Abstract
Purpose
To describe a case of unilateral retinal arterial occlusive vasculitis after multiple intravitreal brolucizumab (IVBr) treatments for diabetic macular edema (DME).
Observations
A 68-year-old Japanese woman who had a 3-year history of insulin-dependent diabetes mellitus presented with decreased vision in the right eye (oculus dexter, OD). After two consecutive IVBr (3 mg) treatments for DME, spaced 6 weeks apart, her best corrected visual acuity improved from 20/32 to 20/28 OD, as central macular thickness (CMT) decreased from 368 μm to 253 μm on optical coherence tomography (OCT). Immediately after the 3rd IVBr, the right intraocular pressure (IOP) increased. One week later, iritis (aqueous flares: 65.0 photon count [PC]/ms) was observed, followed by localized vasculitis 2 weeks later. One month after the 3rd IVBr, extensive vasculitis and vasculitis occluding retinal arterioles were identified. Based on the history of IVBr use and clinical findings, intraocular inflammation (IOI) and subsequent retinal arterial occlusive vasculitis due to IVBr was diagnosed. Topical steroid administration (i.e., eye drops and subtenon injection) resulted in improvement of IOI after 3 months. She subsequently underwent two intravitreal aflibercept injections for DME and panretinal photocoagulation (PRP) to prevent the development of proliferative changes due to diabetic retinopathy. One year after the diagnosis of retinal arterial occlusive vasculitis, the patient had slight loss of vision (20/50) compared to baseline, due to the progression of cataracts, and OCT angiography (OCTA) showed extensive non-perfusion area on the temporal side. However, other examination findings (IOP: 16 mmHg, aqueous flares: 30.5 PC/ms, CMT: 283 μm) were stable.
Conclusions and importance
Diagnosis and treatment at a relatively early stage after the onset of IOI prevented severe visual impairment in this case. Topical betamethasone eye drops reduced anterior chamber inflammation associated with IVBr; however, vascular sheathing worsened when topical drops alone was used. Occlusive retinal vasculitis, diagnosed with fluorescein angiography (FA) and OCTA, appeared to stabilize when subtenon triamcinolone injection was added to topical steroid administration. Because the central macula was not involved, severe vision loss was prevented. It is unknown if topical steroid administration would be adequate to prevent worsening of occlusive vasculitis in other cases. Although not used in this case, oral prednisone is one treatment option that may prevent severe vision loss. However, it requires monitoring of side effects, such as elevated blood glucose levels. PRP is also an option in cases where progression of proliferative changes is a concern, as was done in this case. With these considerations in mind, it is important to diagnose brolucizumab-associated IOI and subsequent retinal arterial occlusive vasculitis in DME patients early and initiate treatment to prevent severe visual impairment. Diagnosing new IOI and subsequent retinal arterial occlusive vasculitis is more difficult in DME than in neovascular age-related macular degeneration because of the inflammatory component often associated vascular occlusions. Therefore, early IOI diagnosis and follow-up using various instruments such as laser flare cell meter, wide-field color imaging, OCT/OCTA, and FA, in addition to usual comprehensive ophthalmologic examinations, is crucial.
Keywords: Brolucizumab, Diabetic macular edema, Intraocular inflammation, Retinal arterial occlusive vasculitis
1. Introduction
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy has become the gold standard for chorioretinal vascular diseases, including neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and retinal vein occlusion. Brolucizumab (Beovu®; Novartis, Basel, Switzerland) is the latest US Food and Drug Administration-approved anti-VEGF agent for DME. In Japan, this agent was launched in May 2020 for nAMD and in June 2022 for DME.
The 96-week results of HAWK and HARRIER, two Phase 3 clinical studies for nAMD, demonstrated the non-inferiority of brolucizumab to aflibercept (Eylea®, Regeneron, Tarrytown, NY) in visual outcomes, with superior anatomical outcomes with dosing every 12 weeks (q12w) or every 8 weeks (q8w).1 The prospective Phase 3 clinical studies for DME, KESTREL, and KITE aim to confirm the non-inferiority of 6 mg brolucizumab to 2 mg aflibercept in functional and morphological levels as well as in the durability of effect over 100 weeks.2 At 52 weeks, as an interim outcome in these studies, 6 mg brolucizumab was noted to be non-inferior to aflibercept in mean change in best corrected visual acuity (BCVA) from baseline, with more subjects achieving central macular thickness (CMT) less than 280 μm, fewer subjects having persistent subretinal and/or intraretinal fluid (SRF and/or IRF, respectively) compared to aflibercept, and with more than 50% of subjects on 6 mg brolucizumab maintained on q12w dosing after loading.3 As such, brolucizumab is expected to have favorable outcomes in nAMD and DME. However, intraocular inflammation (IOI), including retinal vasculitis, and vessel occlusion have been recently reported after intravitreal brolucizumab (IVBr) injection for nAMD.4, 5, 6, 7
To our knowledge, there have not yet been any reports of IOI or retinal vasculitis after IVBr for DME, probably because the use of brolucizumab for DME has only recently been approved. As brolucizumab use for DME is expected to increase in the future, it seems important to share a case in which brolucizumab-related complications were noted, with details of how they were treated. We describe a patient who experienced unilateral retinal arterial occlusive vasculitis following multiple IVBr for DME.
2. Case report
A 68-year-old Japanese woman who had a 3-year history of insulin-dependent diabetes mellitus was referred to our department for decreased vision in the right eye (oculus dexter, OD). The patient had severe non-proliferative diabetic retinopathy in both eyes (Fig. 1, Fig. 2D). Her BCVA was 20/32 OD, with a CMT of 368 μm and IRF on spectral-domain optical coherence tomography (OCT) (Fig. 1G). With her consent, she was enrolled in KESTREL, a Phase 3 clinical study for DME. Because the study was a randomized, double-blind, active controlled trial, neither the investigators nor the patient knew whether she had been randomized to 3 or 6 mg brolucizumab or 2 mg aflibercept. It later became clear that the patient had been randomized to 3 mg brolucizumab. Therefore, for convenience, we refer to this treatment method as IVBr.
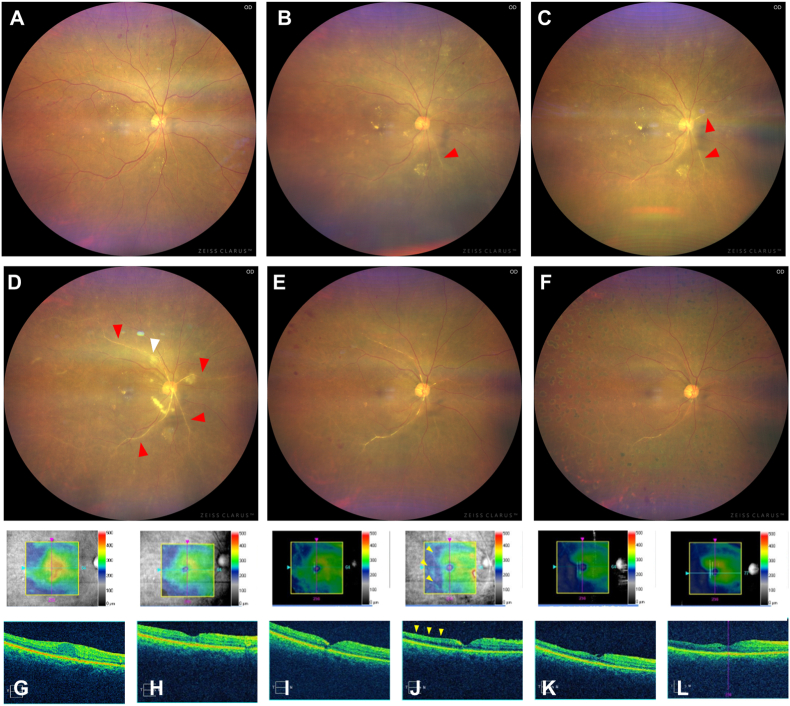
Fig. 1.
Time course of wide-field color imaging (A–F) and optical coherence tomography (OCT) (G-L; upper row: color map, lower row: cross-sectional image) before and after intravitreal brolucizumab injection (IVBr) for diabetic macular edema (DME). Baseline (A, G): Wide-field color image (A) shows severe non-proliferative diabetic retinopathy with multiple retinal hemorrhages and hard exudate in each quadrant. OCT (G) shows center-involved DME. One week after the 3rd IVBr (B, H): Wide-field color image shows no evidence of vitreous opacity or increased retinal hemorrhage (rather, retinal hemorrhage is decreased compared to the initial examination) but shows mild vascular sheathing on nasal retinal arteries (red arrow) (B). OCT (H) reveals complete disappearance of intraretinal fluid (IRF). Two weeks after the 3rd IVBr (C, I): Nasal vascular sheathing (red arrows) is slightly widened (C), although IRF continued to be completely absent on OCT (I). One month after the 3rd IVBr (D, J): Vitreous opacity and cotton wool appearance (white arrow) appears and the vascular sheathing (red arrows) has extended to the entire fundus (D). OCT shows retinal thinning without IRF or subretinal fluid (SRF) (J). Three months after the 3rd IVBr, i.e., 2 months after the diagnosis of retinal arterial occlusive vasculitis (E, K): Vitreous opacity and vascular sheathing are improved. OCT shows residual thinning of the temporal retina. One year after the diagnosis of retinal arterial occlusive vasculitis (F, L): Wide-field color imaging shows photocoagulation scars of panretinal photocoagulation and reduced vitreous opacity and vascular sheath (F). OCT shows continuous thinning of the retina without IRF or SRF (L). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
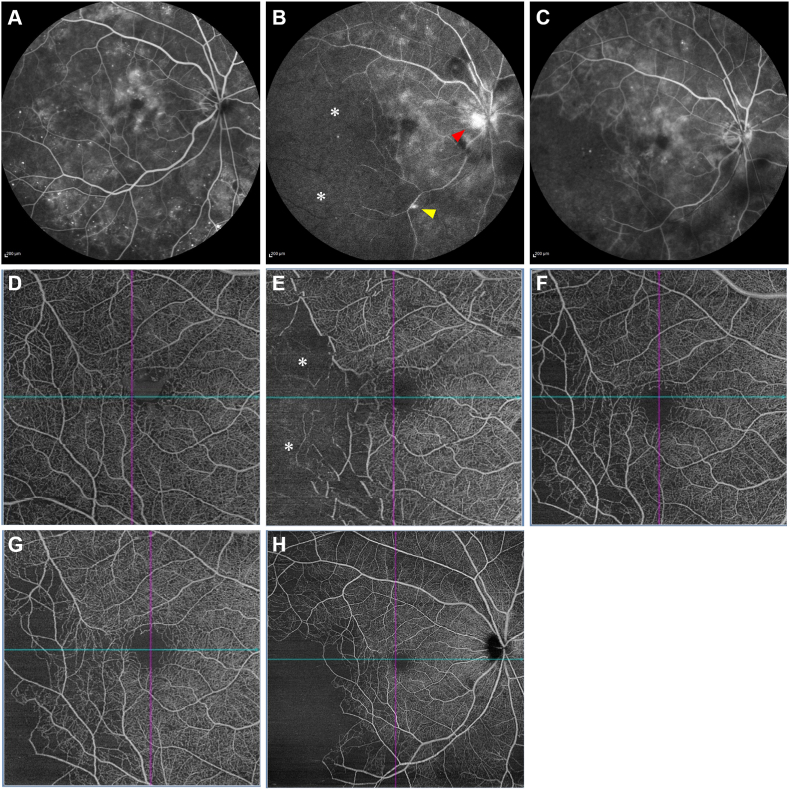
Fig. 2.
Time course of fluorescein angiography (FA) (A–C) and optical coherence tomography angiography (OCTA; D–G: 6 × 6 mm in size, H: 12 × 12 mm in size) (D–H) before and after intravitreal brolucizumab injection (IVBr) for diabetic macular edema (DME). Baseline (A, D): Although FA (A) shows numerous microaneurysms and fluorescein leakage from the microaneurysms in the macula, no extensive non-perfusion areas (NPAs) can be seen. OCTA (B) reveals numerous microaneurysms and local capillary dropouts. One month after the 3rd IVBr (B, E): FA (B) shows extensive NPA (asterisk) associated with temporal artery occlusion, fluorescein leakage from the vein (yellow arrow), and hyper-fluorescence on the optic disc (red arrow). OCTA (E) also shows NPA (asterisk) in the same region. Three months after the 3rd IVBr, i.e., 2 months after the diagnosis of retinal arterial occlusive vasculitis (C, F): FA (C) and OCTA (F) show no increase of NPA; rather, a decreasing trend is observed. One year after the diagnosis of retinal arterial occlusive vasculitis (G, H): The temporal NPA on the 6 × 6 mm OCTA shows more lateral expansion of the temporal NPA in the 12 × 12 mm OCTA, but no neovascularization is observed. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
After two consecutive IVBr treatments spaced 6 weeks apart, her BCVA improved to 20/28 OD, as CMT decreased to 253 μm (Fig. 1H). No evidence of IOI was noted at any of the visits up to this point. Although the intraocular pressure (IOP) was slightly elevated at 25 mmHg before the 3rd IVBr, it was performed as scheduled because there was no evidence of IOI. Immediately after the 3rd IVBr, the IOP increased to 34 mmHg, and the patient was started on dorzolamide hydrochloride/timolol maleate eye drops twice daily for OD.
One week after the 3rd IVBr, the IOP remained elevated at 30 mmHg without BCVA decrease. Slit-lamp examination revealed fine keratic precipitates (KPs) and anterior chamber cells (1+) and flare (1+), but no vitreous cells in OD. The aqueous flares measured by a laser flare cell meter (FM-600; Kowa, Nagoya, Japan) were 65.0 photon count (PC)/ms in OD and 8.7 PC/ms in the left eye. Fundus examination and wide-field color image showed no evidence of vitreous opacity or increased retinal hemorrhage; rather, retinal hemorrhage was noted to have decreased, compared to the level at the initial examination. However, the examination showed mild vascular sheathing on nasal retinal arteries (Fig. 1B, H).
Based on a diagnosis of iritis, the patient was started on 0.1% betamethasone eye drops four times daily for OD. One week later, although vascular sheathing remained, anterior chamber inflammation improved, the IOP in OD was reduced to 15 mmHg, and the aqueous flares were reduced to 45.5 PC/ms in OD (Fig. 1C, I). However, 1 month after the 3rd IVBr, the BCVA decreased to 20/32 without subjective symptoms, and the IOP and aqueous flares in OD were elevated at 26 mmHg and 52.6 PC/ms, respectively. Fundus examination and wide-field color imaging revealed vitreous opacity and severe vascular sheathing extending to the entire fundus (Fig. 1D). OCT showed retinal thinning without IRF or SRF (Fig. 1J). Fluorescein angiography (FA) showed filling defects in temporal retinal arteries and veins, a non-perfusion area (NPA), and segmental leakage from the veins and optic disc (Fig. 2B). OCT angiography (OCTA) indicated that the NPA was in the same location as seen on FA (Fig. 2E).
Based on the history of IVBr use and clinical findings, IOI and subsequent retinal arterial occlusive vasculitis due to brolucizumab injections was diagnosed. We reported this finding as an adverse event to the client of this clinical study. After obtaining her consent and in accordance with study regulations, we decided that the patient should discontinue the study. Subtenon triamcinolone acetonide (20 mg/0.5 mL) injection was administered to the patient, and she was started on brimonidine eye drops twice a day in addition to 0.1% betamethasone eye drops four times a day and dorzolamide hydrochloride/timolol maleate eye drops twice a day for OD. Three months later, although fundus examination revealed residual moderate vascular sheathing, OCTA and FA showed no increase of the NPA; rather, a decreasing trend was observed and the vasculitis was improved (Fig. 1E, K and Fig. 2C, F). She continued to use betamethasone eye drops four times daily and dorzolamide hydrochloride/timolol maleate eye drops two times daily for OD. Thereafter, two intravitreal aflibercept injections were administered for DME, and panretinal photocoagulation (PRP) was applied to prevent the development of proliferative changes due to diabetic retinopathy.
One year after the diagnosis of retinal arterial occlusive vasculitis, the patient had slight loss of vision (20/50) compared to baseline due to the progression of cataracts, and OCTA showed an extensive NPA on the temporal side (Fig. 2G and H). However, other examination findings (IOP: 16 mmHg, aqueous flares: 30.5 PC/ms, CMT: 283 μm) were stable (Fig. 1F, L). As an important note, this case was first reported as an adverse event of retinal vasculitis and was therefore not counted as an event of retinal vascular occlusion later developed in the study report.3
3. Discussion
We described a 68-year-old Japanese woman who developed unilateral retinal arterial occlusive vasculitis after three IVBr treatments for DME. IRF improvement was noted after three IVBr treatments without an associated BCVA improvement. Unfortunately, 1 month after the 3rd IVBr, IOI progressed in the following stages: elevated IOP, iritis, localized vasculitis, widespread vasculitis, and retinal arterial occlusive vasculitis. However, topical steroid administration (i.e., eye drops and subtenon injection) improved the inflammation and prevented severe visual impairment. The current case is the first detailed report of retinal vasculitis associated with brolucizumab therapy for DME.
Brolucizumab (26 kDa), a novel anti-VEGF agent, is the smallest anti-VEGF agent, allowing higher concentrations of molecules to be delivered in the same volume.8 Brolucizumab has been shown to have a prolonged effect and is potentially effective against nAMD, as shown in the HAWK and HARRIER studies.1 However, these reports pointed out that the frequency of IOI after IVBr was 4.6%.9 This is higher than those of ranibizumab and aflibercept (1.5% and 0.5–1.1%, respectively).10 Significant increases in the expression of inflammatory bioactive substances, such as intercellular adhesion molecule (ICAM)-1, interleukin (IL)-6, and monocyte chemotactic protein (MCP)-1, have been reported in the vitreous of patients with DME compared to controls.11 Thus, since DME is an inflammatory disease, there has been concern that IOI would be more frequent in DME than in nAMD. However, the reported IOI rates were 4.7% and 3.7% for the 3-mg and 6-mg brolucizumab groups, respectively, in KESTREL, and 1.7% in the 6-mg brolucizumab group in KITE, which were not significantly different from those in nAMD.3 One possible reason for the difference between the expected and actual results is the difficulty in diagnosing new-onset IOI in eyes with DR. In contrast to nAMD in which lesions are localized to the macula, iritis, retinal hemorrhage, and vasculitis are often present in DR. Therefore, it cannot be denied that several new-onset mild IOIs may have been missed. Furthermore, in eyes with DR, the presence of KP alone may make it difficult to diagnose a new IOI, since previous iritis can sometimes be associated with KP. It is also known that mydriasis can cause a small amount of cells to appear in the anterior chamber. Therefore, even in this case, it was difficult to diagnose IOI by slit-lamp examination and fundus examination alone, and using a laser flare cell meter and wide-field color imaging were useful for diagnosing iritis and vasculitis, respectively.
The usefulness of OCTA in the evaluation of retinal circulation in DR has recently been reported,12 and OCTA was also useful for evaluating vascular occlusion in this case. However, FA was still necessary to evaluate vasculitis because OCTA cannot evaluate leakage from blood vessels, which is different from FA. In the future, diagnosis of new-onset IOI (especially in mild cases) after IVBr for DME is expected to be difficult. In addition to careful examination, it is important to diagnose and treat IOI at an early stage using various devices such as laser flare cell meter, wide-field color image, OCT/OCTA, and FA.
The possibility that nAMD and DME respond differently to brolucizumab should also be noted. Although Mukai et al. reported that the risk factors for IOI after IVBr were older age, female sex, and diabetes,6 the current case is different because the patient is relatively young (68 years). In their report, the number of injections prior to IOI was two or less in 79% (11/14) of the cases, but in our case, IOI occurred after three IVBr treatments. The day of onset of retinal vasculitis in the four cases reported by KESTREL and KITE was also relatively late: days 114 (i.e., 114 days from baseline), 115, 96, and 203. These results suggest that IVBr for DME requires caution because of the risk of IOI, even in patients who have undergone multiple IVBr treatments without complications.
Betamethasone eye drops or subtenon triamcinolone acetonide injection is used for mild cases of brolucizumab-associated IOI in patients with nAMD, and oral prednisolone or intravitreal triamcinolone acetonide is chosen according to the severity.5, 6, 7,13,14 Although not used in this case, systemic administration of corticosteroids may be a treatment option that can prevent severe vision loss for severe brolucizumab-associated IOI in patients with DME, similar to that in AMD. However, worsening glycemic control is known to be an important side effect of corticosteroid use.15 Systemic administration of corticosteroids for severe brolucizumab-related IOIs in patients with DME should be administered with caution and requires close blood glucose monitoring when delivered. It has been reported that PRP can induce inflammation.16 Therefore, in the present case, PRP was applied when the IOI had completely disappeared, i.e., 5 months after the onset of IOI, to prevent the development of proliferative changes. The timing of PRP application is an issue that should be considered in the future. To the best of our knowledge, there are no reports of PRP for retinal occlusion after IVBr for nAMD. It should be noted that nAMD and DME differ in terms of treatment, even for the same brolucizumab-associated IOI.
4. Conclusion
We reported a case of retinal arterial occlusive vasculitis after multiple IVBr for DME. Fortunately, early diagnosis and treatment after disease onset prevented severe visual impairment. There have been many reports of brolucizumab-associated IOI in patients with nAMD, which may also be experienced with DME. However, it is important to note that there are differences in diagnosis and treatment between nAMD and DME, even for the same brolucizumab-associated IOI. In terms of diagnosis, diagnosing new IOI and subsequent retinal arterial occlusive vasculitis in DME would be more difficult than it would be in nAMD because DME has an inflammatory component and often associated vascular occlusions. Therefore, it is important to diagnose IOI and subsequent retinal arterial occlusive vasculitis at an early stage and follow up using various devices such as a laser flare cell meter, wide-field color imaging, OCT/OCTA, and FA. In terms of treatment, it is important to note the differences with nAMD. For severe brolucizumab-associated IOI in patients with nAMD, oral prednisolone is used. However, caution should be taken with the use of oral prednisolone in patients with DME because of the potential for increased blood glucose levels. Further, in cases where there are concerns about progressive proliferative changes, PRP is also an option. With these considerations in mind, it is important to diagnose brolucizumab-associated IOI in patients with DME early and initiate treatment to prevent severe visual impairment, even if visual impairment has begun to develop.
Patient consent
Written consent to publish this case report was obtained from the patient. This case report does not contain personal identifying information.
Funding
This study received no funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
No conflicting relationship exists for any author.
Acknowledgements
None.
References
- 1.Dugel P.U., Singh R.P., Koh A., et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi: 10.1016/j.ophtha.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Garweg J.G. A randomized, double-masked, multicenter, phase III study assessing the efficacy and safety of brolucizumab versus aflibercept in patients with visual impairment due to diabetic macular edema (KITE) Klin Monbl Augenheilkd. 2020;237(4):450–453. doi: 10.1055/a-1101-9126. [DOI] [PubMed] [Google Scholar]
- 3.Brown D.M., Emanuelli A., Bandello F., et al. KESTREL and KITE: 52-week results from two Phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol. 2022;238:157–172. doi: 10.1016/j.ajo.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Haug S.J., Hien D.L., Uludag G., et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18 doi: 10.1016/j.ajoc.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain A., Chea S., Matsumiya W., et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18 doi: 10.1016/j.ajoc.2020.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukai R.G., Matsumoto H.G., Akiyama H.G. Risk factors for emerging intraocular inflammation after intravitreal brolucizumab injection for age-related macular degeneration. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0259879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataoka K., Horiguchi E., Kawano K., et al. Three cases of brolucizumab-associated retinal vasculitis treated with systemic and local steroid therapy. Jpn J Ophthalmol. 2021;65(2):199–207. doi: 10.1007/s10384-021-00818-8. [DOI] [PubMed] [Google Scholar]
- 8.Holz F.G., Dugel P.U., Weissgerber G., et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration: a randomized controlled study. Ophthalmology. 2016;123(5):1080–1089. doi: 10.1016/j.ophtha.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Mones J., Srivastava S.K., Jaffe G.J., et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi: 10.1016/j.ophtha.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Funatsu H., Noma H., Mimura T., Eguchi S., Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Hirano T., Chanwimol K., Weichsel J., Tepelus T., Sadda S. Distinct retinal capillary plexuses in normal eyes as observed in optical coherence tomography angiography axial profile analysis. Sci Rep. 2018;8(1):9380. doi: 10.1038/s41598-018-27536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iesato Y., Hirano T., Yoshida N. Early recovery from vasculitis after brolucizumab with prompt steroid treatment. Ophthalmol Retina. 2022;6(4):325. doi: 10.1016/j.oret.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Hikichi T. Three Japanese cases of intraocular inflammation after intravitreal brolucizumab injections in one clinic. Jpn J Ophthalmol. 2021;65(2):208–214. doi: 10.1007/s10384-021-00819-7. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 16.Shimura M., Yasuda K., Nakazawa T., et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1617–1624. doi: 10.1007/s00417-009-1147-x. [DOI] [PubMed] [Google Scholar]