Abstract
We previously reported that immunization with H antigen from Histoplasma capsulatum did not protect mice against an intravenous challenge with yeasts. Here, we investigated the utility of H antigen to protect mice in a model of pulmonary histoplasmosis. Mice immunized with H antigen and challenged intranasally 4 weeks postvaccination were protected against sublethal and lethal challenges with H. capsulatum yeasts. If the challenge was performed 3 months after vaccination, there was a reduction in fungal burden following sublethal challenge and a modest delay in mortality in mice given a lethal inoculum. Vaccination was associated with production of gamma interferon, granulocyte-macrophage colony-stimulating factor, interleukin-4, and interleukin-10 by splenocytes. Vaccination with H antigen was not accompanied by a major expansion of CD4+ or CD8+ cells in spleens of mice. These results demonstrate that H antigen may be useful as a protective immunogen against pulmonary exposure to H. capsulatum.
Infection with the pathogenic fungus Histoplasma capsulatum ranges from a mild asymptomatic illness to a progressive form which can be life-threatening. Early diagnosis and treatment are essential to a successful outcome for disseminated histoplasmosis. Prior to the advent of antigen detection for disseminated infection, complement fixation and immunodiffusion were the immunodiagnostic tests used to establish a diagnosis. Two antigens, M and H, were identified as specific for histoplasmosis in immunodiffusion (17). The H antigen has been used to discriminate between active and remote infection. The appearance of an H band nearly always signifies active infection (6). The deduced amino acid sequence of this glycoprotein reveals homology to β-glucosidases from other species (7). Subsequent functional studies have validated that H antigen expresses β-glucosidase activity (11, 12).
We previously demonstrated that recombinant H antigen (rH) expressed in Escherichia coli did not confer protection to BALB/c mice challenged intravenously with H. capsulatum yeasts (7). In studies conducted recently in which H antigen was being used as a control protein for vaccination studies, we made the unexpected discovery that immunization with rH provided protection in a murine model of pulmonary histoplasmosis.
MATERIALS AND METHODS
Animals.
Male C57BL/6 and BALB/c mice, 5 weeks old, were purchased from the National Cancer Institute (Frederick, Md.). All animal experiments were done in accordance with the Animal Welfare Act guidelines of the National Institutes of Health.
Preparation of H. capsulatum and infection of mice.
H. capsulatum yeasts (strain G217B) were prepared as described previously (7). Animals were infected intranasally (i.n.) with either 2.5 × 106 (sublethal challenge) or 1.25 × 107 (lethal challenge) yeasts in a 30-μl volume. In C57BL/6 or BALB/c mice, CFU in organs peak at week 1 and are absent beyond week 3 of infection (1, 2).
Organ culture for H. capsulatum.
Recovery of H. capsulatum was performed as described previously (7). The fungal burden was expressed as mean CFU per whole organ ± standard error of the mean (SEM). The limit of detection was 102 CFU.
Vaccination with H antigen.
rH was generated from pET19b as described previously (4) and emulsified in adjuvant containing monophosphoryl lipid A, synthetic trehalose dicorynomycolate, and cell wall skeleton (Ribi Immunochem, Hamilton, Mont.) at a concentration of 1 mg/ml. The recombinant antigen contained less than 5 pg of lipopolysaccharide/mg of protein. Controls received an equal amount of bovine serum albumin (BSA) suspended in adjuvant. Animals were injected subcutaneously with 0.1 ml of emulsion (100 μg of protein) twice. Injections were separated by 2 weeks. Infection of mice was conducted 4 weeks after the last vaccination.
Splenocyte preparation.
Spleens from mice were removed and teased apart between two ground glass slides. Cells were washed three times in Hanks balanced salt solution (BioWhittaker, Walkersville, Md.) and resuspended at a concentration of 2.5 × 106 cells per ml in RPMI containing 10% fetal bovine serum, 5 × 10−5 M 2-mercaptoethanol, 1% sodium pyruvate, 1% nonessential amino acids, 2 mM l-glutamine, and 10 μg of gentamicin per ml.
In vitro generation of cytokine-containing supernatants.
Splenocyte suspensions were prepared from C57BL/6 mice immunized with BSA or rH at 3, 7, and 14 days after each vaccination. One milliliter of suspension was added to each well of a 24-well plate. Cells were exposed to 25 μg of either rH or BSA in a volume of 25 μl. Cells also were incubated with an equal volume of buffer. The cell suspensions were cultured for 24 h at 37°C in 5% CO2, and the supernatants were harvested, filter sterilized, and stored at −70°C until assayed.
Cytokine measurement.
Commercially available enzyme-linked immunosorbent assay kits were used to measure gamma interferon (IFN-γ), interleukin-4 (IL-4), IL-12, tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Endogen, Cambridge, Mass.) and IL-10 (PharMingen, San Diego, Calif.). The data for cytokine measurements were expressed as the change in cytokine level by subtracting the amount of cytokine detected in medium alone from that found in supernatants of antigen-stimulated cells.
Flow cytometric analysis.
Splenocytes were suspended in phosphate-buffered saline (pH 7.4) containing 3% BSA and 0.05% sodium azide (PBSA) at a concentration of 107/ml. One million splenocytes were incubated with saturating concentrations of biotin-conjugated monoclonal antibodies (MAb) to Vβ2, -4, -5.1 and -5.2, -6, -7, -8.1 and -8.2, -9, -10, -11, -12, -13, and -14 (PharMingen) for 30 min at 4°C. Cells were washed three times and incubated with phycoerythrin-conjugated streptavidin for 30 min at 4°C. In addition, equal numbers of splenocytes were incubated with MAb to Vβ3, CD4, or CD8 conjugated to fluorescein isothiocyanate- or phycoerythrin-labeled Vβ8.3 (PharMingen). The cells incubated with direct conjugates were incubated at 4°C for 30 min. All cells were washed three times and resuspended in 1% paraformaldehyde in PBSA until they were analyzed by flow cytometry.
Memory T cells were identified by incubating cells with allophycocyanin-conjugated CD3, fluorescein isothiocyanate-conjugated MAb to CD45, and phycoerythrin-conjugated MAb to CD44 (PharMingen) for 30 min at 4°C. Cells were washed three times with PBSA. Cells that were CD44high, CD45dim were considered to be a memory population.
The mean numbers of splenocytes from each group did not vary significantly from one another. Hence, all data are reported in percentages.
Statistical analyses.
The log rank test was used to analyze differences in survival; Student's t test was employed to analyze differences in cytokine production and fungal burden of organs. If the data were not normally distributed, the Mann-Whitney test was used.
RESULTS
Immunization with rH is protective in mice.
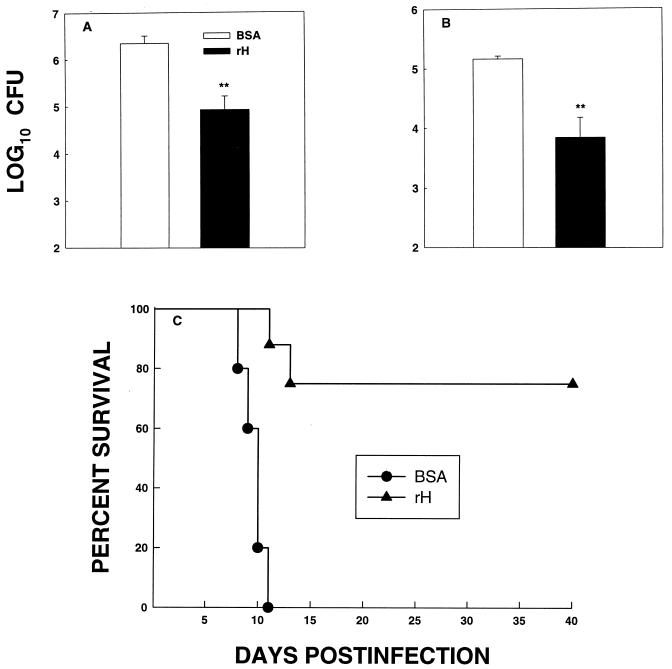
C57BL/6 mice were immunized with rH or BSA and challenged i.n. with 2.5 × 106 H. capsulatum yeasts at 4 weeks postimmunization. At week 1 of infection, mice were sacrificed and fungal recovery from lungs and spleens was determined. Organs from mice immunized with rH contained significantly fewer CFU (P < 0.01) than those from mice immunized with BSA (Fig. 1A and B). Subsequently, we determined whether rH-immunized mice could survive a lethal challenge with yeasts. Four weeks after immunization, mice were exposed to 1.25 × 107 yeasts i.n. and monitored for 40 days. All mice that were injected with BSA succumbed to infection, whereas 75% of those that were vaccinated with rH survived (P < 0.005) (Fig. 1C). At the end of the observation period, surviving mice were sacrificed and lungs and spleens were cultured for H. capsulatum. Organs from all mice contained less than 102 CFU, which is the limit of detection.
FIG. 1.
rH mediates protection in C57BL/6 mice infected i.n. with H. capsulatum. Mice immunized with rH or with BSA were challenged 4 weeks later with 2.5 × 106 H. capsulatum yeasts, and at week 1 of infection, CFU in lungs (A) and spleens (B) were determined. The data represent the mean (± SEM) log10 CFU from at least six animals per group. (C) Immunized mice (n = 8 to 10) also were challenged with 1.25 × 107 yeasts, and survival was monitored. ∗∗, P < 0.01.
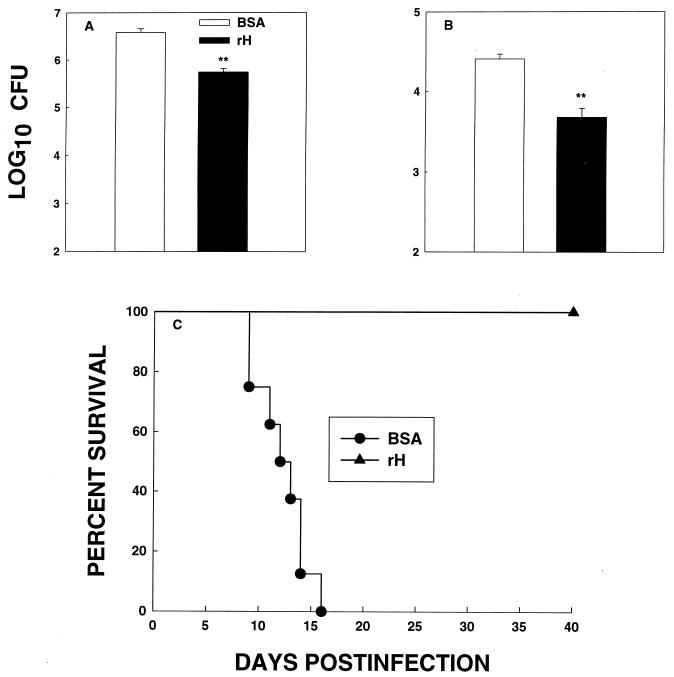
We immunized BALB/c mice with rH or with BSA, and 4 weeks later mice were exposed i.n. to 2.5 × 106 yeasts. The fungal burden in lungs and spleens at week 1 of infection was assessed. In addition, we determined survival of BALB/c mice after a lethal challenge with 1.25 × 107 yeasts. Immunization with rH significantly reduced (P < 0.01) the burden of infection in mice given the lower inoculum compared to controls (Fig. 2A and B), and it improved the survival rate of mice (P < 0.005) given a lethal challenge (Fig. 2C). CFU in lungs and spleens were quantified in the surviving mice. The number of CFU in lungs or spleens was less than 102 in organs of all mice.
FIG. 2.
rH protects BALB/c mice against an i.n. challenge with H. capsulatum. Mice immunized with rH or with BSA were challenged 4 weeks later with 2.5 × 106 H. capsulatum yeasts, and at week 1 of infection, CFU in lungs (A) and spleens (B) were determined. The data represent the mean (± SEM) log10 CFU from at least six animals per group. (C) Immunized mice (n = 8) also were challenged with 1.25 × 107 yeasts, and survival was monitored. Results from one of two experiments are depicted. ∗∗, P < 0.01.
The effect of rH is time dependent.
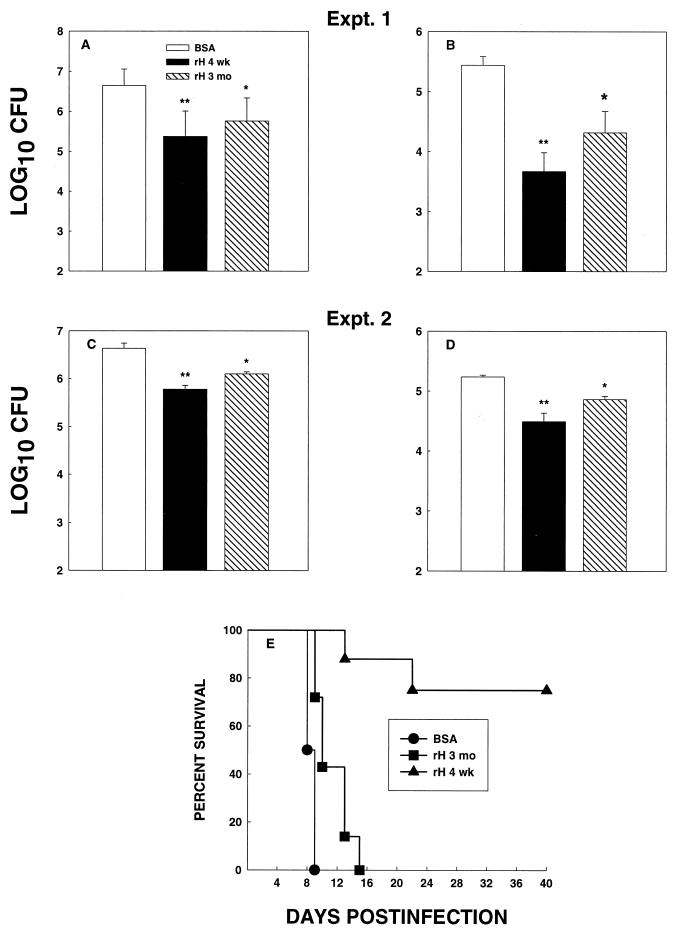
Groups of C57BL/6 mice were immunized with rH antigen or with BSA either 3 months or 4 weeks before infection and then challenged with 2.5 × 106 yeasts. The fungal burden in lungs and spleens was determined at 1 week of infection. In two experiments, CFU in the organs of mice immunized 3 months before challenge were less than those in controls (P < 0.05), but the decrease was not as pronounced as that observed in mice exposed to yeasts 4 weeks postvaccination (Fig. 3A to D). The number of CFU in organs of mice infected 4 weeks postvaccination was significantly lower than that in BSA-injected mice (P < 0.01) but not significantly lower than that in mice immunized 3 months before infection (P > 0.05).
FIG. 3.
Durability of immunization with rH. C57BL/6 mice were immunized with rH and challenged with 2.5 × 106 yeasts either 4 weeks or 3 months postimmunization. CFU in lungs (A and C) and spleens (B and D) were determined. The data represent the mean ± SEM from six animals per group. (E) Mice (n = 6 to 8) were challenged with 1.25 × 107 yeasts, and survival was monitored. ∗, P < 0.05; ∗∗, P < 0.01.
We next determined if a hiatus of 3 months following vaccination would alter the capacity of mice to withstand a lethal challenge. C57BL/6 mice were vaccinated with rH either 3 months or 4 weeks before challenge i.n. with 1.25 × 107 yeasts. Controls were mice immunized with BSA 3 months before infection. There was a modest delay in mortality (P < 0.05) in mice that were immunized with rH 3 months previously, but the salutary effect was not as pronounced as that observed for mice immunized 4 weeks before infection (Fig. 3E).
Cytokine profile of the afferent phase of immunization.
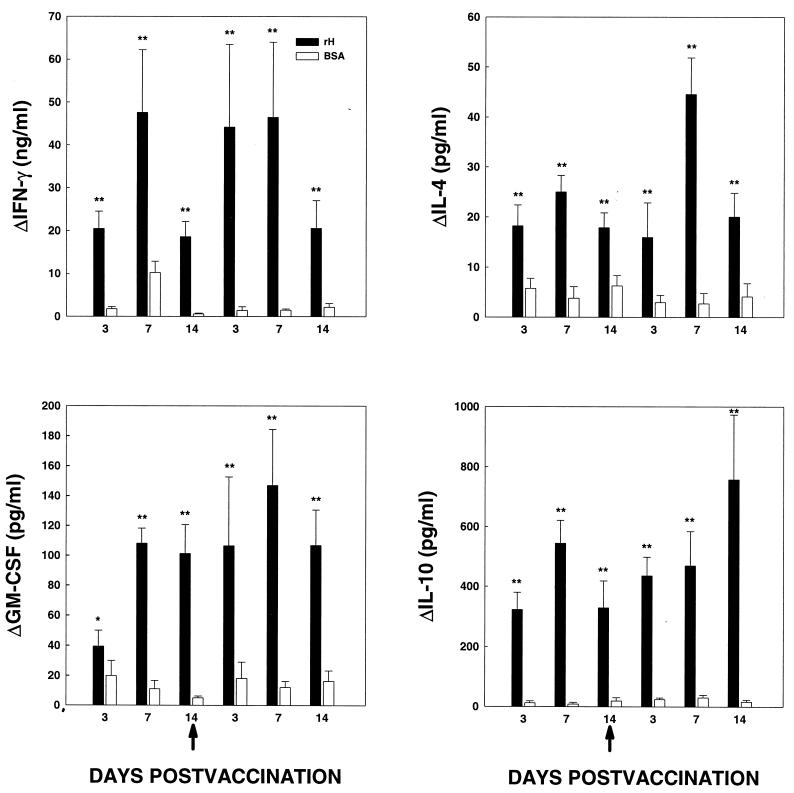
Spleen cells prepared from C57BL/6 mice immunized with rH or BSA were stimulated in vitro with 25 μg of antigen per ml for 24 h. Supernatants were collected and assayed for IFN-γ, IL-4, IL-10, IL-12, TNF-α, and GM-CSF. Cells from mice immunized with rH produced dramatically larger amounts of IFN-γ and GM-CSF than those from mice given BSA on each of the days postvaccination (Fig. 4). The range of difference was 3-fold to as much as 30-fold more IFN-γ (P < 0.01) and 2- to 20-fold for GM-CSF (P <0.01). Moreover, spleen cells from rH-injected mice generated more IL-4 (3- to 7-fold more) and IL-10 (17- to 68-fold more) than cells from BSA-immunized mice (P < 0.01) (Fig. 4). By 4 weeks postvaccination, no differences between the two groups in production of these cytokines were apparent. In both the rH- and BSA-immunized groups, the levels of IL-12 and TNF-α in medium from cells stimulated with cognate antigen were similar to those in medium from unstimulated cells (data not shown).
FIG. 4.
Cytokine production by splenocytes from immunized mice. Supernatants were harvested from immunized mice at the days specified. The arrows indicate the day of second vaccination. The data represent the mean ± SEM from at least six mice per time point. The data are expressed as the change in cytokine level and were calculated by subtracting the amount of cytokine detected in unstimulated splenocytes from that in antigen-stimulated splenocytes. ∗, P < 0.05; ∗∗, P < 0.01.
Phenotypic analysis of splenocytes from H antigen-immunized mice.
Splenocytes from C57BL/6 mice immunized with rH or BSA were analyzed at 3, 7, and 14 days after immunization for surface expression of CD4, CD8, memory, and Vβ. Spleen cells from a group of unimmunized mice were analyzed identically. The percentages of CD4+, CD8+, and CD3+ memory cells from mice immunized with rH did not vary significantly (P >0.05) from those of mice injected with BSA or from those of unimmunized mice, with the single exception that the percentage of CD3+ memory cells from rH-immunized mice was significantly greater (P < 0.01) at day 3 after the first vaccination (Table 1). Furthermore, the percentage and absolute number of Vβ-expressing cells did not vary among the groups (data not shown).
TABLE 1.
Expression of CD4+, CD8+, and CD3+ memory cells by splenocytes from immunized mice
| Immunization | Cell population | % (mean ± SEM) of cells on day:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | After first vaccination
|
After second vaccination
|
||||||
| 3 | 7 | 14 | 3 | 7 | 14 | |||
| None | CD4+ | 15.8 ± 0.8 | ||||||
| CD8+ | 9.9 ± 0.1 | |||||||
| Memorya | 6.1 ± 0.6 | |||||||
| BSA | CD4+ | 11.4 ± 0.5 | 17.8 ± 0.7 | 14.9 ± 1.5 | 9.6 ± 0.5 | 11.2 ± 1.3 | 13.5 ± 2.0 | |
| CD8+ | 6.6 ± 0.4 | 9.3 ± 1.0 | 9.5 ± 1.4 | 6.0 ± 0.5 | 7.6 ± 1.4 | 10.7 ± 1.0 | ||
| Memory | 3.6 ± 0.8 | 8.4 ± 1.6 | 5.7 ± 1.4 | 5.6 ± 1.3 | 5.4 ± 1.3 | 4.8 ± 0.5 | ||
| rH | CD4+ | 11.9 ± 1.1 | 12.7 ± 0.6 | 17.9 ± 1.5 | 8.8 ± 0.6 | 9.2 ± 1.7 | 13.1 ± 1.0 | |
| CD8+ | 6.1 ± 0.6 | 7.7 ± 0.5 | 8.8 ± 0.4 | 5.4 ± 0.4 | 5.3 ± 0.7 | 7.3 ± 0.5 | ||
| Memory | 10.2 ± 0.3b | 9.0 ± 0.7 | 7.3 ± 0.9 | 6.6 ± 0.4 | 8.2 ± 0.9 | 5.0 ± 1.4 | ||
CD3+ memory cells.
P < 0.05 compared to day 0 and BSA-immunized mice.
DISCUSSION
Cellular immunity is paramount to the control of human or experimental infection with H. capsulatum (6, 10, 11). Interventions that promote activation of this arm of immunity are likely to enhance clearance of this fungus from its resident tissues. In this study, we report that H antigen, a β-glucosidase whose utility has been largely confined to immunodiagnosis, may be useful to prevent pulmonary histoplasmosis. The data unequivocally demonstrate that vaccination with the recombinant antigen confers protection against both a sublethal and a lethal i.n. challenge. In fact, the results were unexpected, since a previous report by us indicated that rH was not protective against an intravenous challenge with this fungus (7).
One of the puzzling features of this study is the fact that rH was protective in the pulmonary, but not the systemic, model of histoplasmosis. The reason(s) for this discrepancy is not completely understood. One of the obvious explanations is that the original study examined the response in BALB/c mice. However, rH mediated protection in both C57BL/6 and BALB/c mice exposed i.n. to H. capsulatum. Alternatively, it is possible that the deposit of a larger burden of yeasts within minutes to hours into lymphoid organs following intravenous inoculation impairs the efficacy of clearance. This antagonism may be enhanced by the production of two cytokines, IL-4 and IL-10, within the spleen that have the potential to dampen the protective immune response to H. capsulatum (1, 3, 25). Nevertheless, these results strongly suggest that the efficacy of this protein as an immunogen is dependent on the route of exposure.
The analysis of the correlates of protective immunity mediated by rH was performed only with C57BL/6 mice, although the efficacy of vaccination also was examined with BALB/c mice. Since the two strains react to intravenous or i.n. inoculation with H. capsulatum quite similarly (8, 14), the studies of immune correlates were limited to C57BL/6 mice.
Host control of primary systemic or pulmonary infection with H. capsulatum requires the elaboration of several endogenous cytokines, including IFN-γ, TNF-α, IL-12, and GM-CSF (1, 2, 3, 9, 22, 23, 24, 25). IL-10 and IL-4, on the other hand, are known to exacerbate infection (1, 3, 25). Analysis of the cytokine production during the afferent phase of vaccination revealed that rH triggered elevated levels of cytokines that may improve (IFN-γ and GM-CSF) or exacerbate (IL-4 and IL-10) the course of infection. Since the net effect of vaccination was protection, it may be more useful to analyze the ratio of protective to exacerbating cytokines as a predictor of overall effect. For example, if one examines the ratio of IFN-γ to IL-4 or IL-10, it is evident that the dominant response favors a Th1 or protective response. This analysis, however, may not apply to GM-CSF, since it may not conform to the Th1-Th2 paradigm as do IFN-γ, IL-4, and IL-10. The roles of each of the aforementioned cytokines in the function of rH as a vaccine are being explored. One important pursuit that is being undertaken is to determine if different fragments of this protein stimulate production of particular cytokines. Because control of histoplasmosis requires a Th1-like response (2, 25), identifying the amino acids from rH that induce only IFN-γ may lead to a more potent vaccine.
A leishmanial protein antigen, LACK, when combined with recombinant IL-12, does not provide enduring protection in experimental cutaneous leishmaniasis (16). Mice vaccinated with LACK and IL-12 are protected when challenged 2 weeks, but not 12 weeks, later. In contrast, rH admixed in adjuvant continued to exert protection against the fungus as late as 3 months after initial vaccination. Its efficacy waned after 3 months, but rH still mediated protection against sublethal and lethal challenges. One explanation for the discrepancy is that the host controls of Leishmania and H. capsulatum differ. A second one is that we utilized an adjuvant that may have induced longer-term immunity than that provided by recombinant IL-12. If the protective immune response afforded by rH is dependent on the presence of antigen, it is quite possible that the adjuvant we employed generated a depot in which rH persisted. It is likely, though, that beyond 3 months the protection against H. capsulatum mediated by rH would require boosting.
Controversy exists concerning the requirement of antigen to preserve memory cells, which are the primary reactors in rechallenge experiments (5, 15, 18, 19, 21). It has been proposed that persistent immunity to microbes that depend on a Th1 response for resolution requires the continued presence of antigen (21). Thus, vaccination with plasmid DNA encoding a protein antigen is one means by which to promote the persistence of antigen within tissues and to prolong the efficacy of a vaccine (21). In addition, humoral immunity to protein antigens is capable of providing long-lasting immunity (20, 21). However, there is no published evidence to date that the endogenous humoral immune response to H. capsulatum or to antigens from this organism contributes to protective immunity. In fact, B-cell-deficient mice are no more susceptible than controls to i.n. infection with H. capsulatum yeasts (4).
We examined ex vivo the phenotype of cells in the spleens of mice immunized with H. Except for a single difference at day 3 postimmunization, the percentages and absolute numbers of CD4+, CD8+, Vβ-bearing cells and CD3+ memory cells did not differ between BSA-injected mice and those immunized with H. Thus, no skewing of the T-cell receptor repertoire or expansion of the major T-cell subpopulations could be detected within spleens following vaccination. The failure to identify an increase of a T-cell subpopulation or a Vβ family is not caused by an inability of antigens from this fungus to bias the T-cell response. In this regard, an antigen or antigens from actively replicating yeasts engender overexpression of one family, Vβ4+ cells, in the lungs of mice infected with H. capsulatum (13). We also sought in these studies to ascertain if expansion of a specific T-cell population could be used as a surrogate marker of successful vaccination. Unfortunately, no such marker could be identified in this manner.
In summary, H antigen, heretofore a glycoprotein useful only for serodiagnosis, mediates protective immunity against sublethal and lethal challenges in a model of pulmonary histoplasmosis. Vaccination was efficacious in two strains of mice and was associated with a dominant protective cytokine response, although IL-4 and IL-10 are released by spleen cells. The effect was sustained for at least 3 months postvaccination.
ACKNOWLEDGMENTS
This work was supported by grants AI-34361 and AI-42747 from the National Institutes of Health and by a Merit Review from the Veterans Affairs Hospital.
REFERENCES
- 1.Allendoerfer R, Boivin G P, Deepe G S., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer R, Deepe G S., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allendoerfer R, Deepe G S., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–6082. [PubMed] [Google Scholar]
- 4.Allendörfer R, Brunner G D, Deepe G S., Jr Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J Immunol. 1999;162:7389–7396. [PubMed] [Google Scholar]
- 5.Bachman F M, Kundig T M, Hengarten H, Zinkernagel R M. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cells memory without ‘memory T cells’? Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullock W E. Histoplasma capsulatum, In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1994. pp. 2340–2353. [Google Scholar]
- 7.Deepe G S, Jr, Durose G G. Immunobiological activity of recombinant H antigen from Histoplasma capsulatum. Infect Immun. 1995;63:3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepe G S, Jr, Gibbons R, Brunner G D, Gomez F J. A protective domain of heat shock protein 60 from Histoplasma capsulatum. J Infect Dis. 1996;174:828–834. doi: 10.1093/infdis/174.4.828. [DOI] [PubMed] [Google Scholar]
- 9.Deepe G S, Jr, Gibbons R, Woodward E. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J Immunol. 1999;163:4985–4993. [PubMed] [Google Scholar]
- 10.Deepe G S, Jr, Seder R A. Molecular and cellular determinants of immunity to Histoplasma capsulatum. Res Immunol. 1998;149:407–416. doi: 10.1016/s0923-2494(98)80763-3. [DOI] [PubMed] [Google Scholar]
- 11.Fisher K L, Deepe G S, Jr, Woods J P. Histoplasma capsulatum strain variation in both H antigen production and β-glucosidase activity and overexpression of HAG1 from a telomeric linear plasmid. Infect Immun. 1999;67:3312–3316. doi: 10.1128/iai.67.7.3312-3316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K L, Woods J P. Determination of β-glucosidase enzymatic function of the Histoplasma capsulatum H antigen using a native expression system. Gene. 2000;247:191–197. doi: 10.1016/s0378-1119(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 13.Gomez F J, Cain J A, Gibbons R, Allendoerfer R, Deepe G S., Jr Vβ4+ T cells promote clearance of infection in murine pulmonary histoplasmosis. J Clin Investig. 1998;102:984–995. doi: 10.1172/JCI2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez F J, Gomez A M, Deepe G S., Jr Protective efficacy of an antigen, HIS-62, from the cell wall and cell membrane of Histoplasma capsulatum yeasts. Infect Immun. 1991;59:4459–4464. doi: 10.1128/iai.59.12.4459-4464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurunathan S, Prussin C, Sacks D L, Seder R A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med. 1998;4:1409–1416. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 17.Heiner D C. Diagnosis of histoplasmosis using precipitin reactions in agar gel. Pediatrics. 1958;22:616–627. [PubMed] [Google Scholar]
- 18.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 19.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 20.Pirofski L, Casadevall A. Use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev. 1998;11:1–26. doi: 10.1128/cmr.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seder R A, Hill A V S. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 22.Smith J G, Magee D M, Williams D W, Graybill J R. Tumor necrosis factor-α plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990;162:1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- 23.Wu-Hsieh B A, Lee G-S, Franco M, Hofman F M. Early activation of splenic macrophages by tumor necrosis factor alpha is important in determining the outcome of experimental histoplasmosis in mice. Infect Immun. 1992;60:4230–4238. doi: 10.1128/iai.60.10.4230-4238.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P, Miller G, Seder R A. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]
- 25.Zhou P, Sieve M C, Bennett J E, Kwon-Chung J, Tewari R P, Gazinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]