Abstract
Purpose
To evaluate the clinical efficacy and patient satisfaction rates of low-intensity extracorporeal shockwave therapy LIESWT) in men with vasculogenic erectile dysfunction (ED) using Duolith SD1 machine.
Materials and Methods
This prospective, randomized, double-blinded clinical trial included 60 men who were randomly assigned to LIESWT (n=30, active group) or placebo (n=30) over 6 weeks. Patient demographics, change in International Index of Erectile Function (IIEF)-5, Erection Hardness Score (EHS) and Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores, and an overall satisfaction score (on a 5-point scale), were recorded. All patients were reviewed at 1, 3, and 6 months after completion of therapy.
Results
There were 21 (70%) patients in the LIESWT group and 3 (10%) patients in the placebo group who had a 5-point or greater increase in IIEF-5 score (p=0.018). At 6-month study period, the mean IIEF-5 score was 18.8 (standard deviation [SD], 3.8) in the LIESWT group versus 14.8 (SD, 3.6) in the placebo group, difference in means between groups was 4.0 (95% confidence interval, 2.1–5.9; p<0.001). The EHS scores were higher in the LIESWT group with a mean of greater than 1.2 across the 1, 3, and 6 months compared to the placebo group (p<0.05). All patients completed the treatment study and there was no adverse event reported in terms of penile pain, bruising or deformity. There was a positive correlation between men who reported improvement in EF and treatment satisfaction level with LiESWT (p=0.008).
Conclusions
LIESWT improves erectile function in the short-term especially in men with mild to moderate ED, and those without a cardiometabolic disease.
Keywords: Erectile dysfunction, Extracorporeal shockwave therapy, Patient satisfaction, Penile erection
INTRODUCTION
The landmark study on low-intensity extracorporeal shockwave therapy (LIESWT) first published in 2010 [1] has reignited the interest to use shockwave to treat men with erectile dysfunction (ED). Preclinical studies showed that LIESWT can promote tissue angiogenesis through release of vascular endothelial growth factor [2] and play a role in the recruitment and subsequent differentiation of endothelial progenitor cells to enhance neovascularization and tissue repair near the treatment site [3]. Various animal experiments of ED models showed that LIESWT could partially restore corpus cavernosum fibromuscular pathological changes, endothelial dysfunction, and peripheral neuropathy [4,5].
Published systematic reviews and meta-analyses reported encouraging clinical outcomes with some positive effects on erectile function (EF) following LIESWT [6,7,8,9,10]. Furthermore, several clinical guidelines have been published to support the role of LIESWT to treat men with ED in a research setting [11,12]. Patient selection is an important determinant factor for treatment success with published guidelines advocating LIESWT in younger patients with mild-moderate ED and in the absence of significant comorbidities especially those with cavernous nerve injury [11,12]. While existing clinical studies show that the vasculogenic effects and therapeutic mechanisms among various LIESWT machines are quite similar [6,7,8,9,10,11,12], there is general consensus regarding the treatment template with existing treatment protocol often based on manufacturer's guidelines and prior published studies, and it remains largely unknown if one shockwave modality is superior to another given there is no direct comparative study among the numerous LIESWT machines currently in the commercial market [13].
This randomized, double-blinded, placebo-controlled study evaluates the clinical outcomes in terms of EF recovery and patient satisfaction rates in a group of men with vascular ED who received LIESWT using a second-generation Duolith SD1 ultra (Storz Medical AG, Tägerwilen, Switzerland).
MATERIALS AND METHODS
1. Ethics statement
The present study protocol was reviewed and received approval by the Institutional Review Board of the Princess Alexandra Hospital, University of Queensland (approval number: 610). Informed consent was signed by all subjects.
2. Patient population
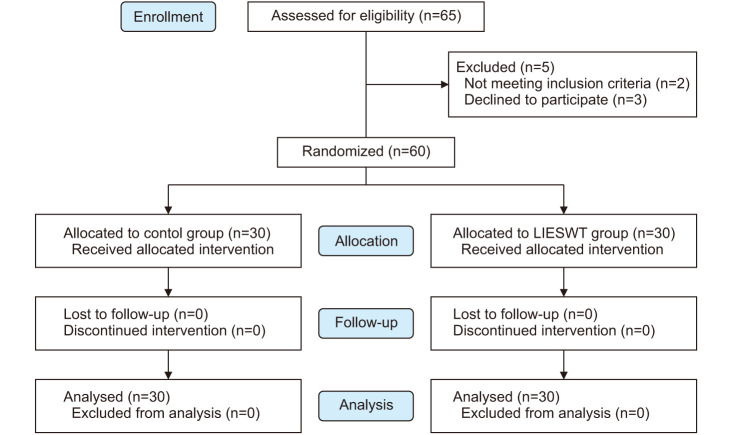
Following internal departmental ethics approval, patients with ED were prospectively enrolled from January 2018 to January 2019 (Fig. 1). Inclusion criteria included patient age ≥18 years, has a poor response to medical therapy, a minimum 6-month history of ED, International Index of Erectile Function (IIEF)-5 score ≥12, and is in a stable sexual relationship for more than 3 months. Poor response to medical therapy is defined as a lack of adequate penile erection for sexual intercourse with the aids of oral phosphodiesterase type-5 inhibitors and/or intracavernosal therapy, with an IIEF-5 score less than 21. Patients who developed ED following prostate cancer treatment such as prostatectomy or radiation, or pre-existing anatomical or neurological conditions were excluded. Patient demographics including comorbid conditions and vascular risk factor and pre-treatment IIEF-5, Erection Hardness Score (EHS) and Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores were recorded. The IIEF-5 questionnaire is the abridged five-item version of the IIEF with a scale from 0 to 25, where a score of 0 to 5 is awarded to each of the 5 questions, where 0 denotes no sexual activity while 5 means almost always or always. On the other hand, the EHS is a validated single-item patient-reported outcome on the hardness of erection ranging from 0 (penis does not enlarge) to 4 (completely hard and fully rigid). The exclusion criteria were patients who have a history of coagulopathy, pelvic (or prostate) surgery, received radiation therapy or androgen deprivation therapy for prostate cancer, uncontrolled diabetes (glycosylated haemoglobin >7%), and those with penile deformity and neurological disorders. The primary outcome of this study was improvement in EF based on IIEF-5 and EHS, while secondary endpoints were safety profile and patient satisfaction rates with LIESWT.
Fig. 1. Flow diagram of study recruitment. LIESWT: low-intensity extracorporial shockwave therapy.
3. Treatment protocol
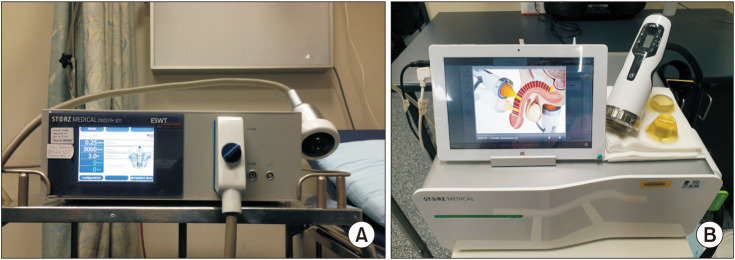
The LiESWT was performed without local or systemic analgesia using the second-generation Duolith SD1 ultra in the outpatient setting. The study protocol was published in the original study [14]. In comparison with first-generation Duolith machine, the second-generation SD1 ultra-machine has a newer housing and handpiece design, a graphical tablet user interface and remote control of energy settings on the handpiece (Fig. 2). The clinically relevant parameters including the actual delivery of shockwave energy are the same between the Duolith machines. Patients were randomized using computer generated random list and each patient was assigned a number so that patient can be tracked throughout the study in a double-blinded manner. Washout of existing erectile medications was performed for 4 weeks prior to entry into the study and patients were refrained from using erectile medications during the study period. In brief, patients received 3,000 shocks at an energy density of 0.25 mJ/mm2 and emission frequency of 6 Hz, twice weekly for 6 weeks. The treatment sites were distal penis (1,000 shockwaves), base of penis (1,000 shockwaves) and corporal bodies on the perineum (500 shockwaves to each crura). The sham treatment was performed using the same medical device and handpiece as in the active LIESWT with the only difference, namely a standoff device at the end of the handpiece that does not transmit any shockwaves despite providing the same sound as the actual LIESWT system. Only the technician operating the shockwave machine was aware of the type of treatment an individual patient had.
Fig. 2. Comparison between (A) 1st and (B) 2nd generation Duolith SD1 ultra (Storz Medical AG, Tägerwilen, Switzerland) LIESWT machine in the outpatient setting. LIESWT: low-intensity extracorporeal shockwave therapy.
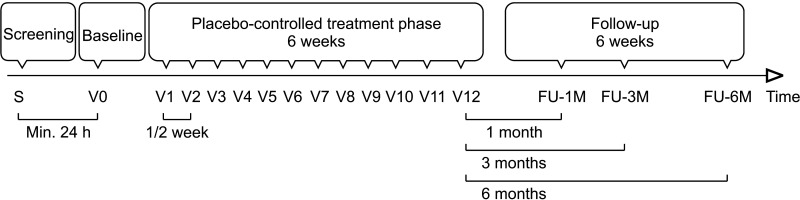
Changes in IIEF-5, EHS and EDITS scores were recorded at 1-, 3-, and 6-months following completion of LIESWT study (Fig. 3). The overall satisfaction rate (on a 5-point scale with 1 being least satisfied and 5 being most satisfied following LIESWT) was documented too. Treatment-related adverse events such as penile pain, bruising, and haematuria were recorded too.
Fig. 3. Study protocol involves each subject undergoing 12 treatments over a 6-week period with follow-up at 1, 3, and 6 months.
An independent third-party was tasked to collect the data to ensure the investigators were blinded to the clinical outcomes and patient satisfaction rates. At the end of the study, the participants were unblinded at 6 months and those in the placebo group were subsequently offered LIESWT to treat their ED.
4. Statistical analysis
An improvement ≥5 points from the baseline IIEF-5 score is considered significant based on change in the severity of ED category [15]. Based on published randomized-controlled placebo trials on LIESWT [6,7,8,9,10] including our previous study [14], assuming for 20% placebo effect and to achieve a power of 80% with an alpha value of 0.05, we calculated a sample size of 23 for each group based on IIEF-5 changes. Further accounting for 15% potential patient dropouts, we recruited 60 patients in total with 30 patients each in the LIESWT and placebo groups.
Statistical analysis was performed with SAS 9.1.3 (SAS Institute, Cary, NC, USA) computer software with values of the study parameters compared using Student t-test or Wilcoxon signed-rank test as appropriate. If there is a significant deviation from the normal distribution, the Mann–Whitney U-test will be used. A chi-square contingency analysis was used to examine the relationship between EF score and treatment satisfaction, with statistical significance set at 5%.
RESULTS
1. Patient demographics
A total of 60 patients were recruited and the mean age was 55.8 years (range, 42–68 y; median, 48 y). Most men (80%) have reported ED for more than 18 months (range, 6–60 mo; median, 33 mo). Both the LIESWT and placebo groups shared a similar number of risk factors and there was no significant difference in medical comorbidities (Table 1). The mean baseline IIEF-5 score was 14.8 (range, 12–18) and most patients have a stratified moderate ED classification (60%). There was no statistically significant difference the types of erectile aids utilized by patients in both groups (p=0.44). There were no significant differences in the mean IIEF-5 (p=0.44) and EHS scores (p=0.36) between the 2 groups.
Table 1. Selected variables on patients' demographics and characteristics.
| Variable | Placebo | LIESWT | |
|---|---|---|---|
| Number of patients | 30 | 30 | |
| Age (y) | 48 (42–63) | 45 (42–68) | |
| Months of ED | 35 (6–60) | 33 (8–59) | |
| Risk factors for ED | |||
| Diabetes | 5 | 7 | |
| Hypertension | 20 | 18 | |
| Dyslipidemia | 14 | 11 | |
| Ischemic heart disease | 1 | 1 | |
| Smoking | 8 | 9 | |
| Baseline IIEF-5 score | 14.8±3.6 | 14.6±3.8 | |
| Baseline EHS | 1.3±0.7 | 1.4±0.6 | |
Values are presented as number only, median (range), or mean±standard deviation.
ED: erectile dysfunction, IIEF: International Index of Erectile Function, EHS: Erection Hardness Score, LIESWT: low-intensity extracorporeal shockwave therapy.
2. Clinical efficacy, safety, and patient satisfaction rate
There were 21 (70%) patients in the LIESWT group and 3 (10%) patients in the placebo group who had a 5-point or greater increase in IIEF-5 score at 6-month review (p=0.028). Spontaneous erection was reported in 18 (60%) patients in the LIESWT group (none in placebo group) at 1-month following completion of study. The mean differences in the IIEF-5 scores between LIESWT and placebo groups were 2.8, 3.6 and 4.0 at 1-, 3-, and 6-month follow-up period (see Table 2). At 6-month study period, the mean IIEF-5 score was 18.8 (standard deviation [SD], 3. in the LIESWT group versus 14.8 (SD, 3.6) in the placebo group, while the difference in means between groups was 4.0 (95% confidence interval [CI], 2.1–5.9; p<0.001). The improved IIEF-5 scores in the LIESWT group remained stable between the 3- and 6-months observation periods. Furthermore, most men in the placebo group reported a return to baseline IIEF-5 scores at 6 months. The EHS scores were higher in the LIESWT group with more than two-thirds of men reporting a score of 4 out of 4. The improvement in EHS was statistically significant in the LIESWT group compared to the placebo group by a mean of greater than 1.2 across the 1-, 3-, and 6-months (p<0.05).
Table 2. Comparing erectile function outcomes between placebo and LIESWT groups.
| Erectile function | Placebo | LIESWT | p-value | |
|---|---|---|---|---|
| IIEF score | ||||
| 1 month | 16.2±3.2 | 19.0±4.5 | 0.028 | |
| 3 months | 15.2±3.8 | 18.8±3.8 | 0.029 | |
| 6 months | 14.8±3.6 | 18.8±3.8 | 0.028 | |
| EHS | ||||
| 1 month | 1.6±0.4 | 2.8±0.6 | 0.029 | |
| 3 months | 1.4±0.6 | 2.6±0.5 | 0.035 | |
| 6 months | 1.3±0.7 | 2.6±0.4 | 0.033 | |
Values are presented as mean±standard deviation.
LIESWT: low-intensity extracorporeal shockwave therapy, IIEF: International Index of Erectile Function, EHS: Erection Hardness Score.
All patients completed the treatment study and there was no adverse event reported in terms of penile pain, bruising or deformity.
Among men who received LIESWT, the mean overall satisfaction score was 4.5 with 25 (83%) patients scored at least 4 out of 5 and would recommend this therapy to other men with ED. An improvement in EDITS Index score >50% were reported in 22 (73%) patients. There was a positive correlation between men who reported improvement in EF and treatment satisfaction level with LIESWT (p=0.008).
DISCUSSION
This study showed statistically significantly greater improvement in EF as evidenced by higher IIEF and EHS scores in the LIESWT group compared with the placebo group, and these positive changes were maintained across the 1-, 3-, and 6-months follow-up period. When comparing the various predictors of success for LIESWT, multiple linear regression analysis showed the improvement in EF score was significantly greater in the subgroup of men with mild to moderate ED, with a mean change in IIEF-5 score of 6.8 (95% CI, 4.4–8.2; p<0.001) in the LIESWT group. Hisasue et al [16] showed that age and the number of concomitant comorbidities were statistically significant predictors for LIESWT efficacy. Our findings concurred with published clinical guidelines on LIESWT that supports better clinical efficacy in men with mild to moderate ED, younger age group, those with minimal cardiovascular comorbidities, and absence of diabetes or cavernous nerve injury [11,12].
Compared to the first LIESWT study which utilized the Omnispec ED 1000 (Medispec, Gaithersburg, MD, USA) machine with a treatment template consisting of 3,000 shockwaves delivered at 0.09 mJ/mm2 to three sites along shaft penis and two at the penile crural levels [1], our study LIESWT treatment template comprised of 12 sessions of twice-weekly LIESWT for 6 weeks with 3,000 shockwaves given at 0.25 mJ/mm2 (1,000 shockwaves were delivered to three sites namely the distal penis, base of penis, and corporal bodies at the perineum). While meta-analyses by Man and Li [6] and Zou et al [7] showed an energy flux density of 0.09 mJ/mm2 appeared to be superior to other protocols, it is important to understand that differences on the shockwave lithotripters and consequently, the actual amount of shockwave energy delivered to the penile tissue [13] may result in another optimal energy setting. Our current treatment template is based our previous experience [14] and has subsequently been adopted by other groups with similar efficacy [17,18].
As expected and akin to other published randomized controlled trials, LIESWT appears to be safe and highly tolerable in our study. All patients completed the treatment course with no drop-out and no patient reported penile pain, bruising, and haematuria during or at subsequent follow-up visits. It is possible that our high patient satisfaction rate was related to high EF scores, and that most patients valued the prospect to regain spontaneous erection without the need for medical therapy following LIESWT. The second-generation SD1 ultra-machine with smaller housing and streamline handpiece for delivery of shockwaves may be more user-friendly and potentially increase patient comfort, although this study is not designed to directly compare patient satisfaction rates between the machines.
We acknowledge several limitations in our study include the lack of objective penile hemodynamic measurements with the use of colour penile duplex ultrasound study, and the relatively short-term follow-up study at 6 months. Our group has published a long-term follow-up study in men following LIESWT and there was a gradual decline in EF over time although this effect appeared to plateau at 48 to 60 months [19]. We utilized common validated questionnaires such as IIEF and EHS to provide objective measurements in EF, and published literature has shown that the improvement in these erectile scores correlated strongly with actual positive penile hemodynamics [11,12,13]. Furthermore, we have complete follow-ups for all participants due to stringent review and ensuring the patients receive optimal care. To our knowledge, this is the first study on LIESWT using the second-generation Duolith SD1 machine, and the first centre to publish the clinical outcomes on a second-generation Duolith electromagnetic LIESWT machine. Unlike most current medical treatments in ED which are reactive and utilized in an on-demand basis, LIESWT appears to be most effective in patients with mild to moderate ED. We agree that further scientific research should be conducted to compare current commercially available LIESWT machines and explore the various pathophysiological mechanisms of LIESWT on actual penile histological changes. While several sexual medicine societies have adopted the use of LIESWT to treat men with ED in a research setting [11,12], caution should be exercised given the lack of clarity regarding the optimal treatment template and modalities of shockwave energy, coupled with over-commercialized and hype of this therapy by many private enterprises. Indeed, additional multi-institutional randomized controlled studies with dose-finding study are needed before LIESWT can be considered a first-line therapy for ED.
CONCLUSIONS
The second generation Duolith SD 1 ultra LIESWT machine improves erectile function in the short-term especially in men with mild to moderate ED, and those without cardiometabolic disease.
Acknowledgements
Storz Medical AG for providing unlimited technical support and the use of Duolith SD1 ultra-machine for the duration of the clinical trial.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: Educational research grant from Storz Medical AG.
- Conceptualization: EC.
- Data curation: WB, JW.
- Formal analysis: EC.
- Funding acquisition: EC.
- Methodology: EC.
- Project administration: WB, JW.
- Supervision: EC.
- Writing — original draft: EC.
- Writing — review & editing: EC, WB, JW.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
- 1.Vardi Y, Appel B, Jacob G, Massarwi O, Gruenwald I. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol. 2010;58:243–248. doi: 10.1016/j.eururo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy as a new and non-invasive angiogenic strategy. Tohoku J Exp Med. 2009;219:1–9. doi: 10.1620/tjem.219.1. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Zhao Y, Wang M, Song W, Li B, Liu W, et al. Defocused low-energy shock wave activates adipose tissue-derived stem cells in vitro via multiple signaling pathways. Cytotherapy. 2016;18:1503–1514. doi: 10.1016/j.jcyt.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016;13:22–32. doi: 10.1016/j.jsxm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Man L, Li G. Low-intensity extracorporeal shock wave therapy for erectile dysfunction: a systematic review and meta-analysis. Urology. 2018;119:97–103. doi: 10.1016/j.urology.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Zou ZJ, Tang LY, Liu ZH, Liang JY, Zhang RC, Wang YJ, et al. Short-term efficacy and safety of low-intensity extracorporeal shock wave therapy in erectile dysfunction: a systematic review and meta-analysis. Int Braz J Urol. 2017;43:805–821. doi: 10.1590/S1677-5538.IBJU.2016.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavijo RI, Kohn TP, Kohn JR, Ramasamy R. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2017;14:27–35. doi: 10.1016/j.jsxm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z, Lin G, Reed-Maldonado A, Wang C, Lee YC, Lue TF. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis. Eur Urol. 2017;71:223–233. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Chang D, Zhang X, Li J, Yang F, Tan K, et al. Effect of low-intensity extracorporeal shock wave on the treatment of erectile dysfunction: a systematic review and meta-analysis. Am J Mens Health. 2019;13:1557988319846749. doi: 10.1177/1557988319846749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capogrosso P, Frey A, Jensen CFS, Rastrelli G, Russo GI, Torremade J, et al. Low-intensity shock wave therapy in sexual medicine-clinical recommendations from the European Society of Sexual Medicine (ESSM) J Sex Med. 2019;16:1490–1505. doi: 10.1016/j.jsxm.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Chung E, Lee J, Liu CC, Taniguchi H, Zhou HL, Park HJ. Clinical practice guideline recommendation on the use of low intensity extracorporeal shock wave therapy and low intensity pulsed ultrasound shock wave therapy to treat erectile dysfunction: the Asia-Pacific Society for Sexual Medicine position statement. World J Mens Health. 2021;39:1–8. doi: 10.5534/wjmh.200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung E, Wang J. A state-of-art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev Med Devices. 2017;14:929–934. doi: 10.1080/17434440.2017.1403897. [DOI] [PubMed] [Google Scholar]
- 14.Chung E, Cartmill R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: an Australian first open-label single-arm prospective clinical trial. BJU Int. 2015;115 Suppl 5:46–49. doi: 10.1111/bju.13035. [DOI] [PubMed] [Google Scholar]
- 15.Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Hisasue S, China T, Horiuchi A, Kimura M, Saito K, Isotani S, et al. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int J Urol. 2016;23:80–84. doi: 10.1111/iju.12955. [DOI] [PubMed] [Google Scholar]
- 17.Olsen AB, Persiani M, Boie S, Hanna M, Lund L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand J Urol. 2015;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 18.Palmieri A, Imbimbo C, Creta M, Verze P, Fusco F, Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie's disease and erectile dysfunction: results from a prospective randomized trial. Int J Androl. 2012;35:190–195. doi: 10.1111/j.1365-2605.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung E, Cartmill R. Evaluation of long-term clinical outcomes and patient satisfaction rate following low intensity shock wave therapy in men with erectile dysfunction: a minimum 5-year follow-up on a prospective open-label single-arm clinical study. Sex Med. 2021;9:100384. doi: 10.1016/j.esxm.2021.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.