Abstract
Our understanding of post‐stroke language function is largely based on older age groups, who show increasing age‐related brain pathology and neural reorganisation. To illustrate language outcomes in the young‐adult brain, we present the case of J., a 23‐year‐old woman with chronic aphasia from a left‐hemisphere stroke affecting the temporal lobe. Diffusion MRI‐based tractography indicated that J.'s language‐relevant white‐matter structures were severely damaged. Employing magnetoencephalography (MEG), we explored J.'s conceptual preparation and word planning abilities using context‐driven and bare picture‐naming tasks. These revealed naming deficits, manifesting as word‐finding difficulties and semantic paraphasias about half of the time. Naming was however facilitated by semantically constraining lead‐in sentences. Altogether, this pattern indicates disrupted lexical‐semantic and phonological retrieval abilities. MEG revealed that J.'s conceptual and naming‐related neural responses were supported by the right hemisphere, compared to the typical left‐lateralised brain response of a matched control. Differential recruitment of right‐hemisphere structures (330–440 ms post‐picture onset) was found concurrently during successful naming (right mid‐to‐posterior temporal lobe) and word‐finding attempts (right inferior frontal gyrus). Disconnection of the temporal lobes via corpus callosum was not critical for recruitment of the right hemisphere in visually guided naming, possibly due to neural activity right lateralising from the outset. Although J.'s right hemisphere responded in a timely manner during word planning, its lexical and phonological retrieval abilities remained modest.
Keywords: alpha oscillations, beta oscillations, event‐related fields, language lateralisation, splenium
Using two tasks, we investigated word production abilities of J., a 23‐year‐old woman with chronic aphasia from a left‐hemisphere stroke. Magnetoencephalography revealed that, compared to the typical left‐lateralised brain response, J.'s neural responses originated in the right hemisphere. Importantly, right‐hemisphere structures were recruited concurrently (330–440 ms post‐picture onset) but spatially differentially during successful naming (right mid‐to‐posterior temporal lobe) and word‐finding attempts (right inferior frontal gyrus).

1. INTRODUCTION
The contribution of the right hemisphere (RH) to language recovery and its ability to compensate for lost function after left‐hemisphere (LH) stroke has long been of interest in aphasia research (Gainotti, 1993; Landis et al., 1982; Papanicolaou et al., 1988; Zaidel, 1983) but remains debated. Evidence suggests that task‐related activity in the RH can be dysfunctional (e.g., Naeser et al., 2005; Postman‐Caucheteux et al., 2010; Selnes, 1999). Other findings, in contrast, suggest a supportive role of the RH (Fernandez et al., 2004; Leff et al., 2002), particularly in the early stages of recovery (Saur et al., 2006; Stockert et al., 2020; for review, see Cocquyt et al., 2017). These results, however, and thus our current understanding of the role of the RH in language after stroke (e.g., Hartwigsen & Saur, 2019; Kiran & Thompson, 2019), are largely based on older age groups. Relatively little is known about these processes in younger adults 1, who constitute at least a 10th of total ischaemic stroke cases (Nedeltchev et al., 2005). In this case report, using magnetoencephalography (MEG), we explore the time course of the RH contribution to word production in a 23‐year‐old woman (J. hereafter) with chronic aphasia developed after an extensive LH infarction.
1.1. Why young stroke sequelae might be different from those in older stroke
Although the risk of stroke and acquired aphasia does increase with age (Engelter et al., 2006; Feigin et al., 2003; Kristensen et al., 1997), ischaemic stroke and silent brain infarcts are not uncommon in the population below 49 years of age (Putaala, 2016; Putaala et al., 2009), with at least 10% of young‐adult stroke survivors having aphasia on long‐term follow‐up (Naess et al., 2009). This young‐adult group seems to be strikingly different from older adults. First of all, the younger brain is not burdened by age‐related adaptive and pathological changes (Mattson & Arumugam, 2018), which also affect language (Peelle, 2019; Shafto & Tyler, 2014). As a result, cerebral tissue can mount a more efficient physiological response to ischaemia—a well‐established finding in animal models (e.g., Buga et al., 2008) and human stroke studies (e.g., Ay et al., 2005). Models of cerebral ischaemia demonstrate that older animals show higher rates of mortality, more severe neurological impairments and more modest recovery (for review, see Popa‐Wagner et al., 2020). This observation is supported clinically: Younger people show better functional outcomes compared to older groups (for review, see Jongbloed, 1986; Weimar et al., 2002; but see Maaijwee et al., 2014), with advanced age predicting more functional deficits independently from stroke severity, lesion characteristics, complications and comorbidities (Knoflach et al., 2012; Macciocchi et al., 1998). Therefore, the young brain presents a unique case for studying cerebral capacity for reorganisation and functional compensation in the absence of age‐related biological changes.
Second, young adults are in a unique position in terms of functional lateralisation, developing throughout ontogenesis (e.g., Everts et al., 2009). Starting from more bilateral patterns at birth (e.g., Perani et al., 2011), language function becomes increasingly lateralised into adulthood (Friederici et al., 2011; Olulade et al., 2020; Ressel, 2008; but see also Groen et al., 2012). On the one hand, more established lateralisation in the young‐adult compared to the child or adolescent brain makes it neurally less flexible (Bates et al., 2001; Newport et al., 2017; for a nuanced perspective, see Anderson et al., 2011; Dennis et al., 2013) and, consequently, more vulnerable to acute focal damage (Esteves et al., 2021). On the other hand, adult neural organisation, characterised by tightly interconnected local networks and pruning of long‐distance interhemispheric connections, emerges as a function of experience (Hervé et al., 2013; Jacobs, 1999; Kosslyn, 1987; Plaut & Behrmann, 2011). Besides experience, functional lateralisation is affected by brain maturation processes, which are determined by biological constraints shaping sensitive and critical periods (Boles et al., 2008). Although the critical period for language acquisition is closed by late adolescence (Hurford, 1991), the brain continues its structural and functional development after adolescence (e.g., Sowell et al., 1999), including ongoing white‐matter maturation (Lebel et al., 2012; Lebel & Beaulieu, 2011). If the neural architecture of language is largely established (direction of lateralisation) but still not fully stabilised (strength of lateralisation) in young adults, it might enable more efficient reorganisation after a focal injury compared to older adults, including involving the contralesional hemisphere, which still retains an echo of early‐life language processing (Martin et al., 2022).
1.2. Theoretical scenarios of RH recruitment after stroke
We consider two scenarios of increased RH activity during language use to be most plausible. The first scenario concerns cases of atypical (strongly right‐lateralised or bilateral) language lateralisation as opposed to the pattern of LH dominance. Because the relation between handedness and the direction of language lateralisation may be at chance level (Mazoyer et al., 2014), premorbid hemispheric dominance for language might be better suggested by the severity of the initial functional impairment. In individuals with strongly right‐lateralised language function, aphasia symptoms tend to be absent after LH stroke damaging perisylvian areas (e.g., Schneck et al., 2021). In cases of more symmetrical organisation, language deficits should be expected, with severity driven, among other things, by a combination of lesion characteristics and degree of lateralisation.
The second scenario involves the RH engaging compensation mechanisms. Unlike restitution (or recovery) of function, which is mainly determined by spontaneous physiological processes and is time restricted, substitution of function typically depends on reorganisation (Rothi & Horner, 1983), which continues into chronic stages (e.g., Holland et al., 2017; Meinzer et al., 2004; Pulvermüller et al., 2005) and involves both hemispheres (e.g., Mohr et al., 2016). Better outcomes could be expected for functions that can be either mediated by multiple neural circuits (e.g., Overgaard & Mogensen, 2011; Price & Friston, 2002; Stefaniak et al., 2020) or are subserved by more distributed networks (Murphy & Corbett, 2009) even in mainly left‐lateralised individuals. For instance, semantic processes (Binder et al., 2009) and pre‐articulatory planning (Tourville & Guenther, 2011), as well as mapping sound to meaning (Hickok & Poeppel, 2007), seem to be bilaterally supported. By contrast, core language abilities (e.g., morphosyntax, Bozic et al., 2010) remain strongly linked to the LH in the majority of the population (Butler et al., 2014; Woodhead et al., 2021).
Interestingly, when language production activity is bilaterally distributed, only left‐handers demonstrate increased interhemispheric connectivity via the corpus callosum (CC), whereas right‐handers show LH dominance and intrinsic connectivity via the CC similar to strongly lateralised individuals (Risse et al., 1997; Tzourio‐Mazoyer et al., 2016). The CC, the main axonal pathway connecting the hemispheres (for review, see Bloom & Hynd, 2005), is considered to play a major role in the development of hemispheric asymmetry (e.g., Aboitiz & Montiel, 2003; Gazzaniga, 2000), including language (Hinkley et al., 2016). Moreover, its integrity has been implicated in interhemispheric compensation and language reorganisation (Piai et al., 2017; Yu et al., 2018). Some discrepancies in the literature on the role of the contralesional hemisphere in recovery might be related to the differences in interhemispheric communication (Bartolomeo & Thiebaut de Schotten, 2016). This includes aphasia, when the activity in the RH may be less efficient when it is isolated or receives degraded input from the left‐lateralised lesioned language networks.
1.3. Time course of spoken word production with MEG and post‐stroke naming ability
Influential models consider spoken word production to be a staged process, with associated time courses (Indefrey & Levelt, 2004; Levelt et al., 1999). Typically, word planning starts with conceptual preparation resulting in retrieval of the target lexical concept within the first 175 ms after picture onset. It is followed by lexical selection (‘lemma retrieval’) and phonological code retrieval around 200–300 ms. Phonological encoding follows (with variable timing depending on the number of phonemes). The phonological code is transformed into an articulatory score around 150 ms prior to articulation (Indefrey, 2011; Indefrey & Levelt, 2004). Unlike more bilaterally supported early visual processing (occipital regions), conceptualisation (ventrotemporal regions) and preparation for articulatory output (left inferior frontal gyrus [IFG] and bilateral sensorimotor areas), lexical selection and phonological code retrieval seem to be firmly grounded in the LH (Indefrey, 2011; Indefrey & Levelt, 2004).
In the aphasia literature, several repetitive transcranial magnetic stimulation (rTMS) studies suggest that suppression of activity in right frontal areas leads to improved naming performance (Naeser et al., 2005; Turkeltaub et al., 2012). Likewise, a functional MRI (fMRI) investigation of patients with left‐lateralised frontoparietal lesions showed activity peaking in the lesion‐homologous areas in the RH only on incorrect naming attempts (Postman‐Caucheteux et al., 2010). There is, however, a scarcity of MEG naming studies in stroke aphasia (e.g., Sörös et al., 2003).
Studying spoken word production with MEG has several advantages. The excellent temporal resolution of the MEG signal enables one to examine brain activity during language use, allowing for a more informed functional interpretation (Hari & Parkkonen, 2015), for example, by differentiating brain activity during early, conceptual stages from activity during self‐monitoring upon hearing one's own incorrect utterance. This characteristic distinguishes the present case study from many previous studies, which have predominantly used methodologies that do not allow for temporal scrutiny. Furthermore, when testing people after stroke, MEG can reveal the neural activity that underpins language function without cerebrovascular pathology violating the underlying assumptions of haemodynamic measures (Archila‐Meléndez et al., 2020; Hillis, 2005; Marshall, 2004; Rossini, 2004).
1.4. Present study
To illustrate the young‐adult brain's neural dynamics during language use after acute vascular trauma, we investigated the word production abilities of a young, right‐handed adult (J.) with chronic aphasia. We focused on J.'s word production for two main reasons. First, production abilities show a less marked recovery after stroke compared to comprehension (e.g., Mazzoni et al., 1992; Prins et al., 1978), with naming difficulties prevailing among people with various aphasia profiles and severity (Garrett, 1992; Goodglass, 1980; Kohn & Goodglass, 1985). Second, in the neurotypical population, the generation of meaningful utterances is highly left lateralised in both right‐ and left‐handers (Tzourio‐Mazoyer et al., 2016; Woodhead et al., 2021), making word retrieval abilities particularly vulnerable to neural loss due to LH stroke.
We used bare picture‐naming and context‐driven picture‐naming tasks (Figure 1) that enabled us to study J.'s word production in more detail. These tasks have replicable behavioural and neural effects (see below). Bare picture naming, where participants name pictures of objects presented on screen, provides a classical measure that correlates well with overall aphasia severity (e.g., Thye & Mirman, 2018) as it taps into all retrieval stages described in models of word naming (Indefrey & Levelt, 2004). Context‐driven naming requires picture naming to complete a sentence, which is either contextually constraining (participant hears ‘The farmer milked the’, followed by the picture of cow) or neutral and unconstrained (‘The child drew a’, picture: cow). This task requires sentence integration for comprehension and taps into conceptual and lexical retrieval, triggered in constraining sentences prior to the picture presentation (Hustá et al., 2021; Piai et al., 2015, 2020). Thus, the context‐driven naming task allowed us to explore whether contextual (semantic and structural) information contained in the sentences facilitated J.'s word production compared to bare picture naming.
FIGURE 1.

Outline of the picture‐naming tasks. Example of a trial in the constrained and unconstrained conditions of the context task and of a trial in the bare picture‐naming task. Both tasks required participants to name the picture as soon as it was displayed. Reproduced with permission from the authors from doi:10.6084/m9.figshare.19224609
We used deterministic tractography based on diffusion MRI to investigate J.'s connectivity in the left and right hemispheres and MEG to explore her brain function in a temporally informed manner. To obtain spatial information from MEG data, we used a sophisticated approach that takes into consideration the lesion's effects on the signal conductivity, thus improving the precision of source localisation (for more details, see Piastra et al., 2018, 2022). Importantly, MEG signatures of both picture naming (Ala‐Salomäki et al., 2021; Levelt et al., 1998; Salmelin et al., 1994; Sörös et al., 2003) and context‐driven word production (Piai et al., 2015; Roos & Piai, 2020) are well established in previous literature, showing replicability of LH sources within individuals and across studies, with a well‐characterised time course. During picture naming, evoked activity is consistently observed in visual areas during the first 200 ms after picture presentation, followed by middle and posterior temporal and parietal regions (sometimes bilaterally, but with test–retest reliability only in the LH) from 200 ms onwards. Around 400 ms onwards, activity is observed in ventral precentral gyrus and IFG (sometimes bilaterally, but with test–retest reliability only in the LH, Ala‐Salomäki et al., 2021; Liljeström et al., 2009; Salmelin et al., 1994; Sörös et al., 2003; Vihla et al., 2006). According to models of word production (e.g., Indefrey & Levelt, 2004), early activity in temporo‐parietal areas reflects conceptual, lexical and phonological retrieval, and later, frontal and sensorimotor activity is associated with phonological and phonetic encoding and articulation.
In context‐driven word production, decreases in the alpha–beta frequency range (10–25 Hz) measured in the period before picture presentation are consistently found over the left mid to posterior inferior, middle and superior temporal regions, extending into the left inferior parietal lobule. In addition, alpha–beta power decreases in the left anterior temporal lobe and left IFG are found (with weak test–retest reliability, Piai et al., 2015; Roos & Piai, 2020). The alpha–beta decreases have been suggested to reflect conceptual and lexical retrieval during word planning (Hustá et al., 2021; Piai et al., 2020). The replicability of LH sources and their time courses aids interpretation of the neural activity differences in the neurotypical and lesioned brain, as well as interpreting J.'s behavioural performance in the light of these differences.
In sum, the literature on post‐stroke recruitment of the contralesional RH for language is dominated by studies on relatively older individuals, a population with an increased risk of vascular and neurodegenerative disease (Mattson & Arumugam, 2018), undergoing neural reorganisation (Cabeza et al., 2002; Chan et al., 2014; Rossi et al., 2004), including in the language domain (Peelle, 2019). Although this is a necessary bias, as it reflects the underlying distribution of stroke in the population, from a theoretical point of view, it provides a skewed perspective on language plasticity. Little is known about the ability of the non‐lesioned RH to contribute to language functioning in young‐adult individuals, who are typically in a unique position in terms of physiological health and relatively recently established (and, possibly, insufficiently stabilised) language lateralisation. Hence, the aim of this case study was to characterise the neural dynamics of word production in a young adult (J.) with chronic post‐stroke aphasia, with a particular focus on interhemispheric connections and contralesional RH, using both spatial and temporal information.
2. MATERIALS AND METHODS
The protocol was approved by the CMO region Arnhem–Nijmegen Ethics Committee (NL58437.091.17), following the Declaration of Helsinki. J. was tested at 2 years and 9 months after stroke. She and her sex‐, age‐ and education‐matched healthy control (23 years old, right‐handed, completed higher general secondary education, and no history of neurological disorders or substance abuse) attended two MEG sessions 1 week apart to perform the context (Session 1) and bare picture‐naming (Session 2) tasks. Additionally, the participants underwent the MRI scanning in Session 1, and the Dutch Comprehensive Aphasia Test (CAT‐NL, Visch‐Brink et al., 2014) and the Amsterdam‐Nijmegen Everyday Language Test (ANELT, Blomert et al., 1994) were administered to J. in Session 2. The sessions took place at the Donders Centre for Cognitive Neuroimaging (Radboud University, Nijmegen, the Netherlands). The participants gave written informed consent and received monetary compensation.
2.1. Case study
At the age of 21, J. suffered a left‐side ischaemic stroke for which emergency treatment did not arrive in time. She stayed in hospital for 3 weeks until her condition stabilised, after which rehabilitation was initiated. During this initial period, she had little language output (‘yes’ and ‘no’). J. sustained extensive damage to temporo‐parietal regions, as well as to the subcortical structures in the LH (Figure 2, upper row). MRI scanning at 2 years 9 months after onset revealed extensive neural tissue loss in the left temporal regions (about 40% of the superior temporal gyrus, 20% of the middle temporal gyrus and 25% of the superior temporal pole), left angular gyrus (about 10%) and left insula (over 50%), whereas frontal areas were damaged to a much lesser extent (about 2% of IFG). Subcortically, structures such as the left basal ganglia (about 50%), hippocampus (10%) and thalamus (10%) were damaged (for details, see the supporting information). J. presented with severe aphasia acutely, and chronic comprehension and production deficits. She received speech and language therapy continuously following the event, initially following a more intensive schedule to twice a week in the last 1.5 years leading up to participation in our experiment. Clinical observations by a trained language pathologist at the time of testing classified J. as having severe aphasia with ‘Broca‐like’ characteristics. According to CAT‐NL, J. was impaired on spoken sentence comprehension, complex word repetition, noun naming and verb naming, with (relatively) spared word repetition and spoken word comprehension (Table 1). Additionally, she scored 20/60 points, or 33% (Ruiter et al., 2011) on ANELT, indicating poor functional language abilities. This profile together with J.'s right‐handedness suggests it is likely that she was premorbidly LH dominant for language.
FIGURE 2.

Structural magnetic resonance image (MRI) and tractography results. (a) Structural MRI (T1 weighted) depicting the extent of the lesion in sagittal, coronal and axial plane (top). Deterministic, in vivo tractography of language‐relevant tracts and corpus callosum (CC) in J. (bottom). (b) Deterministic, in vivo tractography of language‐relevant tracts and CC in the Control. Tractography results depicting a subdivision of the CC: genu, anterior midbody, posterior midbody + isthmus and splenium in J. (a′) and in Control (b′). Axial slices of CC subdivisions are depicted in the superior view (looking from the top of the head, left) and inferior view (looking from the bottom, right). AF, arcuate fasciculus; FAT, frontal aslant tract; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus. Images are depicted in neurological convention (i.e., left hemisphere on the left‐hand side). Reproduced with permission from the authors from doi:10.6084/m9.figshare.19228119
TABLE 1.
J.'s performance on select subtests of the CAT‐NL
| Tested ability (CAT‐NL subtest) | Spoken word comprehension | Spoken sentence comprehension | Word repetition | Complex word repetition | Nonword repetition | Naming: nouns | Naming: verbs |
|---|---|---|---|---|---|---|---|
| Raw score/total score | 26/30 | 23/32 | 28/32 | 3/6 | 4/10 | 23/48 | 4/10 |
Abbreviation: CAT‐NL, Dutch version of the Comprehensive Aphasia Test.
2.2. Design and materials
Pictures representing objects or entities (all concrete nouns) for both tasks were selected from the BOSS database (Brodeur et al., 2010) or from the internet. The context‐driven naming task (Figure 1, top and middle rows) consisted of 78 colour photographs, each combined with a constraining and an unconstraining sentence, amounting to 156 trials in total, presented in six blocks. Sentences were recorded by a native speaker of Dutch spoken at a relatively slow pace (materials were partly taken from Piai et al., 2015) and lasted between 1.8 and 3.6 s. Cloze probabilities (i.e., percentage of people completing the sentence with the same target word) for the picture name following the sentence ranged between 0–39% for unconstrained and 60–100% for constrained sentences (t(77) = 51.236, p < 0.001). Cloze probabilities have been collected over the years from multiple studies in our group with the young‐adult population in the same age range as the two participants of the present study.
The bare picture‐naming task (Figure 1, bottom row) consisted of 88 colour photographs, representing 16 semantic categories (e.g., musical instruments, fruit and body parts) containing five to six exemplars each. Each picture appeared three times throughout the experiment, amounting to 264 trials in total, over eight blocks. Across the tasks, 52/78 pictures for the context‐driven naming and 72/88 pictures for bare naming had norming scores available (Decuyper et al., 2021). There were no significant differences between pictures in naming agreement as measured by H value (mean bare = 0.62, mean context‐driven = 0.68, t(123) = 0.55, p = 0.6) and in word frequency as measured by log‐transformed frequency per million words (mean bare = 2.7, mean context‐driven = 2.8, t(123) = 0.72, p = 0.5). The presentation of stimuli was pseudorandomised using Mix (van Casteren & Davis, 2006), with one unique list per participant.
2.3. Procedure
Each of the two MEG sessions started with instructions and familiarisation with stimuli (pictures and their names), as is commonly done in picture‐naming studies (e.g., Alario et al., 2004). Participants were instructed to keep central fixation and move in the dewar as little as possible. Localisation coils were placed at the nasion and the left and right ear canals. Head localisation was performed in real time (Stolk et al., 2013). The head position was kept as constant as possible for each session. Additional details are provided in the supporting information.
The presentation of the experimental stimuli and the recording of responses were controlled by presentation software (Neurobehavioral Systems). The stimuli were projected on a screen in front of the participants. Vocal responses were recorded with a microphone time‐locked to each picture presentation onset. Both tasks started with a short practice block. For the context task, trials started with a 1000 ms fixation cross, followed by the sentence played through ear tubes in participants' ears. The picture was presented 800 ms after sentence offset for 1000 ms. During the bare picture‐naming task, each trial began with a fixation cross presented for 1000 ms, followed by the presentation of a picture for 2500 ms. For both tasks, after picture offset, three asterisks were presented for a jittered interval of 1.25–1.5 s. For both tasks, participants determined the duration of the breaks.
2.4. Behavioural data analysis
Two raters blinded for context condition analysed errors and calculated response times (RTs) manually using praat (Boersma & Weenink, 2013). The first naming attempt at each trial was scored. Erroneous responses were classified as (1) semantic paraphasia (e.g., violin instead of flute), (2) phonological/phonemic error (e.g., tlufe, instead of flute), (3) word‐finding difficulty (I don't know, I know it or an attempt at pronunciation followed by I don't know), (4) no response (silence), (5) not categorisable (technical failure or non‐intelligible), (6) premature response (starting prior to picture presentation) and (7) correct response after an initial phonemically erroneous onset (i.e., correct articulation was preceded by an attempt containing apraxia‐like acoustic patterns, Kent & Rosenbek, 1983). Trials when the picture was named with the definite article were considered correct, with RTs marked at the article onset. Correct trials after a phonemically erroneous onset (both tasks) and premature responses (context task) were included as correct for MEG analysis because these errors were unlikely to affect early visual processing and lexical‐access stages of picture naming. Interrater reliability for RTs on correct trials as measured by concordance correlation coefficient was 0.98 and 1 (calculated with 95% confidence interval [CI]) for bare and context‐driven naming, respectively, and for errors as measured by Cohen's kappa 0.96 and 0.95, respectively.
We combined correct single trials from both tasks in one data set for statistical analysis of RTs. RT data were log‐transformed to meet the normality of residuals requirement. Using lm() function in r, we fitted a fixed‐effects‐only linear regression model with participant (Control and J.), condition (bare picture naming, context task constrained and context task unconstrained) and their interaction as independent variables. Factor levels were successively tested against each other with repeated contrast coding (package ‘mass’, Ripley et al., 2020), producing the following comparisons: (1) bare picture naming with unconstrained naming, that is, the effect of structure; (2) unconstrained with constrained naming, that is, the effect of context; (3) Control with J., that is, the effect of participant; (4) structure effect differences between participants; and (5) context effect differences between participants. To determine the relation between all three naming conditions for each participant, a series of independent‐samples t tests over trials were run post hoc. For error analysis, chi‐squared tests were conducted to elucidate, first of all, whether J.'s response distributions (all types, see Table 2b) were independent of task and condition and, next, whether predominant responses (i.e., correct naming, correct naming with apraxic patterns, word finding and semantic paraphasias) were differential between bare versus unconstrained and constrained versus unconstrained naming. Holm correction to control for the family‐wise error rate was applied to all p values reported for t and chi‐squared tests.
TABLE 2.
RTs and types of responses
| Task and condition | |||
|---|---|---|---|
| Context task: constrained (n = 78) | Context task: unconstrained (n = 78) | Bare picture naming (n = 264) | |
| (a) Mean RTs, s (SD) a | |||
| Control | 0.73 (0.14) | 0.94 (0.19) | 0.85 (0.12) |
| J. | 1.03 (0.41) | 1.32 (0.35) | 1.44 (0.48) |
| (b) J.: N (%) of total errors and errors by type b | |||
| Total errors | 31 (40%) | 42 (54%) | 140 (53%) |
| Correct after erroneous onset | 5 (6.4%) | 1 (1.3%) | 11 (4.2%) |
| Not categorisable | 0 (0%) | 0 (0%) | 2 (0.8%) |
| Phonological or phonemic | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Response too fast | 2 (2.6%) | 1 (1.3%) | 0 (0%) |
| Semantic paraphasia | 10 (13%) | 4 (5.1%) | 14 (5.3%) |
| No response (silence) | 0 (0%) | 2 (2.6%) | 0 (0%) |
| Word‐finding difficulty (e.g., I don't know) | 14 (18%) | 34 (44%) | 112 (42%) |
Abbreviations: n, number of trials; RTs, response times; SD, standard deviation.
Control and J.'s mean RTs on both tasks per condition.
Number and percentage of incorrect responses produced by J., total and by type. All trials by Control were correct.
2.5. Structural neuroimaging data
2.5.1. MRI acquisition
The MRI acquisition took place in Session 1, after the MEG recording. Anatomical (MP2RAGE and diffusion‐weighted) magnetic resonance images were collected in a single session on a Prisma Fit 3 T scanner with a 32‐channel head coil (Siemens Healthineers, Erlangen, Germany). MP2RAGE images (Marques et al., 2010) were acquired (acquisition duration: 7′32″) using the following parameters: slice thickness 1 mm, voxel size: 1 × 1 × 1 mm, number of slices = 176, repetition time (TR) = 6000 ms, echo time (TE) = 2.34 ms and field of view (FOV) = 256 mm. Diffusion‐weighted images (DWIs) were obtained using a multi‐band echo planar imaging multi‐shell diffusion‐weighted imaging sequence (acquisition duration: 9′30″). Diffusion encoding gradients were applied along 183 directions using multiple shells, that is, 12× b = 0, 86× b = 1250 s/mm2 and 85× b = 2500 s/mm2. Seven b = 0 images with inverted phase‐encoding direction were acquired for susceptibility‐induced distortion correction during processing. The diffusion sequence was acquired in the axial plane with 81 contiguous sections and voxel size of 1.8 × 1.8 × 1.8 mm using multi‐band accelerator factor = 3, TR = 2940 ms, TE = 74.80 ms, with no intersection gap, and FOV = 216 mm.
2.5.2. Diffusion MRI preprocessing
After visual quality control, DWIs were denoised (Veraart, Fieremans, et al., 2016; Veraart, Novikov, et al., 2016), followed by Gibbs ringing artefacts correction (Kellner et al., 2016) using tools from mrtrix3 (https://www.mrtrix.org, Version 3.0.0, Tournier et al., 2019). Then, DWIs were corrected for susceptibility‐induced distortion, eddy current‐induced distortion and head motion using tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (fsl, Version 6.0.1, Jenkinson et al., 2012). For tensor‐based tractography using diffusion toolkit (Wang et al., 2007), only volumes acquired with b = 0 and b = 1250 s/mm2 were selected for further analyses and used to estimate the tensor model (Functional Magnetic Resonance Imaging of the Brain [FMRIB] Software Library [FSL],‘dtifit’). Tracking was run using the dtk setting at a 45° angle threshold, with fibre assignment through continuous tracking (FACT) propagation algorithm and applying the spline filter with fractional anisotropy (FA) as a masking image with 0.2 threshold.
2.5.3. Tractography analysis
Anatomical delineation of the language‐relevant tracts (i.e., arcuate fasciculus [AF], inferior fronto‐occipital fasciculus [IFOF], inferior longitudinal fasciculus [ILF], uncinate fasciculus [UF], frontal aslant tract [FAT] and CC; Figure 2) was performed in trackvis (Wang et al., 2007) using FA maps as anatomical reference and by setting two‐dimensional regions of interest (ROIs) driven by individual brain anatomy. Language‐relevant tracts were delineated according to commonly established anatomical locations (for more details, see François et al., 2016). For the CC, first, to get a general tract overview, was drawn in a sagittal plane midline slice following its shape and outline on a FA map (Figure 2a,b). The anatomical parcellation of the CC was defined according to the guidelines by Hofer and Frahm (2006), where the genu represented connections between prefrontal lobes, the anterior midbody joined premotor and presupplementary motor areas, and the splenium connected parietal, occipital and temporal lobes. Additionally, we defined a conjoined ROI for posterior midbody and isthmus to represent commissural connections between primary motor and primary sensory cortices. Anatomically implausible fibres were removed using exclusion masks. In J.'s LH, the ROIs were enlarged to explore possible tissue displacement, but ROIs were kept anatomically restrained in the RH. The methodology used for J.'s RH was reproduced in both of Control's hemispheres (Figure 2b,b′).
2.6. MEG methodology
MEG data were acquired with a 271 axial gradiometer system (CTF Systems Inc., VSM MedTech Ltd.) at a sampling rate of 1200 Hz. Each task took around 30 min to perform, and participants spent around 1 h in the laboratory including preparation time.
MEG analyses were conducted in MATLAB 2018b using fieldtrip (Oostenveld et al., 2011). For the bare picture‐naming task, the data were segmented into epochs time‐locked to the picture presentation, defined from 500 ms pre‐stimulus to 900 ms post‐stimulus onset. A baseline correction was applied, with the averaged pre‐stimulus 500 ms interval subtracted from the signal. Then, the data were downsampled to 600 Hz and low‐pass filtered at 55 Hz. The context task data underwent a similar procedure. Trials were segmented to span 500 ms before sentence onset until 300 ms after picture presentation. Then, a baseline correction was applied using the 500 ms pre‐sentence interval, followed by a resegmentation of the trials to yield picture‐locked intervals (from −1000 to 300 ms relative to picture onset). Only accurate trials were processed further for the context task and both correct and word‐finding difficulty trials for bare naming.
Artefact correction and rejection were performed on the data with the experimenter blinded for condition. Independent component analysis (ICA) was used to remove eye movements (Jung et al., 2000, as implemented in fieldtrip). Then, single trials were again inspected manually to reject additional trials and/or sensors that retained excessive noise. Finally, the data sets were separated by condition: for constrained versus unconstrained for the context task (54 and 39 trials for J. and 78 and 78 trials for Control), and for bare naming, correct trials for Control (N = 263), correct for J. (N = 125) and word‐finding difficulty for J. (N = 109). All statistical analyses reported below were performed using non‐parametric cluster‐based permutation tests (Maris & Oostenveld, 2007).
2.6.1. Scalp‐level analyses
For the context task, time‐frequency representations (TFRs) of power were calculated between 5 and 40 Hz with an adaptive sliding time window of three cycles' length, advanced in 10 ms and 1 Hz steps (e.g., Piai et al., 2018). The data in each window were multiplied with a Hanning taper, followed by the Fourier transform of the tapered signal. To guide the selection of a time‐frequency window of interest for source localisation, cluster‐based permutation was performed for both participants separately, comparing single trials between the two context conditions. The resulting t values provided a weighted measure of the context effect. The time‐frequency window with the highest absolute t values in both participants was selected for the source localisation (see below).
For bare picture naming, event‐related fields (ERFs) were calculated by averaging the signal over trials, after applying a low‐pass filter of 30 Hz. For the Control, three surrogate conditions were created by randomly selecting one third of the trials without replacement, resulting in ERFs based on 87 to 89 trials. This step was performed to better estimate the variability of the ERFs given a similar number of trials as for J. For J., ERFs were calculated for the correct and word‐finding difficulty trials. All ERFs were then baseline corrected using the averaged signal of the 500 ms baseline period. Finally, planar gradients were calculated for all sensor‐level analyses (Bastiaansen & Knösche, 2000).
We used the well‐known characteristics of visual evoked fields (Ahlfors et al., 1992; Tobimatsu, 2005) and the timing estimates of word production processes (Indefrey & Levelt, 2004) to guide the ERF analyses. Visual evoked fields are prominent during the first 150 ms post‐visual stimulus onset. Conceptual preparation is thought to take place within the first 200 ms after picture presentation. From around 200 ms onward, lexical and phonological processes follow, finally leading to phonetic encoding starting around 200 to 150 ms before speech onset. The presence of visual evoked fields in each participant was confirmed using cluster‐based permutation within‐participant between trials (200 ms baseline vs. 0 to 200 ms time‐locked to picture presentation, performed over all occipital sensors). To guide source analyses, a time point with the largest amplitude across trials within the window identified by cluster‐based permutation was selected, with a window of 30 ms around that peak. Note that, due to this procedure, the timing of the peaks for the two participants differed. Following the time estimates for naming (Indefrey & Levelt, 2004), a second, naming‐related peak was identified for J. using cluster‐based permutation, comparing single trials between the two naming conditions. The resulting t values were used to provide an indication of the time window for source localisation (see below).
2.6.2. MEG source localisation
All analyses were conducted in participants' native space and individually. In order to account for the lesion in the source localisation, we built MRI‐based volumetric headmodels and applied the finite element method (FEM) to compute MEG leadfields (Bertrand et al., 1991; Piastra et al., 2018; Schimpf et al., 2002). This approach takes the lesion's effects on the signal conductivity into consideration, improving the precision of source localisation (for more details, see Piastra et al., 2022). The same FEM‐based approach was taken for both participants. For that, the MRI (T1 weighted [T1w]) was segmented into four different tissues (brain, cerebrospinal fluid [CSF], skull and scalp) using the spm12 software (Penny et al., 2011; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). For J., the lesion resulted in being modelled as a CSF‐filled cavity, in agreement with previous literature (e.g., Minjoli et al., 2017). We then built a 2 mm resolution hexahedral mesh and assigned each tissue a fixed conductivity value following the literature (e.g., Baumann et al., 1997; Dannhauer et al., 2011; Ramon et al., 2004). The source model comprises point‐like dipolar sources (Hämäläinen et al., 1993) positioned in the centroid of the brain elements in the mesh and with x,y,z orientation. The MEG forward problem was solved by applying the FEM implemented in the duneuro software (Schrader et al., 2021; validated in Piastra et al., 2018). The leadfields were consequently used to compute the MEG inverse solution using two beamforming approaches.
For the context task, a frequency‐domain beamformer was used (Dynamic Imaging of Coherent Sources, Gross et al., 2001) over the 800 ms pre‐picture interval. A cross‐spectral density matrix was computed by combining the data of the two context conditions at 18 Hz with a frequency smoothing of 8 Hz around the peak frequency (based on sensor‐level data). For bare picture naming, a time‐domain beamformer was used (Linearly Constrained Minimum Variance, van Veen et al., 1997). The stimulus‐based time windows (visual evoked and naming‐related responses) identified at the sensor level were used, and a baseline period of similar duration was selected (ending at 0 ms picture onset). The covariance matrices were based on the same time windows. For both tasks, using the respective covariance matrices and the leadfields, common spatial filters were constructed (context: over both conditions; bare picture naming: over baseline and stimulus‐based periods), followed by the application of the common spatial filters to the data from each respective condition separately at the single‐trial level. For both tasks and participants separately, cluster‐based permutation tests were performed over trials. A threshold t value of ±2 was used for inferential statistics (corresponding to p < 0.05). Effect size measures are provided in addition (Cohen's d, calculated as the mean difference between conditions divided by the pooled standard deviation), thresholded at ±0.2 (corresponding to larger than small effect sizes). For the Control, a random selection of 110 trials was used for inferential statistics, as to equate the number of available trials between J. and Control. For visualisation, thresholded source maps were interpolated to the participants' MRIs.
3. RESULTS
3.1. Behavioural results
J.'s naming errors were consistent with her performance on CAT‐NL, with differential error distributions across the experimental tasks/conditions (χ 2(14) = 37, p = 0.002). The amount (Table 2) and distribution (Figure 3) of erroneous responses were comparable in the bare picture naming and the unconstrained condition of the context task (around 55%; χ 2(3) = 1.44, p = 0.7), whereas error patterns in the constraining compared to unconstraining condition were independent (χ 2(3) = 15, p = 0.004). Notably, the constraining condition elicited fewer incorrect responses overall (40%). Word‐finding difficulty was the predominant error (Figure 3 and Table 2), constituting almost half of the responses in unconstrained and bare naming but only a fifth of total responses in constrained naming. Conversely, J. made twice as many semantic paraphasias (13% of total responses) in the constrained compared to unconstrained and bare picture naming. Other types of errors were rare.
FIGURE 3.

Behavioural results. (a) Distribution of correct responses and most common error types produced by J. compared across tasks and conditions. Because bare picture naming contained more trials, the number of errors was calculated proportionally. Note that all trials by Control were correct. (b) Distribution of response times across tasks and conditions by Control (bottom panel, only correct responses) and by J. (three top panels) on correct and incorrect trials (semantic paraphasias and word‐finding difficulties). Reproduced with permission from the authors from doi:10.6084/m9.figshare.19224594
RT results are shown in Figure 3 and Table 2, and inferential statistics are presented in Table 3. Descriptively, J. was slower than Control in all conditions on correct trials. However, during constrained naming, her semantic paraphasias were as fast as her correct responses. RTs were overall the slowest when she experienced word‐finding difficulties. The results of the linear regression analysis revealed a significant main effect of participant, with J. responding slower than Control (p < 0.001) and a main effect of context, with pictures named faster after constrained than unconstrained sentences (p < 0.001). No main effect of structure was found; that is, overall naming was equally fast after unconstrained sentences as it was during bare picture naming. Regarding the interaction effects, the structure effect across participants was significant (p < 0.001), whereas context effect differences between participants were not found. Post hoc analyses confirmed the context effect (t(141) = −7.9, p < 0.001, for Control; t(80) = −3.5, p = 0.002, for J.). There was also a significant difference between unconstrained and bare‐naming conditions (t(114) = −6.8, p < 0.001, for Control; t(95) = −5.6, p < 0.001, for J.). However, Control was faster in the bare compared to the unconstrained naming (t(96) = −4, p < 0.001), whereas J. showed similar RTs in both conditions (t(76) = 1.7, p = 0.09).
TABLE 3.
Results of the fixed‐effects linear regression
| Estimates | SE | 95% CI | t value | p value | |
|---|---|---|---|---|---|
| Intercept | −0.01 | 0.01 | −0.03, −0.01 | −1.10 | 0.271 |
| Main effects | |||||
| Structure effect: unconstrained context vs. bare picture naming | 0.01 | 0.03 | −0.04, 0.06 | 0.26 | 0.793 |
| Context effect: constrained vs. unconstrained naming | −0.27 | 0.03 | −0.33, −0.21 | −8.76 | <0.001 |
| Participant effect: control vs. J. | 0.36 | 0.02 | 0.32, 0.41 | 16.47 | <0.001 |
| Interaction effects | |||||
| Participant and structure effect | −0.17 | 0.05 | −0.27, −0.07 | −3.35 | 0.001 |
| Participant and context effect | −0.04 | 0.06 | −0.16, 0.08 | −0.64 | 0.522 |
| Observations = 627 | |||||
| R 2 adjusted = 0.488 | |||||
Note: The model was run with log‐transformed response times as a dependent variable and condition (context‐unconstrained naming, context‐constrained naming and bare picture naming) and participant (patient and control) as predictors, including their interaction. The contrast scheme was repeated sum coding.
Abbreviations: CI, confidence interval; SE, standard error.
3.2. Tractography results
Deterministic tractography (Figure 2a) revealed that the lesion completely damaged the left fronto‐temporal portion of the AF (also called ‘long segment’). Shorter AF connections between frontal and parietal lobes were severely affected; in particular, the connection with the IFG did not reach the parietal cortex. The only AF portion that was preserved was the connection between the parietal and temporal cortices. The left ventral tracts (IFOF, ILF and UF) as well as FAT were completely damaged by the lesion. In the RH, FAT and all ventral connections were preserved. Although AF was spared, the parieto‐temporal fibres were not prominent. Regarding CC (Figure 2a′), only the connections reaching inferior frontal cortices as well as the parietal and occipital lobes (portions of genu and splenium) were, at least partially, preserved. Connections crossing through the midbody and isthmus were damaged. The tapetum was not prominent in either hemisphere. As to be expected, all canonical language tracts and CC (including the forceps major and tapetum) were present in Control and are depicted in Figure 2b,b′ for comparison.
3.3. MEG results
3.3.1. Context‐driven picture naming
For the context task, sensor‐level analyses replicated our previous findings of alpha–beta power decreases for constrained relative to unconstrained contexts prior to picture presentation in both participants (Piai et al., 2015; Roos & Piai, 2020; see also Gastaldon et al., 2020; Hustá et al., 2021; Klaus et al., 2020; Piai et al., 2014, 2017, 2018). Results of source localisation of power decreases in the 10–26 Hz range prior to picture onset are shown in Figure 4. A t value threshold of ±2 proved suboptimal for the source‐level analysis for J. Therefore, for both J. and Control, source‐level results are shown thresholded at ±1.5. In Control, the sources were found predominantly in left IFG, extending to precentral and postcentral gyri (with the largest effect sizes) and parietal areas, and the middle portion of the left middle temporal gyrus. In J., similar frontal and temporal lobe sources as for Control were found in the RH, with the largest effect sizes in frontal areas.
FIGURE 4.

Source localisation of the context effect. Relative power decreases (expressed in t values, top two rows) for constrained relative to unconstrained contexts in the 10–26 Hz range prior to picture onset for Control (a, first row) and J. (a, second row). Power was thresholded at ±1.5. (b) Corresponding Cohen's d maps for Control (first row) and J. (second row), thresholded at ±0.2. Images are depicted in neurological convention (i.e., left hemisphere on the left‐hand side). L, left; R, right. Reproduced with permission from the authors from doi:10.6084/m9.figshare.19228146
3.3.2. Bare picture naming
Figure 5 shows the ERFs for both participants for the bare picture‐naming task, time‐locked to picture onset, averaged over occipital sensors. In line with the literature, visual evoked responses were identified in both participants, albeit with different timing. For Control (Monte Carlo p = 0.004 for visual evoked field vs. baseline), it is clear how the timing is consistent over trials (90 to 130 ms, in line with the timing of visual evoked fields, Tobimatsu, 2005), with a clear overlap between all three surrogate conditions (blue ERFs in Figure 5, top). The sources of this evoked field (with t values more extreme than ±2) were localised to the left occipital cortex (with medium effect size, see Figure 5), including the left cuneus, and right precuneus, in addition to bilateral cerebellum. From sagittal slices, sources are also observed in anterior and mid portions of the left temporal lobe. Sources in the RH were less widespread and had smaller effect sizes. For J., the first visual evoked field with a clear peak was around 150 to 180 ms, which was very similar for both naming (purple ERFs, Monte Carlo p = 0.016 for visual evoked field vs. baseline) and word‐finding (green ERFs, Monte Carlo p = 0.016 for visual evoked field vs. baseline) trials (Figure 5, bottom). The sources of this evoked field were localised to right occipital cortex for both types of trials (albeit differently in terms of power increases/decreases relative to baseline), similar to the occipital areas in Control's LH. Additionally, power increased relative to baseline in the right inferior temporal gyrus, similarly for both naming and word‐finding trials with similar effect sizes, and right inferior parietal lobule, with this latter only prominent for word‐finding trials.
FIGURE 5.

Event‐related fields (ERFs) and sources of early visual evoked responses. ERFs (from planar gradients) for Control (a) and J. (b), averaged over occipital sensors. For Control, the three surrogate conditions are shown in blue, in addition to the overall average in red. For J., ERFs for naming (purple) and word‐finding (green) trials are shown, in addition to Control's average (red) for comparison. The ERFs are displayed between −200 and 700 ms. The time windows selected for topographical maps and source localisation are indicated for each participant. For the source maps, shown below the ERFs, values were thresholded at t more extreme than ±2 and Cohen's d more extreme than ±0.2 (white masks in the left colour bars). Coronal slices are depicted in neurological convention (i.e., left hemisphere on the left‐hand side). L, left; R, right. Reproduced with permission from the authors from doi:10.6084/m9.figshare.19228161
For the naming‐related peak, source localisation was performed in the time window of 330 to 440 ms, identified for J. by the cluster‐based permutation test as having the maximum differences between correct naming and word‐finding trials (Figure 6). Evoked power was increased for naming trials relative to word‐finding trials (warm colours in Figure 6, positive values) in the right mid and posterior portions of the temporal lobe with medium effect sizes. For word‐finding relative to naming trials (cold colours in Figure 6, negative values), evoked power was increased in right IFG with medium effect sizes.
FIGURE 6.
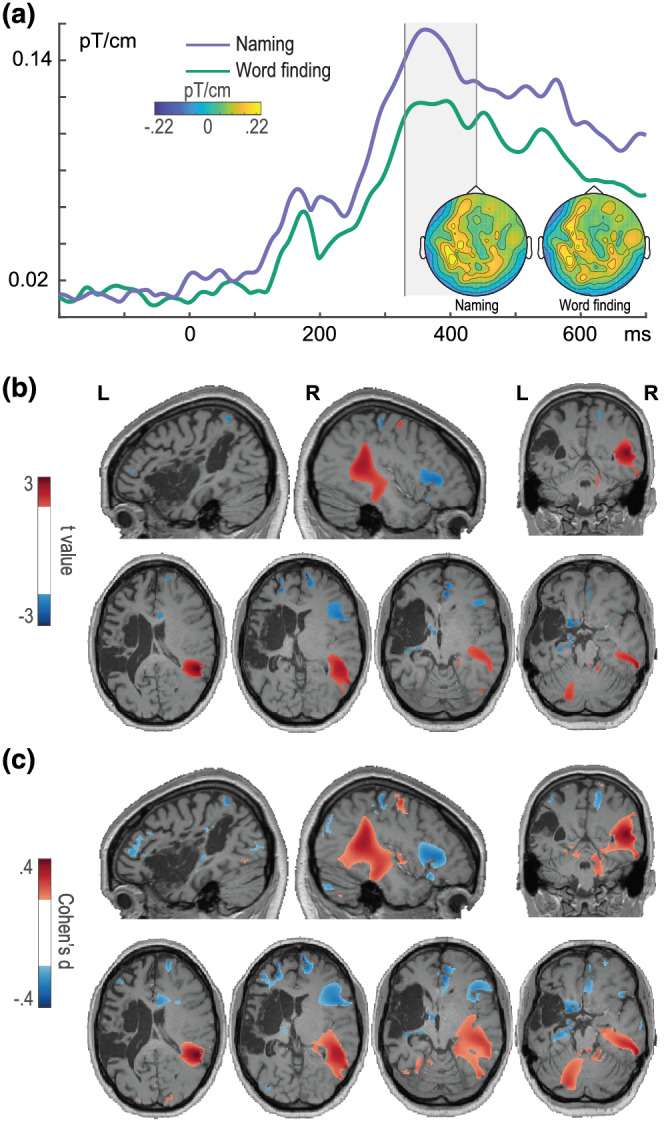
Event‐related fields (ERFs) and sources of naming‐related responses. (a) ERFs for J.'s naming (purple) and word‐finding (green) trials (from planar gradients), averaged over posterior left and right sensors. The ERFs are displayed between −200 and 700 ms. The time window selected for the topographical maps and source analysis is indicated by the shaded area (identified by the cluster‐based permutation analysis). For the source maps (b, c), values were thresholded at t values (b) more extreme than ±2 and Cohen's d (c) more extreme than ± 0.2 (white masks in the left colour bars). Positive values indicate stronger responses for naming relative to word‐finding; negative values indicate stronger responses for word‐finding relative to naming. Slices are depicted in neurological convention (i.e., left hemisphere on the left‐hand side). L, left; R, right. Reproduced with permission from the authors from doi:10.6084/m9.figshare.19228194
4. DISCUSSION
In the present case study, we described the word production abilities of J., a young, right‐handed adult with chronic aphasia following extensive damage to the LH. MEG analyses and source localisation indicated that J.'s production at the conceptual, lexical and phonological retrieval stages was largely supported by activity in the contralesional, RH, with a temporally aligned spatial distinction between successful and unsuccessful naming attempts. Interhemispheric connections through the CC were particularly affected at the level of the temporal lobes, whereas the forceps major connecting occipital lobes was present. Based on J.'s severe aphasia in the (sub‐)acute stage, it is safe to assume that her premorbid language function was strongly left lateralised, indicating that, in her case, the RH recruitment most likely reflects the ‘substitution of function’ scenario, involving reorganisation and/or compensation mechanisms (Anderson et al., 2011; Rothi & Horner, 1983).
4.1. Behavioural and structural MRI findings
Both standardised language tests and experimental tasks revealed impaired word production with relatively spared word comprehension and repetition. J. was considerably slower in naming than Control, and she had serious word‐finding difficulties in both tasks. Word‐finding difficulties (also called omissions or anomic errors) are considered by most accounts to reflect a failure in completing the access to a phonological representation from a target abstract lexical representation (Burke et al., 1991; Dell et al., 1997). Consistent with prior findings (e.g., Piai et al., 2018), J. and Control demonstrated context facilitation effects, that is, faster naming for constrained compared to unconstrained contexts. The additional semantic information helped J. to produce more correct responses, but simultaneously induced more semantic paraphasias, which were as fast as correct responses exclusively in the constraining condition. Semantic errors are typically attributed to dysfunction in lexical selection: As the spreading activation engages a number of related representations, a semantically related word might exceed the target word's activation and get erroneously selected (Dell et al., 1997; Roelofs, 1992). In sum, we take the presence of a context effect in J. as evidence of sufficient comprehension abilities for the simple sentences we employed. Her pattern of semantic paraphasias and word‐finding difficulty together indicate a deficit at both the lexical and phonological retrieval stages. The extent to which concept retrieval is intact, relatively spared or dysfunctional cannot be addressed with the present data.
These behavioural deficits can be explained by the extensive damage to perisylvian brain areas, including most of the middle and superior temporal gyri, temporo‐parietal junction (TPJ) and anterior insula. Temporal regions have been linked to lexical and phonological retrieval stages of word production (Indefrey & Levelt, 2004; Roelofs, 2014). Furthermore, deterministic tractography showed that the major part of J.'s language‐relevant tracts was severely damaged, encompassing left dorsal (fronto‐temporal and fronto‐parietal sections of the AF) and left ventral tracts (IFOF, ILF and UF) as well as FAT. A body of research supports the notion that these tracts are relevant for various aspects of language function, including semantic processing (IFOF, ILF and UF), speech initiation (FAT), verbal fluency (FAT and AF) and phonological processing (AF), with different segments of AF being crucial for naming and comprehension abilities (Dick et al., 2019; Ivanova et al., 2021; Sierpowska et al., 2019; for review, see Dick & Tremblay, 2012). Importantly, the posterior temporal lobe and TPJ together with the underlying white matter, all damaged in J.'s brain, have been suggested to form a structural bottleneck critical for language (e.g., Griffis et al., 2017; Heiss et al., 1999; Rosso et al., 2015). The interhemispheric connectivity was compromised as a large section of CC (midbody and isthmus) was also damaged.
4.2. Functional spatiotemporal findings
Evidence from MEG was substantiated both through the use of t values from single‐trial statistics and through effect sizes. Replicating previous results (Piai et al., 2015, 2017, 2018; Roos & Piai, 2020), both J. and Control showed alpha–beta power decreases in the constrained compared to unconstrained contexts, in line with J.'s relatively preserved context effect found behaviourally. In Control, the sources of the context power‐decrease effect were largely in line with prior findings (e.g., Piai et al., 2015; Roos & Piai, 2020), with an overall LH bias for the temporal lobe. In J., the context effect was localised to the RH.
The time course of bare picture naming revealed differences between the participants, starting with J.'s longer (but within the typically reported range, e.g., Salmelin et al., 1994; Sörös et al., 2003) latencies of the visual evoked responses relative to Control. In line with previous MEG (picture‐naming) findings (Salmelin et al., 1994; Sörös et al., 2003; Tobimatsu, 2005), early evoked responses were localised to visual areas in both participants, with an LH bias for Control and RH bias for J. J.'s successful and anomic naming attempts were in general similar at the visual/conceptual processing stage before 200 ms (with a source in the right inferior parietal lobule only present for word‐finding trials) but then diverged, reaching a maximum difference around 330 to 440 ms. Anomic responses were accompanied by power increases in right IFG with medium effect sizes relative to naming trials, whereas successful naming was accompanied by power increases in the mid and posterior portions of the right temporal lobe with medium effect sizes, compared to anomia trials.
The extent to which right IFG involvement aids or hinders word production is highly debated (e.g., Naeser et al., 2005; Postman‐Caucheteux et al., 2010; Turkeltaub et al., 2012). One limitation of existing literature is the overreliance on methods with poor temporal resolution. In this respect, one cannot know whether the right IFG involvement occurred during a word‐finding state or after it. Our temporally resolved results indicate that the modulation of neural activity in the right IFG for word‐finding difficulty trials occurs during the word planning stages for production, in a window possibly corresponding to lexical and/or phonological retrieval. Moreover, in the same time window, modulation of activity in the right mid‐posterior temporal lobe is apparent for trials with successful naming.
Finally, in terms of interhemispheric connectivity, J.'s connections between the temporal lobes in particular were extensively damaged, yet reliable activity in the RH was observed: Around 150 ms upon seeing a picture, RH areas involved in object recognition were already active (see Figure 5). In this respect, it appears that the temporal lobes need not be directly connected through the temporal portion of CC for the right temporal lobe to receive signals further downstream in the processing chain if the activity is already right lateralised upon stimulus presentation. This point nicely illustrates the gains from taking the temporal dimension into account in the discussion of whether interhemispheric connections are (strictly) needed.
4.3. RH recruitment in young‐adult stroke
Although the RH undeniably contributes to different aspects of language processing (Lindell, 2006), its role in language recovery after stroke remains debated (Stefaniak et al., 2021; Wilson & Schneck, 2020). In the literature based on the typical samples of stroke individuals (i.e., older age groups), RH recruitment has been suggested to be the last resort effort in cases when the left‐lateralised networks are severely damaged (Heiss et al., 1999; Selnes, 1999). In a recent review, Wilson and Schneck (2020) cautioned against interpreting the involvement of the contralesional hemisphere as a sign of large‐scale reorganisation. However, little is known about the role of the RH in the young‐adult aphasia group. It stands to reason that the RH's ability to support language after stroke depends on a multitude of factors, including the language function it is supposed to support or the amount of cellular dysregulation, which accumulates with advancing age even in healthy individuals (López‐Otín et al., 2013; Mattson & Arumugam, 2018). The older brain seems to be at a cellular disadvantage (Mattson & Arumugam, 2018), leading, among other things, to an altered post‐stroke cerebral response (Ay et al., 2005; Zhang et al., 2005; for review, see Popa‐Wagner et al., 2007). Animal models show that the role of the contralesional hemisphere in response to ischaemia is more prominent in younger animals (Buga et al., 2008), and our results tentatively indicate that, at least for some young adults, this also might be the case for post‐stroke RH engagement in language.
Involvement of right frontal and temporo‐parietal homologous areas is known to be common after perinatal stroke and left hemispherectomy in children (Hertz‐Pannier et al., 2002; Staudt, 2002), with secondary right temporo‐parietal stroke later in life leading to aphasia (Guerreiro et al., 1995). Even in older adults with aphasia, greater grey matter volumes found in right temporo‐parietal regions correlate positively with spontaneous speech and naming ability (Xing et al., 2016). These patterns resemble J.'s recruitment of the right temporo‐parietal cortex during successful naming. Although semantic systems are more bilaterally distributed (Binder et al., 2009), which might explain the ability of J.'s RH to support conceptual preparation after stroke (Piai et al., 2017) without appeal to neural reorganisation, lexical and phonological retrieval processes by contrast are strongly associated with the left temporo‐frontal cortices (Indefrey & Levelt, 2004). In light of J. having strongly left‐lateralised premorbid language function, her brain's ability to rely on the RH during word retrieval resulted, most likely, from stroke‐induced reorganisation.
At the same time, J.'s case illustrates once again that what reorganisation and/or compensation can achieve is largely defined by the lesion characteristics (Benghanem et al., 2019; Gleichgerrcht et al., 2015; Griffis et al., 2017; Thye & Mirman, 2018). Likely rooted in evolutionary development, the plasticity inherent for the mammalian central nervous system seems to be specialised for learning and not directly for dealing with trauma (Delgado‐García & Gruart, 2004; Nieto‐Sampedro, 2004). As such, despite the likelihood that both types of plastic changes are governed by overlapping biological mechanisms and principles at the cellular level (Nieto‐Sampedro, 2004), plasticity critically depends on the number and diversity of the available cortical structures (Mercado, 2008). In other words, even in cases such as J.'s, when the cellular machinery enables the most optimal response to ischaemia (Buga et al., 2008; Popa‐Wagner et al., 2011), focal injury by definition constrains plasticity. Age at stroke prognosticates functional outcome inconsistently (for review, see Watila & Balarabe, 2015) for a number of reasons such as the discrepancy between the chronological age and the brain age (Cole & Franke, 2017), or individual differences in collateral circulation and the brain/cognitive reserve (Rabinstein et al., 2019). Due to this, the severity of the initial neurological impairment remains one of the most reliable language improvement predictors (Benghanem et al., 2019; Engelter et al., 2006; Lazar et al., 2010), including for a young‐adult patient group (15–49 years old, mean age 42 years old, Naess et al., 2009).
4.4. Limitations and future directions
This study is limited by the spatial resolution of MEG source localisation, which may not have the precision to separate sources within millimetres, even though we used an optimised approach for building the forward model in the presence of stroke lesions (Piastra et al., 2022). This limits the extent to which we can confidently further specify the exact parts of the right IFG that were recruited. At the behavioural level, the two tasks that we used did not allow for disentangling J.'s conceptual preparation abilities from lexical selection. In the future, adding a more ‘pure’ semantic task (such as the Pyramids and Palm Trees Test, Howard & Patterson, 1992) could help achieve this.
The cross‐sectional nature of our study did not allow us to study the longitudinal course of post‐stroke language recovery in young adults. Comparing the functional outcomes between young adults with a stroke and those in older age groups would present a clearer picture of the impact that brain health might have on the prognosis. Finally, the role of the CC integrity in restitution and substitution of function requires further investigation at the individual and group level, ideally, with tasks tapping a variety of cognitive functions.
As a final note, with this case study, we would like to not only add to the discussion of brain organisation for language but also raise awareness of young‐adult stroke. Although its incidence has been growing (Putaala, 2016), young stroke is routinely missed by clinicians, leading to lack of timely intervention (Sultan & Elkind, 2013). These individuals experience significant reduction of quality of life, functional dependence and inability to return to work (Maaijwee et al., 2014; Naess et al., 2006; Schaapsmeerders et al., 2013; Synhaeve et al., 2014). The young‐adult brain follows a different recovery path, so lack of knowledge about young stroke has consequences for the unique rehabilitation needs of this group. In our interactions with J., she often remarked that she shared therapy sessions with people above 60 and asked us whether that was the right approach in her case. Hopefully, future research will be able to inform whether language therapy should be given differently and/or with additional manipulations to boost trauma‐related plasticity mechanisms.
5. CONCLUSION
From investigating temporally resolved neural activity, we learned that J.'s production abilities were largely supported by RH structures. The extent of disconnection of J.'s left and right temporal lobes suggests that spared interlobar connectivity of these areas might not be a necessary requirement for RH recruitment in visually guided (i.e., confrontation) or context‐driven naming if neural activity is right lateralised from the outset. Finally, concurrent activity during word planning in the RH dissociates between successful (mid‐posterior temporal lobe) and unsuccessful (IFG) naming attempts. In J.'s case, however, the ability of the RH to facilitate naming remains modest, and the degree of the chronic functional impairment seems to be primarily determined by the lesion size and location.
PARTICIPANT CONSENT STATEMENT
The participants gave written informed consent and received monetary compensation for participation.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
All figures by the authors are reproduced with permission from the authors according to a CC BY 4.0 licence.
AUTHOR CONTRIBUTIONS
Irina Chupina: Conceptualization; formal analysis; writing—original draft; writing—review and editing; visualization. Joanna Sierpowska: Formal analysis; writing—original draft; writing—review and editing; visualization. Xiaochen Zheng: Formal analysis; writing—review and editing. Anna Dewenter: Formal analysis; writing—review and editing. Maria‐Carla Piastra: Software; formal analysis; writing—review and editing. Vitória Piai: Conceptualization; methodology; formal analysis; investigation; writing—original draft; writing—review and editing; visualization; supervision; project administration; funding acquisition.
CONFLICTS OF INTEREST
None.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15813.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
We are truly indebted to J. for her participation in this study and to her and other young individuals with aphasia for inspiring us. We are also thankful to Marina Ruiter and Hilde Bosschers for aphasia‐related input; Ileana Camerino for help with testing; Robert Oostenveld, Jan‐Mathijs Schoffelen and Britta Westner for valuable input on electrophysiological methods; Rosemarije Weterings for help with behavioural data analysis; and Christian Letter for his contribution in creating the CC subdivision.
Chupina, I. , Sierpowska, J. , Zheng, X. Y. , Dewenter, A. , Piastra, M.‐C. , & Piai, V. (2022). Time course of right‐hemisphere recruitment during word production following left‐hemisphere damage: A single case of young stroke. European Journal of Neuroscience, 56(8), 5235–5259. 10.1111/ejn.15813
Irina Chupina and Vitória Piai contributed equally to this study.
Funding information This study was supported by grants from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek [NWO]) to V. P. (451‐17‐003 and VI.Vidi.201.081) and to the Language in Interaction Consortium (024‐001‐006). M.‐C. P. was supported by an NWO grant from the Applied and Engineering Sciences domain (14902) and by a FLAG‐ERA grant (NeuronsReunited, NWO 680‐91‐318).
Edited by: Edmund Lalor
Funding information Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Numbers: 680‐91‐318, 14902, 024‐001‐006, 451‐17‐003, VI.Vidi.201.081
Footnotes
For the remainder of this article, we will use the term ‘young adult’ to refer to adults aged between 18 and 49 years old. Although this age group remains heterogeneous (as very young post‐adolescent adults are neurally different from older middle‐aged adults, e.g., Kennedy et al., 2015; Kodiweera et al., 2016), literature on ‘young stoke’ typically includes participants of this age range.
DATA AVAILABILITY STATEMENT
The data are not publicly available given that anonymity of the participants cannot be guaranteed.
REFERENCES
- Aboitiz, F. , & Montiel, J. (2003). One hundred million years of interhemispheric communication: The history of the corpus callosum. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 36(4), 409–420. 10.1590/s0100-879x2003000400002 [DOI] [PubMed] [Google Scholar]
- Ahlfors, S. P. , Ilmoniemi, R. J. , & Hämäläinen, M. S. (1992). Estimates of visually evoked cortical currents. Electroencephalography and Clinical Neurophysiology, 82(3), 225–236. 10.1016/0013-4694(92)90172-E [DOI] [PubMed] [Google Scholar]
- Alario, F. X. , Ferrand, L. , Laganaro, M. , New, B. , Frauenfelder, U. H. , & Segui, J. (2004). Predictors of picture naming speed. Behavior research methods, instruments, & computers: a journal of the Psychonomic Society, Inc, 36(1), 140–155. 10.3758/bf03195559 [DOI] [PubMed] [Google Scholar]
- Ala‐Salomäki, H. , Kujala, J. , Liljeström, M. , & Salmelin, R. (2021). Picture naming yields highly consistent cortical activation patterns: Test–retest reliability of magnetoencephalography recordings. NeuroImage, 227, 117651. 10.1016/j.neuroimage.2020.117651 [DOI] [PubMed] [Google Scholar]
- Anderson, V. , Spencer‐Smith, M. , & Wood, A. (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain, 134(8), 2197–2221. 10.1093/brain/awr103 [DOI] [PubMed] [Google Scholar]
- Archila‐Meléndez, M. E. , Sorg, C. , & Preibisch, C. (2020). Modeling the impact of neurovascular coupling impairments on BOLD‐based functional connectivity at rest. NeuroImage, 218, 116871. 10.1016/j.neuroimage.2020.116871 [DOI] [PubMed] [Google Scholar]
- Ay, H. , Koroshetz, W. J. , Vangel, M. , Benner, T. , Melinosky, C. , Zhu, M. , Menezes, N. , Lopez, C. J. , & Sorensen, A. G. (2005). Conversion of ischemic brain tissue into infarction increases with age. Stroke, 36(12), 2632–2636. 10.1161/01.STR.0000189991.23918.01 [DOI] [PubMed] [Google Scholar]
- Bartolomeo, P. , & Thiebaut de Schotten, M. (2016). Let thy left brain know what thy right brain doeth: Inter‐hemispheric compensation of functional deficits after brain damage. Neuropsychologia, 93(Pt B), 407–412. 10.1016/j.neuropsychologia.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Bastiaansen, M. C. M. , & Knösche, T. R. (2000). Tangential derivative mapping of axial MEG applied to event‐related desynchronization research. Clinical Neurophysiology, 111(7), 1300–1305. 10.1016/S1388-2457(00)00272-8 [DOI] [PubMed] [Google Scholar]
- Bates, E. , Reilly, J. , Wulfeck, B. , Dronkers, N. , Opie, M. , Fenson, J. , Kriz, S. , Jeffries, R. , Miller, L. R. , & Herbst, K. (2001). Differential effects of unilateral lesions on language production in children and adults. Brain and Language, 79(2), 223–265. 10.1006/brln.2001.2482 [DOI] [PubMed] [Google Scholar]
- Baumann, S. B. , Wozny, D. R. , Kelly, S. K. , & Meno, F. M. (1997). The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Transactions on Biomedical Engineering, 44(3), 220–223. 10.1109/10.554770 [DOI] [PubMed] [Google Scholar]
- Benghanem, S. , Rosso, C. , Arbizu, C. , Moulton, E. , Dormont, D. , Leger, A. , Pires, C. , & Samson, Y. (2019). Aphasia outcome: The interactions between initial severity, lesion size and location. Journal of Neurology, 266(6), 1303–1309. 10.1007/s00415-019-09259-3 [DOI] [PubMed] [Google Scholar]
- Bertrand, O. , Thevenet, M. , & Perrin, F. (1991). 3D finite element method in brain electrical activity studies. In Nenonen J., Rajala H. M., & Katila T. (Eds.), Biomagnetic Localization and 3D Modelling, Report of the Department of Technical Physics, Helsinski University (pp. 154–171). [Google Scholar]
- Binder, J. R. , Desai, R. H. , Graves, W. W. , & Conant, L. L. (2009). Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomert, L. , Kean, M. L. , Koster, C. , & Schokker, J. (1994). Amsterdam—Nijmegen Everyday Language Test: Construction, reliability and validity. Aphasiology, 8(4), 381–407. 10.1080/02687039408248666 [DOI] [Google Scholar]
- Bloom, J. S. , & Hynd, G. W. (2005). The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychology Review, 15(2), 59–71. 10.1007/s11065-005-6252-y [DOI] [PubMed] [Google Scholar]
- Boersma, P. , & Weenink, D. (2013). Praat: Doing phonetics by computer [computer program]. Version 5.3. 51. http://www.praat.org
- Boles, D. B. , Barth, J. M. , & Merrill, E. C. (2008). Asymmetry and performance: Toward a neurodevelopmental theory. Brain and Cognition, 66(2), 124–139. 10.1016/j.bandc.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Bozic, M. , Tyler, L. K. , Ives, D. T. , Randall, B. , & Marslen‐Wilson, W. D. (2010). Bihemispheric foundations for human speech comprehension. Proceedings of the National Academy of Sciences, 107(40), 17439–17444. 10.1073/pnas.1000531107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur, M. B. , Dionne‐Dostie, E. , Montreuil, T. , & Lepage, M. (2010). The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS ONE, 5(5), e10773. 10.1371/journal.pone.0010773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga, A.‐M. , Sascau, M. , Pisoschi, C. , Herndon, J. G. , Kessler, C. , & Popa‐Wagner, A. (2008). The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. Journal of Cellular and Molecular Medicine, 12(6b), 2731–2753. 10.1111/j.1582-4934.2008.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, D. M. , MacKay, D. G. , Worthley, J. S. , & Wade, E. (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579. 10.1016/0749-596X(91)90026-G [DOI] [Google Scholar]
- Butler, R. A. , Lambon Ralph, M. A. , & Woollams, A. M. (2014). Capturing multidimensionality in stroke aphasia: Mapping principal behavioural components to neural structures. Brain, 137(12), 3248–3266. 10.1093/brain/awu286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R. , Anderson, N. D. , Locantore, J. K. , & McIntosh, A. R. (2002). Aging gracefully: Compensatory brain activity in high‐performing older adults. NeuroImage, 17(3), 1394–1402. 10.1006/nimg.2002.1280 [DOI] [PubMed] [Google Scholar]
- Chan, M. Y. , Park, D. C. , Savalia, N. K. , Petersen, S. E. , & Wig, G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111(46), E4997–E5006. 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquyt, E. M. , De Ley, L. , Santens, P. , Van Borsel, J. , & De Letter, M. (2017). The role of the right hemisphere in the recovery of stroke‐related aphasia: A systematic review. Journal of Neurolinguistics, 44, 68–90. 10.1016/j.jneuroling.2017.03.004 [DOI] [Google Scholar]
- Cole, J. H. , & Franke, K. (2017). Predicting age using neuroimaging: Innovative brain ageing biomarkers. Trends in Neurosciences, 40(12), 681–690. 10.1016/j.tins.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Dannhauer, M. , Lanfer, B. , Wolters, C. H. , & Knösche, T. R. (2011). Modeling of the human skull in EEG source analysis. Human Brain Mapping, 32(9), 1383–1399. 10.1002/hbm.21114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuyper, C. , Brysbaert, M. , Brodeur, M. B. , & Meyer, A. S. (2021). Bank of Standardized Stimuli (BOSS): Dutch names for 1400 photographs. Journal of Cognition, 4(1), 33. 10.5334/joc.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐García, J. M. , & Gruart, A. (2004). Neural plasticity and regeneration: Myths and expectations. In Herdegen T. & Delgado‐García J. (Eds.), Brain damage and repair (pp. 259–273). Springer. 10.1007/1-4020-2541-6_17 [DOI] [Google Scholar]
- Dell, G. S. , Schwartz, M. F. , Martin, N. , Saffran, E. M. , & Gagnon, D. A. (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801–838. 10.1037/0033-295X.104.4.801 [DOI] [PubMed] [Google Scholar]
- Dennis, M. , Spiegler, B. J. , Juranek, J. J. , Bigler, E. D. , Snead, O. C. , & Fletcher, J. M. (2013). Age, plasticity, and homeostasis in childhood brain disorders. Neuroscience & Biobehavioral Reviews, 37(10), 2760–2773. 10.1016/j.neubiorev.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, A. S. , Garic, D. , Graziano, P. , & Tremblay, P. (2019). The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex, 111, 148–163. 10.1016/j.cortex.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, A. S. , & Tremblay, P. (2012). Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain, 135(12), 3529–3550. 10.1093/brain/aws222 [DOI] [PubMed] [Google Scholar]
- Engelter, S. T. , Gostynski, M. , Papa, S. , Frei, M. , Born, C. , Ajdacic‐Gross, V. , Gutzwiller, F. , & Lyrer, P. A. (2006). Epidemiology of aphasia attributable to first ischemic stroke: Incidence, severity, fluency, etiology, and thrombolysis. Stroke, 37(6), 1379–1384. 10.1161/01.STR.0000221815.64093.8c [DOI] [PubMed] [Google Scholar]
- Esteves, M. , Ganz, E. , Sousa, N. , & Leite‐Almeida, H. (2021). Asymmetrical brain plasticity: Physiology and pathology. Neuroscience, 454, 3–14. 10.1016/j.neuroscience.2020.01.022 [DOI] [PubMed] [Google Scholar]
- Everts, R. , Lidzba, K. , Wilke, M. , Kiefer, C. , Mordasini, M. , Schroth, G. , Perrig, W. , & Steinlin, M. (2009). Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human Brain Mapping, 30(2), 473–483. 10.1002/hbm.20523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin, V. L. , Lawes, C. M. , Bennett, D. A. , & Anderson, C. S. (2003). Stroke epidemiology: A review of population‐based studies of incidence, prevalence, and case‐fatality in the late 20th century. The Lancet Neurology, 2(1), 43–53. 10.1016/S1474-4422(03)00266-7 [DOI] [PubMed] [Google Scholar]
- Fernandez, B. , Cardebat, D. , Demonet, J. F. , Joseph, P. A. , Mazaux, J. M. , Barat, M. , & Allard, M. (2004). Functional MRI follow‐up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke, 35(9), 2171–2176. 10.1161/01.STR.0000139323.76769.b0 [DOI] [PubMed] [Google Scholar]
- François, C. , Ripollés, P. , Bosch, L. , Garcia‐Alix, A. , Muchart, J. , Sierpowska, J. , Fons, C. , Solé, J. , Rebollo, M. , Gaitán, H. , & Rodriguez‐Fornells, A. (2016). Language learning and brain reorganization in a 3.5‐year‐old child with left perinatal stroke revealed using structural and functional connectivity. Cortex, 77, 95–118. 10.1016/j.cortex.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. , Brauer, J. , & Lohmann, G. (2011). Maturation of the language network: From inter‐ to intrahemispheric connectivities. PLoS ONE, 6(6), e20726. 10.1371/journal.pone.0020726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti, G. (1993). The riddle of the right hemisphere's contribution to the recovery of language. International Journal of Language & Communication Disorders, 28(3), 227–246. 10.3109/13682829309060038 [DOI] [PubMed] [Google Scholar]
- Garrett, M. (1992). Disorders of lexical selection. Cognition, 42(1‐3), 143–180. 10.1016/0010-0277(92)90042-G [DOI] [PubMed] [Google Scholar]
- Gastaldon, S. , Arcara, G. , Navarrete, E. , & Peressotti, F. (2020). Commonalities in alpha and beta neural desynchronizations during prediction in language comprehension and production. Cortex, 133, 328–345. 10.1016/j.cortex.2020.09.026 [DOI] [PubMed] [Google Scholar]
- Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain, 123(7), 1293–1326. 10.1093/brain/123.7.1293 [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht, E. , Kocher, M. , Nesland, T. , Rorden, C. , Fridriksson, J. , & Bonilha, L. (2015). Preservation of structural brain network hubs is associated with less severe post‐stroke aphasia. Restorative Neurology and Neuroscience, 34(1), 19–28. 10.3233/RNN-150511 [DOI] [PubMed] [Google Scholar]
- Goodglass, H. (1980). Disorders of naming following brain injury: Observation of the effects of brain injury adds another dimension to our understanding of the relations between neurological and psychological factors in the naming process. American Scientist, 68(6), 647–655. [PubMed] [Google Scholar]
- Griffis, J. C. , Nenert, R. , Allendorfer, J. B. , & Szaflarski, J. P. (2017). Damage to white matter bottlenecks contributes to language impairments after left hemispheric stroke. NeuroImage: Clinical, 14, 552–565. 10.1016/j.nicl.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen, M. A. , Whitehouse, A. J. , Badcock, N. A. , & Bishop, D. V. (2012). Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain and Behavior: A Cognitive Neuroscience Perspective, 2(3), 256–269. 10.1002/brb3.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. , Kujala, J. , Hämäläinen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences, 98(2), 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro, M. , Castro‐Caldas, A. , & Martins, I. P. (1995). Aphasia following right hemisphere lesion in a woman with left hemisphere injury in childhood. Brain and Language, 49(3), 280–288. 10.1006/brln.1995.1034 [DOI] [PubMed] [Google Scholar]
- Hämäläinen, M. , Hari, R. , Ilmoniemi, R. J. , Knuutila, J. , & Lounasmaa, O. V. (1993). Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics, 65(2), 413–497. 10.1103/RevModPhys.65.413 [DOI] [Google Scholar]
- Hari, R. , & Parkkonen, L. (2015). The brain timewise: How timing shapes and supports brain function. Philosophical Transactions of the Royal Society, B: Biological Sciences, 370(1668), 20140170. 10.1098/rstb.2014.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen, G. , & Saur, D. (2019). Neuroimaging of stroke recovery from aphasia—Insights into plasticity of the human language network. NeuroImage, 190, 14–31. 10.1016/j.neuroimage.2017.11.056 [DOI] [PubMed] [Google Scholar]
- Heiss, W. D. , Kessler, J. , Thiel, A. , Ghaemi, M. , & Karbe, H. (1999). Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 45(4), 430–438. [DOI] [PubMed] [Google Scholar]
- Hertz‐Pannier, L. , Chiron, C. , Jambaqué, I. , Renaux‐Kieffer, V. , Van de Moortele, P.‐. F. , Delalande, O. , Fohlen, M. , Brunelle, F. , & Bihan, D. L. (2002). Late plasticity for language in a child's non‐dominant hemisphere: A pre‐ and post‐surgery fMRI study. Brain, 125(2), 361–372. 10.1093/brain/awf020 [DOI] [PubMed] [Google Scholar]
- Hervé, P. Y. , Zago, L. , Petit, L. , Mazoyer, B. , & Tzourio‐Mazoyer, N. (2013). Revisiting human hemispheric specialization with neuroimaging. Trends in Cognitive Sciences, 17(2), 69–80. 10.1016/j.tics.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Hillis, A. E. (2005). Can shift to the right be a good thing? Annals of Neurology, 58(3), 346–348. 10.1002/ana.20621 [DOI] [PubMed] [Google Scholar]
- Hinkley, L. B. , Marco, E. J. , Brown, E. G. , Bukshpun, P. , Gold, J. , Hill, S. , Findlay, A. M. , Jeremy, R. J. , Wakahiro, M. L. , Barkovich, A. J. , Mukherjee, P. , Sherr, E. H. , & Nagarajan, S. S. (2016). The contribution of the corpus callosum to language lateralization. Journal of Neuroscience, 36(16), 4522–4533. 10.1523/JNEUROSCI.3850-14.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer, S. , & Frahm, J. (2006). Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage, 32(3), 989–994. 10.1016/j.neuroimage.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Holland, A. , Fromm, D. , Forbes, M. , & MacWhinney, B. (2017). Long‐term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31(2), 152–165. 10.1080/02687038.2016.1184221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, D. , & Patterson, K. E. (1992). The Pyramids and Palm Trees Test.
- Hurford, J. R. (1991). The evolution of the critical period for language acquisition. Cognition, 40(3), 159–201. 10.1016/0010-0277(91)90024-X [DOI] [PubMed] [Google Scholar]
- Hustá, C. , Zheng, X. , Papoutsi, C. , & Piai, V. (2021). Electrophysiological signatures of conceptual and lexical retrieval from semantic memory. Neuropsychologia, 161, 107988. 10.1016/j.neuropsychologia.2021.107988 [DOI] [PubMed] [Google Scholar]
- Indefrey, P. (2011). The spatial and temporal signatures of word production components: A critical update. Frontiers in Psychology, 2, 255. 10.3389/fpsyg.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey, P. , & Levelt, W. J. M. (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- Ivanova, M. V. , Zhong, A. , Turken, A. , Baldo, J. V. , & Dronkers, N. F. (2021). Functional contributions of the arcuate fasciculus to language processing. Frontiers in Human Neuroscience, 15, 672665. 10.3389/fnhum.2021.672665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, R. A. (1999). Computational studies of the development of functionally specialized neural modules. Trends in Cognitive Sciences, 3(1), 31–38. 10.1016/S1364-6613(98)01260-1 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jongbloed, L. (1986). Prediction of function after stroke: A critical review. Stroke, 17(4), 765–776. 10.1161/01.STR.17.4.765 [DOI] [PubMed] [Google Scholar]
- Jung, T.‐P. , Makeig, S. , Westerfield, M. , Townsend, J. , Courchesne, E. , & Sejnowski, T. J. (2000). Removal of eye activity artifacts from visual event‐related potentials in normal and clinical subjects. Clinical Neurophysiology, 111(10), 1745–1758. 10.1016/S1388-2457(00)00386-2 [DOI] [PubMed] [Google Scholar]
- Kellner, E. , Dhital, B. , Kiselev, V. G. , & Reisert, M. (2016). Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic Resonance in Medicine, 76(5), 1574–1581. 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- Kennedy, K. M. , Rodrigue, K. M. , Bischof, G. N. , Hebrank, A. C. , Reuter‐Lorenz, P. A. , & Park, D. C. (2015). Age trajectories of functional activation under conditions of low and high processing demands: An adult lifespan fMRI study of the aging brain. NeuroImage, 104, 21–34. 10.1016/j.neuroimage.2014.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, R. D. , & Rosenbek, J. C. (1983). Acoustic patterns of apraxia of speech. Journal of Speech, Language, and Hearing Research, 26(2), 231–249. 10.1044/jshr.2602.231 [DOI] [PubMed] [Google Scholar]
- Kiran, S. , & Thompson, C. K. (2019). Neuroplasticity of language networks in aphasia: Advances, updates, and future challenges. Frontiers in Neurology, 10, 295. 10.3389/fneur.2019.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus, J. , Schutter, D. J. , & Piai, V. (2020). Transient perturbation of the left temporal cortex evokes plasticity‐related reconfiguration of the lexical network. Human Brain Mapping, 41(4), 1061–1071. 10.1002/hbm.24860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach, M. , Matosevic, B. , Rucker, M. , Furtner, M. , Mair, A. , Wille, G. , Zangerle, A. , Werner, P. , Ferrari, J. , Schmidauer, C. , Seyfang, L. , Kiechl, S. , Willeit, J. , & For the Austrian Stroke Unit Registry Collaborators . (2012). Functional recovery after ischemic stroke—A matter of age: Data from the Austrian Stroke Unit Registry. Neurology, 78(4), 279–285. 10.1212/WNL.0b013e31824367ab [DOI] [PubMed] [Google Scholar]
- Kodiweera, C. , Alexander, A. L. , Harezlak, J. , McAllister, T. W. , & Wu, Y. C. (2016). Age effects and sex differences in human brain white matter of young to middle‐aged adults: A DTI, NODDI, and q‐space study. NeuroImage, 128, 180–192. 10.1016/j.neuroimage.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, S. E. , & Goodglass, H. (1985). Picture‐naming in aphasia. Brain and Language, 24(2), 266–283. 10.1016/0093-934X(85)90135-X [DOI] [PubMed] [Google Scholar]
- Kosslyn, S. M. (1987). Seeing and imagining in the cerebral hemispheres: A computational approach. Psychological Review, 94(2), 148–175. 10.1037/0033-295X.94.2.148 [DOI] [PubMed] [Google Scholar]
- Kristensen, B. , Malm, J. , Carlberg, B. , Stegmayr, B. , Backman, C. , Fagerlund, M. , & Olsson, T. (1997). Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke, 28(9), 1702–1709. 10.1161/01.str.28.9.1702 [DOI] [PubMed] [Google Scholar]
- Landis, T. , Graves, R. , & Goodglass, H. (1982). Aphasic reading and writing: Possible evidence for right hemisphere participation. Cortex, 18(1), 105–112. 10.1016/S0010-9452(82)80022-1 [DOI] [PubMed] [Google Scholar]
- Lazar, R. M. , Minzer, B. , Antoniello, D. , Festa, J. R. , Krakauer, J. W. , & Marshall, R. S. (2010). Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke, 41(7), 1485–1488. 10.1161/STROKEAHA.109.577338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , & Beaulieu, C. (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31(30), 10937–10947. 10.1523/JNEUROSCI.5302-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Leff, A. , Crinion, J. , Scott, S. , Turkheimer, F. , Howard, D. , & Wise, R. (2002). A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Annals of Neurology, 51(5), 553–558. 10.1002/ana.10181 [DOI] [PubMed] [Google Scholar]
- Levelt, W. J. , Praamstra, P. , Meyer, A. S. , Helenius, P. , & Salmelin, R. (1998). An MEG study of picture naming. Journal of Cognitive Neuroscience, 10(5), 553–567. 10.1162/089892998562960 [DOI] [PubMed] [Google Scholar]
- Levelt, W. J. , Roelofs, A. , & Meyer, A. S. (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Liljeström, M. , Hultén, A. , Parkkonen, L. , & Salmelin, R. (2009). Comparing MEG and fMRI views to naming actions and objects. Human Brain Mapping, 30(6), 1845–1856. 10.1002/hbm.20785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell, A. K. (2006). In your right mind: Right hemisphere contributions to language processing and production. Neuropsychology Review, 16(3), 131–148. 10.1007/s11065-006-9011-9 [DOI] [PubMed] [Google Scholar]
- López‐Otín, C. , Blasco, M. A. , Partridge, L. , Serrano, M. , & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaijwee, N. A. M. M. , Rutten‐Jacobs, L. C. A. , Schaapsmeerders, P. , van Dijk, E. J. , & de Leeuw, F.‐E. (2014). Ischaemic stroke in young adults: Risk factors and long‐term consequences. Nature Reviews Neurology, 10(6), 315–325. 10.1038/nrneurol.2014.72 [DOI] [PubMed] [Google Scholar]
- Macciocchi, S. N. , Diamond, P. T. , Alves, W. M. , & Mertz, T. (1998). Ischemic stroke: Relation of age, lesion location, and initial neurologic deficit to functional outcome. Archives of Physical Medicine and Rehabilitation, 79(10), 1255–1257. 10.1016/S0003-9993(98)90271-4 [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Marques, J. P. , Kober, T. , Krueger, G. , van der Zwaag, W. , Van de Moortele, P.‐F. , & Gruetter, R. (2010). MP2RAGE, a self bias‐field corrected sequence for improved segmentation and T1‐mapping at high field. NeuroImage, 49(2), 1271–1281. 10.1016/j.neuroimage.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Marshall, R. S. (2004). The functional relevance of cerebral hemodynamics: Why blood flow matters to the injured and recovering brain. Current Opinion in Neurology, 17(6), 705–709. 10.1097/00019052-200412000-00010 [DOI] [PubMed] [Google Scholar]
- Martin, K. C. , Seydell‐Greenwald, A. , Berl, M. M. , Gaillard, W. D. , Turkeltaub, P. E. , & Newport, E. L. (2022). A weak shadow of early life language processing persists in the right hemisphere of the mature brain. Neurobiology of Language, 3(3), 1–49. 10.1162/nol_a_00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M. P. , & Arumugam, T. V. (2018). Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metabolism, 27(6), 1176–1199. 10.1016/j.cmet.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer, B. , Zago, L. , Jobard, G. , Crivello, F. , Joliot, M. , Perchey, G. , Mellet, E. , Petit, L. , & Tzourio‐Mazoyer, N. (2014). Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLoS ONE, 9(6), e101165. 10.1371/journal.pone.0101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, M. , Vista, M. , Pardossi, L. , Avila, L. , Bianchi, F. , & Moretti, P. (1992). Spontaneous evolution of aphasia after ischaemic stroke. Aphasiology, 6(4), 387–396. 10.1080/02687039208248609 [DOI] [Google Scholar]
- Meinzer, M. , Elbert, T. , Wienbruch, C. , Djundja, D. , Barthel, G. , & Rockstroh, B. (2004). Intensive language training enhances brain plasticity in chronic aphasia. BMC Biology, 2, 20. 10.1186/1741-7007-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado, E. I. I. I. (2008). Neural and cognitive plasticity: From maps to minds. Psychological Bulletin, 134(1), 109–137. 10.1037/0033-2909.134.1.109 [DOI] [PubMed] [Google Scholar]
- Minjoli, S. , Saturnino, G. B. , Blicher, J. U. , Stagg, C. J. , Siebner, H. R. , Antunes, A. , & Thielscher, A. (2017). The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. NeuroImage: Clinical, 15, 106–117. 10.1016/j.nicl.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, B. , MacGregor, L. J. , Difrancesco, S. , Harrington, K. , Pulvermüller, F. , & Shtyrov, Y. (2016). Hemispheric contributions to language reorganisation: An MEG study of neuroplasticity in chronic post stroke aphasia. Neuropsychologia, 93(Pt B), 413–424. 10.1016/j.neuropsychologia.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Murphy, T. H. , & Corbett, D. (2009). Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews Neuroscience, 10(12), 861–872. 10.1038/nrn2735 [DOI] [PubMed] [Google Scholar]
- Naeser, M. , Martin, P. , Nicholas, M. , Baker, E. , Seekins, H. , Kobayashi, M. , Theoret, H. , Fregni, F. , Mariatormos, J. , & Kurland, J. (2005). Improved picture naming in chronic aphasia after TMS to part of right Broca's area: An open‐protocol study. Brain and Language, 93(1), 95–105. 10.1016/j.bandl.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Naess, H. , Hammersvik, L. , & Skeie, G. O. (2009). Aphasia among young patients with ischemic stroke on long‐term follow‐up. Journal of Stroke and Cerebrovascular Diseases, 18(4), 247–250. 10.1016/j.jstrokecerebrovasdis.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Naess, H. , Waje‐Andreassen, U. , Thomassen, L. , Nyland, H. , & Myhr, K.‐M. (2006). Health‐related quality of life among young adults with ischemic stroke on long‐term follow‐up. Stroke, 37(5), 1232–1236. 10.1161/01.STR.0000217652.42273.02 [DOI] [PubMed] [Google Scholar]
- Nedeltchev, K. , der Maur, T. A. , Georgiadis, D. , Arnold, M. , Caso, V. , Mattle, H. P. , Schroth, G. , Remonda, L. , Sturzenegger, M. , Fischer, U. , & Baumgartner, R. W. (2005). Ischaemic stroke in young adults: Predictors of outcome and recurrence. Journal of Neurology, Neurosurgery & Psychiatry, 76(2), 191–195. 10.1136/jnnp.2004.040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport, E. L. , Landau, B. , Seydell‐Greenwald, A. , Turkeltaub, P. E. , Chambers, C. E. , Dromerick, A. W. , Carpenter, J. , Berl, M. M. , & Gaillard, W. D. (2017). Revisiting Lenneberg's hypotheses about early developmental plasticity: Language organization after left‐hemisphere perinatal stroke. Biolinguistics, 11, 407–422. [PMC free article] [PubMed] [Google Scholar]
- Nieto‐Sampedro, M. (2004). Neural plasticity and central nervous system lesion repair. In Herdegen T. & Delgado‐García J. (Eds.), Brain damage and repair (pp. 323–348). Springer. 10.1007/1-4020-2541-6_17 [DOI] [Google Scholar]
- Olulade, O. A. , Seydell‐Greenwald, A. , Chambers, C. E. , Turkeltaub, P. E. , Dromerick, A. W. , Berl, M. M. , Gaillard, W. D. , & Newport, E. L. (2020). The neural basis of language development: Changes in lateralization over age. Proceedings of the National Academy of Sciences, 117(38), 23477–23483. 10.1073/pnas.1905590117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J.‐M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 1–9. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard, M. , & Mogensen, J. (2011). A framework for the study of multiple realizations: The importance of levels of analysis. Frontiers in Psychology, 2, 79. 10.3389/fpsyg.2011.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou, A. C. , Moore, B. D. , Deutsch, G. , Levin, H. S. , & Eisenberg, H. M. (1988). Evidence for right‐hemisphere involvement in recovery from aphasia. Archives of Neurology, 45(9), 1025–1029. 10.1001/archneur.1988.00520330117020 [DOI] [PubMed] [Google Scholar]
- Peelle, J. E. (2019). Language and aging. In de Zubicaray G. I. & Schiller N. O. (Eds.), The Oxford handbook of neurolinguistics, Oxford Handbooks. Oxford Academic. 10.1093/oxfordhb/9780190672027.013.12 [DOI] [Google Scholar]
- Penny, W. D. , Friston, K. J. , Ashburner, J. T. , Kiebel, S. J. , & Nichols, T. E. (Eds.) (2011). Statistical parametric mapping: The analysis of functional brain images. Elsevier. 10.1016/B978-0-12-372560-8.X5000-1 [DOI] [Google Scholar]
- Perani, D. , Saccuman, M. C. , Scifo, P. , Anwander, A. , Spada, D. , Baldoli, C. , Poloniato, A. , Lohmann, G. , & Friederici, A. D. (2011). Neural language networks at birth. Proceedings of the National Academy of Sciences, 108(38), 16056–16061. 10.1073/pnas.1102991108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai, V. , Klaus, J. , & Rossetto, E. (2020). The lexical nature of alpha‐beta oscillations in context‐driven word production. Journal of Neurolinguistics, 55, 100905. 10.1016/j.jneuroling.2020.100905 [DOI] [Google Scholar]
- Piai, V. , Meyer, L. , Dronkers, N. F. , & Knight, R. T. (2017). Neuroplasticity of language in left‐hemisphere stroke: Evidence linking subsecond electrophysiology and structural connections. Human Brain Mapping, 38(6), 3151–3162. 10.1002/hbm.23581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai, V. , Roelofs, A. , & Maris, E. (2014). Oscillatory brain responses in spoken word production reflect lexical frequency and sentential constraint. Neuropsychologia, 53, 146–156. 10.1016/j.neuropsychologia.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Piai, V. , Roelofs, A. , Rommers, J. , & Maris, E. (2015). Beta oscillations reflect memory and motor aspects of spoken word production. Human Brain Mapping, 36(7), 2767–2780. 10.1002/hbm.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai, V. , Rommers, J. , & Knight, R. T. (2018). Lesion evidence for a critical role of left posterior but not frontal areas in alpha–beta power decreases during context‐driven word production. European Journal of Neuroscience, 48(7), 2622–2629. 10.1111/ejn.13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piastra, M. C. , Nüßing, A. , Vorwerk, J. , Bornfleth, H. , Oostenveld, R. , Engwer, C. , & Wolters, C. H. (2018). The discontinuous Galerkin finite element method for solving the MEG and the combined MEG/EEG forward problem. Frontiers in Neuroscience, 12, 30. 10.3389/fnins.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piastra, M.‐C. , Oostenveld, R. , Schoffelen, J.‐M. , & Piai, V. (2022). Estimating the influence of stroke lesions on MEG source reconstruction. NeuroImage, 260. 10.1016/j.neuroimage.2022.119422 [DOI] [PubMed] [Google Scholar]
- Plaut, D. C. , & Behrmann, M. (2011). Complementary neural representations for faces and words: A computational exploration. Cognitive Neuropsychology, 28(3–4), 251–275. 10.1080/02643294.2011.609812 [DOI] [PubMed] [Google Scholar]
- Popa‐Wagner, A. , Buga, A.‐M. , & Kokaia, Z. (2011). Perturbed cellular response to brain injury during aging. Ageing Research Reviews, 10(1), 71–79. 10.1016/j.arr.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Popa‐Wagner, A. , Carmichael, S. T. , Kokaia, Z. , Kessler, C. , & Walker, L. C. (2007). The response of the aged brain to stroke: Too much, too soon? Current Neurovascular Research, 4(3), 216–227. 10.2174/156720207781387213 [DOI] [PubMed] [Google Scholar]
- Popa‐Wagner, A. , Petcu, E. B. , Capitanescu, B. , Hermann, D. M. , Radu, E. , & Gresita, A. (2020). Ageing as a risk factor for cerebral ischemia: Underlying mechanisms and therapy in animal models and in the clinic. Mechanisms of Ageing and Development, 190, 111312. 10.1016/j.mad.2020.111312 [DOI] [PubMed] [Google Scholar]
- Postman‐Caucheteux, W. A. , Birn, R. M. , Pursley, R. H. , Butman, J. A. , Solomon, J. M. , Picchioni, D. , McArdle, J. , & Braun, A. R. (2010). Single‐trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience, 22(6), 1299–1318. 10.1162/jocn.2009.21261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , & Friston, K. J. (2002). Degeneracy and cognitive anatomy. Trends in Cognitive Sciences, 6(10), 416–421. 10.1016/S1364-6613(02)01976-9 [DOI] [PubMed] [Google Scholar]
- Prins, R. S. , Snow, C. E. , & Wagenaar, E. (1978). Recovery from aphasia: Spontaneous speech versus language comprehension. Brain and Language, 6(2), 192–211. 10.1016/0093-934X(78)90058-5 [DOI] [PubMed] [Google Scholar]
- Pulvermüller, F. , Hauk, O. , Zohsel, K. , Neininger, B. , & Mohr, B. (2005). Therapy‐related reorganization of language in both hemispheres of patients with chronic aphasia. NeuroImage, 28(2), 481–489. 10.1016/j.neuroimage.2005.06.038 [DOI] [PubMed] [Google Scholar]
- Putaala, J. (2016). Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. European Stroke Journal, 1(1), 28–40. 10.1177/2396987316629860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala, J. , Kurkinen, M. , Tarvos, V. , Salonen, O. , Kaste, M. , & Tatlisumak, T. (2009). Silent brain infarcts and leukoaraiosis in young adults with first‐ever ischemic stroke. Neurology, 72(21), 1823–1829. 10.1212/WNL.0b013e3181a711df [DOI] [PubMed] [Google Scholar]
- Rabinstein, A. A. , Albers, G. W. , Brinjikji, W. , & Koch, S. (2019). Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. International Journal of Stroke, 14(1), 23–31. 10.1177/1747493018799979 [DOI] [PubMed] [Google Scholar]
- Ramon, C. , Schimpf, P. , Haueisen, J. , Holmes, M. , & Ishimaru, A. (2004). Role of soft bone, CSF and gray matter in EEG simulations. Brain Topography, 16(4), 245–248. 10.1023/b:brat.0000032859.68959.76 [DOI] [PubMed] [Google Scholar]
- Ressel, V. (2008). Increases in language lateralization in normal children as observed using magnetoencephalography. Brain and Language, 106(3), 167–176. 10.1016/j.bandl.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Ripley, B. , Venables, B. , Bates, D. M. , Hornik, K. , Gebhardt, A. , Firth, D. , & Ripley, M. B. (2020). Package ‘mass’. R package version 7.3‐52.
- Risse, G. L. , Gates, J. R. , & Fangman, M. C. (1997). A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain and Cognition, 33(1), 118–132. 10.1006/brcg.1997.0887 [DOI] [PubMed] [Google Scholar]
- Roelofs, A. (1992). A spreading‐activation theory of lemma retrieval in speaking. Cognition, 42(1‐3), 107–142. 10.1016/0010-0277(92)90041-F [DOI] [PubMed] [Google Scholar]
- Roelofs, A. (2014). A dorsal‐pathway account of aphasic language production: The WEAVER++/ARC model. Cortex, 59, 33–48. 10.1016/j.cortex.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Roos, N. M. , & Piai, V. (2020). Across‐session consistency of context‐driven language processing: A magnetoencephalography study. European Journal of Neuroscience, 52(5), 3457–3469. 10.1111/ejn.14785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S. , Miniussi, C. , Pasqualetti, P. , Babiloni, C. , Rossini, P. M. , & Cappa, S. F. (2004). Age‐related functional changes of prefrontal cortex in long‐term memory: A repetitive transcranial magnetic stimulation study. Journal of Neuroscience, 24(36), 7939–7944. 10.1523/JNEUROSCI.0703-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini, P. M. (2004). Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain, 127(1), 99–110. 10.1093/brain/awh012 [DOI] [PubMed] [Google Scholar]
- Rosso, C. , Vargas, P. , Valabregue, R. , Arbizu, C. , Henry‐Amar, F. , Leger, A. , Lehéricy, S. , & Samson, Y. (2015). Aphasia severity in chronic stroke patients: A combined disconnection in the dorsal and ventral language pathways. Neurorehabilitation and Neural Repair, 29(3), 287–295. 10.1177/1545968314543926 [DOI] [PubMed] [Google Scholar]
- Rothi, L. J. , & Horner, J. (1983). Restitution and substitution: Two theories of recovery with application to neurobehavioral treatment. Journal of Clinical Neuropsychology, 5(1), 73–81. 10.1080/01688638308401152 [DOI] [PubMed] [Google Scholar]
- Ruiter, M. B. , Kolk, H. H. J. , Rietveld, T. C. M. , Dijkstra, N. , & Lotgering, E. (2011). Towards a quantitative measure of verbal effectiveness and efficiency in the Amsterdam‐Nijmegen Everyday Language Test (ANELT). Aphasiology, 25(8), 961–975. 10.1080/02687038.2011.569892 [DOI] [Google Scholar]
- Salmelin, R. , Hari, R. , Lounasmaa, O. V. , & Sams, M. (1994). Dynamics of brain activation during picture naming. Nature, 368(6470), 463–465. 10.1038/368463a0 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Lange, R. , Baumgaertner, A. , Schraknepper, V. , Willmes, K. , Rijntjes, M. , & Weiller, C. (2006). Dynamics of language reorganization after stroke. Brain, 129(6), 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Schaapsmeerders, P. , Maaijwee, N. A. M. , van Dijk, E. J. , Rutten‐Jacobs, L. C. A. , Arntz, R. M. , Schoonderwaldt, H. C. , Dorresteijn, L. D. A. , Kessels, R. P. C. , & de Leeuw, F.‐E. (2013). Long‐term cognitive impairment after first‐ever ischemic stroke in young adults. Stroke, 44(6), 1621–1628. 10.1161/STROKEAHA.111.000792 [DOI] [PubMed] [Google Scholar]
- Schimpf, P. H. , Ramon, C. , & Haueisen, J. (2002). Dipole models for the EEG and MEG. IEEE Transactions on Biomedical Engineering, 49(5), 409–418. 10.1109/10.995679 [DOI] [PubMed] [Google Scholar]
- Schneck, S. M. , Entrup, J. L. , Duff, M. C. , & Wilson, S. M. (2021). Unexpected absence of aphasia following left temporal hemorrhage: A case study with functional neuroimaging to characterize the nature of atypical language localization. Neurocase, 27(1), 97–105. 10.1080/13554794.2021.1886309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader, S. , Westhoff, A. , Piastra, M.‐C. , Miinalainen, T. , Pursiainen, S. , Vorwerk, J. , Brinck, H. , Wolters, C. H. , & Engwer, C. (2021). DUNEuro—A software toolbox for forward modeling in bioelectromagnetism. PLoS ONE, 16(6), e0252431. 10.1371/journal.pone.0252431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selnes, O. A. (1999). Recovery from aphasia: Activating the “right” hemisphere. Annals of Neurology, 45(4), 419–420. [DOI] [PubMed] [Google Scholar]
- Shafto, M. A. , & Tyler, L. K. (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. 10.1126/science.1254404 [DOI] [PubMed] [Google Scholar]
- Sierpowska, J. , Gabarrós, A. , Fernández‐Coello, A. , Camins, À. , Castañer, S. , Juncadella, M. , François, C. , & Rodríguez‐Fornells, A. (2019). White‐matter pathways and semantic processing: Intrasurgical and lesion‐symptom mapping evidence. NeuroImage: Clinical, 22, 101704. 10.1016/j.nicl.2019.101704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörös, P. , Cornelissen, K. , Laine, M. , & Salmelin, R. (2003). Naming actions and objects: Cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. NeuroImage, 19(4), 1787–1801. 10.1016/S1053-8119(03)00217-9 [DOI] [PubMed] [Google Scholar]
- Sowell, E. R. , Thompson, P. M. , Holmes, C. J. , Jernigan, T. L. , & Toga, A. W. (1999). In vivo evidence for post‐adolescent brain maturation in frontal and striatal regions. Nature Neuroscience, 2(10), 859–861. 10.1038/13154 [DOI] [PubMed] [Google Scholar]
- Staudt, M. (2002). Right‐hemispheric organization of language following early left‐sided brain lesions: Functional MRI topography. NeuroImage, 16(4), 954–967. 10.1006/nimg.2002.1108 [DOI] [PubMed] [Google Scholar]
- Stefaniak, J. D. , Alyahya, R. S. W. , & Lambon Ralph, M. A. (2021). Language networks in aphasia and health: A 1000 participant activation likelihood estimation meta‐analysis. NeuroImage, 233, 117960. 10.1016/j.neuroimage.2021.117960 [DOI] [PubMed] [Google Scholar]
- Stefaniak, J. D. , Halai, A. D. , & Lambon Ralph, M. A. (2020). The neural and neurocomputational bases of recovery from post‐stroke aphasia. Nature Reviews Neurology, 16(1), 43–55. 10.1038/s41582-019-0282-1 [DOI] [PubMed] [Google Scholar]
- Stockert, A. , Wawrzyniak, M. , Klingbeil, J. , Wrede, K. , Kümmerer, D. , Hartwigsen, G. , Kaller, C. P. , Weiller, C. , & Saur, D. (2020). Dynamics of language reorganization after left temporo‐parietal and frontal stroke. Brain: A Journal of Neurology, 143(3), 844–861. 10.1093/brain/awaa023 [DOI] [PubMed] [Google Scholar]
- Stolk, A. , Todorovic, A. , Schoffelen, J.‐M. , & Oostenveld, R. (2013). Online and offline tools for head movement compensation in MEG. NeuroImage, 68, 39–48. 10.1016/j.neuroimage.2012.11.047 [DOI] [PubMed] [Google Scholar]
- Sultan, S. , & Elkind, M. S. V. (2013). The growing problem of stroke among young adults. Current Cardiology Reports, 15(12), 421. 10.1007/s11886-013-0421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synhaeve, N. E. , Arntz, R. M. , Maaijwee, N. A. M. , Rutten‐Jacobs, L. C. A. , Schoonderwaldt, H. C. , Dorresteijn, L. D. A. , de Kort, P. L. M. , van Dijk, E. J. , & de Leeuw, F.‐E. (2014). Poor long‐term functional outcome after stroke among adults aged 18 to 50 years: Follow‐Up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation (FUTURE) study. Stroke, 45(4), 1157–1160. 10.1161/STROKEAHA.113.004411 [DOI] [PubMed] [Google Scholar]
- Thye, M. , & Mirman, D. (2018). Relative contributions of lesion location and lesion size to predictions of varied language deficits in post‐stroke aphasia. NeuroImage: Clinical, 20, 1129–1138. 10.1016/j.nicl.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu, S. (2005). Visual evoked magnetic fields and magnetic stimulation of visual cortex. In Handbook of clinical neurophysiology (Vol. 5) (pp. 143–166). Elsevier. 10.1016/S1567-4231(09)70205-9 [DOI] [Google Scholar]
- Tournier, J.‐D. , Smith, R. , Raffelt, D. , Tabbara, R. , Dhollander, T. , Pietsch, M. , Christiaens, D. , Jeurissen, B. , Yeh, C.‐H. , & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tourville, J. A. , & Guenther, F. H. (2011). The DIVA model: A neural theory of speech acquisition and production. Language & Cognitive Processes, 26(7), 952–981. 10.1080/01690960903498424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Coslett, H. B. , Thomas, A. L. , Faseyitan, O. , Benson, J. , Norise, C. , & Hamilton, R. H. (2012). The right hemisphere is not unitary in its role in aphasia recovery. Cortex, 48(9), 1179–1186. 10.1016/j.cortex.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Joliot, M. , Marie, D. , & Mazoyer, B. (2016). Variation in homotopic areas' activity and inter‐hemispheric intrinsic connectivity with type of language lateralization: An FMRI study of covert sentence generation in 297 healthy volunteers. Brain Structure & Function, 221(5), 2735–2753. 10.1007/s00429-015-1068-x [DOI] [PubMed] [Google Scholar]
- van Casteren, M. , & Davis, M. H. (2006). Mix, a program for pseudorandomization. Behavior Research Methods, 38(4), 584–589. 10.3758/BF03193889 [DOI] [PubMed] [Google Scholar]
- van Veen, B. D. , van Drongelen, W. , Yuchtman, M. , & Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering, 44(9), 867–880. 10.1109/10.623056 [DOI] [PubMed] [Google Scholar]
- Veraart, J. , Fieremans, E. , Jelescu, I. O. , Knoll, F. , & Novikov, D. S. (2016). Gibbs ringing in diffusion MRI. Magnetic Resonance in Medicine, 76(1), 301–314. 10.1002/mrm.25866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart, J. , Novikov, D. S. , Christiaens, D. , Ades‐aron, B. , Sijbers, J. , & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihla, M. , Laine, M. , & Salmelin, R. (2006). Cortical dynamics of visual/semantic vs. phonological analysis in picture confrontation. NeuroImage, 33(2), 732–738. 10.1016/j.neuroimage.2006.06.040 [DOI] [PubMed] [Google Scholar]
- Visch‐Brink, E. , Vandenborre, D. , de Smet, H. J. , & Mariën, P. (2014). Comprehensive Aphasia Test—Nederlandse bewerking—Handleiding. Pearson. [Google Scholar]
- Wang, R. , Benner, T. , Sorensen, A. G. , & Wedeen, V. J. (2007). Diffusion toolkit: A software package for diffusion imaging data processing and tractography. Proceedings of the International Society for Magnetic Resonance in Medicine, 15, 3720. [Google Scholar]
- Watila, M. M. , & Balarabe, S. A. (2015). Factors predicting post‐stroke aphasia recovery. Journal of the Neurological Sciences, 352(1‐2), 12–18. 10.1016/j.jns.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Weimar, C. , Ziegler, A. , König, I. R. , & Diener, H.‐C. (2002). Predicting functional outcome and survival after acute ischemic stroke. Journal of Neurology, 249(7), 888–895. 10.1007/s00415-002-0755-8 [DOI] [PubMed] [Google Scholar]
- Wilson, S. M. , & Schneck, S. M. (2020). Neuroplasticity in post‐stroke aphasia: A systematic review and meta‐analysis of functional imaging studies of reorganization of language processing. Neurobiology of Language, 2(1), 22–82. 10.1162/nol_a_00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead, Z. V. J. , Thompson, P. A. , Karlsson, E. M. , & Bishop, D. V. M. (2021). An updated investigation of the multidimensional structure of language lateralization in left‐ and right‐handed adults: A test–retest functional transcranial Doppler sonography study with six language tasks. Royal Society Open Science, 8(2), 200696. 10.1098/rsos.200696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, S. , Lacey, E. H. , Skipper‐Kallal, L. M. , Jiang, X. , Harris‐Love, M. L. , Zeng, J. , & Turkeltaub, P. E. (2016). Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain, 139(1), 227–241. 10.1093/brain/awv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. , Yang, W. , Liu, Y. , Wang, H. , Chen, Z. , & Yan, J. (2018). Changes in the corpus callosum during the recovery of aphasia: A case report. Medicine, 97(24), e11155. 10.1097/MD.0000000000011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel, E. (1983). A response to Gazzaniga: Language in the right hemisphere, convergent perspectives. American Psychologist, 38(5), 542–546. 10.1037/0003-066X.38.5.542 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, R. L. , Wang, Y. , Zhang, C. , Zhang, Z. G. , Meng, H. , & Chopp, M. (2005). Functional recovery in aged and young rats after embolic stroke: Treatment with a phosphodiesterase type 5 inhibitor. Stroke, 36(4), 847–852. 10.1161/01.STR.0000158923.19956.73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data Availability Statement
The data are not publicly available given that anonymity of the participants cannot be guaranteed.


