Highlights
-
•
Vascular cognitive impairment patient shows sustained spatial deficits which are undetected using conventional neuropsychological assessments three-years on from diagnosis.
-
•
DTI suggests reduced FA to SLF parietal segments co-exists with spatial orientation impairment in VCI.
-
•
Egocentric and allocentric orientation hold clinical utility for detecting and monitoring VCI.
Keywords: Vascular cognitive impairment, Vascular dementia, Spatial navigation, Egocentric orientation, Vascular dementia case report
Abbreviations: SHQ, Sea Hero Quest; VST, Virtual Supermarket Test; COT, Clock Orientation Test; WMH, white matter hyperintensities
Abstract
Vascular cognitive impairment (VCI) is the second most prevalent form of dementia, but little is known about the early cognitive and neuroimaging markers. Spatial navigation deficits are an emerging marker for Alzheimer's disease (AD), yet less is known about spatial orientation deficits sensitive to VCI. This case report follows up on the first VCI patient identified to have an egocentric orientation deficit. The study aimed to examine the patient's spatial deficits three years on and gain insights from the addition of the patient's MRI brain scan. A battery of spatial navigation tasks were administered following neuropsychological assessment. Results continue to show spatial orientation deficits. Critically, these changes appear stable and are sensitive to novel spatial tests. Whereas conventional screening tools demonstrate patient recovery. MRI DTI analysis indicates a non-significant trend towards loss of structural integrity to the posterior tracts of the longitudinal superior fasciculus (SLF), while the medial temporal lobe, typically implicated in spatial navigation, is unaffected. This finding potentially reflects reduced network connectivity in posterior to anterior white matter tracts co-existing with spatial orientation deficits. Findings have clinical utility and show spatial orientation as a potential sensitive cognitive marker for VCI.
Abbreviations
- AD
Alzheimer's disease;
- VCI
vascular cognitive impairment;
- SLF
superior longitudinal fasciculus;
- MRI
magnetic resonance imaging;
- DTI
diffusion tensor imaging,
- FA
fractional anisotropy
1. Introduction
Vascular cognitive impairment (VCI) is thought to account for at least 20% of the clinical population affected by dementia [1], second in prevalence only to Alzheimer's disease [2]. Despite this, the cognitive features specific to VCI are still being explored. Multidomain impairments are apparent but executive dysfunction is most frequently associated with the condition [3]. This cognitive heterogeneity is likely due to the diverse nature of VCI, ranging from microinfarcts to white matter hyperintensities (WMH) [4], [5], [6]. Microinfarcts in particular can affect several cortical and subcortical regions [7], accounting for the cognitive heterogeneity in VCI patients. By contrast, WMH are more consistent in their location and show a propensity to the superior longitudinal fasciculus [8], [9], [10].
The Superior longitudinal fasciculus (SLF) is a major white matter pathway in the brain, connecting frontal and parietal brain regions. We have previously hypothesised that disruption of the SLF should not only result in subtle frontally-mediated executive change but also medial parietal-mediated, egocentric spatial orientation changes [11]. Changes to the way we navigate can be a key marker for neurodegeneration [12,13]. Fronto-parietal mediated egocentric orientation is concerned with the spatial relation between objects or landmarks and the self. It is focused on processing spatial information from a person-centred perspective. While our allocentric frame of reference involves the spatial relation between the position of objects and landmarks relative to each other and is focused on map-based representations. Both frames are informed by spatial information from multi-faceted person-to-object centred and environmental cues [14]. Both are required for everyday navigation with egocentric and allocentric processes shifting in response to navigational demands [15].
Indeed, a previous publication of ours shows that egocentric spatial navigation tests are highly sensitive to VCI and distinguish it reliably not only from healthy people but also Alzheimer's disease [16], though see [17]. These egocentric spatial orientation measures might provide a promising cognitive diagnostic and treatment outcome measure for WMH in VCI, specifically. However, at this stage it is not clear how those WMH changes co-exist with egocentric spatial orientation changes over time. This current study tries to address this outstanding question by testing a single VCI case over a three-year period. RK, the 67-year-old VCI patient first presented to our memory service in 2017. At that time RK presented with egocentric and heading direction deficits [18].
Here, we now present the follow-up assessment of RK, along with SLF neuroimaging, to determine if egocentric spatial orientation deficits exist in RK after a three-year period and how they relate to WMH to the SLF. This will inform how consistent spatial orientation changes are in VCI and explore how they relate to the underlying brain integrity. The study aims to inform future cognitive diagnostic and treatment outcome measures for VCI.
2. Method
2.1. Participant
We previously reported the case of RK, a now 67-year-old married man, with six years of secondary education (12 years of education total). RK received a diagnosis of vascular dementia (aka VCI) in March 2017 at our dementia research clinic. Diagnosis was classified by a consultant at the Norfolk and Suffolk Foundation Trust by interviewing the patient, patients medical history, examining neuropsychological assessment scores and clinical CT scan which met the diagnostic criteria for vascular dementia (VCI) [19]. His medical history included hypercholesterolaemia, stage 2 hypertension, an elevated BMI, life-long cigarette smoking, as well as a strong family history of hypercholesterolaemia and heart disease. RK and his care-giver report advancing memory problems and an increased need for daily-living assistance from time 1 (t1) to time 2 (t2), although he remains independent in leaving the house to walk to the local shop daily (see, Table 1 for changes in physical characteristics from time 1 to time 2). We have compared new case control participants as time 2 follow up data was unavailable from the original cohort. The new case controls (N = 14) were recruited from the local community and had a mean age of 67.79 (SD = 3.17), nine males and five females. Their cardiovascular health indicative of VCI pathology, was assessed using QRisk®3–2018 [20] to calculate their cardiovascular disease risk (heart attack or stroke in the next 10 years), all controls had a score of <10%. The controls underwent a battery of cognitive tests (see, Table 2) and demonstrated healthy cognitive function. The research was approved by the UK National Research Ethics Service (NRES: 16/LO/1366) and written informed consent was obtained prior to research activities.
Table 1.
Patient physical characteristics time 1 to time 2.
| Time 1 | Time 2 | Patient change | |
|---|---|---|---|
| Age | 65 | 67 | +2 |
| Height (cm) | 175 | 175 | 0 |
| Weight (kg) | 91 | 90 | −1 |
| BMI | 30 | 29 | −1 |
| BP SYS | 165 | 162 | −3 |
| BP DIA | 100 | 93 | −7 |
| Pulse | 55 | 64 | +9 |
| Medical Management | Simvastatin 40 mg, Bendroflumethiazide 2.5 mg, Clopidogrel 75 mg, Losartan 100mg | Simvastatin 40 mg, Bendroflumethiazide 2.5 mg, Bisoprolol 1.25 mg, Clopidogrel 75 mg, Losartan 100 mg, Levothyroxine 100 mg, Finasteride 5mg | +3 |
*For patient change + represents increase and - represents decrease.
Table 2.
Patient v Control's Neuropsychological Assessment t1 to t2.
|
Time 1 |
Time 2 |
||||
|---|---|---|---|---|---|
| General cognition | Patient Score | Control Score | Patient Score | Control Score | Patient Change |
| ACE total | 82 | 92 (4.7) | 94 | 95.45 (4.37) | +12 |
| ACE: Attention | 18 | 17 (1.9) | 17 | 17.54 (0.93) | −1 |
| ACE: Memory | 18 | 23 (2.7) | 24 | 24.45 (2.66) | +6 |
| ACE: Fluency | 4 | 12 (2.0) | 11 | 13.09 (1.45) | +7 |
| ACE: Language | 26 | 25 (0.9) | 26 | 25.64 (0.5) | |
| ACE: Visuospatial | 16 | 14 (1.0) | 16 | 14.73 (1.1) | |
| Visuospatial function | |||||
| VOSP: Dot Counting | 9 | 10 | +1 | ||
| VOSP: Position discrim | 20 | 20 | |||
| VOSP: Cube analysis | 10 | 10 | |||
| ROCF: Copy | 25 | 33.7 (1.6) | 29 | 33 (2.87) | +4 |
| ROCF: Recall | 9 | 19 (4.5) | 12 | 22.1 (5.6) | +3 |
| Episodic memory | |||||
| FCSRT: Free recall | 15 | 14 | −1 | ||
| FCSRT: Cued recall | 33 | 32 | −1 | ||
| FCSRT: Free delayed recall | 6 | 4 | −2 | ||
| FCSRT: Cued delayed recall | 10 | 10 | |||
| Executive function | |||||
| INECO total | 16.5 | 20 | +3.5 | ||
| Motor series | 3 | 3 | |||
| Interference sensitivity | 2 | 3 | +1 | ||
| Inhibitory control | 2 | 3 | +1 | ||
| Digit backwards | 2 | 2 | |||
| Verbal working memory | 1 | 2 | +1 | ||
| Spatial working memory | 1 | 1 | |||
| Proverbs | 0.5 | 0.5 | |||
| Hayling test | 5 | 5 | |||
| Working memory index | 3 | 3 | |||
| Trial Making Test | Part A | Part B | Part A | Part B | |
| Trial making test: Time | 79 | 117 | 66 | 92 | A: −13, B: −25 |
| Trial making test: Errors | 0 | 2 | 0 | 0 | A: 0, B: −2 |
*ACE, Addenbrooke's cognitive examination; VOSP, Visual Object and Space Perception battery; FCSRT, Free and Cued Selective Reminding Test; INECO frontal screen. Patients raw scores are reported and mean scores for controls.
2.2. Procedure
RK underwent initial cognitive assessment (including neuropsychology and spatial navigation) in March 2017. Findings from this assessment were previously published [18]. RK received an anatomical and diffusion weighted brain scan in August 2019 as part of a longitudinal follow up to identify the vascular abnormalities underlying RK's symptoms of reduced information processing and spatial disorientation and a follow-up physical, cognitive, and spatial orientation assessment was conducted in February 2020 to measure the change in symptoms over three years (from 2017 to 2020) (time 2 in Table. 2). The spatial orientation measures consisted of the Virtual Supermarket Test and the Clock Orientation Test, both of which were sensitive to RK's egocentric impairments at t1, and the addition of Sea Hero Quest was introduced at t2 as a further assessment of egocentric and allocentric spatial ability. The iPad tasks were completed with participants sitting approximately 30 cms from the screen which was situated flat on a desk in front of them. MRI scanning took place by radiologists at the Norfolk and Norwich University Hospital. Tests were administered in the same order for each participant and breaks were given as requested. Testing sessions took approximately 1 h to complete.
2.3. Tasks
2.3.1. Cognitive screening
RK underwent neuropsychological screening, including; Addenbrooke's cognitive examination (ACE-III) [21] (version A at t1 and version C at t2 to minimise test re-test effects), Rey–Osterrieth Complex Figure Test (RCFT) copy and with 3-min delayed recall [22], Cube Analysis, Dot Counting and Position Discrimination from the Visual Object and Space Perception Battery (VOSP) [23], Free and Cued Selective Reminding Test (FCSRT) [24], INECO Frontal Screen Test [25] and the Trail Making Test (TMT) part A and part B [26] (see, Table 2). Controls underwent neuropsychological screening using; ACE-III, RCFT and TMT.
2.3.2. The virtual supermarket test
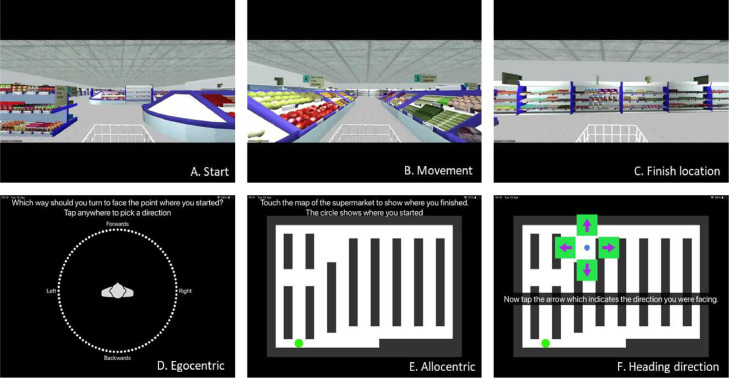
RK and controls underwent spatial testing using the Virtual Supermarket Test (VST). The VST has been developed by our group previously and used in symptomatic mild cognitive impairment (MCI), AD, frontotemporal dementia (FTD) and VCI patients [16,18,27,28]. The VR task is an ecological test of spatial navigation abilities designed to simulate navigating through a real-world supermarket. While at t1, a paper version of the supermarket map (allocentric measure) was used, an alternative form of this measure was employed 3 years later at re-test (t2), to facilitate electronic and automatic recording of participant responses. VST trials [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14] in both versions were identical (see, Fig. 1.A-C). At t1 a paper version of the supermarket was used to record responses (identical to that shown in Fig. 1D-F), which has now been automated. The task consists of 14 trials and takes approximately 15 min to complete. An iPad 9.7 (Apple Inc.,) was used to show participants 20–40 s video clips of a moving shopping trolley in the virtual supermarket. Before the trials begin players are required to demonstrate they can successfully follow the instructions and use the commands during three test runs before being permitted to play. The task avoids the use of landmarks or salient features within the environment and limits the demand on episodic memory, reflecting similar tasks in the literature [29], [30], [31] and taps into path integration processes via three core spatial processes: (i) egocentric self-reference navigation; (ii) allocentric map-based navigation and (iii) heading direction. Performance is calculated using the distance between this and the coordinates of the actual finishing location. This map-based component provides an assessment of geocentric encoding of the virtual environment.
Fig. 1.
Virtual Supermarket Test. Videos were presented in a first-person perspective and participants are provided with optic flow cues from the moving shopping trolley and changing scenery as it followed different routes to reach a different end point in each trial (1A-C). Once the video clip stops, participants indicate in real-life the direction of their starting point (egocentric orientation; 1D). In a second step, participants indicate their finishing location on a birds-eye view map of the supermarket (allocentric orientation; 1E). Lastly, participants are asked to indicate which direction they were facing at the finishing location (heading direction; 1F). Participants indicate their responses by taping their response location on the screen.
2.3.3. The clock orientation test
RK and controls underwent spatial testing using the Clock Orientation Test (COT). The COT has also been developed by our lab [16,18] as a bedside clinical test for egocentric orientation. It requires participants to imagine they are standing in the centre of a large clock, facing a particular number, e.g., the number 3. Participants are then asked, “which number is directly behind you?” (Answer: number 9). Next participants are asked to point, in real-life, to the positions of different numbers on the clock face in relation to the number that they are currently facing. For example, “You are facing number 12, can you point to the number 3?” (Answer: pointing right). The questions increase in complexity across the test and require medial parietal mediated mental imagery, rotation and egocentric processes, with no episodic memory demand. The test consists of 12 trials and takes 5–10 min to complete.
2.3.4. Sea hero quest
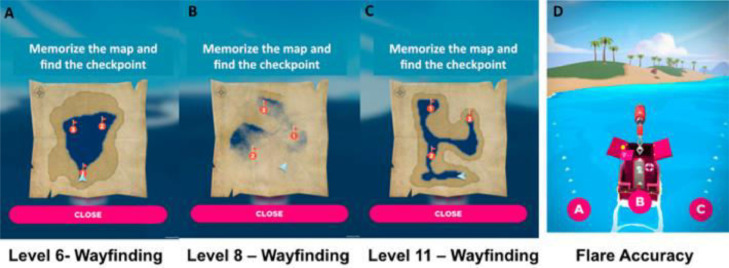
RK and controls underwent spatial testing using Sea hero Quest (SHQ). SHQ is an app- based cognitive task that enables the collection of spatial navigation data and is used in large scale population based studies [32] and has been shown to differentiate egocentric and allocentric frames of reference, exhibiting selective ‘wayfinding’ allocentric deficits in prodromal Alzheimer's disease [33]. SHQ goal-orientated wayfinding (allocentric) levels 6, 8 and 11 were selected due to their previous sensitivity to identifying pre-clinical AD [34]. Players initially see a map featuring a start location and several checkpoints to find in a set order. Participants study a map of the level for a recorded number of seconds. When participants exit the map view, they are asked to immediately find the checkpoints in the order indicated on the map under timed conditions. As participants navigate the boat, they must keep track of their location using self-motion and environmental landscape cues such as water– land separation. If the participant takes more than a set time, an arrow appears pointing in the direction along the Euclidean line to the goal to aid navigation. In flare accuracy (egocentric) levels (here, levels 14, 19, 24, 34 and 44), participants are not provided with an allocentric map. Instead, they immediately navigated along a river to find a flare gun. Once they find the flare gun at the end of the river, the boat rotates by 180°, and participants are asked to choose one of three possible directions (right, front, and left) that they believe points to the starting point. This level requires participants to (i) form an accurate representation of the starting point relative to their position and (ii) integrate this representation with a representation of the direction they are facing after the rotation. Depending on their performance, players receive one, two, or three stars (Fig. 2).
Fig. 2.
SHQ Wayfinding (allocentric) and Flare Accuracy (egocentric) levels.
2.4. MRI acquisition
MRI data was obtained from RK and controls using a 3 tesla Discovery 750 w widebore system (GE Healthcare, Milwaukee, WI, USA) with a 12-channel phased-array head coil for signal reception. After localizers, the T1-weighted (T1w) structural data was acquired using a whole-head 3D inversion-recovery fast spoiled gradient recalled echo sequence with the following parameters: repetition time ¼ 7.7 ms; echo time ¼ 3.1 ms; inversion time ¼ 400 ms; field-of view ¼ 256 mm; acquired matrix ¼ 256 256; 200 sagittal sections of 1 mm thickness; flip angle ¼ 11 ; and an ASSET acceleration factor of 2 in the phase-encoding direction. Furthermore, a 3D T2- weighted fluid attenuated inversion recovery (T2w FLAIR) sequence was prescribed as follows: repetition time ¼ 4800 ms; echo time ¼ 129 ms; inversion time ¼ 1462 ms; field-of-view ¼ 256 mm; acquired matrix ¼ 256 256; 182 sagittal sections of 1mmthickness; flip angle ¼ 90; an ARC acceleration factor of 2 in the phase encoding direction; and a “HyperSense” compressed sensing subsampling factor of 2.
DTI (diffusion-weighted single shot spin-echo planar imaging sequence, TR = 6.7 s,TE = XX ms, 59 axial slices, resolution = 0.937 × 0.937 × 2.499 mm, no cardiac gating, 61 images with diffusion weighting, b = 2000s/mm2, four images without diffusion-weighting, b = 0 s/mm2, subsequently referred to as b0 images) scans were acquired for all participants. MRIs were screened by a consultant neurologists and analysed using FSL (v6.0.0) and Freesurfer (v11.4.2) software.
2.5. White matter hyperintensities, infarcts and perivascular space
The Fazekas scale [35] was used to quantify the amount of white matter T2 hyperintense lesions in RK as it is the most widely used system for describing white matter disease severity in publications. The scale divides the white matter in periventricular and deep white matter, and each region is given a grade depending on the size and confluence of lesions (periventricular hyperintensities (0–3), White matter hyperintensities (0–3), PV (periventricular hyperintensities)= 0 (absence), 1 ("caps" or pencil-thin lining), 2 (smooth "halo"), or 3 (irregular PV extending into the deep white matter), and WM (white matter hyperintensities) = 0 (absence), 1 (punctate foci), 2 (beginning confluence of loci), or 3 (large confluent areas)). Infarct, and perivascular space were quantified by visual inspection using FSL on T2-weighted fluid-attenuated inversion recovery (FLAIR) and on T1-weighted (T1w) structural MRI. Topographical location of each lesion was reported using atlases in FSL eyes. The rater was blinded to all cognitive and spatial navigational results.
2.6. TRActs constrained by under lying anatomy (TRACULA)
Tractography were performed within Freesurfer (TRACULA version 1.56), DTI data was processed using the ENIGMA DTI pre-processing steps (http://enigma.ini.usc.edu/protocols/dti-protocols/). In particular, we used the first b0image as a reference for co-registration of subsequent b0 images (FSL FLIRT [36]). The resulting co-registered b0 images were averaged and served as a reference image during motion correction on the diffusion-weighted images. The gradient table information was adjusted accordingly [37]. Subsequently the data was processed in order to account for geometric distortions, this was performed on the mean b0 image via the T1-w scan. In order to achieve distortion correction, the T1-w scan was rigidly aligned with the mean b0 image [36] and the mean b0 image was nonlinearly registered to this T1-w scan in diffusion space using Advanced Normalization Tools (http://stnava.github.io/ANTs/). The resulting nonlinear registration information was used to unwarp subsequent diffusion-weighted images in native diffusion space. TRACULA's default tensor fitting and tract reconstruction pipelines using the ball-and-stick model were applied to the pre-processed data. The DTI data images were assessed visually. We visually appraised TRACULA's performance in terms of tract reconstruction for temporal and parietal divisions of the superior longitudinal fasciculus as per standard TRACULA segmentation (using global probabilistic tractography with anatomical prior information of predefined pathways). We investigated tract DTI-derived scalar metrics fractional anisotropy (FA) for the tract previously hypothesised to be affected in this patient. Regions of interest consisted of the SLF as well as medial temporal lobe and entorhinal cortex volume to discount Alzheimer's disease pathology.
2.7. Statistical analysis
RK's spatial orientation ability and dMRI mean FA (fractional anisotropy) of the superior longitudinal fasciculus were contrasted against the controls (N = 14) using a Crawford and Howell's (1998) modified paired sample t-test [38] resulting in a Z-case-control (Zcc) score as an interval estimate of the effect size. Two-tailed p values are reported.
3. Results
3.1. RK's neuropsychological assessment
Follow up neuropsychological assessment from time 1 to time 2 suggest a slight increase in scores on the ACE in domains of memory and fluency. Copy and Recall domains of the Rey Complex Figure have also improved, along with a slight lift in INECO scores of executive function in domains of inference sensitivity, inhibitory control and verbal working memory. All other neuropsychological assessment seems relatively stable from time 1 to time 2 (see Table 2).
3.2. Spatial navigation assessment
Scores indicate an uplift in RK's spatial processing but remains to show significant deficits in egocentric measures of VST, COT and SHQ as well as allocentric measures of the VST compared to controls (see, Table 3).
Table 3.
Total scores, standard deviations (SD), Z-case control (Zcc) scores, confidence intervals from a modified paired samples t-test for patient and control group on the Spatial Battery at Time 2.
| Time 1 |
Time 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Condition | Patient Score | Patient Score | Control mean (n = 14) | SD | t-value | p value | size effects (Z_cc) | 95% CI | Normal population falling below RK's score |
| Virtual Supermarket Test (VST) | Egocentric | 4 | 5* | 7.8 | 1.2 | 2.31 | 0.021 | −2.33 | −3.35:1.29 | 2.10% |
| Allocentric | 1.5 | 4* | 13.82 | 5.46 | −1.74 | 0.052 | −1.72 | 2.64: −0.92 | 5.29% | |
| Heading | 6 | 11 | 10 | 3.01 | 0.32 | 0.37 | 0.33 | 0.21: 0.86 | 62.30% | |
| The Clock Test | Cardinal | 1 | 4 | 3.77 | 0.599 | 0.377 | 0.41 | 0.39 | 0.16:0.93 | 64.37% |
| Right Angle | 1 | 1* | 3.09 | 0.76 | −2.66 | 0.01 | −2.75 | 3.9: −1.57 | 0.98% | |
| Mixed | 1 | 1* | 2.77 | 1.09 | −1.61 | 0.07 | 1.62 | −2.42:0.8 | 7% | |
| Total | 3 | 6* | 9.62 | 1.04 | −3.41 | 0.002 | −3.48 | 4.9:2.05 | 0.25% | |
| Sea Hero Quest | Flare Accuracy | – | 10* | 11.3 | 0.67 | 1.98 | 0.042 | −1.94 | 2.83:1.02 | 4.17% |
| Flare Duration | – | 41.1 | 43.15 | 12.89 | 0.15 | 0.44 | −0.16 | 0.63:0.37 | 44.10% | |
| Flare Distance | – | 361.7 | 374.92 | 112.7 | −0.21 | 0.42 | −0.22 | 0.73:0.32 | 41.85% | |
| Wayfinding Distance | – | 520.5 | 800.23 | 227.4 | −0.51 | 0.31 | −0.53 | −1.08:0.04 | 30.76% | |
| Wayfinding Duration | – | 430 | 91.34 | 43.65 | −0.78 | 0.23 | −0.8 | −1.4:−0.18 | 22.61% | |
Significant differences for RK compared to controls are marked in bold. Trend towards significance are marked in italics. P values represents a two-tailed probability that case score differs from controls.
3.3. MRI analysis
3.3.1. Visual rating of signal hyperintensities
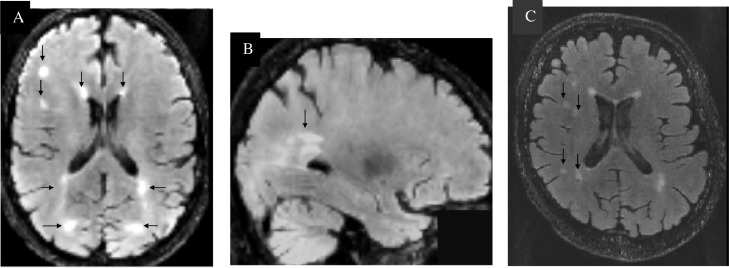
Periventricular hyperintensities and white matter hyperintensities were observed with up to five lesions present, positioning RK as Fazekas grade 2 (see Fig. 3). The Fazekas scale was used to assess severity of WMH [35]. Periventricular WMH and deep WMH were evaluated separately and totalled together as Fazekas scores. The degree of WMH severity was rated by Fazekas scores (mild: 0–2; moderate: 3–4; severe: 5–6).
Fig. 3.
Visual rating of RK's FLAIR imaging. A. White matter hyperintensities on FLAIR MRI (in MNI space) predominately in the right frontal lobe of the cerebral cortex and cerebral white matter. Periventricular white matter lesions are present near the left and right ventricles and close to the white matter colossal body and the lateral occipital cortex, superior division. B. While matter hyperintensity in the occipital lobe. C. Signs of punctuate deep white matter lesions beginning confluence.
3.3.2. White matter differences
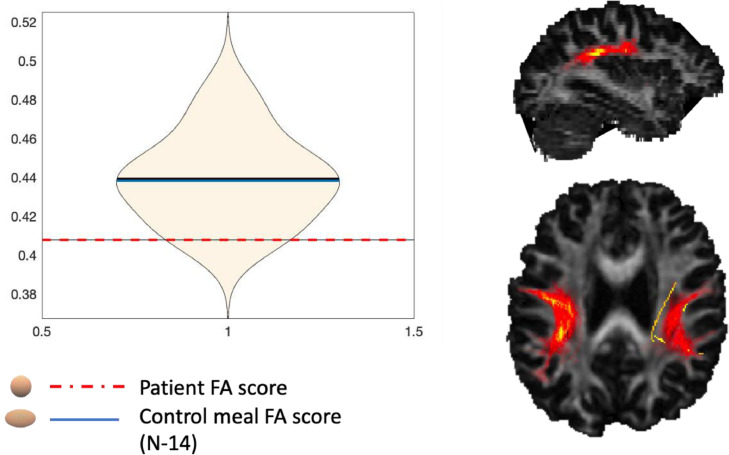
The SLF diffusion properties were assessed using mean fractional anisotropy (FA) and divided into four parameters right hemisphere superior longitudinal fasciculus parietal (rh SLFP); left hemisphere superior longitudinal fasciculus parietal (lh SLFP); right hemisphere superior longitudinal fasciculus temporal (rh SLFT); left hemisphere superior longitudinal fasciculus temporal (rh SLFT). Mean FA was reduced in all tracts of the SLF for RK compared to case controls, although none of which reach statistical significance (see, Fig. 4). FA was most reduced in the left hemisphere superior longitudinal fasciculus parietal (lh SLFP) for RK compared to the case controls, where this appeared to be approaching significance (p = 0.059) (see, Table 4). No changes were reported to medial temporal lobe and entorhinal cortex volumes (p > 0.05).
Fig. 4.
SLF parietal division RK and controls FA mean score.
Table 4.
SLF Tracts of Interest Patient vs Controls.
| rh SLFP | lh SLFP | rh SLFT | lh SLFT | Motion | |
|---|---|---|---|---|---|
| Patient RK | 0.41 | 0.41 | 0.44 | 0.45 | 0.28 |
| Controls | 0.44 (0.02) | 0.44 (0.03) | 0.45 (0.02) | 0.47 (0.02) | 0.34 (0.07) |
| Significance | 0.08 | 0.06 | 0.26 | 0.17 | 0.17 |
| Size effects (Z_cc) | −1.65 | −1.73 | −0.63 | −1.04 | −1.01 |
| Normal population falling below RK's score | 10.1%% | 5.90% | 26.04% | 17.80% | 17.26% |
*Standard deviation in parenthesis. Rh SLFP, right hemisphere superior longitudinal fasciculus parietal; lh SLFP, left hemisphere superior longitudinal fasciculus parietal; rh SLFT, right hemisphere superior longitudinal fasciculus temporal; lh SLFT, left hemisphere superior longitudinal fasciculus temporal.
4. Discussion
The current case report replicates our previous findings in showing egocentric spatial orientation deficits in VCI. More importantly, we demonstrate that spatial orientation deficits persist while other cognitive scores show no changes or even improvement over time. At the same time, spatial orientation deficits appear to co-exist with changes to the posterior SLF, making them highly specific to the WMH in VCI in that region. In particular, fractional anisotropy suggests potential reduced white matter integrity to the left and right SLF parietal sections may appear linked to spatial orientation deficits in VCI.
Results suggest RK's general cognition improved from t1 to t2 in measures of the ACE-III, VOSP, INECO and TMT. The FCSRT remained stable from t1 to t2 for RK. Interestingly, RK's scores would only indicate a clinical cut-off for dementia on the INECO, ROCF-recall and FCSRT (see, Appendix. B). Although, the uplift in scores may be due to test re-test effects which are a common problem in neuropsychological assessment [39]. It is important to consider changes in RK's medical management. Since t1, RK's hypothyroidism is being medically managed which may explain an uplift in his cognitive abilities as hypothyroidism has been associated with deficits to memory and executive function which is reversible with effective treatment [40].
Despite some uplift in results, spatial tests suggest RK remains to have an egocentric deficit when assessed by the Virtual Supermarket Test, the Clock Orientation Test and Sea Hero Quest compared to healthy controls. However, RK now shows an allocentric deficit at follow-up testing compared to controls in the Virtual Supermarket Test, despite showing improvements from t1. This stands in contrast to RK's general cognition which remained stable or improved at follow-up when assessed by standard clinical neuropsychological assessment in domains of attention, fluency, language, and visuospatial processing (see Table 2). Perhaps suggesting RK has consistently had both egocentric and allocentric deficits with instead inconsistencies occurring between the control groups. There are large standard variations in performance for the control groups at both t1 to t2 for allocentric ability on the VST, with the controls at t2 scoring worse on the spatial tasks than the controls at t1 (see, Appendix. A). This confound is difficult to explain, although we know as age increases spatial abilities become poorer [41,42]. The mean age at t1 was 63 years old (SD = 4.8), compared to the mean age of 67.79 years old (SD = 3.17) at t2. Suggesting the controls at t2 were better matched to RK's age and therefore may show greater integrity for comparison of RK's performance than scores at time one.
In sum, it appears RK's spatial impairments are apparent in both egocentric and allocentric spatial frames. This finding is consistent with recent evidence suggesting reduced spatial orientation performance in the in prodromal individuals predicts dementia onset in multiple dementia sub-types (VCI, AD, Lewy body dementia, frontotemporal dementia) [13]. Critically, in this study egocentric and allocentric changes were detected using novel spatial testing and were undetected when using standard neuropsychological assessments specifically targeting spatial processing when comparing RK's scores from t1 to t2 (see, Table 2). Perhaps due to the greater sensitivity than pen and paper testing, as well as strong test-retest reliability [43].
It is commonly recognised that egocentric and allocentric reference frames use separate neurocognitive pathways mediated by different neural structures [44], [45], [46], [47], but these frames can work in parallel with one another [48]. Egocentric frames can be used alone but there is evidence to suggest allocentric representations require the translation of sensory and imagery input from transient egocentric representations [49]. Rodent models of spatial processing suggest egocentric sensory information travels through parietal cortex in order to elicit ‘place cell’ firing in the hippocampus, which is key to the translation of map based allocentric representations [50], it is also suggested human allocentric map based hippocampal representations are driven from inputs from dorsal and ventral visual pathways overlapping posterior parietal regions [51]. As such, our results could be viewed in the context of this integrated model of allocentric representations, which may explain reduced allocentric performance due to egocentric posterior parietal damage in patient RK. In support of this, the demands of allocentric metric of the Virtual Supermarket Test require the participant to view a map of the supermarket and indicate their current position. As well as medial temporal map-based processing, this may also call on visual imagery and short-term spatial memory mediated by medial parietal structures [52,53] to reconstruct their ‘steps’ taken to arrive at the end location. Although, allocentric deficits were not observed when tested using SHQ, RK took considerably longer to complete the Wayfinding (allocentric) levels compared to controls. As such, the egocentric mechanisms affected in RK could be interfering with the formation of allocentric representations. Future studies in a larger sample of mild to moderate VCI patients are required to explore this potential explanation further.
MRI analysis using FLAIR imaging indicates RK has white matter hyperintensities to the right hemisphere frontal lobe of the cerebral cortex and cerebral white matter, as well as periventricular white matter lesions near the lateral ventricles, close to the colossal body and the superior division of the lateral occipital cortex. Although WMH are also common in healthy ageing [55], the MRI profile is consistent with typical subcortical ischaemic presentation of VCI [4]. However, further DTI analysis was used to assess white matter integrity in SLF and results suggest FA was reduced in all four SLF tracts for RK compared to controls. Although differences did not reach statistical significance, a trend towards significance was strongest for the left and right SLF parietal sections. This findings is not conclusive, but supports the notion that reduced integrity to the posterior tracts of the SLF may be a potential hallmark of VCI [11], and could manifest as this apparent egocentric orientation deficit.
Neuroimaging studies emphasise the role of medial and posterior parietal regions in computing egocentric spatial representations [49,56] and research examining the application of virtual reality assessing early neurodegeneration suggest reduced right hemisphere precuneus volumes are associated with poorer egocentric performance [57]. These findings are paralleled by our present results and may suggest posterior regions of the SLF connecting the parietal-to-frontal structures may be implicated in this apparent egocentric decline in spatial orientation observed in RK and our previous VCI patient group study [16].
This case report comes as further evidence to support the use of spatial navigation testing in clinical settings and serves as a sensitive diagnostic tool in the assessment of VCI. The continued sensitivity of The Virtual Supermarket test and the Clock Orientation Test to RK's condition, in light of improved scores on conventional cognitive assessments (ACE-III, VOSP, INECO, TMT), suggest these simple and quick to administer spatial tasks would also be a favourable sensitive cognitive measure for use in VCI intervention studies.
Despite these exciting findings, our study has limitations, RK's MRI scan took place three years after his initial diagnosis. Therefore RK's brain imaging should be interpreted with caution when associating findings with his initial (t1) test scores. Although, reduced integrity to the posterior SLF is of interest concerning his egocentric spatial deficits, FLAIR MRI indicates multiple sites of white matter hyperintensities to suggest axonal loss more typical of a subcortical ischaemic VCI [4]. Type one errors are also identified when using small control groups and Crawford and Howell's (1998) modified paired sample t-test [58]. In addition, pre-existing healthy WMH load has also been shown to exhibit as reduced FA in the SLF [59]. As such, we cannot be clear on the order of the pathogenesis of these injuries, thus, a link between egocentric deficits in VCI and posterior SLF integrity is not conclusive.
Finally, although the utility of app-based spatial navigation tools such as the Virtual Supermarket Test and Sea Hero Quest to detect VCI pathology is clear, the task demands are multidimensional requiring a host of cognitive abilities (visuomotor, executive, working memory, language comprehension) that may be challenging in more advanced disease stages. RK's results should also be discussed in the context of dysexecutive syndrome [54], whereby fronto-parietal function is impaired but medial temporal function remains intact. Results from INECO and FCSRT free recall tests suggest frontal executive problems and fronto-parietal functions including visual imagery, planning and spatial working memory are critical to spatial navigation. Therefore, future studies should consider the syndrome in the context of VCI.
Critically, these injuries observed in RK's MRI could be sensitive to novel spatial tests but go undetected on visuospatial measures of neuropsychological assessment. Future studies are needed to explore potential parietal-to-frontal network disruption in the early stages of VCI to inform the pathogenesis of spatial disruptions.
4.1. Conclusions
This case study illustrates the potential for the application of spatial orientation assessment in clinical settings. This is especially critical for disease monitoring, given that standard neuropsychological assessment has poor selectivity in discriminating dementia sub-types and lacks sensitivity for disease monitoring overtime. In sum, the Virtual Supermarket Test, the Clock Orientation Test and Sea Hero Quest appear to be sensitive to the detection of vascular cognitive impairment.
Funding
This work was supported by the University of East Anglia.
Declaration of Competing Interest
All of the contributing authors have no competing interests.
Ackowledgments
We would like to thank RK and his family for their participation in this study.
Contributor Information
Ellen Lowry, Email: e.lowry@uea.ac.uk.
Michael Hornberger, Email: m.hornberger@uea.ac.uk.
APPENDICES
Supplementary information
Appendix A. Total scores, standard deviations (SD), Z-case control (Zcc) scores, confidence intervals from a modified paired samples t-test for patient and control group on the Spatial Battery at Time 1.
| Measure | Condition | Patient Score | Control sample mean(n = 14) | SD | t-value | p value | size effects(Z_cc) | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
| Virtual Supermarket Test (VST) | Egocentric | 4* | 12.9 | 0.9 | −9.529 | <0.001 | −9.889 | −13.83 to −5.95 | |
| Allocentric | 1.5 | 8.1 | 3.2 | −0.201 | NS | −0.206 | −3.03 to −1.07 | ||
| Heading | 6* | 12.5 | 2.1 | −2.983 | <0.01 | −3.095 | −4.42 to −1.75 | ||
| The Clock Test | Cardinal | 1* | 3.9 | 0.9 | −3.105 | <0.01 | −3.222 | −4.59 to −1.83 | |
| Right Angel | 1* | 3.6 | 0.6 | −4.176 | <0.001 | −4.333 | −6.12 to −2.53 | ||
| Mixed | 1 | 3.9 | 1.7 | −1.644 | NS | −1.706 | −2.56 to −0.83 | ||
| Total | 3* | 11 | 2.6 | −2.965 | 0.01 | −3.077 | −4.4 to −1.74 | ||
| Statue Test | Wall easy | 4 | 4 | 0 | 0 | NS | −3.077 | −0.54 to 0.54 | |
| Wall medium | 1* | 2.6 | 0.5 | −3.085 | 0.01 | −3.16 | −4.51 to 1.79 | ||
| Wall hard | 0 | 0.3 | 0.6 | 0 | NS | 0 | −1.08 to 0.09 | ||
| Stool easy | 0 | 3.7 | 0.4 | −0.723 | NS | −0.75 | 0.12 to 1.36 | ||
| Stool medium | 4* | 2.2 | 0.8 | −0.482 | 0.02 | −2.687 | −3.87 to −1.48 | ||
| Stool hard | 2 | 0.2 | 0.6 | −0.482 | NS | 0.5 | −1.07 to 0.09 | ||
Appendix B. RK's neuropsychological assessment scores alongside normative and clinical cut-off scores. As per reviewer's request.
| Test | RK's Score | Normative sample mean score | Reference |
|---|---|---|---|
| TMT A | 66 | 39.14 (11.84) | Tombaugh, T. N. (2004). Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology, 19(2), 203–214. |
| TMT B | 92 | 91.32 (28.89) | |
| VOSP: dot counting | 9 | 9.9 (0.2) | Elizabeth K.. Warrington, & James, M. (1991). VOSP: the visual object and space perception battery. Pearson.. |
| VOSP: position discrim | 20 | 19.6 (0.9) | |
| VOSP: cube analysis | 10 | 19.2 (1.2) | |
| Clinical mean score | |||
| ACE-III | 82 | <82 cut off for mild dementia | Beishon, L. C., Batterham, A. P., Quinn, T. J., Nelson, C. P., Panerai, R. B., Robinson, T., & Haunton, V. J. (2019). Addenbrooke's Cognitive Examination III (ACE‐III) and mini‐ACE for the detection of dementia and mild cognitive impairment. Cochrane Database of Systematic Reviews, (12). |
| FCSRT: Free recall | 15 | 22.1 (4) VaD mean score | Grober, E., Sanders, A. E., Hall, C., & Lipton, R. B. (2010). Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer disease and associated disorders, 24(3), 284. |
| FCSRT: Cued recall | 33 | 45.8 (4) VaD mean score | |
| INECO total | 20 | <25 cut off for dementia | Sierra Sanjurjo, N., Saraniti, A. B., Gleichgerrcht, E., Roca, M., Manes, F., & Torralva, T. (2019). The IFS (INECO Frontal Screening) and level of education: Normative data. Applied Neuropsychology: Adult, 26(4), 331–339. |
| ROCF: Copy | 29 | >99 percentile | Knight, J. A. (Ed.). (2003). The handbook of Rey-Osterrieth Complex Figure usage: Clinical and research applications. Psychological Assessment Resources, Incorporated. |
| ROCF: Recall | 12 | 27th percentile |
References
- 1.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. https://www.ncbi.nlm.nih.gov/pubmed/21778438 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B., et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. https://www.karger.com/Article/FullText/109998 [Internet]Nov [cited 2021 Dec 9]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev P.S., Kalaria R.N., O'Brien J., Skoog I., Alladi S., Black S.E., et al. Diagnostic criteria for vascular cognitive disorders: a VASCOGstatement. Alzheimer Dis. Assoc. Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dichgans M., Leys D. Vascular cognitive impairment. Circ. Res. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 5.Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. https://www.ncbi.nlm.nih.gov/pubmed/24267647 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman G.C., Erkinjuntti T., Wallin A., Pantoni L., Chui H.C. Subcortical ischemic vascular dementia. Lancet Neurol. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Xia Y., Wang | Xiaoxiao, Wang Y., Zhang D., Benedictor |, et al. White matter hyperintensities induce distal deficits in the connected fibers The effect of WMH on the microstructural integrity of WM. 2021; Available from: https://afni.nimh. [DOI] [PMC free article] [PubMed]
- 9.Chen H.F., Huang L.L., Li H.Y., Qian Y., Yang D., Qing Z., et al. Microstructural disruption of the right inferior fronto-occipital and inferior longitudinal fasciculus contributes to WMH-related cognitive impairment. 2019. [DOI] [PMC free article] [PubMed]
- 10.Veldsman M., Werden E., Egorova N., Khlif M.S., Brodtmann A. Microstructural degeneration and cerebrovascular risk burden underlying executive dysfunction after stroke. Sci. Rep. 2020;10:17911. doi: 10.1038/s41598-020-75074-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry E., Puthusseryppady V., Johnen A.-.K., Renoult L., Hornberger M. Cognitive and neuroimaging markers for preclinical vascular cognitive impairment. Cereb. Circ. Cogn. Behav. 2021;2(October) doi: 10.1016/j.cccb.2021.100029. [Internet]Jan 1 [cited 2021 Oct 13]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coughlan G., Laczo J., Hort J., Minihane A.M., Hornberger M. Spatial navigation deficits - overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018;14(8):496–506. doi: 10.1038/s41582-018-0031-x. https://www.ncbi.nlm.nih.gov/pubmed/29980763 [Internet]Available from: [DOI] [PubMed] [Google Scholar]
- 13.Tangen G.G., Nilsson M.H., Stomrud E., Palmqvist S., Hansson O. Spatial Navigation and Its Association With Biomarkers and Future Dementia in Memory Clinic Patients Without Dementia. Neurology. 2022 doi: 10.1212/WNL.0000000000201106. 0:10.1212/WNL.0000000000201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo D., Serino S., Tuena C., Pedroli E., Dakanalis A., Cipresso P., et al. Egocentric and allocentric spatial reference frames in aging: a systematic review. Neurosci Biobehav Rev. 2017;80(June):605–621. doi: 10.1016/j.neubiorev.2017.07.012. [Internet]Available from: [DOI] [PubMed] [Google Scholar]
- 15.McNaughton B.L., Battaglia F.P., Jensen O., Moser E.I., Moser M.B. Path integration and the neural basis of the “cognitive map. Nat. Rev. Neurosci. 2006;7(8):663–678. doi: 10.1038/nrn1932. https://www.ncbi.nlm.nih.gov/pubmed/16858394 [Internet]Available from: [DOI] [PubMed] [Google Scholar]
- 16.Lowry E., Puthusseryppady V., Coughlan G., Jeffs S., Hornberger M. Path integration changes as a cognitive marker for vascular cognitive impairment?—A pilot study. Front. Hum. Neurosci. 2020;14(April):1–9. doi: 10.3389/fnhum.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moussavi Z., Kimura K., Lithgow B. Egocentric spatial orientation differences between Alzheimer's disease at early stages and mild cognitive impairment: a diagnostic aid. Med. Biol. Eng. Comput. 2022 Feb 1;60(2):501–509. doi: 10.1007/s11517-021-02478-9. https://link.springer.com/article/10.1007/s11517-021-02478-9 [Internet][cited 2022 Apr 20]Available from: [DOI] [PubMed] [Google Scholar]
- 18.Coughlan G., Flanagan E., Jeffs S., Bertoux M., Spiers H., Mioshi E., et al. Diagnostic relevance of spatial orientation for vascular dementia: a case study. Dement. Neuropsychol. 2018;12(1):85–91. doi: 10.1590/1980-57642018dn12-010013. https://www.ncbi.nlm.nih.gov/pubmed/29682239 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachdev P., Kalaria R., O'Brien J., Skoog I., Alladi S., Black S.E., et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. https://www.ncbi.nlm.nih.gov/pubmed/24632990 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hippisley-Cox J., Coupland C., Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357(May):1–21. doi: 10.1136/bmj.j2099. [Internet]May 23 [cited 2022 Mar 10]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh S., Schubert S., Hoon C., Mioshi E., Hodges J.R. Validation of the Addenbrooke's cognitive examination III in frontotemporal dementia and Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2013;36(3–4):242–250. doi: 10.1159/000351671. [DOI] [PubMed] [Google Scholar]
- 22.Lezek N. 2nd. Oxford University Press; New York: 1983. Neuropsychological Assessment. [Google Scholar]
- 23.Warrington E.K., James M. Thames Valley Company; Bury St Edmunds, UK: 1991. The Visual Object and Space Battery Perception. [Google Scholar]
- 24.Buschke H. Cued recall in amnesia. J Clin Exp Neuropsychol. 1984;6(4):433–440. doi: 10.1080/01688638408401233. Nov 1. [DOI] [PubMed] [Google Scholar]
- 25.Torralva T., Roca M., Gleichgerrcht E., López P., Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc. 2009;15(5):777–786. doi: 10.1017/S1355617709990415. [DOI] [PubMed] [Google Scholar]
- 26.Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- 27.Tu S., Wong S., Hodges J.R., Irish M., Piguet O., Hornberger M. Lost in spatial translation - A novel tool to objectively assess spatial disorientation in Alzheimer's disease and frontotemporal dementia. Cortex. 2015;67:83–94. doi: 10.1016/j.cortex.2015.03.016. [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- 28.Tu S., Spiers H.J., Hodges J.R., Piguet O., Hornberger M. Egocentric versus allocentric spatial memory in behavioral variant frontotemporal dementia and Alzheimer's disease. J. Alzheimer's Dis. 2017;59(3):883–892. doi: 10.3233/JAD-160592. https://www.ncbi.nlm.nih.gov/pubmed/28697554 [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- 29.Cushman L.A., Stein K., Duffy C.J. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. 2008. [DOI] [PMC free article] [PubMed]
- 30.Wolbers T., Wiener J.M., Mallot A., Buchel C. Differential recruitment of the hippocampus, medial prefrontal cortex and the human motion complex during path integration in humans. J. Neurosci. 2007;27(35):9408–9416. doi: 10.1523/JNEUROSCI.2146-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morganti F., Stefanini S., Riva G. From allo- to egocentric spatial ability in early Alzheimer's disease: a study with virtual reality spatial tasks. Cogn. Neurosci. 2013;4(3–4):171–180. doi: 10.1080/17588928.2013.854762. https://www.ncbi.nlm.nih.gov/pubmed/24251605 [Internet]Dec [cited 2022 Apr 6]Available from. [DOI] [PubMed] [Google Scholar]
- 32.Coutrot A., Silva R., Manley E., de Cothi W., Sami S., Bohbot V.D., et al. Global determinants of navigation ability. Curr. Biol. 2018;28(17):2861–2866. doi: 10.1016/j.cub.2018.06.009. Sep 10e4. [DOI] [PubMed] [Google Scholar]
- 33.Coughlan G., Coutrot A., Khondoker M., Minihane A.M., Spiers H., Hornberger M. Toward personalized cognitive diagnostics of at-genetic-risk Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2019;116(19):9285–9292. doi: 10.1073/pnas.1901600116. May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlan G., Coutrot A., Khondoker M., Minihane A.M., Spiers H., Hornberger M. Toward personalized cognitive diagnostics of at-genetic-risk Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2019;116(19):9285–9292. doi: 10.1073/pnas.1901600116. https://www.ncbi.nlm.nih.gov/pubmed/31015296 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazekas F., Chawluk J.B., Alavi A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am. J. Neuroradiol. 1987;8(3):421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 36.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. https://onlinelibrary.wiley.com/doi/full/10.1002/mrm.21890 [Internet]Jun 1 [cited 2021 Nov 2]Available from: [DOI] [PubMed] [Google Scholar]
- 38.Crawford J.R., Howell D.C. Comparing an individual's test score against norms derived from small samples. Clin. Neuropsychol. 1998;12(4):482–486. [Google Scholar]
- 39.Aldridge V.K., Dovey T.M., Wade A. Assessing test-retest reliability of psychological measures. Eur. Psychol. 2017;22(4):207–218. https://econtent.hogrefe.com/doi/abs/10.1027/1016-9040/a000298 [Internet]Nov 29 [cited 2021 Dec 9]Available from: [Google Scholar]
- 40.Samuels M.H. Psychiatric and cognitive manifestations of hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(5):377–383. doi: 10.1097/MED.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazova I., Laczó J., Rubinova E., Mokrisova I., Hyncicova E., Andel R., et al. Spatial navigation in young versus older adults. Front. Aging Neurosci. 2013;5(DEC):94. doi: 10.3389/fnagi.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li A.W.Y., King J. Spatial memory and navigation in ageing: a systematic review of MRI and fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 2019;103:33–49. doi: 10.1016/j.neubiorev.2019.05.005. Aug 1. [DOI] [PubMed] [Google Scholar]
- 43.Coughlan G., Puthusseryppady V., Lowry E., Gillings R., Spiers H., Minihane A.M., et al. Test-retest reliability of spatial navigation in adults at-risk of Alzheimer's disease. PLoS ONE. 2020;15(9 September) doi: 10.1371/journal.pone.0239077. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekstrom A.D., Isham E.A. Human spatial navigation: representations across dimensions and scales. Curr. Opin. Behav. Sci. 2017;17:84–89. doi: 10.1016/j.cobeha.2017.06.005. [Internet]Oct 1 [cited 2021 Dec 8]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladyka-Wojcik N., Barense M.D. Reframing spatial frames of reference: what can aging tell us about egocentric and allocentric navigation? Wiley Interdiscip. Rev. Cogn. Sci. 2021;12(3):e1549. doi: 10.1002/wcs.1549. https://onlinelibrary.wiley.com/doi/full/10.1002/wcs.1549 [Internet]May 1 [cited 2021 Dec 8]Available from: [DOI] [PubMed] [Google Scholar]
- 46.Hartley T., Maguire E.A., Spiers H.J., Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37(5):877–888. doi: 10.1016/s0896-6273(03)00095-3. Mar 6. [DOI] [PubMed] [Google Scholar]
- 47.Wolbers T., Weiller C., Buchel C. Neural foundations of emerging route knowledge in complex spatial environments. Brain Res. Cogn. Brain Res. 2004;21(3):401–411. doi: 10.1016/j.cogbrainres.2004.06.013. https://www.ncbi.nlm.nih.gov/pubmed/15511655 [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- 48.Burgess N. Spatial memory: how egocentric and allocentric combine. Trends Cogn. Sci. 2006;10(12):551–557. doi: 10.1016/j.tics.2006.10.005. https://www.ncbi.nlm.nih.gov/pubmed/17071127 [Internet]DecAvailable from. [DOI] [PubMed] [Google Scholar]
- 49.Burgess N., Becker S., O'Keefe J. Memory for events and their spatial context: models abd experiments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356(1413):1493–1503. doi: 10.1098/rstb.2001.0948. king john. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Save E., Paz-Villagran V., Alexinsky T., Poucet B. Functional interaction between the associative parietal cortex and hippocampal place cell firing in the rat. Eur. J. Neurosci. 2005;21(2):522–530. doi: 10.1111/j.1460-9568.2005.03882.x. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1460-9568.2005.03882.x [Internet]Jan 1 [cited 2021 Dec 8]Available from: [DOI] [PubMed] [Google Scholar]
- 51.Byrne P., Becker S., Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 2007;114(2):340–375. doi: 10.1037/0033-295X.114.2.340. https://www.ncbi.nlm.nih.gov/pubmed/17500630 [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fletcher P.C., Shallice T., Frith C.D., Frackowiak R.S.J., Dolan R.J. Brain activity during memory retrieval The influence of imagery and semantic cueing. Brain. 1996;119:1587–1596. doi: 10.1093/brain/119.5.1587. https://academic.oup.com/brain/article/119/5/1587/369207 [Internet][cited 2021 Dec 8]Available from. [DOI] [PubMed] [Google Scholar]
- 53.Wallentin M., Roepstorff A., Glover R., Burgess N. Parallel memory systems for talking about location and age in precuneus, caudate and Broca's region. Neuroimage. 2006;32(4):1850–1864. doi: 10.1016/j.neuroimage.2006.05.002. Oct 1. [DOI] [PubMed] [Google Scholar]
- 54.Townley R.A., Graff-Radford J., Mantyh W.G., Botha H., Polsinelli A.J., Przybelski S.A., et al. Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2020;2(1):1–19. doi: 10.1093/braincomms/fcaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Leeuw F.E., de Groot J.C., Achten E., Oudkerk M., Ramos L.M.P., Heijboer R., et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: the Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neggers S.F., Van der Lubbe R.H., Ramsey N.F., Postma A. Interactions between ego- and allocentric neuronal representations of space. Neuroimage. 2006;31(1):320–331. doi: 10.1016/j.neuroimage.2005.12.028. https://www.ncbi.nlm.nih.gov/pubmed/16473025 [Internet]Available from. [DOI] [PubMed] [Google Scholar]
- 57.Weniger G., Ruhleder M., Lange C., Wolf S., Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49(3):518–527. doi: 10.1016/j.neuropsychologia.2010.12.031. https://www.ncbi.nlm.nih.gov/pubmed/21185847 [Internet]Available from: [DOI] [PubMed] [Google Scholar]
- 58.Crawford J.R., Garthwaite P.H., Azzalini A., Howell D.C., Laws K.R. Testing for a deficit in single-case studies: effects of departures from normality. Neuropsychologia. 2006;44(4):666–677. doi: 10.1016/j.neuropsychologia.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Svä rd D., Nilsson M., rn Lampinen B., Lätt J., Sundgren P.C., Stomrud E., et al. The effect of white matter hyperintensities on statistical analysis of diffusion tensor imaging in cognitively healthy elderly and prodromal Alzheimer's disease. 2017; Available from: doi: 10.1371/journal.pone.0185239. [DOI] [PMC free article] [PubMed]