Abstract
Purpose
To examine how physical activity (PA) and sitting time (ST) are associated with mortality in older Japanese adults.
Methodology
We used the data of 10 233 older Japanese adults aged ≥65 years who provided valid responses to the International Physical Activity Questionnaire‐Short Form (IPAQ‐SF) by a mail survey. Both PA and ST were assessed using the IPAQ‐SF. The results were classified into high or low categories using ≥3.0 metabolic equivalent PA (150 min/week) and ST (300 min/day) into the following four groups: High PA (HPA)/Low ST (LST), HPA/High ST (HST), Low PA (LPA)/LST, and LPA/HST. Mortality data were collected from July 30, 2011, to November 30, 2016. We assessed the interaction of PA and ST status with the risk of all‐cause mortality using the multivariable Cox proportional‐hazards model.
Results
A total of 1014 people were recorded to have died during a median follow‐up period of 5.3 years (51 553 person‐years). After adjustment for confounders, the risk of mortality was higher in the LPA/HST group than in all other groups (HPA/LST: reference; HPA/HST group: hazard ratio [HR] 0.86 (95% confidence interval [CI]: 0.66 to 1.12); LPA/LST group: HR 1.09 (95% CI: 0.88 to 1.35); LPA/HST group: HR 1.36 (95% CI: 1.10 to 1.67); and multiplicative interaction: HR 1.44 (95% CI: 1.07 to 1.94)).
Conclusions
The risk of mortality associated with LPA/HST depends on the level of PA, duration of ST, and their interaction with each other. Our results may be useful in ameliorating the adverse effects leading to mortality in individuals with lower PA, by reducing ST.
Keywords: International Physical Activity Questionnaire‐Short Form, mortality, multiplicative interaction, older adults, physical activity, population‐based longitudinal cohort study, relative excess risk due to interaction, sitting time
1. INTRODUCTION
Insufficient physical activity (PA) is a primary but modifiable factor in shortening lifespans worldwide, 1 , 2 and its prevalence has increased over time in high‐income countries. 3 In 2020, the World Health Organization (WHO) published guidelines on PA and sedentary behavior to promote good health. 4 These guidelines emphasize the importance of engaging in regular moderate‐to‐vigorous PA and reducing sedentary behavior for adults 4 ; however, international comparative studies have revealed that one in three or four adults do not engage in sufficient PA. 3 , 5
Several prospective cohort studies have revealed that PA is negatively associated with the risk of all‐cause mortality in adults via objective 6 , 7 , 8 , 9 , 10 and self‐reported 11 , 12 assessments. In addition, sitting time (ST) or sedentary behavior, according to objective 7 , 8 and self‐reported 11 , 12 , 13 , 14 assessments, is positively associated with the risk of all‐cause mortality. Previous studies using a substitution model reported that in middle‐aged and older adults, replacing ST with PA time is associated with reduced risks of all‐cause mortality 11 , 12 and cardiovascular disease mortality. 11 These results may be due to a close association between PA and ST regarding the risk of mortality.
The number of steps per day has been used as an objective indicator of PA across 111 countries. 15 Recently, a pooled analysis of data from 47 471 adults from 15 international cohorts has reported a negative association between the step counts assessed by an accelerometer and the risk of death. 10 Daily ST was assessed using questionnaires in 20 different countries. 16 Intriguingly, these studies revealed that people in Japan, Hong Kong, and the Czech Republic walk more steps per day and sit for longer periods than people in other countries. 15 , 16 Epidemiological studies on middle‐aged and older adults have not yielded consistent results as to whether PA can reduce the risk of mortality associated with prolonged ST. 11 , 17 , 18 , 19 Also, to our knowledge, the impact of the interaction between PA and ST on the risk of mortality has not been sufficiently examined in older adults aged ≥65 years. Such an investigation may provide findings that are essential for informing public policies regarding PA and ST for the numerous sedentary older adults. An international comparative study of step counts 15 described the lack of a sufficient sample size due to the lower number of older people with wearable devices for collecting objective data on PA compared to young people. Therefore, considering the versatility of PA and ST assessment in older adults, it is necessary to evaluate how self‐reported PA and ST relate with mortality risk. We hypothesize that low PA and high ST are strongly associated with the risk of mortality; therefore, we aimed to examine the interaction between PA and ST regarding the risk of all‐cause mortality in a community‐based longitudinal cohort study of older Japanese adults.
2. MATERIALS AND METHODS
2.1. Study design
The Kyoto–Kameoka Study is a prospective cohort study of older adults aged ≥65 years living in Kameoka, Kyoto, Japan. The details of the study have been described. 20 , 21 , 22 To conduct a complete survey of adults aged ≥65 years living in Kameoka as of July 1, 2011, the person in charge at the City Hall selected eligible candidates based on information such as name, sex, and date of birth taken from basic resident registers managed at the Kameoka City Hall (Figure 1). Among the candidates selected (n = 18 231), 13 294 responded to the survey (response rate: 72.9%). The baseline survey, which was conducted by mail, obtained health‐related information, including PA and ST, via the International Physical Activity Questionnaire‐Short Form (IPAQ‐SF) and medical history, socioeconomic status, smoking status, and alcohol consumption status. We excluded participants with an incomplete IPAQ‐SF (n = 1810), those who needed support level 1 or 2 (n = 667) or long‐term care level 1 or 2 (n = 573), and those who moved out of the city on an unknown date (n = 12). Finally, 10 232 participants were included in the main analysis. This manuscript is in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement. 23
FIGURE 1.
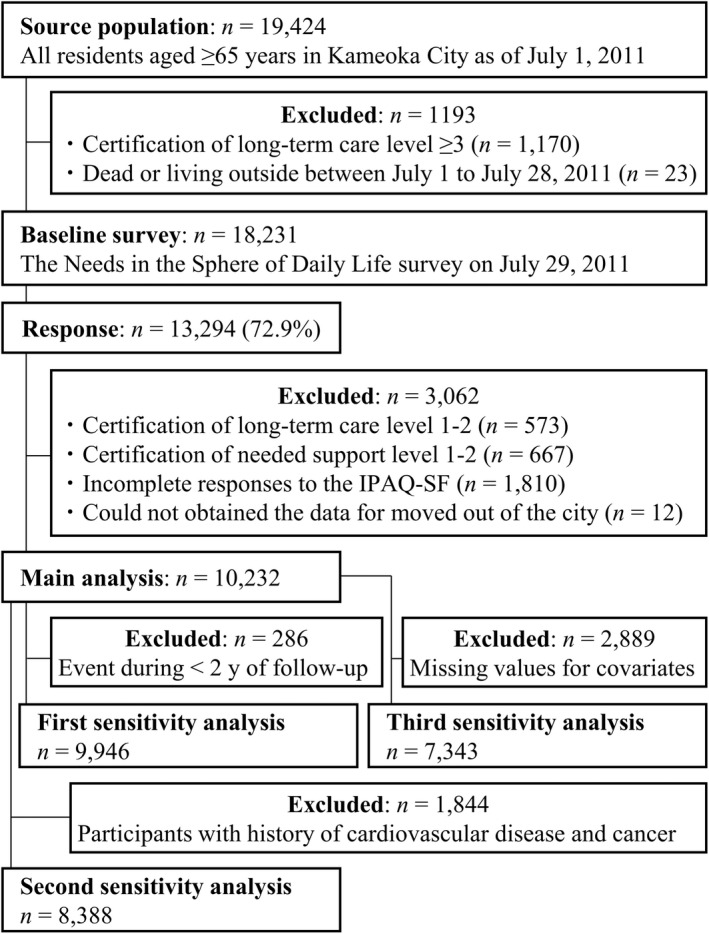
Participant flow diagram for the analysis of physical activity and sitting time and mortality in the Kyoto–Kameoka study. IPAQ‐SF, The International Physical Activity Questionnaire‐Short Form.
The study was conducted according to the guidelines laid down in the 1964 Declaration of Helsinki, and all procedures involving research study participants were approved by the Research Ethics Committee of Kyoto Prefectural University of Medicine (RBMR‐E‐363), the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN‐76‐2), and the Kyoto University of Advanced Science (No. 20–1). We obtained informed consent from all participants at the time of their response to the mail survey.
2.2. Assessment of PA and ST
We assessed PA and ST over the previous average week using the IPAQ‐SF, which has been verified as valid and reproducible in 12 countries, including Japan. 24 The IPAQ‐SF considers only PA performed for bouts of approximately 10 min in a single session. We calculated PA (min/week) for the moderate‐intensity activity of ≥3.0 metabolic equivalents (METs).
For older adults, calculating PA (MET‐min/week) using METs based on the values in the original version of the IPAQ‐SF (vigorous PA = 8.0 METs, moderate PA = 4.0 METs, and walking = 3.3 METs) was done to greatly overestimate PA compared to objective measurements. 25 We calculated PA with PA intensities for older adults used in a previous study (vigorous PA = 5.3 METs, moderate PA = 3.0 METs, and walking = 2.5 METs) 25 and the sum of various activity times (time and frequency). The validity of PA estimated with activity intensities corrected from the IPAQ‐SF was verified in relation to PA estimated with an accelerometer in older Japanese adults aged ≥65 years. 25
We assessed ST with self‐reporting using questions included in the IPAQ‐SF. 24 The reproducibility of ST as estimated from this questionnaire was confirmed in older Japanese adults. 25
2.3. Outcome
The survival status of the cohort participants during the follow‐up period was assessed with information from basic resident registers managed at the Kameoka City Hall. These data were provided by Kameoka City Hall for the period from July 30, 2011, to November 30, 2016. We censored residents whose registers had been invalidated or who had moved out of Kameoka (258 [723 person‐years] out of 10 233 [51 553 person‐years]).
2.4. Statistical analysis
We divided the participants into two groups (≥ or < 150 min of moderate‐intensity activity [≥3.0 METs]/week) according to the WHO guideline‐recommended value for PA. 4 Participants who met the guideline‐recommended PA targets for older adults were allocated to the high PA group. Meanwhile, ST was divided into two groups (≥ or < 300 min/day) according to the cutoff point of a previous study. 13 This cutoff point may be appropriate, considering the median ST of the participants in this study (300 min/day) and the low risk of death in individuals with an ST of <300 min/day in the middle‐aged and older Japanese population. 13 Participants were then classified into the following four categories: High PA (HPA)/Low ST (LST) group (PA: ≥150 min/week and ST: <300 min/day; n = 2047), HPA/High ST (HST) group (PA: ≥150 min/week and ST: ≥300 min/day; n = 1638), Low PA (LPA)/LST group (PA: <150 min/week and ST: <300 min/day; n = 3196), and LPA/HST group (PA: <150 min/week and ST: ≥300 min/day; n = 3351). Descriptive statistics for continuous and categorical variables are presented as mean/standard deviation and number/percentage of participants, respectively. Missing values for covariates were supplemented from five datasets created with multiple imputations using multivariate imputation by chained equations (mice package) in the R software 26 : body mass index (n = 516, 5.0%); family structure (n = 755, 7.4%); socioeconomic status (n = 449, 4.4%); education (n = 1137, 11.1%); smoking status (n = 418, 4.1%); alcohol status (n = 355, 3.5%); self‐reported health (n = 375, 3.7%); sleep times (n = 223, 2.2%); and medications (n = 754, 7.4%). To assume the missing at random (MAR) approximately satisfied, we included all data of the individual characteristics obtained from the Kyoto–Kameoka study baseline survey in the imputation model. All the missing values were presumed to be MAR. In addition, individual characteristics were compared between those included in the present study and those excluded. We assessed the percentages of participants who met the targets for PA for older adults aged ≥65 years in the WHO Physical Activity Guidelines 2020 4 and the Japanese Physical Activity Guidelines for Health Promotion 2013. 27
To adjust for confounders in the association between PA/ST and the risk of all‐cause mortality, we used a multivariate Cox proportional‐hazards model that included baseline covariates. These analyses used time‐on‐study as the time scale. To confirm the assumption for Cox proportional‐hazards models, we conducted the Schoenfeld residuals test and visually confirmed these diagrams. We assumed the proportional‐hazard condition because our data could not reject this test (p‐value = 0.131). Multivariate analysis was performed according to the following two models: Model 1 adjusted for age (continuous), sex (female or male), and population density (≥ 1000 or < 1000 people/km2), while Model 2 additionally adjusted for body mass index (continuous), living alone (yes or no), socioeconomic status (high or low), educational attainment (<9, 10–12, or ≥ 13 years), smoking status (never smoker, past smoker, or current smoker), alcohol drinker (yes or no), self‐reported general health (good or poor), sleep time (continuous), medication use (continuous), and number of chronic diseases (continuous). These adjustment factors were selected in accordance with previous studies. 6 , 9 , 13 We asked the participants for only the number of medications used but not the kinds of drugs. The results of these analyses were calculated as hazard ratios (HRs) and 95% confidence intervals (CI); HRs were also calculated with reference to the HPA/LST group. Regarding the interaction between outcome and exposure, it is best to present both additive and multiplicative measures of interaction. 28 Therefore, we calculated the additive (relative excess risk due to interaction: RERI) and multiplicative interaction using a categorical high/low variable for PA and ST.
We estimated propensity scores for assignment into each group using multivariate logistic regression analysis that included the variables in Model 2, and we created adjusted Kaplan–Meier survival curves using inverse probability weighting. We performed sensitivity analysis according to the following three methods: (1) To eliminate the possibility of reverse causal relationships, we excluded death events recorded in the first two years of follow‐up (194 men and 92 women) and (2) participants with a history of cardiovascular disease and cancer; (3) we performed a similar analysis using a dataset of complete cases, which did not include missing values. In addition, we also created Nelson–Aalen Cumulative Hazard curves for mortality according to PA and ST using age as the time scale. 29
Furthermore, to assess the curves for the relationships between PA/ST and the risk of all‐cause mortality, we used a restricted cubic spline model with three data points based on the distribution of these values. 6 , 21 , 22 To evaluate the association between PA and ST by continuous variable, we calculated the ST–PA time using a continuous variable. Considering that the data were sparse, we truncated the analysis at 5880 METs‐min/week or 1560 min/week in PA, 960 min/day in ST, and 930 min/day in ST–PA time (2% of the distribution). 6 These results were presented as HR and 95% CI, with HR calculated in reference to a complete absence of PA (0 MET‐hours week or min) or ST (0 min). The details of the HR reference point are shown in the Figure 3 legend.
FIGURE 3.
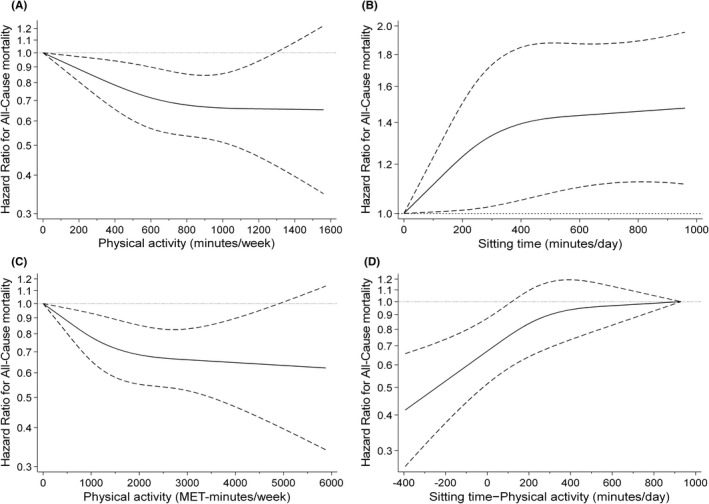
Restricted cubic spline regression model between the physical activity and sitting time status and risk of mortality. Solid lines represent hazard ratios, dashed lines represent 95% confidence intervals, and the hazard ratio based on (A) 0 min/week for ≥3.0METs physical activity (n = 10 082), (B) 0 min/day for sitting time (n = 10 019), (C) 0 MET‐min/week for ≥2.0METs physical activity (n = 10 026), and (D) 930 min/day for sitting time − physical activity (n = 10 025) as reference was calculated. The adjustment factors are age, sex, population density, body mass index, family structure, economic status, educational attainment, smoking status, alcohol consumption status, self‐reported health, sleep times, medication use, and number of chronic diseases.
In statistical analysis, two‐tailed p‐values <0.05 were considered significant. All analyses were performed with STATA MP, version 15.0 (StataCorp LP) and/or R software 3.4.3 (R Core Team).
3. RESULTS
3.1. Participants' demographics
A total of 10 232 participants were ultimately included in the present study. Table 1 presents the characteristics of the participants in each of the four groups by PA and ST status in the cohort analysis. The median ages (interquartile range) were 70 (67 to 74) years in the HPA/LST group, 71 (68 to 75) years in the HPA/HST group, 72 (68 to 77) years in the LPA/LST group, and 74 (70 to 80) years in the LPA/HST group. The LPA/HST group was older and had a higher percentage of women, lower educational attainment, and poorer self‐reported health compared to the HPA/LST group. In addition, the individuals excluded from the study were older and mostly women compared to the participants included in the study (Table S1).
TABLE 1.
Baseline participant characteristics by physical activity and sitting time status
| Total a | PA and ST status | p‐value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPA/LST | HPA/HST | LPA/LST | LPA/HST | ||||||||
| (n = 10 232) | (n = 2047) | (n = 1638) | (n = 3196) | (n = 3351) | |||||||
| Age [years] b | 73.4 | (6.2) | 71.4 | (5.1) | 72.2 | (5.6) | 73.3 | (6.0) | 75.6 | (6.8) | <0.001 |
| 65–74 years [n (%)] c | 6362 | (62.2) | 1554 | (75.9) | 1158 | (70.7) | 1968 | (61.6) | 1682 | (50.2) | <0.001 |
| ≥75 years [n (%)] c | 3870 | (37.8) | 493 | (24.1) | 480 | (29.3) | 1228 | (38.4) | 1669 | (49.8) | |
| Women [n (%)] c | 5342 | (52.2) | 890 | (43.5) | 765 | (46.7) | 1783 | (55.8) | 1904 | (56.8) | <0.001 |
| PD ≥1000 people/km2 [n (%)] c | 4579 | (44.8) | 966 | (47.2) | 764 | (46.6) | 1378 | (43.1) | 1471 | (43.9) | 0.008 |
| Body mass index [kg/m2] b | 22.6 | (3.4) | 22.7 | (3.3) | 22.6 | (3.1) | 22.4 | (3.3) | 22.6 | (3.6) | 0.019 |
| Living alone [n (%)] c | 1174 | (11.5) | 179 | (8.7) | 223 | (13.6) | 329 | (10.3) | 443 | (13.2) | <0.001 |
| HSES [n (%)] c | 3357 | (32.8) | 715 | (34.9) | 559 | (34.1) | 1042 | (32.6) | 1041 | (31.1) | 0.017 |
| Education ≥13 y [n (%)] c | 2142 | (20.9) | 527 | (25.7) | 381 | (23.3) | 625 | (19.6) | 609 | (18.2) | <0.001 |
| Current smoker [n (%)] c | 1167 | (11.4) | 219 | (10.7) | 185 | (11.3) | 381 | (11.9) | 382 | (11.4) | <0.001 |
| Alcohol drinker [n (%)] c | 6696 | (65.4) | 1504 | (73.5) | 1156 | (70.6) | 2033 | (63.6) | 2003 | (59.8) | <0.001 |
| Poor self‐reported health [n (%)] c | 1991 | (19.5) | 202 | (9.9) | 235 | (14.3) | 594 | (18.6) | 960 | (28.6) | <0.001 |
| Sleep times [min/day] b | 406 | (83) | 398 | (73) | 405 | (72) | 399 | (83) | 418 | (93) | <0.001 |
| No medication [n (%)] c | 2222 | (21.7) | 538 | (26.3) | 386 | (23.6) | 697 | (21.8) | 601 | (17.9) | <0.001 |
| Hypertension [n (%)] c | 3802 | (37.2) | 734 | (35.9) | 592 | (36.1) | 1169 | (36.6) | 1307 | (39.0) | 0.056 |
| Stroke [n (%)] c | 373 | (3.7) | 65 | (3.2) | 62 | (3.8) | 97 | (3.0) | 149 | (4.4) | 0.012 |
| Heart disease [n (%)] c | 1241 | (12.1) | 191 | (9.3) | 180 | (11.0) | 383 | (12.0) | 487 | (14.5) | <0.001 |
| Diabetes [n (%)] c | 1040 | (10.2) | 202 | (9.9) | 176 | (10.7) | 301 | (9.4) | 361 | (10.8) | 0.250 |
| Hyperlipidemia [n (%)] c | 947 | (9.3) | 219 | (10.7) | 175 | (10.7) | 259 | (8.1) | 294 | (8.8) | 0.002 |
| Digestive disease [n (%)] c | 486 | (4.8) | 64 | (3.1) | 62 | (3.8) | 145 | (4.5) | 215 | (6.4) | <0.001 |
| Respiratory disease [n (%)] c | 841 | (8.2) | 120 | (5.9) | 159 | (9.7) | 251 | (7.9) | 311 | (9.3) | <0.001 |
| Urological diseases [n (%)] c | 606 | (5.9) | 122 | (6.0) | 111 | (6.8) | 154 | (4.8) | 219 | (6.5) | 0.010 |
| Cancer [n (%)] c | 375 | (3.7) | 64 | (3.1) | 70 | (4.3) | 92 | (2.9) | 149 | (4.4) | 0.002 |
| No. of chronic diseases b , d | 0.9 | (1.0) | 0.9 | (0.9) | 1.0 | (1.0) | 0.9 | (0.9) | 1.0 | (1.0) | <0.001 |
| Physical activity [min/week] b | 229 | (436) | 666 | (601) | 535 | (469) | 15 | (36) | 16 | (36) | <0.001 |
| [MET‐min/week] b | 759 | (1554) | 2270 | (2242) | 1722 | (1714) | 45 | (112) | 47 | (109) | <0.001 |
| Sitting time [min/day] b | 319 | (224) | 162 | (62) | 463 | (191) | 159 | (63) | 498 | (217) | <0.001 |
| Mortality [event/1000 PY] e | 19.7 | 12.0 | 12.6 | 17.5 | 30.2 | ||||||
| [95%CI] e | (18.5 to 20.9) | (10.1 to 14.3) | (10.4 to 15.3) | (15.6 to 19.7) | (27.7 to 33.0) | ||||||
Abbreviations: CI, confidence interval; HSES, high socioeconomic status; HST, high sitting time; HPA, high physical activity; LPA, low physical activity; LST, low sitting time; MET, metabolic equivalents; PA, physical activity; PD, population density; PY, person‐years; and ST, sitting time.
Data for participants with missing values were imputed by multiple imputations: body mass index (n = 516, 5.0%); family structure (n = 755, 7.4%); socioeconomic status (n = 449, 4.4%); education (n = 1137, 11.1%); smoking status (n = 418, 4.1%); alcohol status (n = 355, 3.5%); self‐reported health (n = 375, 3.7%); sleep times (n = 223, 2.2%); medications (n = 754, 7.4%).
Continuous variables are expressed as mean with standard deviation and were analyzed using variance analysis.
Category variables are expressed as the number of cases with percentage and were analyzed using Pearson's chi‐squared test.
From the data obtained on disease status (including the presence of hypertension, stroke, heart disease, diabetes, hyperlipidaemia, digestive disease, respiratory disease, urological diseases, and cancer), the comorbidity scores were summed to obtain a total score ranging from 0 (no comorbidity) to 9 (poor status).
Absolute risk of mortality was calculated using data from July 30, 2011, to November 30, 2016. Mortality risk is shown as rate (95%CI) per 1000 person‐years.
3.2. Prevalence of meeting guideline‐recommended PA targets
Table S2 presents the prevalence of meeting guideline‐recommended PA targets for older adults. The WHO target guideline (≥150 min/week) was met by 36.0% of participants. The prevalence of meeting the guideline was low among individuals aged ≥75 years and among women when these relationships were stratified by age and sex.
3.3. PA and ST on the risk of mortality
Figure 2 and Table 2 present the relationships between PA/ST status and the risk of all‐cause mortality. The median follow‐up period was 5.3 years (51 533 person‐years). During the follow‐up period, 1014 participants (9.9%) died. The risk of all‐cause mortality was higher in the LPA/HST group than in all other groups after adjustment for confounders [HPA/LST: reference; HPA/HST group: HR 0.86 (95% CI: 0.66 to 1.12); LPA/LST group: HR 1.09 (95% CI: 0.88 to 1.35); and LPA/HST group: HR 1.36 (95% CI: 1.10 to 1.67), p < 0.001]. The interaction between LPA and HST accounted for excess risk of mortality in the LPA/HST group [Multiplicative: HR 1.44 (95% CI: 1.07 to 1.94), p = 0.016; Additive: RERI 0.41 (95% CI: 0.12 to 0.71), p < 0.001]. The Nelson–Aalen Cumulative Hazard curves using age as the time scale were similar to the results in these relationships and showed a slope, suggesting an interaction between age and the PA/ST group with regard to their association with mortality (Figure 2B). The same results emerged in a sensitivity analysis (Tables S3–S5) and age‐ and sex‐stratified analyses (Tables S6, S7).
FIGURE 2.
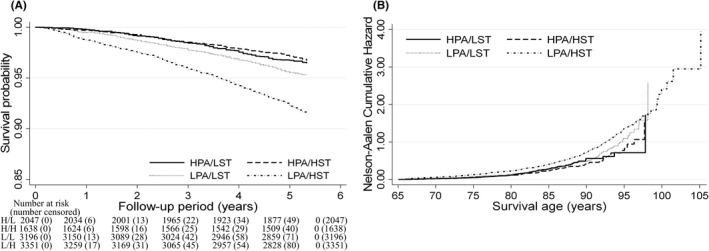
Survival analysis for all‐cause mortality according to physical activity and sitting time status among older adults. (A) Multivariate adjusted Kaplan–Meier survival curves using inverse probability weighting and (B) Nelson–Aalen Cumulative Hazard curves using age as the time‐scale. Four groups stratified by physical activity and sitting time status: HPA/LST, high physical activity/low sitting time (H/L); HPA/HST, high physical activity/high sitting time (H/H); LPA/LST, low physical activity/low sitting time (L/L); and LPA/HST, low physical activity/high sitting time (L/H). The adjustment factors are age, sex, population density, body mass index, family structure, economic status, educational attainment, smoking status, alcohol consumption status, self‐reported health, sleep times, medication use, and number of chronic diseases.
TABLE 2.
Hazard ratios for physical activity and sitting time status and all‐cause mortality calculated using multivariate Cox proportional hazards model
| n | Event | PY | Event/1000 PY | Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rate | 95%CI | HR | 95%CI | HR | 95%CI | ||||
| PA×ST | |||||||||
| HPA/LST | 2047 | 126 | 10 502 | 12.0 | (10.1 to 14.3) | 1.00 | (Ref) | 1.00 | (Ref) |
| HPA/HST | 1638 | 106 | 8399 | 12.6 | (10.4 to 15.3) | 0.93 | (0.72 to 1.21) | 0.86 | (0.66 to 1.12) |
| LPA/LST | 3196 | 284 | 16 184 | 17.5 | (15.6 to 19.7) | 1.23 | (0.99 to 1.52) | 1.09 | (0.88 to 1.35) |
| LPA/HST | 3351 | 498 | 16 467 | 30.2 | (27.7 to 33.0) | 1.71 | (1.39 to 2.09) | 1.36 | (1.10 to 1.67) |
| Interaction | |||||||||
| Additive c | 13.1 | (10.9 to 15.3) | 0.55 | (0.25 to 0.85) | 0.41 | (0.12 to 0.71) | |||
| p‐value | < 0.001 | < 0.001 | < 0.001 | ||||||
| Multiplicative d | 1.65 | (1.22 to 2.21) | 1.49 | (1.11 to 2.00) | 1.44 | (1.07 to 1.94) | |||
| p‐value | 0.001 | 0.008 | 0.016 | ||||||
| PA | |||||||||
| High | 3685 | 232 | 18 902 | 12.3 | (10.8 to 14.0) | 1.00 | (Ref) | 1.00 | (Ref) |
| Low | 6547 | 782 | 32 651 | 24.0 | (22.3 to 25.7) | 1.53 | (1.32 to 1.78) | 1.32 | (1.13 to 1.54) |
| ST | |||||||||
| Low | 5243 | 410 | 26 686 | 15.4 | (13.9 to 16.9) | 1.00 | (Ref) | 1.00 | (Ref) |
| High | 4989 | 604 | 24 867 | 24.3 | (22.4 to 26.3) | 1.28 | (1.12 to 1.45) | 1.14 | (1.00 to 1.30) |
Note: This analysis included 10 232 participants.
Abbreviations: CI, confidence interval; HPA, high physical activity; HR, hazard ratio; HST, high sitting time; LPA, low physical activity; LST, low sitting time; PA, physical activity; PY, person‐years; Ref, reference; RERI, relative excess risk due to interaction; and ST, sitting time.
Model 1: Adjusted for age, sex, and population density.
Model 2: In addition to the factors listed in Model 1, adjusted for body mass index, family structure, economic status, educational attainment, smoking status, alcohol consumption status, self‐reported health status, sleep times, medication use, and number of chronic diseases.
The additive interaction was calculated as the RERI using the following equation: RERI = HR [LPA/HST]–(HR [LPA/LST] + HR [HPA/HST] –1). It is significant (p < 0.05) if the 95% CI of the RERI is not below 0.
It is significant (p < 0.05) if the 95% CI of the multiplicative interaction is not below 1.00.
3.4. Dose–response relationships
To assess the curve relationships of PA and ST to the risk of all‐cause mortality, we used a restricted cubic spline model (Figure 3). We demonstrated that even after adjustment for baseline confounders, with a PA of 0 min or 0 MET‐min/week as a reference, PA was strongly negatively associated with the risk of all‐cause mortality in a dose‐dependent manner up to approximately 800 min/week or approximately 2000 MET‐min/week (about 33 MET‐hour/week); however, no large differences were observed beyond that point (Figure 3A,C). With 0 min of ST as a reference, we demonstrated that ST is strongly positively associated with the risk of all‐cause mortality in a dose‐dependent manner up to 420 min; however, no large differences were observed beyond that point (Figure 3B). We consequently demonstrated that a daily ST–PA time of less than approximately 120 min was negatively associated with the risk of mortality (Figure 3D).
4. DISCUSSION
4.1. Main findings
The present study examined the interaction between PA and ST regarding the risk of all‐cause mortality via a population‐based cohort study of older Japanese adults. We demonstrated that LPA/HST was more strongly associated with the risk of all‐cause mortality than any other combination of PA and ST status. We demonstrated that the interaction between LPA and HST accounted for a relative excess risk of mortality in the LPA/HST group. To the best of our knowledge, this is the first study to examine the interaction between PA and ST regarding the risk of mortality in older adults. The adverse effects between ST and the risk of all‐cause mortality depend on PA, a finding which suggests that both PA and ST need to be assessed.
4.2. PA goals
We observed that 36% of participants met the WHO‐guideline PA target of ≥150 min/week; hence, 64% had insufficient PA. One study reported that 30.3% of older American adults aged ≥60 years met the WHO‐guideline PA target of ≥150 min/week. 30 An international comparative study reported that 60.2% of Japanese do not engage in sufficient PA 5 ; however, a later study reported that the percentage of insufficient PA was 35.7% in the high‐income Asia Pacific area (which includes Japan) and 36.8% among high‐income Western countries. 3 This result differs greatly from the results of our study and previous studies. We previously reported in a 3616‐person sub‐cohort of the present study (mean age 72.3 years) that the mean number of steps (as assessed with an accelerometer) was lower among older adults aged ≥75 years than among older adults aged 65–74 years. 22 This result is consistent with the prevalence of participants who met the WHO‐guideline PA target as estimated from the questionnaire forms. Adhering to PA guidelines is negatively associated with the risk of mortality 30 ; therefore, it is necessary to conduct a study that assesses the prevalence of meeting PA guideline targets using objectively evaluated PA among randomly sampled participants because self‐reported PA can be modified in their desired direction without any actual behavioral change. 31
4.3. Interaction between PA and ST
Our results revealed that HPA/LST may partially reduce the adverse effects between LPA/HST and mortality, and the interaction between LPA and HST may be involved in the risk of all‐cause mortality. Previous studies with middle‐aged and older adults have examined whether PA can reduce the increased risk of mortality associated with HST; however, these results have been inconsistent. 11 , 17 , 18 , 19 Replacing ST with PA time is reported to be more effective for reducing the risk of mortality in middle‐aged and older adults with HST than in those with LST. 11 , 12 Sedentary behavior, as assessed with accelerometers, is approximately 20–30% higher in older adults than in younger adults aged 30–39 years. 32 Our study population comprised older adults aged ≥65 years with longer ST and less PA compared to middle‐aged and older adults in previous studies, which may have made the interaction between PA and ST easier to confirm. Particularly, in a society in which more people are forced to sit for long periods due to job‐related demands, these results may provide further evidence for the benefits of improving PA and ST and could be used for future public health recommendations.
4.4. The dose–response relationship and mechanism of ST − PA regarding the risk of mortality
Our data revealed that a daily ST − PA time < 120 min is negatively associated with the risk of mortality. There are two conceivable reasons for this result. First, the mere act of standing up from a seated position in a chair moves the center of gravity to maintain posture, mobilizing the gastrocnemius (calf muscle) and other muscles, thereby resulting in greater muscle contraction than during sitting. 33 The attendant increases in energy expenditure and heart rate 34 may explain the negative association with the risk of mortality. 35 Second, sedentary behaviors lead to increased blood viscosity and inflammatory markers due to accelerated coagulation of red blood cells in the legs 36 and may be associated with elevated blood pressure 37 and reduced vascular endothelial function, both of which result from increased muscle sympathetic nerve activity. Interrupting sedentary behavior with low‐intensity walking has been demonstrated to improve these adverse effects. 38 Therefore, these studies support our finding revealing that targets for limiting ST depend greatly on individual PA. The finding that setting one's own PA goals leads to increased PA 39 suggests that setting targets for individual ST may contribute to reducing ST.
4.5. Strengths and limitations
The strength of the present study is that it assessed PA and ST using the IPAQ‐SF, 24 which has been validated in many countries, in a large cohort of community‐dwelling older adults. This method enabled us to demonstrate the interaction between PA and ST regarding the risk of mortality in a more accurate and versatile fashion. However, our study also involved several methodological limitations. First, there is the possibility of selection bias due to the different participant characteristics used for inclusion and exclusion. In addition, assessments of self‐reported PA might have included systematic reporting bias. 31 Particularly, PA might have been underestimated if the older adults actually engaged in many physical activities that could not be assessed by IPAQ‐SF. Although we calculated age‐calibrated PA based on PA intensities for older adults used in a previous validation study, self‐reported assessment may inflate estimates of habitual PA. Regardless of this possibility, we confirmed that our results were similar to those of studies that objectively assessed PA and ST. 7 , 8 Second, in our IPAQ‐SF‐based assessment of ST, we could not assess the types of activities performed during ST. The association between ST and the risk of mortality depends on how ST is spent 14 and thus requires the assessment of how the risk of mortality is associated with PA not assessed by the IPAQ‐SF and with the details of ST. It is necessary to re‐evaluate these results using other questionnaires (not bout time) or objectively evaluated PAs because the IPAQ‐SF considers only PA performed for bouts of approximately 10 min in a single session. In addition, it may be necessary to develop a physical activity questionnaire that is easy to respond to for older adults because incomplete IPAQ‐SF responses were one of the main reasons for participant exclusion in this study. Third, the observation period in our study was relatively short. This may be why the 95% CI in spline analysis was broad, as a short observation period means that there are few death events among individuals with high PA. In addition, since we did not obtain data related to the causes of death, we could not examine the association between PA and ST and causes of death. Fourth, although we adjusted for confounders, there may have been residual confounding in the associations between PA/ST and the risk of all‐cause mortality. In addition, although we conducted some sensitivity analyses to eliminate the possibility of reverse causality, direct causal relationships of the observed associations between PA and ST with mortality can be limited. Lastly, a causal interpretation of our results is risky because the HR estimated from our analysis may change over time, and the HR has a built‐in survival bias due to the inclusion of only those who survived during the follow‐up periods. 40 The HR shown in our results can be considered a kind of weighted average of each year‐specific HR during the follow‐up periods. If the hazard risk in the exposed group is higher in the first 5 years and lower afterward, the relationship between exposure factors and event occurrence may be overestimated. However, this should not be a major problem in interpreting the results obtained because we have confirmed the proportional hazards assumption. These limitations can potentially hamper study comparisons and derivation of sufficient doses of PA that would aid guideline development and could hinder the generalizability of our findings. Therefore, our results need to be confirmed with a well‐designed prospective study or randomized controlled trial 41 using a longer follow‐up period.
In conclusion, we demonstrated that LPA and HST are strongly positively associated with the risk of all‐cause mortality. The risk of mortality associated with LPA/HST depends on the level of PA, duration of ST, and their interaction with each other. These findings may be significant when individuals set their own PA goals (a daily ST − PA time <120 min), especially older adults who tend to be sedentary and are unable to engage in PA due to various reasons. In addition, further reduction of sedentary behaviors may help reduce the risk of mortality.
5. PERSPECTIVE
We demonstrated that older adults with both low physical activity and high sitting time have a higher risk of all‐cause mortality than older adults with only one of the above. The interaction between low physical activity and high sitting time may be involved in the risk of all‐cause mortality. Setting sitting time targets based on dose response analysis with consideration of each individual's physical activity regarding the risk of mortality may provide findings that can be used to establish recommended sitting times in PA guidelines in various countries. These results may be useful for ameliorating the adverse effects of reduced PA in older adults, who have been forced by the global COVID‐19 pandemic lockdowns to curtail various activities, reducing their PA. 42 , 43
AUTHOR CONTRIBUTIONS
DW, YH, and MM conceived the concept of the study. YY, HF, and MK managed the project. TY, YY, YW, and MK undertook data acquisition or entry. DW, YY, YH, and MM performed the literature review. DW and YY conducted data analysis. DW performed the graphics. DW and YH contributed to the interpretation of results. DW and YH wrote the first draft of the manuscript. All authors contributed to the writing and revision of the manuscript, and read and approved the final manuscript.
FUNDING INFORMATION
The Kyoto–Kameoka study was conducted with JSPS KAKENHI and was supported by a research grant provided to Misaka Kimura (24240091), Yosuke Yamada (15H05363), and Daiki Watanabe (21 K17699); a grant and administrative support by the Kyoto Prefecture Community‐based Integrated older adults Care Systems Promotion Organization since 2011; Kameoka City under the program of the Long‐term Care Insurance and Planning Division of the Health and Welfare Bureau for the older adults, Ministry of Health, Labour, and Welfare and the World Health Organization (WHO) Collaborating Centre on Community Safety Promotion.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Table S1–S7.
ACKNOWLEDGEMENTS
We appreciate all participants of this study and all individuals involved in the data collection. We acknowledge the several administrative workers of Kameoka city and Kyoto prefecture. We would like to thank the Kyoto–Kameoka Study Group who contributed their resources to the development of this study. We would like to thank Editage (www.editage.jp) for English‐language editing.
Watanabe D, Yamada Y, Yoshida T, et al. Association of the interaction between physical activity and sitting time with mortality in older Japanese adults. Scand J Med Sci Sports. 2022;32:1757‐1767. doi: 10.1111/sms.14234
DATA AVAILABILITY STATEMENT
All data sharing and collaboration requests should be directed to the corresponding author (d2watanabe@nibiohn.go.jp), TY (t-yoshida@nibiohn.go.jp), and YY (yamaday@nibiohn.go.jp).
REFERENCES
- 1. Collaborators GBDRF . Global burden of 87 risk factors in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strain T, Brage S, Sharp SJ, et al. Use of the prevented fraction for the population to determine deaths averted by existing prevalence of physical activity: a descriptive study. Lancet Glob Health. 2020;8:e920‐e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population‐based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077‐e1086. [DOI] [PubMed] [Google Scholar]
- 4. Bull FC, Al‐Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247‐257. [DOI] [PubMed] [Google Scholar]
- 6. Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all‐cause mortality in older women. JAMA Intern Med. 2019;179:1105‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klenk J, Dallmeier D, Denkinger MD, et al. Objectively measured walking duration and sedentary behaviour and four‐year mortality in older people. PLoS One. 2016;11:e0153779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all‐cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saint‐Maurice PF, Troiano RP, Bassett DR Jr, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all‐cause mortality: a meta‐analysis of 15 international cohorts. Lancet Public Health. 2022;7:e219‐e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D. Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol. 2019;73:2062‐2072. [DOI] [PubMed] [Google Scholar]
- 12. Matthews CE, Moore SC, Sampson J, et al. Mortality benefits for replacing sitting time with different physical activities. Med Sci Sports Exerc. 2015;47:1833‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koyama T, Ozaki E, Kuriyama N, et al. Effect of underlying cardiometabolic diseases on the association between sedentary time and all‐cause mortality in a large Japanese population: a cohort analysis based on the J‐MICC study. J Am Heart Assoc. 2021;10:e018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chau JY, Grunseit A, Midthjell K, et al. Sedentary behaviour and risk of mortality from all‐causes and cardiometabolic diseases in adults: evidence from the HUNT3 population cohort. Br J Sports Med. 2015;49:737‐742. [DOI] [PubMed] [Google Scholar]
- 15. Althoff T, Sosic R, Hicks JL, King AC, Delp SL, Leskovec J. Large‐scale physical activity data reveal worldwide activity inequality. Nature. 2017;547:336‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauman A, Ainsworth BE, Sallis JF, et al. The descriptive epidemiology of sitting. a 20‐country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011;41:228‐235. [DOI] [PubMed] [Google Scholar]
- 17. Matthews CE, George SM, Moore SC, et al. Amount of time spent in sedentary behaviors and cause‐specific mortality in US adults. Am J Clin Nutr. 2012;95:437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all‐cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494‐500. [DOI] [PubMed] [Google Scholar]
- 19. Ekelund U, Steene‐Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? a harmonised meta‐analysis of data from more than 1 million men and women. Lancet. 2016;388:1302‐1310. [DOI] [PubMed] [Google Scholar]
- 20. Yamada Y, Nanri H, Watanabe Y, et al. Prevalence of frailty assessed by Fried and Kihon checklist indexes in a prospective cohort study: design and demographics of the Kyoto‐Kameoka longitudinal study. J Am Med Dir Assoc. 2017;18:733.e7‐733.e15. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe D, Yoshida T, Nanri H, et al. Association between the prevalence of frailty and doubly labeled water‐calibrated energy intake among community‐dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76:876‐884. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Group KS . Objectively measured daily step counts and prevalence of frailty in 3,616 older adults. J Am Geriatr Soc. 2020;68:2310‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381‐1395. [DOI] [PubMed] [Google Scholar]
- 25. Tomioka K, Iwamoto J, Saeki K, Okamoto N. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) in elderly adults: the Fujiwara‐kyo Study. J Epidemiol. 2011;21:459‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buuren SV, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Software. 2011;45:1‐67. [Google Scholar]
- 27. Ministry of Health, Labour and Welfare . Japanese Official Physical Activity Guidelines for Health Promotion‐ActiveGuide 2013. https://wwwnibiohngojp/eiken/programs/pdf/active2013‐epdf. Accessed October 12, 2021
- 28. Tyler JV, Mirjam JK. A tutorial on interaction. Epidemiologic Methods. 2014;3:33‐72. [Google Scholar]
- 29. Vyas MV, Fang J, Kapral MK, Austin PC. Choice of time‐scale in time‐to‐event analysis: evaluating age‐dependent associations. Ann Epidemiol. 2021;62:69‐76. [DOI] [PubMed] [Google Scholar]
- 30. Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ. 2020;370:m2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taber DR, Stevens J, Murray DM, et al. The effect of a physical activity intervention on bias in self‐reported activity. Ann Epidemiol. 2009;19:316‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003‐2004. Am J Epidemiol. 2008;167:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655‐2667. [DOI] [PubMed] [Google Scholar]
- 34. Miles‐Chan JL, Dulloo AG. Posture allocation revisited: breaking the sedentary threshold of energy expenditure for obesity management. Front Physiol. 2017;8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171‐179. [DOI] [PubMed] [Google Scholar]
- 36. Hitosugi M, Niwa M, Takatsu A. Rheologic changes in venous blood during prolonged sitting. Thromb Res. 2000;100:409‐412. [DOI] [PubMed] [Google Scholar]
- 37. Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:1673‐1680. [DOI] [PubMed] [Google Scholar]
- 38. Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc. 2015;47:843‐849. [DOI] [PubMed] [Google Scholar]
- 39. Patel MS, Bachireddy C, Small DS, et al. Effect of goal‐setting approaches within a gamification intervention to increase physical activity among economically disadvantaged adults at elevated risk for major adverse cardiovascular events: the ENGAGE randomized clinical trial. JAMA Cardiol. 2021;6:1387‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21:13‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stensvold D, Viken H, Steinshamn SL, et al. Effect of exercise training for five years on all cause mortality in older adults‐the Generation 100 study: randomised controlled trial. BMJ. 2020;371:m3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID‐19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55:1099‐1105. [DOI] [PubMed] [Google Scholar]
- 43. Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS‐CoV‐2 infection, severe COVID‐19 illness and COVID‐19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021;56:901‐912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S7.
Data Availability Statement
All data sharing and collaboration requests should be directed to the corresponding author (d2watanabe@nibiohn.go.jp), TY (t-yoshida@nibiohn.go.jp), and YY (yamaday@nibiohn.go.jp).


