Abstract
Background:
Among adolescents with extremity fractures, individuals with obesity have greater representation compared with individuals of normal-weight, despite having higher areal and volumetric bone mineral density (aBMD, vBMD) than their normal-weight counterparts. The relative increase in BMD in individuals with obesity may thus be insufficient to support the greater force generated upon falling. The load-to-strength ratio is a biomechanical approach for assessing the risk of fracture by comparing applied force to bone strength, with higher load-to-strength ratios indicating higher fracture risk.
Objective:
To assess the load-to-strength ratio at the distal radius in adolescent and young adult females with severe obesity (OB) compared with normal-weight healthy controls (HC). We hypothesized that OB have a higher load-to-strength ratio compared to HC.
Methods:
We examined bone parameters in 65 girls 14–21 years old: 33 OB and 32 HC. We used dual-energy X-ray absorptiometry (DXA) to assess body composition, high resolution peripheral quantitative CT (HR-pQCT) to estimate vBMD, and microfinite element analysis (μFEA) to assess bone strength at the distal radius. To quantify fracture risk, we computed the load-to-strength ratio, where the numerator is defined as the load applied to the outstretched hand during a forward fall and the denominator is the bone strength, as estimated by μFEA.
Results:
Although OB had higher total vBMD than HC (368.3 vs. 319.9 mgHA/cm3, p=0.002), load-to-strength ratio at the radius was greater in OB than HC after controlling for age and race (0.66 vs. 0.54, p<0.0001). In OB, impact force and load-to-strength ratio were associated negatively with % lean mass (r =−0.49; p=0.003 and r =−0.65; p<0.0001 respectively) and positively with visceral fat (r =0.65; p<0.0001 and r =0.36; p =0.04 respectively).
Conclusions:
Adolescent and young adult females with obesity have higher load-to-strength ratio at the distal radius due to higher forces applied to bone in a fall combined with incomplete adaptation of bone to increasing body weight. This is differentially affected by lean mass, fat mass, and visceral fat mass.
Keywords: fracture, obesity, adolescents, radius, load-to-strength ratio
1. Introduction
Obesity is the second leading cause of preventable death in the United States [1]. Yet, the health risks of obesity remain understudied, particularly for the adolescent population. Historically, evidence has pointed to obesity being a protective factor for bone health because higher BMI is associated with higher bone mineral density (BMD), while low BMI is associated with low BMD [2] [3]. However, persons with obesity are paradoxically overrepresented among adolescents with extremity fractures [4].
The risk of fracture depends on bone strength as well as the external force applied to bone. The force of impact of a fall on an outstretched hand can be modeled taking height and body mass into consideration. At the distal radius, bone strength (or failure load) can be estimated non-invasively via application of microfinite element analysis (μFEA) to high-resolution peripheral quantitative computed tomography (HR-pQCT) images [5]. The ratio of load to bone strength provides an estimate of the likelihood of sustaining a fracture [6]. The load-to-strength ratio has previously been applied to understand hip, wrist, forearm, and vertebral fracture risk [7] [8] [9]. Indeed, the load-to-strength ratio is nearly two-fold higher in postmenopausal women with hip fracture than in age-similar women who have not suffered from hip fracture [7]. The load-to-strength ratio is also higher in postmenopausal women with a prevalent vertebral fracture compared to those with no prior fracture [10]. There are few studies that have examined the load-to-strength ratio among adolescents with obesity.
Further, recent findings indicate that the relationship between obesity and bone health is nuanced and depends on measures other than BMI, such as body composition [11]. Hence, we investigated the role of body composition on impact force, bone strength, and load-to-strength ratio. We have previously shown that a higher lean-to-fat mass ratio was positively associated with bone parameters, such as higher vBMD, in adolescent females with obesity [12]. Understanding the role that body composition plays in bone health may drive interventions to optimize bone health and hence is important to decipher. In this study we evaluated (i) the load-to-strength ratio for the distal radius in adolescent and young adult females with obesity compared with normal-weight healthy controls, and (ii) associations of components of the load-to-strength ratio, namely applied force and bone strength, with body composition measures. We thus aimed to explain the paradoxical relationship of the higher bone mineral density (BMD) reported in obesity with the greater risk of fracture. We hypothesized that higher BMD in people with obesity compared to controls is insufficient to support the higher force generated upon falling, given their higher body weight.
2. Methods
2.1. Study design and participants:
We conducted an observational cohort study of adolescents and young adult females, 14–21 years old: 33 with severe obesity (OB) and 32 age and race matched normal-weight healthy controls (HC). Severe obesity was defined in adults as a body mass index (BMI) ≥ 40 kg/m2 and in adolescents as BMI ≥ 120% of the 95th percentile for age and sex [13]. Normal-weight adults had a BMI ≤ 25 kg/m2 and normal-weight adolescents had a BMI ≤ 85% percentile for age and sex. Participants in the group with obesity (OB) were recruited through advertisements of the study at multiple sites, including other tertiary care obesity treatment centers and physician practices, while normal-weight healthy controls (HC) were recruited through only advertisements of the study at multiple sites. Data from this cohort were obtained between 2010–2018 during baseline visits from two ongoing bone studies: an observational study in those with obesity and the observational component of a study that included normal-weight controls. Both studies used the same study instruments. This study received approval from the Partners Healthcare Institutional Review Board. All participants ≥18 years old and parents of participants <18 years old provided informed consent. All participants <18 years old provided informed assent.
Subjects with conditions that could impact bone health, other than conditions directly comorbid with obesity such as polycystic ovary syndrome (PCOS), were excluded. Exclusion criteria included pregnancy, breastfeeding, untreated thyroid disease, smoking > 10 cigarettes/day, glucocorticoid use for more than six weeks, anti-epileptic medication use (that impact vitamin D metabolism), or body weight > 450 lbs due to weight limits on radiology equipment.
2.2. Clinical investigation:
A medical history was conducted to confirm that the subject met inclusion criteria. Subjects self-reported race and ethnicity. Height of each subject was measured 3 times using a wall-mounted stadiometer, and the mean was recorded to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg using an electronic scale.
2.3. High Resolution peripheral quantitative computed tomography (HR-pQCT):
We used first generation HR-pQCT imaging to obtain the vBMD, bone geometry and microarchitecture of the distal radius (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland) for each subject. Scans were performed on the non-dominant arm unless the subject indicated a history of fracture in the non-dominant arm. We set the region of interest to be 9.02 mm long, and the distal boundary to be 9.5 mm proximal to the radius endplate [14]. Imaging was performed with an isotropic voxel size of 82 μm3, delivering a three-dimensional representation of approximately 9 mm in the axial direction. Same-day reproducibility for repeated measurements at our center is 0.2% to 1.4% for volumetric bone mineral density (vBMD) values, 0.3% to 8.6% for trabecular microarchitecture parameters, and 0.6% to 2.4% for cortical microarchitecture parameters. Cortical porosity and thickness were obtained using extended cortical analysis (ECA).
To estimate the failure load of the distal radius, we performed microfinite element analysis (μFEA) on HR-pQCT images [15]. A simulated axial compression force was applied. Estimated failure load was defined as the force at which >2% of the bone tissue experienced >7000 μstrain [16]. The prediction of distal radius failure load using micro-finite element analysis was previously validated [5].
2.4. Calculation of the Load-to-strength Ratio:
We estimated impact force during a forwards fall using a formula derived from a single-spring model. This model was created by Johnston and colleagues to estimate forces on the distal radius resulting from a forward fall on an outstretched arm [6]. The impact force was calculated as demonstrated below, with ‘h’ indicating the height of the fall (in m), ‘m’ being the mass of the subject (in kg), ‘g, 9.81 m/s2’ the gravitational constant, and ‘k’ the stiffness constant (in N/m).
We used half of the subject’s height for ‘h’ to model a fall from standing height [10]. The stiffness constant was experimentally derived by Johnston and colleagues to be 4527 N/m for women [6]. To obtain the load-to-strength ratio, we divided the predicted impact force by the estimated failure load, obtained from μFEA.
2.5. Dual-energy X-ray Absorptiometry (DXA):
Body composition (fat mass, lean mass) was determined using whole-body DXA (Hologic 4500 A, Waltham, MA). The coefficients of variation for fat mass and lean mass for our institution are 2.1%, and 1.0%, respectively.
2.6. Magnetic Resonance Imaging (MRI):
Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were measured using a single axial MR imaging slice through the abdomen at the level of L4 (Siemens Trio, 3T, Siemens Medical Systems, Erlangen, Germany) [17].Coefficients of variation are 1.6% for VAT and 1.1% for abdominal SAT, respectively.
2.7. Statistical Analysis
Data were analyzed using JMP Statistical Discovery Software (JMP Pro Version 15). We reported the results as mean +/− standard deviation (SD) for parametric distribution of data or median (inter quartile range) for non-parametric distribution of data. Depending on data distribution, we applied either the Student’s t-test (for parametric data) or the 2-sample Wilcoxon rank sum test (for non-parametric data) to compare between group differences for continuous variables. Fisher’s Exact test was used to compare proportions. A two-tailed p-value less than 0.05 was considered significant. All data were adjusted for age and race using multivariable regression analysis. Univariate Spearman correlational analyses were used to determine associations of the components of the load-to-strength ratio with demographic, body composition and bone parameters. We further examined interactions by using an interaction term (group* lean mass) in the multivariable regression analysis. To identify the independent contributions of fat mass and lean mass and fat depots (visceral and subcutaneous fat), we ran multiple regression analyses with these variables and checked for collinearity using the variation inflation factor, which was low. We report standardized β weights for each independent variable, which indicates the relative contribution of each variable in that regression model. A p-value of <0.05 was used as the threshold for statistical significance.
3. Results
3.1. Clinical characteristics, body composition, and bone parameters
Baseline characteristics are shown in Table 1. OB and HC subjects did not differ for age, race, or height. In OB group, the age ranged between 15.57 to 21.37 years, and in HC group age ranged from 14.27 to 21.43 years. Eight (out of 33) participants in the OB group had a history of PCOS but currently all participants reported normal menses in the last six months. Fractures reported in both groups were either traumatic or stress fractures and occurred at the extremities (upper/lower).
Table 1:
Baseline Characteristics of Females with Obesity (OB) and Normal-weight Healthy Controls (HC)
| OB N = 33 |
HC N = 32 |
P-value | |
|---|---|---|---|
| Age (years) | 18.23 ± 1.74 | 18.98 ± 1.63 | 0.08 |
| Weight (kg) | 120.71 ± 17.32 | 59.43 ± 6.39 | <.0001 |
| Height (cm) | 164.27 ± 7.11 | 163.77 ± 5.45 | 0.75 |
| BMI (kg/m2) | 44.85 ± 6.81 | 22.19 ± 2.49 | <.0001 |
| Other / not reported | 7 (21.2%) | 8 (25%) | |
| Mixed / Unknown | 1 (3.0%) | 1 (3.1%) | |
| Age of menarche (years) | 11.45 ± 2.53 | 12.52 ± 1.17 | 0.03 |
| Stage 4 | 3.4% | 9.4% | |
| Stage 4 | 3.6% | 6.2% | |
| History of fracture | 6 (18.2%) | 9 (28.1%) | 0.41 |
| % Lean Mass | 50.33 ± 3.09 | 69.10 ± 5.24 | <.0001 |
| % Fat Mass | 49.61 ± 3.14 | 29.52 ± 5.58 | <.0001 |
| Total Lean Mass (kg) | 61.35 ± 7.82 | 41.93 ± 4.22 | <.0001 |
| Total Fat Mass (kg) | 60.94 ± 10.54 | 18.10 ± 4.76 | <.0001 |
| Visceral Adipose Tissue (cm2) | 822.52 ± 242.47 | 180.16 ± 80.22 | <.0001 |
| Subcutaneous Adipose Tissue (cm2) | 665.61 ± 185.05 | 223.43 ± 87.90 | <.0001 |
| Ratio of VAT/SAT | 1.11 (0.94–1.56) | 0.73 (0.66–0.86) | <.0001 |
Data presented as Mean ± SD or Median, (IQR). Bold denotes significant p-value. For continuous variables, between group differences were compared using the Student t-test for parametric data and the 2-sample Wilcoxon rank sum test for non-parametric data. Proportions were compared using the Fisher Exact Test.
By design, OB subjects had higher weight and BMI than HC subjects. OB had higher lean mass, fat mass, % fat mass, VAT, SAT and VAT/SAT ratio than HC. Table 2 shows bone parameters at the distal radius. Total, trabecular, and cortical vBMD were higher in OB than HC after controlling for race and age. OB had higher cortical area and cortical porosity compared with HC. Impact force, failure load and load-to-strength ratio were significantly higher in OB than HC, even after adjusting for age and race (Figure 1). Considering 0.75 as a possible value approaching “high risk” for load-to-strength ratio, 10.8% of OB had a load-to-strength ratio above 0.75 compared with none of the HC (p=0.002).
Table 2:
Bone Parameters at Distal Radius as Assessed by High Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) in Females with Obesity (OB) and Normal-weight Healthy Controls (HC)
| OB N = 33 |
HC N = 32 |
P-value | P-value adjusted for age and race | |
|---|---|---|---|---|
| Total vBMD (mgHA/cm3) | 368.33 ± 65.88 | 319.85 ± 53.14 | 0.002 | 0.0003 |
| Trabecular vBMD (mgHA/cm3) | 202.86 ± 35.21 | 177.93 ± 35.36 | 0.006 | 0.005 |
| Cortical vBMD (mgHA/cm3) | 850.67 ± 45.26 | 828.61 ± 63.84 | 0.11 | 0.005 |
| Total bone area (mm2) | 269.05 ± 58.00 | 262.47 ± 38.21 | 0.59 | 0.92 |
| Cortical thickness (mm) | 0.93 ± 0.19 | 0.76 ± 0.18 | 0.0004 | <0.0001 |
| Cortical area/Total area | 0.24 ± 0.07 | 0.20 ± 0.06 | 0.01 | 0.002 |
| Cortical porosity (%) | 1.26 ± 0.55 | 0.87 ± 0.50 | 0.004 | 0.018 |
| Impact Force (N) | 2961 ± 239 | 2076 ± 125 | <.0001 | <0.0001 |
| Failure Load (N) | 4610.93 ± 769 | 3901.52 ± 641 | 0.0002 | 0.0005 |
| Load-to-Strength Ratio | 0.69 ± 0.11 | 0.54 ± 0.08 | <.0001 | <.0001 |
Data presented as Mean ±SD. Bold denotes significant p-value. Between group differences were compared using the Student t-test for parametric data and the 2-sample Wilcoxon rank sum test for non-parametric data
Figure 1:

Load-to-Strength Ratio in Females with Obesity (OB) and Normal-Weight Healthy Controls (HC) at the Radius. (Line indicates mean)
3.2. Associations of Body Composition with Impact Force (Load), Bone strength and Load-to-strength Ratio
The associations of body composition with impact force, bone strength and load-to-strength ratio are shown in Table 3. Of note, a subset of this dataset demonstrating associations of body composition with bone strength have been previously published [12].
Table 3:
Associations of Impact Force (Load), Failure Load (Strength) and Load-to-strength Ratio with Clinical Characteristics and Body Composition in Females with Obesity (OB) and Normal-weight Healthy Controls (HC)
| Impact Force | Failure Load | Load-to-Strength Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB | HC | OB | HC | OB | HC | |||||||
| R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | |
| BMI (kg/m2) | 0.65 | <.0001 | 0.64 | <.0001 | 0.01 | 0.94 | 0.24 | 0.19 | 0.35 | 0.049 | −0.04 | 0.82 |
| % Lean mass | −0.49 | 0.003 | −0.20 | 0.28 | 0.39 | 0.03 | 0.40 | 0.02 | −0.65 | <.0001 | −0.50 | 0.004 |
| % Fat mass | 0.46 | 0.007 | 0.13 | 0.47 | −0.40 | 0.02 | −0.37 | 0.04 | 0.64 | <.0001 | 0.44 | 0.01 |
| Total lean mass (g) | 0.91 | <.0001 | 0.74 | <.0001 | 0.44 | 0.01 | 0.68 | <.0001 | 0.006 | 0.96 | −0.45 | 0.01 |
| Total fat mass (g) | 0.89 | <.0001 | 0.49 | 0.005 | 0.003 | 0.99 | −0.19 | 0.30 | 0.42 | 0.01 | 0.38 | 0.03 |
| Visceral Adipose Tissue (VAT cm2) | 0.65 | <.0001 | 0.44 | 0.01 | −0.11 | 0.56 | −0.13 | 0.47 | 0.36 | 0.04 | 0.32 | 0.07 |
| Subcutaneous Adipose Tissue (SAT cm2) | 0.51 | 0.002 | 0.38 | 0.11 | 0.08 | 0.65 | −0.23 | 0.35 | 0.17 | 0.35 | 0.35 | 0.14 |
| Ratio of VAT/SAT | 0.17 | 0.34 | 0.03 | 0.89 | −0.16 | 0.38 | −0.46 | 0.048 | 0.17 | 0.36 | 0.53 | 0.02 |
Bolded numbers denote significant p-values.
Impact force was positively associated with BMI, total lean and fat mass, and visceral fat in both OB and HC. In OB, impact force was associated positively with subcutaneous fat and negatively with percent lean mass. On adding total fat mass and total lean mass to a regression model to determine independent contributors to impact force, we saw that both variables contributed positively to impact force in both OB (total lean mass: p<0.0001, β weight 0.53; total fat mass: p<0.0001, β weight 0.52) and HC groups (total lean mass: p<0.0001, β weight 0.76; total fat mass: p<0.0001, β weight 0.59). Similarly, VAT and SAT contributed positively and independently to impact force (p= 0.001, β weight 0.51; and p=0.03, β 0.33 respectively) in OB group.
Failure load was positively associated with total and percent lean mass, and negatively with percent fat mass in both OB and HC. On adding total fat mass and total lean mass to a regression model to determine independent contributors to failure load, we saw that total lean mass contributed positively (p<0.0001, β weight 0.93) and total fat mass contributed negatively (p=0.003, β weight −0.62) to failure load in the OB group. In the HC group, total lean mass contributed positively to failure load (p=0.0007, β weight 0.57). No association was detected with specific fat depots.
Load-to-strength ratio was positively associated with BMI in OB but not HC. In both OB and HC, load-to-strength ratio was associated negatively with percent lean mass (Figure 2) and percent fat mass, and positively with total fat mass. On adding total fat mass and total lean mass to a regression model to determine independent contributors of load-to-strength ratio, total lean mass contributed negatively (p=0.002, β weight −0.67) and total fat mass contributed positively (p=0.0003, β weight 0.83) to load-to-strength ratio in the OB group. In the HC group, total fat mass contributed positively to load-to-strength ratio (p=0.021, β weight 0.39); no association was detected with total lean mass or specific fat depots. In the OB group, load-to-strength ratio was positively associated with VAT (p=0.049, β weight 0.37). Further, we found an interaction between percent lean mass and load-to-strength ratio by group status (OB vs. HC) (β estimate = 0.76; p=0.004). This suggests that a 0.014 greater per unit decrease in load-to-strength ratio by a given increase of 1 % lean mass in OB as compared with HC.
Figure 2:
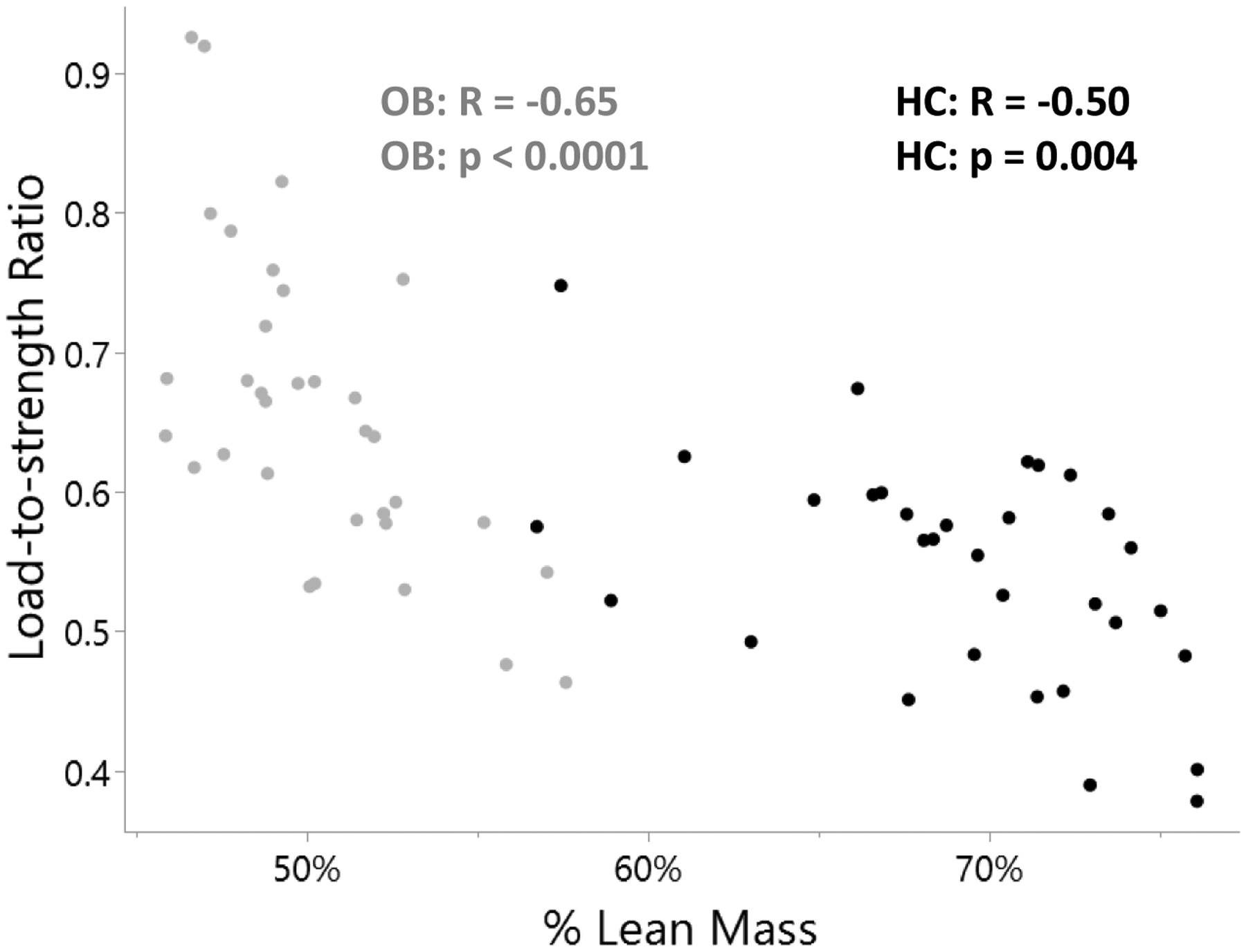
Associations of % Lean Mass with Load-to-strength Ratio
3.2. Associations of Bone Parameters with Impact Force (Load), Bone strength and Load-to-strength Ratio
Impact force was positively associated with total bone area and cortical porosity in HC but not OB. Failure load (bone strength estimate) was positively associated with trabecular vBMD, total bone area and cortical thickness in both OB and HC. In OB and HC, load-to-strength ratio was negatively correlated with trabecular vBMD (Table 4).
Table 4:
Associations of Impact Force (Load), Failure Load (Strength) and Load-to-strength Ratio with Bone Parameters (obtained via HR-pQCT) in Females with Obesity (OB) and Normal-weight Healthy Controls (HC)
| Impact Force | Failure Load | Load-to-Strength Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB | HC | OB | HC | OB | HC | |||||||
| R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | R | P-value | |
| Total vBMD (mgHA/cm3) | 0.09 | 0.63 | −0.03 | 0.85 | 0.34 | 0.05 | 0.47 | 0.007 | −0.27 | 0.13 | −0.55 | 0.001 |
| Trabecular vBMD (mgHA/cm3) | 0.21 | 0.25 | 0.007 | 0.97 | 0.57 | 0.0005 | 0.55 | 0.001 | −0.44 | 0.01 | −0.66 | <.0001 |
| Cortical vBMD (mgHA/cm3) | −0.05 | 0.80 | −0.06 | 0.75 | 0.14 | 0.45 | 0.13 | 0.49 | −0.18 | 0.32 | −0.15 | 0.41 |
| Total bone area (mm2) | 0.09 | 0.63 | 0.45 | 0.01 | 0.47 | 0.008 | 0.36 | 0.04 | −0.46 | 0.01 | −0.21 | 0.25 |
| Cortical thickness (mm) | 0.08 | 0.67 | 0.11 | 0.54 | 0.39 | 0.03 | 0.39 | 0.03 | −0.32 | 0.07 | −0.31 | 0.08 |
| Cortical area/Total area | 0.14 | 0.47 | −0.09 | 0.60 | 0.07 | 0.73 | 0.25 | 0.27 | 0.009 | 0.96 | −0.21 | 0.26 |
| Cortical porosity (%) | 0.22 | 0.23 | 0.37 | 0.04 | 0.08 | 0.65 | 0.25 | 0.18 | 0.09 | 0.62 | 0.25 | 0.18 |
Bolded numbers denote significant p-values.
4. Discussion
Using the load-to-strength biomechanical formula to estimate fracture risk, we demonstrate that adolescents and young adult females with severe obesity have a higher estimated load-to-strength ratio and are therefore at a higher risk of fracture at the distal radius compared to age-matched normal-weight healthy controls. Our findings provide a potential explanation for the observation that despite having a higher average BMD, adolescents with obesity have a greater propensity for fracture than those of normal-weight, especially at the upper extremities [18] [19, 20] [4]. Whereas obesity is associated with higher vBMD [21], this does not fully compensate for the excess mechanical loading sustained by the radius in a fall from standing height. This is despite a higher estimated failure load and total vBMD seen in OB, which suggests that the adaptive increase in bone strength that happens with increasing weight is suboptimal to resist the force generated by a fall in individuals with greater weight. It has previously been shown that adolescent and young adult females with OB do not demonstrate the appropriate adaptation to higher weight for certain bone parameters, such as cortical porosity and the ratio of trabecular rod bone volume to total bone volume, both of which are increased in OB and are associated with lower bone strength [12] [22] [23] [24]. The greater estimated force of impact generated during a fall on an outstretched hand in OB in the setting of suboptimal bone adaptation provides an explanation for the greater load-to-strength ratio and hence greater fracture risk in the upper extremities in females with OB.
In this study, we also evaluated associations of body composition and fat distribution with impact force, bone strength and the load-to-strength ratio. We found that in OB, total lean and fat mass were associated positively and independently with impact force. Furthermore, total fat mass was positively associated with load-to-strength ratio even after controlling for total lean mass. However, total fat mass was associated inversely, while lean mass was associated positively with bone strength. This is consistent with previous literature demonstrating positive effects of lean mass and negative effects of fat mass (particularly visceral fat) on bone endpoints [25]. This also suggests that bone only adapts to lean mass and not fat mass both in the normal-weight and obese states. Furthermore, this implies that with greater increments in fat mass than lean mass as seen in severe obesity, there are diminishing benefits of increasing weight on load-to-strength ratio [1]. Similarly, visceral fat was associated positively with impact force and hence positively with load-to-strength ratio in this study. While increasing BMI is protective against osteoporosis among adults [26], data suggest a differential impact of fat mass vs. lean mass on BMD with lean mass having a greater positive influence on BMD than fat mass in postmenopausal women and young adults [27] [28] [29–31]. Altogether, these findings support reducing fat mass and increasing lean mass as potential strategies to protect bone strength in those with obesity.
Moreover, we found a greater benefit of percent lean mass on load-to-strength ratio in OB vs. HC as suggested by a significant group interaction. These data suggest that one standard unit increase in % lean mass may lead to greater decreases in load-to-strength ratio in OB vs. HC. Thus, targeted interventions in those with obesity, specifically reinforcing benefits of resistance exercise and healthy diet to increase lean mass and decrease fat mass, may promote bone strength, and decrease fracture risk. Changing the body composition (even if keeping the total body weight same) which leads to greater percent lean mass will lead to increases in failure load while keeping the impact force similar, thereby decreasing the load-to-strength ratio. This will decrease the fracture risk in individuals with obesity.
Our study had some limitations. We only examined bone strength at the distal radius in adolescents and young adult females and we know that results could differ by sex, site of the body, and the nature of the fall [32, 33]. Future assessment of axial and appendicular sites in both sexes will provide a comprehensive understanding of fracture risk at weight-bearing sites. Further, we have used a fixed site at distal radius for evaluation of HRpQCT measures which can be less accurate in growing children. However, because our cohort had mostly completed linear growth, this should not affect our results. Also, we reported the biomechanical aspects associated with obesity but have not elaborated on concurrent biochemical alterations which can adversely alter the bone microenvironment [34]. This should also be investigated in future studies.
5. Conclusion
Although obesity is associated with greater BMD, the relative increase in bone strength may not compensate for other fracture-inducing factors. Our study suggests that adolescent females with obesity have a higher load-to strength ratio at the distal radius due to higher forces applied to bone in a fall combined with incomplete adaptation of bone to increasing body weight, and that this is differentially affected by lean mass, fat mass, and visceral fat mass. Longitudinal studies that include both sexes and assess bones in diverse weight-bearing areas are needed to gain a comprehensive understanding of bone health across the weight spectrum.
Funding:
NIH NIDDK R01 DK103946-01A1 (MM, MAB), NIH K23DK110419-01 (VS), K24DK109940 (MAB), K24 HD071843 (MM), NIH P30-DK057521 (VS), NIH-P30 DK040561(VS), S10 OD025248 (MLB), 1UL1TR002541-01
Footnotes
Conflicts of Interest: Authors have no conflicts of interest to disclose relevant to this paper.
References
- 1.Hurt RT, et al. , Obesity epidemic: overview, pathophysiology, and the intensive care unit conundrum. JPEN J Parenter Enteral Nutr, 2011. 35(5 Suppl): p. 4s–13s. [DOI] [PubMed] [Google Scholar]
- 2.Marcus R, et al. , Correlates of bone mineral density in the postmenopausal estrogen/progestin interventions trial. J Bone Miner Res, 1994. 9(9): p. 1467–76. [DOI] [PubMed] [Google Scholar]
- 3.Ravn P, et al. , Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res, 1999. 14(9): p. 1622–7. [DOI] [PubMed] [Google Scholar]
- 4.Goulding A, Grant AM, and Williams SM, Bone and Body Composition of Children and Adolescents With Repeated Forearm Fractures. Journal of Bone and Mineral Research, 2005. 20(12): p. 2090–2096. [DOI] [PubMed] [Google Scholar]
- 5.Macneil JA and Boyd SK, Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone, 2008. 42(6): p. 1203–13. [DOI] [PubMed] [Google Scholar]
- 6.Johnston JD, et al. , A single-spring model predicts the majority of variance in impact force during a fall onto the outstretched hand. J Biomech, 2019. 90: p. 149–152. [DOI] [PubMed] [Google Scholar]
- 7.Bouxsein ML, et al. , Contribution of trochanteric soft tissues to fall force estimates, the factor of risk, and prediction of hip fracture risk. J Bone Miner Res, 2007. 22(6): p. 825–31. [DOI] [PubMed] [Google Scholar]
- 8.Christen D, et al. , Improved fracture risk assessment based on nonlinear micro-finite element simulations from HRpQCT images at the distal radius. J Bone Miner Res, 2013. 28(12): p. 2601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann KN, et al. , Vertebral Volumetric Bone Density and Strength Are Impaired in Women With Low-Weight and Atypical Anorexia Nervosa. J Clin Endocrinol Metab, 2017. 102(1): p. 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ 3rd, et al. , Structural determinants of vertebral fracture risk. J Bone Miner Res, 2007. 22(12): p. 1885–92. [DOI] [PubMed] [Google Scholar]
- 11.Hsu YH, et al. , Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr, 2006. 83(1): p. 146–54. [DOI] [PubMed] [Google Scholar]
- 12.Singhal V, et al. , Suboptimal bone microarchitecure in adolescent girls with obesity compared to normal-weight controls and girls with anorexia nervosa. Bone, 2019. 122: p. 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner AC and Skelton JA, Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr, 2014. 168(6): p. 561–6. [DOI] [PubMed] [Google Scholar]
- 14.Whittier DE, et al. , Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos Int, 2020. 31(9): p. 1607–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutroy S, et al. , Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res, 2008. 23(3): p. 392–9. [DOI] [PubMed] [Google Scholar]
- 16.Pistoia W, et al. , Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone, 2002. 30(6): p. 842–8. [DOI] [PubMed] [Google Scholar]
- 17.Singhal V, et al. , Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone, 2015. 77: p. 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard MB, et al. , Obesity during childhood and adolescence augments bone mass and bone dimensions. The American Journal of Clinical Nutrition, 2004. 80(2): p. 514–523. [DOI] [PubMed] [Google Scholar]
- 19.Wetzsteon RJ, et al. , Bone structure and volumetric BMD in overweight children: a longitudinal study. J Bone Miner Res, 2008. 23(12): p. 1946–53. [DOI] [PubMed] [Google Scholar]
- 20.Goulding A, et al. , Bone mineral density in girls with forearm fractures. J Bone Miner Res, 1998. 13(1): p. 143–8. [DOI] [PubMed] [Google Scholar]
- 21.Hoy CL, Macdonald HM, and McKay HA, How does bone quality differ between healthy-weight and overweight adolescents and young adults? Clin Orthop Relat Res, 2013. 471(4): p. 1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bala Y, et al. , Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res, 2014. 29(6): p. 1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, et al. , Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures. Bone, 2016. 88: p. 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu XS, et al. , Quantification of the Roles of Trabecular Microarchitecture and Trabecular Type in Determining the Elastic Modulus of Human Trabecular Bone. Journal of Bone and Mineral Research, 2006. 21(10): p. 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sornay-Rendu E, et al. , In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res, 2013. 28(7): p. 1679–87. [DOI] [PubMed] [Google Scholar]
- 26.Dimitri P, et al. , Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone, 2012. 50(2): p. 457–66. [DOI] [PubMed] [Google Scholar]
- 27.Ho-Pham LT, et al. , Contributions of lean mass and fat mass to bone mineral density: a study in postmenopausal women. BMC Musculoskelet Disord, 2010. 11: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilesanmi-Oyelere BL, et al. , Lean Body Mass in the Prediction of Bone Mineral Density in Postmenopausal Women. Biores Open Access, 2018. 7(1): p. 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HG, et al. , Lean mass and peak bone mineral density. Osteoporos Sarcopenia, 2020. 6(4): p. 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell DM, et al. , Trabecular Bone Morphology Correlates With Skeletal Maturity and Body Composition in Healthy Adolescent Girls. J Clin Endocrinol Metab, 2018. 103(1): p. 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr JN, et al. , Body composition during childhood and adolescence: relations to bone strength and microstructure. J Clin Endocrinol Metab, 2014. 99(12): p. 4641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler J, et al. , Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res, 2013. 471(4): p. 1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JE, et al. , Childhood obesity as a risk factor for bone fracture: a mechanistic study. Obesity (Silver Spring), 2013. 21(7): p. 1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savvidis C, Tournis S, and Dede AD, Obesity and bone metabolism. Hormones (Athens), 2018. 17(2): p. 205–217. [DOI] [PubMed] [Google Scholar]


