversed by a family of SUMO/Sentrin‐specific proteases (Chen, Zhang, et al., 2021). Deficient SUMOylation results in protein accumulation, aggregation, alteration of synapses and ion channels (Henley et al., 2020; Moon et al., 2021). SUMOylation affects PRPH at Lys398 (Hornbeck et al., 2015).
4.3.16. Nf Farnesylation
Farnesylation adds a lipid group to Cys residues which increases hydrophobicity. Farnesylation can be permanent or reversible and is modified by farnesyltransferase inhibitors (Young et al., 2013).
5. NF HETEROPOLYMER FORMATION
Once expressed and modified through PTMs, the Nf isoforms co‐assemble to form a very stable threadlike heteropolymer (Figure 3). The process of the assembly is understood to require only a small central section within each of the Nf isoforms. This central section has a preserved structure. Simplified, Nf hetero‐polymerisation can be visualised as a 10–15 nm tight knot tied with the central part with two loose, flexible ends of the thread extending another 50–100 nm (represented as ribbons in Figure 3a–e) (Janmey et al., 2003). The C‐ and N‐terminal regions contain intrinsically unstructured domains which are governed by electrostatic forces (Ghosh et al., 2022). These electrostatic forces are responsible for the outward radiation of the charged, C‐terminal Nf tail regions from the core of the assembled Nf heteropolymer (Figure 3l–m). On a microscopic level, this results in charge‐repulsion and change of axonal diameter.
FIGURE 3.
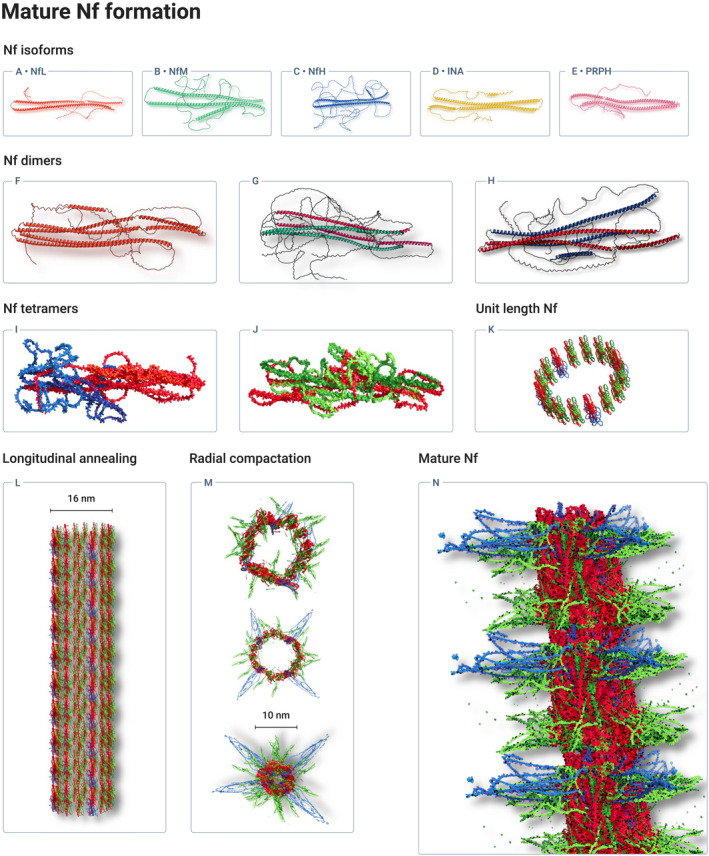
Neurofilaments are key building blocks of the neuro‐axonal cytoskeleton. The Nf heteropolymer is formed by parallel alignment of Nf isoforms to dimers, followed by antiparallel alignment of dimers to tetramers. Tetrameters assemble to a unit length filament. Through longitudinal annealing a ≈ 16 nm filament assembles which is finally radially compacted to the major ≈10 nm neurofilament. Structure models of the Nf isoforms are shown as a protein cartoon for (a) NfL, (b) NfM, (c) NfH, (d) INA and (e) PRPH. Dimer formation requires the presence of NfL and are shown here including visualisation of sidearm chains targeted by PTMs for (f) NfL:NfL, (g) NfL:NfM and (h) NfL:NfH. The interaction between the sidearms becomes more complex with formation of tetramers here shown as surface plots for (i) NfL:NfL with NfL:NfH and (j) for NfL:NfM with NfL:NfM. (k) Shows a single unit length Nf filament which is built from 16 tetramers (4 × (i) and 12 × (j)). (l) Longitudinal annealing of the unit length Nf filaments elongates the filament by 60 nm steps with each unit length added. (m) Radial compaction (sagittal view) of the 16 nm diameter of the unit length Nf to the final diameter of 10 nm (=10.2 Å measurement in pymol). During radial compaction, PTMs support the structural changes of the Nf side‐arms (coloured as in Figure 2). There is progressive emergence of Nf isoform side‐arms from the centre, radiating to the periphery. (n) Mature Nf.
The Nf rod domain is subdivided into four helical domains: 1A, 1b, 2A, 2B. The helical domains are separated by linkers: L1, L12, L2. The 1B domain is flanked by an A11 mode of interaction pocket (N‐terminal) and A11 mode of interaction knob (C‐terminal). This knob‐pocket interaction is the key for alignment of Nf dimers (Figure 3f–h) to tetramers (Figure 3i, j) in phase with the hydrophobic 1B coil domain (Eldirany et al., 2021). Polymer stability mostly comes from the A11 knob‐pocket interactions. The Nf tetramers generally consist of NfL together with one of the other Nf isoforms. The need for one NfL per unit is explained by in vitro data showing that of the human Nf isoforms only NfL can self‐assemble in vitro (Carter et al., 1998). The heteropolymer is formed through co‐assembly of NfL, NfM and NfH (Yuan & Nixon, 2021). INA is added in the brain and PRPH in the peripheral nerve.
6. FUNCTION
Initially, it was proposed that Nf would only affect an organism indirectly through the structure and function of neurons (Lasek et al., 1985). This view is, in its core, still correct but has been expanded:
Action potential conduction speed through axonal diameter (Lawson et al., 1993)
Aldosterone secretion (Maniero et al., 2017)
Axonal diameter (Jones & Safinya, 2008; Lasek et al., 1983; Lasek et al., 1985; Marszalek et al., 1996; Monaco et al., 1989)
Axonal flow and stasis (Balaratnasingam et al., 2007; Vial, 1958)
Axonal transport (Lasek, 1967; Mutalik & Ghose, 2020; Nixon & Logvinenko, 1986)
Epigenetic regulation in cancer (Calmon et al., 2015; Hasan et al., 2021; Li et al., 2020)
Evolution of the nervous system across species (Lasek et al., 1985)
Interaction with mitochondria (Gentil et al., 2011; Wagner et al., 2003; Zhu et al., 2022)
Interaction with myelin proteins (Dashiell et al., 2002)
Mechanical stability (Kim et al., 2011)
Modulation of the endoplasmic reticulum (Rao et al., 2011)
Synaptic modeling and startle response through activity at the presynaptic terminal (Bullock & Horridge, 1965)
Viscoelastic properties (Herrmann et al., 2007; Srinivasan & Kumar, 2012)
7. AGGREGATION
Nf are prone to aggregation (Eldirany et al., 2021). The concept that Nf aggregation is relevant for disease originates in experimental studies on ALS (Julien, 2001). Nf aggregation causes slowing and disruption of axonal transport (Collard et al., 1995) which is followed by loss of neuronal function and disease (Didonna & Opal, 2019; Julien, 2001; Rebelo et al., 2016). Consistent with the experimental data, there is evidence for Nf aggregates in the blood of subjects with ALS (Adiutori et al., 2018). Future studies on Nf aggregation should consider the possibility that a limited degree of aggregate formation is physiological and reversible (Murray et al., 2022). The alignment of aggregation‐prone domains is facilitated by a steric zipper region and their side‐chain interdigitation. The reversibility of this limited aggregate formation is governed by mutations and PTMs (Murray et al., 2022). Irreversibility is increased by mutations introducing Cys, amyloidogenic elements and PTMs such as hyper‐phosphorylation (Murray et al., 2022; Rebelo et al., 2016; Xiao et al., 2006). It has been proposed that Nf aggregate formation is also a relevant factor for long‐term protein preservation (Petzold et al., 2020). Extremely tight folding is mechanically possible because of the large proportion of Lys, and intrinsically unstructured domains.
8. NF ISOFORM CLEAVAGE AND STABILITY
Pioneering experimental work on enzymatic cleavage of intermediate filaments used six enzymes enabling amino acid sequencing as a means of characterisation of the proteolytic breakdown products (Geisler & Weber, 1981). The enzymes employed were 2‐nitro‐5‐thiocyanobenzoic acid, trypsin, thermolysin, chymotrypsin, Staphylococcus aureus V8 protease and carboxypeptidase. In a second step, major Nf fragments were subjected to double chemical cleavage with NTCB and BNPS‐skatole (Geisler et al., 1982). This approach revealed a highly specific 5 K NfL cleavage product (Geisler et al., 1982). The amino acid sequence of this 5 K NfL cleavage product was as follows: Arg‐Ala‐Ala‐Lys‐Asp‐Glu‐Val‐Ser‐Glu‐Ser‐Arg‐Arg‐Leu‐Leu‐Lys‐Ala‐Lys‐Thr‐Leu‐Glu‐Ile‐Glu‐Ala‐Cys (Geisler et al., 1982). Further experiments showed that digestion of NfH revealed fragments of high stability which resisted prolonged treatment with enzymes (Autiliogambetti et al., 1986). The explanation for the different stability of the Nf isoforms to enzymatic digestion was through phosphorylation (Sternberger & Sternberger, 1983). Because NfH is more heavily phosphorylated than NfL it was found to be the more stable Nf isoform (Goldstein et al., 1987).
For clinical biomarker research stability is important. A stable biomarker can more readily be processed in routine laboratory practice. In contrast, a biomarker which is not stable and degrades rapidly requires fast and careful processing an immediate storage at −80°C (Teunissen et al., 2009). In this context, it needs to be acknowledged that early experimental studies (Autiliogambetti et al., 1986; Geisler et al., 1982; Sternberger & Sternberger, 1983) led to an insightful scientific exchange on Nf biomarker stability (Gunnarsson et al., 2011; Koel‐Simmelink et al., 2011). The data used for the two sides of the argument were, at the time, based on the assumption that the tests used quantified the full‐length NfL and NfH proteins. Both Nf isoforms could be shown in fresh blood and CSF samples, but the NfL signal was diminished in blood and abolished in CSF after storage of only 18 h at room temperature. The findings from the immunoblot were consistent with data from ELISA (Koel‐Simmelink et al., 2011). There was a decrease in the concentration of the full‐length NfL within 24 h which progressed to non‐measurable levels within 4 days. In contrast, full length, phosphorylated NfH persisted for up to 290 weeks (Koel‐Simmelink et al., 2011). These experiments were consistent with what was expected from the earlier literature (Autiliogambetti et al., 1986; Geisler & Weber, 1981; Goldstein et al., 1987; Sternberger & Sternberger, 1983).
How could the finding of higher NfL stability using a different ELISA (Gunnarsson et al., 2011) be explained? It was the serendipitous finding of very long‐term stability of NfL from an ancient human brain (Petzold et al., 2020) which stimulated re‐visitation of the question in a joint experiment (Altmann et al., 2021). This included the two groups involved in the scientific letter exchange on NfL stability (Gunnarsson et al., 2011; Koel‐Simmelink et al., 2011). The conclusion from these experiments is that the discussion has moved from stability of the full‐length protein to stability of protein fragments, likely to arise with pathology. The presumed NfL fragment currently quantified is of sufficient stability to be useful for routine laboratory sample handling, transport and storage (Altmann et al., 2021). The data confirm the 7‐day stability of NfL as a biomarker within a 4.4–5.5 pg/ml 95% CI in a Bland–Altman plot (Altmann et al., 2021). Western blot data (Figure S3, Brureau et al., 2017) revealed the absence of full‐length NfL from the CSF sample, but the capture of NfL fragments which is consistent with the Western blotting shown by (Goldstein et al., 1987; Koel‐Simmelink et al., 2011; Sternberger & Sternberger, 1983).
Notably, there has been progress on further characterisation of Nf cleavage products (Geisler et al., 1982). For NfL, it was shown that the full‐length protein was absent from Western blot (Brureau et al., 2017). Instead, a NfL‐specific cleavage product was found around 10 kDa (Brureau et al., 2017). The detailed supplementary materials also show binding of the monoclonal antibody used in (Gunnarsson et al., 2011) to other NfL breakdown products around 50, 40 and 20 kDa which were not specific for NfL because mass spectroscopy showed sequence overlap with Vimetin (P20152) and Desmin (P31001) (Brureau et al., 2017). The elegant methodological approach chosen was to use the commercially available ELISA coated with the capture antibody 47:3 (Figure 2, lane 8 Norgren et al., 2002) to collect protein from CSF which then were subjected to nanoLC Mass spectrometry analysis (Brureau et al., 2017).
These observations are consistent with data demonstrating that calpain mediates cleavage of axonal Nf during Wallerian degeneration (Ma et al., 2013). Proteolytic breakdown fragments for NfL were found around 22, 40 and 55 kDa. Of these, the 55 kDa fragment was least stable (Ma et al., 2013). The 53 and 57 kDa proteolytic NfL fragments have been demonstrated following traumatic brain injury (TBI) in vivo and in vitro (Nixon & Sihag, 1991; Posmantur et al., 1994; Posmantur et al., 1998). In addition, a 30 kDa NfL proteolytic fragment was described in blood samples (Adiutori et al., 2018). A combined immunoprecipitation and mass spectroscopy analysis of NfL cleavage products permitted to characterises NfL based on antibody‐targeted selectivity of head, rod and tail domains (Budelier et al., 2022). Data from brain and CSF samples were compared. The full‐length NfL protein was present in the brain only. The brain also contained a C‐terminal fragment (NfL530−540). In the blood, three NfL fragments dominated, NfL92−224, NfL324−360, NfL530−540. The latter was best for separating samples from individuals with controls. The supplementary data to this report provide a detailed list of the antibody and mass spectroscopy data which show that there are many more NfL fragments in the CSF (Budelier et al., 2022).
In TBI, proteolytic breakdown products for NfH have been described in a human brain tissue microdialysis study from the interstitial fluid (Petzold, Tisdall, et al., 2011). Cleavage was due to activation of an axonal membrane‐bound Ser protease. The enzyme cleaved NfH into the fragments NfH476−986 (56 kDa), NfH476−1026 (60 kDa), NfH1−476 (53 kDa), NfH835−1026 (21 kDa) and NfH852−986 (15 kDa). These in vivo characterised NfH fragments were different to earlier reported calpain cleavage products at 120 and 146 kDa (Greenwood et al., 1993).
Taken together, it is likely that there are many Nf isoform cleavage products which, because of their stability and solubility, are of advantage for use as a biomarker in clinical studies compared to the full‐length Nf proteins.
9. STOICHIOMETRY
Stoichiometry describes the quantitative relationship of several substances in a defined compartment. The stoichiometry of Nf proteins is noted as an averaged value for NfL:NfM:NfH in the following reports:
4:2:1 according to post‐mortem data from bovine spinal cords (Scott et al., 1985)
7:3:2 in mature axons (Janmey et al., 2003)
24:5:2 according to CSF data and Monte Carlo simulations in multiple system atrophy (Kim et al., 2011)
16:11:4 according to CSF data and Monte Carlo simulations in relapsing–remitting multiple sclerosis (Kim et al., 2011)
24:2.4:1.6 in the plasma of individuals with ALS (Zucchi et al., 2018)
These data suggest that, on an averaged group level, there is a change in Nf stoichiometry in disease. But the observation is contested (Yuan & Nixon, 2021) because it was not possible to demonstrate an isolated up‐regulation of a single Nf isoform gene (Robinson et al., 1994; Wong et al., 2000) or protein expression (Ashton et al., 2019) in ALS and AD. Limitations of these studies were, however, the artefacts due to the post‐mortem interval and averaging of data over a large histological area. This criticism aside, indeed selective suppression of NfL mRNA (p0.05) was shown in the lateral horn of post‐mortem cases with ALS if compared to controls (Wong et al., 2000). Therefore, Wong et al. concluded that the stoichiometry of IF expression is markedly disrupted, but cautioned against interpreting this as an ‘abortive regenerative response’. In another study, selective perturbation of NfH miRNAs is reported (Maciotta et al., 2013). Future studies will need to move to single cell level.
In vitro, hydrogel experiments provide further evidence that the Nf heteropolymer remains stable across a large range of Nf stoichiometries (Beck et al., 2012). Consistent with other studies, the ground truth was defined as NfL:NfM:NfH = 7:3:2 (; Beck et al., 2012; Janmey et al., 2003; Kim et al., 2011; Zucchi et al., 2018). The range of NfL:NfM:NfH stoichiometries studied in hydrogels were: 7:3:2; 80:20:0; 82:0:18; 90:10:0; 90:20:0; 92:0:8; 92:0:8; 97:0:3; 97:3:0; 100:0:0. These stoichiometries were tested at different salt concentrations: 48, 96 and 240 mM (Beck, Deek, Choi, et al., 2010). These hydrogel‐based findings (Beck, Deek, Choi, et al., 2010; Beck, Deek, Jones, & Safinya, 2010) reveal a relationship between Nf heteropolymer stiffness which depends on stoichiometry and salt concentration. An increase in NfL or NfM, but not NfH, in the stoichiometry results in a stiffer Nf heteropolymer. It was proposed that there is a stoichiometric governed competition between entropic Nf sidearm repulsion and electrostatic ionic bridging (Kumar et al., 2002). The experimental model is well suited for studying the effect of Nf PTMs changing sidearm charge reviewed here (Table 2, Figure 2). The need to fill this gap in knowledge on Nf has been highlighted as a research priority for informing future computational approaches and clinical studies (Khan et al., 2020; Zucchi et al., 2020).
TABLE 2.
Post‐translational modifications (PTM) and their main target amino acid residue. The reversal of these PTMs are not included in the table and are discussed in the main text as relevant for Nf isoforms, for example Nf dephosphorylation to reverse Nf phosphorylation
| PTM | Mechanism |
|---|---|
| ADP‐ribosylation | Adds ADP‐ribose which is a form of glycosylation. Affected are Gglu, Asp, sSr, Arg, Cys, Lys, diphthamide, phosphoserine, Asn |
| Acetylation | Acetylates Lys |
| C‐terminal | Amination (adds an amine group), glycosyl phosphatidylinositol attachment, phosphorylation |
| Carbonylation | Adds carbon monoxide |
| Carboxylation | Adds a carboxyl group to glutamate |
| Citrullination | Synonymous to deimination, converts Argto Cit |
| Farnesylation | Adds a lipid group |
| Glutamylation | Adds glutamate |
| Glycation | Adds a sugar aldehyde or ketone, the late stage is characterised by formation of advanced glycation end products (AGEs) |
| Glycosylation | Similar to glycation only that this is enzyme mediated |
| Glycylation | Adds glycine |
| Hydroxylation | Changes Pro to Hyp, also works with Lys |
| Isomerisation | Transformation into an isomer which changes the chemical structure |
| Methylation | Add a methyl group to Arg |
| N‐terminal | Arginylation (adds Argi), formylation (adds a formyl group), pyroglutamate (N‐terminal Glu forms a pyroglutamate group) |
| Nitrosylation | Adds nitric oxide |
| Oxidation | Adds oxygen species |
| Phosphorylation | Adds phosphorus to Ser and to a lesser degree also to Thr and Tyr |
| SUMOylation | Adds a small ubiquitin‐related modifier (SUMO) |
| Sulphation | Adds sulphate to Tyr |
| Tyrosination | Adds Tyr |
| Ubiquitination | Adds ubiquitin to Lys |
Another limitation is that the knowledge on Nf stoichiometry is based on averaged data. Figure 3 illustrates that the unit length filament is constructed by 16 tetramers. This calculates to 64 individual Nf isoforms. It is likely that the Nf isoform stoichiometry varies between within the unit length and over the lengths of the longitudinally annealed filament.
For the treatment of neurodegeneration, the observations on Nf stoichiometry could be relevant. It was proposed that an adaptive Nf stoichiometry represents an endogenous mechanism to slow down the progression of neurodegeneration (Zucchi et al., 2018). A switch from the more resource demanding larger proteins, NfH and NfM, to a higher concentration of the smaller NfL in the heteropolymer comes at practically no risk to Nf heteropolymer stability (Kim et al., 2011), but helps the motor neuron to save time and energy (ADP) (Zucchi et al., 2018). Modulation of Nf stoichiometry should be of interest for gene silencing methods (McCampbell et al., 2018; Miller et al., 2020). The idea is to transiently and reversibly lower the expression of individual Nf isoforms. Such experiments should test if a gently applied gene silencing treatment approach can support an endogenous strategy of adaptive protein stoichiometry. Does the gentle down‐regulation of NfM and NfH prolong survival in motor neuron disease? And if so, can this be of benefit for treatment of other neurodegenerative conditions? Or use of Nf aggregation inhibitors.
10. QUANTIFICATION
The impressive advance from first‐generation immuno‐assays to fourth‐generation immuno‐assays had been subject to reviews with a strong focus on the quantification of NfL with SIMOA (Khalil et al., 2018; Li & Mielke, 2019). The main advantage of the, still very expensive, SIMOA approach is an increased detection limit and analytical range if compared to the relative cost‐effective, in house, enzyme‐linked immunosorbent assay (ELISA) (Gaetani et al., 2018; Norgren et al., 2003; Petzold et al., 2003; Rosengren et al., 1996; Shaw et al., 2005; Van Geel et al., 2005). The reported detection limits are 0.18 fg/ml for the immunomagnetic reduction (IMR) assay, 0.241–0.62 pg/ml for the SIMOA assay, 2.70 pg/ml for the Ella assay, 15.6 pg/ml for the ECL assay and 78.0 pg/ml for the ELISA (Gauthier et al., 2021; Kuhle et al., 2016; Liu, Lin, et al., 2020). A lower limit of quantification of 3.9 pg/ml has been advertised for Acridinium Ester (AE) technology (Center of Disease Control, 2022).
Head‐to‐head method comparisons demonstrate general agreement on pathological compared to normal NfL concentrations (Gauthier et al., 2021) or NfH concentrations (Petzold & Shaw, 2007), with a clear point for the higher sensitivity of the NfL SIMOA platform if compared to the NfL ELISA or an NfL electrochemiluminescence immunoassay (Kuhle et al., 2016). A limitation of some comparisons is that the protein standard curve used in the assays was not of identical origin (Gauthier et al., 2021; Kuhle et al., 2016). This has two implications; first test comparisons regarding the lower limit of detection are only indirect; second, it complicates the interpretation of Bland–Altman plots which are interpreted to show that the SIMOA assay underestimates lower range NfL concentrations by about 17% (Gauthier et al., 2021). Another high‐throughput immunoassay has been developed by Siemens Healthineers (Uzgiris et al., 2022). To address these points and downstream effects experts at the interface between pharmacy and regulatory authorities have come together in a very relevant White‐paper (Hersey et al., 2022). This is an important development to move from research use only assays to clinically approved assays. Regarding analytical issues the white paper highlights the need for achieving parallelism (Hersey et al., 2022; Lu et al., 2011) and notes situations where there is a lack of parallelism:
Calibrator and analyte are not recognised in the same way which can be the case in presence of aggregates or diversity of antibodies in the endogenous matrix sample
Endogenous binding partners interfere
A matrix effect
Assuming appropriate analytical validation recommendations were made for clinical biomarkers as surrogate endpoints of for patient selection/stratification (Hersey et al., 2022):
To understand the behaviour of a biomarker in the study population
To provide analytical and clinical data for proposing what is the best biomarker for providing a clinical endpoint
To engage early with regulatory agencies
To carefully and rigorously address the quality control strategy which needs to be sustainable
To validate the method pre‐analytically (sample collection and processing), analytically (sensitivity, specificity, precision, stability, accuracy, cutoff point) and clinically (agencies requirements, specific context of use, study risk)
The pre‐analytical, analytical and quality control points of this white paper were the subject of the first part of this review. In the following, the clinical aspects are reviewed to address above points regarding clinical studies.
11. DISEASES
There has been an impressive increase in diseases and conditions in which Nf have been studied over the past 4 years alone. In this section, the neurological conditions will be reviewed first followed by other medical subspecialties.
11.1. Neurological conditions
11.1.1. ALS
This is a rapidly progressive neurodegenerative condition, mostly leading to death within a couple of years. Nf concentrations in the CSF are among the highest observed for any disease (De Schaepdryver et al., 2018; Rossi et al., 2018). Nf stoichiometry changes in ALS (Khalil et al., 2018). The result of adaptive Nf stoichiometry provides a potentially elegant strategy for neurons to save time and energy while maintaining structural integrity (Zucchi et al., 2018). Presymptomatic individuals with known mutations causing ALS to show an increase in blood NfL levels at least 1–3.5 years before clinical onset (Benatar et al., 2019). CSF and blood Nf concentrations permit to identify individuals with rapid and slow disease progression (Brettschneider, Petzold, Suessmuth, et al., 2006; Gille et al., 2018; Lu et al., 2015). Consequently, Nf became a secondary endpoint for two clinical trials in ALS (Miller et al., 2020; Paganoni et al., 2020).
11.1.2. Multiple sclerosis
MS is a chronic inflammatory disease (Thompson et al., 2018). The relevance of axonal pathology in MS was recognised from human post‐mortem samples. This observation heralded much of the pioneering work on Nf biomarker data (Petzold, 2005b). Cumulative, the large number of publications on Nf concentrations indicate that Nf concentrations rise already up to 6 years prior to onset of disease, called the presymptomatic phase. Likewise, CSF and blood NfL and NfH levels indicate the conversion from clinically isolated syndrome (CIS) to RRMS (Brettschneider, Petzold, Junker, & Tumani, 2006; van der Vuurst de Vries et al., 2018). Individuals with the highest Nf concentrations become, undisputedly, more rapidly disabled on a short‐term basis (Brune et al., 2022; Cantó et al., 2019; Petzold, 2005a). They will also experience more severe atrophy on imaging of their brain, spinal cord and retina (Bsteh et al., 2019; Chitnis et al., 2018; Lie et al., 2022; Petzold et al., 2016). The long‐term prognostic value of Nf in MS is more controversial (Manouchehrinia, Stridh, et al., 2020; Sellebjerg et al., 2018). There seems almost no aspect of the known MS pathology left which has not yet been correlated with body fluid levels of Nf isoforms which renders it impossible to include all findings in this review. An elegant recent observation is that elevated blood levels of NfL were related to the presence of paramagnetic rim MRI lesions (Maggi et al., 2021). This observation is consistent with translational evidence for a build up of Nf brain tissue concentrations in the perilesional white matter (Petzold et al., 2008). Release of Nf from ongoing inflammation leading to axonal injury, and proteolytic breakdown of Nf isoforms, in this area is indeed a convenient model to explain high blood Nf levels in individuals with MS in absence of novel contrast‐enhancing lesions. The other explanation is of course retrograde trans‐synaptic axonal degeneration. This has been studied most extensively in the visual system. Credit goes to Jens Kuhle and his team for pioneering a percentile‐based approach guiding the statistical analysis of body fluid Nf levels (Barro et al., 2018; Cantó et al., 2019; Maggi et al., 2021). It is likely that this approach will gain momentum because it permits to eloquently pick out those individuals with pathological Nf levels. Such an understanding is important on many levels including neurotoxicity. Neurotoxicity arising from an intervention can be recognised by Nf (Petzold, Mondria, et al., 2010). Because individuals with MS suffer from an impaired blood–brain barrier (BBB) they may be particularly vulnerable to potentially neurotoxic treatments.
For future study, all of these associations, key challenges are now to clarify how frequently blood Nf levels require measurement and establishment of a clinically meaningful level of change in concentration (Bittner et al., 2021). In addition to the safety, prognostic and monitoring value of Nf in MS there is now phase 3 trial evidence for Nf as a reliable secondary endpoint for immunosuppression (Hauser et al., 2020). The choice of neurofilaments as a successful secondary outcome measure in treatment trials contributed to FDA approval of Ofatunumab for the treatment of individuals suffering from multiple sclerosis on August 20 2020. The use of the NfL Z‐scores also suggests a novel role for plasma NfL levels for remyelination strategies in MS (Abdelhak et al., 2022).
To conclude, there is a relationship between elevated NfL and NfH levels in MS with:
Disease severity: higher Nf levels indicate more active and progressive disease
Relapses: higher NfL levels are found for about 3 months after a relapse
Brain atrophy: higher NfL and NfH levels predict brain atrophy over the following 10–15 years
Clinical disability scales: correlations with the clinical scales for cognitive and physical functioning which are strongest for involvement of the pyramidal and motor system, presumably due to degeneration of large Nf‐rich axons
Radiological disease activity: correlations with T2 lesion number and total T2 lesion volume, T1 black holes, contrast enhancing and rim lesions
Presymptomatic phase of MS: an increase of NfL and NfH levels predict conversion to clinical definite MS
Conversion of CIS to clinical definite MS
Overall prognostic: individuals with Nf levels in the higher percentiles have a poorer prognosis than those with Nf levels in the lower percentiles
Treatment response: successful treatment is followed by a decrease in Nf levels
Safety laboratory: an elevation of Nf levels during an intervention indicates the potential for neurotoxic side effects. This may be particularly relevant in individuals with NS and a broken BBB.
11.1.3. Neuromyelitis optica
Neuromyelitis Optica Spectrum Disease (NMOSD) is an autoimmune channelopathy directed at the water channel Aquaporin 4 (AQP4) located on astrocytes. Damage in NMOSD is complement mediated and occurs during an acute relapse. NfH and NfL concentrations were found to be higher in NMOSD than in MS and to be of prognostic value (Mariotto et al., 2019; Miyazawa et al., 2007). Recently novel biologics became available for the treatment of NMOSD. There is a potential role for Nf for surveillance of individuals on these very expensive drugs. Not only for safety, but also for discovery of sub‐clinical disease activity.
11.1.4. Myelin oligodendrocyte glycoprotein antibody disease
The other novel disease which has entered the clinical arena is Myelin Oligodendrocyte Glycoprotein Antibody Disease (MOGAD) (Marignier et al., 2021). Similar to NMOSD damage to the nervous system is relapse related and Nf concentrations are increased (Mariotto et al., 2019).
11.1.5. Stroke
Acute stroke affects 700,000 people in the US annually (Powers, 2020). Successful implementation of pharmacological thrombolysis and mechanical thrombectomy has substantially improved the outcome. One challenge for clinical trials is that at the time of inclusion one cannot say how much brain tissue has been damaged irreversibly. The pragmatic approach is therefore to make decisions mainly based on the time interval elapsed between onset of stroke and admission. For selecting patients, longer time frames may apply. NfL and NfH concentrations from blood and CSF correlated with survival, short‐ and long‐term clinical outcome, cognitive function and radiological scales (Gendron et al., 2020; Petzold, Worthington, et al., 2011; Sellner et al., 2011; Tiedt et al., 2018; Zhou et al., 2022). Likewise, serum NfL levels were elevated in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Gravesteijn et al., 2018). There is a role for Nf to optimise planning of patient care pathways and improve on clinical trial efficiency (Gendron et al., 2020).
11.1.6. SAH
In subarachnoid haemorrhage (SAH), Nf isoforms (NfL, NfH) are elevated acutely (Garland et al., 2021; Petzold, Keir, et al., 2006; Petzold, Rejdak, et al., 2005). Longitudinal Nf concentrations also permit for early recognition of secondary complications, vasospasm and hydrocephalus, as a change from individual baseline values is observed (Gendron et al., 2020; Petzold, Keir, et al., 2006).
11.1.7. Dementia
One of the biggest challenges for healthcare systems is the increase of dementias in the ageing population, with an estimated prevalence of 45,956,000 patients worldwide (Feigin et al., 2017). The prevalence is expected to rise to 131 million by 2050 and reach 82% in those over 85 years old. CSF biomarkers can precede the onset of dementia by 10–20 years (Bateman et al., 2012). Among the four main clinical categories, Alzheimer's disease (AD), Frontotemporal lobe dementia (FTLD), vascular dementia (SVD), dementia with Lewy Bodies (Pilotto et al., 2021) and minimal cognitive deficit (MCI), Nf concentrations were consistently found to be highest in FTLD (Petzold et al., 2007; Ende et al., 2019).
11.1.8. FTLD
Because early treatment is key, it was recommended for FTLD trials to include NfL as part of a data‐driven selection tool (Ende et al., 2021). The increase in blood NfL levels precedes the onset of FTLD by at least an average of 1.3 years (Gendron et al., 2022). The clinical spectrum of FTLD is heterogeneous and plasma NfL data were highest in FTLD‐ALS compared to individuals with non‐fluent and agrammatic variant primary progressive aphasia (nfvPPA), semantic variant PPA (svPPA). The quantitative, longitudinal, relationship of the serum concentration of NfL and NfH is described in an elegant and carefully conducted multicentre study (Wilke et al., 2021). One question arising from these data relates to the Nf stoichiometry. Presently the reported concentration of NfL in controls (6.6 pg/ml) is about seven times lower than for NfH (47.6 pg/ml), rather than the expected other way round. This observation goes beyond the analytical discussion of the protein standard concentrations used in immuno‐assays. There are four aspects to this finding:
Is there evidence for endogenous binding of Nf which affects the three isoforms in a different way? This relates, for example to the literature on Nf isoform‐specific autoantibodies in neurological disease?
Is there a potential role for quantification of NfM which has been absent from most of the studies hitherto reported?
Can the use of Nf stoichiometry be of use to circumvent statistical issues related to physiological ageing?
How does modelling of the Nf stoichiometry project on the longitudinal dynamics of Nf isoform concentrations? This with particular reference to the left tail of the polynomial function of the z‐score, twice crossing the zero‐line (Figure 3 A&C in Wilke et al., 2021)
Addressing these and other questions will require a biologically and mathematically logical approach.
11.1.9. AD
In AD PTMs lead to a four to eightfold increase of phosphorylation of NfH and NfM compared to controls (Rudrabhatla et al., 2011). In AD it has been shown that elevated NfL levels are associated with disease progression (Moscoso et al., 2021; Santangelo et al., 2021). In section 7 the relevance of NfL breakdown products, notably in the 10 kDa range (Brureau et al., 2017). Candidate sequences were NfL92−224, NfL324−360, NfL530−540 (Brureau et al., 2017).
Accelerated cognitive decline
Higher in presymptomatic individuals with A‐plaques
Predicts clinical disease onset by 15–20 years
Related to Aβ‐plaques independent disease progression
Predicts fast progression
Improves diagnostic accuracy of AD if added to biomarker panel
11.1.10. DLB
In individuals with Diffuse Lewy Body dementia (DLB) plasma NfL levels are increased if compared to controls (Karantali, Kazis, Chatzikonstantinou, et al., 2021; Pilotto et al., 2021). There is a correlation with cognitive decline.
11.1.11. SVD
Intriguingly in SVD, there is a vasculocentric staining pattern for phosphorylated NfH which was suggested as an alternative passage of the Nf from the brain to the blood (Anad et al., 2022). The increase of Nf concentrations was also more in adult individuals with Down Syndrome and related to disease severity including cognitive function (Fortea et al., 2020; Thwaites et al., 2021).
11.1.12. Huntington's disease
(HD) is caused by an expanded CAG repeat in the Huntingtin gene which leads to progressive neurodegeneration. The number of CAG repeats correlated with Nf concentrations (Byrne et al., 2017). Elevated Nf concentrations predicted brain atrophy (Johnson et al., 2018) and disease onset by 24 years (Scahill et al., 2020). In two other studies, the prognostic value of blood Nf levels was, however less convincing (Parkin et al., 2021; Wild et al., 2007). This may be related to timing of sampling and longitudinal dynamics of Nf levels (Rodrigues et al., 2020). An increase in serum NfL levels was also associated with increased brain network connectivity in pre‐symptomatic individuals known to harbour HD mutations (McColgan et al., 2022). Finally, Nf has been used as a secondary trial endpoint for a novel antisense oligonucleotide trial in HD (Tabrizi et al., 2019).
11.1.13. Prion disease
In Prion Disease (Creutzfeldt Jakob Disease), the process of neurotoxic protein aggregation is so rapid and brain degeneration so widespread that CSF Nf concentrations in Prion disease even eclipse what is observed in ALS (Zerr et al., 2018). Therefore, Nf concentrations are of diagnostic value in the right clinical context (Hermann et al., 2021). Plasma NfL levels were shown to be increased in fatal familial insomnia (Hermann et al., 2022). The highest NfL levels were associated with methionine homozygosity at codon 129 PRNP. Higher NfL levels were of prognostic relevance regarding time to death (Hermann et al., 2022). There is a potential role as a diagnostic biomarker and secondary outcome for treatment trials (Zerr, 2022).
11.1.14. Guillain–Barré syndrome
GBS is a post‐infectious disease affecting the peripheral nervous system (Laman et al., 2022). The disease course of GBS is typically monophasic and can be severe acutely, but most patients make a reasonably good recovery. This is because demyelination in the peripheral nervous system recovers better than in the central nervous system. In a small proportion of individuals with GBS autoantibodies are deposited along the nerve axons (Laman et al., 2022). Overall only a small proportion of individuals with GBS will experience axonal degeneration (Feasby et al., 1986; Petzold, Hinds, et al., 2006). In these patients, elevated CSF Nf concentrations are a poor prognostic sign (Petzold et al., 2002; Petzold, Brettschneider, et al., 2009). It is understood that high CSF Nf concentrations indicate proximal axonal damage at the level of the nerve root (Laman et al., 2022; Petzold, Hinds, et al., 2006). Proximal axonal damage of peripheral motor nerves is less likely to recover than distal axonal damage where axonal sprouting readily occurs (Petzold, Brettschneider, et al., 2009). For this reason, more precise prognostic information is gained from CSF rather than blood Nf levels in acute GBS. For monitoring, however, which requires serial sampling, there is an advantage in quantification of blood Nf levels. This permits early detection of the development of secondary complications (Körtvelyessy et al., 2020; Martín‐Aguilar et al., 2020). Taken together, the Nf data (Körtvelyessy et al., 2020; Martín‐Aguilar et al., 2020; Petzold et al., 2002; Petzold, Brettschneider, et al., 2009; Petzold, Hinds, et al., 2006) contribute to introducing a paradigm shift for the diagnostic workup of diseases seen in a peripheral nerve clinic. It takes 2–3 weeks for neurophysiological tests to diagnose with confidence axonal damage in GBS (see references in Petzold, Brettschneider, et al., 2009), CSF Nf levels provide this information already at onset.
11.1.15. Peripheral neuropathies
Above pioneering work on acute GBS has opened the field of Nf research for other peripheral neuropathies. As a rule of thumb, blood Nf levels are higher with acute than with chronic pathology to the peripheral nerve. Blood and CSF Nf levels are helpful with the diagnostic workup, guiding clinical management and trial design. This includes critical illness neuropathy (CIN) (Sandelius et al., 2018), giant axonal neuropathy (GAN) (Bomont et al., 2000), Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) (Kapoor et al., 2022; Karam, 2022; Lieverloo et al., 2019; Mariotto et al., 2018; Petzold, Brettschneider, et al., 2009). There may be a role for plasma Nf isoform levels to interrogate response to treatment (Kapoor et al., 2022). There was a small increase in blood Nf concentrations in individuals with multifocal motor neuropathy (MMN) (Kmezic et al., 2022), paraproteinaemia‐related demyelinating polyneuropathy (Kmezic et al., 2022), Chemotherapy Induced Peripheral Neuropathy (CIPN) (Huehnchen et al., 2022; Karam, 2022; Kim, Choi, et al., 2020) and a range of inherited neuropathies (Kapoor et al., 2019; Millere et al., 2021; Rossor et al., 2016). The highest blood NfL levels were found in individuals with Hereditary transthyretin amyloidosis (ATTRm) and neurological symptoms (65.8 pg/ml) compared to controls (15.5 pg/ml) (Kapoor et al., 2019). These data are very comparable to those obtained from a treatment trial in ATTRm with patisiran (69.4 pg/ml in diseased individuals compared to 16.3 pg/ml in controls) (Ticau et al., 2020). The experience with Charcot–Marie‐Tooth (CMT) illustrates the relevance of increased analytical sensitivity of the immunoassay. The second‐generation ELISA for NfH was found not to be useful (Rossor et al., 2016), while the fourth‐generation SIMOA for NfL was useful (Rossor et al., 2021). There are no data yet on the stoichiometry of NfL:NfM:NfH in these chronic conditions using highly sensitive assays for all Nf isoforms. This may be relevant because it remains to be seen if the effect size for a single Nf isoform from blood samples will be large enough to be considered as a secondary endpoint for treatment trials in CMT, but may be considered for ATTRm (Ticau et al., 2020). This point is not trivial for the ambitious aim for blood Nf levels to join neurophysiology as an equal partner in the diagnostic workup and management of patients with a disease of the peripheral nervous system.
11.1.16. Spinal cord injury
Following spinal cord injury, NfL and NfH levels are increased and appear to respond to treatment (Kuhle et al., 2015). They are also of diagnostic value in the critical initial days after spinal cord injury (Leister et al., 2021). Elevated Nf levels were found helpful for the differential diagnosis between myelitis and spinal cord infarction (Sechi et al., 2021).
11.1.17. Movement disorders
Clinically, the differential diagnosis of extrapyramidal syndromes can be challenging. The disease course is slower in Parkinson's disease (PD) which progresses over decades if compared to Progressive Supranuclear Palsy (PSP) or Corticobasal Degeneration (CBD) Multisystem Atrophy (MSA) which leads to death in a substantial number of patients in about 3 years. Of the latter, there are again two subtypes, cerebellar (MSA‐C) and pyramidal (MSA‐P). CSF NfL levels are also increased in an extremely rare form of PD in carriers with the E46K‐SNCA mutation (Murueta‐Goyena et al., 2022). The variable penetrance of the PD phenotype in these neurodegenerative disorders renders delineating biomarkers of exceptional value.
Nf help with the differential diagnosis because they are higher in the more severe forms, highest in MSA‐P (Jabbari et al., 2020) and lowest in PD (Lin et al., 2020) with PSP being in between. An early clinical symptom is pure autonomic failure at which time CSF NfL levels are already elevated, preceding conversion to MSA, PD or DLB (Singer et al., 2021). Elevated Nf have prognostic value in rarer movement disorders such as Friedreich Ataxia (Hayer et al., 2020) and Wilson disease (Shribman et al., 2020; Wang, Xu, et al., 2022) which is a curable condition of the copper metabolism. Spinocerebellar Ataxias (SCA) comprise a large number of genetically determined subgroups. Neurofilaments were pathologically elevated in SCA2 and were correlated with disease severity (Yang et al., 2021).
In PD, there are NfL data supported evidence for uncontrolled diabetes mellitus accelerating the progress of neurodegeneration (Uyar et al., 2022). Elevated blood NfL levels were found to precede the onset of PD by at least 5 years (Halloway et al., 2022). The odds ratio for incident PD was 2.54 (95% CI 1.70–3.81) if blood NfL levels were twofold elevated in a model which adjusted for demographic data (Halloway et al., 2022). Blood NfL levels were correlated with disease severity cross‐sectionally and predicted disease progression of cognitive and motor symptoms longitudinally (Lin et al., 2019; Ye et al., 2021). Predictive modelling includes dopamine transporter (DAT) imaging of the putamen (Ye et al., 2021).
11.1.18. Epilepsy
One concern for individuals suffering from Epilepsy is neurodegeneration either resulting from seizure activity or as a neurotoxic treatment side‐effect. Indeed, an increase in Nf concentrations has been observed with acute and prolonged seizure activity (Abaroa et al., 2013; Rejdak et al., 2012).
11.1.19. Metastatic brain lesions
Finally, Nf are elevated in structural brain lesions (Hepner et al., 2019; Winther‐Larsen et al., 2020). Elevated NfL levels were related to tumour activity (Hepner et al., 2019). The potential role for monitoring longitudinal Nf blood concentrations to enable early diagnosis of metastatic brain disease (Lin, Lu, et al., 2021; Winther‐Larsen et al., 2020) still needs to be confirmed prospectively.
11.1.20. TBI
Traumatic Brain Injury is, with 27.08 million (95% CI 24.30–30.30) new cases alone in 2016, a major cause of death and disability worldwide (James et al., 2019). In severe TBI elevated NfL and NfH concentrations give higher odds ratios than clinical scales for predicting morbidity and mortality (Gao et al., 2020a; Kahouadji et al., 2022; Otani et al., 2020; Petzold, Tisdall, et al., 2011; Tisdall & Petzold, 2012). In one study elevation of blood NfL levels persisted for up to 5 years following TBI (Newcombe et al., 2022).
Clinically, it remains challenging to recognise brain damage in mild TBI. Neurofilaments acutely elevated in mild TBI (Shahim et al., 2018) and acutely elevated NfL improve on sensitivity with an area under the receiver operating characteristic curve of 0.80 and additional prognostic information (Shahim et al., 2018). There is a role for body fluid Nf isoform levels in TBI to:
Predict mortality
Predict poor functional outcome
Early recognition of secondary complications, particularly relevant in a sedated and ventilated patient
Inform on severity of high impact injury
Improve sensitivity to detect low impact injury
Correlate with diffuse axonal injury
Consequently also correlate with MRI tractography findings
11.1.21. Concussion
Finally, concussions, particularly repeated, pose a challenge to sport and military medicine because of long‐term consequences and disability in exposed individuals (Blennow et al., 2012). A severe, cumulative, consequence is dementia pugilistica or chronic traumatic encephalopathy (CTE). Elevated NfL concentrations are of diagnostic value in the acute and sub‐acute phase. Regarding sport‐related concussion research on Nf included American football, soccer, ice‐hockey, rugby and contact sports (Joseph et al., 2019; Pattinson et al., 2020). The evidence is however not yet conclusive at population level (James et al., 2021) or targeted at top professional football players (Cornali et al., 2022). A word of caution also comes from post‐mortem data which highlight the likelihood of multiple neuropathologies as source for increased body fluid Nf levels (Asken et al., 2022).
11.2. Infectious diseases
11.2.1. COVID‐19
During the COVID‐19 pandemic, there have been several reports on elevated Nf concentrations in blood and CSF (Cooper et al., 2020; Edén et al., 2020; Espíndola et al., 2021; Prudencio et al., 2021; Sutter et al., 2021; Virhammar et al., 2021). As a rule of thumb, high body fluid levels of Nf are a prognostically poor sign (Kuhle & Petzold, 2011; Petzold, 2007). Consistent with this notion, longitudinal data on elevated Nf levels predict poor outcome and mortality in COVID‐19 disease (Aamodt et al., 2021; Lorenzo et al., 2021; Prudencio et al., 2021). Regarding research on the neuro‐invasive potential of SARS‐CoV2 there is a role for Nf. This includes research on long COVID‐19 disease.
11.2.2. West Nile Virus
Predating COVID‐19 by about a decade, similar questions surrounded the outbreak of West Nile Virus (WNV) (Burki, 2018). This arbovirus infection involved the central and peripheral nervous system. Post‐mortem data showed an inflammatory, perivascular cellular reaction, particularly in the lower brainstem. There was also a relationship between post‐mortem evidence for neuronal death in the brain and spinal cord with CSF Nf concentrations (Petzold, Groves, et al., 2010). This was most notably for motor neurons packed in excess with Nf. There are data to suggest that flaviviridae, to which WNV belongs, interact with the cytoskeleton including Nf (Zhang et al., 2019).
11.2.3. Malaria
There is excellent post‐mortem evidence for axonal injury in individuals suffering from cerebral malaria who had been infected with Plasmodium falciparum (Medana et al., 2002). The immunohistochemical images show both diffuse and focal axonal injury. This is similar to the two patterns observed in sepsis (Ehler et al., 2017). There was a relationship of the extent of axonal injury with clinical data such as the Glasgow Outcome Score. More recently investigation of this important observation has been translated to investigating plasma NfL levels in a rodent model of malaria (Wai et al., 2022). An association of plasma NfL levels with cerebral oedema was observed, but not related to outcome (Wai et al., 2022). Future human studies building on this work should include clinical signs, such as seizures and coma, which are associated with a 15–20% mortality (Medana et al., 2002). In addition to oedema, such studies should also may also consider the contribution of hypoxia, hypoglycaemia, haemorrhages and inflammation in addition to cerebral oedema (Medana et al., 2002).
11.2.4. Herpes, Lyme, tick‐borne encephalitis, PML & HIV
There are at least five more infectious diseases where elevated Nf concentrations have been associated with neuro‐axonal damage and poorer clinical outcome; Herpes encephalitis (HE) (Eckerström et al., 2020; Li et al., 2019; Sellner et al., 2014), Lyme disease, HIV (Alagaratnam, Francesco, et al., 2021; Gisslén et al., 2016; Mellgren et al., 2007), tick‐borne encephalitis (Fortova et al., 2022) and Progressive multifocal leukoencephalopathy (PML) (Costa et al., 2019; Toorop et al., 2020). Longitudinal Nf concentrations permit also to monitor development of PML in patients that are immunosuppressed (Costa et al., 2019; Loonstra et al., 2019). There is a role for Nf monitoring potential neurodegeneration in the evolving landscape of treatment strategies for HIV which have successfully reduced the likelihood of AIDS‐related dementia (Mellgren et al., 2007). Regarding HIV and AIDS there is a role for body fluid Nf levels to:
Recognise HIV‐associated dementia, which has become less frequent with effective HIV treatment
Correlate with cognitive performance
Recognise HIV‐related polyneuropathy
Aid in identifying potential neurodegenerative pathology which may occur with HIV treatment strategies
As a surrogate outcome in HIV treatment trials
11.3. Psychiatry
It is crucial to avoid the misdiagnosis of a primary psychiatric disease from a primary neurodegenerative disease. This is one important diagnostic role for Nf (Ducharme et al., 2020). Higher Nf concentrations are observed with neurodegeneration. It is, therefore, interesting to note that there is a small increase in Nf concentrations in Depression (Bavato et al., 2021; Chen, Liu, et al., 2021; Zhao et al., 2020), Schizophrenia (Bavato et al., 2021; Rodrigues‐Amorim et al., 2020) and with Psychosis (Katisko et al., 2019). Future research will need to investigate how much of this is due to neuro‐axonal loss or potential side effects of treatments. Normal Nf concentrations following electro‐convulsive therapy for severe depression have been helpful for monitoring safety of this treatment option (Besse et al., 2020).
11.4. Cardiology
11.4.1. Cardiac arrest
Cardiac arrest causes neurological disability among survivors in about a third. Blood NfH and NfL concentrations are of prognostic value in predicting survival and anticipating neurological impairment (Hunziker et al., 2021; Moseby‐Knappe et al., 2019; Rundgren et al., 2012) and cognitive impairment (Nordström et al., 2022). Recognised to be more sensitive compared to other blood biomarkers, Nf have been included as secondary outcome measure in two trials aiming for an increased mean arterial pressure (Perkins et al., 2021). These studies also highlighted that a pre‐existing neurological comorbidity is a relevant bias. Contemporary recommendations are to include Nf as a blood biomarker for brain injury in trials alongside the clinical examination, electrophysiology and brain imaging (Perkins et al., 2021).
11.4.2. Arrhythmia and surgery
Extending on cardiac arrest there is a prognostic role for Nf isoforms (NfL and NfH) in Atrial fibrillation (Polymeris et al., 2020) and in cardiac surgery (DiMeglio et al., 2019; Hunziker et al., 2021; Rundgren et al., 2012). Elevated Nf isoforms in the CSF or blood are a poor prognostic sign in cardiac arrest. Monitoring Nf concentrations aided in assessing safety parameters for cardiopulmonary bypass (CPB) surgery (Wiberg et al., 2020).
11.5. Critical care
Sepsis and septic shock are serious problems in critical care because of the high level of morbidity and mortality. Recognition of septic encephalopathy is difficult in the sedated, intubated and ventilated patient. Because of this and the frequently unstable haemodynamic situation, transport of patients to a scanner for brain imaging is logistically extremely challenging and frequently not possible because of safety concerns. There is translational research spanning from experimental models of sepsis, over human post‐mortem examination to Nf analysis which strongly suggests Nf to be a specific biomarker for the two patterns of brain damage occurring in sepsis (Ehler et al., 2017). First, there are small embolic insults which can also be observed on brain imaging (Ehler et al., 2019). Second, there is diffuse axonal damage which is not captured by imaging but a likely source of elevated blood Nf concentrations arising from proteolytic breakdown products (Ehler et al., 2019; Orhun et al., 2021). The findings of body fluid Nf isoform levels relating to severity of brain function impairment seen in septic encephalopathy expands to a milder form, termed delirium (Fong et al., 2020; Halaas et al., 2018; Inoue et al., 2017; Mietani et al., 2019).
11.6. Gastroenterology
There is an important link between impaired liver function and neurological symptoms caused by metabolic encephalopathy. Serum NfL levels are a sensitive biomarker for minimal hepatic encephalopathy (Labenz et al., 2021). The median levels were about threefold above the normal control values of 24.3 pg/ml. The association of diarrhoea with chronic systemic symptoms involving other medical specialities is a ‘red flag’ for amyloidosis (Cabot et al., 1985). Pathological blood NfL concentrations in amyloidosis are of diagnostic value for involvement of the nervous system (Louwsma et al., 2020; Ticau et al., 2020). In oesophageal cancer surgery, an elevation of serum NfH levels was predictive of post‐surgery delirium (Mietani et al., 2022). Elevated plasma NfL levels have been reported anorexia nervosa (Nilsson et al., 2019; Wentz et al., 2020).
11.7. Ophthalmology
In acute optic neuritis (ON) higher Nf blood concentrations were related to poorer visual function and treatment response (Petzold et al., 2004). In the Optic Neuritis Treatment Trial, there was a similar, albeit weaker association for blood NfH levels collected in the later stage of ON (Pasol et al., 2010). In ON, the release of Nf from axons originates from axons along the entire visual pathways through the human brain. The highest degree of optic disc pallor developed in those individuals with the highest blood Nf concentrations (Petzold, Boer, et al., 2010). This clinical observation has since been confirmed by state‐of‐the‐art optical coherence tomography studies (Bsteh et al., 2019; Mariotto et al., 2019; Seitz et al., 2021; Tavazzi et al., 2020). There is experimental evidence for early up‐regulation of retinal tissue Nf levels after axonal damage in ON (Weissert et al., 2022). Next, anterograde axonal transport of Nf isoforms, and their proteolytic fragments, from the retina to the optic nerve contributes to the sustained high blood Nf isoforms concentrations (Mariotto et al., 2019; Petzold et al., 2004).
The other body fluid compartments adjacent to the retinal axons are the vitreous body and anterior chamber fluid. Elevated Nf concentrations can also be observed in these compartments (Petzold, Junemann, et al., 2009; Subramanian et al., 2020; Woltsche et al., 2022). Observations made in Retinal Detachment (RD) (Petzold, Junemann, et al., 2009), Age‐related Macular degeneration (AMD) (Nielsen et al., 2019) and glaucoma (Woltsche et al., 2022) are of potential interest for investigation of longitudinal Nf concentrations from these compartments in other ophthalmological conditions. Big public health issues such as glaucoma and diabetic retinopathy are obvious candidates.
11.8. Obstetrics and gynaecology
The advance in surgical techniques permitted for prenatal repair of myelomeningocele (Adzick et al., 2011). The approach stimulated an extensive debate and Nf may contribute to addressing open questions. Experimentally amniotic fluid Nf concentrations were related to the extent of prenatal spinal cord injury in an animal model with naturally occurring Menignomyelocele (Petzold, Stiefel, & Copp, 2005). Longitudinal blood Nf concentrations provide a much more convenient way for potential screening of candidates eligible for surgery and monitoring of progress. Blood Nf concentrations were already successfully quantified in pre‐eclampsia (Evers et al., 2018). The data on Nf concentrations in normal pregnancies in this study, however, imply that there will have to be separate normal Nf values for pregnant women.
11.9. Oncology
Neurodegeneration is a recognised feature of paraneoplastic disease. It cannot be overemphasised how important the newly described association of increased blood Nf levels in this context might be (Mizenko et al., 2021; Zoccarato et al., 2021). This is because a challenge for neuro‐immunology remains the limited number of validated paraneoplastic antibodies, but frequently a high clinical suspicion of an association remains in individuals who do not harbour any of the known paraneoplastic antibodies. Future studies are required to test for a potential role of CSF and blood Nf levels as an additional paraclinical test for paraneoplastic disorders causing neurodegeneration of the central and or peripheral nervous system.
The role of Nf levels in oncology, more generally, is likely to be limited to structural lesions (Hepner et al., 2019; Winther‐Larsen et al., 2020). For example a recent study on chemotherapy in breast cancer did not find that there was an increase in blood Nf levels (Argyriou et al., 2021). Earlier work suggested that a requirement for chemotherapy to induce neurodegeneration is an impaired blood–brain barrier (Petzold, Mondria, et al., 2010).
11.10. Paediatrics
The role of Nf in paediatric disease recapitulates largely what has been observed for adults. The main laboratory difference is the need for age‐adjusted Nf normal values. The paediatric disease spectrum broadens to include many genetic disorders.
Specifically, Nf blood levels have been studied in Mitochondrial disorders (Varhaug et al., 2021), CLN3 (Do et al., 2020), Autism Spectrum Disorder (He et al., 2020), Spinal muscular atrophy (SMA) (Darras et al., 2019; Faravelli et al., 2020; Kong et al., 2021; Nitz et al., 2021), Congenital Heart Disease (CHD) (Lee et al., 2018), Beta‐propeller protein‐associated neurodegeneration (BPAN) (Takano et al., 2017), Gangliosidosis (Welford et al., 2022), Febrile seizures (Evers et al., 2020), myotonic dystrophy (Nicoletti et al., 2022), Ceroid lipofuscinosis type 2 (CLN2) (Ru et al., 2019) and Langerhans cell histiocytosis (Sveijer et al., 2022). Plasma NfL levels are over 10‐fold elevated in the gangliosidosis GM1 and GM2 if compared to controls (Welford et al., 2022). Plasma NfL levels were highest in the first decade of life, probably owing to a more severe phenotype at this age. Samples taken in the thirties were only marginally elevated compared to healthy controls.
With novel treatment options (e.g. SMA, CLN2) and questions on long‐term clinical outcomes, there is an important role for Nf to contribute with long‐term monitoring. There are direct implications for the use of NfL and NfH levels for the monitoring of diseases progression in children with SMA who receive nusinersen (Darras et al., 2019; Ru et al., 2019). The long‐term decrease of CSF NfH levels is small, and was not significant for CSF NfL or either Nf isoform in the serum (Wel et al., 2022).
11.11. Pulmonology
Because of their high metabolic demand neurons are vulnerable to hypoxia. Obstructive sleep apnoea syndrome (OSAP) is a known risk factor for ischaemic damage to the retina and brain. Is is therefore interesting to learn that in OSAP averaged serum NfL levels were at group level minimally higher if compared to controls (Arslan et al., 2021).
11.12. Rheumatology
Recognition of rheumatological treatment‐related complications (e.g. PML) using Nf was already discussed. In addition, Nf concentrations were found to be elevated and to be of prognostic relevance in Sjogren's syndrome and Lupus (Tjensvoll et al., 2020; Zervides et al., 2022) and Vasculitis (Bischof et al., 2017; Pawlitzki et al., 2019).
11.13. Recreational drug use
The use of ‘Ecstasy’ or 3,4‐Methylenedioxymethamphetamine (MDMA) has been linked to neurotoxicity clinically and experimentally with elevated NfL levels providing an indirect means for measurements (Bavato, Stamatakos, et al., 2022). Likewise, there is an increase in blood NfL levels with ketamine dependence (Liu et al., 2021) and in chronic cocaine use (Bavato, Kexel, et al., 2022). Reduction of use in cocaine was monitored by cocaine hair concentrations and was correlated over a 4‐month period with reduction of blood NfL concentrations (Bavato, Kexel, et al., 2022).
11.14. Space flight
There is experimental evidence for space flight inducing a decrease in Nf immunoreactivity in mechanoreceptors (Proshchina et al., 2022). This observation has been linked to the hypogravitatational motor syndrome (HMS), a subform of the space adaptation syndrome (SAS). Neuronal and axonal changes to the staining pattern for NfH were observed already within 12 days after space flight (Proshchina et al., 2022). Interestingly, space flight also changes the gene for NfL in plants (Arabidopsis) (Shymanovich et al., 2022). There are data indicating a minute increase in blood NfL levels in five cosmonauts from 11.4 pg/ml preflight to 15.2 pg/ml on return (p = 0.04) and a further increase over the following week to 17.2 pg/ml (p = 0.04) (Eulenburg et al., 2021). The group average remains within the normal range and it remains to be seen if the small variation over time of 3.8–5.8 pg/ml are over and above to what can be expected from physiological variation of between days 6.6 pg/ml (range 2.6–17.5) or variation within the same day averaging at 7 pg/ml (Hviid et al., 2021). Therefore, future, prospective, longitudinal studies are required to answer if these observations are caused by brain injury (Eulenburg et al., 2021), changes to Nf gene expression (Shymanovich et al., 2022), or release of Nf proteins from the periphery (Proshchina et al., 2022).
11.15. Other factors affecting Nf values
The interpretation of Nf concentrations in the elderly can be complex because, in addition to age, comorbidities such as impaired renal function, body mass index and presence of diabetes mellitus might be relevant (Akamine et al., 2020; Bakovic et al., 2018; Fitzgerald et al., 2022; Manouchehrinia, Piehl, et al., 2020; Uyar et al., 2022). Others did not find such an association with renal function in individuals with secondary progressive MS (Williams et al., 2021). Haemodialysis increased plasma Nf levels (Chen et al., 2022). Elevated Nf levels are present too little (Wentz et al., 2020) and too much nourishment (Beydoun et al., 2021; Manouchehrinia, Piehl, et al., 2020). The longitudinal concentration of Nf normalised with successful regain of weight (Doose et al., 2021). Related to at least diabetes mellitus, an association was found between elevated serum NfL levels that were predictive of all‐cause mortality in women (Beydoun et al., 2022).
12. META‐ANALYSES
There have been 33 meta‐analyses on the wealth of data on Nf as a biomarker for neurodegeneration over the past 15 years (Agah et al., 2018; Alagaratnam, Widekind, et al., 2021; Angelopoulou et al., 2021; Arneth & Kraus, 2022; Cai & Huang, 2018; Choong et al., 2019; Cong et al., 2021; Forgrave et al., 2019; Gao et al., 2020b; Ge et al., 2018; Hoiland et al., 2022; Hu et al., 2017; Jin et al., 2019; Karantali, Kazis, Chatzikonstantinou, et al., 2021; Karantali, Kazis, McKenna, et al., 2021; Katayama et al., 2020; Koychev et al., 2021; Li et al., 2016; Liu et al., 2022; Liu, Chen, et al., 2020; Martin et al., 2019; Mondello et al., 2021; Peng et al., 2022; Petzold et al., 2007; Rübsamen et al., 2022; Sako et al., 2015; Sferruzza et al., 2021; Wang et al., 2019; Wang et al., 2020a; Wang et al., 2020b; Xu et al., 2016; Zhang et al., 2022; Zhao et al., 2019; Zhou et al., 2021).
12.1. Dementia
12.1.1. AD
Regarding AD (n = 3138) CSF NfL were of higher concentration than in healthy control subjects (n‐1230) with a good ratio of means (ROM) of 2.12 and narrow 95% CI (1.85–2.42) of the 29 studies included (Forgrave et al., 2019). The ROM was smaller (1.18; 95% CI 1.11–1.25) for the comparison of individuals with AD (n = 442) to those with MCI (n = 545) in data from eight studies (Forgrave et al., 2019). Unfortunately, CSF NfL levels were higher in a group of AD disease mimics (n = 1647) if compared to AD (n = 2404) with a ROM of 0.87 (95% CI 0.70–1.08) (Forgrave et al., 2019). The last finding highlights a problem with the interpretation of CSF NfL levels as a diagnostic biomarker for dementia.
Similar was observed for blood NfL levels, higher in AD (n = 471) if compared to cognitively unimpaired controls (n = 518) with a ROM of 2.61 (95% CI 1.54–4.44) (Forgrave et al., 2019). For the comparison of AD (n = 313) with with MCI (n = 381) the ROM (1.30) was not significant with the 95% CI crossing the value ‘one’ (0.86–1.95) (Forgrave et al., 2019).
12.1.2. FTLD
The signal for CSF NfL levels was found to be highest for patients with FTLD (n = 56) compared to controls (n = 98, 1.38; 0.99–1.77), with a very small effect size for CSF NfL only to separate FTLD (n = 58) from AD (n = 99, 0.62; 95% CI 0.26–0.97) (Petzold et al., 2007). For CSF NfH the effects were smaller than for CSF NfL for FTLD (n = 59) compared to controls (n = 108, 0.74; 95% CI 0.40–1.08) and not significant for FTLD compared to AD (Petzold et al., 2007). The main findings were confirmed subsequently on larger patient numbers in a meta‐analysis focused on NfL only (Karantali, Kazis, Chatzikonstantinou, et al., 2021). The effect size for CSF NfL in FTLD (n = 942) compared to controls (n = 696) was very high 2109.10 (95% CI 1527.69–2690.50). More concerning, 13 studies on FTLD were not included in one meta‐analysis (Figure 4 in (Karantali, Kazis, Chatzikonstantinou, et al., 2021)) 2 years after these data were compiled (Figure 3a in (Forgrave et al., 2019)) and four studies were not included in a meta‐analysis 14 years earlier (Figure 3b in (Petzold et al., 2007)). Therefore, the 2019 meta‐analysis should presently be regarded as the most authoritative one with an effect size of 3.41 (95% 2.96–3.93) comparing FTLD (n = 1827) with controls (n = 1113) (Forgrave et al., 2019).
12.1.3. MCI
One meta‐analysis specifically addressed the issue of MCI collected from seven studies (Zhang et al., 2022). Published 3 years after a meta‐analysis based on blood NfL levels in CIS (n = 381) (Forgrave et al., 2019), the 2022 meta‐analysis 1 pooled CSF NfL and blood NfL data from subjects with MCI (n = 676) and controls (n = 504) (Zhang et al., 2022). The effect size was 0.36 (95% CI 0.04–0.68) (Zhang et al., 2022). Subsequent subgroup analysis did confirm a significant effect for CSF NfL levels 0.61 (95% CI 0.21–1.01), but did not find blood NfL levels to be of diagnostic value in MCI (Zhang et al., 2022).
12.2. Movement disorders
12.2.1. PD
Four meta‐analyses included data on PD (Angelopoulou et al., 2021; Ge et al., 2018; Hu et al., 2017; Sako et al., 2015). Another two meta‐analyses are planned (Wang et al., 2020a; Wang et al., 2020b). A potentially diagnostically interesting increase of CSF NfL in individuals with PD (n = 1035) from those with atypical parkinsonian syndromes (n = 930) was found (Angelopoulou et al., 2021). The difference of 1.26 standard deviations is of interest for studies operating on a group level and expands on an earlier meta‐analysis on four studies (1.60; 95% CI 1.22–1.98) (Sako et al., 2015). The differential use of a fixed‐effect model ( 50%) versus random‐effect model ( 50%) was announced as depending on the degree of statistical heterogeneity in one protocol for blood NfL (Wang et al., 2020b) which reads similar to the protocol published for CSF NfL (Wang et al., 2020a). 2
One study (Ge et al., 2018) made use of a hierarchical summary receiver‐operating characteristic model which helps to visualise sensitivity and specificity levels of a test (Rutter & Gatsonis, 2001). This meta‐analysis included 8 studies and found a diagnostic sensitivity for NfL to separate individuals with PD from atypical parkinsonian syndromes of 0.82 (95% CI 0.64–0.91). The corresponding diagnostic specificity was 0.85 (95% CI 0.79–0.89) (Ge et al., 2018).
The comparison of PD with MSA subject to the remaining meta‐analyses is summarised in the next paragraph (Hu et al., 2017).
12.2.2. MSA
MSA (Cong et al., 2021; Hu et al., 2017). There was a highly significant and robust increase of CSF NfL (2500.99, 95% CI 2212.28–2789.70) in MSA (n = 142) if compared to controls (n = 215) from five studies between 2015 and 2018 (Cong et al., 2021). Likewise, CSF NfL was significantly higher (805.23, 95% CI 672.37–938.10) in MSA (n = 77) if compared to PD (n = 86) using a fixed model on the mean difference (Cong et al., 2021). If one uses instead a random model on the mean differences (Hu et al., 2017), the findings are largely confirmed, but the reported effect size is smaller. CSF NfL levels were higher in MSA (n = 212) if compared to PD (n = 373) at 1.56 (1.12–2.00) based on nine studies (Hu et al., 2017).
12.2.3. CJD
Unique among the meta‐analysis discussed here is the network meta‐analysis approach used for CJD (Rübsamen et al., 2022). The method is elegant as it permits ready comparison of sensitivity and specificity values for a range of biomarkers across studies, but does not require that all studies had to include all of the biomarkers. Inclusion criteria were strict, drilling down to the analytical techniques used, cutoff levels reported and details of the patient cohorts. The point was made that while CSF NfL had the highest diagnostic sensitivity (0.99) but not specificity for different diagnostic categories of CJD (Rübsamen et al., 2022). The use of plasma and CSF NfL in CJD has been validated for the early disease course (Schmitz et al., 2022).
12.2.4. Genetic ataxia
Out of 11 studies on blood NfL levels in genetic ataxias, eight used fourth‐generation (SIMOA) immuno‐assays and were included to a meta‐analysis (Peng et al., 2022). The highest effect size was found for ataxia telangiectasia (n = 38) if compared to healthy controls (n = 20, 1.70; 95% CI 1.41–2.00) (Peng et al., 2022). This was followed by individuals with SCA3 (n = 464) where blood NfL levels were higher (1.23; 95% CI 1.18–1.29) if compared to healthy controls (n = 828) (Peng et al., 2022). The effect sizes were smaller for SCA1 (n = 23) compared to healthy controls (n = 25, 1.00; 95% CI 0.39–1.60); Friedreich ataxia (n = 217) compared to healthy controls (n = 62, 0.62; 95% CI 0.21–1.03) and SCA2 (n = 16) compared to healthy controls (n = 22, 0.59; 95% CI 0.27–0.91) (Peng et al., 2022). For SCA3, higher blood NfL levels were found in young mutation carriers (Garcia‐Moreno et al., 2022).
12.3. ALS
Five meta‐analyses included data on ALS (Forgrave et al., 2019; Li et al., 2016; Sferruzza et al., 2021; Xu et al., 2016; Zhou et al., 2021). Similar to PD (Wang et al., 2020a, 2020b) there is an ongoing, protocolised meta‐analysis (Agah et al., 2018). The most complete meta‐analysis on disease (n = 16 studies) comparison demonstrated higher blood NfL levels in individuals with ALS (n = 930) if compared to cognitively unimpaired controls (n = 593) with an effect size of 9.64 (95% CI 6.65–13.99) (Forgrave et al., 2019). The effect size was smaller (3.35; 95% CI 2.19–5.12) for blood NfL levels in ALS (n = 1239) if compared to disease mimics (n = 806, 11 studies) (Forgrave et al., 2019). The first meta‐analysis was based on sensitivity and specificity levels (both 0.8) including 11 studies (Li et al., 2016). Detailed subgroup analysis were performed in a meta‐analysis including 15 studies for NfL (4 studies) and NfH (11 studies) in (Xu et al., 2016). The effect size for blood NfL levels was 1.45 (95% CI 1.24–1.66) comparing individuals with ALS with controls. This effect size is smaller than what was found in the later, larger dataset to which more of the fourth generation immuno‐assays contributed (Forgrave et al., 2019). A subsequent meta‐analysis 3 included only 12 studies (Sferruzza et al., 2021), which is less than what was included earlier (Forgrave et al., 2019; Xu et al., 2016). This meta‐analysis also reports lower effect sizes for blood NfL (1.59; 95% CI 1.25–1.92) levels in ALS (n = 1074) compared to healthy controls (n = 707) (Sferruzza et al., 2021) with respect to the earlier performed meta‐analysis on a larger dataset with an about six times larger effect size of 9.64 (Forgrave et al., 2019). The effect size is lower 0.72 (95% CI −0.39 to 1.83) for the comparison of ALS (n = 539) with other neurological disease controls (n = 216); or of ALS (n = 655) with ALS mimics (n = 358) at 0.95 (95% CI 0.42–1.48) (Sferruzza et al., 2021).
Finally, one meta‐analysis calculated the risk for disease progression and earlier death in ALS (Zhou et al., 2021). This meta‐analysis included 14 studies and 1619 individuals with ALS. Nf levels were grouped according to quartiles. There was an increased risk of faster disease progression and earlier death with higher NfL and pNfH levels. The effect size for faster disease progression was 0.59 (95% CI 0.49–0.69) for NfL and 0.56 (95% CI 0.25–0.88) for pNfH (Zhou et al., 2021).
12.4. MS
There are two meta‐analyses on NfL data in MS (Cai & Huang, 2018; Martin et al., 2019). A 0.88 (95% CI 0.05–1.26) increase in CSF NfL was found in MS (n = 469) if compared to controls (n = 326) in an analysis including 10 studies. Data from these studies were based on a range of different assays spanning 21 years (1996–2021) of research (Cai & Huang, 2018). In contrast, data on blood NfL concentrations were based on the same test used by all five studies included resulting in a lesser degree of heterogeneity with a 0.47 (0.24–0.71) increase in MS (n = 1196) compared to controls (n = 660). These authors did not find matching groups for to be a major factor either in the random‐effect model or meta‐regression (Cai & Huang, 2018). Gender however was, and so was sample size, but paradoxically inverse to the expected (Cai & Huang, 2018). The second meta‐analysis 4 found a roughly comparable effect size for CSF NfL levels (0.61; 95% CI 0.48–0.73) in relapsing–remitting MS (n = 746) if compared to a mix of healthy and diseased controls (n = 435) from data based on 13 studies (Martin et al., 2019). These authors also confirmed by meta‐analysis the observation that CSF NfL levels are higher during a relapse in MS if compared to patients who remain in remission (Martin et al., 2019). The effect size is increased (0.96; 95% CI 0.72–1.20) if only patients with a progressive disease course (n = 158), secondary or primary, are compared to controls (n = 172) (Martin et al., 2019). The treatment effect of natalizumab on NfL levels was meta‐analysed from seven trials (Liu et al., 2022). NfL levels were lower in natalizumab treated (n = 947) compared to pre‐treatment levels (n = 959). The effect size was 0.73 (Liu et al., 2022).
12.5. Brain injury, TBI and concussion
Brain injury and TBI were subject to two meta‐analyses (Gao et al., 2020b; Mondello et al., 2021). The outcomes of these two meta‐analyses are diametrically opposed. The provocative conclusion of the 2021 meta‐analysis was that ‘There is insufficient evidence to support the clinical validity of initial circulating c‐Tau or neurofilament protein concentrations for the management of patients with mTBI’. (Mondello et al., 2021). In contrast, the 2020 meta‐analysis 5 found NfL levels to be increased (2.48; 95% CI 1.52–3.43) in TBI (n = 569) if compared to controls (n = 549) based on data from nine studies (Gao et al., 2020b). A subgroup analysis revealed that ethnicity was relevant. Further subgroup analyses showed that the effect sizes were larger in earlier samples after the event 2.89 (48 h), 2.98 (6–10 days) and 0.28 (1–3 months). Interestingly using a second‐generation immuno‐assay gave an overall larger effect size of 4.06 (95% CI 1.22–6.91) if compared to the fourth‐generation immuno‐assay (1.35; 95% CI 0.79–1.92), but this seems to be driven by two out of six studies, with an overall more homogeneous data distribution of the fourth‐generation immuno‐assay (Figure 3b in (Gao et al., 2020b)). Not surprisingly the effect size was larger for CSF NfL levels (4.03; 95% CI 0.45–7.61) if compared to either serum (2.35; 95% CI 0.45–4.26) or plasma (1.06; 95% CI 0.50–1.61) (Gao et al., 2020b).
12.6. Stroke
One meta‐analysis included five studies on blood NfL levels in stroke using third‐ and fourth‐generation immuno‐assays (Liu, Chen, et al., 2020). The meta‐analysis was focused on poor functional outcome. The odds ratio for increased NfL levels predicting poor functional outcome in individuals with stroke (n = 1346) was 1.71 (95% CI 1.17–2.49).
12.7. Cardiac arrest
One meta‐analysis tested the prognostic value of a range of biomarkers in cardiac arrest (Hoiland et al., 2022). The summary receiver operating curve characteristic analyses (Figure 2a–f in (Hoiland et al., 2022)) showed an AUC of 0.92 for blood NfL levels (n = 935) for predicting unfavourable outcome. This was a better AUC compared to NSE (0.84, n = 4898), S100B (0.85, n = 1724), GFAP (0.77, n = 911), tau (0.89, n = 792) or Ubiquitin carboxyl hydrolase L1 (0.88, n = 702). These data are consistent with an AUC of 0.96 for NfL in a smaller study (Meyer et al., 2022). As demonstrated before for TBI (Gao et al., 2020b), timing of sampling was relevant with blood NfL levels continuing to rise from 24 to 72 h after the event (Figure 3d in (Hoiland et al., 2022)).
12.8. Relationship of CSF and blood NfL levels
A pooled correlation coefficient meta‐analysis showed that there is a strong association between CSF NfL and blood NfL concentrations with R = 0.723 (95% CI 0.540–0.840) based on data from 36 studies (n = 3961 samples) (Alagaratnam, Widekind, et al., 2021). There was a significant heterogeneity between the studies included.
12.9. Meta‐analysis summary
Taken together most meta‐analyses were focused on NfL in the CSF and blood. They consistently confirm that higher NfL values are found in the experimental (diseased) group. Factors found to influence these data were age, disease duration and disease severity. For future meta‐analyses, it is advised to strive for completeness of inclusion of studies (Forgrave et al., 2019; Karantali, Kazis, Chatzikonstantinou, et al., 2021; Sferruzza et al., 2021). It was unexpected to find differences as large as a factor of six for the effect sizes reported by different meta‐analyses on the same disease published only 2 years apart (Forgrave et al., 2019; Sferruzza et al., 2021). There is an unfortunate lack of later meta‐analyses referring to earlier work on the same disease. There is a need for justification of using a fixed‐effect versus random‐effect model (Wang et al., 2020b) and a ROM‐based approach is preferred (Forgrave et al., 2019). The ROM approach overcomes some of the statistical limitations of other meta‐analytical approaches (Forgrave et al., 2019). The ROM can be understood as a x‐fold change of Nf concentrations, with values above one (this is in difference to the effect size for which this value would be zero) indicating higher values in the group of interest. This approach permits to correct for errors identified by the authors, such as: ‘several studies were observed to misreport units for NfL […]’ (Forgrave et al., 2019). This ROM‐based meta‐analysis also carefully reviewed the analytical methods used for quantification of Nf, importantly including the pair of Nf‐antibodies used for capture and detection. There are good reasons for reporting these antibodies and inclusion to a nomenclature was proposed (Petzold et al., 2003), which was adopted for a period of time (Gaiottino et al., 2013; Kuhle et al., 2010; Petzold & Shaw, 2007). Worryingly, 13/65 (20%) studies included did not report which Nf antibodies were used (Forgrave et al., 2019) which makes it very difficult to compare results other than by the ROM approach.
There will be a need for future meta‐analyses to compare the performance of different biomarkers (Rübsamen et al., 2022). This is because health care sustainability requires an economic use of resources and tests which ultimately will depend on the clinical utility expressed as sensitivity and specificity. A network‐based meta‐analysis approach seems promising for head‐to‐head comparisons which include different Nf isoforms, Nf phosphoforms and Nf proteolytic breakdown products (Rübsamen et al., 2022). In addition, the meta‐analyses demonstrated that there are now more data on blood than on CSF Nf concentrations. This is a clear win attributed to the excellent detection limit of fourth‐generation immuno‐assays. Analyses of longitudinal data become possible. The meta‐analyses also remind us that neither CSF nor blood Nf levels are diagnostic for one disease. Accepting that Nf is not a disease‐specific biomarker, it remains relevant for prognosis (Zhou et al., 2021). Therefore, a pertinent question put to future meta‐analyses is: ‘what is the prognostic value of elevated Nf levels’? and expanding on this ‘what is the prognostic value of sustained or rising NfL levels over time’? For conducting such future meta‐analyses it will be relevant to have well‐defined clinical outcome measures to interrogate prognosis.
The large number of studies on body fluid biomarkers has made it almost impossible to cover all relevant data in a future single review. What will remain possible are systematic reviews on select disease groups or reviews drawing on meta‐analyses. The data reviewed here imply that there is a need for developing reporting guidelines for robust meta‐analyses which take into account relevant pre‐analytical, analytical and clinical factors. An alphabetical overview of the 87 conditions included to this review is presented in Table 3.
TABLE 3.
Diseases and conditions in which Nf isoforms have been quantified in the human brain interstitial fluid, CSF, plasma, serum, amniotic fluid, anterior chamber fluid and vitreous. This review included 87 conditions and their clinical subtypes. This list is expected to expand
| AD | DLB | MS |
| AF | Epilepsy | MSA |
| AIDS | Oesophageal cancer surgery | Myotonic dystrophy |
| ALS | Febrile seizures | NMOSD |
| AMD | Friedreich Ataxia | ON |
| Amyloidosis | FTLD | OSAP |
| Anorexia nervosa | FTLD‐ALS | Paraneoplastic disease |
| ATTRm | GAN | PD |
| Autism Spectrum Disorder | Gangliosidosis | PML |
| BPAN | GBS | PPA |
| Cardiac arrest | Glaucoma | Pre‐eclampsia |
| Cardiac surgery | HD | PSP |
| CBD | Hepatic encephalopathy | Psychosis |
| CHD | Herpes encephalitis | RD |
| Chemotoxicity | High BMI | SAH |
| CIDP | HIV | SAS |
| CIN | HMS | SCA |
| CIPN | Ketamine dependence | Schizophrenia |
| CJD | Kidney function impairment | Sepsis |
| CLN2 | Langerhans cell histiocytosis | Sjogren's syndrome |
| CLN3 | Lupus | SMA |
| CMT | Lyme | Spinal cord injury |
| Cocaine use | Malaria | Stroke |
| Concussion | MCI | SVD |
| COVID‐19 | MDMA use | TBI |
| CTE | Menignomyelocele | Tick‐borne encephalitis |
| Dementia | Metastatic brain lesions | Vasculitis |
| Depression | MMN | Wilson disease |
| Diabetes mellitus | MOGAD | WNV |
13. STATISTICS AND TRIALS
There is a need to further develop the statistical approach. A likely influential paper incorporated for example NfL, age and the body mass index (BMI) values into z‐scores. One advantage of this approach is that bold comparisons can be made. The NfL Z‐score data strongly suggest a differential effect of treatment options on blood NfL levels in MS (Benkert et al., 2022). There is no doubt that Nf levels are useful on a group level. The question now is if they will become useful on an individual subject level?
For clinical trial design, it will be relevant to note that all neuroprotective treatment trials in MS which had failed on these primary outcome measures, now retrospectively showed significant effects regarding Nf levels (Preziosa et al., 2020).
Power calculations for trial design will need to include data on the inter‐ and intra‐assay CV, physiological variation of Nf isoforms. A large analytical error, caused for example by a large intra‐assay CV will mask small changes in Nf levels over time. While Z‐scores can correct for demographic data and comorbidities, they remain vulnerable to analytical errors.
14. OUTLOOK
To continue its trajectory, the ‘dazzling rise of Nf […]’ (Bomont, 2021) needs further acceleration. On top of the increasing quantity of clinical studies focused on the subject of this review, there are fundamental questions to basic sciences.
There is half a century of research on the presence of autoantibodies directed at Nf (Petzold, 2005b). Several PTMs of Nf isoforms reviewed act as a trigger for autoimmunity. Until now, the role of such autoantibodies remains unclear. Clinically, it will be important to find out if they are of pathological relevance or only an epiphenomenon? Methodologically, it will be relevant to find out if they interfere with the reliability of the immunoassay by abolishing parallelism (Hersey et al., 2022; Khalil et al., 2018).
Tissue specificity. The assumption on which this review is based is that Nf are 100% specific for the neuronal and axonal compartment. This is not entirely true. Very small quantities of Nf can also be found in blood cells (erythrocytes, T lymphocytes), podocytes, testis, oocytes, cancerous tissue, stem cells and thymus. Quantitatively this may not matter as the Nf concentration measured from 50 neurons is more than what can be measured for 56.39 kg of CD34+ cells or erythrocytes (Petzold, Mondria, et al., 2010; Petzold, Tisdall, et al., 2011). Yet, why do these cells employ Nf? Has this to do with structure and stability?
Cancerous cells also express Nf as part of their cytoskeleton. Nf are an epigenetic factor in oncology (Calmon et al., 2015; Hasan et al., 2021; Li et al., 2020). Why is this the case? Do Nf help cancer to improve resistance against treatment? Or does it increase the risk of cancer? Does this open novel treatment strategies in oncology? Likewise, is there evidence for Nf to contribute to transport and propagation of certain neuroinvasive viruses (Gajdusek, 1985; Zhang et al., 2019)?
The need for a reliable test for Nf as a human body fluid biomarker for neurodegeneration has, because of analytical advantages, narrowed the field down to small proteolytic fragments of NfL. This line of research does not permit investigating the relevance of aggregate formation and PTMs of the larger protein subunits. Nf aggregate formation occurs spontaneously with impaired Nf stoichiometry for example in stem cells (Chao et al., 2007), transgenic models (Didonna & Opal, 2019; Julien, 2001; Rebelo et al., 2016), human disease (Adiutori et al., 2018), taphonomy and archaeology (Petzold et al., 2020). Future research should consider investigating into more detail the role of PTMs given the link with autoimmunity and aggregate formation (Zecha et al., 2022). For example there must be a reason why NfH is the most extensively phosphorylated protein of the human body. Why are Nfs to a large part intrinsically unstructured? Why are some Nfs able to self‐assemble into polymers and form intra‐ and extracellular aggregates in pathology? How does the averaged Nf stoichiometry vary between different regions in the human nervous system?
Most studies draw conclusions on CNS involvement based on pathological blood Nf concentrations. This may not always be correct (see Figure 1). NfL, NfH and NfM are also expressed in the PNS. Future method development work should include INA and PRPH to permit for more accurate separation of CNS from PNS injury. This is of relevance, for example for the interpretation of athletics related to increase in Nf levels.
The relationship between Nf isoforms and axonal conduction velocity should be revisited. Is axonal conduction speed only amplified through increase in axonal diameter, or does axonal Nf stoichiometry and PTMs such as phosphorylation also play a role? And related to this, if Nf isoforms are relevant to speed of information processing in the neuronal network, are they also relevant to understanding neuronal network memory?
-
Quality control and normal values for Nf concentrations. In order to become suitable for clinical routine, there is a need to capitalise on the current enthusiasm and consolidate reproducibility. The facts are that there remains a poor level of agreement between laboratories for reporting absolute Nf concentrations, even if using the same immunoassay. This error is frequently reported in the form of a metric called the coefficient of variation (CV). The inter‐laboratory CV was as high as 59% in the first global validation study (Petzold, Altintas, et al., 2010). This has not yet sufficiently improved in smaller‐scale repeat validation studies to give confidence to divert from the consensus recommendation for Nf batch analysis in expert laboratories (Petzold, Altintas, et al., 2010). It will be important to participate in External Quality control schemes to help individual laboratories to improve and continue gaining trust from the wider medical community in this test. This includes for laboratories to be internally consistent about cutoff levels and normal ranges reported in the literature. As reviewed in the section on FTLD, there is also a need for Nf isoform protein standard curves to be calibrated logically. This will include a revision of the Nf isoform protein sources which presently come from a range of rodents, pigs and bovine sources. It will be of advantage for the study of human diseases using human Nf protein. Such an approach will also be of help to more systematically investigate the presence of autoantibodies mentioned above.
Next, the need for human biofluid‐specific normative data on Nf levels cannot be underestimated. Age, BMI and neurological comorbidities are all relevant. Likewise, a standardised approach to interpretation of these values is required. Absolute values, percentiles and Z‐scores are all used and each approach has advantages and limitations. On a group level, there remains a large overlap between health and disease. Overcoming these issues will pave the way for application of Nf to individualised, personal medicine.
The causal relationship of Nf body fluid levels with age should be investigated in more detail (Paris et al., 2022). The challenge is to separate physiological ageing from accelerated neurodegeneration. There are at least two approaches to this. One approach will be driven clinically by identifying relevant associations, comorbidities and lifestyle which drive age‐related neurodegeneration. The other approach will be motivated neurochemically by investigating Nf stoichiometry, PTMs, autoantibodies, proteolytic breakdown products and aggregates in order to better understand how the human body attempts on a molecular level to adapt to ageing.
There is a need for post‐mortem studies to better understand the distribution of Nf isoforms, PTMs, proteolytic breakdown products and aggregates in the human body. Such studies should include analysis of the reticuloendothelial system as likely relevant to the turn‐over of Nf isoforms in the human body. Such studies will also permit shedding more light on the previous point on the relationship of Nf body fluid concentrations with physiological ageing and pathology. For example are the differences in the Nf proteome found in the spleen of an individual who died from ALS compared to old age? Regarding the human brain, much more detailed region‐specific characterisation of Nf isoforms will be required (Budelier et al., 2022; Magliozzi et al., 2022; Sjölin et al., 2022). Expanding from post‐mortem studies to archaeology, Nf can contribute to investigating the evolutionary descent of the nervous system (Lasek et al., 1985; Petzold et al., 2020; Zalc & Colman, 2000).
Finally, there is an open field for biomarker discovery beyond Nf. Having been validated, Nf can be used as a guide for this line of research. Human body fluid samples with high Nf isoform concentrations taken in an acute clinical event can be selected for in‐depth analytical search of other biomarkers. Data derived from such experiments can be matched from the biomarker discovery profiles found in samples taken in chronic conditions, exacerbation of relapsing conditions, after treatment, from different age groups and from normal control individuals. In conclusion, samples with high Nf isoform concentrations also contain traces of other biomarkers helpful to further investigate neurodegeneration.
15. CONCLUSIONS
It can be concluded that Nf have become the first non‐disease‐specific biomarkers for neurodegeneration which can be quantified on an industrial scale from blood samples and many other body fluids. Consequently, there has been an exponential growth of Nf studies covering all medical specialities. Because Nf are such a sensitive biomarker for neurodegeneration they should not be used as a first‐line diagnostic test for a specific disease. Instead, they provide valuable information through the longitudinal dynamics of Nf concentrations on the background of a well‐defined clinical scenario. The interpretation of Nf concentrations will require adjusted normal ranges for age. Constitutional factors such as obesity, endocrinological problems such as diabetes mellitus, nephrological issues such as renal function and neurological comorbidities will also need to be considered. There is important information for prediction of disease severity and outcome with higher Nf concentrations indicating a poorer prognosis. Nf body fluid levels can increase prior to clinical onset of disease in individuals which harbour pathogenic mutations. The successful use of Nf as a secondary endpoint in several treatment trials already started to influence clinical trial design. In this context, batch analysis of Nf in experienced laboratories is strongly recommended. Finally, for patient safety, unexpected rise of Nf concentrations can alert clinicians to potential neurotoxic complications of interventions.
AUTHOR CONTRIBUTION
This review was conceived, researched and written by Axel Petzold.
CONFLICT OF INTEREST
The author declares no conflict of interest.
OPEN RESEARCH BADGES
This article has been awarded Open Materials Badge for making the components of the research methodology needed to reproduce the reported procedure and analysis publicly available in the Supplements. More information about the Open Science Badges can be found at Open Science Framework
Supporting information
Supplementary material S1
ACKNOWLEDGMENTS
I am grateful to the Royal College of Physicians, London, UK for the generous reward of the 2022 Lady Estelle Wolfson Lectureship. The lecture was given at the Medicine 2022 conference on 01.04.2022. This review was not funded. Figures were re‐drawn by Victoria Morus on the basis of a draft provided by the author. The molecular structures were created in Pymol (https://pymol.org/2/).
Petzold, A. (2022). The 2022 Lady Estelle Wolfson lectureship on neurofilaments. Journal of Neurochemistry, 163, 179–219. 10.1111/jnc.15682
Endnotes
The 2022 meta‐analysis (Zhang et al., 2022) did not refer to the 2019 meta‐analysis (Forgrave et al., 2019).
There was no response from the authors on the progress of this meta‐analysis when contacted by email on MAY 03 2022.
This meta‐analysis was submitted to the Journal on Jul 14 2021, but did not refer to the 2‐year earlier meta‐analysis which was more complete (Forgrave et al., 2019).
DATA AVAILABILITY STATEMENT
Data are shared as Supplementary Material.
REFERENCES
- Aamodt, A. H. , Høgestøl, E. A. , Popperud, T. H. , Holter, J. C. , Dyrhol‐Riise, A. M. , Tonby, K. , Stiksrud, B. , Quist‐Paulsen, E. , Berge, T. , Barratt‐Due, A. , Aukrust, P. , Heggelund, L. , Blennow, K. , Zetterberg, H. , & Harbo, H. F. (2021). Blood neurofilament light concentration at admittance: A potential prognostic marker in COVID‐19. Journal of Neurology, 268, 3574–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaroa, L. , Rodrguez‐Quiroga, S. A. , Melamud, L. , Arakaki, T. , Garretto, N. S. , & Villa, A. M. (2013). Tonic spasms are a common clinical manifestation in patients with neuromyelitis optica. Arquivos de Neuro‐Psiquiatria, 71, 280–283. [DOI] [PubMed] [Google Scholar]
- Abdelhak, A. , Cordano, C. , Boscardin, W. J. , Caverzasi, E. , Kuhle, J. , Chan, B. , Gelfand, J. M. , Yiu, H. H. , Oertel, F. C. , Beaudry‐Richard, A. , Montes, S. C. , Oksenberg, J. R. , Lago, A. L. , Boxer, A. , Rojas‐Martinez, J. C. , Elahi, F. M. , Chan, J. R. , & Green, A. J. (2022). Plasma neurofilament light chain levels suggest neuroaxonal stability following therapeutic remyelination in people with multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 18, 158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiutori, R. , Aarum, J. , Zubiri, I. , Bremang, M. , Jung, S. , Sheer, D. , Pike, I. , & Malaspina, A. (2018). The proteome of neurofilament‐containing protein aggregates in blood. Biochemistry and Biophysics Reports, 14, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzick, N. S. , Thom, E. A. , Spong, C. Y. , Brock, J. W. , Burrows, P. K. , Johnson, M. P. , Howell, L. J. , Farrell, J. A. , Dabrowiak, M. E. , Sutton, L. N. , Gupta, N. , Tulipan, N. B. , D’Alton, M. E. , & Farmer, D. L. (2011). A randomized trial of prenatal versus postnatal repair of myelomeningocele. New England Journal of Medicine, 364, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah, E. , Saleh, F. , Sanjari, M. H. , Saghazadeh, A. , Tafakhori, A. , & Rezaei, N. (2018). CSF and blood biomarkers in amyotrophic lateral sclerosis: Protocol for a systematic review and meta‐analysis. Systematic Reviews, 7, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamine, S. , Marutani, N. , Kanayama, D. , Gotoh, S. , Maruyama, R. , Yanagida, K. , Sakagami, Y. , Mori, K. , Adachi, H. , Kozawa, J. , Maeda, N. , Otsuki, M. , Matsuoka, T. , Iwahashi, H. , Shimomura, I. , Ikeda, M. , & Kudo, T. (2020). Renal function is associated with blood neurofilament light chain level in older adults. Scientific Reports, 10, 20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaratnam, J. , Francesco, D. D. , Zetterberg, H. , Heslegrave, A. , Toombs, J. , Kootstra, N. A. , Underwood, J. , Gisslen, M. , Reiss, P. , Fidler, S. , & Sabin, C. A. (2021). Correlation between cerebrospinal fluid and plasma neurofilament light protein in treated HIV infection: Results from the COBRA study. Journal of Neurovirology, 28, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaratnam, J. , Widekind, S. , von Francesco, D. D. , Underwood, J. , Edison, P. , Winston, A. , Zetterberg, H. , & Fidler, S. (2021). Correlation between CSF and blood neurofilament light chain protein: A systematic review and meta‐analysis. BMJ, 3, e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann, P. , Ponleitner, M. , Rommer, P. S. , Haslacher, H. , Mucher, P. , Leutmezer, F. , Petzold, A. , Wotawa, C. , Lanzenberger, R. , Berger, T. , Zetterberg, H. , & Bsteh, G. (2021). Seven day pre‐analytical stability of serum and plasma neurofilament light chain. Scientific Reports, 11, 11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anad, A. , Barker, M. K. , Katanga, J. A. , Arfanakis, K. , Bridges, L. R. , Esiri, M. M. , Isaacs, J. D. , Mihevc, S. P. , Pereira, A. C. , Schneider, J. A. , & Hainsworth, A. H. (2022). Vasculocentric axonal NfH in small vessel disease. Journal of Neuropathology and Experimental Neurology, 81, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, M. , Higuchi, Y. , Okamoto, Y. , Yuan, J. , Yoshimura, A. , Takei, J. , Taniguchi, T. , Hiramatsu, Y. , Sakiyama, Y. , Hashiguchi, A. , Matsuura, E. , Nakagawa, H. , Sonoda, K. , Yamashita, T. , Tamura, A. , Terasawa, H. , Mitsui, J. , Ishiura, H. , Tsuji, S. , & Takashima, H. (2022). An NEFH founder mutation causes broad phenotypic spectrum in multiple Japanese families. Journal of Human Genetics, 67, 399–403. [DOI] [PubMed] [Google Scholar]
- Angelopoulou, E. , Bougea, A. , Papadopoulos, A. , Papagiannakis, N. , Simitsi, A.‐M. , Koros, C. , Georgakis, M. K. , & Stefanis, L. (2021). CSF and circulating NfL as biomarkers for the discrimination of Parkinson disease from atypical Parkinsonian syndromes: Meta‐analysis. Neurology Clinical Practice, 11, e867–e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou, A. A. , Karteri, S. , Bruna, J. , Mariotto, S. , Simo, M. , Velissaris, D. , Kalofonou, F. , Cavaletti, G. , Ferrari, S. , & Kalofonos, H. P. (2021) Serum neurofilament light chain levels as biomarker of paclitaxel‐induced cognitive impairment in patients with breast cancer: A prospective study. [DOI] [PubMed]
- Arneth, B. , & Kraus, J. (2022). Laboratory biomarkers of multiple sclerosis (MS). Clinical Biochemistry, 99, 1–8. [DOI] [PubMed] [Google Scholar]
- Arslan, B. , Şemsi, R. , İriz, A. , & Dinçel, A. S. (2021) The evaluation of serum brain‐derived neurotrophic factor and neurofilament light chain levels in patients with obstructive sleep apnea syndrome. [DOI] [PMC free article] [PubMed]
- Ashton, N. J. , Leuzy, A. , Lim, Y. M. , Troakes, C. , Hortobágyi, T. , Höglund, K. , Aarsland, D. , Lovestone, S. , Schöll, M. , Blennow, K. , Zetterberg, H. & Hye, A. (2019). Increased plasma neurofilament light chain concentration correlates with severity of post‐mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathologica Communications, 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asken, B. , Tanner, J. A. , VandeVrede, L. , Casaletto, K. B. , Staffaroni, A. M. , Mundada, N. , Fonseca, C. , Iaccarino, L. , La Joie, R. , Tsuei, T. , Mladinov, M. , Grant, H. , Shankar, R. , Wang, K. W. W. , Xu, H. , Cobigo, Y. , Rosen, H. , Gardner, R. C. , Perry, D. C. , … Gil, D. , (2022). Multimodal biomarkers of repetitive head impacts and traumatic encephalopathy syndrome: A clinico‐pathological case series. Journal of Neurotrauma. 10.1089/neu.2022.0060 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autiliogambetti, L. , Crane, R. , & Gambetti, P. (1986). Binding of Bodian silver and monoclonal‐antibodies to defined regions of human neurofilament subunits ‐ Bodian silver reacts with a highly charged unique domain of neurofilaments. Journal of Neurochemistry, 46, 366–370. [DOI] [PubMed] [Google Scholar]
- Bakovic, M. , Filipovic, N. , Ferhatovic, H. L. , Kunac, N. , Zdrilic, E. , Vitlov, U. M. , Kostic, S. , Puljak, L. , & Vukojevic, K. (2018). Changes in neurofilament 200 and tyrosine hydroxylase expression in the cardiac innervation of diabetic rats during aging. Cardiovascular Pathology: the Official Journal of the Society for Cardiovascular Pathology, 32, 38–43. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam, C. , Morgan, W. H. , Bass, L. , Matich, G. , Cringle, S. J. , & Yu, D. Y. (2007). Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure. Investigative Ophthalmology & Visual Science, 48, 3632–3644. [DOI] [PubMed] [Google Scholar]
- Barro, C. , Benkert, P. , Disanto, G. , Tsagkas, C. , Amann, M. , Naegelin, Y. , Leppert, D. , Gobbi, C. , Granziera, C. , Yaldizli, Ö. , Michalak, Z. , Wuerfel, J. , Kappos, L. , Parmar, K. , & Kuhle, J. (2018). Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain: A Journal of Neurology, 141, 2382–2391. [DOI] [PubMed] [Google Scholar]
- Bateman, R. J. , Xiong, C. , Benzinger, T. L. S. , Fagan, A. M. , Goate, A. , Fox, N. C. , Marcus, D. S. , Cairns, N. J. , Xie, X. , Blazey, T. M. , Holtzman, D. M. , Santacruz, A. , Buckles, V. , Oliver, A. , Moulder, K. , Aisen, P. S. , Ghetti, B. , Klunk, W. E. , McDad, E. , … Morris, J. C. (2012). Clinical and biomarker changes in dominantly inherited Alzheimerś disease. New England Journal of Medicine, 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavato, F. , Cathomas, F. , Klaus, F. , Gütter, K. , Barro, C. , Maceski, A. , Seifritz, E. , Kuhle, J. , Kaiser, S. , & Quednow, B. B. (2021). Altered neuroaxonal integrity in schizophrenia and major depressive disorder assessed with neurofilament light chain in serum, 140, 141–148. [DOI] [PubMed] [Google Scholar]
- Bavato, F. , Kexel, A. K. , Kluwe‐Schiavon, B. , Maceski, A. , Baumgartner, M. R. , Seifritz, E. , Kuhle, J. , & Quednow, B. B. (2022) A longitudinal investigation of blood neurofilament light chain levels in chronic cocaine users. [DOI] [PMC free article] [PubMed]
- Bavato, F. , Stamatakos, S. , Ohki, C. M. Y. , Seifritz, E. , Romualdi, P. , Grünblatt, E. , & Quednow, B. B. (2022). Brain‐derived neurotrophic factor protects serotonergic neurons against 3,4‐methylenedioxymethamphetamine (“ecstasy”) induced cytoskeletal damage. Journal of Neural Transmission, 129, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, R. , Deek, J. , Choi, M. C. , Ikawa, T. , Watanabe, O. , Frey, E. , Pincus, P. , & Safinya, C. R. (2010). Unconventional salt trend from soft to stiff in single neurofilament biopolymers. Langmuir, 26, 18595–18599. [DOI] [PubMed] [Google Scholar]
- Beck, R. , Deek, J. , Jones, J. B. , & Safinya, C. R. (2010). Gel‐expanded to gel‐condensed transition in neurofilament networks revealed by direct force measurements. Nature Materials, 9, 40–46. [DOI] [PubMed] [Google Scholar]
- Beck, R. , Deek, J. , & Safinya, C. R. (2012). Structures and interactions in ‘Bottlebrush’ neurofilaments: The role of charged disordered proteins in forming hydrogel networks. Biochemical Society Transactions, 40, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Benatar, M. , Wuu, J. , Lombardi, V. , Jeromin, A. , Bowser, R. , Andersen, P. M. , & Malaspina, A. (2019). Neurofilaments in pre‐symptomatic ALS and the impact of genotype. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 20, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert, P. , Meier, S. , Schaedelin, S. , Manouchehrinia, A. , Yaldizli, Ö. , Maceski, A. , Oechtering, J. , Achtnichts, L. , Conen, D. , Derfuss, T. , Lalive, P. H. , Mueller, C. , Müller, S. , Naegelin, Y. , Oksenberg, J. R. , Pot, C. , Salmen, A. , Willemse, E. , Kockum, I. , … Zecca, C. (2022). Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. The Lancet Neurology, 21, 246–257. [DOI] [PubMed] [Google Scholar]
- Besse, M. , Belz, M. , Folsche, T. , Vogelgsang, J. , Methfessel, I. , Steinacker, P. , Otto, M. , Wiltfang, J. , & Zilles, D. (2020). Serum neurofilament light chain (NFL) remains unchanged during electroconvulsive therapy. The World Journal of Biological Psychiatry, 21, 148–154. [DOI] [PubMed] [Google Scholar]
- Beydoun, M. A. , Hooten, N. N. , Maldonado, A. I. , Beydoun, H. A. , Weiss, J. , Evans, M. K. , & Zonderman, A. B. (2021) Body mass index and allostatic load are directly associated with longitudinal increase in plasma neurofilament light among urban middle‐aged adults. [DOI] [PMC free article] [PubMed]
- Beydoun, M. A. , Hooten, N. N. , Weiss, J. , Beydoun, H. A. , Hossain, S. , Evans, M. K. , & Zonderman, A. B. (2022). Plasma neurofilament light and its association with all‐cause mortality risk among urban middle‐aged men and women. BMC Medicine, 20, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof, A. , Manigold, T. , Barro, C. , Heijnen, I. , Berger, C. T. , Derfuss, T. , Kuhle, J. , & Daikeler, T. (2017). Serum neurofilament light chain: A biomarker of neuronal injury in vasculitic neuropathy. Annals of the Rheumatic Diseases, 77, 1093–1094. [DOI] [PubMed] [Google Scholar]
- Bittner, S. , Oh, J. , Havrdová, E. K. , Tintoré, M. , & Zipp, F. (2021) The potential of serum neurofilament as biomarker for multiple sclerosis. [DOI] [PMC free article] [PubMed]
- Blennow, K. , Hardy, J. , & Zetterberg, H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron, 76, 886–899. [DOI] [PubMed] [Google Scholar]
- Bomont, P. (2021). The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Current Opinion in Cell Biology, 68, 181–191. [DOI] [PubMed] [Google Scholar]
- Bomont, P. , Cavalier, L. , Blondeau, F. , Ben, H. C. , Belal, S. , Tazir, M. , Demir, E. , Topaloglu, H. , Korinthenberg, R. , Tuysuz, B. , Landrieu, P. , Hentati,, F. , & Koenig, M. (2000). The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nature Genetics, 26, 370–374. [DOI] [PubMed] [Google Scholar]
- Brettschneider, J. , Petzold, A. , Junker, A. , & Tumani, H. (2006). Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Multiple Sclerosis Journal, 12, 143–148. [DOI] [PubMed] [Google Scholar]
- Brettschneider, J. , Petzold, A. , Suessmuth, S. , Ludolph, A. , & Tumani, H. (2006). Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology, 66, 852–856. [DOI] [PubMed] [Google Scholar]
- Briot, J. , Simon, M. , & Méchin, M.‐C. (2020). Deimination, intermediate filaments and associated proteins. International Journal of Molecular Sciences, 21, 8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune, S. , Høgestøl, E. A. , de Rodez Benavent, S. A. , Berg‐Hansen, P. , Beyer, M. K. , Leikfoss, I. S. , Bos, S. D. , Sowa, P. , Brunborg, C. , Andorra, M. , Valdeolivas, I. P. , Asseyer, S. , Brandt, A. , Chien, C. , Scheel, M. , Blennow, K. , Zetterberg, H. , de Rosbo, N. K. , Paul, F. , … Harbo, H. F. (2022). Serum neurofilament light chain concentration predicts disease worsening in multiple sclerosis. Multiple Sclerosis Journal. 10.1177/13524585221097296 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brureau, A. , Blanchard‐Bregeon, V. , Pech, C. , Hamon, S. , Chaillou, P. , Guillemot, J.‐C. , Barneoud, P. , Bertrand, P. , Pradier, L. , Rooney, T. , & Schussler, N. (2017). NF‐l in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiology of Disease, 104, 73–84. [DOI] [PubMed] [Google Scholar]
- Bsteh, G. , Berek, K. , Hegen, H. , Teuchner, B. , Buchmann, A. , Voortman, M. M. , Auer, M. , Wurth, S. , Zinganell, A. , Di Pauli, F. , Deisenhammer, F. , Khalil, M. , & Berger, T. (2019). Serum neurofilament levels correlate with retinal nerve fiber layer thinning in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England), 26, 1682–1690. [DOI] [PubMed] [Google Scholar]
- Budelier, M. M. , He, Y. , Barthelemy, N. R. , Jiang, H. , Li, Y. , Park, E. , Henson, R. L. , Schindler, S. E. , Holtzman, D. M. , & Bateman, R. J. (2022). A map of neurofilament light chain species in brain and cerebrospinal fluid and alterations in Alzheimer's disease. Brain Communications, 4, fcac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock, T. , & Horridge, G. A. (1965) Structure and function in the nervous systems of invertebrates.
- Burki, T. (2018). Increase of West Nile Virus cases in europe for 2018. The Lancet, 392, 1000. [DOI] [PubMed] [Google Scholar]
- Butinar, D. , Starr, A. , Zidar, J. , Koutsou, P. , & Christodoulou, K. (2008). Auditory nerve is affected in one of two different point mutations of the neurofilament light gene. Clinical Neurophysiology, 119, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, L. M. , Rodrigues, F. B. , Blennow, K. , Durr, A. , Leavitt, B. R. , Roos, R. A. C. , Scahill, R. I. , Tabrizi, S. J. , Zetterberg, H. , Langbehn, D. , & Wild, E. J. (2017). Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntingtons disease: A retrospective cohort analysis. The Lancet Neurology, 16, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot, R. C. , Scully, R. E. , Mark, E. J. , McNeely, B. U. , Spechler, S. J. , & Stone, G. C. (1985). Case 43‐1985. New England Journal of Medicine, 313, 1070–1079.3930962 [Google Scholar]
- Cai, L. , & Huang, J. (2018). Neurofilament light chain as a biological marker for multiple sclerosis: A meta‐analysis study. Neuropsychiatric Disease and Treatment, 14, 2241–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmon, M. F. , Jeschke, J. , Zhang, W. , Dhir, M. , Siebenkäs, C. , Herrera, A. , Tsai, H.‐C. , O'Hagan, H. M. , Pappou, E. P. , Hooker, C. M. , Fu, T. , Schuebel, K. E. , Gabrielson, E. , Rahal, P. , Herman, J. G. , Baylin, S. B. , & Ahuja, N. (2015). Epigenetic silencing of neurofilament genes promotes an aggressive phenotype in breast cancer. Epigenetics, 10, 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó, E. , Barro, C. , Zhao, C. , Caillier, S. J. , Michalak, Z. , Bove, R. , Tomic, D. , Santaniello, A. , Häring, D. A. , Hollenbach, J. , Henry, R. G. , Cree, B. A. C. , Kappos, L. , Leppert, D. , Hauser, S. L. , Benkert, P. , Oksenberg, J. R. , & Kuhle, J. (2019). Association between serum neurofilament light chain levels and long‐term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurology, 76, 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Sun, D. , & Whitaker, J. N. (1998). Citrullinated myelin basic protein induces experimental autoimmune encephalomyelitis in Lewis rats through a diverse t cell repertoire. Journal of Neuroimmunology, 88, 21–29. [DOI] [PubMed] [Google Scholar]
- Carden, M. J. , Schlaepfer, W. W. , & Lee, V. M. (1985). The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. The Journal of Biological Chemistry, 260, 9805–9817. [PubMed] [Google Scholar]
- Carter, J. , Gragerov, A. , Konvicka, K. , Elder, G. , Weinstein, H. , & Lazzarini, R. A. (1998). Neurofilament (NF) assembly; divergent characteristics of human and rodent NF‐l subunits. Journal of Biological Chemistry, 273, 5101–5108. [DOI] [PubMed] [Google Scholar]
- Center of Disease Control (2022) Serum neurofilament light chain ‐ serum (SSSNFL_H) . https://wwwn.cdc.gov/Nchs/Nhanes/2013‐2014/SSSNFL_H.htm
- Chao, Y. X. , He, B. P. , Cao, Q. , & Tay, S. S. W. (2007). Protein aggregate‐containing neuron‐like cells are differentiated from bone marrow mesenchymal stem cells from mice with neurofilament light subunit gene deficiency. Neuroscience Letters, 417, 240–245. [DOI] [PubMed] [Google Scholar]
- Chen, J.‐B. , Chang, C.‐C. , Moi, S.‐H. , & Li, L.‐C. (2022). A profile of nanoparticle‐based plasma neurodegenerative biomarkers for cognitive function among patients undergoing hemodialysis. International Journal of General Medicine, 15, 6115–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Tong, J. , & Yang, M. (2021). Photoelectrochemical sensor for detection of serum neurofilament light chain based on organic semiconductor modified electrode, 305, 130798. [Google Scholar]
- Chen, M.‐H. , Liu, Y.‐L. , Kuo, H.‐W. , Tsai, S.‐J. , Hsu, J.‐W. , Huang, K.‐L. , Tu, P.‐C. , & Bai, Y.‐M. (2021). Neurofilament light chain is a novel biomarker for major depression and related ExecutiveDysfunction. International Journal of Neuropsychopharmacology, 25, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Zhang, Y. , Wang, Q. , Qin, Y. , Yang, X. , Xing, Z. , Shen, Y. , Wu, H. , & Qi, Y. (2021). The function of SUMOylation and its crucial roles in the development of neurological diseases. The FASEB Journal, 35, e21510. [DOI] [PubMed] [Google Scholar]
- Cheung, W. D. , & Hart, G. W. (2008). AMP‐activated protein kinase and p38 MAPK activate o‐GlcNAcylation of neuronal proteins during glucose deprivation. Journal of Biological Chemistry, 283, 13009–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis, T. , Gonzalez, C. , Healy, B. C. , Saxena, S. , Rosso, M. , Barro, C. , Michalak, Z. , Paul, A. , Kivisakk, P. , Diaz‐Cruz, C. , Sattarnezhad, N. , Pierre, I. V. , Glanz, B. I. , Tomic, D. , Kropshofer, H. , Häring, D. , Leppert, D. , Kappos, L. , Bakshi, R. , … Kuhle, J. (2018). Neurofilament light chain serum levels correlate with 10‐year MRI outcomes in multiple sclerosis. Annals of Clinical Translational Neurology, 5, 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong, A. M. T. L. , Wee, I. J. Y. , Almond, M. , Muratani, M. , Kovari, F. , Russai, R. , & Jenkins, M. P. (2019). A systematic review of the use of biochemical markers in the assessment of spinal cord ischemia in thoracoabdominal aortic aneurysm repair. Vascular and Endovascular Surgery, 53, 230–241. [DOI] [PubMed] [Google Scholar]
- Chou, S. M. , Wang, H. S. , Taniguchi, A. , & Bucala, R. (1998). Advanced glycation endproducts in neurofilament conglomeration of motoneurons in familial and sporadic amyotrophic lateral sclerosis. Molecular Medicine, 4, 324–332. [PMC free article] [PubMed] [Google Scholar]
- Cloos, P. A. , & Christgau, S. (2004). Post‐translational modifications of proteins: Implications for aging, antigen recognition, and autoimmunity. Biogerontology, 5, 139–158. [DOI] [PubMed] [Google Scholar]
- Collard, J.‐F. , Côté, F. , & Julien, J.‐P. (1995). Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature, 375, 61–64. [DOI] [PubMed] [Google Scholar]
- Cong, S. , Xiang, C. , Wang, H. , & Cong, S. (2021). Diagnostic utility of fluid biomarkers in multiple system atrophy: A systematic review and meta‐analysis. Journal of Neurology, 268, 2703–2712. [DOI] [PubMed] [Google Scholar]
- Cooper, J. , Stukas, S. , Hoiland, R. L. , Fergusson, N. A. , Thiara, S. , Foster, D. , Mitra, A. , Stoessl, A. , Panenka, W. J. , Sekhon, M. S. , & Wellington, C. L. (2020). Quantification of neurological blood‐based biomarkers in critically ill patients with coronavirus disease 2019. Critical Care Explorations, 2, e0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornali, C. , Amaddeo, P. , Benussi, A. , Perrone, F. , Manes, M. , Zanardini, R. , Benussi, L. , Belotti, F. , Bellini, G. , Bruzzone, A. , Bruzzone, M. , Morelli, D. , Archetti, S. , Latronico, N. , Padovani, A. , Fontanella, M. M. , Ghidoni, R. , & Borroni, B. (2022). Serum neurofilament light in professional soccer players: Goal on safety. Neurological Sciences, 43, 5087–5090. [DOI] [PubMed] [Google Scholar]
- Costa, G. D. , Martinelli, V. , Moiola, L. , Sangalli, F. , Colombo, B. , Finardi, A. , Cinque, P. , Kolb, E.‐M. , Haghikia, A. , Gold, R. , Furlan, R. , & Comi, G. (2019). Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab‐treated multiple sclerosis patients. Annals of Neurology, 85, 606–610. [DOI] [PubMed] [Google Scholar]
- Couillard‐Despres, S. , Zhu, Q. , Wong, P. , Price, D. , Cleveland, D. , & Julien, J. (1998). Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proceedings of the National Academy of Sciences of the United States of America, 95, 9626–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras, B. T. , Crawford, T. O. , Finkel, R. S. , Mercuri, E. , Vivo, D. C. D. , Oskoui, M. , Tizzano, E. F. , Ryan, M. M. , Muntoni, F. , Zhao, G. , Staropoli, J. , McCampbell, A. , Petrillo, M. , Stebbins, C. , Fradette, S. , Farwell, W. , & Sumner, C. J. (2019). Neurofilament as a potential biomarker for spinal muscular atrophy. Annals of Clinical Translational Neurology, 6, 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiell, S. M. , Tanner, S. L. , Pant, H. C. , & Quarles, R. H. (2002). Myelin‐associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. Journal of Neurochemistry, 81, 1263–1272. [DOI] [PubMed] [Google Scholar]
- De Schaepdryver, M. , Jeromin, A. , Gille, B. , Claeys, K. G. , Herbst, V. , Brix, B. , Van Damme, P. , & Poesen, K. (2018). Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 89, 367–373. [DOI] [PubMed] [Google Scholar]
- Deng, Y. , Li, B. , Liu, F. , Iqbal, K. , Grundke‐Iqbal, I. , Brandt, R. , & Gong, C.‐X. (2007). Regulation between o‐GlcNAcylation and phosphorylation of neurofilament‐m and their dysregulation in Alzheimer disease. The FASEB Journal, 22, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didonna, A. , & Opal, P. (2019). The role of neurofilament aggregation in neurodegeneration: Lessons from rare inherited neurological disorders. Molecular Neurodegeneration, 14, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMeglio, M. , Furey, W. , Hajj, J. , Lindekens, J. , Patel, S. , Acker, M. , Bavaria, J. , Szeto, W. Y. , Atluri, P. , Haber, M. , Diaz‐Arrastia, R. , & Laudanski, K. (2019). Observational study of long‐term persistent elevation of neurodegeneration markers after cardiac surgery. Scientific Reports, 9, 7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, A. N. D. , Sinaii, N. , Masvekar, R. R. , Baker, E. H. , Thurm, A. E. , Soldatos, A. G. , Bianconi, S. E. , Bielekova, B. , & Porter, F. D. (2020). Neurofilament light chain levels correlate with clinical measures in CLN3 disease. Genetics in Medicine, 23, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donev, R. (2021) Apoptosis in health and disease. Elsevier Science & Technology. [Google Scholar]
- Dong, D. , Xu, Z. , Chevrier, M. , Cotter, R. , Cleveland, D. , & Hart, G. (1993). Glycosylation of mammalian neurofilaments. Localization of multiple O‐linked N‐acetylglucosamine moieties on neurofilament polypeptides l and m. Journal of Biological Chemistry, 268, 16679–16687. [PubMed] [Google Scholar]
- Dong, D. , Xu, Z. , Hart, G. , & Cleveland, D. (1996). Cytoplasmic o‐GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament‐h. The Journal of Biological Chemistry, 271, 20845–20852. [DOI] [PubMed] [Google Scholar]
- Doose, A. , Hellerhoff, I. , Tam, F. I. , King, J. A. , Seidel, M. , Geisler, Dipl.‐I. D. , Plähn, H. C. I. , Roessner, V. , Akgün, K. , Ziemssen, T. , & Ehrlich, S. (2021) Neural and glial damage markers in women after long‐term weight‐recovery from anorexia nervosa. 105576. [DOI] [PubMed]
- Ducharme, S. , Dols, A. , Laforce, R. , Devenney, E. , Kumfor, F. , Stock, J. v. d. , Dallaire‐Théroux, C. , Seelaar, H. , Gossink, F. , Vijverberg, E. , Huey, E. , Vandenbulcke, M. , Masellis, M. , Trieu, C. , Onyike, C. , Caramelli, P. , de Souza, L. C. , Santillo, A. , Waldö, M. L. , … Pijnenburg, Y. (2020). Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain, 143, 1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutour‐Provenzano, G. , & Etienne‐Manneville, S. (2021). Intermediate filaments. Current Biology, 31, R522–R529. [DOI] [PubMed] [Google Scholar]
- Eckerström, M. , Nilsson, S. , Zetterberg, H. , Blennow, K. , & Grahn, A. (2020). Cognitive impairment without altered levels of cerebrospinal fluid biomarkers in patients with encephalitis caused by varicella‐zoster virus: A pilot study. Scientific Reports, 10, 22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén, A. , Kanberg, N. , Gostner, J. , Fuchs, D. , Hagberg, L. , Andersson, L.‐M. , Lindh, M. , Price, R. W. , Zetterberg, H. , & Gisslén, M. (2020). CSF biomarkers in patients with COVID‐19 and neurological symptoms. Neurology, 96, e294–e300. [DOI] [PubMed] [Google Scholar]
- Ehler, J. , Barrett, L. K. , Taylor, V. , Groves, M. , Scaravilli, F. , Wittstock, M. , Kolbaske, S. , Grossmann, A. , Henschel, J. , Gloger, M. , Sharshar, T. , Chretien, F. , Gray, F. , Nöldge‐Schomburg, G. , Singer, M. , Sauer, M. , & Petzold, A. (2017). Translational evidence for two distinct patterns of neuroaxonal injury in sepsis: A longitudinal, prospective translational study. Critical Care (London, England), 21, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler, J. , Petzold, A. , Wittstock, M. , Kolbaske, S. , Gloger, M. , Henschel, J. , Heslegrave, A. , Zetterberg, H. , Lunn, M. P. , Rommer, P. S. , Grossmann, A. , Sharshar, T. , Richter, G. , Nöldge‐Schomburg, G. , & Sauer, M. (2019). The prognostic value of neurofilament levels in patients with sepsis‐associated encephalopathy a prospective, pilot observational study. PLoS One, 14, e0211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldirany, S. A. , Lomakin, I. B. , Ho, M. , & Bunick, C. G. (2021). Recent insight into intermediate filament structure. Current Opinion in Cell Biology, 68, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMBL‐EBI (2022) UniProt .
- van der Ende, E. L. , Bron, E. E. , Poos, J. M. , Jiskoot, L. C. , Panman, J. L. , Papma, J. M. , Meeter, L. H. , Dopper, E. G. P. , Wilke, C. , Synofzik, M. , Heller, C. , Swift, I. J. , Sogorb‐Esteve, A. , Bouzigues, A. , Borroni, B. , Sanchez‐Valle, R. , Moreno, F. , Graff, C. , Laforce, R. , … the GENFI consortium (2021). A data‐driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain, 145, 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende, E. L. , Meeter, L. H. , Poos, J. M. , Panman, J. L. , Jiskoot, L. C. , Dopper, E. G. P. , Papma, J. M. , de Jong, F. J. , Verberk, I. M. W. , Teunissen, C. , Rizopoulos, D. , Heller, C. , Convery, R. S. , Moore, K. M. , Bocchetta, M. , Neason, M. , Cash, D. M. , Borroni, B. , Galimberti, D. , … Bessi, V. (2019). Serum neurofilament light chain in genetic frontotemporal dementia: A longitudinal, multicentre cohort study. The Lancet Neurology, 18, 1103–1111. [DOI] [PubMed] [Google Scholar]
- Espíndola, O. M. , Brandão, C. O. , Gomes, Y. C. P. , Siqueira, M. , Soares, C. N. , Lima, M. A. S. D. , Leite, A. C. C. B. , Torezani, G. , Araujo, A. Q. C. , & Silva, M. T. T. (2021). Cerebrospinal fluid findings in neurological diseases associated with COVID‐19 and insights into mechanisms of disease development. International Journal of Infectious Diseases, 102, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg, P. Z. , Buchheim, J.‐I. , Ashton, N. J. , Vassilieva, G. , Blennow, K. , Zetterberg, H. , & Choukér, A. (2021). Changes in blood biomarkers of brain injury and degeneration following long‐duration spaceflight. JAMA Neurology, 78, 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers, K. S. , Atkinson, A. , Barro, C. , Fisch, U. , Pfister, M. , Huhn, E. A. , Lapaire, O. , Kuhle, J. , & Wellmann, S. (2018). Neurofilament as neuronal injury blood marker in preeclampsia. Hypertension, 71, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Evers, K. S. , Hügli, M. , Fouzas, S. , Kasser, S. , Pohl, C. , Stoecklin, B. , Bernasconi, L. , Kuhle, J. , & Wellmann, S. (2020). Serum neurofilament levels in children with febrile seizures and in controls. Frontiers in Neuroscience, 14, 579958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli, I. , Meneri, M. , Saccomanno, D. , Velardo, D. , Abati, E. , Gagliardi, D. , Parente, V. , Petrozzi, L. , Ronchi, D. , Stocchetti, N. , Calderini, E. , D’Angelo, G. , Chidini, G. , Prandi, E. , Ricci, G. , Siciliano, G. , Bresolin, N. , Comi, G. P. , Corti, S. , … Govoni, A. (2020). Nusinersen treatment and cerebrospinal fluid neurofilaments: An explorative study on spinal muscular atrophy type 3 patients. Journal of Cellular and Molecular Medicine, 24, 3034–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasby, T. , Gilbert, J. , Brown, W. , Bolton, C. , Hahn, A. , Koopman, W. , & Zochodne, D. (1986). An acute axonal form of guillain‐barré polyneuropathy. Brain, 109, 1115–1126. [DOI] [PubMed] [Google Scholar]
- Feigin, V. L. , Abajobir, A. A. , Abate, K. H. , Abd‐Allah, F. , Abdulle, A. M. , Abera, S. F. , Abyu, G. Y. , Ahmed, M. B. , Aichour, A. N. , Aichour, I. , Aichour, M. T. E. , Akinyemi, R. O. , Alabed, S. , Al‐Raddadi, R. , Alvis‐Guzman, N. , Amare, A. T. , Ansari, H. , Anwari, P. , Ärnlöv, J. , … Vos, T. (2017). Global, regional, and national burden of neurological disorders during 1990‐2015: A systematic analysis for the global burden of disease study 2015. The Lancet Neurology, 16, 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, K. C. , Sotirchos, E. S. , Smith, M. D. , Lord, H.‐N. , DuVal, A. , Mowry, E. M. , & Calabresi, P. A. (2022). Contributors to serum NfL levels in people without neurologic disease. Annals of Neurology. 10.1002/ana.26446 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, T. G. , Vasunilashorn, S. M. , Ngo, L. , Libermann, T. A. , Dillon, S. T. , Schmitt, E. M. , Pascual‐Leone, A. , Arnold, S. E. , Jones, R. N. , Marcantonio, E. R. , & Inouye, S. K. (2020). Association of plasma neurofilament light with postoperative delirium. Annals of Neurology, 88, 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgrave, L. M. , Ma, M. , Best, J. R. , & DeMarco, M. L. (2019). The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta‐analysis. Alzheimer's & Dementia (Amsterdam, Netherlands), 11, 730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortea, J. , Vilaplana, E. , Carmona‐Iragui, M. , Benejam, B. , Videla, L. , Barroeta, I. , Fernández, S. , Altuna, M. , Pegueroles, J. , Montal, V. , Valldeneu, S. , Giménez, S. , González‐Ortiz, S. , Muñoz, L. , Estellés, T. , Illán‐Gala, I. , Belbin, O. , Camacho, V. , Wilson, L. R. , … Lleó, A. (2020). Clinical and biomarker changes of Alzheimers disease in adults with down syndrome: A cross‐sectional study. The Lancet, 395, 1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortova, A. , Hönig, V. , Palus, M. , Salat, J. , Pychova, M. , Krbkova, L. , Vyhlidalova, T. , Kriha, M. F. , Chrdle, A. , & Ruzek, D. (2022). Serum and cerebrospinal fluid phosphorylated neurofilament heavy subunit as a marker of neuroaxonal damage in tick‐borne encephalitis. Journal of General Virology, 103. [DOI] [PubMed] [Google Scholar]
- Gaetani, L. , Höglund, K. , Parnetti, L. , Pujol‐Calderon, F. , Becker, B. , Eusebi, P. , Sarchielli, P. , Calabresi, P. , Di Filippo, M. , Zetterberg, H. , & Blennow, K. (2018). A new enzyme‐linked immunosorbent assay for neurofilament light in cerebrospinal fluid: Analytical validation and clinical evaluation. Alzheimer's Research & Therapy, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiottino, J. , Norgren, N. , Dobson, R. , Topping, J. , Nissim, A. , Malaspina, A. , Bestwick, J. P. , Monsch, A. U. , Regeniter, A. , Lindberg, R. L. , Kappos, L. , Leppert, D. , Petzold, A. , Giovannoni, G. , & Kuhle, J. (2013). Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One, 8, e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek, D. C. (1985). Hypothesis: Interference with axonal transport of neurofilament as a common pathogenetic mechanism in certain diseases of the central nervous system. New England Journal of Medicine, 312, 714–719. [DOI] [PubMed] [Google Scholar]
- Gao, W. , Zhang, Z. , Lv, X. , Wu, Q. , Yan, J. , Mao, G. , & Xing, W. (2020a). Neurofilament light chain level in traumatic brain injury. Medicine, 99, e22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Zhang, Z. , Lv, X. , Wu, Q. , Yan, J. , Mao, G. , & Xing, W. (2020b). Neurofilament light chain level in traumatic brain injury: A system review and meta‐analysis. Medicine, 99, e22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Moreno, H. , Prudencio, M. , Thomas‐Black, G. , Solanky, N. , Jansen‐West, K. R. , AL‐Shaikh, R. H. , Heslegrave, A. , Zetterberg, M. , Santana, M. M. , de Almeida, L. P. , Vasconcelos‐Ferreira, A. , Januário, C. , Infante, J. , Faber, J. , Klockgether, T. , Reetz, K. , Raposo, M. , Ferreira, A. F. , Lima, M. , … Giunti, P. (2022). Tau and neurofilament light‐chain as fluid biomarkers in spinocerebellar ataxia type 3. European Journal of Neurology, 29, 2439–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland, P. , Morton, M. , Zolnourian, A. , Durnford, A. , Gaastra, B. , Toombs, J. , Heslegrave, A. J. , More, J. , Zetterberg, H. , Bulters, D. O. , & Galea, I. (2021). Neurofilament light predicts neurological outcome after subarachnoid haemorrhage. Brain, 144, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, A. , Viel, S. , Perret, M. , Brocard, G. , Casey, R. , Lombard, C. , Laurent‐Chabalier, S. , Debouverie, M. , Edan, G. , Vukusic, S. , Lebrun‐Frénay, C. , Sèze, J. D. , Laplaud, D. A. , Castelnovo, G. , Gout, O. , Ruet, A. , Moreau, T. , Casez, O. , Clavelou, P. , … Thouvenot, E. (2021). Comparison of SIMOATM and ELLATM to assess serum neurofilament‐light chain in multiple sclerosis. Annals of Clinical Translational Neurology, 8, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, F. , Ding, J. , Liu, Y. , Lin, H. , & Chang, T. (2018). Cerebrospinal fluid NFL in the differential diagnosis of Parkinsonian disorders: A meta‐analysis. Neuroscience Letters, 685, 35–41. [DOI] [PubMed] [Google Scholar]
- Geisler, N. , Plessmann, U. , & Weber, K. (1982). Related amino acid sequences in neurofilaments and non‐neuronal intermediate filaments. Nature, 296, 448–450. [DOI] [PubMed] [Google Scholar]
- Geisler, N. , & Weber, K. (1981). Comparison of the proteins of two immunologically distinct intermediate‐sized filaments by amino acid sequence analysis: Desmin and vimentin. Proceedings of the National Academy of Sciences, 78, 4120–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron, T. F. , Badi, M. K. , Heckman, M. G. , Jansen‐West, K. R. , Vilanilam, G. K. , Johnson, P. W. , Burch, A. R. , Walton, R. L. , Ross, O. A. , Brott, T. G. , Miller, T. M. , Berry, J. D. , Nicholson, K. A. , Wszolek, Z. K. , Oskarsson, B. E. , Sheth, K. N. , Sansing, L. H. , Falcone, G. J. , Cucchiara, B. L. , … Petrucelli, L. (2020). Plasma neurofilament light predicts mortality in patients with stroke. Science Translational Medicine, 12, eaay1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron, T. F. , Heckman, M. G. , White, L. J. , Veire, A. M. , Pedraza, O. , Burch, A. R. , Bozoki, A. C. , Dickerson, B. C. , Domoto‐Reilly, K. , Foroud, T. , Forsberg, L. K. , Galasko, D. R. , Ghoshal, N. , Graff‐Radford, N. R. , Grossman, M. , Heuer, H. W. , Huey, E. D. , Hsiung, G.‐Y. R. , Irwin, D. J. , … Wong, B. (2022). Comprehensive cross‐sectional and longitudinal analyses of plasma neurofilament light across FTD spectrum disorders. Cell Reports Medicine, 3, 100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil, B. J. , Minotti, S. , Beange, M. , Baloh, R. H. , Julien, J.‐P. , & Durham, H. D. (2011). Normal role of the low‐molecular‐weight neurofilament protein in mitochondrial dynamics and disruption in Charcot‐Marie‐Tooth disease. The FASEB Journal, 26, 1194–1203. [DOI] [PubMed] [Google Scholar]
- Ghosh, K. , Huihui, J. , Phillips, M. , & Haider, A. (2022). Rules of physical mathematics govern intrinsically disordered proteins. Annual Review of Biophysics, 51, 355–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille, B. , Schaepdryver, M. D. , Goossens, J. , Dedeene, L. , Vocht, J. D. , Oldoni, E. , Goris, A. , Bosch, L. V. D. , Depreitere, B. , Claeys, K. G. , Tournoy, J. , Damme, P. V. , & Poesen, K. (2018). Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with amyotrophic lateral sclerosis. Neuropathology and Applied Neurobiology, 45, 291–304. [DOI] [PubMed] [Google Scholar]
- Gisslén, M. , Price, R. W. , Andreasson, U. , Norgren, N. , Nilsson, S. , Hagberg, L. , Fuchs, D. , Spudich, S. , Blennow, K. , & Zetterberg, H. (2016). Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross‐sectional study. eBioMedicine, 3, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, M. , Sternberger, N. , & Sternberger, L. (1987). Phosphorylation protects neurofilaments against proteolysis. Journal of Neuroimmunology, 14, 149–160. [DOI] [PubMed] [Google Scholar]
- Grant, P. , & Pant, H. (2000). Neurofilament protein synthesis and phosphorylation. Journal of Neurocytology, 29, 843–872. [DOI] [PubMed] [Google Scholar]
- Gravesteijn, G. , Rutten, J. W. , Verberk, I. M. W. , Böhringer, S. , Liem, M. K. , Grond, J. v. d. , Aartsma‐Rus, A. , Teunissen, C. E. , & Oberstein, S. A. J. L. (2018). Serum neurofilament light correlates with CADASIL disease severity and survival. Annals of Clinical Translational Neurology, 6, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, J. , Troncoso, J. , Costello, A. , & Johnson, G. (1993). Phosphorylation modulates calpain‐mediated proteolysis and calmodulin binding of the 200‐kDa and 160‐kDa neurofilament proteins. Journal of Neurochemistry, 61, 191–199. [DOI] [PubMed] [Google Scholar]
- Gunnarsson, M. , Malmeström, C. , Rosengren, L. , Lycke, J. , & Svenningsson, A. (2011). Reply. Annals of Neurology, 69, 1066–1067. [DOI] [PubMed] [Google Scholar]
- Halaas, N. B. , Blennow, K. , Idland, A.‐V. , Wyller, T. B. , Ræder, J. , Frihagen, F. , Staff, A. C. , Zetterberg, H. , & Watne, L. O. (2018). Neurofilament light in serum and cerebrospinal fluid of hip fracture patients with delirium. Dementia and Geriatric Cognitive Disorders, 46, 346–357. [DOI] [PubMed] [Google Scholar]
- Halloway, S. , Desai, P. , Beck, T. , Aggarwal, N. , Agarwal, P. , Evans, D. , & Rajan, K. B. (2022). Association of neurofilament light with the development and severity of parkinson disease. Neurology, 98, e2185–e2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, F. , Bulman, D. E. , Panisset, M. , & Grimes, D. A. (2005). Neurofilament m gene in a French‐Canadian population with parkinson's disease. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques, 32, 68–70. [DOI] [PubMed] [Google Scholar]
- Hasan, N. M. , Sharma, A. , Ruzgar, N. M. , Deshpande, H. , Olino, K. , Khan, S. , & Ahuja, N. (2021). Epigenetic signatures differentiate uterine and soft tissue leiomyosarcoma. Oncotarget, 12, 1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, S. L. , Bar‐Or, A. , Cohen, J. A. , Comi, G. , Correale, J. , Coyle, P. K. , Cross, A. H. , de Seze, J. , Leppert, D. , Montalban, X. , Selmaj, K. , Wiendl, H. , Kerloeguen, C. , Willi, R. , Li, B. , Kakarieka, A. , Tomic, D. , Goodyear, A. , Pingili, R. , … Kappos, L. (2020). Ofatumumab versus teriflunomide in multiple sclerosis. New England Journal of Medicine, 383, 546–557. [DOI] [PubMed] [Google Scholar]
- Hayer, S. N. , Liepelt, I. , Barro, C. , Wilke, C. , Kuhle, J. , Martus, P. , & Schöls, L. (2020). NfL and pNfH are increased in Friedreich's ataxia. Journal of Neurology, 267, 1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Zhang, X. , Zhang, Y. , & Zhang, W. (2020). Elevated serum neurofilament light chain in children autism spectrum disorder: A case control study. Neurotoxicology, 80, 87–92. [DOI] [PubMed] [Google Scholar]
- Henley, J. M. , Seager, R. , Nakamura, Y. , Talandyte, K. , Nair, J. , & Wilkinson, K. A. (2020). SUMOylation of synaptic and synapse‐associated proteins: An update. Journal of Neurochemistry, 156, 145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner, A. , Porter, J. , Hare, F. , Nasir, S. S. , Zetterberg, H. , Blennow, K. , & Martin, M. G. (2019). Serum neurofilament light, glial fibrillary acidic protein and tau are possible serum biomarkers for activity of brain metastases and gliomas. World Journal of Oncology, 10, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, P. , Appleby, B. , Brandel, J.‐P. , Caughey, B. , Collins, S. , Geschwind, M. D. , Green, A. , Haïk, S. , Kovacs, G. G. , Ladogana, A. , Llorens, F. , Mead, S. , Nishida, N. , Pal, S. , Parchi, P. , Pocchiari, M. , Satoh, K. , Zanusso, G. , & Zerr, I. (2021). Biomarkers and diagnostic guidelines for sporadic Creutzfeldt‐Jakob disease. The Lancet Neurology, 20, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, P. , Canaslan, S. , Villar‐Piqué, A. , Bunck, T. , Goebel, S. , Llorens, F. , Schmitz, M. , & Zerr, I. (2022). Plasma neurofilament light chain as a biomarker for fatal familial insomnia. European Journal of Neurology, 29, 1841–1846. [DOI] [PubMed] [Google Scholar]
- Herrmann, H. , Bär, H. , Kreplak, L. , Strelkov, S. V. , & Aebi, U. (2007). Intermediate filaments: From cell architecture to nanomechanics. Nature Reviews. Molecular Cell Biology, 8, 562–573. [DOI] [PubMed] [Google Scholar]
- Hersey, S. , Keller, S. , Mathews, J. , King, L. , Bandukwala, A. , Berisha, F. , Birchler, M. , Bower, J. , Clausen, V. , Duarte, J. , Garofolo, F. , Hopper, S. , Kar, S. , Mabrouk, O. , Marshall, J.‐C. , McGuire, K. , Naughton, M. , Saito, Y. , Schuhmann, I. , … Zoghbi, J. (2022). 2021 White paper on recent issues in bioanalysis: ISR for biomarkers, liquid biopsies, spectral cytometry, inhalation/oral; multispecific biotherapeutics, accuracy/LLOQ for flow cytometry (Part 2 ‐ recommendations on biomarkers/CDx assays development; validation, cytometry validation; innovation, biotherapeutics PK LBA regulated bioanalysis, critical reagents & positive controls generation). Bioanalysis. 10.4155/bio-2022-0080 [DOI] [PubMed] [Google Scholar]
- Higuchi, Y. , & Takashima, H. (2022). Clinical genetics of Charcot‐Marie‐Tooth disease. Journal of Human Genetics. 10.1038/s10038-022-01031-2 [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ho, T. C. S. , Chan, A. H. Y. , & Ganesan, A. (2020). Thirty years of HDAC inhibitors: 2020 insight and hindsight. Journal of Medicinal Chemistry, 63, 12460–12484. [DOI] [PubMed] [Google Scholar]
- Hoiland, R. L. , Rikhraj, K. J. K. , Thiara, S. , Fordyce, C. , Kramer, A. H. , Skrifvars, M. B. , Wellington, C. L. , Griesdale, D. E. , Fergusson, N. A. , & Sekhon, M. S. (2022). Neurologic prognostication after cardiac arrest using brain biomarkers: A systematic review and meta‐analysis. JAMA Neurology, 79, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga, A. , Laurà, M. , Jaunmuktane, Z. , Jerath, N. U. , Gonzalez, M. A. , Polke, J. M. , Poh, R. , Blake, J. C. , Liu, Y.‐T. , Wiethoff, S. , Bettencourt, C. , Lunn, M. P. , Manji, H. , Hanna, M. G. , Houlden, H. , Brandner, S. , Züchner, S. , Shy, M. , & Reilly, M. M. (2017). Genetic and clinical characteristics of NEFL‐related Charcot‐Marie‐Tooth disease. Journal of neurology . Neurosurgery; Psychiatry, 88, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck, P. V. , Zhang, B. , Murray, B. , Kornhauser, J. M. , Latham, V. , & Skrzypek, E. (2015). PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Research, 43, D512–D520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Yang, Y. , & Gong, D. (2017). Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to parkinson's disease: A meta‐analysis. Neurological sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 38, 407–414. [DOI] [PubMed] [Google Scholar]
- Huehnchen, P. , Schinke, C. , Bangemann, N. , Dordevic, A. D. , Kern, J. , Maierhof, S. K. , Hew, L. , Nolte, L. , Körtvelyessy, P. , Göpfert, J. C. , Ruprecht, K. , Somps, C. J. , Blohmer, J.‐U. , Sehouli, J. , Endres, M. , & Boehmerle, W. (2022). Neurofilament proteins as a potential biomarker in chemotherapy‐induced polyneuropathy. JCI Insight, 7, e154395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker, S. , Quinto, A. , Ramin‐Wright, M. , Becker, C. , Beck, K. , Vincent, A. , Tisljar, K. , Disanto, G. , Benkert, P. , Leppert, D. , Pargger, H. , Marsch, S. , Sutter, R. , Peters, N. , & Kuhle, J. (2021). Serum neurofilament measurement improves clinical risk scores for outcome prediction after cardiac arrest: Results of a prospective study. Critical Care, 25, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid, C. V. B. , Madsen, A. T. , & Winther‐Larsen, A. (2021). Biological variation of serum neurofilament light chain. Clinical Chemistry and Laboratory Medicine (CCLM), 60, 569–575. [DOI] [PubMed] [Google Scholar]
- Inoue, R. , Sumitani, M. , Ogata, T. , Chikuda, H. , Matsubara, T. , Kato, S. , Shimojo, N. , Uchida, K. , & Yamada, Y. (2017). Direct evidence of central nervous system axonal damage in patients with postoperative delirium: A preliminary study of pNF‐H as a promising serum biomarker. Neuroscience Letters, 653, 39–44. [DOI] [PubMed] [Google Scholar]
- Jabbari, E. , Holland, N. , Chelban, V. , Jones, P. S. , Lamb, R. , Rawlinson, C. , Guo, T. , Costantini, A. A. , Tan, M. M. X. , Heslegrave, A. J. , Roncaroli, F. , Klein, J. C. , Ansorge, O. , Allinson, K. S. J. , Jaunmuktane, Z. , Holton, J. L. , Revesz, T. , Warner, T. T. , Lees, A. J. , … Morris, H. R. (2020). Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurology, 77, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, S. L. , Theadom, A. , Ellenbogen, R. G. , Bannick, M. S. , Montjoy‐Venning, W. , Lucchesi, L. R. , Abbasi, N. , Abdulkader, R. , Abraha, H. N. , Adsuar, J. C. , Afarideh, M. , Agrawal, S. , Ahmadi, A. , Ahmed, M. B. , Aichour, A. N. , Aichour, I. , Aichour, M. T. E. , Akinyemi, R. O. , Akseer, N. , … Murray, C. J. L. (2019). Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990‐2016: A systematic analysis for the global burden of disease study 2016. The Lancet Neurology, 18, 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, S.‐N. , Nicholas, J. M. , Lane, C. A. , Parker, T. D. , Lu, K. , Keshavan, A. , Buchanan, S. M. , Keuss, S. E. , Murray‐Smith, H. , Wong, A. , Cash, D. M. , Malone, I. B. , Barnes, J. , Sudre, C. H. , Coath, W. , Prosser, L. , Ourselin, S. , Modat, M. , Thomas, D. L. , … Fox, N. C. (2021). A population‐based study of head injury, cognitive function and pathological markers. Annals of Clinical Translational Neurology, 8, 842–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey, P. A. , Leterrier, J.‐F. , & Herrmann, H. (2003). Assembly and structure of neurofilaments. Current Opinion in Colloid & Interface Science, 8, 40–47. [Google Scholar]
- Jin, M. , Cao, L. , & Dai, Y.‐P. (2019) Role of neurofilament light chain as a potential biomarker for Alzheimer's disease: A correlative meta‐analysis. [DOI] [PMC free article] [PubMed]
- Johnson, E. B. , Byrne, L. M. , Gregory, S. , Rodrigues, F. B. , Blennow, K. , Durr, A. , Leavitt, B. R. , Roos, R. A. , Zetterberg, H. , Tabrizi, S. J. , Scahill, R. I. , Wild, E. J. , & T.‐H. S. Group (2018). Neurofilament light protein in blood predicts regional atrophy in Huntington disease. Neurology, 90, e717–e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. B. , & Safinya, C. R. (2008). Interplay between liquid crystalline and isotropic gels in self‐assembled neurofilament networks. Biophysical Journal, 95, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, J. R. , Swallow, J. S. , Willsey, K. , Lapointe, A. P. , Khalatbari, S. , Korley, F. K. , Oppenlander, M. E. , Park, P. , Szerlip, N. J. , & Broglio, S. P. (2019). Elevated markers of brain injury as a result of clinically asymptomatic high‐acceleration head impacts in high‐school football athletes. Journal of Neurosurgery, 130, 1642–1648. [DOI] [PubMed] [Google Scholar]
- Julien, J. (2001). Amyotrophic lateral sclerosis unfolding the toxicity of the misfolded. Cell, 104, 581–591. [DOI] [PubMed] [Google Scholar]
- Kahouadji, S. , Bouillon‐Minois, J.‐B. , Oris, C. , Durif, J. , Pereira, B. , Pinguet, J. , Rozand, A. , Schmidt, J. , Sapin, V. , & Bouvier, D. (2022). Evaluation of serum neurofilament light in the early management of mTBI patients. Clinical Chemistry and Laboratory Medicine (CCLM), 60, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Kapoor, M. , Carr, A. S. , Foiani, M. S. , Heslegrave, A. J. , Zetterberg, H. , Malaspina, A. , Compton, L. , Hutton, E. , Rossor, A. , Reilly, M. M. , & Lunn, M. P. (2022). Association of plasma neurofilament light chain with disease activity in chronic inflammatory demyelinating polyradiculoneuropathy. European Journal of Neurology. 10.1111/ene.15496 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, M. , Foiani, M. , Heslegrave, A. , Zetterberg, H. , Lunn, M. P. , Malaspina, A. , Gillmore, J. D. , Rossor, A. M. , & Reilly, M. M. (2019). Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. Journal of the Peripheral Nervous System, 24, 314–319. [DOI] [PubMed] [Google Scholar]
- Karam, C. (2022). Chronic inflammatory demyelinating polyradiculoneuropathy: Five new things. Neurology: Clinical Practice, 12, 258–262. 10.1212/CPJ.0000000000001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantali, E. , Kazis, D. , Chatzikonstantinou, S. , Petridis, F. , & Mavroudis, I. (2021). The role of neurofilament light chain in frontotemporal dementia: A meta‐analysis. Aging Clinical and Experimental Research, 33, 869–881. [DOI] [PubMed] [Google Scholar]
- Karantali, E. , Kazis, D. , McKenna, J. , Chatzikonstantinou, S. , Petridis, F. , & Mavroudis, I. (2021). Neurofilament light chain in patients with a concussion or head impacts: A systematic review and meta‐analysis. European Journal of Trauma and Emergency Surgery: Official Publication of the European Trauma Society, 48, 1555–1567. [DOI] [PubMed] [Google Scholar]
- Katayama, T. , Sawada, J. , Takahashi, K. , & Yahara, O. (2020). Cerebrospinal fluid biomarkers in parkinson's disease: A critical overview of the literature and meta‐analyses. Brain Sciences, 10, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katisko, K. , Cajanus, A. , Jääskeläinen, O. , Kontkanen, A. , Hartikainen, P. , Korhonen, V. E. , Helisalmi, S. , Haapasalo, A. , Koivumaa‐Honkanen, H. , Herukka, S.‐K. , Remes, A. M. , & Solje, E. (2019). Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. Journal of Neurology, 267, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, M. , Teunissen, C. E. , Otto, M. , Piehl, F. , Sormani, M. P. , Gattringer, T. , Barro, C. , Kappos, L. , Comabella, M. , Fazekas, F. , Petzold, A. , Blennow, K. , Zetterberg, H. , & Kuhle, J. (2018). Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology, 14, 577–589. [DOI] [PubMed] [Google Scholar]
- Khan, M. I. , Hasan, F. , Mahmud, K. A. H. A. , & Adnan, A. (2020). Recent computational approaches on mechanical behavior of axonal cytoskeletal components of neuron: A brief review. Multiscale Science and Engineering, 2, 199–213. [Google Scholar]
- Kikuchi, S. (2003). Glycation ‐ a sweet tempter for neuronal death. Brain Research Reviews, 41, 306–323. [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Kim, S. B. , Kim, H. S. , Kwon, H. M. , Park, J. H. , Lee, A. J. , Lim, S. O. , Lim, S. , Nam, H. , Hong, Y. B. , Chung, K. W. , & Choi, B.‐O. (2022). Phenotypic heterogeneity in patients with NEFL‐related Charcot Marie Tooth disease. Molecular Genetics & Genomic Medicine, 10, e1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. , Lee, C. H. , & Park, C. B. (2020). Chemical sensing platforms for detecting trace‐level Alzheimers core biomarkers. Chemical Society Reviews, 49, 5446–5472. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Chang, R. , Teunissen, C. , Gebremichael, Y. , & Petzold, A. (2011). Neurofilament stoichiometry simulations during neurodegeneration suggest a remarkable self‐sufficient and stable in vivo protein structure. Journal of the Neurological Sciences, 307, 132–138. [DOI] [PubMed] [Google Scholar]
- Kim, S.‐H. , Choi, M. K. , Park, N. Y. , Hyun, J.‐W. , Lee, M. Y. , Kim, H. J. , Jung, S. K. , & Cha, Y. (2020). Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin‐induced peripheral neuropathy. Scientific Reports, 10, 7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmezic, I. , Samuelsson, K. , Finn, A. , Upate, Z. , Blennow, K. , Zetterberg, H. , & Press, R. (2022). Neurofilament light chain and total tau in the differential diagnosis and prognostic evaluation of acute and chronic inflammatory polyneuropathies. European Journal of Neurology, 29, 2810–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koel‐Simmelink, M. J. A. , Teunissen, C. E. , Behradkia, P. , Blankenstein, M. A. , & Petzold, A. (2011). The neurofilament light chain is not stable in vitro. Annals of Neurology, 69, 1065–1066. [DOI] [PubMed] [Google Scholar]
- Kong, L. , Valdivia, D. O. , Simon, C. M. , Hassinan, C. W. , Delestrée, N. , Ramos, D. M. , Park, J. H. , Pilato, C. M. , Xu, X. , Crowder, M. , Grzyb, C. C. , King, Z. A. , Petrillo, M. , Swoboda, K. J. , Davis, C. , Lutz, C. M. , Stephan, A. H. , Zhao, X. , Weetall, M. , … & Sumner, C. J. (2021). Impaired prenatal motor axon development necessitates early therapeutic intervention in severe SMA. Science Translational Medicine, 13, eabb6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körtvelyessy, P. , Kuhle, J. , Düzel, E. , Vielhaber, S. , Schmidt, C. , Heinius, A. , Leypoldt, F. , Schraven, B. , Reinhold, D. , Leppert, D. , & Goihl, A. (2020). Ratio and index of neurofilament light chain indicate its origin in guillain‐barré syndrome. Annals of Clinical Translational Neurology, 7, 2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koychev, I. , Jansen, K. , Dette, A. , Shi, L. , & Holling, H. (2021). Blood‐based ATN biomarkers of Alzheimer's disease: A meta‐analysis. Journal of Alzheimer's disease: JAD, 79, 177–195. [DOI] [PubMed] [Google Scholar]
- Krüger, R. , Fischer, C. , Schulte, T. , Strauss, K. M. , Müller, T. , Woitalla, D. , Berg, D. , Hungs, M. , Gobbele, R. , Berger, K. , Epplen, J. T. , Riess, O. , & Schöls, L. (2003). Mutation analysis of the neurofilament m gene in Parkinsons disease. Neuroscience Letters, 351, 125–129. [DOI] [PubMed] [Google Scholar]
- Kuhle, J. , Barro, C. , Andreasson, U. , Derfuss, T. , Lindberg, R. , Sandelius, Å. , Liman, V. , Norgren, N. , Blennow, K. , & Zetterberg, H. (2016). Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and SIMOA. Clinical Chemistry and Laboratory Medicine, 54, 1655–1661. [DOI] [PubMed] [Google Scholar]
- Kuhle, J. , Gaiottino, J. , Leppert, D. , Petzold, A. , Bestwick, J. P. , Malaspina, A. , Lu, C.‐H. , Dobson, R. , Disanto, G. , Norgren, N. , Nissim, A. , Kappos, L. , Hurlbert, J. , Yong, V. W. , Giovannoni, G. , & Casha, S. (2015). Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. Journal of Neurology, Neurosurgery, and Psychiatry, 86, 273–279. [DOI] [PubMed] [Google Scholar]
- Kuhle, J. , & Petzold, A. (2011). What makes a prognostic biomarker in CNS diseases: Strategies for targeted biomarker discovery? Part 1: Acute and monophasic diseases. Expert Opinion on Medical Diagnostics, 5, 333–346. [DOI] [PubMed] [Google Scholar]
- Kuhle, J. , Regeniter, A. , Leppert, D. , Mehling, M. , Kappos, L. , Lindberg, R. , & Petzold, A. (2010). A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. Journal of Neuroimmunology, 220, 114–119. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Yin, X. , Trapp, B. D. , Hoh, J. H. , & Paulaitis, M. E. (2002). Relating interactions between neurofilaments to the structure of axonal neurofilament distributions through polymer brush models. Biophysical Journal, 82, 2360–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labenz, C. , Nagel, M. , Kämper, P. , Engel, S. , Bittner, S. , Kaps, L. , Galle, P. R. , Schattenberg, J. M. , Wörns, M.‐A. , & Lüssi, F. (2021). Association between serum levels of neurofilament light chains and minimal hepatic encephalopathy in patients with liver cirrhosis. Clinical and Translational Gastroenterology, 12, e00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoursiere, R. E. , Hadi, D. , & Shaw, G. S. (2022). Acetylation, phosphorylation, ubiquitination (oh my!): Following post‐translational modifications on the ubiquitin road. Biomolecules, 12, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman, J. D. , Huizinga, R. , Boons, G.‐J. , & Jacobs, B. C. (2022). Guillain‐barré syndrome: Expanding the concept of molecular mimicry. Trends in Immunology, 43, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek, R. J. (1967). Bidirectional transport of radioactively labelled axoplasmic components. Nature, 216, 1212–1214. [DOI] [PubMed] [Google Scholar]
- Lasek, R. J. , Oblinger, M. M. , & Drake, P. F. (1983). Molecular biology of neuronal geometry: Expression of neurofilament genes influences axonal diameter. Cold Spring Harbor Symposia on Quantitative Biology, 48(Pt 2), 731–744. [DOI] [PubMed] [Google Scholar]
- Lasek, R. J. , Phillips, L. , Katz, M. J. , & Autilio‐Gambetti, L. (1985). Function and evolution of neurofilament proteins. Annals of the New York Academy of Sciences, 455, 462–478. [DOI] [PubMed] [Google Scholar]
- Lavedan, C. , Buchholtz, S. , Nussbaum, R. L. , Albin, R. L. , & Polymeropoulos, M. H. (2002). A mutation in the human neurofilament m gene in Parkinson's Disease that suggests a role for the cytoskeleton in neuronal degeneration. Neuroscience Letters, 322, 57–61. [DOI] [PubMed] [Google Scholar]
- Lawson, S. N. , Perry, M. J. , Prabhakar, E. , & McCarthy, P. W. (1993). Primary sensory neurones: Neurofilament, neuropeptides and conduction velocity. Brain Research Bulletin, 30, 239–243. [DOI] [PubMed] [Google Scholar]
- Lee, M. , & Cleveland, D. (1996). Neuronal intermediate filaments. Annual Review of Neuroscience (Palo Alto, CA), 19, 187–217. [DOI] [PubMed] [Google Scholar]
- Lee, T. , Chikkabyrappa, S. M. , Reformina, D. , Mastrippolito, A. , Chakravarti, S. B. , Mosca, R. S. , Shaw, G. , & Malhotra, S. P. (2018). Ubiquitin c‐terminal hydrolase 1 and phosphorylated axonal neurofilament heavy chain in infants undergoing cardiac surgery: Preliminary assessment as potential biomarkers of brain injury. World Journal for Pediatric and Congenital Heart Surgery, 9, 412–418. [DOI] [PubMed] [Google Scholar]
- Lee, Y. M. , He, W. , & Liou, Y.‐C. (2021). The redox language in neurodegenerative diseases: Oxidative post‐translational modifications by hydrogen peroxide. Cell Death & Disease, 12, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister, I. , Altendorfer, B. , Maier, D. , Mach, O. , Wutte, C. , Grillhösl, A. , Arevalo‐Martin, A. , Garcia‐Ovejero, D. , Aigner, L. , & Grassner, L. (2021). Serum levels of glial fibrillary acidic protein and neurofilament light protein are related to the neurological impairment and spinal edema after traumatic spinal cord injury. Journal of Neurotrauma, 38, 3431–3439. [DOI] [PubMed] [Google Scholar]
- Leung, C. L. , He, C. Z. , Kaufmann, P. , Chin, S. S. , Naini, A. , Liem, R. K. H. , Mitsumoto, H. , & Hays, A. P. (2006). A pathogenic peripherin gene mutation in a patient with amyotrophic lateral sclerosis. Brain Pathology, 14, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , & Mielke, M. M. (2019). An update on blood‐based markers of Alzheimer's disease using the SiMoA platform. Neurology and Therapy, 8, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. , Shen, D. , Tai, H. , & Cui, L. (2016). Neurofilaments in CSF as diagnostic biomarkers in motor neuron disease: A meta‐analysis. Frontiers in Aging Neuroscience, 8, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Gu, Y. , An, H. , Zhou, Z. , Zheng, D. , Wang, Z. , Wen, Z. , Shen, H.‐Y. , Wang, Q. , & Wang, H. (2019). Cerebrospinal fluid light and heavy neurofilament level increased in anti‐N‐methyl‐d‐aspartate receptor encephalitis. Brain and Behavior: A Cognitive Neuroscience Perspective, 9, e01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Bai, L. , Yu, H. , Cai, D. , Wang, X. , Huang, B. , Peng, S. , Huang, M. , Cao, G. , Kaz, A. M. , Grady, W. M. , Wang, J. , & Luo, Y. (2020). Epigenetic inactivation of ‐internexin accelerates microtubule polymerization in colorectal cancer. Cancer Research, 80, 5203–5215. [DOI] [PubMed] [Google Scholar]
- Lie, I. A. , Kaçar, S. , Wesnes, K. , Brouwer, I. , Kvistad, S. S. , Wergeland, S. , Holmøy, T. , Midgard, R. , Bru, A. , Edland, A. , Eikeland, R. , Gosal, S. , Harbo, H. F. , Kleveland, G. , Sørenes, Y. S. , Øksendal, N. , Varhaug, K. N. , Vedeler, C. A. , Barkhof, F. , & Vrenken, H. (2022) Serum neurofilament as a predictor of 10‐year grey matter atrophy and clinical disability in multiple sclerosis: A longitudinal study. Journal of Neurology, Neurosurgery, and Psychiatry, 93, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieverloo, G. G. A. , Wieske, L. , Verhamme, C. , Vrancken, A. F. J. , Doorn, P. A. , Michalak, Z. , Barro, C. , Schaik, I. N. , Kuhle, J. , & Eftimov, F. (2019). Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. Journal of the Peripheral Nervous System, 24, 187–194. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐H. , Li, C.‐H. , Yang, K.‐C. , Lin, F.‐J. , Wu, C.‐C. , Chieh, J.‐J. , & Chiu, M.‐J. (2019). Blood NfL: A biomarker for disease severity and progression in parkinson disease. Neurology, 93, e1104–e1111. [DOI] [PubMed] [Google Scholar]
- Lin, F. , Lin, W. , Zhu, C. , Lin, J. , Zhu, J. , Li, X.‐Y. , Wang, Z. , Wang, C. , & Huang, H. (2021). Sequencing of neurofilament genes identified NEFH Ser787Arg as a novel risk variant of sporadic amyotrophic lateral sclerosis in chinese subjects. BMC Medical Genomics, 14, e1104–e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W.‐C. , Lu, C.‐H. , Chiu, P.‐Y. , & Yang, S.‐Y. (2020). Plasma total ‐synuclein and neurofilament light chain: Clinical validation for discriminating parkinson's disease from normal control. Dementia and Geriatric Cognitive Disorders, 49, 401–409. [DOI] [PubMed] [Google Scholar]
- Lin, X. , Lu, T. , Deng, H. , Liu, C. , Yang, Y. , Chen, T. , Qin, Y. , Xie, X. , Xie, Z. , Liu, M. , & Ouyang, M. (2021). Serum neurofilament light chain or glial fibrillary acidic protein in the diagnosis and prognosis of brain metastases. Journal of Neurology, 269, 815–823. [DOI] [PubMed] [Google Scholar]
- Liu, D. , Chen, J. , Wang, X. , Xin, J. , Cao, R. , & Yang, S.‐Y. (2020). Serum neurofilament light chain as a predictive biomarker for ischemic stroke outcome: A systematic review and meta‐analysis. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 29, 104813. [DOI] [PubMed] [Google Scholar]
- Liu, H.‐C. , Lin, W.‐C. , Chiu, M.‐J. , Lu, C.‐H. , Lin, C.‐Y. , & Yang, S.‐Y. (2020). Development of an assay of plasma neurofilament light chain utilizing immunomagnetic reduction technology. PLoS One, 15, e0234519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Sun, M. , Zhang, W. , Sun, J. , Gong, P. , Wang, H. , & Wang, M. (2022). Prognostic value of neurofilament light chain in natalizumab therapy for different phases of multiple sclerosis: A systematic review and meta‐analysis. Journal of Clinical Neuroscience, 101, 198–203. [DOI] [PubMed] [Google Scholar]
- Liu, Y.‐L. , Bavato, F. , Chung, A.‐N. , Liu, T.‐H. , Chen, Y.‐L. , Huang, M.‐C. , & Quednow, B. B. (2021). Neurofilament light chain as novel blood biomarker of disturbed neuroaxonal integrity in patients with ketamine dependence. The World Journal of Biological Psychiatry, 22, 713–721. [DOI] [PubMed] [Google Scholar]
- Loonstra, F. C. , Verberk, I. M. W. , Wijburg, M. T. , Wattjes, M. P. , Teunissen, C. E. , Oosten, B. W. , Uitdehaag, B. M. J. , Killestein, J. , & Kempen, Z. L. E. (2019). Serum neurofilaments as candidate biomarkers of natalizumab‐PML. In Annals of neurology (Vol. 86, pp. 322–324). Wiley. [DOI] [PubMed] [Google Scholar]
- Lorenzo, R. D. , Loré, N. I. , Finardi, A. , Mandelli, A. , Cirillo, D. M. , Tresoldi, C. , Benedetti, F. , Ciceri, F. , Rovere‐Querini, P. , Comi, G. , Filippi, M. , Manfredi, A. A. , & Furlan, R. (2021). Blood neurofilament light chain and total tau levels at admission predict death in COVID‐19 patients. Journal of Neurology, 268, 4436–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwsma, J. , Brunger, A. F. , Bijzet, J. , Kroesen, B. J. , Roeloffzen, W. W. H. , Bischof, A. , Kuhle, J. , Drost, G. , Lange, F. , Kuks, J. B. , & Gans, R. O. (2020). Neurofilament light chain, a biomarker for polyneuropathy in systemic amyloidosis. Amyloid, 28, 50–55. [DOI] [PubMed] [Google Scholar]
- Lu, C.‐H. , Kalmar, B. , Malaspina, A. , Greensmith, L. , & Petzold, A. (2011). A method to solubilise protein aggregates for immunoassay quantification which overcomes the neurofilament hook effect. Journal of Neuroscience Methods, 195, 143–150. [DOI] [PubMed] [Google Scholar]
- Lu, C.‐H. , Macdonald‐Wallis, C. , Gray, E. , Pearce, N. , Petzold, A. , Norgren, N. , Giovannoni, G. , Fratta, P. , Sidle, K. , Fish, M. , Orrell, R. , Howard, R. , Talbot, K. , Greensmith, L. , Kuhle, J. , Turner, M. R. , & Malaspina, A. (2015). Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology, 84, 2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, M. , Ferguson, T. A. , Schoch, K. M. , Li, J. , Qian, Y. , Shofer, F. S. , Saatman, K. E. , & Neumar, R. W. (2013). Calpains mediate axonal cytoskeleton disintegration during Wallerian degeneration. Neurobiology of Disease, 56, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciotta, S. , Meregalli, M. , & Torrente, Y. (2013). The involvement of microRNAs in neurodegenerative diseases. Frontiers in Cellular Neuroscience, 7, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacTaggart, B. , & Kashina, A. (2021). Posttranslational modifications of the cytoskeleton. Cytoskeleton, 78, 142–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi, P. , Kuhle, J. , Schädelin, S. , van der Meer, F. , Weigel, M. , Galbusera, R. , Mathias, A. , Lu, P.‐J. , Rahmanzadeh, R. , Benkert, P. , Rosa, F. L. , Cuadra, M. B. , Sati, P. , Théaudin, M. , Pot, C. , van Pesch, V. , Leppert, D. , Stadelmann, C. , Kappos, L. , … Granziera, C. (2021). Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology, 97, e543–e553. 10.1212/WNL.0000000000012326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi, R. , Fadda, G. , Brown, R. A. , Bar‐Or, A. , Howell, O. W. , Hametner, S. , Marastoni, D. , Poli, A. , Nicholas, R. , Calabrese, M. , Monaco, S. , & Reynolds, R. (2022). “Ependymal‐in” gradient of thalamic damage in progressive multiple sclerosis. Annals of Neurology. 10.1002/ana.26448 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniero, C. , Garg, S. , Zhao, W. , Johnson, T. I. , Zhou, J. , Gurnell, M. , & Brown, M. J. (2017) NEFM (neurofilament medium) polypeptide, a marker for zona glomerulosa cells in human adrenal, inhibits D1R (dopamine D1 receptor)–mediated secretion of aldosterone. Hypertension, 70, 357–364. [DOI] [PubMed] [Google Scholar]
- Manouchehrinia, A. , Piehl, F. , Hillert, J. , Kuhle, J. , Alfredsson, L. , Olsson, T. , & Kockum, I. (2020). Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Annals of Clinical Translational Neurology, 7, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manouchehrinia, A. , Stridh, P. , Khademi, M. , Leppert, D. , Barro, C. , Michalak, Z. , Benkert, P. , Lycke, J. , Alfredsson, L. , Kappos, L. , Piehl, F. , Olsson, T. , Kuhle, J. , & Kockum, I. (2020). Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology, 94, e2457–e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignier, R. , Hacohen, Y. , Cobo‐Calvo, A. , Pröbstel, A.‐K. , Aktas, O. , Alexopoulos, H. , Amato, M.‐P. , Asgari, N. , Banwell, B. , Bennett, J. , & Brilot, F. (2021). Myelin‐oligodendrocyte glycoprotein antibody‐associated disease. The Lancet Neurology, 20, 762–772. [DOI] [PubMed] [Google Scholar]
- Mariotto, S. , Farinazzo, A. , Magliozzi, R. , Alberti, D. , Monaco, S. , & Ferrari, S. (2018). Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. Journal of the Peripheral Nervous System, 23, 174–177. [DOI] [PubMed] [Google Scholar]
- Mariotto, S. , Ferrari, S. , Gastaldi, M. , Franciotta, D. , Sechi, E. , Capra, R. , Mancinelli, C. , Schanda, K. , Alberti, D. , Orlandi, R. , Bombardi, R. , Zuliani, L. , Zoccarato, M. , Benedetti, M. D. , Tanel, R. , Calabria, F. , Rossi, F. , Pavone, A. , Grazian, L. , … Gajofatto, A. (2019) Neurofilament light chain serum levels reflect disease severity in MOG‐AB associated disorders . BMJ, England. [DOI] [PubMed]
- Marszalek, J. R. , Williamson, T. L. , Lee, M. K. , Xu, Z. , Hoffman, P. N. , Becher, M. W. , Crawford, T. O. , & Cleveland, D. W. (1996). Neurofilament subunit NF‐H modulates axonal diameter by selectively slowing neurofilament transport. The Journal of Cell Biology, 135, 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S.‐J. , McGlasson, S. , Hunt, D. , & Overell, J. (2019). Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: A meta‐analysis of case‐control studies. Journal of Neurology, Neurosurgery, and Psychiatry, 90, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Aguilar, L. , Camps‐Renom, P. , Lleixà, C. , Pascual‐Goñi, E. , Díaz‐Manera, J. , Rojas‐García, R. , Luna, N. D. , Gallardo, E. , Cortés‐Vicente, E. , Muñoz, L. , Alcolea, D. , Lleó, A. , Casasnovas, C. , Homedes, C. , Gutiérrez‐Gutiérrez, G. , Jimeno‐Montero, M. C. , Berciano, J. , Sedano‐Tous, M. J. , Garcıa‐Sobrino, T. , … Querol, L. (2020). Serum neurofilament light chain predicts long‐term prognosis in guillain‐barré syndrome patients. Journal of Neurology, Neurosurgery & Psychiatry, 92, 70–77. [DOI] [PubMed] [Google Scholar]
- McCampbell, A. , Cole, T. , Wegener, A. J. , Tomassy, G. S. , Setnicka, A. , Farley, B. J. , Schoch, K. M. , Hoye, M. L. , Shabsovich, M. , Sun, L. , Luo, Y. , Zhang, M. , Comfort, R. I. , Tabrizi, N. , Wang, B. , Amacker, J. , Thankamony, S. , Salzman, D. W. , Cudkowicz, M. , Miller, T. M. (2018). Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. Journal of Clinical Investigation, 128, 3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColgan, P. , Gregory, S. , Zeun, P. , Zarkali, A. , Johnson, E. B. , Parker, C. , Fayer, K. , Lowe, J. , Nair, A. , Estevez‐Fraga, C. , Papoutsi, M. , Zhang, H. , Scahill, R. I. , Tabrizi, S. J. , & Rees, G. (2022). Neurofilament light associated connectivity in young‐adult huntington's disease is related to neuronal genes. Brain. 10.1093/brain/awac227 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana, I. M. , Day, N. P. , Hien, T. T. , Mai, N. T. H. , Bethell, D. , Phu, N. H. , Farrar, J. , Esiri, M. M. , White, N. J. , & Turner, G. D. (2002). Axonal injury in cerebral malaria. The American Journal of Pathology, 160, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren, A. , Price, R. W. , Hagberg, L. , Rosengren, L. , Brew, B. J. , & Gisslen, M. (2007). Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV‐1 infection. Neurology, 69, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Meyer, M. , Wiberg, S. , Grand, J. , Obling, L. , Isse, Y. , Frikke‐Schmidt, R. , Kjaergaard, J. , & Hassager, C. (2022). Performance of neuron‐specific enolase and neurofilament light chain in predicting death after hospital admittance from out‐of‐hospital cardiac arrest ‐ a sub study of the IMICA trial. European Heart Journal Acute Cardiovascular Care, 11, zuac041.164. [Google Scholar]
- Mietani, K. , Hasegawa‐Moriyama, M. , Yagi, K. , Inoue, R. , Ogata, T. , Kurano, M. , Shimojo, N. , Seto, Y. , Sumitani, M. , & Uchida, K. (2022). Preoperative detection of serum phosphorylated neurofilament heavy chain subunit predicts postoperative delirium: A prospective observational study. Journal of Gerontology and Geriatrics, 1–9. 10.36150/2499-6564-N488 [ePub ahead of print]. [DOI] [Google Scholar]
- Mietani, K. , Sumitani, M. , Ogata, T. , Shimojo, N. , Inoue, R. , Abe, H. , Kawamura, G. , & Yamada, Y. (2019). Dysfunction of the blood‐brain barrier in postoperative delirium patients, referring to the axonal damage biomarker phosphorylated neurofilament heavy subunit. PLoS One, 14, e0222721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A.‐M. , Rutkowska, A. , Bahl, J. M. , Herukka, S.‐K. , Koel‐Simmelink, M. J. , Kruse, N. , Mollenhauer, B. , Siloaho, M. , Skinningsrud, A. , Zetterberg, H. , Teunissen, C. E. , & Lawlor, B. A. (2016). Multicenter immunoassay validation of cerebrospinal fluid neurofilament light: A biomarker for neurodegeneration. Bioanalysis, 8, 2243–2254. [DOI] [PubMed] [Google Scholar]
- Miller, T. , Cudkowicz, M. , Shaw, P. J. , Andersen, P. M. , Atassi, N. , Bucelli, R. C. , Genge, A. , Glass, J. , Ladha, S. , Ludolph, A. L. , Maragakis, N. J. , McDermott, C. J. , Pestronk, A. , Ravits, J. , Salachas, F. , Trudell, R. , Damme, P. V. , Zinman, L. , Bennett, C. F. , … Ferguson, T. A. (2020). Phase 12 trial of antisense oligonucleotide tofersen for SOD1 ALS. New England Journal of Medicine, 383, 109–119. [DOI] [PubMed] [Google Scholar]
- Millere, E. , Rots, D. , Simrén, J. , Ashton, N. J. , Kupats, E. , Micule, I. , Priedite, V. , Kurjane, N. , Blennow, K. , Gailite, L. , Zetterberg, H. , & Kenina, V. (2021). Plasma neurofilament light chain as a potential biomarker in Charcot‐Marie‐Tooth disease. European Journal of Neurology, 28, 974–981. [DOI] [PubMed] [Google Scholar]
- Mirdita, M. , Schütze, K. , Moriwaki, Y. , Heo, L. , Ovchinnikov, S. , & Steinegger, M. (2021) ColabFold—Making protein folding accessible to all. [DOI] [PMC free article] [PubMed]
- Miyazawa, I. , Nakashima, I. , Petzold, A. , Fujihara, K. , Sato, S. , & Itoyama, Y. (2007). High CSF neurofilament heavy chain levels in neuromyelitis optica. Neurology, 68, 865–867. [DOI] [PubMed] [Google Scholar]
- Mizenko, C. , Bennett, J. L. , Owens, G. , Vollmer, T. L. , & Piquet, A. L. (2021). A longitudinal, observational analysis of neuronal injury biomarkers in a case report of a patient with paraneoplastic anti‐CRMP5 antibody‐associated transverse myelitis, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco, S. , Autilio‐Gambetti, L. , Lasek, R. J. , Katz, M. J. , & Gambetti, P. (1989). Experimental increase of neurofilament transport rate: Decreases in neurofilament number and in axon diameter. Journal of Neuropathology and Experimental Neurology, 48, 23–32. [DOI] [PubMed] [Google Scholar]
- Mondal, S. , & Thompson, P. R. (2021). Chemical biology of protein citrullination by the protein a arginine deiminases. Current Opinion in Chemical Biology, 63, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello, S. , Sorinola, A. , Czeiter, E. , Vámos, Z. , Amrein, K. , Synnot, A. , Donoghue, E. , Sándor, J. , Wang, K. K. W. , Diaz‐Arrastia, R. , Steyerberg, E. W. , Menon, D. K. , Maas, A. I. R. , & Buki, A. (2021). Blood‐based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: A living systematic review and meta‐analysis. Journal of Neurotrauma, 38, 1086–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S. P. , Balana, A. T. , & Pratt, M. R. (2021). Consequences of post‐translational modifications on amyloid proteins as revealed by protein semisynthesis. Current Opinion in Chemical Biology, 64, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso, A. , Grothe, M. J. , Ashton, N. J. , Karikari, T. K. , Rodríguez, J. L. , Snellman, A. , Suárez‐Calvet, M. , Blennow, K. , Zetterberg, H. , Schöll, M. , Weiner, M. W. , Aisen, P. , Petersen, R. , Jack, J. C. R. , Jagust, W. , Trojanowki, J. Q. , Toga, A. W. , Beckett, L. , Green, R. C. , … Hsiao, J. (2021) Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurology 78, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseby‐Knappe, M. , Mattsson, N. , Nielsen, N. , Zetterberg, H. , Blennow, K. , Dankiewicz, J. , Dragancea, I. , Friberg, H. , Lilja, G. , Insel, P. S. , Rylander, C. , Westhall, E. , Kjaergaard, J. , Wise, M. P. , Hassager, C. , Kuiper, M. A. , Stammet, P. , Wanscher, M. C. J. , Wetterslev, J. , … Cronberg, T. (2019). Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurology, 76, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, K. A. , Hughes, M. P. , Hu, C. J. , Sawaya, M. R. , Salwinski, L. , Pan, H. , French, S. W. , Seidler, P. M. , & Eisenberg, D. S. (2022). Identifying amyloid‐related diseases by mapping mutations in low‐complexity protein domains to pathologies. Nature Structural & Molecular Biology, 29, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murueta‐Goyena, A. , Cipriani, R. , Carmona‐Abellán, M. , Acera, M. , Ayo, N. , Pino, R. D. , Tijero, B. , Fernández‐Valle, T. , Gabilondo, I. , Zallo, F. , Matute, C. , Sánchez‐Pernaute, R. , Khurana, V. , Cavaliere, F. , Capetillo‐Zarate, E. , & Gómez‐Esteban, J. C. (2022). Characterization of molecular biomarkers in cerebrospinal fluid and serum of E46K‐SNCA mutation carriers. Parkinsonism & Related Disorders, 96, 29–35. [DOI] [PubMed] [Google Scholar]
- Mutalik, S. P. , & Ghose, A. (2020). Axonal cytomechanics in neuronal development. Journal of Biosciences, 45, 64. [PubMed] [Google Scholar]
- Newcombe, V. F. J. , Ashton, N. J. , Posti, J. P. , Glocker, B. , Manktelow, A. , Chatfield, D. A. , Winzeck, S. , Needham, E. , Correia, M. M. , Williams, G. B. , Simrén, J. , Takala, R. S. K. , Katila, A. J. , Maanpää, H.‐R. , Tallus, J. , Frantzén, J. , Blennow, K. , Tenovuo, O. , Zetterberg, H. , & Menon, D. K. (2022). Post‐acute blood biomarkers and disease progression in traumatic brain injury. Brain, 145, 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti, T. F. , Rossi, S. , Vita, M. G. , Perna, A. , Guerrera, G. , Lino, F. , Iacovelli, C. , Natale, D. D. , Modoni, A. , Battistini, L. , & Silvestri, G. (2022). Elevated serum neurofilament light chain (NfL) as a potential biomarker of neurological involvement in myotonic dystrophy type 1 (DM1). Journal of Neurology, 269, 5086–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, M. K. , Subhi, Y. , Molbech, C. R. , Sellebjerg, F. , & Sørensen, T. L. (2019). Serum neurofilament light chain in healthy elderly and in patients with age‐related macular degeneration. Acta Ophthalmologica, 98, e393–e394. [DOI] [PubMed] [Google Scholar]
- NIH (2022) Gene .
- Nilsson, I. A. K. , Millischer, V. , Karrenbauer, V. D. , Juréus, A. , Salehi, A. M. , Norring, C. , Hausswolff‐Juhlin, Y. v. , Schalling, M. , Blennow, K. , Bulik, C. M. , Zetterberg, H. , & Landén, M. (2019). Plasma neurofilament light chain concentration is increased in anorexia nervosa. Translational Psychiatry, 9, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz, E. , Smitka, M. , Schallner, J. , Akgün, K. , Ziemssen, T. , Hagen, M. , & Tüngler, V. (2021). Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Annals of Clinical Translational Neurology, 8, 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon, R. A. , & Logvinenko, K. B. (1986). Multiple fates of newly synthesized neurofilament proteins: Evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. The Journal of Cell Biology, 102, 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon, R. A. , & Sihag, R. K. (1991). Neurofilament phosphorylation: A new look at regulation and function. Trends in Neurosciences, 14, 501–506. [DOI] [PubMed] [Google Scholar]
- Nordström, E. B. , Lilja, G. , Ullén, S. , Blennow, K. , Friberg, H. , Hassager, C. , Kjærgaard, J. , Mattsson‐Carlgren, N. , Moseby‐Knappe, M. , Nielsen, N. , Vestberg, S. , Zetterberg, H. , & Cronberg, T. (2022). Serum neurofilament light levels are correlated to long‐term neurocognitive outcome measures after cardiac arrest. Brain Injury, 36, 800–809. [DOI] [PubMed] [Google Scholar]
- Norgren, N. , Karlsson, J.‐E. , Rosengren, L. , & Stigbrand, T. (2002). Monoclonal antibodies selective for low molecular weight neurofilaments. Hybridoma and Hybridomics, 21, 53–59. [DOI] [PubMed] [Google Scholar]
- Norgren, N. , Rosengren, L. , & Stigbrand, T. (2003). Elevated neurofilament levels in neurological diseases. Brain Research, 987, 25–31. [DOI] [PubMed] [Google Scholar]
- Orhun, G. , Esen, F. , Yilmaz, V. , Ulusoy, C. , Şanlı, E. , Yıldırım, E. , Gürvit, H. , Özcan, P. E. , Sencer, S. , Bebek, N. , & Tüzün, E. (2021) Elevated sTREM2 and NFL levels in patients with sepsis associated encephalopathy. 1–7. [DOI] [PubMed]
- Otani, N. , Morimoto, Y. , Kinoshita, M. , Ogata, T. , Mori, K. , Kobayashi, M. , Maeda, T. , & Yoshino, A. (2020). Serial changes in serum phosphorylated neurofilament and value for prediction of clinical outcome after traumatic brain injury. Surgical Neurology International, 11, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter, J. S. , & Liem, R. K. H. (1984). The differential appearance of neurofilament triplet polypeptides in the developing rat optic nerve. Developmental Biology, 103, 200–210. [DOI] [PubMed] [Google Scholar]
- Paganoni, S. , Macklin, E. A. , Hendrix, S. , Berry, J. D. , Elliott, M. A. , Maiser, S. , Karam, C. , Caress, J. B. , Owegi, M. A. , Quick, A. , Wymer, J. , Goutman, S. A. , Heitzman, D. , Heiman‐Patterson, T. , Jackson, C. E. , Quinn, C. , Rothstein, J. D. , Kasarskis, E. J. , Katz, J. , … Cudkowicz, M. E. (2020). Trial of sodium phenylbutyratetaurursodiol for amyotrophic lateral sclerosis. New England Journal of Medicine, 383, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakh, S. , Spencer, D. M. , Halloran, M. A. , Soo, K. Y. , & Atkin, J. D. (2013). Redox regulation in amyotrophic lateral sclerosis. Oxidative Medicine and Cellular Longevity, 2013, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, A. , Bora, P. , Parolo, S. , Monine, M. , Tong, X. , Eraly, S. , Masson, E. , Ferguson, T. , McCampbell, A. , Graham, D. , Domenici, E. , Nestorov, I. , & Marchetti, L. (2022). An age‐dependent mathematical model of neurofilament trafficking in healthy conditions. CPT: Pharmacometrics & Systems Pharmacology, 11, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin, G. M. , Corey‐Bloom, J. , Snell, C. , Castleton, J. , & Thomas, E. A. (2021). Plasma neurofilament light in huntingtons disease: A marker for disease onset, but not symptom progression. Parkinsonism & Related Disorders, 87, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasol, J. , Feuer, W. , Yang, C. , Shaw, G. , Kardon, R. , & Guy, J. (2010). Phosphorylated neurofilament heavy chain correlations to visual function, optical coherence tomography, and treatment. Multiple Sclerosis International, 542691. 10.1155/2010/542691 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattinson, C. L. , Meier, T. B. , Guedes, V. A. , Lai, C. , Devoto, C. , Haight, T. , Broglio, S. P. , McAllister, T. , Giza, C. , Huber, D. , Harezlak, J. , Cameron, K. , McGinty, G. , Jackson, J. , Guskiewicz, K. , Mihalik, J. , Brooks, A. , Duma, S. , Rowson, S. , … Gill, J. M. (2020). Plasma biomarker concentrations associated with return to sport following sport‐related concussion in collegiate athletesa concussion assessment, research, and education (CARE) consortium study. JAMA Network Open, 3, e2013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlitzki, M. , Butryn, M. , Kirchner, F. , Färber, J. , Beuing, O. , Minnerup, J. , Meuth, S. G. , & Neumann, J. (2019). CSF neurofilament light chain level predicts axonal damage in cerebral vasculitis. Annals of Clinical Translational Neurology, 6, 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. , Wang, S. , Chen, Z. , Peng, Y. , Wang, C. , Long, Z. , Peng, H. , Shi, Y. , Hou, X. , Lei, L. , Wan, L. , Liu, M. , Zou, G. , Shen, L. , Xia, K. , Qiu, R. , Tang, B. , Ashizawa, T. , Klockgether, T. , & Jiang, H. (2022). Blood neurofilament light chain in genetic ataxia: A meta‐analysis. Movement Disorders: Official Journal of the Movement Disorder Society, 37, 171–181. [DOI] [PubMed] [Google Scholar]
- Perkins, G. D. , Callaway, C. W. , Haywood, K. , Neumar, R. W. , Lilja, G. , Rowland, M. J. , Sawyer, K. N. , Skrifvars, M. B. , & Nolan, J. P. (2021). Brain injury after cardiac arrest. The Lancet, 398, 1269–1278. [DOI] [PubMed] [Google Scholar]
- Petzold, A. (2005a). Axonal damage accumulates in the progressive phase of multiple sclerosis: Three year follow up study. Journal of Neurology, Neurosurgery, and Psychiatry, 76, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. (2005b). Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. Journal of the Neurological Sciences, 233, 183–198. [DOI] [PubMed] [Google Scholar]
- Petzold, A. (2007). CSF biomarkers for improved prognostic accuracy in acute CNS disease. Neurological Research, 29, 691–708. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Altintas, A. , Andreoni, L. , Bartos, A. , Berthele, A. , Blankenstein, M. A. , Buee, L. , Castellazzi, M. , Cepok, S. , Comabella, M. , Constantinescu, C. S. , Deisenhammer, F. , Deniz, G. , Erten, G. , Espiño, M. , Fainardi, E. , Franciotta, D. , Freedman, M. S. , Giedraitis, V. , … Teunissen, C. E. (2010). Neurofilament ELISA validation. Journal of Immunological Methods, 352, 23–31. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Boer, J. F. d. , Schippling, S. , Vermersch, P. , Kardon, R. , Green, A. , Calabresi, P. A. , & Polman, C. (2010). Optical coherence tomography in multiple sclerosis: A systematic review and meta‐analysis. The Lancet Neurology, 9, 921–932. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Brettschneider, J. , Jin, K. , Keir, G. , Murray, N. M. F. , Hirsch, N. P. , Itoyama, Y. , Reilly, M. M. , Takeda, A. , & Tumani, H. (2009). CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain‐Barré yndrome. Muscle & Nerve, 40, 42–49. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Groves, M. , Leis, A. A. , Scaravilli, F. , & Stokic, D. S. (2010). Neuronal and glial cerebrospinal fluid protein biomarkers are elevated after West Nile Virus infection. Muscle & Nerve, 41, 42–49. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Gveric, D. , Groves, M. , Schmierer, K. , Grant, D. , Chapman, M. , Keir, G. , Cuzner, L. , & Thompson, E. J. (2008). Phosphorylation and compactness of neurofilaments in multiple sclerosis: Indicators of axonal pathology. Experimental Neurology, 213, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , Hinds, N. , Keir, G. , Thompson, E. , Chapman, M. , Murray, N. , Hirsch, N. , Manji, H. , & Reilly, M. (2002). CSF neurofilament protein levels predict the primary pathology (axonal versus demyelinating) in Guillain–Barré syndrome. Journal of the Neurological Sciences, 199(Suppl 1), S119. [Google Scholar]
- Petzold, A. , Hinds, N. , Murray, N. M. , Hirsch, N. P. , Grant, D. , Keir, G. , Thompson, E. J. , & Reilly, M. M. (2006). CSF neurofilament levels: A potential prognostic marker in Guillain‐Barré syndrome. Neurology, 67, 1071–1073. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Junemann, A. , Rejdak, K. , Zarnowski, T. , Thaler, S. , Grieb, P. , Kruse, F. E. , Zrenner, E. , & Rejdak, R. (2009). A novel biomarker for retinal degeneration: Vitreous body neurofilament proteins. Journal of Neural Transmission, 116, 1601–1606. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Keir, G. , Green, A. J. E. , Giovannoni, G. , & Thompson, E. J. (2003). A specific ELISA for measuring neurofilament heavy chain phosphoforms. Journal of Immunological Methods, 278, 179–190. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Keir, G. , Kay, A. , Kerr, M. , & Thompson, E. J. (2006). Axonal damage and outcome in subarachnoid haemorrhage. Journal of Neurology, Neurosurgery, and Psychiatry, 77, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , Keir, G. , Warren, J. , Fox, N. , & Rossor, M. N. (2007). A systematic review and meta‐analysis of CSF neurofilament protein levels as biomarkers in dementia. Neuro‐Degenerative Diseases, 4, 185–194. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Lu, C.‐H. , Groves, M. , Gobom, J. , Zetterberg, H. , Shaw, G. , & O'Connor, S. (2020). Protein aggregate formation permits millennium‐old brain preservation. Journal of the Royal Society, Interface, 17, 20190775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , Mondria, T. , Kuhle, J. , Rocca, M. A. , Cornelissen, J. , Boekhorst, P. T. , Lowenberg, B. , Giovannoni, G. , Filippi, M. , Kappos, L. , & Hintzen, R. (2010). Evidence for acute neurotoxicity after chemotherapy. Annals of Neurology, 68, 806–815. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Rejdak, K. , Belli, A. , Sen, J. , Keir, G. , Kitchen, N. , Smith, M. , & Thompson, E. J. (2005). Axonal pathology in subarachnoid and intracerebral hemorrhage. Journal of Neurotrauma, 22, 407–414. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Rejdak, K. , & Plant, G. (2004). Axonal degeneration and inflammation in acute optic neuritis. Journal of Neurology, Neurosurgery & Psychiatry, 75, 1178–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , & Shaw, G. (2007). Comparison of two ELISA methods for measuring levels of the phosphorylated neurofilament heavy chain. Journal of Immunological Methods, 319, 34–40. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Steenwijk, M. D. , Eikelenboom, J. M. , Wattjes, M. P. , & Uitdehaag, B. M. (2016). Elevated CSF neurofilament proteins predict brain atrophy: A 15‐year follow‐up study. Multiple Sclerosis (Houndmills, Basingstoke, England), 22, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Stiefel, D. , & Copp, A. J. (2005). Amniotic fluid brain‐specific proteins are biomarkers for spinal cord injury in experimental myelomeningocele. Journal of Neurochemistry, 95, 594–508. [DOI] [PubMed] [Google Scholar]
- Petzold, A. , Tisdall, M. M. , Girbes, A. R. , Martinian, L. , Thom, M. , Kitchen, N. , & Smith, M. (2011). In vivo monitoring of neuronal loss in traumatic brain injury: A microdialysis study. Brain, 134, 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold, A. , Worthington, V. , Appleby, I. , Kerr, M. E. , Kitchen, N. , & Smith, M. (2011). Cerebrospinal fluid ferritin level, a sensitive diagnostic test in late‐presenting subarachnoid hemorrhage. Journal of Stroke and Cerebrovascular Diseases, 20, 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto, A. , Imarisio, A. , Carrarini, C. , Russo, M. , Masciocchi, S. , Gipponi, S. , Cottini, E. , Aarsland, D. , Zetterberg, H. , Ashton, N. J. , Hye, A. , Bonanni, L. , & Padovani, A. (2021). Plasma neurofilament light chain predicts cognitive progression in prodromal and clinical dementia with lewy bodies. Journal of Alzheimers Disease, 82, 913–919. [DOI] [PubMed] [Google Scholar]
- Pipis, M. , Cortese, A. , Polke, J. M. , Poh, R. , Vandrovcova, J. , Laura, M. , Skorupinska, M. , Jacquier, A. , Juntas‐Morales, R. , Latour, P. , Petiot, P. , Sole, G. , Fromes, Y. , Shah, S. , Blake, J. , Choi, B.‐O. , Chung, K. W. , Stojkovic, T. , Rossor, A. M. , & Reilly, M. M. (2021). Charcot‐Marie‐Tooth disease type 2CC due to NEFH variants causes a progressive, non‐length‐dependent, motor‐predominant phenotype. Journal of neurology . Neurosurgery; Psychiatry, 93, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeris, A. A. , Coslovksy, M. , Aeschbacher, S. , Sinnecker, T. , Benkert, P. , Kobza, R. , Beer, J. , Rodondi, N. , Fischer, U. , Moschovitis, G. , Monsch, A. U. , Springer, A. , Schwenkglenks, M. , Wuerfel, J. , Marchis, G. M. D. , Lyrer, P. A. , Kühne, M. , Osswald, S. , Conen, D. , … Bonati, L. H. (2020). Serum neurofilament light in atrial fibrillation: Clinical, neuroimaging and cognitive correlates. Brain Communications, 2, fcaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posmantur, R. M. , Zhao, X. , Kampfl, A. , Clifton, G. L. , & Hayes, R. L. (1998). Immunoblot analyses of the relative contributions of cysteine and aspartic proteases to neurofilament breakdown products following experimental brain injury in rats. Neurochemical Research, 23, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Posmantur, R. , Hayes, R. L. , Dixon, C. E. , & Taft, W. C. (1994). Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. Journal of Neurotrauma, 11, 533–545. [DOI] [PubMed] [Google Scholar]
- Powers, W. J. (2020). Acute ischemic stroke. New England Journal of Medicine, 383, 252–260. [DOI] [PubMed] [Google Scholar]
- Preziosa, P. , Rocca, M. A. , & Filippi, M. (2020). Current state‐of‐art of the application of serum neurofilaments in multiple sclerosis diagnosis and monitoring. Expert Review of Neurotherapeutics, 20, 747–769. [DOI] [PubMed] [Google Scholar]
- Proshchina, A. , Gulimova, V. , Kharlamova, A. , Krivova, Y. , Barabanov, V. , & Saveliev, S. (2022). Cytoskeleton markers in the spinal cord and mechanoreceptors of thick‐toed geckos after prolonged space flights. Lifestyles, 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio, M. , Erben, Y. , Marquez, C. P. , Jansen‐West, K. R. , Franco‐Mesa, C. , Heckman, M. G. , White, L. J. , Dunmore, J. A. , Cook, C. N. , Lilley, M. T. , Song, Y. , Harlow, C. F. , Oskarsson, B. , Nicholson, K. A. , Wszolek, Z. K. , Hickson, L. J. , O’Horo, J. C. , Hoyne, J. B. , Gendron, T. F. , … Petrucelli, L. (2021). Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID‐19. Science Translational Medicine, 13, eabi7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M. V. , Mohan, P. S. , Kumar, A. , Yuan, A. , Montagna, L. , Campbell, J. , Veeranna, E. E. M. , Julien, J. P. , & Nixon, R. A. (2011). The myosin va head domain binds to the neurofilament‐l rod and modulates endoplasmic reticulum (ER) content and distribution within axons. PLoS One, 6, e17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo, A. P. , Abrams, A. J. , Cottenie, E. , Horga, A. , Gonzalez, M. , Bis, D. M. , Sanchez‐Mejias, A. , Pinto, M. , Buglo, E. , Markel, K. , Prince, J. , Laura, M. , Houlden, H. , Blake, J. , Woodward, C. , Sweeney, M. G. , Holton, J. L. , Hanna, M. , Dallman, J. E. , … Zuchner, S. (2016). Cryptic amyloidogenic elements in the 3' UTRs of neurofilament genes trigger axonal neuropathy. The American Journal of Human Genetics, 98, 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejdak, K. , Kuhle, J. , Rüegg, S. , Lindberg, R. L. P. , Petzold, A. , Sulejczak, D. , Papuc, E. , Rejdak, R. , Stelmasiak, Z. , & Grieb, P. (2012). Neurofilament heavy chain and heat shock protein 70 as markers of seizure‐related brain injury. Epilepsia, 53, 922–927. [DOI] [PubMed] [Google Scholar]
- Robinson, C. A. , Clark, A. W. , Parhad, I. M. , Fung, T. S. , & Bou, S. S. (1994). Gene expression in Alzheimer neocortex as a function of age and pathologic severity. Neurobiology of Aging, 15, 681–690. [DOI] [PubMed] [Google Scholar]
- Rodrigues, F. B. , Byrne, L. M. , Tortelli, R. , Johnson, E. B. , Wijeratne, P. A. , Arridge, M. , Vita, E. D. , Ghazaleh, N. , Houghton, R. , Furby, H. , Alexander, D. C. , Tabrizi, S. J. , Schobel, S. , Scahill, R. I. , Heslegrave, A. , Zetterberg, H. , & Wild, E. J. (2020). Mutant huntingtin and neurofilament light have distinct longitudinal dynamics in Huntington's disease. Science Translational Medicine, 12, eabc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues‐Amorim, D. , Rivera‐Baltanás, T. , Carmen, V.‐C. M. d. , Rodriguez‐Jamardo, C. , Heras, E. d. l. , Barreiro‐Villar, C. , Blanco‐Formoso, M. , Fernández‐Palleiro, P. , Álvarez‐Ariza, M. , López, M. , Garcıa‐Caballero, A. , Olivares, J. M. , & Spuch, C. (2020). Plasma ‐III tubulin, neurofilament light chain and glial fibrillary acidic protein are associated with neurodegeneration and progression in schizophrenia. Scientific Reports, 10, 14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren, L. , Karlsson, J. , Karlsson, J. , Persson, L. , & Wikkelso, C. (1996). Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. Journal of Neurochemistry, 67, 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rossi, D. , Volanti, P. , Brambilla, L. , Colletti, T. , Spataro, R. , & La Bella, V. (2018). CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. Journal of Neurology, 265, 510–521. [DOI] [PubMed] [Google Scholar]
- Rossor, A. M. , Kapoor, M. , Wellington, H. , Spaulding, E. , Sleigh, J. N. , Burgess, R. W. , Laura, M. , Zetterberg, H. , Bacha, A. , Wu, X. , Heslegrave, A. , Shy, M. E. , & Reilly, M. M. (2021). A longitudinal and cross‐sectional study of plasma neurofilament light chain concentration in scpcharcot‐marie‐tooth/scp disease. Journal of the Peripheral Nervous System, 27, 50–57. [DOI] [PubMed] [Google Scholar]
- Rossor, A. M. , Liu, C.‐H. , Petzold, A. , Malaspina, A. , Laura, M. , Greensmith, L. , & Reilly, M. M. (2016). Plasma neurofilament heavy chain is not a useful biomarker in Charcot‐Marie‐Tooth disease. Muscle & Nerve, 53, 972–975. [DOI] [PubMed] [Google Scholar]
- Ru, Y. , Corado, C. , Soon, R. K. , Melton, A. C. , Harris, A. , Yu, G. K. , Pryer, N. , Sinclair, J. R. , Katz, M. L. , Ajayi, T. , Jacoby, D. , Russell, C. B. , & Chandriani, S. (2019). Neurofilament light is a treatment‐responsive biomarker in CLN2 disease. Annals of Clinical Translational Neurology, 6, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen, N. , Pape, S. , Konigorski, S. , Zapf, A. , Rücker, G. , & Karch, A. (2022). Diagnostic accuracy of cerebrospinal fluid biomarkers for the differential diagnosis of sporadic Creutzfeldt‐Jakob disease: A (network) meta‐analysis. European Journal of Neurology, 29, 1366–1376. [DOI] [PubMed] [Google Scholar]
- Rudrabhatla, P. , Jaffe, H. , & Pant, H. C. (2011). Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): Phosphoproteomics of Alzheimers NFTs. The FASEB Journal, 25, 3896–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundgren, M. , Friberg, H. , Cronberg, T. , Romner, B. , & Petzold, A. (2012). Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Critical Care, 16, R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter, C. M. , & Gatsonis, C. A. (2001). A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine, 20, 2865–2884. [DOI] [PubMed] [Google Scholar]
- Sako, W. , Murakami, N. , Izumi, Y. , & Kaji, R. (2015). Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson's disease from atypical parkinsonism: Evidence from a meta‐analysis. Journal of the Neurological Sciences, 352, 84–87. [DOI] [PubMed] [Google Scholar]
- Sandelius, Å. , Zetterberg, H. , Blennow, K. , Adiutori, R. , Malaspina, A. , Laura, M. , Reilly, M. M. , & Rossor, A. M. (2018). Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology, 90, e518–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo, R. , Agosta, F. , Masi, F. , Spinelli, E. G. , Cecchetti, G. , Caso, F. , Mandelli, A. , Cardamone, A. , Barbieri, R. , Furlan, G. M. , & Filippi, M. (2021). Plasma neurofilament light chain levels and cognitive testing as predictors of fast progression in Alzheimer's disease. European Journal of Neurology, 28, 2980–2988. [DOI] [PubMed] [Google Scholar]
- Scahill, R. I. , Zeun, P. , Osborne‐Crowley, K. , Johnson, E. B. , Gregory, S. , Parker, C. , Lowe, J. , Nair, A. , O’Callaghan, C. , Langley, C. , Papoutsi, M. , McColgan, P. , Estevez‐Fraga, C. , Fayer, K. , Wellington, H. , Rodrigues, F. B. , Byrne, L. M. , Heselgrave, A. , Hyare, H. , … Tabrizi, S. J. (2020). Biological and clinical characteristics of gene carriers far from predicted onset in the Huntingtons disease young adult study (HD‐YAS): A cross‐sectional analysis. The Lancet Neurology, 19, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, M. , Canaslan, S. , Espinosa, J. C. , Fernández‐Borges, N. , Villar‐Piqué, A. , Llorens, F. , Varges, D. , Maass, F. , Torres, J. M. , Hermann, P. , & Zerr, I. (2022). Validation of plasma and CSF neurofilament light chain as an early marker for sporadic Creutzfeldt‐Jakob disease. Molecular Neurobiology. 10.1007/s12035-022-02891-7 [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Schrödinger, LLC (2015) The PyMOL molecular graphics system, version 1.8.
- Scott, D. , Smith, K. E. , O'Brien, B. J. , & Angelides, K. J. (1985). Characterization of mammalian neurofilament triplet proteins. Subunit stoichiometry and morphology of native and reconstituted filaments. The Journal of Biological Chemistry, 260, 10736–10747. [PubMed] [Google Scholar]
- Sechi, E. , Mariotto, S. , McKeon, A. , Krecke, K. N. , Pittock, S. J. , Ferrari, S. , Monaco, S. , Flanagan, E. P. , Zanzoni, S. , Rabinstein, A. A. , Wingerchuk, D. M. , Nasr, D. M. , & Zalewski, N. L. (2021). Serum neurofilament to magnetic resonance imaging lesion area ratio differentiates spinal cord infarction from acute myelitis. Stroke, 52, 645–654. [DOI] [PubMed] [Google Scholar]
- Seitz, C. B. , Steffen, F. , Muthuraman, M. , Uphaus, T. , Krämer, J. , Meuth, S. G. , Albrecht, P. , Groppa, S. , Zipp, F , Bittner, S. , & Fleischer, V. (2021). Serum neurofilament levels reflect outer retinal layer changes in multiple sclerosis, 14, 175628642110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellebjerg, F. , Royen, L. , Sørensen, P. S. , Oturai, A. B. , & Jensen, P. E. H. (2018). Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase‐3‐like‐1 in newly diagnosed patients with multiple sclerosis, 25, 1444–1451. [DOI] [PubMed] [Google Scholar]
- Sellner, J. , Davies, N. W. , Howard, R. S. , & Petzold, A. (2014). Neurofilament heavy chain as a marker of neuroaxonal pathology and prognosis in acute encephalitis. European Journal of Neurology, 21, 845–850. [DOI] [PubMed] [Google Scholar]
- Sellner, J. , Patel, A. , Dassan, P. , Brown, M. M. , & Petzold, A. (2011). Hyperacute detection of neurofilament heavy chain in serum following stroke: A transient sign. Neurochemical Research, 36, 2287–2291. [DOI] [PubMed] [Google Scholar]
- Sferruzza, G. , Bosco, L. , Falzone, Y. M. , Russo, T. , Domi, T. , Quattrini, A. , Filippi, M. , & Riva, N. (2021). Neurofilament light chain as a biological marker for amyotrophic lateral sclerosis: A meta‐analysis study. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 23, 446–457. [DOI] [PubMed] [Google Scholar]
- Shahim, P. , Tegner, Y. , Marklund, N. , Blennow, K. , & Zetterberg, H. (2018). Neurofilament light and tau as blood biomarkers for sports‐related concussion. Neurology, 90, e1780–e1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, G. , & Weber, K. (1982). Differential expression of neurofilament triplet proteins in brain development. Nature, 298, 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, G. , Yang, C. , Ellis, R. , Anderson, K. , Mickle, J. P. , Scheff, S. , Pike, B. , Anderson, D. K. , & Howland, D. R. (2005). Hyperphosphorylated neurofilament NF‐H is a serum biomarker of axonal injury. Biochemical and Biophysical Research Communications, 336, 1268–1277. [DOI] [PubMed] [Google Scholar]
- Shribman, S. , Heller, C. , Burrows, M. , Heslegrave, A. , Swift, I. , Foiani, M. S. , Gillett, G. T. , Tsochatzis, E. A. , Rowe, J. B. , Gerhard, A. , Butler, C. R. , Masellis, M. , Bremner, F. , Martin, A. , Jung, L. , Cook, P. , Zetterberg, H. , Bandmann, O. , Rohrer, J. D. , & Warner, T. T. (2020). Plasma neurofilament light as a biomarker of neurological involvement in Wilsons disease. Movement Disorders, 36, 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shymanovich, T. , Vandenbrink, J. P. , Herranz, R. , Medina, F. J. , & Kiss, J. Z. (2022). Spaceflight studies identify a gene encoding an intermediate filament involved in tropism pathways. Plant Physiology and Biochemistry, 171, 191–200. [DOI] [PubMed] [Google Scholar]
- Sihag, R. K. , Inagaki, M. , Yamaguchi, T. , Shea, T. B. , & Pant, H. C. (2007). Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Experimental Cell Research, 313, 2098–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, W. , Schmeichel, A. M. , Shahnawaz, M. , Schmelzer, J. D. , Sletten, D. M. , Gehrking, T. L. , Gehrking, J. A. , Olson, A. D. , Suarez, M. D. , Misra, P. P. , Soto, C. , & Low, P. A. (2021). Alpha‐synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Annals of Neurology, 89, 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölin, K. , Kultima, K. , Larsson, A. , Freyhult, E. , Zjukovskaja, C. , Alkass, K. , & Burman, J. (2022). Distribution of five clinically important neuroglial proteins in the human brain. Molecular Brain, 15, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider, N. T. , & Omary, M. B. (2014). Post‐translational modifications of intermediate filament proteins: Mechanisms and functions. Nature Reviews. Molecular Cell Biology, 15, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, N. , & Kumar, S. (2012). Ordered and disordered proteins as nanomaterial building blocks. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology, 4, 204–218. [DOI] [PubMed] [Google Scholar]
- Srour, N. , Khan, S. , & Richard, S. (2022). The influence of arginine methylation in immunity and inflammation. Journal of Inflammation Research, 15, 2939–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger, L. A. , & Sternberger, N. H. (1983). Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proceedings of the National Academy of Sciences of the United States of America, 80, 6126–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, M. L. , Vig, V. , Chung, J. , Fiorello, M. G. , Xia, W. , Zetterberg, H. , Blennow, K. , Zetterberg, M. , Shareef, F. , Siegel, N. H. , Ness, S. , Jun, G. R. , & Stein, T. D. (2020). Neurofilament light chain in the vitreous humor of the eye. Alzheimer's Research & Therapy, 12, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter, R. , Hert, L. , Marchis, G. M. D. , Twerenbold, R. , Kappos, L. , Naegelin, Y. , Kuster, G. M. , Benkert, P. , Jost, J. , Maceski, A. M. , Rüegg, S. , Siegemund, M. , Leppert, D. , Tschudin‐Sutter, S. , & Kuhle, J. (2021). Serum neurofilament light chain levels in the intensive care unit: Comparison between severely ill patients with and without Coronavirus Disease 2019. Annals of Neurology, 89, 610–616. [DOI] [PubMed] [Google Scholar]
- Sveijer, M. , Greenwood, T. B. , Jädersten, M. , Kvedaraite, E. , Zetterberg, H. , Blennow, K. , Lourda, M. , Gavhed, D. , & Henter, J.‐I. (2022). Screening for neurodegeneration in Langerhans cell histiocytosis with neurofilament light in plasma. British Journal of Haematology, 198, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi, S. J. , Leavitt, B. R. , Landwehrmeyer, G. B. , Wild, E. J. , Saft, C. , Barker, R. A. , Blair, N. F. , Craufurd, J. P. , Rickards, H. , Rosser, A. , Kordasiewicz, H. B. , Czech, C. , Swayze, E. E. , Norris, D. A. , Baumann, T. , Gerlach, I. , Schobel, S. A. , Paz, E. , … Lane, R. M. (2019). Targeting huntingtin expression in patients with Huntington's disease. New England Journal of Medicine, 380, 2307–2316. [DOI] [PubMed] [Google Scholar]
- Takano, K. , Goto, K. , Motobayashi, M. , Wakui, K. , Kawamura, R. , Yamaguchi, T. , Fukushima, Y. , & Kosho, T. (2017). Early manifestations of epileptic encephalopathy, brain atrophy, and elevation of serum neuron specific enolase in a boy with beta‐propeller protein‐associated neurodegeneration. European Journal of Medical Genetics, 60, 521–526. [DOI] [PubMed] [Google Scholar]
- Tavazzi, E. , Jakimovski, D. , Kuhle, J. , Hagemeier, J. , Ozel, O. , Ramanathan, M. , Barro, C. , Bergsland, N. , Tomic, D. , Kropshofer, H. , Leppert, D. , Michalak, Z. , Lincoff, N. , Dwyer, M. G. , Benedict, R. H. B. , Weinstock‐Guttman, B. , & Zivadinov, R. (2020). Serum neurofilament light chain and optical coherence tomography measures in MS: A longitudinal study. Neurology(R) Neuroimmunology and Neuroinflammation, 7, e737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen, C. E. , Petzold, A. , Bennett, J. L. , Berven, F. S. , Brundin, L. , Comabella, M. , Franciotta, D. , Frederiksen, J. L. , Fleming, J. O. , Furlan, R. , Hintzen, R. Q. , Hughes, S. G. , Johnson, M. H. , Krasulova, E. , Kuhle, J. , Magnone, M. C. , Rajda, C. , Rejdak, K. , Schmidt, H. K. , … Deisenhammer, F. (2009). A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology, 73, 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen, F. , Anderton, R. S. , Mastaglia, F. L. , James, I. , Bedlack, R. , & Akkari, P. A. (2022) Intronic NEFH variant is associated with reduced risk for sporadic ALS and later age of disease onset. [DOI] [PMC free article] [PubMed]
- Thompson, A. J. , Baranzini, S. E. , Geurts, J. , Hemmer, B. , & Ciccarelli, O. (2018). Multiple sclerosis. The Lancet, 391, 1622–1636. [DOI] [PubMed] [Google Scholar]
- Thwaites, N. A. , Khan, O. A. , Liu, D. , Steinbach, M. , Gilbert, C. , Cambronero, F. E. , Moore, E. E. , Bown, C. W. , Acosta, L. , Spirko, K. O. , Pechman, K. R. , Hohman, T. J. , Blennow, K. , Zetterberg, H. , Jefferson, A. L. , & Gifford, K. A. (2021). A‐4 cerebrospinal fluid and plasma neurofilament light in relation to longitudinal objective and subjective cognitive decline in older adults, 36, 1043. [Google Scholar]
- Ticau, S. , Sridharan, G. V. , Tsour, S. , Cantley, W. L. , Chan, A. , Gilbert, J. A. , Erbe, D. , Aldinc, E. , Reilly, M. M. , Adams, D. , Polydefkis, M. , Fitzgerald, K. , Vaishnaw, A. , & Nioi, P. (2020). Neurofilament light chain as a biomarker of hereditary transthyretin‐mediated amyloidosis. Neurology, 96, e412–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt, S. , Duering, M. , Barro, C. , Kaya, A. G. , Boeck, J. , Bode, F. J. , Klein, M. , Dorn, F. , Gesierich, B. , Kellert, L. , Ertl‐Wagner, B. , Goertler, M. W. , Petzold, G. C. , Kuhle, J. , Wollenweber, F. A. , Peters, N. , & Dichgans, M. (2018). Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology, 91, e1338–e1347. [DOI] [PubMed] [Google Scholar]
- Tisdall, M. , & Petzold, A. (2012) Comment on "Chronic traumatic encephalopathy in blast‐exposed military veterans and a blast neurotrauma mouse model". Science Translational Medicine 4, 157le8. [DOI] [PubMed] [Google Scholar]
- Tjensvoll, A. B. , Lauvsnes, M. B. , Zetterberg, H. , Kvaløy, J. T. , Kvivik, I. , Maroni, S. S. , Greve, O. J. , Beyer, M. K. , Hirohata, S. , Putterman, C. , Alves, G. , Harboe, E. , Blennow, K. , Gøransson, L. G. , & Omdal, R. (2020). Neurofilament light is a biomarker of brain involvement in lupus and primary sjögren's syndrome. Journal of Neurology, 268, 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toorop, A. A. , Lierop, Z. Y. G. v. , Strijbis, E. E. M. , Teunissen, C. E. , Petzold, A. , Wattjes, M. P. , Barkhof, F. , de Jong, B. A. , van Kempen, Z. L. E. , & Killestein, J. (2020). Mild progressive multifocal leukoencephalopathy after switching from natalizumab to ocrelizumab. Neurology—Neuroimmunology Neuroinflammation, 8, e904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpin, S. , Mixon, A. E. , Stapels, M. D. , Kim, M.‐Y. , Spencer, P. S. , & Deinzer, M. L. (2004). Identification of endogenous phosphorylation sites of bovine medium and low molecular weight neurofilament proteins by tandem mass spectrometry. Biochemistry, 43, 2091–2105. [DOI] [PubMed] [Google Scholar]
- Uyar, M. , Lezius, S. , Buhmann, C. , Pötter‐Nerger, M. , Schulz, R. , Meier, S. , Gerloff, C. , Kuhle, J. , & Choe, C. (2022). Diabetes, glycated hemoglobin (HbA1c), and neuroaxonal damage in Parkinson's disease (MARK‐PD study). Movement Disorders, 37, 1299–1304. [DOI] [PubMed] [Google Scholar]
- Uzgiris, A. J. , Calabresi, P. A. , Lee, S. , Stevenson, L. , Graham, D. , Matias, M. , Rudick, R. A. , Calabresi, P. A. , Stevenson, L. , Graham, D. , Raitcheva, D. , Green, C. , Matias, M. , & Uzgiris, A. J. (2022). Development of a highly sensitive serum neurofilament light chain assay on an automated immunoassay platform. medRxiv [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geel, W. , Rosengren, L. , & Verbeek, M. (2005). An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. Journal of Immunological Methods, 296, 179–185. [DOI] [PubMed] [Google Scholar]
- Varhaug, K. N. , Hikmat, O. , Nakkestad, H. L. , Vedeler, C. A. , & Bindoff, L. A. (2021). Serum biomarkers in primary mitochondrial disorders. Brain Communications, 3, fcaa222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul, M. K. , Veelen, P. A. v. , Delft, M. A. M. v. , Ru, A. d. , Janssen, G. M. C. , Rispens, T. , Toes, R. E. M. , & Trouw, L. A. (2018). Pitfalls in the detection of citrullination and carbamylation. Autoimmunity Reviews, 17, 136–141. [DOI] [PubMed] [Google Scholar]
- Vial, J. (1958). The early changes in the axoplasm during Wallerian degeneation. The Journal of Biophysical and Biochemical Cytology, 4, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella, G. , Thorpe, S. R. , Alderson, N. L. , Stephan, E. M. , Atchley, D. , Wagner, F. , & Lopes‐Virella, M. F. (2003). Autoimmune response to advanced glycosylation end‐products of human LDL. Journal of Lipid Research, 44, 487–493. [DOI] [PubMed] [Google Scholar]
- Virhammar, J. , Nääs, A. , Fällmar, D. , Cunningham, J. L. , Klang, A. , Ashton, N. J. , Jackmann, S. , Westman, G. , Frithiof, R. , Blennow, K. , Zetterberg, H. , Kumlien, E. , & Rostami, E. (2021). Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID‐19 and associated with neurological symptoms and disease severity. European Journal of Neurology, 28, 3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vuurst de Vries, R. M. , Wong, Y. Y. M. , Mescheriakova, J. Y. , van Pelt, E. D. , Runia, T. F. , Jafari, N. , Siepman, T. A. , Melief, M.‐J. , Wierenga‐Wolf, A. F. , van Luijn, M. M. , Samijn, J. P. , Neuteboom, R. F. , & Hintzen, R. Q. (2018). High neurofilament levels are associated with clinically definite multiple sclerosis in children and adults with clinically isolated syndrome. Multiple Sclerosis (Houndmills, Basingstoke, England), 25, 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, O. , Lifshitz, J. , Janmey, P. , Linden, M. , McIntosh, T. K. , & Leterrier, J.‐F. (2003). Mechanisms of mitochondria‐neurofilament interactions. The Journal of Neuroscience, 23, 9046–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai, C. H. , Jin, J. , Cyrklaff, M. , Genoud, C. , Funaya, C. , Sattler, J. , Maceski, A. , Meier, S. , Heiland, S. , Lanzer, M. , & Frischknecht, F. (2022). Neurofilament light chain plasma levels are associated with area of brain damage in experimental cerebral malaria. Scientific Reports, 12, 10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wang, W. , Shi, H. , Han, L. , & Pan, P. (2020a). Cerebrospinal fluid and blood levels of neurofilament light chain in Parkinson disease: A protocol for systematic review and meta‐analysis. Medicine, 99, e21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wang, W. , Shi, H. , Han, L. , & Pan, P. (2020b). Blood neurofilament light chain in Parkinson disease and atypical parkinsonisms: A protocol for systematic review and meta‐analysis. Medicine, 99, e21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Chen, H. , Tang, J. , Guo, Z. , & Wang, Y. (2022). Peptidylarginine deiminase and Alzheimer's disease. Journal of Alzheimers Disease, 85, 473–484. [DOI] [PubMed] [Google Scholar]
- Wang, R.‐M. , Xu, W.‐Q. , Zheng, Z.‐W. , Yang, G.‐M. , Zhang, M.‐Y. , Ke, H.‐Z. , Xia, N. , Dong, Y. , & Wu, Z.‐Y. (2022). Serum neurofilament light chain in Wilson's disease: A promising indicator but unparallel to real‐time treatment response. Movement Disorders, 37, 1531–1535. [DOI] [PubMed] [Google Scholar]
- Wang, S.‐Y. , Chen, W. , Xu, W. , Li, J.‐Q. , Hou, X.‐H. , Ou, Y.‐N. , Yu, J.‐T. , & Tan, L. (2019). Neurofilament light chain in cerebrospinal fluid and blood as a biomarker for neurodegenerative diseases: A systematic review and meta‐analysis. Journal of Alzheimer's disease: JAD, 72, 1353–1361. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhou, W. , Gao, Z. , & Lv, X. (2022). Mass spectrometry analysis of S‐nitrosylation of proteins and its role in cancer, cardiovascular and neurodegenerative diseases. TrAC Trends in Analytical Chemistry, 152, 116625. [Google Scholar]
- Warnecke, A. , Sandalova, T. , Achour, A. , & Harris, R. A. (2014). PyTMs: A useful PyMOL plugin for modeling common post‐translational modifications. BMC Bioinformatics, 15, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissert, R. , Hugosson, T. , & Petzold, A. (2022). Upregulated retinal neurofilament expression in experimental optic neuritis. Neuro‐Ophthalmology, 46, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wel, B. D. , Schaepdryver, M. D. , Poesen, K. , & Claeys, K. G. (2022). Biochemical and clinical biomarkers in adult SMA 3‐4 patients treated with nusinersen for 22 months. Annals of Clinical Translational Neurology, 9, 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford, R. W. D. , Farine, H. , Steiner, M. , Garzotti, M. , Dobrenis, K. , Sievers, C. , Strasser, D. S. , Amraoui, Y. , Groenen, P. M. , Giugliani, R. , & Mengel, E. (2022). Plasma neurofilament light, glial fibrillary acidic protein and lysosphingolipid biomarkers for pharmacodynamics and disease monitoring of GM2 and GM1 gangliosidoses patients. Molecular Genetics and Metabolism Reports, 30, 100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz, E. , Dobrescu, S. R. , Dinkler, L. , Gillberg, C. , Gillberg, K. , Blennow, K. , Råstam, M. , & Zetterberg, H. (2020). Thirty years after anorexia nervosa onset, serum neurofilament light chain protein concentration indicates neuronal injury. European Child & Adolescent Psychiatry, 30, 1907–1915. 10.1007/s00787-020-01657-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg, S. , Holmgaard, F. , Blennow, K. , Nilsson, J. C. , Kjaergaard, J. , Wanscher, M. , Langkilde, A. R. , Hassager, C. , Rasmussen, L. S. , Zetterberg, H. , & Vedel, A. G. (2020). Associations between mean arterial pressure during cardiopulmonary bypass and biomarkers of cerebral injury in patients undergoing cardiac surgery: Secondary results from a randomized controlled trial. Interactive Cardio Vascular and Thoracic Surgery, 32, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, E. J. , Petzold, A. , Keir, G. , & Tabrizi, S. J. (2007). Plasma neurofilament heavy chain levels in Huntington's disease. Neuroscience Letters, 417, 231–233. [DOI] [PubMed] [Google Scholar]
- Wilke, C. , Reich, S. , Swieten, J. C. , Borroni, B. , Sanchez‐Valle, R. , Moreno, F. , Laforce, R. , Graff, C. , Galimberti, D. , Rowe, J. B. , Masellis, M. , Tartaglia, M. C. , Finger, E. , Vandenberghe, R. , Mendonça, A. , Tagliavini, F. , Santana, I. , Ducharme, S. , Butler, C. R. , … Synofzik, M. (2021). Stratifying the presymptomatic phase of genetic frontotemporal dementia by serum NfL and pNfH: A longitudinal multicentre study. Annals of Neurology, 91, 33–47. [DOI] [PubMed] [Google Scholar]
- Williams, T. , Zetterberg, H. , & Chataway, J. (2021). Serum neurofilament light concentrations are not associated with renal function in secondary progressive multiple sclerosis, 100044. [Google Scholar]
- Winther‐Larsen, A. , Hviid, C. V. B. , Meldgaard, P. , Sorensen, B. S. , & Sandfeld‐Paulsen, B. (2020). Neurofilament light chain as a biomarker for brain metastases. Cancers, 12, 2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltsche, N. , Valentin, K. , Hoeflechner, L. , Guttmann, A. , Horwath‐Winter, J. , Schneider, M. R. , Ivastinovic, D. , Lindner, M. , Schmetterer, L. , Singh, N. , Riedl, R. , Buchmann, A. , Khalil, M. , & Lindner, E. (2022). Neurofilament light chain: A new marker for neuronal decay in the anterior chamber fluid of patients with glaucoma. British Journal of Ophthalmology [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wong, N. K. , He, B. P. , & Strong, M. J. (2000). Characterization of neuronal intermediate filament protein expression in cervical spinal motor neurons in sporadic amyotrophic lateral sclerosis (ALS). Journal of Neuropathology and Experimental Neurology, 59, 972–982. [DOI] [PubMed] [Google Scholar]
- Xiao, S. , McLean, J. , & Robertson, J. (2006). Neuronal intermediate filaments and ALS: A new look at an old question. Biochimica et Biophysica Acta, 1762, 1001–1012. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Henderson, R. D. , David, M. , & McCombe, P. A. (2016). Neurofilaments as biomarkers for amyotrophic lateral sclerosis: A systematic review and meta‐analysis. PLoS One, 11, e0164625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. , Qiao, L. , Peng, H. , Liu, A. , Wu, J. , & Huang, J. (2020). A novel missense pathogenic variant in NEFH causing rare Charcot‐Marie‐Tooth neuropathy type 2CC. Neurological Sciences, 42, 757–763. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Shao, Y.‐R. , Li, X.‐Y. , Ma, Y. , Dong, Y. , & Wu, Z.‐Y. (2021) Association of the level of neurofilament light with disease severity in patients with spinocerebellar ataxia type 2. 10.1212/WNL.0000000000012945. [DOI] [PMC free article] [PubMed]
- Ye, R. , Locascio, J. J. , Goodheart, A. E. , Quan, M. , Zhang, B. , & Gomperts, S. N. (2021). Serum NFL levels predict progression of motor impairment and reduction in putamen dopamine transporter binding ratios in de novo Parkinsons disease: An 8‐year longitudinal study. Parkinsonism & Related Disorders, 85, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, Y. , & Li, H. (2022). Recent progress in the analysis of protein deamidation using mass spectrometry. Methods, 200, 42–57. [DOI] [PubMed] [Google Scholar]
- Young, S. G. , Yang, S. H. , Davies, B. S. J. , Jung, H.‐J. , & Fong, L. G. (2013). Targeting protein prenylation in progeria. Science Translational Medicine, 5, 171ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K. , & Proost, P. (2022). Insights into peptidylarginine deiminase expression and citrullination pathways. Trends in Cell Biology, 32, 746–761. [DOI] [PubMed] [Google Scholar]
- Yuan, A. , & Nixon, R. A. (2021). Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Frontiers in Neuroscience, 15, fnins.2021.689938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa, S. A. , Shan, X. , Macauley, M. S. , Clark, T. , Skorobogatko, Y. , Vosseller, K. , & Vocadlo, D. J. (2012). Increasing o‐GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature Chemical Biology, 8, 393–399. [DOI] [PubMed] [Google Scholar]
- Zalc, B. , & Colman, D. R. (2000). Origins of vertebrate success. Science, 288, 271. [DOI] [PubMed] [Google Scholar]
- Zarranz, J. J. , Alegre, J. , Gómez‐Esteban, J. C. , Lezcano, E. , Ros, R. , Ampuero, I. , Vidal, L. , Hoenicka, J. , Rodriguez, O. , Atarés, B. , Llorens, V. , Tortosa, E. G. , del Ser, T. , Muñoz, D. G. , & de Yebenes, J. G. (2003). The new mutation, E46K, of ‐synuclein causes Parkinson and Lewy body dementia. Annals of Neurology, 55, 164–173. [DOI] [PubMed] [Google Scholar]
- Zecha, J. , Gabriel, W. , Spallek, R. , Chang, Y.‐C. , Mergner, J. , Wilhelm, M. , Bassermann, F. , & Kuster, B. (2022). Linking post‐translational modifications and protein turnover by site‐resolved protein turnover profiling. Nature Communications, 13, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr, I. (2022). Laboratory diagnosis of Creutzfeldt Jakob Disease. New England Journal of Medicine, 386, 1345–1350. [DOI] [PubMed] [Google Scholar]
- Zerr, I. , Schmitz, M. , Karch, A. , Villar‐Piqué, A. , Kanata, E. , Golanska, E. , Díaz‐Lucena, D. , Karsanidou, A. , Hermann, P. , Knipper, T. , Goebel, S. , Varges, D. , Sklaviadis, T. , Sikorska, B. , Liberski, P. P. , Santana, I. , Ferrer, I. , Zetterberg, H. , Blennow, K. , … Llorens, F. (2018). Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: Evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 14, 751–763. [DOI] [PubMed] [Google Scholar]
- Zervides, K. A. , Janelidze, S. , Nystedt, J. , Gullstrand, B. , Nilsson, P. , Sundgren, P. C. , Bengtsson, A. A. , Hansson, O. , & Jönsen, A. (2022) Plasma and cerebrospinal fluid neurofilament light concentrations reflect neuronal damage in systemic lupus erythematosus. [DOI] [PMC free article] [PubMed]
- Zetterberg, H. , & Blennow, K. (2018). From cerebrospinal fluid to blood: The third wave of fluid biomarkers for Alzheimer's disease. Journal of Alzheimer's disease: JAD, 64, S271–S279. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Cheng, H. , Liu, W. , Li, H. , Song, Y. , & Jia, L. (2022). Neurofilament light chain in cerebrospinal fluid or blood as a biomarker for mild cognitive impairment: A systematic review and meta‐analysis. Medicine, 101, e28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Gao, W. , Li, J. , Wu, W. , & Jiu, Y. (2019). The role of host cytoskeleton in flavivirus infection. Virologica Sinica, 34, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Mo, M. , Miao, C. , Li, L. , Yang, H. , Liu, Y. , & Yang, G. (2020). Association of serum biomarker neurofilament light concentration with post‐stroke depression: A preliminary study. General Hospital Psychiatry, 64, 17–25. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Xin, Y. , Meng, S. , He, Z. , & Hu, W. (2019). Neurofilament light chain protein in neurodegenerative dementia: A systematic review and network meta‐analysis. Neuroscience and Biobehavioral Reviews, 102, 123–138. [DOI] [PubMed] [Google Scholar]
- Zhou, F.‐Y. , Chen, D.‐W. , Li, H.‐Y. , Zhu, C. , Shen, Y.‐Y. , Peng, Z.‐Y. , Li, L. , Bu, X.‐L. , Zeng, G.‐H. , Zhang, M. , Wang, Y.‐J. , & Jin, W.‐S. (2022). The association of serum neurofilament light chain and acute ischaemic stroke is influenced by effective revascularization. Disease Markers, 2022, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y.‐N. , Chen, Y.‐H. , Dong, S.‐Q. , Yang, W.‐B. , Qian, T. , Liu, X.‐N. , Cheng, Q. , Wang, J.‐C. , & Chen, X.‐J. (2021). Role of blood neurofilaments in the prognosis of amyotrophic lateral sclerosis: A meta‐analysis. Frontiers in Neurology, 12, 712245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, P.‐P. , Hung, H.‐F. , Batchenkova, N. , Nixon‐Abell, J. , Henderson, J. , Zheng, P. , Renvoisé, B. , Pang, S. , Xu, C. S. , Saalfeld, S. , Funke, J. , Xie, Y. , Svara, F. , Hess, H. F. , & Blackstone, C. (2022). Transverse endoplasmic reticulum expansion in hereditary spastic paraplegia corticospinal axons. Human Molecular Genetics. 10.1093/hmg/ddac072 [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccarato, M. , Grisold, W. , Grisold, A. , Poretto, V. , Boso, F. , & Giometto, B. (2021). Paraneoplastic neuropathies: Whats new since the 2004 recommended diagnostic criteria, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi, E. , Bonetto, V. , Sorarù, G. , Martinelli, I. , Parchi, P. , Liguori, R. , & Mandrioli, J. (2020). Neurofilaments in motor neuron disorders: Towards promising diagnostic and prognostic biomarkers. Molecular Neurodegeneration, 15, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi, E. , Lu, C.‐H. , Cho, Y. , Chang, R. , Adiutori, R. , Zubiri, I. , Ceroni, M. , Cereda, C. , Pansarasa, O. , Greensmith, L. , Malaspina, A. , & Petzold, A. (2018). A motor neuron strategy to save time and energy in neurodegeneration: Adaptive protein stoichiometry. Journal of Neurochemistry, 146, 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material S1
Data Availability Statement
Data are shared as Supplementary Material.


