Abstract
Active immunization with Streptococcus mutans glucan binding protein B (GBP-B) has been shown to induce protection against experimental dental caries. This protection presumably results from continuous secretion of salivary antibody to GBP-B, which inhibits accumulation of S. mutans within the oral biofilm. The purpose of this study was to explore the influence of short-term (9- or 24-day) passive oral administration of antibody to S. mutans GBP-B on the longer-term accumulation and cariogenicity of S. mutans in a rat model of dental caries. Preimmune chicken egg yolk immunoglobulin Y (IgY) or IgY antibody to S. mutans GBP-B was supplied in lower (experiment 1) and higher (experiment 2) concentrations in the diet and drinking water of rats for 9 (experiment 1) or 24 (experiment 2) days. During the first 3 days of IgY feeding, all animals were challenged with 5 × 106 streptomycin-resistant S. mutans strain SJ-r organisms. Rats remained infected with S. mutans for 78 days, during which rat molars were sampled for the accumulation of S. mutans SJ-r bacteria and total streptococci. Geometric mean levels of S. mutans SJ-r accumulation on molar surfaces were significantly lower in antibody-treated rats on days 16 and 78 of experiment 2 and were lower on all but the initial (day 5) swabbing occasions in both experiments. Relative to controls, the extent of molar dental caries measured on day 78 was also significantly decreased. The decrease in molar caries correlated with the amount and duration of antibody administration. This is the first demonstration that passive antibody to S. mutans GBP-B can have a protective effect against cariogenic S. mutans infection and disease. Furthermore, this decrease in infection and disease did not require continuous antibody administration for the duration of the infection period. This study also indicates that antibody to components putatively involved only in cellular aggregation can have a significant effect on the incorporation of mutans streptococci in dental biofilm.
The molecular pathogenesis of mutans streptococcus-associated dental caries involves a series of binding events that eventually lead to the accumulation of sufficient numbers of these cariogenic bacteria to cause disease (6). The initial binding event appears to involve the interaction of bacterial cell surface adhesins (antigen I/II) with receptors in the salivary pellicle. These cariogenic streptococci then accumulate in the dental biofilm following enzymatic synthesis of extracellular glucans which provide multiple binding sites for glucan binding proteins (GBPs) associated with the bacterial cell. At least six proteins with glucan binding properties (i.e., the ability to bind α-1,6-glucan) have been identified in Streptococcus mutans (2, 21, 23) and Streptococcus sobrinus (10, 16, 26, 31), the two mutans streptococcal species most closely associated with human disease. Three of these GBPs, all from S. mutans, have been cloned and sequenced (GBP-A, GBP-B, and GBP-C). Their sequences bear little similarity (2, 8a, 21). Several lines of evidence suggest that GBP-A contributes to S. mutans biofilm development (2, 4, 8). The roles of GBP-B and GBP-C in the molecular pathogenesis of S. mutans, however, are yet to be completely resolved.
Immunization strategies employing streptococcal adhesins or glucosyltransferases (GTFs) have been shown to effectively interfere with the molecular pathogenesis of mutans streptococci (reviewed in reference 28). Immune blockade of the glucan binding activity of S. mutans GBPs may serve as another effective strategy, given the apparent central role of these proteins in the accumulation phase of cariogenic microbiota in dental biofilms. At present, S. mutans GBP-B is of the greatest immunological interest in this regard. This protein bears little antigenic similarity to other S. mutans or S. sobrinus GBPs. Saliva of young children often contains immunoglobulin A (IgA) antibody to S. mutans GBP-B (25), indicating that initial infection with S. mutans can lead to induction of immune responses to this protein in humans. GBP-B appears to be significantly more immunogenic than GBP-A in rodents (24). Furthermore, active immunization with S. mutans GBP-B induces immune responses that result in lower levels of S. mutans colonization and in reduced dental caries caused by experimental infection with S. mutans (27). Similar active immunization with GBP-A did not demonstrate these protective effects (24). Thus, antibody to S. mutans GBP-B appears to have the potential to modulate infection and disease caused by S. mutans.
Passive immunization approaches have been used with success to interfere with mutans streptococcal infection and resulting dental caries. Experiments using intravenous, suckling (17; reviewed in reference 28), dietary (18), or mouthwash (3, 7) transfer of antibody raised to intact mutans streptococcal cells have shown each of these methods to result in protection. Passive administration of chicken egg yolk IgY antibody to mutans streptococcal GTF (5) has protected rats from experimental dental caries. Topical administration of mouse (11, 13, 15) or transgenic (14) monoclonal antibody to antigen I/II has also been shown to modify mutans streptococcal infection of humans or nonhuman primates. Since active immunization with S. mutans GBP-B has been shown to induce a protective response in rats, and since this component is theoretically implicated only in the accumulation phase of mutans streptococcal colonization, it was of interest to explore the effect of passive administration of antibody to GBP-B in this model. In the present set of experiments, we show that short-term dietary administration of IgY antibody to S. mutans GBP-B diminishes the accumulation of cariogenic mutans streptococci and the resulting dental disease on molar surfaces of rats.
MATERIALS AND METHODS
GBP.
GBP-B was purified from culture supernatant of S. mutans strain SJ by anion-exchange chromatography. Bacteria were cultivated in sucrose-free chemically defined medium (S. Socransky, C. Smith, and A. Manganello, J. Dent. Res. 52:88, 1973) and subsequently removed by centrifugation. The supernatant was clarified by 0.45-μm-pore-size filtration as previously described (23, 27). The filtrate was brought to pH 6.5 with sodium hydroxide, and 0.02% sodium azide was added. This filtrate was concentrated by tangential flow ultrafiltration using a Pellicon cassette (Millipore Corp., Bedford, Mass.) and further concentrated on a 30-kDa-cutoff Minitan tangential flow ultrafilter (Millipore Corp.). The low-molecular-weight components were removed by diafiltration on the Minitan with 0.02 M sodium phosphate (pH 6.5), 0.02% sodium azide, and 3.3 mM cysteine, the last being used to prevent protein aggregation. The retained protein was subsequently centrifuged at 12,000 × g, and the supernatant was used for further enrichment.
The first stage of anion-exchange chromatography was performed on a 21.5-ml Q Sepharose HP HiLoad 16/10 column with a fast protein liquid chromatography system (Amersham Pharmacia Biotech, Piscataway, N.J.). The low-salt buffer was composed of 0.07 M NaCl, 4 M urea (molecular biology grade; Sigma), and 0.02 M bis-Tris (Ultrol grade; Calbiochem Corp., La Jolla, Calif.), pH 6.5. The high-salt buffer was the same except for the NaCl concentration (0.124 M). The gradient used was 0.15 mM NaCl per ml with a flow rate of 1.0 to 1.5 ml/min. The sample was dialyzed against low-salt buffer containing 1 mM dithiothreitol (DTT) (Sigma) prior to application to the column. The GBP-B peak eluted at approximately 55 ml. A 1 mM concentration of DTT was added to the GBP-B pooled peak. The sample was concentrated to 2 ml by ultrafiltration at 1,500 rpm in an RC3B refrigerated centrifuge (DuPont Instruments) using a CentriPrep YM-30 centrifugal device (Millipore Corp.). The concentrated sample was dialyzed against 1 mM DTT in 6 M urea and 0.02 M bis-Tris (pH 6.5), and then applied to a 1-ml anion-exchange Mono-Q HR 5/5 column (Amersham Pharmacia Biotech). GBP-B was eluted in a gradient of 0 to 0.2 M NaCl at 6.7 mM/ml, as previously described (23). A yield of 4.5 mg of GBP-B was obtained from an 8-liter culture. S. mutans GBP-B prepared in this manner migrates as a single protein band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and was used to immunize chickens.
Immunization of hens.
White Leghorn hens (Abendroth, Waterloo, Wis.) were injected subcutaneously with 6 to 12 μg of purified GBP-B mixed with 75 μg of Quil A adjuvant (Accurate Chemical and Scientific Corp., Westbury, N.Y.) in a volume of 0.5 ml. For experiment 1, hens were immunized eight times at 4-week intervals, followed by 4 months with no immunizations. For experiment 2, the same hens were boosted four more times at 4-week intervals. A total of 244 eggs were collected for antibody purification during the final 16-week phases of each immunization regimen.
IgY preparation.
Yolk antibody (IgY) was purified from the eggs by ammonium sulfate precipitation (29). Briefly, approximately 4.8 liters of yolk was diluted with 6 volumes of water at 4°C. Precipitated material was allowed to settle for 16 h. The supernatant (28 liters) was removed, clarified by the addition of sodium carbonate, (pH 9) to 10 mM, and concentrated to 4.6 liters by diafiltration (Pellicon 50-kDa-cutoff filters, 0.1 mm2; Millipore Corp.). Antibody was precipitated with 20% (wt/vol) ammonium sulfate (Amersham Pharmacia Biotech) and resuspended in 1 liter of phosphate-buffered saline (PBS). Residual salts were removed by buffer exchange with PBS, and the antibody was lyophilized. The final mass was 29 g, containing approximately 14 g of IgY (based on optical density at 280 nm and enzyme-linked immunosorbent assay [ELISA] comparison to affinity-purified IgY). Preimmune antibody was similarly isolated from yolks of nonimmunized hens.
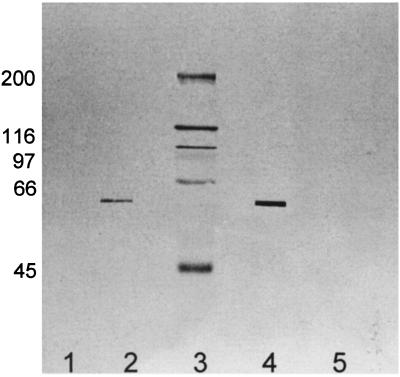
Both preimmune IgY and GBP-B antibody IgY preparations were evaluated for specificity in a Western blot (Fig. 1) against purified GBP-B and components from S. mutans SJ culture supernatant that had been bound to Sephadex G150 and eluted with 3 M guanidine HCl. The latter preparation contained all components with specificity for α-1,6-linked glucan, including GBP-A, GBP-B, GTF-C, and GTF-D. Proteins were first separated by SDS-PAGE, which was performed for 1 h at 17 mA/gel on 7% polyacrylamide gels containing 0.01% SDS with a 4% stacking gel in an air-cooled slab-gel apparatus (Mighty Small, Hoefer Scientific Instruments, San Francisco, Calif.) as previously described (23). For Western blot analysis, SDS-PAGE-separated proteins were electrophoretically transferred to nitrocellulose for 1 h at 200 mA. After blocking, the blotted proteins were incubated for 3 h with preimmune and postimmune chicken IgY reagents (30 μg/lane). Reactive bands were revealed with biotin-rabbit anti-chicken/turkey IgG (Zymed), followed by streptavidin-horseradish peroxidase (Zymed), followed by the addition of 0.05% 4-chloro-1-naphthol, 16.7% methanol, and 0.015% hydrogen peroxide. Only one band of reactivity was observed when the purified GBP-B or the guanidine-eluted mixture of components was exposed to IgY antibody. No reaction with either preparation was observed after exposure to the preimmune IgY.
FIG. 1.
Western blot analysis of preimmune IgY (lanes 1 and 5; 30 μg/ml) or GBP-B IgY (lanes 2 and 4; 30 μg/ml) preparations with purified GBP-B in lanes 4 and 5 (0.04 μg/lane) and Sephadex-purified components from S. mutans SJ culture supernatant in lanes 1 and 2 (10 μl of the concentrated eluate per lane from 500 μl of culture). This eluate includes GBP-A, GBP-B, GTF-C, and GTF-D. Lane 3 contains molecular mass standards, whose sizes (in kilodaltons) are indicated at left.
ELISA.
Antibody titers were measured by sandwich ELISA. Purified GBP-B (100 ng in 0.1 ml) was used to coat Maxisorp microtiter plates (Nalge Nunc, Rochester, N.Y.) for 16 h at 4°C. Plates subsequently were blocked with 1% gelatin (type B; Sigma) in PBS for 1 h at room temperature and washed with 0.1% Tween 20 in PBS. Anti-GPB antibody was added at 200, 100, 50, and 25 ng/ml in PBS containing 0.1% Tween 20 and 0.1% gelatin. After washing, the plates were incubated for 1 h at room temperature with alkaline phosphatase-labeled goat anti-chicken IgG at 0.5 μg/ml in PBS–0.1% Tween 20–0.1% gelatin (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.). The plates were washed again and incubated with Blue Phos phosphatase substrate (Kirkegaard and Perry Laboratories). The anti-GBP signal was twofold above background at 100 ng/ml, whereas preimmune IgY is typically unreactive up to 25 μg/ml.
Experimental protocol.
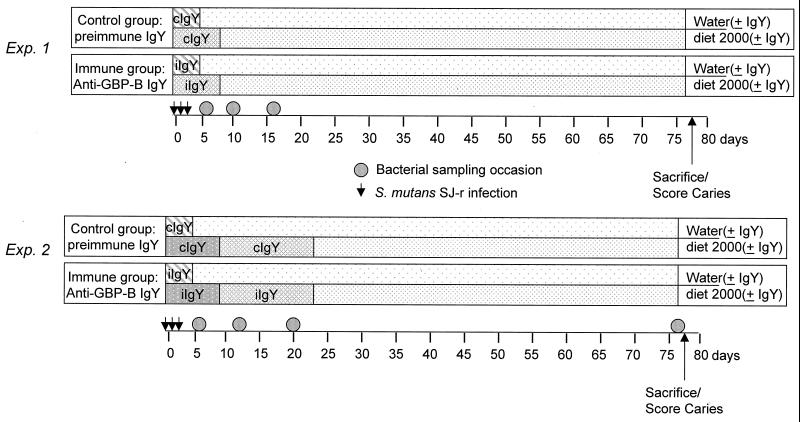
Two experiments were performed to test the effects on S. mutans colonization, accumulation, and resulting dental caries of short-term passive oral administration of IgY antibody to S. mutans GBP-B (Fig. 2). The two experiments differed in the amount of IgY in the diet and in the duration of IgY supplementation. Both experiments used male Sprague-Dawley rats from Charles River Laboratories (Kingston, N.Y.) that were 28 days old at the start of the experiments. In preliminary experiments, no indigenous mutans streptococci could be cultivated from rats obtained from this facility and maintained on high-sucrose Diet 2000 (9).
FIG. 2.
protocols for passive immunization experiments 1 and 2. Control preimmune IgY (cIgY) and GPB-B IgY (iIgY) were administered at 0.17% of the total diet weight (IgY plus Diet 2000) in the first experiment and at 0.44% (first 9 days) or 0.30% (next 14 days) of the total diet weight (IgY plus Diet 2000) in the second experiment. Drinking water contained 75 μg of the respective IgY per ml for the indicated times. Groups of 10 28-day-old rats were used in the first experiment, and 13 rats per group were used in the second experiment.
Both experiments used for infection an S. mutans strain (SJ-r) that was resistant to streptomycin sulfate at a concentration of 0.2 mg/ml. This strain was obtained by plating 0.12 ml of a 10-fold-concentrated anaerobic Todd-Hewitt (Becton Dickinson Co., Sparks, Md.; 0.3% glucose added) broth culture of strain SJ (wild type) onto mitis salivarius (MS) agar (Becton Dickinson) containing 0.2 mg of streptomycin sulfate (Sigma Chemical Co., St. Louis, Mo.) per ml. After 2 days of anaerobic incubation, resistant colonies were recultured in broth and purified by streaking onto tryptic soy agar containing 5% sheep blood (Becton Dickinson). Single colonies were then recultured in broth and stored in 50% glycerol at −20°C. Immediately prior to the experiment, broth cultures of S. mutans SJ-r were streaked onto MS agar plates with or without streptomycin and cultured anaerobically for 2 days at 37°C to confirm streptomycin resistance. Preliminary experiments indicated that the acquired antibiotic resistance did not diminish the cariogenicity of this bacterial strain in the Sprague-Dawley rat.
Rats were housed individually in suspended caging. Additional preliminary experiments using differing doses of S. mutans SJ-r to infect rats of this age revealed that a dose of 5 × 106 organisms was required to establish an infection in all animals. This level of infection was thus selected to examine the effect of dietary antibody to GBP-B on S. mutans accumulation.
Two groups of 10 rats each were used in the first experiment. Upon arrival, the 28-day-old rats were placed on Diet 2000-IgY. The control group (cIgY) received Diet 2000 supplemented with 0.17% preimmune IgY. The anti-GBP-B group (GBP-IgY) received Diet-2000 supplemented with 0.17% IgY containing antibody to S. mutans GBP-B. Rats were fed ad libitum for 9 days, consuming approximately 10 to 15 g of diet each day. On day 10, the supplemented diet was removed and rats in both groups were placed on Diet 2000 for the remaining 69 days of the experiment. On the first 3 days of the experiment, the cIgY and GBP-IgY rat groups received distilled drinking water that contained 75 μg of control or immune IgY/ml, respectively, together with 0.1% sodium benzoate. On day 4, the supplemented drinking water was removed and rats in both groups were placed on distilled water alone. All rats in the first experiment were orally infected with 5 × 106 S. mutans SJ-r bacteria on days 0, 1, and 2. The subsequent accumulations of the infecting strain, as well as total streptococcal microbiota, were sampled on days 5, 9, and 16. All animals were sacrificed on day 78 to measure the extent of dental caries.
The second experiment included two groups of 13 rats each and followed a format similar to that of experiment 1 (Fig. 2) except for the amount and duration of IgY feeding. Rats were placed on Diet 2000-IgY 1 day after arrival. For the first 9 days of experiment 2, the control group (cIgY) received Diet 2000 supplemented with 0.44% preimmune IgY. During this 9-day period, the anti-GBP-B group (GBP-IgY) received Diet 2000 supplemented with 0.44% IgY containing antibody to S. mutans GBP-B. On day 10, the amounts of IgY in the control and immune diets were decreased to 0.30% preimmune IgY or anti-GBP-B IgY, respectively. The animals were maintained on this diet until day 24, at which time the supplemented diet was removed and replaced by Diet 2000 for the remaining 54 days of the experiment. On the first 5 days of the experiment, the cIgY and GBP-IgY rat groups received distilled drinking water that contained 75 μg of control or immune IgY/ml, respectively, together with 0.1% sodium benzoate. On day 5, the supplemented drinking water was removed and rats in both groups were placed on distilled water alone. As in the first experiment, all rats in the second experiment were orally infected with 5 × 106 S. mutans SJ-r organisms on days 0, 1, and 2. The subsequent accumulations of the infecting strain, as well as total streptococcal microbiota, were sampled on days 5, 13, 20, and 78. All animals were sacrificed on day 78 to measure the extent of dental caries.
Bacterial recoveries.
Systematic swabbing of teeth, sonication, and plating to determine bacterial cell counts were all performed as previously described (27). Appropriate dilutions were placed on MS agar to count total streptococci and on MS agar with 0.2 mg of streptomycin sulfate (MSS)/ml to count the experimentally inoculated S. mutans SJ-r organisms. The numbers of mutans streptococci on these plates, as identified by colony morphology, are presented as percentages of the total number of streptococci.
Caries assessment.
The extent and depth of carious lesions in all rat molar teeth (caries scores) were microscopically evaluated by a modified Keyes' method as previously described (27). These combined caries scores were determined separately on buccal, lingual, and occlusal dental surfaces.
Statistical analysis.
The differences in the median values among the treatment groups were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey pairwise multiple comparison test when data were normally distributed. Alternatively, data were analyzed by Kruskal-Wallis one-way ANOVA on ranks, followed by Dunn's multiple comparison procedure when nonparametric distributions were encountered (e.g., bacterial CFU and caries scores).
RESULTS
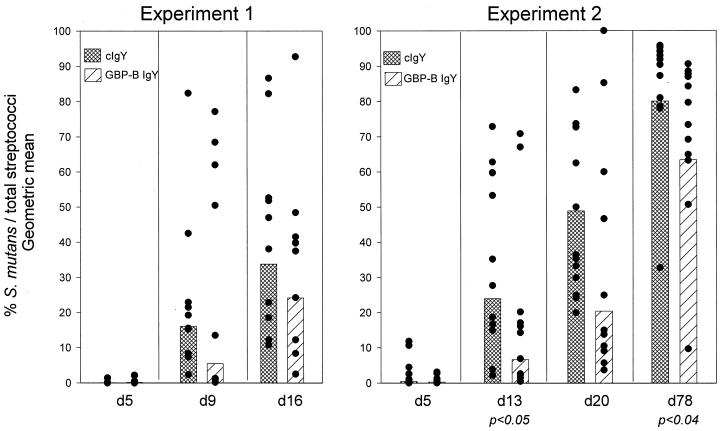
Two experiments using different dietary concentrations of antibody were performed to test the hypothesis that short-term exposure to antibody to S. mutans GBP-B can influence the accumulation of S. mutans in the rodent oral flora. In the first passive immunization experiment (experiment 1), rats were fed a dietary concentration of 1.7% preimmune control IgY (cIgY) or IgY antibody to S. mutans GBP-B (GBP-IgY) for 9 days. In addition, distilled drinking water was supplemented with cIgY or GBP-IgY in control or antibody-fed rats, respectively (Fig. 2). Rats were infected with 5 × 106 S. mutans SJ-r bacteria on days 0, 1, and 2 of the first experiment. The molars of all rats were systematically sampled for streptomycin-resistant S. mutans and for total cultivable streptococci twice during the 9-day dietary administration of antibody (days 5 and 9) and once soon after IgY was removed from the diet (day 16). The percentages of S. mutans (CFU on MSS agar) relative to total streptococci (CFU on MS agar) are shown in Fig. 3 for both groups on all sampling occasions.
FIG. 3.
Accumulation of S. mutans SJ-r on molar surfaces of rats in experiments 1 and 2 after infection with S. mutans. The days after initial infection on which the surfaces were sampled are indicated below each set of data. The level of S. mutans accumulation is shown as a percentage of the total streptococcal CFU (see Materials and Methods). Bars indicate geometric means, and closed circles represent the percentage of CFU for each animal. The levels of statistical significance (Kruskal-Wallis ANOVA) are shown below pairs of cIgY and GBP-B IgY bars.
Five days after the start of infection, S. mutans comprised less than 1% of the total streptococcal flora in both cIgY-fed (geometric mean = 0.07%) and GBP-IgY-fed (geometric mean = 0.17%) groups. Streptomycin-resistant S. mutans could be detected in all but three rats at this time. Differences between groups were not statistically significant.
Four days later (experimental day 9), S. mutans SJ-r showed appreciable accumulation in all cIgY- and GBP-IgY-fed rats (Fig. 3). The geometric mean percentage S. mutans relative to total streptococcal flora was 16.1% in the cIgY-fed rats versus 5.5 percent in the GBP-IgY-fed rats. Accumulation of S. mutans continued in both groups such that on experimental day 16, S. mutans SJ-r represented a geometric mean of 33.8% of the total streptococcal flora in the cIgY group, in contrast to a geometric mean of 24.2% in the GBP-IgY group. Thus, relative to the total streptococcal flora, S. mutans appeared to accumulate to lower levels in the GBP-IgY-fed rats than in the cIgY-fed rats on days 9 and 16, although these differences did not achieve statistical significance.
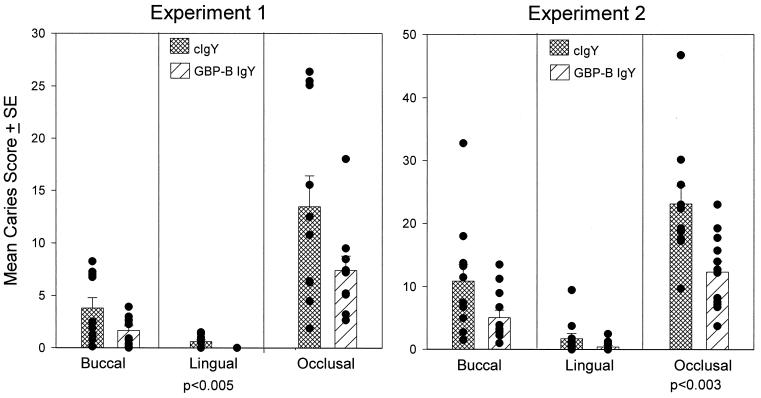
Figure 4 illustrates the extent of dental caries on buccal, lingual, and occlusal surfaces after 78 days of infection of cIgY-fed and GBP-IgY-fed rats in experiment 1. Mean caries scores on each of these surfaces of GBP-IgY-fed rats were approximately half of those observed among controls. These differences were highly significant (P < 0.005) on lingual surfaces and were nearly significant (P = 0.061) on buccal surfaces. Taken together, these data suggest that early exposure to dietary IgY antibody against S. mutans GBP-B may diminish the extent of eventual dental disease and possibly of bacterial accumulation prior to observable disease.
FIG. 4.
Buccal, lingual, and occlusal caries scores on molar surfaces of rats after 78 days of infection with S. mutans SJ-r in experiments 1 and 2. Bars and error bars indicate the means and standard errors of the individual caries scores, which are represented by closed circles. The levels of statistical significance (Kruskal-Wallis ANOVA) are shown below pairs of cIgY and GBP-B IgY bars.
To test whether increased exposure to GBP-IgY can further inhibit S. mutans accumulation or disease progression, a second passive immunization experiment was performed (experiment 2). In this experiment, the duration of exposure to cIgY or GBP-IgY was increased to 24 days, and the concentration of dietary IgY was increased to 4.4% during the initial 9 days of feeding and to 3.0% during the next 15 days of feeding. As in the first experiment, 28-day-old rats were infected with 5 × 106 S. mutans SJ-r organisms on days 0, 1, and 2. The molars of all rats were systematically sampled for streptomycin-resistant S. mutans and for total cultivable streptococci three times during the dietary administration of antibody (days 5, 13, and 20) and at the end of the experiment (day 78). The geometric mean percentage of S. mutans (CFU on MSS agar) relative to total streptococci (CFU on MS agar) are shown in Fig. 3 for both groups on all sampling occasions.
Five days after the start of infection, the geometric mean levels of S. mutans infection within the two groups were similar (cIgY = 0.43%; GBP-IgY = 0.27%), although in two of the cIgY rats, S. mutans comprised more than 10% of the total streptococcal flora. Streptomycin-resistant S. mutans could not be detected in one antibody-fed rat at this time.
Eight days later (day 13), S. mutans SJ-r showed appreciable accumulation in all cIgY- and GBP-IgY-fed rats. The mean percentage of S. mutans relative to total streptococcal flora was 24.0% in the cIgY-fed rats and 6.6% in the GBP-IgY-fed rats. These differences were significant at a P value of <0.05. S. mutans accumulation continued in both groups during dietary administration of cIgY or GBP-IgY. On day 20, S. mutans SJ-r represented a mean of 48.9% in the cIgY group in contrast to a mean of 20.4% in the GBP-IgY group. Although differences on day 20 did not achieve statistical significance, the presence of dietary IgY antibody to S. mutans GBP-B appeared to inhibit the accumulation of S. mutans SJ-r in the dental biofilm.
Most rats were heavily colonized with S. mutans at the end of the experiment (day 78). However, the percentage of S. mutans relative to total streptococcal flora was still significantly lower (P < 0.04) in the group of rats that had been initially fed GBP-IgY (geometric mean = 63.4%) than in the cIgY-fed group (mean = 80.1%), despite the fact that no dietary antibody supplements were given during the last 54 days of the experiment.
Figure 4 illustrates the extent of dental caries on buccal, lingual, and occlusal surfaces after 78 days of infection in cIgY- and GBP-IgY-fed rats. Mean caries scores on each of these surfaces of GBP-IgY-fed rats were approximately half of those observed on all comparable surfaces of rats that were fed IgY. These differences were highly significant (P < 0.003) on occlusal surfaces. Differences on smooth surfaces did not reach significance at a P value of <0.05 (buccal, P = 0.051; lingual, P = 0.086).
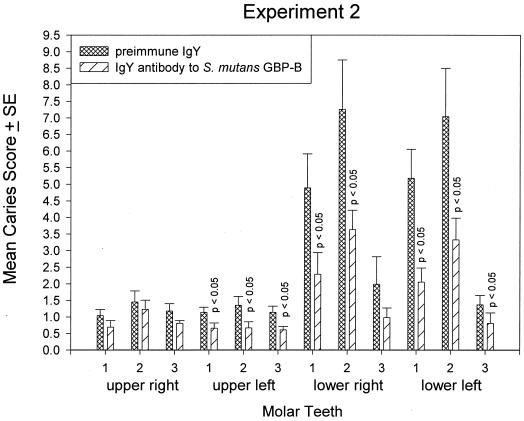
Rats in both experiments were infected 29 days after birth. The third molars of the rat erupt into the oral cavity on day 35 (22). Thus, the colonization potential of these nascent surfaces by S. mutans SJ-r may be different from that of the earlier-erupting first and second molars, which had been initially colonized in the absence of this strain. To examine whether molar eruption in the presence or absence of S. mutans infection and/or antibody ultimately influenced the extent of disease, the caries scores of all molar teeth of the rats in experiments 1 and 2 were calculated with respect to individual teeth. The mean caries scores for individual teeth in both experiments were lower in the group given dietary GBP-IgY, supporting the modulating effect of antibody on disease seen when data were calculated with respect to surface (Fig. 4). Differences in caries scores were significant in 2 of 12 teeth in experiment 1 and 8 of 12 teeth in experiment 2 (Fig. 5), indicating that increasing the amount and duration of dietary antibody application increased the protective effect. Significant effects were most often observed in mandibular molars in both experiments. The earlier-erupting first (day 19) and second (day 22) molars were more significantly protected by dietary antibody than were the third molars, which erupted during infection.
FIG. 5.
Mean caries scores on molar teeth of rats in experiment 2 after 78 days of infection with S. mutans SJ-r. Bars and error bars indicate the means and standard errors of the respective cIgY or GBP-B IgY group. Control and immune molar pairs whose group caries scores differ significantly (P < 0.05 [Kruskal-Wallis ANOVA]) are indicated.
DISCUSSION
The results of this study indicate that passive administration of antibody to S. mutans GBP-B can inhibit the accumulation of S. mutans in the dental biofilm and can provide a level of protection from dental caries caused by these organisms. This finding parallels the effectiveness of active immunization with GBP-B, via systemic (27) or mucosal (24) routes, in modulating mutans streptococcal accumulation and disease. The present study, however, is the first to demonstrate that antibody to GBP-B can be protective via passive administration. Furthermore, the protective effect was achieved with the administration of antibody for less than one-third of the infection period, in contrast to other rat model studies in which antibody to intact cells (18) or to GTF (5) was continuously administered throughout the infection period. The protective effect of GBP-B antibody was improved by increasing the concentration and duration of dietary exposure to antibody. Hamada and coworkers (5) found that the protective effects of IgY antibody to cell-associated GTF occurred at or above IgY dietary concentrations of 0.1%, consistent with our observations that protection could be observed with 0.17 or 0.44% IgY antibody in the diet.
Previous studies by Ma and coworkers (13, 14) also demonstrated a prolonged protective effect from infection and disease well after exposure to antibody had ceased. In those studies, the teeth were made aseptic using topical antibacterial mouthwash. The surfaces were then discontinuously exposed for 3 weeks to high concentrations of mouse (13) or plant (14) antibody to S. mutans antigen I/II adhesin during mutans streptococcal recolonization. Detectable recolonization with indigenous mutans streptococci was prevented for up to 4 months after the topical antibody treatment. Although the mechanism for the long-term effects of the 3-week topical monoclonal antibody exposure is unclear, the inhibition of recolonization in the presence of monoclonal antibody can be assumed to result from blocking of mutans streptococcal adhesin, thus preventing the initial colonization phase in which antigen I/II participates. Accessory immune effects of monoclonal antibody treatment were unlikely, since F(ab′)2 fragments of the mouse IgG monoclonal antibody were also effective, as was the transgenic secretory IgA monoclonal antibody, which is not expected to fix complement (14).
The present study also demonstrated a long-term protective effect after limited (9- or 24-day) exposure to IgY antibody against S. mutans GBP-B, another component putatively involved in the molecular pathogenesis of dental caries. This study lends further support to the concept that short-term passive treatment with antibody of appropriate specificity may have immunotherapeutic efficacy for dental caries. Others have suggested that passive immunization can increase active immune responses to infective antigens. Ramisse and coworkers (19) reported that passive intranasal administration of polyvalent human immunoglobulin to mice, given during a Streptococcus pneumoniae infection, resulted in a convalesent immune response to S. pneumoniae antigens that was higher than that in infected-only controls.
The present IgY anti-GBP-B study differs from the monoclonal antiadhesin studies of Ma and coworkers (13–15) on two main points. Firstly, antibody interactions with newly colonizing mutans streptococci were far more favorable in the monoclonal study. The total bacterial load, including the indigenous mutans streptococci, had been drastically lowered by chlorhexidine treatment prior to antibody exposure. Subsequent bacterial challenge either by external exposure or by surviving indigenous mutans streptococci would be expected to be quite modest, thus favoring the inhibiting effect of monoclonal antibody that periodically bathed the chemically treated teeth during the 3-week period. In contrast, in the present experiments dietary IgY antibody to GBP-B functioned in an untreated oral environment and in the presence of a fairly vigorous (three doses of 5 × 106 bacteria) mutans streptococcal challenge.
In the present experiments, passive antibody was present during the eruption of the rats' third molars, which were exposed both to the indigenous microbiota and to the exogenous S. mutans SJ-r infecting strain. This condition may be expected to favor the inhibitory effects of dietary IgY antibody if uncolonized surfaces are more readily protected than those surfaces on which a dental biofilm is already fully developed. However, analysis of the effect of passive antibody treatment with respect to dental caries on individual teeth did not support this notion (Fig. 5). Rather, these results suggested a greater influence on the first molars, which had erupted earliest and were thus exposed to the indigenous microbiota prior to treatment with IgY. This observation would then suggest that interfering with the colonization or accumulation of superinfecting mutans streptococcal challenge may be easier than interfering with the initial challenge, at least in this model system.
Secondly, the mechanism(s) of protection with antibody to GBP-B is likely to be different from that with antibody to antigen I/II (13–15) or other components of S. mutans. The antiadhesin specificity of the monoclonal antibodies presumably blocks initial colonization events. Other studies, using passive immunization with antibody to GTF, have shown a protective effect on bacterial accumulation and subsequent dental caries in the rat model (5). Since GTF has both catalytic and glucan binding domains (1, 12, 20, 30), antibody to this component could be expected to block the synthetic ability as well as other aspects of protein-glucan interactions. The glucan binding domains of GTF may function primarily in glucan branching and chain lengthening, rather than in attachment of mutans streptococci to the glucan matrix. Antibody to S. mutans GBP-B, used in the present study, has specificity for a glucan binding protein whose primary sequence (8a) is quite different from those found in the putative glucan binding domains of GTF or of the constitutively secreted S. mutans GBP-A, which has sequence similarity with the putative glucan binding domains of GTF (2). Thus, the inhibitory activity of antibody to GBP-B on mutans streptococcal accumulation would be expected to be independent of that due to antibody to antigen I/II or GTF. It cannot be excluded, however, that antibody to GTF, GBP-A, GBP-B, or antigen I/II functions in whole or in part by promoting immune aggregation of planktonic mutans streptococci, thus decreasing or eliminating their ability to incorporate into the dental biofilm. Support for this concept derives from the observation that S. mutans grown in the presence of monoclonal antibody forms long chains and is associated with clumping of cells (15). Similarly, Hamada and coworkers (5) found that IgY antibody to cell-associated GTF also aggregates S. mutans in vitro.
These studies support the use of oral passive immunization to interfere with S. mutans accumulation and subsequent development of dental caries. Furthermore, S. mutans GBP-B is identified as a new target, thus increasing the number of pathways by which passive immunization can achieve a protective effect.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DE-06153 from the National Institute of Dental and Craniofacial Research and by Ophidian Pharmaceuticals, Inc.
We also acknowledge the expert technical assistance of Leigh Barnes.
REFERENCES
- 1.Abo H, Matsumura T, Kodama T, Ohta H, Fukui K, Kato K, Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase) J Bacteriol. 1991;173:989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas J A, Russell R R B, Ferretti J J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filler S J, Gregory R L, Michalek S M, Katz J, McGhee J R. Effect of immune bovine milk on Streptococcus mutans in human dental plaque. Arch Oral Biol. 1991;36:41–47. doi: 10.1016/0003-9969(91)90052-v. [DOI] [PubMed] [Google Scholar]
- 4.Haas W, Banas J A. Ligand-binding properties of the carboxyl-terminal repeat domain of Streptococcus mutans glucan-binding protein A. J Bacteriol. 2000;182:728–733. doi: 10.1128/jb.182.3.728-733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada S, Horikoshi T, Minami T, Kawabata S, Hiraoka J, Fujiwara T, Ooshima T. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect Immun. 1991;59:4161–4167. doi: 10.1128/iai.59.11.4161-4167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, Hirasawa M, Katz J, Childers N K, Michalek S M. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997;31:268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- 8.Hazlett K R O, Mazurkiewicz J E, Banas J A. Inactivation of the gbpA gene of Streptococcus mutans alters structural and functional aspects of plaque biofilm which are compensated by recombination of the gtfB and gtfC genes. Infect Immun. 1999;67:3909–3914. doi: 10.1128/iai.67.8.3909-3914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Jin S, Duncan M J, Taubman M A, Smith D J. Cloning of the gbpB gene from Streptococcus mutans. J Dent Res. 2000;79:224. [Google Scholar]
- 9.Keyes P, Jordan H V. Periodontal lesions in the Syrian hamster. III. Findings related to an infectious and transmissible component. Arch Oral Biol. 1964;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- 10.Landale E C, McCabe M M. Characterization by affinity electrophoresis of an α-1,6-glucan-binding protein from Streptococcus sobrinus. Infect Immun. 1987;55:3011–3016. doi: 10.1128/iai.55.12.3011-3016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehner T, Caldwell J, Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985;50:796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lis M, Shiroza T, Kuramitsu H K. Role of the C-terminal direct repeating units of the Streptococcus mutans glucosyltransferase-S in glucan binding. Appl Environ Microbiol. 1995;61:2040–2042. doi: 10.1128/aem.61.5.2040-2042.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J K, Lehner T. Prevention of colonization of Streptococcus mutans by topical application of monoclonal antibodies in human subjects. Arch Oral Biol. 1990;35(Suppl.):115S–122S. doi: 10.1016/0003-9969(90)90140-6. [DOI] [PubMed] [Google Scholar]
- 14.Ma J K, Hikmat B Y, Wycoff K, Vine N D, Chargelegue D, Yu L, Hein M B, Lehner T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998;4:601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- 15.Ma J K-C, Hunjan M, Smith R, Kelly C, Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect Immun. 1990;58:3407–3414. doi: 10.1128/iai.58.10.3407-3414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Lassiter M O, Banas J A, Galperín M Y, Taylor K G, Doyle R J. Multiple glucan-binding proteins of Streptococcus sobrinus. J Bacteriol. 1996;178:1572–1577. doi: 10.1128/jb.178.6.1572-1577.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalek S M, McGhee J R. Effective immunity to dental caries: passive transfer to rats of antibodies to Streptococcus mutans elicits protection. Infect Immun. 1977;17:644–650. doi: 10.1128/iai.17.3.644-650.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otake S, Nishahara Y, Makinura M, Hatta H, Kim M, Yamamoto T, Hirasawa T. Protection of rats against dental caries by passive immunization with hen-egg yolk antibody (IgY) J Dent Res. 1991;70:162–166. doi: 10.1177/00220345910700030101. [DOI] [PubMed] [Google Scholar]
- 19.Ramisse F, Binder P, Szatanik M, Alonso J M. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J Infect Dis. 1996;173:1123–1128. doi: 10.1093/infdis/173.5.1123. [DOI] [PubMed] [Google Scholar]
- 20.Russell R R B, Shiroza T, Kuramitsu H K, Ferretti J J. Homology of glucosyltransferase gene and protein sequences from Streptococcus sobrinus and Streptococcus mutans. J Dent Res. 1988;67:543–547. doi: 10.1177/00220345880670030401. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Yamamoto Y, Kizaki H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1997;65:668–675. doi: 10.1128/iai.65.2.668-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schour I, Massler M. The teeth. In: Farris E, Griffith J, editors. The rat in laboratory investigation. New York, N.Y: Hafner Publishing Co.; 1962. p. 106. [Google Scholar]
- 23.Smith D J, Akita H, King W F, Taubman M A. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1994;62:2545–2552. doi: 10.1128/iai.62.6.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D J, Heschel R, Melvin J, King W F, Pereira M B B, Taubman M A. Streptococcus mutans glucan binding proteins as dental caries vaccines. In: Husband A J, Beagley K W, Clancy R L, Collins A M, Cripps A W, Emery D L, editors. Mucosal solutions: advances in mucosal immunology. Vol. 2. Sydney, Australia: University of Sydney Press; 1997. pp. 367–377. [Google Scholar]
- 25.Smith D J, King W F, Akita H, Taubman M A. Association of salivary IgA antibody and initial mutants streptococcal infection. Oral Microbiol Immunol. 1998;13:278–285. doi: 10.1111/j.1399-302x.1998.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith D J, King W F, Wu C D, Shen B I, Taubman M A. Structural and antigenic characteristics of Streptococcus sobrinus glucan binding proteins. Infect Immun. 1998;66:5565–5569. doi: 10.1128/iai.66.11.5565-5569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D J, Taubman M A. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–3073. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith D J, Taubman M A. Vaccines against dental caries infection. In: Levine M M, Woodrow G C, Kaper J B, Coban G S, editors. New generation vaccines. 2nd. ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 914–930. [Google Scholar]
- 29.Svendsen L, Crowley A, Ostergaard L H, Stodulski G, Hau J. Development and comparison of purification strategies for chicken antibodies from egg yolk. Lab Anim Sci. 1995;45:89–93. [PubMed] [Google Scholar]
- 30.Wong C, Hefta S A, Paxton R J, Shively J E, Mooser G. Size and subdomain architecture of the glucan-binding domain of sucrose: 3-α-d-glucosyltransferase from Streptococcus sobrinus. Infect Immun. 1990;58:2165–2170. doi: 10.1128/iai.58.7.2165-2170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu-Yuan C D, Gill R E. An 87-kilodalton glucan-binding protein of Streptococcus sobrinus B13. Infect Immun. 1992;60:5291–5293. doi: 10.1128/iai.60.12.5291-5293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]