Abstract
The colonization of intestinal and systemic tissues by Salmonella enterica serovars with different host specificities was determined 7 days after inoculation of 1 to 2-month-old lambs. Following oral inoculation, S. enterica serovars Abortusovis, Dublin, and Gallinarum were recovered in comparable numbers from the intestinal mucosa, but serovar Gallinarum was recovered in lower numbers than the other serovars from systemic sites. The pattern of bacterial recovery from systemic sites following intravenous inoculation was similar. The magnitude of intestinal invasion was evaluated in ovine ligated ileal loops in vivo. Serovars Dublin and Gallinarum and the broad-host-range Salmonella serovar Typhimurium were recovered in comparable numbers from ileal mucosa 3 h after loop inoculation, whereas the recovery of serovar Abortusovis was approximately 10-fold lower. Microscopic analysis of intestinal mucosae infected with serovars Typhimurium and Dublin showed dramatic morphological changes and infiltration of inflammatory cells, whereas mucosae infected with serovars Abortusovis and Gallinarum were indistinguishable from uninfected mucosae. Together these data suggest that Salmonella serovar specificity in sheep correlates with bacterial persistence at systemic sites. Intestinal invasion and avoidance of the host's intestinal inflammatory response may contribute to but do not determine the specificity of serovar Abortosovis for sheep. Intestinal invasion by serovar Abortusovis was significantly reduced after mutation of invH but was not reduced following curing of the virulence plasmid, suggesting that the Salmonella pathogenicity island 1 influences but the virulence plasmid genes do not influence the ability of serovar Abortusovis to invade the intestinal mucosa in sheep.
Serovars of Salmonella enterica subspecies I are associated mainly with warm-blooded vertebrates and are responsible for most Salmonella infections in humans and domesticated animals. Salmonella serovars differ in the range of hosts they can infect and in the nature of disease that may result; this difference is referred to as serovar-host specificity. Some Salmonella serovars, for example, Typhimurium and Enteritis, can infect a wide range of hosts and are termed ubiquitous. They are usually associated with a relatively mild enteric disease, although in some hosts, such as mice, the disease can be systemic and severe. Other serovars are very restricted in their host range, causing severe systemic disease in only one host. For example, Salmonella serovar Typhi is restricted to infections in humans and Salmonella serovar Abortusovis to infections in sheep (8, 24). A third group of serovars is associated predominately with disease in one species but may also infect a limited number of other hosts. For example, Salmonella serovar Dublin is usually associated with cattle, but natural infection by this serotype may also occur in other animals, including humans and sheep (15, 28). The nature of disease associated with this third group of serovars is variable, depending on the specific combination of serovar and host, although in the predominant serovar-host combination the disease is usually systemic.
Ovine salmonellosis may occur with a range of different symptoms of variable severity, depending mainly on the particular serovar involved (15). Serovars Abortusovis, Dublin, and Typhimurium, which each have different degrees of host restriction, are associated with disease in sheep (15). Serovar Abortusovis is the most common causative agent of ovine salmonellosis in southern Europe (14, 15). Infection becomes clinically evident with abortion in the last 6 weeks of pregnancy in the absence of other clinical symptoms (14). After abortion, salmonellae can be isolated from the vaginal discharge for up to a week. Infected ewes may also deliver weak lambs that quickly die or lambs may be born that are apparently healthy but suddenly become ill and die in the first few days of life, with lesions typical of pneumonia (15).
Serovar Dublin can cause both enteritis and abortion in adult sheep, and the disease is often associated with metritis, anorexia, and loss of wool (11, 15). Newborn lambs may experience enteritis with a high mortality rate. It has been possible to infect sheep experimentally with serovar Dublin by the oral route, and this procedure has resulted in enteric disease and systemic dissemination of salmonellae (22). Abortion occurred following inoculation with large doses of inocula and was always associated with death of the ewe. Serovar Typhimurium can also cause disease in sheep, and infected animals show general malaise, with enteritis and death (15). Serovar Typhimurium is not associated with abortion in sheep (15).
The biological basis of Salmonella serovar-host specificity remains unclear, largely due to the paucity of information on the pathogenesis of host-restricted Salmonella serovars in animal species other than mice. The ability of Salmonella to enter and/or persist in intestinal mucosa has been implicated in resistance of mice to infection with serovars Gallinarum and Typhi (25). However, in pigs and cattle the magnitude of intestinal invasion does not correlate with the specificity of serovars Choleraesuis and Dublin (5). Serovar-host specificity for mice has also been correlated to bacterial survival within macrophages (21, 31), whereas in pigs there is no such correlation (36).
Two virulence gene clusters, Salmonella pathogenicity island 1 (SPI-1) and the spv genes, influence Salmonella intestinal invasion and persistence in vivo, respectively. SPI-1 encodes a type III secretion system that translocates secreted effector proteins into the cytoplasm of host cells. These effector proteins, including SopE, SopE2, and SptP, induce cytoskeletal rearrangements resulting in bacterial internalization in vitro (reviewed in 32). Mutation of SPI-1 reduces the intestinal invasiveness and enteropathogenesis of serovars Typhimurium and Dublin in bovine ligated ileal loops (10, 35) and serovar Typhimurium virulence in orally inoculated calves (29, 34). Although SPI-1 has been shown to be required for full virulence of serovar Typhimurium in orally inoculated mice, its role in pathogenesis of other serovar-host combinations is relatively unknown. The spv operon is located in the virulence plasmid of several Salmonella serovars, including Abortusovis, Typhimurium, Dublin, Choleraesuis, and Gallinarum but not Typhi (26). The role of spv in pathogenesis remains poorly understood. The spv operon has a profound impact on the virulence of serovars Typhimurium and Dublin for mice and it influences the net growth of Salmonella serovars in an intracellular niche in mice (12). The role of spv in Salmonella-induced enteritis is less clear. In cattle, a virulence plasmid-cured strain of serovar Dublin was attenuated for systemic but not enteric salmonellosis, and it was fully invasive and enteropathogenic in bovine ligated ileal loops (33). However, an spvR mutant of serovar Dublin was attenuated for both enteric and systemic disease in calves (20), whereas an spvR mutant of serovar Typhimurium was fully virulent (29). The reasons for these conflicting observations are not clear. The virulence plasmid also encodes other potential virulence factors, including plasmid-encoded fimbriae (9). Plasmid genes have been implicated in influencing the invasiveness of serovar Gallinarum in chickens (3). The virulence plasmid of serovar Abortusovis influences the virulence of this serovar for mice (30). However, the contributions of SPI-1 and the virulence plasmid genes to the interaction of serovar Abortusovis with ovine intestinal mucosa are not known.
To gain insights into the biological basis of Salmonella serovar specificity in sheep, the pathogenesis of Salmonella serovars with different degrees of host restriction was evaluated in experimentally infected lambs. The virulence of serovars Abortusovis, Dublin and Gallinarum was correlated with invasion in a quantitative ovine intestinal invasion model in vivo. The involvement of SPI-1 and virulence plasmid genes in intestinal invasion was also determined.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Serovar Gallinarum strains G9 and J91 were kindly provided by J. E. Olsen (Royal Veterinary and Agricultural University, Copenhagen, Denmark). Serovar Abortusovis strain 15/5 was kindly provided by F. Lantier (Institut National de la Recherche Agronomique, Tours-Nouzilly, France). Several of the strains used in this study have been extensively characterized in many different types of virulence assays and in comparison to other strains of the same serovar and appear to be good representative strains for their serovar (2, 3, 5, 33, 36). The strains were maintained as frozen cultures until use. For DNA recombination and genetic analysis, bacteria were grown in Luria-Bertani (LB) medium. P22 HT105/1 was used to transduce a mutation in invH from serovar Typhimurium 4/74 InvH to serovar Abortusovis SS44, as described previously (35). Strain 4/74 InvH carries a TnphoA insertion within invH that has been previously isolated and characterized in our laboratory (35).
TABLE 1.
Bacterial strains used in this work
| Salmonella serovar and strain | Description | Source |
|---|---|---|
| Typhimurium | ||
| 4/74 | Wild-type isolate | 16 |
| 4/74 InvH | invH201::TnphoA derivative of 4/74 | 35 |
| Abortusovis | ||
| 15/5 | Wild-type isolate | 19 |
| SS44 | Wild-type isolate | 6 |
| SU40 | Plasmid-cured derivative of SS44 | 30 |
| SS44 InvH | invH201::TnphoA derivative of SS44 | This study |
| Dublin | ||
| SD2229 | Wild-type isolate | 33 |
| SD3246 | Wild-type isolate | 35 |
| Gallinarum | ||
| G9 | Wild-type isolate | J. E. Olsen |
| J91 | Wild-type clinical isolate | J. E. Olsen |
Infection of lambs.
One- to two-month-old Berrichon crossbred lambs, with no cultural or serological evidence of Salmonella, were used. Groups consisting of two or three lambs each were infected orally with approximately 5 × 108 CFU or intravenously with approximately 5 × 106 CFU of each serovar. Inocula were obtained by growing the strains at 37°C statically for 18 h. For oral inoculation, the bacterial suspensions (1 ml) were mixed with an antiacid solution [5% (wt/vol) Mg(SiO3)3, 5% (wt/vol) NaHCO3, 5% (wt/vol) MgCO3] and were administered orally to animals immediately before the morning feeding. The Salmonella strains were able to survive in the antiacid solution for up to 60 min (data not shown). The lambs were monitored by recording the rectal temperature and checking for the presence of diarrhea every 24 h. At 7 days postinfection, the animals were killed by pentobarbitone overdose. Samples of approximately 1 g each were taken in triplicate from of the all sites analyzed. The systemic samples were taken first to avoid contamination with the intestinal contents. The luminal surfaces of the intestinal samples were washed thoroughly with sterile phosphate-buffered saline (PBS) to remove nonadherent bacteria. Tissues were homogenized, and dilutions were plated out in modified brilliant green agar plates (Difco Laboratories, Detroit, Mich.) in triplicate.
Ovine ileal loop invasion assay.
This assay was based on an intestinal invasion assay developed in calves (35). Four to five-month-old ewes were anesthetized with pentobarbitone (0.44 mg/kg) for the duration of the experiment. The abdominal wall was opened with a midline incision, the distal ileum was exteriorized, and the lumen was flushed with PBS. Loops 9 cm in length were constructed with 1-cm spaces using braided surgical silk. Loop inocula were prepared as follows. Log-phase Salmonella cells were harvested by centrifugation (2,500 × g at 4°C for 10 min) and resuspended in 10 ml of LB broth. Nine milliliters of Salmonella suspension containing approximately 109 CFU was injected into each loop. Sterile LB broth was used as a negative control. Loops were again exteriorized at 1 h postinoculation, and 5 ml of solution GC/Tcm10 (1, 35) containing 300 μg of gentamicin per ml was injected. When the medium was diluted with the loop contents, the working concentration of gentamicin was approximately 150 μg/ml. The loops were returned to the abdominal cavity, and the wound was repaired. After a further hour the ileum was exteriorized, and the individual loops were cut out. The animal was killed by an overdose of pentobarbitone. Loops were opened longitudinally and placed in approximately 50 ml of ice-cold GC/Tcm10 solution to dilute the gentamicin. The tissue was gently washed with saline, and six circular biopsies, each of 6-mm radius, were removed from the central area of the loop. Three of these samples contained Peyer's patches. Each of the biopsies was placed in 3 ml of PBS containing 1% Triton X-100 and homogenized, and counts of the viable bacteria were performed as described above. The stringency of the ligated ileal loop assay has been addressed previously using a noninvasive strain of Escherichia coli, and it is predicted that the gentamicin treatment will kill between 90 and 99% of extracellular bacteria (5, 35).
Microscopic analysis.
The ligated loops were fixed in situ by injecting 5 ml of 0.1 M phosphate-buffered 2.5% glutaraldehyde (pH 7.5). Samples were postfixed in 1% OsO4 for 45 min, dehydrated through graded ethanol solutions, and embedded and sectioned in epoxidic resin. The sections were stained with hematoxylin and eosin and examined in a blinded fashion with an Axioscope Zeiss microscope.
Statistical analysis.
For all statistical analyses, the viable bacteria count data were normalized by logarithmic transformation. P values of less than 0.05 were considered significant. A one-way analysis of variance was carried out on the cell invasion assays to compare all strains and their mutants, using Minitab statistical software (Minitab Inc., State College, Pa.). In the event of a significant difference, a Student's t test was applied. Analysis of the intestinal loop assays was performed with the SAS statistical package (SAS Institute Inc., Cary, N.C.). The data were treated as appropriate for a split plot, and the analysis took into account the variation between sheep.
RESULTS
Virulence of different Salmonella serovars following oral inoculation of lambs.
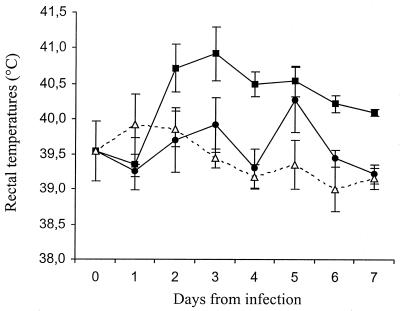
The virulence of Salmonella serovars Abortusovis, Dublin, and Gallinarum was assessed by orally inoculating lambs with approximately 5 × 108 CFU of each serovar. All three serovars induced increases in rectal temperatures in the infected lambs, but these increases were of various severities. Serovar Dublin induced a high and prolonged increase in temperature, whereas the temperature of lambs infected with serovar Gallinarum had returned to normal by 3 days postinoculation (Fig. 1). Serovar Abortusovis elicited lower rectal temperatures than serovar Dublin, and by the end of the experiment the temperatures in animals infected with serovar Abortusovis were similar to those detected in the animals infected with serovar Gallinarum (Fig. 1). None of the infected animals experienced diarrhea.
FIG. 1.
Rectal temperatures of lambs following oral inoculation with 5 × 108 CFU of Salmonella serovars Abortusovis (closed circles), Dublin (closed squares), or Gallinarum (open triangles). Each datum point is the mean of temperatures from five animals plus or minus the standard error of the mean (SEM).
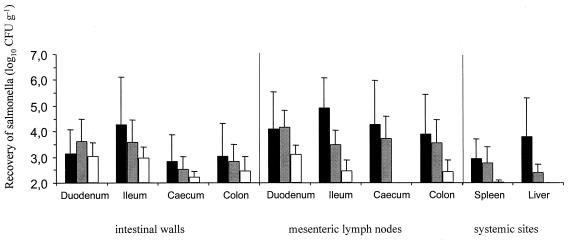
Seven days after inoculation, all three serovars were recovered from each of the intestinal mucosal tissues in comparable numbers (Fig. 2). Serovars Dublin and Abortusovis were reproducibly recovered in higher numbers than serovar Gallinarum from the various mesenteric lymph nodes (MLNs). Serovar Gallinarum was not recovered from liver tissue, and its recovery from spleen tissue was in significantly lower amounts than that of serovars Dublin and Abortusovis (P = 0.02 and P = 0.03, respectively). Taken together, these data suggest that although serovar Gallinarum is able to colonize ovine intestinal tissues, it is unable to establish a systemic infection in lambs.
FIG. 2.
Recovery of Salmonella serovars from intestinal walls, MLNs, and systemic sites of lambs infected orally with 5 × 108 CFU of Salmonella serovars Dublin (black bars), Abortusovis (grey bars), and Gallinarum (white bars). Each bar represents the mean of triplicate samples from five animals ± SEM. Recovery of serovar Gallinarum from spleen was significantly lower than that of Salmonella serovars Dublin (P = 0.02) and Abortusovis (P = 0.03).
Virulence of different Salmonella serovars following intravenous inoculation of lambs.
To evaluate whether the extent of Salmonella colonization of the ovine intestine might affect subsequent bacterial systemic dissemination, the virulence of the different Salmonella serovars was assessed after intravenous (i.v.) inoculation. Lambs were inoculated with 5 × 106 CFU, and the recovery of bacteria at intestinal and systemic sites was enumerated at 7 days postinfection.
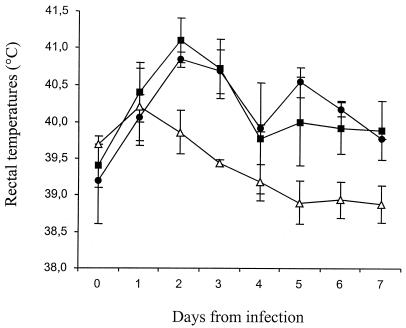
Serovars Dublin and Abortusovis induced a rapid increase in rectal temperature, which peaked at 2 days postinoculation (Fig. 3). Serovar Gallinarum induced a mild and transient increase in rectal temperature. Two of five, one of five, and none of five of the animals infected with serovars Dublin, Abortusovis, and Gallinarum, respectively, experienced diarrhea and fecal shedding of the corresponding serovar.
FIG. 3.
Rectal temperatures of lambs inoculated i.v. with 5 × 106 CFU of Salmonella serovars Abortusovis (closed circles), Dublin (closed squares) or Gallinarum (open triangles). Each datum point represents the mean of five animals ± SEM.
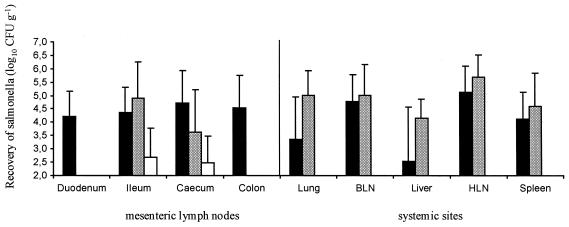
Bacterial recovery of Salmonella serovars from the intestinal mucosae was more variable and these bacteria were in lower numbers than those recovered from orally inoculated lambs (data not shown), with the exception of serovar Abortusovis in the ileal wall (4.0 ± 1.8 log10 CFU/g of tissue). Serovar Dublin colonized the MLNs, draining all regions of the intestines. Serovars Abortusovis and Gallinarum were recovered from ileal and caecal lymph nodes only; serovar Gallinarum was recovered in the lowest numbers, but there were no significant differences between the three serovars (P > 0.05) at these sites (Fig. 4). Serovar Abortusovis was recovered in higher numbers than serovar Dublin from all systemic sites, but this difference was not statistically significant (P > 0.05). It is interesting that serovar Gallinarum was not recovered from any systemic site (Fig. 4).
FIG. 4.
Recovery of Salmonella serovars from MLNs and systemic sites of lambs infected i.v. with 5 × 106 CFU of Salmonella serovars Dublin (closed bars), Abortusovis (stippled bars), and Gallinarum (open bars). BLN, bronchial lymph node; HLN, hepatic lymph node. Each bar represents the mean of triplicate samples from five animals ± SEM.
Intestinal invasion of different Salmonella serovars in ovine ligated ileal loops.
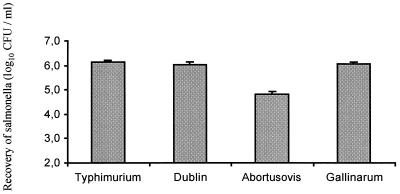
To evaluate the role of intestinal colonization in the pathogenesis of ovine salmonellosis, the invasiveness of serovars Abortusovis (strain SS44), Dublin (strain SD2229), and Gallinarum (strain G9) was assessed in ovine ligated ileal loops. Serovar Typhimurium strain 4/74 was included to allow comparison to the established bovine ligated ileal loop assay. Three hours after inoculation of loops, intracellular bacteria were enumerated with a gentamicin protection assay (Fig. 5). The invasiveness of serovar Typhimurium was comparable to that of serovars Dublin and Gallinarum (P = 0.353 and 0.653, respectively). In contrast serovar Abortusovis was recovered in significantly lower numbers than the other serovars (P < 0.001) (Fig. 5). Similar levels of invasiveness were observed following inoculation of loops with serovar Abortusovis strain 15/5 (5.67 ± 0.10 CFU/ml), serovar Dublin strain SD3246 (6.23 ± 0.03 CFU/ml; P = 0.002 versus 15/5), and serovar Gallinarum strain J91 (6.05 ± 0.09 CFU/ml; P = 0.02 versus 15/5). Each strain was tested in triplicate.
FIG. 5.
Relative invasiveness of Salmonella serovars Typhimurium (strain 4/74), Dublin (strain SD2229), Abortusovis (strain SS44), and Gallinarum (strain G9) in ovine ileal loops; each bar represents the mean from 21, 12, 21, and 12 loops tested ± SEM, respectively. Three samples were analyzed from each loop.
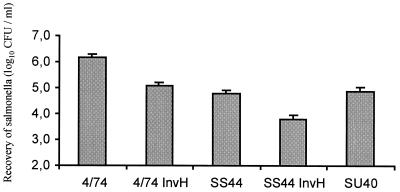
The role of SPI-1 and the virulence plasmid genes in influencing serovar Abortusovis invasion of the ovine intestinal mucosa was evaluated. Wild-type and invH mutants of serovars Typhimurium strain 4/74 and Abortusovis strain SS44 and a plasmid-cured strain (SU40) of serovar Abortusovis SS44 were inoculated into ovine ileal loops. Both serovars Typhimurium InvH (P < 0.001) and Abortusovis InvH (P = 0.002) were recovered in significantly lower numbers than their respective wild-type strains, showing that invasion of ovine intestinal epithelium was mediated by a SPI-1–dependent mechanism (Fig. 6). The wild-type and the plasmid-cured strains of serovar Abortusovis were recovered in similar numbers, demonstrating that the virulence plasmid genes did not influence intestinal invasion (P = 0.406) (Fig. 6).
FIG. 6.
Relative levels of invasiveness of Salmonella serovars Typhimurium (strains 4/74 and 4/74 InvH) and Abortusovis (strains SS44, SS44 InvH, and plasmid-cured SU40) in ovine ileal loops. The bars represent the mean bacterial recoveries from nine loops tested ± SEM. Three samples were analyzed from each loop. SS44 versus SS44 InvH, P = 0.002; SS44 versus SU40, P = 0.406; 4/74 versus 4/74 InvH, P < 0.001.
Histological analysis of Salmonella-infected ovine ileal mucosa.
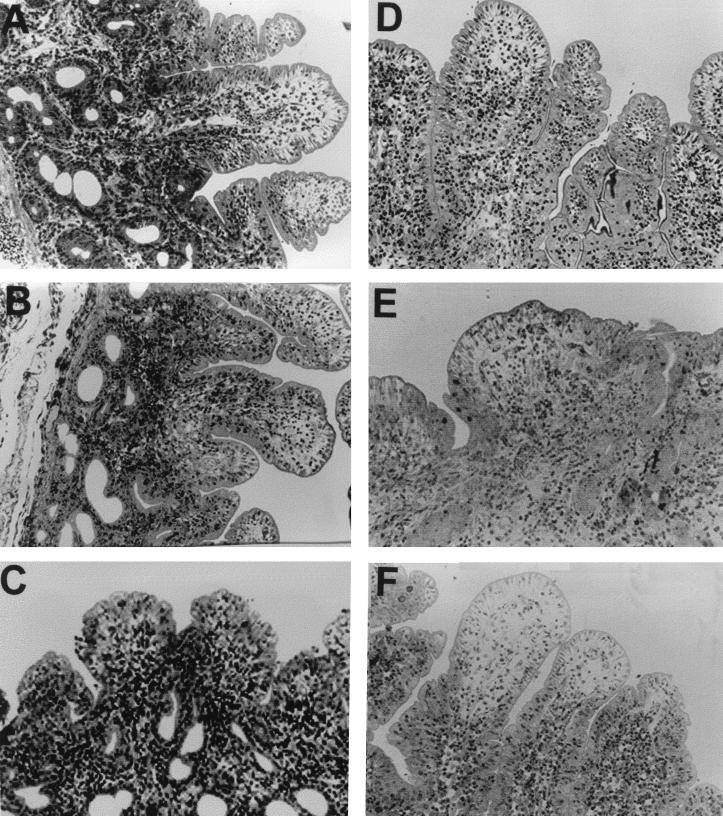
Morphological changes induced by the different Salmonella serovars were assessed by microscopic analysis of sections of infected ileal mucosa stained with hematoxylin and eosin. Within 3 h of loop inoculation, serovars Dublin (SD2229) and Typhimurium (4/74) induced considerable villous atrophy, with associated extrusion of enterocytes and infiltration of inflammatory cells into the submucosa and epithelium (Fig. 7C and E). The architecture of mucosa from loops infected with serovars Abortusovis (SS44) and Gallinarum (G9) was intact (Fig. 7B and D) and indistinguishable from that of uninfected control loops (Fig. 7A). The invH mutation reduced the severity of the damage induced by serovar Typhimurium (Fig. 7F). Hystological analysis of ovine ileal mucosa infected with strains 15/5, SD3246, and J91 showed morphological changes that were identical to those observed with strains SS44, SD2229, and G9, respectively (data not shown).
FIG. 7.
Cross sections of intestinal mucosa from ovine ileal loops stained with hematoxylin and eosin and incubated for 3 h with Salmonella serovars. (A) Untreated control; intestinal mucosa infected with (B) serovars Abortusovis (strain SS44), (C) Dublin (strain SD2229), (D) Gallinarum (strain G9), (E) Typhimurium strain 4/74, and (F) Typhimurium strain 4/74 InvH. A minimum of 10 sections were examined for each loop infected (n = 6) with each Salmonella strain. Representative sections are shown. Magnification, ×400.
Taken together, these results suggest that the relative invasiveness of serovars Dublin, Abortusovis, Gallinarum, and Typhimurium does not correspond to their ability to cause pathological changes in the ovine intestinal mucosa and that other factors, independent of Salmonella internalization, are involved in the disease process.
DISCUSSION
We have investigated the pathogenesis of different Salmonella serovars, possessing different degrees of host restriction, in order to evaluate the basis of serovar-host specificity in sheep. Young animals (lambs) were used because they are more susceptible than adult sheep to symptomatic salmonellosis following inoculation with serovars Abortusovis and Dublin (15). Infection of lambs with serovar Abortusovis resulted in symptoms of salmonellosis, including an increase in temperature and bacterial dissemination to systemic tissues. This finding confirms the association of serovar Abortusovis with sheep and the virulence of the strain chosen for study. Serovar Gallinarum caused relatively mild disease, which confirmed its lack of association with sheep, despite the fact that the same strain is virulent in chickens (3). Serovar Dublin was virulent in sheep, confirming its association with ovine salmonellosis.
The apparent specificity of a serovar for a particular host or range of hosts as defined by epidemiological data is influenced not only by bacterial virulence but also by the ability of the serovar to circulate within the population of the host. It has been proposed that the specificity of a serovar for one host may be unrelated to its degree of virulence in other hosts (18). The characterization of the host range of serovar Dublin, which is virulent in cattle (33) and sheep (22; this study) but is relatively avirulent in pigs (36) and chickens (P. A. Barrow, P. Wigley, and M. Jones, personal communication), demonstrates that there is a correlation between its virulence in a range of hosts and the incidence of natural disease in these hosts. This finding certainly does not prove that virulence is the only factor determining serovar-host specificity, but it illustrates that virulence may be important and justifies our experimental approach, i.e., determining why S. enterica serovars differ in their virulence for different hosts as a means of increasing our understanding of serovar-host specificity.
The ability of S. enterica serovars to invade and/or persist within the ovine intestinal mucosa did not correlate with their degree of virulence for sheep. Orally inoculated serovar Gallinarum showed considerable colonization of ovine intestinal tissues 7 days after oral inoculation and was recovered from ovine ligated ileal loops in greater numbers than serovar Abortusovis despite its inability to cause disease in sheep. Furthermore, serovar Abortusovis was recovered in significantly lower numbers than serovar Dublin from ileal loops, despite being as virulent as serovar Dublin in orally inoculated sheep. Serovar Dublin but not serovars Abortusovis or Gallinarum induced an inflammatory response and dramatic changes in villus architecture. This differential induction of damage has implications for the interpretation of the recovery data, as discussed previously (5). It is probable that the recovery of serovar Dublin was reduced by shedding of infected enterocytes and the uptake of gentamicin by damaged tissue. It is therefore possible that the difference between serovars Dublin and Abortusovis is greater than that measured and that serovar Dublin is relatively more invasive than serovar Gallinarum. Since, neither serovar Gallinarum nor Abortusovis induced damage to the intestines, the lower number of serovar Abortusovis cells recovered is probably an accurate reflection of its relative invasiveness. A lack of correlation between the magnitude of intestinal invasion and virulence has been previously reported in pigs; serovars Dublin and Choleraesuis were recovered in comparable numbers from ileal loops (5) despite the inability of serovar Dublin to cause disease in pigs (36). In contrast, Pascopella et al. (25) reported a correlation between the inability of serovar Gallinarum to invade murine intestines and its avirulence in mice. Taken together, these results suggest that invasion of the intestinal mucosa is necessary for host-restricted serovars to access systemic sites, but this phenotype alone does not determine Salmonella serovar host specificity.
Serovar Abortusovis did not induce mucosal damage or inflammation in ovine ligated ileal loops, despite its virulence in sheep. This is analogous to the pathogenesis of the host-restricted serovars Gallinarum and Pullorum in chickens, in which systemic spread occurs rapidly and with relatively little intestinal inflammation (13, 27). The low level of intestinal inflammation in infected chickens has been correlated to a low induction of proinflammatory cytokines in enterocytes infected with serovar Gallinarum (17). A similar result was obtained in an in vitro human cell assay, in which serovar Typhi induced a relatively low migration of polymorphonuclear cells across an epithelial monolayer when compared to Salmonella serovars associated with enteritis in humans (23). An acute intestinal inflammatory response may act to contain an intestinal infection. Therefore, avoidance of the induction of an intestinal inflammatory response may facilitate the systemic spread of highly host-restricted serovars like Abortusovis. However, this property is unlikely to be the sole factor determining host specificity, since serovar Gallinarum invades ovine intestines and induces a similarly small inflammatory response but is avirulent in sheep.
Serovar Dublin was recovered in comparable numbers and induced changes to the intestinal mucosa similar to those induced by serovar Typhimurium in ovine ligated ileal loops. This similarity between serovars Dublin and Typhimurium has also been reported in bovine and porcine ligated ileal loops (6, 35), and both serovars induce a large influx of fluid and inflammatory cells into bovine ligated ileal loops (35). The induction of an inflammatory response and mucosal damage by serovars Dublin and Typhimurium may be due to a mechanism that is absent in serovar Abortusovis. SPI-1 is a major virulence locus that influences intestinal invasion and enteropathogenesis of serovars Typhimurium and Dublin (10, 35). This locus is conserved in many different Salmonella serovars (7), but its function in the different serovars has received little study. Recent data suggest that SPI-1 is not an important virulence factor in serovar Gallinarum, since mutation of spaS does not affect the virulence of this serotype in chickens following oral challenge (P. A. Barrow, P. Wigley, and M. Jones, personal communication). We have shown that SPI-1 is functional in serovar Abortusovis and influences intestinal invasion in ovine ligated ileal loops. Expression by serovar Abortusovis of secreted effectors that influence enteropathogenesis, such as SopD and SopB (32), warrants further investigation. The mucosal damage induced in ovine ileal loops by serovar Typhimurium is impaired by disruption of the inv-spa-encoded type III protein secretion system encoded by SPI-1.
The evolution of host-specific Salmonella serovars is considered to be associated with an increase in pathogenicity for the specific host (4). This hypothesis is based on the fact that broad-range serovars (i.e., serovars Typhimurium and Enteritidis) are generally associated with severe disease only in young animals, whereas host-restricted serovars cause high mortality in both young and adult hosts. In this respect, the specificity of serovar Abortusovis to sheep appears to be different. Serovar Abortusovis shows low virulence in adult sheep, a fact that becomes particularly evident during abortion. When serovar Abortusovis reaches high counts in the fetal organs, infection of the maternal tissues is limited, and the ewe appears healthy (G. S. Leori et al., unpublished data) (14). It therefore appears that the specificity of serovar Abortusovis to sheep is associated with evolution toward reduced virulence in adult sheep but with the retention of virulence for fetal and newborn lambs. These observations are consistent with a strategy of “stealth” to facilitate bacterial dissemination into the environment and infection of other hosts. The broad-host-range enteropathogenic serovars such as Typhimurium elicit acute diarrheal disease through the acquisition of SPI-1 and associated secreted effector proteins (32). Diarrheal disease results in the prolonged shedding of large numbers of Salmonella cells into the environment. Here we have shown that serovar Abortusovis invades the intestinal mucosa in a SPI-1–dependent mechanism but in relatively low numbers and that it fails to elicit enteritis. Yet via infection of unborn lambs, this serovar is able to disseminate into the environment in high numbers. The molecular genetic characterization of these apparently divergent evolutionary mechanisms still awaits clarification.
ACKNOWLEDGMENTS
This work was supported by grants Ministero dell'Università e della Ricerca Scientifica e Technologia (S.R.), Ministry for Agriculture, Fishery, and Food grant OZ0315, and Biotechnology and Biological Sciences Research Council grant 201/S10274 (T.S.W.) and European Union grant Fair3 CT96-1743 (S.R. and T.S.W.).
We are grateful to A. Montella for critical advice on microscopy analysis. We are also indebted to M. P. Satta and A. Fiori for their invaluable technical assistance.
REFERENCES
- 1.Amin I I, Douce G R, Osbourne M P, Stephen J. Quantitative studies of invasion of rabbit ileal mucosa by Salmonella typhimurium strains which differ in virulence in a model of gastroenteritis. Infect Immun. 1994;62:569–578. doi: 10.1128/iai.62.2.569-578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird G D, Manning E J, Jones P W. Evidence for related virulence sequences in plasmids of Salmonella dublin and Salmonella typhimurium. J Gen Microbiol. 1985;131:1815–1823. doi: 10.1099/00221287-131-7-1815. [DOI] [PubMed] [Google Scholar]
- 3.Barrow P A, Huggins M B, Lovell M A. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect Immun. 1994;62:4602–4610. doi: 10.1128/iai.62.10.4602-4610.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler A J, Tsolis R M, Ficht T A, Adams L G. Evolution of host adaptation in Salmonella enterica. Infect Immun. 1998;66:4579–4587. doi: 10.1128/iai.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton A J, Osborne M P, Wallis T S, Stephen J. Interaction of Salmonella choleraesuis, Salmonella dublin and Salmonella typhimurium with porcine and bovine terminal ileum in vivo. Microbiology. 1999;145:2431–2441. doi: 10.1099/00221287-145-9-2431. [DOI] [PubMed] [Google Scholar]
- 6.Colombo M M, Leori G, Rubino S, Barbato A, Cappuccinelli P. Phenotypic features and molecular characterization of plasmids in Salmonella abortusovis. J Gen Microbiol. 1992;138:725–731. [Google Scholar]
- 7.Darwin K H, Miller V L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12:405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edsall G, Gaines S, Landy M. Studies on infection and immunity in experimental typhoid fever. I. Typhoid fever in chimpanzees orally infected with Salmonella typhosa. J Exp Med. 1960;112:143–166. doi: 10.1084/jem.112.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmerth M, Goebel W, Miller S I, Hueck C J. Genomic subtraction identifies Salmonella typhimurium prophages, F-related plasmid sequences, and a novel fimbrial operon, stf, which are absent in Salmonella typhi. J Bacteriol. 1999;181:5652–5661. doi: 10.1128/jb.181.18.5652-5661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 11.Gitter M, Sojka W J. S. dublin abortion in sheep. Vet Rec. 1970;87:775–778. doi: 10.1136/vr.87.25.775. [DOI] [PubMed] [Google Scholar]
- 12.Gulig P A, Doyle T J. The Salmonella typhimurium virulence plasmid increases the growth rate of salmonellae in mice. Infect Immun. 1993;61:504–511. doi: 10.1128/iai.61.2.504-511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson S C, Bounous D I, Lee M D. Early events in the pathogenesis of avian salmonellosis. Infect Immun. 1999;67:3580–3586. doi: 10.1128/iai.67.7.3580-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack E J. Salmonella abortusovis: an atypical Salmonella. Vet Rec. 1968;82:558–561. [Google Scholar]
- 15.Jack E J. Salmonella abortion in sheep. Vet Annu. 1971;12:57–63. [Google Scholar]
- 16.Jones P W, Dougan G, Hayward C, Mackensie N, Collins P, Chatfield S N. Oral vaccination of calves against experimental salmonellosis using a double aro mutant of Salmonella typhimurium. Vaccine. 1991;9:29–34. doi: 10.1016/0264-410x(91)90313-u. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser P, Rothwell L, Galyov E E, Barrow P A, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 18.Kingsley R A, Baumler A J. Host adaptation and the emergence of infectious disease: the Salmonella paradigm. Mol Microbiol. 2000;36:1006–1014. doi: 10.1046/j.1365-2958.2000.01907.x. [DOI] [PubMed] [Google Scholar]
- 19.Lantier F, Pardon P, Marly J. Immunogenicity of a low-virulence vaccinal strain against Salmonella abortusovis infection in mice. Infect Immun. 1983;40:601–607. doi: 10.1128/iai.40.2.601-607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby S L, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lissner C R, Weinstein D L, O'Brien A D. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J Immunol. 1985;135:544–547. [PubMed] [Google Scholar]
- 22.McCaughey W J, Kavanagh P J, McClelland T G. Experimental Salmonella dublin infection in sheep. Br Vet J. 1971;127:557–566. doi: 10.1016/s0007-1935(17)37232-9. [DOI] [PubMed] [Google Scholar]
- 23.McCormick B A, Miller S I, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardon P, Sanchis R, Marly J, Lantier F, Pepin M, Popoff M. Ovine salmonellosis caused by Salmonella abortus ovis. Ann Rech Vet. 1988;19:221–235. [PubMed] [Google Scholar]
- 25.Pascopella L, Raupach B, Ghori N, Monack D, Falkow S, Small P L. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun. 1995;63:4329–4335. doi: 10.1128/iai.63.11.4329-4335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popoff M Y, Miras I, Coynault C, Lasselin C, Pardon P. Molecular relationships between virulence plasmids of Salmonella serotypes typhimurium and dublin and large plasmids of other Salmonella serotypes. Ann Microbiol. 1984;135A:389–398. doi: 10.1016/s0769-2609(84)80080-0. [DOI] [PubMed] [Google Scholar]
- 27.Smith H W. The susceptibility of different chicken breeds to Salmonella gallinurum infection. Poult Sci. 1956;35:701–705. [Google Scholar]
- 28.Taylor D N, Bied J M, Munro J S, Feldman R A. Salmonella dublin infections in the United States, 1979–1980. J Infect Dis. 1982;146:322–327. doi: 10.1093/infdis/146.3.322. [DOI] [PubMed] [Google Scholar]
- 29.Tsolis R M, Adams L G, Ficht T A, Baumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzzau S, Gulig P A, Paglietti B, Leori G, Stocker B A D, Rubino S. Role of the Salmonella abortusovis virulence plasmid in the infection of BALB/c mice. FEMS Microbiol Lett. 2000;188:15–18. doi: 10.1111/j.1574-6968.2000.tb09161.x. [DOI] [PubMed] [Google Scholar]
- 31.Vladoianu I R, Chang H R, Pechere J C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog. 1990;8:83–90. doi: 10.1016/0882-4010(90)90072-x. [DOI] [PubMed] [Google Scholar]
- 32.Wallis T S, Galyov E E. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- 33.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson P R, Galyov E E, Paulin S M, Jones P W, Wallis T S. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun. 1998;66:1432–1438. doi: 10.1128/iai.66.4.1432-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson P R, Paulin S M, Bland A P, Jones P W, Wallis T S. Characterization of intestinal invasion by Salmonella typhimurium and Salmonella dublin and effect of a mutation in the invH gene. Infect Immun. 1995;63:2743–2754. doi: 10.1128/iai.63.7.2743-2754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson P R, Paulin S M, Jones P W, Wallis T S. Interaction of Salmonella serotypes with porcine macrophages in vitro does not correlate with virulence. Microbiology. 2000;146:1639–1649. doi: 10.1099/00221287-146-7-1639. [DOI] [PubMed] [Google Scholar]