Abstract
In modern 24 h society, circadian disruption is pervasive, arising from night shift work, air travel across multiple time zones, irregular sleep schedules, and exposure to artificial light at night. Disruption of the circadian system is associated with many adverse health consequences, including mood disorders. Here we investigate whether inducing circadian misalignment using a phase advance protocol interferes with the ability to cope with a stressor, thereby increasing susceptibility to the negative consequences of stress. Male rats were maintained on a standard 12:12 light: dark (LD) cycle or subjected to a chronic phase advance (CPA) protocol involving 4 weekly 6 h phase shifts (earlier light onset) of the LD cycle. Rats were then exposed to escapable stress (ES), inescapable stress (IS), or no stress (home cage control; HC) and performance on juvenile social exploration and active escape learning in the two-way shuttlebox test was assessed 24 h and 48 h following stress, respectively. CPA alone had no effect on pre-stress juvenile social exploration, and it also did not interfere with the protective effect of ES on the stress-induced reduction in juvenile social exploration. In contrast, CPA impaired escape learning in the two-way shuttlebox to the same extent as IS in all subjects, regardless of stress history. Additionally, CPA produced somatic alterations that included increased body mass, increased epididymal adiposity, and decreased adrenal mass. These data indicate that CPA differentially modulated the stress-protective effects of behavioral control depending on the type of affective behavior examined.
Keywords: Circadian misalignment, Shift work, Stress, Resilience, Behavioral control
1. Introduction
Mammalian physiology and behavior are temporally organized across the 24 h day. Daily rhythms are coordinated by the circadian system, which is comprised of a hierarchy of biological clocks present throughout the brain and body. The master clock, located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, receives light information from specialized cells in the retina and communicates with subordinate clocks via neural and humoral routes [1]. A key feature of the circadian system is its ability to adapt to abrupt environmental changes (i.e. phase shifts). However, short-term misalignment between internal physiology and external time (termed ‘jet lag’) can induce transient symptoms of fatigue, impaired cognition, gastro-intestinal distress, and diminished mood [2]. Moreover, mounting epidemiological evidence suggests that chronic circadian disruption, as with shift work, is linked to long-term health consequences, including increased risk of cancer, metabolic syndrome, cardiovascular disease, infertility, and mood disorders [3].
There is a significant body of research linking the circadian system to mood disturbances. Mood disorders are associated with alterations in daily rhythms such as the sleep-wake cycle, body temperature, neurotransmitter release, and hormone levels [4]. For example, many depressed patients exhibit blunted cortisol rhythm amplitude and a shift in peak nocturnal cortisol secretion [5]. Furthermore, chronobiological interventions such as bright light therapy can rapidly, albeit temporarily, improve depressive symptoms [6]. However, it is unclear whether these circadian abnormalities are causal or simply symptomatic of mood disorders. In support of a causative relationship, genetic and environmental manipulations of the circadian system are associated with changes in affective behavior in rodent models. For example, mice with a mutation in core circadian regulatory gene circadian locomotor output cycles kaput (CLOCK) display mania-like behaviors [7]. Furthermore, exposure to disruptive light: dark (LD) cycles is associated with increases in depressive-like responses in both diurnal and nocturnal rodents [8–10]. Additionally, mice entrained to a shortened 20-h LD cycle exhibit morphological changes in prefrontal cortical neurons [11], a region important for emotional control.
Importantly, roughly 15–20% of the labor force in industrialized countries participates in evening or rotating shift work [12]. Non-traditional work schedules interfere with normal circadian function by way of mistimed meals, exposure to light at night, and forced activity during the rest period. Interestingly, there is a greater prevalence of mood disorders such as major depressive disorder (MDD) in shift workers during and following their shift work employment, and risk increases as a function of employment duration [13,14]. This suggests that circadian misalignment and/or disruption might make individuals more prone to alterations in mood.
Shift workers report high levels of psychological stress [15], and stress is a major risk factor for the development of MDD [16]. One active coping mechanism that mitigates stressor outcomes is the degree to which an individual can exert behavioral control over aspects of the stressor (e.g. onset/offset, duration, pattern). Behavioral control is studied in rodents using a triadic design. One subject is able to terminate each of a series of electrical tail shocks by turning a wheel located on the front wall of the chamber (escapable stress, ES), while a yoked partner passively receives the same duration of tail shocks (inescapable stress, IS); turning the wheel has no consequence for the IS subject. A third subject is left undisturbed in the home cage and serves as a non-stressed control (HC). Exposure to IS produces behavioral sequelae indicative of an anxiety and depression-like phenotype, including enhanced fear, increased drug taking, reduced social interest, and poor escape learning [17]. In contrast, these effects are absent in ES subjects and prior ES can even buffer against subsequent uncontrollable stressors [18].
Taken together, this evidence led us to hypothesize that circadian misalignment might interfere with the ability to cope with a stressor and thereby increase susceptibility to the negative consequences of stress. To test this question, we exposed rats to a chronic phase advance (CPA) protocol in which the LD cycle was shifted by 6 h (earlier light onset) once per week for four consecutive weeks. Rats were then exposed to ES, IS, or no stress (HC), and behavior was assessed on the juvenile social exploration and active escape learning tasks, 24 and 48 h after stressor exposure (Fig. 1).
Fig. 1.
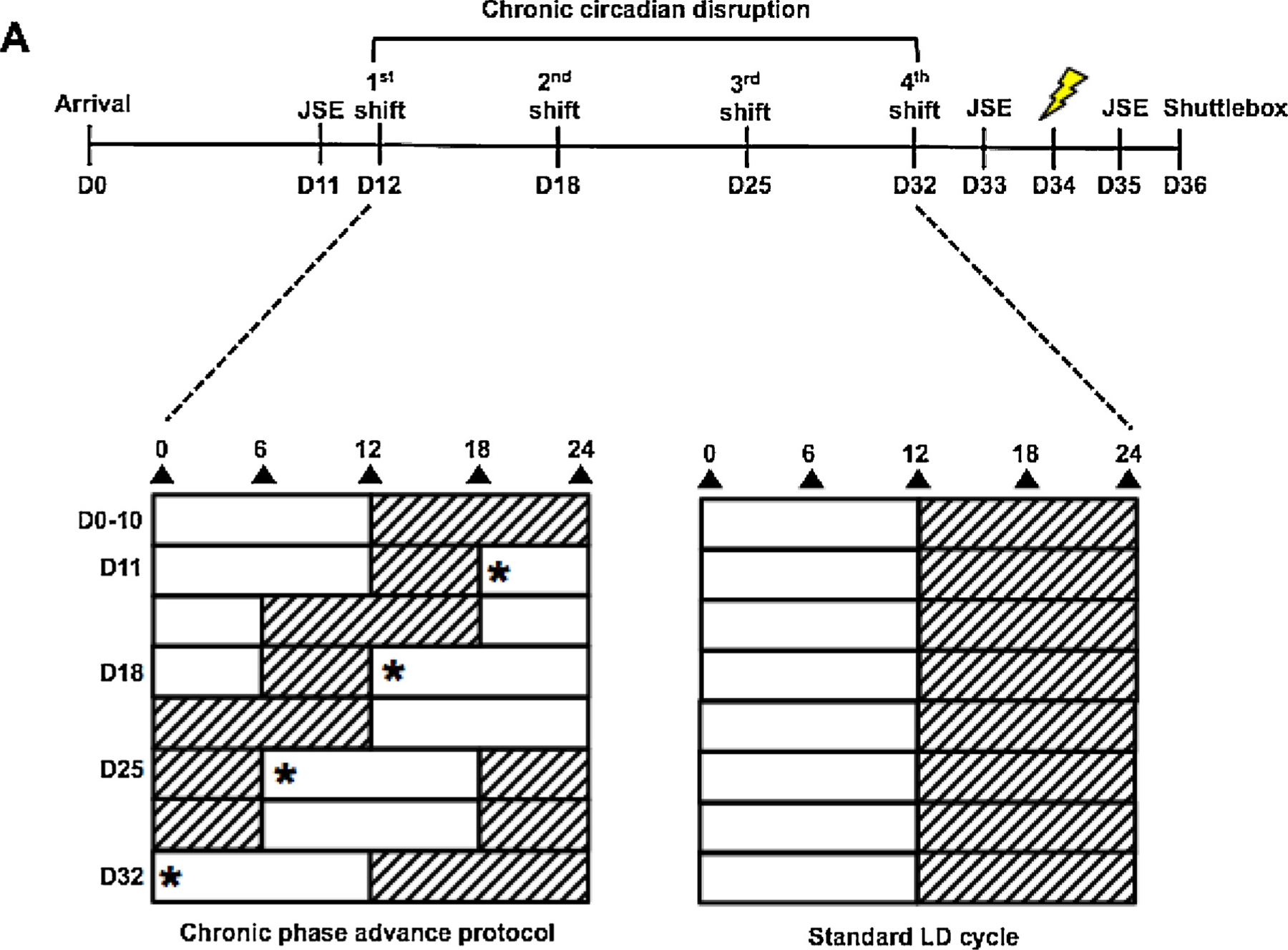
Experimental design. Upon arrival, rats were housed in a standard LD cycle for 10 days before experimentation. On Day 10, rats were evaluated on a baseline juvenile social exploration (JSE) test. On Day 12, half of the rats were subjected to a chronic phase advance (CPA) protocol, which consisted of 4 weekly 6 h phase advances of the LD cycle (2nd shift = Day 18, 3rd shift = Day 25, 4th shift = Day 32), * represents the new ZT following each shift. On Day 33, rats were evaluated on another JSE test. On Day 34, rats were exposed to 100 trials of ES, IS, or HC. 24 and 48 h following stress, rats were evaluated on a JSE and shuttlebox tests, respectively, to determine whether chronic circadian disruption would prevent learned stressor resistance.
2. Methods and materials
2.1. Animals
Subjects were male Sprague-Dawley rats (Envigo, Indianapolis, IN) weighing 200–225 g upon arrival. Rats were pair-housed in poly-carbonate cages (47 × 23 × 20 cm) in light-, temperature-, and humidity-controlled colony rooms. Food and water were available ad libitum, except during stress procedures and behavioral testing. All experimental procedures were conducted in accordance with the University of Colorado’s Institutional Animal Care and Use Committee.
2.2. Experimental design
Rats were entrained to a standard 12:12 h light: dark (LD) cycle with lights on at 0700 [Zeitgeber time (ZT0)]. Control rats were maintained on the LD cycle while half of the subjects were subjected to a chronic phase advance (CPA) protocol (described in Section 2.3). 24 h prior to the first phase shift, all rats were evaluated for baseline juvenile social exploration (Section 2.5). 24 h after the final phase shift, all rats were evaluated for post-CPA juvenile social exploration. 48 h later the final phase shift, all rats then underwent ES, IS, or HC treatment (Section 2.4) during the first half of the light phase. All rats were then evaluated for post-stress juvenile social exploration and escape learning in the two-way shuttlebox task (Section 2.6), 24 and 48 h respectively. Behavioral testing also occurred during the first half of the light phase (Fig. 1).
2.3. Chronic phase advance protocol
The chronic phase advance (CPA) protocol was as described previously [19]. Briefly, rats were subjected to a 6 h phase advance of the LD cycle once every 7 d for 4 consecutive shifts. Each shift was achieved by shortening the previous dark period. Cage changes were performed between ZT2-4 for both groups. Shifted subjects were returned to the same LD cycle as control subjects during stress procedures and behavioral testing.
2.4. Escapable and inescapable stress procedures
Rats received escapable stress (ES), inescapable stress (IS), or remained undisturbed in their home cage (HC). ES and IS rats were restrained in a Plexiglas box (14 × 11 × 17 cm) with a wheel mounted to the front and an acrylic rod extending from the rear. Rats were secured in the box by taping the tail to a rod. Electrical shocks were administered to the tail via copper electrodes (spaced approximately 2.5 cm apart) and delivered by a Precision Animal Shocker (Coulbourn Instruments, Whitehall, PA, USA). The session consisted of 100 trials of tail shock (33 × 1.0 mA, 33 × 1.3 mA, and 34 × 1.6 mA) presented on a variable schedule with a 60 s average inter-trial interval. ES and IS subjects were yoked such that tail shock was terminated for both subjects when the ES subject completed the appropriate wheel turn (i.e. escape) response requirement. The initial response requirement was a single quarter wheel turn. The response requirement increased by one quarter wheel turn when each of three consecutive trials was met in < 5 s. Subsequent latencies under 5 s increased the requirement by 50% to a maximum of four wheel turns. If the requirement was not achieved in < 30 s, the tail shock was terminated and the requirement was reset to a single quarter wheel turn. This protocol is used to ensure that the ES subject acquires an operant response. The wheel was fixed for the IS subject. Thus, yoked subjects receive the same number, intensity, and duration of tail shocks. Subjects were returned to the colony immediately following the stress procedure.
2.5. Juvenile social exploration test
The juvenile social exploration test was conducted as described previously [20]. Briefly, rats were acclimated to a novel cage for 60 min before the test. A 28 ± 3 day-old juvenile conspecific was introduced to the cage. Social exploratory behaviors (sniffing, pinning, allogrooming, and following) initiated by the experimental rat were timed by an observer blind to experimental conditions. After 3 min, the juvenile was removed and the experimental rat was returned to its home cage. Juveniles were used for multiple tests but were never used more than once for the same experimental rat.
2.6. Shuttlebox escape test
The shuttlebox escape test was conducted as described previously [21], but without testing for shock-elicited freezing. Briefly, rats were placed into a two-way shuttlebox (Coulbourn Instruments, Whitehall, PA) and after a 5 min acclimation period, rats received 5 trials in which a single cross of the shuttlebox (FR-1) terminated an ongoing 0.8 mA scrambled foot shock. These were followed by 25 trials in which two crossings of the shuttle box were required to terminate foot shock (FR-2). Trials terminated automatically if the required response had not occurred by 30 s. Crossings were determined by infrared photo switches and latency to escape foot shocks were computed by Graphic State 3.0 software (Coulbourn Instruments, Whitehall, PA).
2.7. Statistical analyses
The study utilized a 2 × 3 factorial design. Experiment 2 utilized an unpaired between groups design. Comparisons for behavior analyses were conducted using an independent t-test, two-way analysis of variance (ANOVA), and repeated measures ANOVA with Fisher’s LSD post hoc. The significance level was set a p < 0.05. Data are shown as mean ± standard error of the mean (SEM). Scores that were ± 2 standard deviations away from the group mean were deemed outliers and excluded from statistical analysis. All analyses and graphs were created using GraphPad Prism (v. 4, GraphPad Software, La Jolla, CA) except for repeated measures ANOVA, which was performed using Statview (v. 5, SAS Institute, Cary, NC). Two rats were injured during the stress procedure and therefore excluded from further testing.
3. Results
3.1. Somatic measures
In rodents, exposure to non 24-h LD cycles, constant light, and dim light at night (dLAN) can all induce somatic alterations [22]. Average body mass was comparable among all groups at the beginning of the study (p < 0.05). However, CPA rats gained significantly more weight than did rats housed in a standard LD cycle prior to stress (t56 = 3.429, p < 0.01, Fig. 2A). Furthermore, there were significant effects of LD cycle and stress treatment on paired epididymal adipose mass (LD cycle: F (1, 50) = 7.485, p < 0.01 and stress: F (2, 50)= 6.919, p < 0.01; Fig. 2B) and paired adrenal mass (LD cycle: F (1, 50) = 4.774, p < 0.05 and stress treatment: F (2, 50)= 3.488, p < 0.05; Fig. 2C). Post hoc analyses revealed that CPA increased adiposity and reduced adrenal mass, whereas stress decreased adiposity and increased adrenal mass.
Fig. 2.
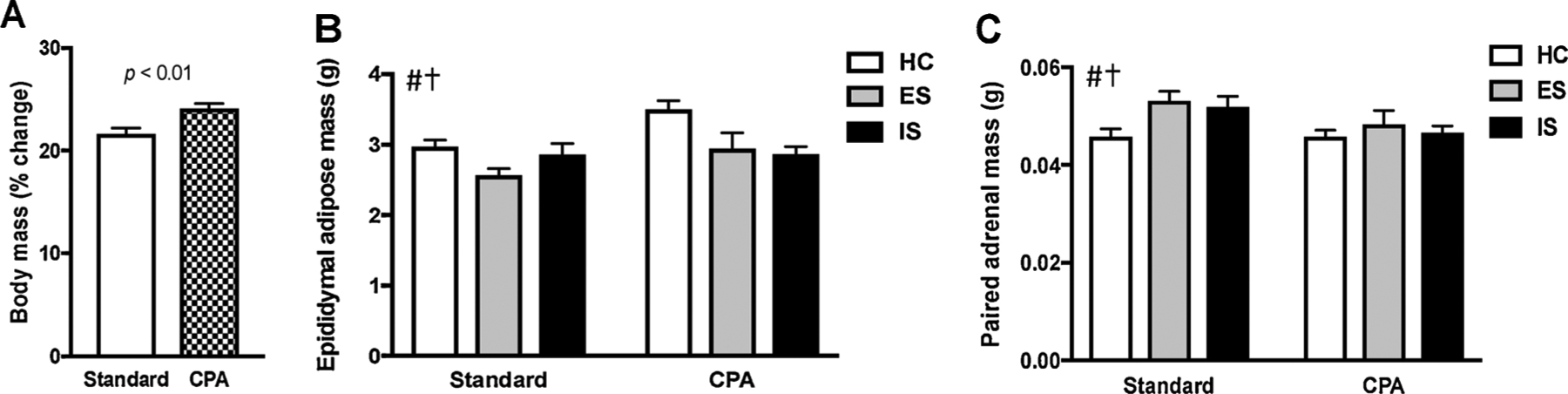
Chronic circadian disruption and stress induced somatic alterations. (A) CPA rats gained significantly more weight than rats housed on a standard LD cycle rats prior to stress, p > 0.01. (B) CPA rats exhibited significantly greater epididymal adipose tissue post mortem. (C) CPA rats also exhibited greater adrenal mass. Each bar represents the mean ± SEM, # represents a simple main effect of LD cycle, † represents a simple main effect of stress, p < 0.05 unless otherwise indicated.
3.2. Acquisition of behavioral control
CPA did not interfere with operant responding during ES. CPA rats learned the controlling escape response at a rate equivalent to rats maintained in a standard LD cycle (p > 0.05, Fig. 3A). Furthermore, there was no difference in the number of trials to reach maximum response criterion (16 quarter turns of the wheel) between groups (p > 0.05, Fig. 3B) and there was no difference in the number of non-contingent (error) responses between groups (p > 0.05, data not shown).
Fig. 3.
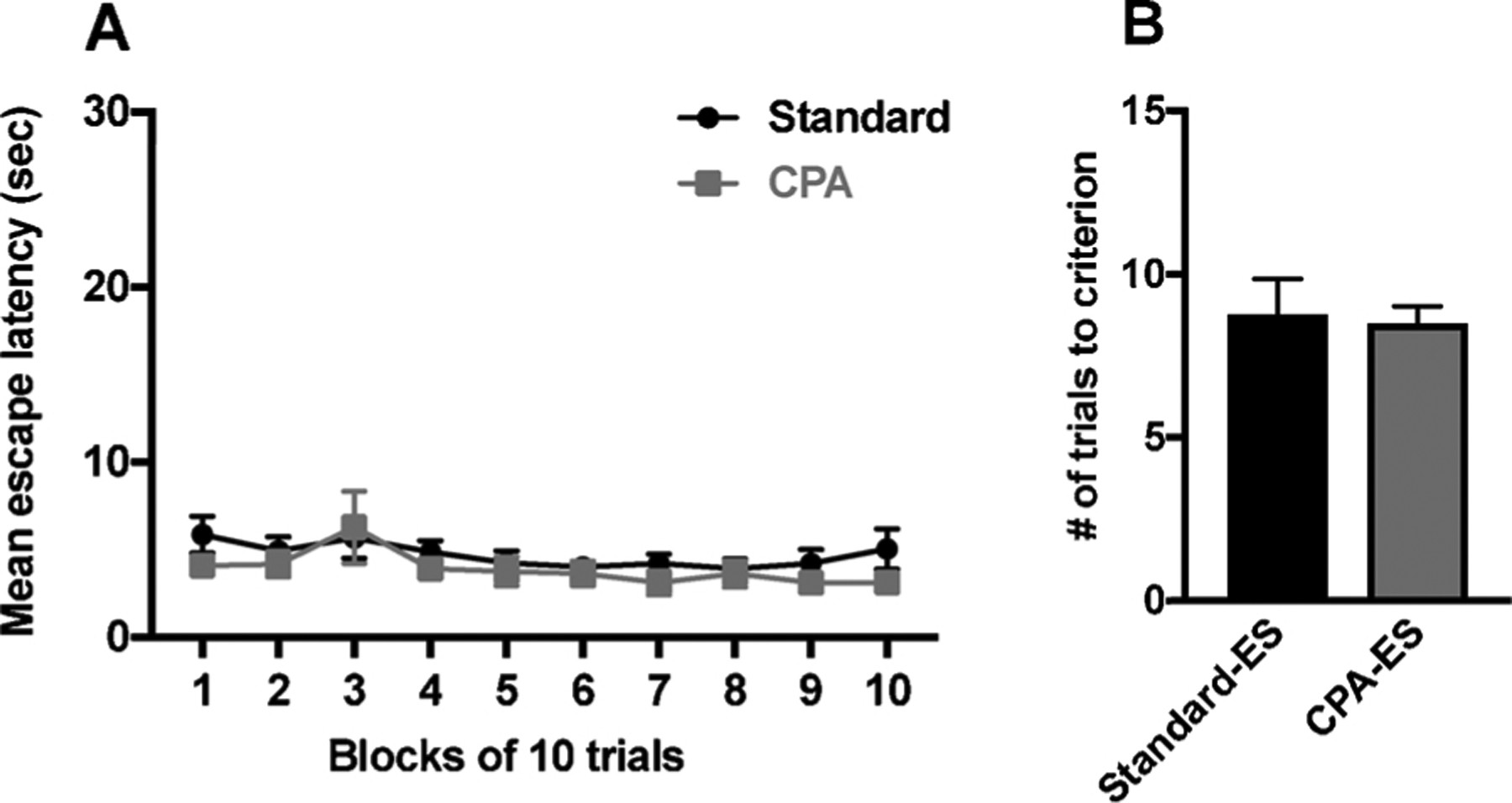
Chronic circadian disruption does not interfere with the acquisition of behavioral control. (A) Response latencies across all trials were equivalent between control LD cycle and CPA rats that received ES. (B) Similarly, the number of trials to reach FR-16 was also equivalent between groups. Each circle or bar represents the mean ± SEM, p < 0.05 unless otherwise indicated.
3.3. Juvenile social exploration
CPA had no effect on juvenile social exploration prior to stress (p > 0.05, Fig. 4A). As expected, ES rats housed in a standard LD cycle were protected from the typical IS-induced reduction in social investigation [F (2,46) = 12.53, p < 0.0001]. However, CPA did not interfere with the protective effect of ES on juvenile social exploration (p > 0.05, Fig. 4B).
Fig. 4.
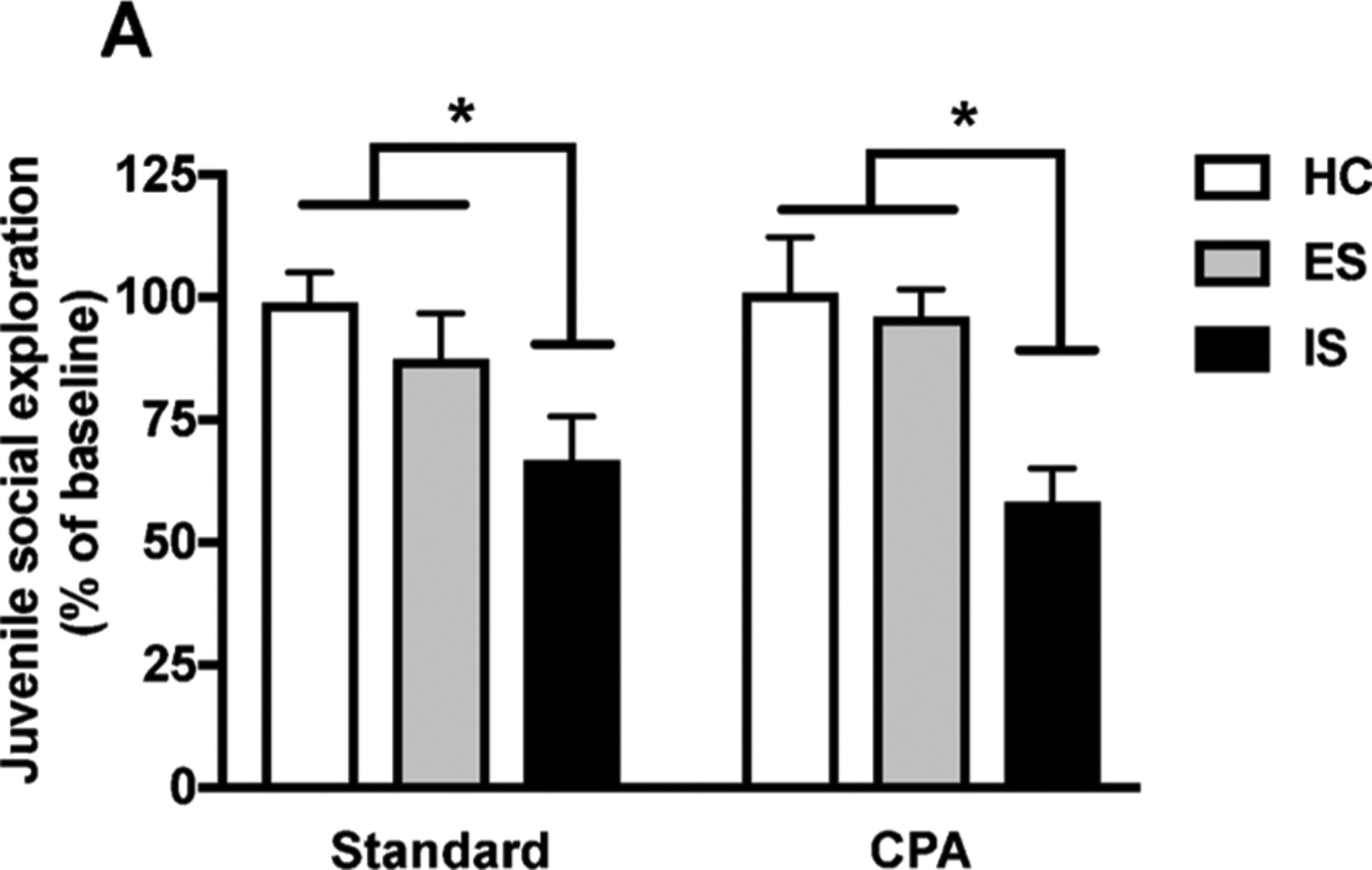
Chronic circadian disruption does not interfere with protective effects of behavioral control on juvenile social exploration. CPA had no effect on social exploration time (expressed as % of baseline). ES prevented the stress-induced reduction in social interaction. Each bar represents the mean ± SEM, p < 0.05 unless otherwise indicated.
3.4. Two-way shuttlebox escape
There was an interaction between light cycle and stress on active escape learning [F (1,36) = 5.144, p > 0.05]. As expected, ES rats housed in a standard LD cycle were protected from the typical IS-induced increase in escape latencies during FR-2 trials in the shuttlebox. However, CPA increased escape latencies in HC during FR-2 trials compared to rats housed in a standard LD cycle (Fig. 5). That is, CPA itself produced failure to learn to escape in the shuttlebox. These data suggest that CPA selectively interferes with shuttlebox FR-2 contingency learning but not shuttlebox FR-1 contingency learning or the controlling (wheel-turn) response during ES training.
Fig. 5.
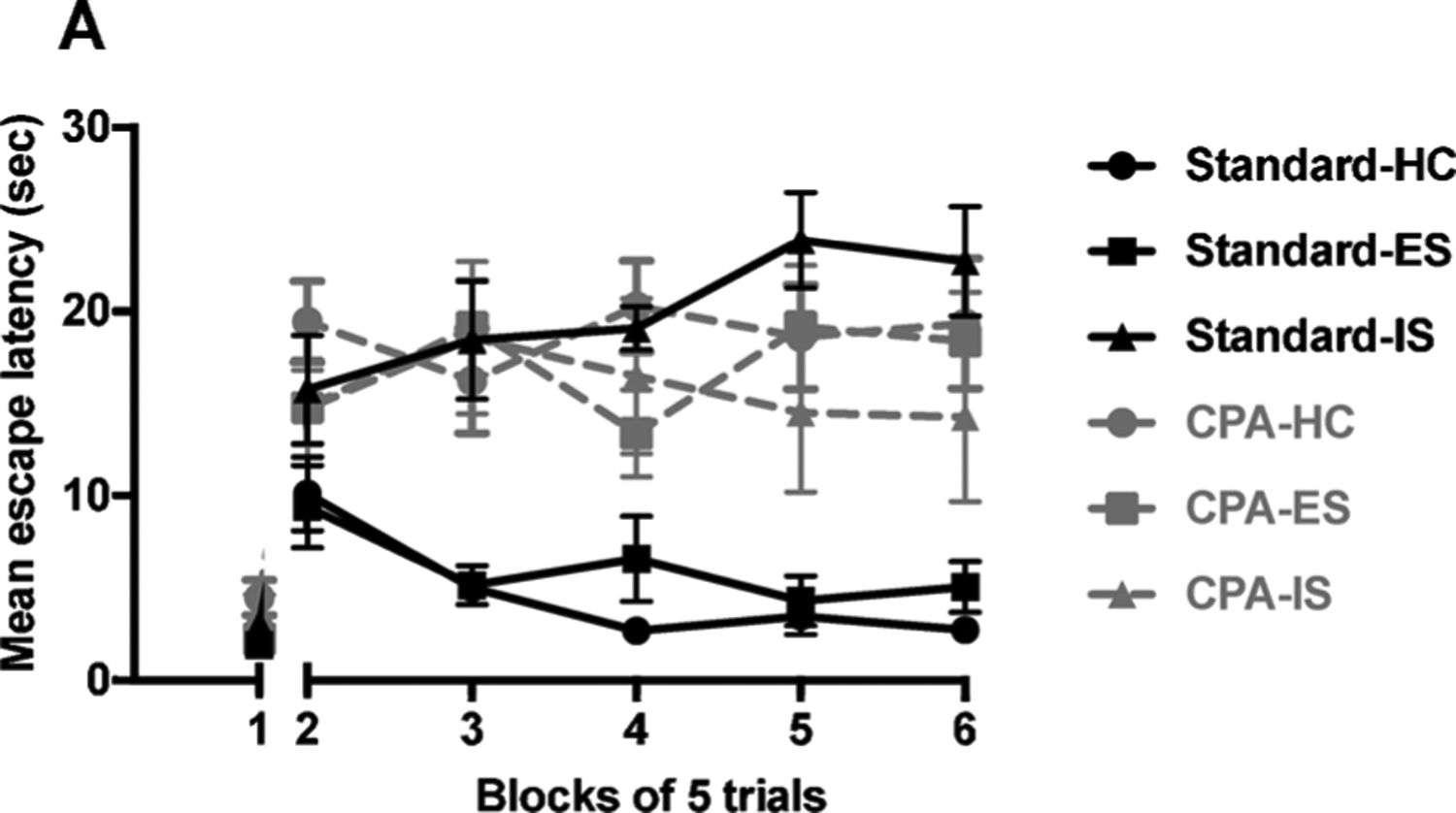
Chronic circadian disruption interferes with active escape learning in the shuttlebox. (A) Escape latencies were equivalent between HC and ES groups maintained on a standard LD cycle. Escape latencies for CPA groups were similar indicating that CPA impaired active escape learning. Each block is comprised of 5 trials; block 1 represents FR-1 responses and blocks 2–6 represent subsequent FR-2 responses. Each circle represents the mean ± SEM, p < 0.05 unless otherwise indicated.
4. Discussion
Stress is a major predisposing factor in the etiology of mood disorders; however, there are large individual differences in the outcome of stressor exposure. Therefore, it is valuable to investigate environmental factors that may contribute to stress susceptibility. In recent decades, the widespread use of electrical lighting has coincided with an increasing prevalence of MDD [23]. Exposure to unnatural and mis-timed light sources is commonplace in modern 24-h society. Shift workers, in particular, are at a high risk for mood disorders [24]. Thus, the present study evaluated whether inducing circadian misalignment: (1) interferes with a known stress-protective factor, behavioral control, and (2) increases susceptibility to the behavioral consequences that are produced by stress.
First, we determined whether circadian misalignment altered propensity to engage in a social encounter 24 h following stress. The juvenile social exploration test provides an ethologically relevant measure of anxiety in rodents [25]. CPA had no effect on social exploratory behavior prior to stress. In contrast, Reijmers and colleagues [26] showed that a single 6 h phase advance reduced overall social exploration time in rats, but did not impair the ability to discriminate between conspecifics (i.e. social discrimination). In that study, however, behavioral testing occurred 9 h after the phase shift [26], whereas in the present study, testing occurred 24 h following the last of four weekly phase shifts. This suggests that the effect of circadian misalignment on social behavior may be dependent on the number of phase shifts and proximity of testing. Furthermore, it is possible that social behavior is not under circadian control as Reijmers and colleagues (2001) also demonstrated that social behavior did not fluctuate across the LD cycle in non-shifted rats [26]. IS, but not ES, reliably produces a significant reduction (~30%) in social exploration for a number of days [20,27,28]. In the present study, as expected, ES subjects housed in a standard LD cycle were protected from the IS-induced reduction in juvenile social exploration 24 h following stress. However, contrary to our hypothesis, prior CPA did not interfere with the stress-buffering effects of ES on this measure; ES and HC rats that underwent CPA spent equivalent amounts of time engaged with the juvenile conspecific. This finding suggests that the protection provided by stressor controllability on social exploratory behavior is quite robust, as CPA did not reduce it. We next determined whether circadian disruption altered subsequent escape performance in the two-way shuttlebox task 48 h following stress. Typically, prior IS, but not ES, results in increased latency to escape foot shock specifically during FR-2 trials [29]. In the present study, CPA itself produced an escape deficit in all subjects, regardless of stress history, such that they were indistinguishable from standard LD cycle subjects that received prior IS. However, CPA also did not increase escape latencies above IS, which could reflect a ceiling effect in the shuttlebox task. This finding is similar to that of Landgraf et al. [30] in which SCN-selective knockdown of core clock gene, Bmal1, increased escape latencies and number of escape failures in the shuttlebox test in non-stressed mice. Thus, both environmental and genetic models of circadian disruption can, even in the absence of stress, produce an escape-learning deficit. However, the present results also indicate that CPA does not impair all forms of escape learning, given that rats exposed to CPA were able to rapidly and reliably learn the controlling (wheel-turn) response during ES training, and were also able to learn the FR-1 task during the initial phase of shuttlebox training. Instead, it appears that CPA impairs the more difficult learning component of the shuttlebox task. This result is similar to the effect of IS on shuttlebox performance; rats exposed to IS are able to learn the initial shuttlebox FR-1 contingency but show profound deficits in FR-2 contingency learning. Maier and colleagues [29] have suggested that rats acquire FR-1 contingencies reflexively, whereas FR-2 contingencies are acquired via a more instrumental goal-directed process. In support, average escape latencies on the very first shuttlebox FR-1 trial, before any prior learning, are typically less than 2 s (here the average escape latency for trial 1 in CPA-HC rats was 1.51 s). In contrast, the FR-2 contingency is not even learned at all without prior FR-1 training [29]. Thus, more complex learning tasks may be especially susceptible to circadian disruption.
One major limitation of the present study is that we did not assess the endogenous biological time (circadian time, CT) of CPA rats. In mice, rhythmic expression of light-inducible clock genes (e.g. Per1/2) resets rapidly (within one cycle) following a single 6 h phase advance in the SCN, whereas expression in peripheral tissues is gradual [31]. Furthermore, overt behavioral rhythms such as locomotor activity can take at least 7 days to re-entrain following a single 6 h phase advance [32]. However, there is some evidence that the rate of re-entrainment accelerates following repeated phase shifts of the LD cycle [33]. Interestingly, Castanon-Cervantes showed that mice subjected to an identical CPA protocol were re-synchronized by day six following the final phase shift [34]. However, earlier time points were not evaluated in this study and so it is unclear exactly how long it takes the circadian system to establish a stable phase relationship to the LD cycle following CPA. Here the post-stress juvenile social exploration and shuttlebox tests occurred 72 and 96 h after the final phase shift, respectively. Thus, it is likely that CPA rats were not fully re-entrained to the new LD cycle at the time of behavioral testing. Importantly, behavioral tests were conducted at approximately the same ZT for all rats (i.e. during the first half of the light phase), and order was counterbalanced to minimize potential time of day effects. As mentioned above, there does not appear to be diurnal variation in baseline social exploratory behavior [33]. However, to the best of our knowledge, there are no studies that have examined whether escape performance in the shuttlebox varies across the LD cycle.
Another limitation to the present study is that we cannot conclude whether our results are due to acute circadian misalignment caused by the final phase shift or to the chronic nature of repeated shifts (CPA). This is an important consideration because acute and repeated manipulations of the LD cycle can produce very different outcomes. For example, mice subjected to CPA exhibit an exaggerated inflammatory response to an immune challenge, whereas mice subjected to a single 6 h phase shift exhibit a similar response to non-shifted controls [34]. Furthermore, a history of circadian disruption can modulate the effects of acute circadian misalignment. For example, prior exposure to 4 weekly alternating phase advances and delays reverses impaired recall of a conditioned fear memory caused by a single 6 h phase advance [35]. Thus, future work should aim to evaluate the impact of acute circadian misalignment versus chronic circadian disruption on the stress-protective effects of behavioral control.
Circadian misalignment likely influences mood related behavior through systems that are downstream of the SCN which have well-established roles in mood regulation [36]. The SCN has limited direct neural connections and therefore must employ other routes (e.g. humoral) to communicate timing information to extra-SCN clocks. Glucocorticoids serve as an important entrainment factor for some extra-SCN clocks [37], and can modulate the resetting kinetics in response to phase shifts in a tissue-dependent manner [38]. Additionally, light can influence mood through non-circadian mechanisms as clock genes have pleiotropic functions [39]. Thus, one explanation for the selective effect of CPA on shuttlebox escape performance but not social exploratory behavior is that CPA differentially affected clock function in brain circuits that serve as the proximate effectors of these behaviors. Indeed, the IS-induced reduction in juvenile social exploration is mediated, at least in part, by 5-hydroxytryptamine 2C (5-HT2C) receptors in the basolateral amygdala [27], whereas IS-induced shuttlebox impairments are mediated by 5-HT2C receptors in the dorsal striatum [40]. Thus, it is likely that IS and CPA-induced shuttlebox impairments are driven by independent mechanisms.
A growing body of research strongly implicates disruption of the circadian system with metabolic dysregulation and obesity [22]. Therefore, we also assessed metabolic factors including weight, epididymal adiposity, and adrenal mass. CPA rats exhibited increased body mass, increased epididymal fat pad mass (an index of overall adiposity), and reduced adrenal mass compared to standard LD cycle rats. These findings are in agreement with previous work by Selgado-Delgado and colleagues (2010) that showed that rats exposed to a chronic shiftwork protocol also exhibited greater body mass and adiposity than non-shifted controls, an effect that was driven by a shift in food consumption to the inactive phase, and not by overall increased food intake [41].
It should be noted that the CPA protocol used in rodent studies might be less disruptive than circadian disruption that is typically experienced by humans. For example, most shift workers have familial and social obligations that prevent them from fully entraining to the LD cycle dictated by their work schedule (termed ‘social jet lag’). In contrast, in mice, CPA does not produce a deficit in total sleep or forced activity during the light (inactive) phase [34] as is the case with shift workers. Future studies should consider this limitation of the CPA protocol and assess the effects of other types of circadian disruption on behavioral control.
5. Conclusions
Overall, these findings indicate that an environmental model of circadian misalignment produces impairment of a type of escape learning that is also reliably impaired by prior uncontrollable stress. However, this type of circadian misalignment does not interfere with at least one of the protective outcomes normally conferred by behavioral control.
Acknowledgments
This work was supported by NIH grant MH050479 awarded to S.F.M. and L.R.W.
L.K.F. was supported by NIH grant F32AG048672.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbr.2018.10.013.
References
- [1].Reppert SM, Weaver DR, Coordination of circadian timing in mammals, Nature 418 (6901) (2002) 935–941. [DOI] [PubMed] [Google Scholar]
- [2].Hastings MH, Reddy AB, Maywood ES, A clockwork web: circadian timing in brain and periphery, in health and disease, Nat. Rev. Neurosci 4 (8) (2003) 649–661. [DOI] [PubMed] [Google Scholar]
- [3].Evans JA, Davidson AJ, Health consequences of circadian disruption in humans and animal models, Prog. Mol. Biol. Transl. Sci 119 (2013) 283–323. [DOI] [PubMed] [Google Scholar]
- [4].Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, Barchas JD, Schatzberg AF, Myers RM, Watson SJ, Akil H, Bunney WE, Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder, Mol. Psychiatry 20 (1) (2015) 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bunney BG, Bunney WE, Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms, Biol. Psychiatry 73 (12) (2013) 1164–1171. [DOI] [PubMed] [Google Scholar]
- [6].Dallaspezia S, Suzuki M, Benedetti F, Chronobiological therapy for mood disorders, Curr. Psychiatry Rep 17 (12) (2015) 95. [DOI] [PubMed] [Google Scholar]
- [7].Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA Jr., McClung CA, Mania-like behavior induced by disruption of CLOCK, Proc. Natl. Acad. Sci. U. S. A 104 (15) (2007) 6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fonken LK, Kitsmiller E, Smale L, Nelson RJ, Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent, J. Biol. Rhythms 27 (4) (2012) 319–327. [DOI] [PubMed] [Google Scholar]
- [9].Fonken LK, Nelson RJ, Dim light at night increases depressive-like responses in male C3H/HeNHsd mice, Behav. Brain Res 243 (2013) 74–78. [DOI] [PubMed] [Google Scholar]
- [10].LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S, Aberrant light directly impairs mood and learning through melanopsin-expressing neurons, Nature 491 (7425) (2012) 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS, Disruption of circadian clocks has ramifications for metabolism, brain, and behavior, Proc. Natl. Acad. Sci. U. S. A 108 (4) (2011) 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McMenamin TM, A time to work: recent trends in shift work and flexible schedules, U.S. Bureau of Labor Statistics, Monthly Labor Review, 2007, pp. 3–15. [Google Scholar]
- [13].Cole RJ, Loving RT, Kripke DF, Psychiatric aspects of shiftwork, Occup. Med. (Philadelphia, Pa) 5 (2) (1990) 301–314. [PubMed] [Google Scholar]
- [14].Scott AJ, Monk TH, Brink LL, Shiftwork as a risk factor for depression: a pilot study, Int. J. Occup. Environ. Health 3 (Supplement 2) (1997) S2–s9. [PubMed] [Google Scholar]
- [15].Costa G, Shift work and health: current problems and preventive actions, Saf. Health Work 1 (2) (2010) 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kendler KS, Karkowski LM, Prescott CA, Causal relationship between stressful life events and the onset of major depression, Am. J. Psychiatry 156 (6) (1999) 837–841. [DOI] [PubMed] [Google Scholar]
- [17].Maier SF, Seligman ME, Learned helplessness at fifty: insights from neuroscience, Psychol. Rev 123 (4) (2016) 349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF, Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus, Nat. Neurosci 8 (3) (2005) 365–371. [DOI] [PubMed] [Google Scholar]
- [19].Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD, Chronic jet-lag increases mortality in aged mice, Curr. Biol. : CB 16 (21) (2006) 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF, The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration, Behav. Brain Res 193 (1) (2008) 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amat J, Paul E, Zarza C, Watkins LR, Maier SF, Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex, J. Neurosci 26 (51) (2006) 13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fonken LK, Nelson RJ, The effects of light at night on circadian clocks and metabolism, Endocr. Rev 35 (4) (2014) 648–670. [DOI] [PubMed] [Google Scholar]
- [23].Bedrosian TA, Nelson RJ, Influence of the modern light environment on mood, Mol. Psychiatry 18 (7) (2013) 751–757. [DOI] [PubMed] [Google Scholar]
- [24].Dumont M, Beaulieu C, Light exposure in the natural environment: relevance to mood and sleep disorders, Sleep Med. 8 (6) (2007) 557–565. [DOI] [PubMed] [Google Scholar]
- [25].File SE, Seth P, A review of 25 years of the social interaction test, Eur. J. Pharmacol 463 (1–3) (2003) 35–53. [DOI] [PubMed] [Google Scholar]
- [26].Reijmers LG, Leus IE, Burbach JP, Spruijt BM, van Ree JM, Social memory in the rat: circadian variation and effect of circadian rhythm disruption, Physiol. Behav 72 (3) (2001) 305–309. [DOI] [PubMed] [Google Scholar]
- [27].Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF, 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress, Biol. Psychiatry 67 (4) (2010) 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Christianson JP, Thompson BM, Watkins LR, Maier SF, Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat, Stress 12 (5) (2009) 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maier SF, Albin RW, Testa TJ, Failure to learn to escape in rats previously exposed to inescapable shock depends on nature of escape response, J. Comp. Physiol. Psychol 85 (3) (1973) 581–592. [Google Scholar]
- [30].Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, Welsh DK, Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice, Biol. Psychiatry 80 (11) (2016) 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H, Resetting central and peripheral circadian oscillators in transgenic rats, Science 288 (5466) (2000) 682–685. [DOI] [PubMed] [Google Scholar]
- [32].Reddy AB, Field MD, Maywood ES, Hastings MH, Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag, J. Neurosci 22 (17) (2002) 7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yan L, Structural and functional changes in the suprachiasmatic nucleus following chronic circadian rhythm perturbation, Neuroscience 183 (2011) 99–107. [DOI] [PubMed] [Google Scholar]
- [34].Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ, Dysregulation of inflammatory responses by chronic circadian disruption, J. Immunol 185 (10) (2010) 5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS, Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice, PLoS One 5 (9) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McCarthy MJ, Welsh DK, Cellular circadian clocks in mood disorders, J. Biol. Rhythms 27 (5) (2012) 339–352. [DOI] [PubMed] [Google Scholar]
- [37].Spencer RL, Chun LE, Hartsock MJ, Woodruff ER, Glucocorticoid hormones are both a major circadian signal and major stress signal: how shared signal contributes to a dynamic relationship between the circadian and stress systems, Front. Neuroendocrinol 49 (2018) 52–71. [DOI] [PubMed] [Google Scholar]
- [38].Kiessling S, Eichele G, Oster H, Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag, J. Clin. Invest 120 (7) (2010) 2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gonzalez MM, Aston-Jones G, Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats, Proc. Natl. Acad. Sci. U. S. A 105 (12) (2008) 4898–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN, 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior, Neuroscience 197 (2011) 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C, Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work, Endocrinology 151 (3) (2010) 1019–1029. [DOI] [PubMed] [Google Scholar]


