Abstract
Purpose
To determine whether microRNA could be a therapy target of erectile dysfunction (ED) and the underlying mechanisms.
Materials and Methods
Eight-week-old fasting male SD rats were intraperitoneally injected with streptozotocin to construct diabetic rat models. Diabetic ED rats were treated with miRNA-92a inhibitor. The cavernous nerves were electrically stimulated to measure the intracavernous pressure and mean arterial pressure of rats in each group. After the detection, the penile cavernous tissues are properly stored for subsequent experiments. Rat aortic endothelial cells were used in in vitro studies.
Results
The expression of miR-92a was significantly increased in the corpus cavernosum of Streptozocin (STZ)-induced diabetic rats and injection of miR-92a antagomir into the corpus cavernosum of diabetic rats significantly increased eNOS/NO/cGMP signaling pathway activities, cavernous endothelial cell proliferation, endothelial cell-cell junction protein expression and decreased the levels of oxidative stress. These changes restored erectile function in STZ-induced diabetic rats. Moreover, in vitro study demonstrated that the miR-92a expression increased significantly in endothelial cells treated with high glucose, inhibiting AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathways in rat aortic endothelial cells via targeting Prkaa2, causing endothelial dysfunction and overactive oxidative stress, miR-92a inhibitor can improve the above parameters.
Conclusions
miRNA-92a inhibitor could exert an inhibition role on oxidative stress and endothelial dysfunction to improve diabetic ED effectively.
Keywords: Endothelial cells, Erectile dysfunction, microRNA, Molecular targeted therapy, Oxidative stress
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability of the penis to reach or maintain sufficient rigidity to complete satisfactory sexual intercourse [1]. ED is a common male disease, and there are hundreds of millions of men worldwide with varying degrees of ED [2,3]. ED has a close relationship with diabetes mellitus, the incidence of ED in male diabetic patients is as high as 75% [4], and the symptoms in these patients appear earlier and are more severe. The prevalence of diabetes is increasing rapidly. The International Diabetes Federation predicts that by 2040, the number of patients with diabetes worldwide will increase to 642 million, and the number of men with diabetes-related ED will also increase significantly [5]. At present, phosphodiesterase 5 inhibitors (PDE5is) can significantly improve erectile function and quality of life in most ED patients. This targeted therapy restores erectile function mainly by inhibiting the degradation of cGMP in smooth muscle cells of the corpus cavernosum (CC), promoting continuous relaxation of smooth muscle and thus maintaining the erection. However, PDE5is are less effective in diabetic ED (DMED) patients [6]. Studies have shown that the effective rate of treatment with PDE5is in nondiabetic ED patients is 85%, while the effective rate of first-time treatment with PDE5is in patients with type 2 diabetes ED is only 56% [7]. Therefore, there is an urgent need to explore new targets for ED treatment in diabetes.
MicroRNAs (miRNAs) are a class of noncoding and highly conserved small-molecule RNAs with a length of approximately 20 to 25 nucleotides [8]. By complementary base pairing with the 3′ untranslated region (3'UTR) of the target mRNA, it degrades target mRNA or inhibits protein translation, thus regulating the expression of the target gene. Numerous studies have proven that miRNAs play important roles in the development of diabetes mellitus [9,10,11], especially in diabetic angiopathies such as diabetic retinopathy, diabetic nephropathy and coronary arterial disease [12]. ED is a common complication of diabetes mellitus and the pathogenesis has a close relationship with diabetic angiopathies. It has been reported that miRNAs can regulate some key procedures during erection. For example, miRNAs can regulate nitric oxide synthase (NOS) activity, miR-92a was reported to decrease NOS activity [13], thereby affecting the bioavailability of nitric oxide (NO); miRNAs can regulate the function of endothelial cells and smooth muscle cells, inhibition of miR-92a could improve re-endothelialization and prevent neointima formation [14]; and miRNAs can also regulate the function of androgen receptors, miR-30b-3p and miR-30d-5p were reported as direct regulators of androgen receptor [15]. Therefore, the biological effects of miRNAs are highly related to the pathological mechanism of ED. However, only limited studies have focused on the function of miRNAs in DMED. In this study, we tried to identify the key miRNAs that regulate the development of DMED, to clarify the underlying mechanisms and to find a potential therapeutic method.
MATERIALS AND METHODS
1. Animals and treatments
Eighty 8-week-old male Sprague-Dawley rats were used in this study. Streptozocin (60 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and the vehicle (0.1 mol/L citrate-phosphate buffer, pH=4.2) were injected intraperitoneally into 70 rats and 10 rats, respectively. At 3 and 7 days after the injection, the blood glucose levels were measured. Only rats with ≥16.7 mmol/L fasting glucose levels at both measurements were considered to have diabetes. At 12 weeks, 62 of the 70 rats injected with streptozotocin had survived. Then we conducted an apomorphine (APO) test [16] to determine the rats with DMED. Briefly, each rat was injected subcutaneously of APO (80 mg/kg; Sigma-Aldrich) in the loose skin of neck. Erectile responses, determined as a distended and congested gland, were recorded for 30 minutes. Rats that did not exhibit an erectile response were considered DMED rats. Thirty rats with DMED were selected and divided into 3 groups, the DMED group, the DMED+miRNA-92a antagomir group, and the DMED+miRNA-92a antagomir control group, n=10 in each group. DMED rats were anesthetized via pentobarbital sodium (40 mg/kg, intraperitoneally) and placed in a supine position. After the penis was exposed, an elastic band was tied at the base of the penis. A 10 nmol miRNA-92a antagomir or antagomir control (total volume 50 µL; RiboBio, Guangzhou, China) was injected into the CC. The elastic band was removed 3 minutes after the injection. The injection was administered every 3 days for 2 weeks.
2. Evaluation of erectile function
The rats were anesthetized via intraperitoneal injections of pentobarbital sodium (40 mg/kg). A PE-50 tube intubated into the carotid artery was used for continuous monitoring of the mean arterial pressure (MAP), and a 25-gauge needle inserted into the right crura was used to monitor the intracavernous pressure (ICP). Cavernous nerves were then isolated and stimulated continuously for 1 minute at a frequency of 15 Hz and voltages of 2.5 V and 5 V, with a 3-minute interval before the next stimulation. The maximal ratio of ICP to MAP and the area under the ICP curve (AUC) were calculated and analyzed to evaluate erectile function. After the measurement, the CC of each rat was cut into 4 to 5 pieces, which were immediately frozen in liquid nitrogen and stored at -80℃ or fixed overnight in 4% paraformaldehyde (Beyotime Biotechnology, Shanghai, China) and then were embedded in paraffin for further experiments.
3. Microarray analysis of miRNAs
The microarray analysis was used to compare the expression profiles of miRNAs in the CC of DMED rats and normal rats. Specifically, the ligation mixture was added to the dephosphorylated RNA samples and incubated for 2 hours. After the samples were dehydrated and re-dissolved, we added GE Blocking Agent (10×) and Hi-RPM Hybridization Buffer (2×) (Aligent, Santa Clara, CA, USA) to the samples and incubated them in a 100℃ dry thermostat for 6 minutes. Then, we used 45 µL of the mixture to assemble the SureHyb chamber (Aligent, Santa Clara, CA, USA) and placed it on the hybridization oven for 20 hours with 55℃ and 20 rpm. After hybridization the chips were washed and put into the slide holder, and Agilent Scan Control Software was used to scan the chips and obtain data.
4. Cell line and reagents
Rat aortic endothelial cells were obtained from American Type Culture Collection (ATCC) and maintained in DMED cell culture medium supplemented with 10% fetal bovine serum in a 37℃ cell culture incubator with 5% CO2. The miR-92a mimic and miR-92a inhibitor were from RiboBio. The transfection was conducted using the complexes of Lipofectamine 3000 (Invitrogen, Waltham, MA, USA) and miR-92a inhibitor (100 nM)/mimic (50nM) or negative controls according to the manufacturer’s instructions. Dorsomorphin (compound C) was obtained from SelleckChem (Houston, TX, USA).
5. Prediction of targeted genes via bioinformatics
The miRanda database [17] (http://www.microrna.org/microrna/home.do) and the TargetScan database [18] (http://www.targetscan.org/vert_72/) were used to predict the target genes of miR-92a, and the two prediction results were intersected (Supplemetary Table 3), which were further enriched by Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway analysis (https://www.kegg.jp), and the genes in the most relevant pathways were selected for further analysis.
6. Luciferase activity assay
HEK293 cells were cotransfected with the miR-92a or the control vector and wild type or the mutant Prkaa1/Prkaa2 3'UTR plasmid combined with pcDNA-R124P-HA or pcDNA-Scramble-HA. Cells lysates were used for detecting firefly and Renilla luciferase activities by using a dual-luciferase reporter assay kit (Promega, Madison, WI, USA) according to the manufacturer's protocol. The normalized values (Renilla/firefly activity) were used for the analysis. Experiments were performed in triplicate.
7. Statistical analysis
Data are presented as the mean±SEM. The Wilcoxon rank-sum test was used for data with non-nonnormal distributions or data with small sample sizes such as quantitative real-time polymerase chain reaction (qRT-PCR) analyses. Statistical analyses were carried out using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer’s test for post hoc comparisons. Data were analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). Differences among groups were considered statistically significant at p<0.05.
8. Ethics statement
All animal experiments were conducted in accordance with the accepted standards of humane animal care approved by the Academic Administration Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. All animal experiments were conducted based on the guidelines of the Academic Animal Care and Use Committee. Institutional animal care and use committee (IACUC) approval is required (No. 81501246).
RESULTS
1. Metabolic parameter
The body weights and blood glucose levels of each group are shown in Table 1. The results showed that before streptozocin instead injection the body weights of each group ranged from 182 g to 214 g, and blood glucose ranged from 3.8 mmol/L to 5.6 mmol/L. No difference was observed among all rats. Before harvesting, the body weights of DMED rats, including the DMED, DMED+miR-92a antagomir, and DMED+miR-92a antagomir control groups, were significantly compared with those of the control group, with no differences among all the three DMED groups. In contrast, the fasting blood glucose level increased significantly in all the three DMED groups compared with the control group.
Table 1. Metabolic parameters.
| Group | Initial weight (g) | Final weight (g) | Initial fasting glucose (mmol/L) | Final fasting glucose (mmol/L) |
|---|---|---|---|---|
| Control | 193.59±7.21 | 451.2±40.71 | 4.79±0.72 | 4.83±0.48 |
| DMED | 200.26±8.80 | 224.11±18.17* | 4.98±0.48 | 27.24±3.36* |
| DMED+miR-92a antagomir | 197.06±10.64 | 224.93±20.02* | 5.25±0.37 | 27.42±2.89* |
| DMED+miR-92a antagomir control | 200.91±10.82 | 222.18±19.35* | 4.85±0.48 | 28.48±2.93* |
Values are present as mean±SEM.
DMED: diabetic erectile dysfunction.
*p<0.05 when compared with the control group (n=10 per group).
2. The miR-92a expression increased in the corpus cavernosum of diabetic erectile dysfunction rats and diabetic endothelial cells
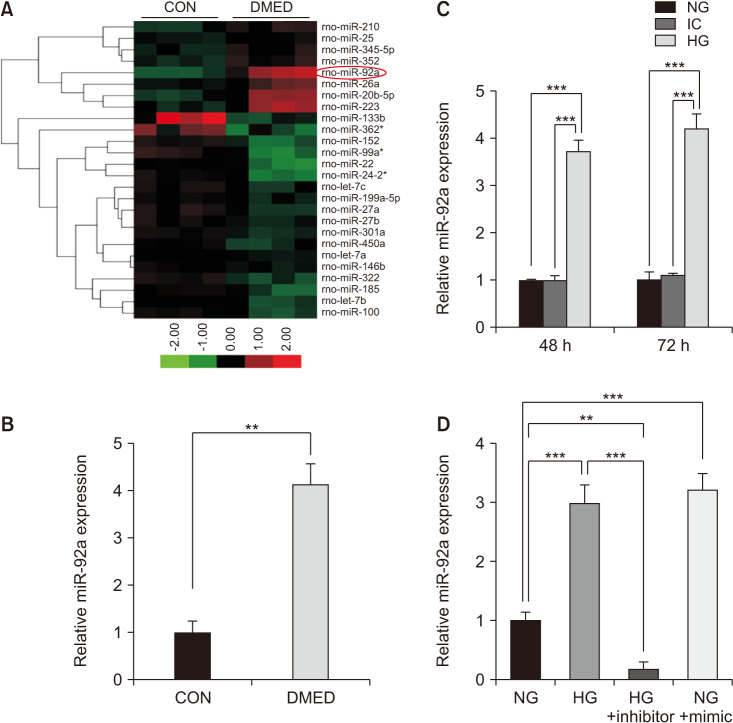
The RNA expression profiling analyzed by Agilent Rat miRNA V18.0 showed that 13 miRs changed significantly (2-fold above or 1/2 below) in the DMED group compared with the control group, in which 3 miRs changed 3-fold above or 1/3 below (Fig. 1A). All of the normalized reads and fold changes of the 13 miRs are shown in Supplement File 1 (Supplement Table 1, 2). Using qRT-PCR analysis, it was validated that the expression of miR-92a increased significantly in the CC of the DMED group (Fig. 1B). Similar results were also observed in the endothelial cells treated with high glucose (HG; 48 h and 72 h after treatment). Moreover, the expression of miR-92a was higher in the endothelial cells treated with HG for 72 hours than that at 48 hours, however, the difference was not significant (Fig. 1C). It was also determined that the miR-92a inhibitor significantly inhibited the expression of miR-92a in the endothelial cells, and the miR-92a mimic increased the expression of miR-92a, similar to the endothelial cells treated with HG (Fig. 1D).
Fig. 1. Elevation of miR-92a in the corpus cavernosum of DMED rats and diabetic endothelial cells. (A) Expression of miRNAs changed 3-fold above or 1/3 below in the corpus cavernosum of diabetic rats compared with CON group (n=4 per group). (B) qRT-PCR results of miR-92a expression in control group and DMED group (n=5). (C) qRT-PCR analysis showing increased expression of miR-92a in the endothelial cells treated with HG (48 h and 72 h after treatment) (n=4–5). (D) miR-92a inhibitor treatment decreased the expression of miR-92a in the endothelial cells treated with HG, while miR-92a mimic induced the expression of miR-92a in the endothelial cells treated with NG. Data are mean±SEM. **p<0.01, ***p<0.001. qRT-PCR: quantitative real-time polymerase chain reaction, CON: control, DMED: diabetic erectile dysfunction, NG: normal glucose (5 mmol/L), IC: isotonic control, HG: high glucose (30 mmol/L).
3. The miR-92a antagomir protected against diabetic erectile dysfunction
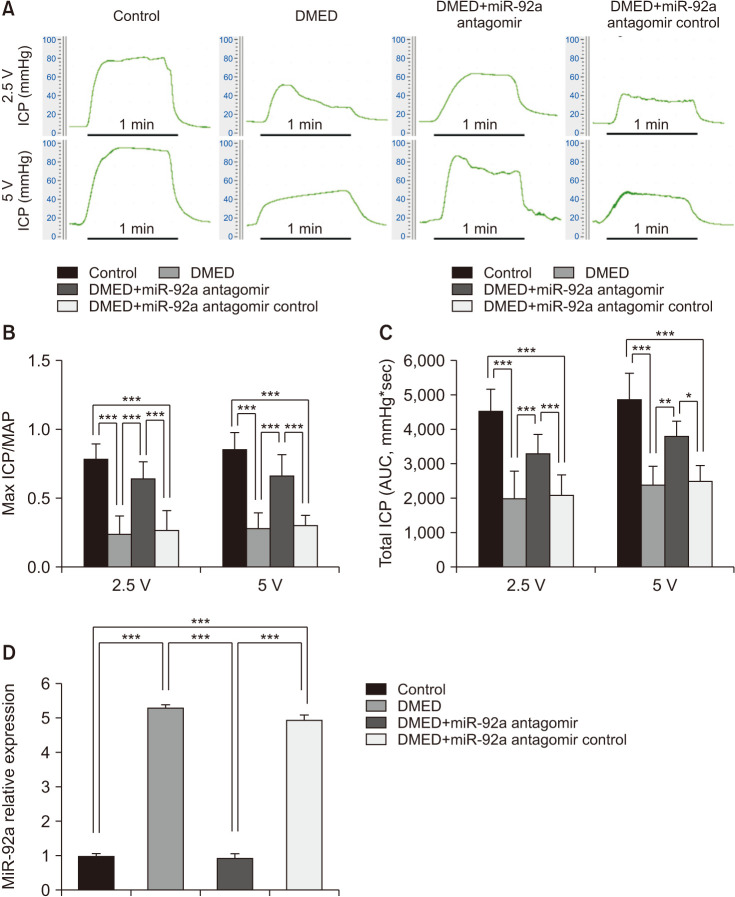
The ratio of maximum ICP/MAP in the DMED group was sharply attenuated compared with that of the control group (p<0.001). However, erectile function was partially restored in the miR-92a antagomir treatment group, although it was still lower than that in the control group, while the miR-92a antagomir control showed no protective effect (Fig. 2A, 2B). The AUCs at 2.5 and 5 V in all 4 groups exhibited the same trend as the maximum ICP/MAP values (Fig. 2C). Moreover, qRT-PCR analysis showed that the expression of miR-92a decreased significantly in the miR-92a antagomir treatment group compared with the DMED group, and the expression level was similar to the control group. The expression level of miR-92a in the antagomir control treatment group showed no differences from that in the DMED group (Fig. 2D).
Fig. 2. The miR-92a antagomir improved erectile function via decreased the expression of miR-92a in the corpus cavernosum of DMED rats. (A) Representative ICP tracing through stimulation of 2.5 volts and 5 volts for 1 minute, respective, in each group (n=10 per group). (B) The max ratio of ICP to MAP of different vols stimulation to cavernous nerve in each group (n=8–10 per group). (C) The total ICP (AUC) of different vols stimulation to cavernous nerve in each group (n=8–10 per group). (D) qRT-PCR analysis showing decreased expression of miR-92a in the corpus cavernosum of DMED rats treated with miR-92a antagomir (n=4–5 per group). Data are mean±SEM. *p<0.05, **p<0.01, ***p<0.001. DMED: diabetic erectile dysfunction, ICP: intracavernous pressure, MAP: mean arterial pressure, AUC: area under curve.
4. miR-92a antagomir improved erectile function via amelioration of endothelial function
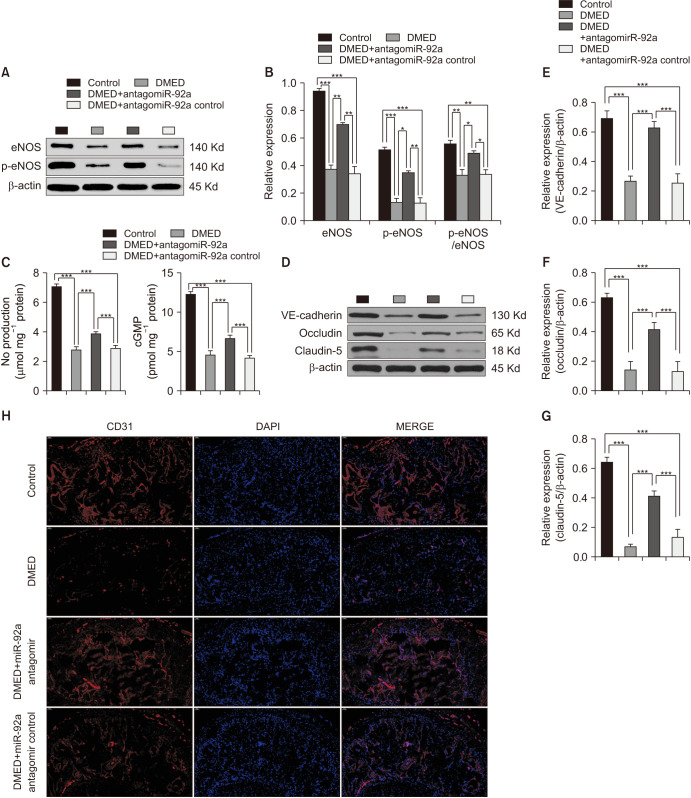
Western blotting was performed to test the expression of eNOS and p-eNOS (Ser1177). The results demonstrated a lower expression of eNOS and p-eNOS (Ser1177) in the DMED group than in the control group. However, the miR-92a antagomir partly enhanced the expression of eNOS and p-eNOS (Ser1177) compared with the DMED group (Fig. 3A, 3B). In addition, NO and cGMP levels were also tested in the CC of all four groups, which showed similar trends as the expression of eNOS and p-eNOS (Fig. 3C). Moreover, immunofluorescence and western blotting were both conducted to determine the expression of target proteins, which indicated the function of endothelial junctions. We found that target protein (VE-cadherin, occludin, and claudin-5) expression was lower in the DMED group than in the control group, while there was a great increase in the DMED+miR-92a antagomir group compared with the DMED group (Fig. 3D-3G). Immunofluorescence result of cavernous tissues with an antibody against CD31 was performed in all four groups, and a similar trend was observed for the expression of endothelial junction proteins (Fig. 3H). Together, miR-92a antagomir improved erectile function by ameliorating the endothelial function. Furthermore, as the cavernous nerve function was as important as the endothelial function in the process of erection, the cavernous nerve function was also determined in the CC of all four groups. Western blotting and immunofluorescence results indicated that the miR-92a antagomir might exert a neuroprotective effect on the cavernous nerve function, as the miR-92a antagomir could partly restore the expression of nNOS in the CC of DMED rats (Supplement Fig. 1). An in vitro study showed similar results in endothelial cells. Inhibition of miR-92a improved endothelial function via amelioration of the AMPK/eNOS signaling pathway (Supplement Fig. 2A, 2B, 2D).
Fig. 3. The miR-92a antagomir improved erectile function via amelioration of eNOS/NO/cGMP signaling pathway and endothelial cell-to-cell junctions. (A) Representative western blot results of eNOS and p-eNOS in the corpus cavernosum of all rats. (B) The expression levels of eNOS and p-eNOS, with β-actin as the loading control, and the relative ratio of p-eNOS/eNOS in the corpus cavernosum of each group are presented as bar graphs (n=6–8 per group). (C) NO and cGMP levels were determined in all four groups (n=4–5 per group). (D) Representative western blot results of VE-cadherin, occludin, and claudin-5 in the corpus cavernosum of all rats. (E-G) The expression levels of VE-cadherin, occludin, and claudin-5, with β-actin as the loading control, in the corpus cavernosum of each group are presented as bar graphs (n=6–8 per group). (H) Representative immunofluorescence result of CD31 in the corpus cavernosum of rats of all four groups. Representative immunofluorescence result of CD31 in the corpus cavernosum of rats of all four groups at ×200 magnification. Data are mean±SEM. *p<0.05, **p<0.01, ***p<0.001. DMED: diabetic erectile dysfunction, NO: nitric oxide.
5. The miR-92a antagomir improved erectile function via inhibition of oxidative stress
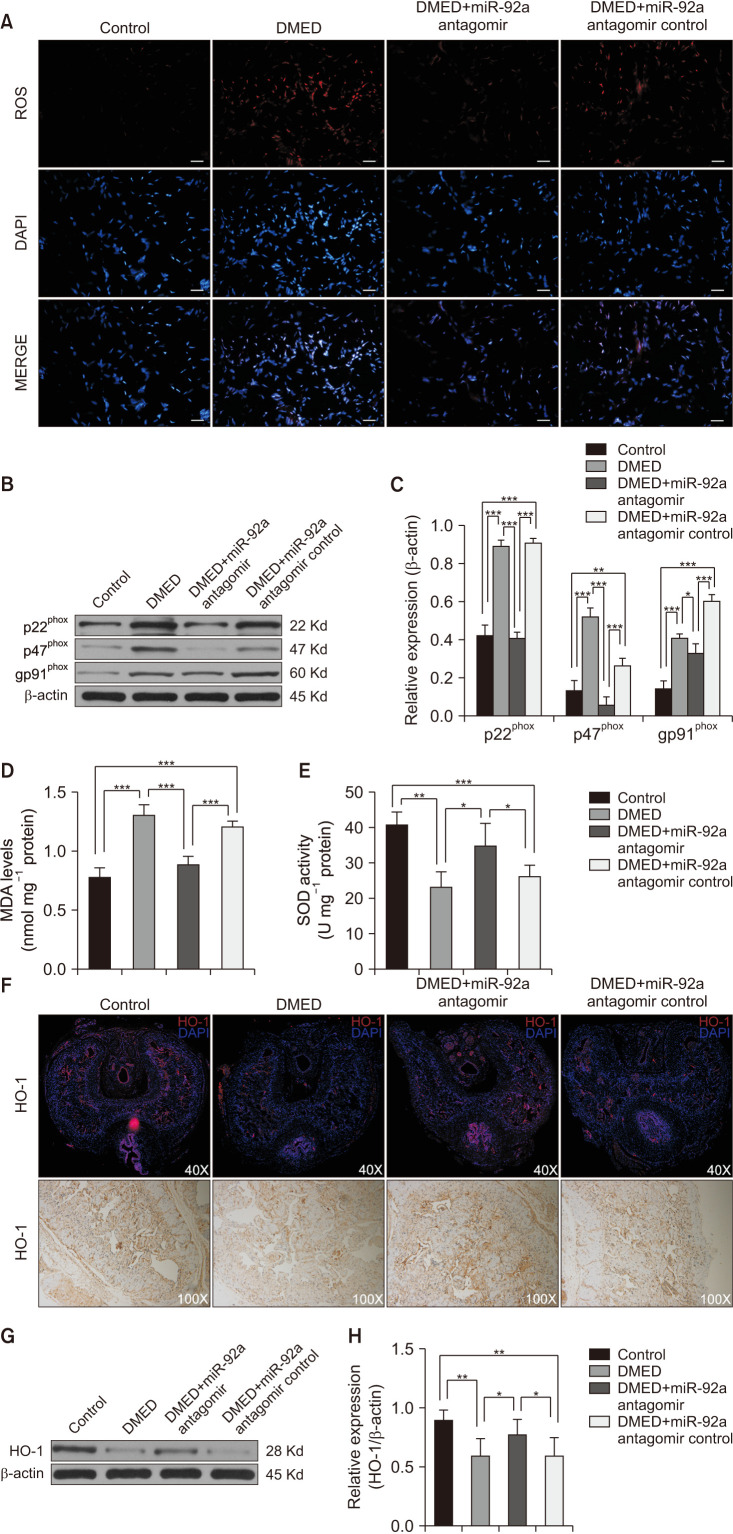
The intracellular reactive oxygen species (ROS) of CC was detected by a fluorescent probe. The levels of ROS increased significantly in the DMED group and were reduced by miR-92a antagomir administration (Fig. 4A). The expression levels of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) subunits were also detected, including p22phox, p47phox, and gp91phox, the expression levels of which in all groups showed a similar trend as those of ROS (Fig. 4B, 4C). The levels of malondialdehyde (MDA) and activities of superoxide dismutase (SOD) were also determined. The MDA levels were higher in the DMED group and DMED+miR-92a antagomir control group than that in the control group and the miR-92a antagomir treatment group (Fig. 4D), while the SOD activities showed an opposite trend (Fig. 4E). Immunofluorescence, IHC and western blotting analyses showed that the expression level of HO-1 was significantly lower in the DMED group than in the control group. The miR-92a antagomir administration activated the expression of HO-1, which was significantly higher than those in the DMED group and DMED+miR-92a antagomir control group (Fig. 4F-4H). An in vitro study showed similar results in endothelial cells. Inhibition of miR-92a improved oxidative stress by ameliorating the AMPK/Nrf2/HO-1 signaling pathway (Supplement Fig. 2A, 2C, 2D). Together, the miR-92a antagomir improved erectile function partly by ameliorating oxidative stress.
Fig. 4. The miR-92a antagomir improved erectile function via inhibition of oxidative stress and restoration of HO-1 expression. (A) Representative images of the ROS fluorescent probe in the corpus cavernosum of all rats at ×400 magnification. The scale bar represents 50 µm. (B, C) Representative western blot results of p22phox, p47phox, and gp91phox in the corpus cavernosum of all rats. Expressions of p22phox, p47phox, and gp91phox with β-actin as the loading control in all rats are presented as bar graphs (n=5–6 per group). (D) MDA levels determined by enzyme-linked immunosorbent assay (ELISA) in the corpus cavernosum of all rats (n=4–5 per group). (E) SOD activities determined by ELISA in the corpus cavernosum of all rats (n=4–5 per group). (F) Representative immunofluorescence and immunohistochemistry result of HO-1 in the corpus cavernosum of rats of all four groups at ×40 and ×100 magnification, respectively. (G) Representative western Blot results of HO-1 in the corpus cavernosum of all rats. (H) The expression levels of HO-1, with β-actin as the loading control, in the corpus cavernosum of each group are presented as bar graphs (n=6 per group). Data are mean±SEM. *p<0.05, **p<0.01, ***p<0.001. DMED: diabetic erectile dysfunction, ROS: reactive oxygen species, MDA: malondialdehyde, SOD: superoxide dismutase.
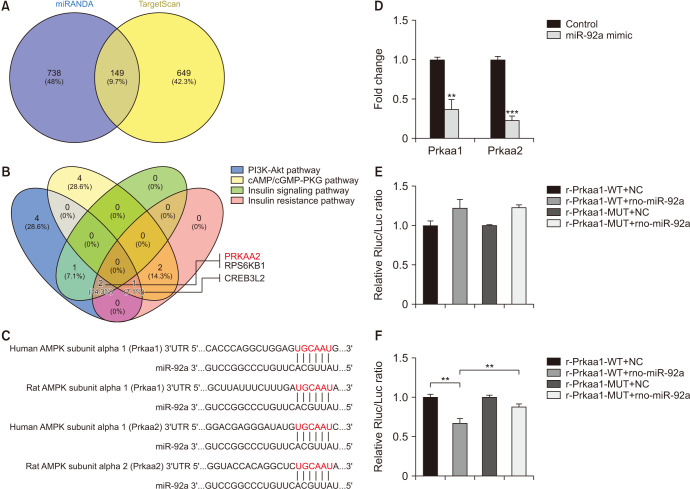
6. Prkaa2 was one of the genes targeted by miR-92a
The miRANDA and TargetScan predicted 149 genes targeted by miR-92a in total (Fig. 5A), which is shown in Supplement Table 3. All the potential genes were further explored via KEGG pathway analysis, and four signaling pathway that were most relevant to diabetes mellitus, were selected for choosing the potential target genes. Prkaa2 was predicted as a putative target of miR-92a (Fig. 5B). We also brought Prkaa1 into further study, and the sequence bound to miRNA-92a in the Prkaa1/Prkaa2 3'UTR was highly conserved in rats and humans (Fig. 5C). qRT-PCR analysis validated that both Prkaa1 and Prkaa2 decreased significantly in the endothelial cells treated with the miR-92a mimic, and the expression of Prkaa2 was even lower than that of Prkaa1 (Fig. 5D). Dual luciferase reporter gene experiments verified that Prkaa2 is a direct target gene of miRNA-92a, while Prkaa1 was unlikely to be a direct target gene of miRNA-92a (Fig. 5E, 5F).
Fig. 5. Prkaa2 was one of the genes targeted by miR-92a. (A, B) Bioinformatic analysis showing Prkaa2 was predicted as one of the genes targeted by miR-92a. (C) Predicted target region on the 3′UTR of Prkaa1 and Prkaa2 as well as the seed sequence of miR-92a by bioinformatic analysis. (D) Decreased expression of both Prkaa1 and Prkaa2 in the endothelial cells treated with miR-92a mimic. (E) Dual luciferase assay showing neither WT nor MUT Prkaa1 3′UTR activity was suppressed in the endothelial cells transfected with rno-miR-92a. (F) Dual luciferase assay showing WT but not MUT Prkaa2 3′UTR activity was suppressed in the endothelial cells transfected with rno-miR-92a. Data are mean±SEM. **p<0.01, ***p<0.001. 3′UTR: 3′ untranslated region, WT: wild type, MUT: mutant, NC: normal control.
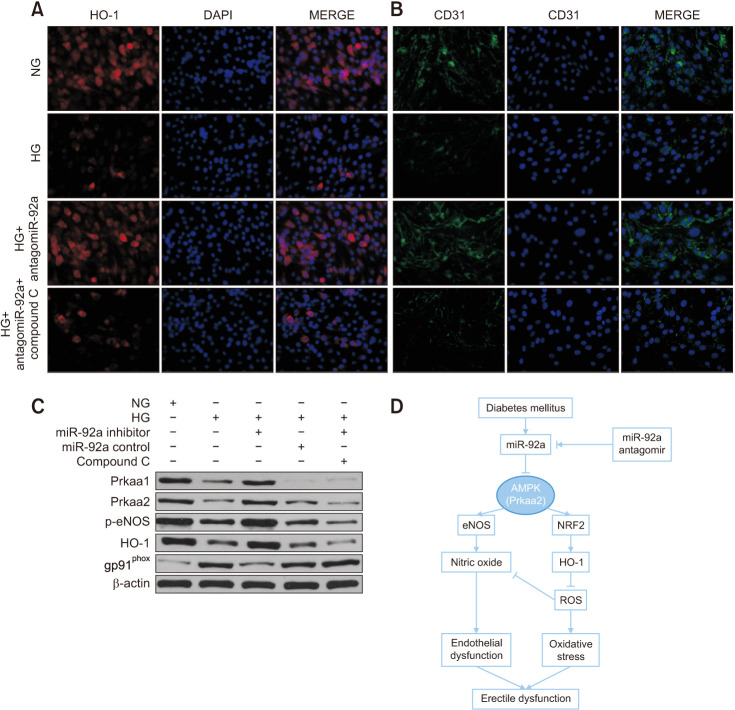
7. AMPK inhibition by compound C reversed the ameliorative effect of miR-92a inhibition on endothelial function and oxidative stress
To determine whether AMPK mediated the effect of miR-92a inhibition on improving endothelial function and attenuating oxidative stress, we used compound C to inhibit AMPK activity in endothelial cells. As shown by immunofluorescence, the activation of HO-1 and CD31 induced by miR-92a inhibition was reversed by compound C in the endothelial cells treated with HG (Fig. 6A, 6B). Consistently, western blotting showed lower expression of p-eNOS and HO-1 and higher expression of gp91phox, which were reversed by compound C (Fig. 6C).
Fig. 6. HO-1 inhibition by compound C reversed the ameliorative effect of miR-92a inhibition in vitro. (A, B) Representative immunofluorescence result of HO-1 and CD31 of endothelial cells of all four groups at ×200 magnification, showing Compound C reversed the effect of miR-92a inhibitor on restoration of HO-1 and CD31 in endothelial cells. (C) Representative western blot results of Prkaa1, Prkaa2, p-eNOS, HO-1, and gp91phox of endothelial cells in each group. (D) Schematic diagram showing miR-92a-AMPK signaling axis in regulation of endothelial function and oxidative stress, and thus regulating erectile function in diabetes. NG: normal glucose, HG: high glucose, ROS: reactive oxygen species.
DISCUSSION
The relationship between miR-92a and eNOS has been tentatively elucidated in the cardiovascular system. miR-92a downregulates the expression level and activity of eNOS, while inhibition of miR-92a can improve cardiac function and promote vascular regeneration by increasing the activity of eNOS [19]. Under diabetic conditions, nNOS expression is downregulated in multiple tissue types [20]; reduced nNOS levels are also found in CC tissues from patients with neurogenic ED or DMED [21]. In our study, the miR-92a antagomir improved ED by upregulating the expression levels of eNOS, p-eNOS (Ser1177), and nNOS, promoting their activity and increasing NO production, and preserving the CD31-positive endothelial area and endothelial cell-to-cell junctions in the CC of diabetic rats, thereby increasing downstream cGMP concentrations and improving erectile function.
Hyperglycemia-induced over-activated oxidative stress disrupt penile endothelial function and vascular homeostasis [22], cause oxidative/antioxidative imbalance, impede the regulatory role of the endothelial system in vascular and smooth muscle contraction, weaken cavernous vasodilator function, and affect blood flow perfusion and erectile function [23,24]. Increased ROS production is a hallmark of oxidative stress and reacts with NO to interfere with NO bioavailability [25,26]. Endogenous ROS are primarily produced by NADPH oxidase, a multi-subunit enzyme that includes subunits of p47phox, p67phox, gp91phox, p22phox, and p40phox in cell membranes [27], mitochondria, peroxisomes, and the endoplasmic reticulum. The expression levels of NADPH oxidase subunits are altered in response to changes in oxidative stress levels. Malondialdehyde (MDA) is a toxic substance produced by lipid peroxidation of the cell membrane, which can indirectly reflect the level of oxidative stress; SOD is an important scavenger of oxidative free radicals in the body, and its activity can also indirectly reflect the level of oxidative stress. HO-1 is an important antioxidant enzyme that catalyzes the metabolism of heme to ferrous iron, carbon monoxide and bilirubin. It has been shown that the expression level of HO-1 is decreased under hyperglycemia condition [28]; in the homocysteine-induced rat ED model, the level of oxidative stress is significantly increased and the expression level of HO-1 is significantly decreased [29]. In our study, the ROS content, the MDA concentration and the expression of NADPH oxidase subunit of penile cavernous tissue of the DMED group rats were significantly higher than that of the Control group, while the SOD activity was significantly decreased, reflecting the significant upregulation of oxidative stress in the DMED rats. The above parameters were improved after treatment with the miRNA-92a inhibitor.
Prkaa2 was initially predicted to be a possible target gene of miRNA-92a using a bioinformatics approach, and Prkaa1 was also found to be a possible target gene of miRNA-92a when comparing the sequences of miRNA-92a with those of potential target genes. Moreover, Prkaa1 and Prkaa2 are genes encoding the catalytic subunits α1 and α2 of AMPK, respectively. AMPK is one of the main cellular energy sensors, and the regulator of metabolic homeostasis is a heterotrimeric enzyme consisting of a catalytic subunit (α1 or α2) and two regulatory subunits (β1 or β2 and γ1, γ2, or γ3) [30]. The metabolic effects of AMPK activation, in particular the metabolic switch it causes from fat synthesis to fat oxidation as well as its ability to promote muscle glucose uptake, making it a potential therapeutic target for diabetes [30]. We observed a decrease in the phosphorylation level of AMPK and a significant down-regulation of the direct substrate acetyl-CoA carboxylase (ACACA) when we cultured endothelial cells in HG medium, and when treated with miRNA-92a mimic in NG (normal glucose) medium, p-AMPK and p-ACACA of endothelial cells showed similar changes. Moreover, miRNA-92a mimic significantly reduced intracellular Prkaa1 and Prkaa2 mRNA expression levels, suggesting that Prkaa1 and Prkaa2 may be target genes of miRNA-92a.
AMPK can directly phosphorylate serine at site 1177 of the eNOS protein (S1177) [31]. Several studies have demonstrated that the AMPK/eNOS signaling pathway plays a key role in regulating endothelial function [32,33]. We found that the AMPK/eNOS signaling pathway was significantly down-regulated in endothelial cells of the HG group and NG+miRNA-92a group. In contrast, miRNA-92a inhibitor significantly activated the AMPK/eNOS signaling pathway and improved endothelial function. The cellular response to oxidative stress is associated with multiple factors, of which the Nrf2 pathway is considered to be the most important factor. Nrf2 is a nuclear transcription factor that binds to the antioxidant response element and can directly regulate HO-1 promoter activity, thereby regulating HO-1 expression levels [34]. In addition to antioxidant stress effects, overexpression of HO-1 plays a defensive and protective role against cardiovascular diseases such as atherosclerosis and myocardial ischemia-reperfusion injury [35]. Thus, the Nrf2/HO-1 signaling pathway plays a key role in resisting oxidative stress and protecting the vasculature. In our study, we found that the AMPK/Nrf2/HO-1 signaling pathway was significantly downregulated in endothelial cells in both the HG and NG+miRNA-92a mimic groups, while the miRNA-92a inhibitor, on the other hand, significantly activated the AMPK/Nrf2/HO-1 signaling pathway and resisted oxidative stress levels.
To verify further whether the miRNA-92a regulates endothelial function and oxidative stress through AMPK, we applied the AMPK inhibitor compound C to detect its effect. Compound C reversed the ameliorative effect of the miRNA-92a inhibitor, further suggesting that the miRNA-92a regulates endothelial cell function via AMPK. Based on bioinformatics predictions, we verified by dual luciferase reporter gene experiments that Prkaa2 is a direct target gene of miRNA-92a and that the sequence bound to miRNA-92a in the Prkaa2 3'UTR is highly conserved in rats and humans. Interestingly, although the sequences binding to miRNA-92a were also found in the Prkaa1 3'UTR, dual-luciferase reporter gene experiments showed that Prkaa1 is unlikely to be a direct target gene of miRNA-92a. This finding indicates that sequence matching is not a determining condition for finding miRNA-92a target genes.
Our study provided evidence, we believe for the first time, that miR-92a inhibitor was effective in partially restoring erectile function in DMED rats, However, our ED rat models of type 1 diabetes may not necessarily represent the pathologic processes that occur in ED induced by type 2 diabetes or other risk factors, and we explore endothelial cells in vitro, which is only one part of CC, other structures could also be further studied.
CONCLUSIONS
Our study provides the first line of evidence that the expression of miR-92a is increased in the CC of DMED rats. The inhibition of miR-92a improved endothelial function and oxidative stress by enhancing AMPK expression by directly targeting Prkaa2 (Fig. 6D). Thus, targeting the miR-92a-Prkaa2 cascade can be an alternative therapeutic strategy for ameliorating DMED.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by grants from the National Natural Science Foundation of China (NSFC #81501246 & #81501020).
- Conceptualization: ZT, JY.
- Data curation: ZY, JS.
- Formal analysis: ZT, KC.
- Funding acquisition: JY.
- Investigation: ZT, YJ.
- Methodology: ZT, ZY.
- Project administration: TW, SW.
- Resources: JL.
- Software: YL.
- Supervision: TW, SW.
- Validation: JY, JL.
- Visualization: ZT, JS.
- Writing – original draft: ZT.
- Writing – review & editing: JY, JL.
Data Sharing Statement
The data used to support the findings of the present study are available from the corresponding author upon request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.210177.
Details of the antibodies
Primer sequences for qRT-PCR
Predicted target genes
The miR-92a antagomir improved erectile function via amelioration of nNOS expression. (A) Representative immunofluorescence result of nNOS in the corpus cavernosum of rats of all four groups at ×100 magnification. (B) Representative western blot results of nNOS in the corpus cavernosum of all rats. (C) The expression levels of nNOS, with β-actin as the loading control, in the corpus cavernosum of each group are presented as bar graphs (n=4 per group). Data are mean±SEM. *p<0.05, **p<0.01, ***p<0.001. DMED: diabetic erectile dysfunction group.
Inhibition of miR-92a improved erectile function via amelioration of the AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathway in vitro. (A) Representative western blot results of AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathway in endothelial cells in each group. (B, C) The expression levels of eNOS, p-eNOS, and HO-1, with β-actin as the loading control, and the relative ratio of p-eNOS/eNOS in the corpus cavernosum of each group are presented as bar graphs (n=4–5 per group). (D) qRT-PCR analysis showing miR-92a inhibitor reactivated the expression of eNOS and HO-1 (n=4–5 per group). *,#p<0.05 versus the control group. qRT-PCR: quantitative real-time polymerase chain reaction, NG: normal glucose (5 mmol/L), HG: high glucose (30 mmol/L).
References
- 1.Mitidieri E, Cirino G, d'Emmanuele di Villa Bianca R, Sorrentino R. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. doi: 10.1016/j.pharmthera.2020.107493. [DOI] [PubMed] [Google Scholar]
- 2.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12 Suppl 4:S6–S11. doi: 10.1038/sj.ijir.3900567. [DOI] [PubMed] [Google Scholar]
- 4.Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129–136. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Kamenov ZA. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123:141–158. doi: 10.1055/s-0034-1394383. [DOI] [PubMed] [Google Scholar]
- 7.Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care. 2003;26:1093–1099. doi: 10.2337/diacare.26.4.1093. [DOI] [PubMed] [Google Scholar]
- 8.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for MicroRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. 2017;38:145–168. doi: 10.1210/er.2016-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Fan J, Zhao Y, Zhang X, Dai B, Zhan J, et al. Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ Res. 2019;125:1106–1120. doi: 10.1161/CIRCRESAHA.119.314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247–260. doi: 10.1016/j.redox.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iaconetti C, Polimeni A, Sorrentino S, Sabatino J, Pironti G, Esposito G, et al. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res Cardiol. 2012;107:296. doi: 10.1007/s00395-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 14.Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103:564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar B, Khaleghzadegan S, Mears B, Hatano K, Kudrolli TA, Chowdhury WH, et al. Identification of miR-30b-3p and miR-30d-5p as direct regulators of androgen receptor signaling in prostate cancer by complementary functional microRNA library screening. Oncotarget. 2016;7:72593–72607. doi: 10.18632/oncotarget.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen Z, Wang T, Yang J, Li R, Wang S, et al. Treatment of diabetes mellitus-induced erectile dysfunction using endothelial progenitor cells genetically modified with human telomerase reverse transcriptase. Oncotarget. 2016;7:39302–39315. doi: 10.18632/oncotarget.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam JW, Rissland OS, Koppstein D, Abreu-Goodger C, Jan CH, Agarwal V, et al. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol Cell. 2014;53:1031–1043. doi: 10.1016/j.molcel.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 20.Gangula PR, Mukhopadhyay S, Ravella K, Cai S, Channon KM, Garfield RE, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–G699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dashwood MR, Crump A, Shi-Wen X, Loesch A. Identification of neuronal nitric oxide synthase (nNOS) in human penis: a potential role of reduced neuronally-derived nitric oxide in erectile dysfunction. Curr Pharm Biotechnol. 2011;12:1316–1321. doi: 10.2174/138920111798280965. [DOI] [PubMed] [Google Scholar]
- 22.Castela A, Gomes P, Domingues VF, Paíga P, Costa R, Vendeira P, et al. Role of oxidative stress-induced systemic and cavernosal molecular alterations in the progression of diabetic erectile dysfunction. J Diabetes. 2015;7:393–401. doi: 10.1111/1753-0407.12181. [DOI] [PubMed] [Google Scholar]
- 23.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24(6 Suppl):S17–S37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 24.Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med. 2009;6:2390–2404. doi: 10.1111/j.1743-6109.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 25.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 197-8. [DOI] [PubMed] [Google Scholar]
- 26.Ryu JK, Kim DJ, Lee T, Kang YS, Yoon SM, Suh JK. The role of free radical in the pathogenesis of impotence in streptozotocin-induced diabetic rats. Yonsei Med J. 2003;44:236–241. doi: 10.3349/ymj.2003.44.2.236. [DOI] [PubMed] [Google Scholar]
- 27.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353(Pt 2):411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gou L, Zhao L, Song W, Wang L, Liu J, Zhang H, et al. Inhibition of miR-92a suppresses oxidative stress and improves endothelial function by upregulating heme oxygenase-1 in db/db mice. Antioxid Redox Signal. 2018;28:358–370. doi: 10.1089/ars.2017.7005. [DOI] [PubMed] [Google Scholar]
- 29.Tang Z, Song J, Yu Z, Cui K, Ruan Y, Wang T, et al. Melatonin treatment ameliorates hyperhomocysteinemia-induced impairment of erectile function in a rat model. J Sex Med. 2019;16:1506–1517. doi: 10.1016/j.jsxm.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32 Suppl 4:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 31.Nagata D, Hirata Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens Res. 2010;33:22–28. doi: 10.1038/hr.2009.187. [DOI] [PubMed] [Google Scholar]
- 32.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. 2011;31:2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, et al. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8:71–85. doi: 10.1007/s12012-008-9016-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the antibodies
Primer sequences for qRT-PCR
Predicted target genes
The miR-92a antagomir improved erectile function via amelioration of nNOS expression. (A) Representative immunofluorescence result of nNOS in the corpus cavernosum of rats of all four groups at ×100 magnification. (B) Representative western blot results of nNOS in the corpus cavernosum of all rats. (C) The expression levels of nNOS, with β-actin as the loading control, in the corpus cavernosum of each group are presented as bar graphs (n=4 per group). Data are mean±SEM. *p<0.05, **p<0.01, ***p<0.001. DMED: diabetic erectile dysfunction group.
Inhibition of miR-92a improved erectile function via amelioration of the AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathway in vitro. (A) Representative western blot results of AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathway in endothelial cells in each group. (B, C) The expression levels of eNOS, p-eNOS, and HO-1, with β-actin as the loading control, and the relative ratio of p-eNOS/eNOS in the corpus cavernosum of each group are presented as bar graphs (n=4–5 per group). (D) qRT-PCR analysis showing miR-92a inhibitor reactivated the expression of eNOS and HO-1 (n=4–5 per group). *,#p<0.05 versus the control group. qRT-PCR: quantitative real-time polymerase chain reaction, NG: normal glucose (5 mmol/L), HG: high glucose (30 mmol/L).
Data Availability Statement
The data used to support the findings of the present study are available from the corresponding author upon request.