Abstract
Australian Animal Disease Spread (AADIS) epidemiological simulation modelling of potential foot‐and‐mouth disease outbreaks in the state of Victoria, Australia examined the targeted use of limited vaccine supplies in combination with varying surveillance resources. Updated, detailed estimates of government response costs were prepared based on state level data inputs of required and available resources. Measures of outbreak spread such as duration and numbers of animals removed through depopulation of infected and vaccinated herds from the epidemiological modelling were compared to summed government response costs. This comparison illustrated the trade‐offs between targeted control strategies combining vaccination‐to‐remove and varying surveillance capacities and their corresponding costs. For this intensive cattle and sheep producing region: (1) Targeting vaccination toward intensive production areas or toward specialized cattle operations had outbreak control and response cost advantages similar to vaccination of all species. The median duration was reduced by 27% and response costs by 11%. (2) Adding to the pool of outbreak surveillance resources available further decreased outbreak duration and outbreak response costs. The median duration was reduced by an additional 13% and response costs declined by an additional 8%. (3) Pooling of vaccine resources overcame the very early binding constraints under proportional allocation of vaccines to individual states with similar reductions in outbreak duration to those with additional surveillance resources. However, government costs rose substantially by over 40% and introduced additional risk of a negative consumer response. Increased knowledge of the outbreak situation obtained from more surveillance led to better‐informed vaccination deployment decisions in the short timeframe they needed to be made.
Keywords: AADIS, foot‐and‐mouth disease, model, surveillance, vaccination, vaccine allocation
The delivery of strain‐appropriate vaccines in sufficient quantities to control foot‐and‐mouth disease (FMD) outbreaks around the world requires efficient management and the use of vaccines. The trade‐limiting effects of using vaccines in an FMD control program reinforce the need for judicious use. These effects arise because, under World Organization for Animal Health guidelines, 1 whether removing or retaining vaccinated animals from the population at the end of an outbreak, it takes longer to regain FMD‐free status compared with situations where stamping out alone is used. This situation is exacerbated if vaccinated animals are retained as it will delay the period until FMD‐free status is regained under international guidelines and add additional complexity to the post‐outbreak surveillance programs aimed at supporting the re‐establishment of FMD‐free status.
Several modelling studies have found disease control benefits from FMD vaccination applied to large outbreaks in formerly FMD‐free countries where vaccinated animals are removed from the population toward the end of, or shortly after the outbreak, and not allowed to enter the food supply (also known as vaccinate‐to‐remove). 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Australian and New Zealand based studies have added that targeted suppressive vaccination of cattle‐only or animals in intensive production areas has offered similar benefits to vaccination of all susceptible species, with fewer animals needing to be vaccinated and lower response costs. 9 , 11 , 12 Additional consideration of response resource constraints 13 in combination with vaccination has reinforced these results.
The objective of this work was to explore vaccine requirements and whether combinations of targeting FMD vaccines by area or species and additional surveillance resources would lead to more efficient use of available vaccine supplies. Using the Australian Animal Disease Spread model (AADIS), 14 a large hypothetical FMD outbreak was simulated starting on a dairy farm in coastal southwestern Victoria in spring. Control programs based on stamping out with or without vaccination were used, and in the former case, a vaccinate‐to‐remove policy was assumed. A fixed national supply of vaccine doses was allocated in two manners – proportionally to individual states and under a shared vaccine pool arrangement. As previous work had shown that the availability of response resources, particularly the ability to find and remove infected premises, is a key determinant of control programs' effectiveness, 13 , 15 , 16 we also ran simulations varying surveillance resources. Trade‐offs between outbreak extents' effects on economic impact and changes in updated estimates of government response costs were determined.
Materials and methods
Epidemiological modelling
The AADIS model version 2.46.6 14 , 17 is a national‐scale epidemiological model used by animal health authorities in Australia to support FMD planning and preparedness. It provides a spatiotemporal agent‐based simulation of the spread and control of an emergency animal disease. The herd is the epidemiological unit, where a herd is defined as a group of co‐mingling animals of the same species under the same production system. A farm may have one or more herds (e.g., a mixed beef‐sheep farm would be made up of a beef cattle herd and a sheep herd).
AADIS has a hybrid architecture that combines mathematical and agent‐based modelling techniques. The spread of disease within a herd is represented by a Susceptible, Exposed, Infectious, Recovered compartmental model implemented as a system of ordinary differential equations (ODEs). It models the herd's infected, infectious, serological, and clinical prevalence over time, taking into account species, production system, and virus strain. For simplicity, the number of animals in any given herd is assumed to be constant, that is, deaths and transfers out are equivalent to births and transfers in. A summary of the herd types used in the AADIS‐FMD model is provided in Table 1. The herd dataset used in the AADIS‐FMD model is derived from a blend of agricultural census data, industry reports and expert opinion. The spread of disease between herds is modelled with a stochastic and spatially explicit agent‐based approach. The herd agents interact in a model environment that stochastically spreads disease across multiple spread pathways such as direct contacts, indirect contacts, saleyard spread, airborne transmission and local spread. Details of the AADIS‐FMD spread pathways can be found in References 14 and 18.
Table 1.
Herd types used in the AADIS‐FMD model
| Herd type | Description | Number of herds | Average herd size | Herd size distribution (min, median, max) |
|---|---|---|---|---|
| Beef extensive | Extensive beef cattle production primarily on large acreages in northern Australia | 3,993 | 1,909 | 400, 1,426, 15,525 |
| Beef intensive | Intensive beef cattle production | 53,458 | 247 | 20, 114, 10,596 |
| Feedlot | Intensive production system where beef cattle are fattened to slaughter weight | 482 | 2057 | 2, 1,041, 45,593 |
| Mixed beef | Beef cattle production within a mixed beef sheep farm | 21,521 | 194 | 1, 100, 14,451 |
| Mixed sheep | Sheep meat or wool production within a mixed beef sheep farm | 21,330 | 1,336 | 1, 800, 69,764 |
| Dairy | Dairy cattle production | 9,628 | 256 | 1, 214, 5,744 |
| Pigs small | Small‐scale pig production system with less than 100 sows and less than 1000 animals. Includes breeding to finish, grower, and breeding to weaner operations. Movements off farms are typical to other farms or sale yards. | 2,379 | 142 | 1, 70, 999 |
| Pigs large | Large‐scale pig production system with 100 or more sows or 1000 or more animals. Includes single and multi‐site breeding to finish, breeding to weaner, grower and integrated operations. Movements off farms are typical of abattoirs. Farms are assumed to have very good biosecurity measures in place. | 320 | 5,884 | 1,000, 3,503, 179,475 |
| Sheep | Broadacre sheep meat or wool production systems | 25,917 | 1,311 | 20, 1,003, 44,000 |
| Smallholders | Small number of mixed‐species animals kept primarily for non‐commercial or micro‐scale commercial purposes. Arbitrarily defined as less than 20 animals on less than 20 ha | 95,498 | 6 | 1, 5, 19 |
| Total | 234,526 |
Baseline control measures are based on the Australian Veterinary Emergency Plan for FMD. 19 In brief, Australia's response to an FMD incursion involves an initial national livestock standstill and subsequent movement restrictions around infected premises (IPs), surveillance and tracking, and stamping out of IPs. Movement restrictions will apply to Restricted Areas (RAs) and Control Areas (CAs). An RA will be a relatively small, declared area (minimum radius 3 km) around IPs and dangerous contact premises (DCPs), and will be subject to intensive surveillance and movement controls. Movement out of the area will be prohibited except under strict permit conditions. The CA will be a larger declared area around the RA(s) – possibly as large as the state or territory in which the outbreak occurs – where restrictions will reduce the risk of disease spreading from the RA. The boundary will be adjusted as confidence about the extent of the outbreak increases but will have a minimum radius of 10 km. In this study we assumed large RAs and CAs initially, that would be reduced in size at two weeks and four weeks into the control program, respectively. Vaccination may also be considered in Australia as part of a response if authorities believe that it would be beneficial in containing and managing the outbreak. Like an actual outbreak response, the simulated control measures can be dynamically constrained by the available resources (e.g., labour and consumables such as vaccine), the accuracy of reports of clinical disease, inefficiencies in tracing systems, and non‐compliance with movement restrictions.
This paper builds on recent AADIS FMD modelling studies enhancing the examination of improved surveillance tools and sampling designs for post‐outbreak management of vaccinated animals. 3 , 20 , 21 An FMD incursion scenario was developed in consultation with the local Victorian animal health authorities. 5 The scenario involved infection being introduced into a dairy farm in coastal southwestern Victoria in spring, under conditions expected to favour the establishment and spread of FMD. Victoria is Australia's largest food and fibre exporting state and is the centre of Australia's dairy production. Victoria has more intensive farming and higher livestock densities than much of the rest of Australia with 0.4 herds and 546 head of livestock per square kilometre in the three local government areas surrounding the seed herd simulatedhere. Because of the mild climate, higher stocking rates, relatively high human population density and proximity to airports and seaports, Victoria is considered a higher risk area of Australia for the introduction, establishment, spread, and economic impact of FMD. 22
The model was run initially without any control measures to the end of a fixed 21‐day ‘silent spread’ phase (i.e., the period until first detection). Previous modelling studies have shown that the time delay until FMD is first detected is a key determinant of the size of an outbreak, feasibility of eradication and cost of the response. 23 , 24 The assumption of 21 days to detection was based on the estimated median time to detection of FMD in Australia should it be introduced. 25 This period was consistent with previously FMD‐free countries that have experienced outbreaks of FMD, with time delays to detection in the range of 15–29 days. 26 , 27 , 28 , 29 A representative iteration at this point in time was selected. This run served as the basis (i.e., the starting situation) for subsequently comparing control strategies. The advantage of this approach was any differences associated with variability in spread prior to the first detection were removed, and the control programs all started from the same infection situation on the day the first case was found.
Two approaches to control were considered, stamping out without vaccination (SO1) and stamping out plus emergency vaccination in a ring around infected premises. Three vaccination options were compared:
all susceptible species (SO3)*;
limited to farms in intensive, high density, production areas (SO4);
targeted to cattle‐only enterprises (feedlots, dairy, and intensive beef farms, but excluding mixed beef‐sheep farms; SO6).
Where vaccination was used, a vaccinate‐and‐remove policy for managing vaccinated animals was simulated. Due to constraints associated with formulating and delivering vaccines to Australia, it was assumed that the earliest vaccination could begin is 14 days into the control program. It was also assumed that emergency ring vaccination would only be triggered once a specified number of IPs (n = 5) had been reached. Thus, vaccination would be triggered on or after day 14 of the control program once five IPs had been found. We assumed all species would be vaccinated according to the following order: 1 (highest priority) = beef feedlot, dairy herds; 2 = intensive beef herds, cattle and sheep on mixed beef‐sheep establishments, sheep; 3 = small and large piggeries, smallholders. Large extensive beef herds, which are only found in northern Australia, were excluded from vaccination. Key response parameter settings used for this study are listed in Table 2.
Table 2.
Key control measures used for this study
| Parameter | Setting |
|---|---|
| Time to detect first infected property | 21 days |
| National livestock standstill period | 3 days |
| Control area (CA) initial size | Whole state/territory |
| Restricted area (RA) initial size | Local government area (LGA) |
| Days into the control program before CA and RA are reduced in size | 14 days and a further 14 days |
| Control area (CA) subsequent size | Radial zone around IP of 50 km radius, then 10 km radius |
| Restricted area (RA) subsequent size | Radial zone around IP of 10 km, then 3 km radius |
| Vaccination start | ≥14 days into control program conditional on at least 5 IPs being reported |
In addition, increasing resources available for surveillance visits and two approaches to vaccine distribution were considered. To test how sensitive the results were to the ability to find and remove infected herds, the number of surveillance teams in Victoria was increased from current estimates of available resources of 50 teams to 75 teams for SO1 and each of the vaccination control strategies, SO3, SO4, and SO6. States and territories either had access to up to 100,000 doses of FMD vaccine each, or they were able to access a shared national pool of vaccines up to 500,000 doses. Cattle and pigs each received one dose, while small ruminants received half of a cattle dose. Under the 100,000‐dose constraint, limiting vaccination to the one heavily infected state, Victoria, to bolster the implementation of trading zones was examined.
The full set of 14 control options is summarised in Table 3. For each simulation, the model was run for 500 iterations. Preliminary work had shown that this provided a high level of convergence for key outbreak metrics like size, duration, and control costs. For a set of model runs, convergence provided an indication as to how close the sample mean was to the theoretical population mean. 30
Table 3.
Control strategies examined under Victorian incursion scenario (FMD3)
| SO1 Base a | SO1 1.5xSurv a | 100K b | 100K VIC b | 100K 1.5xSurv b | 500K b | |
|---|---|---|---|---|---|---|
| Control | Stamping out only | Stamping out plus emergency ring vaccination around infected premises | ||||
| Species vaccinated | None | FMD susceptible species (SO3) | ||||
| High risk, livestock dense areas (SO4) | ||||||
| Feedlot, dairy, and intensive beef cattle, excluding mixed beef‐sheep farms (SO6) | ||||||
| Vaccination ring size | None | 5 km | ||||
| Vaccination distribution | None | 100,000 dose maximum per state | 500,000 dose national shared pool maximum | |||
| Vaccination limited to Victoria only | X | |||||
| Number of surveillance teams | 50 | 75 | 50 | 50 | 75 | 50 |
One scenario run for each stamping out option.
Three scenarios run for each vaccination option with species vaccinated varying.
FMD, foot‐and‐mouth disease.
Economic impact, resource requirements and cost calculations
Previous AADIS work and additional studies have included measures of economic, or market, impacts of FMD outbreaks by estimating changes in trade revenues or by modelling the price and quantity changes caused by disruptions in production, consumption, and international trade. The extent of these potential economic impacts can influence countries' decision‐making on how to determine response resources for protecting producers in the event of outbreaks. Due to Australia's positions in exporting beef, lamb, dairy products, sheep meat, and wool, longer outbreak durations are expected to cause large additional economic losses ranging into billions of dollars. 4 , 31 Potential economic losses for producers of $27.7 billion (AUD) were modelled by Seitzinger et al. 32 under control by stamping out alone with the application of trading zones for a similar Victoria, Australia incursion scenario. A vaccination strategy applied to all susceptible species combined with application of trading zones decreased the losses 12.6% to $24.2 billion as simulated outbreak duration at the 75th percentile fell from 170 to 118 days. Rather than relying on OIE guidelines for declarations of return to FMD free status as the determinant of export recovery time, the modelling applied the length of export recovery as estimated from historical outbreaks of FMD based on outbreak length. The change in estimated avoided economic losses under vaccination is used to provide context of the potential economic impacts for the additional vaccination option scenarios simulated for this paper.
Government response costs, and the associated financial decision making, occur in assigning the allocated, response resources to specific control measures during an outbreak. Although typically much smaller than estimates of FMD's economic impact on exporting countries, they play an increased role when response strategies become more targeted and government budgets become constrained.
The AADIS model supports a range of resource requirements and constraints as well as government response costs for key control operations. Australian jurisdictions were requested to provide updates in Australian dollars to the ongoing estimation of resources and costs for the various control activities as developed previously in References 3, 15, 20, 33. Updates were received from all seven jurisdictions, with Victoria and New South Wales (NSW) providing the most detailed estimates. The efficacy, efficiency and eventual success of any control operation depended on the availability of resources. AADIS explicitly modelled the human resources required to complete field operations as ‘teams’, where a team could be comprised of up to one veterinarian and two support staff depending on the assigned task. AADIS also allocated more than one team to a given farm for field operations and therefore reduced the time taken to complete the activity if these resources were available.
Surveillance, depopulation, disposal, decontamination, vaccination, and compensation were included as discrete field operational activities. IPs were found through passive (farmer) reporting of clinical cases, tracing of movements onto or off known IPs and active surveillance (scheduled inspections) of all properties with susceptible livestock in RAs. All of these processes generated the need for a visit by a surveillance team in a prioritized order. Once FMD had been confirmed the property was managed by an IP operations (IPOPs) team. If available teams were insufficient to accommodate all the operational activities scheduled for a given day, a backlog built up and was carried over to the next day. The estimated times required to complete field activities are provided in Table 4 with more detail on their calculation found in Supporting Information.
Table 4.
Days to complete field operations for slaughter, disposal, surveillance, and vaccination by enterprise
| Culling (days/team/farm) | Disposal (days/team/farm) | Surveillance (days/team/farm) | Vaccination (days/team/farm) | |
|---|---|---|---|---|
| Beef extensive | 14 | 7 | 3 | 3 |
| Beef intensive | 1.6 | 1.5 | 1 | 1 |
| Feedlot | 9 | 9 | 1 | 3 |
| Dairy | 1.6 | 1.5 | 0.7 | 1 |
| Pigs small | 1 | 0.5 | 0.5 | 1 |
| Pigs large | 3 | 3 | 1 | 2 |
| Sheep | 2 | 2 | 1 | 2 |
| Smallholders | 0.3 | 0.3 | 0.3 | 0.3 |
It was unclear from estimates provided by animal health staff whether the private sector or the international animal health reserve were considered when estimating IPOPs and surveillance resources because several jurisdictions appeared to underestimate the maximum resource potentially available based on recent emergency animal disease experiences. For example, the number of teams available to carry out surveillance activities during the equine influenza outbreak in Australia in 2007 was significantly higher than those estimated by states for this study. In the equine influenza response, the private sector provided a significant contribution. 34 Consequently, the existing estimates in AADIS for these activities were used and cross‐referenced with animal health staff estimates for any major discrepancies. Table 5 lists the availability of teams at the state and national levels as well as the number of days to ramp up from initial to maximum resource levels. This study assumed both farmers and animal health workers conducted vaccination, so human resources were not constrained for this activity.
Table 5.
Initial and maximum number of response teams available by Australian state and days to maximum availability of teams
| State | NSW | VIC | QLD | SA | WA | TAS | NT | National |
|---|---|---|---|---|---|---|---|---|
| Surveillance teams | ||||||||
| Initial number | 5 | 3 | 5 | 5 | 4 | 4 | 2 | 17 |
| Maximum number | 60 | 50 | 40 | 20 | 30 | 15 | 15 | 138 |
| First day of availability | 3 | 3 | 3 | 4 | 3 | 4 | 4 | 3 |
| Days to maximum availability | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Cull teams | ||||||||
| Initial number | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 5 |
| Maximum number | 30 | 25 | 25 | 15 | 20 | 10 | 10 | 81 |
| First day of availability | 3 | 3 | 3 | 4 | 3 | 4 | 3 | 3 |
| Days to maximum availability | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 28 |
| Disposal teams | ||||||||
| Initial number | 5 | 5 | 4 | 4 | 4 | 3 | 3 | 17 |
| Maximum number | 40 | 40 | 28 | 20 | 22 | 14 | 16 | 108 |
| First day of availability | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Days to maximum availability | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Disinfection teams | ||||||||
| Initial number | 10 | 10 | 8 | 7 | 5 | 5 | 5 | 30 |
| Maximum number | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 420 |
| First day of availability | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Days to maximum availability | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Vaccination teams | ||||||||
| Initial number | 20 | 12 | 20 | 15 | 10 | 15 | 5 | 58 |
| Maximum number | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 2100 |
| First day of availability | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Days to maximum availability | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Post outbreak surveillance teams | 75 | 63 | 50 | 25 | 38 | 19 | 19 | 289 |
Australia State Abbreviations: NSW, New South Wales; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
The operational control costs used in this study included those for the field operations described above and for running disease control centres. The same cost estimates for field activities were applied to the post‐outbreak period for surveillance and removal of vaccinated animals from the population. This meant vaccinated animals were assumed to be culled on‐farm rather than being transported to abattoirs. Their calculation is described in more detail in Supporting Information. Estimating total government response costs for each of the simulated outbreaks allowed identification of tradeoffs between changes in the outbreak extent for stamping out alone and vaccination options and the associated response cost estimates, as well as the distributions of these model outputs.
Results
In Figure 1, box and whisker plots show the middle 50% of the results as a box with the median indicated with a line through the box. The whiskers indicate the data within 1.5 times the length of the box. Compared to the baseline stamping out strategy results at the left of Figure 1, vaccination reduced the duration of outbreaks, defined as 28 days after the last day on which control measures took place, and the number of animals culled. Under stamping out alone (SO1 base), the median outbreak duration was 164 days with a range between 142 and 190 days from the 25th to the 75th percentile, respectively (Figure 1). The median number of animals culled was 318,822 and ranged between 245,762 to 427,190. Even though the Victorian vaccine supplies were typically exhausted by the 20th day of the outbreak under a vaccination control strategy where each state was constrained to 100,000 doses being administered to all susceptible species (SO3 100K), the median duration was reduced by 27%, from 164 to 120 days, in the second vertical panel of Figure 1. The median number of animals culled dropped by 35% to 205,919. However, the reduction in animals culled was accompanied by the need to remove 102,050 vaccinates at the median with a range from 46,794 to 118,996. Therefore, although outbreak duration was shortened markedly by use of vaccines, the total median numbers of animals which required removal was similar under vaccination and stamping out.
Figure 1.
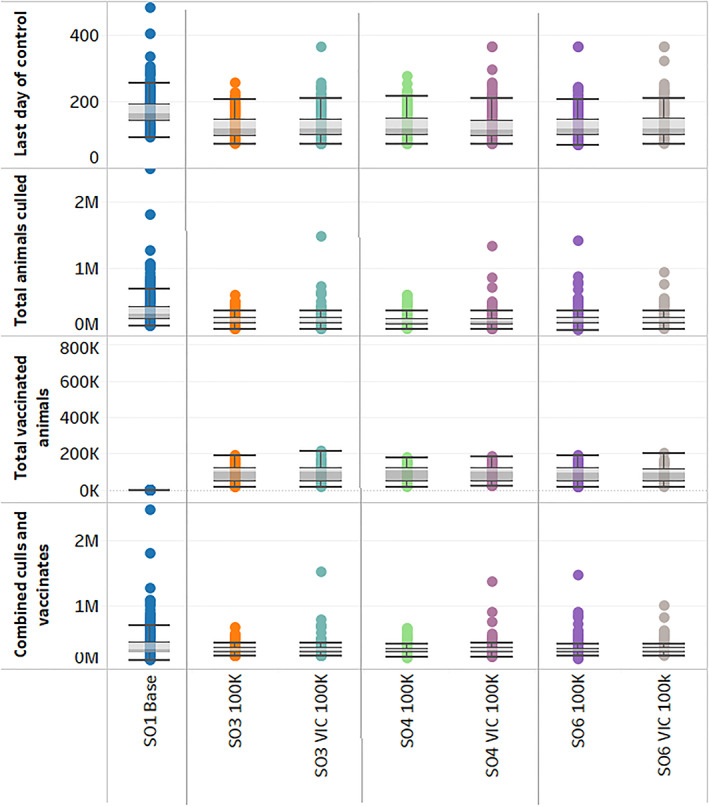
Distributions of AADIS outbreak results comparing stamping out alone with FMD vaccination options targeted by species, geographic areas, and ring size. The naming convention for each of the control strategies specifies: (A) vaccine strategy: no vaccination (SOI), vaccination of FMD susceptible species (SO3), high risk, livestock dense areas (SO4); feedlot, dairy, and intensive beef cattle, excluding mixed beef‐sheep farms (SO6). (B) Vaccine is limited to Victoria (VIC). (C) Vaccine dose allocation strategy: 100,000 dose maximum per state (100K); 500,000 dose national shared pool maximum (500K). AADIS, Australian Animal Disease Spread; FMD, foot‐and‐mouth disease.
Targeting vaccination toward intensive production areas (SO4 100K) shown in the third panel of Figure 1 had the same median duration of vaccinating all species, but there was a reduction in the number of animals culled by 3% and an increase in the number of animals vaccinated by 5%. This increase is due to changes in the composition of the vaccinated population with more small ruminants being vaccinated under SO4 compared to SO3. Since small ruminants require only half a vaccine dose, more animals in total are vaccinated. Targeting specialized cattle operations (SO6 100K) in the right panel of Figure 1 led to only slight changes in the median duration of outbreaks and the number of animals culled and decreased the median number of animals vaccinated by 4%. The similarities in these results reflected the species and production intensity characteristics of Victoria's livestock populations.
Due to the potential advantages of the establishment of trading zones, limiting vaccine use geographically to Victoria alone was also of interest. Under the 100,000‐dose constraint (VIC 100K), slightly fewer animals were required to be vaccinated to achieve similar median durations under each of the vaccine options (SO3, SO4, and SO6). The number of animals culled were also similar to those for wider geographic use of vaccination. However, the upper end of outbreak distributions was longer by several days when vaccination only occurs in Victoria.
Figure 2 shows the results of creating a combined 500,000 vaccine dose pool (500K) relative to the stamping out only and vaccination strategies already described. The median duration of outbreaks fell to their lowest levels among all the simulations. Median duration dropped by 45% to 91 days, with a smaller range between 84 and 100 days for the 25th and 75th percentiles, respectively, where all susceptible species were vaccinated (SO3 500K) as shown in the second vertical panel of Figure 2. The number of animals culled was 49% lower at 161,618 with a range from 140,566 to 185,341. These outcomes were similar for vaccines targeted to intensive production areas and to specialized cattle operations (SO4 500K and SO6 500K) as shown in the third and fourth panels of Figure 2. However, the median number of vaccinated animals climbed 493% to 587,652 with a range from 508,272 to 666,515 when all susceptible species were included, using the entire national supply of vaccines in the pool. Advantages to targeted vaccination of intensive production areas and specialized cattle operations under the 500,000 pooled doses were evident with the median number of vaccinates falling by almost one quarter.
Figure 2.
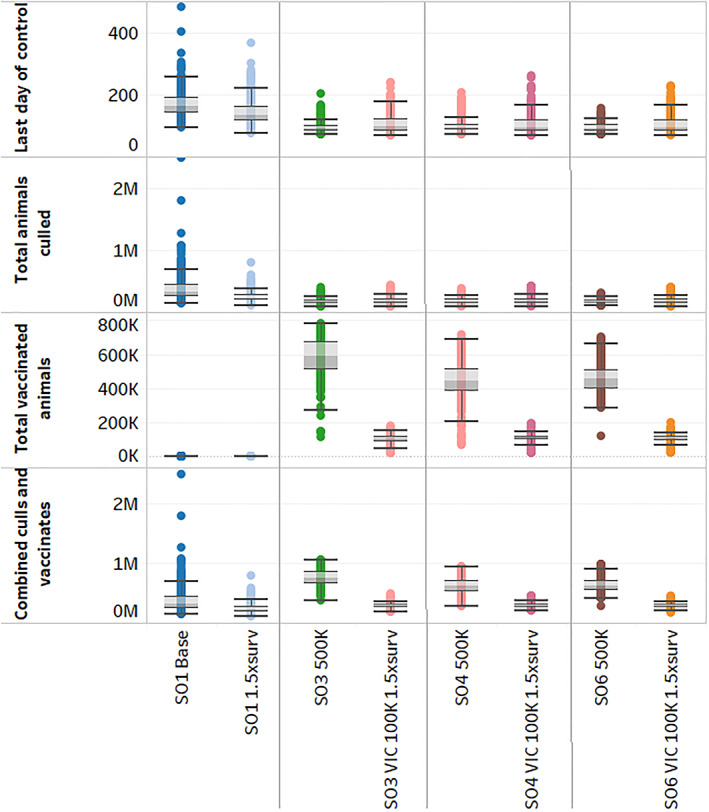
Distributions of AADIS outbreak results in pooled vaccine doses and increased surveillance resources. The naming convention for each of the control strategies specifies: (A) vaccine strategy: no vaccination (SO1), vaccination of FMD susceptible species (SO3); high risk, livestock dense areas (SO4), feedlot, dairy, and intensive beef cattle, excluding mixed beef‐sheep farms (SO6). (B) Vaccine is limited to Victoria (VIC). (C) Vaccine dose allocation strategy: 100,000 dose maximum per state (100K), 500,000 dose national shared pool maximum (500K). (D) Additional surveillance resources (1.5xsurv). AADIS, Australian Animal Disease Spread; FMD, foot‐and‐mouth disease
Because the results for stamping out alone and the vaccination options showed large deficits in covering surveillance visits (Figures 3A and 4A), sensitivity analysis was also conducted by increasing the number of surveillance teams from 50 to 75 (SO1 1.5xsurv). The median duration compared to stamping out alone with baseline surveillance resources was 18% lower at 134 days, and the median number of animals culled decreased by 29% to 226,225 (Figure 2, first vertical panel). The change in surveillance visit deficits due to increased surveillance resources is shown in Figure 3B for the first 100 days of the simulations. The lower bound on surveillance visit deficits dipped slightly compared to Figure 3A, while the distribution of visit deficits became much shorter.
Figure 3.
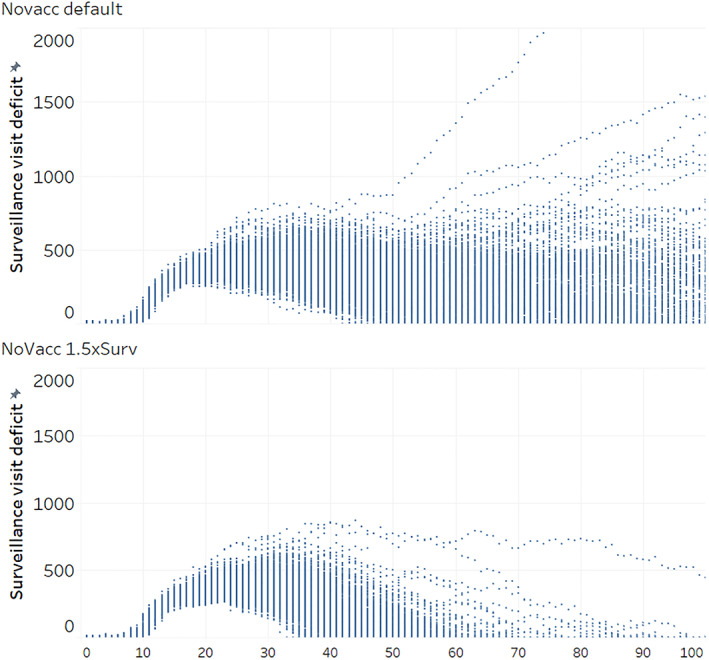
(A, B) Surveillance visit deficits for first 100 outbreak days of 500 iterations of AADIS under stamping out alone (SO1) and current surveillance resource estimate versus 50% increase in surveillance resources (1.5xsurv). Surveillance visit deficit: number of surveillance visits which could not be completed for each day of simulated outbreak. AADIS, Australian Animal Disease Spread.
Figure 4.
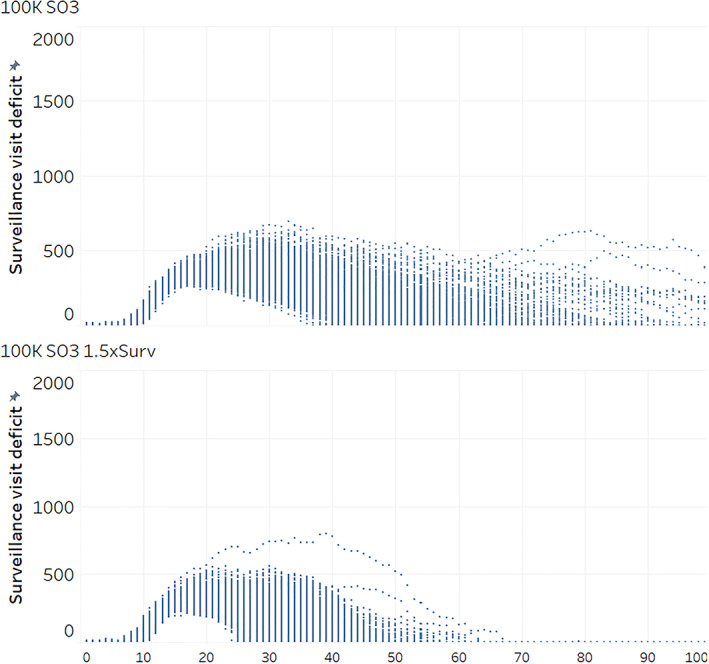
(A, B) Surveillance visit deficits for first 100 outbreak days of 500 iterations of AADIS under vaccination to remove all susceptible species (SO3) and current surveillance resource estimate versus 50% increase in surveillance resources (1.5xsurv). Surveillance visit deficit: number of surveillance visits which could not be completed for each day of simulated outbreak. AADIS, Australian Animal Disease Spread.
The additional surveillance resources led to a 27% decline in the median durations for each of the groups vaccinated in Victoria relative to the stamping out alone control option with additional surveillance (SO3 VIC 100K 1.5xsurv, SO4 VIC 100K 1.5xsurv, and SO6 VIC 100K 1.5xsurv; Figure 2). The median number of animals culled fell by 24%. In each of these three cases, a narrowing in the ranges of vaccinated animal numbers occurred with the additional surveillance resources. Figure 4B illustrates the further narrowing in the surveillance visit deficit distribution which took place when combining vaccination with additional surveillance resources, with all susceptible species vaccination as the example.
Increasing surveillance resources whether stamping out alone or stamping out with vaccination consistently had the most favourable impact on geographic disease spread. In each case, the spread was limited to Victoria up through the 75th percentile of runs. All other control strategies except for vaccines targeted at specialized cattle under SO3 100K showed two infected states at the 75th percentile.
The reductions in outbreak durations between the no vaccination and the vaccination to remove scenarios in Figure 1 are very close to those used to model the $3.5 billion decrease in potential economic impact cited above. 32 The additional similar declines in outbreak durations achieved in the simulation results presented in Figure 2 for either a national vaccine pool or increased surveillance resources would be estimated to provide another $3.5 billion in avoided economic losses. With these economic losses far outweighing the range of total response costs presented for these control strategies in Figure 5, the application of these vaccination options is considered beneficial compared to a policy of stamping out alone. Calculation of their government response costs provides information further delineating the two options.
Figure 5.
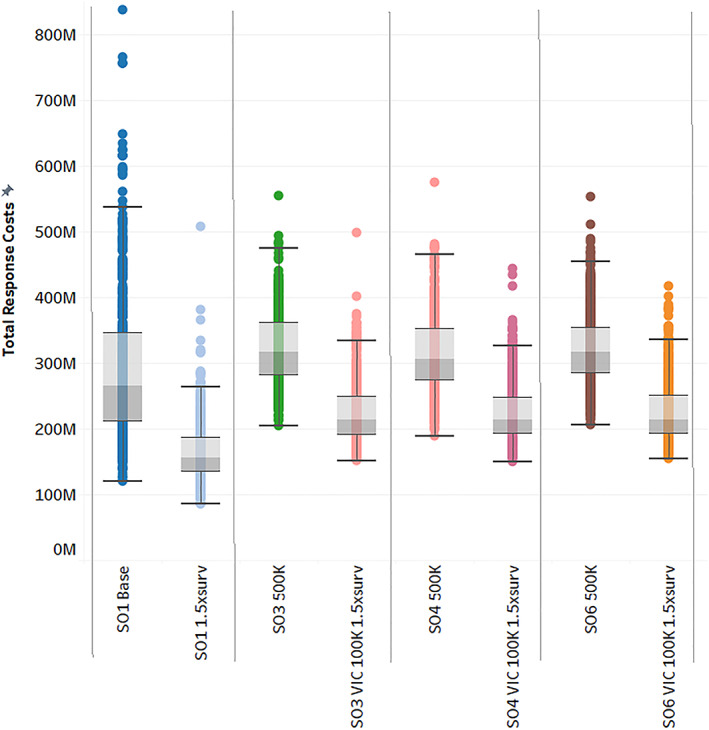
Distributions of AADIS total response costs with pooled vaccine doses and increased response costs. AADIS, Australian Animal Disease Spread.
Table 6 lists the per‐unit costs of individual response activities. Comparison of this paper's updated government response cost estimates with those found in Reference 3 reveals similar results when scaled to the size of outbreaks under study. This occurs even though per unit costs across production types incorporated in AADIS for this work vary much more for smallholder operations in particular.
Table 6.
Estimated per unit Australian outbreak response costs
| AUD/head | AUD/operation | AUD/farm/day | AUD/head | |||
|---|---|---|---|---|---|---|
| Culling | Disposal | Decontamination | Surveillance | Vaccination | Compensation | |
| Beef extensive | 6.50 | 5.00 | 15,000 | 3100 | 2.68 | 1074 |
| Beef intensive | 15.40 | 20.00 | 15,000 | 2500 | 6.64 | 1074 |
| Feedlot | 13.10 | 10.00 | 50,000 | 2500 | 3.80 | 1677 |
| Mixed beef | 15.40 | 20.00 | 15,000 | 2500 | 6.64 | 1074 |
| Mixed sheep | 15.40 | 2.50 | 10,000 | 2500 | 2.20 | 111 |
| Dairy | 14.50 | 20.00 | 50,000 | 2500 | 6.38 | 1600 |
| Pigs small | 20.60 | 29.00 | 10,000 | 1400 | 19.65 | 202 |
| Pigs large | 3.40 | 5.00 | 70,000 | 2500 | 3.00 | 202 |
| Sheep | 3.20 | 2.50 | 10,000 | 2500 | 2.20 | 111 |
| Smallholders | 180.00 | 200.00 | 2500 | 1300 | 92.00 | 462 |
Per animal compensation costs were at least sixfold higher than per animal culling, disposal, and vaccination costs. Similarly, compensation costs dominated decontamination and surveillance costs per operation even for much smaller than average Australian herd sizes (Appendix S1). Because compensation costs accounted for more than 50% of median total response costs in all scenarios, the range of total government response costs was heavily influenced by the total numbers of animals culled and vaccinated. Figure 5 shows increasing surveillance resources from 50 to 75 teams in the first vertical panel decreased median total response costs under stamping out alone by 41%, or $109 million. It also markedly narrowed the distribution of costs. This lowest‐cost control option was due to shortening the median outbreak duration by 30 days and lowering the median number of animals culled by over 92,000.
Access to a pool of 500,000 vaccine doses increased median total government response costs to between $306 million and $318 million, 20% above stamping out alone. Vaccination options combined with increased surveillance resources in the remaining panels of Figure 5 (SO3 VIC 100K 1.5xsurv, SO4 VIC 100K 1.5xsurv, and SO6 VIC 100K 1.5xsurv) lay about midway between stamping out alone and stamping out alone with increased surveillance resources at median total response costs between $213 million and $215 million. With the rise in surveillance resources, limiting vaccine use to Victoria while targeting intensive livestock production areas introduced slightly more variability in the duration and cost ranges, but less in the range of animal numbers removed whether for disease control or due to vaccination. The economic impact benefits of reducing the outbreak were much higher than the increased government response costs from paying for either pooled vaccine doses or additional surveillance combined with targeted vaccination. However, any consumer reaction due to animal welfare concerns from a 167% increase in animals removed could be expected to offset the benefits of the additional 8‐day reduction under the 500,000‐dose vaccine pool in comparison to the combination of additional surveillance resources with targeted vaccination.
Discussion
Modelling studies in New Zealand, 16 Denmark, 35 and Scotland 8 also focused on further targeting vaccine doses and varying response resources during a potential FMD outbreak. 16 found that the odds of a large outbreak were decreased by 22% by allowing dynamic outbreak statistics that are predictive of outbreak extent to influence the triggering of vaccination. This adaptive strategy administered a median of almost 37,000 doses of vaccine with a range up to almost 557,000 doses. The odds of a large outbreak were reduced by 27% when constraints on the availability of veterinarians for surveillance were lifted above 200 visits per day. The number of vaccine doses required fell to almost 21,000 at the median with the upper range declining to 429,050 doses. Although costs were not estimated in this work, administering vaccines more efficiently dovetailed with making the additional surveillance visits possible in terms of effectively managing overall response resource constraints and reducing the impact of managing vaccinated animals.
Assuming a vaccinate‐to‐live FMD control strategy for Scotland combined with the culling of infected premises and dangerous contacts where the situation is conducive to the development of large outbreaks, Porphyre et al. 8 found that 200,000 vaccine doses would be enough to maximise the epidemiological and economic benefits of vaccination. In contrast, with only 100,000 doses of vaccine initially available and re‐stocking taking more than two weeks, the costs of controlling outbreaks rapidly increased. Additionally, vaccine stocks greater than 500,000 doses (equivalent to 30% of Scotland's cattle inventory) did not further reduce the probability of direct costs exceeding 500 million pounds. Under the vaccinate‐to‐live assumption, producer compensation payments were not affected by changes in numbers vaccinated, and the limited export of live animals and animal products from Scotland to other countries kept trade impacts of implementing vaccination low.
Willeberg et al. 35 also pointed to the tradeoffs between disease control and economic impacts under FMD vaccination control strategies when examining early decision indicators of outbreak extent. For Denmark, they state ‘risk managers might tolerate up to a moderate likelihood of a high number of outbreaks in order to avoid these economic consequences of vaccination [related to delayed return to export markets]. However, if the decision tool predicts vaccination to spare a relative[ly] large number of outbreaks, the added costs may appear acceptable, also considering the welfare benefits of a limited culling after implementation of a suppressive vaccination strategy.’ This paper also addressed the need for being able to communicate modelling results based on early indicators effectively to decision‐makers in the time frame required for optimal use of vaccination strategies but without estimating the costs of response.
The results presented here as well as previous literature confirmed that increasing surveillance resources in combination with vaccination are economically and response cost‐effective. Increased knowledge of the outbreak situation provided by additional surveillance led to better‐informed vaccination deployment decisions in the short timeframe they needed to be made. Further work is required to define more precisely the indicated middle ground of vaccine supplies needed to best support a decision to deploy vaccines.
While the benefits of combining FMD vaccination with increased surveillance resources were presented here, in practice this may not be easy to achieve to the extent needed. The resource levels used in this study were based on best estimates by jurisdictional animal health staff and reflect the expected availability of vaccine supplies and veterinary and paraprofessional personnel. International vaccine supply contractual arrangements have improved, and increased engagement from the Australian government has developed more consistent arrangements for the employment of private veterinary practitioners in emergency animal disease responses. Veterinary personnel from other countries can also be drawn upon through Australia's membership in the International Animal Health Emergency Reserve. 36 Sharing of critical resources across veterinary and human medicine during the COVID‐19 pandemic under sudden, extreme need has revealed further approaches to increasing the pool of response resources. 37 However, these options are all subject to the needs of other ongoing and emergency animal health events. As a result, new work is already commencing on managing constrained response resources in a One Health approach, particularly for sudden events.
Results of this work highlight the need for periodic updates of a decision support tool's underlying data for livestock populations, livestock movements, and response cost estimates at jurisdictional levels to refine vaccination response strategies. Applying cost parameters to smaller geographical areas that are the source of estimates is expected to further enhance understanding of individual targeted vaccine strategies' tradeoffs and offer the potential for examining new tools for more effectively managing response costs. 38 Frustration in the lack of up‐to‐date, consistent data for estimation of government response costs is expressed across the above‐cited literature and in additional work in the United States. In particular, examination of possible vaccination to retain strategies for countries with larger exports of livestock and livestock products requires updated, and more refined, industry and location‐specific cost estimates.
Future research focuses on how to gain industry, jurisdictional and Australian government, and public acceptance of targeted vaccination strategies not only through estimates of their value in lowering government response costs but also for the benefits they offer in maintaining Victorian business continuity during outbreaks.
Finally, the value of livestock disease surveillance demonstrated here provides greater motivation for examining advances in FMD point‐of‐care diagnostics and bulk testing for their potential contributions to improving accuracy and reducing the time and costs involved in outbreak surveillance. In turn, this information could be used to determine more efficient use of the FMD vaccine. Differential time periods for delaying return to OIE recognition of FMD freedom are being challenged 39 and new diagnostic approaches offer the potential that surveillance might be able to provide acceptable levels of confidence in the infection status of vaccinated populations in the future. While the declaration of OIE disease freedom influences trade recovery, it is increasingly recognised to be only one of many factors affecting the return to export markets.
Acknowledgements
We would like to acknowledge numerous contributions from the Australian jurisdictions' government staff and industry representatives who generously contributed their knowledge and experience as inputs into this research. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Conflicts of interest and sources of funding
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This project is supported by Meat & Livestock Australia (MLA), through funding from the Australian Government Department of Agriculture, Water, and the Environment as part of its Rural R&D for Profit program, and by producer levies from Australian FMD‐susceptible livestock (cattle, sheep, goats and pigs) industries and Charles Sturt University (CSU), leveraging significant in‐kind support from the research partners. The research partners for this project are the Commonwealth Science and Industrial Research Organisation (CSIRO), CSU through the Graham Centre for Agricultural Innovation, the Bureau of Meteorology (BOM) and the Australian Department of Agriculture, Water and the Environment, supported by Animal Health Australia (AHA).
Supporting information
Appendix S1. Supporting information.
Seitzinger, AH. , Garner, MG. , Bradhurst, R. , Roche, S. , Breed, AC. , Capon, T. , Miller, C. and Tapsuwan, S. , FMD vaccine allocation and surveillance resourcing options for a potential Australian incursion. Aust Vet J. 2022;100:550–561. 10.1111/avj.13195
Endnote
References to control strategies are not sequential due to maintaining consistency with the larger project's scenario naming conventions.
References
- 1. OIE, W.O.f.A.H . Terrestrial animal health code chapter 8.8 infection with foot and mouth disease virus. Paris, 2019. Available at: https://rr-europe.oie.int/wp-content/uploads/2020/08/oie-terrestrial-code-2_2019_en.pdf. Accessed March 8, 2022.
- 2. Barratt AS, Rich KM, Eze JI et al. Framework for estimating indirect costs in animal health using time series analysis. Front Vet Sci 2019;6:190. 10.3389/fvets.2019.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradhurst R, Garner G, East I et al. Management strategies for vaccinated animals after an outbreak of foot‐and‐mouth disease and the impact on return to trade. Plos One 2019;14:e0223518. 10.1371/journal.pone.0223518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buetre, B , Wicks, S , Kruger, H , et al. Potential socio‐economic impacts of an outbreak of foot‐and‐mouth disease in Australia. ABARES research report, Canberra, ACT, 95, 2013. Available at: https://www.awe.gov.au/sites/default/files/abares/documents/RR13.11PotSocEcoImpctOfFMD_v1.0.0.pdf. Accessed 12 January 2022.
- 5. Capon TR, Garner MG, Tapsuwan S et al. A simulation study of the use of vaccination to control foot‐and‐mouth‐disease outbreaks across Australia. Front Vet Sci 2021;2021(8):1–15. 10.3389/fvets.2021.648003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng S, Patton M, Davis J. Market impact of foot‐and‐mouth disease control strategies: a UK case study. Front Vet Sci 2017;4:129. 10.3389/fvets.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller M, Liu L, Shwiff S et al. Macroeconomic impact of foot‐and‐mouth disease vaccination strategies for an outbreak in the Midwestern United States: a computable general equilibrium. Transbound Emerg Dis 2019;66:156–165. 10.1111/tbed.12995. [DOI] [PubMed] [Google Scholar]
- 8. Porphyre T, Rich KM, Auty HK. Assessing the economic impact of vaccine availability when controlling foot and mouth disease outbreaks. Front Vet Sci 2018;5:47. 10.3389/fvets.2018.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanson RL, Rawdon T, Owen K et al. Evaluating the benefits of vaccination when used in combination with stamping‐out measures against hypothetical introductions of foot‐and‐mouth disease into New Zealand: a simulation study. N Z Vet J 2017;65:124–133. 10.1080/00480169.2016.1263165. [DOI] [PubMed] [Google Scholar]
- 10. Schroeder TC, Pendell DL, Sanderson MW et al. Economic impact of alternative Fmd emergency vaccination strategies in the Midwestern United States. J Agric Appl Econ 2015;47:47–76. 10.1017/aae.2014.5. [DOI] [Google Scholar]
- 11. Rawdon TG, Garner MG, Sanson RL et al. Evaluating vaccination strategies to control foot‐and‐mouth disease: a country comparison study. Epidemiol Infect 2018;146:1138–1150. 10.1017/S0950268818001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roche SE, Garner MG, Sanson RL et al. Evaluating vaccination strategies to control foot‐and‐mouth disease: a model comparison study. Epidemiol Infect 2014a;143:1256–1275. 10.1017/S0950268814001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roche SE, Garner MG, Wicks RM et al. How do resources influence control measures during a simulated outbreak of foot and mouth disease in Australia? Prev Vet Med 2014b;113:436–446. 10.1016/j.prevetmed.2013.12.0. [DOI] [PubMed] [Google Scholar]
- 14. Bradhurst RA, Roche SE, East IJ et al. A hybrid modeling approach to simulating foot‐and‐mouth disease outbreaks in Australian livestock. Front Environ Sci 2015;3(17):1‐20. 10.3389/fenvs.2015.00017. [DOI] [Google Scholar]
- 15. Garner MG, Bombarderi N, Cozens M et al. Estimating resource requirements to staff a response to a medium to large outbreak of foot and mouth disease in Australia. Transbound Emerg Dis 2016;63:109–121. 10.1111/tbed.12239. [DOI] [PubMed] [Google Scholar]
- 16. Sanson RL, Yu ZD, Rawdon TG et al. Investigations into a trigger‐based approach for initiating emergency vaccination to augment stamping‐out of foot‐and‐mouth disease in New Zealand: a simulation study. N Z Vet J 2021;69:313–326. 10.1080/00480169.2021.1921069. [DOI] [PubMed] [Google Scholar]
- 17. Bradhurst RA, Roche SE, East IJ et al. Improving the computational efficiency of an agent‐based spatiotemporal model of livestock disease spread and control. Environ Model Softw 2016;77:1–12. 10.1016/j.envsoft.2015.11.015. [DOI] [Google Scholar]
- 18. Bradhurst R. Modelling the spatiotemporal spread and control of viral disease in livestock using a hybrid equation‐based and agent‐based approach [PhD thesis]. University of New England, 2015. Available at: https://rune.une.edu.au/web/bitstream/1959.11/19661/5/open/SOURCE03.pdf. Accessed 8 March 2022.
- 19. Animal Health Australia . Disease strategy: foot‐and‐mouth disease (version 3.4). Canberra, ACT, 2014. Available at: https://animalhealthaustralia.com.au/ausvetplan/. Accessed 12 January 2022.
- 20. Bradhurst R, Garner G, East I et al. Post‐outbreak surveillance strategies to support proof‐of‐freedom from foot‐and‐mouth disease. BioRxiv 2021;2021. 10.1101/2021.04.27.441714. [DOI] [Google Scholar]
- 21. Garner MG, Vosloo W, Tapsuwan S et al. Comparing surveillance approaches to support regaining free status after a foot‐and‐mouth disease outbreak. Prev Vet Med 2021;194:105441. 10.1016/j.prevetmed.2021.105441. [DOI] [PubMed] [Google Scholar]
- 22. East IJ, Wicks RM, Martin PAJ et al. Use of a multi‐criteria analysis framework to inform the design of risk based general surveillance systems for animal disease in Australia. Prev Vet Med 2013;112:230–247. 10.1016/j.prevetmed.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 23. Carpenter TE, O'Brien JM, Hagerman AD et al. Epidemic and economic impacts of delayed detection of foot‐and‐mouth disease: a case study of a simulated outbreak in California. J Vet Diagn Invest 2011;23:26–33. 10.1177/104063871102300104. [DOI] [PubMed] [Google Scholar]
- 24. East IJ, Martin PAJ, Langstaff I et al. Assessing the delay to detection and the size of the outbreak at the time of detection of incursions of foot and mouth disease in Australia. Prev Vet Med 2016;123:1–11. 10.1016/j.prevetmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 25. Martin PA, Langstaff I, Iglesias RM et al. Assessing the efficacy of general surveillance for detection of incursions of livestock diseases in Australia. Prev Vet Med 2015;121:215–230. 10.1016/j.prevetmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 26. Anderson I. Foot and mouth disease 2001: lessons to be learned inquiry report. London, England, The Stationery Office, HCC888, 2002. Available at: https://www.jesip.org.uk/uploads/media/incident_reports_and_inquiries/Foot%20and%20Mouth%20Disease%202001%20Inquiry%20Report.pdf. Accessed 8 March 2022.
- 27. Bouma A, Elbers A, Dekker A et al. The foot‐and‐mouth disease epidemic in The Netherlands in 2001. Prev Vet Med 2003;57:155–166. 10.1016/S0167-5877(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 28. ProMED‐mail . Foot & mouth disease ‐ Japan: (MZ) update. ProMED‐mail 2010. Accessed 23 October 2015.
- 29. Yoon H, Yoon S‐S, Kim Y‐J et al. Epidemiology of the foot‐and‐mouth disease serotype O epidemic of November 2010 to April 2011 in the Republic of Korea. Transbound Emerg Dis. 2013;62:252–263. 10.1111/tbed.12109. [DOI] [PubMed] [Google Scholar]
- 30. Driels MR, Shin YS. Determining the number of iterations for Monte Carlo simulations of weapon effectiveness (no. NPS‐MAE‐04‐005). Naval Postgraduate School, Monterey California, Department of Mechanical and Astronomical Engineering, 2004. Available at: http://hdl.handle.net/10945/798. Accessed 8 March 2022.
- 31. Hafi A, Addai D, Breed AC et al. Economic benefits of implementing trading zones for Australian livestock disease outbreaks of limited duration. Aust Vet J 2022;100:150–161. 10.1111/avj.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seitzinger, AH , Hafi, A , Addai, D et al. Reducing the potential impact of foot and mouth disease in Australia: the economic benefits of targeted response strategies, Prev Vet Med. 2022;204;105636. 10.1016/j.preventmed.2022.105636 [DOI] [PubMed]
- 33. Abdalla A, Beare S, Cao L et al. Foot and mouth disease: evaluating alternatives for controlling a possible outbreak in Australia, ABARE eReport 05.6, Canberra, April, 2005. Available at: https://webarchive.nla.gov.au/awa/20050623073511/http://pandora.nla.gov.au/pan/32832/20050623-0000/PC13123.pdf. Accessed 12 January 2022.
- 34. Webster WR. Overview of the 2007 Australian outbreak of equine influenza. Aust Vet J 2011;89(1):3–4. 10.1111/j.1751-0813.2011.00721.x. [DOI] [PubMed] [Google Scholar]
- 35. Willeberg PW, AlKhamis M, Boklund A et al. Semiquantitative decision tools for FMD emergency vaccination informed by field observations and simulated outbreak data. Front Vet Sci 2017;4:43. 10.3389/fvets.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vallis R. Renewed commitment to international animal health emergency reserve. Aust Vet J 2012;90:N15. [Google Scholar]
- 37. Sumption K, Knight‐Jones TJD, McLaws M et al. Parallels, differences and lessons: a comparison of the management of foot‐and‐mouth disease and COVID‐19 using UK2001/2020 as points of reference. Proc R Soc B 2020;287:20200906. 10.1098/rspb.2020.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seeger RM, Hagerman AD, Johnson KK et al. When poultry take a sick leave: response costs for the 2014–2015 highly pathogenic avian influenza epidemic in the USA. Food Policy 2021;102:102068. 10.1016/j.foodpol.2021.102068. [DOI] [Google Scholar]
- 39. Hagerman, AD , Johnson, KK , Holmstrom, L et al. Saving our bacon without hamstringing the industry: sensitivity of economic losses to post‐outbreak management of foot‐and‐mouth disease vaccinated animals in a simulated US outbreak. 2018 Agricultural & Applied Economics Association Annual Meeting, Washington, DC, 2018. 10.22004/ag.econ.273866. [DOI]
- 40. Geale DW, Barnett PV, Clarke GW et al. A review of OIE country status recovery using vaccinate‐to‐live versus vaccinate‐to‐die foot‐and‐mouth disease response policies II: waiting periods after emergency vaccination in FMD free countries. Transbound Emerg Dis 2015;62:388–406. 10.1111/tbed.12165. [DOI] [PubMed] [Google Scholar]
- 41. Animal Health Australia . Emergency animal disease response agreement (EADRA). Version 20/01‐08/20, 2002. Available at: https://animalhealthaustralia.com.au/eadra/. Accessed 8 March 2022.
- 42. Animal Health Australia . AUSVETPLAN operational manual: decontamination (Version 3.2). Australian Veterinary Emergency Plan (AUSVETPLAN), edition 3, Canberra, ACT, 2008. Available at: https://animalhealthaustralia.com.au/ausvetplan/. Accessed March 8, 2022.
- 43. Animal Health Australia . AUSVETPLAN operational manual: destruction of animals (Version 3.2). Australian Veterinary Emergency Plan (AUSVETPLAN), edition 3, Canberra, ACT, 2015. Available at: https://animalhealthaustralia.com.au/ausvetplan. Accessed January 12, 2022.
- 44. Animal Health Australia . AUSVETPLAN operational manual: disposal (Version 3.2). Australian Veterinary Emergency Plan (AUSVETPLAN), edition 3, Canberra, ACT, 2020. Available at: https://animalhealthaustralia.com.au/ausvetplan/. Accessed 8 March 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


