Abstract
Synchronizing circadian (24 h) rhythms in physiology and behavior with the environmental light-dark cycle is critical for maintaining optimal health. Dysregulation of the circadian system increases susceptibility to numerous pathological conditions including major depressive disorder. Stress is a common etiological factor in the development of depression and the circadian system is highly interconnected to stress-sensitive neurotransmitter systems such as the serotonin (5-hydroxytryptamine, 5-HT) system. Thus, here we propose that stress-induced perturbation of the 5-HT system disrupts circadian processes and increases susceptibility to depression. In this review, we first provide an overview of the basic components of the circadian system. Next, we discuss evidence that circadian dysfunction is associated with changes in mood in humans and rodent models. Finally, we provide evidence that 5-HT is a critical factor linking dysregulation of the circadian system and mood. Determining how these two systems interact may provide novel therapeutic targets for depression.
Keywords: Circadian rhythms, Mood, Depression, Serotonin, Resilience
1. Introduction
The circadian and stress systems are complementary regulatory networks that are important for homeostasis. Life on Earth has evolved under a highly consistent light: dark (LD) cycle and as a result, most organisms have adopted an internal time keeping mechanism, which enables them to anticipate predictable events within the environment. The output of this system is daily (~24 h) rhythms in physiology and behavior that are synchronized with the light-dark cycle to optimize survival. In addition, organisms have adapted another system which allows them to either confront or escape unexpected homeostatic challenges, termed “stressors.” The stress system is primarily comprised of the sympathetic nervous system and the hypothalamic-pituitarya-drenal (HPA) axis (Helfrich-Forster, 2017). The stress response initiates complex changes that are highly adaptive under most circumstances, permitting organisms to recuperate from ongoing distress and even buffer against future adverse events (Packard et al., 2016). These two systems are critical for anticipating both predictable (circadian) and unpredictable (stress) environmental events.
The circadian and stress systems are highly interconnected. Under basal conditions, the stress system is under circadian control (Spencer and Deak, 2017), and is primed during the organism’s active phase to optimize interactions with the environment (Spencer et al., 2018). For example, components of the stress system are increased during an organism’s active phase to maintain vigilance and these stress signals decrease towards the end of the active phase to allow the organism to sleep/rest, and thereby restore depleted resources. Reciprocally, the stress system can feed back onto the circadian system and provide timing information to the rest of the body (Spencer et al., 2018).
Understanding the relationship between these two systems is of clinical importance because perturbation of either system is strongly implicated in the development of mood disorders [reviewed in (Bedrosian and Nelson, 2013; Helfrich-Forster, 2017)]. Major depressive disorder (MDD) is a debilitating psychiatric disorder affecting approximately 6% of the adult population worldwide (Otte et al., 2016). The World Health Organization (WHO) predicts that MDD will become the single greatest contributor to the global burden of disease by the year 2030 (WHO, 2017). MDD is associated with diminished quality of life (Hofmann et al., 2017) and increased risk for mortality (Gilman et al., 2017). MDD is characterized by recurrent episodes of anhedonia and depressed mood, as well as a subset of heterogeneous symptoms involving physiological, behavioral, and cognitive impairments (American Psychiatric Association, 2013). Stress is the greatest known risk factor in the etiology of MDD (Kendler et al., 1999); however, not all individuals who encounter seemingly similar traumatic life events go to develop MDD. Thus, other environmental factors and genetic risk are likely involved in regulating stress resistance and/or resilience.
Many of the symptoms of depression are related to dysregulation of the circadian system. For example, alterations in daily rhythms such as the sleep-wake cycle, body temperature, neurotransmitter and hormone levels, among others, are prevalent among depressed individuals (Bunney et al., 2015). Additionally, several human genetic studies have linked circadian genes to mood disorders including MDD (Buoli et al., 2018). Thus, considerable epidemiological evidence implicates circadian abnormalities in the pathophysiology and maintenance of depression. However, the link between the circadian system and depression is not fully understood.
In this review, we will present evidence that disruption of the circadian system can increase vulnerability to MDD. While significant circadian alterations are present in other mood disorders such as bipolar disorder and seasonal affective disorder [for review see (Logan and McClung, 2016) and (Wirz-Justice, 2018) respectively], here we will focus on non-seasonal unipolar depression (i.e. MDD) because impaired monoamine neurotransmission (i.e. serotonin, dopamine, and norepinephrine) is strongly linked to the pathophysiology and current treatment strategy for this disorder. In particular, the serotonin (5-hydroxytryptaime, 5-HT) system is reciprocally connected with the circadian system and regulates circadian function.
In order to understand how perturbations of the circadian system can have profound consequences for mood and mental health, we first provide an overview of the basic components of the circadian system (Section 2). Next, we discuss evidence showing that circadian dysfunction is prevalent among depressed individuals (Section 3). We also review preclinical studies, which show that various stressors can disrupt the circadian system and induce affective behavioral changes analogous to human depression (Section 4). Further, we summarize evidence that 5-HT is an important intermediate between stress-induced disruption of the circadian system and depression (Section 5). Finally, we conclude with discussion on alternative treatment approaches for MDD that target the circadian system (Section 6).
2. The circadian system
2.1. Circadian rhythms
The daily rotation of the Earth about its axis relative to a stationary light source, the Sun, provides a predictable cycle of alternating light and dark. In response, most organisms ranging from cyanobacteria to humans evolved an internal timekeeping mechanism. The output of this system is approximately 24 h oscillations in nearly all physiological processes and behaviors. The circadian system is highly adaptive: there is selection pressure against animals with non-24 h circadian clocks (Spoelstra et al., 2016). The circadian system allows organisms to anticipate day and night and thereby respond to stimuli in a proactive, rather than reflexive manner in order to anticipate food availability, mating opportunities, and time of peak predation (Vaze and Sharma, 2013). Thus, proper synchronization with the environmental LD cycle is essential for survival (Spoelstra et al., 2016). Disruption of the circadian system is associated with adverse health outcomes including cardiovascular disease, metabolic syndrome, obesity, cancer, as well as mood disorders [reviewed in (Bedrosian et al., 2016)].
In mammals, the circadian system has traditionally been described as consisting of three main components: (1) afferent synchronizing pathways which receive and transmit photic information to a central oscillator; (2) a central oscillator (or “master clock”) responsible for generating circadian rhythms; and (3) efferent pathways which synchronize rhythms throughout the body (Hastings et al., 2018). It is now clear, however, that the circadian system is more complex than this simplified model. For example, while the SCN is somewhat buffered against behavioral feedback, it does receive afferent non-photic input. For example, Section 5.2 of this review discusses afferent serotonin input to the SCN. Moreover, efferent pathways synchronize rhythms throughout the body, but these peripheral oscillators also generate self-sustaining oscillations in the absence of SCN input (Tahara and Shibata, 2018).
Overall, there are criteria a biological rhythm must meet to be classified as circadian. First, the rhythm must have a period of approximately 24 h, which persists even in the absence of external time cues [e.g., persists in continuous darkness, DD; (Aschoff, 1965)]. Second, the rhythm must exhibit temperature compensation, meaning that, unlike most biochemical reactions, its periodicity is buffered against large fluctuations in ambient temperature (Reyes et al., 2008). Third, the rhythm resets (i.e. phase shifts) in response to external time cues within a specific range, a process referred to as entrainment (Aschoff, 1960).
2.2. The master clock
The bilateral suprachiasmatic nuclei (SCN) of the anterior hypothalamus comprise the primary oscillator for the circadian system. The SCN autonomously generates an intrinsic rhythm that is sustained even in the absence of photic information (Herzog et al., 2017). The SCN is also responsible for regulating rhythms in extra-SCN clocks present throughout the body. Lesion of the SCN abolish the expression of most circadian rhythms (Eastman et al., 1984; Moore and Eichler, 1972; Stephan and Zucker, 1972). The SCN is comprised of a relatively small number of neurons (approximately 8000–20,000 neurons in rodents and 50,000 in humans) (Lydic et al., 1980; Van den Pol, 1980; Lydic et al., 1982), the majority of which express the neurotransmitter gamma-aminobutyric acid [GABA; (Moore and Speh, 1993; Abrahamson and Moore, 2001)] and co-localize one or more neuropeptides. The two primary subdivisions of the SCN are the ventrolateral “core” region which is characterized by vasoactive intestinal polypeptide (VIP) immunoreactivity (Samson et al., 1979), and the dorsomedial “shell” region, which requires arginine vasopressin (AVP) signaling (Vandesande et al., 1975; Edwards et al., 2016). However, the neuropeptide distribution of the SCN is heterogeneous across species (Morin, 2013).
2.3. Molecular machinery of the master clock
Daily oscillations of core “clock” genes and proteins in the SCN are necessary to generate circadian rhythms. The master clock is driven by interlocked transcriptional-translational feedback loops, each of which is composed of a positive arm that behaves as the system activator and a negative arm that acts as the repressor. In the primary feedback loop, in mammals, brain and muscle arnt-like protein 1 (BMAL1), and circadian locomotor output cycles kaput (CLOCK) dimerize and bind to E-box cis-regulatory enhancer sequences (CACGTG) in the promoter region of target clock genes thus driving expression of period (Per1, Per2, and Per3) and cryptochrome (Cry1 and Cry2) genes (van der Horst et al., 1999; Zheng et al., 2001). These proteins accumulate in the cytoplasm and upon reaching sufficient levels, translocate back into the nucleus to associate with the CLOCK-BMAL1 complex and thereby repress their own transcription (Kume et al., 1999). This entire process takes approximately 24 h to complete (Fig. 1). The timing of this feedback loop is fine-tuned by posttranslational modifications; clock proteins are subject to phosphorylation, ubiquitination, small ubiquitin-related modification, acetylation, as well as others (Gallego and Virshup, 2007; Duguay and Cermakian, 2009). For example, Per and Cry proteins can be phosphorylated by casein kinase δ1 and ε (CK1δ/ε), which tags them for degradation via ubiquitination or for translocation into the nucleus, respectively (Lee et al., 2001; Reppert and Weaver, 2002). Indeed, disruptions in CK1δ/ε function in SCN GABAergic neurons can lengthen the period of the SCN clock (van der Vinne et al., 2018). Overall, the molecular machinery of the master clock is highly complex, involving accessory transcriptional-translational feedback loops and regulatory proteins that influence the speed, precision, and function of the master clock [reviewed in (Partch et al., 2014)].
Fig. 1.
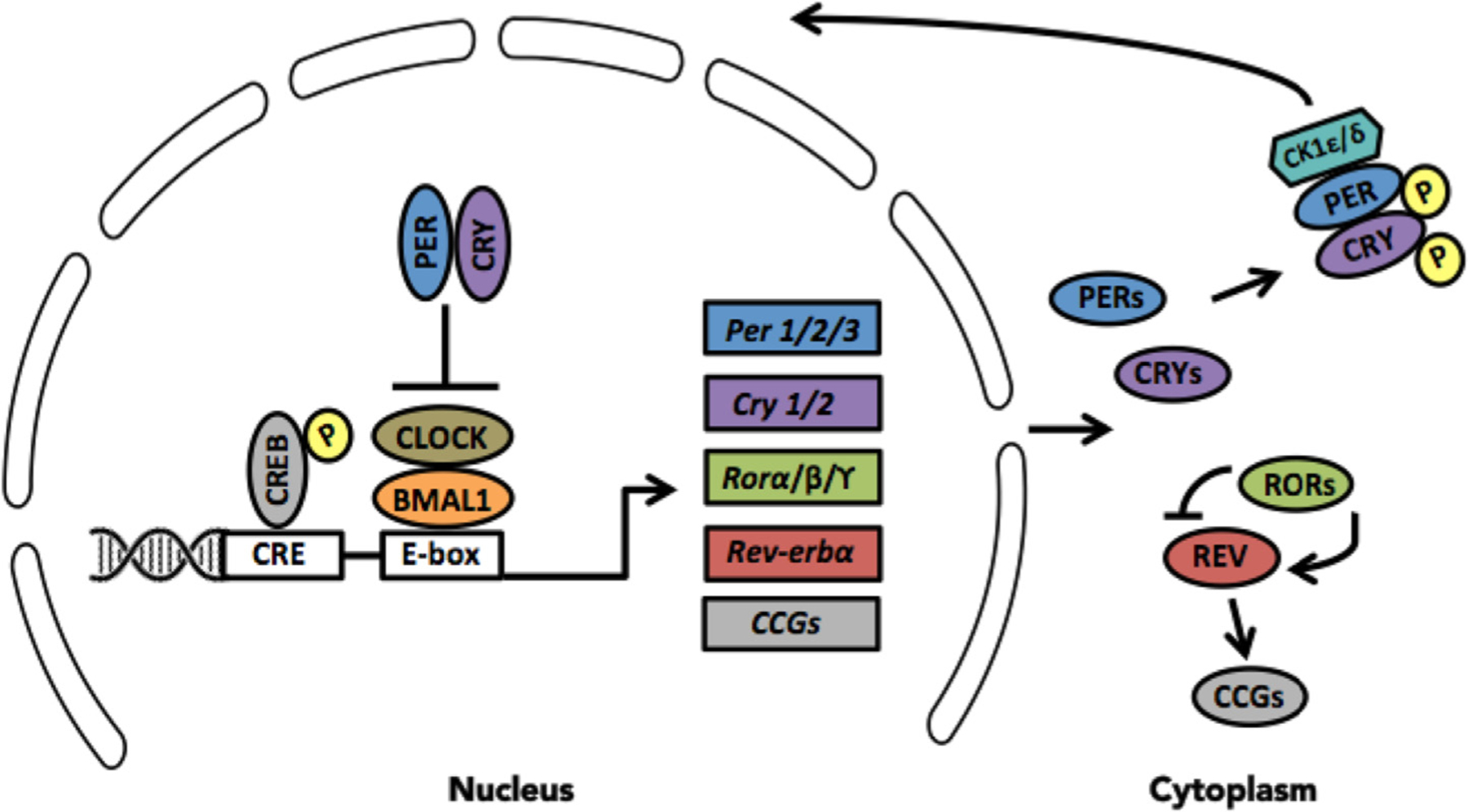
Molecular machinery of the circadian clock. In mammals, the circadian clock is driven by a primary transcriptional-translational feedback loop, which is composed of a positive arm that acts as the system activator and a negative arm that acts as the system repressor. Brain muscle arnt like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK) proteins form heterodimers, which bind to the E-box enhancer unit of three period (PER1/2/3) and two cryptochrome (CRY1/2) genes thereby driving their transcription. Over the course of the subjective day, PER and CRY proteins accumulate in the cytoplasm and upon reaching sufficient levels, dimerize and associate with CK1ε/δ, thereby phosphorylating them and allowing translocation back into the nucleus to inhibit BMAL1/CLOCK-mediated transcription. In a similar fashion, BMAL1/CLOCK activates some nuclear orphan receptor genes (e.g. Rorα/Rorβ/Rorϒ or Reverbα), which repress or activate Bmal1 transcription, respectively to form an auxiliary feedback loop that stabilizes the primary loop. BMAL1/CLOCK can also activate a multitude of clock-controlled genes (CCGs).
It is estimated that at least 10% of the mammalian transcriptome is under circadian control (Panda et al., 2002; Reddy et al., 2006) and possibly as high as 40% of transcripts cycle in some organ systems in the body (Zhang et al., 2014). Furthermore, a recent transcriptomic analysis in primate tissue revealed that 82% of genes coding for druggable targets show cyclic changes in transcription in at least one tissue (Mure et al., 2018). These rhythmic transcripts are almost entirely distinct across tissue types indicating that the role of extra-SCN clocks is unique, and might reflect tissue-specific functions. Oscillatory genes regulate diverse functions such as metabolism, transcription, translation, protein turnover, cell cycle, cell death, vesicle trafficking, transport, and signal transduction (Li and Zhang, 2015) emphasizing the importance of the circadian system in homeostasis.
2.4. Photic entrainment of the master clock
In the absence of time cues (i.e. constant dim light or darkness), the circadian system is ‘free-running’. The period of the free-running circadian system is close to, but not exactly 24 h, and so, the master clock must reset every cycle in order to maintain synchronicity with the environment (Patton and Hastings, 2018). Light serves as the most potent entrainment factor (or zeitgeber, German for “time-giver”) to the master clock. Light enters the inner retina and activates intrinsically photosensitive retinal ganglion cells (ipRGCs) that contain the photo-pigment melanopsin, which is primarily sensitive to blue wavelength light in the range of 470–480 nm (Gooley et al., 2001; Berson et al., 2002; Hattar et al., 2002; Bailes and Lucas, 2013). The SCN receives non-image forming information from rods, cones, and ipRGCs; however, all photic information is transmitted through ipRGCs (Freedman et al., 1999; Panda et al., 2002; Guler et al., 2008). Excitation of ipRGCs is relayed to the ventrolateral portion of the SCN via the retinohypothalamic tract through direct and indirect projections (Moore, 1973; Levine et al., 1991). The electrical signal is then converted to a chemical signal. Glutamate (Castel et al., 1993) and/or pituitary adenylate cyclase-activating polypeptide (Hannibal et al., 2000) are released directly onto SCN neurons to initiate calcium-dependent second messenger signaling (O’Neill and Reddy, 2012). This results in binding of phosphorylated cyclic adenosine monophosphase (cAMP) response element binding protein to cAMP-response elements on the promoter region of the Per1 gene, thereby inducing Per1 expression [Fig. 2; (Albrecht et al., 1997)]. In SCN neurons, Per1 expression is high during the middle of the subjective day and declines to reach its nadir in the subjective night, in anti-phase with Bmal1 expression. During the subjective day, Per1 expression is maximal so light has little to no effect on the master clock. However, during the subjective night, Per1 expression is minimal, and photic information can produce phase shifts of the master clock (Miyake et al., 2000; Schwartz et al., 2011). Thus, photic sensitivity of the master clock is circadian gated, meaning that the response to light varies across the LD cycle and responses to light can vary. Other factors such as light intensity and duration can influence the phase shifting capacity of a light pulse (Sharma et al., 1999; Comas et al., 2008; Dewan et al., 2011).
Fig. 2.
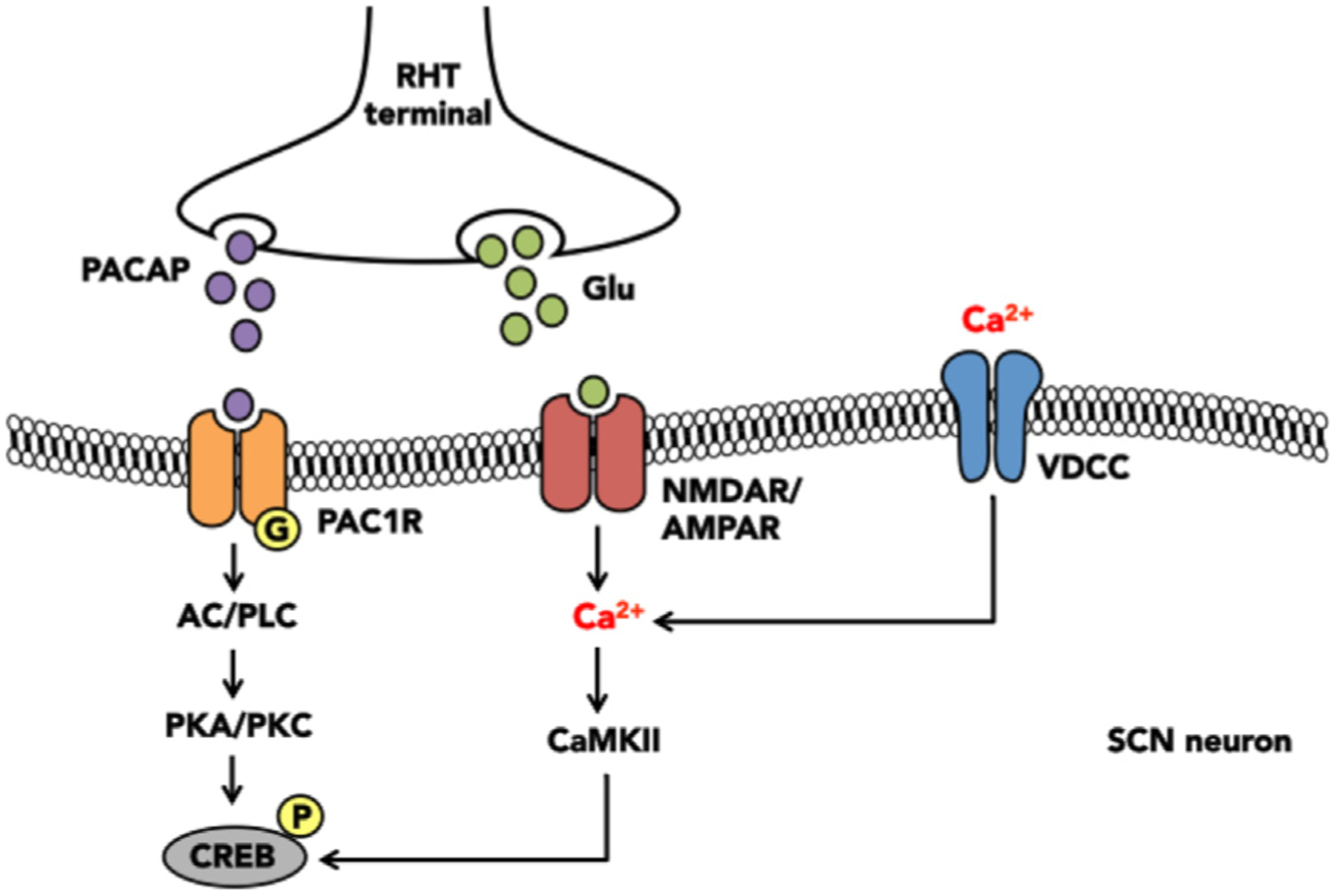
Photic entrainment of the master clock. Photic information received by the retina is relayed to the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (RHT). RHT axon terminals release excitatory molecules such as pituitary adenylate cyclase-activating peptide (PACAP) and/or glutamate (glu) onto ventrolateral SCN neurons. PACAP then binds to the G-coupled PAC type 1 receptor (PAC1R) and glutamate binds to N-methyl-d-aspartate receptor (NMDAR) on the post-synaptic cell. Activation of these receptors initiate distinct signaling second messenger signaling cascades, which ultimately result in phosphorylation of the transcription factor cyclic-AMP response element binding protein (CREB) in the promotor region of the Per gene.
2.5. Extra-SCN clocks
In mammals, circadian clocks exist in virtually every cell type within the body (Mure et al., 2018). Moreover, these extra-SCN clocks appear to possess the same molecular machinery as does the master clock (Yagita et al., 2001). Cells isolated from the liver, lung, skeletal muscle, etc. continue to oscillate in vitro, but dampen after a few cycles, whereas cells isolated from the SCN can oscillate presumably indefinitely (Yamazaki et al., 2000). Because most cells are not retino-recipient, they are not directly entrained by photic signals. Rather, extra-SCN clocks can be entrained by photic information that is indirectly conveyed via SCN-mediated relay pathways. For example, the master clock relays timing information to extra-SCN clocks via neuronal projections and through the coordinated release of various neuropeptides and hormones [see below; (Buijs and Kalsbeek, 2001; Dibner et al., 2010)]. Extra-SCN clocks can also respond to non-photic zeitgebers that provide salient tissue specific information. For example, extra-SCN clocks can respond directly and robustly to cyclic non-photic stimuli such as feeding behavior, social interaction, exercise, and stress independently of the SCN [for review see (Tahara and Shibata, 2018)].
SCN efferent projections are relatively sparse and most notably project to hypothalamic regions involved in hormone synthesis and secretion, including the paraventricular nucleus (PVN), the sub-paraventricular zone, and the dorsomedial hypothalamic nucleus (Morin, 2013), Rhythms generated by extra-SCN clocks in the periphery lag behind that of the SCN and vary in a tissue-specific manner (Yamazaki et al., 2000; Abe et al., 2002; Chun et al., 2015), supporting the idea that the master clock synchronizes these rhythms.
2.6. Glucocorticoid-dependent entrainment of extra-SCN clocks
Entrainment of some extra-SCN clocks depends on glucocorticoids (CORT; primarily cortisol in humans, corticosterone in rodents) (Balsalobre et al., 2000). CORT is the primary (systemic) effector hormone of the HPA axis and is critically involved in the stress response (Spencer-Segal and Akil, 2018). During stress, corticotropin-releasing factor (CRF) is released from neurons in the paraventricular nucleus (PVN) of the hypothalamus into the hypophyseal portal system where it stimulates release of adrenocorticotropic hormone (ACTH) into the circulation. ACTH then initiates release of glucocorticoids from the adrenal gland. Glucocorticoids can feedback and inhibit the HPA axis at the level of the PVN and anterior pituitary. Under basal conditions, CORT exhibits a diurnal pattern of secretion with peak levels coinciding with the onset of active phase in vertebrate species (Spencer and Deak, 2017). The SCN is necessary for generation of CORT rhythmicity (Moore and Eichler, 1972). As mentioned earlier, the SCN projects to the PVN, and so the master clock can regulate CORT at this level or independently of the HPA axis, via splanchnic nerve innervation of the adrenal glands (Buijs and Kalsbeek, 2001), the site of CORT synthesis.
As a steroid hormone, CORT can readily pass through phospholipid membranes to bind its receptor and influence transcription of circadian-related genes. Intracellularly, CORT binds to cytosolic glucocorticoid receptors (GRs), allowing translocation of the CORT-GR complex into the nucleus where it can act as a transcription factor at glucocorticoid response elements (GREs). Core clock genes Per1 and Per2 contain GREs in their promoter regions (Yamamoto et al., 2004; Reddy et al., 2007; So et al., 2009; Cheon et al., 2013). Administration of CORT or GR agonists to tissue explants ex vivo acutely increases gene expression of some core clock genes (Balsalobre et al., 2000; Reddy et al., 2007; So et al., 2009). Furthermore, removal of endogenous CORT via adrenalectomy produces arrhythmic or phase-shifted clock gene expression in some extra-SCN clocks (Amir et al., 2004; Woodruff et al., 2016), an effect that can be restored with in-phase CORT replacement (Woodruff et al., 2016). GRs are ubiquitously expressed in most tissues (De Kloet et al., 1998), with the SCN as a notable exception (Rosenfeld et al., 1988). This suggests that the SCN is largely insensitive to glucocorticoids but that CORT may serve as an entrainment factor for some extra-SCN clocks.
3. The circadian system and mood
3.1. Circadian variation in mood and depressive symptomatology
Mood exhibits daily fluctuations in healthy individuals. Mood is typically dissociated into two separate dimensions, positive and negative affect. A recent study evaluating over 800 million Twitter messages revealed a sharp morning peak in words associated with positive affect and a late evening peak in words associated with sadness and anger (Dzogang et al., 2017). This study supports previous results indicating that positive and negative affect fluctuate independently across the 24 h cycle (Golder and Macy, 2011; Peeters et al., 2006). Interestingly, diurnal variation in negative affect appears to be more stable whereas rhythms in positive affect vary across the week and season (Golder and Macy, 2011).
Diurnal variation in mood is altered in a subset of depressed individuals. Not surprisingly, depressed individuals report overall lower levels of positive affect (i.e. anhedonia), with peak values occurring later in the day compared to healthy controls (Peeters et al., 2006). However, negative affect exhibits a robust circadian rhythm with more moment-to-moment variability. Furthermore, in depressed individuals, peak negative affect occurs in the late morning and steadily declines throughout the day (Peeters et al., 2006). Diurnal variation of symptom severity was also reported in roughly 22% of depressed patients enrolled in the Sequenced Treatment Alternatives to Relieve Depression (STAR-D) study (Morris et al., 2007). Of these, 32% experienced worsening of symptoms in the morning, 20% in the afternoon, and 49% in the evening (Morris et al., 2007). Overall, there is inter-study variability in timing of peak negative and positive affect. For example, the STAR-D study reported a higher percentage of individuals with worsening of symptoms in the evening which contrasts with the earlier study by Peeters et al. It is possible that methodological differences, such as tools for assessing mood and the study populations, may account for some of this variability. It should also be noted that none of the aforementioned studies utilized an experimental design that permits unmasking of endogenous circadian rhythms (e.g. constant routine or forced desynchrony protocols), and so the timing of these rhythms are likely impacted by several exogenous factors.
Diurnal variation in mood can be influenced by an individual’s chronotype, which describes an individual’s biological predisposition for morning, afternoon, or evening activities (Roenneberg et al., 2007). Chronotype is considered to be a relatively stable trait that varies across developmental periods. Interestingly, depressed individuals with a late chronotype (i.e. evening preference) are more likely to experience worsening of symptoms in the morning (Antypa et al., 2017), more frequent and severe depressive episodes (Hasler et al., 2010; Hidalgo et al., 2009 Kim et al., 2010; Kitamura et al., 2010; Au and Reece, 2017), reduced responsiveness to antidepressants (McGlashan et al., 2018), as well as greater suicidality (Bahk et al., 2014). These findings indicate that a subset of depressed individuals exhibit an altered circadian rhythm of mood. Furthermore, non-depressed individuals with an early chronotype score higher on self-report measures of psychological resilience than do late chronotypes (Jeon et al., 2017), suggesting that circadian factors may contribute to susceptibility to mood disorders.
3.2. Circadian rhythms are disrupted in depression
In addition to rhythms in mood, various other circadian rhythms are disrupted in depression. For example, changes in the sleep-activity cycle are common in depression. One main diagnostic criterion for MDD is insomnia or hypersomnia (American Psychiatric Association, 2013), and up to 90% of depressed individuals experience other sleep-wake cycle disturbances including poor sleep quality, increased rapid-eye movement (REM; or paradoxical) sleep, and early morning awakening (Murphy and Peterson, 2015). Sleep disturbances are associated with an increased risk of suicide (Bernert et al., 2015), highlighting the importance of treating sleep in depressed patients. Furthermore, a large scale (91,105 participants) study measuring activity levels by a wrist-worn accelerometer, reported that reductions in the amplitude of activity rhythms are associated with increased risk of MDD and bipolar disorder, greater mood instability, higher neuroticism, more subjective loneliness, lower happiness, and lower health satisfaction (Lyall et al, 2018).
Another common circadian rhythm abnormality involves the HPA axis. In healthy individuals, maximal CORT secretion occurs in the morning prior to waking, and progressively declines across the day (Spencer and Deak, 2017). Depressed individuals, however, often exhibit a 2–3 h phase advance of the morning CORT peak and elevated levels across the day (Koenigsberg et al., 2004 Linkowski et al., 1994). Elevated CORT concentrations can also present as a flattened amplitude of the diurnal cortisol rhythm in depressed individuals and impaired suppression of cortisol following dexamethasone administration (Jarcho et al., 2013). Importantly, flattened cortisol rhythms or elevated CORT concentrations can persist in individuals in remission from MDD (Doane et al., 2013) and may leave individuals susceptible to subsequent episodes of depression.
Many other physiological circadian rhythms are altered in MDD including melatonin, norepinephrine, thyroid stimulating hormone, blood pressure, and pulse [reviewed in (McClung, 2007)]. Furthermore, the degree of circadian misalignment is positively correlated with symptom severity (Emens et al., 2009). Global changes in gene expression rhythms may underlie the disruptions in circadian rhythms in physiology and behavior in patients with MDD. Rhythms in human gene expression were determined in six major cortical and limbic brain regions based on time-of-death from patients with or without a history of MDD. Several hundred transcripts in each region showed 24-h cyclic patterns in controls (including a number of canonical clock genes). Cyclic patterns were, however, much weaker in the brains of patients with MDD (Li et al., 2013).
The observation that so many diverse rhythms are altered in MDD suggests that such disturbances may not be unique to one rhythm but may instead have a central origin (i.e. dysfunction of the master clock itself) and/or an alteration in a key extra-SCN entrainment factor (e.g. glucocorticoids or time of food intake). It remains unclear whether these disturbances induce MDD or are simply symptomatic of MDD. However, in rodents, alterations in circadian entrainment can precede the onset of depression-like behavior suggesting that circadian alterations may be causal and/or predictive of the onset of depression (Spulber et al., 2015).
3.3. Circadian disruption impacts mood
Given the common occurrence of rhythm disturbance in MDD, it is not surprising that environmental perturbations can produce alterations in mood. This section will highlight evidence from humans and animal models linking circadian disruption to changes in negative affect.
3.3.1. Epidemiological evidence
3.3.1.1. Jet lag.
Air travel across multiple time zones can produce a transient period of circadian misalignment (termed “jet lag”) in which internal time is not synchronized with the new environmental LD cycle (Sack, 2010). Similarly, “social” jet lag occurs when an individual shifts schedules to align the timing of their sleep-wake cycle with social obligations (most commonly by using an alarm clock on workdays) (Foster et al., 2013). Jet lag is characterized by a constellation of symptoms including: fatigue, irritability, gastrointestinal issues, reduced mental and physical acuity, and poor mood (Arendt, 2018). The circadian clock likely resets more rapidly following travel in the Westward direction (i.e. phase delay) compared to travel in the Eastward direction (i.e. phase advance) because the phase response curve to light is asymmetrical such that the delay zone is larger than the advance zone (Hilaire et al., 2012; Minors et al, 1991). Furthermore, the endogenous period of the human circadian clock is slightly longer than 24 h (Czeisler, 1999) and this may also contribute to speed of re-entrainment, albeit to a lesser degree. A number of other factors can also influence the severity of jet lag symptoms, including: the number of time zones crossed, ability to sleep during travel, availability and intensity of local circadian time cues, as well as coping ability (Sack, 2010). There is some evidence that long-haul flights can precipitate depressive episodes in individuals with a history of mental illness (Jauhar and Weller, 1982; Katz et al., 2002; Young, 1995). This finding suggests that the circadian misalignment induced by a rapid phase shift can trigger a depressive episode in individuals predisposed to alterations in mood. Furthermore, chronic jet lag, as experienced by flight attendants and crew, produces temporal lobe atrophy and cognitive deficits (Cho, 2001). Interestingly, the degree of cognitive impairment is positively correlated with salivary CORT concentrations (Cho, 2001). Altered CORT patterns have also been observed following acute jet lag (Doane et al., 2010). Interestingly, glucocorticoids modulate the resetting kinetics after abrupt shifts in the LD cycle in rodents (Kiessling et al., 2010; Mohawk et al., 2005), suggesting that changes in CORT may serve as a compensatory mechanism during jet lag recovery.
3.3.1.2. Shift work.
Approximately one fifth of the U.S. labor force engages in evening or rotating shift work (McMenamin, 2007). The symptoms of shift work are similar to jet lag, but the overall impact on health is more severe because shift workers are typically unable to synchronize with their new LD cycle due to frequent work schedule changes and shifted sleep-wake patterns on non-work days (Rajaratnam and Arendt, 2001). Shift workers report poor mood and exhibit an increased risk for development of MDD (Angerer et al., 2017; Asaoka et al., 2013; Dumont and Beaulieu, 2007; Scott et al., 1997). Furthermore, duration of shift work history is positively correlated with severity of depressive symptoms (Lee et al., 2017). Even student nurses who work a single evening shift, experience alterations in mood (Healy et al., 1993). There are multiple factors associated with shift work that could produce affective changes including: exposure to light at night, mistimed meals, and sleep loss.
3.3.2. Evidence from animal models
The epidemiological evidence presented above indicates that the circadian system and mood are linked. In parallel, mounting preclinical research indicates that environmental perturbations of the circadian system can lead to depressed mood. For instance, exposing animals to aberrant lighting environments misaligns internal biological processes from the external environment, and can lead to affective behavioral changes.
An extreme example of this involves experiments that use continuous exposure to light (constant light). Unlike constant darkness or constant dim light, which reveal the free running circadian cycle in rodents, exposure to constant light can desynchronize mammalian clock neurons resulting in arrhythmicity (Ohta et al., 2005; Fonken et al., 2010; Tapia-Osorio et al., 2013). Constant light increases depressive-like behaviors in adult mice and rats (Fonken et al., 2009; Tapia-Osorio et al., 2013) and has a lasting impact on mood related behaviors when mice are exposed during early life (Coleman et al., 2016).
Less extreme disruptions of the environmental light cycle can also have important implications for mood related behaviors. Rodents exposed to dim light during the dark phase (12:12 light:dim cycle) maintain circadian rhythms in activity (Fonken et al., 2010; Fonken et al., 2012; Bedrosian et al., 2013a) but show blunted rhythms in clock gene expression in the SCN and periphery (Bedrosian et al., 2013a; Fonken et al., 2013). Additionally, exposure to dim light during the dark phase increases depressive-like behaviors in several rodent models (Bedrosian et al., 2011; Fonken et al., 2012; Bedrosian et al., 2013b; Fonken and Nelson, 2013) although the effects of dim light at night may be strain specific (Martynhak et al., 2017; Cleary-Gaffney and Coogan, 2018). Interestingly, decreasing the level of daytime illumination that rodents are exposed to can also affect components of the HPA axis and lead to changes in mood related behaviors (Ikeno et al., 2016).
Studies using non-24 h light cycles also support a role for circadian disruption impacting mood and cognitive behaviors. Mice housed in a shortened light cycle (10:10 light:dark) exhibit metabolic changes, impaired cognition, and decreased neuronal complexity compared to mice housed on a 24 h LD cycle (Karatsoreos et al., 2011). Furthermore, exposure to a T7 light cycle (3.5 h light:3.5 h dark) increases depressive-like behaviors in mice, which are reversible with administration of the antidepressant drugs fluoxetine or desipramine (LeGates et al., 2012).
Other models of circadian disruption involve repeated phase shifts of the LD cycle to mimic chronic jet lag or shift work schedules. For example, rats subjected to 4 weekly 6 h phase advances of the LD cycle exhibit impaired escape learning (i.e. learned helplessness), and this deficit is not prevented by behavioral control, a robust stress-protective factor (Daut et al., 2019). Taken together, these studies highlight the importance of light rhythms and intensity, and challenges to the circadian system in regulating behavior. Disrupting the alignment between endogenous biological rhythms and the external lighting environment can have profound consequences for mood.
3.4. Clock genes in depression
3.4.1. Epidemiological evidence
Genetic factors undoubtedly play a role in the onset of MDD as the heritability estimate ranges from 31 to 42% (Flint & Kendler, 2014). Human genetic studies have identified several variants or single nucleotide polymorphisms (SNPS) in clock genes and their modifiers that are linked to mood disorders. SNPs in the Cry1, Npas2 (Soria et al., 2010), and Cry2 (Kovanen et al., 2017) genes are strongly associated with MDD. Additionally, individuals with a history of depression exhibit higher expression of Clock, Per1, and Bmal1 mRNA levels compared to healthy controls as measured in peripheral blood leukocytes in the morning (Gouin et al., 2010). Furthermore, a SNP in the 3′-flanking region of Clock is correlated with sleep profiles, sleep-wake cycle, and depressive episode relapse (Benedetti et al., 2003; Benedetti et al., 2007). Interestingly, some clock gene variants (Clock, Per3, and Npas2) are sex-dependently associated with MDD (Shi et al., 2016). This finding has clinical relevance because MDD prevalence is 2–3 times greater in females than males (Albert, 2015), and there is pre-clinical evidence that aspects of the circadian system are sexually dimorphic (Bailey & Silver, 2014; Chun et al., 2015; Mahoney et al., 2009). Furthermore, several clock genes exhibit weaker cyclic expression patterns in mood-related brain regions in postmortem tissue of depressed individuals compared to non-depressed controls (Li et al., 2013); rhythms were also phase shifted and desynchronized from external time in depressed individuals (Li et al., 2013). Additionally, SNPs in Clock predict response to SSRIs (Kishi et al., 2009). Thus, it appears that variation in clock genes and their promoters may predispose individuals to develop MDD (Desan et al., 2000).
3.4.2. Evidence from animal models
Direct manipulation of the molecular circadian clock in mice provides further support for a role of the circadian system in mood disorders. Roybal et al. (2007) were the first to report that a global mutation in the Clock gene produces manic-like behaviors in mice including hyperactivity, decreased sleep, reduced anxiety, and increased reward valence (Roybal et al., 2007). Subsequent work revealed that global mutations in other clock genes including Per3 (Zhang et al., 2016a; Martynhak et al., 2017), Cry1 (Schnell et al., 2015), and Arntl (BMAL1-coding gene) (Leliavski et al., 2014), alter anxiety and depressive-like responses. Importantly, clock genes act as transcription factors for a number of gene products throughout the lifespan so it is unclear from these types of global clock mutant manipulations if changes in behavior are caused by ongoing disruptions to the circadian system. More targeted mutations of clock genes, however, support a role for disruption of the circadian clock in mood. For example, challenging the circadian system with an SCN-specific knockdown of BMAL1 disrupts circadian rhythms in the SCN and increases depressive-like behavior. BMAL1-knockdown mice showed increases in learned helplessness in the forced swim and tail suspension tests as well as a reduction in sucrose preference (Landgraf et al., 2016). BMAL1-knockdown was also associated with an altered diurnal CORT rhythm and suppressed CORT release in response to stress (Landgraf et al., 2016). Furthermore, knockdown of both Per1 and Per2 in the nucleus accumbens with RNA interference increases anxiety-like responses in mice (Spencer et al., 2013).
3.5. Circadian based treatments of depression
The potential circadian basis for mood disorders is further supported by evidence that circadian strategies can alleviate depressive symptomatology.
3.5.1. Sleep deprivation therapy
One night of total sleep deprivation produces a rapid improvement in mood that persists for up to 36 h in 40–60% of depressed patients (Giedke & Schwarzler, 2002; Wirz-Justice & Van den Hoofdakker, 1999). Partial sleep deprivation (restricted to the second half of the night) is also effective, although not to the same degree (Wirz-Justice et al., 2005). In healthy control subjects, sleep deprivation increases fatigue and irritability with little to no effect on mood (Wirz-Justice, 2008). Importantly, the clinical utility of this treatment is limited because patients typically relapse following sleep recovery. The antidepressant effect of sleep deprivation may be mediated by non-circadian mechanisms. For example, acute sleep deprivation increases plasma cortisol levels, especially during the trough of the CORT rhythm, whereas chronic sleep deprivation reduces overall CORT (Wright et al., 2015). Therefore, sleep deprivation may act as an acute stressor by increasing arousal hormones until sleep homeostasis is restored (McEwen, 2006). This may explain the transient nature of sleep deprivation’s antidepressant effect.
3.5.2. Bright light therapy
Bright light therapy was originally developed to treat seasonal affective disorder (SAD), a mood disorder associated with reduced light exposure during short photoperiod months. More recently, bright light therapy has shown clinical promise in treating non-seasonal depression. Standard bright light therapy involves exposing patients to a light box (10,000 lx) for 30–90 min, typically in the morning shortly after waking (Terman and Terman, 2005). A meta-analysis by Golden et al. (2005) revealed that the effect size produced by bright light therapy is similar to those reported in clinical trials for pharmacological antidepressants. Repeated daily bright light therapy produces a 1 h phase advance in body temperature rhythms in depressed patients (Burgess et al., 2004). Blue light may be more effective than other wavelengths of light in phase shifting circadian rhythms and producing antidepressant effects (Glickman et al., 2006) because human melanopsin-containing ipRGCs are most strongly stimulated by blue light (~480 nm) and minimally influenced by red light (> 600 nm) (Brainard et al., 2001). Furthermore, bright light therapy co-administered with antidepressants is significantly more effective than either treatment alone (Benedetti et al., 2003a, 2003b; Lam et al., 2016). This suggests that circadian based therapies and 5-HTergic drugs have synergistic effects on mood disorders.
3.5.3. Agomelatine
Agomelatine is a potent melatonin receptor (MT1 and MT2) agonist and 5-HT2C receptor antagonist that has antidepressant properties in both humans and animal models of depression (Kennedy and Emsley, 2006; Loo et al., 2002; Papp et al., 2003) with comparable efficacy to other antidepressant drugs (Lemoine et al., 2007 Loo et al., 2002). Agomelatine does improve aspects of sleep, but it is especially effective in treating circadian rhythm disruption in depression. In particular, agomelatine accelerates resynchronization of circadian rhythms following abrupt shifts of the LD cycle in both humans and rodents (Weibel et al., 2000). Interestingly, melatonin administration during the day produces dysphoria and worsens depressive symptoms indicating that melatonin itself does not have antidepressant properties (Carman et al., 1976). This suggests that the antidepressant effects of agomelatine may be due, in part, to action at the 5-HT2C receptor (see below for further details).
4. Stress and the circadian system
As noted above, the circadian and stress systems are complementary regulatory networks that prepare organisms for both predictable (circadian) and unpredictable (stressors) daily challenges; these systems work to restore homeostasis in response to a continuously changing external environment. Importantly, the stress and circadian systems are also highly interrelated. For example, circulating glucocorticoid concentrations exhibit robust regulation by the circadian system and are a primary output of the stress response (via activation of the HPA axis) [reviewed in (Oster et al., 2017)]. Here we will review research on crosstalk between the circadian system and stress.
4.1. Stress disrupts the circadian system
The “social zeitgeber theory of depression” proposes that stressors disrupt the circadian system and precipitate depressive episodes in vulnerable individuals (Grandin et al., 2006). The occurrence of significant life events (e.g. retirement, bereavement, birth of a child, shift work) can impact daily habits which normally serve as auxiliary entrainment factors for the circadian system, and thereby disrupt circadian rhythms including mood (Luan et al., 2011). Bipolar subjects report fewer daily activities than healthy controls and the degree of social rhythm irregularity is predictive of depressive and manic episodes (Shen et al., 2008). Thus, disruption of the circadian system may not only be an epiphenomenon but may actually play a causal role in the development of mood disorders.
In rodents, exposure to an acute traumatic stressor or chronic mild stress elicits a variety of behavioral changes analogous to human depression including reductions in locomotion, exploration, social interaction, sexual motivation, as well as food and water consumption [reviewed in (Maier et al., 2006; Willner, 2005)]. Furthermore, many acute and chronic stress paradigms induce circadian abnormalities. For example, chronic variable stress induces brain region-specific alterations in circadian clock gene rhythms that directly correlate with mood related behaviors (Logan et al., 2015). Furthermore, mice subjected to a chronic social defeat protocol exhibit marked changes in body temperature and locomotor activity rhythms (Krishnan et al., 2007). Interestingly, a subset of resilient mice that do not develop a depression-like phenotype following stress also do not exhibit the same circadian changes as the susceptible mice (Krishnan et al., 2007). In contrast, perceived behavioral control over a stressor produces stress resilience/resistance in rats, but does not protect against stress-induced changes in diurnal rhythms. Both controllable and uncontrollable stressors altered locomotor activity and core body temperature diurnal rhythms to the same extent in rats (Thompson et al., 2013)
Stress-induced changes in circadian rhythms are concomitant with disruptions of clock genes. Animal studies utilizing acute stress paradigms consistently report a rapid (30–60 min) stress-induced increase of Per1 mRNA in extra-SCN clocks in the brain and periphery, but not the master clock itself (Takahashi et al., 2001). As stated above, the SCN lacks GRs (Balsalobre et al., 2000), and so stress-induced glucocorticoids can impact extra-SCN clocks but leave the master clock relatively unperturbed. Stress can, however, increase Per1 expression in a CORT-independent manner (Al-Safadi et al., 2015; Bohacek et al., 2015; Chun et al., 2018). This suggests that a different stress-induced signal is likely responsible for driving Per1 expression in extra-SCN clocks. Furthermore, the Per1 gene also contains a functional cAMP response element (CRE) (Tischkau et al., 2003). Thus, Per1 may act as an immediate early gene during stress (Spencer et al., 2018).
Studies using predictable chronic mild stress paradigms show that chronic stress can serve as an entrainment factor for extra-SCN clocks. For example, rats exposed to several days of restraint or social defeat stress exhibit phase shifted Per2 rhythms in extra-SCN clocks (Tahara et al., 2015). Furthermore, the time of day during which stress occurred determined the direction of the shift (Tahara et al., 2015). Thus, the entraining ability of stress may be an adaptive feature of the circadian system to anticipate and buffer against recurring challenges. In contrast, studies using unpredictable chronic stressors have a more disruptive effect and can even alter clock genes in the SCN (Jiang et al., 2011; Logan et al., 2015).
4.2. Circadian variation in stress susceptibility
In addition to stress altering the circadian system, there is evidence that the circadian system regulates the stress response and stress-induced comorbidities. For example, the time of day during which a stressor is encountered can potently modulate its outcome (Fonken et al., 2016; Johnson et al., 2003). For example, prior stress can increase vulnerability to inflammatory challenges (Fonken et al., 2018). However, the timing of the stressor is critical for regulating the subsequent response to immune challenge: rats exposed to inescapable tail shock stress during the middle of their inactive (light) phase exhibited an exaggerated immune response, whereas rodents exposed to stress during their active (dark) phase, did not (Fonken et al., 2016). Furthermore, rats exposed to stressors during their inactive phase develop an anxiety- and depressive-like phenotype, whereas rodents stressed during the beginning of the active phase, do not (Aslani et al., 2014; Cohen et al., 2015; Fonken et al., 2016; Retana-Márquez et al., 2003; Rybkin et al., 1997). Taken together, these findings suggest that organisms may be more resistant/resilient to stressors during their active phase. This may be an adaptive mechanism whereby an organism up-regulates processes that permit stressor recovery and adaptation during the time of day that it is more likely to encounter a homeostatic threat.
5. Interactions between the circadian system, the serotonin system, and stress
The evidence presented in this review thus far supports a role for the circadian system in regulating mood and vulnerability to depression. However, the mechanism(s) by which the circadian system targets these complex behavioral processes remains unclear. Here we propose that the 5-HT system may serve as the common link between the circadian system, stress, and mood.
One potential mechanism by which stressors might directly impact the circadian system is by way of the 5-HT system. There is bi-directional communication between 5-HT and the circadian system. 5-HT plays a role in producing non-photic phase shifts (Mistlberger et al., 2000) and also opposes the action of light in the SCN (Rea & Pickard, 2000). Conversely, the 5-HT system is under circadian control. The synthesis of 5-HT is driven by the daily rhythmic secretion of glucocorticoids (Malek et al., 2005; Malek et al., 2007), resulting in rhythmic 5-HT release within the SCN (Cagampang and Inouye, 1994) and other limbic projection regions. During exposure to certain types of stressors, 5-HT neurons are under afferent control of stress-responsive brain regions (Chaouloff, 2000). In turn, 5-HT neurons project to regions involved in the regulation of mood (Lowry et al., 2008) as well as the ventrolateral SCN. Thus, altered 5-HT signaling may play a role in circadian dysfunction in MDD. Congruent with this hypothesis, commonly prescribed antidepressants, which increase synaptic 5-HT levels, alter circadian rhythms and clock gene expression (see below).
5.1. The serotonin system
In addition to its role in the circadian system, 5-HT is implicated in a diverse array of physiological processes and behaviors including: the sleep-wake cycle, cardiovascular functions, respiration, thermoregulation, appetite, aggression, sexual behavior, pain, and learning [Reviewed in (Lesch and Waider, 2012)}. The 5-HT system is considered the most architecturally complex and extensive neurotransmitter system in the mammalian central nervous system (Hainer et al., 2015). 5-HT projections are highly collateralized (Waselus et al., 2011): it is estimated that each 5-HT neuron targets at least 500,000 other neurons in the brain (Jacobs and Azmitia, 1992), implicating 5-HT as a modulatory neurotransmitter.
Not all types of stressors produce identical neural activation patterns. “The midbrain raphe nuclei (comprised of the dorsal and median raphe nuclei, DRN and MRN, respectively) are responsive to various types of physical (e.g. forced swim), psychological (e.g. inescapable tailshock, social defeat, immobilization), and metabolic (e.g. hypoglycemia) stressors [reviewed in (Hale et al., 2012)]. The midbrain raphe nuclei are well positioned to modulate fear and anxiety as they innervate many points in the limbic circuit (Graeff et al., 1996). For example, distinct DRN targets include the prefrontal cortex, lateral septum, and amygdala, whereas MRN targets include the medial septum, cingulate, and dorsal hippocampus (Azmitia and Segal, 1978). Moreover, the midbrain raphe nuclei are under afferent control of stress-responsive nuclei. The DRN is the largest 5-HTergic nucleus, containing approximately 50% of all 5-HT synthesizing neurons in the brain (approximately 15,000 neurons in the rat) (Vertes and Crane, 1997). 5-HT signaling is regulated by 17 receptors, the majority of which are G-coupled receptors (Yohn et al., 2017). Dysfunction of the 5-HT system has been implicated in the etiology of MDD since the 1950s (Loomer et al., 1957).”
5.2. Serotonergic regulation of the master clock
A major afferent projection to the SCN originates from the midbrain raphe complex; in hamsters, primarily from the median raphe nucleus [MRN; (Meyer-Bernstein and Morin, 1996; Leander et al., 1998; Yamakawa and Antle, 2010)] and in rats, from both the MRN and DRN [Fig. 3; (Kawano et al., 1996; Moga and Moore, 1997;). In fact, this is one of the densest 5-HT-ergic plexuses in the brain (Beaudet and Descarries, 1979; Bolser and Beaudet, 1985), so it follows that 5-HT is likely critical for circadian function. For example, developmental loss of Pet-1, a transcription factor that determines 5-HT neuron phenotype, disrupts locomotor activity rhythms and lengthens the period and amplitude of SCN neuronal activity ex vivo (Ciarleglio et al., 2011).
Fig. 3.
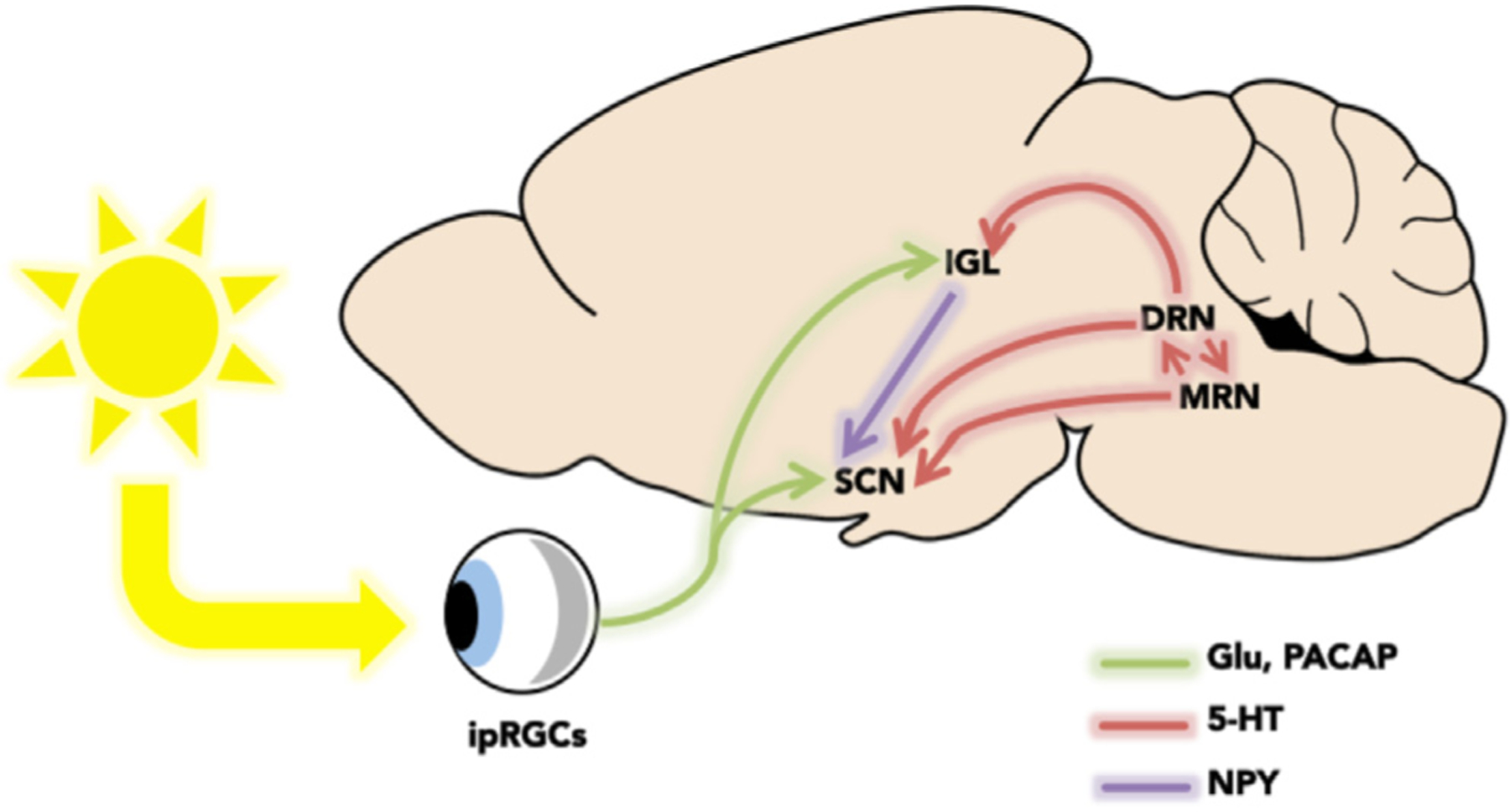
Neural circuitry of the circadian system. Photic information enters the retina and stimulates melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs). Photic information is directly relayed to the suprachiasmatic nucleus (SCN) and intergeniculate leaflet (IGL) via the retinohypothalamic tract (RHT), a glutamatergic and/or pituitary adenylate cyclase-activating peptide (PACAP) pathway. The SCN also receives non-photic information from an NPY-ergic pathway from the as well as from a 5-HTergic pathway from the dorsal raphe and median raphe nuclei (DRN and MRN, respectively).
The circadian role of 5-HT differs across the LD cycle. During the subjective day, 5-HT produces non-photic phase shifts (Mistlberger et al., 2000). For example, application of selective 5-HT receptor agonists during the middle of the light phase shifts SCN activity by approximately 3 h (Lovenberg et al., 1993; Sprouse et al., 2004). While during the subjective night, 5-HT opposes the action of light. 5-HT acts both pre-synaptically on 5-HT terminals and post-synaptically on SCN VIP neurons to inhibit the effect of light on the SCN (Ciarleglio et al., 2011). Furthermore, manipulation of brain 5-HT levels significantly alters endocrine and behavioral circadian rhythms (Smale et al., 1990; Morin and Blanchard, 1991; Whitney et al., 2016).
5.3. Circadian regulation of serotonin
5-HT varies across the LD cycle. 5-HT is highest during the active phase in both nocturnal and diurnal rodents (Challet, 2007). This rhythm persists in constant conditions indicating that this rhythm is endogenously generated. Furthermore, 5-HT transporter mRNA expression and in vivo 5-HT reuptake activity exhibits significant time-dependent changes with higher levels during the dark phase and lower levels during the light phase in midbrain raphe nuclei (Ushijima et al., 2005; Ushijima et al., 2012). Additionally, the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HTIAA), also exhibits diurnal variation in the hamster SCN with greater release occurring during the dark phase (Glass et al., 1992). Taken together, these findings indicate that 5-HT signaling is greater during the active phase.
The circadian regulation of 5-HT requires input from the sympathetic nervous system. For example, adrenergic blockade or ablation of the superior cervical ganglion abolishes the 5-HT circadian rhythm (Snyder et al., 1965; Snyder et al., 1967; Sun et al., 2002). The TPH2 gene, which encodes tryptophan hydroxylase, the rate-limiting enzyme in the synthesis of 5-HT from its precursor L-tryptophan (Walther et al., 2003), exhibits circadian variation within the midbrain raphe nuclei with peak expression occurring 2 h prior to the light-dark transition in rats (Malek et al., 2005; Malek et al., 2007). Furthermore, this rhythm appears to be mediated by the daily glucocorticoid surge as removal of endogenous glucocorticoids via adrenalectomy suppresses tph2 (Malek et al., 2007) and 5-HT (De Kloet et al., 1982; Singh et al., 1990; Azmitia et al., 1993), whereas replacement with exogenous CORT restores the tph2 rhythm (Malek et al., 2007). These findings suggest that glucocorticoids have a stimulatory effect on tph2 expression. Indeed, DRN 5-HT neurons are highly enriched with GRs (Harfstrand et al., 1986). GRs display low affinity for glucocorticoids under basal conditions and so only conditions during which glucocorticoids are secreted to a high degree (e.g. peak of the daily glucocorticoid rhythm, stress) are associated with GR-mediated effects. In support, a variety of acute and chronic stressors (Clark et al., 2005; Donner et al., 2012a; Donner et al., 2018) as well as chronic non-invasive CORT administration (Donner et al., 2012b) abolish the DRN tph2 rhythm. However, it is important to note that the DRN is under afferent control from several brain regions that are also responsive to glucocorticoids and so systemic manipulation of glucocorticoids could impact 5-HT synthesis independently of DRN GRs. Interestingly, Vincent and colleagues (2018) demonstrated that viral-mediated knockdown of DRN GRs in mice increases tph2 at the circadian nadir, indicating an inhibitory effect of glucocorticoids on 5-HT synthesis. These contradictory findings might be explained by the method of glucocorticoid manipulation (i.e. systemic depletion vs. localized inhibition) and/or species-specific differences. Taken together, these data show that stress-induced changes in glucocorticoids could impact the 5-HT system and thereby interfere with normal circadian function.
5.4. The serotonin system is retino-recipient
In addition to the SCN, several mood-related brain regions receive direct retinal input including the amygdala, bed nucleus of the stria terminalis, lateral habenula, as well as the DRN (Hattar et al., 2006). This suggests that light can regulate mood independently of the master clock. The pattern of DRN innervation is similar across all species studied thus far; retinal fibers emerge from the optic tract at the level of the pretectum/anterior superior colliculus and descend into the PAG and terminate in the dorsomedial and lateral subdivisions of the DRN (Fite et al., 1999; Fite and Janusonis, 2001). The majority of these RGCs are described as having alpha-like morphological features and Y type visual response properties (Luan et al., 2011). These can be further subdivided into ‘ON’ or ‘OFF’ type cells. ON-type DRN projecting RGCs (80%) increase firing in response to light increments and preferentially synapse onto GABA neurons. OFF-type DRN projecting RGCs (20%) increase firing in response to light decrements and preferentially synapse onto 5-HT neurons (Luan et al., 2011; Zhang et al., 2016b). These cells may play a role in visual altering responses although their precise function remains unknown (Peichl, 2005).
The fact that the DRN is retino-recipient suggests that the 5-HT system is light sensitive. In gerbils, low frequency light stimulation in the middle of the dark phase significantly increases DRN activity (Fite et al., 2005). In addition, in cats, exposure to intermittent light pulses increases the discharge rate above active waking levels in a sub-population of putative 5-HT neurons located in the midbrain raphe complex (Mosko & Jacobs, 1974). In addition, in rats, 10% of DRN-projecting RGCs are melanopsin-immunopositive and representative of the M1 subtype (Li et al., 2015). Thus, the DRN appears to be involved in photic as well as non-photic circadian functions. In gerbils, selective silencing of DRN-projecting RGCs induces a depression-like phenotype and reduces DRN 5-HT levels (Ren et al., 2013). This suggests that DRN-projecting RGCS may play a role in the regulation of mood. Interestingly, in humans, exposure to blue wavelength light significantly increases activity in the brainstem (Vandewalle et al., 2007; Vandewalle et al., 2009); however, the effect of light-induced stimulation of the DRN on mood remains to be tested.
5.5. Serotonergic drugs impact the circadian system
The three main classes of pharmacological antidepressants that target the serotonin system are: (1) monoamine oxidase inhibitors (MAOIs) that prevent metabolism of monoamines; (2) tricyclic drugs that block the reuptake of monoamines; and (3) selective serotonin reuptake inhibitors (SSRIs) that specifically block the reuptake of 5-HT. Currently, SSRIs are the first line treatment for MDD (Cascade & Kalali, 2007; Kubitz et al., 2013), and prevalence of use continues to rise in the U.S. (Pratt et al., 2017). Originally, SSRIs were thought to exert their antidepressant effects by globally increasing synaptic levels of central 5-HT, but other mechanisms (e.g. hippocampal neurogenesis) may also be involved (Santarelli et al., 2003). SSRIs can also impact the circadian system, and in general, are associated with phase-advancing circadian rhythms. For example, in vitro application of the SSRI, fluoxetine (preloaded with the 5-HT precursor L-tryptophan), significantly phase advances the firing rate of SCN neurons (Ehlen et al., 2001; Sprouse et al., 2006). In addition, intra-SCN administration of 5-HT or specific 5-HT receptor agonists produces phase shifts in locomotor activity rhythms (Dudley et al., 1999; Ehlen et al., 2001). The phase-shifting effects of 5-HTergic drugs are strongest during the middle of the light phase (Horikawa and Shibata, 2004; Prosser, 2003). The 5-HT transporter is highly expressed in the SCN (Amir et al., 1998; Legutko and Gannon, 2001; Sur et al., 1996); therefore, SSRIs likely increase extracellular 5-HT in the SCN and thereby inhibit light-induced phase shifts. Additionally, fibroblasts from Per1 reporter mice treated with SSRIs dose-dependently shortens the period and accelerates the damping rate of Per1 (Nomura et al., 2008). Moreover, acute administration of the SSRI citalopram increases sensitivity of the circadian system in humans (McGlashan et al., 2018). This may explain why bright light therapy is more effective when administered with SSRIs. Importantly, not all antidepressants produce these effects, as non-5-HTergic antidepressants (e.g. imipramine) have no impact on circadian rhythms (Refinetti and Menaker, 1993). Thus, it appears that SSRIs may, in part, exert their therapeutic effects by modulating circadian clockwork through 5-HT-ergic pathways.
6. Conclusion
Proper synchronization with the environmental LD cycle is necessary for optimal health. Environmental disruption of circadian rhythms can impact mood and increase susceptibility to mood disorders. For example, shift workers exhibit high rates of MDD and risk increases as a function of shift work duration (Lee et al., 2017). Exposure to unnatural or mistimed light is pervasive in modern society: nearly 80% of the global population encounters light pollution at night (Falchi et al., 2016). In the present review we summarized several lines of research showing that circadian changes are associated with an increased susceptibility to mood disorders. First, the circadian system controls rhythmicity in numerous physiological processes and behaviors, including both the positive and negative components of mood. Depressed individuals exhibit alterations in the amplitude and/or phase timing of a diverse array of circadian rhythms including mood. Interestingly, these disturbed rhythms often return to normal following relapse from a depressive episode. Second, variations and SNPs in core clock genes predispose individuals to MDD. Third, some circadian-based strategies for treating depression (e.g. bright light therapy, sleep deprivation, agomelatine) can alter circadian rhythms and improve mood, and are as efficacious as SSRIs. Fourth, stress is the dominant predisposing factor for MDD and there is bi-directional regulation between stress and the circadian system. Finally, animal studies using clock gene mutant rodents and models of circadian disruption support a causative relationship between circadian dysfunction and affective behavioral changes.
The exact mechanisms by which disruptions to the circadian system change mood are unclear. Here we provided evidence that modulation of the serotonin system is one of the possible pathways through which the circadian system regulates vulnerability to depression (Fig. 4). The SCN has relatively few direct neural projections, but is reciprocally connected to the 5-HT system via the midbrain raphe nuclei. The midbrain raphe nuclei innervate several brain regions involved in affective regulation. Not surprisingly, 5-HT is important for normal circadian function, and drugs that alter components of 5-HT signaling (e.g. SSRIs) can modulate components of the clock. Therefore, 5-HTergic drugs may contribute to the restoration of circadian function in depressed individuals. Future clinical studies should explore whether novel optimized combinations of circadian and pharmacologic-based treatments help to improve mood. One advantage of circadian based strategies is that they can be tailored to treat individual symptoms. For example, the timing of bright light therapy can be shifted to treat either an advanced or delayed component of circadian rhythms. Ultimately, the etiology and progression of MDD is determined by a complex interaction among genetic, environmental, and neurobiological factors. The 5-HT system may serve as an important intermediate in the circadian regulation of vulnerability to MDD.
Fig. 4.
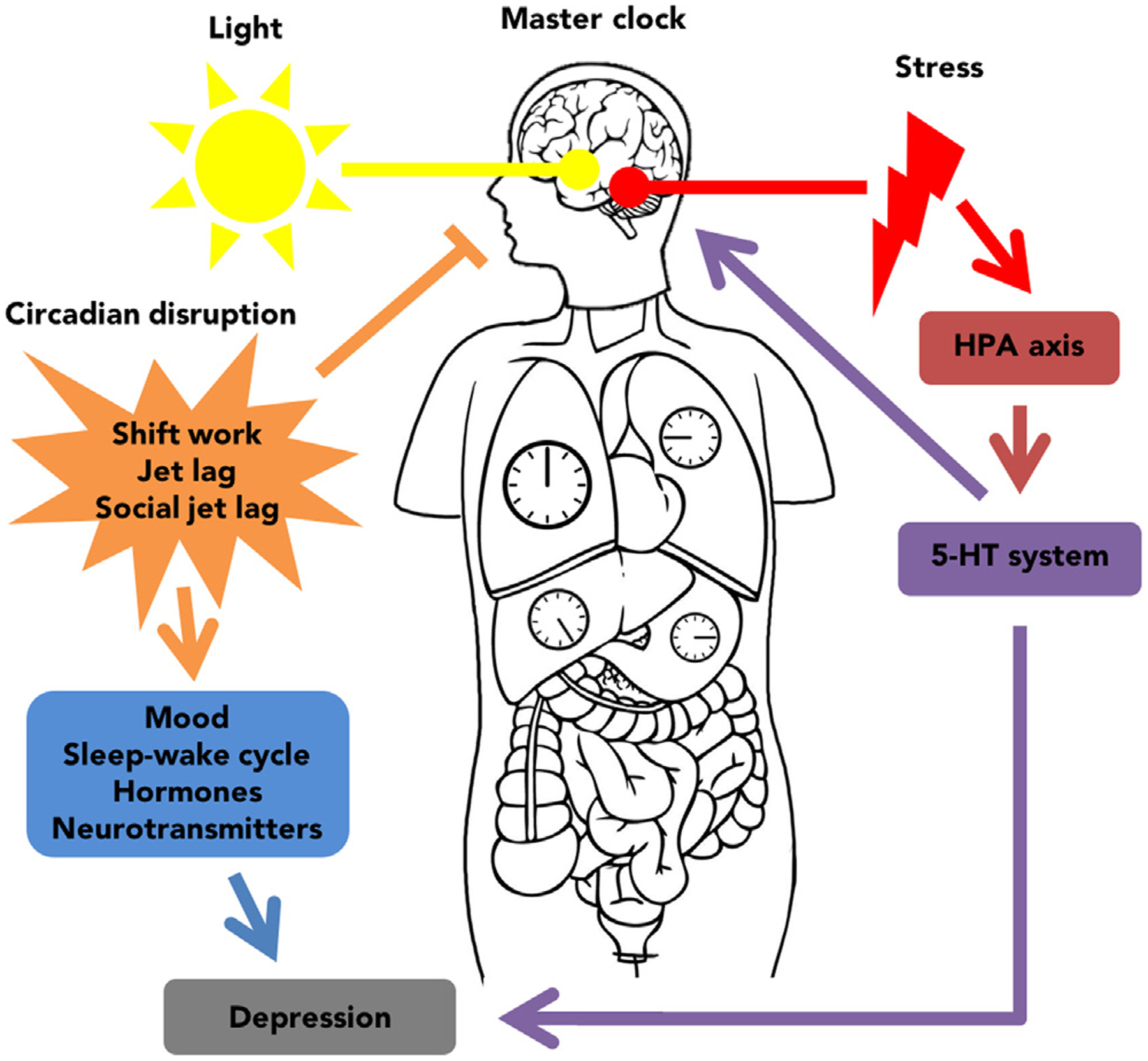
Schematic summarizing the relationship between the circadian system and stress systems in the regulation of mood. The master clock of the circadian system is entrained by light and synchronizes extra-SCN and peripheral clocks using neural and humoral signals. Circadian disruption can dysregulate the master clock resulting in altered circadian rhythms and increased vulnerability to depression. Stress is a risk factor for depression and can impact the 5-HT system. The 5-HT system and the master clock are reciprocally connected and so circadian disruption may exacerbate depressive symptoms through 5-HT innervation of mood-related brain regions.
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD, 2002. Circadian rhythms in isolated brain regions. J. Neurosci 22 (1), 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson EE, Moore RY, 2001. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 889 (1–2), 1–22. [DOI] [PubMed] [Google Scholar]
- Albert PR, 2015. Why is depression more prevalent in women? J. Psychiatry Neurosci 40, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC, 1997. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91 (7), 1055–1064. [DOI] [PubMed] [Google Scholar]
- Al-Safadi S, Branchaud M, Rutherford S, Amir S, 2015. Glucocorticoids and stress-induced changes in the expression of PERIOD1 in the rat forebrain. PLoS ONE 10 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Publishing, 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. 5th ed. Arlington, VA, US, Inc.: xliv, 947–xliv, 947. [Google Scholar]
- Amir S, Robinson B, Ratovitski T, Rea MA, Stewart J, Simantov R, 1998. A role for serotonin in the circadian system revealed by the distribution of serotonin transporter and light-induced Fos immunoreactivity in the suprachiasmatic nucleus and intergeniculate leaflet. Neuroscience 84 (4), 1059–1073. [DOI] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B, Stewart J, 2004. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J. Neurosci 24 (4), 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer P, et al. , 2017. Night work and the risk of depression. Dtsch. Arztebl. Int 114, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antypa N, Verkuil B, Molendijk M, Schoevers R, Penninx B, Van Der Does W, 2017. Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol. Int 34 (8), 1125–1135. [DOI] [PubMed] [Google Scholar]
- Arendt J, 2018. Approaches to the pharmacological management of jet lag. Drugs 78 (14), 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka S, et al. , 2013. Factors associated with shift work disorder in nurses working with rapid-rotation schedules in Japan: the nurses’ sleep health project. Chronobiol. Int 30, 628–636. [DOI] [PubMed] [Google Scholar]
- Aschoff J, 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb. Sympos. Quant. Biol 25, 11–28. [DOI] [PubMed] [Google Scholar]
- Aschoff J, 1965. Circadian rhythms in man. Science 148 (3676), 1427–1432. [DOI] [PubMed] [Google Scholar]
- Aslani S, et al. , 2014. Day and night: diurnal phase influences the response to chronic mild stress. Front. Behav. Neurosci 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au J, Reece J, 2017. The relationship between chronotype and depressive symptoms: a meta-analysis. J. Affect Disord 218, 93–104. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M, 1978. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol 179 (3), 641–647. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Liao B, Chen YS, 1993. Increase of tryptophan hydroxylase enzyme protein by dexamethasone in adrenalectomized rat midbrain. J. Neurosci 13 (12), 5041–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahk YC, Han E, Lee SH, 2014. Biological rhythm differences and suicidal ideation in patients with major depressive disorder. J. Affect. Disord 168, 294–297. [DOI] [PubMed] [Google Scholar]
- Bailes HJ, Lucas RJ, 2013. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc. Biol. Sci 280 (1759), 20122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M, Silver R, 2014. Sex differences in circadian timing systems: implications for disease. Front. Neuroendocrinol 35, 111–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U, 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289 (5488), 2344–2347. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Descarries L, 1979. Radioautographic characterization of a serotonin-accumulating nerve cell group in adult rat hypothalamus. Brain Res. 160 (2), 231–243. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ, 2011. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36 (7), 1062–1069. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Nelson RJ, 2013. Influence of the modern light environment on mood. Mol. Psychiatry 18 (7), 751–757. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ, 2013a. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J. Neuroendocrinol 25 (6), 590–596. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Nelson RJ, 2016. Endocrine effects of circadian disruption. Annu. Rev. Physiol 78, 109–131. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ, 2013b. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J. Neurosci 33 (32), 13081–13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, et al. , 2003a. Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J. Clin. Psychiatry 64, 648–653. [DOI] [PubMed] [Google Scholar]
- Benedetti F, et al. , 2003b. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am. J. Med. Genet. B Neuropsychiatr. Genet 123b, 23–26. [DOI] [PubMed] [Google Scholar]
- Benedetti F, et al. , 2007. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am. J. Med. Genet. B Neuropsychiatr. Genet 144b, 631–635. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Kim JS, Iwata NG, Perlis ML, 2015. Sleep disturbances as an evidence-based suicide risk factor. Curr. Psychiatry Rep 17 (3), 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M, 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295 (5557), 1070–1073. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Manuella F, Roszkowski M, Mansuy IM, 2015. Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology 52, 1–12. [DOI] [PubMed] [Google Scholar]
- Bolser O, Beaudet A, 1985. VIP neurons as prime synaptic targets for serotonin afferents in rat suprachiasmatic nucleus: a combined radioautographic and immunocytochemical study. J. Neurocytol 14 (5), 749–763. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD, 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci 21 (16), 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A, 2001. Hypothalamic integration of central and peripheral clocks. Nat. Rev. Neurosci 2 (7), 521–526. [DOI] [PubMed] [Google Scholar]
- Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, Barchas JD, Schatzberg AF, Myers RM, Watson SJ, Akil H, Bunney WE, 2015. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol. Psychiatry 20 (1), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Serati M, Grassi S, Pergoli L, Cantone L, Altamura AC, Bollati V, 2018. The role of clock genes in the etiology of major depressive disorder. J. Affect. Disord 234, 351–357. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, et al. , 2004. Bright light therapy for winter depression–is phase advancing beneficial? Chronobiol. Int 21, 759–775. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Inouye ST, 1994. Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain Res. 639 (1), 175–179. [DOI] [PubMed] [Google Scholar]
- Carman JS, et al. , 1976. Negative effects of melatonin on depression. Am. J. Psychiatry 133, 1181–1186. [DOI] [PubMed] [Google Scholar]
- Cascade EF, Kalali AH, Thase ME, 2007. Use of antidepressants: expansion beyond depression and anxiety. Psychiatry (Edgmont) 4, 25–28. [PMC free article] [PubMed] [Google Scholar]
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J, 1993. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur. J. Neurosci 5 (4), 368–381. [DOI] [PubMed] [Google Scholar]
- Challet E, 2007. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148, 5648–5655. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, 2000. Serotonin, stress and corticoids. J. Psychopharmacol 14, 139–151. [DOI] [PubMed] [Google Scholar]
- Cheon S, Park N, Cho S, Kim K, 2013. Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Res. 41 (12), 6161–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, 2001. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci 4 (6), 567–568. [DOI] [PubMed] [Google Scholar]
- Chun LE, Woodruff ER, Morton S, Hinds LR, Spencer RL, 2015. Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. J. Biol. Rhythms 30 (5), 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LE, Christensen J, Woodruff ER, Morton SJ, Hinds LR, Spencer RL, 2018. Adrenal-dependent and -independent stress-induced Per1 mRNA in hypothalamic paraventricular nucleus and prefrontal cortex of male and female rats. Stress 21 (1), 69–83. [DOI] [PubMed] [Google Scholar]
- Ciarleglio CM, Resuehr HE, McMahon DG, 2011. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 197, 8–16. [DOI] [PubMed] [Google Scholar]
- Clark JA, Pai LY, Flick RB, Rohrer SP, 2005. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol. Psychiatry 57 (8), 943–946. [DOI] [PubMed] [Google Scholar]
- Cleary-Gaffney M, Coogan AN, 2018. Limited evidence for affective and diurnal rhythm responses to dim light-at-night in male and female C57Bl/6 mice. Physiol. Behav 189, 78–85. [DOI] [PubMed] [Google Scholar]
- Cohen S, et al. , 2015. Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology 40, 774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman G, Gigg J, Canal MM, 2016. Postnatal light alters hypothalamic-pituitary-adrenal axis function and induces a depressive-like phenotype in adult mice. Eur. J. Neurosci 44 (10), 2807–2817. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Hut RA, Daan S, 2008. Circadian phase resetting in response to light-dark and dark-light transitions. J. Biol. Rhythms 23 (5), 425–434. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE, 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284 (5423), 2177–2181. [DOI] [PubMed] [Google Scholar]
- Daut RA, Hartsock MJ, Tomczik AC, Watkins LR, Spencer RL, Maier SF, Fonken LK, 2019. Circadian misalignment has differential effects on affective behavior following exposure to controllable or uncontrollable stress. Behav. Brain Res 359, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Kovacs GL, Szabo G, Telegdy G, Bohus B, Versteeg DH, 1982. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 239 (2), 659–663. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M, 1998. Brain corticosteroid receptor balance in health and disease. Endocr. Rev 19 (3), 269–301. [DOI] [PubMed] [Google Scholar]
- Desan PH, et al. , 2000. Genetic polymorphism at the CLOCK gene locus and major depression. Am. J. Med. Genet 96, 418–421. [DOI] [PubMed] [Google Scholar]
- Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC, 2011. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep 34 (5), 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U, 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol 72, 517–549. [DOI] [PubMed] [Google Scholar]
- Doane LD, Kremen WS, Eaves LJ, Eisen SA, Hauger R, Hellhammer D, Levine S, Lupien S, Lyons MJ, Mendoza S, Prom-Wormley E, Xian H, York TP, Franz CE, Jacobson KC, 2010. Associations between jet lag and cortisol diurnal rhythms after domestic travel. Health Psychol. 29 (2), 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK, 2013. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol 25 (3), 629–642. [DOI] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA, 2012a. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety 29 (4), 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL, Lowry CA, 2012b. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37 (5), 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Kubala KH, Hassell JE Jr., Lieb MW, Nguyen KT, Heinze JD, Drugan RC, Maier SF, Lowry CA, 2018. Two models of inescapable stress increase tph2 mRNA expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus. Neurobiol. Stress 8, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley TE, Dinardo LA, Glass JD, 1999. In vivo assessment of the midbrain raphe nuclear regulation of serotonin release in the hamster suprachiasmatic nucleus. J. Neurophysiol 81 (4), 1469–1477. [DOI] [PubMed] [Google Scholar]
- Duguay D, Cermakian N, 2009. The crosstalk between physiology and circadian clock proteins. Chronobiol. Int 26 (8), 1479–1513. [DOI] [PubMed] [Google Scholar]
- Dumont M, Beaulieu C, 2007. Light exposure in the natural environment: relevance to mood and sleep disorders. Sleep. Med 8 (6), 557–565. [DOI] [PubMed] [Google Scholar]
- Dzogang F, Lightman S, Cristianini N, 2017. Circadian mood variations in Twitter content. Brain. Neurosci. Adv 1 2398212817744501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Mistlberger RE, Rechtschaffen A, 1984. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol. Behav 32 (3), 357–368. [DOI] [PubMed] [Google Scholar]
- Edwards MD, Brancaccio M, Chesham JE, Maywood ES, Hastings MH, 2016. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc. Natl. Acad. Sci. USA 113 (10), 2732–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Grossman GH, Glass JD, 2001. In vivo resetting of the hamster circadian clock by 5-HT7 receptors in the suprachiasmatic nucleus. J. Neurosci 21 (14), 5351–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ, 2009. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiol. Int 23 (3), 474–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R, 2016. The new world atlas of artificial night sky brightness. Sci. Adv 2 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite KV, Janusonis S, 2001. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus). Brain Res. 895 (1–2), 139–145. [DOI] [PubMed] [Google Scholar]
- Fite KV, Janusonis S, Foote W, Bengston L, 1999. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. J. Comp. Neurol 414 (4), 469–484. [DOI] [PubMed] [Google Scholar]
- Fite KV, Wu PS, Bellemer A, 2005. Photostimulation alters c-Fos expression in the dorsal raphe nucleus. Brain Res. 1031 (2), 245–252. [DOI] [PubMed] [Google Scholar]
- Flint J, Kendler KS, 2014. The genetics of major depression. Neuron 81, 484–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ, 2013. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav. Brain Res 243, 74–78. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ, 2009. Influence of light at night on murine anxiety- and depressive-like responses. Behav. Brain Res 205 (2), 349–354. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ, 2012. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythms 27 (4), 319–327. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ, 2013. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythms 28 (4), 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ, 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 107 (43), 18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, Maier SF, 2016. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 66, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, Maier SF, 2018. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain Behav. Immun 73, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, Roenneberg T, 2013. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog. Mol. Biol. Transl. Sci 119, 325–346. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R, 1999. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284 (5413), 502–504. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM, 2007. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell. Biol 8 (2), 139–148. [DOI] [PubMed] [Google Scholar]
- Giedke H, Schwarzler F, 2002. Therapeutic use of sleep deprivation in depression. Sleep Med. Rev 6, 361–377. [PubMed] [Google Scholar]
- Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I, 2017. Depression and mortality in a longitudinal study: 1952–2011. CMAJ 189 (42), E1304–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Randolph WW, Ferreira SA, Rea MA, Hauser UE, Blank JL, De Vries MJ, 1992. Diurnal variation in 5-hydroxyindole-acetic acid output in the suprachiasmatic region of the Siberian hamster assessed by in vivo microdialysis: evidence for nocturnal activation of serotonin release. Neuroendocrinology 56 (4), 582–590. [DOI] [PubMed] [Google Scholar]
- Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC, 2006. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol. Psychiatry 59 (6), 502–507. [DOI] [PubMed] [Google Scholar]
- Golden RN, et al. , 2005. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am. J. Psychiatry 162, 656–662. [DOI] [PubMed] [Google Scholar]
- Golder SA, Macy MW, 2011. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science 333 (6051), 1878–1881. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB, 2001. Melanopsin in cells of origin of the retinohypothalamic tract. Nat. Neurosci 4 (12), 1165. [DOI] [PubMed] [Google Scholar]
- Gouin JP, et al. , 2010. Altered expression of circadian rhythm genes among individuals with a history of depression. J. Affect Disord 126, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff EG, Guimarães ES, De Andrade TG, Deakin JE, 1996. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav 54 (1), 129–141. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY, 2006. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin. Psychol. Rev 26 (6), 679–694. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S, 2008. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453 (7191), 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer C, Mosienko V, Koutsikou S, Crook JJ, Gloss B, Kasparov S, Lumb BM, Alenina N, 2015. Beyond gene inactivation: evolution of tools for analysis of serotonergic circuitry. ACS Chem. Neurosci 6 (7), 1116–1129. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA, 2012. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol. Neurobiol 32 (5), 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J, 2000. PACAP and glutamate are co-stored in the retinohypothalamic tract. J. Comp. Neurol 418 (2), 147–155. [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. , 1986. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc. Natl. Acad. Sci. USA 83 (24), 9779–9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA, 2010. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 176 (2–3), 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M, 2018. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci 19 (8), 453–469. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW, 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295 (5557), 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM, 2006. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol 497 (3), 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D, Minors DS, Waterhouse JM, 1993. Shiftwork, helplessness and depression. J. Affect Disord 29, 17–25. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, 2017. Interactions between psychosocial stress and the circadian endogenous clock. Psych. J 6 (4), 277–289. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH, 2017. Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harb. Perspect. Biol 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML, 2009. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin. Neurosci 63 (3), 283–290. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Curtiss J, Carpenter JK, Kind S, 2017. Effect of treatments for depression on quality of life: a meta-analysis. Cogn. Behav. Ther 46 (4), 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Shibata S, 2004. Phase-resetting response to (+)8-OH-DPAT, a serotonin 1A/7 receptor agonist, in the mouse in vivo. Neurosci. Lett 368 (2), 130–134. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Deats SP, Soler J, Lonstein JS, Yan L, 2016. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus). Behav. Brain. Res 300, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC, 1992. Structure and function of the brain serotonin system. Physiol. Rev 72 (1), 165–229. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM, 2013. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol 93 (1), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhar P, Weller MP, 1982. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Br. J. Psychiatry 140, 231–235. [DOI] [PubMed] [Google Scholar]
- Jeon HJ, Bang YR, Park HY, Kim SA, Yoon IY, 2017. Differential effects of circadian typology on sleep-related symptoms, physical fatigue and psychological well-being in relation to resilience. Chronobiol. Int 34 (6), 677–686. [DOI] [PubMed] [Google Scholar]
- Jiang WG, et al. , 2011. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain Res. 1399, 25–32. [DOI] [PubMed] [Google Scholar]
- Johnson JD, et al. , 2003. Effects of prior stress on LPS-induced cytokine and sickness responses. Am. J. Physiol. Regul. Integr. Comp. Physiol 284, R422–R432. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS, 2011. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. USA 108 (4), 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz G, Knobler HY, Laibel Z, Strauss Z, Durst R, 2002. Time zone change and major psychiatric morbidity: the results of a 6-year study in Jerusalem. Compr. Psychiatry 43 (1), 37–40. [DOI] [PubMed] [Google Scholar]
- Kawano H, Decker K, Reuss S, 1996. Is there a direct retina-raphe-suprachiasmatic nucleus pathway in the rat? Neurosci. Lett 212 (2), 143–146. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156 (6), 837–841. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Emsley R, 2006. Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur. Neuropsychopharmacol 16, 93–100. [DOI] [PubMed] [Google Scholar]
- Kiessling S, Eichele G, Oster H, 2010. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J. Clin. Invest 120, 2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee YJ, Kim H, Cho IH, Lee JY, Cho SJ, 2010. Age as a moderator of the association between depressive symptoms and morningness-eveningness. J. Psychosom. Res 68 (2), 159–164. [DOI] [PubMed] [Google Scholar]
- Kishi T, et al. , 2009. CLOCK may predict the response to fluvoxamine treatment in Japanese major depressive disorder patients. Neuromolecular Med. 11, 53–57. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Kamei Y, Mishima K, 2010. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol. Int 27 (9–10), 1797–1812. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Teicher MH, Mitropoulou V, Navalta C, New AS, Trestman R, Siever LJ, 2004. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. J. Psychiatr. Res 38 (5), 503–511. [DOI] [PubMed] [Google Scholar]
- Kovanen L, et al. , 2017. PRKCDBP (CAVIN3) and CRY2 associate with major depressive disorder. J. Affect Disord 207, 136–140. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131 (2), 391–404. [DOI] [PubMed] [Google Scholar]
- Kubitz N, et al. , 2013. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS One 8, e76882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM, 1999. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98 (2), 193–205. [DOI] [PubMed] [Google Scholar]
- Lam RW, et al. , 2016. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry 73, 56–63. [DOI] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, Welsh DK, 2016. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. Psychiatry 80 (11), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander P, Vrang N, Moller M, 1998. Neuronal projections from the mesencephalic raphe nuclear complex to the suprachiasmatic nucleus and the deep pineal gland of the golden hamster (Mesocricetus auratus). J. Comp. Neurol 399 (1), 73–93. [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM, 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107 (7), 855–867. [DOI] [PubMed] [Google Scholar]
- Lee A, Myung SK, Cho JJ, Jung YJ, Yoon JL, Kim MY, 2017. Night shift work and risk of depression: meta-analysis of observational studies. J. Korean Med. Sci 32 (7), 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S, 2012. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491 (7425), 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legutko R, Gannon RL, 2001. Serotonin transporter localization in the hamster suprachiasmatic nucleus. Brain Res. 893 (1–2), 77–83. [DOI] [PubMed] [Google Scholar]
- Leliavski A, Shostak A, Husse J, Oster H, 2014. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology 155 (1), 133–142. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Nir T, Laudon M, Zisapel N, 2007. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J. Sleep Res 16 (4), 372–380. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Waider J, 2012. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 76 (1), 175–191. [DOI] [PubMed] [Google Scholar]
- Levine JD, Weiss ML, Rosenwasser AM, Miselis RR, 1991. Retinohypothalamic tract in the female albino rat: a study using horseradish peroxidase conjugated to cholera toxin. J. Comp. Neurol 306 (2), 344–360. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ Jr., Akil H, Bunney WE, 2013. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc. Natl. Acad. Sci. USA 110 (24), 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ren C, Huang L, Lin B, Pu M, Pickard GE, So KF, 2015. The dorsal raphe nucleus receives afferents from alpha-like retinal ganglion cells and intrinsically photosensitive retinal ganglion cells in the rat. Invest. Ophthalmol. Vis. Sci 56 (13), 8373–8381. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang L, 2015. Circadian control of global transcription. Biomed. Res. Int 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L’Hermite-Baleriaux M, Leclercq R, Brasseur M, Mendlewicz J, Van Cauter E, 1994. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch. Gen. Psychiatry 51 (8), 616–624. [DOI] [PubMed] [Google Scholar]
- Logan RW, McClung CA, 2016. Animal models of bipolar mania: The past, present, and future. Neuroscience 321, 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA, 2015. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol. Psychiatry 78 (4), 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo H, Hale A, D’Haenen H, 2002. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT(2C) antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int. Clin. Psychopharmacol 17 (5), 239–247. [DOI] [PubMed] [Google Scholar]
- Loomer HP, Saunders JC, Kline NS, 1957. A clinical and pharmacodynamic evaluation of iproniazid as a psychic energizer. Psychiatr. Res. Rep. Am. Psychiatr. Assoc 8, 129–141. [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, et al. , 1993. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11 (3), 449–458. [DOI] [PubMed] [Google Scholar]
- Lowry CA, et al. , 2008. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann. N Y Acad. Sci 1148, 86–94. [DOI] [PubMed] [Google Scholar]
- Luan L, Ren C, Lau BW, Yang J, Pickard GE, So KF, Pu M, 2011. Y-like retinal ganglion cells innervate the dorsal raphe nucleus in the Mongolian gerbil (Meriones unguiculatus). PLoS ONE 6 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, Morales CAC, Biello SM, Mackay D, Ward J, Strawbridge RJ, Gill JMR, Bailey MES, Pell JP, Smith DJ, 2018. Association of disrupted circadian rhyhtmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 5 (6), 507–514. [DOI] [PubMed] [Google Scholar]
- Lydic R, Schoene WC, Czeisler CA, Moore-Ede MC, 1980. Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep 2 (3), 355–361. [DOI] [PubMed] [Google Scholar]
- Lydic R, Albers HE, Tepper B, Moore-Ede MC, 1982. Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J. Comp. Neurol 204 (3), 225–237. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, et al. , 2009. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur. J. Neurosci 30, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, 2006. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci 8 (4), 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek ZS, Dardente H, Pevet P, Raison S, 2005. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur. J. Neurosci 22 (4), 895–901. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Sage D, Pevet P, Raison S, 2007. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 148 (11), 5165–5172. [DOI] [PubMed] [Google Scholar]
- Martynhak BJ, Hogben AL, Zanos P, Georgiou P, Andreatini R, Kitchen I, Archer SN, von Schantz M, Bailey A, van der Veen DR, 2017. Transient anhedonia phenotype and altered circadian timing of behaviour during night-time dim light exposure in Per3(−/−) mice, but not wildtype mice. Sci. Rep 7, 40399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, 2007. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther 114, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 2006. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism 55 (10 Suppl 2), S20–S23. [DOI] [PubMed] [Google Scholar]
- McGlashan EM, Drummond SPA, Cain SW, 2018. Evening types demonstrate reduced SSRI treatment efficacy. Chronobiol. Int 35 (8), 1175–1178. [DOI] [PubMed] [Google Scholar]
- McMenamin TM, Bureau of Labor Statistics, 2007. Time to work: recent trends in shift work and flexible schedules. Monthly Labor Review 130, 3–15 Available online at https://www.bls.gov/opub/mlr/2007/12/art1full.pdf. [Google Scholar]
- Meyer-Bernstein EL, Morin LP, 1996. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J. Neurosci 16 (6), 2097–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A, 1991. A human phase-response curve to light. Neurosci. Lett 133 (1), 36–40. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Glass JD, Miller JD, 2000. Behavioral and serotonergic regulation of circadian rhythms. Biol. Rhythm Res 31 (3), 240–283. [Google Scholar]
- Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, Okamura H, 2000. Phase-dependent responses of Per1 and Per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci. Lett 294 (1), 41–44. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY, 1997. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J. Comp. Neurol 389 (3), 508–534. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Cashen K, Lee TM, 2005. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am. J. Physiol. Regul. Integr. Comp. Physiol 288, R221–R228. [DOI] [PubMed] [Google Scholar]
- Moore RY, 1973. Retinohypothalamic projection in mammals: a comparative study. Brain Res. 49 (2), 403–409. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB, 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42 (1), 201–206. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, 1993. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett 150 (1), 112–116. [DOI] [PubMed] [Google Scholar]
- Morin LP, 2013. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol 243, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Blanchard J, 1991. Serotonergic modulation of the hamster wheelrunning rhythm: response to lighting conditions and food deprivation. Brain Res. 566 (1–2), 186–192. [DOI] [PubMed] [Google Scholar]
- Morris DW, Rush AJ, Jain S, Fava M, Wisniewski SR, Balasubramani GK, Khan AY, Trivedi MH, 2007. Diurnal mood variation in outpatients with major depressive disorder: implications for DSM-V from an analysis of the Sequenced Treatment Alternatives to Relieve Depression Study data. J. Clin. Psychiatry 68 (9), 1339–1347. [PubMed] [Google Scholar]
- Mosko SS, Jacobs BL, 1974. Midbrain raphe neurons: spontaneous activity and response to light. Physiol. Behav 13, 589–593. [DOI] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S, 2018. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359 (6381). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, Peterson MJ, 2015. Sleep disturbances in depression. Sleep Med. Clin 10 (1), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Castanon-Cervantes O, Davidson A, Fukuhara C, 2008. Selective serotonin reuptake inhibitors and raft inhibitors shorten the period of Period1-driven circadian bioluminescence rhythms in rat-1 fibroblasts. Life Sci. 82 (23–24), 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG, 2005. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci 8 (3), 267–269. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Reddy AB, 2012. The essential role of cAMP/Ca2+ signalling in mammalian circadian timekeeping. Biochem. Soc. Trans 40 (1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk D-J, Lightman S, Vgontzas A, Van Cauter E, 2017. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocrine Rev. 38 (1), 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF, 2016. Major depressive disorder. Nat. Rev. Dis. Primers 2, 16065. [DOI] [PubMed] [Google Scholar]
- Packard AE, Egan AE, Ulrich-Lai YM, 2016. HPA axis interactions with behavioral systems. Compr. Physiol 6 (4), 1897–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB, 2002a. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109 (3), 307–320. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA, 2002b. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 298 (5601), 2213–2216. [DOI] [PubMed] [Google Scholar]
- Papp M, et al. , 2003. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology 28, 694–703. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS, 2014. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24 (2), 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AP, Hastings MH, 2018. The suprachiasmatic nucleus. Curr. Biol 28 (15), R816–R822. [DOI] [PubMed] [Google Scholar]
- Peeters F, Berkhof J, Delespaul P, Rottenberg J, Nicolson NA, 2006. Diurnal mood variation in major depressive disorder. Emotion 6 (3), 383–391. [DOI] [PubMed] [Google Scholar]
- Peichl L, 2005. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell Evol. Biol 287 (1), 1001–1012. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Qiuping G, Center for Disease Control, 2017. Antidepressant Use Among Persons Aged 12 and Over: United States, 2011–2014. NCHS data brief, no 283. Hyattsville, MD: National Center for Health Statistics. Available online at https://www.cdc.gov/nchs/data/databriefs/db283.pdf. [Google Scholar]
- Prosser RA, 2003. Serotonin phase-shifts the mouse suprachiasmatic circadian clock in vitro. Brain Res. 966 (1), 110–115. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SM, Arendt J, 2001. Health in a 24-h society. Lancet 358, 999–1005. [DOI] [PubMed] [Google Scholar]
- Rea MA, Pickard GE, 2000. A 5-HT(1B) receptor agonist inhibits light-induced suppression of pineal melatonin production. Brain Res. 858, 424–428. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH, 2006. Circadian orchestration of the hepatic proteome. Curr. Biol 16 (11), 1107–1115. [DOI] [PubMed] [Google Scholar]
- Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, Gonzalez FJ, Lilley KS, Kyriacou CP, Hastings MH, 2007. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 45 (6), 1478–1488. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Menaker M, 1993. Effects of imipramine on circadian rhythms in the golden hamster. Pharmacol. Biochem. Behav 45 (1), 27–33. [DOI] [PubMed] [Google Scholar]
- Ren C, Luan L, Wui-Man Lau B, Huang X, Yang J, Zhou Y, Wu X, Gao J, Pickard GE, So KF, Pu M, 2013. Direct retino-raphe projection alters serotonergic tone and affective behavior. Neuropsychopharmacology 38 (7), 1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, 2002. Coordination of circadian timing in mammals. Nature 418 (6901), 935–941. [DOI] [PubMed] [Google Scholar]
- Retana-Marquez S, et al. , 2003. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 28, 207–227. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Pendergast JS, Yamazaki S, 2008. Mammalian peripheral circadian oscillators are temperature compensated. J. Biol. Rhythms 23 (1), 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M, 2007. Epidemiology of the human circadian clock. Sleep. Med. Rev 11 (6), 429–438. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Van Eekelen JA, Levine S, De Kloet ER, 1988. Ontogeny of the type 2 glucocorticoid receptor in discrete rat brain regions: an immunocytochemical study. Brain Res. 470 (1), 119–127. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, McClung CA, 2007. Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. USA 104 (15), 6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybkin II, et al. , 1997. Effect of restraint stress on food intake and body weight is determined by time of day. Am. J. Physiol 273, R1612–R1622. [DOI] [PubMed] [Google Scholar]
- Sack RL, 2010. Clinical practice. Jet lag. N. Engl. J. Med 362 (5), 440–447. [DOI] [PubMed] [Google Scholar]
- Samson WK, Said SI, McCann SM, 1979. Radioimmunologic localization of vasoactive intestinal polypeptide in hypothalamic and extrahypothalamic sites in the rat brain. Neurosci. Lett 12 (2–3), 265–269. [DOI] [PubMed] [Google Scholar]
- Santarelli L, et al. , 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809. [DOI] [PubMed] [Google Scholar]
- Schnell A, Sandrelli F, Ranc V, Ripperger JA, Brai E, Alberi L, Rainer G, Albrecht U, 2015. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol. Int 32 (8), 1075–1089. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Tavakoli-Nezhad M, Lambert CM, Weaver DR, de la Iglesia HO, 2011. Distinct patterns of period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc. Natl. Acad. Sci. USA 108 (41), 17219–17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Monk TH, Brink LL, 1997. Shiftwork as a risk factor for depression: a pilot study. Int. J. Occup. Environ. Health 3, S2–S9. [PubMed] [Google Scholar]
- Sharma VK, Chandrashekaran MK, Singaravel M, Subbaraj R, 1999. Relationship between light intensity and phase resetting in a mammalian circadian system. J. Exp. Zool 283 (2), 181–185. [DOI] [PubMed] [Google Scholar]
- Shen GH, et al. , 2008. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar Disord. 10, 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SQ, et al. , 2016. Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl. Psychiatry 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VB, Corley KC, Phan TH, Boadle-Biber MC, 1990. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res. 516 (1), 66–76. [DOI] [PubMed] [Google Scholar]
- Smale L, Michels KM, Moore RY, Morin LP, 1990. Destruction of the hamster serotonergic system by 5,7-DHT: effects on circadian rhythm phase, entrainment and response to triazolam. Brain Res. 515 (1–2), 9–19. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Zweig M, Axelrod J, Fischer JE, 1965. Control of the circadian rhythm in serotonin content in the rat pineal gland. Proc. Natl. Acad. Sci. USA 53, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Axelrod J, Zweig M, 1967. Circadian rhythm in the serotonin content of the rat pineal gland: regulating factors. J. Pharmacol. Exp. Ther 158 (2), 206–213. [PubMed] [Google Scholar]
- So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ, 2009. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc. Natl. Acad. Sci. USA 106 (41), 17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria V, et al. , 2010. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology 35, 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, Chun LE, Hartsock MJ, Woodruff ER, 2018. Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front. Neuroendocrinol 49, 52–71. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Deak T, 2017. A users guide to HPA axis research. Physiol. Behav 178, 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, McClung CA, 2013. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur. J. Neurosci 37 (2), 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Akil H, 2018. Glucocorticoids and resilience. Horm. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M, 2016. Natural selection against a circadian clock gene mutation in mice. Proc. Natl. Acad. Sci. USA 113 (3), 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A, 2004. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 46 (1), 52–62. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, Reynolds L, 2006. Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biol. Psychiatry 60 (8), 896–899. [DOI] [PubMed] [Google Scholar]
- Spulber S, Conti M, DuPont C, Raciti M, Bose R, Onishchenko N, Ceccatelli S, 2015. Alterations in circadian entrainment precede the onset of depression-like behavior that does not respond to fluoxetine. Transl. Psychiatry 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW, 2012. Human phase response curve to a 1 h pulse of bright white light. J. Physiol 590 (13), 3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 69(6), 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J, 2002. Circadian 5-HT production regulated by adrenergic signaling. Proc. Natl. Acad. Sci. USA 99 (7), 4686–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Betz H, Schloss P, 1996. Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience 73 (1), 217–231. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Shibata S, 2018. Entrainment of the mouse circadian clock: Effects of stress, exercise and nutrition. Free Radic. Biol. Med 119, 129–138. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Shiraishi T, Kikuchi Y, Haraguchi A, Kuriki D, Sasaki H, Motohashi H, Sakai T, Shibata S, 2015. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci. Rep 5, 11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S, 2001. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology 142 (11), 4910–4917. [DOI] [PubMed] [Google Scholar]
- Tapia-Osorio A, Salgado-Delgado R, Angeles-Castellanos M, Escobar C, 2013. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav. Brain Res 252, 1–9. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS, 2005. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 10 (8), 647–663 quiz 672. [DOI] [PubMed] [Google Scholar]
- Thompson RS, Christianson JP, Maslanik TM, Maier SF, Greenwood BN, Fleshner M, 2013. Effects of stressor controllability on diurnal physiological rhythms. Physiol. Behav 112–113, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischkau SA, Weber ET, Abbott SM, Mitchell JW, Gillette MU, 2003. Circadian clock-controlled regulation of cGMP-protein kinase G in the nocturnal domain. J. Neurosci 23 (20), 7543–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima K, Sakaguchi H, Sato Y, To H, Koyanagi S, Higuchi S, Ohdo S, 2005. Chronopharmacological study of antidepressants in forced swimming test of mice. J. Pharmacol. Exp. Ther 315 (2), 764–770. [DOI] [PubMed] [Google Scholar]
- Ushijima K, Koyanagi S, Sato Y, Ogata T, Matsunaga N, Fujimura A, Ohdo S, 2012. Role of activating transcription factor-4 in 24-hour rhythm of serotonin transporter expression in the mouse midbrain. Mol. Pharmacol 82 (2), 264–270. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, 1980. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J. Comp. Neurol 191 (4), 661–702. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A, 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398 (6728), 627–630. [DOI] [PubMed] [Google Scholar]
- van der Vinne V, Swoap SJ, Vajtay TJ, Weaver DR, 2018. Desynchrony between brain and peripheral clocks caused by CK1delta/epsilon disruption in GABA neurons does not lead to adverse metabolic outcomes. Proc. Natl. Acad. Sci. USA 115 (10), E2437–E2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K, DeMey J, 1975. Identification of the vasopressin-neurophysin producing neurons of the rat suprachiasmatic nuclei. Cell Tissue Res. 156 (3), 377–380. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, Maquet P, 2007. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb. Cortex 17 (12), 2788–2795. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Maquet P, Dijk DJ, 2009. Light as a modulator of cognitive brain function. Trends Cogn. Sci 13 (10), 429–438. [DOI] [PubMed] [Google Scholar]
- Vaze KM, Sharma VK, 2013. On the adaptive significance of circadian clocks for their owners. Chronobiol. Int 30 (4), 413–433. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, 1997. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J. Comp. Neurol 378 (3), 411–424. [DOI] [PubMed] [Google Scholar]
- Vincent MY, Donner NC, Smith DG, Lowry CA, Jacobson L, 2018. Dorsal raphe nucleus glucocorticoid receptors inhibit tph2 gene expression in male C57BL/6J mice. Neurosci. Lett 665, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M, 2003. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299 (5603), 76. [DOI] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ, 2011. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat 41 (4), 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel L, et al. , 2000. A melatonin agonist facilitates circadian resynchronization in old hamsters after abrupt shifts in the light-dark cycle. Brain Res. 880, 207–211. [DOI] [PubMed] [Google Scholar]
- Whitney MS, Shemery AM, Yaw AM, Donovan LJ, Glass JD, Deneris ES, 2016. Adult brain serotonin deficiency causes hyperactivity, circadian disruption, and elimination of siestas. J. Neurosci 36 (38), 9828–9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, 2005. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, et al. , 2005. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol. Med 35, 939–944. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, 2008. Diurnal variation of depressive symptoms. Dialogues Clin. Neurosci 10, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, 2018. Seasonality in affective disorders. Gen. Comp. Endocrinol 258, 244–249. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker RH, 1999. Sleep deprivation in depression: what do we know, where do we go? Biol. Psychiatry 46, 445–453. [DOI] [PubMed] [Google Scholar]
- Woodruff ER, Chun LE, Hinds LR, Spencer RL, 2016. Diurnal corticosterone presence and phase modulate clock gene expression in the male rat prefrontal cortex. Endocrinology 157 (4), 1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2017. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. Available online at http://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf;jsessionid=FF5A7B1D7D63B6455DEA1C5E6E7332B9?sequence=1. [Google Scholar]
- Wright KP Jr., Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA, 2015. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun 47, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, van Der Horst GT, Okamura H, 2001. Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292 (5515), 278–281. [DOI] [PubMed] [Google Scholar]
- Yamakawa GR, Antle MC, 2010. Phenotype and function of raphe projections to the suprachiasmatic nucleus. Eur. J. Neurosci 31 (11), 1974–1983. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T, 2004. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol. Biol 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H, 2000. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288 (5466), 682–685. [DOI] [PubMed] [Google Scholar]
- Yohn CN, Gergues MM, Samuels BA, 2017. The role of 5-HT receptors in depression. Mol. Brain 10 (1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, 1995. Psychiatric morbidity in travelers to Honolulu, Hawaii. Compr. Psychiatry 36 (3), 224–228. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hirano A, Hsu PK, Jones CR, Sakai N, Okuro M, McMahon T, Yamazaki M, Xu Y, Saigoh N, Saigoh K, Lin ST, Kaasik K, Nishino S, Ptacek LJ, Fu YH, 2016a. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc. Natl. Acad. Sci. USA 113 (11), E1536–E1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Huang L, Zhang L, Tan M, Pu M, Pickard GE, So KF, Ren C, 2016b. ON and OFF retinal ganglion cells differentially regulate serotonergic and GABAergic activity in the dorsal raphe nucleus. Sci. Rep 6, 26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB, 2014. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. USA 111 (45), 16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC, 2001. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105 (5), 683–694. [DOI] [PubMed] [Google Scholar]


