Abstract
Abstract
Infants and young children receive the highest exposures to antibiotics globally. Although there is building evidence that early life exposure to antibiotics increases susceptibility to various diseases including gut disorders later in life, the lasting impact of early life antibiotics on the physiology of the gut and its enteric nervous system (ENS) remains unclear. We treated neonatal mice with the antibiotic vancomycin during their first 10 postnatal days, then examined potential lasting effects of the antibiotic treatment on their colons during young adulthood (6 weeks old). We found that neonatal vancomycin treatment disrupted the gut functions of young adult female and male mice differently. Antibiotic‐exposed females had significantly longer whole gut transit while antibiotic‐treated males had significantly lower faecal weights compared to controls. Both male and female antibiotic‐treated mice had greater percentages of faecal water content. Neonatal vancomycin treatment also had sexually dimorphic impacts on the neurochemistry and Ca2+ activity of young adult myenteric and submucosal neurons. Myenteric neurons of male mice were more disrupted than those of females, while opposing changes in submucosal neurons were seen in each sex. Neonatal vancomycin also induced sustained changes in colonic microbiota and lasting depletion of mucosal serotonin (5‐HT) levels. Antibiotic impacts on microbiota and mucosal 5‐HT were not sex‐dependent, but we propose that the responses of the host to these changes are sex‐specific. This first demonstration of long‐term impacts of neonatal antibiotics on the ENS, gut microbiota and mucosal 5‐HT has important implications for gut function and other physiological systems of the host.
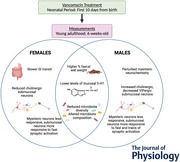
Key points
Early life exposure to antibiotics can increase susceptibility to diseases including functional gastrointestinal (GI) disorders later in life. Yet, the lasting impact of this common therapy on the gut and its enteric nervous system (ENS) remains unclear.
We investigated the long‐term impact of neonatal antibiotic treatment by treating mice with the antibiotic vancomycin during their neonatal period, then examining their colons during young adulthood.
Adolescent female mice given neonatal vancomycin treatment had significantly longer whole gut transit times, while adolescent male and female mice treated with neonatal antibiotics had significantly wetter stools.
Effects of neonatal vancomycin treatment on the neurochemistry and Ca2+ activity of myenteric and submucosal neurons were sexually dimorphic. Neonatal vancomycin also had lasting effects on the colonic microbiome and mucosal serotonin biosynthesis that were not sex‐dependent.
Different male and female responses to antibiotic‐induced disruptions of the ENS, microbiota and mucosal serotonin biosynthesis can lead to sex‐specific impacts on gut function.
Keywords: development, enteric nervous system, microbiota
Abstract figure legend Antibiotics are commonly administered to infants and children around the globe to battle and prevent bacterial infections. We investigated the long‐term repercussions of such early life treatments on the enteric nervous system and the gut. Key findings are highlighted here. Neonatal vancomycin treatment causes long‐lasting changes in the microbiota, lower levels of mucosal serotonin (5‐HT) and wetter stools in young adult mice of either sex. Myenteric and submucosal neuronal excitability caused by synaptic activation was differentially altered between males and females. Only male mice showed perturbed myenteric neurochemistry and only females showed slower gastrointestinal (GI) transit. Our study emphasises the need to address the long‐term, sex‐dependent health implications of early life antibiotic exposure.

Introduction
Pre‐term and low birth weight infants are frequently given prophylactic antibiotics since they are at high risk of developing infections. For example, vancomycin is used to prevent diseases such as necrotising enterocolitis in preterm infants (Langdon et al., 2016; Siu et al., 1998). Despite the benefits of antibiotic therapy, this clinical practice has recently received a lot of negative attention due to its association with paediatric gastrointestinal (GI) disorders (Shaw et al., 2010). Detrimental and long‐lasting effects of antibiotics on the infant have been reported, with increased susceptibility to various diseases including allergy, obesity and inflammatory bowel diseases later in life (Cox et al., 2014; De Vroey et al., 2010; Langdon et al., 2016; Munyaka et al., 2014). Nevertheless, long term impacts of the host of antibiotics given during the critical developmental window immediately following birth remain unclear.
The GI tract is home to the largest and most diverse microbial ecosystem in the human body and is heavily implicated in maintaining host health. In particular, the colon harbours a complex microbial community that is targeted by antibiotics, which results in disease susceptibility (Foong et al., 2020). The extensive network of neurons and glia within the myenteric and submucosal plexus contained within the walls of the GI tract makes up the enteric nervous system (ENS), which regulates vital GI functions including motility and fluid secretion (Bornstein & Foong, 2018; Furness, 2012). Altered microbial community dynamics together with disrupted enteric neuronal excitability are often associated with GI disorders in adults and children (Brierley & Linden, 2014; Foong et al., 2020; Forsythe & Kunze, 2013). Early postnatal life is the major developmental period when commensal microorganisms colonise and have the greatest impact on host physiology (Foong et al., 2020; Langdon et al., 2016). Generally, mice have been central to understanding the physiological significance of host–microbiota interactions and ENS development. In mice, significant anatomical and functional development of enteric neurons and colonic motility occurs postnatally (Foong, 2016; Foong et al., 2015, 2012; Roberts et al., 2010, 2007). We have recently shown that oral administration of vancomycin to mice during their first 10 days of postnatal life significantly disrupted their gut microbiota and the ENS, resulting in colonic dysmotility (Hung et al., 2019). However, it is not yet known whether antibiotics given during this critical developmental window have lasting impacts on the host.
Here, we applied our neonatal antibiotic treatment mouse model and demonstrated that vancomycin given during the infant period induced lasting disruptions in GI function, ENS neurochemistry and activity in a sex‐dependent manner. Although the changes induced by neonatal vancomycin on the levels of mucosal serotonin (5‐HT) and gut microbiota in males and females were indistinguishable, we propose that host responses to these changes are sex‐specific.
Methods
Ethical approval and mice
Wnt1‐Cre (RRID:MGI:2386570) heterozygous mice (Danielian et al., 1998) were mated with R26R‐GCaMP3 homozygous mice (RRID:IMSR_JAX:014538; The Jackson Laboratory, Bar Harbor, ME, USA) to produce progeny that expressed the genetically encoded calcium indicator GCaMP3 in all enteric neurons and glia (Wnt1‐Cre;R26R‐GCaMP3) (Foong et al., 2015; Hennig et al., 2015), or did not express GCaMP3. Newborn mice were designated postnatal day (P)0 animals. All animals had access to food and water ad libitum and were killed humanely by cervical dislocation. All procedures were approved by the University of Melbourne Animal Experimental Ethics Committee (AEC1714195) and conform to The Journal of Physiology’s policy on animal experimentation (Grundy, 2015).
Antibiotic treatment
Mouse pups from each litter were given a dose of sterile water or vancomycin hydrochloride dissolved in sterile water (83 mg/kg/day, total volume of 2.0 μl/g body weight, Sigma‐Aldrich, St Louis, MO, USA) from P0 to P10/11 by oral feeding daily using a micropipette tip (Hansen et al., 2012; Hung et al., 2019). To examine potential long‐term effects of early life vancomycin, litters were separately given single daily doses of water or vancomycin from P0 to P10, in contrast to our previous study where pups in each litter were given either control or antibiotic treatment (Hung et al., 2019). This is to prevent conventionalization by coprophagy in co‐caged littermates (Kim et al., 2017). The pups were then allowed to grow for up to 6 weeks of age (P42–49) with normal animal husbandry protocols. Mice were then euthanized by cervical dislocation, their colons removed and immediately placed in physiological saline (composition in mm: NaCl 118, NaHCO3 25, d‐glucose 11, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaH2PO4 1.0) bubbled with carbogen gas (95% O2–5% CO2) or phosphate buffered saline (PBS).
Whole gut transit assay
A cohort of 73 mice of both sexes (n = 38 control, 35 vancomycin) received 10ml/g body weight oral gavage of 6% (w/v) carmine red dye in 0.5% (w/v) methylcellulose (Yano et al., 2015). They were then individually housed and checked 1 h post‐gavage and every following 15 min for the first 5 h, then every 30 min between 5 and 8 h for the presence of a red faecal pellet. The time it took for animals to defecate the first red pellet was recorded. After the red pellet was observed (or after 8 h if no red pellets are observed), mice were euthanized by cervical dislocation.
Faecal analysis
All formed faecal pellets were collected from isolated colons and were weighed immediately to obtain their wet weight. Then, the pellets were left in a vacuum oven at room temperature for 72−96 h to dry before measuring their dry weight, as previously described (McQuade et al., 2017). Faecal water content was derived by subtracting the dry weights from the wet weights of the faeces from each animal, then expressed as a percentage of the faecal wet weight.
Immunohistochemistry
Segments of mid colon were opened along the mesenteric border, stretched and pinned flat onto Petri dishes lined with a silicone elastomer (Sylgard) and then fixed overnight in 4% formaldehyde in 0.2 M phosphate buffer, pH 7.2, at 4°C. In a small number of cases, opened preparations were fixed for 80 min in 4% formaldehyde (freshly prepared from paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) at room temperature as this fixation is known to provide better labelling of 5‐HT in the myenteric plexus (Chen et al., 2001). Whole‐mount preparations of the submucous plexus and myenteric plexus with adherent longitudinal muscle were obtained from the colon via microdissection as previously described (Foong et al., 2015, 2012; Fung et al., 2017). To investigate 5‐HT cells in the mucosal epithelium, tubes of mid colon were stretched and fixed overnight in 4% formaldehyde in 0.2 M phosphate buffer, pH 7.2, at 4°C. Processed sections (10 μm) were obtained via cryostat as previously described (Gwynne et al., 2009). All preparations, whether whole‐mounts or sections, were incubated for 30 min with 1% Triton X‐100 (ProSciTech, Thuringowa, Queensland, Australia) at room temperature. The tissue was then given three PBS washes, followed by incubation overnight (or maximum 48 h) with a combination of primary antibodies (Table 1) at 4°C. After three PBS washes, the tissue was incubated with a combination of secondary antibodies (Table 1) for about 2.5 h at room temperature. The tissue was given another three washes with PBS, and then mounted onto a slide with Dako fluorescence mounting medium (Carpinteria, CA, USA).
Table 1.
Primary and secondary antisera used
| Raised in | Dilution factor | Source/catalogue no. | |
|---|---|---|---|
| Primary antisera | |||
| Calbindin | Rabbit | 1:1600 | Swant, CB‐38a |
| Calretinin | Goat | 1:1000 | Swant, CG1 |
| ChAT | Goat | 1:100 | Swant, AB144P |
| nNOS | Sheep | 1:1000 | Gift from Dr P. Emson |
| NFM | Rabbit | 1:500 | Merck, AB1987 |
| Hu | Human | 1:5000 | Gift from Dr V. Lennon |
| S100β | Rabbit | 1:1000 | Dako, Z0311 |
| 5‐HT | Rabbit | 1:1000 | Immunostar, 20080 |
| VIP | Rabbit | 1:1000 | Millipore, SB982 |
| Secondary antisera | |||
| Anti‐rabbit AF 647 | Donkey | 1:400 | Thermo Fisher Scientific, A‐31573 |
| Anti‐rabbit AF 594 | Donkey | 1:400 | Thermo Fisher Scientific, A‐21207 |
| Anti‐rabbit AF 488 | Donkey | 1:400 | Thermo Fisher Scientific, A‐21206 |
| Anti‐sheep AF 647 | Donkey | 1:500 | Thermo Fisher Scientific, A‐21448 |
| Anti‐human 594 | Donkey | 1:750 | Jackson Immunoresearch Laboratories, 709‐585‐149 |
Dako, Carpenteria, CA, USA; Immunostar, Hudson, WI, USA; Jackson Immunoresearch Laboratories, West Grove, PA, USA; Merck, Darmstadt, Germany; Millipore, Billerica, MA, USA; Swant, Burgdorf, Switzerland; Thermo Fisher Scientific, Waltham, MA, USA.
Imaging and data analysis
Immunostained preparations were viewed and imaged using a Zeiss fluorescence microscope (Axio Imager M2) or Zeiss LSM800 confocal microscope (Zeiss Microscopy, Jena, Germany). Images were analysed using the Fiji software (ImageJ 1.51s, NIH, Bethesda, MD, USA). For density analysis, images were taken with a ×10 objective and a threshold setting (Auto Local Threshold; Phansalkar) was consistently applied to each image before measuring the density of fluorescent particles in a given area (% area). Cell count analysis was performed on either double or triple labelled preparations imaged with ×20 or ×40 objectives. The total number of cells of each neuronal subtype is divided by the total number of Hu+ neurons (where Hu labels all neurons) to calculate the proportion of each neuronal marker. The proportion is calculated to normalise the data to prevent distortions by different amounts of stretch and slight differences in the number of neurons per ganglion due to location within the colon. Four to six regions of each myenteric plexus preparation and 6–10 regions of each submucous plexus preparation were imaged and analysed. Gut sections stained for 5‐HT were imaged at ×20 using the Zeiss Axio Imager M2 microscope and analysed using the Fiji software. The total number of 5‐HT‐positive cells/area of mucosa (mm2) was counted for each preparation.
Calcium imaging
Tissue preparation
Colonic segments were cut along the mesenteric border and pinned flat, mucosa side up, in an organ bathed lined with Sylgard. Submucosal plexus preparations were obtained by removing the mucosa and the underlying smooth muscle layers by microdissection, after which serosa and longitudinal muscle layers were stripped off to produce myenteric plexus/circular muscle preparations. Submucosal and myenteric plexus preparations were stretched over an Inox ring and clamped with a rubber O‐ring to stabilise the tissue during imaging (Li et al., 2019). The rings were transferred to an organ bath for imaging. The organ bath was constantly superfused (1 ml/min) with 95% O2–5% CO2‐bubbled physiological saline at room temperature throughout the experiment via a gravity‐fed inflow system.
Imaging and data analysis
GCaMP3+ preparations were imaged using a ×20 (NA 1.0) water dipping objective on an upright Zeiss Axio Examiner.Z1 microscope with a Zeiss Axiocam 702 mono camera, and images (1920 × 1216) were acquired at 7 Hz with 20 ms exposure for each frame. Neurons within myenteric/submucosal ganglia were stimulated electrically via a focal stimulating electrode (tungsten wire; 50 μm diameter) placed on an interganglionic fibre tract leading into the imaged ganglion of choice where a single pulse and a train of 20 pulses (20 Hz) were elicited. Focal stimulation of a single interganglionic fibre tract does not activate every input to all neurons within the imaged ganglion (Bornstein et al., 1986; Foong & Bornstein, 2009), and hence it is expected that not all neurons are activated by electrical stimulation.
Analyses were performed using custom‐written directives in IGOR Pro (WaveMetrics, Lake Oswego, OR, USA) (Li et al., 2019). Regions of interest were drawn over a selected area of the cytoplasm for each neuron, excluding the nucleus because GCaMP3 is absent from the nuclei (Tian et al., 2009; Yamada & Mikoshiba, 2012). The fluorescence intensity was calculated as a fraction of the baseline fluorescence, F i/F 0. Neurons were considered responsive only when they exhibited peaks in [Ca2+]i (calcium transients) with signals that had a minimum increase of five or three (for single pulse‐stimulated responses) times the intrinsic noise. The amplitudes of calcium transients were measured as the maximum increase in [Ca2+]i above (ΔF i/F 0). All results were presented as mean ΔF i/F 0 ±SD, where n is the number of animals examined. Further, the number of neurons that were responsive out of all the GCaMP3+ cells recorded in the field of view was noted.
Data presentation and statistics
For each experimental method, mice from at least three separate litters were examined per treatment condition. Data are presented as means ± SD where n is the number of animals examined. Statistical comparisons between vancomycin and water‐fed littermates were performed using Student's unpaired t‐test, the Mann–Whitney test, the Kolmogorov–Smirnov test or the chi‐square test as specified in the text with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA), where P < 0.05 is considered statistically significant.
Sample collection for microbiome analysis and mass spectrometry
Caecal and colonic segments were removed from euthanized animals and cut open along the mesenteric border. Samples of mid‐colonic mucosa and submucosa and full colonic faecal contents were obtained under sterile conditions for mass spectrometry analysis (Hung et al., 2019). Levels of 5‐HT, tryptophan and 5‐hydroxyindoleacetic acid (5‐HIAA; a major metabolite of serotonin) were measured using mass spectrometry–selective reaction monitoring as described elsewhere (Luna et al., 2017). Samples from caecal and colonic content underwent microbial DNA extraction and 16S rDNA sequencing as previously described (Luna et al., 2017). The 16S amplicon data was analysed using the DADA2 pipeline as described earlier (Hung et al., 2020). Spearman's correlation was performed between serotonin concentration and microbiome feature abundance, and significance was adjusted with the Benjamini–Hochberg procedure using R base function.
Results
Neonatal exposure to vancomycin has long‐term sexual dimorphic effects on GI function
We found that 6‐week‐old mice given vancomycin as neonates displayed slower total GI transit when the non‐absorbable dye, carmine red, was administered via oral gavage (Fig. 1A ). Further, the slowed GI transit was mainly prevalent in vancomycin‐treated females rather than males (Fig. 1A ). Nonetheless, when we analysed faecal wet and dry weights, male vancomycin‐treated mice had significantly lower dry weights than controls (Fig. 1 B–C). Both female and male vancomycin‐treated mice had significantly higher percentages of water in their faeces than controls (Fig. 1D ), which suggests an increase in water and electrolyte secretion.
Figure 1. Neonatal exposure to vancomycin has long‐lasting effects on gut function.
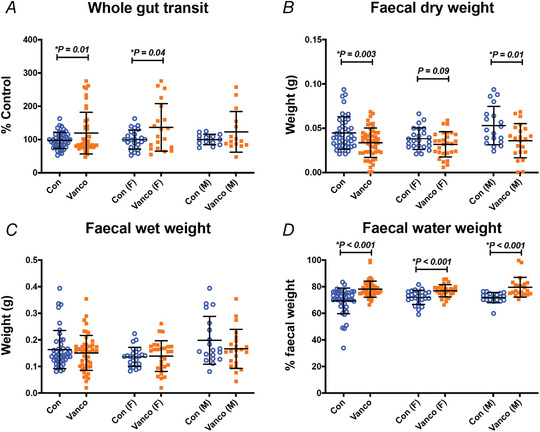
A, whole gut transit of 6‐week‐old mice. The time taken for the expulsion of the first red‐coloured faecal pellet following oral gavage with carmine red dye of each mouse is normalised by the average of the time taken by the control mice experimented on the same day. Control female (n = 23), Vanco female (n = 19), Control male (n = 13), Vanco male (n = 14). Data represented as means ± SD. Treatment effects were assessed with the Kolmogorov–Smirnov test. B–D, dry (B), wet (C) and water (D) weights of faecal pellets taken from colonic segments of 6‐week‐old mice. Control female (n = 24), Vanco female (n = 28), Control male (n = 19), Vanco male (n = 22). Graphs show means ± SD. Treatment effects were assessed with a two‐tailed unpaired t‐test.
Neonatal exposure to vancomycin has lasting sexual dimorphic effects on the neurochemistry of enteric neurons
Various functions of the GI tract including motility are mediated by complex signalling circuitries of enteric neurons. Thus, to investigate the possible causes of disruption of GI function in 6‐week‐old mice, we first examined whether neonatal antibiotic treatment affected the neurochemistry of enteric neurons. We have previously shown that vancomycin‐fed pups at P10/11 had significantly lower Hu+ myenteric neuronal density and no differences in glial S100β+ density compared to control littermates (Hung et al., 2019). When we let the pups mature to 6 weeks of age, we found a lasting reduction in the density of Hu+ neurons due to the antibiotic treatment and an added significant reduction in S100β+ density in the myenteric plexus of male, but not female, mice (Fig. 2 A–C). Female, but not male, 6‐week‐old mice given neonatal vancomycin had significantly fewer Hu+ neurons per ganglion in the submucosal plexus (Fig. 2D and E ).
Figure 2. Neonatal exposure to vancomycin had sexually dimorphic effects on enteric neurons and glia.
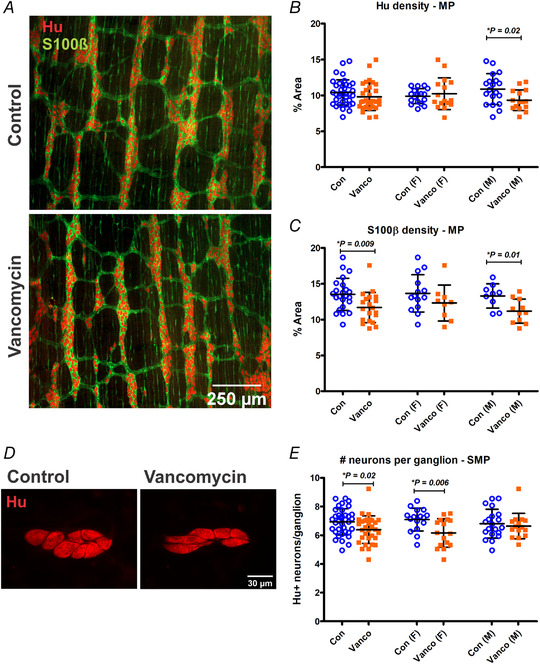
A, representative images of myenteric plexus preparations from the mid colon of control‐ and vancomycin‐treated 6‐week‐old mice stained with the pan‐neuronal marker Hu (red) and glial marker S100β (green). B and C, myenteric neuron (Hu+) (B) and glial (S100β+) density (C) of the mid colon. For Hu density: Control female (n = 17), Vanco female (n = 16), Control male (n = 19), Vanco male (n = 16). For S100β+ density: Control female (n = 14), Vanco female (n = 9), Control male (n = 9), Vanco male (n = 11). D, representative images of submucous plexus preparations from the mid colon of control‐ and vancomycin‐treated 6‐week‐old mice stained with Hu (red). E, number of Hu+ neurons per submucosal ganglion. Control female (n = 16), Vanco female (n = 16), Control male (n = 20), Vanco male (n = 16). All graphs show means ± SD. Treatment groups were compared statistically using unpaired t‐tests.
Enteric neuronal subtypes express unique combinations of markers, known as their neurochemical code, which can be used to deduce their functional roles within the circuitry (Foong et al., 2014; Sang & Young, 1996, 1998). Significant maturation of the neurochemistry of myenteric and submucosal neurons continues after birth with a staggered appearance of various neurochemical subtype markers (Bergner et al., 2014; Laranjeira et al., 2011; Parathan et al., 2020; Pham et al., 1991; Uesaka et al., 2015). We have previously shown a significant impact of vancomycin on the proportion of nitrergic (express neuronal nitric oxide synthase (nNOS)) and calbindin+ myenteric neurons at P10/11 (Hung et al., 2019). Here, we examined the same two neurochemical markers, and expanded our characterization to include a wider range of markers in both enteric plexi of older animals at 6 weeks of age.
We calculated the proportions of various myenteric neuronal subtypes relative to the pan‐neuronal marker, Hu (Fig. 3 A–E). nNOS+ neurons are interneurons and inhibitory motor neurons in the enteric circuitry of guinea‐pigs and mice. Nitrergic neurons are largely separate from cholinergic neurons (which express choline acetyltransferase; ChAT) (Drokhlyansky et al., 2020; Qu et al., 2008; Sang & Young, 1996; Zeisel et al., 2018). There were no significant differences in the proportions of nitrergic or cholinergic myenteric neurons between antibiotic‐treated animals and controls (Fig. 3F and I ). A very small population of myenteric neurons is 5‐HT+ and these are likely to be cholinergic interneurons in the ENS (Sang & Young, 1996). We found that vancomycin‐treated male mice specifically had significantly lower proportions of 5‐HT+ myenteric neurons than control (Fig. 3H ). Most neurofilament M+ (NFM+) neurons have large smooth cell bodies and long smooth processes (Dogiel type II), a shape which is thought to be characteristic of intrinsic sensory neurons (Furness et al., 2004), but we have previously found significant co‐localisation of NFM with nNOS in the myenteric plexus indicating that some NFM+ neurons serve other roles (Parathan et al., 2020). Male, but not female, 6‐week‐old mice exposed to neonatal vancomycin also had significantly lower proportions of NFM+ myenteric neurons (Fig. 3G ). Vancomycin treatment did not appear to impact the overall proportions of calbindin+ or calretinin+ myenteric neurons, both of which are cholinergic. However, calbindin and calretinin are known to be expressed brightly or faintly in the cell bodies of myenteric neurons along the GI tract (Qu et al., 2008; Sang & Young, 1996). When we separated calbindin+ and calretinin+ neurons into brightly and faintly stained groups, we found that male, but not female, mice that were given neonatal vancomycin had significantly lower proportions of faint calbindin+, and higher proportions of bright calretinin+ myenteric neurons compared to controls (Fig. 3 J–M).
Figure 3. Neonatal exposure to vancomycin had sexually dimorphic effects on the neurochemistry of myenteric neurons.
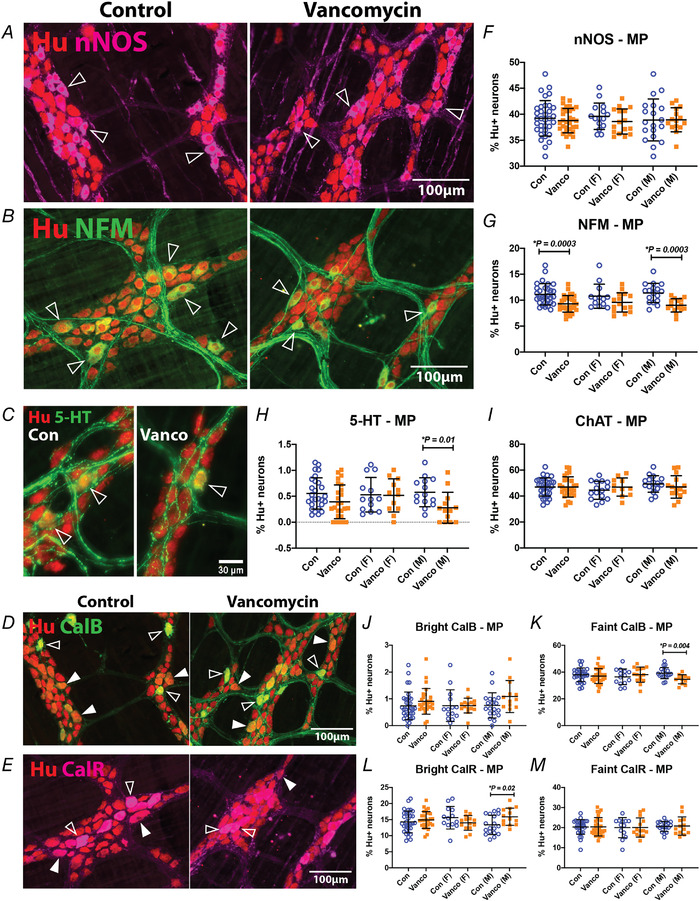
A–E, representative images of myenteric plexus preparations from the mid colon of control‐ and vancomycin‐treated 6‐week‐old mice. A, myenteric neurons were stained with the pan‐neuronal marker, Hu (red) and nNOS (magenta). Some nNOS‐immunoreactive Hu+ neurons are indicated (open arrowheads). Myenteric neurons were stained with Hu (red) and NFM (B), 5‐HT (C) or calbindin (D) in green. Some NFM−, 5‐HT‐ or calbindin‐immunoreactive Hu+ neurons are indicated (open arrowheads). The calbindin antiserum stains some neurons brightly (open arrowheads) and others faintly (closed arrowhead). E, myenteric neurons were stained with Hu (red) and calretinin (magenta). The calretinin antiserum stains some neurons brightly (open arrowheads) and others faintly (closed arrowhead). F–M, quantification of nNOS+ (F), NFM+ (G), 5‐HT+ (H), ChAT+ (I), bright calbindin+ (J), faint calbindin+ (K), bright calretinin+ (L) and faint calretinin+ (M) neurons relative to Hu+ neurons in the mid colon. For Hu/nNOS: Control female (n = 15), Vanco female (n = 16), Control male (n = 19), Vanco male (n = 16). For Hu/NFM: Control female (n = 13), Vanco female (n = 15), Control male (n = 17), Vanco male (n = 15). For Hu/5‐HT: Control female (n = 13), Vanco female (n = 12), Control male (n = 14), Vanco male (n = 14). For Hu/ChAT: Control female (n = 15), Vanco female (n = 11), Control male (n = 16), Vanco male (n = 15). For Hu/calbindin: Control female (n = 14), Vanco female (n = 15), Control male (n = 19), Vanco male (bright: n = 13, faint n = 12). For Hu/calretinin: Control female (n = 13), Vanco female (n = 14), Control male (n = 18), Vanco male (n = 13). Data represented as means ± SD. Treatment groups were compared statistically using unpaired t‐tests or the Kolomogorov–Smirnov test (for panel H specifically).
Because calretinin labels several diverse classes of myenteric neurons in mouse colon, we explored whether calretinin neurons colocalise with NFM which labels a less diverse subset of neurons in this preparation. The results are given in Table 2, which shows that the bright calretinin neurons can be subdivided into NFM+ and NFM− and that neonatal vancomycin has opposite effects on these two populations with the former being increased in young adult males and the latter being decreased. NFM neurons lacking any calretinin were also decreased by the antibiotic treatment. Further, these data show that subtyping faint calretinin neurons does not reveal any effect of neonatal vancomycin in young adult males and that consideration of NFM staining does not identify any changes in females.
Table 2.
Neonatal vancomycin differentially affects NFM+ and CalR+ myenteric neuronal proportions in male mice but not female mice
| All | Female | Male | ||||
|---|---|---|---|---|---|---|
| Con | Vanco | Con | Vanco | Con | Vanco | |
| (n = 30) | (n = 27) | (n = 13) | (n = 14) | (n = 17) | (n = 13) | |
| NFM+/CalR− | 5.8 ± 0.3 | 5.0 ± 0.2* | 5.5 ± 0.4 | 5.3 ± 0.3 | 5.5 ± 0.2 | 4.6 ± 0.2** |
| NFM−/bright CalR+ | 11.1 ± 0.6 | 12.0 ± 0.5 | 12.9 ± 0.8 | 11.2 ± 0.5 | 9.7 ± 0.6 | 12.8 ± 0.8** |
| NFM−/faint CalR+ | 19.1 ± 0.6 | 19.0 ± 0.9 | 19.6 ± 1.0 | 18.6 ± 1.3 | 18.7 ± 0.6 | 19.3 ± 1.2 |
| NFM+/bright CalR+ | 3.7 ± 0.2 | 2.9 ± 0.2** | 3.6 ± 0.3 | 2.7 ± 0.3 | 3.8 ± 0.2 | 3.0 ± 0.3* |
| NFM+/faint CalR+ | 1.6 ± 0.1 | 1.5 ± 0.09 | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.5 ± 0.1 |
*P < 0.05, **P < 0.01, unpaired t‐test. Abbreviations: CalR, calretinin; Con, control; NFM, neurofilament M; Vanco, vancomycin.
Significant maturation of the neurochemistry of submucosal neurons occurs postnatally and can be affected by antibiotic treatment during the post‐weaning period (Foong, 2016; Hung et al., 2020; Parathan et al., 2020). We examined various subtype markers for submucosal neurons relative to Hu (Fig. 4 A–D). In the mouse colon, submucosal secretomotor neurons are typically either cholinergic or non‐cholinergic and immunoreactive for vasoactive intestinal peptide (VIP) (Foong et al., 2014). We found that neonatal vancomycin treatment had different effects on the proportion of cholinergic submucosal neurons in female and male mice at 6 weeks of age. Vancomycin exposed females had significantly lower proportions of ChAT+ neurons, while antibiotic treated males had significantly higher proportions of ChAT+ neurons compared to controls (Fig. 4E ). Neonatal exposure to antibiotics significantly reduced the proportion of VIPergic neurons in males while these neurons were unaffected in females (Fig. 4F ). Some VIPergic submucosal neurons in the mouse colon also express nNOS (Foong et al., 2014), but neonatal vancomycin exposure did not affect nitrergic submucosal neurons (Fig. 4G ). We reported previously that some Hu+ submucosal neurons in the mouse gut express a glial marker, S100β (Parathan et al., 2020). Neonatal exposure to antibiotics did not affect the proportion of S100β+ submucosal neurons (Fig. 4H ).
Figure 4. Neonatal exposure to vancomycin had sexually dimorphic effects on the neurochemistry of submucosal neurons.
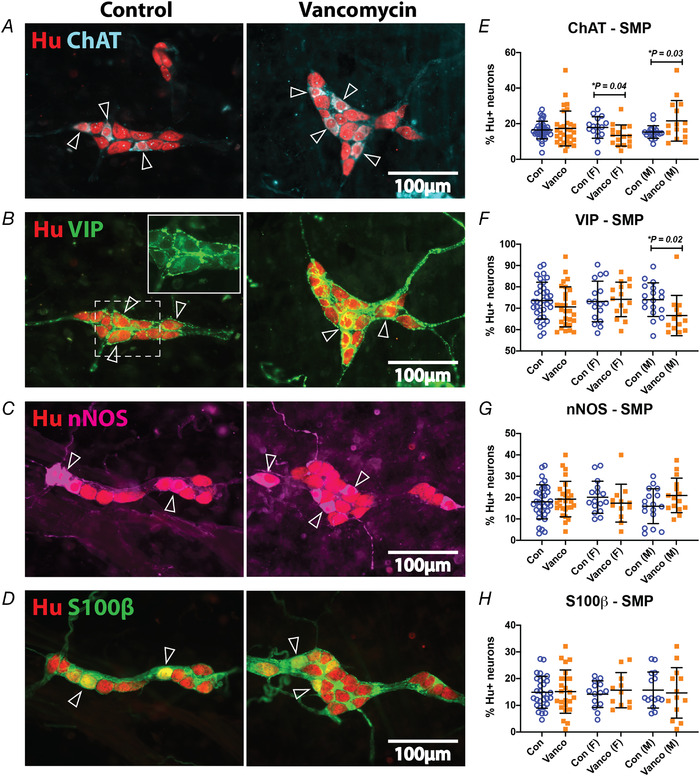
A–D, representative images of submucous plexus preparations from the mid colon of control‐ and vancomycin‐treated 6‐week‐old mice. A, submucosal neurons were stained with the pan‐neuronal marker, Hu (red) and ChAT (cyan). Some ChAT‐immunoreactive Hu+ neurons are indicated (open arrowheads). B, submucosal neurons were stained with Hu (red) and VIP (green). Some VIP‐immunoreactive Hu+ neurons are indicated (open arrowheads). Inset depicts the fainter VIP stain (green) in the Golgi apparatus of neuronal somata and surrounding brighter neuronal terminals and varicosities. C, submucosal neurons were stained with Hu (red) and nNOS (magenta). Some nNOS‐immunoreactive Hu+ neurons are indicated (open arrowheads). D, submucosal neurons were stained with Hu (red) and S100β (green). Some S100β‐immunoreactive Hu+ neurons are indicated (open arrowheads). E–H, quantification of ChAT+ (E), VIP+ (F), nNOS+ (G) and S100β+ (H) neurons relative to Hu+ neurons in the mid colon. For Hu/ChAT: Control female (n = 16), Vanco female (n = 16), Control male (n = 20), Vanco male (n = 15). For Hu/VIP: Control female (n = 17), Vanco female (n = 15), Control male (n = 18), Vanco male (n = 13). For Hu/nNOS: Control female (n = 16), Vanco female (n = 12), Control male (n = 17), Vanco male (n = 15). For Hu/ S100β: Control female (n = 14), Vanco female (n = 9), Control male (n = 9), Vanco male (n = 11). Data represented as means ± SD. Treatment groups were compared statistically using unpaired t‐test.
Neonatal exposure to vancomycin has lasting effects on the activity of enteric neurons
To determine whether the disruption of GI function in 6‐week‐old mice includes alterations in the excitability of enteric neurons, we used the genetically encoded Ca2+ indicator (GCaMP3) to study synaptic transmission to myenteric neurons and to submucosal neurons (Fung et al., 2017; Hung et al., 2019, 2020; Koussoulas et al., 2018; Swaminathan et al., 2019) and identify any significant spatiotemporal changes in the enteric circuitry. Electrical stimulation of interganglionic fibre tracts with a single pulse typically evokes fast excitatory postsynaptic potentials (EPSPs), whereas stimulating with a train of pulses leads to the additional recruitment of slower EPSPs in enteric neurons (Foong et al., 2015, 2012; Gwynne & Bornstein, 2007).
At 6 weeks of age, significantly fewer myenteric neurons from vancomycin‐treated adolescents responded to a single pulse stimulus (Tables 3 and 4 and Supplementary Table S1 and S2). Responses to multipulse stimuli were significantly less common in myenteric neurons from vancomycin‐treated young adult male mice, but there was no change in numbers of responding neurons in vancomycin‐treated females (Tables 3 and 4 and Supplementary Tables S1 and S2). On the other hand, significantly more submucosal neurons from antibiotic‐exposed male and female mice responded to single pulse stimuli but only antibiotic‐exposed males had increased numbers of submucosal neurons responding to multipulse stimuli (Tables 3 and 4 and Supplementary Tables S1 and S2). There were no significant differences in overall amplitudes of responses when they were detected (Supplementary Fig. S1). The unique and identifiable shapes of submucosal ganglia allowed us to perform post hoc immunohistochemical stains, which revealed sex‐dependent differences in amplitudes of responses between VIP and ChAT neurons (Fig. 5 A–C). VIP+ neurons of vancomycin‐treated male mice showed increased amplitudes of [Ca2+]i transients in response to a single stimulus but VIP− neurons from females did not (Fig. 5D ). ChAT+ neurons of vancomycin‐treated female mice showed decreased amplitudes of [Ca2+]i transients evoked by 20‐pulse stimuli but ChAT+ neurons from males did not (Fig. 5G ). Responses of VIP+ neurons to 20 pulse stimuli and ChAT+ neurons to one‐pulse stimuli were similar between control‐ and vancomycin‐treated male and female mice (Fig. 5E and F ).
Table 3.
Treatment induced change in proportion of GCaMP3+ responding enteric neurons – males
| Electrical stimulation | Enteric plexus | Proportion of responders normalised to control |
|---|---|---|
| 1 pulse | MP | 0.7** ** |
| SMP | 2.1** ** | |
| 20 pulse | MP | 0.7** ** |
| SMP | 1.2* * |
**P < 0.01, ****P < 0.0001, Fisher's test.
Table 4.
Treatment induced change in proportion of GCaMP3+ responding enteric neurons – females
| Electrical stimulation | Enteric plexus | Proportion of responders normalised to control |
|---|---|---|
| 1 pulse | MP | 0.8* ** |
| SMP | 2.5** ** | |
| 20 pulse | MP | 1.0 |
| SMP | 1.1 |
***P < 0.001, ****P < 0.0001, Fisher's test.
Figure 5. Neonatal exposure to vancomycin affected the amplitude of electrically evoked [Ca2+]i transients in submucosal plexi.
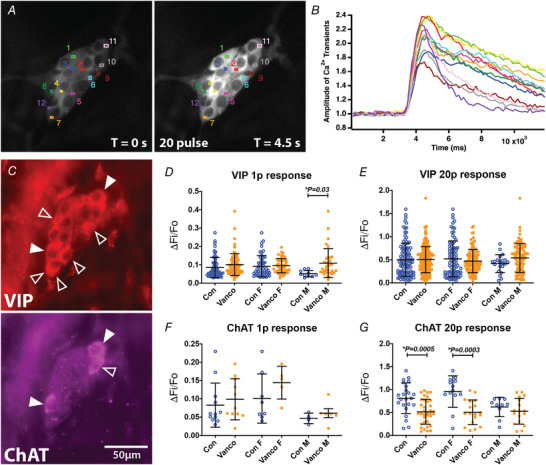
A, regions of interest (ROI 1–12) drawn on representative fluorescence micrographs of the 20 pulse train (20 Hz)‐evoked [Ca2+]i response in submucosal neurons of 6‐week‐old mice given control‐treatments as neonates (GCaMP3 signal at rest (t = 0); and during 20 pulse train stimulation, T = 4.5 s). B, amplitude of [Ca2+]i transients from cells stimulated with a 20 pulse train stimulation. Colours correspond to the ROIs in A. C, post hoc immunohistochemistry labelling vasoactive intestinal peptide (VIP) and choline acetyltransferase (ChAT). Open arrowheads indicate neurons positive for VIP or ChAT. Filled arrowheads indicate neurons positive for both VIP and ChAT. D and E, amplitude of [Ca2+]i transients (ΔF i/F 0) from VIP cells stimulated with a single stimulus (D) and a 20 pulse train (E). F and G, amplitude of [Ca2+]i transients (ΔF i/F 0) from ChAT cells stimulated with a single stimulus (F) and a 20 pulse train (G). All graphs show means ± SD. Treatment effects were assessed with a two‐tailed unpaired t‐test.
The changes in numbers of responsive neurons probably arise because measuring activity in enteric neurons using Ca2+ imaging reflects generation of action potentials in recorded neurons but does not always reflect the presence of subthreshold synaptic potentials. Thus, increased numbers of neurons responding to a stimulus reflects an increase in numbers of neurons whose inputs are suprathreshold. This could arise because each neuron receives more inputs from any single nerve trunk, the amount of transmitter released by active terminals or transmitter efficacy increased, or that the postsynaptic neuron was more excitable, or any combination of these. Conversely, reduced numbers of responsive neurons would reflect reduced numbers of synaptic inputs, reduced transmitter release efficacy or reduced excitability of postsynaptic neurons. These alternatives cannot be distinguished in Ca2+ imaging studies but would require intracellular recording, which is outside the scope of the present study.
Neonatal exposure to vancomycin had lasting effects on levels of mucosal 5‐HT
Over 90% of the body's 5‐HT is synthesised in intestinal enterochromaffin (EC) cells and this neurotransmitter has multiple roles in regulating gut homeostasis by interacting with 5‐HT receptors located on, for example, intrinsic sensory enteric neurons (Gershon, 2013). In the colon, antibiotics modulate 5‐HT levels and gut motility by perturbing microbiota that promote expression of tph1, the gene encoding the rate‐limiting enzyme of mucosal 5‐HT biosynthesis in adult mice (Reigstad et al., 2015; Yano et al., 2015). We investigated whether colonic 5‐HT and its biosynthetic pathway in EC and mast cells are altered by vancomycin exposure during the neonatal period. At P10/11, vancomycin‐fed pups had significantly fewer 5‐HT+ cells in their colonic mucosa than control littermates. Vancomycin‐treated pups had significantly greater levels of faecal and mucosal tryptophan and lower levels of the 5‐HT metabolite, 5‐HIAA (Hung et al., 2019). Here, while neonatal vancomycin treatment did not have a lasting impact on the number of 5‐HT+ cells/mm2 of mucosa of the young adult mid colon (Fig. 6 A‐A′), we found significant lasting impacts of the antibiotic treatment on the levels of tryptophan, 5‐HT and 5‐HIAA in the mucosa and faeces of 6‐week‐old mice (Fig. 6 B–G). Notably, there continued to be significantly lower levels of 5‐HT in the mucosa of the young adult female and male animals that underwent neonatal vancomycin treatment (Fig. 6D ).
Figure 6. Neonatal exposure to vancomycin had lasting effects on mucosal levels of 5‐HT.
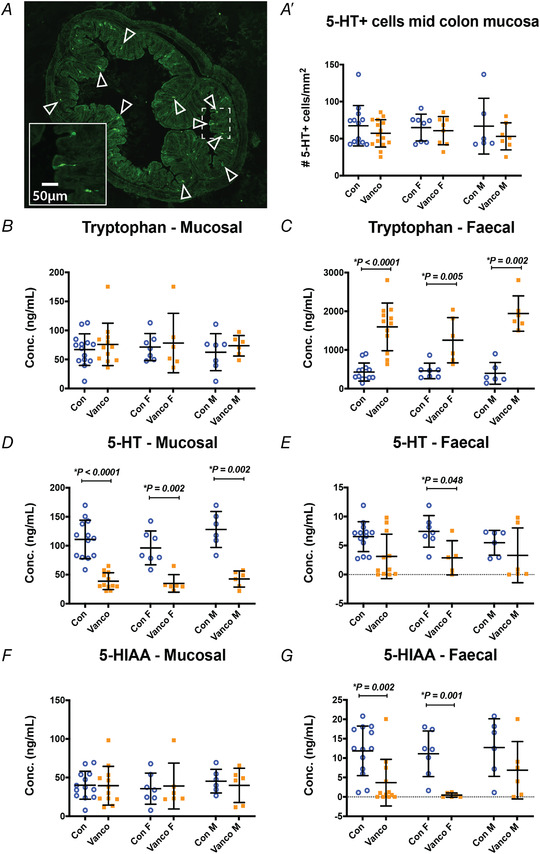
A, representative images of a mid‐colon cross section of a 6‐week‐old mouse immunostained for 5‐HT. A′, number of 5‐HT+ cells in the colonic mucosa of water‐ and vancomycin‐fed animals. Control female (n = 8), Vanco female (n = 8), Control male (n = 6), Vanco male (n = 7). B–G, mass spectrometry analysis measuring the levels of tryptophan (B and C), 5‐HT (D and E) and the metabolite, 5‐HIAA (F and G) in the faeces of all (left), female (F, middle) and male (M, right) control and vancomycin‐treated 6‐week‐old mice. For tryptophan mucosal: Control female (n = 7), Vanco female (n = 6), Control Male (n = 7), Vanco Male (n = 6). For tryptophan faecal: Control female (n = 7), Vanco female (n = 6), Control Male (n = 6), Vanco Male (n = 6). For 5‐HT mucosal: Control female (n = 7), Vanco female (n = 6), Control Male (n = 6), Vanco Male (n = 6). For 5‐HT faecal: Control female (n = 7), Vanco female (n = 5), Control Male (n = 6), Vanco Male (n = 6). For 5‐HIAA mucosal: Control female (n = 7), Vanco female (n = 6), Control Male (n = 6), Vanco Male (n = 6). For 5‐HIAA faecal: Control female (n = 7), Vanco female (n = 6), Control Male (n = 6), Vanco Male (n = 6). Data presented as means ± SD. Treatment groups were compared statistically using two‐tailed unpaired t‐test for panel A' and the Mann–Whitney U‐test for panels B–G.
Neonatal exposure to vancomycin has long‐term effects on GI microbiota diversity and composition
Early postnatal life is a critical period for the maturation of gut microbiota (Hung et al., 2020). We have previously reported the immediate effects of vancomycin treatment on gut microbiota at P10/11, where the antibiotic significantly reduced microbiota diversity and altered microbiota composition (Hung et al., 2019). A similarly low Shannon diversity index was observed in adolescent mice (6 weeks) of either sex treated with neonatal vancomycin indicating a sustained antibiotic effect (Fig. 7A ). Compositional shifts of colonic microbiome were evident between neonate and adult mice in both control and vancomycin groups (Fig. 7B ). At 6‐weeks of age, Akkermansiaceae, Bacteroidaceae, Tannerellaceae and Enterobacteriaceae were expanded in vancomycin‐exposed mice at the expense of Lachnospiraceae and Burkholderiaceae (Fig. 7B ). Principal component analysis demonstrated that the impact of neonatal vancomycin treatment on gut microbiota was not sexually dimorphic (Fig. 7C ). Further, significantly enlarged caeca were found in all 6‐week‐old mice exposed to neonatal vancomycin, suggesting prolonged effects on microbial community dynamics (Fig. 7D ). Interestingly, when we correlated colonic mucosa 5‐HT concentration with colonic family abundance, we found a significant negative association of Akkermansiaeae, Tannerellaceae and Enterobacteriaceae with the level of mucosal serotonin (Fig. 8). Further analysis indicated that those negative associations were highly driven by three dominant amplicon sequence variants (ASVs) including ASV5 (Parabacteroides), ASV21 (Parabacteroides) and ASV6 (Enterobacteriaceae).
Figure 7. Neonatal exposure to vancomycin had long‐lasting effects on gut microbiome community composition.
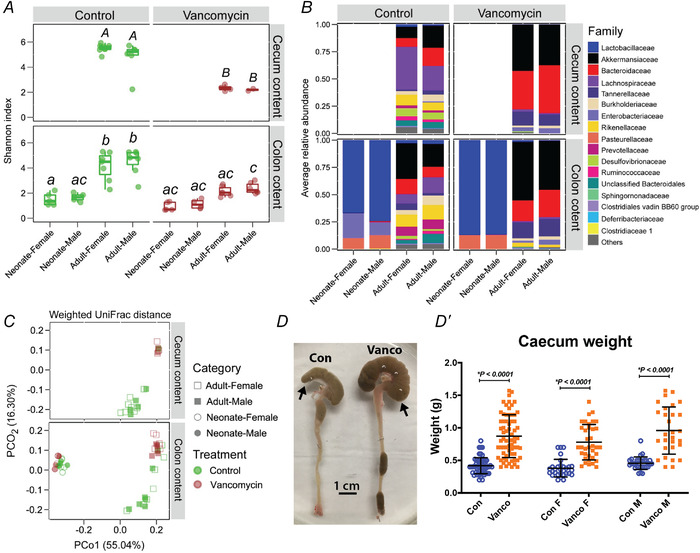
Samples were taken from female and male colonic contents in neonates (P10), and caecum and colonic contents from 6‐week‐old adult mice for microbiota analysis. A, alpha diversity of microbiome community composition in 6‐week‐old and P10/11 mice given neonatal control (green) or vancomycin (brown) treatment. Treatment groups were compared statistically using one‐way ANOVA with Tukey's post hoc test. Uppercase letters (for caecum content): no letters in common indicates a significant difference (P < 0.05) between two groups. Lowercase letters (for colon content): no letters in common indicates a significant difference (P < 0.05) between two groups. B, microbiome composition at the family taxonomic level. C, principal component analysis of samples from female versus male control‐ and vancomycin‐treated mice. D, colons from 6‐week‐old water (con) and vancomycin (vanco) neonatal treated mice (arrow marks caecum). D′, caecum weights of all (left), female (F, middle) and male (M, right) control and vancomycin‐treated 6‐week‐old mice. Control female (n = 30), Vanco female (n = 34), Control male (n = 23), Vanco male (n = 27). All graphs show means ± SD. Treatment effects were assessed with a two‐tailed unpaired t test.
Figure 8. Correlation analysis between colonic mucosa serotonin (5‐HT) concentration and colonic taxonomic (family or ASV) abundance.
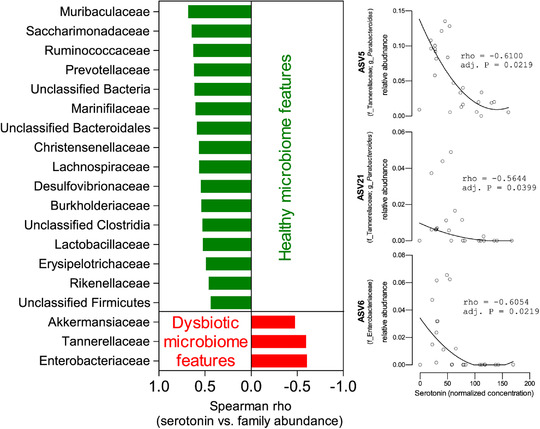
Spearman correlation analysis was performed with the Benjamini–Hochberg procedure applied to adjust raw P‐values for multiple testing correction of the correlation analysis. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Antibiotic exposure during critical developmental windows such as the neonatal period alters the gut microbiome and these changes can be long lasting (Neuman et al., 2018). But whether neonatal antibiotics have lasting impact on associated systems including the ENS and EC cells in the GI tract and how they exert their effects were previously unknown. In this study, we showed that neonatal antibiotic treatment had long‐lasting and sexually dimorphic effects disrupting GI function, neurochemistry and activity of the ENS. Neonatal vancomycin treatment also had prolonged effects on the levels of mucosal 5‐HT and colonic microbial communities that were not sex‐specific.
GI function was differentially affected in antibiotic‐treated male versus female mice
We have previously reported that neonatal vancomycin significantly increased ex vivo colonic motility in mouse pups immediately after treatment (Hung et al., 2019). Colonic motility and presumably its underlying enteric circuitry is still maturing during neonatal life (Foong et al., 2015, 2012; Roberts et al., 2007). In this study, we found that neonatal vancomycin significantly slowed the whole GI transit time of female, but not male, young adult mice suggesting that antibiotic treatment predisposed female mice to constipation. Nevertheless, our data also show elevated water content of the formed faeces in the colons of both female and male mice treated with neonatal vancomycin, which suggests ongoing diarrhoea. This was accompanied by a reduction in the dry weight of formed faeces in the young adult males. Thus, the early life antibiotic treatment produced significant changes in overall GI function whose consequences for homeostatic mechanisms need to be investigated further. It seems, though, that one change induced by the neonatal vancomycin is a net reduction in water absorption from the lumen, perhaps mediated by increased overall secretion as implied by wetter content despite slower transit in the females.
Neonatal antibiotics had sexually dimorphic effects on ENS structure and function
GI functions are mediated by several different subtypes of enteric neurons and glia connected in complex circuits. Although the two main enteric plexi are linked, the myenteric plexus contains the main enteric circuitry underlying GI motility while the submucous plexus contains the motor neurons regulating water and electrolyte secretion (Bornstein & Foong, 2018; Furness, 2012). The ENS undergoes significant maturation between the neonatal period and young adulthood. Substantial numbers of myenteric neurons are still being born during early postnatal stages and there is evidence that a dynamic balance of neurogenesis and turnover of enteric neurons continues into adulthood (Bergner et al., 2014; Foong, 2016; Foong et al., 2020; Kulkarni et al., 2017; Laranjeira et al., 2011; Parathan et al., 2020; Pham et al., 1991). Further, there is developmental plasticity of synaptic connections between myenteric neurons during early postnatal stages (Foong et al., 2015, 2012) with a significant increase in apparent synaptic contacts onto enteric neurons during the post‐weaning period (Parathan et al., 2020). Comparatively little is known about the functional development of the submucous plexus, but maturation of submucosal cells probably occurs later than myenteric cells in mice as they tend to exit the cell cycle and differentiate later than myenteric cells (Jiang et al., 2003; Lasrado et al., 2017; McKeown et al., 2001; Uesaka et al., 2015).
We found that the density and neurochemistry of myenteric neurons in young adult female mice were apparently unaffected by neonatal vancomycin. But there was significant disruption in the myenteric plexus of young adult males with reduced numbers of neurons (and glia) and altered proportions of several subtypes of putative cholinergic neurons, but not nitrergic neurons. Recent single neuron transcriptomic studies (Drokhlyansky et al., 2020; May‐Zhang et al., 2021; Morarach et al., 2021; Wright et al., 2021) and other studies using combined reporter and immunohistochemical analyses (Nestor‐Kalinoski et al., 2022) indicate that neurons can only be subtyped into presumed functional groups by combinations of several key markers. Nevertheless, significant conclusions about the functional identities of the neurons disrupted by neonatal antibiotics can be drawn from our study. The simplest conclusion is that at least one class of descending interneuron, the very small population of 5‐HT neurons (Sang & Young, 1996), is sensitive to neonatal vancomycin. In contrast, calbindin, calretinin and NFM have all been shown in immunohistochemical, reporter and transcriptomic studies to label multiple classes of neurons. All three label myenteric neurons with the typical large smooth cell bodies of intrinsic sensory neurons (Qu et al., 2008), but also label neurons with short lamellar dendrites that are usually identified as either motor neurons or interneurons. The faint calbindin neurons that are reduced in male myenteric plexus also appear to have smooth cell bodies suggesting that they may be intrinsic sensory neurons. This is consistent with the reduction in NFM neurons as this marker has also been found to primarily label neurons with this morphology. Indeed, when we analysed how neurons labelled for both calretinin and NFM, we found that, although bright calretinin neurons increased in male myenteric plexus overall, the bright calretinin neurons that contain NFM were decreased. On the other hand, faint calretinin neurons that express NFM were unaffected by neonatal vancomycin. This strongly suggests that the neonatal antibiotic targets a subset of intrinsic sensory neurons, presumably those that do not have weak calretinin labelling. Unfortunately, whether these neurons also express calbindin could not be explored because the available antisera were not compatible with colocalization studies of calbindin and NFM.
Many bright calbindin or calretinin neurons have lamellar dendrites suggesting that these can be interneurons or in the case of bright calretinin+ neurons excitatory motor neurons to smooth muscle layers (Qu et al., 2008; Sang & Young, 1996). The observed increase in proportion of brightly stained calretinin+ neurons lacking NFM may include interneurons or excitatory motor neurons (Qu et al., 2008; Sang & Young, 1996). A more definitive conclusion about these neurons must await further studies. Nevertheless, it is likely that neonatal vancomycin has lasting effects on several subtypes of myenteric neurons in male mouse colon including 5‐HT interneurons, one or more types of intrinsic sensory neurons and calretinin positive interneurons or excitatory motor neurons.
We have previously shown that the excitability of myenteric neurons in the colon is increased at P10 after vancomycin exposure during the neonatal period (Hung et al., 2019). The data presented here indicate that the excitability of myenteric neurons in young adult males is decreased by neonatal vancomycin, with fewer neurons responding to stimulation of synaptic inputs. In contrast, effects on neurons from young adult females were small in concert with the lack of effects of this treatment on neuron numbers and neurochemistry in this sex.
The lack of effects on myenteric neurons in females was surprising given that it was young adult females that showed altered GI transit after neonatal vancomycin, while young adult males apparently had normal transit. Both sexes, however, had wetter faeces indicating that there were changes in the mechanisms regulating water transport across the mucosa. The submucosal plexus contains the secretomotor neurons that regulate mucosal water electrolyte transport and our data on this plexus support the idea that prolonged changes in GI function due to neonatal antibiotics are due to actions on development of submucosal neurons.
Neonatal vancomycin treatment had sexually dimorphic effects on the neurochemistry of the submucous plexus. The number of Hu+ cells per ganglion and proportions of cholinergic neurons were significantly decreased in female mice given neonatal vancomycin, indicating that antibiotics exposure could affect birth and or survival of these neurons. Vancomycin‐treated females also had more submucosal neurons responding to electrical stimuli which could also cause increase in secretion and faecal water content. On the other hand, there was a significant increase in proportions of cholinergic and a decrease in VIPergic secretomotor neurons in young adult males. How these changes act with the reduced numbers of intrinsic sensory neurons and altered 5‐HT interneurons in the myenteric plexus to result in increased water and electrolyte secretion and enhanced faecal water content remains to be determined. In the mouse, it appears that all the intrinsic sensory neurons are contained within the myenteric plexus (Foong et al., 2014; Mongardi Fantaguzzi et al., 2009), so it is tempting to speculate that the intrinsic sensory neurons affected by neonatal antibiotics are those involved in secretomotor reflexes. Moreover, aside from changes in neurochemistry, there may be other factors such as increased synaptic connectivity, expression and activity of ion channels and neurotransmitter receptors that could affect ENS activity that are outside the scope of the present study (Gwynne & Bornstein, 2007; Hirst et al., 2015; Parathan et al., 2020).
Host response to microbiota and mucosal 5‐HT changes is sex‐specific
The sex‐specific antibiotic‐effects on the ENS could contribute to differences in GI dysfunction observed between male and female mice, but other factors within the GI tract also influence function. The gut microbiota regulates GI functions such as motility and secretion by signalling to the ENS both directly via microbial metabolites and via 5‐HT+ EC cells and regulation of 5‐HT synthesis (Foong et al., 2020). The latter effects can be complex as there is evidence that although mucosal 5‐HT is not essential for colonic motility, changes in 5‐HT and its signalling pathways can both activate and inhibit motility pathways (Balasuriya et al., 2016; Heredia et al., 2013; Keating & Spencer, 2019).
The disruption of early microbial colonisers in the early postnatal gut by vancomycin exposure resulted in prolonged depletion of microbiota diversity and changes in microbiome community dynamics which were similar in young adult males and females. Neonatal exposure to the antibiotic also resulted in prolonged depletion of 5‐HT levels in the colonic mucosa regardless of sex. While the initially reduced number of mucosal 5‐HT+ cells caught up to control levels in post‐weaning/adolescent antibiotic‐exposed mice, the sustained decrease in 5‐HT levels suggests that defects in 5‐HT biosynthesis remain, an effect also reported in previous studies (Reigstad et al., 2015; Yano et al., 2015). Moreover, we found that the antibiotic‐induced expansions of Akkermansiaeae, Tannerellaceae and Enterobacteriaceae families were negatively correlated to mucosal 5‐HT levels. In accordance with literature on adult mice (Ge et al., 2017; Yano et al., 2015), the reduction in 5‐HT levels found in our study could contribute to the slowed GI transit in vancomycin‐treated female mice. Although whole GI transit was not significantly affected in vancomycin males, they are likely to have different defects in GI function, in particular altered mucosal fluid absorption/secretion. Notably, the role of 5‐HT+ cells and impact of the 5‐HT signalling pathway on the behaviour of the hosts can be sex‐dependent. Indeed, the number of 5‐HT+ cells and motility in the colon are affected by the oestrous cycles in female mice (Balasuriya et al., 2016, 2021). Effects of cholera toxin exposure on colonic motility are mediated via 5‐HT3 receptors in female instead of male mice (Balasuriya et al., 2016). It is not yet known whether there are sex differences in 5‐HT receptor expression in the gut, but 5‐HT receptor signalling, particularly involving 5‐HT1A receptors, has been shown to differ between the female and male brains (Stamatakis et al., 2006). The response of 5‐HT EC cells to glucose is impacted in overweight females, but not males (Lumsden et al., 2019). Moreover, females are also more susceptible to irritable bowel syndrome, anxiety and depression disorders, which are linked to dysregulation in 5‐HT signalling (Pretorius & Smith, 2020; So & Savidge, 2021).
Pre‐term and low birth weight infants are frequently given antibiotics, but these treatment practices and types of antibiotics administered are dependent on the severity of their clinical symptoms and are highly variable (Gill et al., 2022; Sadeghirad et al., 2018). Our experimental model tests vancomycin, which is one of the antibiotics given to infants to prevent and treat diseases such as necrotising enterocolitis. In comparison to humans, mice are born immature for gut function such that the mouse gut at birth models a pre‐term human gut, while the P10 gut is closer to full‐term human babies (Dutta & Sengupta, 2016). Furthermore, our group has shown that significant maturation of the mouse ENS occurs between P0 and P10, and by P10 the colon has a functioning albeit still maturing ENS (Foong, 2016). Finally, the human and murine microbiome share similar developmental stages in early life. However, there are other confounding factors in humans besides antibiotics that can impact microbiome development, for example mode‐of‐birth, feeding regimens and hospitalisation, especially in preterm infants who require intense care compared to term babies. Also, because rodent lifespan is significantly shorter than in humans, the longevity of antibiotic exposure on the host could be construed as being prolonged in our murine model, but less so from a microbiome perspective since bacterial renewal rates are similar across species. Therefore, while our mouse model and treatment period capture a critical developmental window for the GI tract, the impact of antibiotics on microbial community structure and its functional development during early life require further study to fully elucidate species differences in long term microbiome–host interactions.
Conclusions
We found that exposing newborn mice to an oral daily dose of vancomycin during the first 10 days of postnatal life has long‐lasting effects on the ENS, EC cells and microbiota of the colon. The prolonged, sexually dimorphic impact of neonatal antibiotic on the ENS and sex‐specific responses to dysbiosis and depleted 5‐HT levels may contribute to different functional GI disorder‐like symptoms in males versus females later in life.
Additional information
Competing interests
All authors had access to the study data and had reviewed and approved the final manuscript. All authors have no competing interests.
Author contributions
J.C.B., T.C.S. and J.P.P.F. designed the study and obtained funding; S.S.B.P., L.Y.H., Q.W., P.P., N.Y., A.H., R.A.L. and J.P.P.F. acquired the data; S.S.B.P., L.Y.H., Q.W. and J.P.P.F. analysed the data; and S.S.B.P., J.C.B., T.C.S. and J.P.P.F. wrote the paper. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported by National Health and Medical Research Council of Australia Project grants APP1099016 to J.P.P.F., J.C.B. and T.C.S., and Australian Research Council grant #DP130101596 to J.C.B., NIDDK P30‐DK56338 and R01DK130517, NIAID U01‐AI24290 and P01‐AI152999, and NINR R01‐NR013497 to T.C.S., and the University of Melbourne International Research and Fee Remission Scholarship to L.Y.H., and the Australian Government Research Training Program Scholarship to S.S.B.P.
Supporting information
Statistical Summary Document
Peer Review History
Table S1
Table S2
Figure S1
Acknowledgements
Open access publishing facilitated by The University of Melbourne, as part of the Wiley – The University of Melbourne agreement via the Council of Australian University Librarians.
Biography
Sabrina Poon is a PhD candidate at the University of Melbourne under the supervision of Dr Jaime Foong and Prof. Joel Bornstein in the Enteric Neuroscience Laboratory. Her research focuses on studying enteric neurodevelopment and how it interacts with the gut microbiota and enteroendocrine cells to impact gut physiology and body metabolism. She is also interested in further characterising the host response to other microbiota insults, as well as examining the underlying mechanism behind sex‐dependent feedback in the enteric nervous system.

Handling Editors: Laura Bennet & Melanie Gareau
Linked articles: This article is highlighted in a Perspective article by Kulkarni. To read this article, visit https://doi.org/10.1113/JP283624.
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP282939#support‐information‐section).
Data availability statement
All data supporting the results presented in the manuscript are included in the manuscript figures and in the Statistical Summary Document as Supporting Information.
References
- Balasuriya, G. K. , Hill‐Yardin, E. L. , Gershon, M. D. , & Bornstein, J. C. (2016). A sexually dimorphic effect of cholera toxin: Rapid changes in colonic motility mediated via a 5‐HT3 receptor‐dependent pathway in female C57Bl/6 mice. Journal of Physiology, 594, 4325–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasuriya, G. K. , Nugapitiya, S. S. , Hill‐Yardin, E. L. , & Bornstein, J. C. (2021). Nitric oxide regulates estrus cycle dependent colonic motility in mice. Frontiers in Neuroscience, 15, 647555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner, A. J. , Stamp, L. A. , Gonsalvez, D. G. , Allison, M. B. , Olson, D. P. , Myers, M. G. , Anderson, C. R. , & Young, H. M. (2014). Birthdating of myenteric neuron subtypes in the small intestine of the mouse. Journal of Comparative Neurology, 522(3), 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein, J. C. , Costa, M. , & Furness, J. B. (1986). Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea‐pig small intestine. Journal of Physiology, 381(1), 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein, J. C. , & Foong, J. P. P. (2018). Enteric neural regulation of mucosal secretion. In Said H. M. (Ed.), Physiology of the gastrointestinal tract (6th ed., pp. 429–451). Academic Press. [Google Scholar]
- Brierley, S. M. , & Linden, D. R. (2014). Neuroplasticity and dysfunction after gastrointestinal inflammation. Nature Reviews. Gastroenterology & Hepatology, 11, 611–627. [DOI] [PubMed] [Google Scholar]
- Chen, J. J. , Li, Z. , Pan, H. , Murphy, D. L. , Tamir, H. , Koepsell, H. , & Gershon, M. D. (2001). Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high‐affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. Journal of Neuroscience, 21(16), 6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, L. M. , Yamanishi, S. , Sohn, J. , Alekseyenko, A. V. , Leung, J. M. , Cho, I. , Kim, S. G. , Li, H. , Gao, Z. , Mahana, D. , Zarate Rodriguez, J. G. , Rogers, A. B. , Robine, N. , Loke, P. , & Blaser, M. J. (2014). Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell, 158(4), 705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian, P. S. , Muccino, D. , Rowitch, D. H. , Michael, S. K. , & McMahon, A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen‐inducible form of Cre recombinase. Current Biology, 8(24), 1323‐S2. [DOI] [PubMed] [Google Scholar]
- De Vroey, B. , De Cassan, C. , Gower‐Rousseau, C. , & Colombel, J. F. (2010). Editorial: Antibiotics earlier, IBD later? American Journal of Gastroenterology, 105(12), 2693–2696. [DOI] [PubMed] [Google Scholar]
- Drokhlyansky, E. , Smillie, C. S. , Van Wittenberghe, N. , Ericsson, M. , Griffin, G. K. , Eraslan, G. , Dionne, D. , Cuoco, M. S. , Goder‐Reiser, M. N. , Sharova, T. , Kuksenko, O. , Aguirre, A. J. , Boland, G. M. , Graham, D. , Rozenblatt‐Rosen, O. , Xavier, R. J. , & Regev, A. (2020). The human and mouse enteric nervous system at single‐cell resolution. Cell, 182(6), 1606–1622.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S. , & Sengupta, P. (2016). Men and mice: Relating their ages. Life Sciences, 152, 244–248. [DOI] [PubMed] [Google Scholar]
- Foong, J. P. (2016). Postnatal development of the mouse enteric nervous system. Advances in Experimental Medicine and Biology, 891, 135–143. [DOI] [PubMed] [Google Scholar]
- Foong, J. P. , & Bornstein, J. C. (2009). mGluR(1) receptors contribute to non‐purinergic slow excitatory transmission to submucosal VIP neurons of guinea‐pig ileum. Frontiers in Neuroscience, 3, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong, J. P. , Hirst, C. S. , Hao, M. M. , McKeown, S. J. , Boesmans, W. , Young, H. M. , Bornstein, J. C. , & Vanden Berghe, P. (2015). Changes in nicotinic neurotransmission during enteric nervous system development. Journal of Neuroscience, 35(18), 7106–7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong, J. P. , Nguyen, T. V. , Furness, J. B. , Bornstein, J. C. , & Young, H. M. (2012). Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. Journal of Physiology, 590(10), 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong, J. P. , Tough, I. R. , Cox, H. M. , & Bornstein, J. C. (2014). Properties of cholinergic and non‐cholinergic submucosal neurons along the mouse colon. Journal of Physiology, 592(4), 777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong, J. P. P. , Hung, L. Y. , Poon, S. , Savidge, T. C. , & Bornstein, J. C. (2020). Early life interaction between the microbiota and the Enteric Nervous System. American Journal of Physiology. Gastrointestinal and Liver Physiology, 319(5), G541–G548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe, P. , & Kunze, W. A. (2013). Voices from within: Gut microbes and the CNS. Cellular and Molecular Life Sciences, 70(1), 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, C. , Boesmans, W. , Cirillo, C. , Foong, J. P. P. , Bornstein, J. C. , & Vanden Berghe, P. (2017). VPAC receptor subtypes tune purinergic neuron‐to‐glia communication in the murine submucosal plexus. Frontiers in Cellular Neuroscience, 11, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness, J. B. (2012). The enteric nervous system and neurogastroenterology. Nature Reviews. Gastroenterology & Hepatology, 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Furness, J. B. , Jones, C. , Nurgali, K. , & Clerc, N. (2004). Intrinsic primary afferent neurons and nerve circuits within the intestine. Progress in Neurobiology, 72(2), 143–164. [DOI] [PubMed] [Google Scholar]
- Ge, X. , Ding, C. , Zhao, W. , Xu, L. , Tian, H. , Gong, J. , Zhu, M. , Li, J. , & Li, N. (2017). Antibiotics‐induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. Journal of Translational Medicine, 15(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon, M. D. (2013). 5‐Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current Opinion in Endocrinology, Diabetes, and Obesity, 20(1), 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, E. M. , Jung, K. , Qvist, N. , & Ellebæk, M. B. (2022). Antibiotics in the medical and surgical treatment of necrotizing enterocolitis. A systematic review. BMC Pediatrics, 22(1), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy, D. (2015). Principles and standards for reporting animal experiments in the Journal of Physiology and Experimental Physiology. Journal of Physiology, 593(12), 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne, R. M. , & Bornstein, J. C. (2007). Synaptic transmission at functionally identified synapses in the enteric nervous system: Roles for both ionotropic and metabotropic receptors. Current Neuropharmacology, 5(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne, R. M. , Ellis, M. , Sjovall, H. , & Bornstein, J. C. (2009). Cholera toxin induces sustained hyperexcitability in submucosal secretomotor neurons in guinea pig jejunum. Gastroenterology, 136(1), 299–308.e4. [DOI] [PubMed] [Google Scholar]
- Hansen, C. H. , Krych, L. , Nielsen, D. S. , Vogensen, F. K. , Hansen, L. H. , Sorensen, S. J. , Buschard, K. , & Hansen, A. K. (2012). Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia, 55(8), 2285–2294. [DOI] [PubMed] [Google Scholar]
- Hennig, G. W. , Gould, T. W. , Koh, S. D. , Corrigan, R. D. , Heredia, D. J. , Shonnard, M. C. , & Smith, T. K. (2015). Use of Genetically Encoded Calcium Indicators (GECIs) combined with advanced motion tracking techniques to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Frontiers in Cellular Neuroscience, 9, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia, D. J. , Gershon, M. D. , Koh, S. D. , Corrigan, R. D. , Okamoto, T. , & Smith, T. K. (2013). Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: In vitro analyses in mice lacking tryptophan hydroxylase 1. Journal of Physiology, 591(23), 5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, C. S. , Foong, J. P. , Stamp, L. A. , Fegan, E. , Dent, S. , Cooper, E. C. , Lomax, A. E. , Anderson, C. R. , Bornstein, J. C. , Young, H. M. , & McKeown, S. J. (2015). Ion channel expression in the developing enteric nervous system. PLoS One, 10(3), e0123436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, L. Y. , Boonma, P. , Unterweger, P. , Parathan, P. , Haag, A. , Luna, R. A. , Bornstein, J. C. , Savidge, T. C. , & Foong, J. P. P. (2019). Neonatal antibiotics disrupt motility and enteric neural circuits in mouse colon. Cellular and Molecular Gastroenterology and Hepatology, 8(2), 298–300.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, L. Y. , Parathan, P. , Boonma, P. , Wu, Q. , Wang, Y. , Haag, A. , Luna, R. A. , Bornstein, J. C. , Savidge, T. C. , & Foong, J. P. P. (2020). Antibiotic exposure postweaning disrupts the neurochemistry and function of enteric neurons mediating colonic motor activity. American Journal of Physiology. Gastrointestinal and Liver Physiology, 318(6), G1042‐G1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Liu, M. T. , & Gershon, M. D. (2003). Netrins and DCC in the guidance of migrating neural crest‐derived cells in the developing bowel and pancreas. Developmental Biology, 258(2), 364–384. [DOI] [PubMed] [Google Scholar]
- Keating, D. J. , & Spencer, N. J. (2019). What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacological Research, 140, 50–55. [DOI] [PubMed] [Google Scholar]
- Kim, D. , Hofstaedter, C. E. , Zhao, C. , Mattei, L. , Tanes, C. , Clarke, E. , Lauder, A. , Sherrill‐Mix, S. , Chehoud, C. , Kelsen, J. , Conrad, M. , Collman, R. G. , Baldassano, R. , Bushman, F. D. , & Bittinger, K. (2017). Optimizing methods and dodging pitfalls in microbiome research. Microbiome, 5(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussoulas, K. , Swaminathan, M. , Fung, C. , Bornstein, J. C. , & Foong, J. P. P. (2018). Neurally released GABA Acts via GABAC receptors to modulate Ca(2+) transients evoked by trains of synaptic inputs, but not responses evoked by single stimuli, in myenteric neurons of mouse ileum. Frontiers in Physiology, 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, S. , Micci, M. A. , Leser, J. , Shin, C. , Tang, S. C. , Fu, Y. Y. , Liu, L. , Li, Q. , Saha, M. , Li, C. , Enikolopov, G. , Becker, L. , Rakhilin, N. , Anderson, M. , Shen, X. , Dong, X. , Butte, M. J. , Song, H. , Southard‐Smith, E. M. , Kapur, R. P. , Bogunovic, M. , & Pasricha, P. J. (2017). Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proceedings of the National Academy of Sciences, USA, 114(18), E3709‐3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon, A. , Crook, N. , & Dantas, G. (2016). The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Medicine, 8(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira, C. , Sandgren, K. , Kessaris, N. , Richardson, W. , Potocnik, A. , Vanden Berghe, P. , & Pachnis, V. (2011). Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. Journal of Clinical Investigation, 121(9), 3412–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasrado, R. , Boesmans, W. , Kleinjung, J. , Pin, C. , Bell, D. , Bhaw, L. , McCallum, S. , Zong, H. , Luo, L. , Clevers, H. , Vanden Berghe, P. , & Pachnis, V. (2017). Lineage‐dependent spatial and functional organization of the mammalian enteric nervous system. Science, 356(6339), 722–726. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Hao, M. M. , Van den Haute, C. , Baekelandt, V. , Boesmans, W. , & Vanden Berghe, P. (2019). Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. eLife, 8, e42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden, A. L. , Martin, A. M. , Sun, E. W. , Schober, G. , Isaacs, N. J. , Pezos, N. , Wattchow, D. A. , de Fontgalland, D. , Rabbitt, P. , Hollington, P. , Sposato, L. , Due, S. L. , Rayner, C. K. , Nguyen, N. Q. , Liou, A. P. , Jackson, V. M. , Young, R. L. , & Keating, D. J. (2019). Sugar responses of human enterochromaffin cells depend on gut region, sex, and body mass. Nutrients, 11(2), 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna, R. A. , Oezguen, N. , Balderas, M. , Venkatachalam, A. , Runge, J. K. , Versalovic, J. , Veenstra‐VanderWeele, J. , Anderson, G. M. , Savidge, T. , & Williams, K. C. (2017). Distinct microbiome‐neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cellular and Molecular Gastroenterology and Hepatology, 3(2), 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May‐Zhang, A. A. , Tycksen, E. , Southard‐Smith, A. N. , Deal, K. K. , Benthal, J. T. , Buehler, D. P. , Adam, M. , Simmons, A. J. , Monaghan, J. R. , Matlock, B. K. , Flaherty, D. K. , Potter, S. S. , Lau, K. S. , & Southard‐Smith, E. M. (2021). Combinatorial transcriptional profiling of mouse and human enteric neurons identifies shared and disparate subtypes in situ. Gastroenterology, 160(3), 755–770.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown, S. J. , Chow, C. W. , & Young, H. M. (2001). Development of the submucous plexus in the large intestine of the mouse. Cell and Tissue Research, 303(2), 301–305. [DOI] [PubMed] [Google Scholar]
- McQuade, R. M. , Stojanovska, V. , Donald, E. L. , Rahman, A. A. , Campelj, D. G. , Abalo, R. , Rybalka, E. , Bornstein, J. C. , & Nurgali, K. (2017). Irinotecan‐induced gastrointestinal dysfunction is associated with enteric neuropathy, but increased numbers of cholinergic myenteric neurons. Frontiers in Physiology, 8, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongardi Fantaguzzi, C. , Thacker, M. , Chiocchetti, R. , & Furness, J. B. (2009). Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell and Tissue Research, 336(2), 179–189. [DOI] [PubMed] [Google Scholar]
- Morarach, K. , Mikhailova, A. , Knoflach, V. , Memic, F. , Kumar, R. , Li, W. , Ernfors, P. , & Marklund, U. (2021). Diversification of molecularly defined myenteric neuron classes revealed by single‐cell RNA sequencing. Nature Neuroscience, 24(1), 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyaka, P. M. , Khafipour, E. , & Ghia, J. E. (2014). External influence of early childhood establishment of gut microbiota and subsequent health implications. Frontiers in Pediatrics, 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor‐Kalinoski, A. , Smith‐Edwards, K. M. , Meerschaert, K. , Margiotta, J. F. , Rajwa, B. , Davis, B. M. , & Howard, M. J. (2022). Unique neural circuit connectivity of mouse proximal, middle, and distal colon defines regional colonic motor patterns. Cellular and Molecular Gastroenterology and Hepatology, 13(1), 309–337.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman, H. , Forsythe, P. , Uzan, A. , Avni, O. , & Koren, O. (2018). Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiology Review, 42, 489–499. [DOI] [PubMed] [Google Scholar]
- Parathan, P. , Wang, Y. , Leembruggen, A. J. , Bornstein, J. C. , & Foong, J. P. (2020). The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Developmental Biology, 458(1), 75–87. [DOI] [PubMed] [Google Scholar]
- Pham, T. D. , Gershon, M. D. , & Rothman, T. P. (1991). Time of origin of neurons in the murine enteric nervous system: Sequence in relation to phenotype. Journal of Comparative Neurology, 314(4), 789–798. [DOI] [PubMed] [Google Scholar]
- Pretorius, L. , & Smith, C. (2020). The trace aminergic system: A gender‐sensitive therapeutic target for IBS? Journal of Biomedical Science, 27(1), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Z. D. , Thacker, M. , Castelucci, P. , Bagyanszki, M. , Epstein, M. L. , & Furness, J. B. (2008). Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and Tissue Research, 334(2), 147–161. [DOI] [PubMed] [Google Scholar]
- Reigstad, C. S. , Salmonson, C. E. , Rainey, J. F., 3rd , Szurszewski, J. H. , Linden, D. R. , Sonnenburg, J. L. , Farrugia, G. , & Kashyap, P. C. (2015). Gut microbes promote colonic serotonin production through an effect of short‐chain fatty acids on enterochromaffin cells. FASEB Journal, 29(4), 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. R. , Ellis, M. , Gwynne, R. M. , Bergner, A. J. , Lewis, M. D. , Beckett, E. A. , Bornstein, J. C. , & Young, H. M. (2010). The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. Journal of Physiology, 588(7), 1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. R. , Murphy, J. F. , Young, H. M. , & Bornstein, J. C. (2007). Development of colonic motility in the neonatal mouse‐studies using spatiotemporal maps. American Journal of Physiology. Gastrointestinal and Liver Physiology, 292(3), G930–G938. [DOI] [PubMed] [Google Scholar]
- Sadeghirad, B. , Florez, I. D. , Chang, Y. , Forutan, F. , Zeraatkar, D. , Morgan, R. L. , Shahid, S. , Bala, M. M. , Beyene, J. , Offringa, M. , Adams‐Webber, T. , Sherman, P. M. , El‐Gouhary, E. , Guyatt, G. H. , & Johnston, B. C. (2018). Comparative effectiveness of prophylactic therapies for necrotizing enterocolitis in preterm infants: Protocol for a network meta‐analysis of randomized trials. International Journal of Preventive Medicine, 9, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang, Q. , & Young, H. M. (1996). Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell and Tissue Research, 284(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Sang, Q. , & Young, H. M. (1998). The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anatomical Record, 251(2), 185–199. [DOI] [PubMed] [Google Scholar]
- Shaw, S. Y. , Blanchard, J. F. , & Bernstein, C. N. (2010). Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. American Journal of Gastroenterology, 105(12), 2687–2692. [DOI] [PubMed] [Google Scholar]
- Siu, Y. K. , Ng, P. C. , Fung, S. C. , Lee, C. H. , Wong, M. Y. , Fok, T. F. , So, K. W. , Cheung, K. L. , Wong, W. , & Cheng, A. F. (1998). Double blind, randomised, placebo controlled study of oral vancomycin in prevention of necrotising enterocolitis in preterm, very low birthweight infants. Archives of Disease in Childhood Fetal and Neonatal Edition, 79(2), F105–F109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So, S. Y. , & Savidge, T. C. (2021). Sex‐bias in irritable bowel syndrome: Linking steroids to the gut‐brain axis. Frontiers in Endocrinology, 12, 684096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, A. , Mantelas, A. , Papaioannou, A. , Pondiki, S. , Fameli, M. , & Stylianopoulou, F. (2006). Effect of neonatal handling on serotonin 1A sub‐type receptors in the rat hippocampus. Neuroscience, 140(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Swaminathan, M. , Hill‐Yardin, E. L. , Bornstein, J. C. , & Foong, J. P. P. (2019). Endogenous glutamate excites myenteric calbindin neurons by activating group i metabotropic glutamate receptors in the mouse colon. Frontiers in Neuroscience, 13, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, L. , Hires, S. A. , Mao, T. , Huber, D. , Chiappe, M. E. , Chalasani, S. H. , Petreanu, L. , Akerboom, J. , McKinney, S. A. , Schreiter, E. R. , Bargmann, C. I. , Jayaraman, V. , Svoboda, K. , & Looger, L. L. (2009). Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods, 6(12), 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka, T. , Nagashimada, M. , & Enomoto, H. (2015). Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. Journal of Neuroscience, 35(27), 9879–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. M. , Schneider, S. , Smith‐Edwards, K. M. , Mafra, F. , Leembruggen, A. J. L. , Gonzalez, M. V. , Kothakapa, D. R. , Anderson, J. B. , Maguire, B. A. , Gao, T. , Missall, T. A. , Howard, M. J. , Bornstein, J. C. , Davis, B. M. , & Heuckeroth, R. O. (2021). scRNA‐Seq reveals new enteric nervous system roles for GDNF, NRTN, and TBX3. Cellular and Molecular Gastroenterology and Hepatology, 11(5), 1548–1592.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, Y. , & Mikoshiba, K. (2012). Quantitative comparison of novel GCaMP‐type genetically encoded Ca(2+) indicators in mammalian neurons. Frontiers in cellular Neuroscience, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, J. M. , Yu, K. , Donaldson, G. P. , Shastri, G. G. , Ann, P. , Ma, L. , Nagler, C. R. , Ismagilov, R. F. , Mazmanian, S. K. , & Hsiao, E. Y. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel, A. , Hochgerner, H. , Lönnerberg, P. , Johnsson, A. , Memic, F. , van der Zwan, J. , Häring, M. , Braun, E. , Borm, L. E. , La Manno, G. , Codeluppi, S. , Furlan, A. , Lee, K. , Skene, N. , Harris, K. D. , Hjerling‐Leffler, J. , Arenas, E. , Ernfors, P. , Marklund, U. , & Linnarsson, S. (2018). Molecular architecture of the mouse nervous system. Cell, 174(4), 999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Peer Review History
Table S1
Table S2
Figure S1
Data Availability Statement
All data supporting the results presented in the manuscript are included in the manuscript figures and in the Statistical Summary Document as Supporting Information.


