Abstract
Human-monocyte-derived dendritic cells (MoDC) are very efficient in the uptake of Listeria monocytogenes, a gram-positive bacterium which is an important pathogen in humans and animals causing systemic infections with symptoms such as septicemia and meningitis. In this work, we analyzed the influence of blood plasma on the internalization of L. monocytogenes into human MoDC and compared the uptake of L. monocytogenes with that of Salmonella enterica serovar Typhimurium and Yersinia enterocolitica. While human plasma did not significantly influence the uptake of serovar Typhimurium and Y. enterocolitica by human MoDC, the efficiency of the uptake of L. monocytogenes by these phagocytes was strongly enhanced by human plasma. In plasma-free medium the internalization of L. monocytogenes was very low, whereas the addition of pooled human immunoglobulins resulted in the internalization of these bacteria to a degree comparable to the highly efficient uptake observed with human plasma. All human plasma tested contained antibodies against the 60-kDa extracellular protein of L. monocytogenes (p60), and anti-p60 antibodies were also found in the commercially available pooled immunoglobulins. Strikingly, in contrast to L. monocytogenes wild type, an iap deletion mutant (totally deficient in p60) showed only a minor difference in the uptake by human MoDC in the presence or the absence of human plasma. These results support the assumption that antibodies against the listerial p60 protein may play an important role in Fc-receptor-mediated uptake of L. monocytogenes by human MoDC via opsonization of the bacteria. This process may have a major impact in preventing systemic infection in L. monocytogenes in immunocompetent humans.
Dendritic cells (DC) are the critical antigen-presenting cells involved in an immune response against microbes (35, 36). DC exist in two functional stages. Immature DC develop from hematopoeitic precursors and are scattered throughout the body in nonlymphoid organs, where they exert sentinel functions. Upon irritation of the tissue DC take up and process antigens. Subsequently, they migrate into lymphoid organs, where maturation of the DC occurs (20, 27). In lymphoid organs they present the antigen epitopes in the context with major histocompatibility complex (MHC) molecules I or II. DC thus play a crucial role in antigen presentation and the initiation of most T-cell-mediated immune responses (2, 7, 29, 32, 41, 42).
There are several identified mechanisms of how antigens are captured by DC. Macropinocytosis is constitutively active in DC (39) and has been shown for DC of mouse, rat, and human origins (26). In addition, immature DC are extremely well equipped with antigen-binding receptors, including FCγ or FCɛ, macrophage mannose receptor, and complement receptors (2). Compared to macropinocytosis, receptor-mediated antigen uptake is more efficient for antigen presentation (2, 43) and results in DC activation (13, 33). We have previously demonstrated that human MoDC are highly competent in the uptake of L. monocytogenes (23), but the mechanism of this uptake remained unclear.
L. monocytogenes, gram-positive bacterium, is an important pathogen of humans and animals due to its capability for invasion of nonphagocytic cells and its replication in the cytosol of these cells (4, 9, 11, 14, 18, 40). A number of virulence determinants involved in the induced processes have been characterized. InlA and InlB, members of the growing family of listerial internalins, trigger the uptake of listeriae by normally nonphagocytic cell types (8, 15). The PrfA-dependent gene cluster (6, 25) present in all L. monocytogenes isolates contains the genes essential for intracellular replication and cell-to-cell spread. Of these gene products, listeriolysin, a pore-forming cytolysin, is required, along with two phospholipases (PlcA and PlcB), for the lysis of the phagosomal membranes, while ActA is involved in the active polymerization process which mediates the mobility of L. monocytogenes within the host cells cytosol. The protein p60, encoded by the gene termed iap, is a major extracellular product secreted by all isolates of L. monocytogenes. This protein has peptidoglycan hydrolase activity but also influences the uptake of L. monocytogenes by nonphagocytic cells (24). Proteins related to p60 are also found in all other Listeria species (5). It has been shown that p60 protein is among the strongest antigens in listeriae for B- and T-cell responses (16, 17).
We show here that the uptake of L. monocytogenes EGD, in contrast to Salmonella enterica serovar Typhimurium and Yersinia enterocolitica by human-monocyte-derived DC (MoDC), is strongly enhanced by human plasma and that Fc-receptor-mediated uptake of antibodies against p60 protein is crucial for this process.
MATERIALS AND METHODS
Bacteria.
All bacterial strains used in this study are described in Table 1. The bacteria were grown in brain heart infusion medium at 30°C (Y. enterocolitica) or 37°C (L. monocytogenes and Salmonella sp.) until they reached the mid-log phase of growth.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Plasmida | Resistance marker | Source or reference |
|---|---|---|---|---|
| L. monocytogenes Sv 1/2a EGD | Wild type | Institute strain collection | ||
| L. monocytogenes Sv 1/2a EGD | Δiap | S. Pilgrim et al. | ||
| L. monocytogenes Sv 1/2a EGD | Wild type | prfA∗ | Erythromycin | This work |
| L. monocytogenes Sv 1/2a EGD | Δiap | prfA∗ | Erythromycin | This work |
| L. monocytogenes DP-L2161 | Δhly | 21 | ||
| L. innocua Sv6a | Wild type | PactA-gfp | Tetracycline | Institute strain collection |
| Y. enterocolitica | Wild type | J. Heesemann | ||
| S. enterica serovar Typhimurium 14028S | Wild type | 28 | ||
| Plasmid pHPS9 | prfA∗ | Erythromycin | J. A. Vazquez-Boland |
prfA∗, Mutant prfA allele from P14-A.
Isolation of human MoDC from peripheral blood.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized leucocyte-enriched buffy coats of healthy adult donors by Lymphoprep (1.077 g/ml; Nycomed, Oslo, Norway) density gradient centrifugation at 400 × g at room temperature. PBMC were plated on tissue culture dishes (3003; Falcon Labware, Oxnard, Calif.) at a density of 5 × 106 cells/ml in RPMI 1640 medium (Gibco), supplemented with l-glutamine (2 mM), 1% autologous human plasma, and 100 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) per ml for 45 min at 37°C. Nonadherent cells were washed free with warm phosphate-buffered saline (PBS), and adherent cells were cultured for 7 days without antibiotics in RPMI 1640 medium, supplemented with 1% autologous human plasma, 2 mM l-glutamine, 1,000 U of recombinant human interleukin-4 (rhIL-4); (PBH, Hanover, Germany) and 800 U of rhGM-CSF (Leukomax; Sandoz, Basel, Switzerland) per ml. Cytokines were replenished every other day.
Phenotypic characterization of human MoDC.
Flow cytometry was used to characterize the surface marker of MoDC. Indirect immunofluorescence was performed according to standard techniques, using murine monoclonal antibodies revealed by Phycoerythrin-conjugated anti-mouse immunoglobulin (Dianova, Hamburg, Germany). The primary antibodies used were: CD1a (OKT6, Rockville, Md.), α-HLA class II DR/DQ (9.3F10) (American Type Culture Collection, Manassas, Va.), CD16 (anti-FcγRIII; clone 3G8), CD32 (anti-FcγRII, clone FLI8.26, 2003; Pharmingen, Hamburg, Germany) and CD64 (anti-FcγRI; clone 10.1; Pharmingen). The stained cells were analyzed on an EPICS XL-MCL (Coulter Immunotech Diagnostics, Krefeld, Germany).
Cellular uptake assay.
On day 7 nonadherent MoDC were collected prior to infection and transferred to new 24-well plates at a density of 5 × 105 cells/ml. MoDC were infected with logarithmically growing bacteria. After two washes with PBS, the bacteria were diluted in RPMI 1640 medium and added at the desired multiplicity of infection (MOI) to each well. The cultures were incubated in RPMI 1640 medium supplemented with different blood factors, including human autologous plasma, human heterologous AB-serum of healthy donors, human serum albumin (BRK), human immunoglobulins (complement-free) (Sandoglobin; Sandoz, Basel, Switzerland), or fetal calf serum (FCS) at 37°C for 1 h. The monolayers were washed twice with PBS. For selective removal of extracellular bacteria, 50 μg (L. monocytogenes) or 100 μg (Salmonella and Yersinia spp.) of gentamicin (Gibco) per ml was added to each well, and the plates were further incubated for 30 min at 37°C. To quantify the uptake of bacteria into MoDC, infected MoDC were centrifuged on cover slides and then visualized by Giemsa staining (see below).
For determination of CFU, the cells were washed for 30 min with PBS after the addition of gentamicin, lysed by the addition of ice-cold distilled water, and incubated for 20 min on ice.
To study the effect of human blood components on the adherence of L. monocytogenes to MoDC, bacteria were incubated with MoDC for 1 h in the presence of 2 μg of cytochalasin D per ml. Cytochalasin D inhibits the bacterial uptake. After 1 h of incubation, the cells were washed six times. The number of adherent L. monocytogenes were determined by determining to CFU.
Determination of bacteria in MoDC by light microscopy after Giemsa staining.
MoDC were infected with bacteria as described above. At different times (30 min, 1 h, 2 h, and 4 h), cells were washed, centrifuged on coverslips, fixed with methanol for 5 min, stained with Giemsa (1:20; Merck, Darmstadt, Germany) for 20 min, and then examined using a Leitz Dialux 20 microscope (oil immersion objective). The number of infected cells and the number of intracellular bacteria per 100 cells were counted in triplicate.
Uptake inhibition studies.
The uptake assay was performed and analyzed as described above, except that the media contained either 5 mg of yeast mannan (Sigma), the competitor of the mannose receptor, per ml or 2 μg of the inhibitor cytochalasin D per ml throughout the duration of the experiment.
In addition, blocking CD16 antibodies (anti-FcγRIII; 3G8) were used to inhibit FcγRIII-mediated phagocytosis in the presence of pooled immunoglobulins (5 mg/ml).
Preadsorbtion of human immunoglobulins.
Pooled human immunoglobulins were adsorbed with 1010 L. monocytogenes for 12 h at 4°C. The preadsorbed immunoglobulins were centrifuged at 6,000 rpm for 10 min at 4°C and added to the infection culture.
Transmission electron microscopy.
MoDC were infected with L. monocytogenes EGD. At 1 h postinfection the cells were washed, fixed in 2.5% glutaraldehyde, postfixed in 2% osmium tetroxide, stained with 0.5% uranyl acetate, dehydrated in graded alcohols, and finally embedded in Lowicryl K4M.
Immunoblotting.
Immunoblotting was performed to analyze Listeria, Salmonella, and Yersinia specific antibodies in human serum or plasma and pooled immunoglobulins. To analyze bacterium-specific antibodies, we used an overnight culture of L. monocytogenes EGD prfA* and a p60 mutant of L. monocytogenes prfA* strain, plus serovar Typhimurium (14028S) and Y. enterocolitica. The Listeria strains were characterized by high expression of the positive regulatory factor PrfA which controls the expression of most of listerial virulence factors.
Surface and supernatant proteins of all strains were prepared as described by Mollenkopf et al. (31) and then precipitated with 10% trichloroacetic acid (Roth) at 4°C for 1 h; proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before being transferred to nitrocellulose (Immunoblot P Membrane; Millipore). For the isolation of surface proteins in the case of L. monocytogenes, the sodium dodecyl sulfate concentration was adjusted to 1%.
The nitrocellulose filters were then blocked with 2% bovine serum albumin in Tris-buffered saline (pH 7.5) and incubated with plasma or sera from healthy individuals (diluted 1:200) or the p60-specific monoclonal mouse antibody K3A7 (38) and pooled human immunoglobulins (15 μg/ml) as controls.
Blots were developed with horseradish peroxidase-conjugated swine immunoglobulins to mouse immunoglobulins (Dako, Elstrup, Denmark) diluted 1:1,000 and horseradish peroxidase-conjugated goat anti-human immunoglobulins (IgA+IgG+IgM, H+L; Dianova, Hamburg, Germany) diluted 1:2,000. We used 4-chloro-1-naphthol (0.02% [wt/vol]) as a substrate.
Statistical analysis.
The data are presented as the means and standard deviations (as indicated by error bars) of representative experiments run at least in triplicate. For statistical comparison, the Student t test and Mann-Whitney U test were performed when appropriate. P values of <0.01 were considered statistically significant.
RESULTS
Uptake of L. monocytogenes by human MoDC is enhanced by human plasma.
Human MoDC were cultured for 7 days in RPMI 1640 medium supplemented with 1% human plasma, rhGM-CSF, and rhIL-4. The cells exhibited the characteristic MoDC morphology and the pattern of surface markers typical of immature MoDC (3, 37). Flow cytometry analysis of cell surface markers showed that more than 80% of these cells expressed CD1a and MHC class II molecules. The 20% of the cells which did not express MoDC markers were predominantly T lymphocytes. Infection experiments with L. monocytogenes and purified human T cells showed that T lymphocytes were not infected with these bacteria (data not shown).
As shown previously, immature MoDC were capable of internalizing L. monocytogenes with high efficiency (23). Electron microscopy showed that most of the intracellular bacteria were located in phago(lyso)somal vacuoles (Fig. 1B). Only a few bacteria escaped into the cytosol. However, our earlier study did not address the mechanism of the uptake of L. monocytogenes by MoDC.
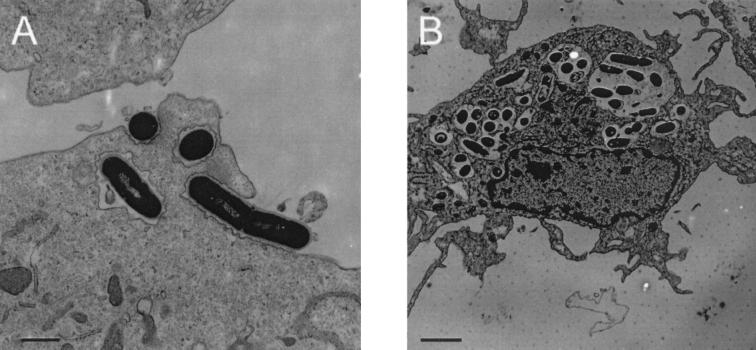
FIG. 1.
(A) Human DC internalize L. monocytogenes. Transmission electron microscopy reveals the uptake process 1 h after incubation of DC with L. monocytogenes EGD in the presence of human plasma. L. monocytogenes are covered by thin folds of plasma membrane (MOI = 50; bar size, 1.1 μm). (B) At 3 h postinfection, L. monocytogenes are located in the phagosome of human DC (MOI = 50; bar size, 2 μm).
First, using transmission electron microscopy we found (Fig. 1A) that the entry of L. monocytogenes into these MoDC occurs by gliding of the plasma membrane over the bacterium. At the position where the bacterial cell is in contact with the host cell, the intimately apposed membrane was undulated. Major morphological changes of the MoDC surface such as membrane ruffles were not observed. Light microscopic visualization of the intracellular bacteria by Giemsa staining showed that L. monocytogenes was very efficiently internalized by MoDC in the presence of 1% human plasma (Fig. 2A). At an MOI of 10 to 50, ca. 80% of the host cells were infected by one or more bacteria. Since the percentage of immature MoDC in the cell population is also about 80%, the data indicate that practically every immature MoDC has taken up L. monocytogenes. Like the L. monocytogenes EGD wild-type strain, the nonpathogenic species L. innocua and an inlA-inlB deletion mutant of L. monocytogenes (23), as well as heat- or gentamicin-killed L. monocytogenes, were effectively internalized in the presence of human plasma. No intracellular bacteria were found when MoDC were incubated at 4°C (data not shown).
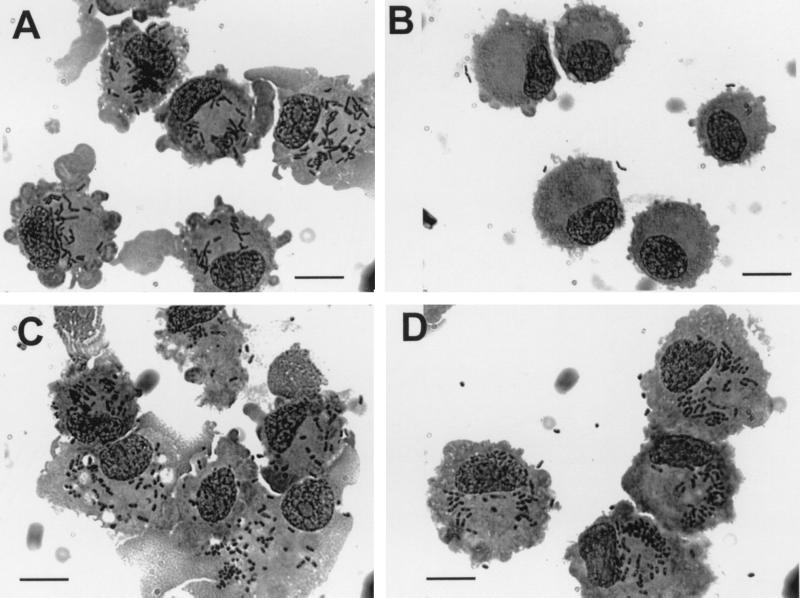
FIG. 2.
Comparison of the uptake of L. monocytogenes EGD and Y. enterocolitica in DC with (A and C) and without (B and D) the presence of human plasma (MOI = 50; L. monocytogenes EGD, upper panel; Y. enterocolitica, lower panel; Giemsa staining was used 1.5 h after the addition of bacteria). Bars, 10 μm.
In contrast to the highly efficient uptake of L. monocytogenes by MoDC in the presence of human plasma, infection carried out in RPMI 1640 alone or in RPMI 1640 supplemented with 10% FCS resulted in a strongly (20-fold) decreased internalization (Fig. 2B and 4). To test whether this difference in uptake was overcome by a longer incubation time, L. monocytoges were incubated with MoDC for 15 min, 30 min, 60 min, 2 h, and 4 h. The difference in the efficiencies of listerial uptake in the presence or absence of human serum remained, however, the same (data not shown).
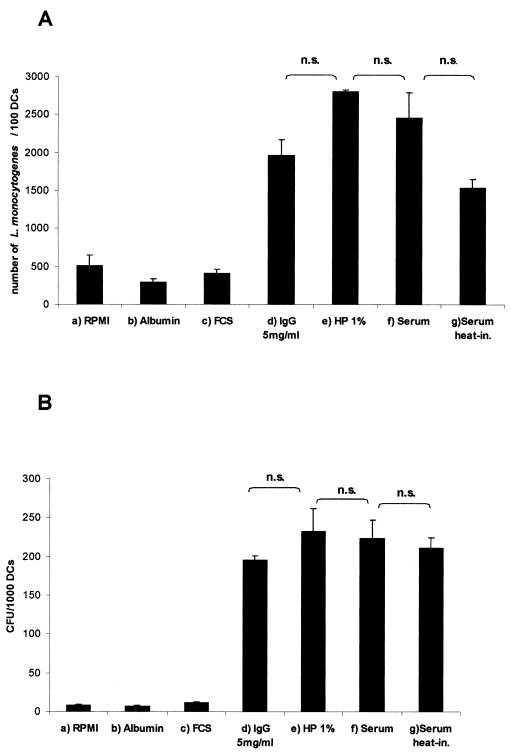
FIG. 4.
Quantification of the uptake of L. monocytogenes into DC 1.5 h after the addition of bacteria with different infection media (L. monocytogenes EGD; MOI = 50). (A) Giemsa-staining. Determination of the number of intracellular bacteria was made per 100 DC. (B) Viable bacteria were determined by counting the CFU per 1,000 lysed DC. Columns: a, RPMI 1640; b, albumin (5%); c, FCS (10%); d, pooled human immunoglobulins (5 mg/ml); e, autologous human plasma (HP; 1%); f, heterologous AB-serum (5%); g, heat-inactivated (20 min, 56°C) heterologous AB-serum 5%. The results are presented as mean values with standard deviation (error bars) of three independent experiments. n.s., Not significant.
In contrast to the increased listerial uptake by MoDC in the presence of human plasma, adherence studies showed no effect of human plasma on the adherence of L. monocytogenes to MoDC (data not shown).
Uptake of serovar Typhimurium and Y. enterocolitica by MoDC is not enhanced by human plasma.
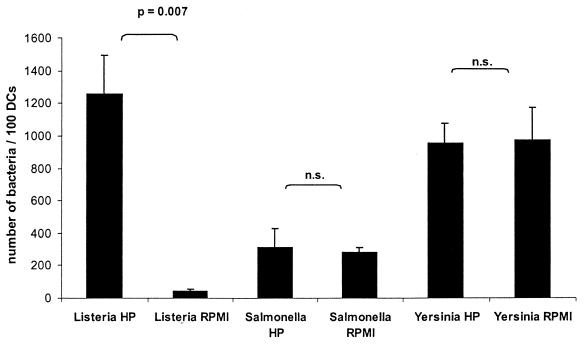
To test whether the strong enhancement of listerial uptake in the presence of human plasma is specific for Listeria sp. or may also be observed for other bacteria, we performed experiments similar to those described above for serovar Typhimurium and Y. enterocolitica. As shown in Fig. 2 and 3, the uptake of both serovar Typhimurium or Y. enterocolitica by MoDC was independent of human plasma. Interestingly, the uptake of serovar Typhimurium and, in particular, Y. enterocolitica by MoDC in RPMI 1640 medium in the absence of human plasma was much higher than that of L. monocytogenes under the same conditions.
FIG. 3.
Quantification of the uptake of L. monocytogenes, serovar Typhimurium, and Y. enterocolitica into DC with or without autologous human plasma by determination of the number of intracellular bacteria per 100 DC after Giemsa staining (1.5 h after the addition of bacteria; MOI = 20). Results are presented as mean values and the standard deviation (error bars) of three independent experiments. n.s., Not significant.
The enhancement in the listerial uptake by human plasma is due to opsonization of Listeria sp. by immunoglobulins.
We next examined which of the major factors present in human plasma may be responsible for the enhancement of listerial uptake. Supplemention of RPMI 1640 medium by serum albumin did not increase the low number of internalized L. monocytogenes obtained after incubation of MoDC with L. monocytogenes in RPMI 1640 alone. No difference in the uptake efficiency was observed between human serum and plasma, excluding an active role of fibrinogen in the internalization of L. monocytogenes. Similarly, heat inactivation of human serum had no significant influence on the uptake rate of L. monocytogenes, excluding a critical role of complement factors (Fig. 4). The large discrepancy between the number of internalized bacteria per DC, as determined by Giemsa staining assay (Fig. 4A) and plating of viable bacteria (Fig.4B), appears to be due to the efficient phagosomal killing of internalized listeriae.
Internalization of L. monocytogenes by MoDC, assayed in the presence or absence of yeast mannan, an established competitive inhibitor of the mannose-fucose receptor, also did not affect the efficiency of uptake, excluding a role for the mannose receptor in the internalization of L. monocytogenes.
Pretreatment of MoDC with cytochalasin D, which inhibits actin filament polymerization, inhibited uptake of L. monocytogenes by MoDC almost completely, indicating that the internalization process requires intact actin microfilament polymerization. The listerial uptake rate into human MoDC in the presence of human plasma without cytochalasin D was about 12 L. monocytogenes per MoDC compared to 0.5 listeria per MoDC after treatment with cytochalasin D (MOI = 20).
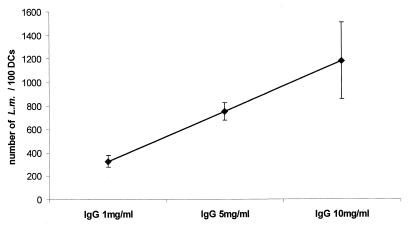
Kaplan (22) provided evidence that actin microfilaments are essential for phagocytosis via the Fc receptor but are much less important for phagocytosis via the C3 receptor. We therefore analyzed the influence of immunoglobulins in the internalization of L. monocytogenes by human MoDC. Increasing concentrations (1, 5, and 10 mg/ml) of pooled human immunoglobulins were added to the RPMI 1640 medium, and internalization of L. monocytogenes was again measured by counting Giemsa-stained intracellular bacteria and viable bacterial cells. The results (Fig. 5) showed a dose-dependent increase of internalized bacteria. Interestingly, high immunoglobulin concentrations (10 mg/ml) resulted in a drop in the number of viable internalized bacteria (data not shown). These data strongly suggest that opsonization of L. monocytogenes with immunoglobulins is responsible for the enhanced uptake of L. monocytogenes by DC; high opsonization of the bacteria may then result in a more efficient elimination of viable bacteria through MoDC.
FIG. 5.
Internalized L. monocytogenes EGD carrying out an infection with different concentrations of pooled human immunoglobulins IgG (L. monocytogenes, MOI = 20, 1.5 h after the addition of bacteria, light microscopy after Giemsa staining). The results are presented as the mean values and the standard deviation (error bars) of three independent experiments.
CD16 monoclonal antibody reduces phagocytosis of L. monocytogenes by MoDC.
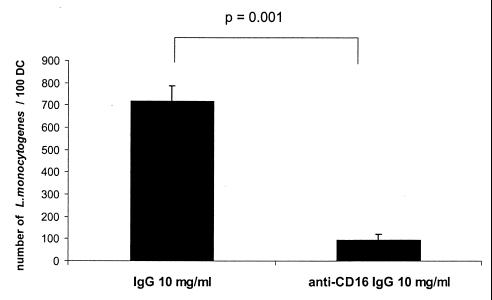
Our data suggest that the uptake of L. monocytogenes is Fc receptor mediated. Human immature MoDC express low amounts of CD16, CD32, and CD64 (data not shown). As shown in Fig. 6, the addition of blocking anti-CD16 antibodies reduces the uptake of L. monocytogenes significantly. These data support the idea that the uptake of L. monocytogenes is FcγRIII receptor mediated.
FIG. 6.
Determination of internalized L. monocytogenes in human MoDC in the presence of pooled IgG (10 mg/ml) with or without blocking anti-CD16 antibodies (MOI = 20, 1.5 h after the addition of bacteria, light microscopy after Giemsa staining). The results are presented as the mean values and the standard deviation (error bars) of three independent experiments.
Antibodies against the listerial p60 protein act as a major opsonin in stimulating the uptake of L. monocytogenes by MoDC.
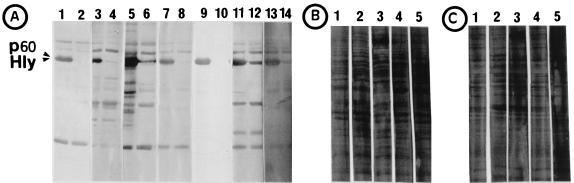
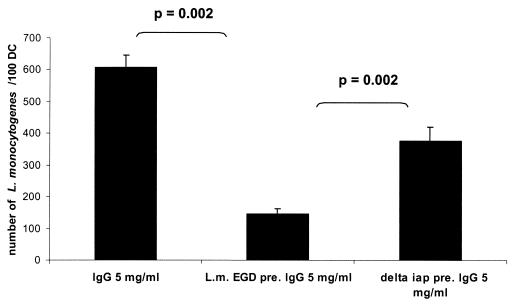
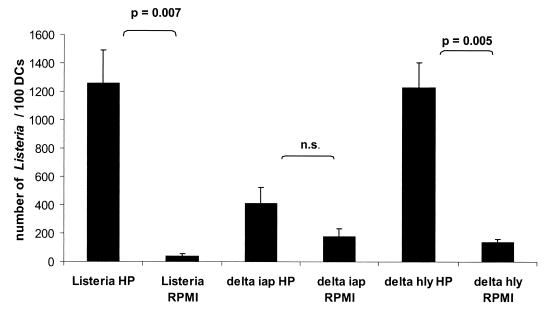
Western blot analysis showed that all used human sera and plasma samples of healthy individuals, as well as the commercially available pooled human immunoglobulins (only tested with Listeria sp.), contained antibodies directed against the three tested bacteria (Fig. 7). However, only Listeria-specific antibodies seemed to be critical for the uptake of L. monocytogenes, while the uptake of serovar Typhimurium and Y. enterocolitica by MoDC was apparently independent of the specific antibodies. The Western blots obtained by testing extracellular and cell-associated proteins of L. monocytogenes with the used human sera showed the p60 protein to be the major immune-reactive component with all serum and plasma samples (Fig. 7A). Human immunoglobulins preadsorbed against L. monocytogenes EGD and added (5 mg/ml) to the RPMI 1640 medium resulted in a significantly (P = 0.002) decreased uptake of L. monocytogenes compared to RPMI 1640 medium supplemented with nonadsorbed immunoglobulins (Fig. 8). Strong support for the assumption that p60 antibodies act as a major opsonin for the uptake of L. monocytogens was obtained by using a recently constructed iap deletion mutant of L. monocytogenes (S. Pilgrim et al., unpublished data) which does not produce p60 at all (Fig. 7A). The addition of iap-preadsorbed immunoglobulins revealed a significant difference in the listerial uptake rate compared to L. monocytogenes wild-type-preadsorbed immunoglobulins (Fig. 8). In addition, internalization of this iap deletion mutant by MoDC showed only minor differences (only a 2-fold increase compared to a 20-fold increase using the L. monocytogenes wild type) in uptake efficiency when it was determined in RPMI 1640 medium with or without human plasma. In contrast, an hly deletion mutant of L. monocytogenes which is unable to produce listeriolysin still showed a very significant difference (10-fold) in the uptake when these two media were used in the uptake assay (Fig. 9). Figure 9 also shows that the internalization of the iap mutant in the absence of human plasma was higher than the internalization of wild-type bacteria. We observed that under the same bacterial culture conditions of L. monocytogenes and its iap deletion mutant, the iap mutant culture contained more dead bacteria. The uptake of dead listeriae was comparable to that of living listeriae (data not shown). In our assay, both dead and living bacteria were counted as internalized by light microscopy after Giemsa staining.
FIG. 7.
Immunoblot analysis of plasma or sera from five healthy individuals by using supernatant and surface proteins of wild-type L. monocytogenes (A; odd-numbered lanes) or p60 mutant L. monocytogenes (A; even-numbered lanes), Y. enterocolitica 14028S (B; lane 1 to 5), and serovar Typhimurium (C; lanes 1 to 5) as antigens. L. monocytogenes EGD prfA* and a p60 mutant of L. monocytogenes prfA* strain are characterized by high expression of the positive regulatory factor PrfA, which controls the expression of most of listerial virulence factors. (A) Lanes 1 and 2 were treated with plasma 1; lanes 3 and 4 were treated with plasma 2, lanes 5 and 6 were treated with plasma 3, lanes 7 and 8 were treated with plasma 4, lanes 9 and 10 were treated with p60-specific monoclonal mouse antibody K3A7 (Rowan 2000) as a control, lanes 11 and 12 were treated with serum, and lanes 13 and 14 were treated with pooled human immunoglobulins (Sandoz). (B and C) In each lane, supernatant and surface proteins isolated from 109 cells were applied. The positions of p60 and listeriolysin (Hly) are indicated on the right side. Lane 1, plasma 1; lane 2, plasma 2; lane 3, plasma 3; lane 4, plasma 4; lane 5, serum.
FIG. 8.
Comparison of the uptake of L. monocytogenes into DC with IgG (5 mg/ml), L. monocytogenes-preadsorbed IgG (5 mg/ml), and Δiap-preadsorbed IgG (5 mg/ml) (MOI = 20; 1.5 h after the addition of bacteria, light microscopy after Giemsa staining).
FIG. 9.
Quantification of the uptake of L. monocytogenes Δiap and Δhly into DC with or without autologous human plasma by determination of the number of intracellular bacteria per 100 DC after Giemsa staining (1.5 h after the addition of bacteria; MOI = 20). The results are presented as the mean values and the standard deviation (error bars) of three independent experiments. n.s., Not significant.
DISCUSSION
Immature human MoDC are highly efficient in taking up L. monocytogenes (23). Uptake by these important antigen-presenting cells occurs by normal phagocytosis since an inlAB deletion mutant, killed listeriae, and the noninvasive species L. innocua are internalized with an efficiency similar to that of virulent L. monocytogenes. The entry of L. monocytogenes into MoDC, when analyzed by electron microscopy, shows that L. monocytogenes cells are engulfed by thin membrane extensions rising from the MoDC membrane enclosing the pathogen tightly, a phagocytosis process resembling a “zipper-like” mechanism (30) without visible morphological changes of the host cell surface.
We have found that the uptake of L. monocytogenes by MoDC is strongly dependent on the presence of human plasma which cannot be replaced by FCS or serum albumin. Internalization of L. monocytogenes in plasma-free medium is very inefficient. In contrast, the uptake of serovar Typhimurium and Y. enterocolitica by MoDC is independent of human plasma, suggesting a major difference in the uptake mechanism of listeriae compared to the two gram-negative bacteria by MoDC.
It has been suggested previously (39) that the mannose receptor present on DC may play a role in the internalization of foreign particles. However, the addition of soluble mannan did not inhibit the uptake of L. monocytogenes. Murine macrophages bind complement-opsonized L. monocytogenes via the complement C3b and C1q receptors (1, 10). DC carry also C3b receptors (2), and C3b generated through activation of the alternative complement pathway by Listeria cell wall fragments may act as an opsonin (19). However, it seems unlikely that complement receptors are essential for the uptake of listeriae by MoDC since heat inactivation of human serum did not significantly reduce phagocytosis. These results thus point to an immunoglobulin-induced uptake via Fc receptors. Indeed, uptake medium supplemented with pooled immunoglobulins resulted in an uptake rate which was comparable to that observed in the presence of human plasma, thus showing that immunoglobulins may be crucial as opsonins in an Fc-mediated uptake of L. monocytogenes by MoDC. DC express several receptors that bind to the Fc portion of immunoglobulins, mediating internalization of the formed antigen-immunoglobulin G (IgG)-complexes (12, 33). Immature MoDC express the Fcγ receptors RI (CD64), RII (CD32), and RIII (CD16). An Fcγ-mediated process was proven by an inhibition experiment with CD16 antibodies. Binding of antibody-opsonized L. monocytogenes to Fc receptors on MoDC may also be responsible for the efficient maturation of MoDC and the upregulation of MHC class II molecules in these MoDC (23) by signaling via the gamma chain of the Fc receptor, which leads to DC activation and antigen presentation by MHC class II molecules (13, 33). Obviously, the uptake of serovar Typhimurium and Y. enterocolitica is not enhanced in the presence of human plasma, although antibodies reacting with surface components of these pathogens were also detected in the human plasma. These results suggest that the uptake of these gram-negative bacteria by MoDC may be different from that of L. monocytogenes.
Western blot analysis showed the presence of antibodies against the 60-kDa extracellular protein of L. monocytogenes (p60) in all human serum and plasma samples tested and in the commercially available pooled human immunoglobulins. Low titers of antilisteriolysin antibodies and other Listeria-specific antibodies were also detected in some human plasma. These antibodies may play a minor role as opsonins for Fc-mediated phagocytosis of L. monocytogenes by MoDC, as suggested by the slightly reduced internalization of the hly deletion mutant in the presence of human plasma. The important role of p60 antibodies for the uptake of L. monocytogenes into human MoDC could be shown by the use of an iap deletion mutant. This iap mutant is a complete null mutation of p60. It was reported that the iap gene encoding p60 is essential for bacterial viability (44). Possibly, our iap mutant strain expresses other enzymes, which may compensate for the lack of p60. Nevertheless, a significant number of nonviable cells are present in a culture of the iap mutant (Pilgrim et al., unpublished). However, preadsorbation of the immunoglobulins with L. monocytogenes and, in particular, the use of the iap mutant, which does not produce any p60, indicated that anti-p60 antibodies act as the predominant listerial opsonin in the Fc-mediated phagocytosis. p60-like proteins containing the same prominent B-cell epitopes carried by L. monocytogenes p60 are produced by all Listeria species (5). The p60-specific antibodies found in the plasma of most immune-competent humans seem to be derived from L. innocua and possibly other environmental nonpathogenic Listeria species to which humans are most likely permanently exposed and not necessarily from exposure to virulent L. monocytogenes (16). These cross-reacting serum p60 antibodies may, however, opsonize virulent L. monocytogenes and enhance the uptake of these pathogens by human MoDC, which are very active in killing L. monocytogenes (23), and thus this mechanism could provide an important barrier against the spreading of virulent L. monocytogenes into the system via the blood stream and may thus contribute to the rather low frequency of human infections by this intracellular pathogen.
ACKNOWLEDGMENTS
This study was supported by a fellowship from the Bundesminisrerium für Bildung und Forschung (AZ01 KS9603) to A.K.-M. within the scope of IZKF Würzburg and by grants from the Fond der Chemischen Industrie.
We thank M. Mäurer for critical reading of the manuscript. We are grateful to A. Bubert for donating the mouse antibody K3A7, J. Heesemann for the Y. enterocolitica strain, and J. A. Vazquez-Boland for plasmid pHPS9 with the mutant prfA allele from P14-A. We thank G. Krohne and C. Gehrig for help with the electron microscopy.
A.K.-M. and S.P. contributed equally to this study.
REFERENCES
- 1.Alvarez-Dominguez C, Carrasco-Marin E, Leyva-Cobian F. Role of complement C1q in phagocytosis of Listeria monocytogenes by murine macrophages-like cell lines. Infect Immun. 1993;61:3664–3672. doi: 10.1128/iai.61.9.3664-3672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells form nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 4.Braun L, Ohayon H, Cossart P. The InIB protein of Listera monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Bubert A, Köhler S, Goebel W. The homologous and heterologous regions within the iap gene allow genus- and species-specific identification of Listeria spp. by polymerase chain reaction. Appl Environ Microbiol. 1992;58:2625–2632. doi: 10.1128/aem.58.8.2625-2632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty T, Leimeister-Wächter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corinti S, Medaglini D, Cavani A, Rescigno M, Pozzi G, Ricciardi-Castagnoli P, Girolomoni G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J Immunol. 1999;163:3029–3036. [PubMed] [Google Scholar]
- 8.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signalling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes requires expression of InIB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 10.Drevets D A, Campbell P A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991;59:2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanger N A, Wardwell K, Shen L, Tedder T F, Guyre P M. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol. 1996;157:541–548. [PubMed] [Google Scholar]
- 13.Fanger N A, Voigtlaender L C D, Swink S, Wardwell K, Fisher J, Graziano R F, Pfefferkorn L C, Guyre P M. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor FcγRI (CD64) expressed on human blood dendritic cells. J Immunol. 1997;158:3090–3098. [PubMed] [Google Scholar]
- 14.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 16.Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Multhaler M, Goebel W, Bubert A. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J Immunol. 1999;162:4781–4789. [PubMed] [Google Scholar]
- 17.Gentschev I, Sokolovic Z, Köhler S, Krohne G F, Hof H, Wagner J, Goebel W. Identification of p60 antibodies in human sera and presentation of this listerial antigen on the surface of attenuated salmonellae by the HlyB-HlyD secretion system. Infect Immun. 1992;60:5091–5098. doi: 10.1128/iai.60.12.5091-5098.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria moncytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington-Fowler L, Henson P M, Wilder M S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981;33:11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol. 1977;6:797–807. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 23.Kolb-Mäurer A, Gentschev I, Fries H W, Fiedler F, Bröcker E-B, Kämpgen E, Goebel W. Listeria monocytogenes-infected human dendritic cells: uptake and host cell response. Infect Immun. 2000;68:3680–3688. doi: 10.1128/iai.68.6.3680-3688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn M, Goebel W. Molecular studies on the virulence of Listeria monocytogenes. Genet Eng. 1995;17:31–51. [PubMed] [Google Scholar]
- 26.Larsson M, Majeed M, Ernst J D, Magnusson K E, Stendahl O, Forsum U. Role of annexins in endocytosis of antigens in immature human dendritic cells. Immunology. 1997;92:501–511. doi: 10.1046/j.1365-2567.1997.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine T P, Chain B M. Endocytosis by antigen presenting cell: dendritic cells are as endocytically active as other antigen-presenting cells. Proc Natl Acad Sci USA. 1992;89:8342–8346. doi: 10.1073/pnas.89.17.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby S J, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang F C, Guiney D G, Songer J G, Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci USA. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellman I, Turley S J, Steinman R M. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 30.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is a receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 31.Mollenkopf H-J, Gentschev I, Bubert A, Schubert P, Goebel W. Extracellular PagC-HlyAs fusion protein for the generation and identification of Salmonella-specific antibodies. Appl Microbiol Biotechnol. 1996;45:629–637. doi: 10.1007/s002530050740. [DOI] [PubMed] [Google Scholar]
- 32.Ni K, O'Neill H C. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223–230. doi: 10.1038/icb.1997.35. [DOI] [PubMed] [Google Scholar]
- 33.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis e Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–203. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- 37.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 38.Rowan N J, Candlish A A G, Bubert A, Anderson J G, Kramer K, McLauchlin J. Virulent rough filaments of Listeria monocytogenes from clinical and food samples secreting wild-type levels of cell-free p60 protein. J Clin Microbiol. 2000;38:2643–2648. doi: 10.1128/jcm.38.7.2643-2648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Mengaut J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 41.Steinman R M, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–567. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 42.Svensson M, Stockinger B, Wick M J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 43.Tan M C A A, Mommaas A M, Drijfhout J W, Jordens R, Onderwater J J M, Verwoerd D, Mulder A A, van der Heiden A N, Scheidegger D, Oomen L C J M, Ottenhoff T H M, Tulp A, Neefjes J J, Koning F. Mannose receptor-mediated uptake of antigens strongly enhance HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 44.Wuenscher M D, Köhler S, Bubert A, Gerike U, Goebel W. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993;175:3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]