Abstract
Background and purpose
Nerve conduction studies (NCS) are the current objective measure for diagnosis of peripheral neuropathy in type 2 diabetes but do not assess nerve structure. This study investigated the utility of peripheral nerve ultrasound as a marker of the presence and severity of peripheral neuropathy in type 2 diabetes.
Methods
A total of 156 patients were recruited, and nerve ultrasound was undertaken on distal tibial and distal median nerves. Neuropathy severity was graded using the modified Toronto Clinical Neuropathy Scale (mTCNS) and Total Neuropathy Score (TNS). Studies were undertaken by a single ultrasonographer blinded to nerve conduction results.
Results
A stepwise increase in tibial nerve cross‐sectional area (CSA) was noted with increasing TNS grade (p < 0.001) and each mTCNS quartile (p < 0.001). Regression analysis demonstrated a correlation between tibial nerve CSA and neuropathy severity (p < 0.001). Using receiver operator curve analysis, tibial nerve CSA of >12.88 mm yielded a sensitivity of 70.5% and specificity of 85.7% for neuropathy detection. Binary logistic regression revealed that tibial nerve CSA was a predictor of abnormal sural sensory nerve action potential amplitude (odds ratio = 1.239, 95% confidence interval [CI] = 1.142–1.345) and abnormal neuropathy score (odds ratio = 1.537, 95% confidence interval [CI] = 1.286–1.838).
Conclusions
Tibial nerve ultrasound has good specificity and sensitivity for neuropathy diagnosis in type 2 diabetes. The study demonstrates that tibial nerve CSA correlates with neuropathy severity. Future serial studies using both ultrasound and NCS may be useful in determining whether changes in ultrasound occur prior to development of nerve conduction abnormalities and neuropathic symptoms.
Keywords: cross‐sectional area, diabetic neuropathy, nerve ultrasound, peripheral nerve
INTRODUCTION
Peripheral neuropathy affects at least 50% of patients with type 2 diabetes [1]. These patients most commonly present with a generalized distal symmetric polyneuropathy, typically with sensory disturbance in the feet. The presence and higher number of features of the metabolic syndrome, including obesity, hypertriglyceridaemia, hypercholesterolaemia, hypertension, and waist circumference, are known to increase the risk of developing a symptomatic peripheral neuropathy [2].
Nerve conduction studies are the current objective measure in diagnosis of peripheral neuropathy but are insensitive to early changes in nerve structure and function [3]. Peripheral nerve ultrasound has had an increasing role in the study of peripheral nerve disorders, in both mono‐ and polyneuropathies [4]. Previous studies in persons with diabetes have demonstrated ultrasound abnormalities when compared to healthy controls [5, 6], particularly in nerve cross‐sectional area (CSA). The present study was undertaken to assess whether assessment of peripheral nerve morphology using ultrasound, performed on a single occasion at the point of care, could provide accurate diagnostic information on the presence and severity of peripheral neuropathy in type 2 diabetes. A further aim of the study was to assess whether nerve ultrasound parameters are able to detect structural changes in patients who do not manifest clinical or nerve conduction abnormalities of neuropathy.
METHODS
STARD (Standards for Reporting of Diagnostic Accuracy Initiative) criteria (Figure 1) were used to evaluate diagnostic accuracy of assessment of neuropathy using ultrasound of the tibial nerve [7]. The study was undertaken in 156 patients with confirmed type 2 diabetes, consecutively recruited between August 2020 and June 2021 from the Diabetes Centre, Prince of Wales Hospital, Sydney. Studies were approved by the Human Research Ethics Committee of the University of New South Wales. All assessments were undertaken at the point of care with informed consent. Exclusion criteria included age < 18 years, inability to provide informed consent, lower limb amputation, peripheral neuropathy due to other causes including vitamin B12 deficiency, alcohol, and prior exposure to neurotoxic medication including immunotherapy and chemotherapy agents. All patients underwent clinical assessment of peripheral neuropathy, including nerve conduction studies, and peripheral nerve ultrasound examination of median and tibial nerves. The sonographer was blinded to nerve conduction study results but not neuropathy symptoms and signs.
FIGURE 1.
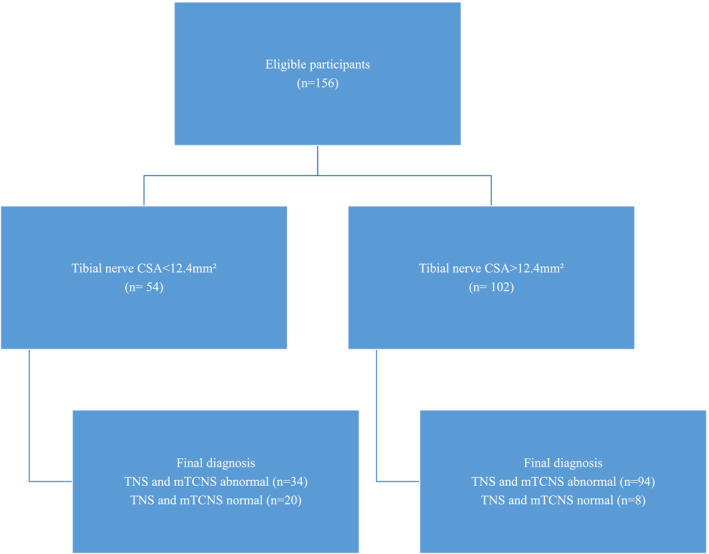
Point‐of‐care assessment of neuropathy with peripheral nerve ultrasound in type 2 diabetes (STARD [Standards for Reporting of Diagnostic Accuracy Initiative] diagram). Index test was ultrasound cross‐sectional area (CSA) measurement of tibial nerve. Reference standard was Total Neuropathy Score (TNS) and modified Toronto Clinical Neuropathy Scale (mTCNS) score >1. [Colour figure can be viewed at wileyonlinelibrary.com
Peripheral neuropathy was assessed using the Total Neuropathy Score (TNS) [8] and the modified Toronto Clinical Neuropathy Scale (mTCNS) [9]. The TNS comprises eight sections: sensory and motor symptoms, pinprick sensation (Neurotip, Owen Mumfor, Oxford, UK), vibration sense (128‐Hz tuning fork), strength examination, deep tendon reflexes, sural sensory amplitude, and tibial motor amplitude on nerve conduction studies (Natus, Middleton, WI, USA). Each section of the TNS provides a maximum score of 4, with total maximum score for the TNS of 32, a higher score representing more severe peripheral neuropathy. The TNS can be subdivided into four grades, with higher grades representing increasing severity of peripheral neuropathy: 0–1 = grade 0, 2–8 = grade 1, 9–16 = grade 2, 17–24 = grade 3, 25–32 = grade 4. The mTCNS comprises of six components of symptom scores and five components of sensory examination scores, each component scored from 0 to 3, with a total possible maximum score of 33 (a higher score representing more severe peripheral neuropathy). Symptom categories include foot pain, numbness, tingling, weakness, ataxia, and upper limb symptoms. Sensory examination categories include pinprick, temperature, light touch, vibration, and position sense.
Standardized ultrasound of median and tibial nerves was performed by a single sonographer. Imaging was performed with a 10–18‐MHz linear array transducer (MyLab One, Esaote, Genoa, Italy) using constant depth, gain, and focus and the musculoskeletal factory preset (acoustic power = 100%, line density set at medium, dynamic range set at 14, and persistence set at 1). All settings were kept constant during participant measurements. To establish the feasibility of ultrasonography in a diabetes clinic, all studies were timed. Peripheral nerve CSA was measured by three free hand traces of the inner margin of the hyperechoic rim, with the mean value used. The probe was maintained at perpendicular plane and removed between each of the three traces. The tibial nerve CSA was assessed at 5 cm proximal to the medial malleolus, away from potential entrapment sites [10]. The nerve was tracked proximally from the medial malleolus along with the tibial vessels. Median nerve CSA was assessed at a point one third of the length of the forearm proximal to the wrist in the participant's dominant hand, away from an entrapment site [11]. The median nerve was initially identified at the wrist at the carpal tunnel inlet and then tracked proximally between the flexor digitorum superficialis and flexor digitorum profundus muscles.
Data analysis
Data were analysed for all measured variables using SPSS Statistics version 26.0 for Windows. Participants were deidentified, and analysis of relevant variables was performed using a coded system. Normality of data was tested with the Shapiro–Wilk test. Normally distributed data were analysed using independent t‐tests. Correlation studies were performed for clinical and ultrasound parameters using Spearman coefficients (rho). Where group comparisons were performed, analysis of variance was used. To assess the influence of confounders, including age, gender, duration of disease, hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR), body mass index, and waist circumference, multiple regression analysis was undertaken. The ability of tibial nerve CSA to detect abnormal neuropathy score, defined as TNS or mTCNS score of >1, was assessed using receiver operator characteristic (ROC) curves and area under the curve (AUC). Binary logistic regression was performed to assess the relationship between tibial nerve CSA and abnormal sural amplitude, chosen as the earliest marker for abnormal nerve conduction studies in diabetic neuropathy. Statistical significance was defined as p < 0.05.
RESULTS
A total of 156 type 2 diabetes participants were consecutively recruited and assessed. All participants had a formal diagnosis of type 2 diabetes for a minimum of 1 month with a mean disease duration of 177 ± 10.61 months. The majority of participants had mild to moderate severity of peripheral neuropathy (Table 1) according to both mTCNS and TNS systems. For TNS, patients were allocated to TNS grades 0–4 [8] as in previous studies [12], with increasing TNS grade indicating more severe peripheral neuropathy. Thirty‐one participants (19.9%) had a TNS score of grade 0 (TNS < 2), 78 participants (50%) had a TNS grade of 1 (TNS = 2–8), 36 participants (23.1%) had a TNS grade of 2, (TNS = 9–16), and 11 participants (7.1%) had a TNS grade of 3 (TNS = 17–24). As noted in previous studies [13], age (p < 0.001) and duration of disease (p = 0.02) were associated with increasing peripheral neuropathy severity, whereas body mass index (BMI; p = 0.769) and waist circumference (p = 0.265) were similar across the different grades of peripheral neuropathy severity (Table 1). There was also a trend toward higher mean HbA1c for higher grade of peripheral neuropathy severity (p = 0.092).
TABLE 1.
Patient demographics
| Characteristic | TNS grade 0 (0–1) | TNS grade 1 (2–8) | TNS grade 2 (9–16) | TNS grade 3 (17–24) | p | Entire cohort |
|---|---|---|---|---|---|---|
| n | 31 | 78 | 36 | 11 | 156 | |
| Age, years | 55.84 ± 2.38 | 66.15 ± 1.25 | 66.56 ± 2.40 | 71.45 ± 3.88 | <0.001 | 64.57 ± 1.05 |
| Men/women | 19/12 | 36/42 | 30/6 | 8/3 | 0.001 | 93/63 |
| BMI, kg/m2 | 31.02 ± 1.20 | 32.35 ± 0.75 | 31.62 ± 1.39 | 33.01 ± 1.82 | 0.769 | 32.0 ± 0.6 |
| Waist circumference, cm | 104.47 ± 2.76 | 108.20 ± 1.71 | 113.16 ± 3.22 | 106.73 ± 11.40 | 0.265 | 108.55 ± 1.48 |
| Disease duration, months | 127.23 ± 14.90 | 176.31 ± 16.03 | 198.83 ± 20.73 | 259.63 ± 49.14 | 0.020 | 177.64 ± 10.61 |
| TNS | 0.2 ± 0.1 | 4.5 ± 0.3 | 11.9 ± 0.4 | 19.1 ± 0.4 | <0.001 | 6.4 ± 0.5 |
| mTCNS | 0.3 ± 0.1 | 4.4 ± 0.3 | 12.0 ± 0.6 | 20.5 ± 1.7 | <0.001 | 6.5 ± 0.5 |
| HbA1c, % | 8.1 ± 0.3 | 8.1 ± 0.2 | 8.5 ± 0.3 | 9.4 ± 0.6 | 0.092 | 8.3 ± 0.1 |
| HbA1c, IFCC units | 65 ± 3 | 65 ± 2 | 69 ± 3 | 79 ± 6 | 67 ± 1 | |
| eGFR, ml/min/1.73 m2 | 72.7 ± 4.0 | 71.2 ± 2.6 | 68.2 ± 4.1 | 64.4 ± 5.9 | 0.675 | 70.3 ± 1.8 |
| Potassium, mmol/L | 4.3 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.4 ± 0.1 | 0.353 | 4.4 ± 0.03 |
| Sural SNAP, μV | 13.0 ± 1.1 | 8.1 ± 1.0 | 2.1 ± 0.5 | 0 | <0.001 | 7.1 ± 0.6 |
| Tibial CMAP, mV | 9.4 ± 0.7 | 7.7 ± 0.6 | 3.8 ± 0.5 | 0.8 ± 0.4 | <0.001 | 6.6 ± 0.4 |
Note: Figures are quoted as mean ± SE. Probability values are denoted for analysis of variance of mean between groups.
Abbreviations: BMI, body mass index; CMAP, compound muscle action potential; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IFCC, International Federation of Clinical Chemistry; mTCNS, modified Toronto Clinical Neuropathy Scale; SNAP, sensory nerve action potential; TNS, Total Neuropathy Score.
The main purpose of the study was to observe whether an assessment of nerve CSA, undertaken on a single occasion, could establish the presence and severity of peripheral neuropathy in participants with type 2 diabetes, even in the mildest grades of peripheral neuropathy and prior to changes on nerve conduction studies. All studies were timed, and tibial and median nerve ultrasound was undertaken at a mean time per participant of 4.7 ± 0.2 min, which included participant positioning, nerve tracking, image capture, and calculation of nerve size (Figure 2). Values obtained in the disease cohort were compared to published reference values, with tibial nerve CSA > 12.4 mm2 considered abnormal [10]. When referenced against that particular value, mean tibial nerve CSA was increased in the cohort with diabetes, with a mean CSA of 14.88 ± 0.42 mm2 (p < 0.001). A tibial nerve CSA of >12.4 mm2 was detected in 65.4% of the participants (n = 102). A subanalysis was undertaken to investigate the effect of increasing peripheral neuropathy severity on tibial nerve CSA. To enable this assessment, mTCNS scores were divided into quartiles, and a stepwise increase in tibial CSA was noted with each increasing quartile of peripheral neuropathy severity (p < 0.001, Figure 3). A similar pattern was noted using TNS grading (p < 0.001, Figure 4). Nerve conduction values are shown in Figure 3 for comparison and demonstrate an increase in tibial nerve CSA seen with decreasing sural sensory nerve action potentials (SNAPs) and tibial nerve compound muscle action potentials (CMAPs).
FIGURE 2.
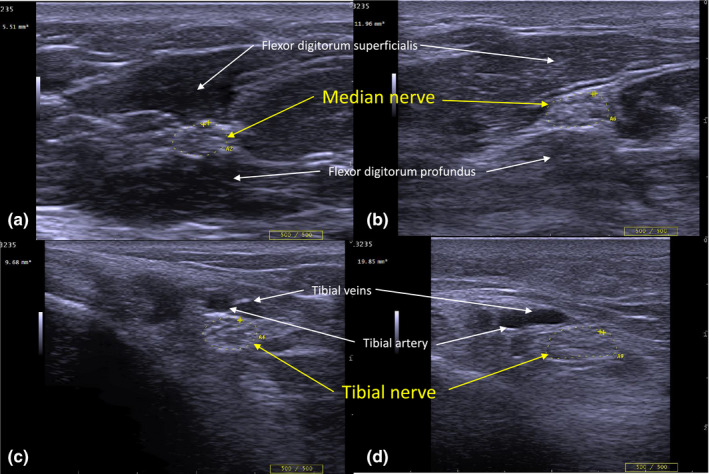
Peripheral nerve ultrasound of median and tibial nerve in diabetes patients with and without neuropathy. Cross‐sectional images of median (a and b) and tibial nerves (c and d) were obtained using peripheral nerve ultrasound. Images a and c are from a patient with type 2 diabetes with a Total Neuropathy Score of 0 and modified Toronto Clinical Neuropathy Score of 0. Images b and d are from a patient with type 2 diabetes with Total Neuropathy Score of 11 and modified Toronto Clinical Neuropathy Score of 16. Nerves on the left‐side panels (a and c) are normal, whereas nerves in the right‐side panels (b and d) are enlarged: median nerve = 11.96 mm2 (normal < 10.01 mm2), tibial nerve = 19.85 mm2 (normal <12.4 mm2). The depth setting for the left‐side image of the tibial nerve is 2 cm, and 3 cm for the right‐side image of the tibial nerve. [Colour figure can be viewed at wileyonlinelibrary.com
FIGURE 3.
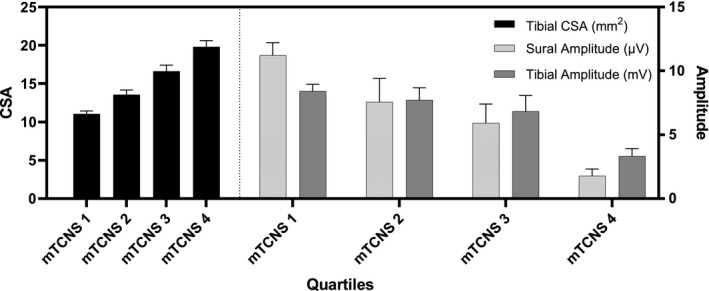
Tibial nerve cross‐sectional area (CSA) and nerve conduction study results in type 2 diabetes patients by modified Toronto Clinical Neuropathy Score (mTCNS). Tibial nerve CSA was measured by peripheral nerve ultrasound for 156 participants with type 2 diabetes and divided into quartiles based on mTCNS. There is a stepwise increase in tibial nerve CSA with increasing neuropathy severity. Mean sural and tibial nerve amplitudes for each quartile of mTCNS demonstrate a stepwise reduction in nerve conduction amplitudes with increasing neuropathy severity.
FIGURE 4.
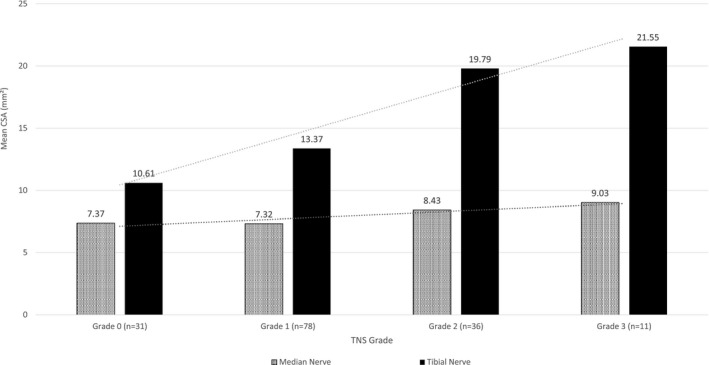
Median and tibial nerve cross‐sectional area (CSA) in type 2 diabetes patients by Total Neuropathy Score (TNS). Median and tibial nerve CSA was measured by peripheral nerve ultrasound for 156 participants with type 2 diabetes based on TNS grades. There is a stepwise increase in median and tibial nerve CSA with increasing peripheral neuropathy severity. This rise is more marked in the tibial nerves.
Data were also analysed according to the presence or absence of nerve conduction abnormalities. In participants who had abnormal nerve conduction studies (40.4%, n = 63), mean tibial CSA was 17.91 ± 0.66 mm2 and abnormal tibial nerve CSA values were recorded in 85.7% of participants. Normal nerve conduction studies were noted in 59.6% of participants (n = 93), and mean tibial nerve CSA was lower than that noted in the abnormal nerve conduction study group (12.84 ± 0.44 mm2, p < 0.001). Of the 93 participants with normal nerve conduction studies, 51.6% of participants were noted to have abnormal tibial CSA values (n = 48). Of these 48 participants, 44.9% had peripheral neuropathy symptoms despite normal nerve conduction studies. Participants were deemed asymptomatic if they did not report any symptoms on TNS or mTCNS assessment. For the group that reported neuropathy symptoms, mean tibial nerve CSA was 17.78 ± 0.67 mm2 (n = 62), compared to 12.83 ± 0.41 mm2 (n = 94) for the group that did not report neuropathy symptoms on the TNS and mTCNS (see Figure 5). For the group with abnormal neurological examination, mean tibial nerve CSA was 16.12 ± 0.46 mm2 (n = 117), compared to 10.73 ± 0.59 mm2 (n = 29) for those who had normal neurological examinations, as recorded on the TNS and mTCNS. Of the total cohort, eight patients had abnormal nerve conduction studies, despite having a normal tibial nerve CSA value (<12.4 mm2), and mean tibial nerve CSA for this group was 11.23 ± 0.22 mm2. In those participants with normal nerve conduction studies, mean tibial nerve CSA was higher in those with neuropathic symptoms compared to those without symptoms (13.97 ± 0.53 mm2, 10.20 ± 0.50 mm2, p < 0.001). Using the literature‐based reference value of 12.4 mm2, tibial nerve CSA by peripheral nerve ultrasound provided a sensitivity of 73.4% and specificity of 71.4% for an abnormal peripheral neuropathy score by TNS or mTCNS (score of >1). ROC analysis was performed for tibial nerve CSA of the 156 patients (AUC = 0.85) using Youden index, and a tibial nerve CSA of 12.89 mm2 yielded a sensitivity of 70.5% and specificity of 85.7%.
FIGURE 5.
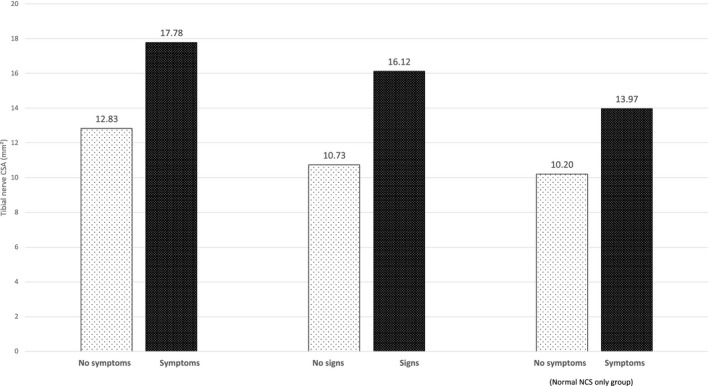
Mean tibial nerve cross‐sectional area (CSA) based on presence of neuropathy symptoms or signs. Mean tibial nerve CSA (mm2) for participants was stratified by neuropathy symptoms or signs, as documented in Total Neuropathy Score and modified Toronto Clinical Neuropathy Scale scores. Tibial nerve CSA is greater in participants who have symptoms or physical signs of neuropathy. In the normal nerve conduction studies (NCS) group, participants with symptoms have greater tibial nerve CSA than those without symptoms.
For median nerve CSA, there was a similar stepwise increase in mean CSA seen with TNS grades (Figure 4). When compared to a published reference value of 10.10 mm2, 8.3% participants (n = 13) had an abnormal median nerve CSA. This subgroup of participants all had neuropathy severity of TNS grade 2 or 3, with a mean median nerve CSA of 11.24 ± 0.34 mm2. Of all participants with abnormal nerve conduction studies (n = 63), mean median CSA was 8.07 ± 0.24 mm2, and for the normal nerve conduction study group (n = 93), mean median CSA was 7.47 ± 0.17 mm.
Correlation between peripheral neuropathy scores and measures of nerve ultrasonography
As a means of establishing the clinical significance of the changes in tibial nerve CSA, correlations were undertaken between tibial nerve CSA values and both TNS and mTCNS. A higher tibial nerve CSA was associated with a higher mTCNS score, representing more severe peripheral neuropathy (r = 0.643, p < 0.001). A similar finding was observed when using the TNS, with higher tibial nerve CSA correlating with higher peripheral neuropathy scores (r = 0.700, p < 0.001). These correlations remained after accounting for potential confounders, including age, HbA1c, and eGFR (r = 0.652, p < 0.001 and r = 0.689, p < 0.001).
Linear regression analysis was also performed to assess the relationship between tibial nerve CSA and peripheral neuropathy severity scores. A regression model revealed a strong relationship between tibial nerve CSA and peripheral neuropathy severity by TNS score (regression coefficient = 0.665, p < 0.001). Using mTCNS, regression analysis again demonstrated a strong relationship between tibial nerve CSA and peripheral neuropathy severity (regression coefficient = 0.634, p < 0.001). These relationships were not affected by age, duration of disease, HbA1c, eGFR, BMI, or waist circumference. Linear regression analysis for median nerve CSA and peripheral neuropathy severity did not reflect the same significance when adjusted for these variables (regression coefficient for TNS = 0.145, p = 0.111; mTCNS = 0.216, p = 0.018).
Binary logistic regression was undertaken to establish the relationship between tibial nerve CSA and sural sensory nerve conduction study values [14, 15]. Binary logistic regression revealed that tibial nerve CSA was a significant predictor of abnormal sural SNAP amplitude (odds ratio = 1.239, 95% confidence interval [CI] = 1.142–1.345, p < 0.001). Binary logistic regression also revealed tibial nerve CSA was a significant predictor of elevated neuropathy score by either TNS or mTCNS (odds ratio = 1.537, 95% CI = 1.286–1.838, p < 0.001).
DISCUSSION
The present study investigated the utility of peripheral nerve ultrasound assessment undertaken at a single point in time as a diagnostic marker of peripheral neuropathy and as a method of establishing its severity in a consecutively recruited cohort of participants with type 2 diabetes, with data collected and analysed at the point of care. Although nerve conduction studies are no longer included in the criteria for diagnosis of neuropathy, ultrasound may be useful as a point‐of‐care assessment tool if it is able to detect neuropathy at an early stage, prior to the onset of established neurological disability [16]. The study has established a significant correlation between nerve CSA and neuropathy severity, and a stepwise increase in tibial CSA with increasing peripheral neuropathy grade, measured using two validated neuropathy scoring systems (TNS and mTCNS). Figure 5 demonstrates that patients with more severe symptoms or signs of diabetic neuropathy had a higher tibial nerve CSA. This study supports that tibial nerve ultrasound measures are more sensitive in detecting neuropathy than median nerve measures, consistent with the length‐dependent nature of neuropathy in diabetes [3]. The study has also established that a high proportion of patients with normal nerve conduction studies have abnormal tibial nerve CSA values. A limitation of the present study is that it was undertaken at a single centre, and further multicentre studies are required to corroborate these findings. As a means of facilitating such studies, nerve CSA was compared to an established literature reference as opposed to values obtained from recruitment of a healthy control cohort. Furthermore, although previous studies have demonstrated high intra‐ and interoperator reliability of nerve ultrasound, these aspects were not assessed specifically in the current study [17, 18]. It is important to note that the finding of an enlarged tibial nerve CSA alone is not diagnostic of the typical length‐dependent polyneuropathy of diabetes. The technique should be used in conjunction with clinical history, examination findings, and possibly nerve conduction studies, to determine whether the patient has a length‐dependent neuropathy due to diabetes or an alternate diagnosis such as immune‐mediated neuropathy, which may also occur in diabetic patients [3, 19, 20]. If clinical review suggests discrepancy between ultrasound measures and clinical features of neuropathy, then an alternative aetiology such as a demyelinating process should be considered. Additional investigations may include serum assessment of B12 levels, paraproteins, and thyroid function, nerve conduction studies, and possibly lumbar puncture to assess cerebrospinal fluid protein concentration [21, 22, 23].
Measurement of CSA is the most sensitive of ultrasound markers for peripheral neuropathy, compared to loss in fascicular pattern or nerve echogenicity [5]. Previous studies have demonstrated evidence of enlarged peripheral nerves in type 2 diabetes [6, 24, 25, 26]. Riazi et al. demonstrated that the distal tibial nerve was enlarged compared to control subjects at three different sites, 1, 3, and 5 cm proximal to the medial malleolus [27]. Pitarokoili et al. also demonstrated enlarged peripheral nerves despite normal neurophysiology and proposed that morphological changes occur prior to electrophysiology [28]. The cause of nerve enlargement has not been fully clarified, but a possible explanation relates to metabolic changes that induce axonal dysfunction. The development of diabetic neuropathy is linked with metabolic derangements induced by hyperglycaemia such as polyol flux, accumulation of advanced glycation end products, and oxidative stress [29]. The conversion of glucose into sorbitol via the aldose reductase conversion process leads to osmotic changes and the development of intraneural oedema [29, 30]. Previous studies have also suggested that Na+/K+ pump dysfunction and intracellular Na+ accumulation in diabetes may contribute to structural changes in patients with peripheral neuropathy [1, 12, 31, 32]. It is known that HbA1c reflecting short‐ to medium‐term glycaemic control does not correlate well with these measures of nerve enlargement, which develop over the long term. Although previous studies have demonstrated that changes in axonal physiology are greater with increasing peripheral neuropathy severity in those with type 2 diabetes, it should also be acknowledged that prior studies have demonstrated an independent association between impaired glucose tolerance and axonal neuropathy, suggesting possible structural and functional nerve changes prior to the diagnosis with type 2 diabetes [12, 31, 33, 34].
From a clinical perspective, disease‐specific pharmacological treatments for diabetic peripheral neuropathy have been difficult to develop, in part due to the lack of a biomarker that allows early detection of peripheral neuropathy. Nerve conduction studies are still the current objective measure for diagnosis of peripheral neuropathy, but require specialized equipment, are often uncomfortable for the patient, and are insensitive to early changes in nerve function [3]. In addition to these factors, sural SNAP and tibial CMAP amplitudes are more subject to variation than conduction velocity, as they are sensitive to electrode placement and impedance variables, which may limit their use as robust biomarkers. In recent years, corneal confocal microscopy has demonstrated impressive sensitivity for early detection of peripheral neuropathy, by assessing changes in corneal nerve fibre length and density [35, 36]. Corneal confocal microscopy does, however, rely on highly specialized equipment, which may only be available at selected centres, and requires a high level of operator expertise. The present study therefore provides a possible additional method of peripheral neuropathy detection, which can be implemented at the point of care.
There is a need for point‐of‐care assessment tools to navigate the current resource‐intensive referral to a neurologist and requirement of specialized electrophysiology equipment for assessment of diabetic peripheral neuropathy. Other techniques that have previously been researched as point‐of‐care tools include the 1‐g monofilament (sensitivity = 66.7%, specificity = 72.0%), DPNCheck (sensitivity = 84.3%, specificity = 68%), and Sudoscan foot electrochemical skin conductance (sensitivity = 77.4%, specificity = 68.3%) [37, 38]. The availability, speed, noninvasive nature, and established diagnostic utility of tibial nerve ultrasound differentiates it from these other techniques and may be able to provide a continuous objective measure that may be used prospectively to assess for neuropathy progression [4]. The availability of extensive reference data also allows for rapid incorporation into selection criteria for clinical trials. Furthermore, previous studies in peripheral neuropathy have demonstrated that nerve ultrasound measures are responsive to interventions such as haemodialysis [39], steroids, and intravenous immunoglobulin administration [40]. This rapid responsiveness to intervention is a contrasting feature to nerve conduction studies, which do not change, even after organ transplantation [41]. The relatively short testing time (4.7 ± 0.2 min) reflects that nerve ultrasonography is an efficient and practical investigation that may be incorporated as a point‐of‐care test in a diabetes clinic. Considering the benefits of nerve ultrasound compared to nerve conduction studies with respect to cost, availability, pain, and noninvasiveness, we would recommend that ultrasonography be added to assessments that are currently recommended at yearly intervals [16]. Current assessment for neuropathy includes careful history, temperature or pinprick sensory examination, vibration sensation assessment with 128‐Hz tuning fork, and 10‐g monofilament testing. Recent studies have demonstrated an important essential role for nerve ultrasound in the diagnosis of carpal tunnel syndrome and ulnar neuropathy, and as a means of guiding interventions such as corticosteroid injections [42]. However, further prospective serial studies in patients with diabetic neuropathy are required to establish whether tibial nerve CSA is responsive to intervention in this patient group. Similarly, further studies are needed in symptomatic patients with normal nerve conduction studies diagnosed with small fibre neuropathy, both with nerve ultrasonography and other measures of small fibre neuropathy assessment such as quantitative sensory and sudomotor testing [43]. In addition to serial measures of distal nerve CSA in diabetic patients, other semiautomated measures such as echogenicity and fascicular counts on nerve ultrasonography should also be considered for future prospective studies [44].
In conclusion, the present study has shown that a single assessment of nerve morphology has the capability to provide information that correlates with the presence and severity of peripheral neuropathy. These findings suggest that nerve ultrasound may have a role in the assessment of patients in diabetes clinics, in a clinical trial recruitment setting or in large community‐based epidemiological studies of peripheral neuropathy.
AUTHOR CONTRIBUTIONS
Roshan Dhanapalaratnam was involved in study design, recruitment, data collection, data interpretation, and manuscript composition. Tushar Issar was involved in study design, data interpretation, discussion, and manuscript composition. Ann M. Poynten and Kerry‐Lee Milner were involved in recruitment, data interpretation, and discussion. Natalie C. G. Kwai was involved in data interpretation and discussion. Arun V. Krishnan was involved in study design, data interpretation, discussion, and manuscript composition. Arun V. Krishnan is the guarantor of this work and as such had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING INFORMATION
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The authors have no other funding or conflicts of interests to declare.
ACKNOWLEDGMENTS
This research was supported by an Australian Government Research Training Program Scholarship. The Total Neuropathy Score was provided to A.V.K. by Professor David Cornblath and John Hopkins University. We are grateful to the staff and patients of the Diabetes Centre at Prince of Wales Hospital, Sydney. Studies were approved by the Human Research Ethics Committee of the University of New South Wales. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Dhanapalaratnam R, Issar T, Poynten AM, Milner K‐L, Kwai NCG, Krishnan AV. Diagnostic accuracy of nerve ultrasonography for the detection of peripheral neuropathy in type 2 diabetes. Eur J Neurol. 2022;29:3571‐3579. doi: 10.1111/ene.15534
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Kamiya H, Murakawa Y, Zhang W, Sima AA. Unmyelinated fiber sensory neuropathy differs in type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2005;21:448‐458. [DOI] [PubMed] [Google Scholar]
- 2. Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 2016;39:801‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of physical medicine and rehabilitation. Neurology. 2005;64:199‐207. [DOI] [PubMed] [Google Scholar]
- 4. Mandeville R, Wali A, Park C, Groessl E, Walker FO, Cartwright MS. Cost‐effectiveness of neuromuscular ultrasound in focal neuropathies. Neurology. 2019;92:e2674‐e2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Telleman JA, Grimm A, Goedee S, Visser LH, Zaidman CM. Nerve ultrasound in polyneuropathies. Muscle Nerve. 2018;57:716‐728. [DOI] [PubMed] [Google Scholar]
- 6. Singh K, Gupta K, Kaur S. High resolution ultrasonography of the tibial nerve in diabetic peripheral neuropathy. J Ultrason. 2017;17:246‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bossuyt PM, Reitsma JB. The STARD initiative. Lancet. 2003;361:71. [DOI] [PubMed] [Google Scholar]
- 8. Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660‐1664. [DOI] [PubMed] [Google Scholar]
- 9. Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto clinical neuropathy score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26:240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisse AL, Katsanos AH, Gold R, Krogias C, Pitarokoili K. Cross‐sectional area reference values for peripheral nerve ultrasound in adults: a systematic review and meta‐analysis‐part II: lower extremity nerves. Eur J Neurol. 2021;28:2313‐2318. [DOI] [PubMed] [Google Scholar]
- 11. Won SJ, Kim BJ, Park KS, Yoon JS, Choi H. Reference values for nerve ultrasonography in the upper extremity. Muscle Nerve. 2013;47:864‐871. [DOI] [PubMed] [Google Scholar]
- 12. Sung JY, Park SB, Liu YT, et al. Progressive axonal dysfunction precedes development of neuropathy in type 2 diabetes. Diabetes. 2012;61:1592‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popescu S, Timar B, Baderca F, et al. Age as an independent factor for the development of neuropathy in diabetic patients. Clin Interv Aging. 2016;11:313‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578‐585. [DOI] [PubMed] [Google Scholar]
- 15. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956‐962. [DOI] [PubMed] [Google Scholar]
- 16. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cartwright MS, Demar S, Griffin LP, Balakrishnan N, Harris JM, Walker FO. Validity and reliability of nerve and muscle ultrasound. Muscle Nerve. 2013;47:515‐521. [DOI] [PubMed] [Google Scholar]
- 18. Alshami AM, Cairns CW, Wylie BK, Souvlis T, Coppieters MW. Reliability and size of the measurement error when determining the cross‐sectional area of the tibial nerve at the tarsal tunnel with ultrasonography. Ultrasound Med Biol. 2009;35:1098‐1102. [DOI] [PubMed] [Google Scholar]
- 19. Fisse AL, Pitarokoili K, Motte J, et al. Nerve echogenicity and intranerve CSA variability in high‐resolution nerve ultrasound (HRUS) in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. 2019;266:468‐475. [DOI] [PubMed] [Google Scholar]
- 20. Fisse AL, Pitarokoili K, Gold R. Nerve ultrasound protocol to detect dysimmune neuropathies. J Vis Exp. 2021;176:2. [DOI] [PubMed] [Google Scholar]
- 21. Brünger J, Motte J, Grüter T, et al. Nerve ultrasound distinguishes non‐inflammatory axonal polyneuropathy from inflammatory polyneuropathy with secondary axonal damage. Front Neurol. 2021;12:809359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European academy of neurology/peripheral nerve society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force‐second revision. J Peripher Nerv Syst. 2021;26:242‐268. [DOI] [PubMed] [Google Scholar]
- 23. American Association of Neuromuscular & Electrodiagnostic Medicine . AANEM policy statement on electrodiagnosis for distal symmetric polyneuropathy. Muscle Nerve. 2018;57:337‐339. [DOI] [PubMed] [Google Scholar]
- 24. Narayan S, Goel A, Singh AK, Thacker AK, Singh N, Gutch M. High resolution ultrasonography of peripheral nerves in diabetic patients to evaluate nerve cross sectional area with clinical profile. Br J Radiol. 2021;94:20200173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal K, Aggarwal P, Gupta M. Ultrasound evaluation of peripheral nerves of the lower limb in diabetic peripheral neuropathy. Eur J Radiol. 2021;145:110058. [DOI] [PubMed] [Google Scholar]
- 26. Breiner A, Qrimli M, Ebadi H, et al. Peripheral nerve high‐resolution ultrasound in diabetes. Muscle Nerve. 2017;55:171‐178. [DOI] [PubMed] [Google Scholar]
- 27. Riazi S, Bril V, Perkins BA, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross‐sectional study. Diabetes Care. 2012;35:2575‐2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pitarokoili K, Kerasnoudis A, Behrendt V, et al. Facing the diagnostic challenge: nerve ultrasound in diabetic patients with neuropathic symptoms. Muscle Nerve. 2016;54:18‐24. [DOI] [PubMed] [Google Scholar]
- 29. Lee D, Dauphinée DM. Morphological and functional changes in the diabetic peripheral nerve: using diagnostic ultrasound and neurosensory testing to select candidates for nerve decompression. J Am Podiatr Med Assoc. 2005;95:433‐437. [DOI] [PubMed] [Google Scholar]
- 30. Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014:674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Issar T, Tummanapalli SS, Borire AA, et al. Impact of the metabolic syndrome on peripheral nerve structure and function in type 2 diabetes. Eur J Neurol. 2021;28:2074‐2082. [DOI] [PubMed] [Google Scholar]
- 32. Huang H, Wu S. Application of high‐resolution ultrasound on diagnosing diabetic peripheral neuropathy. Diabetes Metab Syndr Obes. 2021;14:139‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson M, Pingel R, Lagali N, Dahlin LB, Rolandsson O. Association between HbA(1c) and peripheral neuropathy in a 10‐year follow‐up study of people with normal glucose tolerance, impaired glucose tolerance and type 2 diabetes. Diabet Med. 2017;34:1756‐1764. [DOI] [PubMed] [Google Scholar]
- 34. Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448‐1453. [DOI] [PubMed] [Google Scholar]
- 35. Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non‐invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683‐688. [DOI] [PubMed] [Google Scholar]
- 36. Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61:1856‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Binns‐Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One‐stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high‐risk foot. Diabet Med. 2018;35:887‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JJ, Pribesh SL, Baskette KG, Vinik AI, Colberg SR. A comparison of screening tools for the early detection of peripheral neuropathy in adults with and without type 2 diabetes. J Diabetes Res. 2017;2017:1467213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borire AA, Arnold R, Pussell BA, et al. Haemodialysis alters peripheral nerve morphology in end‐stage kidney disease. Clin Neurophysiol. 2017;128:281‐286. [DOI] [PubMed] [Google Scholar]
- 40. Niu J, Zhang L, Fan J, et al. Nerve ultrasound may help predicting response to immune treatment in chronic inflammatory demyelinating polyradiculoneuropathy. Neurol Sci. 2022;43:3929‐3937. [DOI] [PubMed] [Google Scholar]
- 41. Tavakoli M, Mitu‐Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker FO, Cartwright MS, Alter KE, et al. Indications for neuromuscular ultrasound: expert opinion and review of the literature. Clin Neurophysiol. 2018;129:2658‐2679. [DOI] [PubMed] [Google Scholar]
- 43. Malik RA, Veves A, Tesfaye S, et al. Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27:678‐684. [DOI] [PubMed] [Google Scholar]
- 44. Gamber D, Motte J, Kerasnoudis A, et al. High‐resolution nerve ultrasound to assess nerve echogenicity, fascicular count, and cross‐sectional area using semiautomated analysis. J Neuroimaging. 2020;30:493‐502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


