Abstract
Background
Current guidelines suggest the introduction of early nutrition support within the first 48 h of admission to the intensive care unit (ICU) for patients who cannot eat. In that context, we aimed to describe nutrition practices in the ICU and study the association between the introduction of early nutrition support (< 48 h) in the ICU and patient mortality at day 28 (D28) using data from a multicentre prospective cohort.
Methods
The ‘French-Speaking ICU Nutritional Survey’ (FRANS) study was conducted in 26 ICUs in France and Belgium over 3 months in 2015. Adult patients with a predicted ICU length of stay > 3 days were consecutively included and followed for 10 days. Their mortality was assessed at D28. We investigated the association between early nutrition (< 48 h) and mortality at D28 using univariate and multivariate propensity-score-weighted logistic regression analyses.
Results
During the study period, 1206 patients were included. Early nutrition support was administered to 718 patients (59.5%), with 504 patients receiving enteral nutrition and 214 parenteral nutrition. Early nutrition was more frequently prescribed in the presence of multiple organ failure and less frequently in overweight and obese patients. Early nutrition was significantly associated with D28 mortality in the univariate analysis (crude odds ratio (OR) 1.69, 95% confidence interval (CI) 1.23–2.34) and propensity-weighted multivariate analysis (adjusted OR (aOR) 1.05, 95% CI 1.00–1.10). In subgroup analyses, this association was stronger in patients ≤ 65 years and with SOFA scores ≤ 8. Compared with no early nutrition, a significant association was found of D28 mortality with early enteral (aOR 1.06, 95% CI 1.01–1.11) but not early parenteral nutrition (aOR 1.04, 95% CI 0.98–1.11).
Conclusions
In this prospective cohort study, early nutrition support in the ICU was significantly associated with increased mortality at D28, particularly in younger patients with less severe disease. Compared to no early nutrition, only early enteral nutrition appeared to be associated with increased mortality. Such findings are in contrast with current guidelines on the provision of early nutrition support in the ICU and may challenge our current practices, particularly concerning patients at low nutrition risk.
Trial registration ClinicalTrials.gov Identifier: NCT02599948. Retrospectively registered on November 5th 2015.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04298-1.
Keywords: Clinical nutrition, Intensive care unit, Enteral nutrition, Parenteral nutrition, Critical illness, Clinical nutrition guidelines, Mortality, Early nutrition support
Background
Patients admitted to the ICU suffer from acute critical illness. This induces major catabolic stress, which may result in severe muscle wasting and prolonged impaired functional outcomes [1]. It is commonly accepted that providing adequate nutrition with essential nutrients may help to attenuate the consequences of the catabolic response. However, identifying the appropriate timing, amount and route of nutrition support remains a complex challenge for ICU physicians. Nutritional therapy limits the risk of an energy or protein deficit, which was associated in previous retrospective studies with poor outcomes, such as prolonged ICU and hospital stays, prolonged mechanical ventilation durations and higher incidences of infectious complications [2–4]. The risk of complications is even higher in patients identified with high nutrition risk at ICU admission [5]. However, recent randomised control trials (RCTs) and meta-analyses failed to demonstrate any benefit of the nutritional guideline target adequacy during the first weeks of ICU on the outcomes studied [6–12].
International guidelines recommend the early introduction of hypocaloric enteral nutrition within the first 48 h, except in cases of uncontrolled shock, hypoxemia or acidosis [13–15]. The rationale of these recommendations is based on the results of a meta-analysis reporting the benefit of early enteral nutrition for decreasing the incidence of infectious complications; however, this positive effect was not found when studies involving non-critically ill patients were excluded [14]. The most recent meta-analysis, conducted by the Cochrane group, including seven RCTs published between 1993 and 2012, also reported ‘very low-quality evidence’ in favour of early over delayed enteral nutrition in ICU patients [16]. Furthermore, early initiation with rapid achievement of the energy target has not shown a significant benefit and may even cause harm [12, 17]. In addition, in a recent study by Ortiz‐Reyes et al., early enteral nutrition showed no benefit compared to delayed enteral in ventilated patients receiving vasopressor or inotropic therapies after adjusting for the illness severity [18]. Finally, some concerns have recently emerged about the possible increased risk of digestive complications such as mesenteric ischaemia associated with early enteral feeding [19, 20].
Nutritional practices in the ICU may significantly differ between units as well as between patients. Current prescriptions in the ICU setting and factors influencing caregivers in their choices remain poorly described. We set out to analyse data from a real-life, prospective, multicentric cohort study, to explore the impact of early nutrition, and its route of delivery, on patient mortality. Specifically, the purposes of this observational study were to (1) describe current practices and factors associated with the prescription of early nutrition support within the first 48 h in the ICU, and (2) conduct an adjusted analysis of the association between early nutrition support and 28-day mortality.
Methods
Study design
We performed a multicentre, prospective, observational study specifically designed to explore nutrition practices for critically ill patients during the first 10 days of ICU stay (from day 1 to day 10, with D0 corresponding to the day of ICU admission), the ‘French-Speaking ICU Nutritional Survey’ (FRANS) study. This study was conducted in 23 ICUs in France and three in Belgium. Patients were included over 3 consecutive months, from February to June 2015 for French ICUs and from May to August 2015 for Belgian ICUs. The patients were followed for 28 days. The ethical committee of each institution approved the FRANS study and the trial was retrospectively registered on ClinicalTrials.gov under the reference NCT02599948. Reporting of this study was in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement and guidelines [21].
Study population
Critically ill adult patients with an expected length of stay greater than 3 days in the ICU were included in the FRANS study. The exclusion criteria were as follows: aged under 18 years, the patient or next-of-kin’s refusal, a prior medical decision to limit or discontinue life-sustaining therapies. The study protocol allowed for secondary exclusion after a patient’s inclusion in the study if a decision was made to limit life-sustaining therapy within the first 10 days of their ICU stay.
Data collection
In each participating ICU, a referring physician was responsible for data collection. Data were prospectively collected from medical charts and daily prescriptions using a dedicated case report form.
At the baseline, the following data were recorded: patient demographic characteristics (age, sex, height, weight), admission diagnosis (medical or surgical), Simplified Acute Physiology Score (SAPS) II and use of mechanical ventilation [22]. Organ dysfunction was evaluated with the Sequential Organ Failure Assessment (SOFA) score and the severity of illness with the Acute Physiology and Chronic Health Evaluation (APACHE II) score [23, 24]. Patient weight was measured at ICU admission by weighted beds or Hoyer lift with integrated weighting system. Admission actual weight was used to present intakes in kcal/kg/day and to calculate adjusted body weight for obese patients. Underweight was defined by a body mass index (BMI) < 18 kg/m2, overweight by a BMI between 25 and 30 kg/m2 and obesity by a BMI > 30 kg/m2.
The following nutritional data were collected daily during the 10 first days of ICU stay: volume (mL/day) and route of administration of nutrition support (enteral, parenteral or both), volume of propofol infusion (mL/day) and intravenous glucose (mL/day), prescription of vitamin therapy and trace elements (type and volume in mL). We then calculated the patients’ total energy and protein intakes per kilogram of body weight received by patients, from both nutritional and non-nutritional solutions. Non-nutritional calories were calculated from both the daily propofol and glucose intakes. Propofol accounted for 1.1 kcal/ml and dextrose for 4 kcal/g. Calculated non-nutritional energy was added to energy received through enteral and/or parenteral nutrition and presented as ‘total caloric intake.’ Regarding obese patients, the nutritional intake per kilogram was based on their adjusted body weight (BW) (ideal BW + 0.25 × (actual BW − ideal BW) [25]. The ideal BW was based on the patient's height at a BMI of 25 kg/m2. We considered that patients reached the recommended energy and protein targets if their intakes were above 25 kcal/kg/day and 1.3 g/kg/day, respectively [14]. To consider the progressive rise in energy and protein intakes, instead of using an average intake smoothed over the follow-up, patients were considered to have reached the target if the total daily intake observed during the nutritional follow-up was above the guideline threshold at least once. Early nutrition support was defined as the administration of any nutritional solution, enteral and/or parenteral, during the first 48 h after ICU admission, as described in international guidelines [14, 15].
Specific information concerning organ support and critical care therapies was collected daily: invasive mechanical ventilation, use of neuromuscular blocking agents, vasopressors and sedation. Digestive tract events were also noted every day, including bowel movements, emesis and diarrhoea. Feeding intolerance was defined as the occurrence of emesis and/or diarrhoea concomitant with enteral nutrition administration.
The collected patient outcomes were the total duration of both invasive and noninvasive mechanical ventilation, length of ICU stay, ICU mortality and day-28 (D28) mortality.
Statistical analysis
We first described the demographic characteristics, use of organ support and patient outcomes in the study population. We then studied the nutritional intake received during the nutritional follow-up and the incidence of digestive complications or feeding intolerance. For patients discharged from the ICU, lost to follow-up or who died within the first 10 days of the ICU stay, nutritional data were analysed for the available days. Energy and protein mean intakes were calculated considering the number of days of follow-up. Missing data were marginal in our cohort (< 5%).
Then, we explored factors associated with the prescription of early nutrition support using univariate and multivariable analyses. Early nutrition was studied first as a binary variable (yes/no) and then in three categories (early enteral (EN), early parenteral (PN) and no early nutrition). As patients treated with mixed early nutrition (parenteral and enteral) had the same demographic and nutritional characteristics as those treated with early parenteral alone, they were included in the early parenteral group. The covariates included in the models were selected based on an a priori hypothesis according to the literature and the univariate analysis. For the binary variable (yes/no), we used a multivariable logistic model that included the following factors: age, sex, admission diagnosis, BMI and admission SOFA score. When early nutrition was explored in three categories (EN/PN/none), we used a multinomial, multivariable logistic regression analysis that included the following variables: age, sex, admission diagnosis, early invasive ventilation and early vasopressors. Separate organ support was chosen in this model to assess the specific influence of invasive mechanical ventilation on the choice of nutrition route. The reference category was ‘no early nutrition support.’ Results are reported as crude (ORs) and adjusted odds ratios (aORs) with a 95% confidence interval (95% CI). Multivariable model selection was performed using a two-way stepwise procedure with the aim of minimising the Akaike information criterion (AIC). We assessed multicollinearity between variables by computing the variance inflation factor (VIF) and using the Farrar–Glauber test. The goodness of fit was studied using the Hosmer–Lemeshow test.
Finally, we explored the association between early nutrition support (binary and in three categories) and 28-day mortality with univariable logistic regression and multivariable, multilevel analysis with a random effect on the ICU that admitted the patient (patient in the first level and centre of inclusion in the second). To rule out indication bias concerning early nutrition support, multivariable analyses were also performed using a propensity score. The propensity to belong to the early nutrition group was modelled by the nonparametric gradient boosting machine learning algorithm included in the Twang package [26]. Confounding factors included in the propensity score were age, sex, admission diagnosis, BMI and SOFA score at admission. The balance of the propensity model was assessed by the standardised effect size of the variables (Additional file 1: Fig. S1). Standardised effects of less than 0.20 were considered low (better balance), 0.40 as moderate and 0.60 as large. The weights calculated from the propensity score were used for the weighting of the multivariable logistic regression. In the weighted population, it was possible to assess the association with 28-day mortality in a pseudo-population in which the characteristics of subjects receiving or not receiving early nutrition were balanced. We chose to use the double robust approach to lower the risk of bias relative to the distribution difference of studied cofactors, which may persist even after propensity score weighting [27]. Accordingly, we adjusted our propensity-weighted (PW) regression model for all covariates included in the propensity score model.
Subgroup analyses were performed to assess the strength of the association between early nutrition support and 28-day mortality in specific populations: male/female, age ≤ or > 65 y.o., medical or surgical admission diagnosis, BMI range (underweight, standard, overweight, obese) and admission SOFA score ≤ or > 8. We modified the multivariable multilevel logistic regression analysis in each subgroup analysis by removing the respective subgroup variable. These results are presented as a forest plot.
Variables were compared between groups by Fisher’s exact and Chi-squared tests for categorical variables, and by a Mann–Whitney or Student’s t-test according to the normality of quantitative variables, as assessed by a Shapiro–Wilk test. Comparisons of more than two groups were conducted with one-way ANOVA or the Kruskal–Wallis test. The tests were two-sided, with an alpha risk α = 0.05. Results are given as the median (25th–75th percentiles) or mean (standard deviation) for quantitative variables, as appropriate, and the number of patients (with percentage proportion) for qualitative variables. A P-value less than 0.05 was considered statistically significant. R software (version 4.1.2, GUI 1.77 for Macintosh, GNU and GPL licences, The R Project for Statistical Computing, Vienna, Austria) and the RStudio interface (version 2022.02.0, Boston, MA, USA) were used to perform the statistical analyses.
Results
Characteristics of the study population and nutrition management
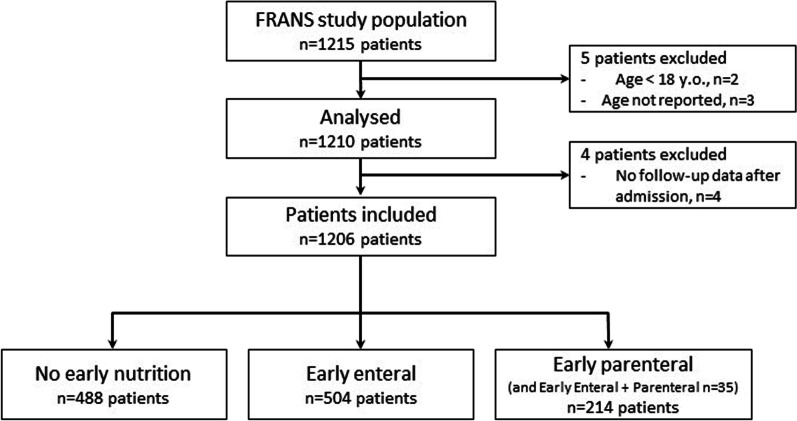
Overall, 1206 patients were included in the present study (Fig. 1). Invasive mechanical ventilation was used for 979 (81.2%) patients for a median duration of 7 [3–15] days. The median ICU length of stay was 10 [6–20] days. The overall ICU mortality was 18.5% (n = 223), and the D28 mortality was 18.8% (n = 226) (Table 1). Our hospital mortality prediction based on the admission SAPS II score was 32%.
Fig. 1.
Flowchart of inclusion and early-nutrition-type distribution
Table 1.
Characteristics of the study population, nutrition management and patients’ overall outcomes according to the 28-day mortality
| Overall (n = 1206) | Survivors (n = 975) | Non-survivors (n = 226) | P value | |
|---|---|---|---|---|
| Patient admission characteristics | ||||
| Age (years) | 62.9 [51.2, 72.8] | 61.6 [50.3, 71.4] | 66.5 [58.1, 76.6] | < 0.001 |
| Height (cm) | 170.0 [165.0, 176.0] | 170.0 [164.0, 176.0] | 170.0 [165.0, 175.0] | 0.712 |
| Weight (kg) | 75.0 [65.0, 87.0] | 75.0 [65.0, 87.0] | 75.0 [63.0, 85.0] | 0.174 |
| Sex (%) | ||||
| Female | 393 (32.6) | 324 (33.2) | 67 (29.6) | 0.338 |
| Male | 813 (67.4) | 651 (66.8) | 159 (70.4) | |
| Admission type (%) | ||||
| Surgical | 603 (50.0) | 535 (54.9) | 65 (28.8) | < 0.001 |
| Medical | 603 (50.0) | 440 (45.1) | 161 (71.2) | |
| BMI (kg/m2) | 25.7 [22.7, 29.7] | 25.7 [22.8, 29.8] | 25.3 [22.7, 28.6] | 0.123 |
| BMI range (%) | ||||
| < 18 | 37 (3.2) | 27 (2.9) | 10 (4.7) | 0.207 |
| 18–25 | 477 (41.1) | 385 (40.9) | 91 (42.5) | |
| 25–30 | 369 (31.8) | 295 (31.3) | 72 (33.6) | |
| > 30 | 277 (23.9) | 234 (24.9) | 41 (19.2) | |
| Country (%) | ||||
| France | 1003 (83.2) | 817 (83.8) | 182 (80.5) | 0.279 |
| Belgium | 203 (16.8) | 158 (16.2) | 44 (19.5) | |
| University hospital (%) | ||||
| Yes | 1060 (87.9) | 870 (89.2) | 187 (82.7) | 0.010 |
| No | 146 (12.1) | 105 (10.8) | 39 (17.3) | |
| Severity and organ support | ||||
| Admission SAPS II score | 44.0 [33.0, 57.0] | 42.0 [32.0, 54.0] | 53.0 [41.8, 65.3] | < 0.001 |
| Admission SOFA score | 8.0 [5.0, 11.0] | 8.0 [4.0, 10.0] | 9.0 [7.0, 12.0] | < 0.001 |
| Admission APACHE II score | 19.0 [13.0, 24.0] | 18.0 [13.0, 23.0] | 22.0 [17.0, 28.0] | < 0.001 |
| Early vasopressors (%) | 754 (62.6) | 572 (58.8) | 180 (79.6) | < 0.001 |
| Early IMV (%) | 870 (72.3) | 684 (70.3) | 182 (80.9) | 0.002 |
| Sedation (%) | 844 (70.0) | 649 (66.6) | 191 (84.5) | < 0.001 |
| NMBA (%) | 216 (17.9) | 156 (16.0) | 59 (26.1) | 0.001 |
| Vasopressors (%) | 818 (67.8) | 615 (63.1) | 200 (88.5) | < 0.001 |
| IMV (%) | 979 (81.2) | 776 (79.6) | 200 (88.5) | 0.003 |
| NIMV (%) | 408 (33.8) | 347 (35.6) | 60 (26.5) | 0.012 |
| Early nutritional intake | ||||
| Timing of enteral after admission (hours) | 37.6 [24.2, 64.6] | 37.1 [24.2, 63.8] | 39.9 [24.5, 72.2] | 0.331 |
| Early nutrition (%) | ||||
| None | 488 (40.5) | 416 (42.7) | 71 (31.4) | 0.020 |
| EN | 504 (41.8) | 392 (40.2) | 110 (48.7) | |
| EN + PN | 35 (2.9) | 26 (2.7) | 8 (3.5) | |
| PN | 179 (14.8) | 141 (14.5) | 37 (16.4) | |
| Total early caloric intake (kcal/kg/day) | 10.03 [4.81, 18.09] | 9.77 [4.59, 17.39] | 11.37 [6.25, 20.53] | 0.006 |
| Early non-nutritional calories (kcal/kg/day) | 3.16 [1.25, 5.63] | 3.13 [1.25, 5.56] | 3.23 [1.29, 6.08] | 0.471 |
| Early protein (g/kg/day) | 0.24 [0.00, 0.64] | 0.21 [0.00, 0.63] | 0.37 [0.00, 0.72] | 0.005 |
| 10-day nutritional intake and adverse effects | ||||
| Enteral nutrition (%) | 753 (62.4) | 579 (59.4) | 170 (75.2) | < 0.001 |
| Parenteral nutrition (%) | 406 (33.7) | 323 (33.1) | 80 (35.4) | 0.567 |
| Total Caloric intake (kcal/kg/day) | 16.94 [7.18, 22.82] | 16.08 [6.17, 22.59] | 18.43 [11.85, 23.89] | < 0.001 |
| Non-nutritional calories (kcal/kg/day) | 2.16 [1.08, 3.92] | 2.11 [1.06, 3.85] | 2.53 [1.21, 4.28] | 0.033 |
| Protein intake (g/kg/day) | 0.62 [0.17, 0.91] | 0.59 [0.09, 0.90] | 0.67 [0.38, 0.98] | 0.002 |
| Diarrhoea (%) | 322 (26.7) | 262 (26.9) | 59 (26.1) | 0.880 |
| Bowel movement (%) | 969 (80.3) | 797 (81.7) | 168 (74.3) | 0.015 |
| Emesis (%) | 186 (15.4) | 144 (14.8) | 40 (17.7) | 0.318 |
| Feeding intolerance (%) | 257 (34.0) | 195 (33.7) | 59 (34.7) | 0.876 |
Results are presented as the median (25th–75th percentiles) for quantitative variables and patient number (column proportion) for qualitative variables
BMI body mass index, SAPS Simplified Acute Physiology Score, SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology and Chronic Health Evaluation, NMBA neuromuscular blocking agents, IMV invasive mechanical ventilation, NIMV noninvasive mechanical ventilation
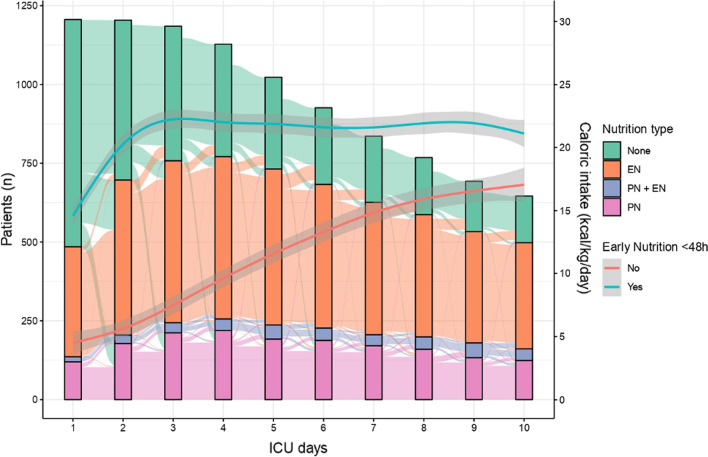
During the follow-up, 954 (79.1%) patients received nutrition support, with 753 (62.4%) patients receiving at least one day of enteral and 406 (33.7%) parenteral nutrition. Mixed nutrition (EN + PN) during the follow-up was provided to 158 patients (13.1%). The rate of delivery of nutrition support increased gradually from day 1 to day 10; enteral nutrition was the predominant route. The median timing of enteral introduction was at 38 [24–65] hours. The maximum number of patients receiving parenteral nutrition was observed on D4 of the ICU stay. When early nutrition was administered, the amount of energy provided increased until day 3 and remained stable thereafter. In the absence of early nutrition, intakes increased linearly and progressively over the follow-up period (Fig. 2). Daily energy and protein targets were reached at least once during the nutritional follow-up for 56% and 30% of all included patients, respectively. Gastrointestinal complications were frequent, with at least one occurrence of emesis or diarrhoea in 15.4% and 26.7% of the overall patients, respectively. Feeding intolerance occurred in 34% of ICU patients receiving enteral nutrition.
Fig. 2.
Ten-day evolution of early nutrition type and caloric intake. Alluvial plot showing the trend for the distribution of the different nutrition types per day for the 10-day follow-up period (left y-axis represents the number of patients). Introduction of nutrition support increased gradually from day 1 to day 10; enteral nutrition was the predominant route. Almost half of the patients received no nutrition support during the first two days. The peak number of patients receiving parenteral nutrition was observed on D4. Mixed nutrition (EN + PN) remained in the minority during the 10-day follow-up. The blue and red curves represent the energy intake trends for patients who received early nutrition support and those who did not, respectively (right y-axis expresses the amount of energy in calories per kilo per day). When early nutrition was administered, an energy intake plateau was reached on day 3 and remained stable thereafter. In the absence of early nutrition, intakes increased linearly and progressively over the 10 days. EN enteral nutrition, EN + PN simultaneous enteral and parenteral nutrition, PN parenteral nutrition, ICU intensive care unit
Early nutrition and associated factors
Among the 718 (59.5%) patients who received early nutrition, initial enteral nutrition was administered to 504 patients (41.8%). Parenteral nutrition was the primary route in 214 patients (17.7%; including 35 patients who had mixed early EN + PN). Compared to those who received initial EN, patients receiving initial PN had higher 48-h energy (19.67 [14.30, 26.88] vs. 14.56 [9.78, 20.77]) and protein intakes (0.75 [0.45, 1.07] vs. 0.49 [0.27, 0.80]) (Additional file 3: Table S1). Overall early non-nutritional energy accounted for 3.16 [1.25, 5.63] kcal/kg/day.
Obese (aOR 0.71, 95% CI 0.52–0.97) and overweight patients (aOR 0.62, 95% CI 0.47–0.83) were less likely than patients with BMIs < 25 kg/m2 to receive early nutrition, whereas the SOFA score was positively associated with early nutrition support (aOR 1.07, 95% CI 1.04–1.1) (Additional file 3: Table S2).
A surgical diagnosis at admission was positively associated with the prescription of early parenteral nutrition (aOR 1.51, 95% CI 1.07–2.11) and negatively associated with enteral nutrition (aOR 0.7, 95% CI 0.53–0.92). Early invasive mechanical ventilation was significantly associated both with early enteral (aOR 9.84, 95% CI 6.54–14.81) and early parenteral nutrition (aOR 1.72, 95% CI 1.17–2.51). Early use of vasopressors was significantly associated with early enteral but not early parenteral nutrition (Additional file 3: Table S3).
Patients receiving early nutrition support by any route had significantly longer durations of invasive mechanical ventilation and longer ICU lengths of stay than patients who did not receive early nutrition (Table 2).
Table 2.
Outcomes of patients with early nutrition and according to the type of early nutrition
| No early nutrition (n = 488) | Early enteral (n = 504) | Early parenteral (n = 214) | P value† | |
|---|---|---|---|---|
| Duration of IMV (days) | 4.0 [2.0, 10.0] | 10.0 [5.0, 18.0] | 7.00 [3.0, 13.0] | < 0.001 |
| Duration of NIMV (days) | 3.0 [2.0, 6.0] | 3.0 [2.0, 4.0] | 3.00 [2.0, 5.0] | 0.017 |
| Ventilator-free days D28 (days) | 5.0 [2.0, 11.0] | 3.0 [1.0, 8.0] | 5.00 [2.0, 10.0] | < 0.001 |
| ICU LOS | 8.0 [5.0, 16.0] | 13.5 [7.0, 24.0] | 12.00 [7.0, 20.0] | < 0.001 |
| 28-day mortality | 71 (14.6) | 110 (21.9) | 45 (21.2) | 0.008 |
| ICU mortality | 61 (12.5) | 119 (23.7) | 43 (20.1) | < 0.001 |
Results are presented as the median (25th–75th percentiles) for quantitative variables and patient number (proportion) for qualitative variables. N = 1206
IMV invasive mechanical ventilation, NIMV noninvasive mechanical ventilation, LOS length of stay
†One-way ANOVA or Kruskal–Wallis between the three groups (no early, early enteral, early parenteral) according to the variable normality
Association between initial nutrition support and patient mortality
Patients who died within 28 days were significantly older, had more frequently a medical admission diagnosis and had significantly higher initial SOFA scores. The proportion of non-survivors at D28 was increased among patients receiving early nutrition by any route, and among those receiving early enteral nutrition, compared to those without nutrition support. ICU survivors had significantly lower early energy and protein intakes (Table 1).
In the univariate analysis, early nutrition, by any route, was significantly associated with increased 28-day mortality. This association remained significant in the multivariable, multilevel analysis (aOR 1.56, 95% CI 1.11–2.2) and the propensity-weighted model (aOR 1.05, 95% CI 1.00–1.10, p = 0.031). Enteral nutrition was significantly associated with D28 mortality in the multilevel analysis (aOR 1.5, 95% CI 1.04–2.17), as well as in the propensity-weighted analysis (aOR 1.06, 95% CI 1.01–1.11, p = 0.03). Early parenteral nutrition, meanwhile, was associated with 28-day mortality in the multilevel analysis (aOR 1.72, 95% CI 1.07–2.77) but not in the propensity-weighted analysis (aOR 1.04, 95% CI 0.98–1.11, p = 0.203) (Table 3).
Table 3.
Association between early nutrition and 28-day mortality
| Variable | Crude* N = 1147 | Multilevel analysis† N = 1147 | Propensity-weighted cohort‡ N = 1147 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Binary early nutrition variable | ||||||
| Early nutrition | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.69 (1.23–2.34) | 0.001 | 1.56 (1.11–2.2) | 0.011 | 1.05 (1–1.1) | 0.031 |
| Model 2 with early nutrition variable including nutrition type | ||||||
| Early nutrition | ||||||
| None | 1 | 1 | 1 | |||
| Enteral | 1.7 (1.22–2.4) | 0.002 | 1.5 (1.04–2.17) | 0.031 | 1.06 (1.01–1.11) | 0.03 |
| Parenteral | 1.66 (1.07–2.55) | 0.021 | 1.72 (1.07–2.77) | 0.027 | 1.04 (0.98–1.11) | 0.203 |
BMI body mass index, SOFA Sequential Organ Failure Assessment
*Univariable logistic regression
†Multilevel, multivariable analysis with a random effect on the centre of inclusion and adjustment of age, sex, admission diagnosis type, BMI range and admission SOFA score
‡Propensity-weighted model with adjustment of age, sex, admission diagnosis type, BMI range and admission SOFA score
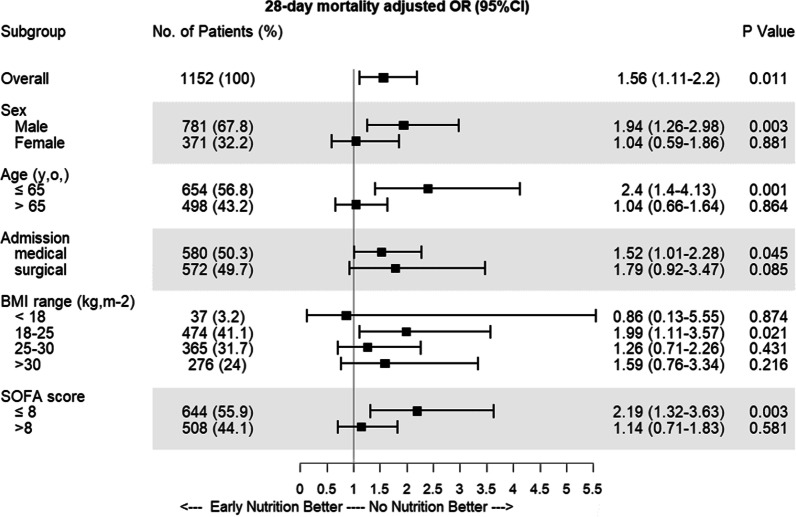
In a subgroup analysis with the multilevel multivariable models, the association between early nutrition and 28-day mortality was strongest in male patients (aOR 1.94, 95% CI 1.26–2.98), those younger than 65 y.o. (aOR 2.4, 95% CI 1.4–4.13), with a BMI between 18 and 25 kg/m2 (aOR 1.99, 95% CI 1.11–3.57) or a SOFA score below 8 (aOR 2.19, 95% CI 1.32–3.63) (Fig. 3).
Fig. 3.
Early nutrition effects in different subgroups. Forest plot depicting the adjusted odds ratios (ORs) from a multilevel, multivariable analysis with a random effect on the centre of inclusion and adjustment of age, sex, admission diagnosis type, body mass index (BMI) range and admission Sequential Organ Failure Assessment (SOFA) score. The association between early nutrition and mortality at day 28 is assessed in subgroups according to sex, age, type of admission, BMI and SOFA score
Regarding early macronutrient intakes, a significantly higher mortality risk was found in patients receiving any amount superior to 6 kcal/kg/d of energy and to 0.3 g/kg/d of protein. A potential dose-dependent effect was observed with early protein intake, with an increase in the mortality risk associated with increasing amount of protein/kg/day (aOR 1.43, 95% CI 1–2.05 for 0.3–0.9 g/kg/day and aOR 1.94, 95% CI 1.25–2.97 for > 0.9 g/kg/day) (Additional file 2: Fig. S2).
Discussion
In this prospective observational study, almost two-thirds of the critically ill patients received early nutrition, primarily by the enteral route, as suggested by nutrition guidelines; however, energy and protein targets were met in only half and one-third of the patients, respectively. Early nutrition was more frequently observed in patients with multiple organ failure and less frequently in overweight and obese patients.
The administration of early nutrition by any route was significantly associated with increased 28-day mortality. This association was stronger in younger patients and those with less severe organ failure. Compared to patients who received no early nutrition, early enteral and parenteral nutrition appeared to be associated with increased mortality in the multivariable multilevel analysis; these associations remained significant in the propensity-weighted analysis for early enteral but not early parenteral nutrition. Situated within the literature, the four key findings of this study are as follows:
First, our study of nutrition practices revealed that more than three-quarters of the patients received nutrition support in the ICU and that their energy and especially protein intakes were below recommended targets. These results are consistent with those reported in recent nutrition trials [28]. A possible explanation might be the difficulties in reaching energy targets via the enteral route, chosen for 60% of our patients, with one-third suffering from feeding intolerance. Furthermore, the overall intakes may have been lower than in previous studies given that the reported volumes were the ones actually received by patients and not just the prescribed ones, even though we have taken into account the non-nutritional calories. Indeed, the difference between prescribed and actual intakes in the literature ranges from 10 to 30% due to digestive intolerance, airway management and organisational constraints [29, 30]. Regarding factors associated with early nutrition prescription, we observed that overweight and obese patients were less likely to receive early nutrition than patients with BMIs < 25 kg/m2. Similar results were previously found in an observational study reporting delayed nutrition support in obese critically ill patients [31]. This might result from the erroneous assumption that overweight and obese patients have sufficient resources to withstand the hypercatabolism associated with ICU stress. However, these patients may suffer from sarcopenic obesity at admission, which can worsen with protein malnutrition and ICU-acquired muscle weakness [32]. Recent data showing a protective effect of adipose tissue on sarcopenia attest to the complexity of energy metabolism in these patients and the need for further research [33–35].
Second, we found that early nutrition support is associated with day-28 mortality. This result could be explained by the risk of overnutrition during the acute phase of critical illness due to the uncontrollable endogenous energy produced by the stressed organism [36]. Indeed, the abrupt rise in intake in the ‘early nutrition’ group, which may be considered as early full-feeding, could then explain the deleterious effects observed. Moreover, in the absence of indirect calorimetry-based prescriptions, the risk of overnutrition in the acute phase is even higher, especially in patients treated with neuromuscular blocking agents, a therapy known to decrease basal metabolic rate. In contrast, after D4, Heidegger et al. demonstrated a beneficial effect of optimising the energy intake by individualising intakes using indirect calorimetry [37]. Despite a low level of evidence, generalisation of indirect calorimetry may help to estimate the intensity of basal metabolism after clinical stabilisation and to prevent the prescription of excessive amounts of energy [36, 38–40]. Furthermore, early nutrition could also inhibit the processes of autophagy—a survival mechanism that ensures the elimination of cellular waste and preservation of mitochondrial functions—and thereby limit the natural stress response to injury [41–43]. Finally, another explanation could be the occurrence of a refeeding syndrome, resulting from the premature introduction of high energy intakes in patients at risk. The latter hypothesis, although impossible to confirm in the absence of biological data in our cohort, seems plausible in view of two recent studies demonstrating the harmful effects of excessive and non-progressive intakes in patients with refeeding hypophosphatemia [44, 45]. Such findings, if confirmed in RCTs, could challenge our current practices on early nutrition in the ICU. In our study, patients not receiving nutrition support in the first 48 h had energy intakes from intravenous dextrose and propofol accounting for nearly 5 kcal/kg/day, close to the levels considered to be permissive underfeeding in recent RCTs [7, 46]. The negative effect of early nutrition seemed to prevail in younger patients and those with lower SOFA scores, which are characteristics of low nutritional risk in the NUTRIC score developed by Heyland et al. [47]. Early nutrition in patients at low nutritional risk (low NUTRIC score) exposes them to the risk of overfeeding and increased morbi-mortality. Our results are in conflict with those of Ortiz-Reyes et al. who found an association between early enteral and a reduced risk of persistent organ dysfunction plus day-28 death in subgroups of patients with a NUTRIC score < 5 or a SOFA < 9 [18]. Beyond obvious differences in study design (nested cohort analysis of an ongoing registry-based RCT) and intervention (early enteral alone), the reasons for these conflicting results may lie in the study population, which was predominantly of medical admission (82%) in this study. Furthermore, no significant association was found between different energy target and mortality in subgroups of NUTRIC score in a post hoc analysis of the PERMIT trial [48]. These data reinforce the need for individualisation of intakes and the need for clinical signs and accurate biomarkers to predict the optimal timing at which the body is able to metabolise external nutrients [49].
Third, we observed increased 28-day mortality for any early macronutrient doses higher than 6 kcal/kg/d of energy and 0.3 g/kg/d of protein. In a post hoc analysis of the EPaNIC trial, Casaer et al. demonstrated that an early low dose of macronutrients was associated with the fastest recovery compared to any higher dose, whether administered parenterally or enterally [50]. This finding is in line with the recent update of the ASPEN guidelines suggesting a lower energy target, between 12 and 20 kcal/kg/d, during the first week of the ICU [11]. A similar observation was made by Servia-Goixart et al. in a multicentre prospective study that included 639 critically ill patients, where a multivariable analysis adjusted for patient characteristics and severity showed that a higher mean energy intake was associated with mortality [51]. However, the authors reported a protective effect associated with the mean protein intake that did not match our results. A possible reason for these divergent findings may be that the protein intake was explored overall during the ICU stay, rather than focusing on the early phase as we did. Yet, early acute-illness-associated stress impairs protein–energy metabolism and its responsiveness to exogenous nutrients [52, 53]. Koekkoek et al. reported, in a recent retrospective study, a time-dependent association of protein intake with mortality: before day 3 in the ICU, a protein intake superior to 0.8 g/kg/day was associated with significantly higher 6-month mortality; after D3, a progressive increase protein provision was associated with an improvement in patients outcomes [54]. Compared to other macronutrients such as glucose and lipids, early administration of amino acids was associated with poor outcomes in a preplanned post hoc analysis of the PEPaNIC study [55]. The soon-to-be-published NUTRIREA-3 trial evaluating early low-energy, low-protein versus standard feeding in severe ICU-ventilated shock patients will add further light on these results [56].
Fourth, enteral nutrition was the most frequent route used to administer early nutrition support. We observed that early nutrition, through enteral and parenteral routes, was associated with higher mortality compared to no early nutrition; however, this effect was not found with early parenteral nutrition in our propensity-weighted analysis. In view of the small difference in OR for 28-day mortality between early enteral and early parenteral nutrition, it is essential to consider that this result may have stemmed from a lack of power due to the small number of patients receiving early parenteral nutrition in our study. Our results should not be misinterpreted as that parenteral nutrition is safe in high dosage and enteral is not. Progressive increase in energy should apply to both routes. The difference we observed between enteral and parenteral only encourages even more vigilance when prescribing early enteral. This association between mortality and early enteral nutrition is in contrast with the findings of recent studies. Indeed, the NUTRIREA-2 study did not find a significant difference in terms of mortality between early enteral and parenteral nutrition in ventilated patients with shock; however, the authors reported a higher incidence of bowel ischaemia and acute colonic pseudo-obstruction in the enteral group [19, 20]. Similarly, in our cohort, enteral intakes may have been pushed too quickly in the 'early nutrition' group, which may explain the detrimental association reported [57]. Pending additional data, early enteral nutrition administration should be thoroughly assessed and monitored in critically ill patients.
The strengths of our investigation include the large number of critically ill patients included in this European, binational, prospective observational study with detailed data on nutritional practices. Moreover, the study population shares similar demographic and clinical characteristics with another large, recent multicentric cohort of ICU patients; this supports the external validity of our observations [58]. Furthermore, the significant number of academic and non-academic participating centres provided a large panel of ICU patients, especially concerning their diagnoses of admission to the ICU. In contrast to registry-based studies, we reported the intakes actually received, not the prescribed ones, as well as the non-nutritional energy intakes. This precision in the acquisition of actual intakes allowed us to estimate, in the most precise manner, the level of macronutrients associated with a poor outcome. In addition, we presented several robust statistical approaches, propensity score weighting and multilevel models, to avoid potential indication bias or centre effect bias, frequently encountered in observational studies.
Nonetheless, the present study has several limitations. First, oral intake data were not collected due to the complexity of accurately estimating the energy intake from each food tray [59, 60]. This lack of data collection may only have led to the underestimation of the energy intake in a few patients given the high invasive mechanical ventilation rate and high severity scores we reported in our cohort. The oral route is barely proposed or used during the acute phase in critically ill patients due to well-known barriers including loss of appetite, dysphagia and general weakness [59, 61]. Second, we lacked the necessary data to estimate the nutritional risk using the Nutrition Risk Screening 2002 or the NUTRIC scores due to its limited use in clinical practice at the time of the survey [47, 62]. However, the report of such score may not have change our results considering recent study from Lew et al., which observed that the association between nutritional adequacy and 28-day mortality was independent of nutritional status [63]. Third, apart from emesis and diarrhoea, no other enteral-nutrition-related adverse events were reported, nor were the management strategies for feeding intolerance. Since the collection of these data, major advances have allowed clinicians to better characterise the severity of these complications using the Gastrointestinal Dysfunction Score and improve their management [64–66]. Fourth, we did not collect data on the staff involved in nutrition care. The presence of critical care dieticians in the ICU is known to be associated with significant improvements in macronutrient provision; however, their integration and precise role remain heterogeneous among units [67, 68]. Fifth, patients’ nutritional follow-up was limited to 10 days and outcomes to day-28. A growing number of articles in the literature insists on the relevance of nutritional support beyond the acute phase and the ICU stay [69, 70]. The definition of core outcomes for ICU nutritional trials now makes it possible to guide the choice of outcomes and to facilitate the comparison and interpretation of results [71]. Last, we did not report the energy requirements estimated by indirect calorimetry for our cohort, though this is recommended by the latest European Guidelines [14]. This choice was made to maximise the inclusion rate given the limited access to this technology in a significant proportion of participating centres.
Conclusions
In this prospective cohort study including 1206 critically ill patients, nutrition support was widely prescribed, mostly by the enteral route. The administration of early nutrition was associated with higher day-28 mortality in a propensity-weighted logistic regression analysis, particularly for the enteral route.
These observations suggest that early provision of high amounts of macronutrients during the ICU stay may be associated with poor outcomes. These data are in contrast with current recommendations, based on low-quality evidence, on early enteral nutrition in the absence of a contraindication. They may inform future RCTs aimed at finding the optimal timing and amount of extrinsic macronutrients during the first days of the ICU stay. Aware of the variability of nutritional practices throughout the hospital stay, future work should also focus on the post-ICU phase to optimise the overall patient pathway and rehabilitation. The ongoing INTENT trial evaluating a whole-hospital nutrition intervention, not limited to the ICU, will provide essential data to shape our future nutritional practices [72].
Supplementary Information
Additional file 1. Fig. S1: Propensity score balance. Comparisons of the absolute standardised mean differences (ASMDs) between the groups receiving early nutrition or not on selected covariates (age, sex, type of admission, BMI range and SOFA score at admission), before and after weighting. After propensity score weighting, the maximum ASMD decreases for all chosen covariates. The statistically significant difference between groups on each covariate is indicated by the solid circle. No significant difference persists after weighting. Standardised effects of less than 0.20 are considered low (better balance), 0.40 as moderate and 0.60 as large. A. Propensity score for the binary variable of early nutrition (yes/no). B. Multinomial propensity score for our three-factor variable (none/EN/PN).
Additional file 2. Fig. S2: Dose-dependent effect of early nutrition. Forest plots presenting the association between increasing the doses of calories (Figure 2A) and protein (Figure 2B) administered during the first 48 hours of the ICU stay and the mortality at 28 days. Adjusted odds ratios (aORs) were calculated using a multivariable logistic regression adjusted for age, sex, admission diagnosis type, BMI range and admission SOFA score; N=1147.
Additional file 3. Table S1: Characteristics according to early nutrition type. Table S2: Multivariable logistic analysis of factors associated with the administration of early nutrition support by any route (<48h). Table S3: Multivariable multinomial analysis of factors associated with the type of early nutrition.
Acknowledgements
We want to sincerely thank all members of the study group for their collaboration and motivation during the inclusion period. FRANS Study group:
Désiré Samba, Jean-Denis Moyer, Philippe Montravers, Nicolas Mongardon, Arnaud Meffert, Audrey De Jong, Fouad Belafia, Jérome Morel, Karim Asehnoune, Pierre-Joachim Mahé, Alain D’Hondt, Nicolas Paquot, Marc Leone, Michel Kaidomar, Ludovic Grech, Eliane Gouteix, Elise Barsam, Jacques Duranteau, Orianne Martinez.
Abbreviations
- aOR
Adjusted odds ratio
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- BMI
Body mass index
- EN
Enteral nutrition
- FRANS
French-Speaking ICU Nutritional Survey
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- NIMV
Noninvasive mechanical ventilation
- NMBA
Neuromuscular blocking agents
- NUTRIC
Nutrition risk in critically ill
- PN
Parenteral nutrition
- RCT
Randomised control trial
- SAPS II
Simplified Acute Physiology Score II
- SOFA
Sequential Organ Failure Assessment
Author contributions
T.L. conceived the study. All authors collected and collated patients’ data. G.T., P.B. and C.A. carried out data verification, cleaning and management. E.P. and M.P.B. conducted the statistical analysis. E.P., T.L., J.C.P., J.M.C. and M.P.B. drafted the manuscript and delivered a critical revision of the manuscript with improvements to the important intellectual content. All authors read and approved the final manuscript.
Funding
This study received funding from Baxter for the printing and distribution of the survey forms. The funder did not participate in designing or executing the trial, in analyses of the data or in writing the manuscript.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The study was approved by the French Committee for the Protection of Human Subjects in Biomedical Research Paris Ile de France IV Saint-Louis (IRB00003835—July 24th 2014) and by the Belgium ethics committee of Erasme Universitary Hospital (P2015/083—May 13th 2015). The access to health information was approved by the French Data Protection Committee (Commission Nationale Informatique et Libertés—915109—DR-2015-304—July 17th 2015). According to French Law, informed consent was not required; a non-opposition statement was obtained after ad hoc information was given to all patients/relatives [73]. Participants' confidentiality was strictly observed throughout the study using the anonymous unique serial number for each subject and restricting data only to the investigators. This study was retrospectively registered on November 5th 2015 (ClinicalTrial.gov, NCT02599948).
Consent for publication
All authors read and approved the final manuscript before publication.
Competing interests
E.P. received a research grant from Nestle Healthscience, teaching fees from Fresenius Kabi and congress reimbursement from Fresenius Kabi and Nutricia. T. L. received consultant’s fees from Fresenius Kabi.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emmanuel Pardo, Email: emmanuel.pardo@aphp.fr.
the FRANS study group:
Désiré Samba, Jean-Denis Moyer, Philippe Montravers, Nicolas Mongardon, Arnaud Meffert, Audrey De Jong, Fouad Belafia, Jérome Morel, Karim Asehnoune, Pierre-Joachim Mahé, Alain D’Hondt, Nicolas Paquot, Marc Leone, Michel Kaidomar, Ludovic Grech, Eliane Gouteix, Elise Barsam, Jacques Duranteau, and Orianne Martinez
References
- 1.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 2.Villet S, Chiolero RL, Bollmann MD, Revelly J-P, Cayeux RNM-C, Delarue J, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Correia MITD, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–239. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 4.Lew CCH, Wong GJY, Cheung KP, Chua AP, Chong MFF, Miller M. Association between malnutrition and 28-day mortality and intensive care length-of-stay in the critically ill: a prospective cohort study. Nutrients. 2017;10:10. doi: 10.3390/nu10010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong DH, Hong SB, Lim CM, Koh Y, Seo J, Kim Y, et al. Relationship between nutrition intake and 28-day mortality using modified NUTRIC score in patients with sepsis. Nutrients. 2019;11:1906. doi: 10.3390/nu11081906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldridge K, Donnelly K, Johnston A. Permissive underfeeding or standard enteral feeding in critically ill adults. J Intensive Care Soc. 2015;16:348–349. doi: 10.1177/1751143715607732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 8.Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr. 2011;93:569–577. doi: 10.3945/ajcn.110.005074. [DOI] [PubMed] [Google Scholar]
- 9.Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr. 2014;100:1337–43. doi: 10.3945/ajcn.114.088609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chelkeba L, Mojtahedzadeh M, Mekonnen Z. Effect of calories delivered on clinical outcomes in critically ill patients: systemic review and meta-analysis. Indian J Crit Care Med. 2017;21:376–90. doi: 10.4103/ijccm.IJCCM_453_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J Parenter Enter Nutr. 2022;46:12–41. doi: 10.1002/jpen.2267. [DOI] [PubMed] [Google Scholar]
- 12.Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017;43:1637–1647. doi: 10.1007/s00134-017-4880-3. [DOI] [PubMed] [Google Scholar]
- 13.Kreymann KG, Berger MM, Deutz NEP, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 15.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) J Parenter Enter Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 16.Padilla PF, Martínez G, Vernooij RWM, Urrútia G, Figuls MRI, Cosp XB. Early enteral nutrition (Within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD012340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz-Reyes L, Patel JJ, Jiang X, Coz Yataco A, Day AG, Shah F, et al. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: a nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit Care. 2022;26:173. doi: 10.1186/s13054-022-04047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reignier J, Boisramé-Helms J, Brisard L, Lascarrou J-B, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2) Lancet. 2018;391:133–143. doi: 10.1016/S0140-6736(17)32146-3. [DOI] [PubMed] [Google Scholar]
- 20.Piton G, Le Gouge A, Boisramé-Helms J, Anguel N, Argaud L, Asfar P, et al. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: a post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. 2022;6:66. doi: 10.1007/s00134-022-06637-w. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American Multicenter Study. J Am Med Assoc. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;66:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 25.Karkeck J. Adjusted body weight for obesity. Am Diet Assoc Ren Pract Gr Newsl. 1984;3:66. [Google Scholar]
- 26.Griffin BA, Ridgeway G, Mccaffrey D, Morral A, Burgette L. Toolkit for weighting and analysis of nonequivalent groups : a tutorial for the twang package [Internet]. 2013. p. 1–30. Available from: http://www.rand.org/statistics/twang.
- 27.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61:962–73. doi: 10.1111/j.1541-0420.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 28.Singer P, Cohen J. Nutrition in the ICU: proof of the pudding is in the tasting. Intensive Care Med. 2015;41:154–156. doi: 10.1007/s00134-014-3537-8. [DOI] [PubMed] [Google Scholar]
- 29.Salciute-Simene E, Stasiunaitis R, Ambrasas E, Tutkus J, Milkevicius I, Sostakaite G, et al. Impact of enteral nutrition interruptions on underfeeding in intensive care unit. Clin Nutr. 2021;40:1310–7. doi: 10.1016/j.clnu.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. 2012;27:702–713. doi: 10.1016/j.jcrc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Borel AL, Schwebel C, Planquette B, Vésin A, Garrouste-Orgeas M, Adrie C, et al. Initiation of nutritional support is delayed in critically ill obese patients: a multicenter cohort study. Am J Clin Nutr. 2014;100:859–866. doi: 10.3945/ajcn.114.088187. [DOI] [PubMed] [Google Scholar]
- 32.Wagenaar CA, Dekker LH, Navis GJ. Prevalence of sarcopenic obesity and sarcopenic overweight in the general population: the lifelines cohort study. Clin Nutr. 2021;40:4422–9. doi: 10.1016/j.clnu.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Segaran E, Wandrag L, Stotz M, Terblanche M, Hickson M. Does body mass index impact on muscle wasting and recovery following critical illness? A pilot feasibility observational study. J Hum Nutr Diet Off J Br Diet Assoc. 2017;30:227–35. doi: 10.1111/jhn.12401. [DOI] [PubMed] [Google Scholar]
- 34.Goossens C, Marques MB, Derde S, Vander Perre S, Dufour T, Thiessen SE, et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle. 2017;8:89–101. doi: 10.1002/jcsm.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019 doi: 10.1186/s13054-019-2506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udin I, Habisreutinger M, Tappy L, Schneider AG, Berger MM. Magnitude of gluconeogenesis and endogenous glucose production: Are they predictable in clinical settings? Clin Nutr. 2021;40:3807–3814. doi: 10.1016/j.clnu.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381:385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 38.Kerklaan D, Hulst JM, Verhoeven JJ, Verbruggen SCAT, Joosten KFM. Use of indirect calorimetry to detect overfeeding in critically ill children: finding the appropriate definition. J Pediatr Gastroenterol Nutr. 2016;63:445–50. doi: 10.1097/MPG.0000000000001197. [DOI] [PubMed] [Google Scholar]
- 39.Duan J-Y, Tsinghua B, Hospital C, Zheng W-H, Zhou H, Xu Y, et al. Energy delivery guided by indirect calorimetry in critically ill patients: a systematic review and meta-analysis. Crit Care. 2021;6:66. doi: 10.21203/rs.3.rs-125406/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nutritional Prescription: Use of indirect calorimetry vs. predictive equations. [cited 2022 Dec 6]. Available from: www.criticalcarenutrition.com.
- 41.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Güiza F, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96:E633–E645. doi: 10.1210/jc.2010-2563. [DOI] [PubMed] [Google Scholar]
- 42.Gunst J, Derese I, Aertgeerts A, Ververs E-J, Wauters A, Van den Berghe G, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41:182–194. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 43.Gunst J. Recovery from critical illness-induced organ failure: the role of autophagy. Crit Care. 2017;21:209. doi: 10.1186/s13054-017-1786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olthof LE, Koekkoek WACK, van Setten C, Kars JCN, van Blokland D, van Zanten ARH. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr. 2018;37:1609–1617. doi: 10.1016/j.clnu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3:943–952. doi: 10.1016/S2213-2600(15)00418-X. [DOI] [PubMed] [Google Scholar]
- 46.The National Heart and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network* L. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed]
- 47.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arabi YM, Aldawood AS, Al-Dorzi HM, Tamim HM, Haddad SH, Jones G, et al. Permissive underfeeding or standard enteral feeding in high-and low-nutritional-risk critically ill adults. Am J Respir Crit Care Med. 2017;195:652–62. doi: 10.1164/rccm.201605-1012OC. [DOI] [PubMed] [Google Scholar]
- 49.Stoppe C, Wendt S, Mehta NM, Compher C, Preiser J-C, Heyland DK, et al. Biomarkers in critical care nutrition. Crit Care. 2020;24:499. doi: 10.1186/s13054-020-03208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial. Am J Respir Crit Care Med. 2013;187:247–255. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 51.Servia-Goixart L, Lopez-Delgado JC, Grau-Carmona T, Trujillano-Cabello J, Bordeje-Laguna ML, Mor-Marco E, et al. Evaluation of nutritional practices in the critical care patient (The ENPIC study): Does nutrition really affect ICU mortality? Clin Nutr ESPEN. 2022;47:325–332. doi: 10.1016/j.clnesp.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Shaw JHF, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg. 1987;205:288–94. doi: 10.1097/00000658-198703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapple L-AS, van Gassel RJJ, Rooyackers O. Protein metabolism in critical illness. Curr Opin Crit Care. 2022;28:367–73. doi: 10.1097/MCC.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 54.Koekkoek WAC (Kristine., van Setten CH (Coralien., Olthof LE, Kars JCN (Hans., van Zanten ARH. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr 2019;38:883–90. [DOI] [PubMed]
- 55.Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, et al. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med. 2017;5:475–483. doi: 10.1016/S2213-2600(17)30186-8. [DOI] [PubMed] [Google Scholar]
- 56.Reignier J, Le Gouge A, Lascarrou JB, Annane D, Argaud L, Hourmant Y, et al. Impact of early low-calorie low-protein versus standard-calorie standard-protein feeding on outcomes of ventilated adults with shock: design and conduct of a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3) BMJ Open. 2021;66:11. doi: 10.1136/bmjopen-2020-045041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClave SA, Codner P, Patel J, Hurt RT, Allen K, Martindale RG. Should we aim for full enteral feeding in the first week of critical illness? Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2016;31:425–431. doi: 10.1177/0884533616653809. [DOI] [PubMed] [Google Scholar]
- 58.Hollinger A, Gayat E, Féliot E, Paugam-Burtz C, Fournier M-C, Duranteau J, et al. Gender and survival of critically ill patients: results from the FROG-ICU study. Ann Intensive Care. 2019;9:43. doi: 10.1186/s13613-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarden RJ, Sutton-Smith L, Boulton C. Oral intake evaluation in patients following critical illness: an ICU cohort study. Nurs Crit Care. 2018;23:179–85. doi: 10.1111/nicc.12343. [DOI] [PubMed] [Google Scholar]
- 60.Peterson SJ, Tsai AA, Scala CM, Sowa DC, Sheean PM, Braunschweig CL. Adequacy of oral intake in critically ill patients 1 week after extubation. J Am Diet Assoc. 2010;110:427–433. doi: 10.1016/j.jada.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Moisey LL, Pikul J, Keller H, Yeung CYE, Rahman A, Heyland DK, et al. Adequacy of protein and energy intake in critically ill adults following liberation from mechanical ventilation is dependent on route of nutrition delivery. Nutr Clin Pract. 2021;36:201–12. doi: 10.1002/ncp.10558. [DOI] [PubMed] [Google Scholar]
- 62.Kondrup J, Ramussen HH, Hamberg O, Stanga Z, Camilo M, Richardson R, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;66:321–36. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 63.Lew CCH, Wong GJY, Cheung KP, Fraser RJL, Chua AP, Chong MFF, et al. The association between nutritional adequacy and 28-day mortality in the critically ill is not modified by their baseline nutritional status and disease severity. Crit Care. 2019;23:222. doi: 10.1186/s13054-019-2500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reintam Blaser A, Padar M, Mändul M, Elke G, Engel C, Fischer K, et al. Development of the Gastrointestinal Dysfunction Score (GIDS) for critically ill patients—a prospective multicenter observational study (iSOFA study) Clin Nutr. 2021;40:4932–40. doi: 10.1016/j.clnu.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Preiser JC, Arabi YM, Berger MM, Casaer M, McClave S, Montejo-González JC, et al. A guide to enteral nutrition in intensive care units: 10 expert tips for the daily practice. Crit Care. 2021;25:1–13. doi: 10.1186/s13054-021-03847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, et al. Monitoring nutrition in the ICU. Clin Nutr. 2019;38:584–593. doi: 10.1016/j.clnu.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med. 2012;40:412–9. doi: 10.1097/CCM.0b013e31822f0ad7. [DOI] [PubMed] [Google Scholar]
- 68.Derouin E, Picard G, Kerever S. Dieticians’ practices in intensive care: a national survey. Clin Nutr ESPEN. 2021;45:245–51. doi: 10.1016/j.clnesp.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship: collaborating to improve post-ICU disability. Am J Respir Crit Care Med. 2011;15(183):962–964. doi: 10.1164/rccm.201012-2042ED. [DOI] [PubMed] [Google Scholar]
- 70.Ridley EJ, Parke RL, Davies AR, Bailey M, Hodgson C, Deane AM, et al. What happens to nutrition intake in the post-intensive care unit hospitalization period? An observational cohort study in critically ill adults. J Parenter Enter Nutr. 2019;43:88–95. doi: 10.1002/jpen.1196. [DOI] [PubMed] [Google Scholar]
- 71.Davies TW, van Gassel RJJ, van de Poll M, Gunst J, Casaer MP, Christopher KB, et al. Core outcome measures for clinical effectiveness trials of nutritional and metabolic interventions in critical illness: an international modified Delphi consensus study evaluation (CONCISE) Crit Care. 2022;26:240. doi: 10.1186/s13054-022-04113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridley EJ, Bailey M, Chapman M, Chapple L-AS, Deane AM, Hodgson C, et al. Protocol summary and statistical analysis plan for Intensive Nutrition Therapy comparEd to usual care iN criTically ill adults (INTENT): a phase II randomised controlled trial. BMJ Open. 2022;12:050153. [DOI] [PMC free article] [PubMed]
- 73.Toulouse E, Lafont B, Granier S, Mcgurk G, Bazin JE. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;66:883–5. doi: 10.1016/j.accpm.2020.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1: Propensity score balance. Comparisons of the absolute standardised mean differences (ASMDs) between the groups receiving early nutrition or not on selected covariates (age, sex, type of admission, BMI range and SOFA score at admission), before and after weighting. After propensity score weighting, the maximum ASMD decreases for all chosen covariates. The statistically significant difference between groups on each covariate is indicated by the solid circle. No significant difference persists after weighting. Standardised effects of less than 0.20 are considered low (better balance), 0.40 as moderate and 0.60 as large. A. Propensity score for the binary variable of early nutrition (yes/no). B. Multinomial propensity score for our three-factor variable (none/EN/PN).
Additional file 2. Fig. S2: Dose-dependent effect of early nutrition. Forest plots presenting the association between increasing the doses of calories (Figure 2A) and protein (Figure 2B) administered during the first 48 hours of the ICU stay and the mortality at 28 days. Adjusted odds ratios (aORs) were calculated using a multivariable logistic regression adjusted for age, sex, admission diagnosis type, BMI range and admission SOFA score; N=1147.
Additional file 3. Table S1: Characteristics according to early nutrition type. Table S2: Multivariable logistic analysis of factors associated with the administration of early nutrition support by any route (<48h). Table S3: Multivariable multinomial analysis of factors associated with the type of early nutrition.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.