Abstract
Purpose
Varicocele is a common problem among infertile men. Varicocele repair (VR) is frequently performed to improve semen parameters and the chances of pregnancy. However, there is a lack of consensus about the diagnosis, indications for VR and its outcomes. The aim of this study was to explore global practice patterns on the management of varicocele in the context of male infertility.
Materials and Methods
Sixty practicing urologists/andrologists from 23 countries contributed 382 multiple-choice-questions pertaining to varicocele management. These were condensed into an online questionnaire that was forwarded to clinicians involved in male infertility management through direct invitation. The results were analyzed for disagreement and agreement in practice patterns and, compared with the latest guidelines of international professional societies (American Urological Association [AUA], American Society for Reproductive Medicine [ASRM], and European Association of Urology [EAU]), and with evidence emerging from recent systematic reviews and meta-analyses. Additionally, an expert opinion on each topic was provided based on the consensus of 16 experts in the field.
Results
The questionnaire was answered by 574 clinicians from 59 countries. The majority of respondents were urologists/uro-andrologists. A wide diversity of opinion was seen in every aspect of varicocele diagnosis, indications for repair, choice of technique, management of sub-clinical varicocele and the role of VR in azoospermia. A significant proportion of the responses were at odds with the recommendations of AUA, ASRM, and EAU. A large number of clinical situations were identified where no guidelines are available.
Conclusions
This study is the largest global survey performed to date on the clinical management of varicocele for male infertility. It demonstrates: 1) a wide disagreement in the approach to varicocele management, 2) large gaps in the clinical practice guidelines from professional societies, and 3) the need for further studies on several aspects of varicocele management in infertile men.
Keywords: Consensus, Disease management, Male infertility, Survey, Varicocele
INTRODUCTION
Varicocele, defined as an abnormal enlargement and tortuosity of veins in the pampiniform plexus, is the most common correctable cause of male infertility [1]. Varicocele affects nearly 15% of the general male population and is diagnosed in 19% to 41% of primary male infertility and 80% of secondary male infertility cases [2]. An epidemiological study from six European countries, involving 7,035 male subjects from the general population, indicated the presence of clinical varicocele (grades I–III) in 15.7% of the population, with more than 50% of the men having a variable degree of semen quality deterioration [3]. Despite the lack of history of infertility in the latter study, impaired semen quality was present even in low grades of varicocele and it was more pronounced in those men with grade III varicoceles. This is keeping with an earlier study from the World Health Organization (WHO) which reported the prevalence of varicocele among infertile patients with normal and abnormal semen parameters as 11.7% and 25.4%, respectively [4].
Using bibliometric analytics, Baskaran et al. reported that the number of publications on male infertility and varicocele demonstrated an increasing trend from 1988 to 2018 [5]. A more recent scientometric study on human varicocele research showed that between the years 1988 and 2020, there were four times more original articles published on surgical approaches compared to non-surgical options [6]. However, despite all these studies, the practical management of varicocele for fertility and non-fertility related indications is not clearly established and many areas of controversy still remain.
Current challenges in the management of subfertile men with varicocele include determining the true benefit of varicocele repair (VR) on pregnancy and live birth rates [7]. The improvement in sperm parameters and reduction in seminal reactive oxygen species (ROS) or sperm DNA fragmentation (SDF) after VR is highly variable and may depend on various factors such as grade of varicocele, age of patient, testes size, pre-treatment sperm parameters and hormone levels. Moreover, the role of VR in the management of infertile men with varicocele and azoospermia or in those with subclinical varicocele remains controversial. Additionally, there is no consensus as to the management of infertile men with varicocele recurrence. As a result, considerable variation and controversy is expected in the worldwide practice patterns of varicocele management for different clinical situations.
The aim of this study was to use a comprehensive online survey to determine the attitudes and practice patterns of clinicians worldwide in the management of varicocele in infertile men, thus identifying divergence and concurrence in global practice patterns, and to compare these with the latest international (American Urological Association/American Society for Reproductive Medicine [AUA/ASRM], European Association of Urology [EAU]) practice guidelines, and with evidence from systematic reviews and recent meta-analyses. Finally, in order to provide further clarity in each area of varicocele evaluation and management, an “Expert Opinion” has been provided based on the consensus of 16 highly-experienced experts from around the globe.
MATERIALS AND METHODS
1. Survey design and participants
One hundred and eight urologists/andrologists from 34 countries were invited to submit multiple-choice questions (MCQs) on varicocele-related clinical topics that they considered most important or controversial and relevant to their practice. A total of 382 questions were received from 60 practicing urologists/andrologists from 23 countries. A team of 9 experienced urologists/andrologists* (RS, PK, AR, NP, EK, NT, MEB, HK, TM) made multiple revisions to merge related or duplicate questions, remove ambiguity, and create a list of questions that were most representative of clinical dilemmas in actual practice.
Questionnaire revisions yielded a final list of 55 MCQs (Supplement File 1), which covered important aspects related to varicocele demographics and diagnosis, indications for VR, technical aspects of VR, and fertility-related outcomes. Eight of the 55 questions addressed VR for indications other than fertility and are not discussed in this paper. An additional 8 questions documented the participants’ demographic data.
This questionnaire was made available online from July 23, 2021 to August 20, 2021 via a secured tool (SelectSurvey) created by the Cleveland Clinic’s Information Technology Department. The initial invitation to take the survey was sent to 200 urologists/andrologists who were part of a global group initiated by the American Center of Reproductive Medicine with the purpose of discussing clinical and research topics related to varicocele. In turn, they forwarded the invitation to their colleagues involved in the care of infertile men through direct communication. The following societies distributed the questionnaire link to their members: Arab Association of Urology, Asia Pacific Society of Sexual Medicine, Association Francaise d’Urologie, Brazilian Association of Assisted Reproduction, Brazilian Society of Urology, Egyptian Society of Andrology, European Association of Urology, Indonesian Urological Association, Indonesian Society of Andrological Urology, Iranian Urological Association, Middle East Society for Sexual Medicine, Sociedad Argentina de Andrologia, Société d'Andrologie de Langue Française, Société Internationale d’Urologie, Spanish Association of Andrology, Sexual and Reproductive Medicine, Turkish Association of Urology, and Urological Society of Australia and New Zealand. Practitioners were informed about the nature and objective of the survey and requested to volunteer to fill out the online questionnaire. The questionnaire was provided in the English language and used standard medical terms. Participants were allowed to omit some questions and could review their answers at the end of the survey before final submission.
2. Statistical analysis
The survey responses were downloaded and saved as comma-separated (CVS) files from the SelectSurvey tool. Duplicate responses were excluded from the analysis. Summary statistics were calculated using MedCalc Statistical Software (version 19.0.5; MedCalc Software, Ostend, Belgium). Since some respondents skipped some questions, and some questions allowed multiple answers, each response was reported as a percentage of the number of respondents for that question. Subgroup analyses were performed using the chi-square test. A p-value of <0.05 was considered statistically significant.
3. International guidelines and other recommendations
The latest guidelines from the EAU [8,9] and the AUA/ASRM [10,11,12] were selected as references since these have been recently updated and are widely referred to. The clinical practices of the survey respondents were compared to these guidelines. Additionally, recent systematic reviews and meta-analyses, and other relevant studies, were referred to for clarification on the diverse practices and opinions expressed by the survey respondents.
4. Expert opinion
Since many of the controversial topics were not addressed by the guidelines, sixteen highly-experienced clinicians† (GMC, AZ, AK, MG, AR, PK, TH, EK, GR, HK, MA, TT, EB, OR, GC, AH) from around the world were invited to comment on each section. Their comments were condensed into an “Expert Opinion” that was representative of the opinion of most experts (75% consensus) and would provide practical guidance to clinicians. The alternative opinions are also presented in addition to the consensus opinion when there was a significant difference of opinion amongst the expert panel.
RESULTS AND DISCUSSION
1. Demographics of survey participants
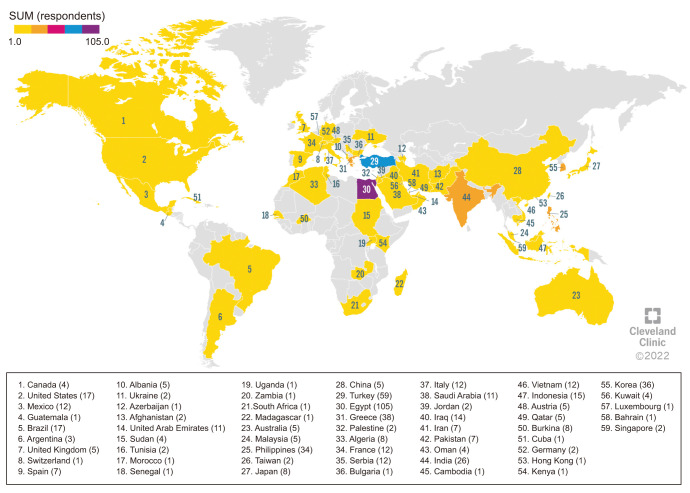
The total number of responses received at the end of the survey was 841. After excluding duplicates and partial responses, a total of 574 responses from 59 countries were considered for analysis (Fig. 1, Supplement File 2).
Fig. 1. Geographical distribution of respondents. The number of respondents is shown in brackets after the name of each country. The violet color indicates the country with the greatest number of respondents (n=105), the blue color are those with a lower number (n=59), the orange color are those with a further reduction in the number of respondents (from 26 to 38), and the yellow color are the countries with the lowest number of respondents (from 1 to 17).
Geographical distribution of the respondents included Asia (n=277, 48.3%), Europe (n=103, 17.9%), South America (n=34, 5.9%), Africa (n=134, 23.3%), North America (n=21, 3.7%), and Australia (n=5, 0.9%).
The majority of the respondents’ age were between 35 and 44 years (n=184, 32.1%) and 45–54 years (n=146, 25.4%), followed by 25–34 years (n=108, 18.8%), 55–64 years (n=94, 16.4%), and over 65 years (n=42, 7.3%).
The respondents were equally divided between general urologists (n=227, 39.5%) and urologists with a special interest in male infertility (n=226, 39.4%). Primary practice was andrology in 15.9% (n=91) and “other” in 4.9% (n=28). Approximately half (n=277, 48.3%) of the respondents had training in clinical andrology. The respondents had a wide range of experience: <5 years, 24.6%; 5 to 10 years, 21.9%; 11 to 20 years, 22.8%; and >20 years, 30.7%.
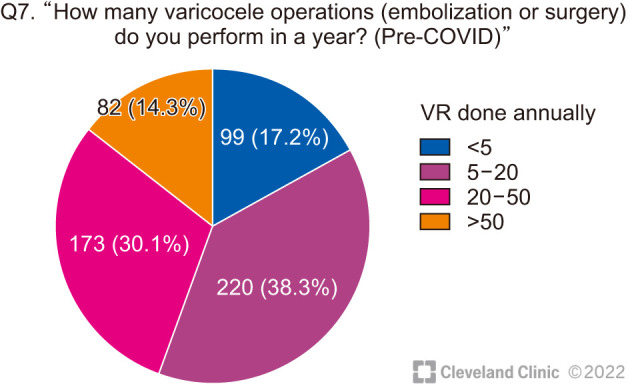
The respondents’ frequency of performing VR was also very varied (Fig. 2)
Fig. 2. Number of varicocele repairs (VRs) done annually by the respondents.
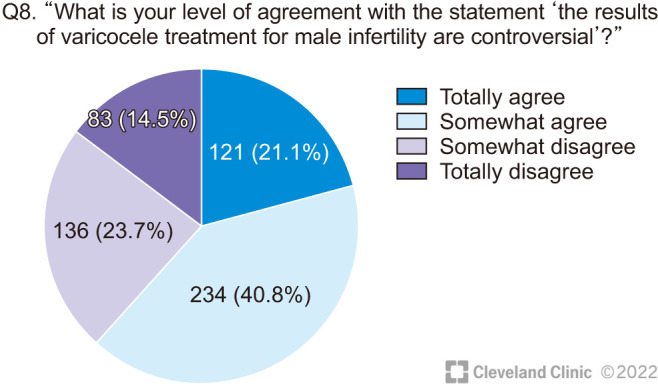
The majority of respondents indicated that they agreed in varying degrees with the premise of this survey that “the results of varicocele treatment for male infertility are controversial” (Fig. 3).
Fig. 3. Degree of agreement of the respondents with the premise of the survey.
2. Diagnosis of varicocele
1) Method of diagnosis of varicocele
(1) Survey results
Majority of respondents (70.4%; 404/574) based their diagnosis of varicocele on a combination of clinical findings and confirmation by duplex Doppler ultrasound (US) whereas 18.6% (107/574) based their diagnosis on physical examination alone. However, 9.9% (57/574) used imaging alone to diagnose a varicocele, and a few used clinical findings combined with thermography or venography.
(2) Guidelines
The AUA/ASRM guidelines (statement-21) state that “routine use of ultrasonography to investigate presumed varicocele is to be discouraged” and recommend that varicocele should be diagnosed based on physical examination alone, with sonography being done only if the physical examination is difficult.
The EAU guidelines (10.3.6.1.2) also state that the “management of varicocele is still mainly based on a physical examination”, but they also suggest the use of US when “palpation is unreliable” or when recurrence or persistence is suspected due to lack of improvement in semen parameters after VR. They also state that “definitive evidence of reflux and venous diameter may be utilised in the decision to treat”.
(3) Discussion
While 18.6% of respondents rely solely on physical examination to establish diagnosis of varicocele, the majority routinely confirm their diagnosis with US even though this is not indicated by the guidelines. Possible reasons for this widepread use of US to confirm a clinically diagnosed varicocele are discussed in the section below (2.1.4).
Interestingly, 10% of the surveyed clinicians still establish a diagnosis of varicocele based on US alone. This is contrary to the guidelines’ recommendations that state that US is not indicated if there is no palpable clinical varicocele, since subclinical varicocele should not be corrected.
(4) Expert opinion
Given the absence of evidence supporting the correction of subclinical varicoceles, the diagnosis of varicocele should be based primarily on physical examination and not on imaging alone. Routine US imaging to look for a varicocele in every man with subfertility, irrespective of physical findings, is not warranted.
US is useful when local examination is difficult (e.g., tight scrotum or thick spermatic cord), and may also be used to confirm clinical recurrence, or to confirm the diagnosis when clinical findings are equivocal (the experts were divided on this: some recommended US to confirm a clinical diagnosis of grade I varicocele, while others felt the diagnosis should be purely clinical). Some experts recommended US to assess the grade of reflux in a clinical varicocele and considered this in their decision to recommend VR. US was also recommended by some experts for objective documentation prior to VR, for insurance re-imbursement and in case of a legal issue.
2) Ultrasound parameters for the diagnosis of varicocele
(1) Survey results
Although the diagnosis of varicocele is based on clinical examination, many clinicians still use US examination for confirmation. About one-third of the respondents do not do US studies themselves. Of the 391 who responded, 56.0% (219 respondents) reported using 3 mm venous diameter for diagnosing varicocele, while 29.4% used 2.5 mm and 11.3% used 2 mm vein diameter as the diagnostic cut-off. A cut-off of >4 mm was used by 3.3%. Sub-group analysis showed that physicians who received specific training in male infertility, or who had more than 10 years experience, were more likely to perform US examinations themselves, and use 3 mm vein diameter as the diagnostic cut-off.
(2) Guidelines
The AUA/ASRM guidelines (statement 21) specify the presence of multiple veins with a diameter >3 mm and reversal of blood flow during Valsalva for US diagnosis of varicocele, but do not make any other technical recommendations.
The EAU (10.4.3.2) has adopted the recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group [13]. Varicocele diagnosis is based on a venous diameter of 3 mm or more of the largest vein measured at any location in the upright position during a Valsalva maneuver and with venous reflux of duration >2 seconds.
(3) Discussion
Since US is widely used to confirm a diagnosis of varicocele, it is important to have a consensus of what venous diameter parameters constitute a US diagnosis of varicocele. However, the size of the veins required to diagnose varicoceles differs in the literature. This variation may be caused by different evaluation positions (supine or upright position), examination either at rest or with Valsalva maneuver, or different measurement sites (relative to the testis or the spermatic cord).
The 3 mm venous diameter threshold during the Valsalva maneuver is widely accepted in the scientific literature for the diagnosis of varicocele [14,15]. However, there is considerable variation in practice with 40.6% of respondents diagnosing varicocele even when vein diameters are less than 3 mm. They are supported by some studies that have validated the diagnostic utility of smaller vein diameters. Karami et al [16] found 2.65 mm as the threshold to differentiate patients with clinical varicocele from normal subjects with high sensitivity and specificity. However, there are no studies that support 2 mm as the cut-off value, which was used by 11.2% of the respondents. The presence of continuous reflux in the spermatic vein has also been suggested as a useful Doppler finding that predicts improvement after VR [17].
(4) Expert opinion
The considerable divergence in the US diagnosis of varicocele needs to be avoided to ensure uniformity of diagnosis and management. Current evidence favors the adoption of the EAU recommendations for the US diagnosis of varicocele as mentioned above. When US evaluation is indicated to diagnose a varicocele, it should be performed in the upright position, during Valsalva, at a fixed location on the cord and with measurement of venous reflux.
Improper use of the US and lack of consensus on the threshold values used to diagnose clinical varicocele could result in over-diagnosis of early varicoceles and would lead to unnecessary surgeries and/or confusion about outcomes after treatment of ‘grade-I’ varicoceles.
3. Incidence and symptoms of varicocele
1) Survey results
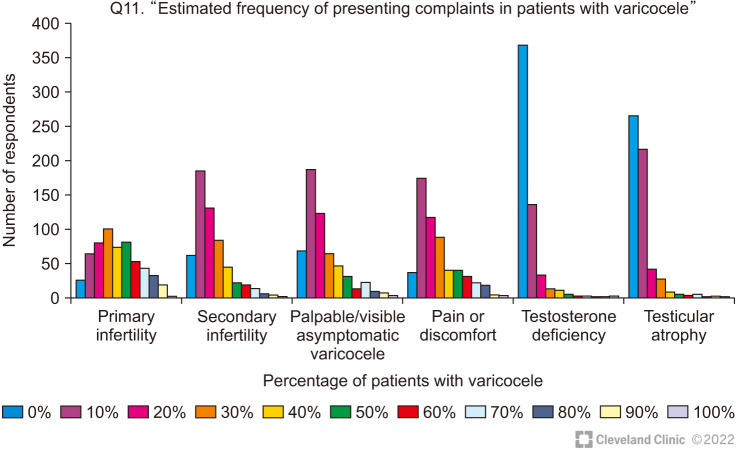
The estimated frequency of presenting symptoms in men (adult and adolescent) with varicocele is presented in Fig. 4.
Fig. 4. Estimated percentage of patients presenting with common symptoms related to varicocele.
Primary infertility was reported as the commonest presenting symptom of varicocele. In response to a question on their estimate of what proportion of men with oligoasthenoteratozoospermia (OAT) had a clinical varicocele, there is a wide divergence in the respondents’ estimates. Thus, while 13.9% of respondents reported an estimated incidence of <10%, a slightly larger percentage (18.8%) reported that >50% of their infertile men had varicoceles. The remaining clinicians were also equally divided between a prevalence of 10%–25% (33.1%) and 25%–50% (34.1%).
2) Guidelines
The EAU guidelines (10.4.3.3.1) quote a prevalence of varicocele in almost 15% of the normal male population, in 25% of men with abnormal semen analysis, and in 35%–40% of men presenting with infertility.
3) Discussion
The marked variation in estimated prevalence of varicocele reported by the survey participants suggests a lack of uniformity in the criteria and methods of establishing a diagnosis of varicocele. This may, in turn, be the reason for significant outcome differences reported by various studies.
4) Expert opinion
There is a need for more studies on the prevalence of varicocele in fertile and infertile men using well-defined criteria. Clinical examination is the mainstay of diagnosis but it is subjective, resulting in varying estimates of varicocele prevalence. A combination of clinical findings and strictly defined US criteria may help to ensure uniformity in varicocele diagnosis.
4. Indications for varicocele repair
The various clinical scenarios in which the respondents would advise VR are listed in Table 1.
Table 1. Indications for varicocele repair in men with infertility.
| Q17. “What are your indications for varicocele repair in an infertile couple?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Infertility with clinical varicocele, abnormal semen analysis, and normal female partner <35 years old | 526 | 91.6 |
| Infertility with clinical varicocele, and abnormal semen analysis or elevated SDF, irrespective of female partner status | 220 | 38.3 |
| Infertility with clinical varicocele, normal semen analysis, normal female partner, but elevated SDF | 199 | 34.7 |
| Clinical varicocele with normal semen analysis, normal SDF, but ipsilateral testicular atrophy | 179 | 31.2 |
| Infertility with clinical varicocele, normal semen analysis, normal SDF, normal female partner, but failed IUI/IVF | 105 | 18.3 |
| Infertility with clinical varicocele, normal semen analysis, normal female partner, but elevated OS | 94 | 16.4 |
| Large asymptomatic, varicocele with normal semen analysis, and normal testicular size | 79 | 13.8 |
| I do not recommend varicocelectomy. I rather prefer to proceed with other treatments (IUI/IVF/ICSI) | 7 | 1.2 |
| Total number of respondents | 574 | |
ICSI: intracytoplasmic sperm injection, IUI: intrauterine insemination, IVF: in vitro fertilization, OS: oxidative stress, SDF: sperm DNA fragmentation.
1) Oligoasthenoteratozoospermia
(1) Survey results
Semen analysis was the most common laboratory test used to evaluate the impact of a clinical varicocele on fertility (n=559, 97.4%), followed by reproductive hormonal assay (n=303, 52.8%), SDF (n=150, 26.1%), and oxidative stress (OS) testing (n=38, 6.6%).
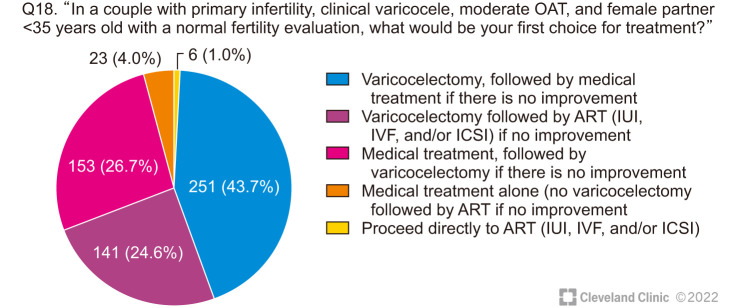
When treating a couple with primary infertility, and moderate OAT with a clinical varicocele, 68.3% of the respondents stated that they would directly proceed with VR as the first line of treatment, and then follow-up with medical therapy or assisted reproductive technology (ART) if there was no improvement. 26.7% would first attempt medical therapy before proceeding for VR and 1.0% would proceed to ART without VR (Fig. 5).
Fig. 5. Therapeutic decisions on the management of a couple with primary infertility and clinical varicocele. OAT: oligoasthenoteratozoospermia, ART: assisted reproductive technology, IUI: intrauterine insemination, IVF: in vitro fertilization, ICSI: intracytoplasmic sperm injection.
This variability in the approach to timing of VR was also reflected in the number of semen analyses the clinicians requested before proceeding for VR. When the first semen analysis was abnormal and a varicocele was detected, 11% of clinicians would advise surgery right away, and 50.3% would advise surgery if another report within a month showed OAT. However, 28% would wait 3 months before doing another semen test and then advise surgery if OAT persisted, while only 10.6% stated that they would consider multiple semen reports before advising surgery.
This trend towards early surgery is also reflected in the time to surgery once the diagnosis and recommendation for VR are made, with 60.5% (328/542) of respondents stating that most of their patients underwent VR within 3 months of diagnosis.
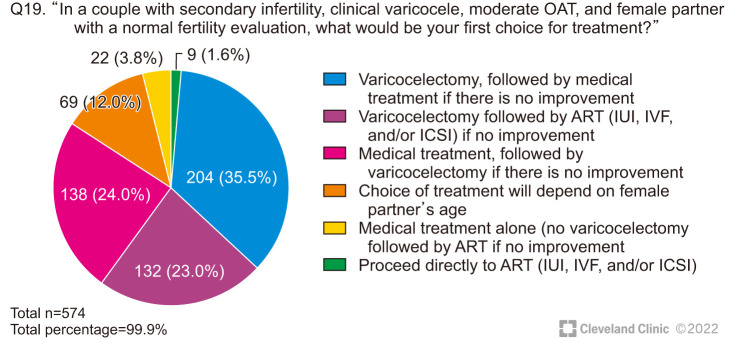
When a couple has secondary infertility, 12.0% of respondents stated that their decision would be affected by the female partner’s age, but the majority (58.5%) still recommended VR as the first step; only 1.6% suggested proceeding directly to ART (Fig. 6).
Fig. 6. Therapeutic decisions on the management of a couple with secondary infertility and clinical varicocele. OAT: oligoasthenoteratozoospermia, ART: assisted reproductive technology, IUI: intrauterine insemination, IVF: in vitro fertilization, ICSI: intracytoplasmic sperm injection.
The severity of OAT was not a deterrent to advising surgery. Responding to a case scenario of severe OAT (<1 mill/mL) associated with grade 2 or 3 varicocele, 73.3% said they would advise VR as the first line of treatment and 17.9% would consider VR if intracytoplasmic sperm injection (ICSI) had failed.
(2) Guidelines
The AUA/ASRM guidelines (statement 25) recommend VR if there is infertility and palpable varicocele associated with “abnormal semen parameters, except for azoospermic men.” The older AUA/ASRM guidelines (2012) [18] also recommend VR in men with abnormal semen analysis even if they are not currently attempting conception. The EAU guidelines (10.4.3.3.2) state that VR results in significant improvement in abnormal semen parameters including in some men with non-obstructive azoospermia (NOA), and recommend VR in men with OAT, and also suggests that couples with “otherwise unexplained subfertility” may also benefit from VR.
(3) Discussion
The latest Cochrane review [7] states that VR for men with OAT may improve pregnancy rates but it is uncertain whether live birth rates increase. Despite this opinion, the majority of respondents favored VR before proceeding to ART, and recommended VR as the first step in treatment, in accordance with the guidelines. A different opinion may have been expressed if the survey population had comprised IVF specialists.
However, one-third of the respondents felt that there was no need to rush into VR and that patients may benefit from an initial trial with medical management before considering surgery. This is in concordance with a recent meta-analysis which suggested that while antioxidants (AOX) did not improve pregnancy rates in men who had VR, AOX may be of benefit in men with unoperated varicoceles [19]. Giving a couple time to conceive naturally without rushing into VR, especially if they are young, is supported by a study that showed similar pregnancy rates between conservative and surgical approaches when the duration of infertility was short, but greater chances of pregnancy after VR if the couple has been infertile for more than 2 years [20].
Thus, there is no consensus or guidelines on the timing of VR. Some clinicians feel that once a varicocele is detected it should be corrected quickly to prevent further deterioration. Others adopt a more conservative approach before advising VR [21].
(4) Expert opinion
VR is a useful procedure for the treatment of men with OAT and this is reflected in global practice patterns and the EAU/AUA/ASRM guidelines.
Considering the natural variations in semen parameters, the possibility of fertility despite the presence of an untreated varicocele, and the uncertainty about VR outcomes, it is reasonable to not rush into VR if the couple is young and the duration of infertility is short (1–2 years). However, other than this, VR can be recommended as the first step when there is OAT and a clinical varicocele, after proper counseling about the likelihood of benefit after VR (see section 7.2).
The role and duration of conservative management in couples with varicocele needs further critical evaluation as this may help identify couples that can be managed medically (avoiding unnecessary VR) from those that are better managed by early VR.
2) Isolated asthenozoospermia
(1) Survey results
VR for men with isolated asthenozoospermia was routinely advised by 63% of the respondents, while 22% would advise it in selected cases.
(2) Guidelines
The EAU and AUA/ASRM guidelines do not specifically mention whether varicocele surgery is indicated in men with isolated asthenozoospermia, though the previous AUA/ASRM guidelines [18] stated “one or more parameters” should be abnormal.
(3) Discussion
“Isolated asthenozoospermia” includes a very diverse group of patients. A man with normal sperm count but only 2% motility has a different etiology and prognosis from a man with normal sperm count and 30% motility. A man with low normal sperm count and asthenozoospermia may represent a different pathology from that of a man with high sperm counts and asthenozoospermia.
It is clear that varicoceles can have a negative impact on sperm motility and total motile sperm count (TMSC), and that TMSC can improve significantly after VR [22]. However, there are only a few retrospective studies on the role of VR in isolated asthenozoospermia and they report mixed outcomes [23,24,25]. They also fail to distinguish between the various sub-groups as discussed above. Thus, there is limited evidence favoring VR in men with isolated asthenozoospermia. However, the high endorsement by the survey respondents for VR in men with isolated asthenozoospermia suggests that, despite lack of reported evidence, the respondents may be seeing a benefit in these patients.
(4) Expert opinion
Further studies are needed to assess the utility of VR in isolated asthenozoospermia. A distinction should be made between different severity and etiologies of asthenozoospermia. Until then, based on the survey respondents’ practices, a guarded recommendation can be made for VR in these cases.
3) Isolated teratozoospermia
(1) Survey results
VR for men with isolated teratozoospermia was routinely advised by 41.1% of the respondents, and another 30.3% would do so in selected cases.
(2) Guidelines
The EAU and AUA guidelines do not specifically mention whether VR is indicated in men with isolated teratozoospermia.
(3) Discussion
Teratozoospermia has become a contentious issue. While earlier studies often attributed significant predictive value to morphology [26], recently there has been criticism that the criteria have become too strict resulting in an excessive diagnosis of teratozoospermia and loss of clinical predictive value [27,28]. Hence, the willingness of the majority of respondents to offer VR for isolated teratozoospermia is surprising.
Very few papers address the benefit of VR in men with isolated teratozoospermia and these studies report mixed results. While a study by Cakiroglu et al [24] demonstrated no improvement in sperm morphology after VR in men with otherwise normal sperm counts, other retrospective studies demonstrated an improvement in sperm morphology [29] as well as pregnancy rates [30] after VR. This improvement was most likely seen with cases of immature spermatozoa and spermatozoa with head abnormalities [31].
(4) Expert opinion
There is little evidence to support VR for men with isolated teratozoospermia. However, since the majority of respondents have indicated that they would suggest VR for this indication, there is a need for further studies so that clear recommendations can be made. Monomorphic teratozoospermia (macrozoospermia and globozoospermia) has a genetic basis [32] and VR is not indicated for these cases.
4) Isolated severe necrozoospermia
(1) Survey results
VR for men with isolated severe necrozoospermia was routinely recommended by 42.5% of the respondents, while another 27.4% did so in selected cases.
(2) Guidelines
The EAU and AUA guidelines do not specifically mention whether varicocele surgery is indicated in men with isolated necrozoospermia.
(3) Discussion
The etiology of necrozoospermia is frequently unclear but varicocele has been hypothesized as a possible cause [33]. However, no published studies have evaluated the direct role of VR to improve isolated necrozoospermia.
(4) Expert opinion
There is no evidence to support VR for this indication. Yet, the majority of clinicians have indicated that they would proceed with treatment. Hence, those who perform VR in men with isolated severe necrozoospermia should document and publish their results so that evidence-based recommendations can be made in the future.
5) Isolated increased sperm DNA fragmentation or oxidative stress
(1) Survey results
Among the survey respondents, 34.7% (199/574) indicated of survey respondents indicated that they would consider VR if SDF was increased even if conventional semen parameters were normal (Table 1), and 16.4% (94/574) of respondents would advise VR if the OS markers alone were elevated while 18.3% (105/574) would advise VR even with normal semen parameters and normal SDF if multiple attempts at intrauterine insemination/in vitro fertilization (IUI/IVF) had failed.
(2) Guidelines
AUA/ASRM guidelines (statement 19) state that “there are no well-controlled studies that VR will reduce risk of recurrent pregnancy loss in men with elevated SDF”.
EAU guidelines (10.4.3.3.4) state that there is “increasing evidence” that VR may improve SDF and ART outcomes and recommends VR for men with raised SDF and failed ART (failure of embryogenesis or implantation, or recurrent pregnancy loss) “after extensive counseling”. They make a weak recommendation for VR in men with increased SDF and otherwise unexplained infertility (10.4.3.5), but also state, “the dilemma is whether varicocele treatment is indicated in men with raised DNA fragmentation and normal semen parameters”.
(3) Discussion
SDF has emerged as an important measure of sperm function and a predictor of reproductive outcomes [34]. VR is associated with an improvement in SDF, including both single-strand and double-strand DNA fragmentation, as well as seminal OS [35], and two recent meta-analyses calculated a mean reduction in SDF after VR of 7.23% [36] and 6.14% [37] respectively.
Men with varicoceles frequently have higher rates of SDF and elevated OS even when a semen analysis shows normal semen parameters [38,39,40,41]. In a small, controlled trial, Fathi et al [42] reported decreased SDF (9% reduction) and higher pregnancy rates in normozoospermia men with high SDF (>25% by sperm chromatin dispersion assay) after VR.
(4) Expert opinion
There is evidence to support VR in men with isolated high SDF. VR may be recommended for such men if other measures to reduce SDF have failed, especially if there is a history of failed ART. Further research is needed to elucidate whether the improvement in SDF and OS after VR in men with a normal semen analysis translates to an increase in natural or ART pregnancy rates.
6) Varicocele repair prior to assisted reproductive technology
(1) Survey results
Interestingly, in a couple planning to undergo IVF, where the man has OAT and varicocele, 62.7% (360/574) of respondents would recommend VR before proceeding to IVF. Some respondents would decide based on the age of the female partner or duration of infertility (Table 2). Another 17.8% (102/574) would recommend VR prior to IVF only if SDF was elevated.
Table 2. Varicocele repair prior to IVF.
| Q23.“If a man has OAT and clinical varicocele and the couple is willing to undergo IVF, what do you recommend?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Recommend varicocele repair before considering IVF | 360 | 62.7 |
| Correct varicocele if IVF fails | 131 | 22.8 |
| Recommend varicocele repair before IVF, only if SDF is high | 102 | 17.8 |
| Proceed directly with IVF | 102 | 17.8 |
| Depends on the female age | 230 | 40.1 |
| Depends on the severity of the varicocele | 132 | 23.0 |
| Depends on the duration of infertility | 83 | 14.5 |
| Depends on the male age | 72 | 12.5 |
| Total number of respondents | 574 | |
IVF: in vitro fertilization, OAT: oligoasthenoteratozoospermia, SDF: sperm DNA fragmentation.
(2) Guidelines
The current AUA/ASRM guidelines do not comment on whether VR prior to IVF will improve pregnancy rates, though they do list VR as an option before IVF when SDF is raised and there is a history of recurrent pregnancy loss (statement 19). However, older AUA/ASRM guidelines [18] state that “VR usually is not indicated as the primary treatment for couples when IVF is necessary”.
The EAU guidelines state that VR may improve ART outcomes in men with OAT (10.4.3.3.2) and also suggest a role for VR before ART when there is elevated SDF (see discussion above in section 4.5.2).
(3) Discussion
Correcting a varicocele before proceeding for IVF-ICSI is a controversial topic, and many ART centres take no cognizance of a varicocele. However, the majority of survey respondents felt that a varicocele should be corrected before ART. Support for VR before ART comes from a meta-analysis by Esteves et al [43] who reported increased clinical pregnancies and birth rate in 3 of 4 reviewed studies, and from a meta-analysis by Kirby et al [44] who reported that VR improved the ART live birth rate in men with oligospermia (odds ratio [OR], 1.699). Thus, some couples may benefit from having VR before ART. Since VR is a minor procedure compared to the time and expense involved in ART, it may be argued that VR should be done for whatever benefit it may confer.
(4) Expert opinion
Recommending VR to all men prior to ART is a delicate decision since it will delay the ART procedure by 3 to 6 months for an unpredictable benefit. There is a need to identify which subgroups of men with clinical varicoceles will have an improvement in the ART outcomes after prior VR. Until such evidence is available, the decision to perform VR before ART should be individualized based on other variables like varicocele grade, SDF levels, history of prior failure, duration of infertility, female partner’s age, etc., and after a thorough discussion with the couple.
When ART is being done for an older woman, and her male partner has OAT with varicocele, some experts suggest that the option of performing both ART and VR can be considered. ART success rate per cycle is low in older women [45], and the possibility of achieving a spontaneous pregnancy if the semen quality improves after VR can never be excluded a priori.
7) When not to recommend varicocele repair
(1) Survey results
Participants listed several clinical situations in which they would not recommend VR (Table 3).
Table 3. Clinical situations where varicocele repair is not advised.
| Q25. “In a man with a clinical varicocele, OAT and infertility, when are you likely to NOT advise varicocele repair?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Grade of varicocele is mild (grade 1) | 242 | 42.2 |
| FSH above normal | 161 | 28.0 |
| Female age >35 years | 159 | 27.7 |
| Small testes (<10 mL) | 149 | 26.0 |
| Will usually advise surgery despite any of the above | 145 | 25.3 |
| Male age >40 years | 105 | 18.3 |
| Severe OAT (<1 mill/mL) | 99 | 17.2 |
| Total number of respondents | 574 | |
FSH: follicle stimulating hormone, OAT: oligoasthenoteratozoospermia.
(2) Guidelines
The AUA/ASRM guidelines recommend against VR for non-palpable varicoceles (statement 26) and also when IVF is indicated due to a female factor (2012 guidelines). The EAU guidelines (10.4.3.3.2) do not recommend VR in men with normal semen analysis and those with a sub-clinical varicocele. However, no exclusions based on any of the other factors are specified.
(3) Discussion
Since a significant proportion of men will not benefit from VR, it is important to individualize the recommendation for VR, based on favorable and unfavorable prognostic factors.
However, there is considerable variation in the clinical factors that the surveyed physicians take into consideration when deciding that VR is unlikely to succeed in a given case. Interestingly, one-fourth of respondents indicated that they would take any chance and would advise surgery despite negative prognostic factors.
The partner’s age was considered important by 27.7% of the respondents who suggested that if the female partner’s age is >35 years, then they would not want to delay ART and hence would not recommend VR. The AUA/ASRM guidelines (statement 1) state that “maternal age is the strongest predictor of fertility outcome”. Therefore, the need for haste is understandable. Similarly, male age >40 years was cited as an exclusion factor by 18.3%. However, a study by Fırat and Erdemir [46] showed that age need not be an exclusion factor. The study evaluated outcomes after VR in 293 couples of different ages. TMSC was found to be significantly increased in all groups after varicocelectomy (p<0.05) and though pregnancy rate after varicocelectomy was higher in group 3 (both partners <35 years old) compared with group 2 (patients ≥35 years old and their spouses <35 years old) and group 1 (both partners ≥35 years old), the differences were not significant (p=0.133). This suggests that even couples with both partners over 35 years of age have a reasonable chance of natural pregnancy after VR, and advanced male age is not a contraindication to VR. A similar conclusion was reached in a study by Hsiao et al [47].
The most common reason (42.2%) for not advising VR was low grade (grade I) of varicocele. Several studies have shown greater improvement and higher pregnancy rates when a higher grade of varicocele was operated. A recent meta-analysis of 20 studies by Asafu-Adjei et al [48], stratified outcomes by varicocele grade and demonstrated that improvements in sperm concentration and overall motility occurred with all grades of varicocele but it was proportional to the grade of varicocele. Thus, the mean sperm concentration improvement in men with grades I, II, II-III and III varicoceles were 5.5, 8.9, 12.7, and 16.0 million sperm/ml, respectively while the mean improvement in the percent of overall motility in men with grades I, II, II-III and III varicoceles was 9.6%, 10.6%, 10.8% and 17.7%, respectively [48].
A significant proportion of respondents listed “follicle stimulating hormone (FSH) above normal” (28% of respondents) or “testes <10 mL” (26% of respondents) as contraindications to VR. While this correlates with a common sense approach that these parameters indicate significant testicular damage and hence improvement after VR is less likely, there is little evidence in the literature to support such a belief. A study by Birowo et al [49] found improvement in sperm retrieval rates after VR in men with NOA across all levels of FSH, while another study showed a reduction in FSH after VR with a correlation between reduction in FSH and an increase in semen parameters [50]. Similarly, while studies have shown an increase in testicular volume after VR in both adolescents [51] and adults [52,53], there is no data is supporting the exclusion of VR in men with small testes and OAT.
Only 17.2% of the respondents felt that VR would not benefit men with severe OAT (<1 mill/mL) and would not recommend VR for this group. While several studies have documented the benefit of VR in men with severe OAT (<5 mill/mL) [54,55], few studies address the possibility of benefit from VR in case of extreme OAT (<1 mill/mL). In a study of 102 men with severe OAT (<5 mill/mL), Enatsu et al [56] reported that 41.1% of men had significant improvement after surgery, but the chances of improvement were greater in men with initial counts of 2–5 mill/mL as compared to those with <2 mill/mL. A study by Dada et al [57] emphasized the need for genetic testing prior to VR in men with extreme OAT.
(4) Expert opinion
There are no robust contra-indications to VR. However, men with very poor baseline sperm parameters (e.g., extreme OAT) and small (grade I) varicoceles are less likely to experience a clinically significant improvement in sperm parameters after VR.
Moreover, couples with advanced maternal age or poor ovarian reserve should be cognizant of the time delay associated with a VR and the unfavorable impact of maternal age on spontaneous pregnancy rates. These couples should consider opting for ART without delay but may consider VR as a simultaneous procedure to improve semen quality for future attempts.
8) Predictors of a successful outcome (pregnancy) after varicocele repair
(1) Survey results
When asked to identify which factors could predict a successful outcome in terms of natural pregnancy after VR, the majority identified higher grade of varicocele, higher pre-operative motile sperm count, larger testicular volume, lower FSH, and bilaterality of the varicocele as key predictors of a higher chance of benefit (Table 4).
Table 4. Predictors of pregnancy after varicocele repair.
| Q 36: “In your experience, which of the following pre surgery parameters predict varicocele repair success in terms of pregnancy?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Higher grade of varicocele | 355 | 68.1 |
| Higher total motile sperm count | 258 | 49.5 |
| Larger testes volume | 232 | 44.5 |
| Higher total sperm count | 214 | 41.1 |
| Lower serum FSH level | 181 | 34.7 |
| Bilateral varicocele | 176 | 33.8 |
| Higher serum testosterone level | 58 | 11.1 |
| Other | 23 | 4.4 |
| Total number of respondents | 521 | |
FSH: follicle stimulating hormone.
(2) Guidelines
The AUA/ASRM guidelines state that “maternal age is the strongest predictor of fertility outcome” (statement 1) but do not identify any patient or varicocele-related characteristics that predict a greater chance of benefit from VR. The EAU guidelines do mention that a higher grade of varicocele is associated with greater improvement (10.4.3.3.2) and this should be considered while counseling a patient.
(3) Discussion
The female partner’s age is the most important predictor of pregnancy. However, this question focused on factors that would predict maximum improvement in semen parameters and thus improve pregancy rates.
In a systematic review of parameters that would predict favorable outcomes after VR, Samplaski and Jarvi [58] reported that the best predictor of improved semen parameters, as well as of natural and ART pregnancy rates, were higher pre-operative semen parameters, and that the greatest improvements were seen in men with larger varicoceles. There was some evidence that higher testosterone, larger testes, and younger age were associated with better outcomes. Predictive normograms have also been described [59]. Kamal et al [60] also reported that natural pregnancy rates after VR were much higher if the initial sperm count was >5 mill/mL. If US Doppler study has been carried out, then the presence of continuous reflux predicts a higher chance of improvement [17].
(4) Expert opinion
The greatest improvement in semen parameters and highest chances of natural pregnancy after VR may be expected when the female partner is young, initial semen analysis shows mild to moderate OAT, varicocele is large (grade III) and bilateral, testicular volume and serum FSH are normal, or there is secondary infertility.
5. Technical aspects of varicocele repair
1) Choice of technique, use of magnification, identification of artery
(1) Survey results
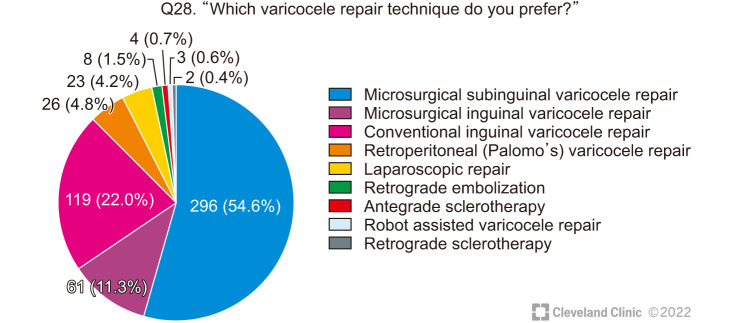
The respondents’ choice of technique for VR is shown in Fig. 7.
Fig. 7. Choice of varicocele repair technique.
Nearly half (43.2%) of the surgeons reported that they routinely used the operating microscope and 26.0% used magnifying loupes for performing VR. The rest listed a variety of reasons for not using magnification (Table 5).
Table 5. Use of operating microscope for varicocele repair.
| Q 29: “Do you perform varicocelectomy using an operating microscope?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Yes, I use it routinely | 234 | 43.2 |
| I use magnifying loupes | 141 | 26.0 |
| I don’t have microscope | 136 | 25.1 |
| I feel comfortable with naked eye | 79 | 14.6 |
| I don’t have microsurgical skills | 61 | 11.3 |
| I feel that a microscope doesn’t make much difference in outcomes | 38 | 7.0 |
| Total number of respondents | 542 | |
Only 13.4% used an intra-operative Doppler to identify the artery. The majority relied on visual identification using either a microscope (33.1%) or loupes (13.4%); 17.4% claimed to identify the artery without the help of magnification, while 24.4% did not identify the artery and just avoided the area of pulsations.
Only a minority of the survey responders (2.6%) preferred retrograde embolization or antegrade or retrograde sclerotherapy as a primary method for VR.
(2) Guidelines
The AUA/ASRM guidelines recommend “surgical varicocelectomy” (statement 25) and quote the highest success with subinguinal microsurgical varicocelectomy as compared to other surgical techniques.
EAU guidelines state that microsurgical varicocelectomy “is the most effective among the different varicocelectomy techniques” and has the lowest complication and recurrence rates. However, adequate support from RCTs is lacking, and “other techniques are also viable options” (10.4.3.4). Radiological techniques are minimally invasive and are widely used but have higher recurrence rates.
(3) Discussion
There are many options for surgical VR, each having its potential advantages and disadvantages. One meta-analysis found no specific VR technique to be the most effective in improving fertility [61], while another one reported the highest spontaneous pregnancy rate following sub-inguinal microsurgical VR (41%) vs. retroperitoneal (37%) vs. inguinal (26%) vs. laparoscopic transperitoneal (26%) vs. percutaneous embolization (36%) [62].
Techniques utilizing optical magnification optimize the surgeon’s ability to preserve the testicular artery and lymphatic channels while ligating all veins to minimize the risk of hydrocele formation or varicocele recurrence. Preservation of the gonadal artery during VR is considered important, and techniques that facilitate this include optical magnification with an operative microscope or operating loupes, administering papaverine locally, using a micro-Doppler [63], or a combination of the above.
Almost one-fourth (24.4%) of the responders do not identify the arteries but just avoid the area of pulsations. This may lead to an increased possibility of missing surrounding veins and a higher risk of varicocele recurrence.
In this context, microsurgical VR is considered the gold standard procedure having the least postoperative complications and lowest recurrence rate [64], and was the technique used by more than half of the respondents. The microsurgical subinguinal approach has the further advantage of a short postoperative recovery compared to the inguinal approach because no muscle or fascia is incised during the procedure. However, the number of veins and arteries encountered in the subinguinal approach is more than in the inguinal approach, rendering dissection more challenging [65]. In adolescents, use of the microscope makes identification of the tiny testicular arteries easier.
Varicocele treatment by venous embolization or antegrade sclerotherapy [66] was not popular with the respondents, but this may reflect a geographical bias. Percutaneous embolization of varicoceles will result in less post-procedural pain than surgical repairs. However, interventional access to the internal spermatic veins (especially the right side) may not be possible due to technical challenges in up to 20% of cases [67], and recurrence rates range from 4% to 27% (EAU guidelines 10.4.3.4).
(4) Expert opinion
Microsurgical subinguinal VR is the standard of care because it is associated with lower complication and recurrence rates, and possibly higher improvement in semen parameters. However, it is technically demanding and needs access to an operating microscope. Not all surgeons are trained in the technique but those who do VR regularly are strongly encouraged to acquire microsurgical skills to be able to perform subinguinal microsurgical VR. Meanwhile, surgeons should utilize the technique that they are competent in and most comfortable with.
2) Ligation of additional veins outside the spermatic cord
(1) Survey results
The majority of respondents ligate the external spermatic or cremasteric veins (always, 33.9%; often, 47.8%).
In contrast, the majority do not ligate the gubernacular veins (never, 25.3%; rarely, 29.3%). Only 15.7% always ligate the gubernacular veins while 16.1% ligate them when operating for recurrence, and 13.7% ligate if there is a grade III varicocele.
(2) Guidelines
There are no specific comments on the need to ligate cremasteric or gubernacular veins.
(3) Discussion
Ligation of the external spermatic or cremasteric veins has been popular for a long time [68], and most respondents reported ligating them during VR. On the other hand, the majority of respondents disputed the suggestion that the gubernacular veins should be ligated.
The concept of ligating the gubernacular veins was proposed by Goldstein in 1992 [69] based on earlier published studies on varicocele recurrence. In a case series of 640 men who underwent microsurgical VR with gubernacular vein ligation, he reported a low recurrence rate of 0.6%. However, there was no control group and the low recurrence rate may have been due to the microsurgical cord dissection. A subsequent paper, from the same institution, which included a control group of men whose testes were not delivered showed similar recurrence rates and improvement in semen parameters in both groups [70]. Similarly, a controlled randomized study by Huo et al [71] found no difference in either recurrence or improvement rates, but observed that gubernacular vein ligation increased the operating time by a mean of 6 minutes and increased the incidence of scrotal edema and testicular engorgement (raising the suspicion that ligation of gubernacular veins resulted in occlusion of normal drainage channels).
On the other hand, a randomised, controlled study by Allameh et al [72] found lower recurrence, greater improvement in sperm motility, and no increase in the complications, in the group that underwent gubernacular vein ligation.
(4) Expert opinion
Ligation of the external spermatic veins is easy, adds minimal operating time, may reduce recurrence, and can be done routinely. Gubernacular vein ligation requires additional steps and a larger incision to deliver the testis. Since the benefit of this extra step is controversial, it should be deemed optional, and as suggested by the survey respondents may be omitted entirely or limited to selected cases of grade III or recurrent varicocele.
3) Unilateral versus bilateral repair
(1) Survey results
In the presence of a left clinical varicocele, 63.8% of respondents would operate the right side only if a clinical varicocele was present on the right side, while 18.8% would correct the right side even if it was subclinical. Besides, 17.4% stated that they did not perform simultaneous bilateral repair.
(2) Guidelines
While both EAU and AUA guidelines are clear that subclinical varicoceles should not be operated upon, there is no specific recommendation regarding a right-side subclinical varicocele when there is a left-sided clinical varicocele.
(3) Discussion
A recent systematic review favored bilateral VR over unilateral VR when there are bilateral clinical varicoceles [73]. However, the choice of procedure becomes less clear when the varicocele on the right side is equivocal or subclinical.
A large meta-analysis of 4 randomized controlled trials (RCTs) with 637 cases compared outcomes following unilateral or bilateral VR in men with left-clinical and right-subclinical varicoceles [74]. The authors reported no significant difference in the increase in sperm concentration and sperm motility in the two groups but the odds ratio for spontaneous pregnancy rate was 1.73 favoring bilateral ligation. The authors concluded that bilateral ligation may be superior but more RCTs were needed.
A prospective, randomized controlled study by Sun et al [75] compared the outcomes at one year following unilateral VR (179 men) versus bilateral VR (179 men) in infertile men with left clinical and right subclinical varicoceles. Both groups showed improvement but the increase in sperm concentration, progressive motility, and morphology were significantly greater, and pregnancy rates were higher in the bilateral group (42.5% vs. 26%).
On the other hand, an earlier, smaller study on 104 men found equal improvement in semen parameters and pregnancy rates following unilateral or bilateral VR in this group of men [76].
(4) Expert opinion
When there is a bilateral clinical varicocele then VR should be performed bilaterally.
However, there is controversy in the medical literature regarding concomitant repair of a subclinical right varicocele at the time of left clinical VR, and a definitive recommendation cannot be made. Taking into consideration the general injunction against VR for a subclinical varicocele, the consensus of expert opinion recommends against VR of the right subclinical varicocele in this situation.
If the clinician is considering bilateral ligation for such a case then it should be a shared decision between the surgeon and patient with the understanding that the benefit of a bilateral procedure is uncertain, and involves extra operative time and an additional incision.
4) Sperm cryopreservation prior to varicocelectomy
(1) Survey results
Opinion was divided on the need for prior sperm cryopreservation. Overall, 32.5% of the clinicians never recommend it, while 32.5% recommend it when there is severe OAT, and 25.5% consider cryopreservation prudent when operating on a solitary testis.
(2) Guidelines
There are no recommendations on when sperm should be cryopreserved before VR.
(3) Discussion
The spermatic artery can be damaged during VR, especially when utilizing non-magnified surgical techniques, and this may further jeopardize an already impaired spermatogenesis with significant consequences if the original sperm count was already very low [75]. Further, men with severe OAT may progress naturally to azoospermia [77], and if that happens after VR, then the VR procedure may be blamed. Not all men undergoing VR will have an improvement in their semen parameters, and sometimes there may be deterioration which may even result in azoospermia in men who had extreme OAT pre-operatively [78]. Hence, it seems prudent to advise sperm cryopreservation before VR in men with extreme oligozoospermia.
Further, testicular atrophy can occur rarely after VR [79], and while this may not have much of an impact if there is a normal contralateral testicle, it would be disastrous in a man with a solitary testicle. Therefore, it would be safer to cryopreserve sperm before VR on a solitary testis.
(4) Expert opinion
Sperm cryopreservation prior to VR is not recommended as a routine procedure since the additional costs are not warranted. However, in keeping with the survey findings, it should be considered when there is a solitary testis or extreme OAT (<100,000/mL). Sperm cryopreservation may also be considered in men with varicocele who have severe OAT and progressively declining semen parameters but are delaying VR.
5) Testicular biopsy at the time of varicocele repair for oligoasthenoteratozoospermia
(1) Survey results
The vast majority (94.1%) of respondents do not perform a testicular biopsy when VR is being done for OAT.
(2) Guidelines
The guidelines do not make any recommendation for testicular biopsy when VR is done for OAT.
(3) Discussion
While it was a practice in the past to do a testicular biopsy at the time of VR for “prognostic purposes”, it is not recommended currently as there is no evidence that the biopsy results influence further management [80], and it may lead to the formation of antisperm antibodies which may affect sperm parameters later on.
(4) Expert opinion
Testicular biopsy is not indicated at the time of VR for management of OAT.
Men who are undergoing VR for infertility, and also have bilateral testicular microlithiasis, may be offered a simultaneous testicular biopsy to look for germ cell neoplasia in situ (GCNIS) and future risk of testicular germ cell tumor (TGCT) (EAU guidelines 10.4.2.2).
6. Follow-up and recurrence after varicocele repair
1) Post varicocele repair follow-up with ultrasound or Doppler ultrasound
(1) Survey
There was considerable divergence of opinion on follow-up, with 14.8% (77/521) advising post-VR US routinely for all patients, and 21.7% (113/521) never asking for post-op US. Others advised it conditionally for reasons listed in Table 6.
Table 6. Doppler ultrasound evaluation for follow-up after varicocele repair.
| Q 42:“Do you perform Doppler or ultrasound evaluation after varicocele repair?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| If physical examination suggests residual varicocele | 216 | 41.5 |
| If semen parameters have not improved | 157 | 30.1 |
| If patient is not relieved of pain | 121 | 23.2 |
| Never | 113 | 21.7 |
| In every case | 77 | 14.8 |
| Total number of respondents | 521 | |
(2) Guidelines
The AUA/ASRM guidelines (statement 21) recommend against routine scrotal US to look for varicocele; there is no specific recommendation about the use of US to monitor follow-up or look for recurrence.
The EAU guidelines (10.4.3.2) recommend scrotal Doppler US to look for residual/recurrent varicoceles if there is no improvement in semen parameters after VR.
(3) Discussion
Routine Doppler US follow-up after VR can act as a quality control measure, helping the clinician to confirm that there is no residual varicocele (failed VR) and that the artery is intact. However, this has several disadvantages apart from the additional cost and time spent. Often a post-VR Doppler US may detect a varicocele that is not clinically significant, and this could lead to a false diagnosis of failed surgery. In a small study on adolescents post-VR, there was marked variation between clinical and US findings, with US reporting residual/recurrent varicocele in 12/15 cases [81]. When US detects a residual “subclinical” varicocele following VR, it could lead to unjustified patient disappointment and anxiety, and may cause a management dilemma.
Post VR Doppler US has been recommended in the EAU guidelines when there is no improvement in semen parameters and a recurrence is suspected. However, as discussed above, it is not clear whether the US detected recurrence is of significance if the clinical examination is normal. A small-study [82] reported a 60% recurrence 5 years after VR when the men were examined with Doppler US, supporting the suggestion that Doppler US may over-estimate post VR recurrence. On the other hand, often there is thickening of the spermatic cord due to thrombosed veins after VR and a Doppler US study can confirm whether there is significant reflux in the thickened cord.
(4) Expert opinion
Routine scrotal Doppler US after VR is not recommended, though some surgeons may choose to do it to monitor the technical success of their VR procedure.
Repeat US may be considered if there is no improvement in semen parameters, or non-resolution of pain, and there are findings on physical examination suggestive of residual or recurrent varicocele.
2) Post-operative complications
(1) Survey results
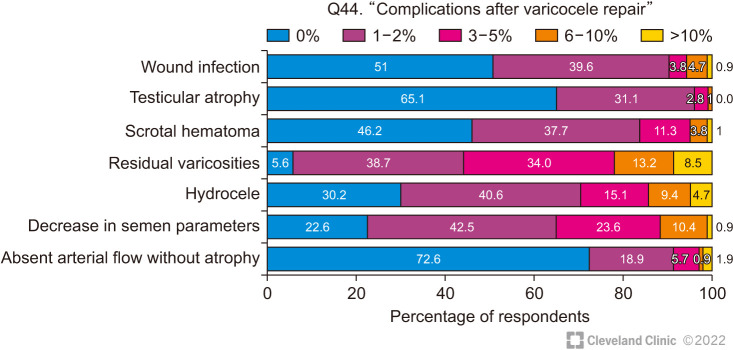
A number of complications of varicocele surgery were reported by the respondents but the overall incidence was less than 10% (Fig. 8).
Fig. 8. Frequency of complications after varicocele repair.
(2) Guidelines
The recent AUA/ASRM guidelines do not comment on post-VR complications, but the 2011 guidelines warn that laparoscopic VR does have some risk of major intraperitoneal complications. The EAU guidelines (10.4.3.4) state that microsurgical varicocelectomy is the most effective with the least complications.
(3) Discussion
The most common reported complications were hydrocele and residual varicocele, both of which are minimized by the use of magnification to preserve lymphatics while ligating all tributaries of the spermatic vein.
A fall in sperm count after treatment was also reported by a fair number of surgeons. This may be due to spontaneous variation in semen parameters or may be due to an undetected damage to the testicular artery which did not result in atrophy but compromised the testicular function. This would be avoided by the use of magnification and other intra-operative measures to preserve the testicular artery during surgery.
Spermatic vein embolization would be free of surgical complications and risk of damage to the testicular artery. However, the technique demands interventional radiologic expertise, is associated with a higher failure rate, and has potential serious complications including vascular perforation, coil migration leading to renal vein thrombosis, and thrombosis of pampiniform plexus [81].
(4) Expert opinion
Subinguinal microsurgical varicocelectomy is associated with the lowest complication rate and is the gold standard for VR. However, surgeons should adhere to the technique that they are comfortable with since a poorly done microsurgical procedure will do more harm than good. Nevertheless, to minimize the complications rate, obtaining formal microsurgical training is highly advisable for surgeons who do VR regularly.
3) Recurrence after varicocele repair
(1) Survey results
The majority of respondents reported that varicocele recurrence occurred within the first year (35.1%) or between the first and second year (41%).
The main factors listed as the cause of recurrence were surgeon’s experience (61%) and/or surgical approach (51.7%), indicating missed tributaries of the spermatic vein as the main cause. However, 29.8% listed cremasteric veins, and 23.2% named gubernacular veins as possible causes of recurrence.
Opinion on the management of recurrence was widely divided. While 14.6% recommended repeating VR for all recurrences, 17.1% said that they do not usually suggest treatment of a recurrent varicocele.
The remaining respondents made their decision based on the outcome of the first surgery but expressed contrasting viewpoints. While 27% would suggest a repeat procedure if there had been no improvement after the first procedure, 28.5% would suggest repeat procedure only if there had been significant improvement after the first procedure followed by a gradual decline, and an additional 12.7% agreed that they would not operate if there had been no benefit after the first surgery.
The choice of procedure also varied widely. For recurrence after a subinguinal microsurgical varicocelectomy, one-third (34.9%) of the surgeons opted for a repeat procedure at the subinguinal level while 28.4% opted to go higher (inguinal, 14.5%; Palomo, 8.7%; laparoscopic, 5.2%). Besides, 15% recommended venographic occlusion while 21.8% opted not to treat the recurrence.
(2) Guidelines
The EAU guidelines (10.4.3.4 – Table 41) state that inguinal/subinguinal microsurgical VR has the lowest recurrence rate compared to other surgical techniques, and that radiological occlusion procedures have significantly higher rates of recurrence. The guidelines (10.4.3.2) recommend Doppler US to look for recurrent varicocele if there is no improvement after VR but do not comment on the likelihood of benefit from VR for recurrent varicoceles.
(3) Discussion
There is a wide range in the incidence of recurrence reported based on surgical approach, technique, duration and method of follow-up [83]. The recurrence of a varicocele is disturbing to both the surgeon and the patient. An early recurrence is a residual varicocele that has enlarged again.
Missed tributaries of the spermatic vein are the primary cause of recurrence and therefore microsurgical VR has the lowest recurrence rate since small tributaries can be identified and ligated [84]. The role of the cremasteric and gubernacular veins is still debated. Franco et al [85] performed left iliac vein venographic studies of 73 men with primary or recurrent varicoceles and failed to demonstrate reflux from the extrafunicular veins into the pampiniform plexus. They observed that the cremasteric vein was always continent even when grossly dilated, and concluded that the cremasteric vein has a limited, if any, role in the pathogenesis of varicocele or its recurrence. However, the majority of survey respondents reported ligating the cremasteric veins (see section 5.2.1).
The diverse practices of the survey respondents would suggest that the benefit from re-operating a recurrent varicocele is unclear. However, a systematic review [83] of men undergoing VR of recurrent varicocele by surgery, retrograde embolization, or antegrade sclerotherapy reported high rates of success in treating the recurrence and highly significant improvement in semen parameters.
(4) Expert opinion
Care should be taken to ligate all tributaries of the spermatic vein to prevent a recurrence. When cremasteric veins are easily accessible (as in low approaches) they should also be ligated. Routine delivery of the testes to ligate gubernacular veins is not recommended (see section 5.2.3).
Surgery for recurrent varicocele may be advised but with a cautionary note about chances of improvement in semen quality. When the previous VR was by a high approach, then the repeat surgery should be at the subinguinal or inguinal level. When the primary surgery was sub-inguinal, then repeat surgery at a lower level can be done but would be significantly more difficult, and hence venographic occlusion, or an inguinal approach, could be considered.
7. Outcomes
1) Parameters of a successful outcome
(1) Survey results
In response to a question as to what the respondents considered the main parameter for determining whether the VR had a successful outcome, only a small percentage stated that increased pregnancy rates (16.3%) or an increase in live birth rate (9.8%) should be the primary outcome measure.
About 58.7% stated that a significant improvement in semen parameters, even if it did not reach normal reference values, would be considered a successful outcome, while 15.2% would consider the VR a success only if the semen parameters improved to normal.
The majority (73.9%) asked for the first post-operative semen report at 3 months, and 19% chose to wait till 6 months, while a few asked for it earlier or later than 3–6 months.
(2) Guidelines
Both AUA/ASRM and the EAU guidelines comment on VR outcomes in terms of both semen parameters and pregnancy rates. The EAU guidelines also state that VR reduces SDF and improves OS.
(3) Discussion
While Cochrane reviews and systematic reviews on the efficacy of VR emphasize spontaneous pregnancy rates and live birth rates [7] as the important outcome measures, the majority of clinicians responding to this survey considered VR successful if semen parameters increased significantly. This may reflect the fact that pregnancy depends on many factors, both male and female, and that the role of VR is to improve one of these factors. Also, VR may help to improve ART outcomes. The counter-argument is that if the live birth rate is not changed, then the improvement brought about by VR is not of significance.
(4) Expert opinion
Patients who undergo VR benefit in terms of improvement in semen parameters and sperm function. However, the impact of this on pregnancy and live birth rates may be limited as they depend on many other factors. Thus, although the improvement in semen parameters after VR may not result in a proportionate increase in live birth rates, improvement in the semen parameters is valid as a primary outcome measure of VR. When sufficient data is available from RCTs, then live birth rates can be considered as the primary outcome measure.
Also, improvement in semen parameters may allow couples to succeed with lower levels of treatment, such as ovulation induction cycles or IUI rather than IVF. Thus, improved semen parameters after VR will be a useful outcome for these patients.
2) Chances of benefit after varicocele repair
(1) Survey results
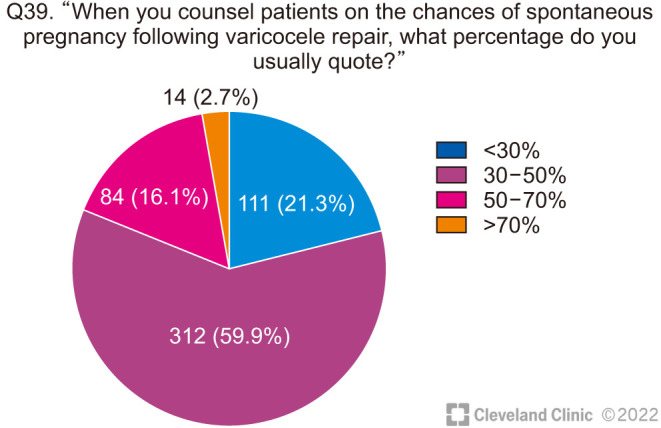
When asked about how they counsel their patients about the chances of benefit from VR, the majority of clinicians quoted moderately optimistic figures with 43.0% of respondents offering a 50% to 70% chance of significant improvement in semen parameters (Fig. 9), and 59.9% clinicians advising a 30% to 50% chance of spontaneous pregnancy (Fig. 10).
Fig. 9. Chances of clinically significant improvement in semen parameters after varicocele repair.
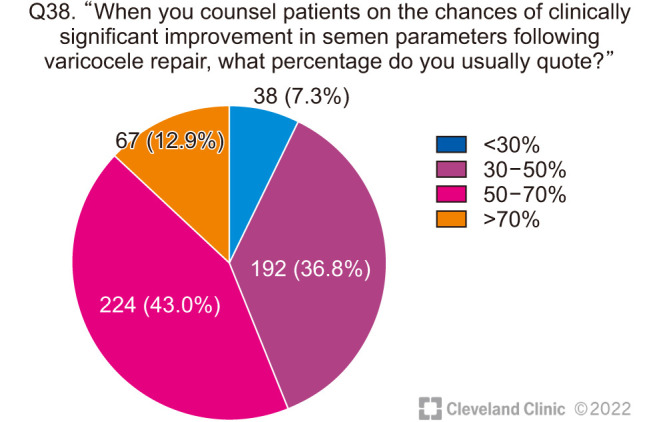
Fig. 10. Chances of spontaneous pregnancy after varicocele repair.
(2) Guidelines
The AUA/ASRM guidelines (statement 25) quote an estimated pregnancy rate of 52% (95% confidence interval [CI], 24%–83%) after subinguinal microsurgical VR. The EAU guidelines (10.4.3.3.2) quote two meta-analyses to suggest that VR improves chances of pregnancy with a combined OR of 2.39 (95% CI, 1.56–3.66)–4.15 (95% CI, 2.31–7.45), with time to improvement up to two spermatogenic cycles and time to spontaneous pregnancy of 6 to 12 months.
(3) Discussion
Several meta-analyses have been published evaluating the benefit of VR on semen parameters and pregnancy rates, and report an improvement in semen parameters and pregnancy rates that are in agreement with the chances that are counseled by the survey respondents [86,87].
(4) Expert recommendation
Patients can be counseled that there is a 50%–70% chance of significant improvement in their semen parameters after VR, and a 30% to 50% chance of a natural pregnancy. However, there is also a need to better define the expected outcomes of VR and provide a more precise, individualized prognosis based on clinical findings, semen parameters, hormonal levels, prognostic biomarkers, duration of infertility, and the couple’s age. A validated prognostic model would be helpful.
3) Time to maximum improvement after varicocele repair
(1) Survey results
There exists considerable variation in the expected time to maximum improvement. About 34% of clinicians opted for 3–6 months, 29.2% for 6 to 9 months, and 26.7% for 9 to 12 months. Lastly, 8.6% suggested waiting for more than 12 months.
(2) Guidelines
Both AUA/ASRM and EAU guidelines suggest that improvement should be expected within 1-2 spermatogenic cycles, that is within 3 to 6 months, and recommend semen testing every 3 months up to one year.
(3) Discussion
The expected time to maximum improvement is clinically important since lack of improvement by that time period would be an indication of failure of benefit from the procedure, and the need to proceed with ART. Waiting too long would result in unnecessary delay in the next step.
A few studies have examined the time to maximum improvement after VR. The majority of these studies suggest that improvement in semen parameters occurs by the first 3 months after varicocelectomy with no further significant improvement seen afterward [88,89,90]. Machen et al [91] stratified the improvement in sperm count by time after VR and reported that 78.8% had improvement at 3 months, 16.9% at 6 months, and 4.2% beyond 6 months. When specifically evaluating men with severe oligospermia pre-operatively, with TMSC of <5 million/mL, the largest improvement may be seen between 3–6 months post-operatively [92].
(4) Expert opinion
Most of the evidence suggests that if improvement occurs it will happen in the first 3 months after surgery which corresponds with the spermatogenesis cycle. However, men with severe OAT may take up to 6 months for maximal improvement. If improvement in semen parameters has not occurred by 12 months postoperatively, then it is not likely that there will be any improvement. On the other hand, examining the semen earlier than 3 months after VR may lead to an erroneous assessment of the outcomes.
8. Subclinical varicocele
1) Survey results
The survey reveals a contradiction in the clinicians’ approach to subclinical varicoceles. Earlier (section 2.1.1), most respondents have stated that they relied on physical examination to diagnose a varicocele. However, when asked how often they did US to look for a varicocele in a man with OAT, even when there was no clinical varicocele, 26.5% answered “always”, 23.9% answered “usually”, and 32.5% asked for it “occasionally”. Thus, only 17.1% abided by the guidelines recommendation that US is not indicated to look for a subclinical varicocele.
Along similar lines, when asked when they would repair a subclinical varicocele, only 44.7% replied “never”. The remaining majority of clinicians were willing to recommend VR, even for subclinical varicoceles, for a variety of clinical indications (Table 7).
Table 7. Varicocele repair for subclinical varicocele.
| Q 50:“When do you repair a bilateral subclinical varicocele?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Never | 230 | 44.7 |
| If OAT has not improved with medical therapy | 180 | 35.0 |
| In all infertile men with a subclinical varicocele | 106 | 20.6 |
| Chronic orchalgia | 80 | 15.6 |
| If semen parameters are normal but SDF is elevated | 73 | 14.2 |
| Total number of respondents | 514 | |
OAT: oligoasthenoteratozoospermia, SDF: sperm DNA fragmentation.
The frequency of performing VR in patients with subclinical varicocele tended to be higher among responders with up to 10 years of clinical experience as compared to those with more than 10 years of experience (212/267 [79.4%] vs. 214/307 [69.7%]; p=0.048). However, the difference in the proportion of responders with and without sub-specialty training in infertility that performed VR in patients with subclinical varicocele was not significant (220/297 [74.1%] vs. 206/277 [74.4%]).
On the other hand, the approach to the management of a grade I varicocele was different. When asked about the management of bilateral clinical grade I varicocele in a man with moderate OAT, half of the respondents stated that they would not recommend surgery (22.5%, never; 22.1%, rarely) and 20% would recommend surgery only occasionally (in 10%–25% cases). Only one-fourth of clinicians would routinely advise surgery in this situation (usually, 16.4%; always, 19%).
2) Guidelines
The AUA/ASRM (statement 26) and EAU (10.4.3.3.2) guidelines are unequivocal in recommending against the treatment of a subclinical varicocele.
3) Discussion
There appears to be considerable confusion and diversity of opinion and practice when it comes to subclinical and grade-I clinical varicoceles.
Thus, half of the clinicians do not believe that correcting a grade-I varicocele is of benefit and usually do not recommend its repair. However, paradoxically, when faced with the frustration of having nothing to offer a man with idiopathic OAT, the majority of clinicians succumb to “clutching at straws” and ask for an US even though there is no suspicion of varicocele on examination, and would offer VR if a varicocele is detected on US.
Though there are studies claiming benefit from repair of subclinical varicoceles [93,94] the consensus, as reflected in the AUA and EAU guidelines, is that subclinical varicoceles should not be repaired, and therefore US should not be performed to look for a varicocele when there is no clinical suspicion of a varicocele.
Correlating benefit from surgery with grade of varicocele, a recent meta-analysis of 20 studies involving 2001 patients, showed that while there was a statistically significant improvement in semen parameters after VR for all grades of varicocele, the improvement after surgery for grade-I varicoceles was small in magnitude, and clinically significant improvement was more likely when the varicocele was large (grade II-III or III) [48].
4) Expert opinion
Based on current evidence, VR for subclinical varicoceles is not recommended, and VR for grade I varicoceles may be considered after discussing the lower likelihood of significant benefit.
However, the practice patterns revealed by this survey suggest a need for a reassessment of the evidence on the utility, or otherwise, and of correcting subclinical and grade-I clinical varicoceles (since the boundary between the two is often blurred) so that clinicians are empowered to take an informed and firm stand on whether or not to treat these cases.
9. Varicocele and non-obstructive azoospermia
1) Survey results
Half of the respondents do not favour VR in men with NOA (never, 15.5%; in <10% of cases, 34.9%), and 16.3% recommend it only in 10%–25% of their azoospermic men. However, one-third of respondents believe that VR has a useful role in men with NOA with 7.5% recommending VR in 25%–50% of their patients, and 25.9% performing VR in >50% of their azoospermic men (Fig. 11) .
Fig. 11. Frequency of varicocele repair in a man with NOA. NOA: non-obstructive azoospermia.
However, there is some discrepancy in responses to a related question in which 58.6% of respondents said they would offer VR prior to microdissection testicular sperm extraction (microTESE) (Fig. 12). This is probably a reflection of the confusion that surrounds the role of VR in NOA.
Fig. 12. Management of patients with clinical varicocele and NOA. NOA: non-obstructive azoospermia, TESE: testicular sperm extraction, ICSI: intracytoplasmic sperm injection.
The respondents were equally divided on the role of a diagnostic testicular biopsy before VR in men with NOA to determine whether VR would be of benefit. For example, 16.9% recommend a prior biopsy in most of their cases, and 28.4% recommended it in selected cases. However, the other physicians recommended it only rarely (19.6%) or never (35.1%).
More than half the respondents perform a testicular biopsy at the time of VR in most, or selected patients, with the goal of cryopreserving sperm, establishing prognosis, and ruling out ITGCN (intratubular germ cell neoplasia) (Table 8).
Table 8. Testicular biopsy during varicocele repair for a man with non-obstructive azoospermia.
| Q 54: “Do you perform testicular biopsy at the time of varicocele repair for a man with non-obstructive azoospermia?” (you can choose multiple options) | ||
|---|---|---|
| Answer | No. of responses | Percentage of respondents |
| Never | 152 | 29.8 |
| Rarely | 70 | 13.7 |
| In select patients | 131 | 25.7 |
| In most cases | 108 | 21.2 |
| For cryopreservation if sperm are found | 104 | 20.4 |
| For prognosis | 46 | 9.0 |
| To rule out ITGCN | 32 | 6.3 |
| Total number of respondents | 510 | |
ITGCN: intratubular germ cell neoplasia.
2) Guidelines
AUA/ASRM guidelines (statement 27) states, “the couple should be informed of the absence of definitive evidence supporting VR prior to ART”.
The EAU (10.4.3.3.3) guidelines state that VR in men with NOA may result in the appearance of sperm in the ejaculate (20.8% to 55%) and is associated with improved surgical sperm retrieval rates (OR, 2.65; 95% CI, 1.69–4.14). However, it cautions that the evidence is based on observational studies and advises that “the risks and benefits of VR must be discussed fully with the patient with NOA and a clinically significant varicocele”.
3) Discussion
The controversy over the role of VR in men with NOA is reflected in the wide divergence in practices reported by the survey respondents.
Despite several meta-analyses [95,96,97] showing that a percentage of men with NOA ranging from 34% to 43.9% will have appearance of a few sperms in the ejaculate after correction of a clinical varicocele, the survey reveals a reluctance on part of the majority of respondents to consider VR in men with NOA. There could be several reasons for this. Firstly, in almost all cases the sperm count achieved is very low, and ICSI is still needed [98], and sometimes the appearance of sperm is only transitory [99,100]. Further, a major criticism of these studies is that none of them are controlled and the appearance of sperm in these men may be due to spontaneous variation and not be due to the VR (AUA/ASRM guideline statement 27). A recent study showed that 23.9% of men diagnosed as azoospermia were found to have rare sperm in the ejaculate when tested with additional centrifugation and staining with nuclear fast picroindigocarmine [101].
Men with NOA are a very heterogeneous group with varying testes size, hormone levels, grades of varicocele, and presence of other etiological factors; since few studies have addressed these variables prospectively the selection of NOA patients for VR remains a matter of personal belief and choice. Also, an increasing number of genetic mutations are being identified as causes of maturation arrest [102] and VR would be unproductive in these cases.
Some support for correcting a varicocele in this group comes from studies that have shown higher sperm retrieval during microdissection TESE if the varicocele has been corrected earlier [103,104] and a meta-analysis showed a strong trend towards improved live birth rates following VR (OR, 2.208; p=0.052) [44].
Studies have shown that testicular histopathology has a predictive value in NOA with men who have late maturation arrest or hypospermatogenesis being the most likely to have sperm in the ejaculate after VR [99], However, this survey shows that clinicians are divided in their opinion on doing a biopsy either prior to or during VR in these cases.
4) Expert opinion
There is limited and controversial evidence supporting VR in men with NOA. Hence, the decision to recommend or not recommend VR for NOA is left to the discretion of the clinician. When it is being offered (in men with large varicoceles, non-atrophied testes, no genetic or known cause for testicular failure) there should be a detailed discussion of the prognosis. If sperm appear in the ejaculate after VR, sperm cryopreservation should be considered since relapse of azoospermia has been reported [99].
At centers performing needle testis biopsy, one approach could be to take a couple of percutaneous biopsies before considering VR: one biopsy can be sent for histopathology and another to the IVF laboratory. If the histology is favorable (late arrest or hypospermatogenesis), then VR can be recommended since chances of benefit are higher; if sperm are found in the IVF laboratory then these can be cryopreserved and the couple can proceed for ICSI.
If VR is opted for without doing prior testicular biopsy, the biopsy may be done as an optional procedure at the time of VR to establish prognosis, rule out premalignant state, and to check for sperm. If sperm are found they can be cryopreserved and the couple may proceed for ICSI without delay.
There is a need for controlled studies that assess outcomes after VR in well-defined subgroups of men with NOA.
LIMITATIONS OF THIS STUDY
While this is the first global survey of this nature, it has several limitations. The survey was conducted in English and this may have restricted participation by clinicians from predominately non-English speaking regions and countries. The survey was distributed through a global group of experts experienced in varicocele management rather than to all male infertility specialists, which could have a created a bias in selection or exclusion of those answering the survey. There is a preponderance of responses from some countries (Turkey, Egypt), while there is marked under-representation from some large countries (Russia, China). Since the total number of invitations to participate in the survey is not known, we are unable to calculate the denominator to the response rate. However, 574 responses received from 59 countries provide a valid and comprehensive perspective of global practices. Though the questionnaire used is not a validated one, it was developed from 382 questions submitted by 60 uro-andrologists from 23 countries and thus is representative of global clinical concerns.
Despite these limitations, the authors feel that the survey findings address many of the important issues about the diagnosis, treatment, and expected outcomes of infertile men with varicoceles. It also raises important questions for future clinical trials to improve and standardize guidelines for the management of these patients.
CONCLUSIONS
The questionnaire used for this survey has been created from questions raised by a large, international group of clinicians, and thus reflects the real-life, practical concerns of physicians dealing with male infertility and varicocele.
The survey responses represent the opinions and practices of 574 clinicians from 59 countries and reveal a marked diversity in all aspects of varicocele management. The survey highlights several areas where there is inconclusive data and the need for more research, and also identifies numerous lacunae in the management guidelines issued by professional bodies (EAU, AUA, ASRM), which need to be addressed in future guidelines.
Besides, this survey serves the useful purpose of allowing clinicians to compare their practices with those of their peers, and against recommended guidelines and the latest research findings, and thus rethink some of their own practices and clinical protocols. This survey invites clinicians to ponder over what they know, what they do, where they go wrong, and what they should do.
Finally, professional societies can survey their membership using such questionnaires to assess the extent to which their guidelines are being followed and to identify areas of dispute or confusion where further clarifications are needed.
Acknowledgements
Authors are thankful to the artist Sarah Rolitsky, from the Cleveland Clinic’s Center for Medical Art & Photography for her help with the illustrations. We would like to acknowledge all the members of Global Andrology Forum: Abderrazak Bouzouita, Abdullah Alarbid, Ahmed El-Sakka, Ahmed M. Harraz, Ahmed Shokeir, Ahmet Asci, Ahmet Gudeloglu, Akira Tsujimura, Ala'a Farkouh, Aldo E. Calogero, Amarnath Rambhatla, Amin Bouker, Amr El Meliegy, Ana Puigvert, Andrea Crafa, Aram Adamyan, Arif Kalkanli, Armand Zini, Armen E. Avoyan, Ashok Agarwal, Ates Kadioglu, Ayad Palani, Aykut Baser, Azin Aghamajidi, Balantine Eze, Bambang Sasongko Noegroho, Basuki Purnomo, Berk Hazir, Bircan Kolbaşı Erkan, Birute Zilaitiene, Carlo Giuloni, Chak-Lam Cho, Christopher C.K. Ho, Ciro Salzano, Colin Teo, Damayanthi Durairajanayagam, Daniel Suslik Zylbersztejn, Davor Jezek, Deniz Kulaksiz, Dimitrios Kafetzis, Dong Sup Lee, Doron Stember, Dung Mai Ba Tien, Edmund Ko, Edoardo Pescatori, Edson Borges, Ege Can Serefoglu, Emine Saïs-Hamza, Emrullah Sogutdelen, Eric Chung, Eric Huyghe, Erman Ceyhan, Ettore Caroppo, Evangelini Evgeni, Fabrizio Castiglioni, Fahmi Bahar, Faisal Alhajeri, Fatih Gokalp, Federica Finocchi, Florence Boitrelle, Fotios Dimitriadis, Francesco Lombardo, Franco Gadda, Fulvio Colombo, Gede Wirya Kusuma Duarsa, George Tsangaris, Germar-Michael Pinggera, Gian Maria Busetto, Giancarlo Balercia, Gianmaria Salvio, Gianmartin Cito, Gideon Blecher, Giorgio Franco, Giorgio Ivan Russo, Giovanni Liguori, Giovanni M. Colpi, Gokhan Calik, Gökhan Çeker, Gustavo Marquesine Paul, Haitham Elbardisi, Hakan Keskin, Hamed Akhavizadegan, Haocheng Lin, Hassan N Sallam, Herik Acosta, Hisanori Taniguchi, Hussein Kandil, Hyun Jun Park, Imad Ziouziou, Ioannis Sokolakis, Israel Maldonado Rosas, Jackson Kirkman-Brown, Jae Il Shin, Jean de la Rosette, Jens Sonksen, Jie Dong, Jim Hotaling, Jo Ben Chua, Joe Lee, Joel Marmar, Jonathan Ramsay, Jose Moreno-Sepulveda, Ju Tae Seo, Juan Alvarez, Juan Manuel Corral Molina, Ka Lun Lo, Kaan Aydos, Kadir Bocu, Kareim Khalafalla, Kashif Siddiqi, Kasonde Bowa, Kavindra Kumar Kesari, Kay-Seong Ngoo, Keisuke Okada, Keshab Karna, Koichi Nagao, Koji Chiba, Konstantinos Makarounis, Landon Trost, Lawrence Jenkins, Lucia Rocco, Lukman Hakim, Mahsa Darbandi, Mara Simopoulou, Marah Hehemann, Marcelo Rodriguez Peña, Marco Alves, Marco Falcone, Marion Bendayan, Marjan Sabbaghian, Marlon Martinez, Marziyeh Tavalaee, Massimiliano Timpano, Mazdak Razi, Mesut Altan, Mesut Berkan Duran, Mikkel Fode, Miroslav Vučinić, Mitsuru Nago, Mohamad Moussa, Mohamed Arafa, Mohamed Elkhouly, Mohamed Khalili, Mohamed S. Al-Marhoon, Mohammad Ali Sadighi Gilani, Mohammad Ayodhia Soebadi, Mohammad Hossein Nasr-Esfahani, Mohan S Kamath, Muhammet Rasit Ugur, Murat Gül, Mustafa Emre Bakırcıoğlu, Nam Cheol Park, Natalio Cruz, Nazim Gherabi, Neel Parekh, Nguyen Ho Vinh Phuoc, Nicholas Tadros, Nicolas Garrido, Nikolaos Sofikitis, Niloofar Sodeifi, Noora Al Khalidi, Oğuzhan Kahraman, Ohad Shoshany, Osvaldo Rajmil, Paksi Satyagraha, Panagiotos Drakopoulos, Paraskevi Vogiatzi, Parisa Dolati, Partha Das, Parviz Kavoussi, Peter Ka-Fung Chiu, Petroula A. Tsioulou, Ponco Birowo, Premal Patel, Priyank Kothari, Puneet Sindhwani, Qaisar Javed, Quang Nguyen, Rafael F. Ambar, Raghavender Kosgi, Rajender Singh, Ralf Henkel, Ramadan Saleh, Ramy Abou Ghayda, Raneen Sawaid Kaiyal, Raphael Henrique Ferreira Santos, Ricky Adriansjah, Rima Dada, Rosita Angela Condorelli, Rossella Cannarella, Rupin Shah, Sakti Brodjonegoro, Saleem Ali Banihani, Samantha Schon, Sami AlSaid, Sandro La Vignera, Sara Darbandi, Sava Micic, Selcuk Sarikaya, Sezgin Gunes, Shannon Hee Kyung Kim, Sheena E Lewis, Sheryl Homa, Shingai Mutambirwa, Shinichiro Fukuhara, Shinnosuke Kuroda, Shubhadeep Roychoudhury, Sijo Parekattil, Siu King Mak, Sofia Ines Leonardi Diaz, SreelathaGopalakrishnan, Stephen Krawetz, Suks Minhas, Sun Tae Ahn, Sunil Jindal, Taha Abo-Almagd Abdel-Meguid, Tan V Le, Taymour Mostafa, Teng Aik Ong, Teppei Takeshima, Thorsten Diemer, Tiago Cesar Mierzwa, Tomer Avidor-Reiss, Toshiyasu Amano, Trenton Barrett, Tsung Yen Lin, Tuncay Toprak, Umut Arslan, Vijay Kumar, Vilvapathy Senguttuvan Karthikeyan, Vineet Malhotra, Wael Ibrahim, Walid Kerkeni, Widi Atmoko, Wongi Woo, Yasushi Yumura, Yiming Yuan, Yoshiharu Morimoto, Yuki Kato, Yukihiro Umemoto, Yu-Sheng Cheng.
The authors are thankful to the following societies and journals for promoting this online survey through the efforts of their members, as listed below:
1) Arab Association of Urology (Hussein Kandil, MD, UAE)
2) Asia Pacific Society of Sexual Medicine (Hyun Jun Park, MD, South Korea)
3) Association Francaise d’Urologie (Eric Huyghe, MD, France)
4) Brazilian Association of Assisted Reproduction (Edson Borges Jr, MD)
5) B razilian Society of Urology (Rafael F. Ambar, MD, Brazil)
6) E gyptian Society of Andrology (Mohamed Arafa, MD, Qatar)
7) E uropean Association of Urology (Fotios Dimitriadis, MD, Greece; Nikolaos Sofikitis, MD, PhD, Greece)
8) Indonesian Urological Association (Ponco Birowo, MD, PhD; Gede Wirya Kusuma Duarsa, MD, PhD; Widi Atmoko, MD)
9) Indonesian Society of Andrological Urology (Ricky Adriansjah, MD)
10) Iranian Urological Association (Mohammad Ali Sadighi Gilani, MD, Iran)
11) Middle East Society for Sexual Medicine (Amr El Meliegy, MD, FECSM, Egypt)
12) Sociedad Argentina de Andrologia (Marcelo Rodriguez Peña MD, PhD)
13) Société d'Andrologie de Langue Française (Florence Boitrelle, MD, France)
14) Société Internationale d’Urologie (Jean de la Rosette, MD PhD)
15) Spanish Association of Andrology, Sexual and Reproductive Medicine (Juan Manuel Corral Molina, MD, PhD, Spain; Juan Alvarez, MD, PhD, Spain)
16) Turkish Association of Urology (Ates Kadioglu, MD, Turkey)
17) Urological Society of Australia and New Zealand (Hyun Jun Park, MD, South Korea)
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This research was supported by the American Center for Reproductive Medicine, Cleveland Clinic (Project Number: IF100066).
- Conceptualization: Ashok Agarwal, Rupin Shah.
- Data curation: Shinnosuke Kuroda, Ahmed M. Harraz.
- Methodology, Project administration: Ashok Agarwal.
- Writing – original draft: Parviz Kavoussi, Amarnath Rambhatla, Ramadan Saleh, Rossella Cannarella, Florence Boitrelle, Shinnosuke Kuroda, Taha Abo-Almagd Abdel-Meguid Hamoda, Armand Zini, Edmund Ko, Gokhan Calik, Tuncay Toprak, Hussein Kandil, Murat Gül.
- Writing – review & editing: All authors.
Author’s Initials: *RS: Rupin Shah, PK: Parviz Kavoussi, AR: Amarnath Rambhatla, NP: Neel Parekh, EK: Edmund Ko, NT: Nicholas Tadros, MEB: Mustafa Emre Bakırcıoğlu, HK: Hussein Kandil, TM: Taymour Mostafa.
†GMC: Giovanni M. Colpi, AZ: Armand Zini, AK: Ates Kadioglu, MG: Murat Gül, AR: Amarnath Rambhatla, PK: Parviz Kavoussi, TH: Taha Abo-Almagd Abdel-Meguid Hamoda, EK: Edmund Ko, GR: Giorgio Ivan Russo, HK: Hussein Kandil, MA: Mohamed Arafa, TT: Tuncay Toprak, MEB: Mustafa Emre Bakırcıoğlu, OR: Osvaldo Rajmil, GC: Gokhan Calik, AH: Ahmed M. Harraz.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220048.
Online Survey on Varicocele Clinical Practice
The countries and regions and of survey participants
References
- 1.Su JS, Farber NJ, Vij SC. Pathophysiology and treatment options of varicocele: an overview. Andrologia. 2021;53:e13576. doi: 10.1111/and.13576. [DOI] [PubMed] [Google Scholar]
- 2.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsgaard J, Joensen UN, Carlsen E, Erenpreiss J, Blomberg Jensen M, Matulevicius V, et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: a study of 7035 healthy young men from six European countries. Eur Urol. 2016;70:1019–1029. doi: 10.1016/j.eururo.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–1293. [PubMed] [Google Scholar]
- 5.Baskaran S, Agarwal A, Leisegang K, Pushparaj PN, Panner Selvam MK, Henkel R. An in-depth bibliometric analysis and current perspective on male infertility research. World J Mens Health. 2021;39:302–314. doi: 10.5534/wjmh.180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Finelli R, Durairajanayagam D, Leisegang K, Henkel R, Salvio G, et al. Comprehensive analysis of global research on human varicocele: a scientometric approach. World J Mens Health. 2022 doi: 10.5534/wjmh.210202. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persad E, O'Loughlin CA, Kaur S, Wagner G, Matyas N, Hassler-Di Fratta MR, et al. Surgical or radiological treatment for varicoceles in subfertile men. Cochrane Database Syst Rev. 2021;4:CD000479. doi: 10.1002/14651858.CD000479.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80:333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–620. doi: 10.1016/j.eururo.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline. Linthicum (MD): American Urology Association; 2020. [Google Scholar]
- 11.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. J Urol. 2021;205:36–43. doi: 10.1097/JU.0000000000001521. [DOI] [PubMed] [Google Scholar]
- 12.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. J Urol. 2021;205:44–51. doi: 10.1097/JU.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 13.Freeman S, Bertolotto M, Richenberg J, Belfield J, Dogra V, Huang DY, et al. members of the ESUR-SPIWG WG. Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur Radiol. 2020;30:11–25. doi: 10.1007/s00330-019-06280-y. [DOI] [PubMed] [Google Scholar]
- 14.Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82–84. doi: 10.1097/00005392-199501000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Pilatz A, Altinkilic B, Kohler E, Marconi M, Weidner W. Color Doppler ultrasound imaging in varicoceles: is the venous diameter sufficient for predicting clinical and subclinical varicocele? World J Urol. 2011;29:645–650. doi: 10.1007/s00345-011-0701-4. [DOI] [PubMed] [Google Scholar]
- 16.Karami M, Mazdak H, Khanbabapour S, Adibi A, Nasr N. Determination of the best position and site for color Doppler ultrasonographic evaluation of the testicular vein to define the clinical grades of varicocele ultrasonographically. Adv Biomed Res. 2014;3:17. doi: 10.4103/2277-9175.124647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavallini G, Scroppo FI, Colpi GM. The clinical usefulness of a novel grading system for varicocoeles using duplex Doppler ultrasound examination based on postsurgical modifications of seminal parameters. Andrology. 2019;7:62–68. doi: 10.1111/andr.12556. [DOI] [PubMed] [Google Scholar]
- 18.An AUA Best Practice Policy, ASRM Practice Committee Report. Report on varicocele and infertility [Internet] Linthicum (MD): American Urological Association; 2012. [Cited 2022 May 27]. Available from: https://www.auanet.org/documents/education/clinical-guidance/Varicocele-Archive.pdf . [Google Scholar]
- 19.Pyrgidis N, Sokolakis I, Palapelas V, Tishukov M, Mykoniatis I, Symeonidis EN, et al. The effect of antioxidant supplementation on operated or non-operated varicocele-associated infertility: a systematic review and meta-analysis. Antioxidants (Basel) 2021;10:106. doi: 10.3390/antiox10071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giagulli VA, Carbone MD. Varicocele correction for infertility: which patients to treat? Int J Androl. 2011;34:236–241. doi: 10.1111/j.1365-2605.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- 21.Nieschlag E, Hertle L, Fischedick A, Abshagen K, Behre HM. Update on treatment of varicocele: counselling as effective as occlusion of the vena spermatica. Hum Reprod. 1998;13:2147–2150. doi: 10.1093/humrep/13.8.2147. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Yu Y, Liu Y, Wang L. Outcome of varicocelectomy on different degrees of total motile sperm count: a systematic review and meta-analysis. Syst Biol Reprod Med. 2019;65:430–436. doi: 10.1080/19396368.2019.1655813. [DOI] [PubMed] [Google Scholar]
- 23.Boman JM, Libman J, Zini A. Microsurgical varicocelectomy for isolated asthenospermia. J Urol. 2008;180:2129–2132. doi: 10.1016/j.juro.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Cakiroglu B, Sinanoglu O, Gozukucuk R. The effect of varicocelectomy on sperm parameters in subfertile men with clinical varicoceles who have asthenozoospermia or teratozoospermia with normal sperm density. ISRN Urol. 2013;2013:698351. doi: 10.1155/2013/698351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okeke L, Ikuerowo O, Chiekwe I, Etukakpan B, Shittu O, Olapade-Olaopa O. Is varicocelectomy indicated in subfertile men with clinical varicoceles who have asthenospermia or teratospermia and normal sperm density? Int J Urol. 2007;14:729–732. doi: 10.1111/j.1442-2042.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 26.Coetzee K, Kruge TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update. 1998;4:73–82. doi: 10.1093/humupd/4.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Danis RB, Samplaski MK. Sperm morphology: history, challenges, and impact on natural and assisted fertility. Curr Urol Rep. 2019;20:43. doi: 10.1007/s11934-019-0911-7. [DOI] [PubMed] [Google Scholar]
- 28.Kohn TP, Kohn JR, Ramasamy R. Effect of sperm morphology on pregnancy success via intrauterine insemination: a systematic review and meta-analysis. J Urol. 2018;199:812–822. doi: 10.1016/j.juro.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 29.Choe JH, Seo JT. Is varicocelectomy useful for subfertile men with isolated teratozoospermia? Urology. 2015;86:1123–1128. doi: 10.1016/j.urology.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Ilktac A, Hamidli S, Ersoz C, Dogan B, Akcay M. Efficacy of varicocelectomy in primary infertile patients with isolated teratozoospermia. A retrospective analysis. Andrologia. 2020;52:e13875. doi: 10.1111/and.13875. [DOI] [PubMed] [Google Scholar]
- 31.Cakan M, Bakirtas H, Aldemir M, Demirel F, Altug U. Results of varicocelectomy in patients with isolated teratozoospermia. Urol Int. 2008;80:172–176. doi: 10.1159/000112609. [DOI] [PubMed] [Google Scholar]
- 32.De Braekeleer M, Nguyen MH, Morel F, Perrin A. Genetic aspects of monomorphic teratozoospermia: a review. J Assist Reprod Genet. 2015;32:615–623. doi: 10.1007/s10815-015-0433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumont A, Barbotin AL, Lefebvre-Khalil V, Mitchell V, Rigot JM, Boitrelle F, et al. [Necrozoospermia: from etiologic diagnosis to therapeutic management] Gynecol Obstet Fertil Senol. 2017;45:238–248. doi: 10.1016/j.gofs.2017.01.010. French. [DOI] [PubMed] [Google Scholar]
- 34.Ribas-Maynou J, Yeste M, Becerra-Tomas N, Aston KI, James ER, Salas-Huetos A. Clinical implications of sperm DNA damage in IVF and ICSI: updated systematic review and meta-analysis. Biol Rev Camb Philos Soc. 2021;96:1284–1300. doi: 10.1111/brv.12700. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Cerrillo S, Gual-Frau J, Benet J, Abad C, Prats J, Amengual MJ, et al. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum Fertil (Camb) 2022;25:135–141. doi: 10.1080/14647273.2019.1711204. [DOI] [PubMed] [Google Scholar]
- 36.Lira Neto FT, Roque M, Esteves SC. Effect of varicocelectomy on sperm deoxyribonucleic acid fragmentation rates in infertile men with clinical varicocele: a systematic review and meta-analysis. Fertil Steril. 2021;116:696–712. doi: 10.1016/j.fertnstert.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Qiu D, Shi Q, Pan L. Efficacy of varicocelectomy for sperm DNA integrity improvement: a meta-analysis. Andrologia. 2021;53:e13885. doi: 10.1111/and.13885. [DOI] [PubMed] [Google Scholar]
- 38.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ., Jr Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–1436. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 39.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod. 2006;21:986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 40.Ammar O, Tekeya O, Hannachi I, Sallem A, Haouas Z, Mehdi M. Increased sperm DNA fragmentation in infertile men with varicocele: relationship with apoptosis, seminal oxidative stress, and spermatic parameters. Reprod Sci. 2021;28:909–919. doi: 10.1007/s43032-020-00311-6. [DOI] [PubMed] [Google Scholar]
- 41.Jeremias JT, Belardin LB, Okada FK, Antoniassi MP, Fraietta R, Bertolla RP, et al. Oxidative origin of sperm DNA fragmentation in the adult varicocele. Int Braz J Urol. 2021;47:275–283. doi: 10.1590/S1677-5538.IBJU.2019.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fathi A, Mohamed O, Mahmoud O, Alsagheer GA, Reyad AM, Abolyosr A, et al. The impact of varicocelectomy on sperm DNA fragmentation and pregnancy rate in subfertile men with normal semen parameters: a pilot study. Arab J Urol. 2021;19:186–190. doi: 10.1080/2090598X.2021.1889746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteves SC, Roque M, Agarwal A. Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl. 2016;18:254–258. doi: 10.4103/1008-682X.163269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016;106:1338–1343. doi: 10.1016/j.fertnstert.2016.07.1093. [DOI] [PubMed] [Google Scholar]
- 45.Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J. 2014;55:305–309. doi: 10.11622/smedj.2014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fırat F, Erdemir F. The effect of age on semen quality and spontaneous pregnancy rates in patients who treated with microsurgical inguinal varicocelectomy. Cureus. 2020;12:e7744. doi: 10.7759/cureus.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao W, Rosoff JS, Pale JR, Greenwood EA, Goldstein M. Older age is associated with similar improvements in semen parameters and testosterone after subinguinal microsurgical varicocelectomy. J Urol. 2011;185:620–625. doi: 10.1016/j.juro.2010.09.114. [DOI] [PubMed] [Google Scholar]
- 48.Asafu-Adjei D, Judge C, Deibert CM, Li G, Stember D, Stahl PJ. Systematic review of the impact of varicocele grade on response to surgical management. J Urol. 2020;203:48–56. doi: 10.1097/JU.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 49.Birowo P, Prasetyo DT, Pujianto DA, Atmoko W, Rasyid N, Sini IR. Effect of varicocele repair on sperm retrieval rate and testicular histopathological patterns in men with nonobstructive azoospermia. Asian J Androl. 2022;24:85–89. doi: 10.4103/aja.aja_29_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantoro U, Catanzariti F, Lacetera V, Quaresima L, Giovanni M, Polito M. Percentage change of FSH value: new variable to predict the seminal outcome after varicocelectomy. Andrologia. 2015;47:412–416. doi: 10.1111/and.12280. [DOI] [PubMed] [Google Scholar]
- 51.Zhou T, Zhang W, Chen Q, Li L, Cao H, Xu CL, et al. Effect of varicocelectomy on testis volume and semen parameters in adolescents: a meta-analysis. Asian J Androl. 2015;17:1012–1016. doi: 10.4103/1008-682X.148075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Culha M, Mutlu N, Acar O, Baykal M. Comparison of testicular volumes before and after varicocelectomy. Urol Int. 1998;60:220–223. doi: 10.1159/000030258. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto H, Saito K, Ogawa Y, Yoshida H. Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology. 2008;71:485–489. doi: 10.1016/j.urology.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 54.Almekaty K, Zahran MH, Zoeir A, Minhas S, Salem K. The role of artery-preserving varicocelectomy in subfertile men with severe oligozoospermia: a randomized controlled study. Andrology. 2019;7:193–198. doi: 10.1111/andr.12580. [DOI] [PubMed] [Google Scholar]
- 55.Majzoub A, ElBardisi H, Covarrubias S, Mak N, Agarwal A, Henkel R, et al. Effect of microsurgical varicocelectomy on fertility outcome and treatment plans of patients with severe oligozoospermia: an original report and meta-analysis. Andrologia. 2021;53:e14059. doi: 10.1111/and.14059. [DOI] [PubMed] [Google Scholar]
- 56.Enatsu N, Yamaguchi K, Chiba K, Miyake H, Fujisawa M. Clinical outcome of microsurgical varicocelectomy in infertile men with severe oligozoospermia. Urology. 2014;83:1071–1074. doi: 10.1016/j.urology.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Dada R, Kumar R, Shamsi MB, Sidhu T, Mitra A, Singh S, et al. Azoospermia factor deletions in varicocele cases with severe oligozoospermia. Indian J Med Sci. 2007;61:505–510. [PubMed] [Google Scholar]
- 58.Samplaski MK, Jarvi KA. Prognostic factors for a favorable outcome after varicocele repair in adolescents and adults. Asian J Androl. 2016;18:217–221. doi: 10.4103/1008-682X.169558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samplaski MK, Yu C, Kattan MW, Lo KC, Grober ED, Zini A, et al. Nomograms for predicting changes in semen parameters in infertile men after varicocele repair. Fertil Steril. 2014;102:68–74. doi: 10.1016/j.fertnstert.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 60.Kamal KM, Jarvi K, Zini A. Microsurgical varicocelectomy in the era of assisted reproductive technology: influence of initial semen quality on pregnancy rates. Fertil Steril. 2001;75:1013–1016. doi: 10.1016/s0015-0282(01)01698-3. [DOI] [PubMed] [Google Scholar]
- 61.Ding H, Tian J, Du W, Zhang L, Wang H, Wang Z. Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int. 2012;110:1536–1542. doi: 10.1111/j.1464-410X.2012.11093.x. [DOI] [PubMed] [Google Scholar]
- 62.Lundy SD, Sabanegh ES., Jr Varicocele management for infertility and pain: a systematic review. Arab J Urol. 2017;16:157–170. doi: 10.1016/j.aju.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cocuzza M, Pagani R, Coelho R, Srougi M, Hallak J. The systematic use of intraoperative vascular Doppler ultrasound during microsurgical subinguinal varicocelectomy improves precise identification and preservation of testicular blood supply. Fertil Steril. 2010;93:2396–2399. doi: 10.1016/j.fertnstert.2009.01.088. [DOI] [PubMed] [Google Scholar]
- 64.Mehta A, Goldstein M. Microsurgical varicocelectomy: a review. Asian J Androl. 2013;15:56–60. doi: 10.1038/aja.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Xia SJ, Liu ZH, Tao L, Ge JF, Xu CM, et al. Inguinal and subinguinal micro-varicocelectomy, the optimal surgical management of varicocele: a meta-analysis. Asian J Androl. 2015;17:74–80. doi: 10.4103/1008-682X.136443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colpi GM, Carmignani L, Nerva F, Piediferro G, Castiglioni F, Grugnetti C, et al. Surgical treatment of varicocele by a subinguinal approach combined with antegrade intraoperative sclerotherapy of venous vessels. BJU Int. 2006;97:142–145. doi: 10.1111/j.1464-410X.2006.05915.x. [DOI] [PubMed] [Google Scholar]
- 67.Cassidy D, Jarvi K, Grober E, Lo K. Varicocele surgery or embolization: which is better? Can Urol Assoc J. 2012;6:266–268. doi: 10.5489/cuaj.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sayfan J, Adam YG, Soffer Y. A new entity in varicocele subfertility: the "cremasteric reflux". Fertil Steril. 1980;33:88–90. doi: 10.1016/s0015-0282(16)44486-9. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148:1808–1811. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 70.Ramasamy R, Schlegel PN. Microsurgical inguinal varicocelectomy with and without testicular delivery. Urology. 2006;68:1323–1326. doi: 10.1016/j.urology.2006.08.1113. [DOI] [PubMed] [Google Scholar]
- 71.Hou Y, Zhang Y, Zhang Y, Huo W, Li H. Comparison between microsurgical subinguinal varicocelectomy with and without testicular delivery for infertile men: is testicular delivery an unnecessary procedure. Urol J. 2015;12:2261–2266. [PubMed] [Google Scholar]
- 72.Allameh F, Hasanzadeh Haddad A, Abedi A, Ranjbar A, Qashqai H, Fadavi B, et al. Varicocelectomy with primary gubernaculum veins closure: a randomised clinical trial. Andrologia. 2018 doi: 10.1111/and.12991. [Epub] [DOI] [PubMed] [Google Scholar]
- 73.Ou N, Zhu J, Zhang W, Liang Z, Hu R, Song Y, et al. Bilateral is superior to unilateral varicocelectomy in infertile men with bilateral varicocele: systematic review and meta-analysis. Andrologia. 2019;51:e13462. doi: 10.1111/and.13462. [DOI] [PubMed] [Google Scholar]
- 74.Niu Y, Wang D, Chen Y, Pokhrel G, Xu H, Wang T, et al. Comparison of clinical outcome of bilateral and unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a meta-analysis of randomised controlled trials. Andrologia. 2018;50:e13078. doi: 10.1111/and.13078. [DOI] [PubMed] [Google Scholar]
- 75.Sun XL, Wang JL, Peng YP, Gao QQ, Song T, Yu W, et al. Bilateral is superior to unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a prospective randomized controlled study. Int Urol Nephrol. 2018;50:205–210. doi: 10.1007/s11255-017-1749-x. [DOI] [PubMed] [Google Scholar]
- 76.Zheng YQ, Gao X, Li ZJ, Yu YL, Zhang ZG, Li W. Efficacy of bilateral and left varicocelectomy in infertile men with left clinical and right subclinical varicoceles: a comparative study. Urology. 2009;73:1236–1240. doi: 10.1016/j.urology.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 77.Chehval MJ, Purcell MH. Deterioration of semen parameters over time in men with untreated varicocele: evidence of progressive testicular damage. Fertil Steril. 1992;57:174–177. doi: 10.1016/s0015-0282(16)54796-7. [DOI] [PubMed] [Google Scholar]
- 78.Addar AM, Nazer A, Almardawi A, Al Hathal N, Kattan S. The yield of microscopic varicocelectomy in men with severe oligospermia. Urol Ann. 2021;13:268–271. doi: 10.4103/UA.UA_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan PT, Wright EJ, Goldstein M. Incidence and postoperative outcomes of accidental ligation of the testicular artery during microsurgical varicocelectomy. J Urol. 2005;173:482–484. doi: 10.1097/01.ju.0000148942.61914.2e. [DOI] [PubMed] [Google Scholar]
- 80.Barazani Y, Nagler HM. Other work has highlighted the limitations of using histopathology to predict success after varicocelectomy. Fertil Steril. 2011;95:487. doi: 10.1016/j.fertnstert.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 81.Lund L, Roebuck DJ, Lee KH, Sørensen HT, Yeung CK. Clinical assessment after varicocelectomy. Scand J Urol Nephrol. 2000;34:119–122. doi: 10.1080/003655900750016733. [DOI] [PubMed] [Google Scholar]
- 82.Akcar Değirmenci N, Turgut M, Ozkan R. [Recurrence rate after varicocelectomy in infertile men] Tani Girisim Radyol. 2004;10:144–146. Turkish. [PubMed] [Google Scholar]
- 83.Rotker K, Sigman M. Recurrent varicocele. Asian J Androl. 2016;18:229–233. doi: 10.4103/1008-682X.171578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: a meta-analysis to define the best technique. J Androl. 2009;30:33–40. doi: 10.2164/jandrol.108.005967. [DOI] [PubMed] [Google Scholar]
- 85.Franco G, Iori F, de Dominicis C, Dal Forno S, Mander A, Laurenti C. Challenging the role of cremasteric reflux in the pathogenesis of varicocele using a new venographic approach. J Urol. 1999;161:117–121. [PubMed] [Google Scholar]
- 86.Kim KH, Lee JY, Kang DH, Lee H, Seo JT, Cho KS. Impact of surgical varicocele repair on pregnancy rate in subfertile men with clinical varicocele and impaired semen quality: a meta-analysis of randomized clinical trials. Korean J Urol. 2013;54:703–709. doi: 10.4111/kju.2013.54.10.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 88.Fukuda T, Miyake H, Enatsu N, Matsushita K, Fujisawa M. Assessment of time-dependent changes in semen parameters in infertile men after microsurgical varicocelectomy. Urology. 2015;86:48–51. doi: 10.1016/j.urology.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Al Bakri A, Lo K, Grober E, Cassidy D, Cardoso JP, Jarvi K. Time for improvement in semen parameters after varicocelectomy. J Urol. 2012;187:227–231. doi: 10.1016/j.juro.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 90.Ghaed MA, Makian SA, Moradi A, Maghsoudi R, Gandomi-Mohammadabadi A. Best time to wait for the improvement of the sperm parameter after varicocelectomy: 3 or 6 months. Arch Ital Urol Androl. 2020;92 doi: 10.4081/aiua.2020.3.259. [DOI] [PubMed] [Google Scholar]
- 91.Machen GL, Johnson D, Nissen MA, Naber E, Sandlow JI. Time to improvement of semen parameters after microscopic varicocelectomy: when it occurs and its effects on fertility. Andrologia. 2020;52:e13500. doi: 10.1111/and.13500. [DOI] [PubMed] [Google Scholar]
- 92.Masterson TA, Greer AB, Ramasamy R. Time to improvement in semen parameters after microsurgical varicocelectomy in men with severe oligospermia. Can Urol Assoc J. 2019;13:E66–E69. doi: 10.5489/cuaj.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seo JT, Kim KT, Moon MH, Kim WT. The significance of microsurgical varicocelectomy in the treatment of subclinical varicocele. Fertil Steril. 2010;93:1907–1910. doi: 10.1016/j.fertnstert.2008.12.118. [DOI] [PubMed] [Google Scholar]
- 94.Cantoro U, Polito M, Muzzonigro G. Reassessing the role of subclinical varicocele in infertile men with impaired semen quality: a prospective study. Urology. 2015;85:826–830. doi: 10.1016/j.urology.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Elzanaty S. Varicocele repair in non-obstructive azoospermic men: diagnostic value of testicular biopsy - a meta-analysis. Scand J Urol. 2014;48:494–498. doi: 10.3109/21681805.2014.932839. [DOI] [PubMed] [Google Scholar]
- 96.Weedin JW, Khera M, Lipshultz LI. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010;183:2309–2315. doi: 10.1016/j.juro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl. 2016;18:246–253. doi: 10.4103/1008-682X.169562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berookhim BM, Schlegel PN. Azoospermia due to spermatogenic failure. Urol Clin North Am. 2014;41:97–113. doi: 10.1016/j.ucl.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Abdel-Meguid TA. Predictors of sperm recovery and azoospermia relapse in men with nonobstructive azoospermia after varicocele repair. J Urol. 2012;187:222–226. doi: 10.1016/j.juro.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 100.Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology. 2007;69:352–355. doi: 10.1016/j.urology.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 101.Sharma RK, Gupta S, Agarwal A, Finelli R, Kuroda S, Saleh R, et al. Role of cytocentrifugation combined with nuclear fast picroindigocarmine staining in detecting cryptozoospermia in men diagnosed with azoospermia. World J Mens Health. 2022 doi: 10.5534/wjmh.210210. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krausz C, Riera-Escamilla A, Moreno-Mendoza D, Holleman K, Cioppi F, Algaba F, et al. Genetic dissection of spermatogenic arrest through exome analysis: clinical implications for the management of azoospermic men. Genet Med. 2020;22:1956–1966. doi: 10.1038/s41436-020-0907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inci K, Hascicek M, Kara O, Dikmen AV, Gurgan T, Ergen A. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol. 2009;182:1500–1505. doi: 10.1016/j.juro.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 104.Haydardedeoglu B, Turunc T, Kilicdag EB, Gul U, Bagis T. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: a retrospective pilot study. Urology. 2010;75:83–86. doi: 10.1016/j.urology.2009.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Survey on Varicocele Clinical Practice
The countries and regions and of survey participants