Abstract
Globally, respiratory syncytial virus (RSV) is the leading cause of bronchiolitis and pneumonia in young children, and the association between severe RSV disease and later recurrent wheeze and asthma is well established. Whilst a causal link between RSV and wheeze/asthma is not yet proven, immunological evidence suggests skewing towards a Th2‐type response, and dampening of IFN‐γ antiviral immunity during RSV infection underpins airway hyper‐reactivity in a subset of susceptible children after RSV infection. Age at primary RSV infection, viral co‐infection and genetic influences may act as effect‐modifiers. Despite the significant morbidity and mortality burden of RSV disease in children, there is currently no licensed vaccine. Recent advancements in RSV preventatives, including long‐acting monoclonal antibodies and maternal vaccinations, show significant promise and we are on the cusp of a new era in RSV prevention. However, the potential impact of RSV preventatives on subsequent wheeze and asthma remains unclear. The ongoing COVID‐19 pandemic and associated public health measures have disrupted the usual seasonality of RSV. Whilst this has posed challenges for health‐care services it has also enhanced our understanding of RSV transmission. The near absence of RSV cases during the first year of the pandemic in the context of strict public health measures has provided a rare opportunity to study the impact of delayed age of primary RSV infection on asthma prevalence. In this review, we summarise current understanding of the association between RSV, recurrent wheeze and asthma with a focus on pathophysiology, preventative strategies and future research priorities.
Keywords: asthma, COVID‐19, palivizumab, respiratory syncytial virus, wheeze
Key Points.
The association between early respiratory syncytial virus (RSV) bronchiolitis and recurrent wheeze and asthma is clear, however a causal mechanism remains unproven and pathophysiology is yet to be fully elucidated.
Existing and potential RSV preventatives, including monoclonal antibodies and paediatric and maternal vaccines, will reduce the burden of acute RSV in the future. Their potential impact on later wheeze and asthma is an important research priority.
Out‐of‐season surges and delays in primary RSV infection due to COVID‐19 public health measures provide a unique opportunity to study the drivers of RSV transmission and any potential impact on future asthma.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of severe acute lower respiratory tract infection (LRTI) in infants and young children, resulting annually in 33 million infections, >3 million hospitalisations and >100 000 deaths in children aged 0–60 months world‐wide, 1 with a mortality rate of up to 9% in low‐resource countries representing 99% of global RSV mortality. 2 , 3 , 4 The vast majority of children have had serologically proven infection with RSV by age 2 years, 4 representing a major health‐care burden particularly during the peak winter season. 5 Risk factors such as socio‐economic status influence the morbidity and mortality from acute RSV infection, with low and middle‐income countries disproportionately impacted. 3 , 5 There are several risk factors common to many studies including preterm birth, younger age at time of infection and co‐infection with other respiratory viruses (Fig. 1). 5 Infants aged under 6 months account for 46% of total RSV mortality 2 , 6 and mortality is increased in children with co‐morbidities (e.g. cardiorespiratory or immune conditions) with approximately 5% mortality in children with congenital heart disease. 7 However, the vast majority of children that develop severe RSV LRTI are otherwise healthy infants born at term. 2
Fig. 1.
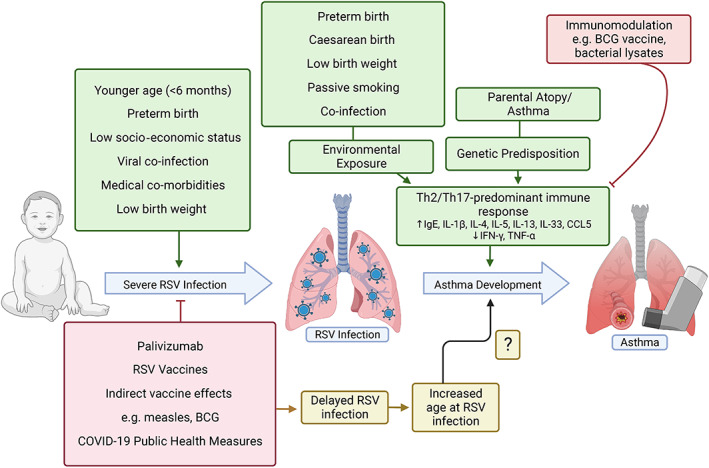
Postulated factors influencing the development of severe respiratory syncytial virus (RSV) infection in infants and children, and how they may relate to the hypothesised mechanism of a Th2/Th17‐predominant immune response linking RSV infection to subsequent asthma development. Depicted in green are factors with possible or proven positive association, and in red factors negatively associated with severe RSV infection or asthma in early life. The impact of RSV preventatives, and consequent later age of primary RSV infection, on asthma development is unknown. Created with BioRender.com.
An association between severe RSV bronchiolitis and subsequent recurrent wheeze and asthma into later childhood has been shown in several studies. 2 , 8 , 9 , 10 Recurrent wheeze and asthma are burdensome conditions that can interfere with a child's quality of life, are associated with frequent health‐care visits and contribute to workplace absences for caregivers. 8 , 11 Previous RSV bronchiolitis has also been associated with more severe asthma. 9 Compared to age‐matched controls without prior hospitalisation with RSV LRTI, hospitalisation with RSV LRTI was associated with more severe asthma as evidenced by three‐fold higher rates of asthma admission and medication use. 9 Acute RSV LRTI alone represents a significant public health issue, however its potential role in recurrent wheezing illnesses and asthma further highlights the need for interventions to reduce the burden of disease. This article will provide an overview of current understanding of the pathophysiological relationship between severe RSV, recurrent wheeze and asthma and discuss future potential preventative strategies. We will also discuss the impact of the SARS‐CoV‐2 (COVID‐19) pandemic on RSV epidemiology and how this may impact on the development of wheeze and asthma in the future.
Causality and Pathophysiology of the RSV–Asthma Relationship
Numerous studies and systematic reviews have established an association between RSV infection in infancy and later chronic wheeze and asthma, 8 however causality is yet to be determined. 2 , 4 , 11 , 12 A World Health Organization report published in 2020 was inconclusive in identifying whether the relationship between RSV and asthma was causal or one of correlation with a shared predisposition. 2 , 4 To conclusively determine the causality of RSV in asthma development, several criteria need to be met. First, a temporal relationship must be established, that is RSV infection should precede the onset of asthma. 12 In a systematic review, Feldman et al. identified several longitudinal studies that demonstrated that acute RSV infection preceded allergic sensitisation and subsequent asthma development. 12 , 13 , 14 Second, a dose‐dependent relationship should be evident, with more severe RSV infection being associated with heightened risk of asthma. Multiple studies have outlined the relationship between the severity of viral respiratory infections in infancy and subsequent asthma risk supporting a dose‐dependent effect. 12 , 13 , 15 Additionally, severe RSV infection in infancy may be associated with more severe forms of asthma in childhood. 9 Third, there must be a plausible biological mechanism. Recently, numerous potential pathophysiological relationships have been described suggesting that aberrant immunological responses during RSV infection may underpin asthma development, particularly in the setting of atopy, genetic predisposition and environmental exposures. 4 Understanding the immunological pathways linking severe RSV infection to asthma is an ongoing focus of research.
A recent review of infant immunity in severe RSV disease found that immature immune regulation may result in pathological skewing away from antiviral IFN‐γ mediated Th1 immunity towards a Th2‐dominant response associated with increased clinical severity of RSV disease and a more reactive, asthma‐prone airway, particularly in preterm infants. 16 Proposed mechanisms include decreased production of type 1 interferon (IFN) by less abundant plasmacytoid dendritic cells, decreased TNF‐α and IFN‐γ production by regulatory and γδ T cells and increased production of IL‐4, IL‐5, IL‐13 and IL‐33 by dendritic cells, Th2 and type 2 innate lymphoid cells (ILC2s) (Fig. 1). 16 , 17 , 18 In both murine models of RSV infection 19 and in vitro studies of RSV‐infected infant tracheal aspirates, 20 a Th2‐predominant pathway with CCL5, IL‐1β and IL‐13 production resulted in histological features similar to asthma. 19 , 20 These include goblet cell hyperplasia, increased mucus production and airway hyperreactivity. 4 A Th2/Th17‐dominant response to RSV infection, with increased IgE, IL‐4, IL‐13 and decreased IFN‐γ is widely reported in the literature as a significant predisposing factor for recurrent wheeze and asthma. 4 , 17 , 18 , 19 , 20 , 21 , 22 However, some study findings have contradicted this, identifying increased IFN‐γ and IFN‐α to be positively associated with asthma risk 21 and further research is needed.
The immune interplay between early RSV infection and development of wheeze and asthma is likely also influenced by genetic and environmental factors. Single nucleotide polymorphisms in genes coding chemokines and cytokines potentially act to sensitise and increase airway reactivity in both severe RSV bronchiolitis and asthma. 4 , 23 Decreased gene expression of suppressor of cytokine signalling (SOCS) proteins (SOCS2, SOCS3 and SOCS5) and TNFRSF25 (leading to decreased NF‐κB and IFN‐γ production), 16 and increased prevalence of the IL‐8‐251A polymorphism 17 may also play a role. Environmental factors may also predispose towards wheeze and asthma post‐RSV infection. Factors such as caesarean section delivery, birthweight, passive smoke exposure, 24 co‐infection with other respiratory viruses, e.g. rhinovirus, 8 , 21 age >6 months at time of infection, 25 , 26 respiratory microbiome, e.g. colonisation with Streptococcus pneumoniae, and parental asthma or atopy 21 have been shown to be important (Fig. 1). Further exploration of the risk factors and effect modifiers is essential in predicting and ultimately preventing subsequent morbidity and mortality. In particular, identification of immune biomarkers and therapeutic targets within immunological pathways enhancing the Th1 immune response may be of benefit. 16
The potential causal relationship between severe RSV infection and asthma is supported by long‐term follow‐up studies of children who received RSV monoclonal antibody prophylaxis. 12 A randomised controlled trial (MAKI trial) of palivizumab versus placebo in 429 healthy preterm infants born at 33–35 weeks resulted in a nearly 50% reduction in recurrent wheeze (11% vs. 21%, P = 0.01) in the first year of life. 27 Similar findings have also been demonstrated in an observational case–control study of ex‐premature infants followed for 3 years, 28 supporting a causal relationship between RSV and subsequent wheeze. However, subsequent follow‐up of the MAKI trial 27 with evaluation of asthma outcomes at 6 years of age demonstrated no significant reduction in physician‐diagnosed asthma or lung function in those infants treated with palivizumab compared to controls, despite a decrease in parent‐reported wheeze. 29 Similarly, a randomised controlled trial of another RSV preventative, motavizumab, in healthy Native American infants identified a reduction in parent‐reported wheeze but no significant impact of motavizumab over placebo on physician‐diagnosed wheezing at 1–3 years of age. 30 A systematic review 11 of palivizumab for prevention of recurrent wheeze and asthma outcomes did not identify any significant benefit. However, subgroup analysis identified a reduction in wheeze in infants born at 32–36 weeks' gestation without a family history of atopy, 11 substantiating a need for further studies into the potential benefits of RSV prophylaxis in late‐preterm infants. It is important to note that many studies of RSV preventatives were not adequately designed or powered to determine asthma outcomes in children at age 6 years, when spirometry (the gold‐standard for asthma diagnosis) can be reliably performed. Despite advancements in our understanding of the RSV–asthma relationship, there is still no consensus on causality. 12
RSV Prevention: Current and Future Interventions
The pathophysiology of asthma is complex and not yet fully elucidated. Targets for intervention are thus a focus of intense research. RSV likely represents a piece of the greater immunopathological puzzle of asthma pathogenesis. Currently, the only licensed preventative for severe acute RSV infection is passive immunoprophylaxis with palivizumab, a monoclonal antibody administered monthly during the RSV season. However, palivizumab is extremely expensive and is thus reserved for infants at the highest risk of severe RSV disease, for example prematurity <32 weeks or those with significant comorbidities. 2 , 11 However, severe bronchiolitis is frequently observed in low‐risk and late‐preterm infants who have a similar risk of developing wheeze and asthma, 25 , 31 hence a wider subgroup of infants would potentially benefit from access to RSV prophylaxis.
Age at primary RSV infection may influence the likelihood of developing asthma. Wang et al. showed that primary RSV infection at age 6–23 months was associated with increased risk of asthma and wheeze compared to primary infection at age 0–6 months. 25 Furthermore, Homaira et al. identified the rate of asthma hospitalisation was two to seven‐fold greater in children who had been hospitalised with RSV at ages >6 months compared to those admitted aged 0–6 months. 26 Moreover, Jackson et al. identified that infection with RSV at 3 years of age, when compared to earlier infection in the first 2 years of life, was more strongly associated with subsequent asthma at 6 years of age. 32 The achievement of early RSV prevention would enormously reduce RSV‐associated morbidity and mortality. 8 , 33 A vaccine with 80% efficacy would potentially prevent up 1.1 million hospitalisations and 22 000 deaths annually world‐wide. 3 However, transient protection against RSV and subsequent delayed age of primary infection may have inadvertent consequences on risk of asthma development. 25 , 26 , 32 Thus, with several new RSV preventatives on the horizon, well‐designed longitudinal studies are needed to determine the impact of early RSV prevention on future risk of asthma.
There are currently a number of paediatric and maternal RSV vaccines approaching clinical translation, with the World Health Organisation predicting vaccine release within 5–10 years 6 and regulatory approval for long‐acting monoclonal antibody prophylaxis expected within 1 year. 34 Important areas for exploration include the development of vaccines for infants <6 months with immunity enduring across childhood and maternal vaccines for transplacental protection. 6 , 32 A systematic review of RSV vaccines in development identified that live‐attenuated vaccines targeted to infants 6–24 months of age had the most promising immunogenicity and safety profiles with a >4‐fold increase (minimum measure of ‘immune‐response’) in neutralising and anti‐F antibodies across 90–95% of recipients. 6 Many candidate vaccines primarily target the prefusion F protein (preF), responsible for viral fusion with human cells, with a number of phase II trials of preF vaccines demonstrating a 9 to 15‐fold increase in RSV‐specific neutralising antibodies. 35 A randomised phase 2b trial of preF RSV vaccines in pregnant women demonstrated an increase of 11–17.5 in 50% RSV neutralising titre ratios compared to placebo and up to 91.5% efficacy against severe disease in infants of immunised pregnant women. 36 A new long‐acting monoclonal antibody, nirsevimab, has been shown to reduce severe RSV disease and associated hospitalisation in healthy preterm infants and requires only a single dose across a 5‐month peak season. 37
In addition to vaccines specifically targeting RSV, several alternative preventative strategies are also being explored. Early immunisation against measles, at 4 months of age rather than 6 months, has recently been suggested as a strategy to prevent severe RSV infection in vulnerable infants under 6 months. 38 Immune mechanisms of indirect protection from RSV include prevention of measles‐induced immunosuppression, epigenetic and metabolic adaptations to enhance innate immune responsiveness and potential B‐ and T‐cell cross‐reactivity to the measles vaccine providing some coverage against RSV infection. 38 , 39 Indirect immunity to many pathogens including RSV has also been described in association with the Bacille Calmette‐Guérin (BCG) vaccine, 40 which has been demonstrated to reduce incidence of RSV infection, 41 hospitalisation and mortality in BCG compared to non‐BCG vaccinated children. 40
It is also important to consider how RSV preventatives may impact upon subsequent risk of wheeze and asthma. Elucidating the pathophysiology of the RSV‐asthma pathway may reveal immunological targets for asthma prevention, such as those influencing the balance of the Th1/Th2 immune response. In addition to its potential role in RSV prevention, the BCG vaccine may also promote predominant Th1/Th17 immune responses to improve antiviral immunity for up to 12 months, 40 and potentially dampen the Th2 allergic‐type response associated with asthma. 16 The promising effects of immunomodulation in reducing asthma and wheeze have also been shown through the use of bacterial lysates, inactivated pathogenic antigens, demonstrated to improve the balance between Th1 and Th2 adaptive immune response to pathogens as summarised in a recent review in Frontiers in Immunology. 42 An alternative therapeutic strategy may involve specific targeting of immunological mediators implicated in the RSV‐asthma pathway, with medications such as allopurinol and anakinra, targeting uric acid and IL‐1β respectively. 20
RSV Infection in the Era of COVID‐19
The epidemiology of RSV disease has been significantly impacted by the ongoing coronavirus‐19 (COVID‐19) pandemic. In Australia, public health measures to mitigate against COVID‐19 initially dramatically reduced the incidence of RSV infections and hospitalisations in the first year of the pandemic, with a near absence of cases throughout its typical peak season in 2020. 43 Interseasonal resurgences of RSV however have since been observed nation‐wide, 44 in some cases exceeding the peak incidence of previous years and displaying a higher average age of infection 45 with an approximately three‐fold increase in hospitalisations in children observed in Victoria, Australia in early 2021 compared to pre‐pandemic levels. 46 A similar effect has been observed world‐wide, 44 in a large number of countries including the USA 47 and China. 48 Although public health restrictions have now largely eased across Australia and internationally the epidemiology of RSV remains difficult to predict in light of future COVID‐19 outbreaks. 44 Williams et al. discussed the challenges inherent to this shift in epidemiology and preparation of health‐care services to meet demands of unseasonal peaks in RSV infections, in addition to disruption to RSV‐related research and vaccine trials. 49 However, this change in epidemiology also presents a unique opportunity to observe RSV transmission dynamics and disease trends as it re‐emerges in the community. 49 Interestingly, interrupted transmission of RSV due to strict COVID‐19 public health measures in the first year of the pandemic was associated with significant reductions in genomic diversity of circulating RSV clades. 44 The successful and rapid development of novel mRNA vaccination platforms, sequencing and tracing technology to combat the COVID‐19 pandemic may also shed light on potential control strategies for outbreaks of other respiratory viruses including RSV. 49
Further considerations include whether a later age of primary RSV infection 45 may result in a greater prevalence of asthma amongst a cohort of infants protected from early‐life RSV infection by COVID‐19 restrictions. 25 This may provide clues as to the likely impact of long‐acting monoclonal agents with respect to asthma outcomes in delaying age of first RSV infection. The potential effects of RSV‐SARS‐CoV‐2 co‐infection on disease severity (and asthma risk) as seen with parainfluenza virus type 3 5 and rhinovirus 21 will be an important focus of ongoing research. 50 This merits ongoing follow‐up of a unique cohort of infants to determine how different factors influence asthma and wheeze outcomes later in life.
Conclusion
Despite significant advancements in our understanding of RSV disease and asthma pathophysiology and prevention, critical knowledge gaps remain. Priority research areas include continued development of RSV preventatives, anti‐viral therapies and identifying immunological targets for therapeutics to treat severe RSV disease. Furthermore, it will be important to consider the impact that protection from RSV in early infancy and subsequent delayed age at RSV infection by vaccination and prophylaxis may have upon potential asthma development. In the context of the ongoing COVID‐19 pandemic and unpredictable surges in RSV, surveillance remains crucial to inform timing of preventative interventions, health service preparedness and clinical trials. Close monitoring of trends in RSV infection and asthma through population‐based ecological studies will form the basis upon which to understand future changes to wheeze and asthma prevalence, further elucidating the relationship between these burdensome conditions.
Acknowledgement
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Conflict of interest: None declared.
References
- 1. Li Y, Wang X, Blau DM et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022; 399: 2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Driscoll AJ, Arshad SH, Bont L et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization‐sponsored meeting. Vaccine 2020; 38: 2435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, McAllister DA, O'Brien KL et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017; 390: 946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esteban I, Stein RT, Polack FP. A durable relationship: Respiratory syncytial virus bronchiolitis and asthma past their golden anniversary. Vaccines 2020; 8: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson J, Oeum M, Verkolf E et al. Factors associated with severe respiratory syncytial virus disease in hospitalised children: A retrospective analysis. Arch. Dis. Child. 2021; 107: 359–64. [DOI] [PubMed] [Google Scholar]
- 6. Shan J, Britton PN, King CL, Booy R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza Other Respir. Viruses 2021; 15: 539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen‐Van‐Tam J, Wyffels V, Smulders M et al. Cumulative incidence of post‐infection asthma or wheezing among young children clinically diagnosed with respiratory syncytial virus infection in the United States: A retrospective database analysis. Influenza Other Respir. Viruses 2020; 14: 730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi T, Ooi Y, Zaw EM et al. Association between respiratory syncytial virus‐associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 2019; 222: S628–S33. [DOI] [PubMed] [Google Scholar]
- 9. Coutts J, Fullarton J, Morris C et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr. Pulmonol. 2020; 55: 1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruotsalainen M, Heikkilä P, Backman K, Korppi M. An increased asthma risk continued until young adulthood after early‐childhood hospitalisation for wheezing. Acta Paediatr. 2021; 111: 157–62. [DOI] [PubMed] [Google Scholar]
- 11. Quinn LA, Shields MD, Sinha I, Groves HE. Respiratory syncytial virus prophylaxis for prevention of recurrent childhood wheeze and asthma: A systematic review. Syst. Rev. 2020; 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early‐life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am. J. Respir. Crit. Care Med. 2015; 191: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escobar GJ, Ragins A, Li SX, Prager L, Masaquel AS, Kipnis P. Recurrent wheezing in the third year of life among children born at 32 weeks' gestation or later: Relationship to laboratory‐confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch. Pediatr. Adolesc. Med. 2010; 164: 915–22. [DOI] [PubMed] [Google Scholar]
- 14. Kotaniemi‐Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus‐induced wheezing in infancy—The first sign of childhood asthma? J. Allergy Clin. Immunol. 2003; 111: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner SW, Young S, Landau LI, Le Souëf PN. Reduced lung function both before bronchiolitis and at 11 years. Arch. Dis. Child. 2002; 87: 417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson J, Do LAH, Wurzel D et al. Severe respiratory syncytial virus disease in preterm infants: A case of innate immaturity. Thorax 2021; 76: 942–50. [DOI] [PubMed] [Google Scholar]
- 17. Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr. Infect. Dis. J. 2003; 22: S76–82. [DOI] [PubMed] [Google Scholar]
- 18. Kitcharoensakkul M, Bacharier LB, Yin‐Declue H et al. Impaired tumor necrosis factor‐α secretion by CD4 T cells during respiratory syncytial virus bronchiolitis associated with recurrent wheeze. Immun. Inflamm. Dis. 2020; 8: 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narayanan D, Grayson MH. Comparing respiratory syncytial virus and rhinovirus in development of post‐viral airway disease. J. Asthma 2020; 59: 434–41. [DOI] [PubMed] [Google Scholar]
- 20. Schuler CF IV, Malinczak C‐A, Best SKK et al. Inhibition of uric acid or IL‐1β ameliorates respiratory syncytial virus immunopathology and development of asthma. Allergy 2020; 75: 2279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raita Y, Pérez‐Losada M, Freishtat RJ et al. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat. Commun. 2021; 12: 3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi T, Li N, He Y et al. Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb. Pathog. 2021; 156: 104867. [DOI] [PubMed] [Google Scholar]
- 23. Drysdale SB, Milner AD, Greenough A. Respiratory syncytial virus infection and chronic respiratory morbidity – Is there a functional or genetic predisposition? Acta Paediatr. 2012; 101: 1114–20. [DOI] [PubMed] [Google Scholar]
- 24. Zhou Y, Tong L, Li M et al. Recurrent wheezing and asthma after respiratory syncytial virus bronchiolitis. Front. Pediatr. 2021; 9: 649003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Li Y, Nair H, Campbell H, RESCEU Investigators . Time‐varying association between severe respiratory syncytial virus infections and subsequent severe asthma and wheeze and influences of age at the infection. J Infect Dis 2021; 226: S38–44. [DOI] [PubMed] [Google Scholar]
- 26. Homaira N, Briggs N, Oei JL et al. Association of age at first severe respiratory syncytial virus disease with subsequent risk of severe asthma: A population‐based cohort study. J. Infect. Dis. 2019; 220: 550–6. [DOI] [PubMed] [Google Scholar]
- 27. Blanken MO, Rovers MM, Molenaar JM et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013; 368: 1791–9. [DOI] [PubMed] [Google Scholar]
- 28. Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões EA. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 2013; 132: 811–8. [DOI] [PubMed] [Google Scholar]
- 29. Scheltema NM, Nibbelke EE, Pouw J et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: A randomised controlled trial. Lancet Respir. Med. 2018; 6: 257–64. [DOI] [PubMed] [Google Scholar]
- 30. O'Brien KL, Chandran A, Weatherholtz R et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy native American infants: A phase 3 randomised double‐blind placebo‐controlled trial. Lancet Infect. Dis. 2015; 15: 1398–408. [DOI] [PubMed] [Google Scholar]
- 31. Mejias A, Wu B, Tandon N et al. Risk of childhood wheeze and asthma after respiratory syncytial virus infection in full‐term infants. Pediatr. Allergy Immunol. 2020; 31: 47–56. [DOI] [PubMed] [Google Scholar]
- 32. Jackson DJ, Gangnon RE, Evans MD et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am. J. Respir. Crit. Care Med. 2008; 178: 667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. PATH . RSV vaccine and mAb snapshot 2019. Available from: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/.
- 34. Mazur NI, Terstappen J, Baral R et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powell K. The race to make vaccines for a dangerous respiratory virus. Nature 2021; 600: 379–80. [DOI] [PubMed] [Google Scholar]
- 36. Simões EAF, Center KJ, Tita ATN et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 2022; 386: 1615–26. [DOI] [PubMed] [Google Scholar]
- 37. Griffin MP, Yuan Y, Takas T et al. Single‐dose nirsevimab for prevention of RSV in preterm infants. N. Engl. J. Med. 2020; 383: 415–25. [DOI] [PubMed] [Google Scholar]
- 38. Do LAH, Toh ZQ, Licciardi PV, Mulholland EK. Can early measles vaccination control both measles and respiratory syncytial virus infections? Lancet Global Health 2021; 10: e288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mina MJ. Measles, immune suppression and vaccination: Direct and indirect nonspecific vaccine benefits. J. Infect. 2017; 74: S10–S7. [DOI] [PubMed] [Google Scholar]
- 40. Moulson AJ, Av‐Gay Y. BCG immunomodulation: From the ‘hygiene hypothesis’ to COVID‐19. Immunobiology 2021; 226: 152052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stensballe LG, Nante E, Jensen IP et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea‐Bissau: A beneficial effect of BCG vaccination for girls: Community based case–control study. Vaccine 2005; 23: 1251–7. [DOI] [PubMed] [Google Scholar]
- 42. Kaczynska A, Klosinska M, Janeczek K, Zarobkiewicz M, Emeryk A. Promising immunomodulatory effects of bacterial lysates in allergic diseases. Front. Immunol. 2022; 13: 907149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeoh DK, Foley DA, Minney‐Smith CA et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 2021; 72: 2199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eden J‐S, Sikazwe C, Xie R et al. Off‐season RSV epidemics in Australia after easing of COVID‐19 restrictions. Nat. Commun. 2022; 13: 2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foley DA, Yeoh DK, Minney‐Smith CA et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019‐related public health measures. Clin. Infect. Dis. 2021; 73: e2829–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McNab S, Ha Do LA, Clifford V et al. Changing epidemiology of respiratory syncytial virus in Australia – Delayed re‐emergence in Victoria compared to WA/NSW after prolonged lock‐down for COVID‐19. Clin. Infect. Dis. 2021; 73: 2365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. RSV National Trends Atlanta: CDC: Centers for Disease Control and Prevention (CDC). Available from: https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html.
- 48. Jia R, Lu L, Su L et al. Resurgence of respiratory syncytial virus infection during COVID‐19 pandemic among children in Shanghai, China. Front. Microbiol. 2022; 13: 938372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams TC, Sinha I, Barr IG, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID‐19 pandemic. Eurosurveillance 2021; 26: 2100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zandi M, Soltani S, Fani M, Abbasi S, Ebrahimi S, Ramezani A. Severe acute respiratory syndrome coronavirus 2 and respiratory syncytial virus coinfection in children. Osong. Public Health Res. Perspect. 2021; 12: 286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


