Abstract
Aims
(a) The aim of this study was to investigate both the formation of dense connective tissue within the dental pulp, and its association with pulpal inflammation in teeth with advanced carious lesions; and (b) to investigate in vitro whether inflammation affects the expression of markers related to chondrogenesis/osteogenesis in pulp cells.
Materials and methods
Radiology and Histology: Forty‐six teeth with advanced carious lesions were radiographically investigated for intra‐pulpal radiodense structures. Specimens were processed for histology and stained with haematoxylin/eosin and proteoglycan‐specific stains. The intra‐pulpal connective tissue was scored as pulp stones or ectopic connective tissue. Cell culture: pulpal cells from human third molars (n = 5) were cultured in chondrogenic medium +/− TLR2/4 agonists. Expression of the genes IL6, TLR2/4, SOX9, COL1A1, COL2A1, TGFB1, RUNX2 and ALPL was assessed by qPCR. Proteoglycan content within cultures was assessed spectrophotometrically.
Results
Radiodense structures were discovered in about half of all pulps. They were associated with ectopic connective tissue (χ2 = 8.932, p = .004, OR = 6.80, 95% CI: [1.84, 25.19]) and with pulp stones (χ2 = 12.274, df = 1, p < .001, OR = 22.167, 95% CI: [2.57, 200.00]). The morphology of the ectopic tissue resembled cartilage and was associated with inflammatory infiltration of the pulp (χ2 = 10.148, p = .002, OR = 17.77, 95% CI: [2.05, 154.21]). After continuous stimulation of cultured cells with TLR2/4 agonists, the expression of two inflammatory markers increased: IL6 at Days 7 (p = .020) and 14 (p = .008); TLR2 at Days 7 (p = .023) and 14 (p = .009). Similarly, expression of chondrogenic markers decreased: SOX9 at Day 14 (p = .035) and TGFB1 at Day 7 (p = .004), and the osteogenic marker COL1A1 at Day 7 (p = .007). Proteoglycan content did not differ between unstimulated and stimulated cells.
Conclusions
Ectopic connective tissue resembling cartilage can form in teeth affected by advanced carious lesions. This tissue type is radiographically visible and is associated with inflammatory infiltration of the pulp. Although TLR2/4 agonists led to an inflammatory response in cell culture of pulp cells, the effect on the expression of osteogenic/chondrogenic markers was limited, suggesting that immune cells are needed for connective tissue formation in vivo.
Keywords: caries, cartilage, dental pulp, inflammation, ossification, pulp stone
INTRODUCTION
A study of teeth with radiographically advanced stages of carious lesions has shown a dense ectopic connective tissue type within the pulpal stroma (Demant et al., 2021). This tissue resembled neither pulp stones nor phenotypes of tertiary dentine (i.e. reactionary or reparative dentine). Furthermore, a histological investigation showed that the ectopic tissue was limited to teeth with extremely deep carious lesions, as defined by the European Society of Endodontology (European Society of Endodontology (ESE) et al., 2019).
Currently, it is not known whether this tissue type is associated with irreversible inflammatory damage to the pulpal tissue, or to what extent it is structurally dense enough to be systematically radiographically visible. If the ectopic tissue is associated with irreversible pulpal damage, and if it can indeed be visualized using conventional radiographic techniques, these findings might be included in the diagnosis of the status of the pulp prior to intervention. Although it is well known that clinical assessment of the pulp is a challenging task (Mejàre et al., 2012), such information would be relevant for diagnostic purposes when investigating the inflammatory status of the pulp.
Hard‐tissue formation within the pulpal tissue is a well‐known phenomenon, and the tissue formed has traditionally been defined either as tertiary dentinogenesis or as pulp stones. Tertiary dentinogenesis is viewed as a response either of the odontoblasts (reactionary dentinogenesis), or in the event of the demise of the odontoblasts, of an odontoblast‐like cell (reparative dentinogenesis; Bjørndal et al., 1998; Bjørndal & Darvann, 1999; Smith et al., 1995). Pulp stones are masses of dense connective tissue that form within the pulpal tissue; their aetiology has been explained as reactions to pulpal damage or as part of normal pulpal development (Moss‐Salentijn & Klyvert, 1988).
The formation of ectopic dense connective tissue within a soft‐tissue compartment has also been reported to occur in other parts of the body, especially after severe soft‐tissue damage. The process, which has been termed heterotopic ossification (Cholok et al., 2018), could also be driven by a mutation of the gene ACVR1 (activin A receptor type 1) in the bone morphogenetic protein (BMP) pathway, as with fibrodysplasia ossificans progressiva (Schoenmaker et al., 2021). Heterotopic ossification is currently believed to be induced by acute inflammation caused by soft‐tissue damage, which leads to the priming of stromal/fibroblastic cells within the local microenvironment and to their differentiation into a cartilage or bone‐like phenotype (Dey et al., 2018). It is therefore tempting to speculate that the formation of ectopic connective tissue within the dental pulp represents a similar process, in that pulpal inflammation caused by the advanced carious lesion may lead to the formation of a tissue much like that seen during heterotopic ossification. However, this would challenge the commonly held concept of the odontoblast/odontoblast‐like cell being the principal driver for hard‐tissue formation within the pulp, and thereby the understanding that the tissue formed should be seen as a variety of dentine. It has also been proposed that the ectopic formation of dense connective tissue within the pulp represents a dystrophic scar‐like matrix caused by the unregulated and passive calcification of damage or necrotic areas of the pulpal tissue (Ricucci et al., 2014).
The odontoblast has been shown to express toll‐like receptors (TLR), which suggests that the odontoblast is a modulator of immune function and thereby serves as a coordinator of the activation of both the innate and adaptive immune system (Farges et al., 2015). It is not known whether direct signalling through the odontoblast leads to the formation of ectopic connective tissue, or whether this phenomenon results from a complex inflammatory modulation of the pulpal microenvironment much like the activity seen in heterotopic ossification. It would therefore be interesting to investigate whether inflammatory stimulation of pulpal stromal cells would bring about changes in the gene expression of connective tissue markers related to osteogenesis and/or chondrogenesis. The first hypothesis (hypothesis i) was that the ectopic connective tissue found in cases of extremely deep carious lesions is associated with irreversible inflammatory damage to the pulpal tissue, and resembles cartilage and/or bone, much like the ectopic tissue found in cases of heterotopic ossification. The second hypothesis (hypothesis ii) was that the expression of genes related to the formation of cartilage and/or bone would be upregulated by culturing pulpal stromal cells under experimental conditions mimicking inflammation and chondrogenesis.
The first aim of the study was to describe the distribution of pulp stones and ectopic connective tissue and any association between these structures and pulp inflammation in carious teeth; and to establish whether the ectopic connective tissue can be described as either cartilage and/or bone. The second aim was to experimentally investigate whether inflammatory stimulation of mesenchymal stromal cells from the dental pulp with TLR2/4 agonists would cause an upregulation of a panel of genes related to inflammation, chondrogenesis and/or osteogenesis, compared with unstimulated cells.
MATERIALS AND METHODS
The manuscript of this laboratory study was written according to preferred reporting items for laboratory studies in Endodontology 2021 guidelines (Nagendrababu et al., 2021); an overview can be seen in Figure 1.
FIGURE 1.

PRILE 2021 flowchart
The histological investigation comprised 46 permanent molars with extensive carious lesions. These had been extracted in a group of young individuals (aged 12–18 years) as part of a dental mission on Mactan Island, the Philippines. The extractions were purely based on therapeutic indications, and not related to this study. The inclusion criteria for the extracted teeth were as follows: (a) teeth with extensive carious lesions, and (b) the extraction could be carried out without damaging the tooth. In the clinical setting, no attempt was made to grade the penetration depth of the carious lesions. The cell‐culturing part of this study was based on mesenchymal stromal cells harvested from healthy third molars extracted as part of relevant treatment also not related to this study. The inclusion of both groups of teeth was undertaken in accordance with ethical principles, including the World Medical Association Declaration of Helsinki (version 2008), and approved by the Department of Education Lapu‐Lapu, Mactan Island, the Philippines. The biological human material was fully anonymized, and the inclusion of the extracted teeth and cell lines in this study was reviewed and approved by the Danish National Committee on Health and Research Ethics under protocol number H‐1‐2012‐FSP.
Histology
After extraction, the teeth were immediately fixed in 4% neutral buffered formalin (VWR) for a minimum of 24 h and prepared for macroscopic photography and histological processing according to the previously published protocol (Demant et al., 2021). Briefly, a radiograph was first obtained of each specimen in a buccal‐lingual direction using a Planmeca Prosensor. HD, Planmeca ProX intraoral X‐ray unit and the Planmeca Romexis software (Plandent). The exposure time was 0.6 s, with 6KV and 8 mA as parameters. Each tooth was then sectioned in half through the central part of the occlusal cavitated lesion in a mesio‐distal direction, using a high‐precision saw (MIKRO‐TRENN). Each sectioned half was photographed, and the specimens were subsequently decalcified in 10% EDTA (pH 6.9) and processed for histology. Each macroscopically sectioned half was sectioned serially into 4 μm sections, starting from the central part of the carious lesion until complete exhaustion of the specimens. The number of obtained sections varied greatly from specimen to specimen due to differences in tooth type and section plane. The sectioning procedure was carried out using a sliding microtome (Leica Biosystems). A detailed description of the tissue processing methods has been described elsewhere (Demant et al., 2021).
Histological evaluation
Haematoxylin and eosin (HE) staining was used to examine tissue architecture, and specifically (a) the presence/absence of a pulp stones, defined as bodies of dense connective tissue with a circular, layered appearance without cellular inclusions; and (b) the presence/absence of ectopic connective tissue, defined as dense, connective tissue resembling neither a classic pulp stone nor tertiary dentine matrix. Finally, the presence or absence of inflammatory cells within the pulpal stroma was scored, as described elsewhere (Demant et al., 2021). Approximately every fifth section was stained with HE. All analyses were carried out based on the section with the most severe tissue reaction.
Although the histological specimens had been demineralized prior to sectioning, the term ‘ectopic mineralized connective tissue’ was used as a general term, as these structures could also be visualized by means of radiology. In order to determine whether the ectopic mineralized connective tissue was related to cartilage or bone, the specimens were stained according to established protocols with Safranin O/Fast Green (Sigma). Safranin O stains proteoglycans intensely orange to red Schmitz et al. (2010). As well as staining with Safranin O/Fast Green, selected specimens were stained with Alcian Blue van Gieson in order to detect the presence of proteoglycans in areas of the specimens that stained blue and to detect the presence of collagen in areas that stained intensely red (Quintarelly et al., 1964; Scott et al., 1964). They were also stained with Toluidine Blue O, where areas of metachromatic staining that caused a change in colour from blue to purple indicated the presence of a cartilaginous matrix (Schmitz et al., 2010). Stained and mounted tissue samples were visualized using an Axio Scan.Z1 slide scanner (Zeiss). The histological scorings were carried out by the first author and SVE. In case of disagreement, the scoring was discussed until concensus was reached.
Radiographic evaluation
Conventional periapical radiographs were used to assess the degree of carious lesion penetration according to the definitions made by the European Society of Endodontology (ESE) et al. (2019). Furthermore, the radiographs were used to score the presence or absence of discrete, radiodense structures within the coronal pulp (Figure 2). Only teeth with clearly visible, well‐demarcated radiodense structures within the coronal pulp were scored as positive (Figure 2a–c). No attempts were made to distinguish between the histologic variables ‘pulp stones’ and ‘dense connective tissue’ radiographically. The association between the presence of radiodense structures and the histological variables specified above was investigated. Radiographic scoring was carried out by the first author and LB.
FIGURE 2.
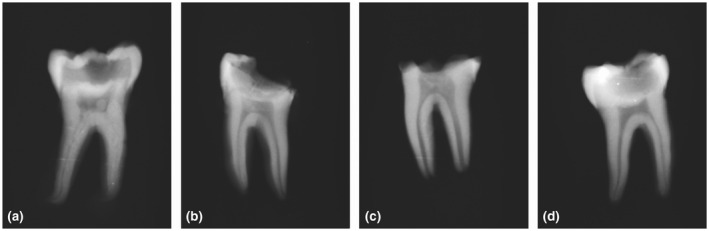
Radiodense structures in the pulp chamber. The radiodense structures lying freely within the pulp stroma were seen either as distinct structures (a, b) or as areas whose radiodense appearance was more diffuse (c). An example of a pulp chamber with no radiodense changes (d)
Cell culture
Upon extraction, the teeth (n = 5) were disinfected with ethanol, and remnants of the periodontal ligament were scraped off with dental scalers. The teeth were wrapped in sterile gauze and subsequently cracked in a vice. The pulpal tissue was removed using sterile tweezers and minced into smaller pieces with a scalpel blade. The pulp tissue was washed once in Hank's Balanced Salt Solution (LIFE technologies) supplemented with 5% (v/v) foetal bovine serum (FBS; FCS, HyClone) and centrifuged at 400g for 10 min. The supernatant was removed, and the pellet digested with 5 ml collagenase/dispase solution (collagenase: 3 mg/ml, dispase: 4 mg/ml dissolved in phosphate‐buffered saline (PBS; Sigma)) for 60 min in a 37°C water bath with vortexing every 15 min in order to help break up the tissue. After digestion, the sample was washed twice in HBBS supplemented with 5% FBS, and dissolved in growth medium (DMEM high glucose (LIFE technologies) supplemented with 10% FBS, 2 mmol/L L‐glutamine (LIFE Technologies) and 100 U/ml penicillin/streptomycin (Sigma)). The cell suspension was then seeded onto 100 mm Petri dishes, expanded in 10 ml growth medium and incubated at 37°C in 5% CO2 and >90% humidity until 80% confluency. Medium was changed twice per week.
Chondrogenic differentiation was induced according to Beier et al. (2019). Pulp cells from five biological replicates were cultured in DMEM supplemented with 100 nmol/L dexamethasone (Sigma); 0.17 mmol/L L‐ascorbate‐2‐phosphate (Sigma); 0.35 mmol/L proline (Sigma); 0.91 mmol/L pyruvate (Sigma); 1 ml ITS‐G Premix (1 mg/ml insulin, 0.55 mg/ml transferrin, 0.67 μg/ml sodium selenite, (LIFE Technologies)); 5 ng/ml TGF‐beta 3 (PeproTech); and 500 ng/ml BMP‐6 (PeproTech). Cells were cultured at 1 × 105 cells per well in a 48‐well plate. For each cell line, each condition was cultured in duplicate.
To investigate the effect of inflammation on chondrogenic differentiation, the following was added to the chondrogenic medium: 1 μg/ml TLR2 agonist, PAM2CSK4 and #14E14‐MM (Invivogen); together with 1 μg/ml TLR4 agonist (LPS from Porphyromonas gingivalis, Ultrapure, Version #14F18‐MM (InvivoGen)). This was done for the duration of the culture at 7 and 14 days according to previously published work (Karlis et al., 2020). Media were refreshed twice a week. RNA samples were collected at the start of the experiment, that is before the addition of chondrogenic medium at baseline (t = 0), then at 7 days (t = 7) and finally at 14 days (t = 14).
Quantitative assessment of proteoglycan
In order to assess the content of proteoglycan (a major component of the extracellular matrix of cartilage) and to evaluate the difference between groups (unstimulated cells vs. cells stimulated with TLR2/4 agonists), the chondrogenic monolayers were washed twice with PBS and fixed with 4% neutral buffered formalin for 10 min. After three washing steps with PBS, the cells were stained with a 0.1% Safranin O staining solution (Safranin O, diluted in dd H2O) for 30 min. After removal of the Safranin O solution, plates were washed thrice with PBS. Absorbed Safranin O was washed out in 200 μl isopropanol per well and incubated for 30 min. with gentle agitation. As each condition was cultured in duplicate, the isopropanolol from each well within the duplicate was pooled. Finally, 100 μl per sample were transferred in triplicates (total 300 μl) to an optical plate, and absorbance was quantified as optical density (O.D.) in a microplate reader at 540 nm. The measured signal was corrected by the DNA concentration of the individual wells as assessed using the CyQuant® Kit (Invitrogen) according to the manufacturer's protocol.
RNA isolation and real‐time quantitative polymerase chain reaction (qPCR)
Cells were cultured for 7 and 14 days in 48‐well plates. Three wells were pooled for each experimental condition. RNA was isolated using the RNeasy Mini Kit (QIAGEN) following the manufacturer's instructions. The reverse transcriptase reaction was performed using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, where according to the manufacturer's protocol, using Oligo(dT)18 and D(N)6 primers). qPCR was performed on the LC480 light cycler (Roche). Three nanogram of cDNA were used in a total volume of 20 μl containing Light Cycler SybrGreen1 Master mix (Roche) and 1 μmol/L of each primer. A standard two‐step QPCR program was performed at an annealing temperature of 60°C. Sequence information for all primers is listed in Table 1. The experimental conditions were not affected by expression of the house‐keeping gene hydroxymethylbilane synthase HMBS (Hydroxymethylbilane synthase, previously known as PBGD [porphobilinogen deaminase]). Samples were normalized for the expression of HMBS by calculating the ΔCt (CtGene‐of‐interest − Ct HMBS ); expression of the various genes relative to the house‐keeping gene was expressed as 2−(ΔCt).
TABLE 1.
Primer sequence for quantitative PCR
| Gene | Sequence 5′–3′ | Amplicon length (bp) | Ensemble gene ID |
|---|---|---|---|
| HMBS |
5′ TgCAgTTTgAAATCATTgCTATgTC 3′ 5′ AACAggCTTTTCTCTCCAATCTTAgA 3′ |
84 | ENSG00000113721 |
| IL6 |
5′ ACAGCCACTCACCTCTTCA 3′ 5′ ACCAGGCAAGTCTCCTCAT 3′ |
207 | ENSG00000136244 |
| TLR2 |
5′ ggCTTCTCTgTCTTgTgACCg 3′ 5′ gAgCCCTgAgggAATggAg 3′ |
75 | ENSG00000137462 |
| TLR4 |
5′ CTgCAATggATCAAggAACCAg 3′ 5′ CCATTCgTTCAACTTCCACCA 3′ |
51 | ENSG00000136869 |
| TGFB1 |
5′ CACCCgCgTgCTAATggT 3′ 5′ CTCggAgCTCTgATgTgTTgAA 3′ |
100 | ENSG00000105329 |
| SOX9 |
5′ CCCAACGCCATCTTCAAGG 3′ 5′ CTGCTCAGCTCGCCGATGT 3′ |
242 | ENSG00000125398 |
| COL2A1 |
5′ TGTCAGGGCCAGGATGT 3′ 5′ CTCCTTTCTGTCCCTTTGG 3′ |
256 | ENSG00000139219 |
| COL1A1 |
5′ TCCAACGAGATCGAGATCC 3′ 5′ AAGCCGAATTCCTGGTCT 3′ |
190 | ENSG00000108821 |
| RUNX2 |
5′ ATGCTTCATCGCCTCAC 3′ 5′ ACTGCTTGCAGCCTTAAAT 3′ |
156 | ENSG00000124813 |
| ALPL |
5′ GCTTCAAACCGAGATACAAGCA 3′ 5′ GCTCGAAGAGACCCAATAGGTAGT 3′ |
101 | ENSG00000162551 |
Abbreviations: HMBS, hydroxymethylbilane synthase; IL6, interleukin 6; TLR2, toll‐like receptor 2; TLR4, toll‐like receptor 4; TGFB1, transforming growth factor, beta 1; SOX9, SRY‐box transcription factor 9; COL2A1, collagen type II alpha 1 chain; COL1A1, collagen type I alpha 1 chain; RUNX2, RUNX family transcription factor 2; ALPL, alkaline phosphatase, biomineralization associated. The first oligonucleotide sequence per gene represents the forward primer, and the second sequence represents the reverse primer.
Data analysis and statistics
Where necessary, associations between dichotomous variables were assessed using Pearson's chi‐squared or Fisher's exact test (degrees of freedom = 1). The effect size was reported by computing the odds ratio (OR) with 95% confidence intervals (CI). For the analysis of gene‐expression data, the paired‐samples t‐test (two‐tailed) was used to test for statistical difference between unstimulated and stimulated cells. Interrater agreement was calculated using the Cohen's Kappa method (κ) and evaluated as described elsewhere (Landis & Koch, 1973). For all mentioned calculations, values were considered to be significantly different when p < .05. All calculations were done out using the SPSS statistics package, edition 28 (IBM Corp.).
RESULTS
Assessment of interrater agreement showed complete agreement (κ = 1.00) for the assessment of ‘pulp stones’ (κ = 1.00; p < .001) and ‘Inflammatory infiltration’ (κ = 1.00; p < .001). The following variables had relatively lower κ‐values: ‘ectopic connective tissue’ (κ = 0.84; p < .001), ‘Radiodense structures’ (κ = 0.83; p < .001) and ‘penetration depth’ (κ = 0.78; p < .001). All results besides penetration depth were evaluated as ‘nearly perfect’ and penetration depth as ‘substantial’.
Radiology and histology: Radiodense structures were identified in 56.5% (n = 26) of the teeth. These structures were almost exclusively found in the coronal part of the pulp (n = 24). Histologically, the total sample contained fewer pulp stones (32.6%, n = 15) than ectopic mineralized connective tissue (45.7%, n = 21). Some teeth contained both a pulp stone and ectopic connective tissue (21.7%, n = 10). In‐depth analysis revealed that the radiodense structures and the presence of ectopic mineralized connective tissue were significantly associated (Χ2 = 18.128, df = 1, p < .001, OR = 24.429, 95% CI: [4.47, 133.53]; Figure 4a,b). The same was true for pulp stones and radiodense structures (Χ2 = 12.274, df = 1, p < .001, OR = 22.167, 95% CI: [2.57, 200.00]). A significant association was shown between inflammatory infiltrates and the presence of an ectopic connective tissue structure (Χ2 = 13.638, df = 1, p < .001, OR = 18.46, 95% CI: [2.14, 159.47]; Figure 4c–f). In contrast, an inflammatory infiltrate was not significantly associated with the presence of a pulp stone. Penetration depth of the carious lesion was neither associated with the presence of radiodense structures, nor with the presence of a pulp stone. However, increased penetration depth was associated with the presence of ectopic connective tissue (Fisher's exact; p = .01).
FIGURE 4.
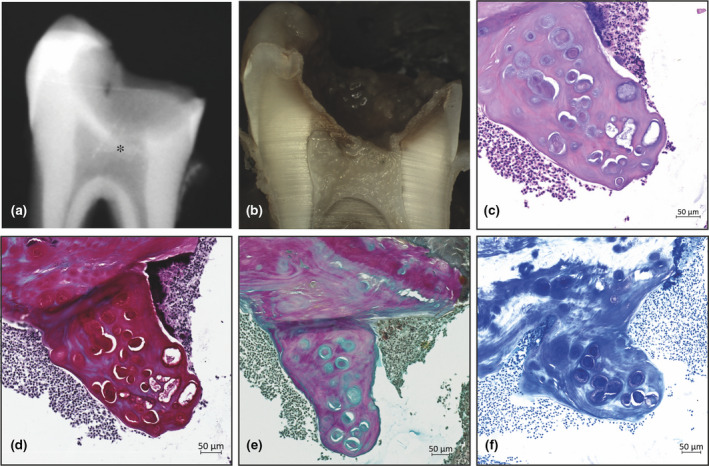
Histological examples of ectopic connective tissue. Ectopic mineralized connective tissue was visible both radiographically (a*) and macroscopically (b). The appearance of the ectopic connective tissue was cartilage‐like, with cells arranged in lacunae (c). The presence of a glycosaminoglycan‐rich matrix was confirmed by staining with Safranin O (d), Alcian Blue van Gieson (e) and Toluidine Blue (f).
The ectopic connective tissue consisted of a dense extracellular matrix in which cells arranged in lacunae were embedded in a manner reminiscent of chondrocytes. The ectopic structures ranged in size from small aggregates composed of a few such lacunae (Figure 3a–c) to large masses consisting of many lacunae (Figure 3d). A minority of lacunae were demonstrated to be arranged as doublets (Figure 3b). Overall, the lacunal unit (Figure 3a) consisted of a centrally positioned basophile cell nucleus surrounded by two types of extracellular matrix: one type a lightly basophilic matrix with a honeycomb‐like or reticular structure, and the other a dense, strongly basophilic matrix somewhat layered in appearance that was arranged as a circular structure around the central nucleus. In some cases, however, this matrix had more of a semi‐lunar morphology. Irrespective of its appearance, the second densely basophilic matrix was always located within the honeycomb‐like matrix. The ectopic connective tissue either lay freely within the stroma, or was attached to the dentine of the pulp chamber wall. In the latter case, there was always a clear line of demarcation between the dentine and the ectopic connective tissue structures.
FIGURE 3.
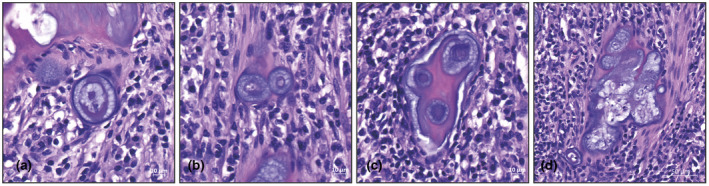
(a) Basic structure of the ectopic connective tissue was the lacunal unit consisting of a central basophilic element in a honeycomb‐like matrix. The unit was surrounded by a dense capsule. (b) Not many units were found as doublets, which could indicate an unstable phenotype. The lacunal units were often arranged in groups that ranged from a small number of lacunae (c) to many units (d). In both cases, the units were separated from each other by an inter‐unit matrix each of various density.
Staining of the ectopic connective tissue structures with Safranin O/Fast green caused the central lacuna and its surrounding matrix to stain intensely red, indicating the presence of proteoglycans (Figure 4d). These findings were confirmed by two rounds of staining. The first used Alcian Blue van Gieson (Figure 4e), in which case the lacunal structure itself stained light blue, indicating the presence of proteoglycans; the surrounding matrix stained intensely red, indicating a collagen‐rich matrix. The second used Toluidine Blue (Figure 4f), where metachromatism of the honeycomb‐like matrix was clear.
Chronic exposure of pulpal cells to TLR2/4 agonists
Quantitative assessment of proteoglycan content
Having shown histologically that ectopic connective tissue was associated with an inflammatory infiltrate, it was next investigated whether pulp stromal cells harvested from sound third molars could, under the influence of an inflammatory stimulus, be induced in vitro to differentiate into structures that resembled cartilage or bone. Pulp stromal cells of passage 4 were cultured in the presence or absence of TLR2/4 agonists mimicking a chronic inflammation. Measurement of the proteoglycan content showed no significant difference, either at Day 7 or at Day 14, between the unstimulated cells and cells stimulated with TLR2/4 agonist (Figure 5).
FIGURE 5.
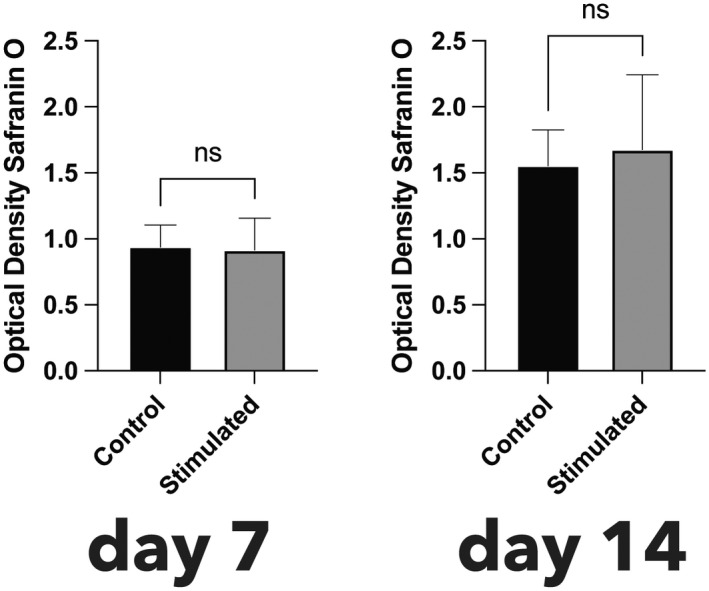
Quantification of proteoglycan content by O.D. of safranin O staining. To take account of differences in cell numbers, the O.D. measurement was normalized against the DNA concentration. Neither at t = 7 days or t = 14 days was there a significant difference in proteoglycan content between cells treated with TLR2/4 agonists and the control.
Gene‐expression analysis
Verification of inflammatory stimulation: The inflammatory activation of the investigated cells was verified by assessing the change in expression of IL6 (Interleukin 6) upon stimulation with the TLR2/4 agonists. This treatment led to a significant increase in expression of IL6 at Day 7 (p = .020, 95% CI: [0.93, 6.23]) and at Day 14 (p = .008, 95% CI [0.5707, 2.017]). Investigation of the ability of the TLR2/4 agonists to induce the expression of their own receptors (TLR2 and TLR4) also showed that the treatment led to a significant increase in expression of TLR2 at Day 7 (p = .020, 95% CI: [0.03, 0.24]) and at Day 14 (p = .009, 95% CI: [0.48, 1.89]). There was no significant change in expression of TLR4 between the control and the stimulated group (Figure 6a). Chondrogenesis: At Day 7, the treatment with TLR2/4 agonists had produced a significant decrease in the expression of TGFB1 (Transforming growth factor, beta 1), a growth factor involved in chondrogenesis (p = .004, 95% CI: [−17.86, −6.43]). At Day 14, the treatment with TLR2/4 agonists had produced a significant decrease in expression of SOX9 (SRY‐box transcription factor 9), a key transcription factor for chondrogenesis (p = .035, 95% CI: [−0.10, −0.01]). At Days 7 and 14, there was no apparent effect of the TLR2/4 agonists on the expression of COL2A1 (Collagen type II alpha 1 chain) (Figure 6b). Osteogenesis: At Day 7, treatment with TLR2/4 agonists had produced a significant decrease in the expression of COL1A1 (Collagen type I alpha 1 chain), the major matrix protein of bone (p = .007, 95% CI: [−204.60, −61.74]). At Days 7 and 14, there was no effect of TLR2/4 agonist treatment on the expression of RUNX2 (RUNX family transcription factor 2). Likewise, at Days 7 and 14, expression of ALPL (Alkaline phosphatase, biomineralization associated) had not been significantly influenced by the inflammatory condition (Figure 6c).
FIGURE 6.
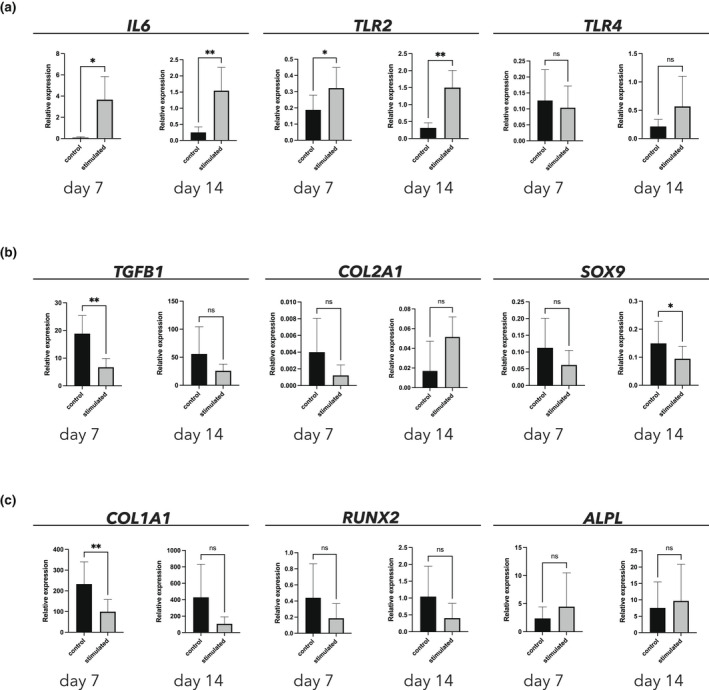
Gene expression of genes relating to inflammation (a), chondrogenesis (b) and osteogenesis (c)
DISCUSSION
This study showed that the formation of hard connective tissue within the pulp can be grouped either into pulp stones or into structures of ectopic mineralized connective tissue that were only seen in cases with inflammatory infiltration of the pulpal stroma. This is in line with previously published results, showing that extremely deep carious lesions are associated with the presence of pulpal mineralization (Demant et al., 2021), and that increased penetration depth is associated with pulpal inflammation (Reeves & Stanley, 1966). Comparision of our radiographic and histological findings showed that the presence of radiodense structures within the coronal pulp as visualized on conventional radiographs was significantly associated with the presence of histologically verified ectopic connective tissue structures as well as with pulp stones; however, only ectopic connective tissue was associated with increased penetration depth and the presence of an inflammatory infiltrate. These findings indicate that it is not clinically possible to discern between pulp stones and ectopic connective tissue based on a conventional periapical radiograph. However, if radiodense structures are present in the coronal pulp on a radiograph of a tooth with a severely progressed carious lesion, it is more probable that these structures are ectopic connective tissue, and that the pulp stroma is inflamed. It is therefore speculated that any findings of radiodense structures within the coronal pulp should be included in preoperative diagnostic assessments of teeth with an initial uncertain pulpal diagnosis, especially in cases of advanced caries. The presence of a radiodense structure in the coronal pulp would indicate irreversible pulpal damage. Such an approach would be in line with earlier published results accompanied by the recommendation that the radiographically defined lesion depth ‘extremely deep’ should be used as a cut‐off level beyond which at least parts of the pulp is believed to be irreversibly damaged (Demant et al., 2021). It is of course important to note that because it is not possible to discern between ectopic connective tissue and pulp stones radiographically, such findings should never stand alone in the assessment of pulpal health. In a tooth with no carious lesion or other obvious signs of damage, the finding of a coronal radiodense structure might be a pulp stone and not a sign of pathology.
Histologically, the ectopic connective tissue described in this study had a distinct morphology, in which cells embedded within the extracellular tissue matrix were arranged in lacunae (Figure 3a–d). The fact that very few of the lacunal structures were found in doublets would seem to indicate that this phenotype is not stable. The lacunae‐like structures described suggest that the ectopic connective tissue structures is related more to cartilage or bone than to dentine. Previous research has suggested that hard‐tissue formation in the pulp after the death of the odontoblast should not be regarded as regeneration of a dentine matrix, since the hard‐tissue matrix thus produced lacks features that are considered to be a hallmark of dentine, such as the presence of dentine tubuli. Instead, it suggests that the phenomenon should be regarded more as repair than regeneration (Ricucci et al., 2014). The current results support the notion that the ectopically formed mineralized connective tissue does not seem to be a variant of dentine. Furthermore, the present results indicate that the tissue is not a passive repair‐like process leading to the simple diffusion‐dependant mineralization of a fibrotic scar, but is a controlled process with distinct developmental steps (Figure 3a–d). A distinct and ordered morphology is a common feature of active biomineralization as compared to passive biological mineralization, which often has an amorphous morphology (Weiner & Dove, 2003).
As the specimens used in this study were demineralized before histological processing, it cannot be concluded whether the ectopic connective tissue represents an intermediate step towards a more stable and mineralized phenotype such as the one seen in heterotopic ossification (Dey et al., 2018).
Although the current finding of cartilage‐like structures within the dental pulp to our knowledge is the first of its kind, it seems possible that pulpal stromal tissue can undergo metaplastic transformation leading to a cartilage‐like phenotype. Pulpal stromal cells have in vitro been shown to able to differentiate into both chondrogenic and osteogenic cells (Karaöz et al., 2011; Laino et al., 2005; Nemeth et al., 2014). This pluripotency mirrors the ectomesenchymal origin of the pulp (Nanci, 2012). The fact that such structures have not been noticed in teeth before might be due to the fact that the specimens of the current investigation are teeth with untreated active carious lesions coming from a group of relatively young individuals.
The fact that the ectopic mineralized connective tissue structures were in some cases attached to the dentinal walls of the pulp chamber—albeit with a clear demarcating line—may indicate that, relative to tertiary dentine (reactive and/or reparative), the tissue represents a different and separate route on a common developmental pathway. This pathway seems to be induced by inflammatory stimulation of the pulpal tissue. The attached and/or embedded denticles described in the literature (Moss‐Salentijn & Klyvert, 1988) might be examples of such attached ectopic connective tissue, but ones that has been noted at another stage of its formation. In this investigation, pulp stones were not significantly associated with inflammation, which corroborates earlier published findings in which pulp stones were reported to be present in nonerupted and noncarious teeth (Moss‐Salentijn & Klyvert, 1983). Together, this would potentially classify pulp stones as a part of normal dental development. To take the differences in aetiology into account, it therefore seems appropriate to apply a general terminology that differentiates between pulp stones and other types of ectopic mineralized connective tissue. The terms used in this article—‘pulp stone’ versus ‘ectopic mineralized connective tissue’—seem convenient.
This study failed to show any clear effect of inflammatory stimulation on the expression of genes related to chondrogenesis (Figure 6b) and osteogenesis (Figure 6c). A main finding of our investigation of the changes in gene expression between stimulated and unstimulated cells was that stimulation with TLR2/4 agonists led first and foremost to significant upregulation of the genes related to inflammation (in both IL6 and TLR2; Figure 6a). The results of the gene‐expression analysis were corroborated by those of the spectrophotometric assessment of proteoglycan content in cell cultures, which showed no significant difference between stimulated and unstimulated cells (Figure 5). Historically, the odontoblast/odontoblast‐like cell has been regarded as responsible for hard‐tissue formation within the pulp; the fact that these cells have been shown to express TLRs (Durand et al., 2006) has made it tempting to assume a binary relationship between the stimulation with an external noxious stimulant and the upregulation of connective tissue‐matrix production. This might be too a simple an explanation. Unlike the inability to show any upregulation of genes related to chondrogenesis and osteogenesis (hypothesis ii), the finding of upregulation of genes related to inflammation is still in line with the literature on heterotopic ossification showing that inflammation and tissue damage are prerequisites for the onset of heterotopic ossification and precede its formation (Dey et al., 2018). It should be emphasized that the relatively simple set‐up of the in vitro cell‐culturing experiments, in which a monoculture of pulpal stromal cells was stimulated with two pure formulations of TLR2/4 agonists, may have been too simple, unlike in vivo inflammation, where inflammatory cells and a multitude of different bacterial strains interact. This simplification may be the reason for the observation of no clear pattern in the upregulation of genes related to chondrogenesis/osteogenesis. Future investigations should be based on more advanced experimental set‐ups, employing co‐cultures of pulpal stromal cells and inflammatory cells under different culturing conditions, and focusing on the ways in which the interaction between mesenchymal stromal cells and inflammatory cells and mediators might translate into hard‐tissue formation. The interaction between the mesenchymal stromal cells (in the pulp or elsewhere) and the resident or homing immune cells and mediators seems to be absolutely imperative for the induction of connective tissue formation. Finally, if the formation of ectopic mineralized connective tissue within the pulp is indeed an example of heterotopic ossification, which the histological part of this investigation could indeed indicate, the tooth might serve as an excellent model for further research into this phenomenon.
CONCLUSION
The formation of ectopic mineralized connective tissue within the dental pulp is associated with severe inflammatory damage to the pulpal stroma. The resulting tissue type does not in any way seem similar to dentine, but instead resembles cartilage or maybe a precursor to bone, much like what is seen in heterotopic ossification, thereby confirming hypothesis i. Although the TLR2/4 agonist stimulation of pulpal mesenchymal stromal cells led to an increase in the expression of inflammatory markers, it had a limited effect on the expression of osteogenic and chondrogenic markers, thereby disproving hypothesis ii. Ectopic mineralized connective tissue seems to be more dependent on complex interactions between immune cells and pulpal mesenchymal cells than on direct stimulation of the pulpal stromal cells.
AUTHOR CONTRIBUTIONS
S. Demant contributed to the study design, scoring of histological and radiographic samples, data analysis and writing. T. Schoen maker contributed to assisting experiments, data analysis and interpretation and proofreading. S.M.G. van Erck w involved in scoring of histological and radiographic samples, data analysis, writing and proofreading. S. Dabelsteen contributed to assisting experiments, data analysis and proofreading. T.J. de Vries contributed to supervising experiments, data analysis and interpretation of results and proofreading. L. Bjørndal contributed to study design, scoring of histological and radiographic samples, data analysis and interpretation of results, and proofreading.
FUNDING INFORMATION
This project was partly funded by the University of Copenhagen, the Danish Endodontic Society and the Danish Dental Association.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICAL APPROVAL
Ethics approval has been obtained.
ACKNOWLEDGEMENTS
The authors acknowledge laboratory technicians Dorrit Nolting (University of Copenhagen) and Jolanda Hogervorst (ACTA) for their invaluable advice and help regarding histological techniques and qPCR and evaluation. This project was partly funded by the University of Copenhagen, the Danish Endodontic Society and the Danish Dental Association.
Demant, S. , Schoenmaker, T. , van Erck, S.M.G. , Dabelsteen, S. , de Vries, T.J. & Bjørndal, L. (2022) Intra‐pulpal connective tissue formation and the advanced carious lesion: Is chondrogenesis and heterotopic ossification a response to pulpal inflammation? International Endodontic Journal, 55, 1212–1224. Available from: 10.1111/iej.13821
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bjørndal, L. & Darvann, T. (1999) A light microscopic study of odontoblastic and non‐odontoblastic cells involved in tertiary dentinogenesis in well‐defined cavitated carious lesions. Journal of Caries Research, 33, 50–60. [DOI] [PubMed] [Google Scholar]
- Bjørndal, L. , Darvann, T. & Thylstrup, A. (1998) A quantitative light microscopic study of the odontoblast and subodontoblastic reactions to active and arrested enamel caries without cavitation. Journal of Caries Research, 32, 59–69. [DOI] [PubMed] [Google Scholar]
- Cholok, D. , Chung, M.T. , Ranganathan, K. , Ucer, S. , Day, D. , Davis, T.A. et al. (2018) Heterotopic ossification and the elucidation of pathologic differentiation. Bone, 109, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demant, S. , Dabelsteen, S. & Bjørndal, L. (2021) A macroscopic and histological analysis of radiographically well‐defined deep and extremely deep carious lesions: Carious lesion characteristics as indicators of the level of bacterial penetration and pulp response. International Endodontic Journal, 54, 319–330. [DOI] [PubMed] [Google Scholar]
- Dey, D. , Wheatley, B.M. , Cholok, D., Agarwal, S., Yu, P.B., Levi, B. et al. (2018) The traumatic bone: trauma‐induced heterotopic ossification. Translational Research, 186, 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand, S. , Flacher, V. , Roméas, A., Carrouel, F., Colomb, E., Vincent, C. et al. (2006) Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. The Journal of Immunology, 176, 2880–2887. [DOI] [PubMed] [Google Scholar]
- European Society of Endodontology (ESE) , Duncan, H.F. , Galler, K.M. , Tomson, P.L. , Simon, S. , El‐Karim, I., Kundzina, R. et al. (2019) European Society of Endodontology position statement: management of deep caries and the exposed pulp. International Endodontic Journal, 52, 923–934. [DOI] [PubMed] [Google Scholar]
- Farges, J.C. , Alliot‐Licht, B. , Renard, E. , Ducret, E. , Gaudin, A. , Smith, A.J. et al. (2015) Dental pulp defence and repair mechanisms in dental caries. Mediators of Inflammation, 2015, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaöz, E. , Demircan, P.C. , Sağlam, O. , Aksoy, A. , Kaymaz, F. & Duruksu, G. (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow‐derived mesenchymal stem cells. Histochemistry and Cell Biology, 136, 455–473. [DOI] [PubMed] [Google Scholar]
- Karlis, G.D. , Schöningh, E. , Jansen, I.D.C. , Schoenmaker, T. , Hogevorst, J.M.A. , van Veen, H.A. et al. (2020) Chronic exposure of gingival fibroblasts to TLR2 or TLR4 agonist inhibits osteoclastogenesis but does not affect osteogenesis. Frontiers in Immunology, 11, 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laino, G. , D'aquino, R. , Graziano, A. , Lanza, V. , Carinci, F. , Naro, F. et al. (2005) A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). Journal of Bone and Mineral Research, 8, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Landis, J.R. & Koch, G.G. (1973) The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. [PubMed] [Google Scholar]
- Mejàre, I.A. , Axelsson, S. , Davidson, T. , Frisk, F. , Hakeberg, M. , Kvist, T. et al. (2012) Diagnosis of the condition of the dental pulp: a systematic review. International Endodontic Journal, 45, 597–613. [DOI] [PubMed] [Google Scholar]
- Moss‐Salentijn, L. & Klyvert, M.H. (1983) Epithelially induced denticles in the pulps of recently erupted noncarious human premolars. Journal of Endodontics, 9, 554–560. [DOI] [PubMed] [Google Scholar]
- Moss‐Salentijn, L. & Klyvert, M.H. (1988) Calcified structures in human dental pulps. Journal of Endodontics, 14, 184–189. [DOI] [PubMed] [Google Scholar]
- Nagendrababu, V. , Murray, P.E. , Ordinola‐Zapata, R. , Peters, O.A. , Rôças, I.N. , Siqueira, J.F., Jr. et al. (2021) PRILE 2021 guidelines for reporting laboratory studies in Endodontology: a consensus‐based development. International Endodontic Journal, 54, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Nanci, A. (2012) Development of the tooth and its supporting tissues. In: Nanci, A. (Ed.) Ten cate's oral histology, 8th edition. Saint Louis, MI: Elsevier, pp. 70–94. [Google Scholar]
- Nemeth, C.L. , Janebodin, K. , Yuan, A.E. , Dennis, J.E. , Reyes, M. & Kim, D.H. (2014) Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG‐GelMA‐HA hydrogels. Tissue Engineering, 20, 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintarelly, G. , Scott, J.E. & Dellovo, M.C. (1964) The chemical and histochemical properties of Alcian Blue. II. Dye binding of tissue polyanions. Histochemie, 4, 86–98. [DOI] [PubMed] [Google Scholar]
- Reeves, R. & Stanley, H.R. (1966) The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surgery, Oral Medicine, Oral Pathology, 22, 59–65. [DOI] [PubMed] [Google Scholar]
- Ricucci, D. , Loghin, S. , Lin, L.M. , Spångberg, L.S.W. & Tay, F.R. (2014) Is hard tissue formation in the dental pulp after the death of the primary odontoblasts a regenerative or a reparative process? Journal of Dentistry, 42, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Ruhl, T. & Beier, J.P. (2019) Quantification of chondrogenic differentiation in monolayer cultures of mesenchymal stromal cells. Analytical Biochemistry, 582, 113356. [DOI] [PubMed] [Google Scholar]
- Schmitz, N. , Laverty, S. , Kraus, V.B. & Aigner, T. (2010) Basic methods in histopathology of joint tissues. Osteoarthritis and Cartilage, 18, 113–116. [DOI] [PubMed] [Google Scholar]
- Schoenmaker, T. , Mokry, M. , Micha, D. , Netelenbos, C. , Bravenboer, N. , Gilijamse, M. et al. (2021) Activin‐a induces early differential gene expression exclusively in periodontal ligament fibroblasts from fibrodysplasia ossificans progressiva patients. Biomedicine, 6, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J.E. , Quintarelli, G. & Dellovo, M.C. (1964) The chemical and histochemical properties of Alcian Blue. I. The mechanism of Alcian Blue staining. Histochemie, 4, 73–85. [DOI] [PubMed] [Google Scholar]
- Smith, A.J. , Cassidy, N. , Perry, H. , Begue‐kirn, C. , Ruch, J.V. & Lesot, H. (1995) Reactionary dentinogenesis. International Journal of Developmental Biology, 39, 273–280. [PubMed] [Google Scholar]
- Weiner, S. & Dove, P.M. (2003) An overview of biomineralization processes and the problem of the vital effect. Reviews in Mineralogy and Geochemistry, 54, 1–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


