Highlights
-
•
Adaptive radiotherapy offers potential clinical benefits for breast cancer patients.
-
•
Advantages of MR-guided adaptive radiotherapy are expected in the neoadjuvant setting.
-
•
Autonomous aritifical intellingence-driven technologies might further enable adaptive radiotherapy.
Keywords: Breast cancer, Adaptive radiotherapy, Partial breast irradiation, MR-linac
Abstract
Research in the field of local and locoregional breast cancer radiotherapy aims to maintain excellent oncological outcomes while reducing treatment-related toxicity. Adaptive radiotherapy (ART) considers variations in target and organs at risk (OARs) anatomy occurring during the treatment course and integrates these in re-optimized treatment plans. Exploiting ART routinely in clinic may result in smaller target volumes and better OAR sparing, which may lead to reduction of acute as well as late toxicities. In this review MR-guided and CT-guided ART for breast cancer patients according to different clinical scenarios (neoadjuvant and adjuvant partial breast irradiation, whole breast, chest wall and regional nodal irradiation) are reviewed and their advantages as well as challenging aspects discussed.
Introduction
Breast cancer is the most common cancer among women, with over 350.000 new cases in Europe in 2020 and an incidence of more than 140 per 100.000 [1]. Patients diagnosed with early stage breast cancers have a very favourable prognosis with overall survival rates of 98 % for stage I and 92 % for stage II, while three quarters of patients diagnosed with stage III are still alive at 5 years [2]. Real-world data report that 63 % of patients diagnosed with local or loco-regional breast cancer receive breast conserving surgery and 37 % undergo mastectomy [3]. As radiotherapy is a standard treatment in the breast conserving approach and a possible indication according to risk factors after mastectomy, most breast cancer patients receive radiotherapy as part of their curative treatment. The number of patients affected by breast cancer needing radiotherapy is expected to increase by 10 % from 2012 to 2025 in Europe [4].
Besides improving oncological outcomes, developments in radiotherapy aim to reduce acute and long-term toxicities, the latter being particularly important for breast cancer patients, given the high rate of curability. Adaptive radiotherapy (ART) intends to adjust the treatment through a re-optimization of the radiation plan taking into account variations occurring during the therapy course, such as changes in patients‘ anatomy, organs at risk (OARs) or treatment target [5]. This has been shown to improve both target coverage and OAR sparing for treatments in the abdominal region, pelvis, lung and head and neck [6] and might allow dose escalation strategies.
External radiotherapy for breast cancer is usually performed over 3 to 6 weeks, whereas new data suggest that five fractions administered over one week might become the new standard [7]. Before irradiation, portal images or cone beam CTs are acquired and used to correct variations in patient positioning. These image guidance methods cannot correct for interfraction variability such as changes in the breast (e.g. swelling, seroma modifications), a different breathing pattern compared to the planning CT or a different arm position. Additionally, intrafractional target motion has also been described [8], [9], [10]. Therefore, safety margins of one cm or more are used to compensate and avoid missing the target. For breast cancers, ART might allow margin reduction and might consequently reduce the volume of lung, heart and chest wall musculature being irradiated. In addition, when performing partial breast irradiation (PBI), it will facilitate volume reduction of healthy breast tissue irradiated. Since the curve of the dose–response for late normal tissue effects for the breast is very steep [11] even a small reduction of the dose to the breast tissue might favourably affect late toxicities and cosmetic outcomes [7]. Regarding the heart, data showed that the rate of major coronary events increases linearly with the mean heart dose by 7.4 % per Gray [12] and might even reach 19 % per Gray [13]. The incidence of clinically relevant radiation-induced lung injury (pneumonitis, fibrosis) after breast irradiation has lowered over time, with recent data indicating rates of 2–3 %, especially when hypofractionated regimens as well as new techniques are used [14]. Besides breast, heart and lung toxicity, additional concerns when treating breast cancer patients are represented by secondary radiation-induced cancers. For breast cancer patients, Surveillance, Epidemiology, and End Results (SEER) data analysis reported an absolute excess risk for a second cancer of 35 per 10,000 patient-years for irradiated patients compared to 23 for non-irradiated patients [15]. Considering these data and that long-term toxicities have no dose threshold, ART represents a potential benefit for breast cancer patients. In this review, we will discuss MR- and CT-based ART for breast cancer patients in distinct clinical scenarios, reviewing advantages as well as challenges in each.
MR-based ART
Adjuvant PBI
In the context of PBI, a correct target visualization is essential. Compared to CT images, MR offers superior soft tissue contrast. In the adjuvant setting, MR might allow better visualization of the tumor bed, namely the postoperative breast tissue changes and the seroma, which appears bright on T2W sequences (Fig. 1). This is particularly important when the resection cavity has no distinct margins (score 2–3, Cavity visualization score [16]), or no cavity can be recognized (score 1) such that it is difficult to correctly delineate the tumor bed on CT images [17]. MR has been shown in different studies to be superior to CT for clearer visualization of post-lumpectomy changes, diminishing interobserver contouring variability and improving clinical target volume (CTV) delineation accuracy [18], [19], also allowing for smaller CTVs [20], [21]. Nevertheless, no benefit by adding MR in terms of consistency of target delineation has been described [21], [22], [23] and some studies showed that the CTVs based on MR might be even larger than those based on CT images, mostly because postoperative breast tissue changes visible on MR imaging could not be recognized on CT images [24], [25]. Therefore, even if MR appears helpful in defining the adjuvant target volumes when no clear resection cavity (i.e. non-visible seroma, clips not present) is present, published data are conflicting and the general value of an MR-based CTV definition remains nowadays controversial.
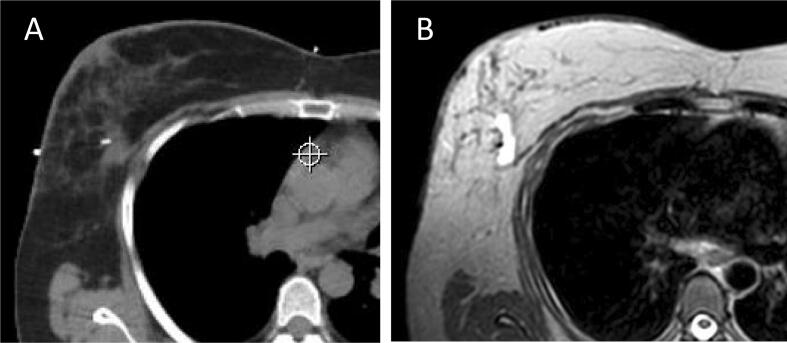
Fig. 1.
A: Planning CT: clip is bright and visible, whereas seroma can hardly be recognized. B: Planning MR T2W sequence: clip is visible as signal void, seroma is bright, can be distinguished from the rest of the gland and pectoral muscle.
MR-based ART might allow for smaller planning target volumes (PTVs). PTV margins up to 10 mm have been calculated as being necessary to take into account variations in tumor bed position during fractionated radiotherapy [26]. These might be reduced when a daily plan adaptation is performed [27]. A PTV margin reduction might be particularly attractive when oncoplastic surgery has been performed following which the CTV is frequently larger than it would be without oncoplastic surgery, because part of the breast gland has been mobilized leading to tissue that was adjacent to the cancer becoming more widely displaced. Another scenario where a daily MR-based plan adaptation might offer advantages is when a seroma cavity is present. Seroma can be well identified on MR, especially in the T2W sequences, while it is rather difficult to recognize it on cone beam CT images (Fig. 2). A shrinkage of the excision cavity volume of more than 60 % mainly due to reduction of the seroma has been observed [28] and dosimetric advantages of a plan adaptation around the middle of the treatment course have been described [29]. Seroma changes are less relevant when applying highly hypofractionated schedules, while are expected to be more important during fractionated RT courses and treatment time of 3–5 weeks. In these cases, off-line MR-based plan adaption i.e. in the middle of the treatment course or weekly might help in reducing PTV margins used to account for interfractional variability.
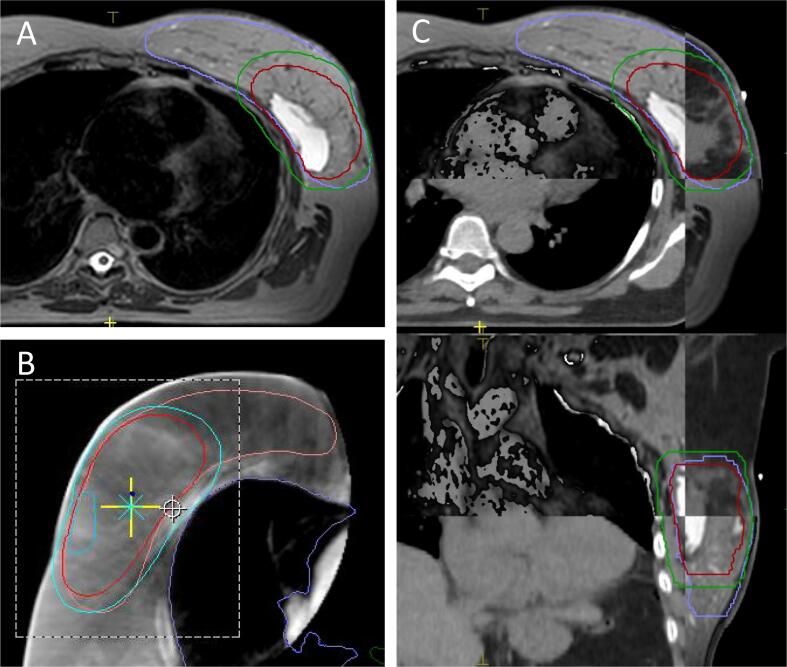
Fig. 2.
A: Daily MR acquired before treatment plan adaptation and treatment delivery at the 1.5 T MR-linac, T2W sequence. The seroma can be exactly recognized as well as the breast tissue. B: On daily cone beam CT acquired before treatment delivery, neither the breast tissue nor the seroma can be recognized. C: Matching between planning CT and daily MR is accurate (in A and C the same patient is displayed, in B a different patient is displayed).
With the aim of adopting MR for treatment planning and image guidance for treatment delivery, hybrid machines combining an MR scanner with a linear accelerator (MR-Linac) have become commercially available (Unity, Elekta AB, Stockholm, Sweden, and MRdian, ViewRay, Oakwood Village, OH). These systems offer MR-based image-guided radiotherapy (IGRT) including online daily MR-based plan adaptation [30], [31]. The first MR-guided adjuvant PBI treatments have been performed in low field MR machines coupled with 60Co sources. Here, no additional margins for the PTV were used, minimal intrafraction motion of the tumor bed was observed [32] and a seroma reduction was documented through repeated MR scanning during the course of radiotherapy [33], suggesting MR as an optimal imaging modality for ART in the adjuvant PBI setting. More recently, MR-guided adjuvant PBI at the 0.35 T (MRdian, ViewRay) [34], [35] and 1.5 T MR-Linac (Unity, Elekta) [36], [37] have been successfully delivered. Treatments could be performed in a short time, with daily plan adaptation taking on average four minutes and a cumulative in-room time of 25 min [36].
When treating on an MR-Linac, due to the Lorentz force, the interaction of secondary electrons with the magnetic field is responsible for the electron stream effect (ESE) and the electron return effect (ERE). For breast cancer patients, the ESE might cause out-of-field dose deposition the patient́s chin [34], [38], and ERE in-field dose deposition on the lung/chest wall and on patient́s skin (air/tissue interfaces) [39], [40], [41], [42], [43], [44]. These effects were investigated in the low-field [34] as well as high field MR-Linac [36], [37]. It was demonstrated that: 1) ESE and ERE can be accurately calculated by dedicated Monte Carlo based dose simulation algorithms (i.e. the treatment planning system Monaco 5.4 (Elekta AB, Stockholm, Sweden, [36], [37]), 2) for laterally located targets the ESE is directed to both the ipsilateral arm and the patient́s chin [36], and 3) the dose due to the ESE is effectively minimized using a 1 cm bolus [34], [36], [37]. Of note, the increase in the maximal dose to the skin due to the ERE was reported to be less than 1 % of the prescribed dose [37] and no relevant acute nor early late toxicity was observed, with good or excellent cosmetic outcomes [36]. Next to ESE and ERE, another challenging aspect for treatments at the MR-Linac is the geometric distortion [45]. The clinical impact of the geometric distortion is still unknown, but as the extent of distortions increases with the distance from the isocenter (up to 2 mm for distances of greater than 17 cm, both for the 1.5 T and the 0.35 T systems [46], [47]), for breast cancer patients it should be taken into account for very laterally located targets.
Several studies for adjuvant PBI at the MR-linac are recruiting, either as breast dedicated trials or as part of umbrella studies for the treatment of different tumor entities, including breast [48], [49], [50], [51].
In summary, through a superior soft tissue contrast compared to CT, MR appears to allow for a more accurate delineation of the CTV in the adjuvant PBI setting, even if its additional value compared to CT-based breast target contouring is still controversial. Additionally, the possibility of daily MR-based online plan adaptation might be exploited to reduce margins for the PTV. Adjuvant PBI at the MR-Linacs has been successfully performed, with promising early results and more studies are opened for recruitment.
Neoadjuvant PBI
In recent years, interest in neoadjuvant radiotherapy has rapidly grown, and studies have started recruiting patients with the goal of either facilitating PBI or delaying or avoiding surgery for low-risk breast cancers or allowing tumor downstaging for breast conserving surgery in patients with more advanced tumor stages [52], [53], [54], [55], [56]. Most of the benefits of MR-guided radiotherapy are expected in the neoadjuvant setting, for which precise visualization of the gross tumor volume (GTV) is essential and relatively difficult on CT images, especially when performed without contrast enhancement. A more accurate target delineation with less inter-observer variability has been shown in the neoadjuvant PBI setting compared to adjuvant PBI [57]. For preoperative target volume delineation, MR has been proven to be superior to CT for visualization of the tumor [58], and consensus guidelines for target delineation on MR images for neoadjuvant PBI are available [59]. During treatment delivery, interfraction and intrafraction target position variability must be carefully considered. Neoadjuvant PBI protocols usually deliver treatment in one or very few fractions. In these cases, intrafraction variability will not be compensated in subsequent fractions. In addition, the duration of each fraction increases, such that intrafraction monitoring of target motion is particularly important [60]. For breast cancers, intrafractional tumor displacement of up to 4 mm has been documented on cine MR [10]. Importantly, the MR-linac allows for imaging during radiation dose delivery, with the advantage of being able to monitor both tumor and OAR motion. Moreover, small margins for the PTV are necessary to minimize toxicity for highly hypofractionated stereotactic neoadjuvant treatments. Small margins, in turn, are possible when the target is clearly visible during the entire procedure. Therefore, treatments on MR-linacs appear to offer advantages compared to conventional linacs when performing neoadjuvant PBI and suggestions for protocol optimization when performing MR-guided breast cancer treatments are available [61]. Preparatory planning studies for MR-linac based neoadjuvant PBI showed promising results [62] and new protocols for fractionated or single-dose neoadjuvant PBI at MR-linacs are open [63], [64], [65]. These studies might also support translational research in breast cancer, facilitating a better understanding of tumor response to radiation [66].
For dose calculation an electron density map is needed, which is only available on CT. Therefore, at present in the clinical routine, for MR-based ART a planning CT is still employed to calculate the reference plan and match with the daily MR for MR-based plan adaptation. To overcome this methodological shortcoming, various approaches for generation of synthetic CTs from MRs are under development and clinical validation [67], [68], [69]. An MR-only workflow is expected to reduce geometrical uncertainties resulting from CT-MR matching and to be more efficient as well as more cost-effective [70]. Additionally, in order to speed up the adaptation procedure, autonomous un-supervised treatment planning systems have been developed and have been shown to be clinically feasible [71].
In summary, neoadjuvant PBI indications are rapidly growing, and MR has been shown superior to CT for target visualization. To date, no clinical experiences of MR-guided online ART in the neoadjuvant setting has been published but protocols have been designed and studies are recruiting. Thus, in the coming years data about feasibility and advantages of MR-based ART in this setting are expected.
CT-based ART
During fractionated adjuvant breast or chest wall irradiation with or without targeting lymphatics, anatomical variations of the target and OARs may occur. They mainly result from changes in the irradiated breast, i.e., swelling and seroma modification, variations in the arm position as well as in the position of the heart with respect to the target according to the breathing pattern (Fig. 3). Without the possibility of online plan adaptation, margins of 10 mm or more may be required to cover for intrafraction and interfraction variability. In fact, variation in the tumor bed position may require margins up to 10 mm [26]. Variation in the position of the heart up to 10 mm in both directions (towards and away from the breast/ chest wall) has been described [72]. Similarly, variability in the arm position has been investigated and margins up to 10 mm appear to be needed to account for it [73], [74]. ART might offer the possibility to personalize the treatment, reducing PTV margins and treatment-related toxicities. Significant changes of seroma and/ or lumpectomy cavity volume CT-documented during the radiotherapy course have been described my many authors [28], [29], [75], [76], [77], [78], [79], [80], [81], [82]. In patients planned for radiotherapy few weeks after surgery and with larger seromas and/or lumpectomy cavity, ART through re-planning CT performed after 2 weeks of radiotherapy enabled a significant reduction of the boost volume [78]. Recently, Lezzi et al considered a total of 75 applied fractions on 5 right-sided breast cancer patients treated with 40.05 Gy in 15 fractions at Ethos (Varian Medical Systems, Palo Alto, CA), without online adaptation [83]. Applying the original plan after off-line rigid registration between the daily CBCT and the planning CT, showed a in 4 fractions a suboptimal but still acceptable target volume coverage and an unacceptable target coverage in 3 fractions. In this study, patients with larger CTVs were those who showed more often under-coverage problems, being therefore those who might benefit the most by an adaptive approach. Based on the current available literature, it appears that patients with larger breast target volumes and boost volumes might benefit the most from ART. Here it should be noticed that significant seroma changes, influencing the boost volume, are more prone to occur in patients treated with conventional fractionation or light hypofractionation, which nowadays are being slowly replaced by highly hypofractionated schedules. Still, the application of high fractional doses suggests a potential benefit of ART, particularly when highly hypofractionated schedules are applied to larger volumes and to targets closed to critical structures, i.e. including lymph nodes regions and/ or left-sided tumours. Here, higher fractional doses to organs at risk such as heart, lung and brachial plexus are relevant especially for late toxicity. Nevertheless, despite the above-described single center observations and multiple efforts to establish standardized criteria to patients for ART [83], [84], it remains very often a clinical decision based on CBCT if, when and how often a patient should undergo replanning.
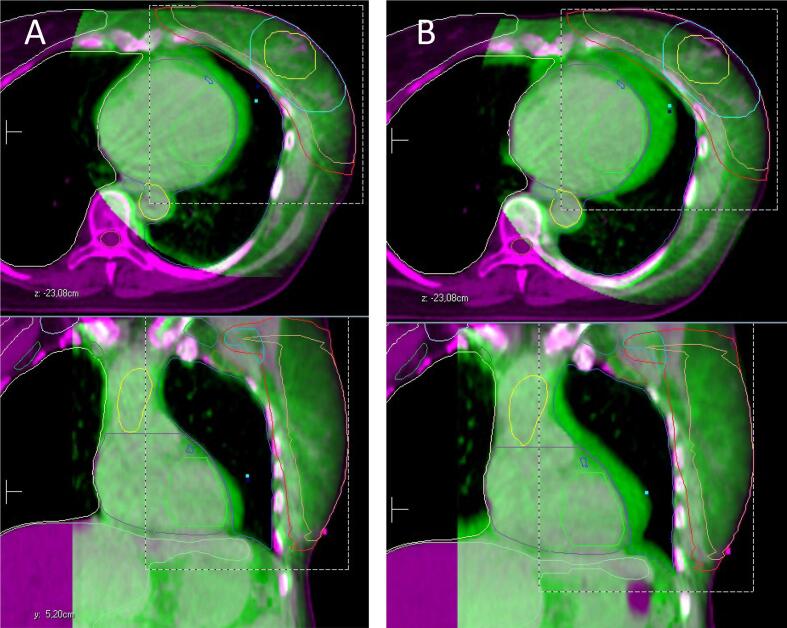
Fig. 3.
Changes of the heart position during radiotherapy for left sided breast cancer. In purple the planning CT (reference), in green the CBCT. Structures displayed: CTV breast, PTV breast, CTV boost (tumor bed), PTV boost, contralateral breast, heart, left ventricle, thoracic aorta. A: Fraction 6. B: Fraction 10.
At the moment, plan adaptation for treatments at conventional linac requires a new planning CT, new target and OARs delineation and the generation of a completely new plan. This process is labor intensive and resource demanding, such that in clinical practice as a pragmatic approach large PTV margins are used to account for interfraction and intrafraction variability.
Novel technologies based on artificial intelligence (AI) are being investigated for their potential application to enable ART based on CBCT [85]. Here, a synthetic CT based on the CBCT is generated and automatic contouring procedures of target and OARs based on rigid and deformable registration with the planning CT are applied. Then, the adapted plan is calculated, verified and finally delivered. For breast cancers, programs for AI-based fast synthetic CT generation have been successfully implemented [86] and deep-learning systems for auto-segmentation for OARs delineation have been described [87], [88], [89], [90]. To allow for a quick procedure for off-line but also potentially on-line plan adaptation, AI-based methods for dose calculations are under development [91]. Linacs with CT-based dedicated ART systems have recently become available on the market. These systems allow for online ART, which, others than offline, enables treatment on a new plan on the same day. The Varian Ethos system (Varian Medical Systems, Palo Alto, CA) has been clinically implemented and adaptive treatments in the pelvic regions with Ethos were successfully performed within 20 min [92], [93], [94]. To date however no CT-based ART experiences for breast cancers have been published, although CT-based ART for breast cancer patients is currently under evaluation [95].
Taking a broader perspective, there are currently clinical developments towards precision radiotherapy. Therapeutic decisions need to take into account patients clinical information as well as complex biological and imaging features of the tumor. AI can facilitate development and implementation of models to predict tumor response [96], [97]. This may allow individual treatment tailoring, including decisions on radiotherapy dose and volumes [98]. For breast cancer radiotherapy, this might specifically have consequences for target volume delineation, dose painting, timing for re-planning and indication for combination with other systemic therapies [99]. In a context where resources for health care are limited and need to be carefully assigned, a reflection about the higher costs of ART is necessary. In spite of AI-driven processes, ART procedures remain time-consuming, at least in this initial phase of their clinical implementation, with consequently higher costs in terms of staff members employed at every level, i.e. radiation therapists, physicists, clinicians. In addition, higher costs for new equipment and technology, especially ART-enabling radiotherapy devices, i.e. MR-Linacs and CT-adaptive Linacs, need to be considered. With the goal to provide data for accurate cost-value analyses for new investments in the field of radiotherapy, the European Society for Radiation Oncology (ESTRO) launched in 2010 the HERO project (Health Economics in Radiation Oncology) [100], [101]. At a national level, the German Cancer Research Centre (DKFZ) established in 2017 a division for health economics. At present, early health economic evaluations for MR guided ART are available for prostate cancer [102], [103], [104] and SBRT [105]. These studies seem to indicate that the higher costs of performing MR guided ART are overcome, especially when ultra hypofractionated scheduled are adopted, by the reduced costs of acute and late toxicities management [106]. Data for breast ART health economics evaluation are not available yet, since complex indicators such as the burden of cancer with its socioeconomic impact, cost-benefit as well as cost-effectiveness analyses of new interventions still need to be assessed. Especially in the context of adjuvant breast ART, these analyses are particularly complex, since costs are immediately evident, while many years are required to assess advantages in terms of better clinical outcomes (i.e. increased tumor control, less toxicity and better quality of life) and consequently potential sparing of secondary health care costs [107].
Conclusions
ART could be of benefit to breast cancer patients in several clinical scenarios, particularly PBI, in which ART could minimize margins and thereby reduce the volume of normal tissue irradiated. Autonomous AI-driven technologies are expected to further enable ART in the near future. Initial clinical experiences are promising and numerous studies are currently recruiting such that further data on likely clinical benefit are expected in the coming years.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The MR-Linac research program is funded by the German Research Council (to D.T. and D.Z., ZI 736/2-1). Radiation Oncology Tübingen receives financial and technical support from Elekta AB, Kaiku Health and TheraPanacea under a research agreement. C.D.C. is supported by the Medical Faculty Tübingen (TüFF). Odense University Hospital has research agreements with Elekta and Philips.
Acknowledgement
We acknowledge support by Open Access Publishing Fund of Universitätsbibliothek - Eberhard Karls Universität Tübingen.
References
- 1.httpsecis.jrc.ec.europa.euexplorer.
- 2.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.httpsprogressreport.cancer.govtreatmentbreast_cancer.
- 4.Borras J.M., Lievens Y., Barton M., Corral J., Ferlay J., Bray F., et al. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis Radiother Oncol. 2016;119:5–11. doi: 10.1016/j.radonc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Yan D., Vicini F., Wong J., Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 6.Glide-Hurst C.K., Lee P., Yock A.D., Olsen J.R., Cao M., Siddiqui F., et al. Adaptive Radiation Therapy (ART) Strategies and Technical Considerations: A State of the ART Review From NRG Oncology. Int J Radiat Oncol Biol Phys. 2021;109:1054–1075. doi: 10.1016/j.ijrobp.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray Brunt A., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekstra N., Habraken S., Swaak-Kragten A., Hoogeman M., Pignol J.P. Intrafraction motion during partial breast irradiation depends on treatment time. Radiother Oncol. 2021;159:176–182. doi: 10.1016/j.radonc.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 9.van Heijst T.C., Philippens M.E., Charaghvandi R.K., den Hartogh M.D., Lagendijk J.J., van den Bongard H.J., et al. Quantification of intra-fraction motion in breast radiotherapy using supine magnetic resonance imaging. Phys Med Biol. 2016;61:1352–1370. doi: 10.1088/0031-9155/61/3/1352. [DOI] [PubMed] [Google Scholar]
- 10.Groot Koerkamp M., vdBH, Philippens MEP, Lagendijk JJW, Howeling AC. OC-0414 Intrafraction displacement of breast tumor (bed) and axillary lymph nodes on cine MRI. Radiother Oncol. 2019;133 [Google Scholar]
- 11.Thames H.D., Jr., Withers H.R., Peters L.J., Fletcher G.H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 12.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 13.Laugaard Lorenzen E., Christian Rehammar J., Jensen M.B., Ewertz M., Brink C. Radiation-induced risk of ischemic heart disease following breast cancer radiotherapy in Denmark, 1977–2005. Radiother Oncol. 2020;152:103–110. doi: 10.1016/j.radonc.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Lee B.M., Chang J.S., Kim S.Y., Keum K.C., Suh C.O., Kim Y.B. Hypofractionated Radiotherapy Dose Scheme and Application of New Techniques Are Associated to a Lower Incidence of Radiation Pneumonitis in Breast Cancer Patients. Front Oncol. 2020;10:124. doi: 10.3389/fonc.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burt L.M., Ying J., Poppe M.M., Suneja G., Gaffney D.K. Risk of secondary malignancies after radiation therapy for breast cancer: Comprehensive results. Breast. 2017;35:122–129. doi: 10.1016/j.breast.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Landis D.M., Luo W., Song J., Bellon J.R., Punglia R.S., Wong J.S., et al. Variability among breast radiation oncologists in delineation of the postsurgical lumpectomy cavity. Int J Radiat Oncol Biol Phys. 2007;67:1299–1308. doi: 10.1016/j.ijrobp.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 17.van Mourik A.M., Elkhuizen P.H., Minkema D., Duppen J.C. Dutch Young Boost Study G, van Vliet-Vroegindeweij C. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol. 2010;94:286–291. doi: 10.1016/j.radonc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hammadi N., Caparrotti P., Divakar S., Riyas M., Chandramouli S.H., Hammoud R., et al. MRI Reduces Variation of Contouring for Boost Clinical Target Volume in Breast Cancer Patients Without Surgical Clips in the Tumour Bed. Radiol Oncol. 2017;51:160–168. doi: 10.1515/raon-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolicoeur M., Racine M.L., Trop I., Hathout L., Nguyen D., Derashodian T., et al. Localization of the surgical bed using supine magnetic resonance and computed tomography scan fusion for planification of breast interstitial brachytherapy. Radiother Oncol. 2011;100:480–484. doi: 10.1016/j.radonc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Mast M., Coerkamp E., Heijenbrok M., Scholten A., Jansen W., Kouwenhoven E., et al. Target volume delineation in breast conserving radiotherapy: are co-registered CT and MR images of added value? Radiat Oncol. 2014;9:65. doi: 10.1186/1748-717X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogson E.M., Delaney G.P., Ahern V., Boxer M.M., Chan C., David S., et al. Comparison of Magnetic Resonance Imaging and Computed Tomography for Breast Target Volume Delineation in Prone and Supine Positions. Int J Radiat Oncol Biol Phys. 2016;96:905–912. doi: 10.1016/j.ijrobp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Giezen M., Kouwenhoven E., Scholten A.N., Coerkamp E.G., Heijenbrok M., Jansen W.P., et al. MRI- versus CT-based volume delineation of lumpectomy cavity in supine position in breast-conserving therapy: an exploratory study. Int J Radiat Oncol Biol Phys. 2012;82:1332–1340. doi: 10.1016/j.ijrobp.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Denh M.D., Philippens M.E., IE VAND, Kleynen CE, Tersteeg RJ, Kotte AN,, et al. Post-lumpectomy CT-guided tumor bed delineation for breast boost and partial breast irradiation: Can additional pre- and postoperative imaging reduce interobserver variability? Oncol Lett. 2015;10:2795–2801. doi: 10.3892/ol.2015.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.B. Sabine DG, P. Peter, J. Clara, P. Bert, K. John. Open Low-Field Magnetic Resonance (MR) Versus CT Scanner (CT) Imaging in Breast Radiotherapy Treatment Planning. Int J Radiat Oncol Biol Phys. 2005;63:232–233. [Google Scholar]
- 25.Kirby A.M., Yarnold J.R., Evans P.M., Morgan V.A., Schmidt M.A., Scurr E.D., et al. Tumor bed delineation for partial breast and breast boost radiotherapy planned in the prone position: what does MRI add to X-ray CT localization of titanium clips placed in the excision cavity wall? Int J Radiat Oncol Biol Phys. 2009;74:1276–1282. doi: 10.1016/j.ijrobp.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Topolnjak R., van Vliet-Vroegindeweij C., Sonke J.J., Minkema D., Remeijer P., Nijkamp J., et al. Breast-conserving therapy: radiotherapy margins for breast tumor bed boost. Int J Radiat Oncol Biol Phys. 2008;72:941–948. doi: 10.1016/j.ijrobp.2008.06.1924. [DOI] [PubMed] [Google Scholar]
- 27.Musunuru H.B., Yadav P., Olson S.J., Anderson B.M. Improved Ipsilateral Breast and Chest Wall Sparing With MR-Guided 3-fraction Accelerated Partial Breast Irradiation: A Dosimetric Study Comparing MR-Linac and CT-Linac Plans. Adv Radiat Oncol. 2021;6 doi: 10.1016/j.adro.2021.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tersteeg R.J., Roesink J.M., Albregts M., Warlam-Rodenhuis C.C., van Asselen B. Changes in excision cavity volume: prediction of the reduction in absolute volume during breast irradiation. Int J Radiat Oncol Biol Phys. 2009;74:1181–1185. doi: 10.1016/j.ijrobp.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 29.Alderliesten T., den Hollander S., Yang T.J., Elkhuizen P.H., van Mourik A.M., Hurkmans C., et al. Dosimetric impact of post-operative seroma reduction during radiotherapy after breast-conserving surgery. Radiother Oncol. 2011;100:265–270. doi: 10.1016/j.radonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Raaymakers B.W., Jurgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A., van Asselen B., et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 31.Kluter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl. Radiat Oncol. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acharya S., Fischer-Valuck B.W., Mazur T.R., Curcuru A., Sona K., Kashani R., et al. Magnetic Resonance Image Guided Radiation Therapy for External Beam Accelerated Partial-Breast Irradiation: Evaluation of Delivered Dose and Intrafractional Cavity Motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Jeon S.H., Shin K.H., Park S.Y., Kim J.I., Park J.M., Kim J.H., et al. Seroma change during magnetic resonance imaging-guided partial breast irradiation and its clinical implications. Radiat Oncol. 2017;12:103. doi: 10.1186/s13014-017-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J.M., Shin K.H., Kim J.I., Park S.Y., Jeon S.H., Choi N., et al. Air-electron stream interactions during magnetic resonance IGRT : Skin irradiation outside the treatment field during accelerated partial breast irradiation. Strahlenther Onkol. 2018;194:50–59. doi: 10.1007/s00066-017-1212-z. [DOI] [PubMed] [Google Scholar]
- 35.Price A.T., Kennedy W.R., Henke L.E., Brown S.R., Green O.L., Thomas M.A., et al. Implementing stereotactic accelerated partial breast irradiation using magnetic resonance guided radiation therapy. Radiother Oncol. 2021;164:275–281. doi: 10.1016/j.radonc.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 36.De-Colle C., Nachbar M., Mnnich D., Boeke S., Gani C., Weidner N., et al. Analysis of the electron-stream effect in patients treated with partial breast irradiation using the 1.5 T MR-linear accelerator. Clin Transl Radiat Oncol. 2021;27:103–108. doi: 10.1016/j.ctro.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachbar M., Monnich D., Boeke S., Gani C., Weidner N., Heinrich V., et al. Partial breast irradiation with the 1.5 T MR-Linac: First patient treatment and analysis of electron return and stream effects. Radiother Oncol. 2020;145:30–35. doi: 10.1016/j.radonc.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Malkov V.N., Hackett S.L., Wolthaus J.W.H., Raaymakers B.W., van Asselen B. Monte Carlo simulations of out-of-field surface doses due to the electron streaming effect in orthogonal magnetic fields. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/ab0aa0. [DOI] [PubMed] [Google Scholar]
- 39.Oborn B.M., Metcalfe P.E., Butson M.J., Rosenfeld A.B. Monte Carlo characterization of skin doses in 6 MV transverse field MRI-linac systems: effect of field size, surface orientation, magnetic field strength, and exit bolus. Med Phys. 2010;37:5208–5217. doi: 10.1118/1.3488980. [DOI] [PubMed] [Google Scholar]
- 40.Kim A., Lim-Reinders S., McCann C., Ahmad S.B., Sahgal A., Lee J., et al. Magnetic field dose effects on different radiation beam geometries for hypofractionated partial breast irradiation. J Appl Clin Med Phys. 2017;18:62–70. doi: 10.1002/acm2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malkov V.N., Hackett S.L., van Asselen B., Raaymakers B.W., Wolthaus J.W.H. Monte Carlo simulations of out-of-field skin dose due to spiralling contaminant electrons in a perpendicular magnetic field. Med Phys. 2019;46:1467–1477. doi: 10.1002/mp.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hackett S.L., van Asselen B., Wolthaus J.W.H., Bluemink J.J., Ishakoglu K., Kok J., et al. Spiraling contaminant electrons increase doses to surfaces outside the photon beam of an MRI-linac with a perpendicular magnetic field. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aaba8f. [DOI] [PubMed] [Google Scholar]
- 43.Raaijmakers A.J., Raaymakers B.W., Lagendijk J.J. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol. 2005;50:1363–1376. doi: 10.1088/0031-9155/50/7/002. [DOI] [PubMed] [Google Scholar]
- 44.van Heijst T.C., den Hartogh M.D., Lagendijk J.J., van den Bongard H.J., van Asselen B. MR-guided breast radiotherapy: feasibility and magnetic-field impact on skin dose. Phys Med Biol. 2013;58:5917–5930. doi: 10.1088/0031-9155/58/17/5917. [DOI] [PubMed] [Google Scholar]
- 45.Walker A., Liney G., Metcalfe P., Holloway L. MRI distortion: considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med. 2014;37:103–113. doi: 10.1007/s13246-014-0252-2. [DOI] [PubMed] [Google Scholar]
- 46.Tijssen R.H.N., Philippens M.E.P., Paulson E.S., Glitzner M., Chugh B., Wetscherek A., et al. MRI commissioning of 1.5T MR-linac systems - a multi-institutional study. Radiother Oncol. 2019;132:114–120. doi: 10.1016/j.radonc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Ginn J.S., Agazaryan N., Cao M., Baharom U., Low D.A., Yang Y., et al. Characterization of spatial distortion in a 0.35 T MRI-guided radiotherapy system. Phys Med Biol. 2017;62:4525–4540. doi: 10.1088/1361-6560/aa6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.B A. Real-time MRI-Guided 3-Fraction Accelerated Partial Breast Irradiation in Early Breast Cancer. Clinical Trialsgov Identifier NCT03936478.
- 49.M R. CONFIRM: Magnetic Resonance Guided Radiation Therapy. Clinical trialsgov Identifier NCT04368702.
- 50.R H. Prospective Evaluation of Radiotherapy Using Magnetic Resonance Image Guided Treatment (PERMIT). Clinical trialsgov Identifier NCT03727698.
- 51.Zips D GC. Feasibility of Online MR-guided Radiotherapy on a 1.5T MR-Linac. ClinicalTrialsgov Identifier: NCT04172753.
- 52.Charaghvandi R.K., van Asselen B., Philippens M.E., Verkooijen H.M., van Gils C.H., van Diest P.J., et al. Redefining radiotherapy for early-stage breast cancer with single dose ablative treatment: a study protocol. BMC Cancer. 2017;17:181. doi: 10.1186/s12885-017-3144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horton J.K., Blitzblau R.C., Yoo S., Geradts J., Chang Z., Baker J.A., et al. Preoperative Single-Fraction Partial Breast Radiation Therapy: A Novel Phase 1, Dose-Escalation Protocol With Radiation Response Biomarkers. Int J Radiat Oncol Biol Phys. 2015;92:846–855. doi: 10.1016/j.ijrobp.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasmel J.E., Charaghvandi R.K., Houweling A.C., Philippens M.E.P., van Asselen B., Vreuls C.P.H., et al. Tumor Response After Neoadjuvant Magnetic Resonance Guided Single Ablative Dose Partial Breast Irradiation. Int J Radiat Oncol Biol Phys. 2020;106:821–829. doi: 10.1016/j.ijrobp.2019.11.406. [DOI] [PubMed] [Google Scholar]
- 55.Bosma S.C.J., Leij F., Vreeswijk S., Maaker M., Wesseling J., Vijver M.V., et al. Five-Year Results of the Preoperative Accelerated Partial Breast Irradiation (PAPBI) Trial. Int J Radiat Oncol Biol Phys. 2020;106:958–967. doi: 10.1016/j.ijrobp.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 56.Guidolin K., Yaremko B., Lynn K., Gaede S., Kornecki A., Muscedere G., et al. Stereotactic image-guided neoadjuvant ablative single-dose radiation, then lumpectomy, for early breast cancer: the SIGNAL prospective single-arm trial of single-dose radiation therapy. Curr Oncol. 2019;26:e334–e340. doi: 10.3747/co.26.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Leij F., Elkhuizen P.H., Janssen T.M., Poortmans P., van der Sangen M., Scholten A.N., et al. Target volume delineation in external beam partial breast irradiation: less inter-observer variation with preoperative- compared to postoperative delineation. Radiother Oncol. 2014;110:467–470. doi: 10.1016/j.radonc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 58.den Hartogh M.D., Philippens M.E., van Dam I.E., Kleynen C.E., Tersteeg R.J., Pijnappel R.M., et al. MRI and CT imaging for preoperative target volume delineation in breast-conserving therapy. Radiat Oncol. 2014;9:63. doi: 10.1186/1748-717X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasmel J.E., Groot Koerkamp M.L., Kirby A.M., Russell N.S., Shaitelman S.F., Vesprini D., et al. Consensus on Contouring Primary Breast Tumors on MRI in the Setting of Neoadjuvant Partial Breast Irradiation in Trials. Pract Radiat Oncol. 2020;10:e466–e474. doi: 10.1016/j.prro.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 60.van Herk M., McWilliam A., Dubec M., Faivre-Finn C., Choudhury A. Magnetic Resonance Imaging-Guided Radiation Therapy: A Short Strengths, Weaknesses, Opportunities, and Threats Analysis. Int J Radiat Oncol Biol Phys. 2018;101:1057–1060. doi: 10.1016/j.ijrobp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Groot Koerkamp M.L., Vasmel J.E., Russell N.S., Shaitelman S.F., Anandadas C.N., Currey A., et al. Optimizing MR-Guided Radiotherapy for Breast Cancer Patients. Front Oncol. 2020;10:1107. doi: 10.3389/fonc.2020.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charaghvandi K.R., Van't Westeinde T., Yoo S., Houweling A.C., Rodrigues A., Verkooijen H.M., et al. Single dose partial breast irradiation using an MRI linear accelerator in the supine and prone treatment position. Clin Transl Radiat Oncol. 2019;14:1–7. doi: 10.1016/j.ctro.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A R. Dose Escalation Study of Single Fraction Early Stage Breast Cancer. Clinical trialsgov Identifier NCT04040569.
- 64.A C. MRI-Based Preoperative Accelerated Partial Breast Irradiation. Clinical trialsgov Identifier NCT02728076.
- 65.HJGD vdB. MRI-Guided Single Dose Preoperative Radiotherapy in Low-risk Breast Cancer. Clinical trialsgov Identifier NCT03863301.
- 66.Lightowlers S.V., Boersma L.J., Fourquet A., Kirova Y.M., Offersen B.V., Poortmans P., et al. Preoperative breast radiation therapy: Indications and perspectives. Eur J Cancer. 2017;82:184–192. doi: 10.1016/j.ejca.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Han X. MR-based synthetic CT generation using a deep convolutional neural network method. Med Phys. 2017;44:1408–1419. doi: 10.1002/mp.12155. [DOI] [PubMed] [Google Scholar]
- 68.Ahunbay E.E., Thapa R., Chen X., Paulson E., Li X.A. A Technique to Rapidly Generate Synthetic Computed Tomography for Magnetic Resonance Imaging-Guided Online Adaptive Replanning: An Exploratory Study. Int J Radiat Oncol Biol Phys. 2019;103:1261–1270. doi: 10.1016/j.ijrobp.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 69.M L Groot Koerkamp YJMdH, M Maspero, C Kontaxis, S Mandija, J E Vasmel, R K Charaghvandi, M E P Philippens, B van Asselen, H J G D van den Bongard. Synthetic CT for single-fraction neoadjuvant partial breast irradiation on an MRI-linac. Phys Med Biol. 20121;66. [DOI] [PubMed]
- 70.Jonsson J., Nyholm T., Soderkvist K. The rationale for MR-only treatment planning for external radiotherapy. Clin Transl Radiat Oncol. 2019;18:60–65. doi: 10.1016/j.ctro.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kunzel L.A., Nachbar M., Hagmuller M., Gani C., Boeke S., Zips D., et al. First experience of autonomous, un-supervised treatment planning integrated in adaptive MR-guided radiotherapy and delivered to a patient with prostate cancer. Radiother Oncol. 2021;159:197–201. doi: 10.1016/j.radonc.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 72.van Haaren P., Claassen-Janssen F., van de Sande I., Boersma L., van der Sangen M., Hurkmans C. Heart position variability during voluntary moderate deep inspiration breath-hold radiotherapy for breast cancer determined by repeat CBCT scans. Phys Med. 2017;40:88–94. doi: 10.1016/j.ejmp.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 73.Kapanen M., Laaksomaa M., Skytta T., Haltamo M., Pehkonen J., Lehtonen T., et al. Residual position errors of lymph node surrogates in breast cancer adjuvant radiotherapy: Comparison of two arm fixation devices and the effect of arm position correction. Med Dosim. 2016;41:47–52. doi: 10.1016/j.meddos.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Laaksomaa M., Kapanen M., Haltamo M., Skytta T., Peltola S., Hyodynmaa S., et al. Determination of the optimal matching position for setup images and minimal setup margins in adjuvant radiotherapy of breast and lymph nodes treated in voluntary deep inhalation breath-hold. Radiat Oncol. 2015;10:76. doi: 10.1186/s13014-015-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho H., Kim C. Volumetric changes in the lumpectomy cavity during whole breast irradiation after breast conserving surgery. Radiat Oncol J. 2011;29:277–282. doi: 10.3857/roj.2011.29.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flannery T.W., Nichols E.M., Cheston S.B., Marter K.J., Naqvi S.A., Markham K.M., et al. Repeat computed tomography simulation to assess lumpectomy cavity volume during whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2009;75:751–756. doi: 10.1016/j.ijrobp.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 77.Hurkmans C., Admiraal M., van der Sangen M., Dijkmans I. Significance of breast boost volume changes during radiotherapy in relation to current clinical interobserver variations. Radiother Oncol. 2009;90:60–65. doi: 10.1016/j.radonc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Hurkmans C.W., Dijckmans I., Reijnen M., van der Leer J., van Vliet-Vroegindeweij C., van der Sangen M. Adaptive radiation therapy for breast IMRT-simultaneously integrated boost: three-year clinical experience. Radiother Oncol. 2012;103:183–187. doi: 10.1016/j.radonc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Mohiuddin M.M., Nichols E.M., Marter K.J., Flannery T.W. Decrease of the lumpectomy cavity volume after whole-breast irradiation affects small field boost planning. Med Dosim. 2012;37:339–343. doi: 10.1016/j.meddos.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Oh K.S., Kong F.M., Griffith K.A., Yanke B., Pierce L.J. Planning the breast tumor bed boost: changes in the excision cavity volume and surgical scar location after breast-conserving surgery and whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2006;66:680–686. doi: 10.1016/j.ijrobp.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 81.Sager O., Dincoglan F., Uysal B., Demiral S., Gamsiz H., Elcim Y., et al. Evaluation of adaptive radiotherapy (ART) by use of replanning the tumor bed boost with repeated computed tomography (CT) simulation after whole breast irradiation (WBI) for breast cancer patients having clinically evident seroma. Jpn J Radiol. 2018;36:401–406. doi: 10.1007/s11604-018-0735-2. [DOI] [PubMed] [Google Scholar]
- 82.Sharma R., Spierer M., Mutyala S., Thawani N., Cohen H.W., Hong L., et al. Change in seroma volume during whole-breast radiation therapy. Int J Radiat Oncol Biol Phys. 2009;75:89–93. doi: 10.1016/j.ijrobp.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 83.Iezzi M., Cusumano D., Piccari D., Menna S., Catucci F., D'Aviero A., et al. Dosimetric Impact of Inter-Fraction Variability in the Treatment of Breast Cancer: Towards New Criteria to Evaluate the Appropriateness of Online Adaptive Radiotherapy. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.838039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zegers C.M.L., Baeza J.A., van Elmpt W., Murrer L.H.P., Verhoeven K., Boersma L., et al. Three-dimensional dose evaluation in breast cancer patients to define decision criteria for adaptive radiotherapy. Acta Oncol. 2017;56:1487–1494. doi: 10.1080/0284186X.2017.1349334. [DOI] [PubMed] [Google Scholar]
- 85.Archambault Y., BCBDea. Making on-line adaptive radiotherapy possible uring artificial intelligence and machine learning for efficient daily re-planning. Medical Physics Internaitonal Journal. 2020:8. [Google Scholar]
- 86.Maspero M., Houweling A.C., Savenije M.H.F., van Heijst T.C.F., Verhoeff J.J.C., Kotte A., et al. A single neural network for cone-beam computed tomography-based radiotherapy of head-and-neck, lung and breast cancer. Phys Imaging Radiat Oncol. 2020;14:24–31. doi: 10.1016/j.phro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung S.Y., Chang J.S., Choi M.S., Chang Y., Choi B.S., Chun J., et al. Clinical feasibility of deep learning-based auto-segmentation of target volumes and organs-at-risk in breast cancer patients after breast-conserving surgery. Radiat Oncol. 2021;16:44. doi: 10.1186/s13014-021-01771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi M.S., Choi B.S., Chung S.Y., Kim N., Chun J., Kim Y.B., et al. Clinical evaluation of atlas- and deep learning-based automatic segmentation of multiple organs and clinical target volumes for breast cancer. Radiother Oncol. 2020;153:139–145. doi: 10.1016/j.radonc.2020.09.045. [DOI] [PubMed] [Google Scholar]
- 89.Zeleznik R., Weiss J., Taron J., Guthier C., Bitterman D.S., Hancox C., et al. Deep-learning system to improve the quality and efficiency of volumetric heart segmentation for breast cancer. NPJ Digit Med. 2021;4:43. doi: 10.1038/s41746-021-00416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schreier J., Attanasi F., Laaksonen H. A Full-Image Deep Segmenter for CT Images in Breast Cancer Radiotherapy Treatment. Front Oncol. 2019;9:677. doi: 10.3389/fonc.2019.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bakx N., Bluemink H., Hagelaar E., van der Sangen M., Theuws J., Hurkmans C. Development and evaluation of radiotherapy deep learning dose prediction models for breast cancer. Phys Imaging Radiat Oncol. 2021;17:65–70. doi: 10.1016/j.phro.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Byrne M., Archibald-Heeren B., Hu Y., Teh A., Beserminji R., Cai E., et al. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J Appl Clin Med Phys. 2021 doi: 10.1002/acm2.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sibolt P., Andersson L.M., Calmels L., Sjostrom D., Bjelkengren U., Geertsen P., et al. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region. Phys Imaging Radiat Oncol. 2021;17:1–7. doi: 10.1016/j.phro.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moazzezi M., Rose B., Kisling K., Moore K.L., Ray X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J Appl Clin Med Phys. 2021;22:82–93. doi: 10.1002/acm2.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verhoeven K. Prospective Validation of 3D Dose Metrics as Selection Criteria for Adaptive Radiotherapy in Breast Cancer Patients, Clinical trialsgov Identifier NCT03385031.
- 96.Mirestean C.C., Volovat C., Iancu R.I., Iancu D.P.T. Radiomics in Triple Negative Breast Cancer: New Horizons in an Aggressive Subtype of the Disease. J Clin Med. 2022;11 doi: 10.3390/jcm11030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia B., Wang H., Wang Z., Qian Z., Xiao Q., Liu Y., et al. A Combined Nomogram Model to Predict Disease-free Survival in Triple-Negative Breast Cancer Patients With Neoadjuvant Chemotherapy. Front Genet. 2021;12 doi: 10.3389/fgene.2021.783513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tran W.T., Jerzak K., Lu F.I., Klein J., Tabbarah S., Lagree A., et al. Personalized Breast Cancer Treatments Using Artificial Intelligence in Radiomics and Pathomics. J Med Imaging Radiat Sci. 2019;50:S32–S41. doi: 10.1016/j.jmir.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 99.Poortmans P.M.P., Takanen S., Marta G.N., Meattini I., Kaidar-Person O. Winter is over: The use of Artificial Intelligence to individualise radiation therapy for breast cancer. Breast. 2020;49:194–200. doi: 10.1016/j.breast.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Defourny N., Dunscombe P., Perrier L., Grau C., Lievens Y. Cost evaluations of radiotherapy: What do we know? An ESTRO-HERO analysis Radiother Oncol. 2016;121:468–474. doi: 10.1016/j.radonc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Lievens Y., Grau C. Health economics in radiation oncology: introducing the ESTRO HERO project. Radiother Oncol. 2012;103:109–112. doi: 10.1016/j.radonc.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 102.Hehakaya C., van der Voort van Zyp JRN, Vanneste BGL, Grutters JPC, Grobbee DE, Verkooijen HM,, et al. Early health economic analysis of 1.5 T MRI-guided radiotherapy for localized prostate cancer: Decision analytic modelling. Radiother Oncol. 2021;161:74–82. doi: 10.1016/j.radonc.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Parikh N.R., Clark M.A., Patel P., Kafka-Peterson K., Zaide L., Ma T.M., et al. Time-Driven Activity-Based Costing of CT-Guided vs MR-Guided Prostate SBRT. Appl Radiat Oncol. 2021;10:33–40. [PMC free article] [PubMed] [Google Scholar]
- 104.Schumacher L.D., Dal Pra A., Hoffe S.E., Mellon E.A. Toxicity reduction required for MRI-guided radiotherapy to be cost-effective in the treatment of localized prostate cancer. Br J Radiol. 2020;93:20200028. doi: 10.1259/bjr.20200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parikh N.R., Lee P.P., Raman S.S., Cao M., Lamb J., Tyran M., et al. Time-Driven Activity-Based Costing Comparison of CT-Guided Versus MR-Guided SBRT. JCO Oncol Pract. 2020;16:e1378–e1385. doi: 10.1200/JOP.19.00605. [DOI] [PubMed] [Google Scholar]
- 106.Australia MSACsCo. Magnetic Resonance Image Guided Radiation Therapy, Assessment report. 2020.
- 107.Lievens Y. Economic evaluation of post-operative radiotherapy in breast cancer: how a local treatment cost-effectively improves survival. Belgian J of. Med Oncol. 2008 [Google Scholar]