Abstract
Background
Deficient endogenous pain modulation and increased nociceptive excitability are key features of central sensitization and can be assessed in humans by conditioned pain modulation (CPM, anti‐nociceptive) and temporal summation of pain (TSP, pro‐nociceptive), respectively. This study aimed to investigate these measures as proxies for central sensitization in subjects with chronic neuropathic pain (NP) after spinal cord injury (SCI).
Methods
In paraplegic subjects with NP (SCI‐NP; n = 17) and healthy controls (HC; n = 17), parallel and sequential sham‐controlled CPM paradigms were performed using pressure pain threshold at the hand, that is, above lesion level, as test stimulus. The conditioning stimulus was a noxious cold (verum) or lukewarm water bath (sham) applied contralaterally. Regarding pro‐nociceptive mechanisms, a TSP protocol with individually‐adjusted pressure pain stimuli at the thenar eminence was used. CPM and TSP magnitudes were related to intensity and spatial extent of spontaneous NP.
Results
Neither the parallel nor sequential sham‐controlled CPM paradigm showed any significant inhibition of above‐level pressure pain thresholds for SCI‐NP or HC. Accordingly, no group difference in CPM capacity was found, however, subjects with more intense spontaneous NP showed lower inhibitory CPM capacity. TSP was observed for both groups but was not enhanced in SCI‐NP.
Conclusions
Our results do not support altered above‐level anti‐ or pro‐nociceptive mechanisms in SCI‐NP compared with HC; however, they also highlight the relevance of spontaneous NP intensity with regards to the capacity of endogenous pain modulation in SCI subjects.
Significance
Central sensitization encompasses deficient endogenous pain modulation and increased nociceptive excitability. These two mechanisms can be assessed in humans by conditioned pain modulation and temporal summation of pain, respectively. Our data demonstrates a lack of descending pain inhibition only in subjects with severe neuropathic pain which may hint towards central sensitization at spinal and/or supra‐spinal levels. Disentangling the mechanisms of endogenous pain modulation and neuronal hyperexcitability might improve mechanism‐based treatment of neuropathic pain in subjects with spinal cord injury.
1. INTRODUCTION
Central neuropathic pain (NP) is a severe and debilitating consequence of spinal cord injury (SCI). Although the underlying mechanisms of NP are not fully understood, deficient descending pain inhibition and enhanced neuronal excitability are discussed as potential key contributors (Yarnitsky et al., 2014). Sophisticated phenotyping in subjects with NP after SCI (SCI‐NP) might help to disentangle decreased anti‐ and increased pro‐nociceptive mechanisms.
Conditioned pain modulation (CPM) paradigms are used to assess the descending pain modulatory system and quantify the net sum of pain inhibition or facilitation in humans (Yarnitsky, 2010). CPM investigates the effect of a heterotopic noxious conditioning stimulus on a test stimulus, for example, pain thresholds or supra‐threshold stimuli. Inhibitory CPM capacity is represented as increased pain thresholds or decreased pain ratings to supra‐threshold stimuli. An overall lack of inhibitory capacity has been reported in different pain conditions (systematically reviewed in Lewis et al., 2012) including SCI‐NP (Albu et al., 2015; Gruener et al., 2016). These two studies in SCI‐NP reported deficient inhibition of supra‐threshold noxious stimuli applied above the lesion level, indicating widespread spinal and/or supra‐spinal sensitization. In contrast, two more recent studies found intact CPM capacity in subjects with NP after SCI (SCI‐NP; Gagne et al., 2020; Gruener et al., 2020). To improve comparability across studies, expert recommendations for CPM paradigms were introduced (Yarnitsky et al., 2015). Here, we used one of the most commonly used test stimuli, that is, pressure pain threshold (PPT; Nuwailati et al., 2020), and a cold water bath as a conditioning stimulus. Seminal studies used a sham condition (e.g. lukewarm water bath) to reduce repeated stimulation bias (Granot et al., 2008; Kennedy et al., 2020) and a review by Yarnitsky et al. (2015) recommended using a sequential rather than a parallel CPM design due to the distraction bias. Hence, our first goal was to investigate the CPM capacity above the level of lesion in SCI‐NP following state‐of‐the‐art recommendations investigating PPT changes using a sham‐controlled CPM paradigm. Several studies reported an association of CPM capacity with NP characteristics, for example, less inhibitory CPM capacity correlated with higher spontaneous burning pain intensity in SCI (Albu et al., 2015), as well as with higher pain intensities in subjects with postherpetic neuralgia (Pickering et al., 2014) and chemotherapy‐induced peripheral neuropathy (Nahman‐Averbuch et al., 2011). Thus, the relationship between pain modulatory capacity and spontaneous NP characteristics, for example, intensity and spatial extent, was explored.
Next to CPM as an assessment of anti‐nociceptive processes, we examined temporal summation of pain (TSP) as a proxy of neuronal hyperexcitability reflecting a pro‐nociceptive mechanism. TSP is considered the psychophysical correlate of the pre‐clinical wind‐up phenomenon in human (Ren, 1994), and describes increased pain perception in response to repeated or prolonged noxious stimulation (Price & Dubner, 1977). Previous studies in subjects with SCI used TSP protocols consisting of repetitive phasic (Defrin et al., 2001; Eide et al., 1996; Gruener et al., 2020; Konopka et al., 2012) or tonic (Albu et al., 2015; Gruener et al., 2016, 2020; Scheuren et al., 2019) noxious stimulation. Some of these studies reported increased TSP in SCI‐NP (Albu et al., 2015; Gruener et al., 2016), whereas others showed no difference in TSP between SCI‐NP and HC (Gruener et al., 2020; Scheuren et al., 2019). Our second goal was to investigate TSP in SCI‐NP using repetitive pressure pain stimuli applied above the level of lesion to identify possible widespread nociceptive hyperexcitability reflecting a pro‐nociceptive mechanism.
2. METHODS
2.1. Subjects
This study was carried out in 34 subjects including a group of subjects with chronic NP after SCI (n = 17) as well as a group of age‐ and sex‐matched HC (n = 17). SCI‐NP subjects were recruited from the Spinal Cord Injury Center at Balgrist University Hospital and the Swiss Spinal Cord Injury Cohort Study database, while HC were recruited via online flyer advertisements. Inclusion criteria for the SCI cohort were a thoracolumbar SCI for at least 1 year and the presence of NP according to the current diagnostic criteria (Finnerup et al., 2016). Exclusion criteria for the SCI cohort were neurological disorders other than SCI, psychiatric or cognitive conditions interfering with the study, and pregnancy. Exclusion criteria for HC comprised of pregnancy, any history or signs of a neurological condition, history of a psychiatric condition, any acute or chronic pain condition, as well as chronic medication intake (except contraceptives). All subjects provided written informed consent and all procedures were in accordance with the Declaration of Helsinki. The study was approved by the local ethics board ‘Kantonale Ethikkomission Zürich, KEK’ (ref.number: EK‐04/2006, PB_2016–02051, clinicaltrials.gov: NCT02138344).
2.2. Clinical assessment and pain phenotyping
The clinical assessment started with sensory integrity testing of the hand, an area above the level of lesion where CPM and TSP paradigms were applied. Light touch, pinprick and vibration (64 Hz Rydel‐Seiffer tuning fork), as well as thermal stimulation using 25°C and 40°C thermorollers (Somedic, Hörby, Sweden) were applied to the hand contralateral to the most painful site in SCI‐NP subjects. For HC, the assessment site was identical to the respective SCI‐NP match. According to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), light touch and pinprick scores of 2 were classified as intact sensory function, together with vibration sensation of >6/8 and correct discrimination of cold and warm thermorollers.
In addition, a set of questionnaires was used to assess potential confounding effects of pain catastrophizing, anxiety and depression on pain experience. The subjects were asked to fill out electronic versions of the Pain Catastrophizing Scale (PCS; Sullivan et al., 1996) and the Hospital Anxiety Depression Scale (HADS; Zigmond & Snaith, 1983).
For SCI‐NP subjects, the American Spinal Injury Association Impairment Scale (AIS) grade (Marino et al., 2003) and the neurological level of injury (Kirshblum et al., 2011) were determined based on a recently performed standard neurological examination. Presence of cold and dynamical mechanical allodynia were tested in all painful body sites with a 25°C thermoroller (Somedic, Hörby, Sweden), or brush and Q‐tip, respectively. The quantification of NP extent was performed via pain drawings on two standardized body charts (frontal and dorsal view) printed in DIN‐A4 format (Rosner et al., 2021). The current spontaneous NP was shaded and further characterized by (a) verbal descriptors (hot, burning, shooting, piercing, stinging, stabbing, sharp, throbbing, cramping) and (b) pain intensity (numeric rating scale, NRS; ‘0’ = no pain, ‘10’ = worst pain imaginable). The experimenter manually outlined the borders of the pain areas. After digitalization, the spatial pain extent was quantified as the sum of the pixel counts on both pain drawings and reported as the percentage of total body area (Rosner et al., 2021). At‐ and below‐level NP was differentiated. While At‐level pain was defined by its presence within one dermatome rostral and three dermatomes caudal to the neurological level of injury (NLI), below‐level NP below three dermatomes caudal to the NLI (Bryce et al., 2012).
2.3. Conditioned pain modulation
The CPM protocol is illustrated in Figure 1. All involved testers underwent and official QST training and were certified by the German Research Network on Neuropathic Pain (DFNS). An analogue pressure algometer (FDN100, Wagner Instruments) with a circular silicone rubber tip (1 cm diameter) was used to assess the PPT at the thenar eminence. The subject placed his/her hand next to the hip to have relaxed arm and hand muscles. During PPT assessment the hand was supported and stabilized by the testers non‐dominant hand. The PPT was recorded using the method of limits with an increase of 0.5 kg/s. This rate of pressure application was practiced beforehand and double‐checked on the algometer scale. After this pre‐assessment of the PPT, the conditioning stimulus was applied by immersion of the contralateral hand up to the wrist for 2 min into a cold (9°C) or lukewarm (32°C) water bath, that is, verum and sham conditioning, respectively. The order of the 9°C cold and the 32°C sham water bath was randomized, and the water baths were separated by at least 20 min. In the circumstance of removing the hand from the cold water bath, a minimal immersion time of 60 s was defined for including in the data analysis (Yarnitsky et al., 2015). The PPT was assessed during the water bath (parallel design, after 30 s of exposure) and directly after the water bath (sequential design). The magnitude of CPM was calculated using two approaches. First, the effect of the cold water bath was calculated as the PPT change during and after the noxious conditioning in comparison with the pre‐assessment (absolute difference and normalized in percentage). Additionally, the PPT changes elicited by the sham water bath were subtracted from the cold water bath effects, resulting in sham‐controlled CPM values. Here, pain inhibition is presented as negative CPM values reflecting anti‐nociceptive mechanisms, whereas positive values depict pro‐nociceptive mechanisms, that is, pain facilitation.
FIGURE 1.
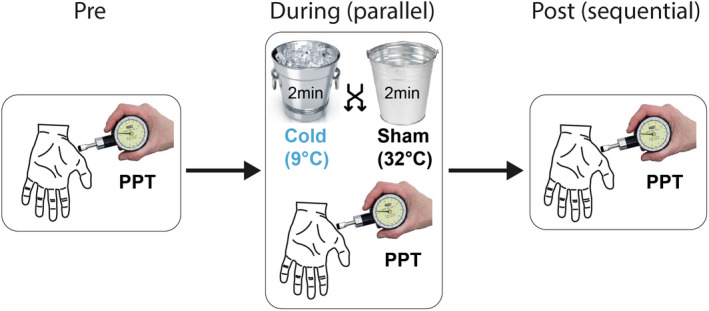
Conditioned pain modulation (CPM) paradigm. Modulation of the pressure pain threshold (PPT) was performed using a sequential and parallel CPM paradigm. PPT was assessed before, during and after the 2‐min conditioning stimuli, that is, either a cold water bath (9°C) or a sham bath (32°C).
2.4. Temporal summation of pain
The assessment of TSP was always performed after the PPT assessment and before the water bath. The same pressure algometer as described above was used to apply repetitive pressure pain stimuli at the thenar eminence. First, the individual pressure stimulation intensity eliciting a pain rating of NRS 4 out of 10 was assessed using an adapted staircase method. Pressure stimuli of 1 s duration were applied starting at an intensity 2 kg above the PPT. Depending on the subject's pain rating, pressure stimulus intensity was increased or decreased until NRS 4/10 was reached. A maximum of four staircase stimuli were applied to reduce sensitization. The TSP protocol consisted of 12 successive stimuli at NRS‐4 applied with a frequency of 0.33 Hz, and the subjects were instructed to verbally rate every stimulus during the inter‐stimulus interval. The magnitude of TSP was calculated as the mean of the last three (stimuli 10–12) normalized in percentage to the mean of the first three pain ratings. Positive values reflect pain summation/facilitation as a pro‐nociceptive mechanism, while negative values reflect pain habituation/inhibition.
2.5. Data analysis and statistics
TSP was only analysed if the repetitive pressure pain stimuli were tolerated, and the analysis of the CPM capacity was dependent on obtaining the PPT values within the safety limit of 10 kg stimulation intensity.
The procedure to calculate the cut‐offs for meaningful CPM and TSP was defined based on a previous publication from Locke et al. (2014) who calculated the standard error of measurement (SEM) using the following formula:
Hence, we calculated the standard deviation (SD) and intraclass correlation coefficient (ICC, two‐way, absolute agreement, single rater/measurement) for CPM based on both pre‐assessment PPTs (cold and sham condition) in HC (Locke et al., 2014). The same calculations were performed for the mean of the first three pain ratings in the TSP protocol (repeated TSP protocol as part of a larger study). The resulting SEM was added to the mean value and converted to a percentage change. Subjects with a change larger than the standard error of measurement were classified as responders, that is, inhibitory or facilitators, whereas subjects with smaller changes were classified as non‐responders (Locke et al., 2014; Vaegter et al., 2018).
Additionally, the SCI‐NP study sample was subdivided into an anti‐nociceptive group and a partial/fully pro‐nociceptive group to further characterize the pain cohort. Subjects of the anti‐nociceptive group showed a meaningful inhibition (responders) in CPM as well as a TSP value below the median. In contrast, subjects of the pro‐nociceptive group showed either a facilitatory or non‐meaningful CPM (non‐responder) and/or a pronounced TSP (above the median).
Statistical analyses were performed using RStudio (version 4.0.4 for windows), with p < 0.05 considered statistically significant. Mann–Whitney U tests (non‐parametric) were performed to compare the subject characteristics between the groups as well as pain characteristics between the anti‐ and pro‐nociceptive subgroups. Normal distribution of the data was tested using the Shapiro–Wilk test and histograms. CPM data were found to be not normally distributed. Therefore, the statistical analyses were performed using one‐sample Wilcoxon signed rank tests against zero to assess effects on a group level, and Mann–Whitney U tests to assess group differences. TSP data were found to be normally distributed. Therefore, one‐sample t‐tests against zero were used for group level analysis, and unpaired t‐tests to assess group differences. Further, Spearman correlations were performed to investigate the association of CPM and TSP magnitudes with NP characteristics, that is, intensity and extent of spontaneous NP.
3. RESULTS
3.1. Subjects
One SCI‐NP subject had to be excluded from the analysis due to clinical evidence of a concomitant polyneuropathy above the level of lesion. This resulted in a final sample of 33 subjects (SCI‐NP: n = 16, HC: n = 17). A total of eight SCI‐NP subjects were under analgesic medication, including antiepileptics, antidepressants, opioids, cannabinoids and non‐steroidal anti‐inflammatory drugs. Table 1 lists the subject characteristics including demographics, SCI‐related and pain‐related attributes. There was neither a difference in age (p = 0.227) nor in the HADS subscores for anxiety (p = 0.467) or depression (p = 0.054) between SCI‐NP and HC. However, higher PCS scores for SCI‐NP compared with HC were revealed (p = 0.010). Importantly, all subjects presented with intact sensory scores, that is, light touch, pinprick, vibration and thermal testing, at the hand.
TABLE 1.
Subject characteristics. Demographics, SCI and neuropathic pain characteristics are reported in mean and standard deviation, as well as full range for pain characteristics
| SCI‐NP | HC | p value | |
|---|---|---|---|
| Gender (f/m) | 2/14 | 4/13 | |
| Age (y) | 56.2 ± 9.4 | 48.9 ± 15.9 | 0.227 |
| PCS (score) | 13.1 ± 8.3 | 6.1 ± 7.5 | 0.010* |
| HADS – Anxiety (score) | 4.2 ± 3.4 | 3.5 ± 2.8 | 0.467 |
| HADS – Depression (score) | 4.5 ± 3.8 | 2.2 ± 2.4 | 0.054 |
| SCI characteristics | |||
| Time since injury (y) | 17.6 ± 8.9 | — | |
| AIS grade | 8 A, 1 B, 1 C, 6 D | — | |
| NLI | Th1‐L3 | — | |
| Aetiology (traumatic/nontraumatic) | 11/5 | — | |
| Pain characteristics | |||
| Current pain intensity (NRS) | 4.3 ± 1.8 (2–7) | — | |
| Pain extent (% of body area) | 13.6 ± 10.3 (2.4–38.7) | — | |
| At‐level pain (yes/no) | 9/7 | — | |
| Below‐level pain (yes/no) | 16/0 | — | |
| Allodynia (yes/no) | 3/13 | — | |
Note: Significance levels are reported for the comparison of SCI‐NP and HC as * for p < 0.10.
Abbreviations: AIS A, sensorimotor complete SCI; AIS B, sensory incomplete SCI; AIS C‐D, sensorimotor incomplete SCI; AIS, American Spinal Injury Association Impairment Scale; HADS, Hospital Anxiety and Depression Scale; HC, healthy controls; NLI, neurological level of injury; PCS, Pain Catastrophizing Scale; SCI, spinal cord injury.
3.2. Conditioned pain modulation
The conditioning stimulus elicited moderate to severe pain (NRS 8 [6–9]) and was tolerated by all subjects except for one HC and one SCI‐NP subject withdrawing the hand after 60 s of the cold water bath. The groups did not differ in terms of pain ratings of the conditioning stimulus (HC: NRS 7.4 ± 2.2, SCI‐NP: NRS 7.0 ± 3.0, p = 0.655, unpaired t‐test). PPT from one SCI‐NP subject during the cold water bath was not recorded due to timing issues. No significant group differences were found for PPT before conditioning (pre‐assessment; HC: 4.4 kg [3.0–4.9 kg], SCI‐NP: 4.4 kg [3.2–6.4 kg], p = 0.843). The ICC for PPT testing in HC was good to excellent (0.89), which resulted in a meaningful CPM capacity at ±10.5% from the pre‐assessment. The CPM data is illustrated in Figures 2 and 3 for the time points during (parallel design) and post water bath (sequential design), respectively. Table 2 shows the raw values for all different CPM test designs.
FIGURE 2.
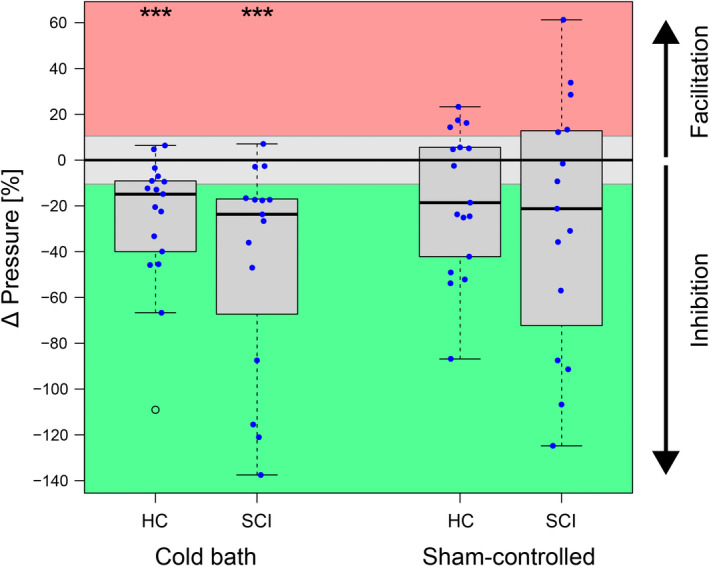
Conditioned pain modulation (CPM) capacity assessed during the conditioning stimulus (parallel design). The CPM capacity is presented as the change in pressure pain threshold during conditioning. Green represents meaningful inhibition, grey reflects no meaningful change, and red stands for meaningful facilitation. Left: CPM effects conditioned using a cold water bath (9°C for 2 min). Right: Sham‐controlled CPM capacity (effect elicited by 32°C sham water bath subtracted from effect elicited by 9°C cold water bath). Abbreviations: HC, healthy controls; SCI, spinal cord injury.
FIGURE 3.
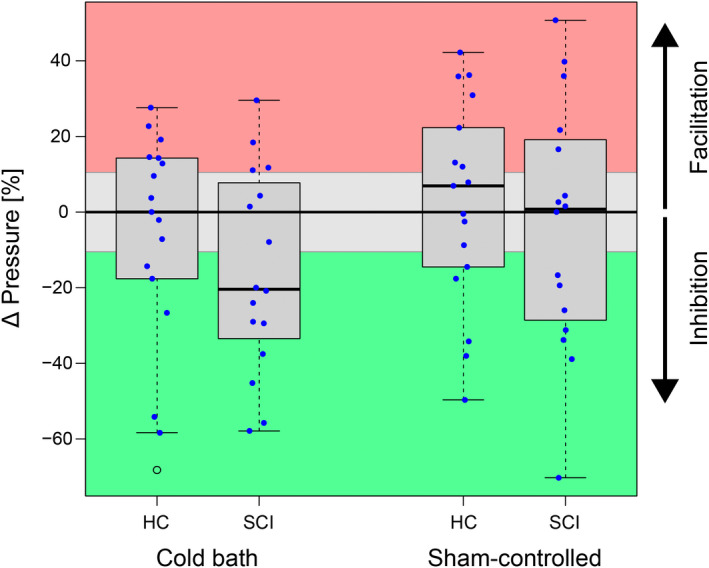
Conditioned pain modulation (CPM) capacity assessed after the conditioning stimulus (sequential design). The CPM capacity is presented as the change in pressure pain threshold after conditioning. Green represents meaningful inhibition, grey reflects no meaningful change, and red stands for meaningful facilitation. Left: CPM effects conditioned using a cold water bath (9°C for 2 min). Right: Sham‐controlled CPM capacity (effect elicited by 32°C sham water bath subtracted from effect elicited by 9°C cold water bath). Abbreviations: HC, healthy controls; SCI, spinal cord injury.
TABLE 2.
Raw values of conditioned pain modulation (CPM) capacity on pressure pain thresholds (PPT). PPTs for the three time points, pre, during and post water bath, are reported along with the CPM magnitudes for both parallel and sequential design
| Cold bath | Sham bath | Sham‐controlled | |||||
|---|---|---|---|---|---|---|---|
| HC | SCI‐NP | HC | SCI‐NP | HC | SCI‐NP | ||
| Pre (kg) | 4.4 (3.0; 4.9) | 4.4 (3.2; 6.4) | 4.3 (3.2; 5.4) | 5.4 (2.9; 6.0) | — | — | |
| Parallel design | During (kg) | 5.2 (4.0; 6.4) | 5.7 (4.5; 7.0) | 4.6 (3.1; 5.4) | 4.9 (3.6; 7.7) | — | — |
| Change (kg) | −0.8** (−1.2; −0.5) | −0.9*** (−1.9; −0.5) | −0.4* (−0.7; 0.1) | −0.8* (−1.4; 0.2) | −0.5 (−1.5; 0.4) | −1.0 (−1.8; 0.6) | |
| Sequential design | Post (kg) | 4.0 (3.7; 5.1) | 4.7 (3.0; 6.4) | 4.3 (3.6; 5.6) | 4.6 (3.7; 7.2) | — | — |
| Change (kg) | 0.0 (−0.6; 0.7) | −0.6 (−1.3; 0.2) | −0.4 (−0.9; −0.1) | −0.5 (−1.3; 0.1) | 0.3 (−0.5; 0.9) | 0.0 (−0.8; 0.7) | |
Note: All values are reported as median and inter‐quartile range. Negative changes depict inhibition, positive changes depict facilitation. Significance levels are reported for the t‐test against zero as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
Abbreviations: CPM, conditioned pain modulation; HC, healthy controls; SCI, spinal cord injury.
There was a significant inhibitory CPM effect on PPTs during noxious conditioning in HC (p < 0.001, 11 inhibitors, 6 non‐responders) and in SCI‐NP (p < 0.001, 12 inhibitors, 3 non‐responders, 1 NA). However, no difference in CPM capacity between SCI‐NP and HC was found (p = 0.295). For sham‐controlled CPM, no significant inhibition of PPTs was seen for HC (p = 0.057, 9 inhibitors, 4 non‐responders, 4 facilitators) nor for SCI‐NP (p = 0.121, 8 inhibitors, 2 non‐responders, 5 facilitators, 1 NA). In addition, no difference between the two groups was detected (p = 0.655). In addition, the randomization into cold or sham condition first did not lead to different CPM effects (cold bath first −28.5% ± 32.8%, sham bath first −18.8% ± 49.4%, p = 0.512).
Compared with the PPT assessment during noxious conditioning (parallel design), the assessment after noxious conditioning (sequential design) resulted in less pronounced pain modulation, that is, non‐meaningful changes of PPT seen for HC (p = 0.660, 6 inhibitors, 5 non‐responders, 6 facilitators) as well as SCI‐NP (p = 0.051, 9 inhibitors, 3 non‐responders, 4 facilitators). Again, no difference in CPM capacity between SCI‐NP and HC was seen (p = 0.292) and sham‐controlled CPM effects were non‐significant for both HC (p = 0.747, 5 inhibitors, 5 non‐responders, 7 facilitators) and SCI‐NP (p = 0.798, 7 inhibitors, 4 non‐responders, 5 facilitators). No difference between the two groups was found for sham‐controlled sequential CPM (p = 0.606).
3.3. Temporal summation of pain
Pain rating increases during the TSP paradigm are shown in Figure 4. Start pain ratings (first 3 averaged) of pressure stimuli were NRS 3.4 ± 1.1 for HC and NRS 3.7 ± 1.2 for SCI‐NP (p = 0.422), which increased to NRS 4.7 ± 1.8 for HC and NRS 4.5 ± 1.6 for SCI‐NP (p = 0.775) at the end of the stimulus train (last 3 averaged). The ICC for pressure pain ratings in HC was moderate (0.53), which resulted in a meaningful TSP effect of ±15.7%. The recorded pain ratings reflect pronounced TSP for HC (p < 0.001, +41.7% ± 38.3%) and for SCI‐NP (p = 0.005, +22.8% ± 28.0%), but no group difference was observed (p = 0.114). The HC group consisted of 12 facilitators, 4 non‐responders and 1 inhibitor, whereas the SCI‐NP group included 9 facilitators, 6 non‐responders, and 1 inhibitor.
FIGURE 4.
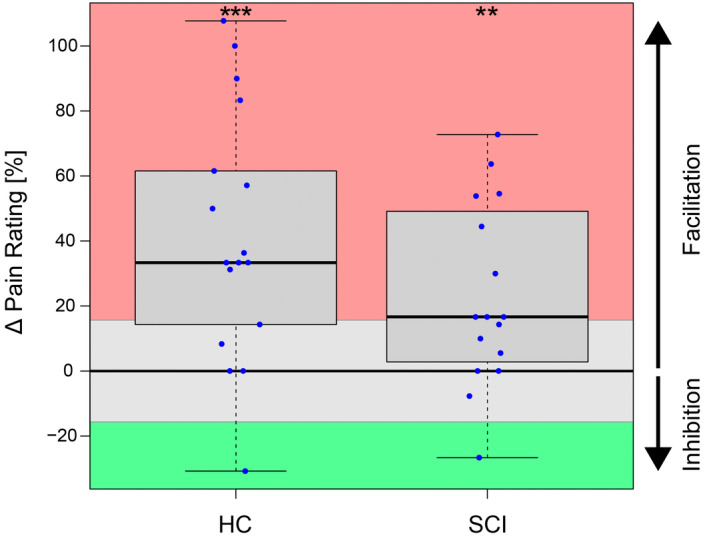
Magnitude of temporal summation of pain (TSP). The TSP magnitude is presented as the change in pain ratings from the start of the stimulus train (first 3 averaged) to the end of the stimulus train (last 3 averaged). Red represents meaningful summation/facilitation, grey reflects no meaningful change, and green stands for meaningful inhibition. Significance levels are reported as **for p < 0.01 and ***for p < 0.001. Abbreviations: HC, healthy controls; SCI, spinal cord injury.
3.4. Association of anti‐ and pro‐nociceptive mechanisms with neuropathic pain characteristics
Correlations of CPM magnitude and spontaneous NP characteristics, that is, intensity and spatial extent, are shown in Figure 5. Interestingly, the sham‐controlled CPM magnitude (parallel design) correlated with NP intensity (rho = 0.609, p = 0.021), but not with NP extent (rho = −0.221, p = 0.427). NP intensity also correlated with CPM magnitude during cold bath only (rho = 0.690, p = 0.006). This highlights that the lower the NP intensity, the more inhibitory the CPM capacity, and the higher the NP intensity, the more facilitatory or deficient the CPM capacity. In contrast, the TSP magnitude neither correlated with the intensity (rho = 0.175, p = 0.533) nor the extent of NP (rho = −0.080, p = 0.769).
FIGURE 5.
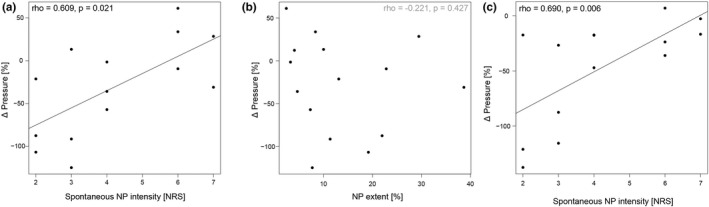
Correlation of conditioned pain modulation (CPM) capacity with neuropathic pain characteristics. The sham‐controlled CPM capacity (effect by the sham water bath subtracted from effect by the cold water bath) is presented as the change in pressure pain thresholds in percent in the parallel CPM paradigm. Negative changes depict inhibition, positive changes depict facilitation. (a) Positive correlation of CPM magnitude with spontaneous neuropathic pain intensity. (b) No significant correlation of CPM magnitude with neuropathic pain extent. (c) Positive correlation of the CPM magnitude during the cold bath only with spontaneous neuropathic pain intensity. Abbreviation: NRS, numeric rating scale.
The SCI‐NP subgroup in a pro‐nociceptive state (n = 8) showed higher NP intensity (NRS 5.3 ± 1.6, p = 0.036) than the subgroup in the anti‐nociceptive state (n = 3, NRS 2.7 ± 0.6). In contrast, NP extent was not different between the two subgroups (p = 0.776; 14.7% ± 13.8% for pro‐, and 13.7% ± 7.4% for anti‐nociceptive group).
4. DISCUSSION
Overall, this study showed no difference in above‐level CPM capacity and TSP between SCI‐NP and HC, but lends further support to a spectrum of anti‐ and pro‐nociceptive mechanisms in both cohorts. Importantly, the association of reduced inhibitory CPM capacity with higher NP intensity emphasizes a potential role of malfunctioning endogenous pain inhibition in subjects with severe NP after SCI.
4.1. The role of conditioned pain modulation paradigms—from pain inhibition to facilitation
The CPM results in the HC cohort underline several important aspects of appropriate study design selection. The findings from our parallel CPM design showing significant pain inhibition during noxious conditioning are in line with reference values from healthy subjects based on a similar paradigm (PPT during cold water immersion; Schliessbach et al., 2019). Nevertheless, around 10% of our HC revealed no inhibition during the parallel CPM paradigm and their PPT changes were found to lie within the standard error of measurement, that is, non‐responders. Although the CPM paradigm was mainly reported to examine descending inhibition, recent reports highlight the spectrum from inhibitory to facilitatory CPM effects in HC (Potvin & Marchand, 2016; Schliessbach et al., 2019). This spectrum of CPM capacity is likely to represent the balance between anti‐ and pro‐nociceptive mechanisms. A recent study highlighted this spread of nociceptive phenotypes from inhibition to facilitation in HC by using an approach of contrasting noxious conditioning to innocuous conditioning, that is, sham‐controlled design, (Huynh et al., 2021). With around two thirds of their study cohort being classified as inhibitors and one third being facilitators, our data revealed similar proportions. Such an observed spread might prevent to reveal overall differences in CPM between pain cohorts and HC and begs the question of how inhibitors and facilitators can be generally distinguished or further characterized. For example, a recent study reported that grey matter volume and resting state connectivity of particular brain regions partially explains the variability in CPM capacity in HC (Huynh et al., 2021).
In contrast to the parallel design, the noxious conditioning effect in the sequential CPM design was non‐significant for HC as well as for SCI‐NP. In general, CPM recommendations suggest performing sequential CPM paradigms, since parallel designs are more likely to be biased by distraction (Do et al., 2020; Nir & Yarnitsky, 2015; Yarnitsky, 2015). In addition, parallel CPM effects are more susceptible to cold water‐induced blood pressure increases which have been discussed to induce analgesic effects (Chalaye et al., 2013; Nir & Yarnitsky, 2015). Then again, it is known that inhibitory effects after ceasing of the conditioning stimulus are smaller compared with during conditioning and rather short‐lived (Yarnitsky et al., 2015). Despite the achievement of a moderate to severe pain intensity during the cold water bath (NRS 8 [6–9]) as recommended (Nir et al., 2011), and the PPT testing performed within 3.5 min after conditioning (Coulombe‐Leveque et al., 2021; Yarnitsky et al., 2015), our CPM effects in the sequential design were marginal. In addition, the sham‐controlled CPM effects were even smaller for both, the parallel and sequential paradigms. This can be mainly attributed to the fact that also the sham conditioning using lukewarm water led to pain inhibition. Although this might be somewhat surprising, there are a few potential explanations for this observation which at the same time pose important arguments for future inclusion of sham conditions into CPM paradigms. First, repeated application of the test stimulus is subject to a measurement error and Kennedy and colleagues showed that a sham condition can reveal this issue of repeated measurement error of the test stimulus. Second, the descending pain modulatory system is not only activated by painful stimuli but also attention/distraction effects play an important role (e.g. review by Moont et al., 2010; Villemure & Bushnell, 2002) and a distracting effect of immersing the hand into a lukewarm water bath is conceivable. Third, PPT modulation during sham condition may indicate a potential residual habituation from repeated application of the stimulus. For all of these reasons, sham conditions are an essential part of state‐of‐the‐art CPM paradigms (Granot et al., 2008; Kennedy et al., 2020) and enable to determine the real change in CPM beyond repeated measurement errors and potential distraction bias.
4.2. Severe neuropathic pain relates to impaired endogenous pain modulation
Independent of the applied CPM paradigm (noxious or sham‐controlled, parallel or sequential designs) no difference in CPM capacity between SCI‐NP and HC was found. Our study is on one hand in line with the study by Gagné and colleagues (2020) reporting an intact inhibitory CPM capacity even in chronic SCI‐NP subjects and with Gruener et al. (2020) revealing no difference in CPM capacity between SCI subjects who develop NP, those who do not develop NP, and HC. Both of these studies investigated CPM above the level of lesion to determine widespread central sensitization in SCI‐NP. On the other hand, our findings are in disagreement with two other studies performed in SCI showing a lack of CPM in SCI‐NP compared with SCI‐nonNP and HC (Albu et al., 2015; Gruener et al., 2016). Methodological differences might account for these inconsistent findings, mainly with regards to testing site in SCI‐NP, intensity and modality of the conditioning as well as test stimuli. Specifically, these two studies used thermal conditioning and test stimuli, whereas we examined a mechanical test stimulus with thermal conditioning. For example, a methodological comparison was performed by Nahman‐Averbuch et al. (2013), applying heat and mechanical test stimuli at pain threshold and supra‐threshold pain intensities. This study revealed the highest CPM responder rate (inhibitors) using PPT as the test stimulus. Further, Kovacevic et al. (2021) reported higher ICC for the CPM effect assessed with PPT compared with heat pain thresholds. These two studies endorse the usage of PPT as the test stimulus.
Interestingly, the sham‐controlled CPM capacity (parallel design) was positively correlated with NP intensity. This finding indicates that those subjects with a lack of descending pain inhibition (i.e. less negative CPM effects) reported more severe NP intensity. Herewith, we substantiate another study in SCI‐NP reporting an association of deficient CPM capacity and higher spontaneous burning pain intensity (Albu et al., 2015). Additionally, deficient CPM capacity has also been shown to correlate with higher pain intensities in subjects with postherpetic neuralgia (Pickering et al., 2014) and chemotherapy‐induced peripheral neuropathy (Nahman‐Averbuch et al., 2011). Taken together, our findings support the potential role of deficient anti‐nociceptive processes, for example, lack of descending inhibition, in severe chronic NP. However, if the endogenous pain modulatory capacity is a cause or consequence of chronic NP cannot be conclusively disentangled with cross‐sectional study designs. In this regard, future longitudinal studies in SCI‐NP are warranted. Interestingly, a study reporting improvements in CPM capacity after surgical relief of painful osteoarthritis hints towards a pain‐induced origin of deficient CPM capacity (Kosek & Ordeberg, 2000). In contrast, acute pain‐free SCI subjects who eventually developed NP presented with less efficient at‐level CPM capacities at this early stage compared with SCI subjects who remained pain‐free (Gruener et al., 2020). The latter study infers a predictive value of early at‐level CPM capacity regarding the risk for NP development, although, above‐level CPM capacity revealed neither a group difference nor a predictive value.
4.3. No elevated temporal summation of pain in spinal cord injury–neuropathic pain
Our results highlighted a pronounced TSP in HC and SCI‐NP, whereby no group differences were found. In SCI‐NP, most TSP paradigms are applied directly within painful body sites at or below the lesion level in studies reporting enhanced TSP in SCI‐NP compared with control groups (Defrin et al., 2001; Eide et al., 1996; Konopka et al., 2012). Similar results are described for other chronic pain conditions, for example, painful knee osteoarthritis (Arendt‐Nielsen et al., 2010) and fibromyalgia (Staud et al., 2001, 2003, 2014). However, investigation of sensory intact sites above the lesion level might provide insights into widespread spinal or supra‐spinal sensitization processes (Carlton et al., 2009). Here, our findings are not in line with the study by Gruener et al. (2016) reporting higher TSP above the lesion level in SCI‐NP compared with SCI without NP and HC. One possible explanation for this discrepancy might be the fundamentally different TSP protocol used: Gruener and colleagues included a single tonic heat stimulus applied to the whole hand (likely leading to extensive spatial pain summation), while we applied repetitive focal mechanical stimulation to the thenar eminence. Another methodological difference concerns the usage of test stimuli to activate deep (algometry) versus superficial nociceptive fibres (heat stimulation). Repetitive activation of deep nociceptive fibres has been shown to be a reliable measure (Cathcart et al., 2009).
The lack of an association between TSP magnitude and NP intensity is in line with a study by Granovsky et al. (2017) and again in contrast to Gruener et al. (2016). The latter study discusses the association of TSP with central NP intensity as a result of either hyperactive ascending tracts and/or “pain generators” in the thalamus and cortex. Further studies are warranted consolidating these working hypotheses.
4.4. Anti‐ and pro‐nociceptive states relate to neuropathic pain intensity
The overall nociceptive state including both anti‐ and pro‐nociceptive aspects as investigated by CPM and TSP protocols has previously been termed “pain modulation profile” (Yarnitsky et al., 2014). We were able to show that subjects in an anti‐nociceptive state, that is, meaningful CPM inhibition and low TSP magnitude, suffered from less intense NP than subjects in a partial or full pro‐nociceptive state. In general, chronic pain cohorts have mostly been positioned on the pro‐nociceptive side of the pain modulation profile which was accompanied by a more severe pain phenotype (Granovsky & Yarnitsky, 2013). Thus, identifying individual pain modulation profiles in acute pain patients, for example, anti‐ versus pro‐nociceptive processes, might have a valuable implication in the prediction of chronic pain development and efficacy of analgesic medication targeting distinct anti‐ or pro‐nociceptive mechanisms.
5. LIMITATIONS
Although the study design for CPM and TSP are based on state‐of‐the‐art recommendations, several limitations need to be mentioned. We tested CPM and TSP by application of noxious stimuli above the lesion level, that is, hand, to investigate a systemic/widespread central sensitization and did not find any group differences for either readout. Testing these two paradigms at the level of lesion might have resulted in other findings as suggested by prior studies (Gruener et al., 2020; Vogel et al., 2017). Therefore, future investigation might benefit from a direct comparison between at‐ and above‐level CPM and TSP with the known drawbacks regarding a potential sensory loss of function at the lesion level. Furthermore, the sample size was rather small which impeded further exploration of pain modulatory profiles and missed a direct comparison with an SCI cohort without NP. Lastly, potential interference of analgesic medication with CPM and TSP was not controlled for.
6. CONCLUSION
Our results did not reveal impaired CPM or enhanced TSP in SCI‐NP compared with HC. Therefore, we claim that overall neither deficient anti‐ nor pro‐nociceptive mechanisms above the lesion level contribute to the presence of NP after SCI. However, we highlighted that a sham‐controlled CPM paradigm reveals a spectrum from pain inhibition to facilitation in both SCI‐NP and HC and provided evidence that the intensity of spontaneous NP is associated with the subject's position on this spectrum. Specifically when tested above the level of lesion, the observed reduction in descending pain inhibition in subjects suffering from severe NP might hint towards central sensitization processes at widespread spinal and/or supra‐spinal levels. Disentangling the processes of descending inhibition and neuronal hyperexcitability on a single subject level might improve pain modulation profiling and thereby facilitate personalized mechanism‐based treatment as well as patient stratification for clinical trials.
FUNDING INFORMATION
This study has been funded by the Clinical Research Priority Program of the University of Zurich (CRPP Pain).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest regarding this work.
ACKNOWLEDGEMENTS
Open access funding provided by Universitat Zurich. [Correction added on 30 November 2022, after first online publication: CSAL funding statement has been added.]
Lütolf, R. , De Schoenmacker, I. , Rosner, J. , Sirucek, L. , Schweinhardt, P. , Curt, A. , & Hubli, M. (2022). Anti‐ and Pro‐Nociceptive mechanisms in neuropathic pain after human spinal cord injury. European Journal of Pain, 26(10), 2176–2187. 10.1002/ejp.2029
REFERENCES
- Albu, S. , Gomez‐Soriano, J. , Avila‐Martin, G. , & Taylor, J. (2015). Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain, 156, 260–272. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Nie, H. , Laursen, M. B. , Laursen, B. S. , Madeleine, P. , Simonsen, O. H. , & Graven‐Nielsen, T. (2010). Sensitization in patients with painful knee osteoarthritis. Pain, 149, 573–581. [DOI] [PubMed] [Google Scholar]
- Bryce, T. N. , Biering‐Sorensen, F. , Finnerup, N. B. , Cardenas, D. D. , Defrin, R. , Lundeberg, T. , Norrbrink, C. , Richards, J. S. , Siddall, P. , Stripling, T. , Treede, R. D. , Waxman, S. G. , Widerstrom‐Noga, E. , Yezierski, R. P. , & Dijkers, M. (2012). International spinal cord injury pain classification: Part I. background and description. March 6‐7, 2009. Spinal Cord, 50, 413–417. [DOI] [PubMed] [Google Scholar]
- Carlton, S. M. , Du, J. , Tan, H. Y. , Nesic, O. , Hargett, G. L. , Bopp, A. C. , Yamani, A. , Lin, Q. , Willis, W. D. , & Hulsebosch, C. E. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain, 147, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart, S. , Winefield, A. H. , Rolan, P. , & Lushington, K. (2009). Reliability of temporal summation and diffuse noxious inhibitory control. Pain Research & Management, 14, 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaye, P. , Devoize, L. , Lafrenaye, S. , Dallel, R. , & Marchand, S. (2013). Cardiovascular influences on conditioned pain modulation. Pain, 154, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Coulombe‐Leveque, A. , Tousignant‐Laflamme, Y. , Leonard, G. , & Marchand, S. (2021). The effect of conditioning stimulus intensity on conditioned pain modulation (CPM) hypoalgesia. Canadian Journal of Pain, 5, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin, R. , Ohry, A. , Blumen, N. , & Urca, G. (2001). Characterization of chronic pain and somatosensory function in spinal cord injury subjects. Pain, 89, 253–263. [DOI] [PubMed] [Google Scholar]
- Do, A. T. L. , Enax‐Krumova, E. K. , Ozgul, O. , Eitner, L. B. , Heba, S. , Tegenthoff, M. , Maier, C. , & Hoffken, O. (2020). Distraction by a cognitive task has a higher impact on electrophysiological measures compared with conditioned pain modulation. BMC Neuroscience, 21, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide, P. K. , Jorum, E. , & Stenehjem, A. E. (1996). Somatosensory findings in patients with spinal cord injury and central dysaesthesia pain. Journal of Neurology, Neurosurgery, and Psychiatry, 60, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup, N. B. , Haroutounian, S. , Kamerman, P. , Baron, R. , Bennett, D. L. , Bouhassira, D. , Cruccu, G. , Freeman, R. , Hansson, P. , Nurmikko, T. , Raja, S. N. , Rice, A. S. , Serra, J. , Smith, B. H. , Treede, R. D. , & Jensen, T. S. (2016). Neuropathic pain: An updated grading system for research and clinical practice. Pain, 157, 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne, M. , Cote, I. , Boulet, M. , Jutzeler, C. R. , Kramer, J. L. K. , & Mercier, C. (2020). Conditioned pain modulation decreases over time in patients with neuropathic pain following a spinal cord injury. Neurorehabilitation and Neural Repair, 34, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot, M. , Weissman‐Fogel, I. , Crispel, Y. , Pud, D. , Granovsky, Y. , Sprecher, E. , & Yarnitsky, D. (2008). Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain, 136, 142–149. [DOI] [PubMed] [Google Scholar]
- Granovsky, Y. , Nahman‐Averbuch, H. , Khamaisi, M. , & Granot, M. (2017). Efficient conditioned pain modulation despite pain persistence in painful diabetic neuropathy. Pain Reports, 2, e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovsky, Y. , & Yarnitsky, D. (2013). Personalized pain medicine: The clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Medical Journal, 4, e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruener, H. , Zeilig, G. , Gaidukov, E. , Rachamim‐Katz, O. , Ringler, E. , Blumen, N. , Engel‐Haber, E. , & Defrin, R. (2020). Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: Results of a long‐term longitudinal study. Pain, 161, 545–556. [DOI] [PubMed] [Google Scholar]
- Gruener, H. , Zeilig, G. , Laufer, Y. , Blumen, N. , & Defrin, R. (2016). Differential pain modulation properties in central neuropathic pain after spinal cord injury. Pain, 157, 1415–1424. [DOI] [PubMed] [Google Scholar]
- Huynh, V. , Lutolf, R. , Rosner, J. , Luechinger, R. , Curt, A. , Kollias, S. , Michels, L. , & Hubli, M. (2021). Descending pain modulatory efficiency in healthy subjects is related to structure and resting connectivity of brain regions. NeuroImage, 247, 118742. [DOI] [PubMed] [Google Scholar]
- Kennedy, D. L. , Kemp, H. I. , Wu, C. , Ridout, D. A. , & Rice, A. S. C. (2020). Determining real change in conditioned pain modulation: A repeated measures study in healthy volunteers. The Journal of Pain, 21, 708–721. [DOI] [PubMed] [Google Scholar]
- Kirshblum, S. C. , Burns, S. P. , Biering‐Sorensen, F. , Donovan, W. , Graves, D. E. , Jha, A. , Johansen, M. , Jones, L. , Krassioukov, A. , Mulcahey, M. J. , Schmidt‐Read, M. , & Waring, W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). The Journal of Spinal Cord Medicine, 34, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, K. H. , Harbers, M. , Houghton, A. , Kortekaas, R. , van Vliet, A. , Timmerman, W. , den Boer, J. A. , Struys, M. M. , & van Wijhe, M. (2012). Somatosensory profiles but not numbers of somatosensory abnormalities of neuropathic pain patients correspond with neuropathic pain grading. PLoS One, 7, e43526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek, E. , & Ordeberg, G. (2000). Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain, 88, 69–78. [DOI] [PubMed] [Google Scholar]
- Kovacevic, M. , Klicov, L. , Vuklis, D. , Neblett, R. , & Knezevic, A. (2021). Test‐retest reliability of pressure pain threshold and heat pain threshold as test stimuli for evaluation of conditioned pain modulation. Neurophysiologie Clinique, 51, 433–442. [DOI] [PubMed] [Google Scholar]
- Lewis, G. N. , Rice, D. A. , & McNair, P. J. (2012). Conditioned pain modulation in populations with chronic pain: A systematic review and meta‐analysis. The Journal of Pain, 13, 936–944. [DOI] [PubMed] [Google Scholar]
- Locke, D. , Gibson, W. , Moss, P. , Munyard, K. , Mamotte, C. , & Wright, A. (2014). Analysis of meaningful conditioned pain modulation effect in a pain‐free adult population. The Journal of Pain, 15, 1190–1198. [DOI] [PubMed] [Google Scholar]
- Marino, R. J. , Barros, T. , Biering‐Sorensen, F. , Burns, S. P. , Donovan, W. H. , Graves, D. E. , Haak, M. , Hudson, L. M. , Priebe, M. M. , & Committee, A. N. S. (2003). International standards for neurological classification of spinal cord injury. The Journal of Spinal Cord Medicine, 26(Suppl 1), S50–S56. [DOI] [PubMed] [Google Scholar]
- Moont, R. , Pud, D. , Sprecher, E. , Sharvit, G. , & Yarnitsky, D. (2010). ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain, 150, 113–120. [DOI] [PubMed] [Google Scholar]
- Nahman‐Averbuch, H. , Yarnitsky, D. , Granovsky, Y. , Gerber, E. , Dagul, P. , & Granot, M. (2013). The role of stimulation parameters on the conditioned pain modulation response. Scandinavian Journal of Pain, 4, 10–14. [DOI] [PubMed] [Google Scholar]
- Nahman‐Averbuch, H. , Yarnitsky, D. , Granovsky, Y. , Sprecher, E. , Steiner, M. , Tzuk‐Shina, T. , & Pud, D. (2011). Pronociceptive pain modulation in patients with painful chemotherapy‐induced polyneuropathy. Journal of Pain and Symptom Management, 42, 229–238. [DOI] [PubMed] [Google Scholar]
- Nir, R. R. , Granovsky, Y. , Yarnitsky, D. , Sprecher, E. , & Granot, M. (2011). A psychophysical study of endogenous analgesia: The role of the conditioning pain in the induction and magnitude of conditioned pain modulation. European Journal of Pain, 15, 491–497. [DOI] [PubMed] [Google Scholar]
- Nir, R. R. , & Yarnitsky, D. (2015). Conditioned pain modulation. Current Opinion in Supportive and Palliative Care, 9, 131–137. [DOI] [PubMed] [Google Scholar]
- Nuwailati, R. , Curatolo, M. , LeResche, L. , Ramsay, D. S. , Spiekerman, C. , & Drangsholt, M. (2020). Reliability of the conditioned pain modulation paradigm across three anatomical sites. Scandinavian Journal of Pain, 20, 283–296. [DOI] [PubMed] [Google Scholar]
- Pickering, G. , Pereira, B. , Dufour, E. , Soule, S. , & Dubray, C. (2014). Impaired modulation of pain in patients with postherpetic neuralgia. Pain Research & Management, 19, e19–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin, S. , & Marchand, S. (2016). Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain, 157, 1704–1710. [DOI] [PubMed] [Google Scholar]
- Price, D. D. , & Dubner, R. (1977). Mechanisms of first and second pain in the peripheral and central nervous systems. The Journal of Investigative Dermatology, 69, 167–171. [DOI] [PubMed] [Google Scholar]
- Ren, K. (1994). Wind‐up and the NMDA receptor: From animal studies to humans. Pain, 59, 157–158. [DOI] [PubMed] [Google Scholar]
- Rosner, J. , Lutolf, R. , Hostettler, P. , Villiger, M. , Clijsen, R. , Hohenauer, E. , Barbero, M. , Curt, A. , & Hubli, M. (2021). Assessment of neuropathic pain after spinal cord injury using quantitative pain drawings. Spinal Cord, 59, 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuren, P. S. , Gagne, M. , Jutzeler, C. R. , Rosner, J. , Mercier, C. , & Kramer, J. L. K. (2019). Tracking changes in neuropathic pain after acute spinal cord injury. Frontiers in Neurology, 10, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliessbach, J. , Lutolf, C. , Streitberger, K. , Scaramozzino, P. , Arendt‐Nielsen, L. , & Curatolo, M. (2019). Reference values of conditioned pain modulation. Scandinavian Journal of Pain, 19, 279–286. [DOI] [PubMed] [Google Scholar]
- Staud, R. , Cannon, R. C. , Mauderli, A. P. , Robinson, M. E. , Price, D. D. , & Vierck, C. J., Jr. (2003). Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain, 102, 87–95. [DOI] [PubMed] [Google Scholar]
- Staud, R. , Vierck, C. J. , Cannon, R. L. , Mauderli, A. P. , & Price, D. D. (2001). Abnormal sensitization and temporal summation of second pain (wind‐up) in patients with fibromyalgia syndrome. Pain, 91, 165–175. [DOI] [PubMed] [Google Scholar]
- Staud, R. , Weyl, E. E. , Riley, J. L., 3rd , & Fillingim, R. B. (2014). Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS One, 9, e89086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M. B. , Scott, R. , & Pivik, J. (1996). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7, 524–532. [Google Scholar]
- Vaegter, H. B. , Petersen, K. K. , Morch, C. D. , Imai, Y. , & Arendt‐Nielsen, L. (2018). Assessment of CPM reliability: Quantification of the within‐subject reliability of 10 different protocols. Scandinavian Journal of Pain, 18, 729–737. [DOI] [PubMed] [Google Scholar]
- Villemure, C. , & Bushnell, C. M. (2002). Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain, 95, 195–199. [DOI] [PubMed] [Google Scholar]
- Vogel, C. , Rukwied, R. , Stockinger, L. , Schley, M. , Schmelz, M. , Schleinzer, W. , & Konrad, C. (2017). Functional characterization of at‐level hypersensitivity in patients with spinal cord injury. The Journal of Pain, 18, 66–78. [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. (2010). Conditioned pain modulation (the diffuse noxious inhibitory control‐like effect): Its relevance for acute and chronic pain states. Current Opinion in Anaesthesiology, 23, 611–615. [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. (2015). Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain, 156(Suppl 1), S24–S31. [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. , Bouhassira, D. , Drewes, A. M. , Fillingim, R. B. , Granot, M. , Hansson, P. , Landau, R. , Marchand, S. , Matre, D. , Nilsen, K. B. , Stubhaug, A. , Treede, R. D. , & Wilder‐Smith, O. H. (2015). Recommendations on practice of conditioned pain modulation (CPM) testing. European Journal of Pain, 19, 805–806. [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. , Granot, M. , & Granovsky, Y. (2014). Pain modulation profile and pain therapy: Between pro‐ and antinociception. Pain, 155, 663–665. [DOI] [PubMed] [Google Scholar]
- Zigmond, A. S. , & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. [DOI] [PubMed] [Google Scholar]


