Abstract
Black men are disproportionately affected by prostate cancer (PCa), with earlier presentation, more aggressive disease, and higher mortality rates versus White men. Furthermore, Black men have less access to PCa treatment and experience longer delays between diagnosis and treatment. In this review, the authors discuss the factors contributing to racial disparities and present solutions to improve access to care and increase clinical trial participation among Black men with PCa. Racial disparities observed among Black men with PCa are multifaceted, evolving from institutional racism. Cultural factors include generalized mistrust of the health care system, poor physician‐patient communication, lack of information on PCa and treatment options, fear of PCa diagnosis, and perceived societal stigma of the disease. In the United States, geographic trends in racial disparities have been observed. Economic factors, e.g., cost of care, recovery time, and cancer debt, play an important role in racial disparities observed in PCa treatment and outcomes. Racial diversity is often lacking in genomic and precision medicine studies. Black men are largely underrepresented in key phase 3 PCa trials and may be less willing to enroll in clinical trials due to lack of awareness, lack of diversity in clinical trial research teams, and bias of health care providers to recommend clinical research. The authors propose solutions to address these factors that include educating clinicians and institutions on the barriers Black men experience, increasing the diversity of health care providers and clinical research teams, and empowering Black men to be involved in their treatment, which are keys to creating equity for Black men with PCa.
Lay summary
Prostate cancer negatively affects Black men more than men of other races.
The history of segregation and mistreatment in the health care system may contribute to mistrust among Black men.
Outcomes are worse for Black men because they are less likely to be screened or to receive treatment for prostate cancer.
Black men also are unlikely to participate in clinical research, making it difficult for investigators to understand how Black men are affected by prostate cancer.
Suggestions for addressing these differences include teaching physicians and nurses about the issues Black men experience getting treatment and improving how Black men get information on prostate cancer.
Keywords: African American, Black men, clinical trial participation, institutional racism, prostate cancer, racial disparity
Short abstract
Racial disparities seen within prostate cancer diagnosis, treatment, and outcomes are multifaceted and evolve from institutional racism. The disparities can be addressed by educating physicians about the barriers faced by Black men receiving health care, identifying the best practices for conveying information on treatments and clinical trials, and increasing diversity among health care professionals.
INTRODUCTION
The incidence of prostate cancer (PCa) is continually increasing, with an estimated 268,490 projected cases in the United States in 2022. 1 Black men are disproportionally affected by PCa; they have the highest PCa incidence in the United States (183.4 new cases per 100,000) 2 , 3 , 4 and, compared with White men, are more likely to develop PCa at almost every stage of the disease continuum and in every age group. 2 , 5 This higher disease burden is reflected in a 2.2‐fold higher risk of death from PCa versus White men. 6 Black men are also more likely to present with PCa at a younger age 7 (an average of 2 years earlier) 8 and to be diagnosed with more aggressive disease and/or diagnosed at later stages, contributing to fewer treatment options and higher morbidity and mortality rates. 8 , 9 , 10 , 11 Despite this, when receiving treatment for PCa, fewer Black men undergo intensive therapy and/or definitive surgery with radical prostatectomy (RP) versus White men. 11 , 12 For these reasons, the American Cancer Society recommends that Black men begin discussing prostate‐specific antigen (PSA) testing with their physician at age 45 years (vs. 45–50 years for average‐risk men) to increase the chances of an early diagnosis. 6 However, although guidelines highlight the age disparity between racial groups, they fail to provide explicit screening recommendations for Black men with PCa.
Participation in clinical research for minority populations is critical to improving disease outcomes, yet Black men are under‐enrolled and underrepresented in key PCa trials. Factors accounting for low enrollment of Black men include an overall mistrust in the health care system and clinical research, a lack of awareness and access to prospective clinical trials, lack of representative diversity in clinical trial research teams, lack of education or bias of health care providers to recommend definitive therapies or access to clinical research, and reluctance to receive medical care (Fig. 1). 13 , 14 , 15 , 16
FIGURE 1.
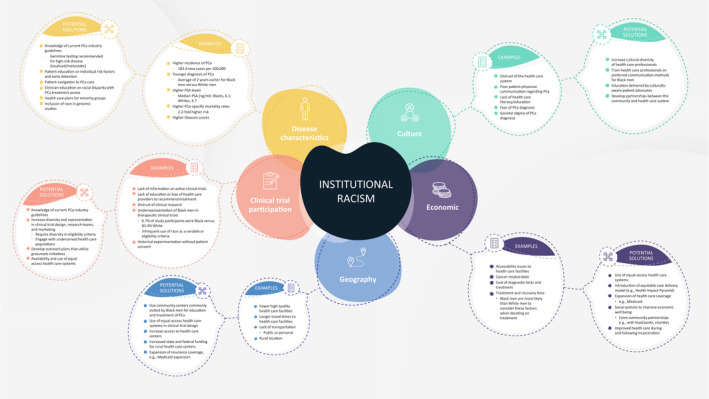
A summary of factors contributing to the racial disparities observed in populations of men with prostate cancer. PCa indicates prostate cancer; PSA, prostate‐specific antigen. Adapted from: Vince RA Jr, Eyrich NW, Mahal BA, Stensland K, Schaeffer EM, Spratt DE. Reporting of racial health disparities research: are we making progress? J Clin Oncol. 2022; 40(1):8–11.
The root cause of racial disparity in the diagnosis, treatment, and outcomes of PCa is multifaceted, complex, and is driven by institutional racism. This review discusses factors contributing to racial disparities to frame solutions for improving access to care and increasing clinical trial participation among Black men with PCa.
OBSERVATIONS
Institutional racism in the health care system
Historically, racial disparities were explained by biologic differences; however, advances in clinical and social research highlight the role of institutional racism contributing to disparities in the health care system. Medical mistreatment of the Black population is deeply rooted in history, including the Transatlantic slave trade, in which Black people were denied medical treatment, and the belief that cancer was unimportant in minority races. 17 Inaccurate biologic differences between races, e.g., higher pain thresholds and reduced risk of injury, were published in medical journals, legitimizing ill‐informed medical judgements. 18 The racism that intertwines the foundations of medical society is evident in the modern day through denial of health and life insurance coverage among Black families with incomes <$75,000 and lower socioeconomic status (SES), leading to lower health care access and resources, ultimately resulting in the underdiagnosis and/or misdiagnosis of cancer. 5 , 19 , 20
Disease characteristics in Black men
Black men exhibit higher PSA levels 21 (median PSA 6.1. ng/ml vs. 4.7 ng/ml for White men) 22 and Gleason scores >6 compared with White men. 23 Despite earlier disease progression, PSA screening rates were lower among Black men versus White men (30.3%; 95% confidence interval [CI], 28.3%–32.3%; vs. 32.3%; 95% CI, 31.7%–32.8%, respectively). 24 PSA screening frequencies declined by 9.5% between 2012 and 2018, with the largest decline seen among Black men (11.6% vs. 9.3% in White men). 24
Although Black men are unlikely to receive PSA screening, the PCa‐specific mortality (PCSM) rate in Black men declined from 81.9 deaths per 100,000 in 1993 to 39.8 deaths per 100,000 between 2012 and 2016. 6 PCSM among White men also declined, although to a lower degree, from 37 deaths per 100,000 in 1993 to 19 deaths per 100,000 during 2012–2016. 6 Nevertheless, the presence of the mortality gap (>2.0‐fold) between Black and White men was consistent between 2012 and 2016. 6
Genomic and precision medicine studies often lack racial diversity, with most data derived from men of European ancestry. 25 A systematic review of key studies performing whole genome sequencing or exome sequencing (2010–2018) reported that only 37% of studies used race as a variable, and, of those studies, only 14% of patients were Black. 26
PCa is one of the most inheritable malignancies 2 and whole genome sequencing has identified individual single‐nucleotide polymorphisms, 2 PCa loci, 2 and allele substitutions 25 associated with an increased risk of PCa. Germline variants, such as rs1447295, rs16901979, rs7000448, and rs6983267, 27 , 28 found on chromosome 8q24 are consistently associated with earlier development of PCa in Black men and disease characteristics such as higher Gleason score, advanced tumor stage, and metastasis. 28 , 29 Although genomic regions have been associated with PCa, no single gene is responsible for the differences between racial groups. In addition, genetic mutations associated with clinical outcomes occur at similar rates across race groups. 30 Updated guidelines recommend germline testing for all men with high‐risk localized and metastatic PCa. 31 , 32 Further study is required into alleles unique to Black ancestry, e.g., at chromosomes 2p21, 11q22, 17p11, 22q12, and Xq21, to understand the genetic link between race and PCa outcomes. 33
Impact of demographics, health care literacy, and population trends on treatment access in Black men
Cultural mistrust of the health care system
Black men experience more issues gaining access to PCa care compared with White men. 5 Barriers to access could be financial, such as lower SES and health care coverage, or nonfinancial, such as poor health‐seeking behaviors and limited knowledge of PCa (Fig. 1). 5 , 34 , 35
Black men report higher levels of general distrust of the health care system compared with other racial groups. 5 , 13 , 36 A study assessing attitudes toward PCa care (2015–2017) reported that Black men express fear that PCa screening will not be thorough and personal test results will be misused. 13 This mistrust is associated with higher degrees of negative opinion and fear of diagnostic outcomes. 37 Consequently, symptomatic Black men are unlikely to seek professional evaluation and advice on PCa. 37
Lower accessibility to clinicians with similar culture may factor into the unwillingness to seek diagnoses and care 38 because more Black men report that similar culture is important compared with White men (49.6% vs. 31.2%, respectively). 39 In 2019, Black men comprised 13.4% of the US population, 40 yet 6.2% of medical school graduates and only 5.0% of active clinicians were Black. 41 Improving cultural diversification of the health care system and developing partnerships with community organizations will improve communication and trust, resulting in better outcomes for Black men with PCa.
Communication
Communication issues offer further explanations for the racial disparities reported in PCa diagnosis and treatment within the United States. 42 Black men were unlikely to report good physician‐patient communication compared with White men (60% vs. 71%, respectively; p < .001), 42 with conversations regarding PSA screening described as infrequent and suboptimal. 35 A retrospective analysis of 1308 men with PCa reported that decisions on PCa and screening among Black men were heavily dependent on information from community members, family, and interpersonal sources, such as barbers and pastors. 43 , 44 The inclusion of women in community outreach programs and health fairs positively impacted attendance, with men remarking on how women emphasize and start conversions about preventative care. 45 Such preferences for receiving information on PCa highlight how health care programs should be culturally tailored to communicate effectively with Black patients.
Knowledge of PCa within the Black community
Nearly one half of Black men report being uneducated about PCa screening (e.g., PSA testing or digital rectal examination). 37 , 46 , 47 Studies evaluating PCa knowledge reported low scores among Black men across various ages, educational levels, and SES levels. 46 , 47 Assessing knowledge of PCa using a 12‐item questionnaire, Ogunsanya et al. reported that 47% of 267 Black patients had no knowledge of the signs of the disease, PSA testing, guidelines for screening age, and posttreatment quality of life. 47 Black men were unlikely to be provided with information essential to shared decision‐making (SDM) before PSA testing 35 and, when provided with information, remained confused compared with White men (13.1% vs. 4.8%; p = .008). 44 In addition, more Black men with aggressive, high‐risk PCa misperceived the severity of their disease compared with White men and thus were unlikely to pursue treatment. 48
Treatment access for Black men
The type of health care facility available to patients can influence equal access to PCa screening and treatment. Studies have reported that, despite having clinical characteristics similar to those of White men, Black men were unlikely to receive treatment 5 , 12 , 23 , 49 , 50 , 51 and were more likely to experience delays between PCa diagnosis and treatment. 36 , 49 National Cancer Institute‐designated Comprehensive Cancer Centers (NCI‐CCCs) follow structured processes to ensure the quality and quantity of research and clinical care. Patients who receive planned treatment at NCI‐CCCs experience better 5‐year overall survival (OS) rates compared with those not treated at these facilities (OS, 64.3% [95% CI, 62.8%–65.8%] vs. 60.7% [95% CI, 60.3%–61.1%], respectively; p < .001). 52 Patients who were uninsured, Black, or had lower SES were unlikely to receive treatment from NCI‐CCCs compared with those who were White, insured, or had higher SES. 52
Furthermore, Black men were unlikely to undergo treatment (adjusted odds ratio [OR], 0.71; 95% CI, 0.67–0.76), 51 and RP (69.0% vs. 91.0%; p < .001). 53 However, when adjusting for the use of equal‐access health care systems (e.g., the Veterans Health Administration or the Veterans Affairs [VA] health care system), Medicare insurance, or receipt of care in clinical trials, disparities in the likelihood or time to treatment were no longer reported. 5 , 21 , 54 Some studies using equal‐access trial designs report that Black men were more likely to receive treatment (OR, 1.05; p < .001) 54 and to have better outcomes compared with White men. 55
Many publications offer suggestions to improve treatment access through communication and education about PCa; however, often these solutions are not race‐focused. Men who are more knowledgeable about PCa treatment options, possible short‐term and long‐term adverse events, and clinical evidence and those who partake in SDM report better understanding, lower anxiety, and overall better quality of life versus those who are ill‐informed. 56 During 2015–2018, the SDM process between patients and physicians increased. 57 This is reflected in the 2018 US Preventive Services Task Force, which revised its 2012 recommendation of not initiating PSA screening to emphasize the importance of the SDM process for patients. 58 However, evidence is lacking that demonstrates any impact of SDM on health outcomes, especially across racial groups, 59 highlighting that PCa management for Black men is an important area on which to focus for future improvement.
Geographic differences
Black men report having less access to high‐quality PCa care 5 compared with White men, depending on geographic location (36.3% vs. 31%; p < .001), 42 reflected in differing OS outcomes between racial groups. 60 Black men experienced higher mortality rates than White men in New Jersey (adjusted hazard ratio [AHR], 2.60; 95% CI, 1.53–4.40), Georgia (AHR, 1.88; 95% CI, 1.10–3.22), and Louisiana (AHR, 1.80; 95% CI, 1.06–3.07). 60 Geographic disparities in PCa care often follow patterns of high Black populations, insurance type, poverty trends, and proximity to health care facilities, with NCI‐CCCs located in areas away from high Black populations. 52 , 61
Travel distance to a medical facility affects the course of diagnosis, treatment, and clinical outcomes. 62 , 63 Black men reported longer travel time to clinics versus White men (25 vs. 20 minutes, respectively) 64 and were unlikely to travel long distances to health care facilities. 63 Longer distance travel for treatment was associated with improved OS for White men but not for Black men (hazard ratio, 0.87 [95% CI, 0.83–0.91] vs. 0.95 [95% CI, 0.85–1.06], respectively; p < .001 vs. p < .33, respectively). 63 OS mediated by long‐distance travel was influenced by baseline economic and facility‐level factors. 63 For example, more Black men reported difficulty offsetting medical expenses (22.1% vs. 7.5%; p < .001) and obtaining insurance approval (21.1% vs. 13.3%; p < .001) based on where they resided compared with White men. 42
Although there is evidence for the geographic disparity in PCa diagnosis and treatment access, studies rely on self‐reported data and geographic information system‐calculated travel times. 64 Geographic information system travel times are closely associated with economic factors and limitations and can be influenced by means of transport, e.g., public transport or private vehicle.
Economic impact on affordability and access to diagnostic tests and treatment
Patients of lower SES have less access to health care resources. 50 , 65 , 66 , 67 In lower income populations, White men had higher odds of receiving PSA monitoring versus Black men (OR, 1.15; 95% CI, 1.08–1.22). 68 Among insured men who had elevated PSA results between 2011 and 2017, Black men were less likely to undergo subsequent prostate magnetic resonance imaging compared with White men. 69 In addition, in real‐world clinical practice, Black men were less likely to receive comprehensive genomic profiling earlier in their treatment course compared with men of European ancestry. 70 In contrast, another retrospective study of 3083 Black men and 1704 White men who underwent RP in multiple VA medical centers reported that Black men of low SES experienced a decreased risk of post‐RP castration‐resistant prostate cancer, metastasis, and PCSM versus White men. 66
Across all cohorts of cancer survivors, financial hardship influences treatment options 71 and potentially differs by race. Black men report having fewer PCa treatment options based on their health insurance plans (35% vs. 25.6%; p < .001). 42 More Black survivors compared with White survivors reported financial hardship from cancer debt (30.5% vs. 18.5%; p < .001), whereas more White survivors reported using assets to pay for cancer treatment (9.3% vs. 4.8%; p = .006). 71 Patients who reported financial hardship were 4.4 times more likely to limit care (95% CI, 2.6–6.6) than those who did not through medication nonadherence (OR, 2.7; 95% CI, 1.5–4.9), not seeking medical advice because of the expense (OR, 4.1; 95% CI, 2.4–6.9), 71 and refusing treatment (OR, 5.9; 95% CI, 2.6–13.7). In a mutually adjusted model, diverting assets from other expenditures and debts was not associated with limiting cancer care when controlling for the other forms of financial hardship. 71
The outcomes for Black men of low SES suggest that complex societal factors may affect clinical outcomes (Fig. 1). 66 Although both Black men and White men with newly diagnosed PCa indicated that a cure was an important decision‐making factor, Black men were significantly more likely than White men to consider factors such as the cost of care (65.8% vs. 32.1%; p < .001), impact on daily activities (73.5% vs. 57.6%; p < .001), treatment time (75.7% vs. 39.0%; p < .001), and recovery time (80.7% vs. 49.5%; p < .001) as important when deciding on PCa treatment. 48
Black enrollment in clinical trials
A relationship exists between clinical trial participation and outcomes in patients with advanced PCa who lack the opportunity to receive experimental therapies. Between 1987 and 2016, Black men in the United States were largely underrepresented in phase 3 PCa trials; of the 72 clinical trials analyzed, 83.4% of the men who participated were White versus 6.7% who were Black. 72 The PROCEED real‐world registry study (ClinicalTrials.gov identifier NCT03064490; 2011–2017) reported that, of the 1976 men enrolled, 86.7% were White and 11.6% were Black 73 (below the 2019 US census, 13.4% 74 ). Clinical trials are vital in the development of novel therapies, and underrepresentation of the Black population may hinder the development of efficacious and safe oncology treatments for real‐world clinical practice. 75 Clinical trials have demonstrated improved outcomes in Black men with PCa; the Abi Race trial (ClinicalTrials.gov identifier NCT01940276) reported longer PSA progression‐free survival for Black men versus White men who received treatment with abiraterone, 55 indicating that opportunities may be missed from low enrollment of Black men.
Complex factors contribute to the underrepresentation of Black men in clinical studies (Fig. 1). Many cultural and economic factors already have been identified, with lower SES strongly associated with poorer health literacy and knowledge of clinical trials. Although Black men express a willingness to participate in studies, 14 they do not receive information on available clinical trials, potentially because of the implicit bias of health care providers during patient interactions or because they are more likely to live in communities with limited access to academic medical centers. 75 , 76 Focus groups on genomic testing and research discovered that Black men often lacked an understanding of medical terminology, were reluctant to seek medical care, and had unfavorable attitudes toward research. 13 Eligibility barriers, such as comorbidities, poor Eastern Cooperative Oncology Group performance scores, multiple malignancies, and previous chemotherapy, can also affect Black representation in trials. 5 , 77 A review of 401 interventional clinical trials assessing OS reported that 47.9% of trials had study design criteria that excluded Black men, 78 identifying a major area of improvement for future PCa research.
US Food and Drug Administration guidance for industry‐funded and government‐funded clinical study designs recommends enrolling participants who reflect the diversity of the clinically relevant target population. 15 To increase recruitment of Black men into clinical trials, the following approaches have been proposed: educate clinicians and clinical trial recruiters regarding racial disparities in enrollment, increase clinician diversity, provide financial assistance to patients, reduce barriers to travel by increasing placement of cancer centers in areas with high minority populations, and engage with participants at commonly used community facilities, such as places of worship. 75 , 79 In addition, the inclusion of equal‐access health care systems and availability for remote visits in future study designs could also increase Black participation. 5 The Prostate Outreach Project provided free PSA screening and PCa education events, which led to the recruitment of 4420 men between 2003 and 2009, 62.8% of whom were Black. 80 Other programs that have successfully increased Black participation in clinical trials used community health fairs, Black PCa survivor‐led speeches, introductory letters from known Black spokespeople, and free PSA screening events. 75
The underrepresentation of minority populations in clinical trials fails to reflect the most vulnerable populations, and consequently trials lack statistical power to expose racial disparities in PCa treatment. 81 Patients with a higher risk of PCa both need and want information on novel and experimental treatments.
DISCUSSION
Black men account for nearly 30% of all PCa deaths in the United States, and any intent to decrease deaths from PCa should include appropriate representation of this at‐risk population. Black men hold a unique perspective on PCa care. Issues of particular concern include younger age and high‐risk disease at diagnosis, multifactorial risk of suboptimal treatment, and a rationale for treatment intensification at earlier stages of disease to prevent or delay progression. Diverse factors contribute to racial disparities among US Black men with PCa, many of which have been exacerbated by the COVID‐19 pandemic. 3 , 17 Cultural factors include generalized mistrust of the health care system, poor physician‐patient communication, and fear of a PCa diagnosis and the accompanying societal stigma (Fig. 1; see also Video S1). 82 Economic disparities are prevalent within the Black community, and financial hardship influences the decision to seek and/or select PCa screening/treatment, in which costs of diagnostic tests and treatment, treatment time, and recovery time are key factors. The advancement and introduction of novel diagnostic technologies will only increase existing disparities; therefore, addressing socioeconomic factors through development of an equitable care delivery model ultimately can affect the outcomes of more patients (e.g., the Health Impact Pyramid). 83 An overriding goal is to educate Black men on the importance of screening, diagnosis, treatment, and follow‐through on PCa care. Physician‐patient relationships must be improved by educating physicians on the true extent of racial disparity within PCa, maintaining current knowledge of industry guidelines (particularly on initiating diagnostic tests and treatment for high‐risk, specific ethnic populations), and increasing diversity among staff. It is crucial that representation of minority races is increased in medical schools, postgraduate education, and academia.
Patient‐physician relationships must be nurtured. Improvements in the information provided to patients on PCa diagnosis and treatment, patient inclusion in the SDM process, and development of strategies with culturally aware patient advocates will aid patient relationships in the health care system. Importantly, physicians and other health care providers must spend extra time asking about and listening to Black patients' concerns regarding their health care. Family and friends (especially women) are more accessible and trusted sources of information among Black men and can be key in changing attitudes and promoting routine prostate monitoring, a strategy that requires optimization.
Grassroots initiatives could offer support for how facilities and clinicians can collaborate with the community to increase knowledge of PCa. These initiatives may also introduce other stakeholders to the battle against PCa, such as nonprofit organizations (e.g., the American Cancer Society) and government health services (e.g., VA). Embedding PSA screening into electronic medical records and routine health maintenance could aid in addressing screening differences that exist between races. The use of next‐generation sequencing technologies and epigenomics in the diagnosis and management of PCa should continue to be developed with a focus on using a racially diverse patient population.
Black men are underrepresented in clinical trials, resulting in insufficient statistical power to compare clinical outcomes of Black men versus White men with PCa. Focused initiatives are required to increase Black enrollment in clinical trials, not because of a lack of willingness among the Black community but because of a lack of information on active clinical trials. To comprehensively address these disparities, areas of development may include identifying and educating physicians on the best practices for conveying information of active clinical trials, improving clinical trial matching and registries, and specific eligibility criteria and study design requirements that focus on patient‐centered end points. Successful strategies to increase access to clinical trials would include clinical study sites in the community (where study assessments can be performed remotely) or health care centers located where Black men are commonly treated. Industry sponsors would have a significant impact on racial disparities in PCa by building clinical trial infrastructure with diverse clinical trial research teams at these sites. Likewise, clinical trial designs that include specific questions regarding race or ancestral differences in the population would also enhance Black patient engagement and improve credibility among sponsors.
The factors influencing PCa awareness, diagnosis, treatment, and outcomes are multifaceted and entrenched within society. It is imperative that the scientific community actively moves away from researching biologic differences between races, focusing instead on policy advocacy that will promote societal change to address institutional racism within health care. Educating clinicians about the barriers that Black patients face, as well as empowering and educating Black men on the importance of routine prostate care, are key to creating equity for Black men with PCa.
AUTHOR CONTRIBUTIONS
James W. Lillard, Jr, Kelvin A. Moses, Brandon A. Mahal, and Daniel J. George: Conceptualization, visualization, writing–original draft, and writing–review and editing.
CONFLICTS OF INTEREST
Kelvin A. Moses reports personal fees from Myovant and Astellas/Pfizer and participation in speakers' bureaus for Astellas/Pfizer and Dendreon outside the submitted work. Brandon A. Mahal reports funding from Prostate Cancer Foundation, the American Society for Radiation Oncology, the US Department of Defense, and the National Institutes of Health; and personal fees from Myovant and the Cancer Study Group outside the submitted work. Daniel J. George reports grants from Calithera, Capio Biosciences, and EMD Serono; grants and personal fees from Astellas, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Novartis, and Pfizer; personal fees from AstraZeneca, Axess Oncology, Flatiron, Ipsen, Merck Sharp & Dohme, Michael J. Hennessey Associates, Millennium Medical Publishing, Modra Pharmaceutical B.V., Myovant Sciences Inc., NCI Genitourinary, Nektar Therapeutics, Physician Education Resource, UroGPO, Vizuri Health Sciences, Platform Q, Propella Therapeutics, RevHealth, Seattle Genetics, WebMD, and Xcures; grants, personal fees, and nonfinancial support from Bayer HealthCare Pharmaceuticals, Exelixis, Inc., and Sanofi; grants from Acerta Pharmaceuticals; personal fees and nonfinancial support from UroToday and other support from the American Association for Cancer Research, all outside the submitted work. James W. Lillard made no disclosures.
FUNDING INFORMATION
Editorial support was provided by Lauren Rainer, BSc; Laura Geuss, PhD; and Julie B. Stimmel, PhD, at Onyx (a Prime Global agency) and was funded by Pfizer.
Supporting information
Video S1 Supporting Information
REFERENCES
- 1. American Cancer Society . Prostate Statistics. Accessed May 1, 2022. https://cancerstatisticscenter.cancer.org/?_ga=2.112629568.531270960.1613386898‐1085770420.1613386898#!/cancer‐site/Prostate
- 2. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8:a030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7‐33. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer . Accessed April 13, 2021. https://seer.cancer.gov/statfacts/html/prost.html
- 5. Chowdhury‐Paulino IM, Ericsson C, Vince R Jr, Spratt DE, George DJ, Mucci LA Racial disparities in prostate cancer among Black men: epidemiology and outcomes Prostate Cancer Prostatic Dis Published online September 2, 2021. doi: 10.1038/s41391-41021-00451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Cancer Society Cancer Facts & Figures for African Americans 2019–2021. American Cancer Society; 2019. Accessed February 15, 2021. https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/cancer‐facts‐and‐figures‐for‐african‐americans/cancer‐facts‐and‐figures‐for‐african‐americans‐2019‐2021.pdf
- 7. National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection . Version 2. 2021. Accessed September 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf
- 8. Pietro GD, Chornokur G, Kumar NB, Davis C, Park JY. Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J. 2016;20(suppl 2):S112‐S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das H, Rodriguez R. Health care disparities in urologic oncology: a systematic review. Urology. 2020;136:9‐18. [DOI] [PubMed] [Google Scholar]
- 11. Naik G, Akinyemiju T. Disparities in hospitalization outcomes among African‐American and White prostate cancer patients. Cancer Epidemiol. 2017;46:73‐79. [DOI] [PubMed] [Google Scholar]
- 12. Beebe‐Dimmer JL, Ruterbusch JJ, Cooney KA, et al. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: a SEER‐Medicare investigation. Cancer Med. 2019;8:3325‐3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers CR, Rovito MJ, Hussein M, et al. Attitudes toward genomic testing and prostate cancer research among Black men. Am J Prev Med. 2018;55(5 suppl 1):S103‐S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan CP, Napoles AM, Narine S, et al. Knowledge and attitudes regarding clinical trials and willingness to participate among prostate cancer patients. Contemp Clin Trials. 2015;45:443‐448. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration (FDA) . Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs. Guidance for Industry. FDA. 2020. Accessed March 29, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/enhancing‐diversityclinical‐trial‐populations‐eligibility‐criteria‐enrollment‐practices‐and‐trial
- 16. McKay RR, Gold T, Zarif JC, et al. Tackling diversity in prostate cancer clinical trials: a report from the Diversity Working Group of the IRONMAN Registry. JCO Glob Oncol. 2021;7:495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ufuah S, Tallman JE, Moses KA. The pursuit of health equity and equality in urologic oncology: where we have been and where we are going. Eur Urol Focus. 2021;7:929‐936. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between Blacks and Whites. Proc Natl Acad Sci U S A. 2016;113:4296‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson B. How structural racism can kill cancer patients: Black patients with breast cancer and other malignancies face historical inequities that are ingrained but not inevitable. In this article, the second of a 2‐part series, we explore the consequences of and potential solutions to racism and inequality in cancer care. Cancer Cytopathol. 2020;128:83‐84. [DOI] [PubMed] [Google Scholar]
- 20. Lent AB, Garrido CO, Baird EH, Viela R, Harris RB. Racial/ethnic disparities in health and life insurance denial due to cancer among cancer survivors. Int J Environ Res Public Health. 2022;19:2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non‐Hispanic White men with prostate cancer in an equal‐access health care system. Cancer. 2020;126:1683‐1690. [DOI] [PubMed] [Google Scholar]
- 22. Moses KA, Chen LY, Sjoberg DD, Bernstein M, Touijer KA. Black and White men younger than 50 years of age demonstrate similar outcomes after radical prostatectomy. BMC Urol. 2014;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moses KA, Orom H, Brasel A, Gaddy J, Underwood W 3rd. Racial/ethnic disparity in treatment for prostate cancer: does cancer severity matter? Urology. 2017;99:76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kensler KH, Pernar CH, Mahal BA, et al. Racial and ethnic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. J Natl Cancer Inst. 2021;113:719‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan SH, Petrovics G, Srivastava S. Prostate cancer genomics: recent advances and the prevailing underrepresentation from racial and ethnic minorities. Int J Mol Sci. 2018;19:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nugent A, Conatser KR, Turner LL, Nugent JT, Sarino EMB, Ricks‐Santi LJ. Reporting of race in genome and exome sequencing studies of cancer: a scoping review of the literature. Genet Med. 2019;21:2676‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haiman CA, Patterson N, Freedman ML, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39:638‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schumacher FR, Feigelson HS, Cox DG, et al. A common 8q24 variant in prostate and breast cancer from a large nested case–control study. Cancer Res. 2007;67:2951‐2956. [DOI] [PubMed] [Google Scholar]
- 29. Sjoblom L, Saramaki O, Annala M, et al. Microseminoprotein‐beta expression in different stages of prostate cancer. PLoS One. 2016;11:e0150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koga Y, Song H, Chalmers ZR, et al. Genomic profiling of prostate cancers from men with African and European ancestry. Clin Cancer Res. 2020;26:4651‐4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Comprehensive Cancer Network . NCCN Guidelines for Prostate Cancer, Version 3.2022. Accessed March 30, 2022. https://www.nccn.org/guidelines/guidelines‐process/transparency‐process‐and‐recommendations/GetFileFromFileManager?fileManagerId=11771
- 32. Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol. 2021;205:14‐21. [DOI] [PubMed] [Google Scholar]
- 33. Baffoe‐Bonnie AB, Kittles RA, Gillanders E, et al. Genome‐wide linkage of 77 families from the African American Hereditary Prostate Cancer study (AAHPC). Prostate. 2007;67:22‐31. [DOI] [PubMed] [Google Scholar]
- 34. Alexis O, Worsley A. An integrative review exploring Black men of African and Caribbean backgrounds, their fears of prostate cancer and their attitudes towards screening. Health Educ Res. 2018;33:155‐166. [DOI] [PubMed] [Google Scholar]
- 35. Leyva B, Persoskie A, Ottenbacher A, et al. Do men receive information required for shared decision making about PSA testing? Results from a national survey. J Cancer Educ. 2016;31:693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinlock BL, Thorpe RJ Jr, Howard DL, et al. Racial disparity in time between first diagnosis and initial treatment of prostate cancer. Cancer Control. 2016;23:47‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woods VD, Montgomery SB, Belliard JC, Ramirez‐Johnson J, Wilson CM. Culture, Black men, and prostate cancer: what is reality? Cancer Control. 2004;11:388‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alsan M, Garrick O, Graziani GC. Does Diversity Matter for Health? Experimental Evidence From Oakland. National Bureau of Economic Research; 2018. Accessed September 14, 2021. https://www.nber.org/papers/w24787
- 39. Butler SS, Winkfield KM, Ahn C, et al. Racial disparities in patient‐reported measures of physician cultural competency among cancer survivors in the United States. JAMA Oncol. 2020;6:152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. United States Census Bureau . United States Census QuickFacts 2019. Accessed June 22, 2021. https://www.census.gov/quickfacts/fact/table/US/IPE120219
- 41. Association of American Medical Colleges (AAMC) . Diversity in Medicine: Facts and Figures 2019. Accessed June 22, 2021. https://www.aamc.org/data‐reports/workforce/interactive‐data/figure‐18‐percentage‐all‐active‐physicians‐race/ethnicity‐2018
- 42. Pollack CE, Armstrong KA, Mitra N, et al. A multidimensional view of racial differences in access to prostate cancer care. Cancer. 2017;123:4449‐4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walsh‐Childers K, Odedina F, Poitier A, Kaninjing E, Taylor G 3rd. Choosing channels, sources, and content for communicating prostate cancer information to Black men: a systematic review of the literature. Am J Mens Health. 2018;12:1728‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hewitt T, Killinger KA, Hiller S, Boura JA, Lutz M. Exploring racial differences surrounding prostate cancer screening: beliefs and attitudes in community dwelling men attending an urban men's health event. Am J Mens Health. 2018;12:1929‐1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schoenfeld ER, Francis LE. Word on the street: engaging local leaders in a dialogue about prostate cancer among African Americans. Am J Mens Health. 2016;10:377‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliver JS, Allen RS, Eichorst MK, et al. A pilot study of prostate cancer knowledge among African American men and their health care advocates: implications for screening decisions. Cancer Causes Control. 2018;29:699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogunsanya ME, Brown CM, Odedina FT, Barner JC, Adedipe TB, Corbell B. Knowledge of prostate cancer and screening among young multiethnic Black men. Am J Mens Health. 2017;11:1008‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gordon BE, Basak R, Carpenter WR, Usinger D, Godley PA, Chen RC. Factors influencing prostate cancer treatment decisions for African American and White men. Cancer. 2019;125:1693‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watson M, Grande D, Radhakrishnan A, Mitra N, Ward KR, Pollack CE. Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethn Dis. 2017;27:201‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Obirieze AC, Moten A, Allen D, Ahaghotu CA. African‐American men with low‐risk prostate cancer: modern treatment and outcome trends. J Racial Ethn Health Disparities. 2015;2:295‐302. [DOI] [PubMed] [Google Scholar]
- 52. Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population‐based study. Cancer. 2015;121:3885‐3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bickell NA, Lin JJ, Abramson SR, et al. Racial disparities in clinically significant prostate cancer treatment: the potential health information technology offers. J Oncol Pract. 2018;14:e23‐e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rude T, Walter D, Ciprut S, et al. Interaction between race and prostate cancer treatment benefit in the Veterans Health Administration. Cancer. 2021;127:3985‐3990. [DOI] [PubMed] [Google Scholar]
- 55. George DJ, Heath EI, Sartor AO, et al. Abi Race: a prospective, multicenter study of Black (B) and White (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP) [abstract]. J Clin Oncol. 2018;36(18 suppl):LBA5009. [Google Scholar]
- 56. Bieber C, Nicolai J, Gschwendtner K, et al. How does a shared decision‐making (SDM) intervention for oncologists affect participation style and preference matching in patients with breast and colon cancer? J Cancer Educ. 2018;33:708‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jiang C, Fedewa SA, Wen Y, Jemal A, Han X. Shared decision making and prostate‐specific antigen based prostate cancer screening following the 2018 update of USPSTF screening guideline. Prostate Cancer Prostatic Dis. 2021;24:77‐80. [DOI] [PubMed] [Google Scholar]
- 58. Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1901‐1913. [DOI] [PubMed] [Google Scholar]
- 59. Martinez‐Gonzalez NA, Neuner‐Jehle S, Plate A, Rosemann T, Senn O. The effects of shared decision‐making compared to usual care for prostate cancer screening decisions: a systematic review and meta‐analysis. BMC Cancer. 2018;18:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fletcher SA, Marchese M, Cole AP, et al. Geographic distribution of racial differences in prostate cancer mortality. JAMA Netw Open. 2020;3:e201839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30. [DOI] [PubMed] [Google Scholar]
- 62. Cobran EK, Chen RC, Overman R, et al. Racial differences in diffusion of intensity‐modulated radiation therapy for localized prostate cancer. Am J Mens Health. 2016;10:399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vetterlein MW, Loppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer. 2017;123:3241‐3252. [DOI] [PubMed] [Google Scholar]
- 64. Wong MS, Grande DT, Mitra N, et al. Racial differences in geographic access to medical care as measured by patient report and geographic information systems. Med Care. 2017;55:817‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nguyen C, Lairson DR, Swartz MD, Du XL. Racial, socioeconomic, and geographic disparities in the receipt, timing to initiation, and duration of adjuvant androgen deprivation therapy in men with prostate cancer. J Racial Ethn Health Disparities. 2019;6:133‐142. [DOI] [PubMed] [Google Scholar]
- 66. Everist MM, Howard LE, Aronson WJ, et al. Socioeconomic status, race, and long‐term outcomes after radical prostatectomy in an equal access health system: results from the SEARCH database. Urol Oncol. 2019;37:289.e211‐289.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kantor ED, Haneuse S, Valdimarsdottir UA, Williams DR, Signorello LB, Rider JR. Socioenvironmental adversity and risk of prostate cancer in non‐Hispanic Black and White men. Cancer Causes Control. 2019;30:997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moses KA, Zhao Z, Bi Y, et al. The impact of sociodemographic factors and PSA screening among low‐income Black and White men: data from the Southern Community Cohort Study. Prostate Cancer Prostatic Dis. 2017;20:424‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abashidze N, Stecher C, Rosenkrantz AB, Duszak R Jr, Hughes DR. Racial and ethnic disparities in the use of prostate magnetic resonance imaging following an elevated prostate‐specific antigen test. JAMA Netw Open. 2021;4:e2132388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sivakumar S, Lee JK, Moore JA, et al. Ancestral characterization of the genomic landscape, comprehensive genomic profiling utilization, and treatment patterns may inform disparities in advanced prostate cancer: a large‐scale analysis [abstract]. J Clin Oncol. 2021;39(15 suppl):5003. [Google Scholar]
- 71. Hastert TA, Banegas MP, Hamel LM, et al. Race, financial hardship, and limiting care because of cost in a diverse cohort of cancer survivors. J Cancer Surviv. 2019;13:429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rencsok EM, Bazzi LA, McKay RR, et al. Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiol Biomarkers Prev. 2020;29:1374‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Higano CS, Armstrong AJ, Sartor AO, et al. Real‐world outcomes of sipuleucel‐T treatment in PROCEED, a prospective registry of men with metastatic castration‐resistant prostate cancer. Cancer. 2019;125:4172‐4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. United States Census Bureau . 2019. American Community Survey 1‐Year Estimates. Accessed May 4, 2021. https://data.census.gov/cedsci/table?q=Black%20or%20African%20American&tid=ACSDT1Y2019.B02001
- 75. Ahaghotu C, Tyler R, Sartor O. African American participation in oncology clinical trials—focus on prostate cancer: implications, barriers, and potential solutions. Clin Genitourin Cancer. 2015;14:105‐116. [DOI] [PubMed] [Google Scholar]
- 76. Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34:2874‐2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp Clin Trials Commun. 2018;11:156‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vastola ME, Yang DD, Muralidhar V, et al. Laboratory eligibility criteria as potential barriers to participation by Black men in prostate cancer clinical trials. JAMA Oncol. 2018;4:413‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hahn AW, Bilen MA, Agarwal N. Successful recruitment of Black men to prostate cancer clinical trials—a lesson in achievement. JAMA Netw Open. 2021;4:e2034652. [DOI] [PubMed] [Google Scholar]
- 80. Ashorobi OS, Frost J, Wang X, et al. Prostate cancer education, detection, and follow‐up in a community‐based multiethnic cohort of medically underserved men. Am J Mens Health. 2017;11:82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Balakrishnan AS, Palmer NR, Fergus KB, et al. Minority recruitment trends in phase III prostate cancer clinical trials (2003 to 2014): progress and critical areas for improvement. J Urol. 2019;201:259‐267. [DOI] [PubMed] [Google Scholar]
- 82. Vapiwala N, Miller D, Laventure B, et al. Stigma, beliefs and perceptions regarding prostate cancer among Black and Latino men and women. BMC Public Health. 2021;21:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Frieden TR. The future of public health. N Engl J Med. 2015;373:1748‐1754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Supporting Information


