Abstract
Bacillus cereus (B. cereus) endophthalmitis is a vision-threatening bacterial infection. Uncontrolled inflammatory responses are the hallmark of this disease which cause irreversible damage to the retina. We recently reported C-X-C chemokines as a vital modulators which impacted the pathogenesis of this disease. Here, we investigated the impact of two highly upregulated C-C chemokines, CCL2 and CCL3, on intraocular inflammation this disease. B. cereus was injected into the eyes of C57BL/6J (WT), CCL2−/−, and CCL3−/− mice to induce endophthalmitis. Infected eyes were examined for bacterial growth, retinal function, and inflammation. Bacterial growth in CCL2−/− and CCL3−/− mice were similar, but retained retinal function was greater in CCL2−/− and CCL3−/− eyes compared to that of C57BL/6J eyes. The retinal architecture of infected eyes of CCL2−/− mice were conserved for a longer period of time than in infected CCL3−/− eyes. Infected CCL2−/− and CCL3−/− eyes had less inflammation than did infected C57BL/6J eyes. Based on these results, we assessed the efficacies of intravitreal anti-CCL2 or anti-CCL3 with or without the antibiotic gatifloxacin. Compared to infected untreated eyes, there was significantly less inflammation and greater retention of retinal function in eyes treated with anti-CCL2 or anti-CCL3 with gatifloxacin. This study showed that B. cereus endophthalmitis in CCL2−/− mice had a better clinical outcome than in CCL3−/− mice. Intravitreal administration of anti-CCL2 and anti-CCL3 with gatifloxacin significantly reduced inflammation and provided protection of retinal function. These results suggest that CCL2 and CCL3 are prospective anti-inflammatory targets that should be tested along with other antibiotics for treating Bacillus and perhaps other forms of endophthalmitis.
Keywords: Bacillus cereus, Ocular infection, Inflammation, Immune responses, Chemokine, Endophthalmitis
1. Introduction
Endophthalmitis is one of the most severe acute microbial infections of the eye. Facilitated by accidental injury, postoperative surgery, or systemic infections, microbes can enter the posterior segment of the eye and cause infection (Callegan et al., 2007; Mursalin et al., 2020c). In this immune privileged site, robust inflammation is triggered via innate activation in response to these microbes, which can result in damage to the visual system (Miller et al., 2019). Clinical symptoms of endophthalmitis vary from manageable ocular inflammation to fulminant, rapidly developing, untreatable and blinding infections (Kitsche et al., 2020; Mirzania et al., 2021). Although microbial endophthalmitis can be initiated by both bacteria and fungi, the vast majority of reported culture-positive cases are caused by Gram-positive bacteria (Callegan et al., 2002b). Among all the organisms reported to be associated with endophthalmitis, cases caused by Bacillus spp. have one of the most severe and rapid courses, causing significant vision loss in one to two days (Mursalin et al., 2020c).
Bacillus spp. are Gram-positive, facultative anaerobes which are rod shaped and motile (Bottone, 2010; Drobniewski, 1993). This microbe’s characteristic spore formation allows Bacillus to disperse widely in nature, and it is commonly found in soil and is associated with plant matter (Ehling-Schulz et al., 2019; Lee et al., 2019). Bacillus as a pathogen is commonly associated with non-life-threatening intestinal diseases, but this organism has also been reported to cause meningitis, bacteremia, endocarditis, wound infections, pneumonia, and septicemia (Enosi Tuipulotu et al., 2021; Logan, 2012). In the eye, Bacillus replicates unimpeded in the vitreous, shedding cell wall components that are highly inflammogenic and secreting toxic products that can damage ocular tissues (Callegan et al., 1999b; Callegan et al., 2005; Coburn et al., 2020, 2021; Livingston et al., 2021). Bacillus cell wall components such as peptidoglycan, pili, and S-layer protein trigger inflammation, significantly contributing to the progression of disease pathogenesis (Callegan et al., 1999a; Callegan et al., 2017; Mursalin et al., 2019; Parkunan et al., 2014). Toxicity and quorum sensing regulation of Bacillus virulence factors also impact pathogenesis (Callegan et al., 2002a; Callegan et al., 2003). From the host point of view, innate receptors (Toll-like receptors TLR2 and TLR4) and their adaptor molecules (TRIF and MyD88) have been reported to be important for disease severity (Novosad et al., 2011; Parkunan et al., 2015). Inflammogenic bacterial components activate TLR2 and TLR4 pathways, which in turn trigger the synthesis of inflammatory mediators (cytokines and chemokines) that are major contributors to inflammation and endophthalmitis pathogenesis (Mursalin et al., 2020a). Mediator expression is elevated in parallel with a declining clinical outcome in infected mouse eyes (Miller et al., 2019; Mursalin et al., 2020c; Ramadan et al., 2006). Among the mediators whose expression parallels evolving blood-retinal barrier (BRB) permeability and inflammation during Bacillus endophthalmitis are interleukins 1-beta (IL-1β) and 6 (IL6), tumor necrosis factor alpha (TNF-α), and a number of C-X-C and C-C chemoattractants (Coburn et al., 2020; Moyer et al., 2009; Ramadan et al., 2008). To date, our studies with transgenic knockout models revealed that TNF-α, CXCL1, CXCL2, and CXCL10 were important intraocular inflammatory mediators during Bacillus endophthalmitis (Mursalin et al., 2021a; Parkunan et al., 2016; Ramadan et al., 2008). A similar cohort of mediators have been detected in human eyes with endophthalmitis (Hao et al., 2016).
As a primary function, chemokines act as recruiters of immune cells to the site of infection (Sokol and Luster, 2015). These immune cells attempt to control the infection by producing toxic products that are detrimental for invading microbes and, unfortunately, can damage ocular tissues (Miller et al., 2019). Polymorphonuclear leukocytes were observed as rapidly as 4 hours following infection with Bacillus, suggesting that recruiting mediators are synthesized prior to this time during infection (Ramadan et al., 2006). As inflammation evolves over time, mediator synthesis continues. In addition to C-X-C chemokine expression, we also reported elevated expression of C-C chemokines in mouse eyes infected with Bacillus (Coburn et al., 2018). At 10 hours following intraocular infection with Bacillus, we detected the expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and macrophage inflammatory protein 1-alpha (MIP-1α/CCL3) (Mursalin et al., 2020b). CCL2 and CCL3 are highly homologous and share similar functions to CXCL2, which, as noted above, contributes to inflammation in Bacillus endophthalmitis (Deshmane et al., 2009; Menten et al., 2002; Zhu et al., 2021).
Although the absence of certain C-X-C chemokines delayed disease progression in Bacillus endophthalmitis, inflammation in these eyes still progressed and eyes lost visual function (Mursalin et al., 2021a). This suggests the contribution of other mediators that might contribute to this response. Therefore, in this study, we hypothesized that during Bacillus endophthalmitis, a deficiency in or inhibition of CCL2 or CCL3 might abate inflammation and reduce infection severity. Our findings suggested that disease severity was significantly reduced in mice deficient in CCL2 or CCL3. Further, a therapeutic strategy utilizing anti-CCL2 or anti-CCL3 and a broad-spectrum bactericidal antibiotic provided a much-improved clinical outcome during experimental Bacillus endophthalmitis. Together, these results highlight the possibility of these C-C chemokines as prospective therapeutic targets.
2. Materials and Methods
2.1. Mice
The following strains of mice were used in these studies: breeding pairs of CCL2−/− (C57BL/6.129S4-Ccl2tm1Rol/J, 004434) and CCL3−/− (C57BL/6.129P2-Ccl3tm1Unc/J, 002687), and wild-type (WT) C57BL/6J (000664) mice (Jackson Laboratories, Bar Harbor ME). All mice were co-housed on a 12-hour on/off light cycle in biosafety level 2 conditions. Purchased and weaned mice of 8-10 weeks of age were co-housed for at least 2 weeks for microbiota equilibration. All procedures were done following guidelines of the Guide for the Care and Use of Laboratory Animals, the University of Oklahoma Health Sciences Center IACUC, and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Primary Neutrophil Isolation
Primary neutrophils were collected from bone marrow of C57BL/6J, CCL2−/−, and CCL3−/− mice. The MACS neutrophil isolation kit (Miltenyl Biotech, Gladbach, Germany) was used according to the manufacturer’s instructions. Briefly, bone marrow from the harvested femurs of C57BL/6J, CCL2−/−, and CCL3−/− mice was collected in separate tubes containing RPMI media (GIBCO/ThermoFisher, Waltham MA) supplemented with 10% fetal bovine serum (Sigma Aldrich, St. Louis MO). Harvested bone marrow was centrifuged at 100g for 10 minutes and washed with PBS (pH 7.2) supplemented with bovine serum albumin (0.5%) and EDTA (2 mM). Then, cells were enumerated and for every 5×107 cells, 200 μL of wash buffer and 50 μL neutrophil biotin-antibody cocktail were added. Cells were mixed well and incubated for 10 minutes at 4°C, then pelleted, resuspended, and incubated again for 4°C for 15 minutes in 100 μL of anti-biotin microbeads in 400 μL of wash buffer. After incubation, cells were washed and resuspended to a concentration of 108 cells per 500 μL of buffer. The MACS column and appropriate separator were selected based on the numbers of total cells and neutrophils. LS columns were placed inside the MACS separator. A collection tube was placed under each LS column and the columns were washed with 3 mL of buffer. Following the wash, new collection tubes were placed under the LS columns, and the entire 500 μL sample was loaded onto the columns. At the end of three washes, neutrophils were collected, counted, centrifuged at 100g for 10 minutes, and resuspended in RPMI medium (Mursalin et al., 2020a, 2021a).
2.3. Bacterial Phagocytosis Assay
The gentamicin exclusion assay was used to measure bacterial internalization. Briefly, the assay used primary neutrophils from C57BL/6J, CCL2−/−, and CCL3−/− mice to assess the effect of the absence of these chemokines on phagocytosis of B. cereus. Approximately 1×105 of neutrophils from each group of mice were incubated for 90 minutes with 20 MOI (~2×106) of B. cereus strain ATCC 14579. One aliquot of neutrophils from each group was washed and treated with gentamicin (200 μg/mL) for 60 minutes to kill any extracellular bacteria. Treated neutrophils were washed and centrifuged to remove residual gentamicin, and were lysed with 0.5% Triton X-100 to release phagocytosed B. cereus for enumeration. Another aliquot of neutrophils from each group of mice was washed, centrifuged, and lysed with 0.5% Triton X-100, and represented both phagocytosed and extracellular bacteria. As a control, approximately 106 B. cereus/mL were incubated with gentamicin (200 μg/mL) for 60 minutes. Colony forming units (CFU) of B. cereus were enumerated by serial dilution and plating (Mursalin et al., 2021a).
2.4. Experimental B. cereus Endophthalmitis
All animal experiments were performed with C57BL/6J, and CCL2−/− and CCL3−/− male and female mice. Mice were sedated using a cocktail of ketamine (85 mg/kg body weight; Ketathesia, Covetrus, Dublin, OH) and xylazine (14 mg/kg body weight; AnaSed, Akorn Inc., Decatur, IL). All mice were infected in the right eye mid-vitreous with 100 CFU Bacillus cereus/0.5 μl brain heart infusion media (BHI) using a sterile glass capillary needle (Mursalin et al., 2021b). At 8, 10, 12, 14 and 16 hours postinfection, electroretinography was performed to measure the retinal function. At these time points, mice were euthanized by CO2 inhalation and infected eyes were harvested for quantitation of viable intraocular B. cereus, neutrophil infiltration, and analysis of ocular architecture by histology, as described below.
2.5. Retinal Function Analysis
Electroretinography (ERG) is used clinically to diagnose disturbances in visual function by quantifying the electrical response of the retina following a light stimulus. Briefly, infected mice were adapted to dark conditions for at least six hours, sedated with ketamine/xylazine, and pupils were dilated with topical phenylephrine (Akorn, Inc.). Two gold wire electrodes were placed onto the corneas and reference electrodes were affixed to the forehead and tail. Retinal responses following stimulation via five flashes of white light (1200 cd s/m2) were recorded as A-wave (photoreceptor cell function) and B-wave (Muller cells, bipolar cells, and second-order neuronal function) amplitudes. Amplitudes of A- and B-waves of infected eyes were compared with that of uninfected fellow eyes of the same animal (Espion E2, Diagnosys LLC, Lowell MA), and reported as percent retinal function retained, as previously described (Mursalin et al., 2019; Novosad et al., 2011; Parkunan et al., 2015; Ramadan et al., 2006).
2.6. Intraocular Bacterial Viability
As previously described, viable intraocular B. cereus were quantified by track dilution. Mice were euthanized and infected eyes from all groups were harvested at specific time points. Eyes were then each homogenized in 400 μl PBS containing sterile 1-mm glass beads (BioSpec Products, Inc., Bartlesville OK). Eye homogenates were then serially diluted 10-fold in PBS and track pipetted onto BHI agar plates. Viable CFU were counted after overnight incubation at 37°C (Mursalin et al., 2019; Novosad et al., 2011; Parkunan et al., 2015; Ramadan et al., 2006).
2.7. Intraocular Inflammatory Cell Influx
As previously described, the extent of inflammatory cell influx into infected mouse eyes was semi-quantified using a myeloperoxidase (MPO) sandwich ELISA (Hycult Biotech, Plymouth Meeting PA). Infected eyes of euthanized mice were harvested at various time points and transferred into PBS supplemented with proteinase inhibitor cocktail (Roche Diagnostics, Indianapolis IN). Harvested eyes were then homogenized using 1-mm sterile glass beads (BioSpec Products, Inc.). Negative controls included homogenates of uninfected eyes (Mursalin et al., 2020b; Parkunan et al., 2014). The lower limit of detection for this assay was 2 ng/ml.
2.8. Histology
As previously described, infected eyes were harvested from euthanized mice at specific times and incubated in High Alcoholic Prefer fixative for 4 hours (Mursalin et al., 2019; Novosad et al., 2011; Parkunan et al., 2015). Eyes were then transferred to 70% ethanol and subsequently embedded in paraffin. The embedded eyes were then sectioned and stained with hematoxylin and eosin, and imaged using a Polaroid slide scanner.
2.9. Neutralization of CCL2 or CCL3 in vivo
As described above, C57BL/6J mice were anesthetized and eyes were infected with B. cereus. At 2 hours postinfection, mice were anesthetized with isoflurane and divided at random into the following treatment groups: Anti-CCL2 monoclonal antibody (Anti-CCL2; 250 ng anti-CCL2/JJE/MCP-1 IgG/0.5μl PBS, clone 123616; MAB479); Anti-CCL3 monoclonal antibody (Anti-CCL3; 250 ngAnti-CCL3/MIP-1alpha IgG/0.5μl PBS, clone 39624, MAB450); 1.25 μg gatifloxacin/0.5 μl PBS (GAT; Zymaxid 0.5%; Allergan, Inc., Irvine CA); 0.5 μl PBS containing 1.25 μg gatifloxacin and 250ng Anti-CCL2 (GAT+Anti-CCL2); 0.5 μl PBS containing 1.25 μg gatifloxacin and 250 ng Anti-CCL3 (GAT+Anti-CCL3), or isotype control antibody (Isotype; 0.5 μg nonspecific control IgG2A/0.5 μl, MAB 006, clone 54447l). All antibodies were purchased from R&D Systems (Minneapolis, MN), and working concentrations were chosen based on previous publications (Mursalin et al., 2021a; Parkunan et al., 2016). Another group of mice was left untreated to serve as controls. At 10 hours postinfection, viable B. cereus, retinal function, and intraocular inflammation were assessed, as described below (LaGrow et al., 2017; Mursalin et al., 2021a).
2.10. Statistics
GraphPad Prism 9 was used for the statistical analysis (GraphPad Software, Inc., La Jolla CA). The Mann-Whitney test was used for statistical comparisons, unless otherwise specified. p-values of < 0.05 were considered significant. Since results in male and female mice of C57BL/6J, CCL2−/−, and CCL3−/− were not different, we combined the male and female data in the results (Mursalin et al., 2021a; Parkunan et al., 2016).
3. Results
3.1. The absence of CCL2 or CCL3 did not affect Bacillus internalization by mouse primary neutrophils.
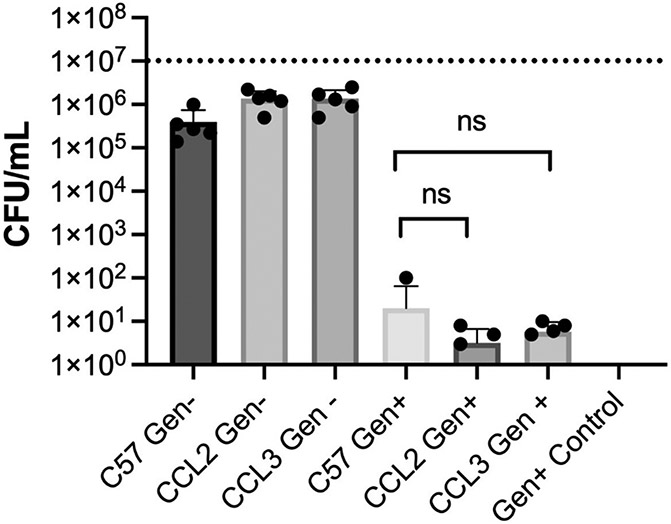
CCL2 and CCL3 chemokines recruit immune cells to an infected area. One key function of these newly recruited immune cells is ridding the infection site of invading microbes and foreign particles by phagocytosis. A gentamicin exclusion assay was used to measure bacterial internalization to determine whether the deficiency of CCL2 and or CCL3 in mice modified the phagocytic function of the neutrophil (Fig. 1). Isolated primary neutrophils from C57BL/6J, and CCL2−/− (P=0.5238) or CCL3−/− (P=0.2827) mice showed similar internalization of B. cereus. No bacteria were recovered upon incubation with gentamicin, suggesting that the B. cereus strain used was susceptible to the antibiotic. Taken together, these findings suggested that the lack of CCL2 and CCL3 did not alter the phagocytic function of mouse primary neutrophils.
Fig. 1: Absence of CCL2 or CCL3 did not affect Bacillus internalization by mouse primary neutrophils.
Primary neutrophils from C57BL/6J, CCL2−/−, and CCL3−/− mice were incubated with B. cereus strain ATCC 14579 for 90 minutes. Cells were then treated with gentamicin (Gen) for 60 minutes to kill external bacteria. Internalization of bacteria by CCL2−/−, and CCL3−/− neutrophils was not significantly different from that of C57BL/6J neutrophils. C57, C57BL/6J; Gen+, Gentamicin treated; Gen-, Gentamicin untreated. Values represent mean ± SEM of N ≥ 5 for at least two separate experiments; *P < 0.05. Dashed lines represent the initial bacterial inoculum. nsP≥0.05.
3.2. The absence of CCL2 or CCL3 did not modify intraocular Bacillus growth.
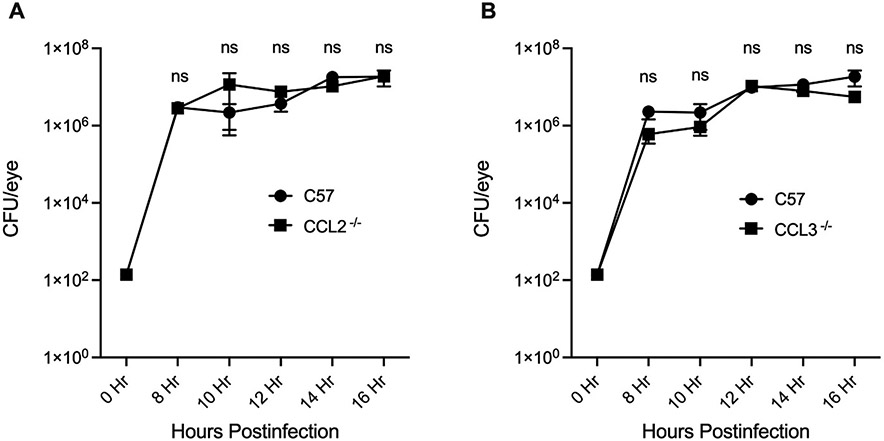
To determine whether a CCL2 or CCL3 deficiency modified the growth of B. cereus during endophthalmitis, viable intraocular B. cereus was quantified during infection in C57BL/6J, CCL2−/−, and CCL3−/− mouse eyes (Fig. 2A and 2B). The number of B. cereus in infected CCL2−/− mice eyes were statistically similar to infected C57BL/6J mouse eyes at all time points (P ≥ 0.05, Fig. 2A). Likewise, the numbers of B. cereus in CCL3−/− and C57BL/6J mouse eyes were also statistically similar at every time point (P ≥ 0.05, Fig. 2B). Together, these findings suggest that CCL2 or CCL3 deficiency did not modify intraocular Bacillus growth in mouse eyes.
Fig. 2: The absence of CCL2 or CCL3 did not modify intraocular Bacillus growth.
Intraocular B. cereus ATCC 14579 growth was not affected by the absence of CCL2 or CCL3. Eyes of CCL2 −/−, CCL3 −/−, and C57BL/6J (C57) male and female mice were infected with 100 CFU B. cereus. Eyes were harvested at designated time points and B. cereus were quantified. No significant differences in B. cereus burden were observed among mouse strains (P ≥ 0.05) (A and B). Values represent means ± SEM of n ≥ 6 eyes at each time point with at least 3 independent experiments. nsP≥0.05.
3.3. The absence of CCL2 or CCL3 delayed A-wave decline, but not B-wave decline, during B. cereus infection.
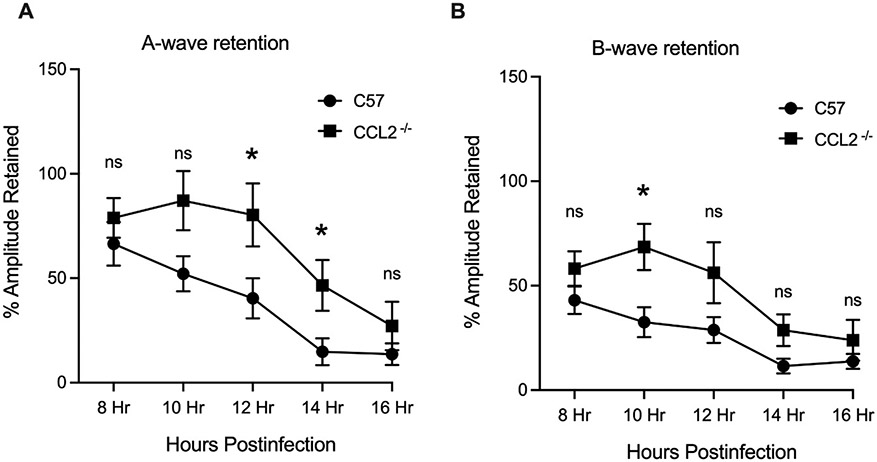
Infection progression, damage to delicate retinal cells, and declines in retinal function are linked. Retinal function analysis of B. cereus infected eyes is described in Figs. 3 and 4. In infected C57BL/6J eyes, A-wave amplitudes declined by nearly 80% from 8 to 16 hours after infection. In infected CCL2−/− mouse eyes, A-wave amplitudes declined by 65% over the same time period. A-wave amplitude retention was greater in CCL2−/− eyes compared to that of C57BL/6J eyes at 12 and 14 hours postinfection (P ≤ 0.05, Fig. 3A). At all other time points, percent retention of A-wave amplitudes in CCL2 and C57BL/6J eyes were similar. Surprisingly, B-wave amplitude retention was greater in CCL2 −/− eyes compared to that of C57BL/6J eyes at 10 hours only (P ≤ 0.05, Fig. 3B). At all other times, percent retention of B-wave amplitudes in CCL2−/− and C57BL/6J was similar (P≥0.05, Fig 3B).
Fig. 3: The absence of CCL2 delayed A-wave decline during B. cereus infection.
Eyes of C57BL/6J and CCL2−/− mice were infected with 100 CFU B. cereus. Retinal function was assessed by ERG at 8, 10, 12, 14 and 16 hours postinfection. In infected CCL2−/− eyes, retained A- wave responses were significantly greater than retained A- wave responses in infected C57BL/6J eyes at 12 (P=0.0348) and 14 (P=0.0308) hours postinfection (Fig 3A). B-wave responses in CCL2−/− eyes were significantly greater than the B-wave responses in the infected C57BL/6J eyes at 10 hours postinfection (P=0.0156, Fig 3B). Values represent means ± SEM of n ≥ 6 eyes at each time point with at least 3 independent experiments. *P ≤ 0.05 and nsP≥0.05.
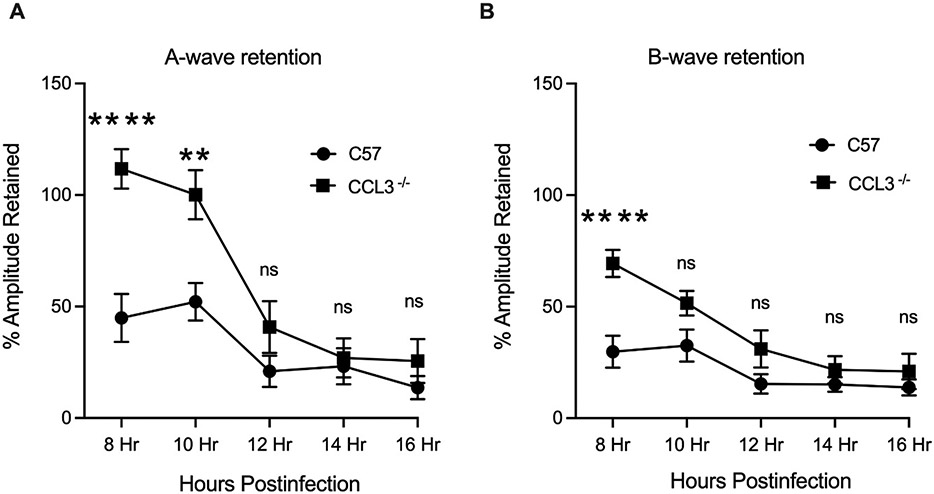
Fig. 4: The absence of CCL3 delayed A-wave decline during B. cereus infection.
Eyes of C57BL/6J (C57) and CCL3−/− and mice were infected with 100 CFU B. cereus. Retinal function was assessed by ERG at 8, 10, 12, 14 and 16 hours postinfection. Compared to infected C57BL/6J mice eyes, A- wave responses were significantly retained at 8 (P<0.0001) and 10 (P=0.0098) hours postinfection (Fig 4A), whereas B-wave responses were significantly retained at 8 hours postinfection ((P<0.0001), Fig 4B). Values represent means ± SEM of n ≥ 6 eyes at each time point with at least 3 independent experiments. *P ≤ 0.05, ****P≤0.0001, and nsP≥0.05.
For infected CCL3−/− mouse eyes, a similar outcome of retained retinal function was observed, but only for earlier timepoints. Percent retention of A-wave amplitudes was significantly greater in CCL3−/− eyes compared to that of C57BL/6J eyes at 8 and 10 hours postinfection (P ≤ 0.0098, Fig. 4A). At all other time points, percent retention of A-wave amplitudes in CCL3−/− and C57BL/6J was similar. Surprisingly, B-wave amplitude retention was greater in CCL3−/− eyes compared to that of C57BL/6J at 8 hours only (P ≤ 0.0001, Fig. 4B). At all other time points, percent retention of B-wave amplitudes in CCL3−/− and C57BL/6J was similar (P≥0.05, Fig. 4B). Taken together, these outcomes indicated that the absence of CCL2 or CCL3 may have only delayed the decline in photoreceptor cell function. Any protection afforded by the absence of CCL2 or CCL3 did not extend to other cells responsible for visual function. The absence of these C-C chemokines influenced retinal function retention regardless of the intraocular load of Bacillus.
3.4. The absence of CCL2 and CCL3 delayed intraocular inflammation and ocular damage during B. cereus endophthalmitis.
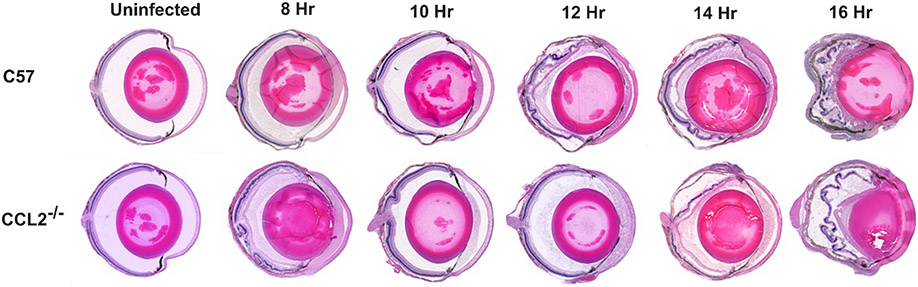
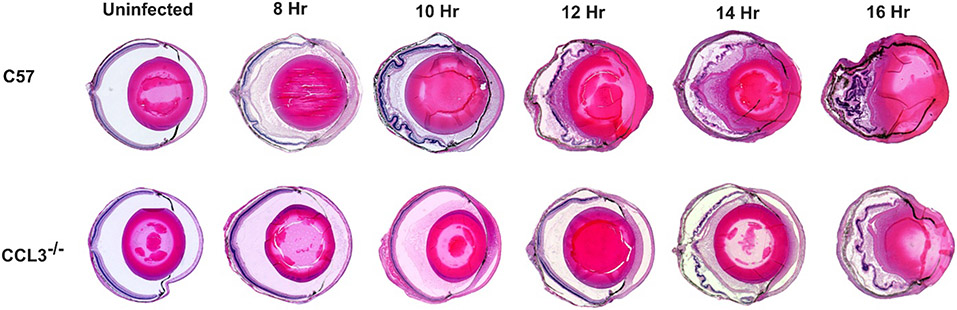
Overall inflammation and ocular tissue damage caused by B. cereus infection in C57BL/6J, CCL2−/−, and CCL3−/− mouse eyes were assessed by histology. Uninfected CCL2−/− and CCL3−/− mouse eyes were structurally comparable to eyes of uninfected C57BL/6J mice (Figs. 5 and 6). There were no noticeable changes in cellular morphology in the cornea or retina, and there were no signs of inflammation in the uninfected groups. At 8 hours postinfection, mild to medium deposition of fibrin and infiltration of immune cells was observed in C57BL/6J eyes. Anterior chambers in these eyes were packed with fibrin and retinas in the posterior segment were partially detached. In contrast, retinal structural integrity and ocular architecture were well-preserved, with minimal deposition of fibrin and minimal inflammation in infected CCL2−/− and CCL3−/− mouse eyes at 8 hours after infection (Figs. 5 and 6). At 10 hours postinfection, medium to moderate fibrin deposition and infiltration of inflammatory cells were visible throughout the infected eyes of C57BL/6J and CCL2−/− mice (Fig. 5). Retinal layers were partially detached and corneas were highly edematous in these eyes. In contrast, retinas and other ocular structures were preserved and inflammation was minimal in the infected eyes of CCL3−/− mice (Fig. 6). At 12 hours postinfection, infected C57BL/6J mouse eyes were severely inflamed and retinas were completely detached. These eyes were also filled with fibrin and inflammatory cells (Figs. 5 and 6). In contrast, CCL2−/− eyes had well-preserved ocular architecture with minimum infiltration of immune cells. Infected CCL2−/− eyes looked quite similar in appearance to C57BL/6J eyes at 8 hours postinfection. At 12 hours postinfection, CCL3−/− eyes were less inflamed than C57BL/6J eyes, and ocular structures were better preserved in CCL3−/− eyes. At 14 hours postinfection CCL2−/− eyes demonstrated partially preserved retinal layers with moderate to high degrees of immune cell infiltration compared to that of C57BL/6J eyes at the same time point. At 16 hours after infection, inflammation was present throughout ocular structures and retinal architecture was completely lost in infected C57BL/6J and CCL2−/− eyes. Similar findings were observed at 14 and 16 hours postinfection in infected CCL3−/− and C57BL/6J eyes. Taken together, these results demonstrated that the damage to retinal architecture and inflammation was delayed in infected eyes of CCL2−/− and CCL3−/− mice. Compared to C57BL/6J mice, ocular architecture was sustained for a prolonged period in the eyes of infected CCL2−/− mice, and more so than in infected eyes of CCL3−/− mice during Bacillus endophthalmitis. These results support the influence of each chemokine on not only inflammation, but also overall endophthalmitis pathogenesis.
Fig. 5: The absence of CCL2 delayed intraocular inflammation and ocular damage during infection.
C57BL/6J (C57) and CCL2 −/− mice eyes were infected with 100 CFU B. cereus. Uninfected and infected globes were harvested at 8, 10, 12, 14, and 16 hours postinfection and processed for H&E staining. Uninfected C57 and CCL2 −/− had with no signs of inflammation, and were architecturally and morphologically similar. At 8 hours postinfection, fibrin deposition was observed in the anterior chamber of all eyes. At 8 and 10 hours postinfection, infected eyes of C57 and CCL2 −/− mice were similarly inflamed, with inflammatory cells in the posterior segment. At 12 and 14 hours postinfection, infected eyes of C57 mice showed retinal detachment and partial dissolution of retinal layers. In contrast, infected eyes of CCL2−/− mice had minimal inflammation and intact retinal layers at the same time points. At 16 hours postinfection infected eyes of C57 and CCL2−/− mice were identical with complete dissolution of retinal layers and fibrin deposition. Sections are representative of 3 eyes per time point with at least 3 independent experiments. Original magnification, ×10.
Fig. 6: The absence of CCL3 delayed intraocular inflammation and ocular damage during infection.
Eyes of C57BL/6J (C57) and CCL3−/− mice were infected with 100 CFU B. cereus. Uninfected and infected globes were harvested at 8, 10, 12, 14, and 16 hours postinfection and processed for H&E staining. Uninfected mice in both groups were not inflamed and were architecturally and morphologically similar. At 8 and 10 hours postinfection, partially detached retinal layers with fibrin deposition was observed in the anterior chamber of C57 eyes. They were also similarly inflamed with inflammatory cells in the posterior segment. In contrast CCL3−/− eyes had minimal to mild inflammation and intact retinal layers at same time points. From 12 hours postinfection both C57 and CCL3−/− mice eyes showed retinal detachment and dissolution of retinal layers. Sections are representative of 3 eyes per time point with at least 3 independent experiments. Original magnification, ×10.
3.5. The absence of CCL2 and CCL3 impeded intraocular inflammation during B. cereus endophthalmitis.
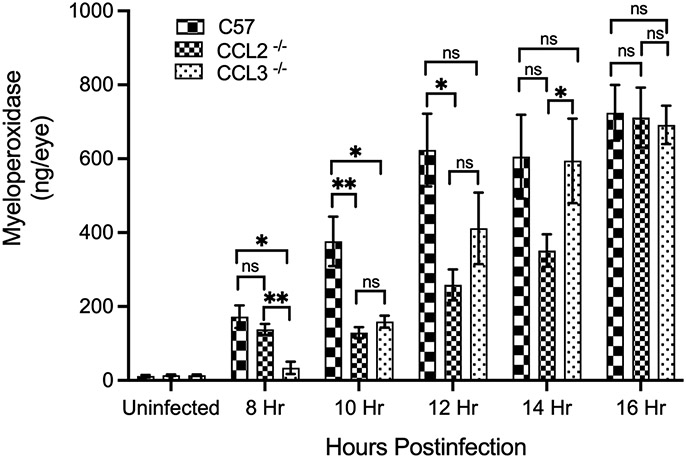
Because neutrophils are the earliest and most numerous infiltrating immune cell in the eye during Bacillus endophthalmitis, we semi-quantified infiltration by measuring myeloperoxidase (MPO) activity in C57BL/6J, CCL2−/−, and CCL3−/− mouse eyes infected with B. cereus (Fig. 7). At 8 hours postinfection, MPO levels in infected CCL2−/− mouse eyes were 19% lower than in infected eyes of C57BL/6J mice, but these values were not statistically significant (P=0.5397). MPO levels in infected CCL2−/− eyes were significantly lower compared to that of infected C57BL/6J eyes at 10 and 12 hours postinfection (P=0.0079 and P=0.0159, respectively). At these time points, MPO levels were 65% and 58% lower than that of C57BL/6J eyes. At 14 hours (P=0.1429) and 16 hours (P=0.8413) postinfection, MPO levels in infected CCL2−/− and C57BL/6J eyes were similar. MPO levels in infected CCL3−/− eyes were significantly lower (P=0.0159, 80% and P=0.0159, 57%) than that of C57BL/6J eyes at 8 and 10 hours postinfection, respectively. At 12 hours postinfection, MPO levels of infected CCL3−/− eyes were 34% lower than that of infected C57BL/6J eyes, but the values were not statistically significant (P=0.1746). MPO levels in infected CCL3−/− eyes were similar to that of infected C57BL/6J eyes at 14 and 16 hours postinfection (P=0.8413 and P=0.8016, respectively). In comparing MPO in CCL2−/− versus CCL3−/− eyes, MPO concentrations in CCL3−/− eyes were 75% lower (P=0.0079) at 8 hours postinfection, but 69% greater (P=0.0476) at 14 hours postinfection than that of CCL2−/− eyes. These results implied a significant delay in neutrophil infiltration in infected CCL2−/− eyes. Despite the initial delay, the amount of MPO in infected CCL2−/− eyes eventually reached the levels of infected C57BL/6J eyes. Taken together, these findings further support our results of reduced ocular pathology and retinal function loss in B. cereus-infected CCL2−/− and CCL3−/− eyes, strongly suggesting that in the immune response to B. cereus endophthalmitis, C-C chemokines play a significant role.
Fig. 7: The absence of CCL2 and CCL3 impeded intraocular inflammation during B. cereus endophthalmitis.
MPO concentrations were significantly reduced in the absence of CCL2 and CCL3 only at earlier times. C57BL/6J (C57), CCL2−/−, and CCL3−/− mouse eyes were injected with 100 CFU B. cereus. At 8, 10, 12, 14, and 16 hours postinfection, infected eyes were harvested and infiltration of PMN was assessed by quantifying MPO in whole eyes by sandwich ELISA. Compared to C57 mouse eyes, MPO levels were significantly reduced in CCL2−/− mouse eyes at 10 and 12 house postinfection. In CCL3−/− mouse eyes MPO levels were significantly reduced at 8 and 10 hours postinfection. No significant difference in MPO levels at 14 and 16 hours postinfection between C57 and, CCL2−/−and CCL3−/− mouse eyes. MPO levels were significantly high in CCL2−/− mouse eyes compared to CCL3−/− eyes at earlier times. However at later times, from 10 to 14 hours postinfection, MPO levels were higher in CCL3−/− eyes compared to CCL2−/− mouse eyes. Values represent means ± SEM for n ≥ 5 eyes per group per time point with at least 3 independent experiments. *P ≤ 0.05, **P≤ 0.01, and nsP≥0.05.
3.6. Treatment with anti-CCL2 or anti-CCL3 improved retention of retinal function and reduced intraocular inflammation.
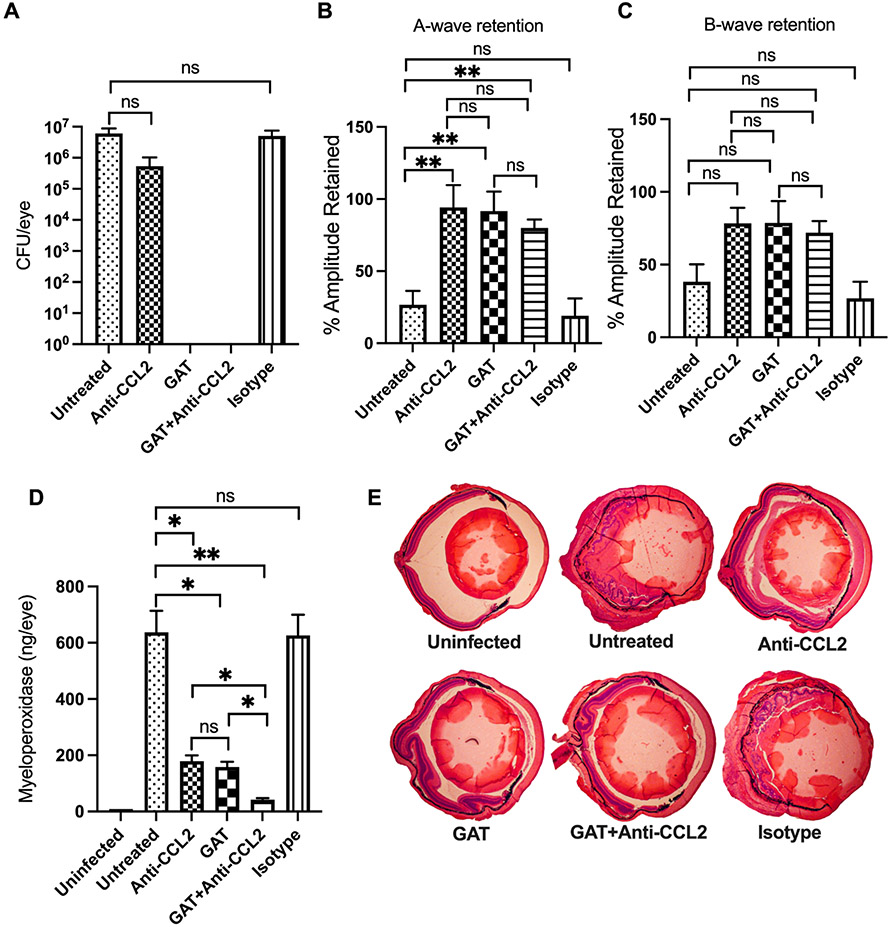
Since the absence of CCL2 or CCL3 blunted inflammation and inflammation-associated injury to the eye during B. cereus endophthalmitis, we examined whether neutralization of these C-C chemokines would provide a therapeutic benefit. C57BL/6J mouse eyes were infected with B. cereus, and experimental groups were treated at 2 hours postinfection with anti-CCL2 or anti-CCL3 antibody, these antibodies with or without gatifloxacin, with an IgG2A isotype control, or with gatifloxacin alone. A group left untreated served as controls. At 10 hours after infection, infected and treated eyes were examined for viable B. cereus, retinal function, and inflammation (Figs. 8 and 9). Gatifloxacin sterilized infected eyes in all groups treated with this antibiotic. There were no differences (P≥0.05) in recovered bacteria, retinal function, or MPO concentration between isotype-treated and untreated eyes (Figs. 8 and 9). In the anti-CCL2 study, A-wave amplitudes were retained to a significantly greater degree in infected eyes treated with anti-CCL2 alone (P=0.0030), gatifloxacin alone (P=0.0047), or gatifloxacin + anti-CCL2 (P=0.0014) than in untreated eyes (Fig. 8B). B-wave retention among these groups was comparable and better than in untreated or isotype-treated groups, but was not statistically significant (P≥0.05, Fig. 8C). In the anti-CCL3 study, A-wave retention was not significant in infected eyes treated with anti-CCL3 alone (P=0.1807), but was significant in the groups treated with gatifloxacin alone (P=0.0260) or gatifloxacin + anti-CCL3 (P=0.0225), compared to untreated eyes (Fig. 9B). Compared to untreated infected eyes, B-wave retention was not significant in infected eyes treated with anti-CCL3 alone (P=0.2949), but was significant in infected eyes treated with gatifloxacin alone (P=0.0043) or gatifloxacin + anti-CCL3 (P=0.0225).
Fig. 8: Treatment with anti-CCL2 improved retention of retinal function and reduced intraocular inflammation.
Treatment with anti-CCL2 antibody alone, or with or without gatifloxacin (GAT) arrests intraocular inflammation and protects retinal function. C57BL/6J mice were intravitreally injected with 100 CFU B. cereus. At 2 hours postinfection, groups of infected eyes were treated with 250 ng/0.5μl anti-CCL2, 1.25ug/0.5μl GAT, 0.5μl PBS containing 250 ng Anti-CCL2 and 1.25μg GAT, and 0.5μg/0.5μl nonspecific isotype IgG2A (Isotype). All eyes were analyzed at 10 hours postinfection. (8A) Gatifloxacin sterilized the eye in both GAT alone and GAT+Anti-CCL2 treated eyes. No differences in bacterial counts were detected between untreated and anti-CCL2 (P=0.0635) or isotype-treated (P=0.7302) eyes. (8B) Retained A-wave function was significantly greater in anti-CCL2 alone (P=0.0087), GAT alone (P=0.0047) and GAT+anti-CCL2 (P=0.0014) treated eyes compared to untreated eyes. (8C) Compared to untreated eyes, B-wave function was not significantly retained in anti-CCL2 alone (P=0.0559), GAT alone (P=0.1471), and GAT+anti-CCL2 (P=0.1088) treated eyes. No differences in A- or B-wave retention was detected between untreated and isotype treated eyes. (8D) Compared to untreated eyes MPO concentrations was significantly less in anti-CCL2 alone (P=0.0159), GAT alone (P=0.0159), and GAT+anti-CCL2 (P=0.0079) treated eyes. Values represent means ± SEM for n ≥ 5 eyes per group per time point with at least 3 independent experiments. *P ≤ 0.05, **P≤0.01, ***P≤0.001, and nsP≥0.05. (8E) H&E staining showed severe inflammation and complete loss of retinal architecture in both untreated and isotype-treated mice. In contrast, mild inflammation and intact retinal layers were found in infected eyes treated with either anti-CCL2 alone, GAT alone, and GAT+anti-CCL2. Original magnification, ×10. Sections are representative of two eyes in each group.
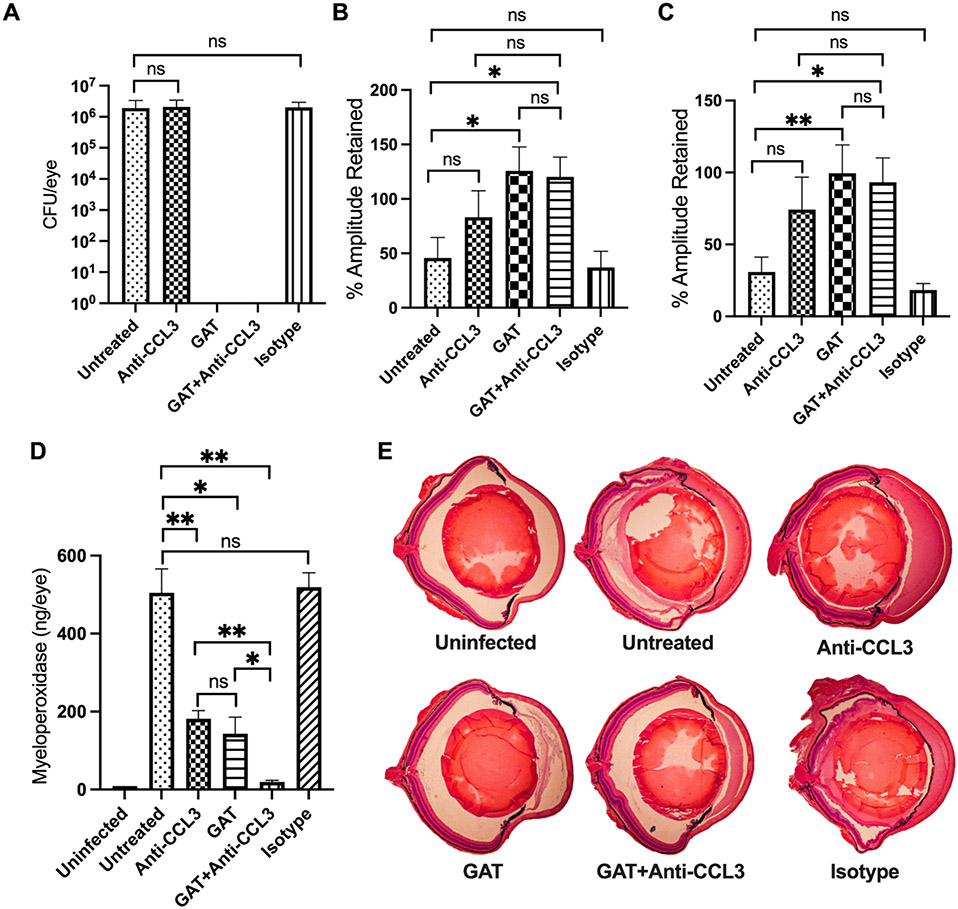
Fig. 9: Treatment with anti-CCL3 improved retention of retinal function and reduced intraocular inflammation.
Treatment with anti-CCL3 antibody alone, with or without gatifloxacin (GAT) arrested intraocular inflammation and retained retinal function. C57BL/6J mice were intravitreally injected with 100 CFU B. cereus. At 2 hours postinfection, groups of infected eyes were treated with 250 ng/0.5μl anti-CCL3, 1.25ug/0.5μl GAT, 0.5μl PBS containing 250 ng Anti-CCL3 and 1.25μg GAT, and 0.5μg/0.5μl nonspecific isotype IgG2A (Isotype). All eyes were analyzed at 10 hours postinfection. (9A) Treatment with gatifloxacin sterilized the eye in both GAT alone and GAT+anti-CCL3 treated eyes. No differences in bacterial counts were observed between untreated and anti-CCL3 (P=0.3282) or isotype-treated (P=0.5358) eyes. (9B) Compared to untreated eyes A-wave function was significantly greater in GAT alone (P=0.026) and GAT+anti-CCL3 (P=0.0225) treated eyes, but not in anti-CCL3 alone (P=0.1807) treated eyes. (8C) Compared to untreated eyes, B-wave function was significantly retained in GAT alone (P=0.0043) and GAT+anti-CCL3 (P=0.0225) treated eyes but not in anti-CCL3 alone (P=0.2949) treated eyes. No differences in A- or B-wave retention was detected between untreated and isotype-treated eyes. (9D) MPO concentrations in infected eyes of mice treated with anti-CCL3 antibody with (P=0.0079) or without antibiotics (P=0.0079) and antibiotics alone (P=0.0159) were significantly less than in untreated eyes. Values represent means ± SEM for n ≥ 5 eyes per group per time point with at least 2 independent experiments. *P ≤ 0.05, **P≤0.01, and nsP≥0.05. (9E) Histology analysis of H&E staining showed stark contrast between uninfected and untreated or isotype-treated mice. Compared to uninfected, untreated or isotype-treated mice eyes were inflamed, with the loss of partial retinal architecture. In contrast, there was mild inflammation and undamaged retinal layers in infected eyes treated with GAT alone and GAT+anti-CCL3. Retinal layers in anti-CCL3 alone treated eyes were intact but eyes were highly inflamed. Original magnification, ×10. Sections are representative of two eyes in each group.
Neutrophil infiltration was assessed following anti-CCL2 or anti-CCL3 treatment by measuring MPO concentrations. In general, no differences in MPO concentrations between isotype control-treated and untreated eyes were noted (P≥0.05, Figs. 8D, 9D). MPO concentrations were significantly reduced in eyes treated with anti-CCL2 alone (72%, P=0.0159), gatifloxacin alone (75%, P=0.0159), or gatifloxacin + anti-CCL2 (96%, P=0.0079), compared to that of untreated infected eyes (Fig. 8D). MPO concentrations were also significantly reduced in eyes treated with gatifloxacin + anti-CCL2 compared to eyes treated with anti-CCL2 alone (88%, P=0.0159) or gatifloxacin alone (87%, P=0.0159). Similarly, MPO concentrations were significantly reduced in eyes treated with anti-CCL3 alone (64%, P=0.0079), gatifloxacin alone (71%, P=0.0159), or gatifloxacin + anti-CCL3 (96%, P=0.0079), compared to untreated infected eyes (Fig. 9D). MPO concentrations were also significantly less in eyes treated with gatifloxacin + anti-CCL3 treated eyes compared to eyes treated with anti-CCL3 alone (89%, P=0.0079) or gatifloxacin alone (86%, P=0.0159) (Fig. 9D).
In our histology analysis, we observed that isotype-treated and untreated eyes were severely damaged and inflamed to degrees similar to that described in Fig. 5 and Fig. 6 (Fig. 8E, 9E). Compared to isotype-treated and untreated eyes, eyes treated with anti-CCL2 alone, gatifloxacin + anti-CCL2, gatifloxacin + anti-CCL3, or gatifloxacin alone were morphologically similar, structurally well-preserved, and had reduced inflammation and inflammatory damage (Fig. 8E, 9E). Eyes treated with anti-CCL3 alone were more inflamed than eyes treated with anti-CCL2, but these eyes retained an intact retinal architecture (Fig. 9E). Overall, these findings showed that anti-CCL2 or anti-CCL3 treatment with gatifloxacin sterilized infected eyes and decreased intraocular inflammation during Bacillus endophthalmitis. Clinically, this outcome is better than that of treatment with antibiotic or antibody alone. This implies that targeting chemokines CCL2 and CCL3 could be beneficial in the treatment of B. cereus endophthalmitis and perhaps endophthalmitis caused by other pathogens.
4. Discussion
Bacillus endophthalmitis is one of the most dangerous forms of microbial endophthalmitis that frequently progresses to vision loss, blindness, and/or removal of the globe (Mursalin et al., 2020c). This is especially true if therapeutic intervention is delayed, as can be the issue in traumatic injuries. This devastating consequence is the result of an interactive process between bacterial and host factors inside the eye during evolving infection (Callegan et al., 2007; Callegan et al., 2002c). These factors trigger adverse inflammatory responses that impair visual function and disrupt tissue architecture and integrity. Bacterial secreted toxins alter the vascular permeability of the eye and damage the retina (Callegan et al., 2002a; Callegan et al., 1999b; Callegan et al., 2005; Moyer et al., 2009). Bacterial cell wall components interact with immune receptors of the ocular innate immune system to trigger the inflammatory responses that recruit inflammatory cells into the eye (Mursalin et al., 2020a; Novosad et al., 2011; Parkunan et al., 2014; Parkunan et al., 2015). Existing treatments for endophthalmitis (antibiotics and corticosteroids) do not target bacterial products which trigger inflammation and damage tissues, and are often ineffective in mitigating damaging inflammation (Alfaro et al., 1996; Liu et al., 2000; Manning, S., et al., 2018; Pan et al., 2017; Sakalar et al., 2011; Slean et al., 2017; Yang et al., 2010). Therefore, novel therapeutics which target both anti-bacterial and anti-inflammatory activities are lacking.
The evolution of ocular changes during bacterial endophthalmitis can vary widely and depends on the organism infecting the eye. Coagulase-negative staphylococci are considered to be relatively avirulent pathogens, and often cause therapeutically responsive bacterial endophthalmitis (Huebner and Goldmann, 1999; Ormerod et al., 1993; Piette and Verschraegen, 2009). Pathogenic bacteria (Bacillus spp. Staphylococcus aureus, streptococci enterococci, Klebsiella spp.) are often linked to complicated cases of endophthalmitis which are often therapeutically refractory (Teweldemedhin et al., 2017). This, coupled with the emergence of multidrug resistance in many of the aforementioned pathogens suggests the importance of identifying new therapeutic targets (Slean et al., 2017). Bacillus spp. are common causative organisms in rapidly blinding endophthalmitis associated with penetrating ocular trauma and in endogenous endophthalmitis cases in adults (Mursalin et al., 2020c). In addition to its inherent beta-lactam antibiotic resistance, Bacillus is able to physically avoid an incoming inflammatory cell onslaught via its motility and arsenal of toxins and highly inflammogenic cell wall components (Callegan et al., 2002a; Callegan et al., 1999b; Callegan et al., 2005; Enosi Tuipulotu et al., 2021; Mursalin et al., 2020a). Together, these factors make Bacillus a formidable threat to the eye and vision.
Toll-like receptors are among the early immune recognition receptors that interact with invading microbial particles to trigger an immune response (Beutler 2009). For Bacillus endophthalmitis, activation of TLRs 2 and 4 impact disease severity as the cell wall components of Bacillus (peptidoglycan, lipoprotein, and S-layer protein) activate one or both of these TLRs, triggering the immune and inflammatory responses in the ocular microenvironment (Coburn et al., 2018; Mursalin et al., 2020b; Novosad et al., 2011). Inflammatory responses impact fibrin deposition, vitreous immune cell infiltration and exudate, and iris adhesion to the lens (posterior synechiae) (Mursalin et al., 2020c). We identified the expression of and explored the in vivo impacts of CXCL1, CXCL2, CXCL10, TNFα, and IL-6 on Bacillus endophthalmitis. The absence of TNFα in TNFα-deficient mice reduced inflammation but promoted B. cereus growth, causing a rapid decline in retinal function and worse infection compared to wild-type mice (Ramadan et al., 2008). The absence of IL6 in IL6-deficient mice had no effect on the course or severity of experimental Bacillus endophthalmitis (Parkunan et al., 2016). Absence of the individual C-X-C mediators noted above in knockout mice blunted neutrophil infiltration and resulted in improved retinal function outcome, implying the significance of these mediator molecules as therapeutic targets in controlling ocular inflammation and as well as endophthalmitis pathogenesis (Mursalin et al., 2021a; Parkunan et al., 2016).
Some inflammatory mediators are chemoattractants which signal via G-protein coupled receptors to drive leukocyte recruitment during immune surveillance and inflammation (Jeong and Lee, 2011; Newton and Dixit, 2012). Chemoattractants form concentration gradients that create directional signals for infiltrating cells to move toward injury sites and aid their arrest and extravasation into the parenchyma (Kumar et al., 2013; Zhang and Liang, 2016). Chemokines are divided into C-X-C and C-C subfamilies based on the location of the first pair of their N-terminal cysteines. These subfamilies have overlapping functions and are best known for stimulating the migration of cells (Hughes and Nibbs, 2018; Miller and Mayo, 2017). The ocular environment contains multifunctional cells (retinal pigment epithelium [RPE], photosensitive cells, astrocytes, retinal microglia, corneal and iris epithelium, and Muller cells) which are good sources of immune mediators (Adamus et al., 2001; Gilger, 2008; Gregerson, 1998; Taylor, 2009). In Bacillus endophthalmitis, inhibition of TLRs 2 and 4 resulted in blunted expression of these mediators, many of whom are chemoattractants in these subfamilies (Mursalin et al., 2020a; Mursalin et al., 2020b). To explore the significance of individual immune mediators and highlight putative anti-inflammatory targets, we focused on the C-X-C chemokines (CXCL1, CXCL2, and CXCL10) that are upregulated during B. cereus endophthalmitis (Mursalin et al., 2021a; Parkunan et al., 2016). We reported that their absence in knockout mice or after treatment with neutralizing antibody minimized the disease severity by delaying negative effects, but did not completely ameliorate inflammation or completely protect the retina. Together, these results suggest that other mediators might be compensating for their absence.
Inflammatory chemokines CCL2 (MCP-1), CCL3 (MIP-1α), and other members of the C-C subfamily are derived from a wide variety of cells such as lymphocytes, endothelium, epithelial cells, neutrophils, mononuclear phagocytes, and NK cells (Deshmane et al., 2009; Mantovani et al., 2004; Menten et al., 2002; Palomino and Marti, 2015; Reichel et al., 2009). CCL2 and CCL3 are highly upregulated in mouse eyes during experimental Bacillus endophthalmitis (Coburn et al., 2018; Mursalin et al., 2020b). CCL2 and CCL3 have two adjacent cysteines near their amino-terminal end. CCL2 binds receptor CCR2, whereas CCL3 interacts with receptors CCR1 and CCR5. CCL2 recruits monocytes, basophils, dendritic cells and memory T cells to the sites of inflammation from injury or infection (Mantovani et al., 2004; Miller and Mayo, 2017; Palomino and Marti, 2015). Recent findings suggest that the scope of CCL2's functions are wide ranging, and may also impact effector molecule secretion, leukocyte behavior, killing, and survival. Recombinant CCL2 treatment increased the efferocytosis (recognition and engulfment) of foreign bodies and apoptotic cells by murine alveolar macrophages, PEMs, and J774 macrophages (Gschwandtner et al., 2019). CCL3 exhibits chemotactic activity and is involved in the acute inflammatory response by recruiting and activating neutrophils, a key cellular player in Bacillus endophthalmitis (Miller et al., 2019). In a mouse pulmonary infection model, the absence of CCL3 significantly reduced the phagocytic activity of alveolar macrophages toward Klebsiella pneumoniae (Lindell et al., 2001). Considering the importance of CCL2 and CCL3 in efferocytosis, we evaluated the bacterial internalization abilities of primary neutrophils derived from C57BL/6J, CCL2−/−, and CCL3−/− mice to investigate whether the lack of these chemoattractants changed this neutrophil function. The number of internalized B. cereus in neutrophils from these mouse strains was similar, suggesting that the absence of these chemokines did not affect phagocytic function.
Rapid intraocular bacterial growth is a unique and important characteristic of Bacillus endophthalmitis (Mursalin et al., 2020c). Vitreous humour is a highly hydrated tissue consisting of various organic and inorganic components with a water content of between 98–99.7% (Kaplan, 2007). Mouse vitreous humour appears to be a good growth medium, as pathogens such as B. cereus, E. faecalis, K. pneumoniae, S. aureus, and S. pneumoniae can replicate inside mouse eyes (Astley et al., 2019; Booth et al., 1998; Coburn et al., 2019; Hunt et al., 2014; LaGrow et al., 2017). In the mouse eye, B. cereus grows faster than S. aureus, S. epidermidis, E. faecalis, and S. pneumoniae (Mursalin et al., 2020c). We also reported that during Bacillus endophthalmitis, the absences of TLR2 or 4, adaptor MyD88, or inflammatory mediators IL-6, CXCL1, CXCL2, or CXCL10 did not impact intraocular bacterial growth (Mursalin et al., 2021a; Novosad et al., 2011; Parkunan et al., 2016; Parkunan et al., 2015). In contrast, the absence of TLR4 adaptor TRIF resulted in slower intraocular Bacillus growth, while the absence of TNFα caused more rapid intraocular Bacillus growth (Parkunan et al., 2015; Ramadan et al., 2008). In the current study, the absence of CCL2 or CCL3 did not impact intraocular Bacillus growth, suggesting that, similar to individual C-X-C chemoattractants, the absence of individual C-C chemoattractants do not affect the growth of Bacillus in the eye (Mursalin et al., 2021a).
The retina has many functions associated with proper vision, including forming a physiological barrier, maintaining the eye's homeostasis, and ensuring proper function of the phototransduction cascade (Grossniklaus et al., 2015; Hoon et al., 2014). Because of the retina's vital role in vision, even minimal damage can cause permanent loss of sight. During Bacillus endophthalmitis, bacteria produce toxins and other products that negatively impact the structural integrity and function of the retina (Parke et al., 2012; Ramadan et al., 2006). Bacillus is motile, allowing the organism to physically move to the retina and directly interact with innate receptors which trigger inflammatory pathways (Callegan et al., 2005; Parkunan et al., 2014). We reported that the absence of CXCL1, CXCL2, or CXCL10 resulted in well-preserved retinal layers and function during Bacillus endophthalmitis (Mursalin et al., 2021a; Parkunan et al., 2016). In the current study, the absence of CCL2 or CCL3 resulted in an overall trend in better retained retinal function, but significance was achieved only at select time points during infection.
As Figs. 3 and 4 show, the delay in retinal function loss was more gradual in mice without CCL2 compared to that of mice without CCL3. In general, retinal function declines correspond to damaged retinal structures during Bacillus endophthalmitis. We described that the absence of CXCL1, CXCL2, or CXCL10 reduced pathological damage to the retina in this infection model (Mursalin et al., 2021a; Parkunan et al., 2016). In the current study, the absence of CCL2 or CCL3 resulted in preservation of ocular structures and retinal layer integrity for an extended period. At 12 and 14 hours postinfection, clear and distinct architectural differences were observed between infected C57BL/6J and CCL2−/− eyes. Infected CCL3−/− eyes looked relatively better from 8 hours to 14 hours postinfection than infected C57BL/6J eyes. Together, these findings suggested that the absence of CCL2 or CCL3 may have resulted in an environment of protection for the retina for a time during Bacillus endophthalmitis. The elevated expression of both CCL2 and CCL3 during Bacillus endophthalmitis could explain why we observed comparatively greater preservation of retinal function and improved ocular pathology when these two chemokines were absent (Coburn et al., 2018; Mursalin et al., 2020b).
In general, C-X-C chemoattractants recruit neutrophils, while C-C chemoattractants tend to attract monocytes and provide critical support in fighting bacterial infections (Miller and Mayo, 2017; Palomino and Marti, 2015). Inflammatory cells respond to microbial stimuli by secreting chemoattractants that target the CCR receptors and traffic to the sites of microbial infection in response to CCL2 and CCL3 secretion. These inflammatory chemokines have been reported to impact the recruitment of inflammatory cells into the eye and CNS in a rat experimental autoimmune uveitis model and impact disease severity (Reichel et al., 2009). Genetic deletion of CCL2 in mice also resulted a failure of monocyte and T-cell recruitment to inflammatory lesions (Gschwandtner et al., 2019; Huang et al., 2001). We recently reported that genetic deletion of CXCL2 and CXCL10 significantly reduced neutrophil infiltration during Bacillus endophthalmitis, as did the absence of CXCL1 (Mursalin et al., 2021a; Parkunan et al., 2016). Taken together with our cumulative retinal function and pathology data in this model, the findings provide further evidence that the damage to the ocular tissues and declined retinal function observed in Bacillus endophthalmitis might, in large part, be due to uninhibited inflammation. In the current study, genetic deletion of CCL2 significantly reduced the neutrophil influx at 10 and 12 hours postinfection. Genetic deletion of CCL3 significantly reduced the infiltration of neutrophils during the earlier phases of infection, but MPO concentrations eventually caught up to that of the infected C57BL/6J eyes starting at 12 hours postinfection. These results relate to our retinal function and histology findings and suggest that the absence of CCL3 may provide early protection, but the absence of CCL2 provides prolonged protection. These C-C chemokines may have a wider role in the pathogenesis of Bacillus endophthalmitis.
Currently, treatment for Bacillus endophthalmitis includes intravitreal antibiotics in an attempt to sterilize the infected eye. As severe intraocular inflammation is a hallmark of this disease, corticosteroids are also often prescribed, but their effectiveness in this disease is controversial (Liu et al., 2000; Pan et al., 2017; Sakalar et al., 2011; Vahey and Flynn, 1991). Thus, finding new and viable anti-inflammatory targets is critical for Bacillus and other types of endophthalmitis. Inflammatory mediators recruit inflammatory cells which cause bystander damage to the infected tissue, suggesting their importance as therapeutic targets (Miller et al., 2019). In Bacillus endophthalmitis, we identified a TLR4-driven cohort of chemokines (Coburn et al., 2018). We reported that treatment of Bacillus-infected mouse eyes with antibody to CXCL1, CXCL2, or CXCL10 significantly reduced inflammation and retinal function loss, but did not completely suppress all detrimental clinical features of endophthalmitis, suggesting that other chemokines contributed to the process (Mursalin et al., 2021a; Parkunan et al., 2016). Based on our results in CCL2−/− and CCL3−/− mice, we tested the anti-inflammatory potential of antibodies to these chemokines in the B. cereus endophthalmitis model.
Several therapeutic strategies have been used to interfere with CCL2 signaling. The importance of CCL2/CCR2 antagonist has been reported in preventing, alleviating, or treating uveitis, rheumatoid arthritis, multiple sclerosis, asthma, CCR2-mediated inflammatory syndrome, and chronic obstructive pulmonary disease (Xia and Sui, 2009). Anti-CCL2 treatment significantly delayed the onset and shortened the duration of both experimental autoimmune uveitis (EAU) and experimental autoimmune encephalomyelitis (EAE) (Adamus et al., 2001; Mahad and Ransohoff, 2003). In contrast, rheumatoid arthritis patients treated with anti-CCL2 monoclonal antibody showed no changes in clinical symptoms and, in some cases, large doses made the outcome worse (Haringman et al., 2006). Here, we tested the therapeutic potential of intravitreal anti-CCL2 with or without gatifloxacin. We chose the 2-hour treatment time because the overall progression of experimental Bacillus endophthalmitis is relatively fast and to mimic treatment in an ocular trauma patient seeking immediate therapy. Administration of anti-CCL2 significantly enhanced retinal function retention and reduced intraocular inflammation and tissue damage. Adding gatifloxacin to anti-CCL2 sterilized the eyes and significantly reduced the intraocular inflammation compared to treatment with the anti-CCL2 alone or antibiotic alone. Targeting CCL3 has shown distinct effects in a mouse model of retinitis pigmentosa (Kohno et al., 2014). Genetic deletion of CCL3 attenuated the severity of acute and chronic retinal degeneration (Kohno et al., 2014). Anti-CCL3 treatment did not affect clinical EAE but inhibited the clinical signs of EAU in rats (Manczak et al., 2002). Another study on anti-CCL3 treatment in the herpes simplex virus-induced mouse model of Behçet's disease reported that anti-CCL3 treatment ameliorated disease symptoms (Sayeed et al., 2019). In the current study, the administration of anti-CCL3 as an early treatment option during Bacillus endophthalmitis did not significantly improve the retinal function unless gatifloxacin was included in the treatment regimen. Administration of anti-CCL3 reduced inflammatory cell influx compared to the untreated group. The anti-inflammatory effect was even greater when gatifloxacin was added.
It is estimated that the rapidly growing number of ocular surgeries will raise the incidence of endophthalmitis cases, which can be medical emergencies that threaten vision. The sight-threatening consequences of bacterial endophthalmitis are the result of a complex interaction between pathogens, their products, and the host response. Bacillus endophthalmitis is rare, but compared to other types of endophthalmitis, is a substantial and imminent threat to vision. Rapid infection progression, poor functional and anatomic outcomes, and excessive therapeutic failure rates are typical of Bacillus endophthalmitis (Mursalin et al., 2020c). Inflammatory chemokines and their receptors are central to the inflammation process; therefore, targets that have the potential to reduce inflammation are attractive therapeutic options. Here, we demonstrated that genetic deletion of CCL2 or CCL3 resulted in a somewhat better ocular pathology, reduced influx of potentially damaging immune cells, and better retinal function retention. The absence of CCL2 resulted in better retinal function and eyes had significantly less pathology and inflammation for a prolonged period. In the absence of CCL3, clinical improvement was observed for a brief period of time. Although we found better clinical outcomes with single anti-chemokine antibodies and gatifloxacin in our current and previous studies, additional studies are required to better conclude the therapeutic potential of chemokine neutralization with antibiotics in Bacillus and other types of endophthalmitis.
5. Acknowledgements and Funding
The authors thank Dr. Feng Li and Mark Dittmar (OUHSC P30 Animal Analysis Core, Dean A. McGee Eye Institute, Oklahoma City, OK, USA), and the OUHSC P30 Cellular Imaging Core (Dean A. McGee Eye Institute, Oklahoma City, OK, USA) for histology expertise. This work was supported by National Institutes of Health grants R21EY028066 and R01EY028810 (to MCC). Our research is also supported in part by National Institutes of Health grants R21EY021802 (to MCC), National Eye Institute Vision Core Grant P30EY021725 (to MCC), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness.
6. References
- Adamus G, Manczak M, Machnicki M, 2001. Expression of CC chemokines and their receptors in the eye in autoimmune anterior uveitis associated with EAE. Invest. Ophthalmol. Vis. Sci 42, 2894–2903. https://iovs.arvojournals.org/article.aspx?articleid=2123258. [PubMed] [Google Scholar]
- Alfaro DV, Davis J, Kim S, Bia F, Bogard JF, Briggs JW, Liggett PE., 1996. Experimental Bacillus Cereus post-traumatic endophthalmitis and treatment with ciprofloxacin. Br. J. Ophthalmol 80, 755–758. 10.1136/bjo.80.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley R, Miller FC, Mursalin MH, Coburn PS, Callegan MC, 2019. An eye on Staphylococcus aureus toxins: Roles in ocular damage and inflammation. Toxins (Basel) 11. 10.3390/toxins11060356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler BA, 2009. TLRs and innate immunity. Blood 113, 1399–1407. 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth MC, Hatter KL, Miller D, et al. , 1998. Molecular epidemiology of Staphylococcus aureus and Enterococcus faecalis in endophthalmitis. Infect. Immun 66, 356–360. 10.1128/iai.66.1.356-360.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone EJ, 2010. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev 23, 382–398. 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Booth MC, Jett BD, Gilmore MS, 1999a. Pathogenesis of Gram-positive bacterial endophthalmitis. Infect. Immun 67, 3348–3356. 10.1128/IAI.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Jett BD, Hancock LE, Gilmore MS, 1999b. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect. Immun 67, 3357–3366. 10.1128/IAI.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Cochran DC, Kane ST, Gilmore MS, Gominet M, Lereclus D, 2002a. Contribution of membrane-damaging toxins to Bacillus endophthalmitis pathogenesis. Infect. Immun 70, 5381–5389. 10.1128/IAI.70.10.5381-5389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Engelbert M, Parke II DW, Jett BD, Gilmore MS, 2002b. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev 15, 111–124. 10.1128/CMR.15.1.111-124.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Gilmore MS, 2002c. Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 21, 367–373. 10.1089/10445490260099647. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer A, Hunt JJ, Novosad B, 2007. Bacterial endophthalmitis: Therapeutic challenges and host-pathogen interactions. Prog. Retin. Eye Res 26, 189–203. 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Gilmore MS, Gominet M, Lereclus D, 2003. Relationship of PLC-R-regulated factors to Bacillus endophthalmitis virulence. Infect. Immun 71, 3116–3124. 10.1128/iai.71.6.3116-3124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Kane ST, Cochran DC, Novasad B, Gilmore MS, Gominet M, Lereclus D, 2005. Bacillus endophthalmitis: Roles of bacterial toxins and motility during infection. Invest. Ophthalmol. Vis. Sci 46, 3233–3238. 10.1167/iovs.05-0410. [DOI] [PubMed] [Google Scholar]
- Callegan MC, Parkunan SM, Randall CB et al. , 2017. The role of pili in Bacillus cereus intraocular infection. Exp. Eye Res 159, 69–76. 10.1016/j.exer.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, Miller FC, LaGrow AL, Parkunan SM, Randall CB, Staats RL, Callegan MC, 2018. TLR4 modulates inflammatory gene targets in the retina during Bacillus cereus endophthalmitis. BMC Ophthalmol. 18, 96. 10.1186/s12886-018-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, LaGrow AL, Land C, et al. , 2019. Disarming pore-forming toxins with biomimetic nanosponges in intraocular infections. mSphere 4. 10.1128/mSphere.00262-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, Miller FC, Enty MA, Land C, LaGrow AL, Mursalin MH, Callegan MC, 2020. Expression of Bacillus cereus virulence-related genes in an ocular infection-related environment. Microorganisms 8. 10.3390/microorganisms8040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, Miller FC, Enty MA, Land C, LaGrow AL, Mursalin MH, Callegan MC, 2021. The Bacillus virulome in endophthalmitis. Microbiology 167 10.1099/mic.0.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE, 2009. Monocyte Chemoattractant Protein-1 (MCP-1): An overview. J. Interferon Cytokine Res 29, 313–326. 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobniewski FA, 1993. Bacillus cereus and related species. Clin. Microbiol. Rev 6, 324–338. 10.1128/CMR.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling-Schulz M, Lereclus D, Koehler TM, 2019. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr 7 10.1128/microbiolspec.GPP3-0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuipulotu E, Mathur A, Ngo C, Man SM, 2021. Bacillus cereus: Epidemiology, virulence factors, and host-pathogen interactions. Trends Microbiol. 29, 458–471. 10.1016/j.tim.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Gilger BC, 2008. Immunology of the ocular surface. Vet. Clin. North Am. Small Anim. Prac.t 38, 223–231, v. 10.1016/j.cvsm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, 1998. Immune privilege in the retina. Ocular Immunology and Inflammation 6, 257–267. 10.1076/ocii.6.4.257.4029. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Geisert EE, Nickerson JM, 2015. Introduction to the retina. Progress in molecular biology and translational science 134, 383–396. 10.1016/bs.pmbts.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Derler R, Midwood KS, 2019. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol 10, 2759. 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Yi C, Wang Y, Li J, Huang F, He L, Chi W, 2016., Identification of intraocular inflammatory mediators in patients with endophthalmitis. Mol. Vis 22, 563–574. https://pubmed.ncbi.nlm.nih.gov/27293374/. [PMC free article] [PubMed] [Google Scholar]
- Haringman JJ, Gerlag DM, Smeets TJM, Boeten D, Bosch FVC, Bresnihan B, Bresnihan B, Breedveld FC, Dinant HJ, Legay F, Gram H, Loetscher P, Schuouder R, Woodworth T, Tak PP,2006. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 54, 2387–2392. 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Santina LD, Wong ROL,, 2014. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res 42, 44–84. 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM, 2001. Absence of Monocyte Chemoattractant Protein-1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in Experimental Autoimmune Encephalomyelitis. J. Exp. Med 193, 713–726. 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner J, Goldmann DA, 1999. Coagulase-negative staphylococci: Role as pathogens. Annu. Rev. Med 50, 223–236. 10.1146/annurev.med.50.1.223. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Nibbs RJB, 2018. A guide to chemokines and their receptors. FEBS J. 285, 2944–2971. 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JJ, Astley R, Wheatley N, Wang J, Callegan MC, 2014. TLR4 contributes to the host response to Klebsiella intraocular infection. Curr. Eye Res 39, 790–802. 10.3109/02713683.2014.883412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong E, Lee JY, 2011. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med. J 52, 379–392. 10.3349/ymj.2011.52.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HJ, 2007. Anatomy and function of the eye. Chemical Immunology and Allergy 92, 4–10. 10.1159/000099236. [DOI] [PubMed] [Google Scholar]
- Kitsche M, Herber R, Pillunat LE, Terai N, 2020. Clinical and visual outcome of endophthalmitis patients: A single-center experience. Graefes Arch. Clin. Exp. Ophthalmol 258, 183–189. 10.1007/s00417-019-04480-2. [DOI] [PubMed] [Google Scholar]
- Kohno H, Maeda T, Perusek L, Pearlman E, Maeha A, 2014. CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration. J. Immunol 192, 3816–3827. 10.4049/jimmunol.1301738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ingle H, Prasad D, Kumar H, 2013. Recognition of bacterial infection by innate immune sensors. Crit. Rev. Microbiol 39, 229–246. 10.3109/1040841x.2012.706249. [DOI] [PubMed] [Google Scholar]
- LaGrow AL, Coburn PS, Miller FC, et al. , 2017. A novel biomimetic nanosponge protects the retina from the Enterococcus faecalis cytolysin. mSphere 2 10.1128/mSphere.00335-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Kim WS, Paik HD, 2019. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol 28, 1297–1305. 10.1007/s10068-019-00691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell DM, Theodore J, Standford TJ, Mancuso P, Leshen ZJ, Huffhagle GB, 2001. Macrophage inflammatory protein-1alpha/ccl3 is required for clearance of an acute Klebsiella pneumoniae pulmonary infection. Infect. Immun 69, 6364–6369. 10.1128/iai.69.10.6364-6369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Way T, Rodrigues M, Steidl SM,, 2000. Effects of intravitreal corticosteroids in the treatment of Bacillus cereus endophthalmitis. Arch. Ophthalmol 118, 803–806. 10.1001/archopht.118.6.803. [DOI] [PubMed] [Google Scholar]
- Livingston ET, Mursalin H, Coburn PS, Astley R, Miller FC, Amayem O, Lereclus D, Callegan MC, 2021. Immune inhibitor a metalloproteases contribute to virulence in Bacillus endophthalmitis. Infect. Immun 89, e0020121. 10.1128/iai.00201-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan NA, 2012. Bacillus and relatives in foodborne illness. J. Appl. Microbiol 112, 417–429. 10.1111/j.1365-2672.2011.05204.x. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM, 2003. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and Experimental Autoimmune Encephalomyelitis (EAE). Semin. Immunol 15, 23–32. 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Manczak M, Jiang S, Orzechowska B, Adams G, 2002. Crucial role of CCL3/MIP-1 alpha in the recurrence of autoimmune anterior uveitis induced with myelin basic protein in lewis rats. J. Autoimmun 18, 259–270. 10.1006/jaut.2002.0591. [DOI] [PubMed] [Google Scholar]
- Manning S, Ugahory C, Lindstedt EW, et al. , 2018. A prospective multicentre randomized placebo-controlled superiority trial in patients with suspected bacterial endophthalmitis after cataract surgery on the adjuvant use of intravitreal dexamethasone to intravitreal antibiotics. Acta Ophthalmologica, 96(4), 348–355. 10.1111/aos.13610 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzoni S, Allavena P, Vecchi A, Locati M, 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Damme JV, 2002. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 13, 455–481. 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Miller FC, Coburn PS, Mursalin MH, LaGrow AL, Livingston E, Callegan MC, 2019. Targets of immunomodulation in bacterial endophthalmitis. Prog. Retin. Eye Res 73, 100763. 10.1016/j.preteyeres.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Mayo KH, 2017. Chemokines from a structural perspective. Int. J. Mol. Sci 18 10.3390/ijms18102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzania D, Fleming TL, Robbins CB, Feng HL, Fekrat S, 2021. Time to presentation after symptom onset in endophthalmitis: Clinical features and visual outcomes. Ophthalmol. Retina 5, 324–329. 10.1016/j.oret.2020.07.027. [DOI] [PubMed] [Google Scholar]
- Moyer AL, Ramadan RT, Novasad BD, Astley R, Callegan MC, 2009. Bacillus cereus-induced permeability of the blood-ocular barrier during experimental endophthalmitis. Invest. Ophthalmol. Vis. Sci 50, 3783–3793. 10.1167/iovs.08-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Coburn PS, Livingston E, Miller FC, Astley R, Fouet A, Callegan MC, 2019. S-layer impacts the virulence of Bacillus in endophthalmitis. Invest. Ophthalmol. Vis. Sci 60, 3727–3739. 10.1167/iovs.19-27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Coburn PS, Livingston E, Miller FC, Astley R, Flores-Mireles AL, Callegan MC, 2020a. Bacillus s-layer-mediated innate interactions during endophthalmitis. Front. Immunol 11 10.3389/fimmu.2020.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Coburn PS, Miller FC, Livingston ET, Astley R, Callegan MC, 2020b. Innate immune interference attenuates inflammation in Bacillus endophthalmitis. Invest. Ophthalmol. Vis. Sci 61, 17. 10.1167/iovs.61.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Livingston ET, Callegan MC, 2020c. The cereus matter of Bacillus endophthalmitis. Exp. Eye Res 193, 107959. 10.1016/j.exer.2020.107959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH Coburn PS Miller FC Livingston ET Astley R Callegan MC, 2021a. C-X-C chemokines influence intraocular inflammation during Bacillus endophthalmitis. Invest. Ophthalmol. Vis. Sci 62, 14. 10.1167/iovs.62.14.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Livingston E, Coburn PS, Miller FC, Astley R, Callegan MC, 2021b. Intravitreal injection and quantitation of infection parameters in a mouse model of bacterial endophthalmitis. J. Vis. Exp 10.3791/61749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dixit VM, 2012. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol 4 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosad BD, Astley R, Callegan MC, 2011. Role of Toll-Like Receptor (TLR) 2 in experimental Bacillus cereus endophthalmitis. PLoS One 6, e28619. 10.1371/journal.pone.0028619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod LD, Becker LE, Cruise RJ, et al. ,, 1993. Endophthahitis caused by the coagulase-negative Staphylococci. Ophthalmology, 100(5), 715–723. 10.1016/s0161-6420(93)31584-8. [DOI] [PubMed] [Google Scholar]
- Palomino DC, Marti LC, 2015. Chemokines and immunity. Einstein 13(3), 469–473. 10.1590/s1679-45082015rb3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Liu Y, Wang R, et al. , 2017. Treatment of Bacillus cereus endophthalmitis with endoscopy-assisted vitrectomy. Medicine 96, e8701. 10.1097/MD.0000000000008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke DW, Pathengay A, Flynn HW, Albini T, Schwartz SG, 2012. Risk factors for endophthalmitis and retinal detachment with retained intraocular foreign bodies. J. Ophthalmol 2012, 758526. 10.1155/2012/758526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkunan SM, Astley R, Callegan MC, 2014. Role of TLR5 and flagella in Bacillus intraocular infection. PLoS One 9, e100543. 10.1371/journal.pone.0100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkunan SM, Randall CB, Astley RA, Furtado GC, Lira SA, Callegan MC, 2016. CXCL1, but not IL-6, significantly impacts intraocular inflammation during infection. J. Leukoc. Biol 100, 1125–1134. 10.1189/jlb.3A0416-173R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkunan SM, Randall CB, Coburn PS, Astley RA, Staats RL, Callegan MC, 2015. Unexpected roles for Toll-Like Receptor 4 and TRIF in intraocular infection with gram-positive bacteria. Infect. Immun 83, 3926–3936. 10.1128/IAI.00502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette A, Verschraegen G, 2009. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol 134, 45–54. 10.1016/j.vetmic.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Ramadan RT, Ramirez R, Novosad BD, Callegan MC, 2006. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr. Eye Res 31, 955–965. 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- Ramadan RT, Moyer AL, Callegan MC, 2008. A role for tumor necrosis factor-alpha in experimental Bacillus cereus endophthalmitis pathogenesis. Invest. Ophthalmol. Vis. Sci 49, 4482–4489. 10.1167/iovs.08-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CA, Rehberg M, Lerchenberger M, Berbeich N, Bihari P, Khandoga AG, Zahler S, Krombach F, 2009. CCL2 and CCL3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arterioscler Thromb. Vasc. Biol 29, 1787–1793. 10.1161/atvbaha.109.193268. [DOI] [PubMed] [Google Scholar]
- Sakalar YB, Ozekinci S, Celen MK., 2011. Treatment of experimental Bacillus cereus endophthalmitis using intravitreal moxifloxacin with or without dexamethasone. J. Ocul. Pharmacol. Ther 27, 593–598. 10.1089/jop.2011.0021. [DOI] [PubMed] [Google Scholar]
- Sayeed HM, Lee ES, Byun HO, Sohn S, 2019. The role of CCR1 and therapeutic effects of anti-CCL3 antibody in herpes simplex virus-induced behçet's disease mouse model. Immunology 158, 206–218. 10.1111/imm.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slean GR, Shorstein NH, Liu L, Paschal JF, Winthrop KL, Herrinton LJ, 2017. Pathogens and antibiotic sensitivities in endophthalmitis. Clin. Exp. Ophthalmol 45, 481–488. 10.1111/ceo.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Luster AD, 2015. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol 7 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, 2009. Ocular immune privilege. Eye 23, 1885–1889. 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teweldemedhin M, Gebreyesus H, Alsbaha AH, Asgedom SW, Saravanan M, 2017. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 17, 212. 10.1186/s12886-017-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey JB, Flynn HW Jr., 1991. Results in the management of Bacillus endophthalmitis. Ophthalmic. Surg 22, 681–686. https://pubmed.ncbi.nlm.nih.gov/1792034. [PubMed] [Google Scholar]
- Xia M, Sui Z, 2009. Recent developments in CCR2 antagonists. Expert Opin. Ther. Pat 19, 295–303. 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lu CK, Lee FL, Hsu WM, Lee VF, Lee SM, 2010. Treatment and outcome of traumatic endophthalmitis in open globe injury with retained intraocular foreign body. Ophthalmologica. 224, 79–85. 10.1159/000235725. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liang C, 2016. Innate recognition of microbial-derived signals in immunity and inflammation. Sci. China Life Sci 59, 1210–1217. 10.1007/s11427-016-0325-6. [DOI] [PubMed] [Google Scholar]
- Zhu S, Liu M, Bennett S, Wang Z, Pfleger KDG, 2021. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J. Cell Physiol 236, 7211–7222. 10.1002/jcp.30375. [DOI] [PubMed] [Google Scholar]