Abstract
Background and purpose
Ictal respiratory disturbances have increasingly been reported, in both generalized and focal seizures, especially involving the temporal lobe. Recognition of ictal breathing impairment has gained importance for the risk of sudden unexpected death in epilepsy (SUDEP). The aim of this study was to evaluate the incidence of ictal apnea (IA) and related hypoxemia during seizures.
Methods
We collected and analyzed electroclinical data from consecutive patients undergoing long‐term video‐electroencephalographic (video‐EEG) monitoring with cardiorespiratory polygraphy. Patients were recruited at the epilepsy monitoring unit of the Civil Hospital of Baggiovara, Modena Academic Hospital, from April 2020 to February 2022.
Results
A total of 552 seizures were recorded in 63 patients. IA was observed in 57 of 552 (10.3%) seizures in 16 of 63 (25.4%) patients. Thirteen (81.2%) patients had focal seizures, and 11 of 16 patients showing IA had a diagnosis of temporal lobe epilepsy; two had a diagnosis of frontal lobe epilepsy and three of epileptic encephalopathy. Apnea agnosia was reported in all seizure types. Hypoxemia was observed in 25 of 57 (43.9%) seizures with IA, and the severity of hypoxemia was related to apnea duration. Apnea duration was significantly associated with epilepsy of unknown etiology (magnetic resonance imaging negative) and with older age at epilepsy onset (p < 0.001).
Conclusions
Ictal respiratory changes are a frequent clinical phenomenon, more likely to occur in focal epilepsies, although detected even in patients with epileptic encephalopathy. Our findings emphasize the need for respiratory polygraphy during long‐term video‐EEG monitoring for diagnostic and prognostic purposes, as well as in relation to the potential link of ictal apnea with the SUDEP risk.
Keywords: central apnea, electroencephalography, hypoxia, sudden unexpected death in epilepsy, temporal lobe epilepsy
Ictal respiratory changes are a frequent clinical phenomenon, more likely to occur in focal epilepsies, although detected even in patients with epileptic encephalopathy. Our findings support the utilization of respiratory polygraphy during long‐term video‐electroencephalographic monitoring for diagnostic and prognostic purposes, and in relation to the potential link of ictal apnea with sudden unexpected death in epilepsy risk.
INTRODUCTION
Ictal respiratory changes encompass central or obstructive apnea, tachypnea, bradypnea, hypoventilation, and hypoxemia [1]. Ictal respiratory disruptions and related hypoxemia have been described for more than a century, in both generalized and focal seizures, with the first clinical description belonging to Hughlings Jackson [2]. Data concerning respiratory impairment associated with seizures have been initially reported in case reports and small case series, in both pediatric [3, 4, 5, 6] and adult patients [7, 8]. More recently, the study of peri‐ictal respiratory disturbances has gained more attention, mainly in relation to the study of factors associated with sudden unexplained death in epilepsy (SUDEP). Multicentric studies have reported that ictal hypoxemia and seizure‐related ictal central apnea occur in 33%–41% and in 36%–40% of focal seizures, respectively [9, 10, 11, 12].
Tonic–clonic seizures are known to be frequently accompanied by breathing impairment and oxygen desaturation [10], which many authors consider a relevant risk factor for SUDEP [7, 10, 12, 13]. The relationship between respiratory inhibition occurring in the terminal phase of tonic–clonic seizures and the risk of SUDEP has been elucidated [14]. However, there is now increasing awareness that ictal respiratory arrest leading to oxygen desaturation can be observed also during focal seizures, mostly of temporal lobe origin [9, 11, 15, 16]. Data derived from intracranial electroencephalographic (EEG) studies in epileptic patients [17, 18, 19] demonstrated that direct electrical stimulation of temporolimbic structures can induce an apneic response. The limbic network (more specifically, the amygdala) appears to play a key role in breathing inhibition throughout the ictal discharge. These findings support that temporal lobe seizures could manifest peri‐ictal respiratory changes more frequently respect with different seizures/epilepsy types [17, 18].
The principal aim of this study was to evaluate the incidence of ictal apnea (IA) and related hypoxemia in consecutive patients who underwent long‐term video‐EEG monitoring with extensive respiratory polygraphy in the epilepsy monitoring unit and to identify possible correlations with demographic and clinical variables of interest.
MATERIALS AND METHODS
Study population
We prospectively enrolled consecutive patients admitted to the epilepsy monitoring unit (EMU) at the Civil Hospital of Baggiovara, Modena Academic Hospital (Modena, Italy) from April 2020 to February 2022. Patients were admitted to the EMU both for diagnostic purpose and for presurgical evaluation. Therefore, we prospectively enrolled different epilepsy patient populations not restricted to focal drug‐resistant epilepsy. Every patient underwent video‐EEG long‐term monitoring (VLTM) with a 10–20 EEG system (Nihon Kohden Neurofax EEG‐1200, Mod JE‐120) integrated with a standard precordial single‐channel electrocardiogram (EKG), pulse oximetry for SpO2 measurement, and thoracoabdominal belt for respiratory inductance plethysmography. Four patients with IA were already described in a previous case series [16].
Inclusion criteria were (i) age older than 14 years at hospitalization and (ii) at least one seizure (focal or generalized) recorded during VLTM with cardiorespiratory polygraphy (EKG, pulse oximetry, and thoracoabdominal respiratory inductance plethysmography). Patients were excluded if no seizure was recorded during EMU admission or if the patient experienced only psychogenic nonepileptic seizures.
For each patient, we collected the following electroclinical and demographic data: age at the time of admission, gender, age at epilepsy onset, disease duration, hemisphere of the epileptic focus, family history of epilepsy, febrile seizures in past medical history, and drug responsiveness. Additionally, we considered the number of seizures recorded, the state of vigilance at the time of seizure onset, seizure frequency, brain magnetic resonance imaging (MRI) findings, and etiology. Seizures' frequency was classified into the following: (i) less than one seizure per year, (ii) more than one seizure per year, (iii) one or more seizure per month, (iv) one or more seizure per week, and (v) daily seizures. According to the last International League Against Epilepsy (ILAE) classification proposal [20], the etiology was classified as structural, genetic, metabolic, autoimmune, or unknown. The drug‐response status of the patient at the time of EMU admission was defined accordingly to the ILAE definition of drug resistance [21].
According to published criteria [11, 12, 22], apnea was considered as a respiratory arrest of ≥5 s visible on the pneumographic channel, preceded and followed by stable breathing for at least 5 s, and confirmed by visual inspection of the recorded video. Postictal apnea was defined as a respiratory arrest starting within 5 s after ictal discharge termination.
Thus, a more thorough data collection regarding patients with seizure‐related apnea was performed, including apnea duration, hypoxemia with further specifications (duration, nadir, and degree of oxygen desaturation), time from apnea to first ictal EEG modification, apnea awareness, and heart rate changes.
Hypoxemia was defined as a drop of SpO2 value to <95% and classified as mild (90%–94%), moderate (75%–89%), or severe (<75%) [11, 12]. For patients who manifested IA, mean oxygen saturation preceding the seizure onset by 5 min was calculated. Tachycardia and bradycardia were respectively defined as heart rate > 100 beats per minute and < 60 beats per minute, or a >20% deviation from baseline [11].
Statistical analyses
First, we investigated whether there was an association between IA occurrence and specific clinical and/or demographic variables (i.e., age at EMU admission, gender, age at epilepsy onset, epilepsy duration, drug resistance, etiology, electroclinical localization, state of vigilance at seizure onset, and seizure frequency and type).
Afterward, we focused on the subgroup of patients with IA. For each patient, all recorded seizures, accompanied or not by apnea, were considered. We explored whether there was any statistically significant relation between the apnea/hypoxemia occurrence and different clinical variables.
After testing for normality of distribution of continuous variables with Shapiro–Wilk test, independent sample t‐test was performed for normally distributed variables, whereas Mann–Whitney and Kruskal–Wallis tests were used for not normally distributed variables. To assess correlations between nonnormally distributed variables, Spearman rho test was calculated. Chi‐squared test was used for analyzing the frequency in dichotomous variables.
The statistical analysis was performed with Statistical Package for Social Science (IBM, v27). Summary statistics are reported as mean ± SD (range). The statistical significance was set at p < 0.05.
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethical committee of Area Vasta Emilia Nord (NET‐2013‐02355313 N.155/14). Patients gave written informed consent for the use of their clinical records in this study. The study was conducted in accordance with the World Medical Association Declaration of Helsinki.
RESULTS
In the study period, 232 patients were admitted to the EMU. According to inclusion criteria, we recruited 63 consecutive patients for a total of 552 seizures. A total of 358 of 552 (65%) recorded seizures had a focal onset, whereas 194 (35%) had a generalized onset. Only one generalized tonic–clonic seizure and one focal to bilateral tonic–clonic seizure were recorded.
IA was observed in 57 of 552 seizures (10.3%) in 16 of 63 patients (25.4%).
Clinical and demographic characteristic of the patients with and without IA are summarized in Table 1. Overall, 52 patients (82.54%) presented focal seizures, whereas 11 (17.46%) had generalized seizures. Among the patients who had focal epilepsy, 32 (52.38%) had a diagnosis of temporal lobe epilepsy (TLE), 18 (28.57%) of frontal lobe epilepsy (FLE), one of parietal lobe epilepsy, and one of occipital lobe epilepsy. There were no significant differences between epilepsy syndrome, seizure frequency, and/or etiology between patients with and without IA.
TABLE 1.
Patients demographic and clinical features
| Characteristic | All patients | IA | No‐IA | K–W/χ2 | p |
|---|---|---|---|---|---|
| n | 63 | 16 | 47 | ||
| Age, years | 35.8 ± 15.5 (14–67) | 34.1 ± 12.8 (14–55) | 36.4 ± 16.3 (14–67) | 0.039K–W | 0.843 |
| Gender, F/M | 25/38 | 6/10 | 20/27 | 0.637χ | 0.425 |
| Age at onset, years | 21.9 ± 14.8 (2 months–62 years) | 23.4 ± 14.2 (6 months–50 years) | 21.4 ± 15.2 (2 months–62 years) | 0.440K–W | 0.507 |
| Epilepsy duration, years | 13.9 ± 14.1 (2 months–64 years) | 10.6 ± 12.4 (2 months–43 years) | 15 ± 14.6 (2 months–62 years) | 1.297K–W | 0.255 |
| Side of epileptic focus, left/right/generalized | 23/29/11 | 7/6/3 | 16/23/8 | 0.668χ | 0.716 |
| Family history of epilepsy, yes/no | 24/39 | 6/10 | 18/29 | 0.003χ | 0.955 |
| Febrile seizures, yes/no | 8/55 | 2/14 | 6/41 | 0.001χ | 0.978 |
| ASM response, yes/no | 22/41 | 5/11 | 17/30 | 0.127χ | 0.721 |
| Seizure frequency, n | 63 | 16 | 47 | 3.713χ | 0.446 |
| <1 per year | 3 | 0 | 3 | ||
| >1 per year | 13 | 3 | 10 | ||
| ≥1 per month | 18 | 7 | 11 | ||
| ≥1 per week | 15 | 4 | 11 | ||
| Daily | 14 | 2 | 12 | ||
| Focal seizures, yes/no | 52/11 | 13/3 | 39/8 | 0.025χ | 0.875 |
| Epilepsy syndrome, n | 63 | 16 | 47 | 3.884χ | 0.143 |
| TLE | 32 | 11 | 21 | ||
| Extra‐TLE | 20 | 2 | 18 | ||
| Others a | 11 | 3 | 8 | ||
| Etiology, n | 63 | 16 | 47 | 2.890χ | 0.576 |
| Structural b | 36 | 11 | 25 | ||
| Unknown | 20 | 5 | 15 | ||
| Genetic | 3 | 0 | 3 | ||
| Metabolic | 2 | 0 | 2 | ||
| Immune | 2 | 0 | 2 |
Note: Data are presented as mean, SD, and range.
Abbreviations: ASM, antiseizure medication; F, female; FCD, focal cortical dysplasia; IA, patients with ictal apnea; K–W, Kruskal–Wallis test; M, male; No‐IA, patients without ictal apnea; TLE, temporal lobe epilepsy.
Seven with epileptic encephalopathy, one with idiopathic generalized epilepsy, one with Lafora disease, two with generalized epilepsy of unknown cause.
Four with FCD (one with left temporal FCD, one with left frontoparietal FCD, and two with right temporal FCDs), two with long‐term epilepsy‐associated tumors (both right temporal), one with hippocampal sclerosis, three with gliosis (one with left frontoinsular gliosis, one with bilateral frontoparietal gliosis, and one with left temporal gliosis), and one with bilateral posterior calcifications.
Also, no significant differences were found in terms of age, gender distribution, age at epilepsy onset, illness duration, side of epileptic focus, family history of epilepsy, febrile seizures in childhood, or antiseizure medication response between patients with and without IA.
IA features
Considering the patients with IA, we recorded 57 (35.2%) seizures with IA over 162 seizures. Therefore, IA was not a constant phenomenon among these patients.
In Table 2 are reported details of patients and seizures with IA.
TABLE 2.
Features of seizures with ictal apnea
| IA patient | IA occurrence in recorded seizures | Mean apnea duration, s | Mean hypoxemia duration, s | Hypoxemia nadir, % | Epilepsy type | IA‐related seizure type | Ictal heart rate changes |
|---|---|---|---|---|---|---|---|
| Subj04 | 1/1 | 16 | 23 | 89% | TLE | FIAS | Tachycardia |
| Subj07 | 8/37 | 5.25 (±0.71) | 11 | 90% | EE | GO (tonic seizure) | Tachycardia |
| Subj24 | 3/3 | 71.7 (±10.7) | 74.3 (±11.4) | 74% | TLE | FIAS | Tachycardia |
| Subj26 | 2/28 | 6.5 (±2.8) | – | – | FLE | FAS | Tachycardia |
| Subj27 | 6/8 | 25.5 (±23.4) | 22.5 (±20.3) | 89% | TLE | FIAS | Tachycardia |
| Subj29 | 4/30 | 10.7 (±3.7) | – | – | EE | GO (tonic seizure) | Bradycardia |
| Subj31 | 1/1 | 13 | 24 | 87% | TLE | FIAS | Tachycardia |
| Subj38 | 4/4 | 25.7 (±10.7) | 9.7 (±9.0) | 92% | TLE | FAS | Tachycardia |
| Subj39 | 2/2 | 31 (±12.79) | 44 | 76% | TLE | FIAS | Bradycardia |
| Subj44 | 3/4 | 46.7 (±47.2) | Not available | 85% | TLE | FIAS, FAS | Tachycardia |
| Subj47 | 1/1 | 11 | – | – | TLE | FIAS | Tachycardia |
| Subj48 | 5/13 | 5 (±0) | – | – | EE | GO (tonic seizure) | Tachycardia |
| Subj52 | 5/14 | 20.4 (±20.4) | 33 (±11.1) | 80% | FLE | FIAS | Tachycardia |
| Subj53 | 1/1 | 30 | – | – | TLE | FIAS | Bradycardia |
| Subj54 | 10/14 | 37.7 (±44.3) | 46 (±18.1) | 87% | TLE | FIAS | Tachycardia |
| Subj56 | 1/1 | 20 | 44 | 83% | TLE | FIAS | Tachycardia |
Note: Data are presented in mean, with SD presented in parentheses.
Abbreviations: EE, epileptic encephalopathy; FAS, focal aware seizure; FIAS, focal impaired awareness seizure; FLE, frontal lobe epilepsy; GO, generalized onset; IA, ictal apnea; TLE, temporal lobe epilepsy.
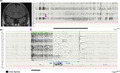
Apnea was mainly observed in focal seizures (40/57; p = 0.002), specifically in patients with seizures involving the temporal lobe (11/16; Figure 1a), and was more frequent in focal seizures with impaired awareness (p < 0.001). Only two patients with frontal epilepsy had seizures accompanied by IA. Notably, apnea agnosia was reported in all IA‐related seizures.
FIGURE 1.
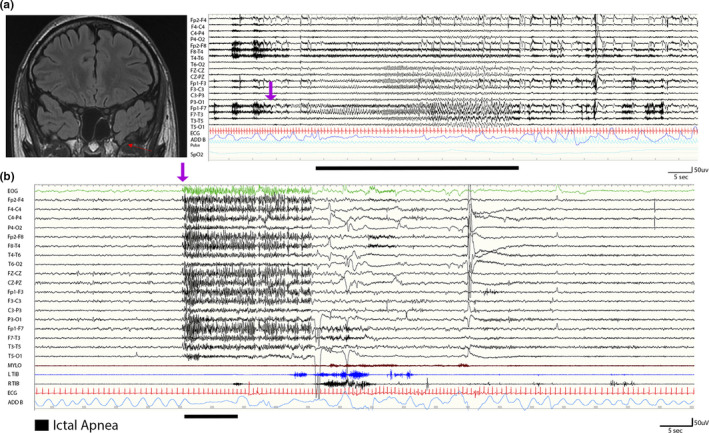
(a) A 20‐year‐old male with ictal apnea seizures. On the left, a coronal T2 fluid‐attenuated inversion recovery magnetic resonance imaging scan shows the presence of a left temporopolar encephalocele (red arrow). On the right, the electroencephalogram (EEG; 120 s) shows a left temporal seizure arising from the frontotemporal channels (Fp1‐F7, F7‐T3), rapidly involving the ipsilateral and contralateral frontal regions including the anterior vertex. The ictal discharge (indicated by the purple arrow) is characterized by low‐voltage fast rhythms evolving in sharply contoured theta and then delta rhythmic activity with diffuse abrupt termination. Ictal apnea starts during the ictal phase in association with bradycardia. Ictal apnea duration is indicated by the black bar. Red channel: electrocardiogram; blue channel: thoracoabdominal respirogram. (b) A 14‐year‐old male patient with Lennox–Gastaut epileptic encephalopathy. During non‐rapid eye movement sleep, the EEG shows abrupt diffuse fast activity (as indicated by the purple arrow) predominant over the frontocentral and vertex regions for 20 s, followed by slow activity. The polygraphy shows ictal tachycardia (red channel) and flattening of the thoracoabdominal respirogram (light blue channel; indicated by the black bar). No significant muscle activity was recorded (mylohyoid and left and right tibialis anterior muscles) during the epileptic discharge [Colour figure can be viewed at wileyonlinelibrary.com
Considering nonfocal seizures, IA was observed only during generalized onset seizures characterized by low‐voltage diffuse fast activities on EEG ictal discharge and in patients with epileptic encephalopathies (Figure 1b).
In relation to the vigilance state, 25 (43.9%) seizures with IA were recorded during wakefulness and 32 (56.1%) during non‐rapid eye movement (NREM) sleep. No seizures arose from rapid eye movement sleep. Thus, apnea was found in seizures occurring in both wakefulness and sleep, without any difference in apnea occurrence in relation to the state of vigilance at seizure onset. Considering the side of seizure onset, the lateralization of the epileptic focus was not found to be associated with apnea occurrence (p = 0.44).
Apnea duration showed a great variability, from 5 to 150 s (mean = 8.7 ± 20.3 s). Apnea duration was significantly longer (i) in seizures with focal onset compared to generalized onset (p < 0.001): 32.5 ± 30.27 versus 6.47 ± 2.96 s, respectively (Figure 2a); and (ii) in seizures recorded in epilepsy of unknown cause (p < 0.05; Figure 2b). Finally, apnea duration correlated with the age at epilepsy onset (R = 0.591, p < 0.001; Figure 2c).
FIGURE 2.
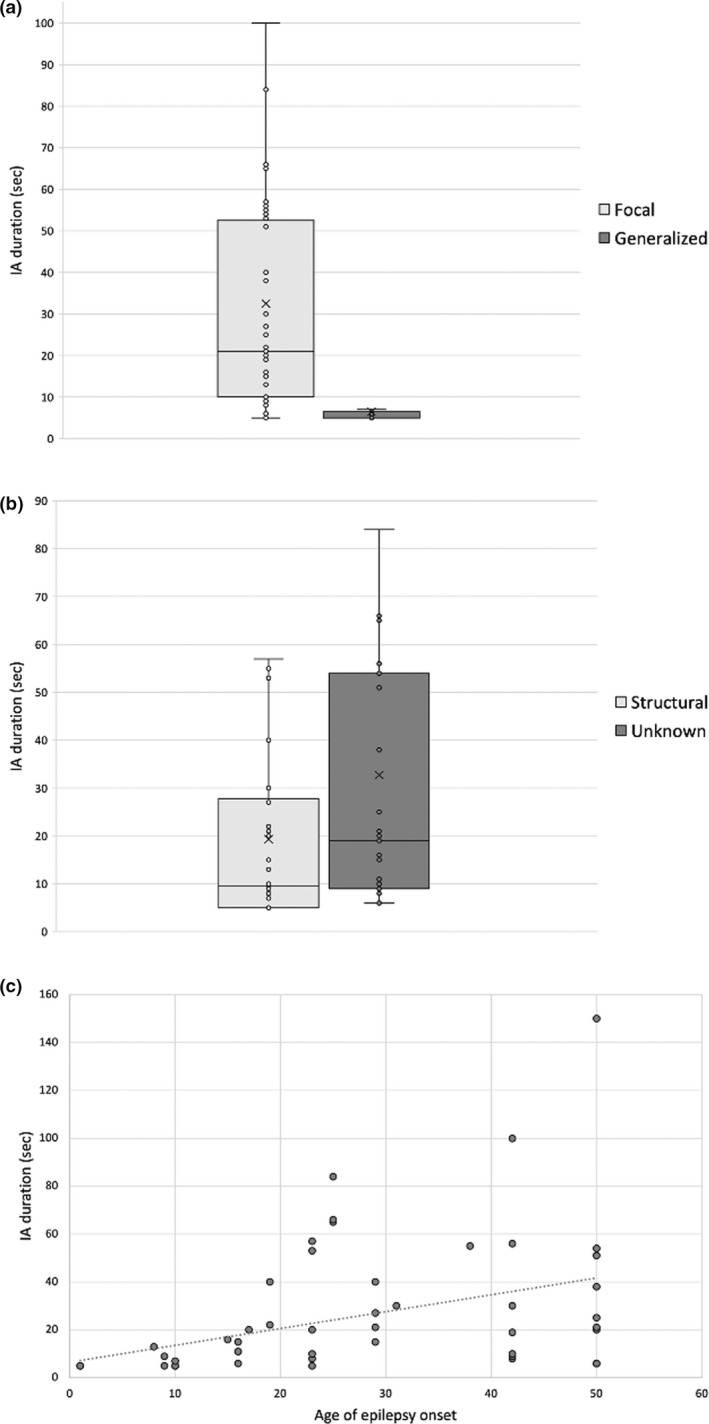
Longer ictal apnea (IA) duration is significantly associated with clinical variables. Box‐and‐whisker plots show the association between the IA duration and (a) seizures with focal onset (p < 0.001), (b) epilepsy with unknown etiology (p < 0.05), and (c) older age of epilepsy onset (R = 0.591, p < 0.001). The central horizontal line of the boxes marks the median of the sample, and the “x” in the middle of each box represents the mean of the sample. The upper and lower edges of the box (the hinges) mark the 25th and 75th percentiles (the central 50% of values fall within the box). Finally, the open circles represent individual patients. Longer IA duration was significantly associated with seizures with focal onset (p < 0.001; a), epilepsy of unknown etiology (p < 0.05; b), and older age of epilepsy onset (R = 0.591, p < 0.001; c)
Considering the apnea onset with respect to the scalp EEG ictal onset, seven focal epilepsy patients showed apnea as the first ictal manifestation before any EEG modification (43.8% of all IA patients). In these events, the mean time between apnea onset and the subsequent ictal EEG onset was 9 ± 7.9 s (range = 1–27). Nine of 16 patients had apnea persisting after the termination of the ictal discharge. In patients with epileptic encephalopathy, IA occurrence was always concomitant with the generalized ictal discharge. In this series, no patient had apnea beginning after the termination of the ictal discharge.
As concerns cardiac frequency changes related to IA, bradycardia was observed in only three patients (18.8%), whereas tachycardia was present in the majority (13/16, 81.2%; χ2[1] = 6.250, p = 0.012).
Ictal hypoxemia
Hypoxemia occurred in 25 of 57 (43.9%) of the recorded seizures with apnea, being mild in eight, moderate in 16, and severe in one. Hypoxemia nadir ranged from 92% to 74%. Ictal hypoxemia length showed great variability, lasting 4–87 s (5.32 ± 15.5). When oxygen desaturation occurred, it was mainly of moderate degree (75%–89%). The severity of hypoxemia was significantly associated with apnea duration (R = −0.620, p < 0.001; Figure 3). In addition, patients with unknown etiology showed ictal hypoxemia more frequently compared with patients with structural etiology (p = 0.022). Also, the nadir of desaturation was associated with epilepsy etiology (p = 0.010); the mean nadir value in seizures recorded in epilepsy of unknown cause was 91.04 ± 8.44%, whereas it was 96.26 ± 6.20% in epilepsy of structural etiology.
FIGURE 3.
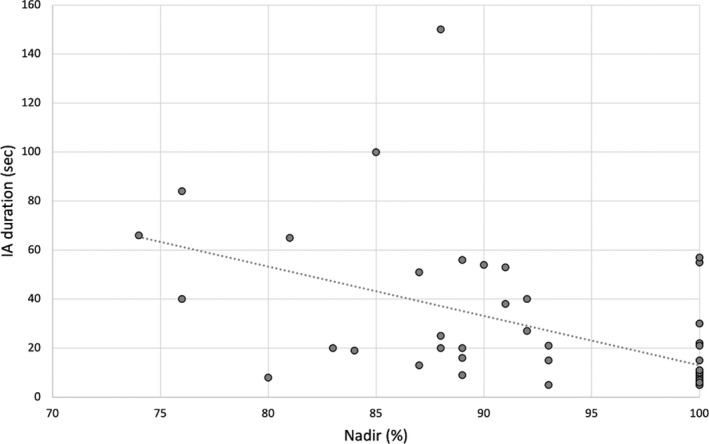
The length of ictal apnea (IA) was inversely associated with hypoxemia nadir (R = −0.620, p < 0.001)
Regarding the epilepsy duration and seizure frequency previous to EMU admission, we have not observed any relationship of these two variables with apnea duration, degree of oxygen desaturation, or oxygen desaturation nadir.
DISCUSSION
In this study, we explored the presence of IA in a cohort of patients investigated in the EMU for diagnostic purposes. Remarkably, while confirming this clinical phenomenon to be frequent in focal epilepsy, we also reported the presence of IA in patients with generalized tonic seizures. The correlation with clinical variables showed that patients with epilepsy of unknown etiology more frequently have ictal hypoxemia, with a more severe degree of oxygen desaturation than other etiological categories. Overall, our findings expand the previous knowledge and confirm the importance of respiratory polygraphic recordings during VLTM.
Occurrence of IA
In our cohort, one fourth (25.4%) of patients had seizures accompanied by IA. Therefore, IA appears to be a considerably frequent clinical phenomenon.
The IA incidence reported here is slightly lower than previously described [9, 11, 12, 15, 23]. This discrepancy could be the result of differences in the clinical characteristics of the recruited patients. Herein, in contrast with previous studies, we included patients with different epilepsy syndromes, not limiting the study of IA to focal drug‐resistant epilepsy cases. Another plausible explanation could be that we recorded only one generalized tonic–clonic seizure and one focal to bilateral tonic–clonic seizure among our patients, which is much lower compared to previous studies [11, 12].
IA was predominant among patients with focal epilepsy (13/16, 81.3%), and in particular with TLE (11/16), in accordance with the data already published [9, 11]. Of note, patients who presented with apnea‐related seizures also had seizures not associated with apnea, suggesting that the epileptic network might be susceptible to a certain degree of interseizure variability. Among extra‐TLE seizures, one case of interest has been represented by one patient who had IA only when the ictal discharge involved the anterior and midtemporal EEG channels with clinical appearance of oroalimentary automatisms. Hence, we suggest that IA could be a sign of temporal lobe involvement, even throughout the development of the epileptic discharge of extratemporal origin.
Pathophysiological mechanisms
When considering IA in focal seizures without convulsive features, it is largely accepted that the underlying pathophysiology is of central origin. There is strong evidence supporting that the regions of the limbic/paralimbic network (i.e., amygdala, hippocampus, anterior parahippocampal gyrus, and anteromesial fusiform gyrus) might be involved in the genesis of ictal central apnea [17, 18, 19]. Recently, some authors [17, 18, 19] investigated the respiratory central network by means of neurophysiological invasive studies. Specifically, they demonstrated that direct electrical stimulation of the amygdala and its specific subnuclei (central and basal) is sufficient to induce a transient apneic phenomenon [18]. The amygdalar subnuclei are highly interconnected with respiratory centers located in the brainstem, namely in the medulla and pons. The involvement of the limbic network could also corroborate that temporal lobe seizures are significantly associated with IA.
By contrast, four patients affected by either epileptic encephalopathy with focal and generalized seizures (3/4 patients) or FLE presented with IA in relation to generalized EEG discharges characterized by fast spikes and polyspikes followed by diffuse brief EEG slowing. In this setting, we were not able to assess whether the apnea was central or obstructive due to the availability of only one thoracoabdominal belt and the absence of nasal/oral thermistor for airflow measurement. However, it could be suggested that the apnea was caused by a tonic contraction of the upper airway, which is known to cause obstructive apnea [24]. It has been proposed that ictal laryngospasm could be driven by tonic seizure discharge spread to cortical areas governing laryngeal motor control (perisylvian motor cortex and anterior insula) [25].
Thus, the underlying physiopathological mechanisms of IA in focal seizures compared to generalized tonic seizures are probably different. To support this assumption, the mean apnea duration appeared to be significantly shorter in patients with generalized discharges, and IA never preceded the EEG ictal onset but instead was strictly limited to the ictal discharge. Apnea occurrence in generalized tonic seizures has not been described in recent literature [11, 12]; this may be because adult patients with generalized epilepsy or with epileptic encephalopathies are rarely evaluated in the setting of an EMU. On the other hand, as regards generalized tonic–clonic seizures, apnea has been studied in relation to the postictal phase and considered a potential risk factor for SUDEP [12, 26].
In focal seizures, IA has the appearance of a complex dynamic neurophysiological phenomenon. In two of our patients with TLE, IA was seen in different phases of the ictal event. Also, respiratory modifications, such as irregular breathing or transient apnea, were observed long after the end of ictal discharge. Respiratory disturbances in the postictal period have been extensively reported in the MORTEMUS study [14], which revealed severe cardiorespiratory impairment emerging up to 3 min after the end of ictal discharge and leading possibly to SUDEP.
Correlation with electroclinical variables
No demographic or clinical variable (i.e., age at VLTM, gender, age at onset, epilepsy duration, family history of epilepsy, drug resistance, seizure frequency) was found to be significantly associated with IA occurrence. In the same way, etiology, lateralization of the epileptic focus, and state of vigilance at seizure onset, were not associated with IA. As a result, these observations do not allow determination of which patients could be more prone to present with ictal respiratory impairment.
Additionally, all patients were agnostic of the occurrence of IA, nor did they have dyspnea, even when apnea lasted >60 s and even when the degree of oxygen desaturation was severe. The agnosia of IA was already reported in other studies [11]. Hence, this finding explains why ictal respiratory changes are generally underrated. This may also be due to the lack of utilization of respiratory polygraphy in the diagnostic assessment of patients with epilepsy. The latest guideline regarding the minimum standards of VLTM consider the use of respiratory polygraphy to be optional [27].
In our study, hypoxemia incidence (43.9%) among IA‐related seizures was similar to previous studies [9, 11, 12]. In particular, we found a statistically significant correlation between IA duration and the severity of hypoxemia. Moreover, desaturations of moderate degree (<90%) were significantly correlated with temporal lobe seizures, as already reported [9].
As a remarkable result, epilepsy of unknown etiology was found to be associated with longer IA duration and more severe hypoxemia compared to structural etiology. This finding is consistent with what Moseley et al. [28] reported in a previous study. They observed that patients with normal brain MRI had higher rates of ictal autonomic disturbances, including ictal tachycardia and hypoxemia [28]. We cannot provide a clear and precise physiopathological explanation; nevertheless, we can speculate that MRI‐negative patients have subtle amygdala/temporomesial alterations that promote the ictal involvement of brainstem respiratory centers leading to IA and hypoxia. Interestingly, evidence from MRI studies point toward increased amygdala volume in some MRI‐negative TLE patients [29, 30]. This hypothesis should be formally tested in future studies.
As concerns ictal heart rate changes in association with IA, concordantly with previous literature [9, 11, 31], ictal tachycardia was far more common than bradycardia.
Peri‐ictal apnea has also been considered as a potential risk factor for SUDEP [7, 12, 14, 32]. It is widely debated whether this ictal manifestation could constitute an effective danger for people with epilepsy. IA appears to be a self‐limited phenomenon secondary to the spread of the ictal discharge to the limbic network, and moreover, severe oxygen desaturation is rarely observed [11], as it is reported in our study. On the other hand, agnosia of IA occurrence may represent a potential danger, especially during sleep. Moreover, we did not find any difference in IA occurrence in relation to vigilance level, seizure frequency, or drug response, which are recognized as risk factors for SUDEP. However, the presence of IA in patients with epileptic encephalopathy, an electroclinical entity that already comprises in its definition features that pose a risk of death to patients, could represent an adjunctive element of concern. Nevertheless, no case of SUDEP has been reported in our population so far.
CONCLUSIONS
Our findings suggest that IA is a frequent ictal manifestation representative of a more extensive autonomic impairment in relation to focal seizures. Ictal respiratory changes and hypoxemia are often overlooked, because they are not reported by patients and are not revealed by an adequate respiratory polygraphic monitoring. We also recommend looking for ictal autonomic disruption in patients with epileptic encephalopathies to quantify the risk for SUDEP, already known to be higher in this population. In focal epilepsy, IA may represent a valuable ictal localizing sign toward the limbic network. Further investigations are needed to confirm these preliminary data and to determine whether IA recurrence might expose patients to higher risk of SUDEP.
AUTHOR CONTRIBUTIONS
Elisa Micalizzi: Conceptualization (equal), writing–original draft (lead), data curation (equal), investigation (equal), methodology (equal). Anna Elisabetta Vaudano: Conceptualization (equal), administration (equal), supervision (equal), writing–review & editing (equal). Alice Ballerini: Data curation (equal), formal analysis (lead), methodology (equal), writing–original draft (supporting). Francesca Talami: Data curation (supporting), investigation (supporting). Giada Giovannini: Data curation (supporting). Giulia Turchi: Data curation (supporting). Maria Cristina Cioclu: Data curation (supporting). Leandra Giunta: Investigation (supporting), data curation (supporting). Stefano Meletti: Conceptualization (equal), project administration (equal), supervision (equal), writing–review & editing (equal).
CONFLICT OF INTEREST
S.M. has received research grant support from the Ministry of Health and the nonprofit organization Fondazione Cassa di Risparmio di Modena; he has received personal compensation as a scientific advisory board member for UCB and Eisai. A.E.V. has received personal compensation as a scientific advisory board member for Angelini Pharma. The other authors report no conflict of interest.
ACKNOWLEDGMENTS
This study was funded by the Ministry of Health under the Ricerca Finalizzata (project code NET‐2013‐02355313) and by MIUR under the Dipartimenti di Eccellenza 2018‐2022 grant. The authors thank all the neurophysiology technicians working in this epilepsy monitoring unit for providing valuable technical support. Open Access Funding provided by Universita degli Studi di Modena e Reggio Emilia within the CRUI‐CARE Agreement.
Micalizzi E, Vaudano AE, Ballerini A, et al. Ictal apnea: A prospective monocentric study in patients with epilepsy. Eur J Neurol. 2022;29:3701‐3710. doi: 10.1111/ene.15547
Elisa Micalizzi and Anna Elisabetta Vaudano are joint first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request from any qualified investigator, maintaining anonymization of the patients.
REFERENCES
- 1. Blum AS. Respiratory physiology of seizures clinical findings of ictal respiratory changes. J Clin Neurophysiol. 2009;26:309‐315. [DOI] [PubMed] [Google Scholar]
- 2. Jackson JH. On asphyxia in slight epileptic paroxysms: on the symptomatology of slight epileptic fits supposed to depend on discharge‐lesions of the uncinate gyrus. Lancet. 1899;1:79‐80. [Google Scholar]
- 3. Coulter DL. Partial seizures with apnea and bradycardia. Arch Neurol. 1984;41:173‐174. http://archneur.jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 4. Singh B, Al Shahwan SA, Al Deeb SM. Partial seizures presenting as life‐ threatening apnea. Epilepsia. 1993;34(5):901‐903. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe K, Kuroyanagi M, Hara K, Miyazaki S. Neonatal seizures and subsequent epilepsy. Brain Dev. 1982;4(5):341‐346. doi: 10.1016/S0387-7604(82)80017-X [DOI] [PubMed] [Google Scholar]
- 6. Miyagawa T, Sotero M, Avellino AM, et al. Apnea caused by mesial temporal lobe mass lesions in infants: report of 3 cases. J Child Neurol. 2007;22(9):1079‐1083. doi: 10.1177/0883073807306245 [DOI] [PubMed] [Google Scholar]
- 7. Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry. 1996;60:297‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee HW, Hong SB, Tae WS, Seo DW, Kim SE. Partial seizures manifesting as apnea only in an adult. Epilepsia. 1999;40(12):1828‐I83I. [DOI] [PubMed] [Google Scholar]
- 9. Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization‐related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(12):3239‐3245. doi: 10.1093/brain/awn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruno E, Maira G, Biondi A, Richardson MP. Ictal hypoxemia: a systematic review and meta‐analysis. Seizure. 2018;63:7‐13. doi: 10.1016/j.seizure.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 11. Lacuey N, Zonjy B, Hampson JP, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia. 2018;59(3):573‐582. doi: 10.1111/epi.14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilella L, Lacuey N, Hampson JP, et al. Incidence, recurrence, and risk factors for peri‐ictal central apnea and sudden unexpected death in epilepsy. Front Neurol. 2019;10:166. doi: 10.3389/fneur.2019.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia. 2000;41:536‐541. [DOI] [PubMed] [Google Scholar]
- 14. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966‐977. doi: 10.1016/S1474-4422(13)70214-X [DOI] [PubMed] [Google Scholar]
- 15. Tio E, Culler GW, Bachman EM, Schuele S. Ictal central apneas in temporal lobe epilepsies. Epilepsy Behav. 2020;112:107434. doi: 10.1016/j.yebeh.2020.107434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Micalizzi E, Vaudano AE, Giovannini G, Turchi G, Giunta L, Meletti S. Case report: ictal central apnea as first and overlooked symptom in temporal lobe seizures. Front Neurol. 2021;4(12):753860. doi: 10.3389/fneur.2021.753860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci. 2015;35(28):10281‐10289. doi: 10.1523/JNEUROSCI.0888-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacuey N, Hampson JP, Harper RM, Miller JP, Lhatoo S. Limbic and paralimbic structures driving ictal central apnea. Neurology. 2019;92:e655‐e669. doi: 10.1212/WNL.0000000000006920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nobis WP, González Otárula KA, Templer JW, et al. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. J Neurosurg. 2020;132(5):1313‐1323. doi: 10.3171/2019.1.JNS183157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522‐530. doi: 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 21. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069‐1077. doi: 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 22. Kanth K, Park K, Seyal M. Severity of peri‐ictal respiratory dysfunction with epilepsy duration and patient age at epilepsy onset. Front Neurol. 2020;11:618841. doi: 10.3389/fneur.2020.618841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacuey N, Hupp NJ, Hampson H, Lhatoo S. Ictal central apnea (ICA) may be a useful semiological sign in invasive epilepsy surgery evaluations. Epilepsy Res. 2019;156:106164. doi: 10.1016/j.eplepsyres.2019.106164 [DOI] [PubMed] [Google Scholar]
- 24. Rubboli G, Zamagni M, Michelucci R, et al. Epileptic intermittent snoring. Neurology. 2001;56:1601‐1602. [DOI] [PubMed] [Google Scholar]
- 25. Subramani K, Paul A. Laryngospasm during subarachnoid block. Br J Anaesth. 2005;94(5):668‐670. doi: 10.1093/bja/aei099 [DOI] [PubMed] [Google Scholar]
- 26. Vilella L, Lacuey N, Jp H, et al. Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology. 2019;92:e171‐e182. doi: 10.1212/WNL.0000000000006785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tatum WO, Mani J, Jin K, et al. Minimum standards for inpatient long‐term video‐electroencephalographic monitoring: a clinical practice guideline of the international league against epilepsy and International Federation of Clinical Neurophysiology. Epilepsia. 2022;63:290‐315. doi: 10.1111/epi.16977 [DOI] [PubMed] [Google Scholar]
- 28. Moseley BD, Wirrel EC, Nickels K, Johnson JN, Ackerman MJ, Britton J. Electrocardiographic and oximetric changes during partial complex and generalized seizures. Epilepsy Res. 2011;95:237‐245. [DOI] [PubMed] [Google Scholar]
- 29. Lv RJ, Sun ZR, Cui T, Guan HZ, Ren HT, Shao XQ. Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy. BMC Neurol. 2014;14:194. doi: 10.1186/s12883-014-0194-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chakravarty K, Ray S, Kharbanda PS, Lal V, Baishya J. Temporal lobe epilepsy with amygdala enlargement: a systematic review. Acta Neurol Scand. 2021;144(3):236‐250. doi: 10.1111/ane.13455 PMID: 33987835. [DOI] [PubMed] [Google Scholar]
- 31. Rocamora R, Kurthen M, Kickfett L, von Oertzen J, Elger EC. Cardiac asystole in epilepsy: clinical and neurophysiologic features. Epilepsia. 2003;44(2):179‐185. [DOI] [PubMed] [Google Scholar]
- 32. Manolis TA, Manolis AA, Melitac H, Manoli AS. Sudden unexpected death in epilepsy: the neuro‐cardio‐respiratory connection. Seizure. 2019;64:65‐73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from any qualified investigator, maintaining anonymization of the patients.


