Abstract
Persistent sciatic artery (PSA) is a rare congenital peripheral artery disorder that is usually detected incidentally on computed tomographic examination. PSA can also cause iliac aneurysm and acute thromboembolism, which are potentially associated with rest pain, claudication, and limb-threatening ischemia. Patients with PSA and leg ischemia should be treated with revascularization and appropriate management of PSA aneurysm. The authors often choose emergent bypass surgery or endovascular intervention for aneurysmal rupture and acute lower-extremity arterial occlusion. This report describes an emergency procedure using catheter-based thrombolysis for acute limb ischemia in a patient with PSA.
Keywords: Thrombolysis, Persistent sciatic artery, Acute limb-threating ischemia
INTRODUCTION
Persistent sciatic artery (PSA) is mostly asymptomatic; however, it can be associated with the development of aneurysms and peripheral artery occlusion [1-3]. The sciatic artery is a branch of the umbilical artery, and supplies blood to the developing lower limbs during the early embryonic phase. In the later embryonic phase, the sciatic artery is completely interrupted and the superficial femoral artery (SFA) alternatively perfuses the lower limbs. If blood flow through the sciatic artery persists, the vessel is required to sustain sufficient perfusion of the lower limbs, possibly due to hypoplasia of the femoral artery system [1-3]. Arterial bypass or endovascular intervention often requires immediate treatment for limb-threatening ischemic PSA, thus emphasizing the need for prompt revascularization [4-8]. PSA causes iliac artery aneurysms in 48% of patients and is potentially associated with rest pain, buttock pain, neuropathy, claudication, and acute limb ischemia (ALI) [4,5]. ALI is mostly caused by thromboembolism in the aneurysm. This case report describes an emergency procedure using catheter-based thrombolysis for ALI in a patient with PSA. Six years after catheter intervention, the patient’s clinical course remained uneventful, and oral antithrombotic drugs were continued. The study was approved by the Institutional Review Board of the Tokoname Municipal Hospital (IRB no. 2022-04) and the written informed consent was obtained from the patient.
CASE
A 74-year-old female was admitted to the hospital for acute-onset left leg coldness and pain that had developed over one day. The patient complained of rest pain and partial loss of motor function. She was a current smoker with a 20-year history of type 2 diabetes mellitus on insulin therapy. Anthropometric characteristics included a height of 156.2 cm and a body weight of 50.3 kg. Popliteal and pedal pulses were absent, and the left foot was mottled and cool. The ankle-brachial index was 1.02 on the right, pulseless on the left, and pulses in the left popliteal, pedal, and posterior tibial arteries were absent. The mean preoperative Rutherford classification score was 4. Enhanced computed tomography angiography revealed a left internal iliac artery (IIA) aneurysm and enhanced collateral flow toward the occluded popliteal artery (PA) below the knee joint distal to a hypoplastic, incomplete SFA (Fig. 1A). She was diagnosed with PSA (type 2b according to the Pillet-Gauffre classification [SFA absent and complete PSA reaching the popliteal artery]) associated with ALI, which required emergency revascularization to salvage the limb [4]. Immediate endovascular procedure was decided after discussing with the patient and her family.
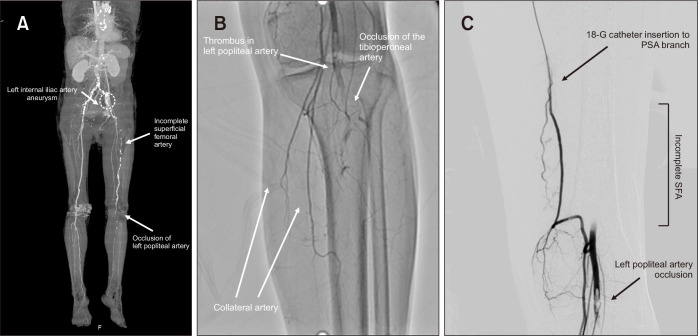
Fig. 1.
(A) Computed tomography angiography showed left superficial femoral artery (SFA) hypoplasia and the thrombus from the left popliteal artery (PA) to the tibioperoneal artery. (B) Conventional angiography revealed a filling defect of left PA. (C) After catheter injection of urokinase, secondary emergent catheter-based thrombolysis was targeted for the left PA thrombus. PSA, persistent sciatic artery.
A retrograde 4-Fr sheath was inserted using the right femoral approach over the aortic bifurcation into the contralateral iliac artery. Digital subtraction angiography (DSA) revealed aneurysmal dilatation of the proximal left IIA and filling defects in the left PA. Total occlusion of the residual thrombus was observed from the left PA to the tibioperoneal trunk (Fig. 1B). After angiography, secondary emergency catheter-directed thrombolysis (CDT) was performed using a multi-sideport 18-gauge infusion catheter (Smiths Medical Inc., Minneapolis, MN, USA) to distribute the lytic agent after administration of a urokinase bolus for 30 minutes (800 IU/mL, total 8,000 U urokinase) (Fig. 1C). A low-dose regimen, including urokinase 120,000 IU, prostaglandin E1 20 μg, and heparin 10,000 units/day, was administered through the tip of the catheter. The patient’s leg pain resolved the next day, and numbness gradually disappeared over the ensuing days. Thrombolytic catheter infusion was continued for 5 days until the next angiography. On the fifth day, DSA detected no residual popliteal thrombi (Fig. 2A). The post-thrombolytic ABI improved to 0.71 on the left side. Postoperative recovery was favorable, and no additional secondary interventions were required. The patient was discharged 11 days after therapeutic anticoagulation with heparin. Six years after the treatment, the patient’s clinical course remained uneventful with continuous administration of oral antiplatelet (clopidogrel 75 mg/day) and low-dose anticoagulant (warfarin 2 mg/day). Six years after the catheter intervention, enhanced computed tomography (CT) revealed no thrombus from the left PA to the tibioperoneal artery (Fig. 2B).
Fig. 2.
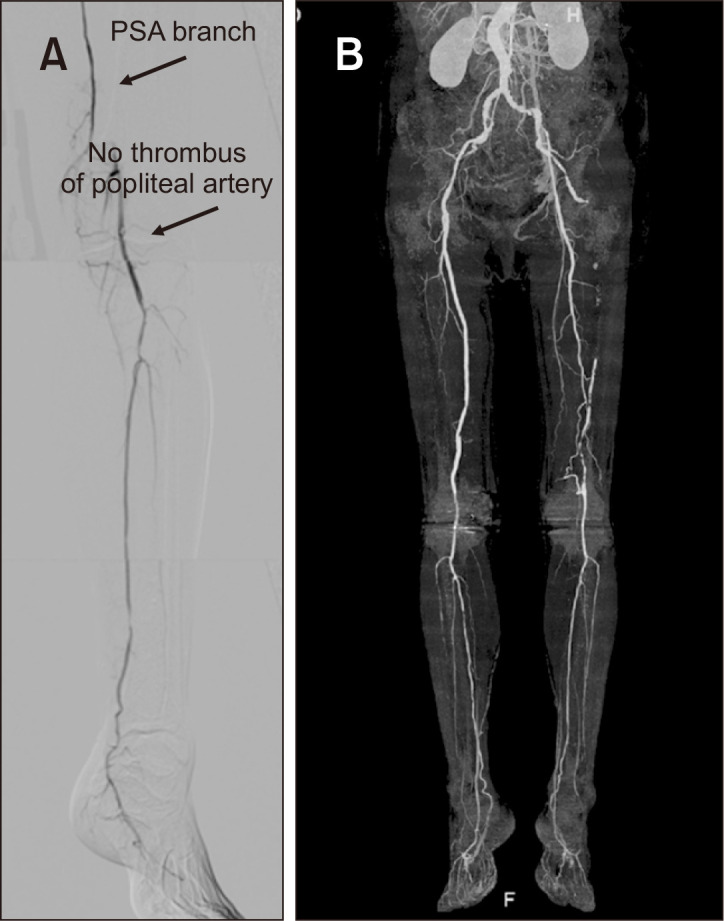
(A) Post-thrombolytic digital substruction angiography revealed no thrombus of left popliteal artery on the postoperative day 5. (B) Six years after the catheter intervention, computed tomography angiography showed patency from left popliteal to the posterior tibial artery. PSA, persistent sciatic artery.
DISCUSSION
. The anatomical location of the PSA differs from that of the SFA, which is occasionally associated with the development of iliac artery aneurysm and peripheral occlusion. The PSA is required to sustain sufficient perfusion of the lower limbs, possibly due to hypoplasia of the SFA [1-3]. In 1994, Gauffre et al. [4] described five different types of PSA; the type 2a subtype (presence of native SFA, but PSA does not reach the popliteal artery) was the most common variant according to the Pillet–Gauffre classification. ALI with PSA is mostly caused by thromboembolism owing to the presence of an IIA aneurysm. In 2016, Ahn et al. [5] advocated a modified classification into five types based on the presence of an iliac aneurysm, and bypass surgery was mostly required for classes III and IV (incomplete SFA is present, but complete or incomplete PSA persisted in the lower part). This case was type IIIa according to the Ahn–Min classification, which was classified according to whether SFA and PSA were complete and aneurysmal status [4,5].
Etiology, pathophysiology, and anatomical factors should be considered in the treatment of PSA. The initial evaluation of patients with limb ischemic PSA and decision making in the immediate management period should be discussed and, among those who undergo endovascular and open surgical revascularization, the techniques are often complementary [5-7]. Meghpara et al. [6] successfully performed CDT in a PSA patient with ALI, who was diagnosed with bilateral PSA and a right PSA aneurysm using endovascular procedures concomitant with stent graft placement, bare metal stent implantation, and CDT. Although endovascular treatment is minimally invasive, stent graft implantation may be required, and the long-term patency rate is slightly lower than open surgery [8-10]. Deng et al. [7] also reported endovascular treatment of PSA with ALI. CT angiography revealed the presence of an incomplete PSA with the absence of an SFA, and CDT and stenting placement were performed. The vessels were successfully opened, and the symptoms resolved. However, six months after the operation, the patient complained of claudication and the distal PSA was found to be occluded. Vakhitov et al. [10] reported that unfavorable long-term patency and management of bleeding are mandatory for secondary thrombolysis. A study from Finland reported that age >75 years and arterial fibrillation were negative independent factors affecting postoperative amputation-free survival, which was 24% at 10 years. Hence, indications for endovascular surgery should be considered with caution.
We performed CDT in a patient with an iliac aneurysm caused by PSA. Owing to maintenance therapy with oral antiplatelet and low-dose anticoagulant, the clinical course was uneventful, and no thrombus was detected in follow-up examinations in the ensuing years. Gandhi et al. [9] reported that low-dose CDT or thrombectomy were also similar in terms of the outcomes of ALI, including periprocedural complications, patency, reintervention, limb salvage, and amputation-free survival with the same thrombolysis time and occlusion length/lesion. As maintenance therapy after CDT to prevent recurrent distal embolic occlusion, an international, randomized, double-blind, placebo-controlled trial reported that major adverse cardiovascular and limb events were significantly lower in those treated with low-dose direct-acting oral anticoagulant (rivaroxaban) plus aspirin compared with aspirin alone [11].
For the immediate treatment of limb-threatening ischemia among patients with type IIIa PSA according to the new Ahn–Min classification (i.e., incomplete SFA, PSA with aneurysm to the popliteal artery), CDT reported herein yielded a favorable outcome. Additionally, the keys to successful thrombolysis include, not only patient age and duration and severity of ischemia, but also the length and size of the anatomical occlusion and appropriate follow-up.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: MN. Writing the article: MN. Critical revision of the article: all authors. Final approval of the article: all authors. Overall responsibility: MN.
REFERENCES
- 1.Green PH. On a new variety of the femoral artery: with observations. Lancet. 1832;17:730–731. doi: 10.1016/S0140-6736(02)83351-7. [DOI] [Google Scholar]
- 2.Brantley SK, Rigdon EE, Raju S. Persistent sciatic artery: embryology, pathology, and treatment. J Vasc Surg. 1993;18:242–248. doi: 10.1016/0741-5214(93)90604-K. [DOI] [PubMed] [Google Scholar]
- 3.Bower EB, Smullens SN, Parke WW. Clinical aspects of persistent sciatic artery: report of two cases and review of the literature. Surgery. 1977;81:588–595. [PubMed] [Google Scholar]
- 4.Gauffre S, Lasjaunias P, Zerah M. Sciatic artery: a case, review of literature and attempt of systemization. Surg Radiol Anat. 1994;16:105–109. doi: 10.1007/BF01627932. [DOI] [PubMed] [Google Scholar]
- 5.Ahn S, Min SK, Min SI, Ha J, Jung IM, Kim SJ, et al. Treatment strategy for persistent sciatic artery and novel classification reflecting anatomic status. Eur J Vasc Endovasc Surg. 2016;52:360–369. doi: 10.1016/j.ejvs.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Meghpara MK, Alaoudi M, Mutyala M. Persistent sciatic artery in a patient with unilateral acute lower extremity ischemia. J Vasc Surg Cases Innov Tech. 2020;7:89–92. doi: 10.1016/j.jvscit.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L, Deng Z, Chen K, Chen Z, Chen G, Xiong G. Endovascular repair of persistent sciatic artery with limb ischemia: a wrong choice? Front Surg. 2020;7:582753. doi: 10.3389/fsurg.2020.582753.495ee889066546f395fe80fa0cbdb1ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwood R, Berridge DC, Kessel DO, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2018;8:CD002784. doi: 10.1002/14651858.CD002784.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi SS, Ewing JA, Cooper E, Chaves JM, Gray BH. Comparison of low-dose catheter-directed thrombolysis with and without pharmacomechanical thrombectomy for acute lower extremity ischemia. Ann Vasc Surg. 2018;46:178–186. doi: 10.1016/j.avsg.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Vakhitov D, Oksala N, Saarinen E, Vakhitov K, Salenius JP, Suominen V. Survival of patients and treatment-related outcome after intra-arterial thrombolysis for acute lower limb ischemia. Ann Vasc Surg. 2019;55:251–259. doi: 10.1016/j.avsg.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]