Summary
The Difficult Airway Society recommends that all patients should be pre‐oxygenated before the induction of general anaesthesia, but this may not always be easy or comfortable and anaesthesia may often be induced without full pre‐oxygenation. We tested the hypothesis that high‐flow nasal oxygen cannulae would be easier and more comfortable than facemasks for pre‐oxygenation. We randomly allocated 199 patients undergoing elective surgery aged ≥ 10 years to pre‐oxygenation using either high‐flow nasal oxygen or facemask. Ease and comfort were assessed by anaesthetists and patients on 10‐cm visual analogue scale and six‐point smiley face scale, respectively. Secondary endpoints included end‐tidal oxygen fraction after securing a definitive airway and time to secure an airway. A mean difference (95%CI) between groups in ratings of ‐0.76 (‐1.25 to ‐0.27) cm for ease of use (p = 0.003) and ‐0.45 (‐0.75 to ‐0.13) points for comfort (p = 0.006), both favoured high‐flow nasal oxygen. A mean difference (95%CI) between groups in end‐tidal oxygen fraction of 3.89% (2.41–5.37%) after securing a definitive airway also favoured high‐flow nasal oxygen (p < 0.001). There was no significant difference between groups in the number of patients with hypoxaemia (SpO2 < 90%) or severe hypoxaemia (SpO2 < 85%) lasting ≥ 1 min or ≥ 2 min; in the proportion of patients with an end‐tidal oxygen fraction < 87% in the first 5 min after tracheal intubation (52.2% vs. 58.9% in facemask and high‐flow nasal oxygen groups, respectively; p = 0.31); or in time taken to secure an airway (11.6 vs. 12.2 min in facemask and high‐flow nasal oxygen groups, respectively; p = 0.65). In conclusion, we found pre‐oxygenation with high‐flow nasal oxygen to be easier for anaesthetists and more comfortable for patients than pre‐oxygenation with a facemask, with no clinically relevant differences in end‐tidal oxygen fraction after securing a definitive airway or time to secure an airway. The differences in ease and comfort were modest.
Keywords: airway management, high‐flow nasal oxygenation, pre‐oxygenation, transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE)
Introduction
The development of a potentially catastrophic ‘can't intubate, can't oxygenate’ situation during anaesthesia is difficult to predict [1, 2, 3, 4]. Thus, guidelines from the Difficult Airway Society (DAS) for management of unanticipated difficult tracheal intubation in adults recommend that “all patients should be pre‐oxygenated before the induction of general anaesthesia” until the end‐tidal oxygen fraction (FETO2) is 0.87–0.9 [5].
With conventional pre‐oxygenation, patients breathe 100% oxygen through a tightly sealed facemask, either for a fixed time (typically 3 min) or until the target FETO2 is reached. This is usually straightforward, but requires the undivided attention of a trained clinician. It may be difficult and uncomfortable for the patient to get a good seal with a facemask. A few breaths of room air (e.g. if the facemask is briefly lifted to allow the patient to say something) might set the process back substantially [6]. Also, if spontaneous ventilation with a supraglottic airway device is planned, the patient may take one or more breaths of room air between removing the facemask and insertion of the airway. Thus, in practice, it seems that anaesthesia is induced in many patients who have not been pre‐oxygenated fully, or even at all [7, 8].
Humidified high‐flow nasal oxygenation (HFNO) emerged as an alternative to continuous positive airway pressure therapy in the first decade of this century [9, 10]. Heated, humidified oxygen is delivered via purpose‐designed nasal prongs at flow rates up to 70 l.min‐1 and concentrations of up to 100% oxygen [9, 10]. Patel and Nouraei first described using HFNO for pre‐oxygenation followed by apnoeic ‘postoxygenation’ until a definitive airway has been secured [11]. The term ‘peroxygenation’ includes pre‐oxygenation and this subsequent period of apnoeic postoxygenation with or without ventilation [12].
Studies of pre‐oxygenation with HFNO have had mixed results [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Technicalities matter: in particular, a closed mouth appears to be important when pre‐oxygenating a patient with HFNO [15, 17]. Also, it is difficult to measure FETO2 with HFNO [15]. Nevertheless, support has emerged for the potential of HFNO to extend safe apnoeic time through peroxygenation [13, 14, 15, 16, 18, 19, 22, 23]. Safe apnoeic time is arguably more relevant to patient safety than FETO2 at the end of pre‐oxygenation [12, 15]. Also, published data reflect study conditions rather than real‐world practice. As noted, although pre‐oxygenation with a facemask is clearly achievable in most patients, in practice this is not always done [7, 8].
From a human factors perspective, improving ease and comfort might result in a greater proportion of patients adequately pre‐oxygenated and thus, potentially, a reduction in the number of patients harmed through oxygen desaturation during airway management. Anecdotally, HFNO seems easier to administer and more comfortable for patients than facemask for pre‐oxygenation. However, at the time of conceiving this study, we could find only one publication to support this impression, and the context was a potential need for re‐intubation of the trachea postoperatively rather than routine pre‐oxygenation [20]. Therefore, we aimed to test the hypothesis that anaesthetists would rate pre‐oxygenation easier and patients would rate it more comfortable with HFNO cannulae than facemasks.
Methods
This study was approved by The Northern B Health and Disability Ethics Committee and Auckland District Health Board Research Review Committee. All participating anaesthetists, patients and/or their parents or legal guardians provided written informed consent.
We conducted a prospective, randomised study of pre‐oxygenation in adult and paediatric patients. We recruited patients from four semi‐independently staffed and directed suites of operating theatres (our study sites); three at Auckland City Hospital for adult patients (undergoing cardiothoracic, general and obstetric and gynaecological surgery, respectively) and one at Starship Children's Hospital for paediatric patients. We defined two primary endpoints. The first was ease of pre‐oxygenation assessed by participating anaesthetists at a convenient time shortly after securing the airway on an anchored 10‐cm visual analogue scale (0, easiest). The second primary endpoint was comfort during pre‐oxygenation assessed by participating patients immediately before induction of anaesthesia (i.e. after pre‐oxygenation, as defined by Patel and El‐Boghdadly [12]) on a six‐point smiley face scale (0, most comfortable) shown to them by a researcher without interrupting the delivery of oxygen. Our smiley face scale (see online Supporting Information, Appendix S1) was a slight modification of a scale modified by Tiruvoipati et al. [20] from the Wong‐Baker Faces® Pain Rating Scale© [24]. Permission for its use has been obtained from Dr Tiruvoipati and the Wong‐Baker FACES Foundation.
Our secondary endpoints were: FETO2 after securing a definitive airway (by intubation of the trachea or insertion of a supraglottic airway; the mean of the first five FETO2 readings taken at 30‐s intervals); the proportion of patients with an episode of hypoxaemia (SpO2 < 90%); the proportion of patients with an episode of severe hypoxaemia (SpO2 ≤ 85%) of ≥ 2 min duration during the study period (defined as the period from the first pulse oximeter reading until 15 min after a definitive secure airway was established); the proportion of patients for whom FETO2 fell below 87% in the 5 min immediately after securing a definitive airway; and the time taken from the first pulse oximeter reading in the operating theatre until a definitive airway was secured. An anaesthetic information management system (SAFERSleep, Safer Sleep LLC, Delaware, USA and Auckland, New Zealand) recorded all physiological data directly from monitors. These records were used to obtain the physiological secondary endpoints. Patients' age, BMI, sex, ASA physical status and ethnicity were obtained from clinical records.
We recruited five anaesthetists in each site (i.e. 20 in total), who each aimed to complete 10 cases (i.e. 200 cases in total). Those recruited received a copy of the study protocol and the DAS 2015 guidelines [5]. They also attended a training session on the study protocol and use of HFNO for pre‐oxygenation.
Recruitment was based on the availability of research staff and participating anaesthetists. We included patients if they were: aged ≥ 10 y; undergoing elective surgery under general anaesthesia with intravenous induction; with a participating anaesthetist; with planned use of a tracheal tube or a supraglottic airway device; and willing to provide informed consent. Our exclusion criteria included: patients undergoing an emergency caesarean section; any known contraindication to HFNO; patients undergoing acute operations for which they had not appropriately fasted; > 50% of the nares occluded by the nasal prongs (as judged by the anaesthetist); bleeding in nose or oropharynx; situations in which continuous positive airway pressure was contraindicated; a pre‐existing nasal obstruction or hypoxaemia; known cyanotic congenital heart disease; on pre‐operative oxygen therapy secondary to chronic lung disease; planned induction of anaesthesia with a volatile anaesthetic; or planned awake tracheal intubation. We randomly allocated each anaesthetist's patients to conventional methods or HFNO in equal numbers. Treatment allocation was established by opening a sealed opaque envelope after informed consent had been given and once the patient was in the operating theatre or, in the case of children, in the pre‐operative area. It was not possible for participants to be blinded to randomisation in this study. Except to the extent that gaseous induction of anaesthesia was excluded, the management of anaesthesia was at the discretion of each anaesthetist.
In the control group, pre‐oxygenation was provided using 100% oxygen via a sealed facemask and a circle‐absorber anaesthetic circuit primed with 100% oxygen by installing a ventilation bag to the mouthpiece filter and ventilating the circuit with 100% oxygen. In the facemask group, anaesthetists were asked to obtain a FETO2 of > 87% before starting induction of anaesthesia, if possible. Anaesthetists were free to carry out bag‐mask ventilation of the lungs once induction medications had been administered. The FIO2 was continued at 100% until at least 5 min after securing a definitive airway.
In the intervention group, pre‐oxygenation was provided using HFNO via Optiflow THRIVE™ (Fisher and Paykel Healthcare Limited, Auckland, New Zealand) until SpO2 on pulse oximetry was > 95% and for at least 3 min in adults. For adults, a flow of 40 l.min‐1 was used until induction agents had been administered, and then increased to 70 l.min‐1. For children, flows were based on weight, as follows: 0–15 kg, 2 l.kg‐1.min‐1; 15–30 kg, 35 l.min‐1; 30–50 kg, 40 l.min‐1; and > 50 kg, 50 l.min‐1 [25]. These flows were checked in the pre‐operative area and decreased for pre‐oxygenation if not tolerated, but after induction of anaesthesia all flows were based on weight. Nasal oxygenation was continued without ventilation of the lungs in both adult and paediatric patients while waiting for neuromuscular blockade, and during placing, replacing or repositioning the airway. Anaesthetists were free to carry out bag‐mask ventilation of the lungs if they considered this necessary to maintain safe oxygen saturations. After securing the airway, the patient was connected to a circle circuit primed with 100% oxygen and the FIO2 was continued at 100% for a period of at least five more minutes. Relevant times were recorded, including start of pre‐oxygenation and start of induction of anaesthesia.
We documented the following predefined adverse events directly until patients left the post‐anaesthesia care unit and from the discharge summary thereafter: unplanned admission to ICU; aspiration of gastric contents into the lungs; damage to dentition; pneumothorax; declared crises on account of difficulty with the airway during the peri‐induction period; stroke as defined by the patient's clinical team and recorded in the discharge summary; in‐hospital death; unexpected admission of a day‐case patient to hospital; and prolongation of expected hospitalisation of 2 days or more. Two investigators reviewed all reported adverse events and categorised them as serious or not serious, and as related to the study, possibly related or not related to the study. A data safety monitoring committee reviewed a summary of adverse events at about the midpoint of the study.
In a short, open‐ended, end‐of‐study survey, anaesthetists were asked whether they would consider using HFNO for pre‐oxygenation in all or only in selected patients, and to give reasons for their answers.
We estimated that 100 participants per study group would be needed to show an improvement in comfort of 0.5 points with 80% power and a two‐tailed p value of 0.05. This was based on variance seen by previous studies that compared observer‐rated comfort scores between traditional facemask and nasal prongs with a six‐point smiley face scale and found mean (SD) comfort scores of 0.93 (1.43) and 0.53 (1.04), respectively [20]. We assumed a 10‐cm visual analogue scale should be at least as sensitive as a six‐point smiley face scale and therefore this should also suffice for our ratings of ease of pre‐oxygenation.
The distributions of the continuous data variables were approximately normal. These outcomes were compared between groups using a general linear model which included group and site as fixed factors. We treated missing data as missing at random and undertook no imputation. We conducted all analyses on an intention‐to‐treat basis. We used R version 4.1.0 and RStudio version 1.4.1717 (R Core Team, Vienna, Austria) to perform analyses. A p value of < 0.05 was considered statistically significant. An independent study monitor conducted regular audits of the study's processes and records.
Results
Patients were recruited between 17 March 2017 and 5 June 2019. One participating anaesthetist withdrew from the study after completing only nine patients, so 199 patients were randomly allocated. Three patients were subsequently excluded, one in the facemask and two in the HFNO group. Thus, 99 patients finally entered the facemask group and 97 the HFNO group (Fig. 1). The groups were well balanced in respect of age, BMI, sex, ASA physical status and ethnicity (Table 1). Tracheal tubes were used in all adult and 22 paediatric patients. Supraglottic airway devices were used in 25 paediatric patients: 15 in the facemask group and 10 in the HFNO group.
Figure 1.

Patients, screened, excluded, consented, randomised and included in the analysis.
HFNO, high‐flow nasal oxygen; AIMS, anaesthetic information management system.
Table 1.
Characteristics of patients receiving pre‐oxygenation by facemask or high‐flow nasal oxygen (HFNO) overall, for adults and children. Values are mean (SD), number (proportion) or median (IQR [range]).
| Overall | Adults | Children | ||||
|---|---|---|---|---|---|---|
| Facemask | HFNO | Facemask | HFNO | Facemask | HFNO | |
| n = 99 | n = 97 | n = 74 | n = 75 | n = 25 | n = 22 | |
| Age; y | 44.2 (23.6) | 47.6 (23.9) | 54.6 (17.6) | 57.6 (17.0) | 14.0 (12.0–15.0 [10.0–16.0] | 13.0 (12.0–15.0 [10.0–16.0]) |
| BMI; kg.m‐2 | 28.9 (10) | 28.1 (7.9) | 30.2 (10.2) | 29.8 (7.5) | 22.0 (18.6–26.7 [14.7–41.0]) | 19.6 (17.6–23.5 [15.1–35.3]) |
| Weight; kg | 77.5 (25.5) | 78.5 (24.8) | 84.3 (23.5) | 85.1 (22.6) | 57.4 (45.0–62.4 [29.2–119.0]) | 55.5 (45.2–67.4 [30.2–98.5]) |
| Sex; female | 55 (55.6%) | 55 (56.7%) | 41 (55.4%) | 41 (54.7%) | 14 (56.0%) | 14 (63.6%) |
| ASA physical status | ||||||
| 1 | 23 (23.2%) | 20 (20.6%) | 9 (12.2%) | 7 (9.3%) | 14 (56.0%) | 13 (59.1%) |
| 2 | 39 (39.4%) | 36 (37.1%) | 29 (39.2%) | 31 (41.3%) | 10 (40.0%) | 5 (22.7%) |
| 3 | 22 (22.2%) | 26 (26.8%) | 21 (28.4%) | 22 (29.3%) | 1 (4.0%) | 4 (18.2%) |
| 4 | 15 (15.2%) | 15 (15.5%) | 15 (20.3%) | 15 (20.0%) | 0 | 0 |
| Ethnicity | ||||||
| European | 68 (68.7%) | 59 (60.8%) | 52 (70.3%) | 46 (61.3%) | 16 (64.0%) | 13 (59.1%) |
| Māori | 5 (5.1%) | 10 (10.3%) | 3 (4.1%) | 7 (9.3%) | 2 (8.0%) | 3 (13.6%) |
| Pacific Peoples | 17 (17.2%) | 16 (16.5%) | 12 (16.2%) | 10 (13.3%) | 5 (20.0%) | 6 (27.3%) |
| Asian | 9 (9.1%) | 11 (11.3%) | 7 (9.5%) | 11 (14.7%) | 2 (8.0%) | 0 |
| Other | 0 | 1 (1.3%) | 0 | 0 | 0 | 0 |
There was a mean difference (95%CI) between groups of ‐0.76 cm (‐1.25 to ‐0.27 cm; p = 0.003) for the ratings of ease of pre‐oxygenation, and of −0.45 (−0.75 to −0.13; p = 0.006) for the ratings of patients' comfort, both favouring HFNO (Tables 2 and 3; Fig. 2). There was a mean difference (95%CI) between groups in FETO2 of 3.89% (2.41–5.37%; p < 0.001) favouring HFNO immediately after securing a definitive airway (Table 3). There was no significant difference between groups in any of the other secondary endpoints (Table 3).
Table 2.
Ratings by anaesthetists for ease of pre‐oxygenation on a 10‐cm visual analogue scale (0, easiest; 10, hardest) and by patients for comfort on a six‐point comfort scale (0, most comfortable; 5, least comfortable), and experience with and tolerance to pre‐oxygenation comparing facemasks with high‐flow nasal oxygen (HFNO) cannulae. Values are mean (SD), mean difference (95%CI) or number (proportion).
| Facemask | HFNO | Mean difference | p value | |
|---|---|---|---|---|
| n = 99 | n = 97 | (95%CI) | ||
| Ease for anaesthetist; cm | 1.62 (2.2) | 0.89 (1.48) | ‐0.76 (‐1.25 to ‐0.27) | 0.003 |
| Experiences noted by anaesthetist | ||||
| Beard nuisance | 3 (3.0%) | – | ||
| Poor facemask fit | 5 (5.1%) | – | ||
|
Supplemented with mask ventilation* |
– | 5 (5.2%) | ||
| Flow returning from the patient's mouth during tracheal intubation | – | 1 (1.0%) | ||
| Fluttering uvula | – | 2 (2.1%) | ||
| Comfort for patients; points | 1.48 (1.2) | 1.03 (0.9) | ‐0.45 (‐0.75 to ‐0.13) | 0.006 |
| Experiences of individual patients | ||||
| Not tolerated, stopped | 3 (3.0%) | 1 (1.0%) | ||
| Not comfortable | 3 (3.0%) | 5 (5.2%) | ||
| Flowrate decreased † | – | 22 (22.7%) | ||
One because patient oxygen saturation dropped to 94% for < 1 min; one because the patient was anxious; two because the anaesthetist inadvertently reverted to habitual practice; and one because of a delay in obtaining the required tracheal tube.
In paediatric patients, flowrate was set interactively in the pre‐operative area to comfortable levels, which in all 22 patients were between 10 and 30 l.min‐1 lower than prescribed; flowrates were set to the prescribed rates after induction of anaesthesia.
Table 3.
Comparison between groups of pre‐oxygenation with facemask or high‐flow nasal oxygen (HFNO) for duration of pre‐oxygenation, time to secure airway, end‐tidal oxygen fraction (FETO2) immediately after securing an airway, numbers of patients with hypoxaemia (defined as an oxygen saturation (SpO2 < 90% of ≥ 1 or ≥ 2 min duration), with severe hypoxaemia (SpO2 < 85%) or with FETO2 < 87% in the 5 min after tracheal intubation, and numbers of patients experiencing adverse events. Values are mean (SD), mean difference (95%CI), number (proportion) or OR (95%CI). Patients could experience more than one adverse event.
| Facemask | HFNO | Mean difference | p value | |
|---|---|---|---|---|
| n = 99 | n = 97 | (95%CI) or OR (95%CI) | ||
| Time to secure airway; min | 11.6 (7.9) | 12.2 (8.0) | 0.46 (‐1.59 to 2.51) | 0.659 |
| Duration of pre‐oxygenation; min | 4.5 (3.9)* | 8.1 (7.2) | 3.5 (2.0–5.1) | <0.001 |
| FETO2 and hypoxaemia | ||||
|---|---|---|---|---|
| FETO2 post‐intubation; % | 89.6 (7.5) † | 93.5 (4.3) † | 3.89 (2.41–5.37) | <0.001 |
| Hypoxaemia (≥ 1 min) ‡ | 8 (8.7%) † | 6 (6.3%) † | 0.71 (0.22–2.15) | 0.545 |
| Severe hypoxaemia (≥ 1 min) ‡ | 4 (4.3%) † | 3 (3.2%) † | 0.72 (0.14–3.43) | 0.679 |
| Hypoxaemia (≥ 2 min) ‡ | 1 (1.1%) † | 1 (1.1%) † | – | – |
| Severe hypoxaemia (≥ 2 min) ‡ | 1 (1.1%) † | 0 † | – | – |
| FETO2 < 87% (5 min post‐intubation) | 48 (52.2%) † | 56 (58.9%) † | 1.37 (0.75–2.52) | 0.311 |
| Adverse events | ||||
|---|---|---|---|---|
| Predefined adverse events | 4 (4.0%) | 1 (1.0%) | 0.24 (0.1–1.65) | 0.202 |
| Any non‐serious adverse events | 25 (25.3%) | 27 (27.8%) | 1.13 (0.59–2.14) | 0.718 |
| Predefined serious adverse events | 7 (7.1%) | 11 (11.3%) | 1.65 (0.61–4.72) | 0.333 |
| Any serious adverse events | 18 (18.2%) | 24 (24.7%) | 1.46 (0.72–2.99) | 0.295 |
| Any (serious or non‐serious) adverse events | 42 (42.4%) | 51 (52.6%) | 1.50 (0.84–2.73) | 0.175 |
Excludes two patients for whom the start of pre‐oxygenation was not recorded.
Excludes nine patients (seven facemask; two HFNO) with missing or incomplete physiological recordings.
Patients with hypoxaemia for ≥ 2 min are a subset of those with hypoxaemia of ≥ 1 min, and those with severe hypoxaemia are a subset of those with hypoxaemia.
Figure 2.
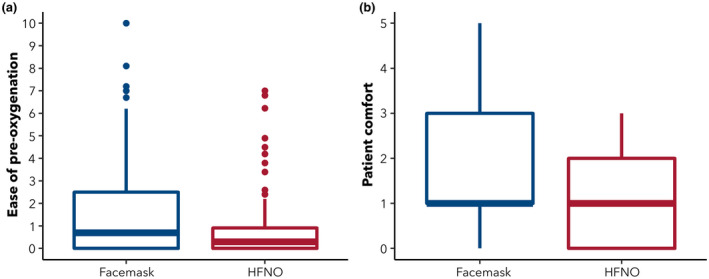
Median (boxes), interquartile range (lines) and outliers of ratings (dots) by (a) anaesthetists for ease of pre‐oxygenation on a 10‐cm visual analogue scale (0, easiest; 10, hardest); and (b) patients for comfort on a six‐point comfort scale (0, most comfortable; 5, least comfortable) comparing pre‐oxygenation with facemask or high‐flow nasal oxygen (HFNO).
Facemask pre‐oxygenation was not tolerated by three patients and another three found it uncomfortable. When used, HFNO was not well tolerated by one patient (who nevertheless did try to use it and was included in the analysis) and an additional five found it uncomfortable. In the pre‐operative area, all children asked for the flow to be reduced from that prescribed by their weight (Table 2), and these reduced flows were used for pre‐oxygenation until they were anaesthetised. The duration of pre‐oxygenation was 3.5 min shorter in the facemask group than in the HFNO group (p < 0.001; Table 3).
In total, 212 adverse events were reported in 93 patients; 42 of these experienced one or more events rated as serious (Table 3 and online Supporting Information, Appendix S2). Most of these adverse events were related to surgery. None were judged as definitely related to HFNO and there was no significant difference between groups in the rates of these events.
The end‐of‐study survey was completed by 19 anaesthetists, all of whom stated that they would prefer to restrict their use of HFNO for pre‐oxygenation to selected patients. Reasons for this were cost (n = 4); wastefulness (n = 3); ease of facemask pre‐oxygenation with no extra device needed (n = 3); preference for feedback from bag‐mask ventilation (n = 1); unnecessary (n = 4); not all patients tolerate nasal prongs (n = 3); better for specific groups (n = 3); and desire to use gas induction in some patients (n = 1).
Discussion
Pre‐oxygenation with HFNO was rated by anaesthetists as easier to use and by patients as more comfortable than facemask pre‐oxygenation. Both methods were rated as easy in most cases and as comfortable by most patients, but there were more patients with more difficult and less comfortable pre‐oxygenation in the facemask group. The study's size was calculated on the basis that a difference in comfort of 0.5 points would be clinically important. The mean difference (95%CI) was −0.45 (−0.75 to −0.13). These confidence limits contain the predefined value of 0.5, but do not definitively demonstrate a difference of at least this size, so the actual difference between groups might not meet our predefined criterion for clinical importance. A few patients in each group stated explicitly that their method was uncomfortable or even intolerable. Almost all the children in the HFNO group needed lower than prescribed flow rates while awake, but this did not preclude increasing the flows once they were anaesthetised. Although many adverse events were reported, there was no difference between groups in this regard and none was considered related to HFNO.
Several studies have evaluated the comfort of pre‐oxygenation with HFNO in various contexts [14, 20, 21, 26, 27, 28, 29, 30]. All but one were published before the start of our study [20]. Taken collectively, the findings of these studies in relation to patient comfort are broadly consistent with ours and generally favour HFNO over mask pre‐oxygenation (and markedly over non‐invasive ventilation). None of these previous studies explicitly compared ease‐of‐use from the perspective of the anaesthetist. Our study also differs from these earlier studies in having more patients and evaluation of comfort and ease of use as its primary objective.
Patients who were pre‐oxygenated with HFNO had, on average, a slightly higher FETO2 immediately after securing a definitive airway than patients who underwent conventional pre‐oxygenation, but there was no significant difference between groups in FETO2 readings below 87% in the first 5 min after tracheal intubation, in time taken to secure an airway and in the number of patients in who experienced arterial hypoxaemia during the peri‐induction period. Thus, the efficacy of pre‐oxygenation with HFNO appeared, at the least, no less than that of pre‐oxygenation with a mask. We did not adequately assess efficiency, which is best measured by the apnoea time to desaturation [12]. The results of previous studies have been mixed in respect of physiological endpoints of this type [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26]. This probably reflects differences in patient groups, in the technicalities of how HFNO was administered and in how the data were obtained. In our study, we measured FETO2 immediately after securing the airway. Thus, we could use the same method of estimating FETO2 in each group. This time‐point may, arguably, also be of greater relevance to the clinical value of pre‐oxygenation than the end of pre‐oxygenation with a facemask, given that difficulty in managing the airway is usually only appreciated after at least one attempt has been made to intubate the trachea or insert a supraglottic airway. However, the clinical implications of minor differences between groups in physiological endpoints obtained under the conditions of studies in which considerable emphasis is placed on achieving adequate pre‐oxygenation may be more relevant to understanding the technicalities of using HFNO effectively than to the potential clinical implications of doing so. Evidence for an increase in safe apnoeic time, which we did not measure in our study, provides more compelling support for the use of HFNO in peroxygenation [13, 14, 15, 16, 18, 19, 22, 23], but the data on this point also reflect study conditions rather than real‐world clinical practice [15]. The question arises as to whether the modest advantages in ease of use and patient comfort demonstrated in our results would translate into a greater proportion of patients adequately pre‐oxygenated in routine clinical practice and hence a smaller number harmed through difficulty in managing the airway. A study to show a difference in complications, including mortality, related to managing patients' airways would need to be very large. Before undertaking such a study, more work is warranted to better understand how to eliminate the entrainment of room air through the mouth when using HFNO [15].
A limitation of our study is that it was conducted in two closely affiliated institutions in a single city in New Zealand. As a result, our findings cannot be generalised to other institutions, cities or countries. We have limited information about reasons for not participating in the study. Furthermore, our findings cannot be applied to children under the age of 10 y. The majority of children below this age undergo an inhalational induction of anaesthesia at Starship Children's Hospital, and including them would have involved asking for a fundamental change in their management. We acknowledge that there are important differences between children, even those aged 10–16 y, and adults, but have not sought to compare results between them because the number of children in our study was relatively small. Rather, these children were included to broaden our study population. There could be selection bias of patients not wanting to participate because they did not want nasal prongs. We also cannot exclude a Hawthorn effect: as noted, we believe study anaesthetists may well have been more focused on pre‐oxygenation than during their normal practice, but this would have applied to both study groups. Because manually collected times were recorded to the nearest minute and automated data were collected every 30 s, there was a variation of up to 2 min in determining the exact time for securing a definitive airway. Again, this applied to both study groups, but for future trials more frequent observations should be considered. The timing of the assessment of ease of use (shortly after the airway had been secured) may have led to recall bias: for example, complications during airway management, including desaturation or difficulty in airway management, may have affected these ratings. However, we thought it important not to interrupt the anaesthetists until this important goal had been achieved. The visual analogue scale used to assess ease of use was not specifically validated for this purpose, but such scales are widely used to assess a range of unidimensional experiences. To assess comfort, we followed the approach of Tiruvoipati et al. in using a modification of the Wong‐Baker Faces® Pain Rating Scale [20]. We did not formally validate this modified scale, either for children or for adults, and the smiley faces were designed to represent different levels of pain rather than different levels of comfort. We have had difficulty finding any tools for assessing patient comfort that are both simple and validated. Furthermore, in its broadest sense, comfort is a multidimensional concept [31, 32]. However, in the context of this study, we think our scale, with its comfort‐related text anchors, has some face validity, and our participants (both adults and children) appeared to understand its use. Nevertheless, this lack of validation is a limitation, and further research is needed on how best to evaluate the ease and comfort of procedures such as pre‐oxygenation.
It is not straightforward to measure FETO2 while using HFNO for pre‐oxygenation, so we pre‐oxygenated patients in the HFNO group until the SpO2 > 95% and for at least 3 min in adults. Three minutes is roughly five half‐lives for a complete exchange of oxygen and, with certain caveats, should produce a FETO2 > 95% (which exceeds that suggested in the DAS guidelines) in a patient with a functional residual capacity of 2500 ml and alveolar ventilation of 3000 ml.min−1 [33]. A SpO2 of > 95% provides little if any assurance of adequate pre‐oxygenation, and some of our patients may have had a larger functional residual capacity than 2500 ml or lower alveolar ventilation than 3000 ml.min‐1. Thus we cannot be sure that we achieved the FETO2 specified in the DAS guidelines in all patients in the HFNO group. Our choice of 3 min reflects a human factors argument for keeping the time required for pre‐oxygenation short enough to facilitate universal compliance with an approach that should ensure all patients are at least reasonably well pre‐oxygenated, but there is a case for using a longer period in selected patients, or perhaps routinely, and some other investigators have done this [11, 34] . In fact, pre‐oxygenation was continued for more than 3 min in many patients in the HFNO group, and, on average, for longer than that in the facemask group, although time from first pulse oximetry reading to securing an airway was not significantly different between groups. This longer duration of pre‐oxygenation with HFNO may reflect the ability to provide pre‐oxygenation conveniently while carrying out other tasks and without the need for a dedicated person to maintain a tight seal with a facemask, which might be one of the primary human factor advantages of this approach to pre‐oxygenation. In our protocol, we specified that episodes of hypoxaemia (SpO2 < 90%) and severe hypoxaemia (SpO2 ≤ 85%) should have a duration of 2 min because our software samples these data every 30 s; thus 2 min provides four consecutive samples, which may make artefactual results less likely than two consecutive samples [7]. However, we have also reported episodes of hypoxaemia of 1 min duration. Finally, industry funding was a potential source of bias. However, the study was investigator initiated, and employees of the sponsor were not involved in the collection or analysis of the data, or in writing the paper, other than by reading and commenting on these. Importantly, an independent study monitor regularly reviewed the study processes and documentation, including the primary data records.
In conclusion, we found pre‐oxygenation with HFNO to be easier for anaesthetists and more comfortable for patients than pre‐oxygenation with a facemask with no clinically relevant differences in FETO2 after securing a definitive airway or in time to secure an airway. The differences in ease and comfort were modest.
Supporting information
Appendix S1. Smiley face scale.
Appendix S2. Adverse events.
Acknowledgements
This study was prospectively registered in the Australian and New Zealand Clinical Trials Registry (ACTRN12616001433493). We would like to thank all of the patients who participated in this study, A. Barber, K. English, D. Harvey and C. Bradfield for their contributions as members of the Data Safety Monitoring Committee, S. Wilkinson and V. Ward for assistance with data collection, A. Potts for her careful monitoring of the trial and C. Kruger for his contribution to the development of the protocol. This investigator‐initiated study was funded by a grant from Fisher and Paykel Healthcare. AM is a director of and has shares in Safer Sleep LLC. He has a consulting contract with Fisher and Paykel Healthcare. MP and GK are employees of Fisher and Paykel Healthcare. No other external funding or competing interests declared. Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians.
References
- 1. Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. British Journal of Anaesthesia 2011; 106: 617–31. [DOI] [PubMed] [Google Scholar]
- 2. Yentis SM. Predicting difficult intubation ‐ worthwhile exercise or pointless ritual? Anaesthesia 2002; 57: 105–9. [DOI] [PubMed] [Google Scholar]
- 3. Yentis SM. Predicting trouble in airway management. Anesthesiology 2006; 105: 871–2. [DOI] [PubMed] [Google Scholar]
- 4. Nørskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrøm LH. Diagnostic accuracy of anaesthesiologists' prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia 2015; 70: 272–81. [DOI] [PubMed] [Google Scholar]
- 5. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mosier J, Reardon RF, DeVries PA, et al. Time to loss of preoxygenation in emergency department patients. Journal of Emergency Medicine 2020; 59: 637–42. [DOI] [PubMed] [Google Scholar]
- 7. Ehrenfeld JM, Funk LM, Van Schalkwyk J, Merry AF, Sandberg WS, Gawande A. The incidence of hypoxemia during surgery: evidence from two institutions. Canadian Journal of Anesthesia 2010; 57: 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baillard C, Depret F, Levy V, Boubaya M, Beloucif S. Incidence and prediction of inadequate preoxygenation before induction of anaesthesia. Annales Francaises d Anesthesie et de Reanimation 2014; 33: e55–e58. [DOI] [PubMed] [Google Scholar]
- 9. de Klerk A. Humidified high‐flow nasal cannula: is it the new and improved CPAP? Advances in Neonatal Care 2008; 8: 98–106. [DOI] [PubMed] [Google Scholar]
- 10. Parke R, McGuinness S, Eccleston M. Nasal high‐flow therapy delivers low level positive airway pressure. British Journal of Anaesthesia 2009; 103: 886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel A, Nouraei SA. Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel A, El‐Boghdadly K. Facemask or high‐flow nasal oxygenation: time to switch? Anaesthesia 2021; 11: 11. [DOI] [PubMed] [Google Scholar]
- 13. Hamp T, Prager G, Baron‐Stefaniak J, Muller J, Bichler C, Plochl W. Duration of safe apnea in patients with morbid obesity during passive oxygenation using high‐flow nasal insufflation versus regular flow nasal insufflation, a randomized trial. Surgery for Obesity and Related Diseases 2021; 17: 347–55. [DOI] [PubMed] [Google Scholar]
- 14. Hua Z, Liu Z, Li Y, Zhang H, Yang M, Zuo M. Transnasal humidified rapid insufflation ventilatory exchange vs. facemask oxygenation in elderly patients undergoing general anaesthesia: a randomized controlled trial. Scientific Reports 2020; 10: 5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyons C, McElwain J, Coughlan MG, et al. Pre‐oxygenation with facemask oxygen vs high‐flow nasal oxygen vs high‐flow nasal oxygen plus mouthpiece: a randomised controlled trial. Anaesthesia 2021; 17: 17. [DOI] [PubMed] [Google Scholar]
- 16. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre‐oxygenation with facemask pre‐oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia 2017; 72: 439–43. [DOI] [PubMed] [Google Scholar]
- 17. Pillai A, Daga V, Lewis J, Mahmoud M, Mushambi M, Bogod D. High‐flow humidified nasal oxygenation vs. standard face mask oxygenation. Anaesthesia 2016; 71: 1280–3. [DOI] [PubMed] [Google Scholar]
- 18. Rajan S, Joseph N, Tosh P, Kadapamannil D, Paul J, Kumar L. Effectiveness of transnasal humidified rapid‐insufflation ventilatory exchange versus traditional preoxygenation followed by apnoeic oxygenation in delaying desaturation during apnoea: a preliminary study. Indian Journal of Anaesthesia 2018; 62: 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sjoblom A, Broms J, Hedberg M, et al. Pre‐oxygenation using high‐flow nasal oxygen vs. tight facemask during rapid sequence induction. Anaesthesia 2021; 76: 1176–83. [DOI] [PubMed] [Google Scholar]
- 20. Tiruvoipati R, Lewis D, Haji K, Botha J. High‐flow nasal oxygen vs high‐flow face mask: a randomized crossover trial in extubated patients. Journal of Critical Care 2010; 25: 463–8. [DOI] [PubMed] [Google Scholar]
- 21. Tremey B, Squara P, De Labarre H, et al. Hands‐free induction of general anesthesia: a randomised pilot study comparing usual care and high‐flow nasal oxygen. Minerva Anestesiologica 2020; 86: 1135–42. [DOI] [PubMed] [Google Scholar]
- 22. Wong DT, Dallaire A, Singh KP, et al. High‐flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesthesia and Analgesia 2019; 129: 1130–6. [DOI] [PubMed] [Google Scholar]
- 23. Zhou S, Zhou Y, Cao X, et al. The efficacy of high flow nasal oxygenation for maintaining maternal oxygenation during rapid sequence induction in pregnancy: a prospective randomised clinical trial. European Journal of Anaesthesiology 2020; 24: 24. [DOI] [PubMed] [Google Scholar]
- 24. Whaley LF, Wong DL. Nursing Care of Infants and Children. 2nd edn. St. Louis: Mosby, 1983. [Google Scholar]
- 25. Humphreys S, Lee‐Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. British Journal of Anaesthesia 2017; 118: 232–8. [DOI] [PubMed] [Google Scholar]
- 26. Ng I, Krieser R, Mezzavia P, Lee K, Tseng C, Douglas NWR, Segal R. The use of Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) for pre‐oxygenation in neurosurgical patients: a randomised controlled trial. Anaesthesia and Intensive Care 2018; 46: 360–7. [DOI] [PubMed] [Google Scholar]
- 27. Lodenius A, Piehl J, Ostlund A, Ullman J, Jonsson FM. Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre‐oxygenation for rapid sequence induction in adults: a prospective randomised non‐blinded clinical trial. Anaesthesia 2018; 73: 564–71. [DOI] [PubMed] [Google Scholar]
- 28. Hanouz JL, Lhermitte D, Gerard JL, Fischer MO. Comparison of pre‐oxygenation using spontaneous breathing through face mask and high‐flow nasal oxygen: a randomised controlled crossover study in healthy volunteers. European Journal of Anaesthesiology 2019; 36: 335–41. [DOI] [PubMed] [Google Scholar]
- 29. Vourc'h M, Baud G, Feuillet F, et al. High‐flow nasal cannulae versus non‐invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine 2019; 13: 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ang KS, Green A, Ramaswamy KK, Frerk C. Preoxygenation using the Optiflow system. British Journal of Anaesthesia 2017; 118: 463–4. [DOI] [PubMed] [Google Scholar]
- 31. Wensley C, Botti M, McKillop A, Merry AF. A framework of comfort for practice: An integrative review identifying the multiple influences on patients' experience of comfort in healthcare settings. International Journal for Quality in Health Care 2017; 29: 151–62. [DOI] [PubMed] [Google Scholar]
- 32. Wensley C, Botti M, McKillop A, Merry AF. Maximising comfort: how do patients describe the care that matters? A two‐stage qualitative descriptive study to develop a quality improvement framework for comfort‐related care in inpatient settings. British Medical Journal Open 2020; 10: e033336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Canadian Journal of Anesthesia 2009; 1: 449–66. [DOI] [PubMed] [Google Scholar]
- 34. Vourc'h M, Asfar P, Volteau C, et al. High‐flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Medicine 2015; 41: 1538–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Smiley face scale.
Appendix S2. Adverse events.


