Abstract
Probiotics can improve animal growth performance and intestinal health. Bacillus species, Lactobacillus species, Bifidobacterium species, yeast etc. are the common types of probiotics. However, understanding the effects of probiotics on the immune status and gut microbiota of weaning piglets and how the probiotics exert their impact are still limited. This study aimed to investigate the effects of Bacillus amyloliquefaciens 40 (BA40) on the performance, immune status and gut microbiota of piglets. A total of 12 litters of newborn piglets were randomly divided into 3 groups. Piglets in control group were orally dosed with phosphate buffered saline; BA40 group and probiotics group were orally gavaged with resuspension BA40 and a probiotics product, respectively. The results showed that BA40 treatment significantly decreased (P < 0.05) the diarrhea incidence (from d 5 to 40), diamine oxidase, D-lactate, interleukin (IL)-1β and interferon-γ concentrations compared with control group and probiotics group. Meanwhile BA40 dramatically increased the total antioxidant capacity, IL-10 and secretory immunoglobulin-A concentrations in contrast to control group. For the microbial composition, BA40 modulated the microbiota by improving the abundance of Bacteroides, Phascolarctobacterium (producing short-chain fatty acids) and Desulfovibrio and reducing the proliferation of pathogens (Streptococcus, Tyzzerella, Vellionella and paraeggerthella). Meanwhile, a metabolic function prediction explained that carbohydrate metabolism and amino acid metabolism enriched in BA40 group in contrast to control group and probiotics group. For correlation analysis, the results demonstrated that BA40-enriched Phascolarctobacterium and Desulfovibrio provide insights into strategies for elevating the health status and performance of weaned piglets. Altogether, BA40 exerted stronger ability in decreasing diarrhea incidence and improved antioxidant activity, gut barrier function and immune status of piglets than the other treatments. Our study provided the experimental and theoretical basis for the application of BA40 in pig production.
Keywords: Bacillus amyloliquefaciens, Piglet, Immune status, Gut microbiota, Metabolic function
1. Introduction
Weaning stress occurred, particularly during the first week after weaning from the sow, always accompanied with a series of problems, such as intestinal system dysfunctions, which can reduce pig health status, feed intake, growth rate (Campbell et al., 2013; Wen et al., 2020a; Wen et al., 2020b). So weaning stress is the most challenging event in a pig's life, and how to effectively decrease the stress caused by weaning and increase the health status of piglets is one of the most important challenges in pig production.
Previous studies demonstrated that probiotics can improve intestinal health (Wang et al., 2017a) and regulate immunity in piglets (Deng et al., 2013), which provide a potential strategy to address weaning stress. Bifidobacterium species, Lactobacillus species, Bacillus species, yeast etc. are the common types of probiotics (Foligne et al., 2013). Bacillus species are preferred as a feed additive as they can tolerate harsh environments (high temperature) through formulation of spores as well as can produce a variety of enzymes such as protease, amylase, and lipase (Hong et al., 2005). Similarly, Bifidobacterium species, Lactobacillus species are used as the feed additives due to their capability to colonize the intestines early (van Zyl et al., 2020). Our previous study found that Bacillus amyloliquefaciens (BA40) also can inhibit the major pathogens found on pig farms (Jiang et al., 2021). Supplementing the diets of growing pigs with Bacillus amyloliquefaciens or Bacillus subtilis could increase growth performance and improve the digestive and absorptive capacity of pigs (Wang et al., 2017a; Hu et al., 2018). However, whether BA40 could alleviate weaning stress and how it would exert a protective effect in weaned piglets are unclear. A probiotics product (Glyco Guard, containing Bifidobacterium infantis, Lactobacillus plantarum and Pediococcus lactis) is a specially formulated direct-fed microbial product used by veterinarians to support digestion and a healthy gastrointestinal system in livestock. It also able to quickly and effectively manage diarrhea and prevent diarrhea becoming more serious. Therefore, the present study was conducted to investigate the effects of Bacillus amyloliquefaciens BA40 and the probiotics product on the performance, immune status and gut microbiota of piglets.
2. Materials and methods
2.1. Animal ethics statement
All the procedures were approved by the Institutional Animal Care and Use Committee at Zhejiang University.
2.2. Bacterial strain preparation
BA40 was isolated from the gastrointestinal track of Jinhua pigs (Chinese panda pigs) by our laboratory. BA40 was preserved in China Center for Type Culture Collection (CCTCC, NO. M2021535). The probiotic product named Glyco Guard was provided by Evolve Biosystems compony as a positive control, and Glyco Gurad contained 3 different probiotics (B. infantis, L. plantarum and P. lactis). In this experiment, BA40 was cultured in Luria–Bertani (LB) medium at 37 °C in a shaking incubator under aerobic conditions for 12 h. BA40 was harvested by centrifugation at 4,000 × g for 10 min at 4 °C, washed 3 times in phosphate buffered saline (PBS) and centrifuged again at 4,000 × g for 10 min. Eventually, a final bacterial concentration of 1.0 × 109 CFU (colony forming units)/mL was obtained.
2.3. Animal experimental design and sample collection
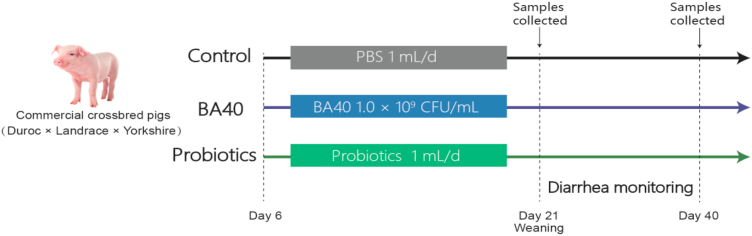
According to the sow breed, litter size, date of birth and the birth weight of piglets, a total of 12 litters (Duroc × Yorkshire × Landrace, 11 to 12 piglets in each litter) of newborn piglets with identical birth dates and parities were picked from a commercial pig farm located in Quzhou, Zhejiang, China. The experimental design and scheme of the animal treatments is presented in Fig. 1. The piglets were randomly subjected to 3 treatments: control group (4 litters), BA40 group (4 litters) and probiotics group (4 litters). After 5 d of adaptation, piglets in control group were orally gavaged with 1 mL PBS once a day. Piglets in BA40 group were orally gavaged with resuspension BA40 (1.0 × 109 CFU/mL) 1 mL once a day. Piglets in the probiotics group were gavaged with resuspension of probiotics product 1 mL (according to the recommended dose of the instructions) once a day. All treatments were applied from d 6 to 18.
Fig. 1.
Experimental design and scheme of the animal treatments. BA40 = Bacillus amyloliquefaciens 40.
The composition and nutritional value of the diets are presented in Table 1. Rectal swab samples (first, one piglet per litter was randomly selected, then 2 litters of the 4 litters were selected at random and one piglet was selected from each litter) for microbial analysis were collected into sterile Eppendorf tubes by sterile cotton swabs at the morning of d 21, frozen immediately in liquid nitrogen, and stored at −80 °C. Six piglets (same operation as above) were randomly picked from each group at d 21. Blood samples were harvested from the anterior vena cava into 10-mL vacutainer tubes, then centrifuged it at 4 °C and 1,000 × g for 10 min and serum were obtained. All serum samples were frozen at −80 °C until analysis.
Table 1.
Ingredient composition and nutrient concentration in the experimental (%, as-fed basis).1
| Ingredients | Content | Nutrition levels | Content |
|---|---|---|---|
| Corn | 48.50 | DE, Mcal/kg | 3.35 |
| Soybean meal | 20.00 | CP | 16.97 |
| Barley | 15.00 | TP | 0.62 |
| Extruded soybean | 6.00 | Ca | 0.72 |
| Wheat shorts | 5.00 | Lysine | 1.11 |
| Soybean oil | 1.50 | Methionine | 0.33 |
| Premix2 | 4.00 | Threonine | 0.72 |
CP = crude protein; DE = digestible energy; TP = total phosphorus; Ca = calcium.
Analyzed values determined in duplicate.
Provided the following amount of vitamins and minerals per kilogram diet: vitamin A, 18,000 IU; vitamin D3, 5,000 IU; vitamin E, 20 mg; vitamin K3, 5 mg; vitamin B1, 3 mg; vitamin B2, 10 mg; vitamin B6, 4 mg; vitamin B12, 30 μg; pantothenic acid, 20 mg; folic acid, 2 mg; nicotinic acid, 35 mg; Fe(FeSO4), 90 mg; Cu (CuSO4), 20 mg; Zn (ZnSO4), 80 mg; Mn (MnSO4), 15 mg; Se (Na2SeO3), 0.3 mg; KI, 0.3 mg.
2.4. Growth performance
After sow delivery, the number of piglets born, the number of piglets live born and the birthweight of piglets were recorded. From birth to weaning, the survival rate and the weaning weight of piglets of each group were recorded and analyzed. The diarrhea incidence of piglets was measured from parturition to weaning. From piglets weaning to d 40, fecal consistency was assessed 4 to 5 times daily, and scored based on previous studies (Huang et al., 2016, 2019): 0, normal feces (solid feces); 1, slight diarrhea (soft and loose feces); 2, moderate diarrhea (semi-liquid feces); 3, severe diarrhea (watery feces). Total diarrhea scores = sum of daily diarrhea scores. Average diarrhea scores = total diarrhea scores/days.
2.5. Serum parameters analysis
Serum levels of D-lactate (DLA) and diamine oxidase (DAO) were quantified with the ELISA kits (Jiangsu Enzyme-Labeled Biological Technology, Jiangsu, China). Meanwhile, serum parameters including IL-1β, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-10, IL-22, IL-23 and fecal secretory immunoglobulin A (sIgA) were also determined with ELISA kits (Jiangsu Enzyme-Labeled Biological Technology, Jiangsu, China). In addition, serum total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity and malonaldehyde (MDA) were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). These protocols were carried out in strict accordance with the manufacturer's instructions.
2.6. Microbial analysis of the feces
Total genomic DNA of the 18 rectal swab samples (6 each group) were extracted by the PowerSoil DNA isolation kit (MoBio Laboratories Inc., USA). The V3–V4 gene region of the bacterial 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR was conducted as follows: 3 min of denaturation at 95 °C; 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C; and final extension at 72 °C for 10 min. PCR products were purified and quantified bt QIAquick gel extraction kit (QIAGEN, Hilden, Germany) and Quant-iT PicoGreen dsDNA assay kit (Life Technologies, Carlsbad, USA), respectively. Illumina Hiseq2500 was conducted according to previous studies (Jiang et al., 2021; Su et al., 2021). The data were processed with the QIIME2 (http://qiime2.org/) pipeline and DADA2 (Version 1.8) was used to denoise (Edgar and Flyvbjerg, 2015). Then the VSEARCH (Version 2.15.0) was applied to identify and remove chimeric sequences (Rognes et al., 2016). An amplicon sequence variant (ASV) table was generated from the effective tags that did not contain non-biological nucleotides. Representative sequences were assigned to taxonomy using the RDP classifier (Version 2.2) based on the SILVA database (silva138/16s_bacteria). The abundance of each taxonomy was constructed by a Perl script and visualized using SVG. The sequence data were deposited in the Sequence Read Archive (SRA) under the accession number PRJNA778041.
The α and β diversities of the bacterial of the bacterial community were calculated in the free online platform of Majorbio Cloud Platform (www.majorbio.com) and online analysis platform (Microbiome Analyst, http://www.microbiomeanalyst.ca/) based on Chao1 and unweighted UniFrac distance (Chong et al., 2020). The main differentially abundant genera were selected by the Liner discriminant analysis Effect Size (LEfSe) method (Nicola Segata et al., 2011). Furthermore, the predicted microbial metabolic functions were conducted by PICRUST2 (https://huttenhower.sph.harvard.edu/galaxy). The heatmap package of R (Version 3.3.1) and Python (Version 2.7) were applied to generate heatmaps.
2.7. Correlation analysis of growth performance, serum parameters, incidence of diarrhea, gut microbiota and prediction of microbial gene functions
Spearman's correlation analysis of growth performance, serum parameters, incidence of diarrhea, differential gut microbiota composition and microbial gene functions was computed by R (Version 3.2.4).
2.8. Statistical analysis
SPSS 20.0 software (SAS Inc., Chicago, IL) was used to analyze data. One-way ANOVA and Duncan's test were used to determine the difference among groups. Data are expressed as the mean ± SD. An arbitrary P-value, i.e., P < 0.05, was considered statistically significant. GraphPad Prism 8 (San Diego, CA, USA) was used to generate bar plots.
3. Results
3.1. Growth performance of piglets
The growth performance of the piglets is presented in Table 2, there were no differences among the number of piglets at birth, at weaning and alive after weaning (P > 0.05) among the 3 groups. Similarly, the weight at birth, weight gain and weight at weaning also did not differ (P > 0.05). In the BA40 group there was an increase in weight gain and weight at weaning compared to the control group (7.07 ± 0.56 kg vs 6.52 ± 0.70 kg, 8.75 ± 0.60 kg vs 8.28 ± 0.60 kg, respectively). The diarrhea incidence of piglets during sucking period in BA40 group decreased significantly (P < 0.05) compared with the other 2 groups.
Table 2.
Effects of treatments on the performance of piglets.
| Item | Control | BA40 | Probiotics | P-value |
|---|---|---|---|---|
| Number at birth, live | 12.25 ± 0.75 | 12.00 ± 0.71 | 11.75 ± 0.47 | 0.867 |
| Number at weaning | 11 ± 0.41 | 11.00 ± 0.71 | 10.50 ± 0.50 | 0.767 |
| Weaning alive rate1, % | 90.8 ± 0.07 | 91.6 ± 0.004 | 90.6 ± 0.04 | 0.948 |
| Litter weight at birth, kg | 20.95 ± 1.06 | 19.10 ± 0.74 | 20.12 ± 0.73 | 0.316 |
| Weight at birth2, kg | 1.76 ± 0.11 | 1.63 ± 0.06 | 1.74 ± 0.12 | 0.623 |
| Litter weight at weaning, kg | 90.46 ± 4.11 | 95.59 ± 6.01 | 88.11 ± 8.94 | 0.728 |
| Weight at weaning3, kg | 8.28 ± 0.60 | 8.75 ± 0.60 | 8.32 ± 0.51 | 0.816 |
| Litter weight gain4, kg | 69.03 ± 4.62 | 76.1 ± 5.67 | 67.79 ± 9.35 | 0.665 |
| Weight gain5, kg | 6.52 ± 0.70 | 7.07 ± 0.56 | 6.63 ± 0.57 | 0.755 |
| Diarrhea incidence6, % | 3.08 ± 0.38a | 1.53 ± 0.21b | 2.85 ± 0.25a | 0.007 |
BA40 = Bacillus amyloliquefaciens 40.
a,bMeans within a row with different superscripts significantly differ (P < 0.05).
Weaning alive rate = [litter size at weaning (live) - litter size at birth (live)]/litter size at birth (live).
Piglet weight at birth = litter weight at birth/litter size at birth (live).
Piglet weight at weaning = litter weight at weaning/litter size at weaning (live).
Litter weight gain = litter weight at weaning - litter weight at birth.
Piglet weight gain = piglet weight at weaning - piglet weight at birth.
Diarrhea incidence = total diarrhea piglets/[litter size at birth (live) × trial days].
3.2. Diarrhea monitoring from d 21 to 40
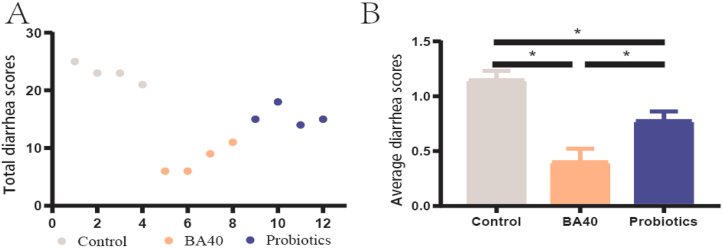
The diarrhea scores were recorded from d 21 to 40 to monitor diarrhea conditions among the 3 treatments. As shown in Fig. 2A, the total diarrhea scores were presented for each litter. After stopping the gavage of PBS, BA40 and probiotics, a significant downward (P < 0.05) trend in average diarrhea scores was observed in the BA40 group (Fig. 2B). It showed that the BA40 treatment decreased the incidence of diarrhea during d 21 to 40.
Fig. 2.
Effect of BA40 administration on diarrhea incidence of pigs during d 21 to 40. (A) Total diarrhea scores in each litter. (B) Average diarrhea scores; each group were expressed as mean (SD). SD = standard deviation. The asterisk (∗) represented significant differences, P < 0.05. All the values contained 4 repetitions. Average diarrhea scores = total diarrhea scores/days. BA40 = Bacillus amyloliquefaciens 40.
3.3. Antioxidant ability and intestinal injury parameters of weaner piglets at serum level
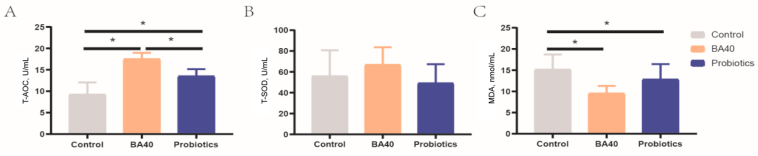
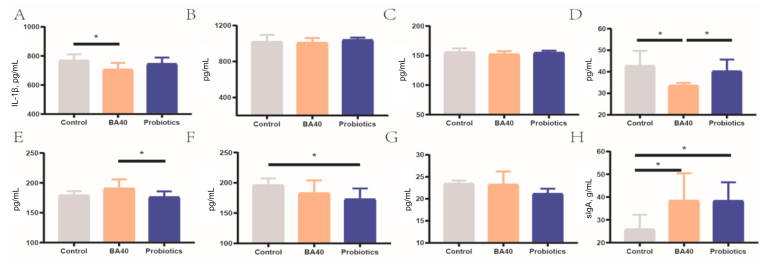
The results for the pig serum antioxidant ability were presented in Fig. 3. Compared to the control group and probiotics group, it was found that T-AOC increased dramatically (P < 0.05) in the BA40 group, while no significantly differences were found for SOD content among the 3 groups (Fig. 3A and B). For MDA, there was a same trend with T-AOC, the MDA concentration in serum level notably decreased (P < 0.05) in the BA40 and probiotics groups compared to the control group (Fig. 3C). However, there was no significant difference between the BA40 group and the probiotics group in MDA concentration.
Fig. 3.
Serum antioxidant ability of weaned piglets. (A) Total antioxidant capacity (T-AOC). (B) Total superoxide dismutase (T-SOD). (C) Malonaldehyde (MDA). Each group were expressed as mean (SD). SD = standard deviation. BA40 = Bacillus amyloliquefaciens 40. The asterisk (∗) represented significant differences, P < 0.05. All the values contained 6 repetitions.
Fig. 4 presented the serum levels of DAO and DLA in piglets. As shown in Fig. 4A and B, changes in DAO activity and DLA concentration in serum were observed among all 3 groups of piglets. The DAO and DLA levels in serum were significantly decreased (P < 0.05) in the BA40 group in comparison to the control group and probiotics group. No significant differences (P < 0.05) were observed between the control group and probiotics group in DAO activity.
Fig. 4.
Serum levels of DAO and DLA in weaned piglets. (A) Diamine oxidase (DAO). (B) D-lactate (DLA). Each group were expressed as mean (SD). SD = standard deviation. BA40 = Bacillus amyloliquefaciens 40. The asterisk (∗) represented significant differences, P < 0.05. All the values contained 6 repetitions.
3.4. Serum inflammatory cytokines and fecal sIgA of piglets
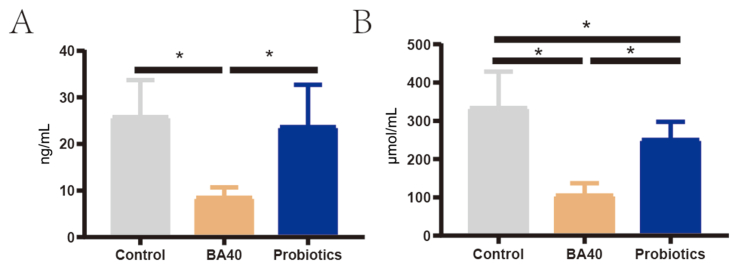
Serum immunity parameters were determined to assess the immune status of the 3 groups of piglets (Fig. 5). For serum inflammatory cytokines, the BA40 group reduced (P < 0.05) the serum IL-1β, INF-γ and improved the IL-10 compared with the control group or probiotics group (Fig. 5A, D and E). Meanwhile, the probiotics group had decreased (P < 0.05) IL-22 level compared with the control group (Fig. 5F). Furthermore, the BA40 and probiotics groups also had a significantly increased (P < 0.05) sIgA concentration in the feces compared to the control group (Fig. 5H). There were no significant differences in IL-6, TNF-α and IL-23 levels between the 3 groups (Fig. 5B, C and G).
Fig. 5.
Serum inflammatory cytokines and fecal immunoglobulin A (sIgA) of weaned piglets. (A to H) Interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-10, IL-22, IL-23, and sIgA. Results are presented as mean (SD). SD = standard deviation. BA40 = Bacillus amyloliquefaciens 40. The asterisk (∗) represented significant differences, P < 0.05. All the values contained 6 repetitions.
3.5. Intestinal bacterial composition
The change in bacterial diversity related to BA40 supplementation was investigated using the 16S rRNA sequencing. As shown in Table 3, the number of recovered reads of each group ranged from 22,261 ± 1,207 to 27,875 ± 3,536 with Good's coverage of all samples (approximately 0.99) and the total number of sequences (72,541) were collected by 16S rRNA sequencing, which suggested that the depth of sequencing was adequate for ongoing analysis. However, the number of observed ASV, Chao1 index, Shannon index and Simpson index had no differences among the 3 groups, indicating that the oral gavage of the additives may not influence the richness index of bacterial community during the piglet suckling stage.
Table 3.
Characteristics of amplicon libraries in the bacteria community.
| Characteristic | Control | BA40 | Probiotics | Total number |
|---|---|---|---|---|
| Number of sequences | 22,261 ± 1,207 | 22,405 ± 525 | 27,875 ± 3,536 | 72,541 |
| Number of observed ASV | 169 ± 11 | 165 ± 14 | 172 ± 15 | 506 |
| Chao1 index | 170 ± 11 | 165 ± 14 | 175 ± 14 | |
| Shannon index | 3.58 ± 0.13 | 3.47 ± 0.17 | 3.67 ± 0.13 | |
| Simpson index | 0.07 ± 0.01 | 0.07 ± 0.11 | 0.06 ± 0.01 |
BA40 = Bacillus amyloliquefaciens 40; ASV = amplicon sequence variant.
All the values contained 6 repetitions.
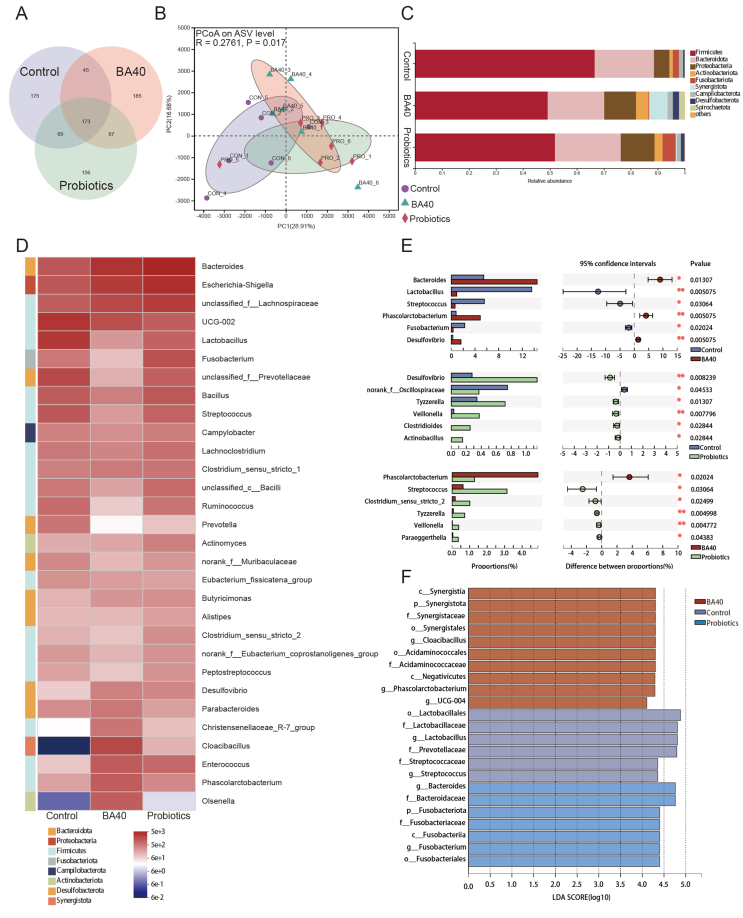
Venn analysis (Fig. 6A) revealed that 173 ASV were shared among all groups, and the BA40 group had the most unique microbes (185 ASV). Furthermore, Fig. 6B shows the principal coordinate analysis (PCoA), and the results demonstrated that the gut microbial community in the BA40 group was significantly separated (P = 0.017) from the control group. The results of Venn and PCoA plot indicated that BA40 worked more efficiently than other treatments in altering microbial composition.
Fig. 6.
Description of the intestinal microbiota composition in weaned pigs. (A) The Venn analysis of amplicon sequence variants (ASV). (B) Principal coordinate analysis (PCoA) plot of the bacterial community based on Euclidean distances. (C) Bar plots at phylum level. (D) Heatmap of top 30 genera (relative abundances) among the groups. (E) False discovery rate (FDR) correction of significantly different genera. All results are expressed as mean (SD) in each group. (F) Liner discriminant analysis (LDA) scores (>4.0) computed for features at the ASV level. Letters represented the taxonomy of the bacteria: p, phylum, c, class; o, order; f, family; g, genus. All the values contained 6 repetitions. BA40 = Bacillus amyloliquefaciens 40.
Furthermore, the taxonomy bar plot in phylum level revealed that Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteriota are the prevailing bacteria in the piglet's intestinal microbiota (Fig. 6C and D), and the results of distinguished genera were further analyzed by statical analysis of metagenomic profiles (STAMP) multiple-test correction (Fig. 6E). BA40 treatments enhanced the Bacteroides (P < 0.05), Phascolarctobacterium (P < 0.01), and Desulfovibrio (P < 0.01) in contrast to the control group, while Lactobacillus (P < 0.05), Streptococcus (P < 0.05) and Fusobacterium (P < 0.05) decreased notably. Compared with the probiotics group, Streptococcus (P < 0.05), Clostridium_sensu_stricto_2 (P < 0.05), Tyzzerella (P < 0.01), Veillonella (P < 0.01), Paraeggerthella (P < 0.05) showed a dramatic reduction in the BA40 group, while the Phascolarctobacterium (P < 0.05) increased significantly.
To further analyze the degree of influence of significantly different species (LDA scores >4.0) from phylum to genus level, LEfSe analysis was applied (Fig. 6F). We found that Synerigistaceae, Acidaminococcaceae, Phascolarctobacterium and UCG-004 were predominant in BA40 group. For probiotics group, Bacteroidaceae, Fusobacteriaceae, Bacteroides and Fusobacterium were the dominant species. Lactobacillaceae, Prevotellaceae and Streotococcaceae were the main components of gut microbiota in the control group.
3.6. Prediction of gut microbiota metabolic functions
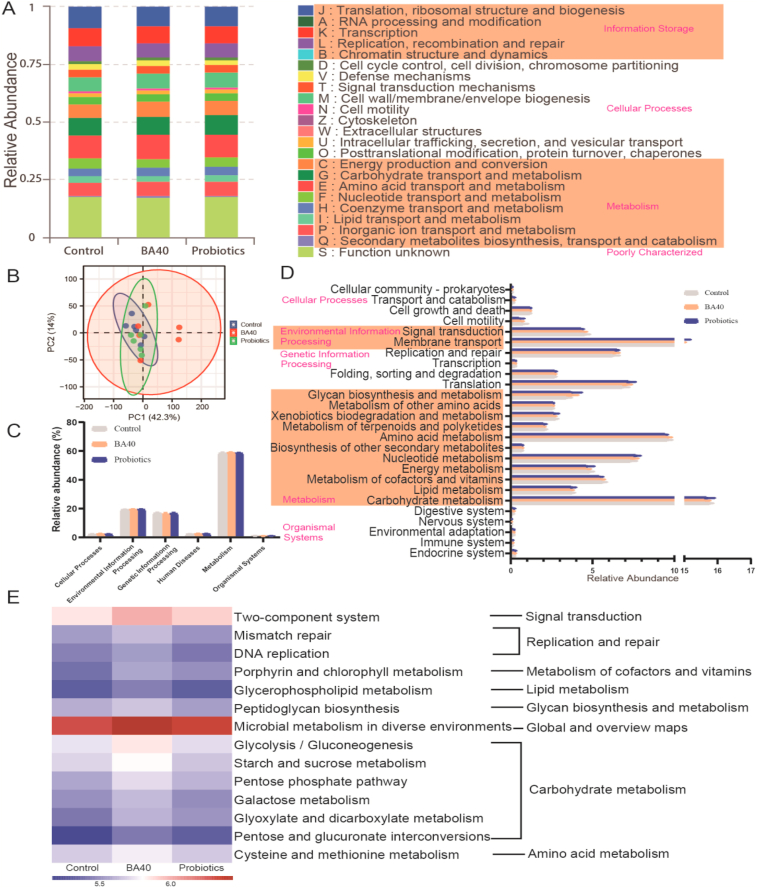
Metabolic function of the gut bacterial communities was predicted using Phylogentic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) based on the Clusters of Orthologous Groups of proteins (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (Fig. 7). As shown in Fig. 7A, the Information Storage (A, B), Cellular Processes (V), Metabolism (G, H, P, Q) were significantly enriched in BA40 group in contrast to control group (P < 0.05). In addition, the probiotics group only shows a dramatic increase in information storage than the control group (B). Other function predictions were exhibited no differences among the 3 groups (P > 0.05).
Fig. 7.
Metagenomic bacterial functional predications analyzed by Phylogentic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2). (A) Clusters of Orthologous Groups of proteins (COG) function classification. (B) Principal component analysis (PCA) plot of bacteria metabolic functions. (C) Bar plot of metabolic pathways at level 1. (D) Bar plot of Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog functional predictions at Level 2. (E) Heatmap of the significantly different microbial functions at level 3. All the values contained 6 repetitions. BA40 = Bacillus amyloliquefaciens 40.
Principal component analysis (PCA) plot showed that the bacterial functions didn't differ among the groups (Fig. 7B). At KEGG function prediction level 1 and level 2 (Fig. 7C and D), no differences (P > 0.05) were observed among the 3 treatments, while 14 pathways at KEGG level 3 notably enriched (P < 0.05) in the BA40 group (Fig. 7E), including signal transduction (two-component system), replication and repair (mismatch repair, DNA replication), metabolism of cofactors and vitamins (porphyrin and chlorophyⅡ metabolism), lipid metabolism (glycerophospholipid metabolism), glycan biosynthesis and metabolism (peptidoglycan biosynthesis), global and overview maps (microbial metabolism in diverse environments), carbohydrate metabolism (glycolysis/gluconeogenesis, starch and sucrose metabolism, pentose phosphate pathway, galactose metabolism, glyoxylate and dicarboxylate metabolism, pentose and glucuronate interconversions), amino acid metabolism (cysteine and methionine metabolism).
3.7. Correlation analysis of gut microbiome, piglet performance, serum and fecal parameters
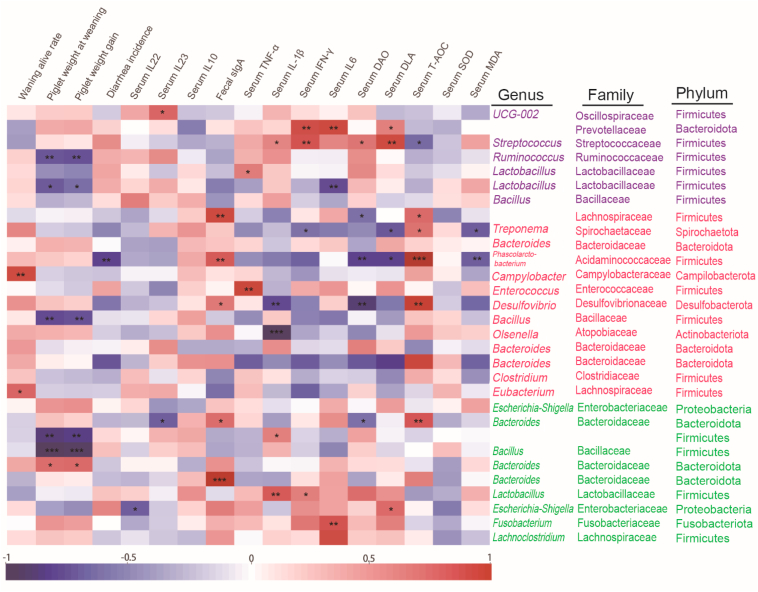
Spearman correlation was further conducted to investigate the relationship among serum and fecal parameters, gut microbiota and piglet growth performance (Fig. 8). As for the control group, certain gut bacteria in piglets (Streptococcus, Ruminococcus, Lactobacillus) were negatively correlated with piglet weight at weaning, piglet weight gain and serum content of T-AOC (P < 0.05), and positively correlated with serum content of IL-22, INF-γ, IF-6, DAO and DLA (P < 0.05). The BA40 treatment enriched Phascolarctobacterium and Dseulfovibro and had a positive relationship with fecal sIgA and serum content of T-AOC (P < 0.05), and a negative relationship with diarrhea incidence, serum content of IL-1β, DAO, DLA and MDA (P < 0.05). Interestingly, Bacillus had a significantly negative correlation with piglet weight at weaning, piglet weight gain (P < 0.05) in both the BA40 and probiotics groups. Bacteroides, Lactobacillus and Fusobacterium had a positive relationship with fecal sIgA, serum inflammatory cytokines (TNF-α, IL-1β, INF-γ) and DLA (P < 0.05).
Fig. 8.
Spearman correlation analysis of gut bacteriome, piglet performance, serum and fecal parameters. Purple: control group; red: BA40 group; green: probiotics group. Significant correlation is represented by ∗∗∗P < 0.001, ∗∗0.001 < P < 0.01, ∗0.01 < P < 0.05 respectively. All the values contained 6 repetitions.
4. Discussion
Due to the emergence of antibiotic-resistant bacteria and antibiotic residues, the use of antibiotics in feed are banned in many countries (Martin et al., 2015). Hence, alternatives to antibiotics, such as probiotics have been widely used in animal feed, because they could maintain the balance of microbiota, enhance digestive capacity, improve the mucosal immunity and inhibit the proliferation of pathogens in the intestine (Callaway et al., 2008; Wang et al., 2016, 2021; Du et al., 2018). Furthermore, the early intervention of intestinal flora is considered to be an emerging method to relieve diarrhea in piglets caused by weaning stress (El Aidy et al., 2013; Dou et al., 2017) and the dietary alternatives also are a preventive strategy for effectively controlling post-weaning diarrhea (Tao et al., 2015; Gresse et al., 2017). In the present study, the supplementation of BA40 during sucking period improved the performance of piglets, whilst relieving diarrhea from d 5 to 40. In addition, BA40 modulated immunity and gut microbial composition of piglets compared to the control group, which was positively associated with a reduction in the incidence of diarrhea.
A large number of studies have investigated the beneficial effects of probiotics on relieving weaning stress and reducing diarrhea (Cao et al., 2019; Wang et al., 2019a; Xin et al., 2020). Our study showed similar results as BA40 could effectively decrease the diarrhea incidence in both sucking piglets and post-weaning pigs compared to the control group and a commercial probiotic product, Glyco Guard. Weaning stress is usually caused by oxidative stress (Campbell et al., 2013), and the major antioxidant defense mechanisms are composed of antioxidant enzymes and biological antioxidants, including T-AOC, SOD (Riedl et al., 2009; Wang et al., 2019b). MDA was a metabolite from lipid peroxidation, and a biomarker for oxidative stress (Chen et al., 2014). The improved T-AOC and SOD concentrations reflect the increase of antioxidant capacity in host. Our study agrees with previous studies (Wang et al., 2017a; Hu et al., 2018; Wu et al., 2020), which suggested that BA40 could improve the T-AOC and reduce MDA concentrations in serum to enhance the antioxidant ability. Probiotics have their own antioxidant enzymatic systems, one of the best-known enzymes is SOD (Wang et al., 2017c). Harmful superoxide could be catalyzed by SOD into hydrogen peroxide and water, so SOD concentration can reflect the antioxidant ability. Previous research implied that Bacillus amyloliquefaciens SC06 elevated the antioxidant enzyme activity in the intestinal porcine epithelial cells-1 (IPEC-1) (Wang et al., 2017b). Meanwhile, many studies have revealed that high levels of DAO and DLA in serum cause intestinal barrier injury or intestinal permeability (Chen et al., 2015; Yi et al., 2016). Also, ZJUAF-4 releases the oxidative stress-induced by diquat in mice due to decreasing the DLA and DAO activity (Hao et al., 2021). These results suggest that BA40 reduces the incidence of diarrhea because the probiotics contained in BA40 may possess their own antioxidant system which helps the host to defense the oxidative stress.
Serum inflammatory Cytokines reflect the physiological and immune status of pigs during the suckling period until weaning. Lower concentrations of IL-1β and INF-γ and high level of IL-10 were observed for piglets supplemented with BA40, compared with the control group, while the probiotics group had no positive effects on serum parameters. Serum IL-1β, TNF-α and INF-γ are indicators of pro-inflammatory reactions, while IL-10, IL-22 suggests an anti-inflammatory effect (Mizoguchi et al., 2018; Zhou et al., 2019). In addition, secretory immunoglobulin A (sIgA) is the most abundant colonic antibody antigen known as “immune exclusion” and could improve the immune status of animals (Palm et al., 2014). We found that both the BA40 group and the probiotics group increased the fecal sIgA concentration. Overall, BA40 has the potential to alleviate inflammation in piglets and improve intestinal immunity.
Probiotics can positively regulate the composition of intestinal microorganisms (Cisek and Binek, 2014; Kristensen et al., 2016; Hu et al., 2017). Similar alpha diversity (Chao1, Shannon, Simpson index) was observed in the gut microbiota of the 3 groups in our study (Table 1). However, there was a significant difference in beta diversity in the BA40 group compared to the control and probiotic groups (p = 0.017, Fig. 6B). Shenglan Hu et al. (2018) reported that there were no differences of Chao 1 and Shannon diversity indexes, while a significant increase of β diversity was demonstrated with dietary supplementation of Bacillus amyloliquefaciens during d 6 to d 18. Fecal microbial communities may consist of several bacteria as biomarkers which could help humans to detect inflammatory bowel disease (Durban et al., 2011). In the phylum level of bacterial composition (Fig. 6C), Synergistota increased dramatically after the BA40 treatment in contrast with the control and probiotics groups. It demonstrates that syneigistota is positively related to the degradation of amino acids (Kuroda et al., 2021). We observed that the relative abundance of Desulfobacterota at the phylum level increased in both the BA40 and probiotics groups compared to the control group. Most members of the Desulfobacterota perform an important role in oxidizing organic substrates completely and some members of it exert another function to form acetate as an end product by incomplete oxidation (Kuever et al., 2015). Acetate, one of the major gut microbial metabolites, can increase the production of IgA in the colon to maintain mucosal homeostasis, so the increase of Desulfobacterota has the positive effect on piglet gut barrier and immunity (Takeuchi et al., 2021).
Gut microbiota of the 3 groups at the genus level can further illustrate the mechanisms by which BA40 relieved the incidence of diarrhea (Fig. 6D and E). Probiotics protect their host from pathogens by altering the gut microbiota composition (competitive exclusion), including occupation of attachment sites, consumption of nutrient sources preferred by pathogens, and production of antimicrobial substances (Sekirov et al., 2010). In our study, compared to the control group, BA40 significantly reduced the relative abundance of pathogens, such as Streptococcus and Fusobacterium. Streptococcus is a diverse genus, infecting different animals as well as humans, with diseases ranging from strep throat to necrotizing fasciitis (Feng et al., 2014). Some research reported that Fusobacterium is widely known and studied as a human and animal pathogen and mice treated with Fusobacterium induced disruption of the colonic architecture, with immune cell infiltration and depleted mucus layers (Creemers-Schild et al., 2014; Yu et al., 2017; Engevik et al., 2021). These harmful pathogens will proliferate excessively when the intestinal microbiota is abnormal, and the endotoxins secreted by these pathogens will enter and injur intestinal cells resulting in reduced intestinal fluid absorption and accelerated intestinal fluid secretion. The potential mechanism of BA40 decreasing the incidence of diarrhea may inhibit the proliferation of pathogens and utilize the nutrient sources preferred by these pathogens, resulting in competitive exclusion. Results of our study are similar to that of Lei et al. (2015) who used dietary supplementation of Bacillus amyloliquefaciens to significantly decrease the relative abundance of Escherichia coli in the cecum and reduce the incidence of diarrhea. In contrast, some bacteria which can produce beneficial metabolites, such as Bacteroides, Phascolarctobacterium and Desulfovibrio, dramatically increased in the BA40 group in contrast to the control group in the current study. The main by-products of Bacteroides anaerobic respiration are acetic acid, isovaleric acid and succinic acid and Bacteroides are involved in many important metabolic activities in the colon, including fermentation of carbohydrates, utilization of nitrogenous substances (Wexler, 2007; Porter et al., 2018). Phascolarctobacterium can produce short-chain fatty acids, including acetate and propionate. Phascolarctobacterium species are distributed widely in the gastrointestinal tract that may utilize succinate generated by other bacteria, such as Bacteroides, to adapt to the intestinal environment (Watanabe et al., 2012; Stackebrandt and Osawa, 2015; Wu et al., 2017; Ikeyama et al., 2020). Desulfovibrio is known as a sulfate reducing bacterium and this microorganism also shows potential for bioremediation (Rowan et al., 2010). Additionally, in the current study, Desulfovibrio, Tyzzerella, Veillonella, Clostridioides and Actinobacillus increased in the probiotics group in contrast to the control group. Tyzzerella increases markedly in the Crohn's disease patients and is related to inflammatory bowel disease (Olaisen et al., 2021). Veillonella has limited pathogenicity and it is often associated with oral infections, bite wounds, head, neck and various soft tissue infections (van den Bogert et al., 2013). For Clostridioides, they are known to produce a variety of toxins, some of which are fatal (Guo et al., 2020). Actinobacillus spp. has been suggested to cause abortion, metritis and decrease litter sizes in pigs (Rycroft and Garside, 2000; Givens and Marley, 2008). Furthermore, the extended bar-plot (Fig. 6E) showed that the Phascolarctobacterium enhanced significantly and the potential pathogens declined notably in the BA40 group. All these results suggest that BA40 may reduce the incidence of diarrhea by inhibiting pathogenic microorganisms in the gut and promoting the proliferation of probiotic bacteria (e.g. short-chain fatty acid producing bacteria) compared to the control group. The LDA plot (Fig. 6F) presented that the Synergistaceae, Cloacibacillus and Phascolarctobacterium made the main contribution in BA40 group. The genus of Cloacibacillus contains a mucin-degrading bacterium from the pig intestinal tract which could ferment amino acid as a probiotic (Looft et al., 2013). However, in the probiotics group, Bacteroides and Fusobacterium became the predominant members of microbial community. Meanwhile, the Lactobacillus and Streptococcus made the greatest contributions in the control group. A study reported that the defensive barrier of the stomach and intestine was impaired as Streptococcus suis became dominant (Su et al., 2008). The high relative abundance of Streptococcus could illustrate the control group had the highest diarrhea incidence from d 5 to 40. In summary, BA40 increased the abundance of Bacteroides, Phascolarctobacterium and Desulfovibrio, and these bacteria can produce various metabolites (short-chain fatty acids) to improve the antioxidant ability and active anti-inflammatory signaling pathway (Zong et al., 2020). Meanwhile, BA40 regulated gut microbiota by reducing the number of potential pathogens (Streptococcus, Tyzzerella, Vellionella and paraeggerthella) to create a stable intestinal environment, leading to decreased incidence of diarrhea.
This study showed changes in the metabolic function prediction of piglet intestinal microbiota when BA40 or a commercial probiotic product was given to weaner piglets. COG and KEGG functional prediction results explained that carbohydrate metabolism and amino acid metabolism increased in the BA40 group, which predicted the improved digestibility of carbohydrate and protein. Especially, the increase in starch and sucrose metabolism, pentose phosphate pathway and cysteine and methionine metabolism in the BA40 group indicated that nutrients (proteins, sugars) were degraded as metabolites to fuel host growth and synthesize essential compounds to defense pathogen invasion (Ruan, 2014; Zhou et al., 2018; Cao et al., 2019). Starch and sucrose metabolism plays critical roles in the stress response, and acts to regulate the expression of transcription factors for crosstalk with hormonal, oxidative and defense signaling (Ruan, 2014). The pentose phosphate pathway (PPP) is one of the main antioxidant defense, and PPP produces metabolites and cofactors necessary for the synthesis of glutathione (GSH), for the detoxification processes, for DNA duplication (Riganti et al., 2012). Methionine metabolism is used for methylation and cysteine production, and it also intervenes in lipid metabolism, activation of endogenous antioxidant enzymes and the biosynthesis of GSH to counteract oxidative stress (Martinez et al., 2017). Some studies show that restricting methionine stimulates the production of GSH and reduces oxidative stress (Sayed Abdoullah Hosseini et al., 2012; Kitada et al., 2021). It is known that beneficial gut microorganisms affect the digestion of proteins and amino acid metabolism, and the efficiency of protein digestion and amino acid absorption is limited by the catabolism of luminal microbes (Albers, 2009; Fang et al., 2010). The function prediction presented the benefits of BA40 which may have improved starch and amino acid metabolism by altering the gut microbial composition or its metabolites. Also, DNA mismatch repair, DNA replication, glycolysis/gluconeogenesis and glycerophospholipid metabolism were significantly upregulated in the BA40 group. BA40 may alter the gut microbial communities to enhance repair and replication by increasing the antioxidant ability because oxidative stress disturbs the balance between prooxidant–antioxidant in cells, resulting in DNA hydroxylation and DNA damage (Schieber and Chandel, 2014). In general, the metabolic function prediction of the BA40 group could illustrate that BA40 could activate oxidative and defense signaling to alter gut microorganisms and enhance amino acid metabolism. This, in turn, could generate GSH to relieve oxidative stress, resulting in a decrease of the incidence of diarrhea.
The correlation analysis demonstrated an underlying effect between the gut microbiota, production performance and serum parameters in weaner piglets. Notably, the results showed that in the BA40 group, the microbiota was positively associated with a decrease in the incidence of diarrhea and modulation of serum indicators. Feed Bacillus subtilis positively re-established the microbiota and reduced the incidence of diarrhea (Hu et al., 2014). These results indicate that BA40 can benefit the microbial community and improve performance. Dietary probiotics including Bacillus subtilis (Hong et al., 2005), Bacillus licheniformis (Yue et al., 2020) and Clostridium butyricum (Cao et al., 2019) were found to promote the immune status and decrease the diarrhea incidence of weaned piglets. The BA40-enriched Phascolarctobacterium and Desulfovibrio could act as one of the solutions to manage the health status and performance of weaner piglets.
5. Conclusion
The present study demonstrated that BA40 decreased the incidence of diarrhea and improved the antioxidant activity, gut barrier function and immune status of piglets. BA40 also regulated microbiota with increasing Bacteroides, Phascolarctobacterium (producing short-chain fatty acids) and Desulfovibrio and decreasing the abundance of pathogens (Streptococcus, Tyzzerella, Vellionella and paraeggerthella). Furthermore, the metabolic function prediction results provided a potential mechanism that BA40 enhanced starch and sucrose metabolism, pentose phosphate pathway and cysteine and methionine metabolism to improve the antioxidant ability.
Author contributions
Zipeng Jiang: Conceptualization, Methodology, Investigation, Writing - original draft. Weifa Su, Wentao Li, Chaoyue Wen and Shuai Du: Investigation, Visualization. Yu Zhang, Huan He and Tao Gong: Formal analysis, Visualization. Xinxia Wang, Yizhen Wang and Mingliang Jin: Writing - review & editing. Zeqing Lu: Resources, Writing - review and editing, Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This research was supported by the fund from Science and Technology Projects of Zhejiang (2021C02008, CTZB-2020080127), China Agricultural Research System (CARS-35), National Center of Technology Innovation for Pigs.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5'-methylthioadenosine. IUBMB Life. 2009;61(12):1132–1142. doi: 10.1002/iub.278. [DOI] [PubMed] [Google Scholar]
- Callaway T.R., Edrington T.S., Anderson R.C., Harvey R.B., Genovese K.J., Kennedy C.N., Venn D.W., Nisbet D.J. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev. 2008;9(2):217–225. doi: 10.1017/S1466252308001540. [DOI] [PubMed] [Google Scholar]
- Cao G., Tao F., Hu Y., Li Z., Zhang Y., Deng B., Zhan X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019;10(5):2926–2934. doi: 10.1039/c8fo02370k. [DOI] [PubMed] [Google Scholar]
- Chen Q., Yu W., Shi J., Shen J., Hu Y., Gong J., Li J., Li N. The effect of extracorporeal membrane oxygenation therapy on systemic oxidative stress injury in a porcine model. Artif Organs. 2014;38(5):426–431. doi: 10.1111/aor.12198. [DOI] [PubMed] [Google Scholar]
- Chen Y., Miao L., Yao Y., Wu W., Wu X., Gong C., Qiu L., Chen J. Dexmedetomidine ameliorate CLP-induced rat intestinal injury via inhibition of inflammation. Mediat Inflamm. 2015;2015 doi: 10.1155/2015/918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Liu P., Zhou G., Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc. 2020;15(3):799–821. doi: 10.1038/s41596-019-0264-1. [DOI] [PubMed] [Google Scholar]
- Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol J Vet Sci. 2014;17(2):385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
- Creemers-Schild D., Spanjaard F.G.L., Visser L.G., Brouwer C.N.M., Kuijper E.J. Fusobacterium necrophorum, an emerging pathogen of otogenic and paranasal infections? New Microbes New Infect. 2014;2:52–57. doi: 10.1002/nmi2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Li Y., Zhang J., Yang Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res Vet Sci. 2013;94(1):62–68. doi: 10.1016/j.rvsc.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Dou S., Gadonna-Widehem P., Rome V., Hamoudi D., Rhazi L., Lakhal L., Larcher T., Bahi-Jaber N., Pinon-Quintana A., Guyonvarch A., Huerou-Luron I.L., Abdennebi-Najar L. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Xu H., Mei X., Cao X., Gong L., Wu Y., Li Y., Yu D., Liu S., Wang Y., Li W. Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benef Microbes. 2018;9(5):743–754. doi: 10.3920/BM2017.0142. [DOI] [PubMed] [Google Scholar]
- Durban A., Abellan J.J., Jimenez-Hernandez N., Ponce M., Ponce J., Sala T., D'Auria G., Latorre A., Moya A. Assessing gut microbial diversity from feces and rectal mucosa. Microb Ecol. 2011;61(1):123–133. doi: 10.1007/s00248-010-9738-y. [DOI] [PubMed] [Google Scholar]
- El Aidy S., Hooiveld G., Tremaroli V., Backhed F., Kleerebezem M. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microb. 2013;4(2):118–124. doi: 10.4161/gmic.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Yao K., Zhang X., Zhao S., Sun Z., Tian G., Yu B., Lin Y., Zhu B., Jia G., Zhang K., Chen D., Wu D. Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids. 2010;39(3):633–640. doi: 10.1007/s00726-010-0502-x. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhang H., Wu Z., Wang S., Cao M., Hu D., Wang C. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence. 2014;5(4):477–497. doi: 10.4161/viru.28595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foligne B., Daniel C., Pot B. Probiotics from research to market: the possibilities, risks and challenges. Curr Opin Microbiol. 2013;16(3):284–292. doi: 10.1016/j.mib.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Rycroft A.N., Garside L.H. Actinobacillus species and their role in animal disease. Vet J. 2000;159:18–36. doi: 10.1053/tvjl.1999.0403. [DOI] [PubMed] [Google Scholar]
- Givens M.D., Marley M.S. Infectious causes of embryonic and fetal mortality. Theriogenology. 2008;70(3):270–285. doi: 10.1016/j.theriogenology.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25(10):851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Guo P., Zhang K., Ma X., He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Cheng Y., Su W., Wang C., Lu Z., Jin M., Wang F., Wang Y. Pediococcus pentosaceus ZJUAF-4 relieves oxidative stress and restores the gut microbiota in diquat-induced intestinal injury. Appl Microbiol Biotechnol. 2021;105(4):1657–1668. doi: 10.1007/s00253-021-11111-6. [DOI] [PubMed] [Google Scholar]
- Hong H.A., Duc le H., Cutting S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev. 2005;29(4):813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hu S., Cao X., Wu Y., Mei X., Xu H., Wang Y., Zhang X., Gong L., Li W. Effects of probiotic Bacillus as an alternative of antibiotics on digestive enzymes activity and intestinal integrity of piglets. Front Microbiol. 2018;9:2427. doi: 10.3389/fmicb.2018.02427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Wang L., Jiang Z. Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept Lett. 2017;24(5):382–387. doi: 10.2174/0929866524666170223143615. [DOI] [PubMed] [Google Scholar]
- Hu Y., Dun Y., Li S., Zhao S., Peng N., Liang Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and Faecal bacterial flora of weaned piglets. Asian-Australas J Anim Sci. 2014;27(8):1131–1140. doi: 10.5713/ajas.2013.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Sun W., Yan Z., Shi H., Yang Q., Wang P., Li S., Liu L., Zhao S., Gun S. Integrative analyses of long non-coding RNA and mRNA involved in piglet ileum immune response to Clostridium perfringens type C infection. Front Cell Infect Microbiol. 2019;9:130. doi: 10.3389/fcimb.2019.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yang Q., Yuan J., Liu L., Sun W., Jiang Y., Zhao S., Zhang S., Huang W., Gun S. Effect of genetic diversity in swine leukocyte antigen-DRA gene on piglet diarrhea. Genes. 2016;7(7) doi: 10.3390/genes7070036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama N., Murakami T., Toyoda A., Mori H., Iino T., Ohkuma M., Sakamoto M. Microbial interaction between the succinate-utilizing bacterium Phascolarctobacterium faecium and the gut commensal Bacteroides thetaiotaomicron. Microbiologyopen. 2020;9(10):e1111. doi: 10.1002/mbo3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Li W., Su W., Wen C., Gong T., Zhang Y., Wang Y., Jin M., Lu Z. Protective effects of Bacillus amyloliquefaciens 40 against Clostridium perfringens infection in mice. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.733591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Joy M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4(19) doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Ogura Y., Monno I., Xu J., Koya D. Effect of methionine restriction on aging: its relationship to oxidative stress. Biomedicines. 2021;9(2) doi: 10.3390/biomedicines9020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuever J., Rainey F.A., Widdel F. 2015. Desulfobacterales ord. nov, bergey's manual of systematics of archaea and bacteria; pp. 1–2. [Google Scholar]
- Kuroda K., Narihiro T., Nobu M.K., Tobo A., Yamauchi M., Yamada M. Ecogenomics reveals microbial metabolic networks in a psychrophilic methanogenic bioreactor treating soy sauce production wastewater. Microb Environ. 2021;36(4) doi: 10.1264/jsme2.ME21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Piao X., Ru Y., Zhang H., Peron A., Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australas J Anim Sci. 2015;28(2):239–246. doi: 10.5713/ajas.14.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looft T., Levine U.Y., Stanton T.B. Cloacibacillus porcorum sp. nov., a mucin-degrading bacterium from the swine intestinal tract and emended description of the genus Cloacibacillus. Int J Syst Evol Microbiol. 2013;63(Pt 6):1960–1966. doi: 10.1099/ijs.0.044719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M.J., Thottathil S.E., Newman T.B. Antibiotics overuse in animal agriculture: a call to action for health Care providers. Am J Publ Health. 2015;105(12):2409–2410. doi: 10.2105/AJPH.2015.302870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Y., Li X., Liu G., Bin P., Yan W., Mas D., Valdivie M., Hu C.A., Ren W., Yin Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49(12):2091–2098. doi: 10.1007/s00726-017-2494-2. [DOI] [PubMed] [Google Scholar]
- Engevik Melinda A., Danhof H.A., Ruan Wenly, Engevik Amy C., Chang-Graham Alexandra L., Engevik Kristen A., Shi Zhongcheng, Zhao Yanling, Brand Colleen K., Krystofiak Evan S., Venable Susan, Liu Xinli, Hirschi Kendal D., Hyser Joseph M., Spinler Jennifer K., Britton Robert A., Versalovic James. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio. 2021;12(2) doi: 10.1128/mbio.02706-20. e02706-02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Yano A., Himuro H., Ezaki Y., Sadanaga T., Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53(4):465–474. doi: 10.1007/s00535-017-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola Segata J.I., Waldron1 Levi, Gevers Dirk, Miropolsky Larisa, Garrett Wendy S., Curtis Huttenhower. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-6-r60. R60doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaisen M., Flatberg A., Granlund A.V.B., Royset E.S., Martinsen T.C., Sandvik A.K., Fossmark R. Bacterial mucosa-associated microbiome in inflamed and proximal noninflamed ileum of patients with crohn's disease. Inflamm Bowel Dis. 2021;27(1):12–24. doi: 10.1093/ibd/izaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm N.W., de Zoete M.R., Cullen T.W., Barry N.A., Stefanowski J., Hao L., Degnan P.H., Hu J., Peter I., Zhang W., Ruggiero E., Cho J.H., Goodman A.L., Flavell R.A. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N.T., Luis A.S., Martens E.C. Bacteroides thetaiotaomicron. Trends Microbiol. 2018;26(11):966–967. doi: 10.1016/j.tim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Riedl M.A., Saxon A., Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130(3):244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C., Gazzano E., Polimeni M., Aldieri E., Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53(3):421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Edgar Robert C., Flyvbjerg H. Error filtering, pair assembly, and error correction for next-generation sequencing reads. Bioinformatics. 2015 doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., Mahe F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4 doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan F., Docherty N.G., Murphy M., Murphy B., Calvin Coffey J., O'Connell P.R. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53(11):1530–1536. doi: 10.1007/DCR.0b013e3181f1e620. [DOI] [PubMed] [Google Scholar]
- Ruan Y.L. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65:33–67. doi: 10.1146/annurev-arplant-050213-040251. [DOI] [PubMed] [Google Scholar]
- Sayed Abdoullah Hosseini M.Z., Lotfollahian Houshang, Shivazad Mahmoud, Moravaj Hussein. Reevaluation of methionine requirement based on performance and immune responses in broiler breeder hens. Jpn Poultry Sci Assoc. 2012;49(1):26–33. doi: 10.2141/jpsa.011021. [DOI] [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Osawa R. 2015. Phascolarctobacterium, bergey's manual of systematics of archaea and bacteria; pp. 1–4. [Google Scholar]
- Su W., Jiang Z., Hao L., Li W., Gong T., Zhang Y., Du S., Wang C., Lu Z., Jin M., Wang Y. Variations of soybean meal and corn mixed substrates in physicochemical characteristics and microbiota during two-stage solid-state fermentation. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.688839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Yao W., Perez-Gutierrez O.N., Smidt H., Zhu W.Y. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol Ecol. 2008;66(3):546–555. doi: 10.1111/j.1574-6941.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Miyauchi E., Kanaya T., Kato T., Nakanishi Y., Watanabe T., Kitami T., Taida T., Sasaki T., Negishi H., Shimamoto S., Matsuyama A., Kimura I., Williams I.R., Ohara O., Ohno H. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature. 2021;595(7868):560–564. doi: 10.1038/s41586-021-03727-5. [DOI] [PubMed] [Google Scholar]
- Tao X., Xu Z., Wan J. Intestinal microbiota diversity and expression of pattern recognition receptors in newly weaned piglets. Anaerobe. 2015;32:51–56. doi: 10.1016/j.anaerobe.2014.12.005. [DOI] [PubMed] [Google Scholar]
- van den Bogert B., Erkus O., Boekhorst J., de Goffau M., Smid E.J., Zoetendal E.G., Kleerebezem M. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol. 2013;85(2):376–388. doi: 10.1111/1574-6941.12127. [DOI] [PubMed] [Google Scholar]
- van Zyl W.F., Deane S.M., Dicks L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microb. 2020;12(1) doi: 10.1080/19490976.2020.1831339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhou Y., Tang L., Zeng Z., Gong L., Wu Y., Li W.F. Effects of Bacillus amyloliquefaciens instead of antibiotics on growth performance, intestinal health, and intestinal microbiota of broilers. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.679368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Cao G., Zhang H., Li Q., Yang C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019;10(12):7844–7854. doi: 10.1039/c9fo01650c. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Wang B., Mei X., Jiang S., Li W. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poultry Sci. 2019;98(8):3138–3149. doi: 10.3382/ps/pez124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu Y., Wang B., Cao X., Fu A., Li Y., Li W. Effects of probiotic Bacillus as a substitute for antibiotics on antioxidant capacity and intestinal autophagy of piglets. Amb Express. 2017;7(1):52. doi: 10.1186/s13568-017-0353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu Y., Wang Y., Fu A., Gong L., Li W., Li Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl Microbiol Biotechnol. 2017;101(7):3015–3026. doi: 10.1007/s00253-016-8032-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu D., Wang Y., Li W. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5) doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.B., Du W., Fu A.K., Zhang X.P., Huang Y., Lee K.H., Yu K., Li W.F., Li Y.L. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef Microbes. 2016;7(4):529–538. doi: 10.3920/BM2015.0099. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Nagai F., Morotomi M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl Environ Microbiol. 2012;78(2):511–518. doi: 10.1128/AEM.06035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Guo Q., Wang W., Duan Y., Zhang L., Li J., He S., Chen W., Li F. Taurine alleviates intestinal injury by mediating tight junction barriers in diquat-challenged piglet models. Front Physiol. 2020;11:449. doi: 10.3389/fphys.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Li F., Guo Q., Zhang L., Duan Y., Wang W., Li J., He S., Chen W., Yin Y. Protective effects of taurine against muscle damage induced by diquat in 35 days weaned piglets. J Anim Sci Biotechnol. 2020;11:56. doi: 10.1186/s40104-020-00463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H.M. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Guo X., Zhang J., Zhang M., Ou Z., Peng Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp Ther Med. 2017;14(4):3122–3126. doi: 10.3892/etm.2017.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu H., Cao X., Liu R., Tang L., Zeng Z., Li W. Bacillus amyloliquefaciens ameliorates H2O2-induced oxidative damage by regulating transporters, tight junctions, and apoptosis gene expression in cell line IPEC-1. Probiotics Antimicrob Proteins. 2020;12(2):649–656. doi: 10.1007/s12602-019-09538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J., Zeng D., Wang H., Sun N., Zhao Y., Dan Y., Pan K., Jing B., Ni X. Probiotic Lactobacillus johnsonii BS15 promotes growth performance, intestinal immunity, and gut microbiota in piglets. Probiotics Antimicrob Proteins. 2020;12(1):184–193. doi: 10.1007/s12602-018-9511-y. [DOI] [PubMed] [Google Scholar]
- Yi H., Zhang L., Gan Z., Xiong H., Yu C., Du H., Wang Y. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci Rep. 2016;6 doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Guo F., Yu Y., Sun T., Ma D., Han J., Qian Y., Kryczek I., Sun D., Nagarsheth N., Chen Y., Chen H., Hong J., Zou W., Fang J.Y. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3) doi: 10.1016/j.cell.2017.07.008. 548–563 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S., Li Z., Hu F., Picimbon J.F. Curing piglets from diarrhea and preparation of a healthy microbiome with Bacillus treatment for industrial animal breeding. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-75207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Xiong X., Yin J., Zou L., Wang K., Shao Y., Yin Y. Dietary lysozyme alters sow's gut microbiota, serum immunity and milk metabolite profile. Front Microbiol. 2019;10:177. doi: 10.3389/fmicb.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Yan Y., Mi J., Zhang H., Lu L., Luo Q., Li X., Zeng X., Cao Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J Agric Food Chem. 2018;66(4):898–907. doi: 10.1021/acs.jafc.7b05546. [DOI] [PubMed] [Google Scholar]
- Zong X., Fu J., Xu B., Wang Y., Jin M. Interplay between gut microbiota and antimicrobial peptides. Anim Nutr. 2020;6(4):389–396. doi: 10.1016/j.aninu.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]