Abstract
Background
Accurate epidemiological and outcomes data regarding cutaneous squamous cell carcinoma (cSCC) extending to the temporal bone is lacking.
Methods
Retrospective analysis of 167 Australian patients with primary and peri‐temporal bone cSCC.
Results
cSCC extending from secondary subsites (93.4%) was 14 times more frequent than primary temporal bone SCC (6.6%). For patients who underwent curative surgery ± post‐operative radiotherapy (n = 146, 87.4%), 5‐year disease‐free survival, locoregional recurrence‐free survival, disease‐specific survival, and overall survival was 53.0%, 59.4%, 67.9%, and 44.7%, respectively. External ear and pre‐auricular tumors, salvage surgery, tumor size (≥40 mm medial‐lateral), nodal disease, and involved margins were negative predictors of survival in multivariable analysis.
Conclusion
In regions of high sun exposure, cSCCs extending to the temporal bone are more common than primary cancers. Outcomes are improved with clear margins, justifying the need for radical resection. Further research regarding pre‐auricular cancers is required given poorer associated survival outcomes.
Keywords: cutaneous squamous cell carcinoma, perineural spread, radiation therapy, skull base surgery, temporal bone
1. INTRODUCTION
Extension of cutaneous squamous cell carcinoma (cSCC) to the temporal bone represents advanced and aggressive disease that can be associated with poor outcomes. Spread to the temporal bone can result from three disease processes: (1) direct invasion of an advanced peri‐auricular cSCC, (2) metastatic extension from an involved peri‐auricular lymph node with cSCC; and (3) facial nerve (VII) perineural spread (PNS) from cSCC. Unlike primary external auditory canal (EAC) (or temporal bone) SCC, an extremely rare entity with an estimated incidence of 1–2 cases per million per year, secondary involvement of the temporal bone by cSCC arising from surrounding structures occurs more frequently. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 This is especially the case in heavily sun exposed areas of the world where keratinocyte carcinoma (KC) is highly prevalent. 10 In a recent publication by our institution of malignancies requiring any form of temporal bone resection (TBR), secondary tumors involving the temporal bone occurred 12 times more commonly than primary tumors; with cSCC the predominant tumor subtype encompassing 75% of the total cohort. 11
Despite this, accurate epidemiological and outcomes data for patients with cSCC extending to the temporal bone is lacking, with the few published studies generally limited by small cohorts and pooling of histopathological subtypes and locations of tumor origin. Given this, we aim to examine the epidemiology, treatment, survival outcomes, and prognostic factors of patients with primary and peri‐temporal bone cSCC. We also aim to draw comparisons between subsite of tumor origin to identify areas where further study is required to improve survival.
2. METHODS
2.1. Patient population
A retrospective case‐series was conducted of consecutive patients managed through the Princess Alexandra Hospital, Brisbane, Australia, between 2000 and 2019. Institutional ethics approval was obtained prior to commencing (LNR/2019/QMS/50709).
Patients were included if they had cSCC requiring any form of TBR. This included tumors originating in the EAC (primary temporal bone) or from surrounding sites (peri‐temporal bone) that directly invaded or extended close to the EAC/temporal bone. Patients with zone 2 or 3 VII PNS according to the Williams et al. classification (disease involving the mastoid VII up to the brain stem) were also included. 12 Tumors of non‐cSCC histopathology were excluded. Patients with zone 1 VII PNS (disease involving peripheral VII branch[es] up to the stylomastoid foramen) without a tumor mass intimately associated with or invading the EAC/temporal bone were excluded. Only patients who underwent curative intent surgery (definitive or salvage) with or without post‐operative radiotherapy (PORT) were included in survival and prognostic factor analyses. Key demographical data was collected including age at diagnosis, gender, immune status, signs and symptoms at presentation, and previous treatment.
2.2. Disease extent
All patients underwent contrast‐enhanced magnetic resonance imaging (MRI) and/or computed tomography (CT) of the head and neck to evaluate the primary tumor. In addition, a full body positron emission tomography (PET) or CT was utilized to assess for regional and distant metastatic disease. All pre‐operative imaging was re‐analyzed by an experienced skull base radiologist (M.G.) who was blinded to outcomes. Data was recorded on tumor location, size, and PNS (including nerve[s] involved and Williams et al. zonal extent). 12
2.3. Treatment
All patients were assessed through the institution's Head and Neck Tumor Board to determine best management and, if applicable, plan surgical intervention. Surgical resection was performed en bloc, including involved nerves if required for PNS, with the aim of removing cancer with adequate margins of healthy tissue (macroscopically >10 mm for the main tumor, >5 mm in nerves with PNS).
TBR was utilized to address both direct EAC or temporal bone invasion and for tumor extension close to the temporal bone, with the extent of bony resection dependent on the medial spread of disease. This was performed as either a mastoidectomy, partial TBR (PTBR), lateral TBR (LTBR), or total TBR using techniques described previously. 2 PTBR was defined as a less radical form of LTBR, such as en bloc excision of the mastoid tip or part of the bony EAC. TBR was combined with ancillary procedures (i.e., parotidectomy, mandibulectomy) as required to clear the peripheral margins. If present, PNS was managed according to the nerve(s) involved and zonal extent. 13
Neck dissection was performed on all patients with clinical nodal disease. Patients with a high probability for occult disease also underwent an elective neck dissection and/or superficial parotidectomy. All patients were offered PORT unless previously given with no scope for further dosing, or the patient had early‐stage disease with favorable histopathological features.
Disease was generally considered unresectable in patients with involvement of the internal carotid artery, jugular bulb, lower cranial nerves, zone 3 PNS, or extensive dural or brain invasion. Unresectable neck disease, distant metastasis, and poor general health were also considered contraindications to surgery. In such cases, palliative surgery was considered for the purposes of improving symptom management and quality of life. Alternatively, definitive or palliative radiotherapy was offered.
Data was collected on treatment intent, operative details, adjuvant therapy, and complications. Treatment was considered salvage in patients who had recurrence in a peri‐auricular nodal bed or recurred after previous local radiotherapy treatment. Histopathology reports were reviewed to record surgical margin status, PNS, differentiation, lymphovascular invasion (LVI), perineural invasion (PNI), structural invasion (cartilage/parotid/bone/dura), and nodal disease.
2.4. Comparative analysis
Patients were stratified into subsite of tumor origin and statistical testing was used to assess for differences between groups. For comparing categorical variables, either the Pearson χ 2 or Fisher exact test was used if there was a count less than five in the crosstab. The Bonferroni correction was used when conducting multiple pairwise comparisons. For comparing categorical with continuous variables, ANOVA was used for normally distributed samples. For non‐parametric data, either the Mann–Whitney U or Kruskal‐Wallis test was used if comparing more than two groups.
2.5. Survival outcomes and prognostic factors analyses
For patients treated with curative intent surgery, the primary end points of interest were disease‐free survival (DFS), locoregional recurrence‐free survival (LRRFS), disease‐specific survival (DSS), and overall survival (OS) at 2‐ and 5‐years after surgery, calculated using Kaplan–Meier survival analysis.
Kaplan–Meier analysis with log‐rank testing and univariable Cox regression was performed for prognostic factors for all outcomes of interest. Variables with a p value <0.2 in univariable Cox analysis and/or of known clinical significance were included in the preliminary Cox model. Multiple Cox regression was performed through backward stepwise elimination based on likelihood ratios (entry p value <0.2, retention p value <0.1), generating the final Cox model.
Results were considered statistically significant at a p value of <0.05. Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Patient population
Two hundred and fifty‐eight patients were assessed for enrolment, of which 91 were excluded due to zone 1 VII PNS alone or non‐cSCC histopathology (Figure 1). The remaining 167 cases of cSCC extending to the temporal bone were analyzed for epidemiology and treatment. For the analysis of survival outcomes and prognostic factors, we excluded a further 21 patients who had either unresectable disease or non‐operative management, leaving 146 patients who underwent curative intent surgical intervention.
FIGURE 1.

Flowchart of the study cohort. Abbreviations: VII PNS, facial nerve perineural spread; cSCC, cutaneous squamous cell carcinoma
The mean age at diagnosis was 68.9 years (range, 35–94 years), with 91.6% of the cohort being male and 19.2% immunosuppressed. Patients with primary EAC SCC, compared to those with peri‐temporal bone cSCC, were more commonly female (45.5% vs. 5.8%; p < 0.001) and immunocompetent (100% vs. 20.5%; p = 0.089).
3.2. Clinical features
As demonstrated in Table 1, the most common presenting symptoms were a skin lesion (42.5%), subcutaneous mass with (10.2%) or without (32.3%) ulceration, new VII palsy (31.7%), pain (28.7%), dysaesthesia or paraesthesia (10.8%), and numbness (10.8%). The mean duration of symptoms to diagnosis was 6.9 months (median, 3 months; range, 0.5–108 months). Significantly higher rates of VII palsy were seen in patients who had pre‐auricular tumors (32.9%) (including skin and nodal lesions) compared to other sites (7.2%; p < 0.001), excluding cases of VII PNS without a tumor mass.
TABLE 1.
Clinical features at diagnosis
| Signs and symptoms | No. of patients (n = 167) | % |
|---|---|---|
| Skin lesion | 71 | 42.5 |
| Subcutaneous mass | 71 | 42.5 |
| With ulceration | 17 | 10.2 |
| Without ulceration | 54 | 32.3 |
| VII palsy | 53 | 31.7 |
| Complete (HB VI) | 37 | 22.2 |
| Partial (HB II‐V) | 16 | 9.6 |
| PNS related | 38 | 22.8 |
| Direct invasion related | 15 | 9.0 |
| Pain | 48 | 28.7 |
| Dysesthesia/paresthesia | 18 | 10.8 |
| Numbness | 18 | 10.8 |
| Bleeding | 13 | 7.8 |
| Discharge | 11 | 6.6 |
| Hearing Loss | 11 | 6.6 |
| Otorrhea | 9 | 5.4 |
| Trismus | 8 | 4.8 |
| Headache | 1 | 0.6 |
| Pruritus | 1 | 0.6 |
Abbreviations: VII, facial nerve; HB, House Brackmann grade; PNS, perineural spread.
Thirty‐eight patients (22.8%) presented with primary untreated disease. Nineteen patients (11.4%) had residual disease at presentation from an inadequate recent excision. The remaining 110 patients had either local (n = 52, 31.1%) or regionally (n = 58, 34.7%) recurrent disease at presentation; the latter including 15 patients (9.0%) with recurrence in the parotid (n = 12), pre‐auricular (n = 2), or post‐auricular (n = 1) nodal bed. In these cases, the average time from initial treatment of the primary to diagnosis was 19.7 months (median, 13 months; range, 1–89 months). Of those with locally residual or recurrent disease (n = 70), 34 (48.6%) had multiple previous attempts at surgical excision.
3.3. Tumor characteristics
Table 2 stratifies patients by tumor origin and provides a comparison of clinicopathological features and survival outcomes. The majority of patients (93.4%) had secondary involvement of the temporal bone from cSCC of surrounding subsites, while 6.6% had a primary temporal bone (EAC) origin. The most common peri‐temporal bone sites of origin were the parotid or pre‐auricular nodes (29.9%) and the external ear (20.4%).
TABLE 2.
Patient characteristics, radiological features, treatment, histopathological features, and survival outcomes by subsite of tumor origin
| Variable | Temporal bone (EAC) | Peri‐temporal bone | External ear | Pre‐auricular skin | Parotid and pre‐auricular LN | Post‐auricular and infra‐auricular skin | Post‐auricular LN | VII PNS alone |
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| n [split] (%) | 11 (6.6) | 156 (93.4) | 34 (20.4) | 23 (13.8) | 50 [42/8] (29.9) | 15 [12/3] (9.0) | 9 (5.4) | 25 [8/17] (15.0) a |
| Mean age, year (range) | 74.2 (51–87) | 68.6 (35–94) | 70.5 (43–92) | 67.7 (45–94) | 69.0 (42–90) | 66.5 (35–80) | 64.7 (47–82) | 68.5 (45–83) |
| Male, % | 54.5 | 94.2 | 97.1 | 95.7 | 96.0 | 93.3 | 100 | 84.0 |
| Immunosuppressed, % | 0.0 | 20.5 | 20.6 | 34.8 | 26.0 | 13.3 | 0.0 | 8.0 |
| Previous local treatment, % | 18.2 | 53.8 | 52.9 | 73.9 | 30.0 | 60.0 | 11.1 | 72.0 |
| VII palsy, % | 9.1 | 33.3 | 5.9 | 21.7 | 38.0 | 13.3 | 0.0 | 96.0 |
| Radiological features | ||||||||
| PNS, % | 9.1 | 34.0 | 17.6 | 26.1 | 26.0 | 20.0 | 0.0 | 100 |
| Max zonal extent (1/2/3), n | 0/1/0 | 8/39/6 | 4/2/0 | 3/3/0 | 1/9/3 | 0/3/0 | 0/0/0 | 0/22/3 |
| Mean max tumor dimension, mm (range) | 20.9 (10–37) | 43.9 (7–127) | 43.3 (10–97) | 51.5 (20–127) | 42.4 (17–90) | 56.6 (37–80) | 44.7 (20–98) | 27.1 (7–89) b |
| Mean medial‐lateral tumor dimension, mm (range) | 11.3 (4–22) | 29.6 (3–77) | 29.6 (3–77) | 31.6 (17–50) | 31.2 (11–61) | 33.1 (11–50) | 28.2 (14–45) | 18.1 (6–40) b |
| Treatment | ||||||||
| Curative intent surgery, % | 100 | 86.5 | 94.1 | 87.0 | 92.0 | 93.3 | 88.9 | 60.0 |
| Histopathological features c | ||||||||
| Involved margins, % | 18.2 | 32.6 | 21.9 | 40.0 | 39.1 | 21.4 | 25.0 | 40.0 |
| Poor differentiation, % | 0.0 | 37.0 | 34.4 | 20.0 | 41.3 | 50.0 | 62.5 | 26.7 |
| Cartilage invasion, % | 36.4 | 46.7 | 84.4 | 50.0 | 34.8 | 50.0 | 37.5 | 0.0 |
| Parotid parenchyma invasion, % | 9.1 | 60.0 | 28.1 | 70.0 | 87.0 | 42.9 | 50.0 | 53.3 |
| Bone invasion, % | 54.5 | 20.7 | 21.9 | 30.0 | 21.7 | 14.3 | 25.0 | 6.7 |
| pN+, % | 0.0 | 34.8 | 12.5 | 25.0 | 63.0 | 28.6 | 50.0 | 6.7 |
| Survival outcomes c | ||||||||
| DFS (2Y/5Y), % | 90.0/90.0 | 62.4/50.5 | 64.5/59.9 | 48.2/34.4 | 54.6/32.9 | 85.1/85.1 | 85.7/57.1 | 71.4/63.5 |
| LRRFS (2Y/5Y), % | 90.0/90.0 | 66.4/57.3 | 64.5/64.5 | 48.2/34.4 | 62.8/51.5 | 85.1/85.1 | 85.7/57.1 | 84.6/68.4 |
| DSS (2Y/5Y), % | 100/100 | 79.1/65.7 | 76.0/70.6 | 72.2/46.6 | 69.7/49.6 | 100/100 | 100/100 | 92.9/82.5 |
| OS (2Y/5Y), % | 100/56.3 | 63.9/43.8 | 58.6/46.9 | 68.4/36.1 | 53.5/25.6 | 85.7/66.7 | 46.9/46.9 | 86.7/77.0 |
Note: Peri‐temporal bone encompasses the external ear, pre‐auricular skin, parotid and pre‐auricular LN, post‐auricular and infra‐auricular skin, post‐auricular LN, and VII PNS alone subsites.
Abbreviations: EAC, external auditory canal; LN, lymph node; VII, facial nerve; PNS, perineural spread; pN+, pathologically involved lymph nodes (excluding main tumor mass); DFS, disease‐free survival; LRRFS, locoregional recurrence‐free survival; DSS, disease‐specific survival; OS, overall survival; 2Y, 2‐year; 5Y, 5‐year.
Split reflects patients who had VII PNS alone with no identifiable tumor mass (n = 8) or VII PNS with nerve expansion or extra‐neural spread (n = 17).
Including only patients who had a radiological identifiable tumor mass associated with VII PNS (nerve expansion or extra‐neural spread) (n = 17).
Percentages are reflective of only those who received curative intent surgery with or without post‐operative radiotherapy.
Radiologically, the average maximal and medial‐lateral tumor dimensions were 42.2 mm (range, 7–127 mm) and 28.2 mm (range, 3–77 mm), respectively, excluding cases of VII PNS with no identifiable tumor mass (n = 8). On average, external ear and pre‐auricular tumors were significantly larger in maximal (p = 0.030) and medial‐lateral dimensions (p = 0.002) than other sites, while primary EAC SCCs were significantly smaller (p < 0.001 and p < 0.001, respectively).
PNS was present on imaging in 54 patients (32.3%), including 29 (17.4%) who had PNS in addition to the main tumor mass relating to the temporal bone. Fifteen patients (9.0%) had VII PNS, 9 (5.4%) had mandibular branch of the trigeminal nerve (V3) PNS, and 30 (18.0%) had simultaneous VII and V3 PNS. Compared to other subsites, pre‐auricular tumors had higher rates of concurrent PNS (36.5% vs. 16.9%; p = 0.073), excluding cases of VII PNS alone.
3.4. Treatment
One hundred and forty‐six patients (87.4%) underwent curative intent surgical resection, including definitive (n = 117, 70.1%) and salvage (n = 29, 17.4%) cases. Eight patients (4.8%) underwent palliative intent surgery. Six patients (3.6%) received radiotherapy alone, with either a curative (n = 2, 1.2%) or palliative (n = 4, 2.4%) intent. Seven patients (4.2%) received supportive cares only.
The operative details for the patients who underwent curative surgery are summarized in Table 3, and a clinical example of a typical patient is provided in Figure 2. All patients underwent TBR, largely in the form of a LTBR (89.7%). Most also underwent some form of parotidectomy (90.4%) or pinnectomy (72.6%), with 46.6% also requiring excision of the temporomandibular joint (TMJ) and/or mandible. Infratemporal fossa (ITF) resection was utilized in 23.3% to address concurrent V3 PNS or direct tumor invasion. Twenty‐one patients (14.4%) also required a lateral craniotomy, including 18 (12.3%) to gain access to the Gasserian ganglion for zone 2 V3 disease, 2 (1.4%) with dural resection for direct tumor extension, and 1 (0.7%) to clear PNS involving the geniculate ganglion and greater superficial petrosal nerve. Eighty patients (54.8%) required VII resection, of whom 16 (11.0%) had preservation of one or more peripheral branches.
TABLE 3.
Operative details of patients who underwent curative intent surgery
| Treatment | No. of patients (n = 146) | % |
|---|---|---|
| Temporal bone resection | 146 | 100 |
| Mastoidectomy | 5 | 3.4 |
| Partial | 9 | 6.2 |
| Lateral a | 131 | 89.7 |
| Total | 1 | 0.7 |
| Pinnectomy | 106 | 72.6 |
| Partial | 51 | 34.9 |
| Total | 55 | 37.7 |
| Parotidectomy | 132 | 90.4 |
| Superficial | 60 | 41.1 |
| Total b | 9 | 6.2 |
| Radical | 63 | 43.2 |
| TMJ ± mandible resection | 68 | 46.6 |
| TMJ/condyle | 20 | 13.7 |
| Ascending mandible | 44 | 30.1 |
| Hemimandible | 4 | 2.7 |
| ITF resection | 34 | 23.3 |
| Craniotomy | 21 | 14.4 |
| With dural resection | 2 | 1.4 |
| Without dural resection | 19 | 13.0 |
| VII resection | 80 | 54.8 |
| Branch/division | 16 | 11.0 |
| Trunk/stylomastoid foramen | 17 | 11.6 |
| Second genu | 30 | 20.5 |
| First genu/geniculate ganglion | 13 | 8.9 |
| Internal auditory canal | 4 | 2.7 |
| V3 resection | 34 | 23.3 |
| ITF (ATN) | 7 | 4.8 |
| Foramen ovale | 9 | 6.2 |
| Gasserian ganglion | 18 | 12.3 |
| Neck dissection | 85 | 58.2 |
| Reconstruction | ||
| Nil | 5 | 3.4 |
| Local (rotation/advancement) | 33 | 22.6 |
| Pedicled | 31 | 21.2 |
| Free flap | 77 | 52.7 |
Abbreviations: TMJ, temporomandibular joint; ITF, infratemporal fossa; VII, facial nerve; V3, trigeminal nerve mandibular branch; ATN, auriculotemporal nerve.
Partial temporal bone resection was defined as a less radical form of lateral temporal bone resection, i.e., en bloc excision of the mastoid tip or part of the bony external auditory canal.
Superficial and total parotidectomy were defined as excision of the superficial or entire parotid gland, respectively, with either preservation of the entire facial nerve or resection of only one peripheral facial nerve branch. Radical parotidectomy was defined as excision of the entire parotid gland with resection of the facial nerve at (or proximal to) its main trunk.
FIGURE 2.

A patient with a right metastatic parotid cutaneous squamous cell carcinoma extending into the conchal bowl and external auditory canal (A–C) (red arrows). Surgical resection (D) involved partial pinnectomy, lateral temporal bone resection, radical parotidectomy, ascending mandibulectomy, infratemporal fossa resection, and modified radical neck dissection. Reconstruction involved an anterolateral thigh free flap and static slings. The lateral (E) and medial (F) aspects of the excised specimen are provided (tie placed on facial nerve) [Color figure can be viewed at wileyonlinelibrary.com]
Reconstruction of the soft tissue defect was most commonly achieved with a free flap (52.7%), primarily from the anterolateral thigh (40.4%). Of the patients who had VII resection (n = 80), 58 (72.5%) underwent static reconstruction intra‐ and/or post‐operatively (i.e., lateral tarsorrhaphy, gold weight insertion, and/or static slings), 11 (13.8%) underwent dynamic reconstruction (nerve grafting) intra‐operatively, and the remaining 11 (13.8%) had no reconstruction.
Of the patients who underwent curative intent surgery, 77.4% (n = 113/146) received PORT. Of those, 109 patients (96.5%) completed their prescribed course with an average dose of 60 Gray (range, 45–69 Gy) in 30 fractions (range, 20–34 fractions). Four patients (3.5%) ceased their treatment prematurely. Of the remaining 33 patients (22.6%) who did not receive PORT, 19 were unable to receive further radiotherapy after previously receiving a full dose to the area, 6 declined, 5 were observed due to early‐stage disease, 2 had early recurrences before radiotherapy could commence, and 1 died post‐operatively.
3.5. Complications
Post‐operative hospital stay varied from 2 to 55 days (mean, 12 days; median, 10 days). Intra‐operative complications were reported in 24 patients (16.4%), including dural tear and/or cerebrospinal fluid (CSF) leak (n = 12), internal jugular vein or jugular bulb (n = 4) injury, oral mucosal injury (n = 2), transection of a VII branch (n = 3) or the hypoglossal nerve (n = 1), free flap congestion requiring revision anastomoses (n = 1), and stapes dislocation (n = 1). These intra‐operative events did not lead to any related post‐operative complications or prolonged hospital admissions.
A summary of post‐operative complications according to the Clavien‐Dindo classification is provided in Table 4. 14 Sixty‐five patients (44.5%) experienced a post‐operative complication, with 27 (18.5%) having multiple complications, for a total of 120 complications. The most frequent post‐surgical complications were related to the wound or free flap reconstruction (n = 78, 65.0%), pneumonia or pulmonary oedema (n = 9, 7.5%), blood transfusion (n = 9, 7.5%), and CSF leak (n = 5, 4.2%). There was one peri‐operative mortality. This patient was a 76 year old male who underwent a LTBR, radical parotidectomy, ascending mandibulectomy, ITF resection, and craniotomy with Gasserian ganglion resection for PNS. He sustained a non‐ST elevated myocardial infarction day 2 post‐operatively and developed biventricular failure requiring hemodynamic support. He then sustained a cardiac arrest and died day 6 post‐operatively.
TABLE 4.
Complications post curative intent surgery, according to the Clavien‐Dindo classification system 14
| Complication details | No. of patients (n = 146) | % |
|---|---|---|
| Post‐operative complication | ||
| No | 80 | 54.8 |
| Unknown | 1 | 0.7 |
| Yes a | 65 | 44.5 |
| Grade I | 10 | 6.8 |
| Grade II | 8 | 5.5 |
| Grade IIIa | 7 | 4.8 |
| Grade IIIb | 34 | 23.3 |
| Grade IV | 5 | 3.4 |
| Grade V | 1 | 0.7 |
| Multiple complications | 27 | 18.5 |
| Complication list b | ||
| Grade I | 15 | 12.5 |
| Wound dehiscence | 7 | 5.8 |
| Seroma drainage | 3 | 2.5 |
| CSF leak requiring prolonged surgical drain | 2 | 1.7 |
| Pulmonary oedema | 2 | 1.7 |
| Failure to void requiring prolonged catheter | 1 | 0.8 |
| Grade II | 29 | 24.2 |
| Blood transfusion | 9 | 7.5 |
| Pneumonia | 5 | 4.2 |
| Wound infection | 5 | 4.2 |
| DVT/PE | 4 | 3.3 |
| AF | 4 | 3.3 |
| Dysphagia requiring NG feeding | 1 | 0.8 |
| NSTEMI | 1 | 0.8 |
| Grade IIIa | 8 | 6.7 |
| Washout ± debridement of flap/donor site under LA | 7 | 5.8 |
| Dysphagia requiring PEG | 1 | 0.8 |
| Grade IIIb | 62 | 51.7 |
| Washout ± debridement of flap/donor site | 53 | 44.2 |
| Free flap exploration ± revision | 3 | 2.5 |
| CSF leak requiring surgical repair + lumbar drain | 3 | 2.5 |
| Long ventilatory wean/failed extubation requiring tracheostomy | 3 | 2.5 |
| Grade IV | 5 | 4.2 |
| APO requiring I&V + HD support | 2 | 1.7 |
| Sepsis requiring HD support | 1 | 0.8 |
| Status epilepticus from cerebritis requiring sedation + I&V | 1 | 0.8 |
| Haemorrhagic shock from UGI bleed requiring HD support | 1 | 0.8 |
| Grade V | 1 | 0.8 |
| NSTEMI + decompensated heart failure resulting in death | 1 | 0.8 |
Abbreviations: CSF, cerebrospinal fluid; DVT, deep vein thrombosis; PE, pulmonary embolism; AF, atrial fibrillation; NG, nasogastric; NSTEMI, non‐ST‐elevation myocardial infarction; LA, local anesthetic; PEG, percutaneous endoscopic gastrostomy; APO, acute pulmonary oedema; I&V, intubation and ventilation; HD, hemodynamic; UGI, upper gastrointestinal.
Numbers according to maximal Clavien‐Dindo complication grade experienced by a patient. 14
Percentages are based on total number of complications experienced by all patients (n = 120).
In patients who had surgical preservation of the entire VII (n = 66), the rate of VII palsy post‐operatively was 42.4% (n = 28), with most being partial (House Brackmann grades [HB] II–V) (n = 23, 34.8%) versus complete (HB VI) (n = 5, 7.6%) palsies. Of these 28 patients, 19 had eventual complete recovery in VII function, 4 had partial recovery, 4 had no recovery, and 1 was unknown. The mean time to maximal VII recovery (partial or complete) was 11.4 months post‐operatively (median, 7.5 months; range 1–48 months).
Fourteen patients (12.4%) experienced late complications following PORT. These included: skull base osteomyelitis (n = 4), osteoradionecrosis of the mandible (n = 3) and EAC (n = 1) (in a patient where mastoidectomy only was performed for VII PNS), wound dehiscence or infection (n = 3), temporal lobe radionecrosis (n = 2), and dysphagia requiring permanent enteral feeding (n = 1).
3.6. Pathological features
As seen in Table 5, most patients who received curative intent surgery were pathologically classified as either T3 (47.3%) or T4b (25.3%) according to the 8th edition AJCC system, given the advanced nature of disease. A high proportion also had N3b disease (42.5%), reflecting the cases of involved peri‐auricular lymph nodes with extranodal extension to the temporal bone. As such, almost all patients were prognostically Stage III (28.1%) or IV (68.5%).
TABLE 5.
Pathological features of patients who underwent curative intent surgery
| Pathological features | No. of patients (n = 146) | % |
|---|---|---|
| Pathological TNM classification and stage (AJCC 8th ed.) | ||
| T | ||
| Tx | 1 | 0.7 |
| rTx | 20 | 13.7 |
| T1 | 3 | 2.1 |
| rT1 | 6 | 4.1 |
| rT2 | 9 | 6.2 |
| T3 | 29 | 19.9 |
| rT3 | 40 | 27.4 |
| T4a | 1 | 0.7 |
| T4b | 14 | 9.6 |
| rT4b | 23 | 15.8 |
| N | ||
| Nx | 1 | 0.7 |
| N0 | 76 | 52.1 |
| N1 | 2 | 1.4 |
| N2a | 3 | 2.1 |
| N2b | 2 | 1.4 |
| N3b | 49 | 33.6 |
| rN3b | 13 | 8.9 |
| M | ||
| M0 | 146 | 100 |
| Stage | ||
| I | 4 | 2.7 |
| III | 41 | 28.1 |
| IV | 100 | 68.5 |
| Unknown | 1 | 0.7 |
| Histopathological features | ||
| Surgical margin | ||
| Clear | 48 | 32.9 |
| Close | 52 | 35.6 |
| Involved | 46 | 31.5 |
| PNS | ||
| No | 115 | 78.8 |
| Unknown | 1 | 0.7 |
| Yes | 30 | 20.5 |
| VII alone | 10 | 6.8 |
| V3 alone | 4 | 2.7 |
| VII + V3 | 16 | 11.0 |
| Zone 1 | 4 | 2.7 |
| Zone 2 | 24 | 16.4 |
| Zone 3 | 2 | 1.4 |
| Differentiation | ||
| Well | 4 | 2.7 |
| Moderate | 87 | 59.6 |
| Poor | 50 | 34.2 |
| Unknown | 5 | 3.4 |
| LVI | ||
| No | 69 | 47.3 |
| Unknown | 35 | 24.0 |
| Yes | 42 | 28.8 |
| PNI | ||
| No | 50 | 34.2 |
| Unknown | 12 | 8.2 |
| Yes | 84 | 57.5 |
| Structural invasion a | ||
| Cartilage | 67 | 45.9 |
| Parotid parenchyma | 82 | 56.2 |
| Bone | 33 | 22.6 |
| Dura | 2 | 1.4 |
| pN+ | ||
| No | 98 | 67.1 |
| Unknown | 1 | 0.7 |
| Yes | 47 | 32.2 |
Abbreviations: AJCC, American Joint Committee on Cancer; PNS, perineural spread; VII, facial nerve; V3, trigeminal nerve mandibular branch; LVI, lymphovascular invasion; PNI, perineural invasion; pN+, pathologically involved lymph nodes (excluding main tumor mass).
Two patients had unknown status within this group.
Pathological margins were reported as clear in 32.9%, close (<5 mm) in 35.6%, and involved in 31.5%. A significantly higher proportion of close and involved margins was seen in pre‐auricular tumors compared to other tumors (78.8% vs. 57.5%; p = 0.006). Recurrent tumors had significantly higher rates of involved margins than primary tumors (38.7% versus 16.7%; p = 0.007).
Thirty patients (20.5%) had pathological evidence of PNS, with the majority having zone 2 disease (n = 24, 16.3%) and simultaneous VII and V3 involvement (n = 16, 11.0%). Most tumors were moderately differentiated (59.6%), with LVI present in 28.8% and PNI in 57.5%. Compared to primary tumors, recurrent tumors had significantly higher rates of LVI (48.6% vs. 19.5%; p = 0.002) and PNI (75.3% vs. 43.8%; p < 0.001). Histopathologically, 45.9% of tumors involved cartilage and 56.2% invaded the parotid parenchyma. Bone invasion was demonstrated in 22.6% (n = 33), with involvement of the temporal bone in 32 patients (21.9%) and mandible in 6 (4.1%). Only 2 patients (1.4%) had dural invasion. There were no statistically significant differences in structural invasion between primary and recurrent tumors.
Forty‐seven patients (32.2%) had pathologically involved lymph nodes in addition to the main tumor mass, including parotid and/or pre‐auricular nodes in 31 (21.2%), post‐auricular nodes in 2 (1.4%), and cervical nodes in 29 (19.9%). The distribution of cervical nodal involvement by level was as follows: level I, 6 (4.1%); level II, 22 (15.1%); level III, 7 (4.8%); level IV, 2 (1.4%); and level V, 2 (1.4%). A significantly higher rate of cervical nodal metastasis was seen with recurrent tumors compared to primary tumors (26.2% vs. 10.2%; p = 0.024), and with tumors located in a peri‐auricular nodal subsite compared to all other subsites (39.3% vs. 10.4%; p < 0.001). Similarly, peri‐temporal bone tumors (22.7%) had higher proportions of cervical nodal involvement than primary temporal bone tumors (0.0%), but this did not reach statistical significance (p = 0.067).
3.7. Survival outcomes
For all patients treated with curative intent surgery with or without PORT (n = 146, 87.4%), the 2‐ and 5‐year DFS was 64.5% and 53.0%, LRRFS was 68.2% and 59.4%, DSS was 80.7% and 67.9%, and OS was 66.4% and 44.7%, respectively (Figure 3). Six patients (4.1%) were lost to follow‐up in the 5 years post‐surgery.
FIGURE 3.
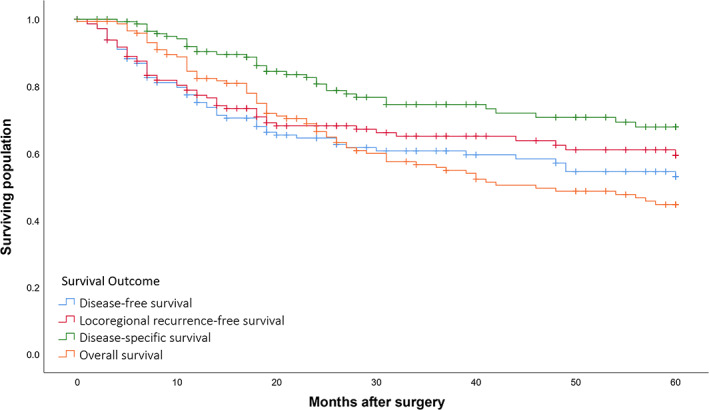
Survival outcomes for patients treated with curative intent surgery with or without post‐operative radiotherapy (n = 146) [Color figure can be viewed at wileyonlinelibrary.com]
Survival rates by subsite of origin are outlined in Table 2, and the Kaplan–Meier plot for DFS is presented in Figure 4. While primary EAC SCCs tended to have better 5‐year outcomes than peri‐temporal bone cSCCs, statistical significance was not reached for DFS (90.0% vs. 50.5%; p = 0.067), LRRFS (90.0% vs. 57.3%; p = 0.110), DSS (100% vs. 65.7%; p = 0.078), or OS (56.3% vs. 43.8%; p = 0.231). External ear and pre‐auricular tumors had significantly worse outcomes compared to all other sites, with a 5‐year DFS of 42.3% versus 74.9% (p = 0.001), LRRFS of 51.4% versus 76.0% (p = 0.004), DSS of 54.9% versus 93.1% (p < 0.001), and OS of 34.6% versus 65.0% (p = 0.001).
FIGURE 4.
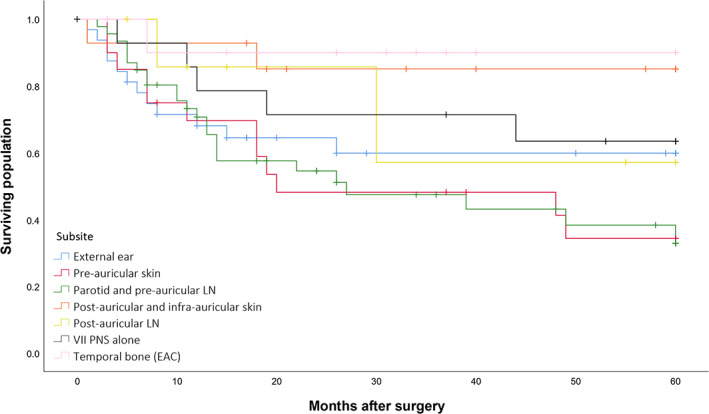
Disease‐free survival by subsite of tumor origin in curative intent surgical cases (n = 146). Abbreviations: LN, lymph node; VII, facial nerve; PNS, perineural spread; EAC, external auditory canal [Color figure can be viewed at wileyonlinelibrary.com]
Fifty‐eight patients (39.7%) had recurrence(s) during the follow‐up period at an average of 14.3 months post‐operatively (median, 10.5 months; range, 1–60 months). Forty‐nine of these patients (33.6%) had locoregional recurrence (local n = 43; regional n = 11), while 17 (11.6%) had distant recurrence. Twelve patients had more than one location of recurrence.
3.8. Prognostic factors
The results of univariable Cox regression analysis are summarized in Supplementary Table S1. Based on a p value of <0.2, immunosuppression, VII palsy, subsite of origin, radiological medial‐lateral tumor dimension, salvage surgery, surgical margin status, differentiation, pathological parotid invasion, and pathological nodal disease were included in the preliminary Cox models. Although not statistically significant in univariable analysis, age, pathological PNS and maximal zonal extent, and pathological bony invasion were also included given their known clinical impact. PNI and LVI were excluded from multivariable analysis given the relatively high numbers of missing data in these groups, resulting in a significant reduction in power of the final Cox models.
The multiple Cox regression models are detailed in Table 6. Subsite of tumor origin retained independent prognostic significance, with external ear and pre‐auricular tumors significantly increasing risk of any recurrence (HR: 2.27 [95% CI: 1.06–4.87], p = 0.035), locoregional recurrence (HR: 2.38 [95% CI: 1.10–5.14], p = 0.027), death from disease (HR: 18.05 [95% CI: 2.44–133.68], p = 0.005), and all‐cause mortality (HR: 2.44 [95% CI: 1.28–4.64], p = 0.007), compared to other sites. After adjusting for other factors, tumors ≥40 mm in medial‐lateral dimension had significantly greater hazard of locoregional recurrence (HR: 2.06 [95% CI: 1.04–4.05], p = 0.037) and disease related death (HR: 2.41 [95% CI: 1.03–5.66], p = 0.043), compared to tumors <40 mm. Salvage surgery also worsened DFS (HR: 2.13 [95% CI: 1.15–3.95], p = 0.016) and LRRFS (HR: 2.71 [95% CI: 1.42–5.15], p = 0.002), compared to definitive surgery.
TABLE 6.
Final multiple Cox regression models for prognostic factors for all survival outcomes in patients who underwent curative intent surgery (n = 146)
| DFS | LRRFS | DSS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic factor | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| Subsite | ||||||||||||
| All other sites | Ref | Ref | Ref | Ref | ||||||||
| Ext. ear + Pre‐auric | 2.27 | (1.06–4.87) | 0.035 | 2.38 | (1.10–5.14) | 0.027 | 18.05 | (2.44–133.68) | 0.005 | 2.44 | (1.28–4.64) | 0.007 |
| Radiological medial‐lateral tumor dimension | ||||||||||||
| <40 mm | Ref | Ref | Ref | Ref | ||||||||
| ≥40 mm | 1.86 | (0.95–3.67) | 0.073 | 2.06 | (1.04–4.05) | 0.037 | 2.41 | (1.03–5.66) | 0.043 | 1.75 | (0.97–3.15) | 0.063 |
| Salvage surgery | ||||||||||||
| No (definitive) | Ref | Ref | ||||||||||
| Yes | 2.13 | (1.15–3.95) | 0.016 | 2.71 | (1.42–5.15) | 0.002 | ||||||
| Surgical margin | ||||||||||||
| Clear | Ref | Ref | ||||||||||
| Close | 2.48 | (1.02–6.01) | 0.044 | 2.93 | (1.18–7.28) | 0.021 | ||||||
| Involved | 3.36 | (1.40–8.07) | 0.007 | 3.89 | (1.54–9.81) | 0.004 | ||||||
| pN | ||||||||||||
| No | Ref | Ref | Ref | |||||||||
| Peri‐auric or Cerv | 1.14 | (0.57–2.26) | 0.719 | 1.16 | (0.50–2.69) | 0.730 | 1.38 | (0.79–2.43) | 0.262 | |||
| Peri‐auric + Cerv | 2.38 | (1.15–4.94) | 0.020 | 3.65 | (1.52–8.75) | 0.004 | 2.23 | (1.17–4.28) | 0.015 | |||
Note: Bolding indicates statistical significance (p value <0.05).
Abbreviations: DFS, disease‐free survival; LRRFS, locoregional recurrence‐free survival; DSS, disease‐specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; Ext. external; Pre‐auric, pre‐auricular (including cutaneous and nodal lesions); pN, pathological nodal disease (including main tumor mass); Peri‐auric, peri‐auricular node(s); Cerv, cervical node(s).
Patients with pathologically involved peri‐auricular and cervical nodes, compared to none, had significantly higher rates of recurrence (HR: 2.38 [95% CI: 1.15–4.94], p = 0.020), death from disease (HR: 3.65 [95% CI: 1.52–8.75], p = 0.004), and death (HR: 2.23 [95% CI: 1.17–4.28], p = 0.015). Involved surgical margins conferred more than a 3‐fold increase in the risk of any recurrence (HR: 3.36 [95% CI: 1.40–8.07], p = 0.007) and locoregional recurrence (HR: 3.89 [95% CI: 1.54–9.81], p = 0.004), compared to clear margins. Similarly, patients with close margins had over two times the risk of recurrence than those with clear margins. Kaplan–Meier plots for the factors comprising the DFS model are provided in Figure 5.
FIGURE 5.

Disease‐free survival in curative intent surgical patients (n = 146), stratified by (A) subsite, (B) radiological medial‐lateral tumor dimension, (C) salvage surgery, (D) surgical margin, and (E) pathological nodal disease (pN). Note: p values calculated using log‐rank testing [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Overall, there is a paucity of studies in the literature that report on cSCC extending to the temporal bone. Furthermore, the accuracy and external validity of these reports are limited by small cohort numbers and grouping of locations of tumor origin and histopathology. Thus, we analyzed a case‐series comprised entirely of patients with primary and peri‐temporal bone cSCC and highlighted key differences in clinicopathological features and survival outcomes between subsite of tumor origin—the largest known study of its kind.
In our study, peri‐temporal bone cSCCs extending to the temporal bone were more than 14 times as common as primary EAC malignancies (93.4% vs. 6.6%). These findings were unsurprising given Australia, and especially Queensland, has world‐leading rates of KC. 10 In addition, the main underlying etiology for primary temporal bone SCC is a chronic inflammatory process leading to metaplastic or neoplastic change, as opposed to UV damage. 15
Secondary cSCCs also had worse survival outcomes compared to primary EAC SCC. In particular, carcinomas originating on the external ear and pre‐auricular region had significantly worse outcomes compared to all else, independent of size, salvage surgery, surgical margins, and pathological nodal status. Peri‐auricular cSCCs are known to be aggressive with higher locoregional recurrence and mortality rates than other head or neck subsites. They also tend to have high rates of incomplete or inadequate primary excision, possibly in efforts to limit morbidity (i.e., iatrogenic VII palsy) or due to an underestimation of medial spread of malignancy. 7 , 16 , 17 This was also reflected in this study, where 77.2% of patients had locoregionally recurrent or residual disease at diagnosis. Furthermore, patients who underwent salvage surgery had significantly worse outcomes than those who had definitive surgery. Appropriate oncological treatment of the primary is thus paramount to control disease in the first instance.
Early recognition and treatment of disease is also crucial, as our results demonstrated that patients with more advanced disease, particularly those with greater medial‐lateral tumor dimensions (≥40 mm) and pathological nodal disease, had significantly higher rates of recurrence and mortality.
Interestingly, the presence of PNS had no significant impact on survival in univariable or multivariable analyses. We suspect that our unit's awareness and appropriate treatment of PNS mitigated the risk of poorer survival. Additionally, only two patients who underwent curative intent surgery had pathological zone 3 disease, with the extent of PNS being underestimated on pre‐operative imaging. Outcomes in such patients are known to be significantly worse and generally considered unresectable at the outset. Nonetheless, clinicians should be aware of the often‐coexistent PNS in these tumors and have a low threshold for performing dedicated MRI to assess for PNS and incorporate this into treatment.
Furthermore, VII palsy had no significant correlation with survival, likely in part due to most patients having VII palsy related to PNS (n = 38/53, 72%), as opposed to direct tumor invasion. Of note, these latter patients demonstrated a trend toward worse survival in univariable analysis, suggesting that these tumors may be more aggressive than those that develop PNS. The literature also remains divided on the prognostic significance of VII palsy. In their small study of 25 Australian patients who underwent LTBR for cSCC largely from peri‐temporal bone sites, Leedman et al. found that clinical or radiological pre‐operative VII involvement significantly worsened OS. 18 However, McRackan et al. found no statistically significant relationship between VII palsy and survival in their 60‐patient cohort, of which 93% were secondary cSCCs. 19 Thus, larger studies are required to further investigate the potential impact of VII palsy.
The advanced and aggressive nature of cSCC involving the temporal bone was demonstrated through our long‐term outcomes, with 2‐ and 5‐year DFS of 64.5% and 53.0%, DSS of 80.7% and 67.9%, and OS of 66.4% and 44.7%, respectively. These outcomes generally compare favorably to other reports in the literature and to other advanced head and neck malignancies. O'Connor et al. demonstrated 5‐year DFS and OS of 28% and 40%, respectively, in their cohort of 47 patients who required LTBR predominately for peri‐temporal bone cSCC (85%). 20 McRacken et al. and Gal et al. revealed recurrence rates of 32% and 57% respectively for cSCCs of the peri‐auricular skin involving the temporal bone, but the average follow‐up was less than 26 months in both. 19 , 21 In their small Australian cohorts, Leedman et al. found a 2‐year LRRFS of 82%, DSS of 75% and OS of 68%, while Kwok et al. had a 2‐year DFS of 79%, DSS of 86%, and OS of 72%. 18 , 22 However, longer‐term follow‐up was lacking in these studies.
While surgical resection and PORT remains the mainstay of treatment for cSCC extending to the temporal bone, treatment strategies have not been well researched. Not only can these malignancies directly invade the EAC and/or temporal bone, but they can also extend to crevices around the lateral skull base and TMJ. Although traditional approaches have often favored soft tissue resection alone, achieving an en bloc clear margin resection in this conservative setting is very difficult with these tumors. 23 Thus, our institution has adopted a more radical approach, utilizing TBR when the tumor is abutting to improve access and facilitate clearance of the posterior and medial margins, even in the absence of direct bony involvement. This can be seen in our cohort, with all patients undergoing TBR, despite less than 25% having pathological temporal bone invasion, resulting in a negative margin resection in almost 70%.
This treatment approach appears justified when considering the significant impact margin status has on survival. Patients with involved margins had almost four times the risk of locoregional recurrence than those with clear margins, while close margins (<5 mm) carried almost three times the risk. Further studies need to be undertaken on modes of spread and fascial planes in the lateral skull base region to improve pre‐operative planning and margin status. This is particularly important for pre‐auricular lesions, which had the highest rates of involved or close margins (78.8%) and the poorest outcomes compared to other sites.
Despite undergoing extensive surgical resections, most of our patients generally tolerated treatment well with an acceptable level of post‐treatment morbidity. Post‐operatively, 27% experienced a Clavien‐Dindo Grade 3 or worse complication, and just 12% had long‐term complications following radiotherapy. 14 However, subjective measures of post‐treatment quality of life were not undertaken in this study. Given its promising results for treatment of cSCC, immunotherapy may soon play a greater role in treatment protocols, with the potential benefit of reducing or avoiding the morbidity associated with current treatments. 24 , 25
The limitations of this study are largely in its retrospective nature, with reliance on existing records and reports. Some results may therefore be subject to information bias. Furthermore, despite examining a large cohort in the context of a rare disease, results are still somewhat limited in accuracy by relatively low patient numbers. Strengths of the study included consecutive inclusion of patients and a small amount of missing data, with only six patients lost to follow‐up. Additionally, only certain factors, such as LVI and PNI, were affected by missing data, resulting in exclusion from the multivariable model. Thus, larger prospective studies are required to elucidate the true effect of these factors on prognosis.
5. CONCLUSION
cSCCs extending to the temporal bone are a problem that deserves more attention, especially in areas of high KC incidence. In this study, secondary cSCCs were more than 14 times as common as primary malignancies and generally demonstrated worse survival outcomes—specifically those originating on the external ear and pre‐auricular region. Most also presented with locoregionally recurrent or residual disease, and those who underwent salvage surgery, as opposed to definitive, had significantly higher recurrence rates. This reinforces the importance of adequate treatment of the primary lesion. Clinicians must also closely examine for PNS and incorporate it into any treatment planning, as appropriate management was shown in this study to mitigate any negative potential survival impact.
The aggressiveness of this disease was demonstrated through our 5‐year DSS and OS rates of 67.9% and 44.7%, which compare favorably to other studies in the literature. Early recognition of disease and prompt treatment is essential, as patients with larger tumors and pathological nodes had significantly worse survival. Surgical resection and PORT remains the mainstay of treatment, with generally acceptable post‐treatment morbidity for the patient. A radical surgical approach is justified given that involved surgical margins increased the risk of locoregional recurrence almost four times. Immunotherapy may soon play a role in the management of these cancers, and results from this large series will help in the design of future clinical trials.
Given the poor prognosis of pre‐auricular tumors and high rates of close or involved margins, further research is a needed into the patterns of tumor spread and fascial planes in this region to improve pre‐operative planning and ultimately patient outcomes.
AUTHOR CONTRIBUTIONS
Michael J. C. Schachtel: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing‐original draft, writing‐review and editing. Mitesh Gandhi: conceptualization, data curation, investigation, methodology, project administration, supervision, validation, writing‐review and editing. James J. Bowman: conceptualization, investigation, methodology, project administration, supervision, validation, writing‐review and editing. Sandro V. Porceddu: conceptualization, investigation, supervision, validation, writing‐review and editing. Benedict J. Panizza: conceptualization, data curation, investigation, methodology, project administration, supervision, validation, visualization, writing‐original draft, writing‐review and editing.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Table S1 Univariable Cox regression for prognostic factors for all survival outcomes in patients who underwent curative intent surgery (n = 146).
ACKNOWLEDGMENTS
The authors thank all those from the Princess Alexandra Hospital head and neck cancer service who were and continue to be involved in the care of these patients. We especially thank the plastic and reconstructive surgeons for their reconstructive work. Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Schachtel MJC, Gandhi M, Bowman JJ, Porceddu SV, Panizza BJ. Epidemiology and treatment outcomes of cutaneous squamous cell carcinoma extending to the temporal bone. Head & Neck. 2022;44(12):2727‐2743. doi: 10.1002/hed.27185
Section Editor: Paul W. Gidley
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Essig GF, Kitipornchai L, Adams F, et al. Lateral temporal bone resection in advanced cutaneous squamous cell carcinoma: report of 35 patients. J Neurol Surg B Skull Base. 2013;74:54‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panizza B, Solares CA, Gleeson MJ. Lateral skull base surgery. In: Watkinson JC, Gilbert RW, eds. Stell and Maran's Textbook of Head and Neck Surgery and Oncology. Hodder Arnold; 2012:779‐790. [Google Scholar]
- 3. Gidley PW, Roberts DB, Sturgis EM. Squamous cell carcinoma of the temporal bone. Laryngoscope. 2010;120:1144‐1151. [DOI] [PubMed] [Google Scholar]
- 4. Chi FL, Gu FM, Dai CF, Chen B, Li HW. Survival outcomes in surgical treatment of 72 cases of squamous cell carcinoma of the temporal bone. Otol Neurotol. 2011;32:665‐669. [DOI] [PubMed] [Google Scholar]
- 5. Lionello M, Stritoni P, Facciolo MC, et al. Temporal bone carcinoma. Current diagnostic, therapeutic, and prognostic concepts. J Surg Oncol. 2014;110:383‐392. [DOI] [PubMed] [Google Scholar]
- 6. Zanoletti E, Marioni G, Stritoni P, et al. Temporal bone squamous cell carcinoma: analyzing prognosis with univariate and multivariate models. Laryngoscope. 2014;124:1192‐1198. [DOI] [PubMed] [Google Scholar]
- 7. Anderson S, Patel P, Panizza B. Squamous cell carcinoma extending to the temporal bone. In: Riffat F, Palme CE, Veness M, eds. Non‐Melanoma Skin Cancer of the Head and Neck. Springer India; 2015:131‐143. [Google Scholar]
- 8. Bowman JJ, Ward M, Panizza B. Management of squamous cell carcinoma involving the temporal bone. Curr Otorhinolaryngol Rep. 2018;6:330‐336. [Google Scholar]
- 9. Gidley PW, Thompson CR, Roberts DB, DeMonte F, Hanna EY. The oncology of otology. Laryngoscope. 2012;122:393‐400. [DOI] [PubMed] [Google Scholar]
- 10. Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R. Incidence and prevalence of non‐melanoma skin cancer in Australia: a systematic review. Australas J Dermatol. 2015;56:258‐267. [DOI] [PubMed] [Google Scholar]
- 11. Schachtel MJC, Gandhi M, Bowman JJ, Erian C, Porceddu SV, Panizza BJ. Malignancies requiring temporal bone resection: an Australian single‐institution experience. ANZ J Surg. 2021;91:1462‐1471. [DOI] [PubMed] [Google Scholar]
- 12. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 13. Solares CA, Mason E, Panizza BJ. Surgical management of perineural spread of head and neck cancers. J Neurol Surg B Skull Base. 2016;77:140‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masterson L, Donnelly NP. Squamous cell carcinoma of the temporal bone. In: Scott‐Brown WG, Watkinson JC, Clarke R, eds. Scott‐Brown's Otorhinolaryngology Head and Neck Surgery. CRC Press; 2019. [Google Scholar]
- 16. Tan PY, Ek E, Su S, Giorlando F, Dieu T. Incomplete excision of squamous cell carcinoma of the skin: a prospective observational study. Plast Reconstr Surg. 2007;120:910‐916. [DOI] [PubMed] [Google Scholar]
- 17. Mirshams M, Razzaghi M, Noormohammadpour P, Naraghi Z, Kamyab K, Sabouri RS. Incidence of incomplete excision in surgically treated cutaneous squamous cell carcinoma and identification of the related risk factors. Acta Med Iran. 2011;49:806‐809. [PubMed] [Google Scholar]
- 18. Leedman S, Wormald R, Flukes S. Lateral temporal bone resection for cutaneous carcinomas of the external auditory canal and peri‐auricular region. J Laryngol Otol. 2021;135:1‐6. [DOI] [PubMed] [Google Scholar]
- 19. McRackan TR, Fang TY, Pelosi S, et al. Factors associated with recurrence of squamous cell carcinoma involving the temporal bone. Ann Otol Rhinol Laryngol. 2014;123:235‐239. [DOI] [PubMed] [Google Scholar]
- 20. O'Connor A, Behan L, Toner M, Kinsella J, Beausang E, Timon C. Evaluating the outcomes of temporal bone resection in metastatic cutaneous head and neck malignancies: 13‐year review. J Laryngol Otol. 2015;129:964‐969. [DOI] [PubMed] [Google Scholar]
- 21. Gal TJ, Futran ND, Bartels LJ, Klotch DW. Auricular carcinoma with temporal bone invasion: outcome analysis. Otolaryngol Head Neck Surg. 1999;121:62‐65. [DOI] [PubMed] [Google Scholar]
- 22. Kwok MM, Choong KWK, Virk J, Kleid S, Magarey MJ. Lateral temporal bone resections for Peri‐auricular cutaneous squamous cell carcinoma ‐ prognostic indicators and radiological predictive values. J Laryngol Otol. 2021;136:1‐22. [DOI] [PubMed] [Google Scholar]
- 23. Shao A, Wong DK, McIvor NP, et al. Parotid metastatic disease from cutaneous squamous cell carcinoma: prognostic role of facial nerve sacrifice, lateral temporal bone resection, immune status and P‐stage. Head Neck. 2014;36:545‐550. [DOI] [PubMed] [Google Scholar]
- 24. Wessely A, Steeb T, Leiter U, Garbe C, Berking C, Heppt MV. Immune checkpoint blockade in advanced cutaneous squamous cell carcinoma: what do we currently know in 2020? Int J Mol Sci. 2020;21:9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nightingale J, Gandhi M, Helena J, et al. Immunotherapy for the treatment of perineural spread in cutaneous head and neck squamous cell carcinoma: time to rethink treatment paradigms. Head Neck. 2022;44:1099‐1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariable Cox regression for prognostic factors for all survival outcomes in patients who underwent curative intent surgery (n = 146).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


