Both nickel (Ni) and cobalt (Co) are common skin sensitizers known to cause contact allergy, 1 not only existing in alloys but also in different consumer products, such as cosmetics, 2 even though not intentionally added. Mineral pigments such as iron‐ (Fe), manganese‐ (Mn) and titanium‐ (Ti) oxides are often blended and used for colouring of cosmetic products.3, 4 These pigments may naturally contain different metal impurities such as Ni and Co. Repetitive use may result in skin accumulation and give rise to allergic contact dermatitis. 5 A recent report 6 on patch test results on 52 000 dermatitis patients in Europe shows 17.6% of the patients to be positive to Ni (the largest fraction) and 5.39% positive to Co. So far, it is unclear whether impurities of Ni or Co are associated with specific pigments in cosmetics. The aim of this study was to assess total amounts of Ni and Co in various inorganic pigments common in facial and powdered cosmetics (e.g., eye shadow cosmetics), and determine the extent of their dissolution into artificial sweat.
METHODS
Samples of 10 colouring pigments used in various facial cosmetic products were kindly provided by a supplier focused on cosmetics. The investigated pigments were all inorganic: (1) Unipure Yellow LC 182, (2) Unipure Red LC 381, (3) Unipure Pink LC 583, (4) Unipure Violet LC 581, (5) Unipure Black LC 990, (6) Zeosafe CL‐07, (7) Mica GH 103, (8) Mica GH 108, (9) Mica GH 603 and (10) Unipure White LC 981. All pigments were supplied as dry powders and covered a range of colours (confirmed by three different people to avoid personal colour bias). Details are compiled in Figure 1.
FIGURE 1.
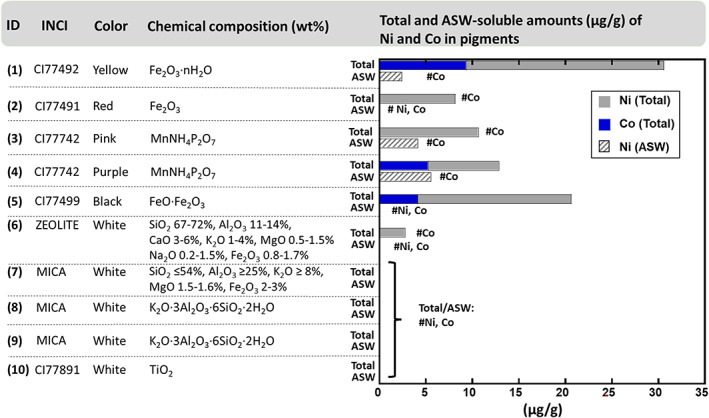
Summary of the investigated pigments (pigment ID number, International Nomenclature Cosmetic Ingredient (INCI) name, colour, chemical composition (supplier information)), and total and artificial sweat (ASW)‐soluble amounts (μg/g) of Ni and Co from each pigment (by means of graphite furnace atomic absorption spectrometry, raw data in Table S1 in Appendix S1) compared with the total content. #Ni or #Co denotes a concentration below the limit of detection (see “Methods” section)
The total Ni and Co content in the pigments were determined after complete pigment digestion in diluted aqua regia 7 (2.5 vol% HCl and 6.5 vol % HNO3, pH <0.1). The sweat‐soluble fraction of Ni and Co from the pigments were investigated after one‐week extraction in artificial sweat (ASW, pH 6.5) at 30 °C, according to EN 1811:2011 + A1:2015. 8 Details are provided in section S1.1 in Appendix S1. All particle‐free solution samples were analysed by means of graphite furnace atomic absorption spectrometry (GF‐AAS) using a PerkinElmer AA800 analyst instrument and triplicate readings of each sample. 9 In diluted aqua regia, the limits of detection were 0.76–0.83 µg/g for Ni and 1.17–1.27 µg/g for Co. In ASW, they were 1.21–1.29 µg/g for Ni and 0.85–0.91 µg/g for Co.
The potential enrichment of Ni and Co in selected pigments were assessed by means of time of flight secondary ion mass spectrometry (ToF‐SIMS), a surface‐sensitive technique with a detection limit in the μg/g range. Details are given in Appendix S1.
RESULTS
Results on soluble amounts of Ni and Co released into artificial sweat from the pigments compared to their total content are summarized in Figure 1. All coloured pigments (IDs 1–5), and one of the white pigments (ID 6), contained detectable amounts of Ni, whereas only the yellow, purple and black pigments (IDs 1, 4, 5) also contained Co. The Co contents were for these pigments lower than the corresponding Ni contents. Only Ni was dissolved into ASW. The ASW‐soluble fractions of Ni were lower than the total contents and were only observed for three pigments (yellow (ID 1), pink (ID 3) and purple (ID 4)), in amounts ranging from 2.4 to 5.5 μg Ni/g pigment. Ni was only soluble into ASW for pigment ID 1, containing Fe2O3·nH2O, but not Fe2O3 (ID 2) or FeO·Fe2O3 (ID 5). This can be explained by different solubilities of these Fe oxides, also influencing the dissolution of impurities.10, 11 The coloured pigments (IDs 1–5) were all rich in Fe or Mn and contained clearly higher total amounts (7.6–21.2 μg/g) of Ni compared with the white pigments (2.7 μg/g—ID 6, <1.17 μg/g—IDs 7–10). Co was an identified constituent in the Fe‐ and Mn‐rich pigments (the yellow, purple and black pigments, IDs 1,4,5), ranging from 4.1 to 9.4 μg/g (total content), though no Co was released into ASW. To further gain insights in the location and distribution of the Co and Ni impurities, investigations were performed on the yellow and black pigments of highest Ni and Co content (IDs 1 and 5) by means of ToF‐SIMS. Neither Ni nor Co was observed (see Figures S1 and S2). This implies that Ni and Co were not locally enriched but rather evenly distributed within the pigments.
DISCUSSION
Findings of this study reveal that impurities of both Ni and Co can be present in inorganic metal oxide pigments used in cosmetics. Their presence was associated with metal oxides rich in Fe and Mn. These pigments are usually darker and/or more colourful compared with less coloured or white pigments containing silicon‐ (Si) and Ti‐rich pigments. A similar trend was observed in a recent study on tattoo inks 3 showing a strong correlation between the content of impurities of Ni and Co from the ink with several metal constituents, including Fe and Mn, and also between each other. Both Ni and Co had considerably lower solubility in artificial sweat than the corresponding total impurity content. The dissolution of Ni into artificial sweat was primarily observed for Fe‐ and Mn‐oxide pigments at a concentration above 1 μg/g, a level that may increase the risk for the development of allergies in sensitized subjects.4, 5 Note that the used concentration of these pigments in some facial cosmetic products, such as eye shadow, can be a significant fraction (a few to more than 50%) of the ingredients. Consistent with a previous study on cosmetic pigments, 12 no dissolution of Co was observed in artificial sweat, which suggests Co in these pigments to be present in insoluble forms or in too low concentrations to be detected.
AUTHOR CONTRIBUTIONS
Xuying Wang planned and conducted the study, and drafted the manuscript; Yolanda S. Hedberg supervised the study and revised the manuscript; Inger Odnevall supervised the study and revised the manuscript.
FUNDING INFORMATION
This work was supported by Wolfe‐Western fellowship, Canada (#2020); the Canada Research Chairs Program (#950‐233099); start‐up funds (#Western 2020); KTH faculty grants (#Odnevall 2019‐2020).
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGEMENTS
We thank Dr. Jonas Hedberg, Dr. Heng‐Yong Nie and Kwang Soak Gabriel O'Donnell, all at the University of Western Ontario, Canada, for ToF‐SIMS measurements and discussions.
Wang X, Hedberg YS, Odnevall I. Presence of impurities of nickel and cobalt in facial cosmetic pigments and their dissolution into artificial sweat. Contact Dermatitis. 2022;87(6):550‐553. doi: 10.1111/cod.14212
Funding information Canada Research Chairs Program (#950‐233099); KTH faculty grants (#Odnevall 2019‐2020); start‐up funds (#Western 2020); Wolfe‐Western fellowship, Canada (#2020)
REFERENCES
- 1. Chen JK, Thyssen JP. Metal Allergy: From Dermatitis to Implant and Device Failure. Springer; 2018:177‐196. [Google Scholar]
- 2. EU . Regulation of the European Parliament and of the Council on Cosmetic Products 2009 . Accessed June 7, 2022. https://data.consilium.europa.eu/doc/document/ST-3623-2009-INIT/en/pdf
- 3. Wang X, Josefsson L, Meschnark S, et al. Analytical survey of tattoo inks—a chemical and legal perspective with focus on sensitizing substances. Contact Dermatitis. 2021;85(3):340‐353. [DOI] [PubMed] [Google Scholar]
- 4. Borowska S, Brzóska MM. Metals in cosmetics: implications for human health. J Appl Toxicol. 2015;35(6):551‐572. [DOI] [PubMed] [Google Scholar]
- 5. Bocca B, Pino A, Alimonti A, Forte G. Toxic metals contained in cosmetics: a status report. Regul Toxicol Pharmacol. 2014;68(3):447‐467. [DOI] [PubMed] [Google Scholar]
- 6. Uter W, Bauer A, Belloni Fortina A, et al. Patch test results with the European baseline series and additions thereof in the ESSCA network, 2015–2018. Contact Dermatitis. 2021;84(2):109‐120. [DOI] [PubMed] [Google Scholar]
- 7. Dulski TR. A Manual for the Chemical Analysis of Metals. ASTM West Conshohocken; 1996. [Google Scholar]
- 8. CEN . Reference test method for release of nickel from all post assemblies which are inserted into pierced parts of the human body and articles intended to come into direct and prolonged contact with the skin, EN 1811:2011+A1:2015; 2015.
- 9. Wang X, Herting G, Wei Z, Odnevall Wallinder I, Hedberg Y. Bioaccessibility of nickel and cobalt in powders and massive forms of stainless steel, nickel‐or cobalt‐based alloys, and nickel and cobalt metals in artificial sweat. Regul Toxicol Pharmacol. 2019;106:15‐26. [DOI] [PubMed] [Google Scholar]
- 10. Baes CF, Mesmer RE. The Hydrolysis of Cations. Robert E. Krieger; 1986:226‐237. [Google Scholar]
- 11. Cornell RM, Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses. Wiley‐vch Weinheim; 2003. [Google Scholar]
- 12. Bruzzoniti MC, Abollino O, Pazzi M, Rivoira L, Giacomino A, Vincenti M. Chromium, nickel, and cobalt in cosmetic matrices: an integrated bioanalytical characterization through total content, bioaccessibility, and Cr (III)/Cr (VI) speciation. Anal Bioanal Chem. 2017;409(29):6831‐6841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


