Abstract
Recently, therapeutics based on messenger RNA (mRNA) have attracted significant interest for vaccines, cancer immunotherapy, and gene editing. However, the lack of biocompatible vehicles capable of delivering mRNA to the target tissue and efficiently expressing the encoded proteins impedes the development of mRNA-based therapies for a variety of diseases. Herein, we report mRNA-loaded polymeric nanoparticles based on diethylenetriamine- substituted poly(aspartic acid) that induce protein expression in the lungs and muscles following intravenous and intramuscular injections, respectively. Animal studies revealed that the amount of polyethylene glycol (PEG) on the nanoparticle surface affects the translation of the delivered mRNA into the encoded protein in the target tissue. After systemic administration, only mRNA-loaded nanoparticles modified with PEG at a molar ratio of 1: 1 (PEG: polymer) induce protein expression in the lungs. In contrast, protein expression was detected only following intramuscular injection of mRNA-loaded nanoparticles with a PEG: polymer ratio of 10: 1. These findings suggest that the PEG density on the surface of poly(aspartic acid)-based nanoparticles should be optimized for different delivery routes depending on the purpose of the mRNA treatment.
Keywords: Polymeric nanoparticle, mRNA delivery, gene therapy, systemic delivery, PEGylation
Graphical Abstract

INTRODUCTION
Messenger RNA (mRNA)-based gene therapies demonstrate a great promise for treating various diseases, especially after the COVID-19 mRNA vaccine led to numerous studies focused on the development of mRNA therapeutics.1–3 mRNA therapeutics are mainly explored in antigen production, protein replacement therapy, and genome engineering.4, 5 In contrast to DNA, intracellularly delivered mRNA can be translated into an encoded protein in the cytoplasm without integrating the host gene, reducing the risk of biosafety. However, the efficient delivery of intact mRNA to the cytoplasm of target cells after systemic administration is a challenging step. It requires delivery systems that improve the stability of mRNA in the blood stream, increase their accumulation in the targeted tissue following systemic administration, and facilitate intracellular internalization and endosomal escape of the delivered mRNA molecules.6, 7 Many studies have developed mRNA carriers by using various materials such as lipids, polymers, dendrimers, and natural biomolecules.8–11 Although lipid nanoparticles currently exhibit the highest mRNA transfection efficiency in vivo, there is considerable interest in the development of polymer-based mRNA carriers. Polymeric nanoparticles consisting of cationic molecules, such as polyethyleneimine (PEI), have been extensively investigated due to their tunable structure, diverse functionalities, and excellent transfection efficiencies.12, 13 Despite these advantages, their toxicity related to poor degradability have restricted their clinical application. Polyamino acid-based polycations have been studied for nucleic acid delivery due to their biodegradability and biocompatibility.14–17 Particularly, polymers composed of poly(aspartic acid) (P(Asp)) can reduce the cytotoxicity of gene carriers. This is due to the fact that the P(Asp) backbone can be cleaved as a result of the self-catalytic reaction between the backbone and the side chain amide nitrogen.18 The biodegradability of P(Asp) is essential to achieving safe and prolonged gene expression by reducing cumulative toxicity caused by cellular retention of polycations. However, studies focused on the systemic delivery of mRNA by P(Asp)-based nanoparticles are still limited.19, 20 Polyethylene glycol (PEG) shielding of the polymeric nanoparticle is also essential in reducing toxicity and inflammatory responses, preventing nanoparticle aggregation in physiological conditions, and improving gene delivery and transfection efficiency.21 However, few studies have explored the effects of PEG shielding on mRNA transfection efficiency of polymeric nanoparticles after different administration routes.
Finally, the majority of previously developed mRNA-loaded nanoparticles predominantly deliver mRNA to the liver following systemic administration.22, 23 Consequently, carriers capable of extrahepatic delivery of mRNA are essential for the application of mRNA therapy to treat a variety of diseases. Recent reports demonstrated that mRNA-loaded lipid nanoparticles can produce the protein of interest in non-liver tissues.24–26 However, only a few studies have shown mRNA delivery to the lung using polymeric nanoparticles following systemic administration.
Herein, we report a polymeric nanoparticle (PNP) for mRNA delivery using diethylenetriamine (DET)-substituted P(Asp) (P[Asp(DET)]). The synthesized PNP was modified with polyethylene glycol (PEG) at different ratios in order to investigate the effects of PEGylation on mRNA transfection efficiency and biocompatibility. In vivo studies in mice revealed that PNP with optimal PEGylation density can deliver mRNA to the lungs and express the encoded protein after intravenous administration. Furthermore, the developed nanoparticles also showed efficient localized protein expression after intramuscular injection.
MATERIALS AND METHODS
Materials.
Triphosgene, n-butyl amine, diethylenetriamine, and β-benzyl L-aspartate (BLA) were purchased from Alfa Aesar (Tewksbury, MA, USA). Enhanced green fluorescent protein (EGFP) mRNA and firefly luciferase (Fluc) mRNA were purchased from TriLink BioTechnologies (San Diego, CA, USA). mPEG-succinimidyl carboxymethyl ester (mPEG(2k)-SCM) was obtained from Biopharma PEG Scientific Inc (Watertown, MA, USA). Calcein AM fluorescent dye was purchased from BD Biosciences (San Jose, CA, USA). NucBlue™ Live ReadyProbes Reagent, D-luciferin sodium salt monohydrate, Quant-it™ RiboGreen RNA Assay Kit, and HEPES buffer solution were purchased from Thermo Fisher Scientific (Waltham, MA, USA). BDP TR NHS ester and Cyanine7.5 NHS ester were obtained from Lumiprobe (Hunt Valley, MD, USA). All other reagents were of analytical grade and were obtained from Sigma-Aldrich Inc (Milwaukee, WI, USA) or Fisher Scientific Inc. (Hampton, NH, USA).
Synthesis and characterization of N-substituted polyaspartamide p[Asp(DET)].
The polymer synthesis process consists of three stages. Stage one resulted in the synthesis of β-benzyl L-aspartate N-carboxyanhydride (βLA-NCA). Stage two involves polymerizing βLA-NCA using n-butyl amine as an initiator. The final stage is an aminolysis reaction, in which the benzyl alcohol groups on the polymer side are substituted by diethylenetriamine (DET). The Fuchs-Farthing method is then employed to synthesize the βLA-NCA.27 Briefly, 5 g of βLA (24.42 mmol) is kept under vacuum pressure for 2 hours and is reacted with 0.4 equivalence of triphosgene (2.9 g for βLA). Next, sixty milliliters of the anhydrous THF are used to suspend the reactant mixture and are refluxed for four hours at 40 °C under an argon atmosphere. The conversion of the suspension into a clear pale yellow indicates the formation of βLA-NCA solution. Next, the βLA-NCA solution is cooled to room temperature, followed by the stepwise addition of 75–85 mL of anhydrous hexane. Then the βLA-NCA in the cosolvent solution is stored at −20 °C for 12 hours to allow for NCA crystallization. Finally, the NCA is collected and further purified by recrystallization from THF and hexane. Poly (β-benzyl-L-aspartate) (PBLA) is synthesized using the previously described method.18, 28 Ring-opening polymerization is used to polymerize βLA-NCA (6 g, 30 mmol), and this was dissolved in 10 mL anhydrous DMSO and mixed with 90 mL anhydrous dichloromethane. The polymerization reaction is initiated by n-butylamine (0.3 mL, 0.30 mmol). The reaction is carried out under an argon atmosphere at 45 °C for 48 hours. PBLA is collected by precipitation from diethyl ether, and the filtrate is dried under a vacuum. The last stage in the synthesis scheme is the aminolysis reaction in which the benzyl alcohol groups on the polymer side chain are substituted with DET. The PBLA polymer is dissolved in anhydrous N-methyl-2-pyrrolidone (100 mg/mL). The distilled DET (10 equivalence of the benzyl alcohol polymerized unit) was then added to the reaction mixture, and the reaction was performed at room temperature for 48 hours. The polymer solution is dialyzed against 0.1 M HCl, 0.01 M HCl, and finally against ddH2O for 24 hours in each step using regenerated cellulose dialysis tubing (MWCO 5000). Finally, the polymer P[Asp(DET)] is collected by freeze-drying.
The 1H NMR spectra of the synthesized polymer are recorded using a Bruker 400 MHz Advanced III spectrometer (Bruker, Billerica, MA) using deuterated dimethyl sulfoxide (DMSO). The molecular weight and chemical composition are determined from the measured spectra. The number of the repeating units in PBLA is calculated to be 87 units and the molecular weight of the polymer (Mn) is calculated to be 17,926 Da. The aminolysis conversation of PBLA into P[(Asp(DET)] is confirmed by NMR, and the MW of the polymer is calculated to be 17,494 Da.
Preparation and Characterization of mRNA-loaded Polymeric Nanoparticles.
The synthesized polymer (5mg/mL) was dissolved in 10 mM HEPES buffer. The ratio of primary amines in polymer to phosphate groups in mRNA (N/P) was 32 when the solutions of polymer and mRNA were combined. The reaction mixture was stirred at 1500 rpm for 1 hour at room temperature. To modify the surface of the mRNA-loaded polymeric nanoparticles with polyethylene glycol (PEG), mPEG-succinimidyl carboxymethyl ester (mPEG(2k)-SCM) was added to the mixture and then stirred at 1500 rpm for 1 hour at room temperature. The molar ratios of mPEG(2k)-SCM to polymer were 1: 1 and 10: 1. The synthesized PEG-PNP was purified by a 10 kDa centrifugal filter. The hydrodynamic size and zeta potential of nanoparticles were measured by ZetaSizer NanoSeries (Malvern, Worcestershire, UK) as previously described.29, 30 The mRNA encapsulation efficiency was measured using a modified Quant-iT RiboGreen RNA assay.31 For tracking the polymeric nanoparticle, Red fluorescent dye (BDP TR) and Cy7.5 dye were conjugated to the P[Asp(DET)] polymer before the synthesis of polymeric nanoparticles with mRNA. The free dye is removed by a 10 kDa centrifugal filter after PNP synthesis.
Cell Viability Assay.
To assess the cytotoxicity of the synthesized mRNA-loaded polymeric nanoparticles, Huh7 cells were seeded at a density of 1×104 cells per well in 96-well plates. The cells were exposed to PNP, PEG(1x)-PNP, and PEG(10x)-PNP containing 3 μg/mL EGFR mRNA for 48 hours (3 wells per group). Cell viability was assessed by measuring the fluorescence intensity of living cells using the Calcein AM assay according to the manufacturer’s protocol.32 Control cells were cultured in fresh media. Data are displayed as a relative percentage (%) of the values from control cells that were not treated with polymeric nanoparticles.
Cellular Internalization and EGFP mRNA Transfection Assay.
To evaluate cellular internalization of nanoparticles and EGFP mRNA transfection efficiency, Huh7 cells were plated at a density of 2.5×104 cells per well in 24-well plates and cells were incubated with red fluorescent dye (BDP TR) conjugated PNP, PEG(1x) – PNP, and PEG(10x) – PNP (3 wells per group). The mRNA concentration was 3 μg/mL in each well. After 48 hours, cells were washed with DPBS and stained with NucBlue. The fluorescence images were obtained using a Keyence BZ-X Fluorescence Microscope (Keyence, Osaka, Japan). The fluorescence signals in the microscopic images were quantified using ImageJ software (National Institutes of Health (NIH), MD, USA).
In vivo evaluation of the Luciferase mRNA-loaded Polymeric Nanoparticles.
PEG(1x)-PNP and PEG(10x)-PNP loaded with Firefly luciferase (Fluc) mRNA at a dose of 0.44 mg/kg were intravenously administered to 11 week-old female Swiss Webster mice (four mice per group). After 6 hours, mice were injected with D-luciferin solution (150 mg/kg in 200μL PBS) intraperitoneally. Luminescence was captured with the IVIS Lumina XRMS imaging system (Perkin Elmer, Hopkinton, MA) after 15 min. Luminescence images were analyzed using Living Image software (Perkin Elmer, Hopkinton, MA). To evaluate luciferase expression after intramuscular injection, mice were intramuscularly injected with 50 μL of the Fluc mRNA-loaded PEG(1x)-PNP and PEG(10x)-PNP at a mRNA dose of 0.22 mg/kg (four mice per group). Luminescence images were captured using the IVIS Lumina XRMS imaging system 6 hours after the injection as described above.
Statistical Analysis.
GraphPad Prism v9 (GraphPad Software, CA, USA) was used for statistical analyses. For statistical significance, the t-test was used to compare the means of two separate groups, and one-way analysis of variance (ANOVA) was employed when there were more than two independent groups. The standard error of measurement is indicated as standard deviation (SD). Differences between groups were considered statistically significant at P < 0.05. The following symbols represent statistical significance: *P < 0.05, ** P < 0.01, and *** P < 0.001.
RESULTS AND DISCUSSION
Preparation and Characterization of mRNA-loaded Polymeric Nanoparticles
N-substituted polyaspartamide P[Asp(DET)] was synthesized as represented in Figure 1. Briefly, β-benzyl L-aspartate N-carboxyanhydride (βLA-NCA) is synthesized by the Fuchs-Farthing method using β-benzyl L-aspartate and triphosgene.27 Ring-opening polymerization is used to obtain poly(β-benzyl-L-aspartate) (PBLA).18, 28 The last step is the aminolysis reaction of PBLA with diethylenetriamine (DET), which results in the synthesis of P[Asp(DET)]91. The polymerization degree of the P[Asp(DET)], 91 was determined by nuclear magnetic resonance (NMR) (Figures S1 and S2). We paid significant attention to endolysosomal escape when choosing P[Asp(DET)] as a delivery vehicle for mRNA. Previous research demonstrated that DET side chains in the polyamino acid-based polycations significantly improved gene delivery efficiency.16, 33–36 This efficiency has been attributed to the unique DET structure, where the mono-protonated form of DET at neutral pH is converted to the di-protonated form in the acidic pH environment of the endosome and lysosome.33, 37 This conversion increases the internal osmotic pressure of endosomes and lysosomes and disrupts their membrane. The pH-selective membrane destabilization enables the efficient escape of polymeric nanoparticles into the cytoplasm with minimal cytotoxicity. Polyplexes based on (P[Asp(DET)]) display efficient endosomal escape and high transfection efficiency with minimal toxicity when compared to PEI.18, 35
Figure 1.
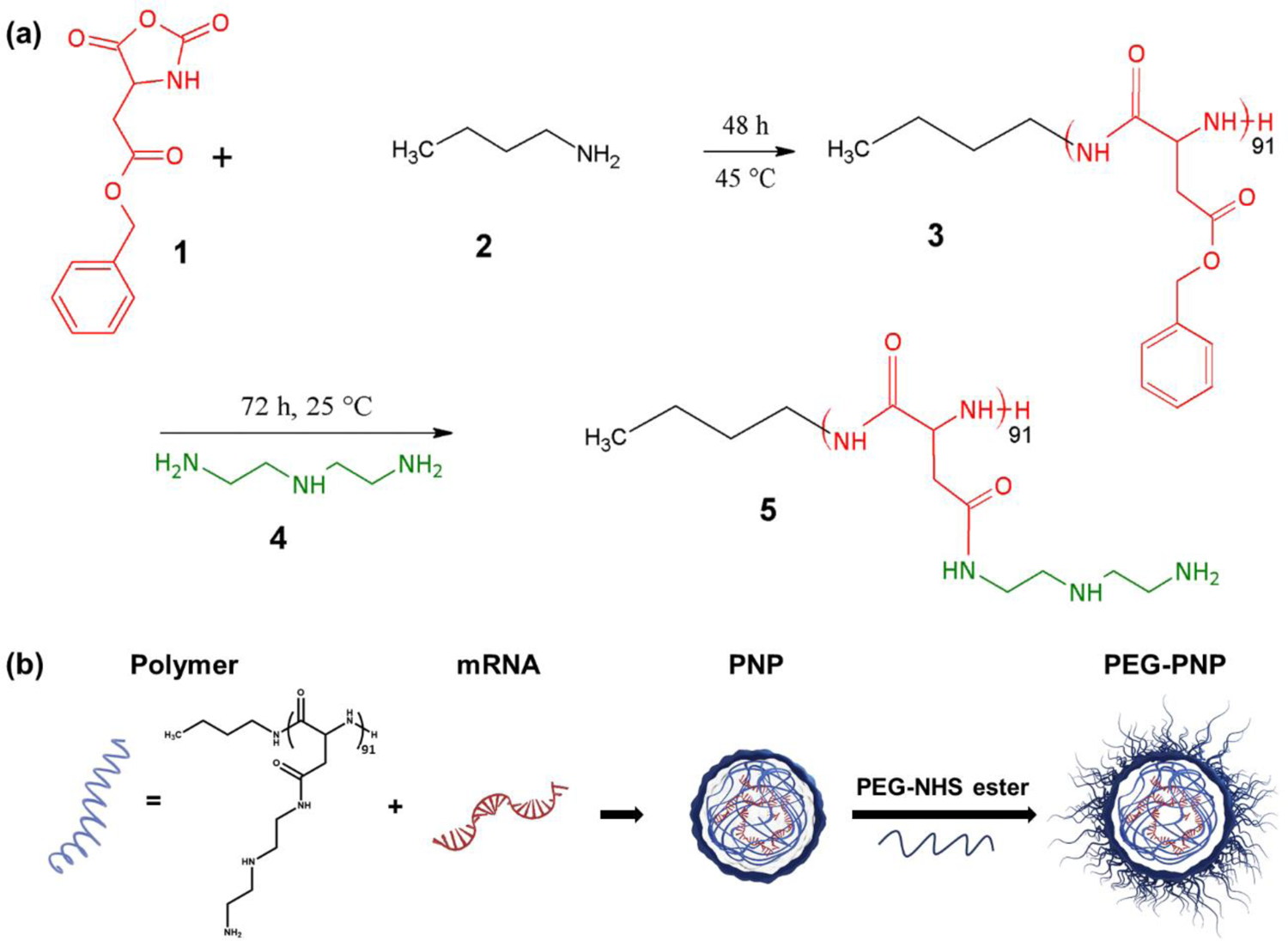
(a) Scheme for the synthesis of poly(β-benzyl-L-aspartate) (PBLA, 3) using ring-opening polymerization with β-benzyl L-aspartate N-carboxyanhydride (βLA-NCA, 1) initiated by n-butylamine(2). N-substituted polyaspartamide P[Asp(DET)] (5) is synthesized by the aminolysis reaction with diethylenetriamine (DET) (4). (b) Schematic illustration of the synthesis of the mRNA-loaded polymeric nanoparticles (PNP) and modification of the PNP by polyethylene glycol conjugation on the nanoparticle surface via amide bonds.
Polymeric nanoparticles (PNP) were prepared by mixing the synthesized P[Asp(DET)]91 polymer with EGFP mRNA in 10 mM HEPES buffer (Figure 1). PNP was formulated via electrostatic interactions between the positively charged polymer and the negatively charged mRNA. The ratios of amine group in DET of the polymer to phosphate group in the mRNA (N/P) were examined from 4 to 32 (Figure S3). For N/P ratio 32, the hydrodynamic diameter of PNP was 179 ± 1 nm (polydispersity index (PDI) = 0.20) (Figure 2). Z-potential measurements indicated that PNP has a positive surface charge, 13.2 ± 1.49 mV. The mRNA encapsulation efficiency was 99.7%.31 To increase colloidal stability and minimize non-specific interactions in the biological environment, we introduced 2 kDa poly(ethylene glycol)(PEG) to the surface of PNP via NHS ester and amine group reaction, creating an amide bond (Figure 1). PEG was added to the PNP at 1: 1 and 10: 1 molar ratios to the polymer of PNP, which correspond to 11.5% and 23% of the amine groups in the polymer, respectively. PEG-PNP showed reduced zeta potentials; 7.35 ± 1.37 mV for 1:1 ratio (PEG(1x)-PNP), and 3.22 ± 0.74 mV for 10:1 ratio (PEG(10x)-PNP). The zeta potential values decreased as the PEG to polymer ratio increased because positively charged amine groups were employed for PEG conjugation. Dynamic light scattering (DLS) analysis revealed that the average hydrodynamic diameters of PEG(1x)-PNP (215 ± 3 nm, PDI = 0.28) and PEG(10x)-PNP (220 ± 5 nm, PDI = 0.29) were slightly larger than PNP (Figure 2).
Figure 2.
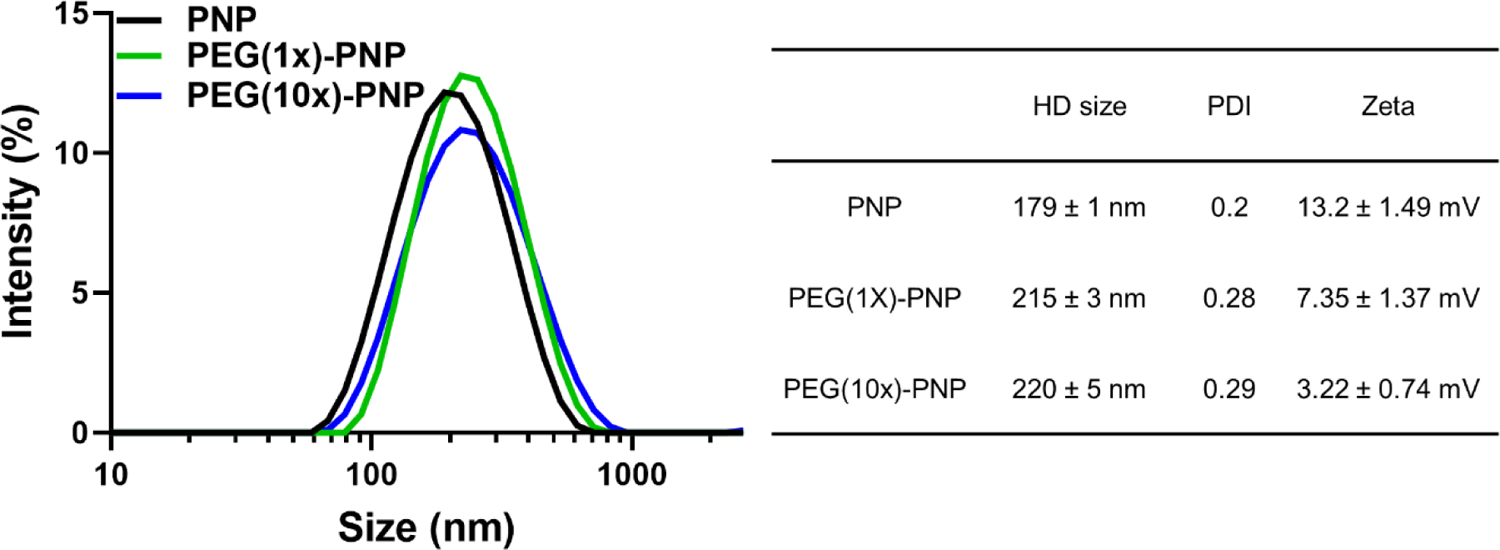
Representative dynamic light scattering profiles of PNP, PEG(1x)-PNP, and PEG(10x)-PNP (N/P 32) and the summary of parameters for nanoparticles.
In vitro mRNA Transfection Efficiency and Cytotoxicity of mRNA-loaded Polymeric Nanoparticles
The Huh7 hepatocyte-derived cellular carcinoma cell line was used to evaluate the cytotoxicity and transfection efficiencies of EGFP mRNA-loaded PNP, PEG(1x)-PNP, and PEG(10x)-PNP prepared at an N/P of 32. The cultured cells were incubated with red fluorescent dye (BDP TR) -labeled polymeric nanoparticles (3 μg/mL EGFP mRNA) for 48 hours. The red fluorescence intensities showed that PNP, PEG(1x)-PNP, and PEG(10x)-PNP are internalized by these cells and distributed throughout the cytoplasm (Figure 3a). The average red fluorescence intensities in cells treated with the three nanoparticles are comparable but slightly decreases as the PEG ratio increases. The difference in cellular uptake could be explained by the presence of a PEG layer on the nanoparticle’s surface, which reduces the interaction between the positively charged nanoparticle and the negatively charged cellular membrane. The green fluorescence intensity was also measured in cells to evaluate the ability of EGFP mRNA delivered by the tested nanoparticles to be translate into the encoded protein (Figure 3b). PEG(1x)-PNP showed a higher EGFP expression compared to PNP, which is due to possible aggregation of PNP in the biological environment.21, 38, 39 PEG(10x)-PNP with higher PEG content demonstrated a lower EGFP expression than PEG(1x)-PNP. It may be reasonable to presume that PEGylation not only aids in protecting mRNA against RNase-mediated degradation but also impedes the release of mRNA in the cells. Grun et al. evaluated mRNA transfection efficiency of mRNA-loaded polymeric nanoparticles with various surface PEG densities.38 The transfection efficiencies of nanoparticles with low PEG content were similar to, or somewhat higher than, that of non-PEGylated polymeric nanoparticles, but decreased at a certain percentage of PEG content, which confirmed a similar pattern to our findings. The negative effect of high PEG content on gene transfection may be due to reduced electrostatic interactions between nanoparticles and cells. Additionally, in our case, the amine group of DET has been utilized for PEG conjugation and, as a result, the endosomal escape of PEG(10x)-PNP could be reduced.33, 37
Figure 3.
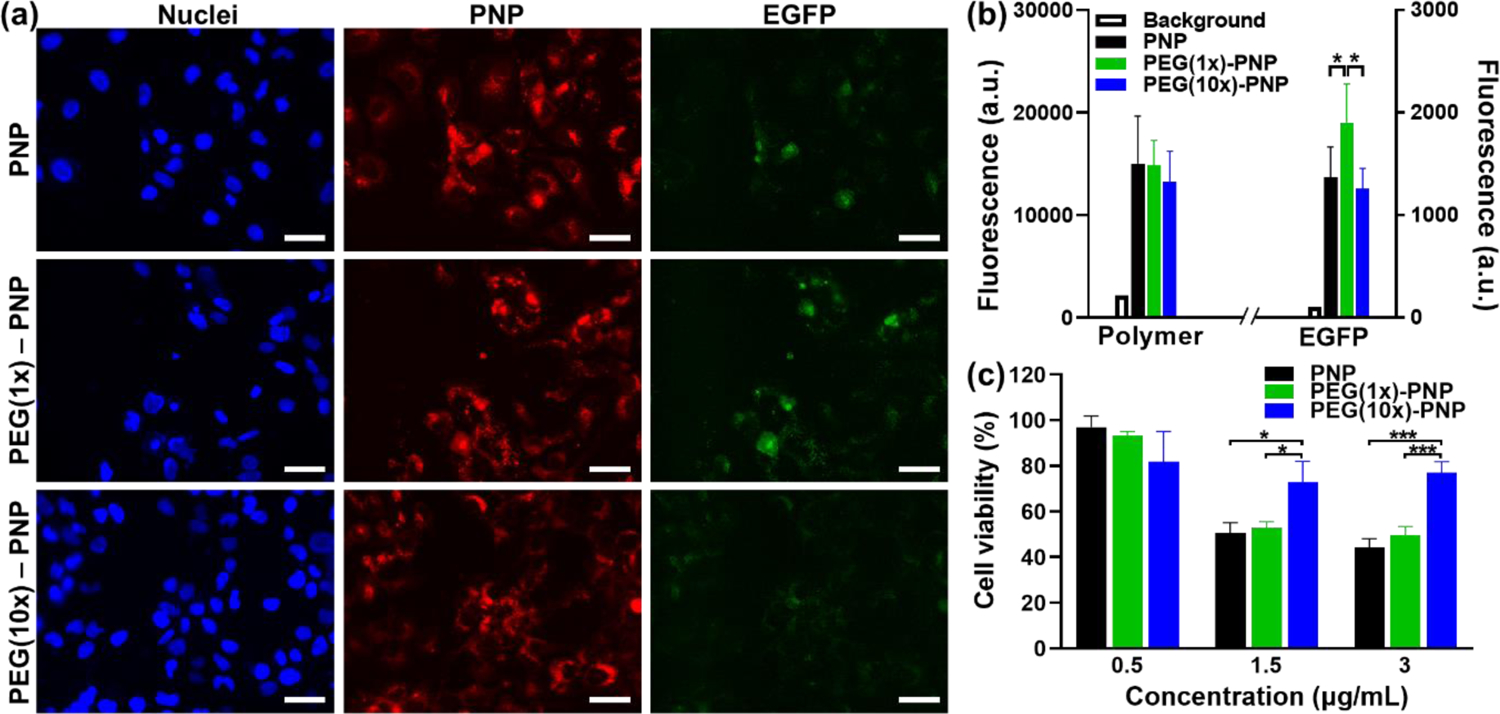
(a) Representative fluorescence microscopy images of cells after 48 hours of incubation with PNP, PEG(1x) – PNP, and PEG(10x) – PNP. The blue pseudo color represents the fluorescence signal created by nuclei staining, the red pseudo color represents polymer, and the green pseudo color is generated by EGFP. Scale bars = 50 μm. (b) Background signals and mean fluorescence intensities in Huh7 human hepatocytes incubated with PNP, PEG(1x)-PNP, and PEG(10x)-PNP for 48 hours (EGFR mRNA 3 μg/mL) (n = 3). The fluorescence signal in the microscopy images was quantified using Image J software. (c) Cell viability of Huh7 cells treated for 48 hours with PNP, PEG(1x)-PNP, and PEG(10x)-PNP at EGFR mRNA concentrations of 0.5, 1.5, and 3 μg/mL (n = 3). The results of the experiment were presented as mean values ± standard deviation. The p-values, *P < 0.05, and *** P < 0.001, were used to designate statistical significance.
Cytotoxicity of nanoparticles loaded with various mRNA concentrations (0.5 – 3 μg/mL) was also evaluated under the same experimental conditions used in the EGFP transfection assay (Figure 3c). The cell viability decreased in a dose-dependent manner. At a lower concentration (0.5 μg/mL mRNA), none of the polymeric nanoparticles showed any noticeable cytotoxicity. However, positively charged PNP (13.2 ± 1.49 mV) and PEG(1x)-PNP (7.35 ± 1.37 mV) decreased cell viability to 50% at doses of 1.5 μg/mL and 3 μg/mL mRNA. Cationic nanocarriers are well known for cytotoxicity because positively charged nanoparticles interact with cellular components and inhibit the normal cellular process.40 On the contrary, PEG(10x)-PNP with a slightly positive charge can markedly reduce cytotoxicity at higher concentrations (1.5 μg/mL and 3 μg/mL mRNA, Figure 3c). Taken together, these data indicate that greater PEG content improves the biocompatibility of PNP while potentially decreasing the efficiency of mRNA delivery.
Evaluation of Luciferase Protein Production Efficiency of mRNA-loaded Polymeric Nanoparticles In vivo after Systemic and Local Administrations.
We next evaluated Firefly luciferase (Fluc) mRNA delivery efficiency of PEG(1x)-PNP, and PEG(10x)-PNP in vivo. PNP was not included because of concerns about its toxicity in mice.31, 41 Fluc mRNA was chosen because mRNA translation into the encoded protein, Fluc, can be visualized by the bioluminescence signals in live mice, which are generated following the intraperitoneal injection of D-luciferin substrate.42 To assess in vivo transfection efficiency of Fluc mRNA following systemic administration, PEG-PNP were injected intravenously via the tail vein at a dose of 0.44 mg/kg mRNA. Bioluminescence images demonstrated that luciferase is primarily expressed in the lungs 6 hours after intravenous injection of PEG(1x)-PNP (Figure 4a). While PEG(10x)-PNP, which has higher PEG density and less positively charged surface, did not generate observable luminescence signals, suggesting inefficient Fluc mRNA translation into the protein (Figure 4b). Therefore, these results suggest that both zeta potential and the degree of PEGylation of PEG-PNP must be controlled precisely for mRNA delivery and translation in the lungs.
Figure 4.
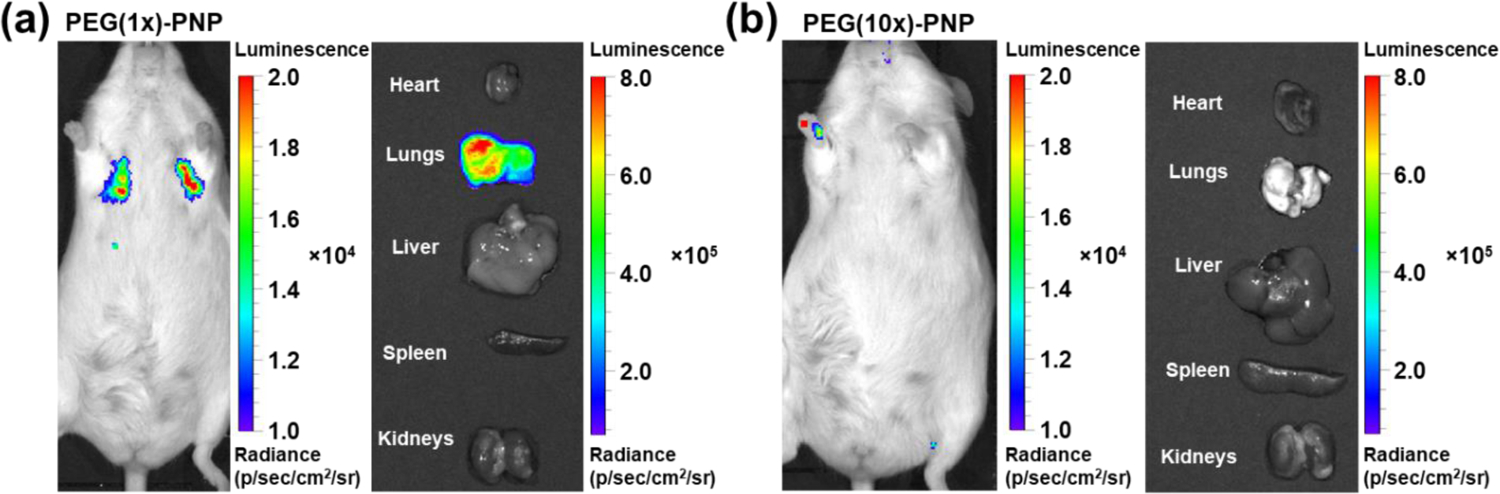
Representative bioluminescence images of mice and excised organs 6 hours after intravenous injection of (a) PEG(1x)-PNP and (b) PEG(10x)-PNP at a dose of 0.44 mg Fluc mRNA kg−1. Luciferase expressions of PEG(1x)-PNP are shown in the lungs. In mouse treated with PEG(1x)-PNP, the mean luminescence signals of the lungs in live mouse and excised lungs are 1.24 × 104 and 5.16 × 105, respectively.
Next, mRNA-loaded PEG(1x)-PNP was also formulated with Cy7.5 tagged polymer and injected intravenously to investigate the biodistribution of the systematically delivered nanoparticles. Although firefly luciferase is primarily produced in the lungs after the injection of PEG(1x)-PNP, Cy7.5 fluorescence is observed in other major organs, and the liver showed the highest accumulation 6 hours after injection (Figure 5 and Figures S4). It is known that systemically administrated nanoparticles are mostly captured in the liver after systemic administration because nanoparticles are subjected to Apolipoprotein E (ApoE) mediated uptake.43 According to several studies, mRNA-loaded nanoparticles accumulated in various organs including the liver, but the mRNA was exclusively translated in the lungs as shown by our results. Kaczmarek et al. showed, for the first time, that poly(β-amino ester) (PBAE) -based polymer–lipid nanoparticles were detected broadly in all organs but capable of functional delivery of mRNA to the lungs after intravenous administration in mice.31 They also identified that the transfected cell types in lungs were lung endothelial cells and immune cells.44 Moreover, polyester-based carriers, ionizable amino-polyester-lipid nanoparticles, PEI-based nanoparticles, and lipid-based nanoparticles have been investigated for mRNA transfection in the lungs.25, 45–48 Notably, those nanoparticles do not require active targeting ligands to deliver mRNA to the lungs. It is poorly understood what caused the differences in uptake and preferential mRNA translational into the encoded protein in the lung. Siegwart and colleagues demonstrated that their lipid nanoparticle could be utilized for selective organ targeting e.g., lung and spleen, by manipulating the lipid components.25 They also showed that the nanoparticle’s physicochemical properties such as global pKa and the resulting binding of distinct proteins in serum led to interactions with the specific receptors of particular tissues.49 A recent report also confirmed that positively charged lipid nanoparticles with cationic lipids could shift lipid nanoparticle specificity to the lungs.26 Previous reports and our results suggest that positive surface charge can contribute to lung-specific mRNA delivery.
Figure 5.
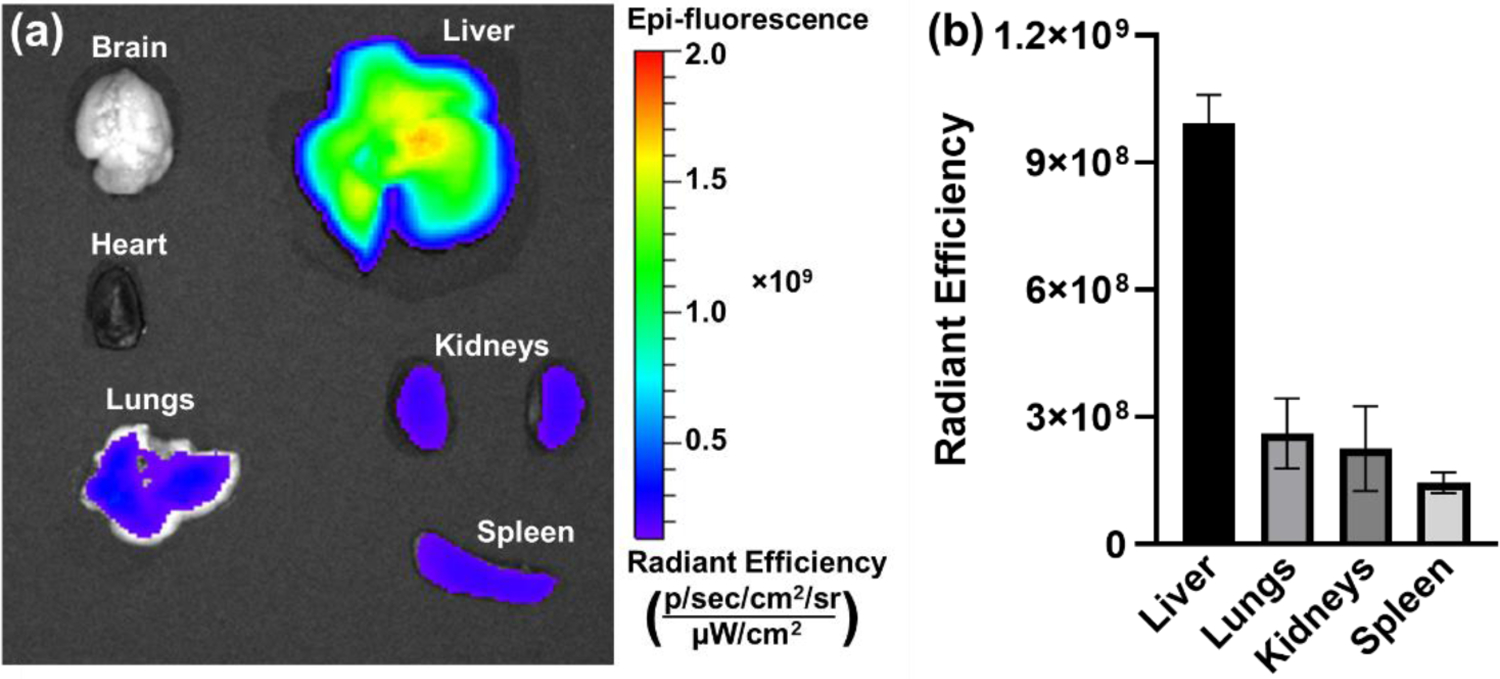
(a) Representative Cy7.5 fluorescence image of mouse organs after injection of Cy7.5 tagged PEG-PNP at 6 hours post-injection. (b) The mean fluorescence intensities of Cy7.5 of excised organs from mice given Cy7.5-tagged PEG-PNP intravenously.
We also demonstrated that PEG-PNP can produce luciferase protein via intramuscular administration to explore their potential use as a vaccine carrier (Figure 6). After 6 hours post-injection, luminescence signals of PEG(10x)-PNP were detected in the hind limb (Figure 6b). The luminescence signals are one order of magnitude higher than after intravenous injection. Despite efficient in vitro and in vivo systemic delivery, PEG(1x)-PNP did not mediate mRNA expression after intramuscular injection (Figure 6a). In vivo-jetPEI, a commercially available cationic polymer for nucleic acid delivery, demonstrated similar results to PEG(1x)-PNP (Figure S5). Bioluminescence was observed in the lungs following intravenous injection of luciferase mRNA complexed with in vivo-jetPEI. However, intramuscular injection of in vivo-jetPEI produced no bioluminescence, whereas PEG(10x)-PNP successfully mediated luciferase expression following intramuscular injection. This outcome shows how the degree of PEGylation may significantly impact the expression of proteins locally.38 Qi et al. evaluated in vivo mRNA transfection efficiency of mRNA-loaded PAMAM polyplexes with various PEG contents from 0% to 15% via intramuscular injection.41 The transfection efficiencies of nanoparticles with low PEG content were increased as PEG content increased. 8% PEG showed the highest transfection efficiency, and 15% PEG showed reduced transfection efficiency than 8% PEG. This result confirmed that certain content of PEG help mRNA expression in intramuscular injection. However, it is currently unclear how the PEG contents and the surface charge of nanoparticles affect the transfection efficacy in muscle tissue. We hypothesized that depending on the delivery route, the nanoparticle comes into contact with various biological environments, such as different proteins in biological fluids and different cells including extracellular matrices. The surface charge of the nanoparticle can influence the interaction of protein/cell with the nanoparticle.49 Carrasco et al. demonstrated that intramuscularly injected lipid nanoparticle exhibit off-target systemic expression of mRNA in the liver depending on the surface charge of nanoparticles because certain nanoparticles can pass into the vasculature rather than remain in muscle tissue after injection.50 In addition, nanoparticles with different physicochemical properties induced different humoral and cellular responses.51 These factors lead to distinct nanoparticle fates and varying protein expression levels in the targeted tissue. As a result, the surface characteristics of the mRNA carrier must be appropriately adjusted for therapeutic purposes.
Figure 6.
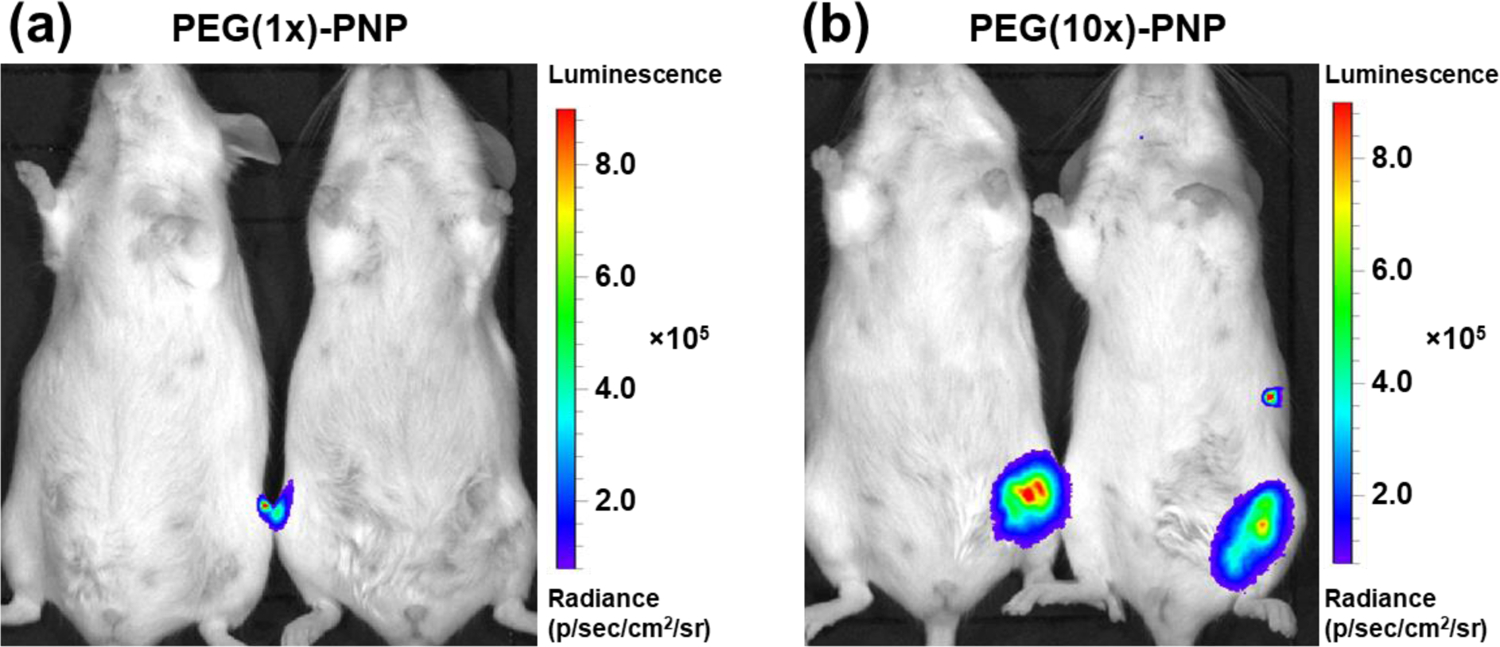
Representative luminescence image of mice following intramuscular injection of (a) PEG(1x)-PNP and (b) PEG(10x)-PNP at a dose of 0.22 mg mRNA kg−1. For mice treated with PEG(10x)-PNP, the mean luminescence signal of the hind limb of treated mice is 4.47 × 105.
CONCLUSIONS
In conclusion, we have developed poly(aspartic acid)-based polymeric nanoparticles which deliver mRNA and mediate protein expression both in vitro and in vivo. Polyethylene glycol is added to the surface of polymeric nanoparticles to increase their utility and allow for biocompatible in vivo expression of mRNA. The increased cell viability resulting from treatment with PEGylated polymeric nanoparticles in comparison to non-PEGylated nanoparticles is the most apparent indication of the influence on cells from the neutralization of positive charge mediated by PEGylation. In addition, a low degree of PEGylation significantly increased transfection efficiency compared to the non-PEGylated nanoparticles. We also demonstrated that the PEGylated polymeric nanoparticle can deliver mRNA to the lungs and express encoded protein after systemic administration in mice. It is worth noting that PEG-PNP do not require any targeting modification, relying only on the polymeric nanoparticles’ physicochemical properties to mediate protein expression in the lung following intravenous injection. This PEGylated polymeric nanoparticle demonstrates significant potential to be exploited in lung-targeted therapies for a number of disorders. Furthermore, PEGylated polymeric nanoparticles with a high degree of PEG exhibited efficient localized protein expression following intramuscular injection. Our results confirmed that the PEG density must be tailored for different delivery routes in accordance with the purpose of the mRNA treatment.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R37CA234006 and R01CA237569. Support was also received from the College of Pharmacy at Oregon State University. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
ABBREVIATIONS
- PNP
polymeric nanoparticle
- p(Asp)
poly(aspartic acid)
- PEG
polyethylene glycol
- PDI
polydispersity index
- SD
standard deviation
- EGFP
Enhanced green fluorescent protein
- Fluc
firefly luciferase (Fluc)
- DLS
dynamic light scattering
Footnotes
Supporting Information.
NMR results of synthesized poly (β-benzyl-L-aspartate)(PBLA) and P[Asp(DET)](PDF)
REFERENCES
- 1.Barbier AJ; Jiang AY; Zhang P; Wooster R; Anderson DG, The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol 2022, 40, 840–854. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary N; Weissman D; Whitehead KA, mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov 2021, 20, 817–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardi N; Hogan MJ; Porter FW; Weissman D, mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov 2018, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibba ML; Ciccone G; Esposito CL; Catuogno S; Giangrande PH, Advances in mRNA non-viral delivery approaches. Adv. Drug Del. Rev 2021, 177, 113930. [DOI] [PubMed] [Google Scholar]
- 5.Qin S; Tang X; Chen Y; Chen K; Fan N; Xiao W; Zheng Q; Li G; Teng Y; Wu M; Song X, mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther 2022, 7, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y; Hu Y; Tian H; Chen X, Opportunities and Challenges for mRNA Delivery Nanoplatforms. J. Phys. Chem. Lett 2022, 13, 1314–1322. [DOI] [PubMed] [Google Scholar]
- 7.Yen A; Cheng Y; Sylvestre M; Gustafson HH; Puri S; Pun SH, Serum Nuclease Susceptibility of mRNA Cargo in Condensed Polyplexes. Mol. Pharm 2018, 15, 2268–2276. [DOI] [PubMed] [Google Scholar]
- 8.Uchida S; Perche F; Pichon C; Cabral H, Nanomedicine-Based Approaches for mRNA Delivery. Molecular Pharmaceutics 2020, 17, 3654–3684. [DOI] [PubMed] [Google Scholar]
- 9.Tenchov R; Bird R; Curtze AE; Zhou Q, Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [DOI] [PubMed] [Google Scholar]
- 10.Hajj KA; Whitehead KA, Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater 2017, 2, 17056. [Google Scholar]
- 11.Meng C; Chen Z; Li G; Welte T; Shen H, Nanoplatforms for mRNA Therapeutics. Adv. Ther 2021, 4, 2000099. [Google Scholar]
- 12.Kumar R; Santa Chalarca CF; Bockman MR; Bruggen CV; Grimme CJ; Dalal RJ; Hanson MG; Hexum JK; Reineke TM, Polymeric Delivery of Therapeutic Nucleic Acids. Chem. Rev 2021, 121, 11527–11652. [DOI] [PubMed] [Google Scholar]
- 13.Piotrowski-Daspit AS; Kauffman AC; Bracaglia LG; Saltzman WM, Polymeric vehicles for nucleic acid delivery. Adv. Drug Del. Rev 2020, 156, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie J-J; Dou X-B; Hu H; Yu B; Chen D-F; Wang R-X; Xu F-J, Poly(aspartic acid)-based Degradable Assemblies for Highly Efficient Gene Delivery. ACS Appl. Mater. Interfaces 2015, 7, 553–562. [DOI] [PubMed] [Google Scholar]
- 15.Uchida S; Kinoh H; Ishii T; Matsui A; Tockary TA; Takeda KM; Uchida H; Osada K; Itaka K; Kataoka K, Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials 2016, 82, 221–228. [DOI] [PubMed] [Google Scholar]
- 16.Schumann C; Nguyen DX; Norgard M; Bortnyak Y; Korzun T; Chan S; Lorenz AS; Moses AS; Albarqi HA; Wong L; Michaelis K; Zhu X; Alani AWG; Taratula OR; Krasnow S; Marks DL; Taratula O, Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics 2018, 8, 5276–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masago K; Itaka K; Nishiyama N; Chung U.-i.; Kataoka K, Gene delivery with biocompatible cationic polymer: Pharmacogenomic analysis on cell bioactivity. Biomaterials 2007, 28, 5169–5175. [DOI] [PubMed] [Google Scholar]
- 18.Itaka K; Ishii T; Hasegawa Y; Kataoka K, Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials 2010, 31, 3707–3714. [DOI] [PubMed] [Google Scholar]
- 19.Matsui A; Uchida S; Ishii T; Itaka K; Kataoka K, Messenger RNA-based therapeutics for the treatment of apoptosis-associated diseases. Sci. Rep 2015, 5, 15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q; Osada K; Ge Z; Uchida S; Tockary TA; Dirisala A; Matsui A; Toh K; Takeda KM; Liu X; Nomoto T; Ishii T; Oba M; Matsumoto Y; Kataoka K, Polyplex micelle installing intracellular self-processing functionalities without free catiomers for safe and efficient systemic gene therapy through tumor vasculature targeting. Biomaterials 2017, 113, 253–265. [DOI] [PubMed] [Google Scholar]
- 21.Uchida S; Itaka K; Chen Q; Osada K; Ishii T; Shibata M-A; Harada-Shiba M; Kataoka K, PEGylated Polyplex With Optimized PEG Shielding Enhances Gene Introduction in Lungs by Minimizing Inflammatory Responses. Mol. Ther 2012, 20, 1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni JA; Cullis PR; Meel R. v. d., Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [DOI] [PubMed] [Google Scholar]
- 23.Blanco E; Shen H; Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 2015, 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loughrey D; Dahlman JE, Non-liver mRNA Delivery. Acc. Chem. Res 2022, 55, 13–23. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q; Wei T; Farbiak L; Johnson LT; Dilliard SA; Siegwart DJ, Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol 2020, 15, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LoPresti ST; Arral ML; Chaudhary N; Whitehead KA, The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J. Control. Release 2022, 345, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daly WH; Poché D, The preparation of N-carboxyanhydrides of α-amino acids using bis(trichloromethyl)carbonate. Tetrahedron Lett. 1988, 29, 5859–5862. [Google Scholar]
- 28.Rao DA; Mishra G; Doddapaneni BS; Kyryachenko S; Wierzbicki IH; Ngyuen DX; Shah V; Al Fatease AM; Alany RG; Alani AWG, Combinatorial Polymeric Conjugated Micelles with Dual Cytotoxic and Antiangiogenic Effects for the Treatment of Ovarian Cancer. Chem. Mater 2016, 28, 6068–6079. [Google Scholar]
- 29.Dani RK; Schumann C; Taratula O; Taratula O, Temperature-tunable iron oxide nanoparticles for remote-controlled drug release. AAPS PharmSciTech 2014, 15, 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taratula O; Schumann C; Naleway MA; Pang AJ; Chon KJ; Taratula O, A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol Pharm 2013, 10, 3946–58. [DOI] [PubMed] [Google Scholar]
- 31.Kaczmarek JC; Patel AK; Kauffman KJ; Fenton OS; Webber MJ; Heartlein MW; DeRosa F; Anderson DG, Polymer–Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew. Chem. Int. Ed 2016, 55, 13808–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taratula O; Dani RK; Schumann C; Xu H; Wang A; Song H; Dhagat P; Taratula O, Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int J Pharm 2013, 458, 169–80. [DOI] [PubMed] [Google Scholar]
- 33.Kanayama N; Fukushima S; Nishiyama N; Itaka K; Jang W-D; Miyata K; Yamasaki Y; Chung U.-i.; Kataoka K, A PEG-Based Biocompatible Block Catiomer with High Buffering Capacity for the Construction of Polyplex Micelles Showing Efficient Gene Transfer toward Primary Cells. ChemMedChem 2006, 1, 439–444. [DOI] [PubMed] [Google Scholar]
- 34.Akagi D; Oba M; Koyama H; Nishiyama N; Fukushima S; Miyata T; Nagawa H; Kataoka K, Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther. 2007, 14, 1029–1038. [DOI] [PubMed] [Google Scholar]
- 35.Uchida S; Itaka K; Uchida H; Hayakawa K; Ogata T; Ishii T; Fukushima S; Osada K; Kataoka K, In Vivo Messenger RNA Introduction into the Central Nervous System Using Polyplex Nanomicelle. PLOS ONE 2013, 8, e56220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C-Y; Perche F; Ikegami M; Uchida S; Kataoka K; Itaka K, Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J. Control. Release 2016, 235, 268–275. [DOI] [PubMed] [Google Scholar]
- 37.Miyata K; Oba M; Nakanishi M; Fukushima S; Yamasaki Y; Koyama H; Nishiyama N; Kataoka K, Polyplexes from Poly(aspartamide) Bearing 1,2-Diaminoethane Side Chains Induce pH-Selective, Endosomal Membrane Destabilization with Amplified Transfection and Negligible Cytotoxicity. J. Am. Chem. Soc 2008, 130, 16287–16294. [DOI] [PubMed] [Google Scholar]
- 38.Grun MK; Suberi A; Shin K; Lee T; Gomerdinger V; Moscato ZM; Piotrowski-Daspit AS; Saltzman WM, PEGylation of poly(amine-co-ester) polyplexes for tunable gene delivery. Biomaterials 2021, 272, 120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha D; Kumar S; Ray D; Mata J; Aswal VK, Structure and stability of biodegradable polymer nanoparticles in electrolyte solution. Materials Letters: X 2021, 10, 100066. [Google Scholar]
- 40.Lv H; Zhang S; Wang B; Cui S; Yan J, Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [DOI] [PubMed] [Google Scholar]
- 41.Qi R; Gao Y; Tang Y; He R-R; Liu T-L; He Y; Sun S; Li B-Y; Li Y-B; Liu G, PEG-conjugated PAMAM Dendrimers Mediate Efficient Intramuscular Gene Expression. The AAPS Journal 2009, 11, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billingsley MM; Singh N; Ravikumar P; Zhang R; June CH; Mitchell MJ, Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering. Nano Letters 2020, 20, 1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akinc A; Querbes W; De S; Qin J; Frank-Kamenetsky M; Jayaprakash KN; Jayaraman M; Rajeev KG; Cantley WL; Dorkin JR; Butler JS; Qin L; Racie T; Sprague A; Fava E; Zeigerer A; Hope MJ; Zerial M; Sah DWY; Fitzgerald K; Tracy MA; Manoharan M; Koteliansky V; Fougerolles A. d.; Maier MA, Targeted Delivery of RNAi Therapeutics With Endogenous and Exogenous Ligand-Based Mechanisms. Molecular Therapy 2010, 18, 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczmarek JC; Kauffman KJ; Fenton OS; Sadtler K; Patel AK; Heartlein MW; DeRosa F; Anderson DG, Optimization of a Degradable Polymer–Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018, 18, 6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y; Xiong H; Zhang X; Cheng Q; Siegwart DJ, Systemic mRNA Delivery to the Lungs by Functional Polyester-based Carriers. Biomacromolecules 2017, 18, 4307–4315. [DOI] [PubMed] [Google Scholar]
- 46.Schrom E; Huber M; Aneja M; Dohmen C; Emrich D; Geiger J; Hasenpusch G; Herrmann-Janson A; Kretzschmann V; Mykhailyk O; Pasewald T; Oak P; Hilgendorff A; Wohlleber D; Hoymann H-G; Schaudien D; Plank C; Rudolph C; Kubisch-Dohmen R, Translation of Angiotensin-Converting Enzyme 2 upon Liver- and Lung-Targeted Delivery of Optimized Chemically Modified mRNA. Mol. Ther. Nucleic Acids 2017, 7, 350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowalski PS; Capasso Palmiero U; Huang Y; Rudra A; Langer R; Anderson DG, Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery. Advanced Materials 2018, 30, 1801151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ke X; Shelton L; Hu Y; Zhu Y; Chow E; Tang H; Santos JL; Mao H-Q, Surface-Functionalized PEGylated Nanoparticles Deliver Messenger RNA to Pulmonary Immune Cells. ACS Applied Materials & Interfaces 2020, 12, 35835–35844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dilliard SA; Cheng Q; Siegwart DJ, On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrasco MJ; Alishetty S; Alameh M-G; Said H; Wright L; Paige M; Soliman O; Weissman D; Cleveland TE; Grishaev A; Buschmann MD, Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Communications Biology 2021, 4, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderluzzi G; Lou G; Woods S; Schmidt ST; Gallorini S; Brazzoli M; Johnson R; Roberts CW; O’Hagan DT; Baudner BC; Perrie Y, The role of nanoparticle format and route of administration on self-amplifying mRNA vaccine potency. J. Controlled Release 2022, 342, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


