Abstract
Purpose
Seminal oxidative stress (OS) is a recognized factor potentially associated with male infertility, but the efficacy of antioxidant (AOX) therapy is controversial and there is no consensus on its utility. Primary outcomes of this study were to investigate the effect of AOX on spontaneous clinical pregnancy, live birth and miscarriage rates in male infertile patients. Secondary outcomes were conventional semen parameters, sperm DNA fragmentation (SDF) and seminal OS.
Materials and Methods
Literature search was performed using Scopus, PubMed, Ovid, Embase, and Cochrane databases. Only randomized controlled trials (RCTs) were included and the meta-analysis was conducted according to PRISMA guidelines.
Results
We assessed for eligibility 1,307 abstracts, and 45 RCTs were finally included, for a total of 4,332 infertile patients. We found a significantly higher pregnancy rate in patients treated with AOX compared to placebo-treated or untreated controls, without significant inter-study heterogeneity. No effects on live-birth or miscarriage rates were observed in four studies. A significantly higher sperm concentration, sperm progressive motility, sperm total motility, and normal sperm morphology was found in patients compared to controls. We found no effect on SDF in analysis of three eligible studies. Seminal levels of total antioxidant capacity were significantly higher, while seminal malondialdehyde acid was significantly lower in patients than controls. These results did not change after exclusion of studies performed following varicocele repair.
Conclusions
The present analysis upgrades the level of evidence favoring a recommendation for using AOX in male infertility to improve the spontaneous pregnancy rate and the conventional sperm parameters. The failure to demonstrate an increase in live-birth rate, despite an increase in pregnancy rates, is due to the very few RCTs specifically assessing the impact of AOX on live-birth rate. Therefore, further RCTs assessing the impact of AOX on live-birth rate and miscarriage rate, and SDF will be helpful.
Keywords: Antioxidants, Male infertility, Meta-analysis, Pregnancy, Semen parameters, Sperm DNA fragmentation
INTRODUCTION
Infertility affects about one in six couples worldwide, and approximately 50% of infertility is due to male factor [1,2]. Many causes have been attributed to male factor infertility, such as varicocele, endocrine disturbances, genetic abnormalities, immunological factors, urogenital abnormalities or infections, lifestyle, malignancy, systemic disease, gonadotoxins, or obstruction of the reproductive tract [1]. About 30% to 40% of cases are labeled as idiopathic male infertility (IMI) which is diagnosed when there are normal findings on physical examination, and on genetic and hormonal evaluation, but semen analysis reveals abnormal parameters with failure to achieve fatherhood despite unprotected sexual intercourse [3].
Seminal oxidative stress (OS) is now recognized as a potential contributing factor to the various causes of infertility, including IMI. Seminal reactive oxygen species (ROS) are produced by immature sperm, leukocytes, and as by-products of metabolic pathways, and are needed for the normal function of spermatozoa [4]. Excess production of ROS occurs under several circumstances, including smoking, alcohol, other lifestyle factors, varicocele, radiation exposure, and several other conditions [5]. OS occurs when there is a high concentration of ROS leading to an imbalance between ROS and antioxidants (AOXs) [5]. Several studies have suggested that OS plays a significant role in male infertility [6,7,8]. OS can interfere with capacitation, sperm DNA integrity, and cause sperm membrane damage and this can impact the fertilization process [9,10]. Spermatozoa are particularly susceptible to the action of high concentrations of ROS due to the presence of a large amount of polyunsaturated fatty acids in their plasma membrane as well as a low concentration of enzymes in the cytoplasm that can neutralize ROS [11]. Recently, the term Male Oxidative Stress Infertility (MOSI) was proposed by Agarwal et al [12] to encompass men with abnormal semen analysis and high OS, who were previously classified as having IMI.
The rational for AOX therapy is based on the understanding that AOXs may neutralize these potentially harmful oxidants. An AOX is a substance that neutralizes or protects the cells against the detrimental effects of oxidation and free radicals. The AOX system has enzymatic or non-enzymatic factors. Enzymatic AOXs include superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. Non-enzymatic AOXs include glutathione, cysteine, N-acetylcysteine (NAC), carotenoids, vitamin C, vitamin E, carnitine, ferritin, L-arginine, transferrin, coenzyme Q10 (CoQ10), myo-inositol, lycopene, selenium, zinc, and folate [13,14]. The mechanism of action of these AOXs includes free radical scavenging and neutralization as well as preserving sperm DNA integrity and mitochondrial transport [15].
In 2021, a systematic review by Agarwal et al [16] identified 97 clinical trials (52 uncontrolled, 12 unblinded and 33 blinded randomized controlled trials [RCTs]) evaluating the efficacy of a single or combined AOXs for treatment of male infertility. By conducting a qualitative analysis of the evidence, they suggested that a review of the guidelines is needed, as the role of AOXs should be supported in case of (1) abnormal semen quality (grade C recommendation), (2) varicocele (grade C recommendation), and (3) idiopathic and unexplained male infertility (grade B recommendation) [16]. Studies on single supplements or on specific clinical contexts have demonstrated the positive role of AOXs on semen quality and reproductive outcomes. Thus, two recent meta-analyses of RCTs showed a significant positive effect of NAC and L-carnitine/L-acetyl-carnitine (LC/LAC) on semen parameters [17,18]. In addition, a meta-analysis of six studies with a total of 576 patients, found that the administration of AOXs after varicocelectomy resulted in a greater improvement in sperm concentration (p<0.0001), total sperm motility (p=0.03), progressive sperm motility (p<0.00001), and sperm morphology (p<0.00001) compared to placebo [19]. Furthermore, AOXs improved progressive sperm motility and sperm vitality, and significantly reduced sperm DNA fragmentation (SDF) when used in patients undergoing sperm freezing and thawing [20].
The 2019, Cochrane review assessed the benefit of different AOXs on pregnancy, live-birth, and miscarriage rates [14]. Despite the low quality of the evidence, this latest Cochrane review validated the efficacy of AOXs in improving the pregnancy rate (odds ratio [OR] 2.97, 95% confidence interval [CI] 1.91–4.63). However, the review failed to provide evidence of improvement of live-birth rate because of the low quality of the studies and limited amount of data available.
Despite these potential benefits, the role of AOX treatment in male infertility is still controversial. Major scientific societies, including the European Association of Urology (EAU) [21] and the American Urological Association/American Society for Reproductive Medicine (AUA/ASRM) [22], are not supporting the routine use of AOXs in infertile men, mainly due to the heterogeneity of data. Hence, there is a need for further studies.
The aim of the present systematic review and meta-analysis is to provide an updated, analysis on the impact of AOX therapy for male infertility as compared to placebo or no treatment. Spontaneous clinical pregnancy rate, live-birth, and miscarriage rates were selected as primary outcomes, while conventional sperm parameters, SDF, and seminal OS indices were considered as secondary outcomes. To the best of our knowledge, this is the first study conducting a meta-analysis of seminal OS indices in infertile men after AOX therapy.
MATERIALS AND METHODS
1. Protocol and outcome measures
This meta-analysis was conducted on previously published articles investigating the role of AOX therapy in the management of male infertility and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocols [23]. The PRISMA checklist has been included as Supplement Table 1. The primary outcome was defined as the impact of AOXs on spontaneous clinical pregnancy rate, live-birth rate, and miscarriage rate. Secondary outcomes were the impact on basic semen parameters (sperm concentration, progressive sperm motility, total sperm motility, sperm morphology using the percentage of normal sperm forms), SDF, and indices of seminal OS such as seminal total AOX capacity (TAC) and malondialdehyde acid (MDA). This protocol has been registered in the PROSPERO database (PROSPERO registration number: CRD42022304600).
2. Eligibility criteria
This systematic review and meta-analysis included all English-language RCTs on male factor subfertility/infertility published until July 2021 that reported spontaneous pregnancy outcomes, and conventional semen parameters, SDF, or seminal OS in AOX-treated patients vs. placebo-treated or untreated controls. AOX is defined as a supplement (containing a single or a combination of compounds) that can be obtained without a prescription, is not regulated as a pharmaceutical drug, and has an AOX effect. Combined AOXs are those that are composed of two or more AOXs. All RCT studies were included regardless of the type or dose of the oral AOX. Studies that include AOXs plus a plant extract were included if the AOX was the main compound used in the intervention group. All different study designs other than RCTs, animal studies, in vitro studies, case reports, case series, studies of plant extracts or herbal substances, and studies enrolling men taking hormonal or any other fertility drugs were excluded.
3. Search strategy
A systematic search was conducted using Scopus, PubMed, Ovid, Embase, and Cochrane databases. The initial query string on Scopus search was: ( ( ( TITLE-ABS-KEY ( antioxidant ) AND TITLE-ABS-KEY ( infertil* ) AND TITLE-ABS-KEY ( ( male ) OR ( man ) OR ( men ) ) ) AND NOT ( TITLE-ABS-KEY ( ( mouse ) OR ( mice ) OR ( rat ) OR ( animal ) ) ) ) AND NOT ( TITLE-ABS-KEY ( ( meta-analysis ) OR ( meta-analysis ) ) ) ) AND ( TITLE-ABS-KEY ( ( year*) OR ( month* ) OR ( day* ) OR ( year* ) OR ( time ) OR ( duration ) ) ) AND ( LIMIT-TO ( DOCTYPE,"ar") ) AND ( EXCLUDE ( SUBJAREA,"AGRI" ) OR EXCLUDE ( SUBJAREA,"ENVI" ) ) AND ( EXCLUDE ( SUBJAREA,"CENG" ) ) AND ( EXCLUDE ( SUBJAREA,"ENGI" ) ) AND ( EXCLUDE ( SUBJAREA,"COMP") ) AND ( EXCLUDE ( SUBJAREA,"IMMU" ) ) AND ( EXCLUDE ( SUBJAREA,"HEAL" ) ) AND ( EXCLUDE ( SUBJAREA,"SOCI" ) ) AND ( EXCLUDE ( SUBJAREA,"MATE" ) ) AND ( EXCLUDE ( SUBJAREA,"MULT" ) OR EXCLUDE ( SUBJAREA,"VETE" ) ) AND ( EXCLUDE ( LANGUAGE,"Portuguese" ) ) AND ( EXCLUDE ( LANGUAGE,"Turkish" ) OR EXCLUDE ( LANGUAGE,"Chinese" ) OR EXCLUDE ( LANGUAGE,"Dutch" ) OR EXCLUDE ( LANGUAGE,"German" ) ) AND ( EXCLUDE ( LANGUAGE,"Hungarian" ) ) AND ( EXCLUDE ( LANGUAGE,"French" ) ) AND ( EXCLUDE ( LANGUAGE,"Persian" ) ). PubMed, Ovid, Embase, and Cochrane’s search string used was: ( (antioxidant ) AND ( infertil* ) AND ( ( male ) OR ( man ) OR ( men) ) ) AND ( ( ( year* ) OR ( month* ) OR ( day* ) OR ( year* ) OR ( time ) OR ( duration ) ) ).
All the eligible studies were selected following the PICO (Population, Intervention, Comparison/Comparator, Outcomes) model (Supplement Table 2). Abstracts of the retrieved articles were independently screened and assessed to confirm their eligibility by four researchers (GS, AF, SK, AR). They worked in pairs, and disagreements were resolved by the fifth researcher (RC).
4. Assessment of quality of included studies
To evaluate the quality of evidence (QoE) of the included studies three tools were used: the Cochrane Risk of Bias of RCTs [24], the JADAD score [25], and CONSORT guidelines [26]. The QoE was assessed by four researchers (GS, AF, SK, AR) who worked in pairs. Disagreements were resolved by a fifth researcher (RC).
5. Data extraction
Data extraction was conducted for all eligible articles with full-text availability. Extracted information included the following: first author, year of publication, country, study design, the total number of patients, types of AOX, duration of treatment in months, semen parameters (volume, total motility, progressive motility, concentration, and morphology), OS indices (TAC and MDA), SDF, and pregnancy-related outcomes (pregnancy rate, live-birth rate, and miscarriage rate).
6. Accuracy of data collection
To ensure accuracy of search results and to reduce potential errors due to manual collection, screening for eligibility, data extraction, and quality assessment were done in duplicates and cross-matched in each subgroup. In case of discrepancy between screener and verifier, the results in dispute were verified by a third senior author to make a final decision.
7. Statistical analysis
The meta-analysis was performed using the random effect model. Measures of heterogeneity included Cochrane-Q test and I2 statistics. The weight of each individual study was determined using the inverse variance method and the Mantel–Haenszel methods for continuous and binary data, respectively. The publication bias was assessed with the color-contoured funnel plot and the asymmetry of the plot was tested by the Egger and Harbord tests for mean difference (MD) and log-OR respectively. For the SDF, we used the standardized mean difference (SMD), as this outcome was evaluated using different protocols. A subgroup analysis was performed as a sensitivity analysis and to determine the importance of subgroups on the pooled effect size. All p-values lower than 0.05 were considered statistically significant. The analysis was performed using RevMan software v. 5.4 (Cochrane Collaboration, Oxford, UK) and the IBM v 25 statistical software (IBM Corp., Armonk, NY, USA) and R-programing language v 4.1.0.
RESULTS
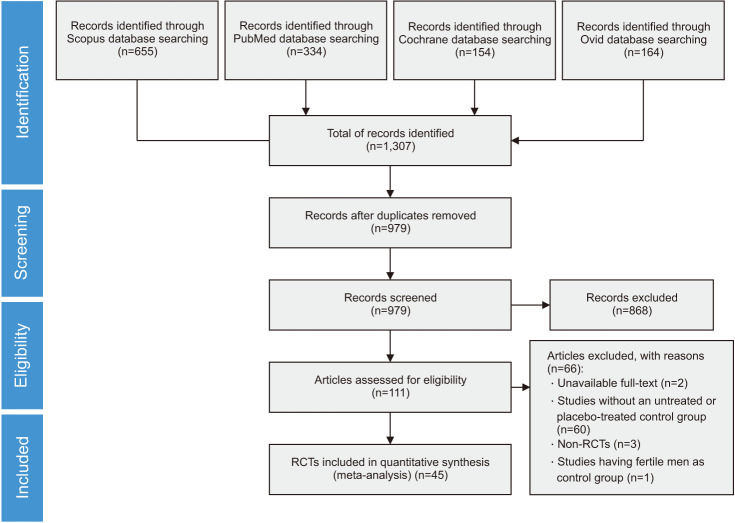
Using the above-mentioned search strategy, we extracted 1,307 abstracts. After the exclusion of 328 duplicates, the remaining 979 abstracts were assessed for inclusion in the meta-analysis. Of these, 868 were judged not eligible based on their title and the abstract, or because they were narrative reviews, comments, systematic reviews and meta-analyses, or letters to the editor. Among the remaining 111 articles that were initially deemed eligible, 66 were excluded due to unavailable full-text, absence of untreated or placebo-treated control group, non-RCT study design, not extractable data or presence of fertile men in the control group. Finally, 45 RCTs met the study inclusion criteria and were included in the meta-analysis, for a total of 4,332 infertile patients (Fig. 1).
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the included studies. RCT: randomized controlled trial.
Six of the included studies were performed in an infertile population receiving AOX or no treatment following varicocele repair [27,28,29,30,31,32]. Therefore, for each outcome which included a varicocele repair group we performed a sub-analysis after exclusion of these studies. The main characteristics of the included studies are shown in Table 1. The duration of AOX therapy of the analyzed studies ranged from 1 to 12 months. The quality of the included studies is shown in Table 2. The inclusion and exclusion criteria for each included study are detailed in Supplement Table 3.
Table 1. Main characteristics of the 45 randomized controlled trials included in this meta-analysis.
| Reference | Year | Population | Intervention | Control | Duration (mo) | Outcome |
|---|---|---|---|---|---|---|
| Eslamian et al [51] | 2020 | Idiopathic asthenozoospermic men | 465 mg DHA plus 600 IU vitamin E (n=41), or 465 mg DHA plus vitamin E–resembling placebo (n=41), or 600 IU vitamin E plus DHA-resembling placebo (n=45) | Placebo (n=45) | 3 | Basic semen parameters, OS |
| Kopets et al [38] | 2020 | Patients with IMI | LC/LAC 1,990 mg, coQ10 40 mg, L-arginine 250 mg, glutathione 100 mg, Zinc 7.5 mg, vitamin B9 234 μg, vitamin B12 2 μg, Selenium 50 μg (n=42) | Placebo (n=41) | 6 | Basic semen parameters, natural pregnancy |
| Steiner et al [42] | 2020 | Patients with OAT or high SDF | 500 mg of vitamin C, 400 mg of vitamin E, 0.20 mg of selenium, 1,000 mg of LC, 20 mg of zinc, 1,000 mcg of folic acid, 10 mg of lycopene daily (n=85) | Placebo (n=86) | 6 | Semen parameters, SDF, live-birth rate |
| Ardestani Zadeh et al [27] | 2019 | Infertile patients with varicocele undergone to varicocele repair | Folic acid 5 mg, Selenium 200 μg, vitamin E 400 IU (n=27) | No treatment (n=30) | 6 | Semen parameters |
| Kızılay and Altay [28] | 2019 | Infertile patients with OAT and varicocele, following varicocele repair | LC fumarate 2 g, LAC 1 g, fructose 2 g, citric acid 100 mg, vitamin C 180 mg, Zinc 20 mg, Folic acid 400 mcg, Selenium 100 mcg, CoQ10 40 mg, vitamin B12 3 mcg (n=62) | No treatment (n=28) | 6 | Basic semen parameters, natural pregnancy/live-birth |
| Nouri et al [60] | 2019 | Idiopathic asthenozoospermic men | Lycopene 25 mg daily (n=17) | Placebo (n=19) | 3 | Basic semen parameter, OS |
| Blomberg Jensen et al [69] | 2018 | Men part of an infertile couple with impaired semen quality | Vitamin D 1,400 IU+calcium 500 mg plus vitamin D 300,000 IU oil once orally (n=136) | Placebo (n=133) | 5 | Semen parameters, live-birth rate (ART)b |
| Busetto et al [36] | 2018 | Infertile men with oligo- and/or astheno- and/or teratozoospermia, divided into patients with varicocele grade I–III, and patients without varicocele | LC 1,000 mg, fumarate 725 mg, LAC 500 mg, fructose 1,000 mg, CoQ10 20 mg, vitamin C 90 mg, zinc 10 mg, folic acid 200 μg and vitamin B12 1.5 μg/daily (n=49) | Placebo (n=45) | 6 | Basic semen parameters, natural pregnancy/live-birth, miscarriage |
| Lu et al [55] | 2018 | Infertile men with left-sided clinical varicocele | 400 mg melatonin daily (n=27) | Placebo (n=27) | 3 | Basic semen parameters, OS |
| Stenqvist et al [43] | 2018 | Subfertile men | Vitamin C 3 mg, vitamin E 5 mg, vitamin B12 0.5 μg, LC 750 mg, CoQ10 10 mg, folic acid 100 μg, zinc 5 mg, selenium 25 μg (n=37) | Placebo (n=40) | 6 | Basic semen parameters, SDF, pregnancy rate |
| Busetto et al [35] | 2017 | Infertile patients with OAT | 2 sachets of Proxeed Plus daily (1,000 mg l-carnitine, 725 mg fumarate, 500 mg LAC, 1,000 mg fructose, 20 mg CoQ10, 90 mg vitamin C, 10 mg zinc, 200 μg folic acid and1.5 μg vitamin B12) (n=52) | Placebo (n=52) | 6 | Basic semen parameters, natural pregnancy/live-birth |
| Barekat et al [29] | 2016 | Subfertile men with varicocele grade 2–3 following varicocele repair | NAC 200 mg (n=20) | No treatment (n=20) | 3 | Basic semen parameters, OS, SDF, natural pregnancy/live-birth |
| Ener et al [30] | 2016 | Infertile men with a left-sided clinical varicocele following to varicocele repair | Vitamin E 600 mg (n=22) | No treatment (n=23) | 12 | Basic semen parameters, natural pregnancy/live-birth |
| Boonyarangkul et al [46] | 2015 | Men with abnormal semen analysis | Tamoxifen citrate 20 mg (n=15)a or Folate 5 mg (n=15) or Tamoxifen citrate 20 mg plus Folate 5 mg (n=15)a | Placebo (n=15) | 3 | Basic semen parameters |
| Cyrus et al [31] | 2015 | Infertile men with palpable varicocele grade 2–3 underwent to varicocele repair | Vitamin C 500 mg (n=46) | Placebo (n=69) | 3 | Basic semen parameters |
| Haghighian et al [53] | 2015 | Infertile men with idiopathic asthenozoospermia | ALA 600 mg (n=23) | Placebo (n=21) | 3 | Basic semen parameters, OS |
| Moslemi Mehni et al [57] | 2014 | Infertile men with idiopathic OAT | Pentoxifylline 800 mg+L-carnitine 1,000 mg (n=58) or Pentoxifylline 800 mg+Placebo (n=59) or LC 1,000 mg+Placebo (n=59) | Placebo (n=59) | 3 | Basic semen parameters |
| Azizollahi et al [32] | 2013 | Infertile men with varicocele grade III following varicocele repair | Zinc 66 mg (n=32) or Folic acid 5 mg (n=26) or Zinc 66 mg+Folic acid 5 mg (n=29) | Placebo (n=25) | 6 | Sperm concentration, morphology, motility, forward progressive motility |
| da Silva et al [49] | 2013 | Subfertile men | Folic acid 5 mg daily (n=23) | Placebo (n=26) | 3 | Basic semen parameters |
| Gopinath et al [37] | 2013 | Idiopathic OA | 2 tablets of CoQ10 50 mg+L-carnitine 500 mg+lycopene 2.5 mg+zinc 12.5 mg (n=46) or 1 tablet (n=43) | Placebo (n=36) | 6 | Basic semen parameters, spontaneous pregnancy rate |
| Safarinejad et al [64] | 2012 | Infertile men with primary infertility for at least 2 years | CoQ10 (Ubiquinol) 200 mg (n=114) | Placebo (n=114) | 6.5 | Basic semen parameters, seminal plasma antioxidant status |
| Nadjarzadeh et al [59] | 2011 | Infertile men with OAT who have been trying for pregnancy for >1 year of unprotected intercourse | CoQ10 200 mg (n=23) | Placebo (n=24) | 3 | Basic semen parameters, OS |
| Safarinejad [65] | 2011 | Infertile men with idiopathic OAT | Omega-3 fatty acids: EPA and DHA, 1.84 g per day (n=119) | Placebo (n=119) | 8 | Basic semen parameters, OS |
| Dimitriadis et al [50] | 2010 | Infertile men with OA | Vardenafil 10 mg (n=23)a or Sildenafil 50 mg (n=25)a or LC 1000 mg (n=26) | No treatment (n=22) | 5.5 | Basic semen parameters |
| Martinez-Soto et al [56] | 2010 | Infertile men | DHA 1,000 mg+EPA 135 mg (n=35) | Placebo (n=29) | 2.5 | SDF, basic semen parameters, antioxidant capacity |
| Morgante et al [58] | 2010 | Infertile men with asthenospermia | L-arginine 1,660 mg+carnitine 150 mg+LAC 50 mg+ginseng 200 mg in one vial (n=90) | No treatment (n=90) | 3 | Basic semen parameters |
| Peivandi et al [62] | 2010 | Infertile men | LC 2 g (n=15) | Placebo (n=15) | 2 | Basic semen parameters |
| Balercia et al [34] | 2009 | Infertile men with idiopathic asthenozoospermia | CoQ10 200 mg (n=30) | Placebo (n=30) | 6 | Basic semen parameters, spontaneous pregnancy rate |
| Ciftci et al [47] | 2009 | IMI | NAC (600 mg daily) (n=60) | Placebo (n=60) | 3 | Basic semen parameters, OS |
| Safarinejad and Safarinejad [66] | 2009 | Men with idiopathic OAT, asthenospermia or teratospermia of 2 years duration | Selenium 200 µg (n=116) or NAC 600 mg (n=118) or Selenium 200 µg+NAC 600 mg (n=116) | Placebo (n=118) | 6.5 | Basic semen parameters |
| Safarinejad [67] | 2009 | Subfertile men with idiopathic OAT | CoQ10 300 mg (n=106) | Placebo (n=106) | 6.5 | Basic semen parameters |
| Stanislavov et al [68] | 2009 | Subfertile patients | 80 mg Pycnogenol and 3 g L-arginine aspartate (n=25) | Placebo (n=25) | 1 | Basic semen parameters |
| Omu et al [61] | 2008 | Men with asthenozoospermia attending infertility and andrology clinic | Zinc 400 mg (n=11) or Zinc 400 mg+vitamin E 20 mg (n=12) or Zinc 400 mg+vitamin E 20 mg+vitamin C 10 mg (n=14) | No treatment (n=8) | 3 | Basic semen parameters, spontaneous pregnancy rate, live-birth rate, miscarriage |
| Paradiso Galatioto et al [40] | 2008 | Men with persistent oligospermia (5–20 million/mL) 6 months after retrograde embolization | NAC 600 mg, vitamin C 180 mg, vitamin E 12 mg, vitamin A 3.6 IU, thiamine 24 mg, riboXavin 6 mg, piridoxin 12 mg, nicotinamide 60 mg, pantothenate 12 mg, biotin 2.4 mg, cyanocobalamin 6 mg, ergocalciferol 480 IU, calcium 60 mg, magnesium 21.5 mg, phosphate 27 mg, iron 12 mg manganese 0.6 mg, copper 1.2 mg, zinc 0.6 mg (n=20) | No treatment (n=22) | 3 | Basic semen parameters, natural pregnancy |
| Sigman et al [71] | 2006 | Subfertile men aged 18 to 65 years | LC 2,000 mg+LAC 1,000 mg (n=12) | Placebo (n=9) | 4 | Basic semen parameters, pregnancy rate (ART)b |
| Balercia et al [33] | 2005 | Subfertile men with idiopathic asthenozoospermia | LC 3 g (n=15) vs. LAC 3 g (n=15) vs. LC 2 g+LAC 1 g (n=14) | Placebo (n=15) | 6 | Basic semen parameters, spontaneous pregnancy rate |
| Greco et al [52] | 2005 | Subfertile men | Vitamin C 1 g and Vitamin E 1 g (n=32) | Placebo (n=32) | 2 | Basic semen parameters, SDF |
| Lenzi et al [54] | 2003 | Subfertile men with OAT | LC 2 g+LAC 1,000 mg (n=43) | Placebo (n=43) | 2 | Basic semen parameters, spontaneous pregnancy rate |
| Závaczki et al [45] | 2003 | Subfertile men | Magnesium 3,000 mg (n=10) | Placebo (n=10) | 3 | Basic semen parameters, spontaneous pregnancy rate |
| Conquer et al [48] | 2000 | Healthy asthenozoospermic men who were patients of an infertility clinic | DHA 400 mg (n=9) or DHA 800 mg (n=10) | Placebo (n=9) | 3 | Basic semen parameters |
| Rolf et al [63] | 1999 | Men with infertility for over one year | Vitamin C 1,000 mg+vitamin E 800 mg (n=15) | Placebo (n=16) | 2 | Basic semen parameters and pregnancy rate |
| Omu et al [39] | 1998 | Men with asthenozoospermia attending infertility and andrology clinic | Zinc 500 mg (n=49) | No therapy (n=48) | 3 | Basic semen parameters, natural pregnancy/live-birth |
| Scott et al [41] | 1998 | Men attending subfertility clinic with low sperm motility | Selenium 100 mg daily alone (n=16) or selenium 100 mg plus selenium combined with vitamins A (1 mg), C (10 mg), and E (15 mg) supplements daily (n=30) | Placebo (n=18) | 3 | Basic semen parameters, natural pregnancy/live-birth |
| Suleiman et al [44] | 1996 | Asthenozospermic men attending a fertility center | Vitamin E 100 mg (n=52) | Placebo (n=35) | 6 | Sperm motility, spontaneous live-birth, pregnancy, miscarriage and MDA levels |
| Dawson et al [70] | 1990 | Men with sperm agglutination | Ascorbic acid 1,000 mg (n=10) or Ascorbic acid 200 mg (n=10) | Placebo (n=10) | 0.75 | Basic semen parameters |
ALA: alpha-lipoic acid, ART: assisted reproductive technique, CoQ10: coenzyme Q10, DHA: docosahexaenoic acid, EPA: eicosapentaenoic, IMI: idiopathic male infertility, LAC: L-acetyl-carnitine, LC: L-carnitine, MDA: malondialdehyde acid, NAC: N-acetylcysteine, OA: oligo-asthenoteratozoospermia, OAT: oligo-asthenoteratozoospermia, OS: oxidative stress, SDF: sperm DNA fragmentation.
aThis group was excluded from the analysis.
bOutcome excluded from the present meta-analysis, since it does not assess spontaneous pregnancy rate.
Table 2. Quality of evidence assessment results of the Cochrane Risk of Bias for Randomized Controlled Trials [21], CONSORT guidelines [23], and JADAD score [22].
| Reference | Year | Cochrane Risk of Bias for Randomized Controlled Trials | CONSORT guideline (1–25) | JADAD (1–5) | Total quality score (9–51) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection bias Random sequence generationa | Selection bias Allocation concealmenta | Reporting bias Selective reportinga | Other bias Other sources of biasa | Performance bias Blinding (participants and personnel)a | Detection bias Blinding (outcome assessment)a | Attrition bias Incomplete outcome dataa | Total score (7–21) | |||||
| Eslamian et al [51] | 2020 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 17 | 22 | 5 | 44 |
| Kopets et al [38] | 2020 | 3 | 3 | 3 | 2 | 3 | 2 | 2 | 18 | 18 | 5 | 41 |
| Steiner et al [42] | 2020 | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 17 | 15 | 3 | 35 |
| Ardestani Zadeh et al [27] | 2019 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 16 | 15 | 3 | 34 |
| Kızılay and Altay [28] | 2019 | 3 | 2 | 2 | 2 | 1 | 1 | 3 | 14 | 12 | 3 | 29 |
| Nouri et al [60] | 2019 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 20 | 17 | 5 | 42 |
| Blomberg Jensen et al [69] | 2018 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 24 | 4 | 48 |
| Busetto et al [36] | 2018 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 20 | 4 | 44 |
| Lu et al [55] | 2018 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 14 | 3 | 37 |
| Stenqvist et al [43] | 2018 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 20 | 21 | 5 | 46 |
| Busetto et al [35] | 2017 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 16 | 17 | 4 | 37 |
| Barekat et al [29] | 2016 | 2 | 2 | 3 | 2 | 1 | 1 | 3 | 14 | 13 | 2 | 29 |
| Ener et al [30] | 2016 | 2 | 2 | 1 | 2 | 1 | 1 | 3 | 12 | 13 | 2 | 27 |
| Boonyarangkul et al [46] | 2015 | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 16 | 13 | 3 | 32 |
| Cyrus et al [31] | 2015 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 19 | 21 | 5 | 45 |
| Haghighian et al [53] | 2015 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 22 | 5 | 47 |
| Moslemi Mehni et al [57] | 2014 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 11 | 13 | 2 | 26 |
| Azizollahi et al [32] | 2013 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 12 | 10 | 3 | 25 |
| da Silva et al [49] | 2013 | 3 | 3 | 2 | 2 | 3 | 2 | 3 | 18 | 17 | 5 | 40 |
| Gopinath et al [37] | 2013 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 14 | 17 | 3 | 34 |
| Safarinejad et al [64] | 2012 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21 | 20 | 5 | 46 |
| Nadjarzadeh et al [59] | 2011 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 19 | 19 | 4 | 42 |
| Safarinejad [65] | 2011 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 20 | 19 | 5 | 44 |
| Dimitriadis et al [50] | 2010 | 2 | 1 | 2 | 2 | 1 | 1 | 3 | 12 | 14 | 2 | 15 |
| Martinez-Soto et al [56] | 2010 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 19 | 15 | 4 | 38 |
| Morgante et al [58] | 2010 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 12 | 9 | 2 | 23 |
| Peivandi et al [62] | 2010 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 18 | 8 | 3 | 29 |
| Balercia et al [34] | 2009 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 17 | 12 | 2 | 31 |
| Ciftci et al [47] | 2009 | 3 | 3 | 3 | 1 | 2 | 2 | 3 | 17 | 14 | 4 | 35 |
| Safarinejad and Safarinejad [66] | 2009 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 20 | 4 | 44 |
| Safarinejad [67] | 2009 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 20 | 20 | 4 | 44 |
| Stanislavov et al [68] | 2009 | 1 | 3 | 2 | 2 | 3 | 3 | 3 | 17 | 14 | 3 | 34 |
| Omu et al [61] | 2008 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 9 | 9 | 2 | 20 |
| Paradiso Galatioto et al [40] | 2008 | 3 | 3 | 2 | 3 | 1 | 1 | 3 | 16 | 18 | 3 | 37 |
| Sigman et al [71] | 2006 | 2 | 2 | 2 | 2 | 2 | 1 | 3 | 14 | 15 | 3 | 32 |
| Balercia et al [33] | 2005 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 14 | 12 | 3 | 29 |
| Greco et al [52] | 2005 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 15 | 13 | 3 | 31 |
| Lenzi et al [54] | 2003 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 16 | 15 | 3 | 34 |
| Závaczki et al [45] | 2003 | 2 | 2 | 2 | 3 | 1 | 2 | 1 | 13 | 8 | 2 | 23 |
| Conquer et al [48] | 2000 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 16 | 16 | 3 | 35 |
| Rolf et al [63] | 1999 | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 17 | 16 | 3 | 36 |
| Omu et al [39] | 1998 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 11 | 13 | 1 | 25 |
| Scott et al [41] | 1998 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 17 | 14 | 4 | 35 |
| Suleiman et al [44] | 1996 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 12 | 6 | 3 | 21 |
| Dawson et al [70] | 1990 | 2 | 2 | 2 | 1 | 3 | 2 | 3 | 15 | 8 | 2 | 28 |
aHigh risk of bias=1; Unclear risk of bias=2; Low risk of bias=3.
1. Spontaneous pregnancy rate
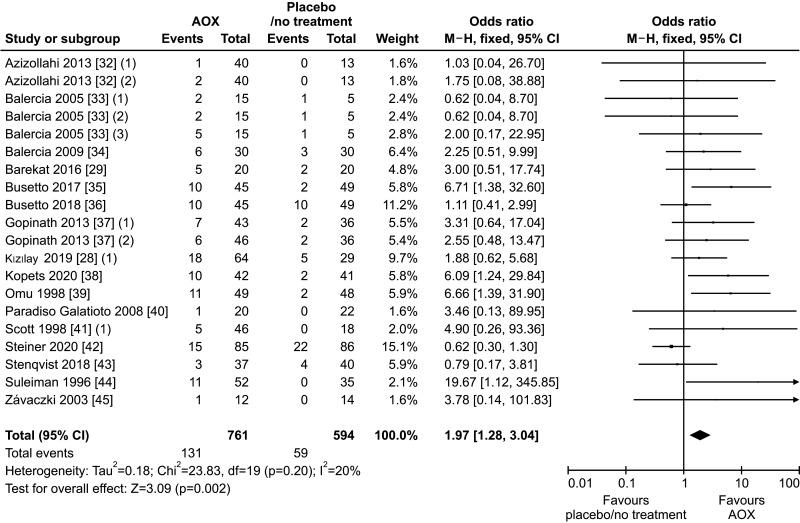
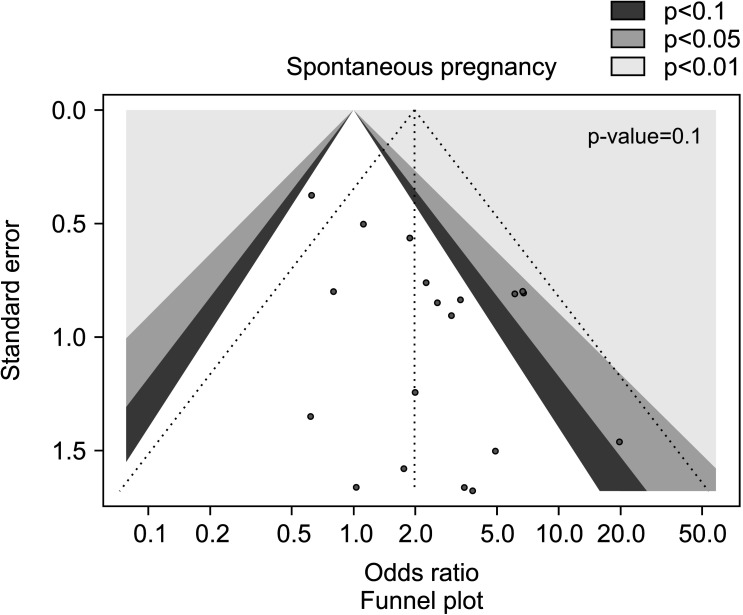
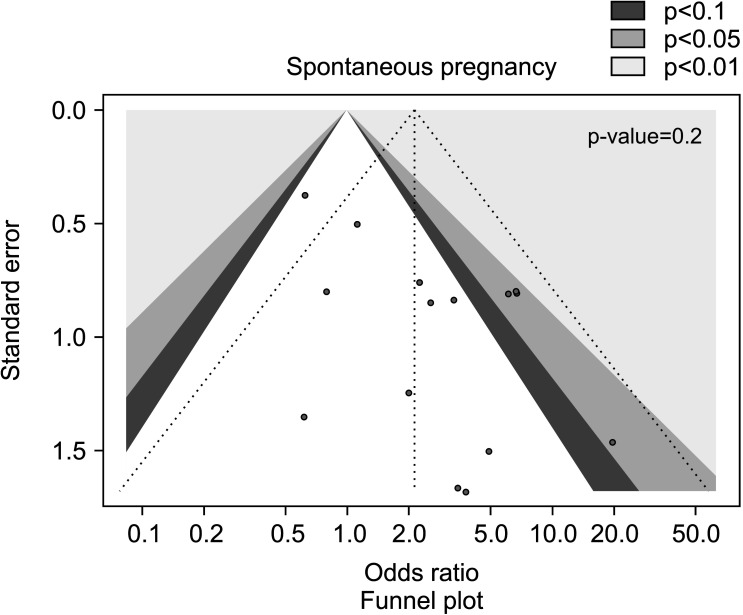
Sixteen studies [28,29,32,33,34,35,36,37,38,39,40,41,42,43,44,45] assessed spontaneous pregnancy rate in association with AOX, including 1,355 infertile patients (761 in the AOX-treated group and 594 in the untreated or placebo-treated control group) and 190 pregnancies were recorded. We found a positive effect of AOX treatment on spontaneous pregnancy rate (OR 1.97 [95% CI: 1.28, 3.04]; p<0.01), with absence of significant inter-study heterogeneity (I2=20%; χ2 p=0.20) (Fig. 2). There was no significant publication bias (Fig. 3).
Fig. 2. Forest plot of the spontaneous pregnancy rate in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
Fig. 3. Funnel plot of the spontaneous pregnancy rate in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
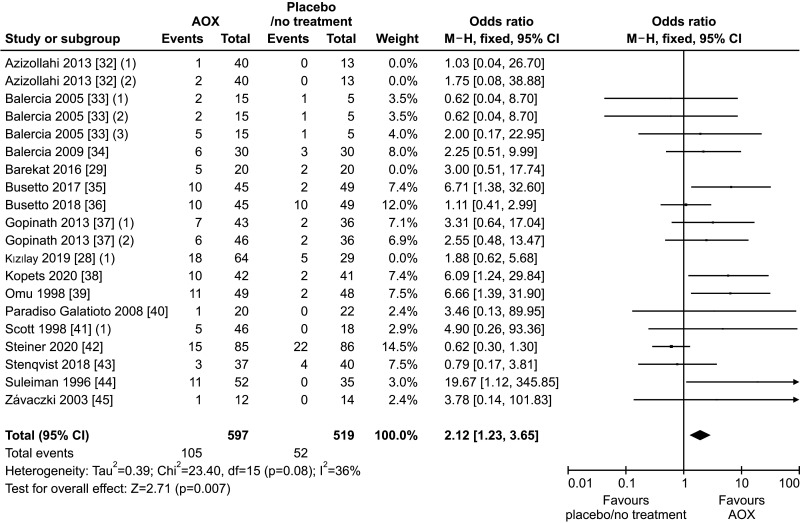
The sub-analysis performed after the exclusion of the studies administering AOX or no treatment following varicocele repair confirmed the benefit of AOX administration on spontaneous pregnancy rate (OR 2.12 [95% CI: 1.23, 3.65]; n=157 events; p<0.01] (Fig. 4), in the absence of significant inter-study heterogeneity (I2=36%; χ2 p=0.08), and no significant publication bias as confirmed by the funnel plot (Fig. 5).
Fig. 4. Forest plot of the spontaneous pregnancy rate in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
Fig. 5. Funnel plot of the spontaneous pregnancy rate in infertile patients treated with antioxidants compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
2. Live-birth rate
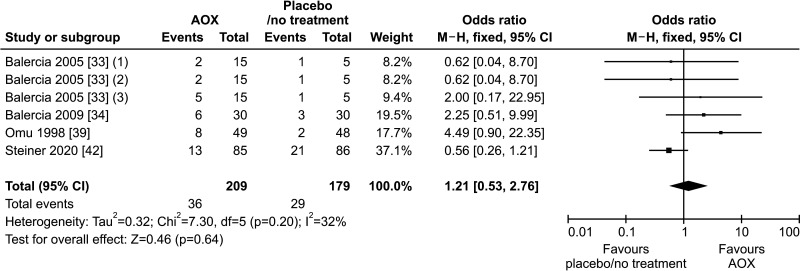
Only four studies could be included in the analysis of live-birth rate [33,34,39,42], overall including 388 infertile patients (209 in the AOX-treated group and 179 in the control one) and with 65 events (live-birth) recorded.
The analysis revealed no effect of AOX treatment on live-birth rate (OR 1.21 [95% CI: 0.53, 2.76]; p=0.64) (Fig. 6).
Fig. 6. Forest plot of the live-birth rate in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
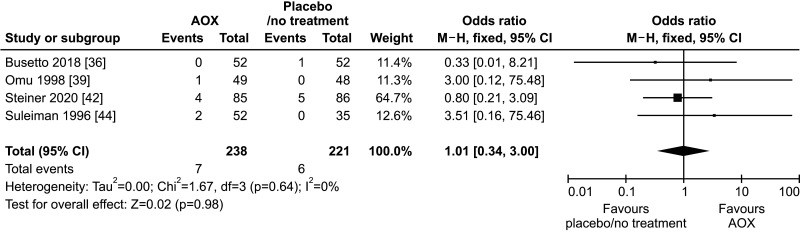
3. Miscarriage rate
Four studies reported data on the miscarriage rate and could be included in the analysis [36,39,42,44]. We found no effect of AOX treatment on miscarriage rate (OR 1.01 [95% CI: 0.34, 3.00]; n=13 events; p=0.98), in a total population of 459 infertile patients (Fig. 7).
Fig. 7. Forest plot of the miscarriage rate in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
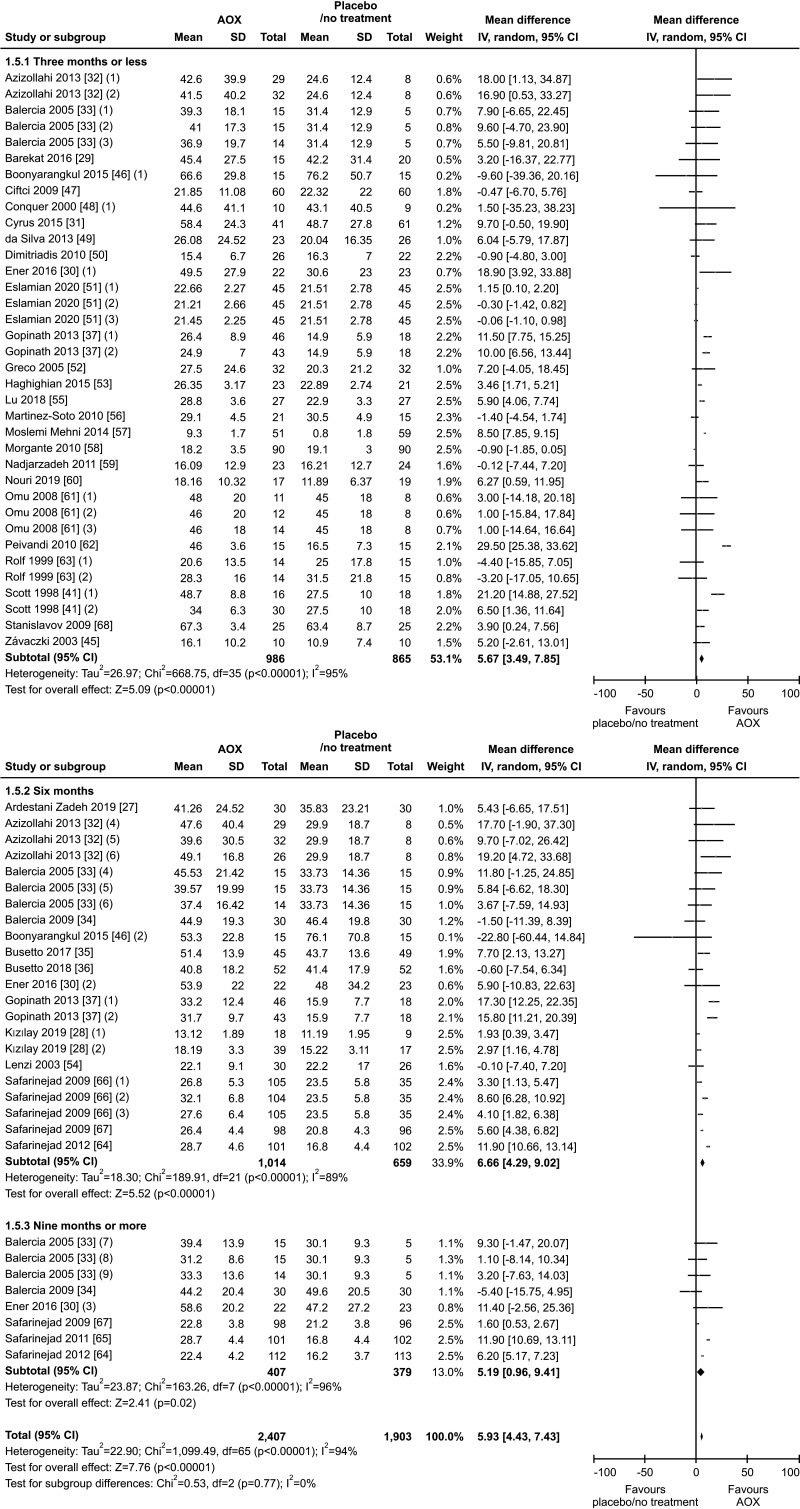
4. Sperm concentration
A total of thirty-six studies were included [27,28,29,30,31,32,33,34,35,36,37,41,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68], allowing to analyze this outcome in 4,310 infertile patients (2,407 AOX-treated patients and 1,903 placebo-treated or untreated controls).
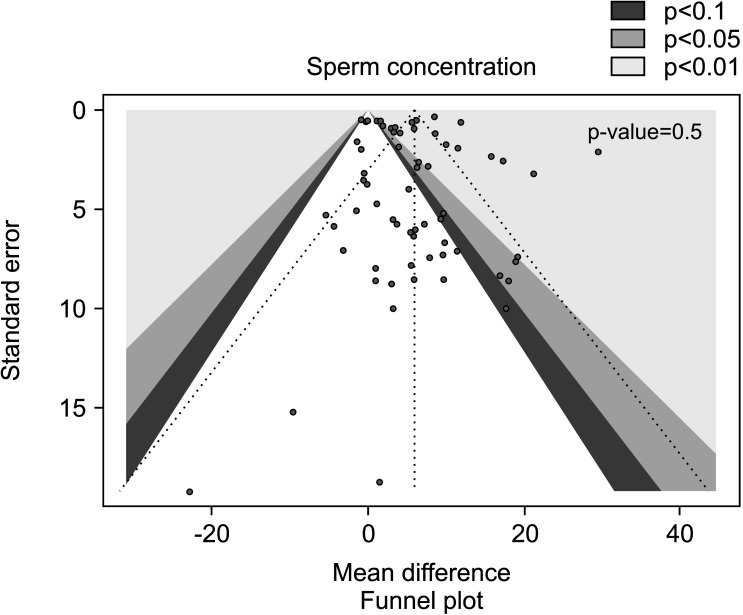
We found a significantly positive effect of AOX treatment on sperm concentration (weighted MD 5.93 mil/mL [95% CI: 4.43, 7.43]; p<0.01), with the presence of significant inter-study heterogeneity (I2=94%; χ2 p<0.01). There was no significant publication bias (p=0.5) (Fig. 8, 9).
Fig. 8. Forest plot of the sperm concentration in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
Fig. 9. Funnel plot of the sperm concentration in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
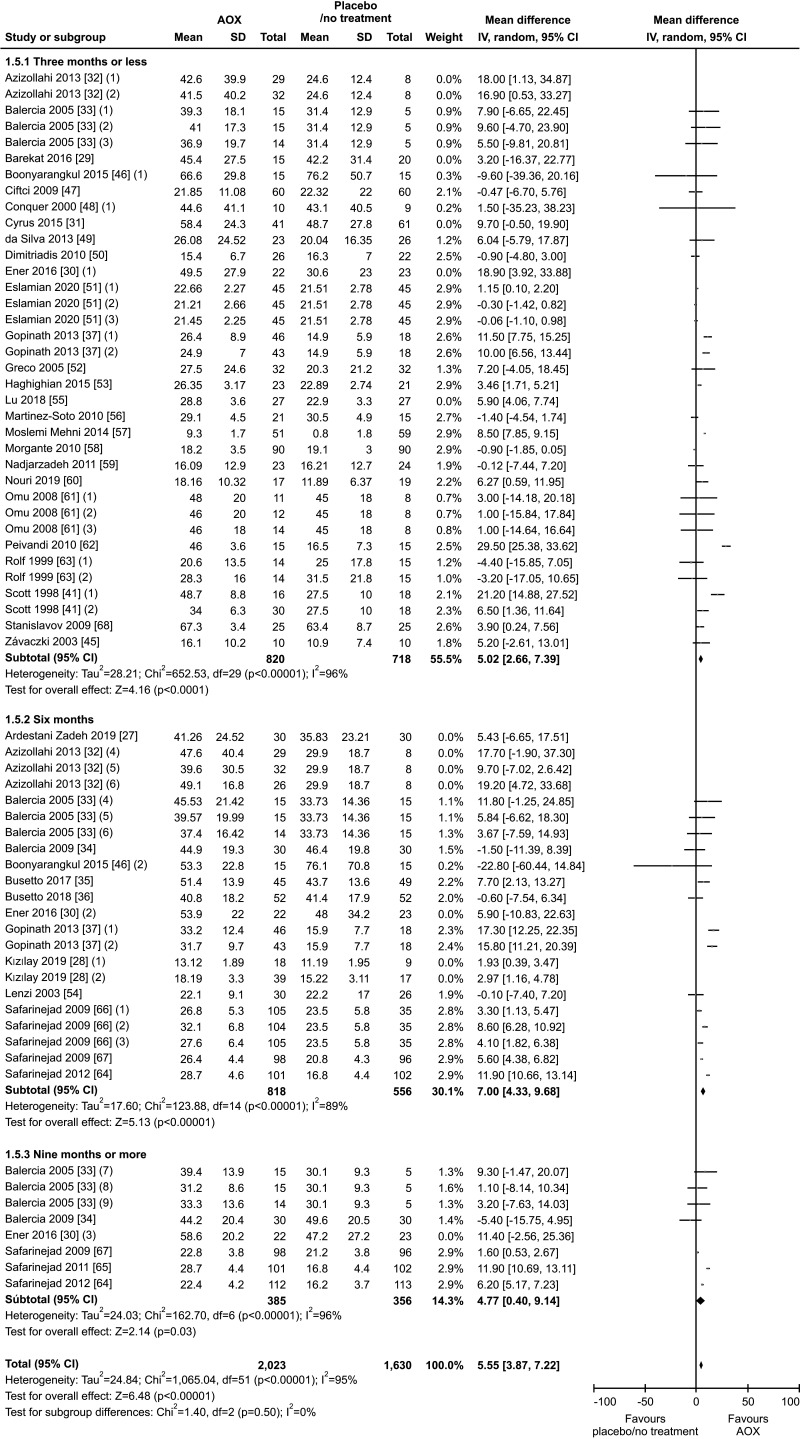
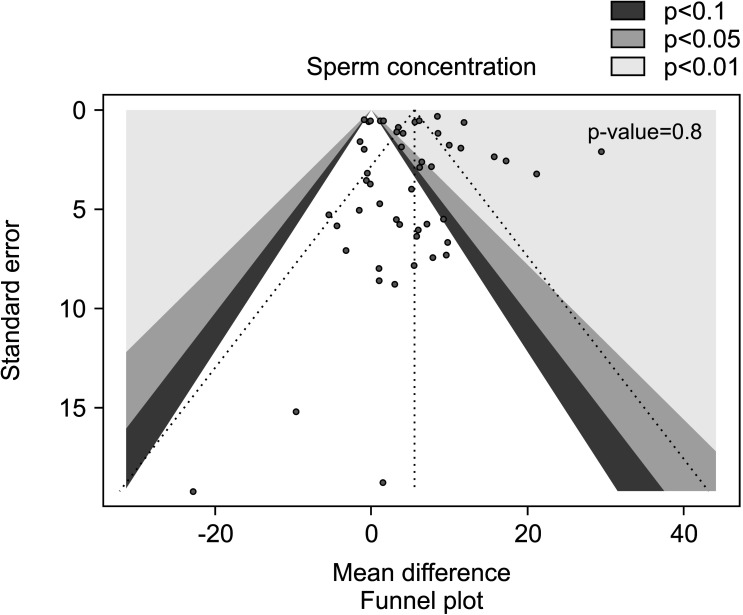
The results of sub-group analysis performed after exclusion of 7 studies on patients with varicocele that underwent varicocele repair followed by AOX or placebo/no treatment [27,32,55] on a total of 3,653 infertile men, indicated a significant persistent positive effect of AOX supplementation on sperm concentration (weighted MD 5.55 mil/mL [95% CI: 3.87, 7.22]; 2,023 patients vs. 1,630 controls; p<0.01). A significant inter-study heterogeneity was also found in this subgroup analysis (I2=95%; χ2 p<0.01), with no significant publication bias (p=0.8) (Fig. 10, 11).
Fig. 10. Forest plot of the sperm concentration in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
Fig. 11. Funnel plot of the sperm concentration in infertile patients treated with antioxidants compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
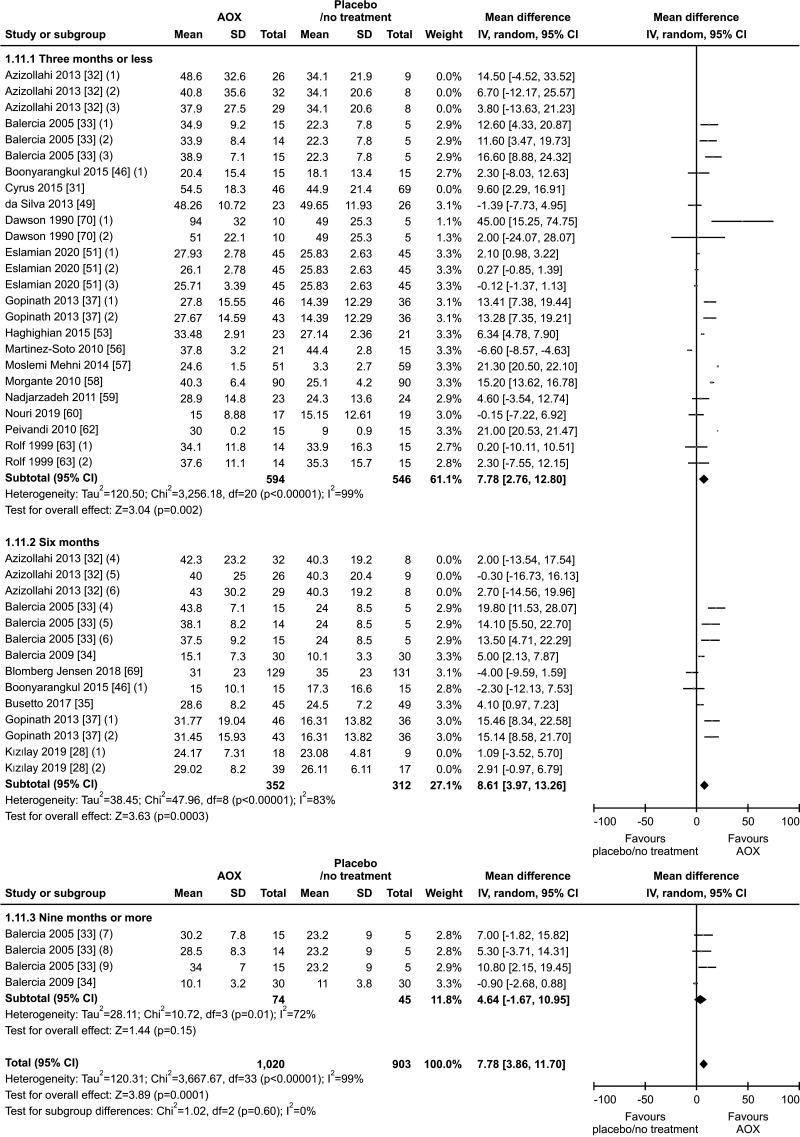
5. Progressive sperm motility
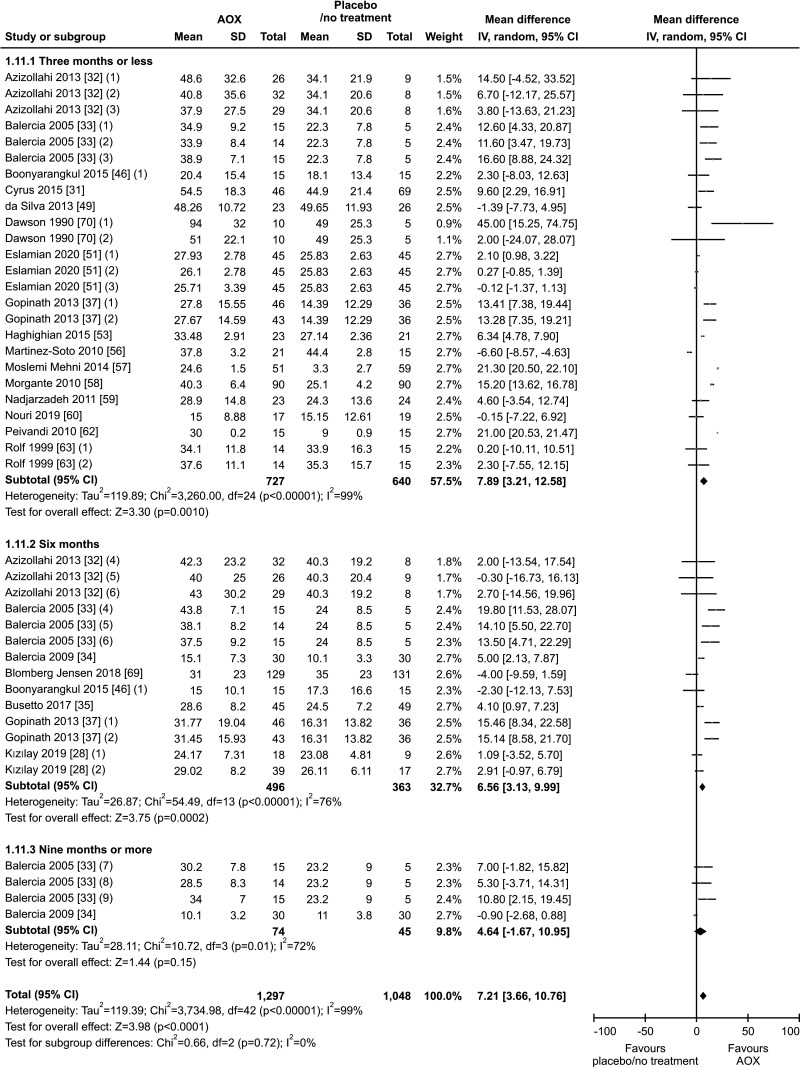
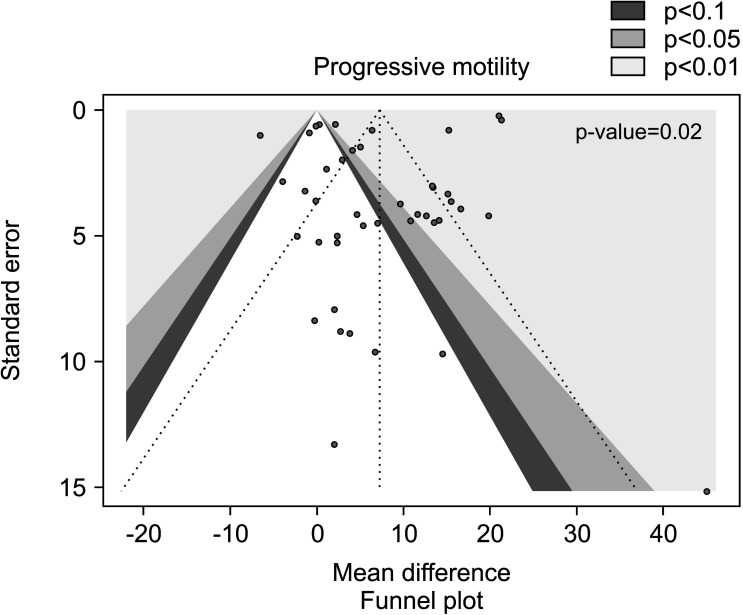
Twenty studies have been included in the analysis of the effect of AOX on progressive sperm motility [28,31,32,33,34,35,37,46,49,51,53,56,57,58,59,60,62,63,69,70], overall including 2,345 infertile patients (1,297 AOX-treated patients and 1,048 placebo-treated or untreated controls). The analysis showed a significant positive effect of AOX on progressive sperm motility (weighted MD 7.21% [95% CI: 3.66, 10.76]; p<0.01). Significant inter-study heterogeneity was observed (I2=99%; χ2 p<0.01). The Funnel plot was significantly asymmetrical denoting the presence of publication bias (p=0.02) (Fig. 12, 13).
Fig. 12. Forest plot of the sperm progressive motility in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
Fig. 13. Funnel plot of the sperm progressive motility in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
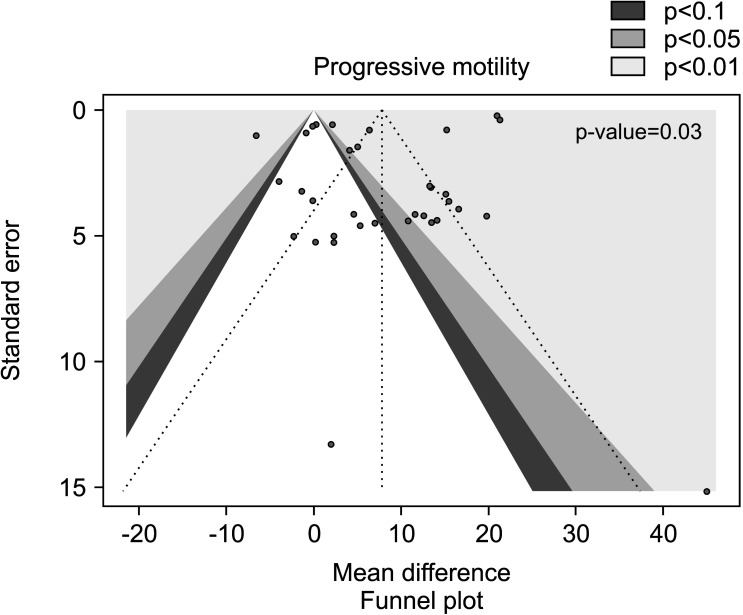
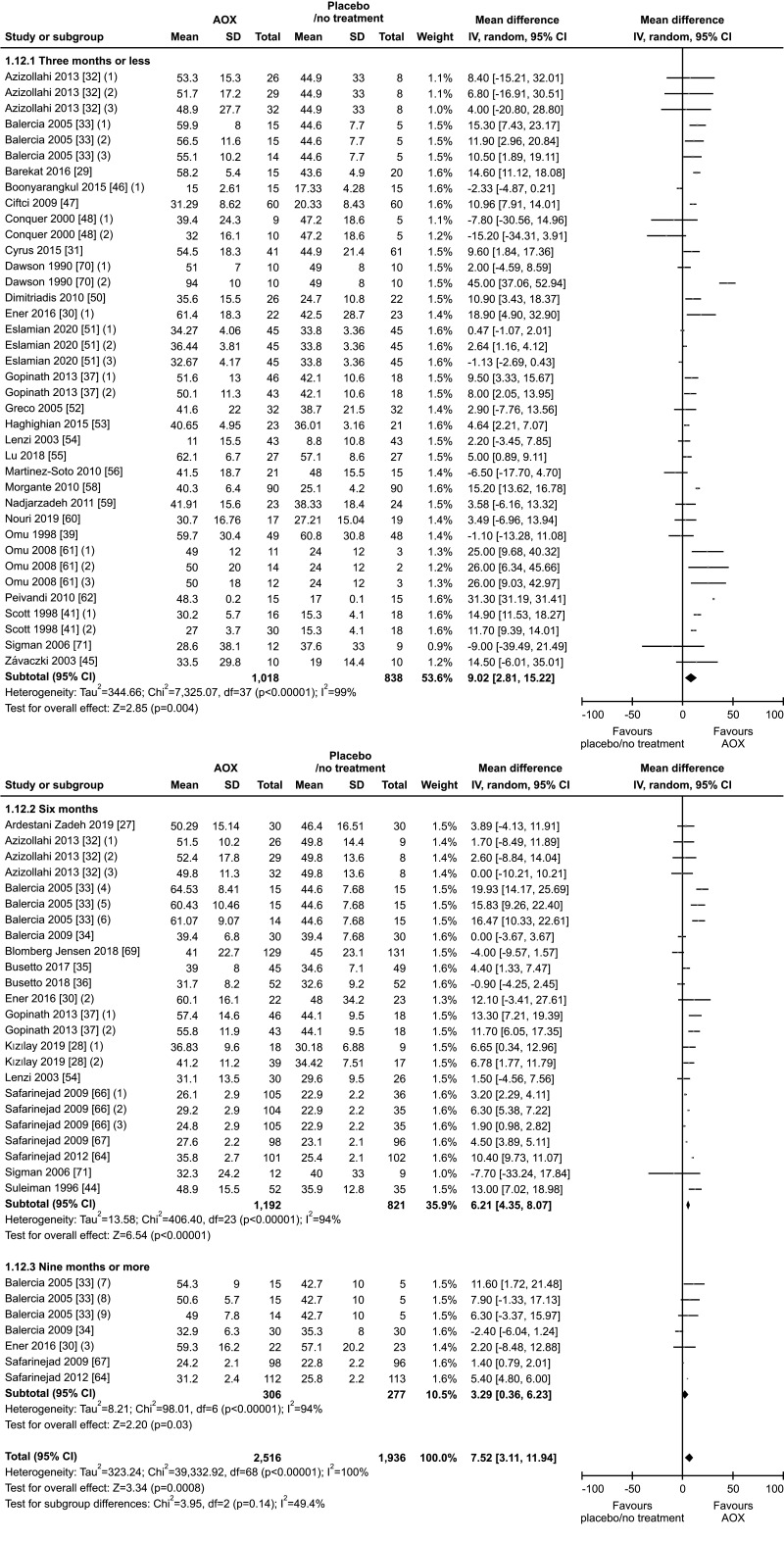
The benefit of AOX supplementation on progressive sperm motility was also confirmed by the analysis performed after the exclusion of studies carried out in patients with varicocele or treated with varicocele repair and AOX or placebo/no treatment [28,31,32], including a total of 1,923 infertile men (weighted MD 7.78% [95% CI: 3.86, 11.70]; 1,020 patients vs. 903 controls; p<0.01). We found significant inter-study heterogeneity (I2=99%; χ2 p<0.01) Also, significant publication bias persisted even after the exclusion of the studies with varicocele repair (Fig. 14, 15).
Fig. 14. Forest plot of the progressive sperm motility in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
Fig. 15. Funnel plot of the progressive sperm motility in infertile patients treated with antioxidants compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
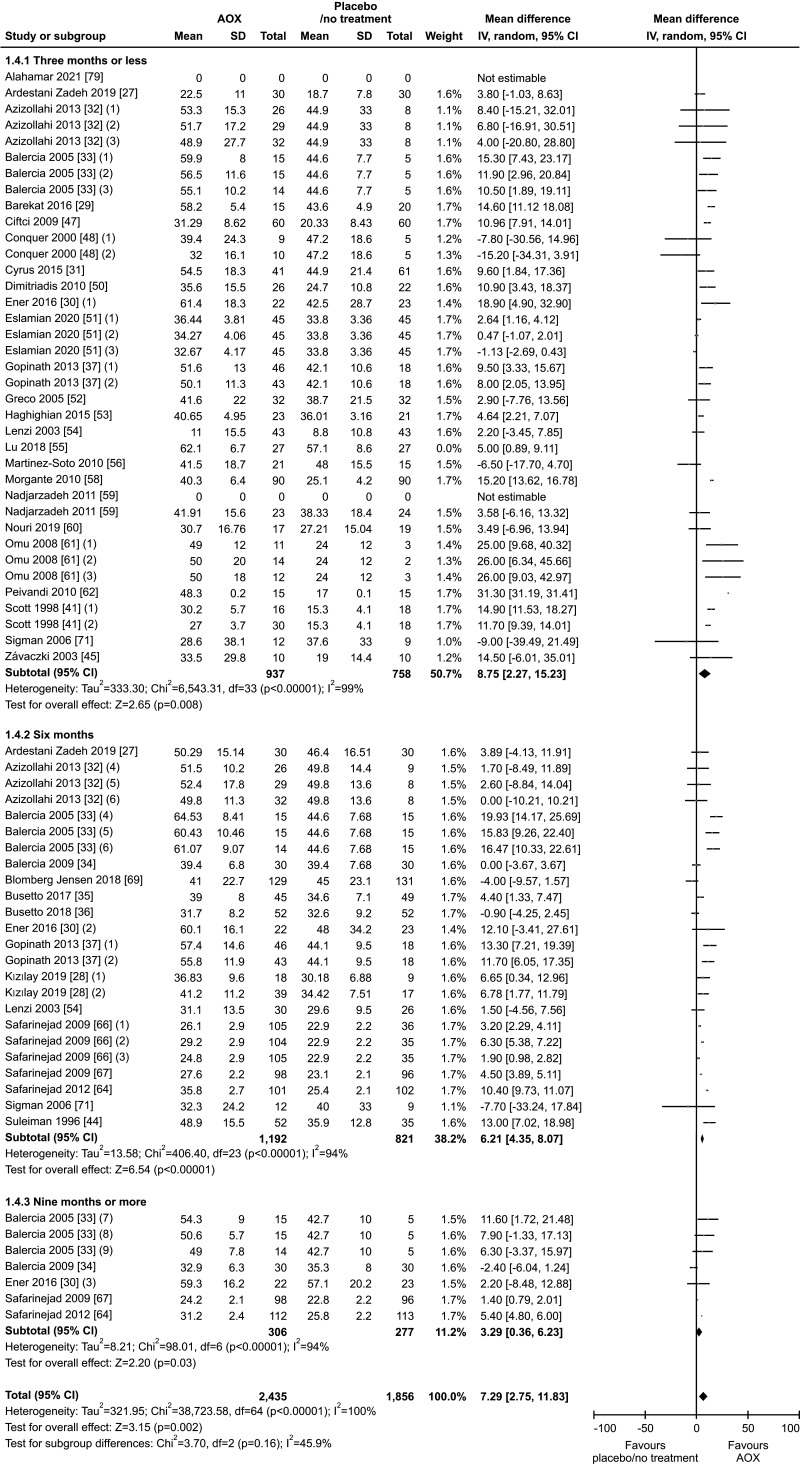
6. Total sperm motility
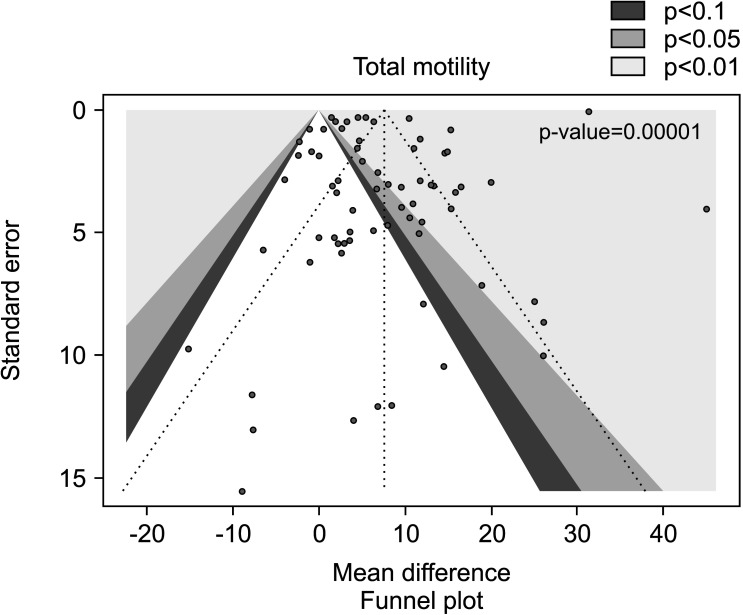
Thirty-six studies with a total of 4,452 infertile patients (2,516 AOX-treated patients and 1,936 placebo-treated or untreated controls) were included in the analysis of total sperm motility [27,28,29,30,31,32,33,34,35,36,37,39,41,44,45,46,47,48,50,51,52,53,54,55,56,58,59,60,61,62,64,66,67,69,70,71]. We found a significant positive effect of AOX on total sperm motility (weighted MD 7.52% [95% CI: 3.11, 11.94]; p<0.01).
Significant inter-study heterogeneity was observed (I2=100%; χ2 p<0.01). The asymmetry of the Funnel plot denoted significant publication bias (p<0.001) (Fig. 16, 17).
Fig. 16. Forest plot of the total sperm motility in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
Fig. 17. Funnel plot of the sperm total motility in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
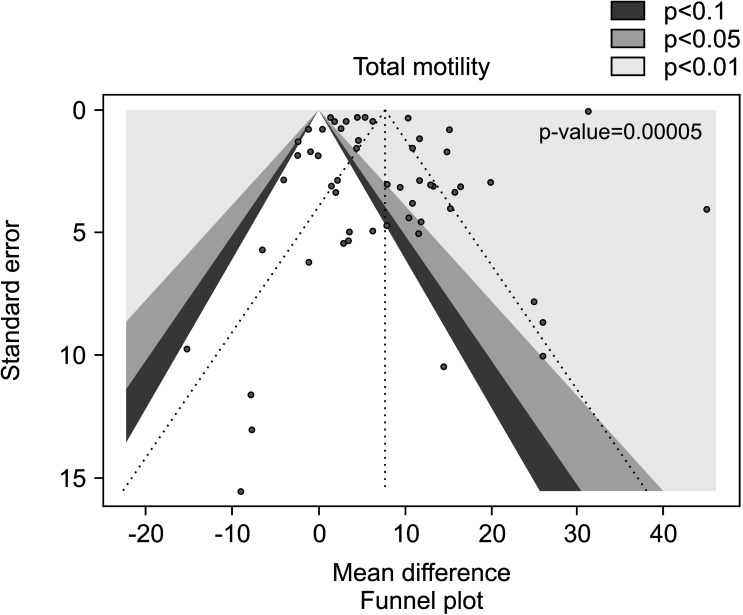
We also found a positive effect of AOX supplementation on total sperm motility after the exclusion of studies carried out in patients with varicocele or treated with varicocele repair and AOX or placebo/no treatment [27,28,29,30,31,32,50,55], including a total of 4,291 infertile men (MD 7.29% [95% CI: 2.75, 11.83]; 2,435 patients vs. 1,856 controls; p<0.01).
The analysis resulted in a significant inter-study heterogeneity (I2=100%; χ2 p<0.01) and the publication bias persisted even after the exclusion of the studies with varicocele repair (p<0.001) (Fig. 18, 19).
Fig. 18. Forest plot of the sperm total motility in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
Fig. 19. Funnel plot of the total sperm motility in infertile patients treated with antioxidants compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
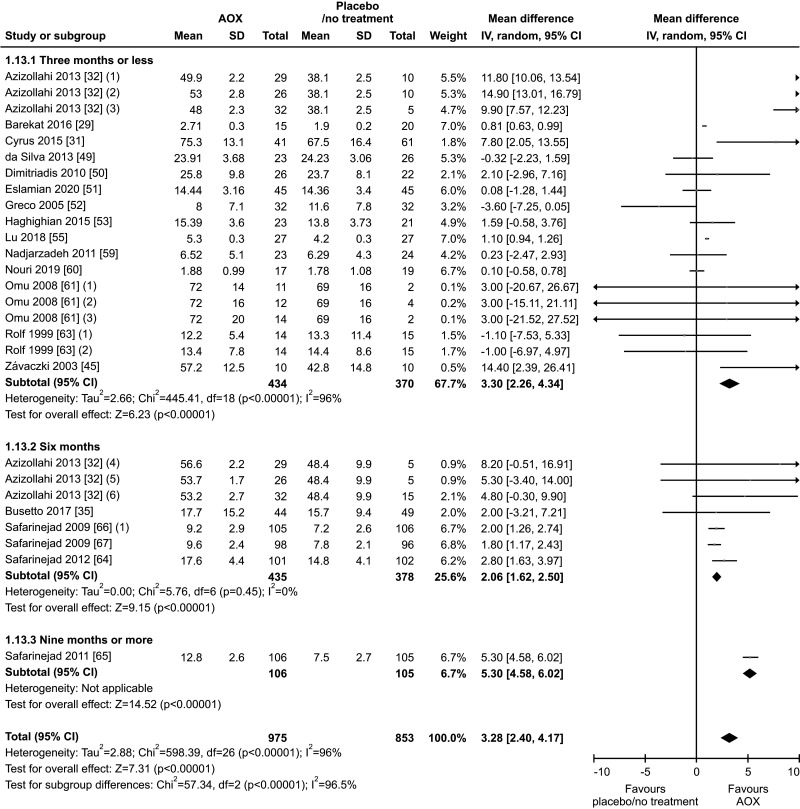
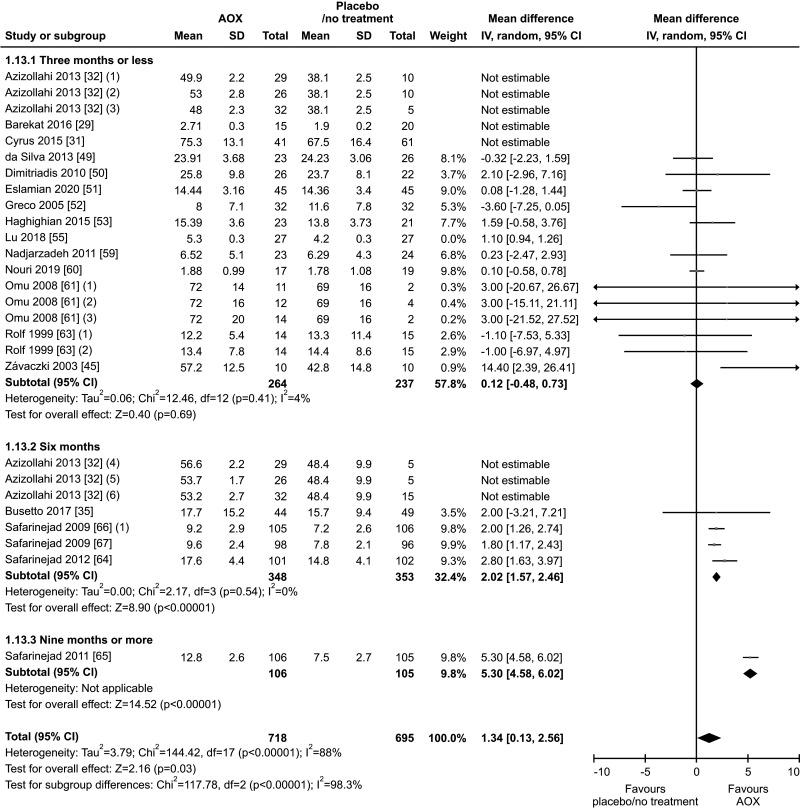
7. Sperm morphology
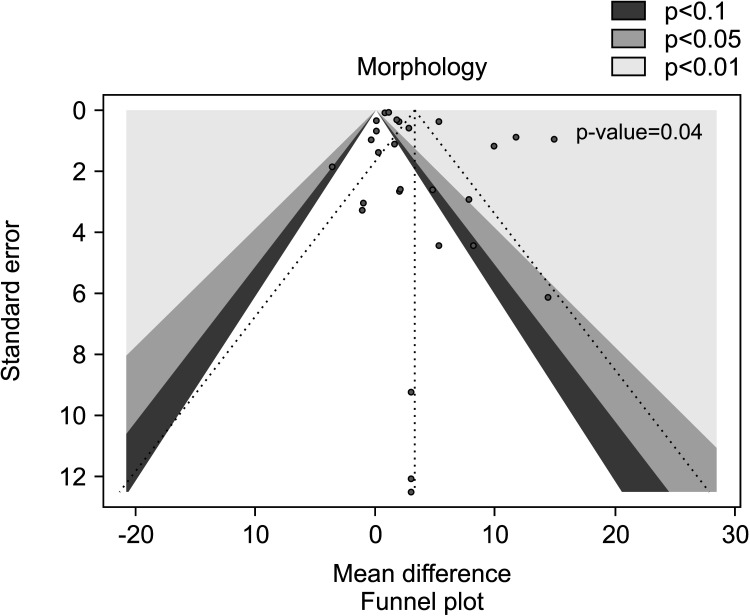
Eighteen studies, with a total of 1828 infertile men (975 in the AOX-treated group and 853 in the placebo-treated or untreated one), were included in the analysis of sperm morphology [29,31,32,35,45,49,50,51,52,53,55,59,60,61,63,64,66,67]. We found a significant positive effect of AOX on sperm morphology (weighted MD 3.28% [95% CI: 2.40, 4.17]; p<0.01). Significant inter-study heterogeneity was observed (I2=96%; χ2 p<0.01). In addition, a significant funnel plot asymmetry was found (p=0.04), thus consistent with the presence of publication bias (Fig. 20, 21).
Fig. 20. Forest plot of the sperm morphology in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
Fig. 21. Funnel plot of the sperm morphology in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
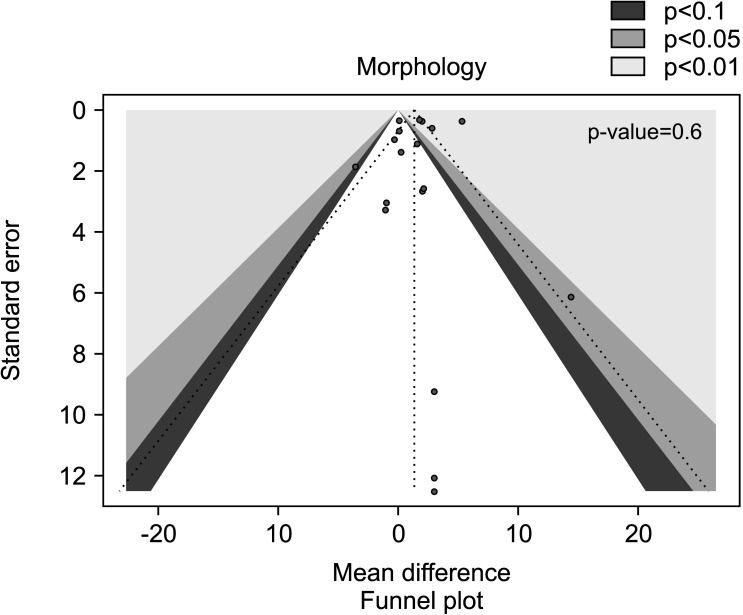
We also found a positive effect of AOX supplementation on sperm morphology after the exclusion of studies carried out in patients with varicocele or treated with varicocele repair and AOX or placebo/no treatment [29,31,32,50,55], including a total of 1,413 infertile men (weighted MD 1.34% [95% CI: 0.13, 2.56]; 718 patients vs. 695 controls; p=0.03).
The analysis showed significant inter-study heterogeneity (I2=88%; χ2 p<0.01). The publication bias disappeared after the exclusion of varicocele repair studies (p=0.6) (Fig. 22, 23).
Fig. 22. Forest plot of the sperm morphology in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
Fig. 23. Funnel plot of the sperm morphology in infertile patients treated with antioxidants compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
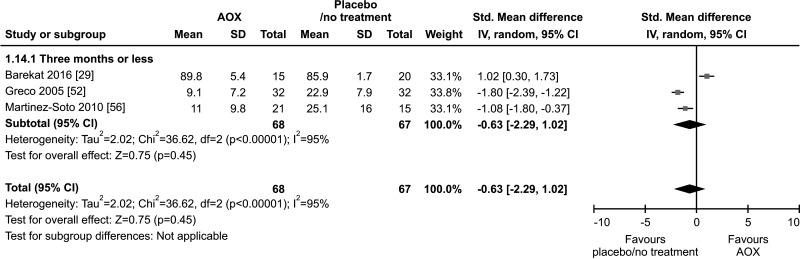
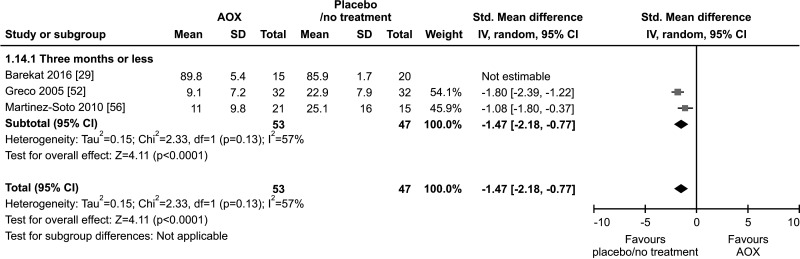
8. SDF
Only three studies, with a total of 68 AOX-treated patients and 67 placebo-treated or untreated controls, were included in the analysis of SDF [29,52,56]. All three studies used the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) test. No effect of AOX on SDF was found compared to placebo or no treatment (SMD -0.63 [95% CI: -2.29, 1.02]; p=0.45) (Fig. 24).
Fig. 24. Forest plot of the sperm DNA fragmentation in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
We found a significant lower SDF in patients on AOX compared to controls when the analysis was repeated after the exclusion of one study carried out in patients treated with varicocele repair and AOX or placebo/no treatment [29] (SMD -1.47 [95% CI: -2.18, -0.77]; 53 patients vs. 47 controls; p<0.01), although this analysis was performed in only two studies.
Furthermore, the analysis showed a significant inter-study heterogeneity both overall (I2=95%; χ2 p<0.01), and in subgroup analysis (I2=95%; χ2 p<0.01) (Fig. 25).
Fig. 25. Forest plot of the sperm DNA fragmentation in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls, after the removal of studies including patients with varicocele undergoing varicocele repair.
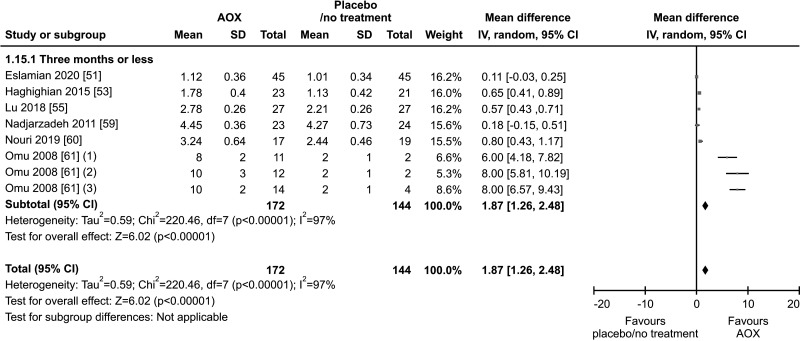
9. Seminal TAC
Six studies, with a total of 172 AOX-treated patients and 144 placebo-treated or untreated controls, were included in the analysis of seminal TAC [51,53,55,59,60,61]. Seminal levels of TAC were significantly higher in patients compared to controls (weighted MD 1.87 mmol Trolox/L [95% CI: 1.26, 2.48; p<0.01]). The analysis demonstrated in a significant inter-study heterogeneity (I2=97%; χ2 p<0.01) (Fig. 26).
Fig. 26. Forest plot of the total antioxidant (AOX) capacity in infertile patients treated with antioxidants compared to placebo or untreated infertile controls.
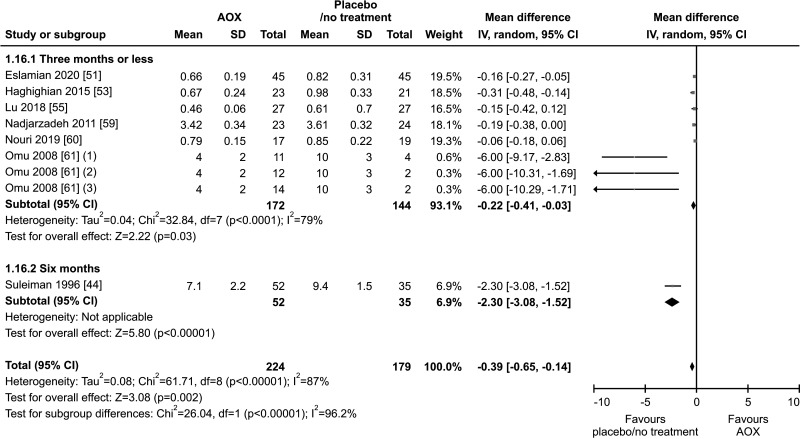
10. Seminal MDA
Seven studies, with a total of 224 patients and 179 controls, analyzed levels of seminal MDA [44,51,53,55,59,60,61]. Seminal levels of MDA were significantly lower in patients treated with AOX compared to placebo-treated or untreated controls (weighted MD -0.39 nmol/mL [95% CI: -0.65, -0.14; p<0.01]). The analysis showed a significant inter-study heterogeneity (I2=96.2%; χ2 p<0.01) (Fig. 27).
Fig. 27. Forest plot of the malondialdehyde in infertile patients treated with antioxidants (AOXs) compared to placebo or untreated infertile controls.
DISCUSSION
1. Impact of AOX therapy on spontaneous pregnancy outcomes
When treating infertile men with AOXs, the main desired outcomes include an improvement in clinical pregnancy and live-birth rates, and a reduction in miscarriage rates. According to the results of the current meta-analysis, the odds for a spontaneous clinical pregnancy are almost double (OR 1.97 [95% CI: 1.28, 3.04]; p<0.01) in infertile men after treatment with AOX compared to controls who have received placebo or no treatment. These results are in line with the latest 2019 Cochrane review and meta-analysis by Smits et al [14], that included 11 studies and reported an increased clinical pregnancy rate with various AOX treatments (OR 2.97, p<0.0001). The latter study only analyzed 105 events from the 11 RCTs as compared to 190 events from 16 RCTs of 1,355 patients in our study.
Another meta-analysis specifically evaluating combined LC/LAC supplementation in infertile men with idiopathic oligo-asthenoteratozoospermia found significantly higher clinical pregnancy rates in the treatment group compared to the control group (OR 3.76, p=0.002) [18]. A beneficial effect of AOX therapy on clinical pregnancy after spontaneous or assisted reproduction has also been concluded by several reviews [72,73,74]. Conversely, the recent MOXI trial did not report a favorable effect of AOX treatment in infertile men in terms of improving clinical pregnancy, showing similar clinical pregnancy rates in both the treatment and placebo groups (9% in both groups, p=0.98) [42]. This is likely due to evaluation of outcome after only 3 months of treatment and a small sample size. Furthermore, a meta-analysis on the effect of CoQ10 in infertile men did not report higher pregnancy rates in the treatment group despite improvement in semen parameters [75]. The recent Cochrane review also included 3 studies that found no difference in miscarriage rates between AOX and placebo or untreated groups [14]. The same meta-analysis reported significantly higher live-birth rates among the treatment group (OR 1.79, p=0.005) [14]. The MOXI trial, however, did not demonstrate any benefit of AOX in improving live-birth rates [42]. In our meta-analysis, no impact on miscarriage and live-birth rates following AOX therapy in infertile men was observed, despite higher pregnancy rates. This is likely due to the small number of studies and events.
In light of these mixed findings, there is a need for further RCTs specifically assessing these outcomes with adequate follow up as these events may not occur soon after the start of therapy. In addition, as suggested by Steiner et al [42], the lack of effect on these parameters could result from the lack of selection of patients who should receive AOXs as well as confounding factors affecting pregnancy rates. In fact, it may be hypothesized that the main beneficiaries of AOX therapy are those with high seminal OS. Future RCTs should be designed to include mainly patients with elevated OS markers.
2. Impact of AOX therapy on basic semen parameters
Semen analysis is the cornerstone of the male infertility work up and often the first laboratory test ordered. The ultimate measure of success for treatment of infertility is a clinical pregnancy or live-birth but these outcomes may need up to 10 months to manifest. In the meantime, semen parameters can be monitored to determine if the prescribed treatment is having a positive effect in improving the couple’s chance to conceive. In our meta-analysis, treatment with AOX (either single or combined) significantly improved sperm concentration, progressive and total motility, and sperm morphology. Similarly, a previously published systematic review reported that vitamin E, vitamin C, NAC, carnitines, CoQ10, lycopene, selenium, and zinc were associated with improved sperm concentration, motility, and morphology [73]. The latest Cochrane database systematic review stated that there was high heterogeneity in published studies and reliable conclusions could not be drawn regarding the effect of AOX on sperm concentration, total motility, and progressive motility [14]. However, the authors suggested that carnitines and combined AOX led to improvement in sperm motility and polyunsaturated fats while zinc improved sperm concentration when compared to placebo or no treatment [14]. When taking the data from previous studies and from our new analysis together, there appears to be benefit of AOX on semen analysis parameters, regardless of the supplement used. In contrast to previous studies, our meta-analysis focused on the use of AOX in general, and not on specific molecules or combinations, for which the studies are very heterogenous.
3. Impact of AOX therapy on SDF
Given the well-established role of OS in the pathogenesis of SDF [76], we aimed to identify articles that have investigated the effect of AOX therapy on SDF. The very limited number of controlled studies on AOXs and SDF, likely precluded our ability to further evaluate the effect of AOX on SDF. However, several prospective studies reported a significant reduction in SDF from baseline after AOX therapy in infertile men, regardless of the assay used or the type of AOX. For example, supplementation of NAC for three months resulted in significant reductions in DNA fragmentation as measured by TUNEL assay (from 19.3% to 15.1%, p=0.01) [77]. Supplying a combination AOX of vitamin C, vitamin E, and CoQ10 resulted in significant improvement in DNA fragmentation index (DFI) as measured by Sperm Chromatin Structure Assay (SCSA) [78]. Significant reductions in SDF percentage as measured by Sperm Chromatin Dispersion (SCD) were also reported after three months of CoQ10 treatment [79]. Two trials investigated AOX therapy after varicocelectomy in men with clinical varicocele; both reported no additional benefit of AOX in reduction of SDF after varicocelectomy in these men compared to varicocelectomy alone, signifying the importance of addressing the underlying condition when possible, rather than empiric AOX supplementation [29,80]. One trial on astaxanthin supplementation reported no reduction in SDF compared to placebo [81], while another on folic acid reported SDF improvement only in carriers of MTHFR gene 677 thymidine/thymidine polymorphism [82]. Conversely, a recent multicenter RCT administering folic acid and zinc or placebo to 2,370 infertile men for six months reported a significantly lower SDF in patients compared to controls [83]. Therefore, it is difficult to reach a firm conclusion as to the impact of AOX on levels of SDF due to many factors including the small number of available studies, the variable AOXs regimens, the different assays used for SDF, and the different conditions associated with SDF. Additional well-designed studies are warranted.
4. Impact of AOX therapy on seminal OS indices
The most pragmatic use of AOX in male infertility is in cases of elevated seminal OS for men classified as having MOSI. Normally there is a homeostasis between ROS and AOX. If the scale tips towards ROS, then dietary supplementation with AOX may help restore this balance and improve seminal quality. TAC is a measure of total AOX present in the seminal plasma and provides a measure of reductive potential [84]. Studies have demonstrated that infertile men have lower TAC when compared to fertile men, and semen parameters such as concentration, motility, and morphology have been positively correlated with TAC [85]. Our analysis identified that seminal TAC improved after treatment with AOX. There was also a significant decrease in seminal MDA, an indicator of lipid peroxidation, after treatment with AOX. However, significant inter-study heterogeneity was found. Several earlier studies have demonstrated improvement in OS and a decrease in MDA after treatment with vitamin C, vitamin E, beta-carotene, zinc, selenium, and NAC used either alone or in combination [44,47,61,86,87]. Our meta-analysis is consistent with the results of these previous studies and, as far as we know, this is the first meta-analysis assessing the impact of AOX on TAC and MDA. However, while AOXs seem to improve seminal OS indices, clinicians need also to be aware that over treatment with AOXs can lead to toxicity and reductive stress [88]. Thus, identification and selection of patients with high risk for MOSI could be useful to maximize the benefits of AOXs on sperm quality and prevent reductive stress toxicity. When the assessment of seminal OS becomes more standardized, this test could be important to identify those who could benefit from AOX.
5. What could this study change?
Currently, AOX therapy is being widely used in male infertility management, even though there is only limited evidence and no practical guidelines on the duration or even the type of AOX to be used [73]. Our meta-analysis has provided encouraging evidence, demonstrating the positive impact of AOX therapy on clinical pregnancy rate, seminal parameters, and OS levels in men for whom a careful diagnostic evaluation has excluded major comorbidities, genetic, anatomical, inflammatory, traumatic or testicular cause of male infertility, or associated female infertility (Supplement Table 3). Some of the included studies were performed in a population of men with varicocele who underwent varicocele repair and were subsequently given AOX or placebo or untreated. The exclusion of these varicocele studies did not change the results, suggesting that the effect of AOX on the analyzed outcomes is independent of varicocele repair.
Compared to the last Cochrane review [14], the present study increased the number of RCTs, allowing the study of the largest population analyzed so far (Table 3). This allowed us to confirm the Cochrane’s study findings and upgrade the level of evidence of many of the investigated outcomes [14,75,89,90,91] (Table 3). Moreover, to the best of our knowledge, this is the first study to offer a meta-analytic investigation of seminal levels of TAC and MDA in patients with male infertility after AOX administration and to confirm a positive effect on both of these outcomes.
Table 3. Comparison of the findings of the present study with the last Cochrane meta-analysis [14].
| Outcome | Present meta-analysis | Cochrane meta-analysis [14] | ||
|---|---|---|---|---|
| Summary of the results | Number of participants (n studies) | Summary of the results | Number of participants (n studies) | |
| Spontaneous pregnancy ratea | OR 1.97 (95% CI: 1.28, 3.04); n=190 events; p<0.01; I2=20%; χ2 p=0.20 (Fig. 2) | 1,355 (16 RCTs) | OR 2.97 (95% CI: 1.20, 2.67); n=105 events; p<0.05; I2=0% | 786 (11 RCTs) |
| Live-birth ratea | OR 1.21 (95% CI: 0.53, 2.76); n=65 events; p=0.64 (Fig. 6) | 388 (4 RCTs) | OR 1.79 (95% CI: 1.20, 2.67); n=124 events; p<0.05; I2=40% | 750 (7 RCTs) |
| Miscarriage ratea | OR 1.01 (95% CI: 0.34, 3.00); n=13 events; p=0.98 (Fig. 7) | 459 (4 RCTs) | OR 1.74 (95% 0.40, 7.60); n=8 events; p=0.46 | 247 (3 RCTs) |
| Sperm concentration | MD 5.93 mil/mL (95% CI: 4.43, 7.43); p<0.01; I2=94%; χ2 p<0.01 (Fig. 8) | 4,310 (36 RCTs) | Overall effect not assessedb | 3,456 (26 RCTs) |
| Progressive sperm motility | MD 7.21% (95% CI: 3.66, 10.76); p<0.01; I2=99%; χ2 p<0.01 (Fig. 12) | 2,345 (20 RCTs) | Overall effect not assessedb | 1,523 (15 RCTs) |
| Total sperm motility | MD 7.52% (95% CI: 3.11, 11.94); p<0.01; I2=100%; χ2 p<0.01 (Fig. 16) | 4,452 (36 RCTs) | Overall effect not assessedb | 3,456 (25 RCTs) |
| Sperm morphology | MD 3.28% (95% CI: 2.40, 4.17); p<0.01; I2=96%; χ2 p<0.01 (Fig. 20) | 1,828 (18 RCTs) | Not assessed | Not assessed |
| Sperm DNA fragmentation | SMD -0.63 (95% CI: -2.29, 1.02); p=0.45 (Fig. 24) | 135 (3 RCTs) | MD -5.0 (95% CI: -12.61, 2.61); p=0.20 | 254 (6 RCTs) |
| Seminal TAC levels | MD 1.87 (95% CI: 1.26, 2.48; p<0.01; I2=97%; χ2 p<0.01 (Fig. 26) | 316 (6 RCTs) | Not assessed | Not assessed |
| Seminal MDA levels | MD -0.39 (95% CI: -0.65, -0.14; p<0.01; I2=87%; χ2 p<0.01 (Fig. 27) | 403 (7 RCTs) | Not assessed | Not assessed |
CI: confidence interval, MD: mean difference, MDA: malondialdehyde acid, OR: odds ratio, RCT: randomized controlled trial, SMD: standardized mean difference, TAC: total antioxidant capacity.
aOnly spontaneous events were considered in the present meta-analysis.
bSubgroup analysis based on treatment duration was performed.
6. Comparison with other studies
The MOXI trial has investigated the impact of AOX therapy on male infertility [42]. The authors conclude that AOX therapy neither improves semen parameters and DNA integrity, nor improves in vivo pregnancy or live-birth rates among men with male factor infertility. However, its study design had some limitations.
First, only 144 of the required 790 couples were recruited to achieve 80% power for the primary outcome (live-birth rates). Secondly, at baseline, the placebo group had a higher proportion of men with secondary infertility and the AOX group had a lower percentage of morphologically normal sperm. Third, the participants were assigned to either AOX or placebo based on semen parameters and female partner’s age, while other important issues related to the underlying etiological factors for diagnoses of male infertility were ignored. This may be an additional source of selection bias since some genital abnormalities such as varicocele may impact the outcome of AOX therapy of infertile men [36,92].
Fourth, the MOXI trial used a combined AOX formula containing vitamin C (500 mg), vitamin E (400 mg), selenium (0.20 mg), LC (1,000 mg), zinc (20 mg), folic acid (1,000 mg), lycopene (10 mg), and vitamin D (2,000 IU). The authors state that they selected this formulation based on the finding of a previous Cochrane systematic review reporting that each individual component has a positive impact on sperm structure or function and/or pregnancy rates after assisted reproductive technique (ART). However, it remains unclear whether the formulation chosen in the MOXI trial is appropriate or not.
Fifth, the authors of the MOXI trial state that AOX therapy was given for at least 3 months and up to 6 months. The US Food and Drug Administration (FDA) recommends a minimum duration of 26 weeks for assessing the impact of a therapeutic agent used for the treatment of male factor infertility. Using different durations of AOX therapy may add an important confounder that may impact the interpretation of the results. Lastly, ovarian stimulation with clomiphene citrate followed by intrauterine insemination (IUI) was used for couples who had not conceived after a trial with AOX vs. placebo. Thus, there is a concern of having heterogeneous responses due to combining AOX supplementation with IUI.
Several meta-analyses have evaluated the potential benefit of AOX for treatment of male infertility. Despite being considered the gold standard, the last Cochrane meta-analysis [14] has some objective limitations, that the present study has overcome (Table 3). Compared to the last Cochrane review, our study increased the number of RCTs, and investigated the largest population analyzed so far on this topic. This allowed us to score the evidence on clinical pregnancy as moderate-quality and, accordingly, the analysis of this outcome resulted in minimal inter-study heterogeneity (Table 3). Regarding live-birth rate, the population analyzed in the present study is smaller than that assessed in the Cochrane meta-analysis, since we focused only on spontaneous pregnancies. Also, some of the outcomes of the present study, such as sperm morphology, seminal levels of TAC and of MDA, were not analyzed in the study by Smits et al [14]. In addition, concerning the conventional sperm parameters (sperm concentration, progressive and total motility), the Cochrane review provided the results of the sub-group analysis only (e.g., effect of a single AOX, or of a specific time of assessment), without analyzing the overall effect (hence, independently from the time and the specific AOX used). The detailed analysis of the differences in the sample size, in the number of RCTs included for each outcome, and in the results between the present study and the Cochrane meta-analysis is detailed in Table 3.
Pitfalls of the Cochrane and several other meta-analyses are highlighted in the strengths and weakness analysis provided in Table 4. If we consider the previous meta-analyses, the number of analyzed studies has generally been limited most of the time [75,90], thus providing very-low or low-quality evidence. Furthermore, some meta-analyses evaluated the impact of only one or two AOX, failing to provide a comprehensive picture on their use in male infertility [75,90] (Table 4). Comprehensively, the present study analyzed the largest cohort so far, which allowed to upgrade the level of the evidence.
Table 4. Summary of the strengths and weakness of the meta-analyses on AOX published in the last ten years.
| Society | Year | Strengths | Weakness |
|---|---|---|---|
| Present study | 2022 | Evidence based on the highest number of RCTs and on the largest population analyzed so far Highest level of evidence provided so far Absence of inter-study heterogeneity for clinical pregnancy Evidence on seminal indices of OS (TAC and MDA) Sub-analysis performed after the exclusion of patients with varicocele Evidence on RCTs only Score of the QoE using the GRADE system |
Limited number of controlled trials on live-birth rate, miscarriage and SDF Inter-study heterogeneity for secondary outcomes No subgroup analysis according to the type of AOX, and heterogeneity in the formulation of AOX analyzed |
| [14] | 2019 | Subgroup analysis according to the type of AOX Evidence on RCTs only Score of the QoE using the GRADE system High number of studies Absence of inter-study heterogeneity for clinical pregnancy |
Limited number of controlled trials on live-birth rate, miscarriage, and SDF Inter-study heterogeneity for secondary outcomes Only the Cochrane Risk of Bias of RCTs is used for QoE No details on the training of researchers are provided Patients with varicocele are included in the analysis Evidence on seminal indices of OS (e.g., TAC and MDA) is not provided Low, very-low QoE |
| [89] | 2021 | Evidence on RCTs only High selected cohort (idiopathic asthenozoospermia) Absence of heterogeneity in the formulation of AOX analysed |
The idiopathic etiology is not clearly mentioned in the inclusion criteria of the included studies Only two AOXs are analyzed (N-acetyl-cysteine, L-carnitine/L-acetyl-carnitine) Limited number of studies (n=7) Only the Cochrane Risk of Bias of RCTs is used for QoE No details on the training of researchers are provided |
| [90] | 2021 | Evidence on RCTs only High selected cohort (idiopathic infertility) Absence of heterogeneity in the formulation of AOX analyzed |
Limited number of studies (n=3) Only the Cochrane Risk of Bias of RCTs is used for QoE No details on the training of researchers are provided Inter-study heterogeneity Only one antioxidant is analyzed (N-Acetyl-cysteine) |
| [91] | 2014 | Evidence on RCTs only Score of the QoE using the GRADE system |
Low sample size for each assessed outcome Only the Cochrane Risk of Bias of RCTs is used for QoE No details on the training of researchers are provided Inter-study heterogeneity Low, very-low QoE |
| [75] | 2013 | Evidence on RCTs only Absence of heterogeneity in the formulation of AOX analysed |
Limited number of studies (n=3) Only the Cochrane Risk of Bias of RCTs is used for QoE No details on the training of researchers are provided Only one AOX is analyzed (Co-enzyme Q10) Inter-study heterogeneity |
AOX: antioxidant, GRADE: Grading of Recommendations Assessment, Development and Evaluation, MDA: malondialdehyde acid, OS: oxidative stress, RCT: randomized controlled trial, QoE: quality of evidence, TAC: total antioxidant capacity.
7. Limitations of the study
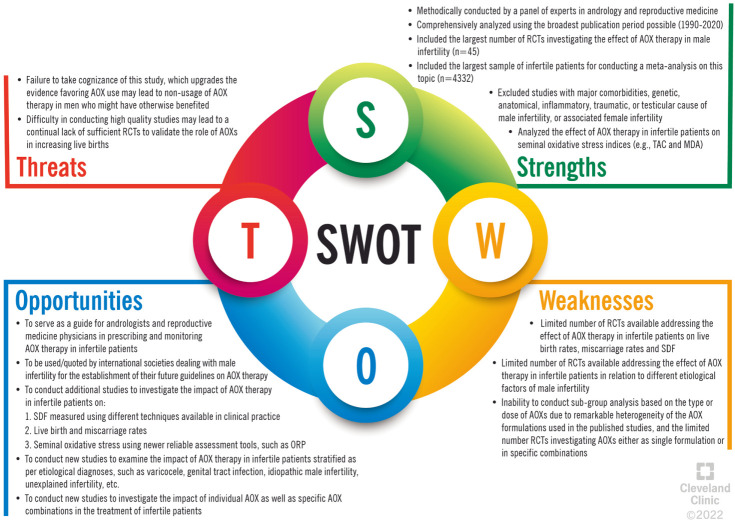
The present meta-analysis demonstrates the positive impact of AOX on spontaneous pregnancy, seminal OS indices and basic sperm parameters. However, the limited number of controlled studies investigating the effect of AOXs on live-birth rate and SDF prevented us from reaching a firm conclusion about these outcomes. This study is also not evaluating the effects of AOXs based on subgroup analysis. The heterogeneity in the formulations studied in the literature also resulted in the presence of outliers encountered during our analysis, but we were able to adjust for this in our statistical approach. This foreseeable and expected outcome represents the limitation of the present study, based on the scarcity of well-designed studies to be included in such a meta-analysis. Beside these limitations, it is important to underline that until now there are no robust studies that have investigated the relationship between micronutrient patterns and infertility and have highlighted the role of AOXs in those patients with specific nutrient deficiency.
Despite this, our study was still capable of generating statistical results that are consistent with the scientific narrative. However, we need more well-designed large RCTs to reach more definitive conclusions. These points are highlighted in the Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis, which is shown in Fig. 28.
Fig. 28. Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis of the present study. AOX: antioxidant, MDA: malondialdehyde acid, ORP: oxidation-reduction potential, RCT: randomized controlled trial, SDF: sperm DNA fragmentation, TAC: total antioxidant capacity.
CONCLUSIONS
Our study included the largest population analyzed for the impact of AOX therapy on male infertility. The current meta-analysis demonstrates low to moderate evidence on the positive impact of AOX therapy on spontaneous pregnancy rate, and conventional sperm parameters in infertile men. Additionally, our results provide a very low to low evidence on the potential positive effect of AOX therapy on levels of seminal TAC and MDA in infertile men. The exclusion of studies that examined patients treated with AOX or placebo or untreated after varicocele repair did not change the results. This suggests that the effect of AOX on the analyzed outcomes is independent of varicocele repair. Compared to the last Cochrane review in 2019 [14], our study increased the number of RCTs included in the meta-analysis, creating the largest population analyzed with regards to the benefits of AOXs on male fertility. Also, some of the outcomes of the present study, such as sperm morphology, seminal levels of TAC and of MDA, had not been analyzed in the 2019 Cochrane review [14].
Finally, the present analysis provides additional evidence in favor of recommending the use of AOX therapy in male infertility (Table 5). The results of the current meta-analysis may help update those guidelines of the scientific societies that do not express a clear position on the use of AOX for the treatment of the infertile male due to the lack of evidence [22,93,94,95] (Table 6). For the clinical use of AOX to be standardized, further studies are needed, as at present there is not a specific AOX or AOX combination with a particular dosing that can be recommended for infertile men due to the heterogeneity of the available data.
Table 5. Summary of the findings of the present study.
| Outcome | Results | Number of participants (n studies) | Level of the evidence (scored using the GRADEa system) |
|---|---|---|---|
| Spontaneous pregnancy rate | OR 1.97 (95% CI: 1.28, 3.04); n=190 events; p<0.01; I2=20%; χ2 p=0.20 (Fig. 2) | 1,355 (16 RCTs) | ⊕⊕⊕⊝ (Moderate)b |
| Live-birth rate | OR 1.21 (95% CI: 0.53, 2.76); n=65 events; p=0.64 (Fig. 6) | 388 (4 RCTs) | ⊕⊝⊝⊝ (Very low)b,c,d |
| Miscarriage rate | OR 1.01 (95% CI: 0.34, 3.00); n=13 events; p=0.98 (Fig. 7) | 459 (4 RCTs) | ⊕⊝⊝⊝ (Very low)b,c,d |
| Sperm concentration | MD 5.93 mil/mL (95% CI: 4.43, 7.43); p<0.01; I2=94%; χ2 p<0.01 (Fig. 8) | 4,310 (35 RCTs) | ⊕⊕⊕⊝ (Moderate)b |
| Progressive sperm motility | MD 7.21% (95% CI: 3.66, 10.76); p<0.01; I2=99%; χ2 p<0.01 (Fig. 12) | 2,345 (20 RCTs) | ⊕⊕⊕⊝ (Moderate)b |
| Total sperm motility | MD 7.52% (95% CI: 3.11, 11.94); p<0.01; I2=100%; χ2 p<0.01 (Fig. 16) | 4,452 (36 RCTs) | ⊕⊕⊕⊝ (Moderate)b |
| Sperm morphology | MD 3.28% (95% CI: 2.40, 4.17); p<0.01; I2=96%; χ2 p<0.01 (Fig. 20) | 1,828 (18 RCTs) | ⊕⊕⊝⊝ (Low)b,c |
| Sperm DNA fragmentation | SMD -0.63 (95% CI: -2.29, 1.02); p=0.45 (Fig. 24) | 135 (3 RCTs) | ⊕⊝⊝⊝ (Very low)b,c,d |
| Seminal TAC levels | MD 1.87 (95% CI: 1.26, 2.48; p<0.01; I2=97%; χ2 p<0.01 (Fig. 26) | 316 (6 RCTs) | ⊕⊕⊝⊝ (Low)b,c |
| Seminal MDA levels | MD -0.39 (95% CI: -0.65, -0.14; p<0.01; I2=87%; χ2 p<0.01 (Fig. 27) | 403 (7 RCTs) | ⊕⊕⊝⊝ (Low)b,c |
CI: confidence interval, GRADE: Grading of Recommendations Assessment, Development and Evaluation, MD: mean difference, MDA: malondialdehyde acid, OR: odds ratio, RCT: randomized controlled trial, SMD: standardized mean difference, TAC: total antioxidant capacity.
aHigh level: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate level: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low level: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low level: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
bDowngraded one level for risk of bias: lack of allocation concealment, lack of blinding and incomplete accounting of patients and outcome events.
cDowngraded one level for serious imprecision: small sample size.
dDowngraded one level for serious imprecision: crossing the line of no effect.
Table 6. Summary of the Societies’ recommendations on the use of AOX for the treatment of male infertility.
| Society | Year | Recommendation |
|---|---|---|
| European Academy of Andrology (EAA) [92] | 2018 | Recommendation 9. According to the current evidence, we cannot recommend either for or against antioxidants, and for antiestrogens (tamoxifen or clomiphene) or aromatase inhibitors (2ØOOO) |
| American Urological Association (AUA)/American Society for Reproductive Medicine (ASRM) [22] | 2021 | Recommendation 43. Clinicians should counsel patients that the benefits of supplements (e.g., antioxidants, vitamins) are of questionable clinical utility in treating male infertility. Existing data are inadequate to provide recommendation for specific agents to use for this purpose (Conditional Recommendation; Evidence Level: Grade B) Current data suggest that they are likely not harmful, but it is questionable whether they will provide tangible improvements in fertility outcomes |
| Italian Society of Andrology and Sexual Medicine (SIAMS) [93] | 2021 | We recommend against treatment with nutraceuticals/antioxidants in unselected infertile men to increase sperm parameters (Expert Opinion) We suggest considering the use of nutraceuticals/antioxidants in selected patients with idiopathic oligozoospermia and/or asthenozoospermia and/or clear signs of high OS, since in some cases they might improve sperm parameters (2, ØOOO) We cannot recommend either for or against the use of nutraceuticals/antioxidants to increase pregnancy rate (Expert Opinion) |
| European Association of Urology – Sexual and Reproductive Health Guidelines [94] | 2022 | No clear recommendation can be made for treatment of patients with idiopathic infertility using antioxidants, although antioxidant use may improve semen parameters (weak) |
AOX: antioxidant, OS: oxidative stress.
Accordingly, based on our results, the following suggestions can be made on the use of AOX for the treatment of patients with male infertility:
1. We suggest the use of AOX to improve spontaneous pregnancy rates in patients diagnosed with IMI after a careful diagnostic work-up and the exclusion of major causes of infertility, and in those with varicocele following varicocele repair (2 ØØØO).
2. We suggest the use of AOX to improve conventional sperm parameters in couples diagnosed with IMI after a careful diagnostic work-up and the exclusion of other causes of infertility, and in those with varicocele following varicocele repair (2 ØØØO).
3. We suggest the use of AOX to improve the seminal indices of OS (TAC and MDA) in patients diagnosed with IMI and OS (2 ØØOO).
The present study identifies the need for further RCTs assessing the impact of AOX on live-birth rate, miscarriage rate, and SDF, as only few studies have analyzed these outcomes in infertile patients treated with AOX compared to placebo-treated or untreated controls. Finally, the ideal treatment duration, as well as best therapeutic regimen (single vs. combined AOX) remains unknown and further studies will be needed to address these issues.
Acknowledgements
Authors are thankful to the Global Andrology Forum for its support of this study.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: AA, RS.
- Data curation: R.Sa, RC, AH.
- Formal analysis: RC, AH.
- Funding acquisition: None.
- Investigation: GS, SK, AF.
- Methodology: R.Sa, RC, AH.
- Project administration: AA, RS.
- Supervision: FB, TAAMH.
- Validation: AR, AZ, GC, MG, PK, EK, GC, TT, GP, HJP, RAG, SM, GMB, MEB, AK, EC, GIR, AEC, RFA, CNJ.
- Writing – original draft: RC, GS, R.Sa, FB, AF.
- Writing – review & editing & final approval: AA, RS.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220067.
PRISMA checklist.
Eligibility criteria based on the PICOS (Population, Intervention, Comparison/Comparator, Outcomes, Study design) model
Inclusion and exclusion criteria of the patients enrolled in the studies included in this meta-analysis
References
- 1.Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, et al. Male infertility. Lancet. 2021;397:319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 2.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13 Suppl 1:33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 3.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and reproductive health. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80:333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 5.Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, et al. Oxidative stress and male infertility. Reprod Med Biol. 2020;20:41–52. doi: 10.1002/rmb2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Mulgund A, Alshahrani S, Assidi M, Abuzenadah AM, Sharma R, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol. 2014;12:126. doi: 10.1186/1477-7827-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- 8.Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. CEJU. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A, Cho CL, Esteves SC, Majzoub A. Reactive oxygen species and sperm DNA fragmentation. Transl Androl Urol. 2017;6(Suppl 4):S695–S696. doi: 10.21037/tau.2017.05.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84:1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barati E, Nikzad H, Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol Life Sci. 2020;77:93–113. doi: 10.1007/s00018-019-03253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali M, Martinez M, Parekh N. Are antioxidants a viable treatment option for male infertility? Andrologia. 2021;53:e13644. doi: 10.1111/and.13644. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, et al. Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence. World J Mens Health. 2021;39:233–290. doi: 10.5534/wjmh.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei G, Zhou Z, Cui Y, Huang Y, Wan Z, Che X, et al. A Meta-Analysis of the Efficacy of L-Carnitine/L-Acetyl-Carnitine or N-Acetyl-Cysteine in Men With Idiopathic Asthenozoospermia. Am J Mens Health. 2021;15:15579883211011371. doi: 10.1177/15579883211011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia. 2020;52:e13470. doi: 10.1111/and.13470. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang T, Ding W, Wu J, Wu G, Wang Y, et al. Efficacy of antioxidant therapy on sperm quality measurements after varicocelectomy: a systematic review and meta-analysis. Andrologia. 2019;51:e13396. doi: 10.1111/and.13396. [DOI] [PubMed] [Google Scholar]
- 20.Bahmyari R, Zare M, Sharma R, Agarwal A, Halvaei I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: a systematic review and meta-analysis. Andrologia. 2020;52:e13514. doi: 10.1111/and.13514. [DOI] [PubMed] [Google Scholar]
- 21.Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioğlu A, et al. Sexual and reproductive health. Vol. 282 Arnhem: European Association of Urology; 2022. [Google Scholar]
- 22.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. J Urol. 2021;205:36–43. doi: 10.1097/JU.0000000000001521. [DOI] [PubMed] [Google Scholar]
- 23.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardestani Zadeh A, Arab D, Kia NS, Heshmati S, Amirkhalili SN. The role of vitamin E - selenium - folic acid supplementation in improving sperm parameters after varicocelectomy: a randomized clinical trial. Urol J. 2019;16:495–500. doi: 10.22037/uj.v0i0.4653. [DOI] [PubMed] [Google Scholar]
- 28.Kızılay F, Altay B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: a randomized controlled trial. Int J Impot Res. 2019;31:424–431. doi: 10.1038/s41443-018-0109-4. [DOI] [PubMed] [Google Scholar]
- 29.Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10:120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ener K, Aldemir M, Işık E, Okulu E, Özcan MF, Uğurlu M, et al. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: a randomised controlled study. Andrologia. 2016;48:829–834. doi: 10.1111/and.12521. [DOI] [PubMed] [Google Scholar]
- 31.Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–238. doi: 10.1590/S1677-5538.IBJU.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azizollahi G, Azizollahi S, Babaei H, Kianinejad M, Baneshi MR, Nematollahi-mahani SN. Effects of supplement therapy on sperm parameters, protamine content and acrosomal integrity of varicocelectomized subjects. J Assist Reprod Genet. 2013;30:593–599. doi: 10.1007/s10815-013-9961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005;84:662–671. doi: 10.1016/j.fertnstert.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 34.Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009;91:1785–1792. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 35.Busetto GM, Virmani A, Antonini G, Ragonesi G, Del Giudice F, Agarwal A, et al. Effect of antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double blind place controlled (DBPC) study. Eur Urol Suppl. 2017;16:e782. doi: 10.1111/and.12927. [DOI] [PubMed] [Google Scholar]
- 36.Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia. 2018;50:e12927. doi: 10.1111/and.12927. [DOI] [PubMed] [Google Scholar]
- 37.Gopinath PM, Kalra B, Saxena A, Malik S, Kochhar K, Kalra S, et al. Fixed dose combination therapy of antioxidants in treatment of idiopathic oligoasthenozoospermia: results of a randomized, double-blind, placebo-controlled clinical trial. IJIFM. 2013;4:6–13. [Google Scholar]
- 38.Kopets R, Kuibida I, Chernyavska I, Cherepanyn V, Mazo R, Fedevych V, et al. Dietary supplementation with a novel l-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: a randomized clinical study. Andrology. 2020;8:1184–1193. doi: 10.1111/andr.12805. [DOI] [PubMed] [Google Scholar]
- 39.Omu AE, Dashti H, Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod Biol. 1998;79:179–184. doi: 10.1016/s0301-2115(97)00262-5. [DOI] [PubMed] [Google Scholar]
- 40.Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 41.Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998;82:76–80. doi: 10.1046/j.1464-410x.1998.00683.x. [DOI] [PubMed] [Google Scholar]
- 42.Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, et al. Reproductive Medicine Network. The effect of antioxidants on male factor infertility: the males, antioxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113:552–560.e3. doi: 10.1016/j.fertnstert.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenqvist A, Oleszczuk K, Leijonhufvud I, Giwercman A. Impact of antioxidant treatment on DNA fragmentation index: a double-blind placebo-controlled randomized trial. Andrology. 2018;6:811–816. doi: 10.1111/andr.12547. [DOI] [PubMed] [Google Scholar]
- 44.Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- 45.Závaczki Z, Szöllõsi J, Kiss SA, Koloszár S, Fejes I, Kovács L, et al. Magnesium-orotate supplementation for idiopathic infertile male patients: a randomized, placebo-controlled clinical pilot study. Magnes Res. 2003;16:131–136. [PubMed] [Google Scholar]
- 46.Boonyarangkul A, Vinayanuvattikhun N, Chiamchanya C, Visutakul P. Comparative study of the effects of tamoxifen citrate and folate on semen quality of the infertile male with semen abnormality. J Med Assoc Thai. 2015;98:1057–1063. [PubMed] [Google Scholar]
- 47.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74:73–76. doi: 10.1016/j.urology.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 48.Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids. 2000;35:149–154. doi: 10.1007/BF02664764. [DOI] [PubMed] [Google Scholar]
- 49.da Silva TM, Maia MCS, Arruda JT, Approbato FC, Mendonça CR, Approbato MS. Folic acid does not improve semen parametrs in subfertile men: a double-blin, randomized, placebo-controlled study. JBRA Assist Reprod. 2013;17:152–157. [Google Scholar]
- 50.Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: a randomized trial. BJU Int. 2010;106:1181–1185. doi: 10.1111/j.1464-410X.2010.09243.x. [DOI] [PubMed] [Google Scholar]
- 51.Eslamian G, Amirjannati N, Noori N, Sadeghi MR, Hekmatdoost A. Effects of coadministration of DHA and vitamin E on spermatogram, seminal oxidative stress, and sperm phospholipids in asthenozoospermic men: a randomized controlled trial. Am J Clin Nutr. 2020;112:707–719. doi: 10.1093/ajcn/nqaa124. [DOI] [PubMed] [Google Scholar]
- 52.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 53.Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104:318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L, Dondero F, et al. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril. 2003;79:292–300. doi: 10.1016/s0015-0282(02)04679-4. [DOI] [PubMed] [Google Scholar]
- 55.Lu XL, Liu JJ, Li JT, Yang QA, Zhang JM. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: a placebo-controlled, double-blind trial. Andrologia. 2018;50:e13033. doi: 10.1111/and.13033. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Soto JC, Domingo JC, Cordobilla B, Palbero L, Pellicer A, Landeras J. Effect of dietary DHA supplementation on sperm DNA integrity. Fertil Steril. 2010;94:S235–S236. [Google Scholar]
- 57.Moslemi Mehni N, Ketabchi AA, Hosseini E. Combination effect of Pentoxifylline and L-carnitine on idiopathic oligoasthenoteratozoospermia. Iran J Reprod Med. 2014;12:817–824. [PMC free article] [PubMed] [Google Scholar]
- 58.Morgante G, Scolaro V, Tosti C, Di Sabatino A, Piomboni P, De Leo V. [Treatment with carnitine, acetyl carnitine, L-arginine and ginseng improves sperm motility and sexual health in men with asthenopermia] Minerva Urol Nefrol. 2010;62:213–218. [PubMed] [Google Scholar]
- 59.Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest. 2011;34:e224–e228. doi: 10.3275/7572. [DOI] [PubMed] [Google Scholar]
- 60.Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33:3203–3211. doi: 10.1002/ptr.6493. [DOI] [PubMed] [Google Scholar]
- 61.Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract. 2008;17:108–116. doi: 10.1159/000112963. [DOI] [PubMed] [Google Scholar]
- 62.Peivandi S, Karimpour A, Moslemizadeh N. Effects of L-carnitine on infertile men’s spermogram; a randomized clinical trial. J Reprod Infertil. 2010;10:245–251. [Google Scholar]
- 63.Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–1033. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- 64.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 65.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 66.Safarinejad MR, Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009;181:741–751. doi: 10.1016/j.juro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–248. doi: 10.1016/j.juro.2009.02.121. [DOI] [PubMed] [Google Scholar]
- 68.Stanislavov R, Nikolova V, Rohdewald P. Improvement of seminal parameters with Prelox: a randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res. 2009;23:297–302. doi: 10.1002/ptr.2592. [DOI] [PubMed] [Google Scholar]
- 69.Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jørgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103:870–881. doi: 10.1210/jc.2017-01656. [DOI] [PubMed] [Google Scholar]
- 70.Dawson EB, Harris WA, Powell LC. Relationship between ascorbic acid and male fertility. World Rev Nutr Diet. 1990;62:1–26. doi: 10.1159/000417532. [DOI] [PubMed] [Google Scholar]
- 71.Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2006;85:1409–1414. doi: 10.1016/j.fertnstert.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 72.Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. 2014;2014:426951. doi: 10.1155/2014/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–124. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–723. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. 2013;30:1147–1156. doi: 10.1007/s10815-013-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muratori M, Marchiani S, Tamburrino L, Baldi E. Sperm DNA fragmentation: mechanisms of origin. Adv Exp Med Biol. 2019;1166:75–85. doi: 10.1007/978-3-030-21664-1_5. [DOI] [PubMed] [Google Scholar]
- 77.Jannatifar R, Cheraghi E, Nasr-Esfahani MH, Piroozmanesh H. Association of heat shock protein A2 expression and sperm quality after N-acetyl-cysteine supplementation in asthenoterato-zoospermic infertile men. Andrologia. 2021;53:e14024. doi: 10.1111/and.14024. [DOI] [PubMed] [Google Scholar]
- 78.Salehi P, Zahra Shahrokhi S, Kamran T, Ajami A, Taghiyar S, Reza Deemeh M. Effect of antioxidant therapy on the sperm DNA integrity improvement; a longitudinal cohort study. Int J Reprod Biomed. 2019;17:99–106. doi: 10.18502/ijrm.v17i2.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alahmar AT, Sengupta P, Dutta S, Calogero AE. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med. 2021;48:150–155. doi: 10.5653/cerm.2020.04084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pourmand G, Movahedin M, Dehghani S, Mehrsai A, Ahmadi A, Pourhosein M, et al. Does L-carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Urology. 2014;84:821–825. doi: 10.1016/j.urology.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Kumalic SI, Klun IV, Bokal EV, Pinter B. Effect of the oral intake of astaxanthin on semen parameters in patients with oligo-astheno-teratozoospermia: a randomized double-blind placebo-controlled trial. Radiol Oncol. 2020;55:97–105. doi: 10.2478/raon-2020-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang WJ, Lu XL, Li JT, Zhang JM. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: a double-blind, randomized, placebo-controlled trial. Andrology. 2020;8:110–116. doi: 10.1111/andr.12652. [DOI] [PubMed] [Google Scholar]
- 83.Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA. 2020;323:35–48. doi: 10.1001/jama.2019.18714. Erratum in: JAMA 2020;323:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta S, Finelli R, Agarwal A, Henkel R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia. 2021;53:e13624. doi: 10.1111/and.13624. [DOI] [PubMed] [Google Scholar]
- 85.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17:87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–165. doi: 10.1054/plef.2000.0174. [DOI] [PubMed] [Google Scholar]
- 87.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 88.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 89.Wei G, Zhou Z, Cui Y, Huang Y, Wan Z, Che X, et al. A meta-analysis of the efficacy of L-carnitine/L-acetyl-carnitine or N-acetyl-cysteine in men with idiopathic asthenozoospermia. Am J Mens Health. 2021;15:15579883211011371. doi: 10.1177/15579883211011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Z, Cui Y, Zhang X, Zhang Y. The role of N-acetyl-cysteine (NAC) orally daily on the sperm parameters and serum hormones in idiopathic infertile men: a systematic review and meta-analysis of randomised controlled trials. Andrologia. 2021;53:e13953. doi: 10.1111/and.13953. [DOI] [PubMed] [Google Scholar]
- 91.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;12:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 92.Su JS, Farber NJ, Vij SC. Pathophysiology and treatment options of varicocele: an overview. Andrologia. 2021;53:e13576. doi: 10.1111/and.13576. [DOI] [PubMed] [Google Scholar]
- 93.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of Andrology guideline management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–524. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 94.Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, et al. Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): endorsing organization: Italian Society of Embryology, Reproduction, and Research (SIERR) J Endocrinol Invest. 2022;45:1085–1113. doi: 10.1007/s40618-022-01741-6. [DOI] [PubMed] [Google Scholar]
- 95.European Association of Urology. Sexual and reproductive health [Internet] Arnhem: European Association of Urology; c2022. [cited 2022 Apr 12]. Available from: https://uroweb.org/guidelines/sexual-and-reproductive-health. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
Eligibility criteria based on the PICOS (Population, Intervention, Comparison/Comparator, Outcomes, Study design) model
Inclusion and exclusion criteria of the patients enrolled in the studies included in this meta-analysis