Abstract
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) comprises group of small vessel vasculitides, including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). In 2022, the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) jointly proposed new classification criteria for AAV (the 2022 ACR/EULAR criteria). In this review, we briefly summarize the 2022 ACR/EULAR criteria for GPA, MPA, and EGPA, and introduce our clinical experience with applying them to patients who were previously diagnosed with AAV based on three criteria: firstly, the classification criteria for GPA and EGPA proposed by the ACR in 1990; secondly, the algorithm for the classification of AAV and polyarteritis nodosa proposed by the European Medicines Agency algorithm in 2007 (the 2007 EMA algorithm); and thirdly, the revised International Chapel Hill Consensus Conference nomenclature of vasculitides in 2012 (the 2012 CHCC definitions). We found that concordance rate was highest in patients with MPA (96.6%), followed by those with EGPA (86.3%) and GPA (73.8%). In addition, compared to previous criteria, we noted several issues of the undervalued or overvalued items in the 2022 ACR/EULAR criteria for classifying AAV and provided several suggestions. To increase the diagnostic accuracy and reduce the discordance rate among the new and previous criteria for AAV, we suggest that the previous criteria should be considered together with the 2022 ACR/EULAR criteria when applying the classification criteria for AAV to patients suspected of AAV.
Keywords: Antineutrophil cytoplasmic antibody-associated vasculitis, ACR/EULAR, classification criteria, 2022
INTRODUCTION
Vasculitides are currently divided into three categories according to the size of affected vessels, major organs involved, and underlying diseases. Among the vasculitides classified based on the size of affected vessels, small vessel vasculitis is composed of two groups, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and immune complex vasculitis. Unlike immune complex vasculitis, AAV is characterized by necrotizing vasculitis with no or few immune complexes and is associated with ANCA.1,2,3 AAV is further divided into three subtypes, including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA), according to clinical, laboratory, radiological and histological features.1,3,4 In addition, researchers have also attempted to categorize AAV into three groups according to the presence of ANCA type, such as myeloperoxidase (MPO)-ANCA vasculitis, proteinase 3 (PR3)-ANCA vasculitis and ANCA-negative vasculitis because ANCA plays a key role in the pathogenesis of AAV.5,6,7
The clinical features of AAV are not easily differentiated from those of other systemic diseases in the early phase, and the frequency of serious complications or all-cause mortality is as high as chronic medical conditions in refractory cases.8,9,10,11 Therefore, the importance of accurate early diagnosis for early intervention according to guidelines for the management of AAV is emphasized.12 So far, the classification criteria for AAV have included three criteria: firstly, the classification criteria for GPA and EGPA proposed by the American College of Rheumatology (ACR) in 1990 (the 1990 ACR criteria);13,14 secondly, the algorithm for the classification of AAV and polyarteritis nodosa proposed by the European Medicines Agency algorithm in 2007 (the 2007 EMA algorithm);4 and thirdly, the revised International Chapel Hill Consensus Conference nomenclature of vasculitides in 2012 (the 2012 CHCC definitions).1
We modified and introduced a table combining the 1990 ACR criteria for EGPA, the 2007 EMA algorithm, and the 2012 CHCC definitions in our previous review article.3 This table used the same GPA surrogate markers as those suggested by the 2007 EMA algorithm. The order of applying these criteria moves from the left. If a patient meets the 1990 ACR criteria for EGPA, then the patient can be classified as having EGPA, and the classification process stops. If not, the patient can be classified as having GPA by the following three conditions: 1) histopathological features with granulomatous inflammation suggestive of GPA; 2) histopathological features without granulomatous inflammation suggestive of MPA and the presence of GPA surrogate markers; and 3) no biopsy performed, the presence of GPA surrogate markers, and ANCA positivity. If not, the patient can be classified as having MPA by the following two conditions: 1) histopathological features and the absence of GPA surrogate markers; 2) no biopsy performed, the absence of GPA surrogate markers, ANCA positivity, and suspected renal vasculitis.3,4 Histopathological features of GPA and MPA depend on the 2012 CHCC definitions, and suspected renal vasculitis is defined as hematuria red blood cell (RBC) cast or >10% RBC dysmorphism or hematuria ≥2+ and proteinuria ≥2+ on urine stick (Table 1).4
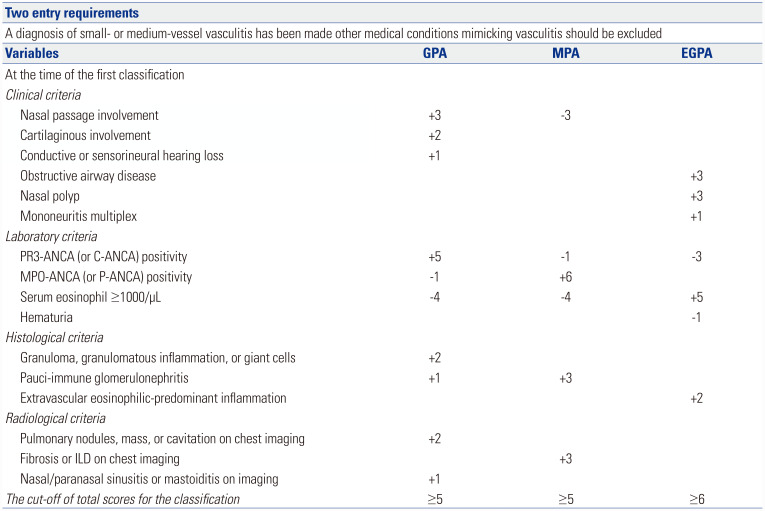
Table 1. 2007 EMA Algorithm Modified with 2012 CHCC Definitions (Flowchart from Left to Right).
| Conditions | ACR for EGPA (1990 ACR) | Histology compatible with 2012 CHCC definition for GPA | Histology compatible with 2012 CHCC definition for MPA and GPA surrogate markers |
No histology and GPA surrogate markers and PR3- or MPO-ANCA positivity |
Clinical and Histology compatible with 2012 CHCC definition for MPA and No GPA surrogate markers |
No histology and No GPA surrogate markers and PR3- or MPO-ANCA positivity and renal vasculitis |
Histology compatible with 2012 CHCC definition for cPAN or typical angiographic features of cPAN |
| Classified as | EGPA | GPA | GPA | GPA | MPA | MPA | cPAN |
| Comments | Necrotizing granuloma with eosinophil infiltrate 1) history of asthma 2) eosinophil >10% 3) mono- or polyneuropathy 4) migratory non-fixed pulmonary infiltrates 5) paranasal sinusitis 6) extravasation of eosinophil on histology (4 of 6) |
Necrotizing granuloma without eosinophil infiltrate | Necrotizing vasculitis without granuloma without eosinophil infiltrate with few immune deposit Upper respiratory markers or Lower respiratory markers |
Upper respiratory markers or Lower respiratory markers |
Necrotizing vasculitis without granuloma without eosinophil infiltrate with few or no immune deposit | No GN Rare ANCA |
EMA, European Medicine Agency; CHCC, Chapel Hill Consensus Conference; ACR, American College of Rheumatology; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; PR3, proteinase 3; MPO, myeloperoxidase; ANCA, antineutrophil cytoplasmic antibody; cPAN, classic polyarteritis nodosa; GN, glomerulonephritis.
In 2022, the ACR and The European Alliance of Associations for Rheumatology (EULAR) jointly proposed the new classification criteria for AAV (the 2022 ACR/EULAR criteria). When applying new criteria to patients suspected of AAV, two mandatory requirements to apply the 2022 ACR/EULAR classification criteria for AAV should be met: a diagnosis of small- or medium-vessel vasculitis has been made, and other medical conditions mimicking vasculitis have been excluded.15,16,17 There are two distinct differences between the 2022 ACR/EULAR criteria and the previous criteria. One is that the new criteria include items classified into clinical, laboratory, radiological and histological categories. The other is that they assign differently weighted points to each item, and furthermore, they provide cut-off values of the total score for the classification of GPA, MPA, and EGPA.1,4,13,14,15,16,17 Recently, we applied the 2022 ACR/EULAR criteria for GPA, MPA, and EGPA to patients who were previously classified as having GPA, MPA, and EGPA based on the previous criteria, respectively, and reported concordance rates between the new and previous criteria and issues of the discordance between the two criteria.18,19,20
In this review, we briefly summarize the 2022 ACR/EULAR criteria for GPA, MPA, and EGPA and introduce our clinical experience with applying them to patients who were previously diagnosed with AAV based on the 1990 ACR criteria, the 2007 EMA algorithm, and the 2012 CHCC definitions.
PATIENTS INCLUDED IN THE THREE PREVIOUS STUDIES
Inclusion criteria among the three previous studies were mostly similar. The inclusion criteria in the present study were 1) patients who were enrolled in the Severance Hospital ANCA-associated Vasculitides (SHAVE) cohort (an observational cohort of patients with AAV at a single centre); 2) patients who were classified as having AAV at the Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital between May 2000 and June 2021; 3) patients who met the criteria modified by the three criteria, the 1990 ACR criteria for EGPA, the 2007 EMA algorithm, and the 2012 CHCC definitions; 4) patients who had medical records with sufficient data enough to apply the 2022 ACR/EULAR criteria; 5) patients who were followed-up for at least 3 months; 6) patients who had no concomitant medical conditions such as serious infectious diseases, malignancies and systemic vasculitides other than AAV at diagnosis; 7) patients who had never been exposed to glucocorticoids equivalent to prednisolone >20 mg/day before the first diagnosis of AAV.18,19,20
APPLICATION OF THE 2022 ACR/EULAR CLASSIFICATION CRITERIA FOR GPA TO PATIENTS WHO WERE PREVIOUSLY DIAGNOSED WITH GPA
The 2022 ACR/EULAR criteria for GPA consist of 10 items, among which three, three, two, and two items are included in the clinical, laboratory, histological, and radiological criteria, respectively. In terms of the clinical criteria, +3, +2, and +1 points are assigned to nasal passage involvement, cartilaginous involvement, and conductive or sensorineural hearing loss, respectively. In terms of the laboratory criteria, +5, -1, and -4 points are assigned to PR3-ANCA (or C-ANCA) positivity, MPO-ANCA (or P-ANCA) positivity, and serum eosinophil ≥1000/µL, respectively. In terms of the histological criteria, +2, and +1 points are assigned to granuloma, granulomatous inflammation or giant cells, and pauci-immune glomerulonephritis, respectively. In terms of the radiological criteria, +2, and +1 points are assigned to pulmonary nodules, mass, or cavitation on chest imaging, and nasal/paranasal sinusitis or mastoiditis on imaging. When a total score is more than 5, GPA can be classified (Fig. 1).15
Fig. 1. Summary of the 2022 ACR/EULAR classification criteria for GPA, MPA, and EGPA. ACR, American College of Rheumatology; EULAR, European Alliance of Associations for Rheumatology; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis.
We applied the 2022 ACR/EULAR criteria for GPA to 65 patients who were previously diagnosed with GPA. Among the 65 patients, 48 were reclassified as having GPA, which provided a concordance rate between the new and the previous criteria of 73.8% (Table 2). Among the 17 patients who were not reclassified as GPA by the new criteria, 16 could be classified as having MPA according to the 2022 ACR/EULAR criteria for MPA. MPO-ANCA (or P-ANCA) positivity was the most critical contributing factor in the classification of MPA. One patient did not meet the 2022 ACR/EULAR criteria for GPA, MPA, or EGPA, and was classified as having unclassifiable vasculitis.4,15,16,17,18
Table 2. Concordance Rates and Issues Regarding the Discordance between the 2022 ACR/EULAR Criteria and the Previous Criteria for AAV in Patients Who Were Previously Diagnosed with Each AAV.
| GPA (n=65) | MPA (n=117) | EGPA (n=51) | |
|---|---|---|---|
| Concordance rate | 73.8% | 96.6% | 86.3% |
| Discordance issues | • Undervalued item of granuloma, granulomatous inflammation, or giant cells on biopsy. • Undervalued item of cartilaginous involvement. • Overvalued item of PR3-ANCA (or C-ANCA) positivity. |
• Ignored MPA-specific histopathological findings of major organs except for pauci-immune glomerulonephritis. • Overvalued item of MPO-ANCA (or P-ANCA) positivity. • Controversial issue regarding the item of fibrosis or ILD on chest imaging. |
• No item of non-fixed pulmonary infiltration. • Negatively overvalued item of PR3-ANCA (or C-ANCA) positivity. • Negatively overvalued item of hematuria. |
ACR, American College of Rheumatology; EULAR, European Alliance of Associations for Rheumatology; AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; PR3, proteinase 3; C, cytoplasmic; MPO, myeloperoxidase; P, perinuclear; ILD, interstitial lung disease.
We noted several issues affecting the discordance between the new and previous criteria. First, the item of granuloma, granulomatous inflammation, or giant cells on biopsy, which is a typical histologic feature of GPA,1,2,4 might have been undervalued. Since only +2 points are assigned to the item, 4 patients were reclassified as having MPA despite a finding of necrotising vasculitis of small vessels with granuloma on biopsy. Second, the item of cartilaginous involvement, which is another typical clinical feature of GPA among the three subtypes of AAV,4,21 might have been undervalued as well. One patient exhibited GPA-related endobronchial stenosis (+2), pulmonary nodule (+2), pauci-immune glomerulonephritis on biopsy (+1), and MPO-ANCA (or P-ANCA) positivity (-1), but could not be reclassified as having GPA due to a total score of 4. Third, the item of PR3-ANCA (or C-ANCA) positivity might have been overvalued. One patient had hearing loss due to chronic otitis media (+1), MPO-ANCA (or P-ANCA) positivity (-1), pulmonary nodules (+2), and paranasal sinusitis (+1) could not be reclassified as having GPA due to a total score of 3. According to the 2022 ACR/EULAR criteria, if ANCA is not detected even when a patient with clear evidence of necrotizing vasculitis in small vessels together with granuloma on biopsy is suspected of GPA, it may be impossible to classify this patient as having GPA unlike the 2007 EMA algorithm or the 2012 CHCC definitions (Table 2).1,4,15
Therefore, we carefully suggest that the points assigned to the items of granuloma, granulomatous inflammation, or giant cells on biopsy, and cartilaginous involvement should be upgraded, and biopsy should be performed in cases strongly suspected of having GPA despite the absence of PR3-ANCA (or C-ANCA). In addition, the weighted points assigned to the items of MPO-ANCA (or P-ANCA) and PR3-ANCA (or C-ANCA) positivity might need further discussion.
APPLICATION OF THE 2022 ACR/EULAR CLASSIFICATION CRITERIA FOR MPA TO PATIENTS WHO WERE PREVIOUSLY DIAGNOSED WITH MPA
The 2022 ACR/EULAR criteria for MPA consist of one, three, one, and one clinical, laboratory, histological, and radiological criteria, respectively. Regarding the clinical criteria, nasal passage involvement is assigned -3 points. Regarding the laboratory criteria, -1, +6, and -4 points are assigned to PR3-ANCA (or C-ANCA) positivity, MPO-ANCA (or P-ANCA) positivity, and serum eosinophil ≥1000/µL, respectively. Regarding the histological criteria, +3 points are assigned to pauci-immune glomerulonephritis, which is highly valued compared to the 2022 ACR/EULAR criteria for GPA.15 Regarding the radiological criteria, considerable points (+3) are assigned to fibrosis or interstitial lung disease (ILD) on chest imaging. A total score of more than 5 can classify MPA16 (Fig. 1).
We applied the 2022 ACR/EULAR criteria for MPA to 117 the patients who were previously diagnosed with MPA. Among the 117 patients, 113 patients were reclassified as having MPA, and the concordance rate between the new and the previous criteria was as high as 96.6% (Table 2).19 The remaining 4 patients were reclassified as having unclassifiable vasculitis, and MPO-ANCA (or P-ANCA) negativity was a major factor in the failure of reclassifying them as having MPA in three of them. In contrast, 3 patients who had MPO-ANCA (or P-ANCA) and PR3-ANCA (or C-ANCA) could be reclassified as having both MPA and GPA based on the 2022 ACR/EULAR criteria for MPA and GPA.
We highlighted several distinct aspects of the 2022 ACR/EULAR criteria compared to previous criteria. First, MPA-specific histological findings of major organs except for pauciimmune glomerulonephritis did not contribute significantly to the classification of MPA including ANCA-associated glomerulonephritis or renal limited vasculitis.4,22,23 Three of 4 patients who could not be reclassified as having MPA showed MPA-specific histological features based on both the 2007 EMA algorithm and 2012 CHCC definitions.1,4 Second, the weight of MPO-ANCA (or P-ANCA) positivity might have been overvalued. No MPO-ANCA (or P-ANCA) was detected in 3 patients who were not reclassified as having MPA despite a typical histological finding of MPA. Third, the item of fibrosis or ILD on chest imaging may be expected to cause confusion in actual clinical practice due to its diverse causes.24 Contrary to the cases mentioned above, it may be controversial to judge lung fibrosis and ILD in patients in whom lung biopsy was not performed as clinical results by MPA only with MPO-ANCA (or P-ANCA) positivity. This is because it is difficult to exclude the possibility of fibrosis or ILD caused by various causes regardless of MPO-ANCA (or P-ANCA) positivity (Table 2).
Therefore, we suggest that a new item of histological features of MPA based on the 2012 CHCC definitions should be added and that the points assigned to the items of MPO-ANCA (or P-ANCA) positivity (+6) should be downgraded, given the existence of various antigenic epitopes of MPO-ANCA (or P-ANCA) and false positivity of P-ANCA.7,25 In addition, we also insisted that other causes of lung lesions should be evaluated when applying the item of fibrosis or ILD on chest imaging, and in particular, more attention should be paid to the classification of MPA when there is no clinical clue suggesting MPA other than MPO-ANCA (or P-ANCA) positivity.
APPLICATION OF THE 2022 ACR/EULAR CLASSIFICATION CRITERIA FOR EGPA TO PATIENTS WHO WERE PREVIOUSLY DIAGNOSED WITH EGPA
The 2022 ACR/EULAR criteria for GPA consist of three, three, and one clinical, laboratory, and histological criteria, respectively, and there are no radiological criteria. As for the clinical criteria,+3, +3, and +1 points are assigned to obstructive airway disease, nasal polyp, and mononeuritis multiplex, respectively. As for the laboratory criteria, -3, +5, and -1 points are assigned to PR3-ANCA (or C-ANCA) positivity, serum eosinophil ≥ 1000/µL, and hematuria, respectively. No points were assigned to MPO-ANCA (or P-ANCA) positivity. As for the histological criteria, +2 points are assigned to extravascular eosinophilic-predominant inflammation. When a total score of more than 6 is achieved, EGPA can be classified17 (Fig. 1).
We applied the 2022 ACR/EULAR criteria for EGPA to 51 the patients who were previously diagnosed with EGPA. Among the 51 patients, 44 patients were reclassified as having GPA, and the concordance rate between the new and the previous criteria was 86.3% (Table 2).20 Among the 7 patients who were not reclassified as EGPA by the new criteria, 3, 1, and 3 patients were reclassified as having MPA, GPA, and unclassifiable vasculitis, respectively. Furthermore, among the 44 patients who were reclassified as having EGPA, 6 patients were reclassified as having both EGPA and MPA and one patient as having both EGPA and GPA, respectively.
We noticed several issues with the discordance between the new and previous criteria. First, the absence of an item of non-fixed pulmonary infiltration, and the absence of an item of paranasal sinusitis might have been important factors in the failure to reclassify EGPA, although migratory pulmonary infiltration is known as a typical radiological feature of the allergic stage of EGPA.1,26,27 This is because all three patients who were reclassified as having unclassifiable vasculitis exhibited asthma history, non-fixed pulmonary infiltrates, and paranasal sinusitis. Second, although PR3-ANCA has been emphasized as a factor suggestive of GPA by the associated group,28 the negatively weighted PR3-ANCA (or C-ANCA) positivity (-3) might have been overvalued. This situation is expected to challenge the concept of ANCA-positive EGPA in the future.29 Among the 7 patients who were not reclassified as having EGPA by the new criteria, 2 patients achieved total scores of 5 and 4 due to PR3-ANCA (or C-ANCA) positivity. If the point assigned had been downgraded to -1 point, they could have been reclassified as having EGPA. Third, hematuria in EGPA patients was ignored, and further, a negative point (-1) is assigned to hematuria. Among the 7 patients who were not reclassified as having EGPA, 1 patient achieved a total score of 5 and was not reclassified as having EGPA due to hematuria (-1). Given that hematuria can be caused by renal involvement of EGPA,29,30 if no point was assigned to hematuria, this patient could have been reclassified as having EGPA (Table 2).
Therefore, we carefully suggest that the addition of at least a new item of non-fixed pulmonary infiltrates should be reconsidered and that the overweighted points assigned to PR3-ANCA (or C-ANCA) positivity should be downgraded. In addition, we also suggested that the deletion of the item of hematuria should be reconsidered when EGPA is strongly suspected.
VIRTUAL CASES TO DISCUSS
In order to better understand the above, we present a situation in which the new criteria can be appropriately applied in actual clinical practice through two virtual cases. The first virtual case is a patient who exhibits ILD on chest imaging and in whom MPO-ANCA is detected. According to the 2022 ACR/EULAR criteria for MPA, the patient gets +3 and +6 points by fibrosis or ILD on chest imaging. Since the patient does not exhibit other clinical, laboratory, or radiological symptoms and signs, there are no additional plus or minus points given. Therefore, the patient achieves a total score of +9 and can be classified as having MPA. Conversely, according to the 2007 EMA algorithm and the 2012 CHCC definitions, the patient has MPO-ANCA positivity but does not have evidence of either a surrogate marker suggestive of GPA or suspected renal vasculitis. A lung biopsy cannot be performed because the lung lesion cannot be easily biopsied. Therefore, the patient could not be classified as having AAV. Given that there are various etiologies of ILD, the possibility that ILD is one thing and MPO-ANCA positivity is another cannot be ruled out. Therefore, an aggressive biopsy is necessary in this case, otherwise, the classification has a conflict among three criteria.
The second virtual case is another patient who exhibits a saddle nose and paranasal sinusitis. Paranasal sinusitis and granulomatous inflammation are confirmed by computed tomography and paranasal sinus biopsy, respectively. Also, MPO-ANCA is detected. According to the 2022 ACR/EULAR criteria for GPA, the patient gets +2, +2, and +1 points by cartilaginous involvement, granulomatous inflammation on biopsy, and paranasal sinusitis on imaging, respectively, whereas the patient gets -1 due to MPO-ANCA (or P-ANCA) positivity. Therefore, the patient achieves a total score of +4 and cannot be classified as having GPA. In contrast, according to the 2007 EMA algorithm and the 2012 CHCC definitions, the patient can be classified as having GPA through GPA-indicating histopathological patterns, a GPA surrogate marker, and cartilaginous involvement regardless of ANCA positivity. This discrepancy may be due to the undervalued items of histopathological patterns and cartilaginous involvement of the 2022 ACR/EULAR criteria. Therefore, at the time of diagnosis, we suggest that physicians should apply the 2007 EMA algorithm and the 2012 CHCC definitions as well as the 2022 ACR/EULAR criteria to the patient who is suspected of AAV but in whom the classification of its subtype is not clear. Moreover, we also suggest that more than two experts should participate in the decision in difficult cases like this patient.
ISSUES TO FURTHER DISCUSS
While applying the 2022 ACR/EULAR criteria for AAV to the patients who were previously diagnosed with each AAV, we found three issues that warrant further discussion. First, the 2007 EMA algorithm provides the classification order of EGPA, GPA, MPA, and unclassifiable vasculitis,4 while the 2022 ACR/EULAR criteria do not mention the classification order.15,16,17 Therefore, when AAV is suspected, the 2022 ACR/EULAR criteria for GPA, MPA, and EGPA are all applied, so the simultaneous classification of two AAVs may occur. We wonder what treatment recommendations should be applied to patients classified as having two AAVs at the same time. This is because the therapeutic regimens for GPA and MPA are quite different from those for EGPA.12,31 Second, a few patients who were previously diagnosed with AAV were reclassified as having unclassifiable vasculitis. In these patients, we wonder whether the classification should be changed, and further, whether the treatment recommendations for AAV should be applied to these patients. Third, it is well known that clinical features are different between patients with ANCA-positive and those with ANCA-negative EGPA.26,29 However, the classification rate of ANCA-positive EGPA will be reduced according to the 2022 ACR/EULAR criteria because the weights of the items of MPO-ANCA or (P-ANCA) and PR3-ANCA (or C-ANCA) positivity for MPA and GPA are upgraded.18,19 In addition, it is hoped that, in the near future, when a revised version of the 2022 ACR/EULAR criteria for AAV is proposed, new biomarkers for diagnosis in addition to ANCA positivity, serum eosinophilia, and urinalysis will be included in the new criteria.32
CONCLUSION
In this review, we introduced the results of previous studies on the application of the 2022 ACR/EULAR criteria for AAV to the patients who were previously diagnosed with AAV. We found that the concordance rate was highest in patients with MPA (96.6%), followed by those with EGPA (86.3%) and GPA (73.8%). In addition, we raised several issues for further discussion and provided several suggestions. To increase the diagnostic accuracy and reduce the discordance rate among the new and previous criteria for AAV, we suggest that the 2007 EMA algorithm and the 2012 CHCC definitions should be considered together with the 2022 ACR/EULAR criteria when applying the classification criteria for AAV to patients suspected of AAV.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang-Won Lee.
- Data curation: Jung Yoon Pyo, Lucy Eunju Lee, and Sang-Won Lee.
- Formal analysis: Jung Yoon Pyo, Lucy Eunju Lee, and Sang-Won Lee.
- Investigation: Jung Yoon Pyo, Lucy Eunju Lee, and Sang-Won Lee.
- Methodology: Yong-Beom Park and Sang-Won Lee.
- Project administration: Sang-Won Lee.
- Resources: Jung Yoon Pyo, Lucy Eunju Lee, and Sang-Won Lee.
- Software: Jung Yoon Pyo, Lucy Eunju Lee, and Sang-Won Lee.
- Supervision: Sang-Won Lee.
- Validation: Yong-Beom Park.
- Visualization: Jung Yoon Pyo and Lucy Eunju Lee.
- Writing—original draft: Jung Yoon Pyo and Sang-Won Lee.
- Writing—review & editing: Lucy Eunju Lee, Yong-Beom Park, and Sang-Won Lee.
- Approval of final manuscript: all authors.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 3.Choi CB, Park YB, Lee SW. Antineutrophil cytoplasmic antibody-associated vasculitis in Korea: a narrative review. Yonsei Med J. 2019;60:10–21. doi: 10.3349/ymj.2019.60.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016;12:570–579. doi: 10.1038/nrrheum.2016.123. [DOI] [PubMed] [Google Scholar]
- 6.Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 7.Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10:463–473. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- 8.Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–1010. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]
- 9.Chaigne B, Guillevin L. Unsolved questions and concerns about treatment of anti-neutrophil cytoplasm antibody-associated vasculitides. Clin Exp Rheumatol. 2016;34(3 Suppl 97):S121–S128. [PubMed] [Google Scholar]
- 10.Elefante E, Monti S, Bond M, Lepri G, Quartuccio L, Talarico R, et al. One year in review 2017: systemic vasculitis. Clin Exp Rheumatol. 2017;35 Suppl 103:5–26. [PubMed] [Google Scholar]
- 11.Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis. 2017;76:1566–1574. doi: 10.1136/annrheumdis-2016-210942. [DOI] [PubMed] [Google Scholar]
- 12.Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021;73:1366–1383. doi: 10.1002/art.41773. [DOI] [PubMed] [Google Scholar]
- 13.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 14.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–1100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 15.Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:315–320. doi: 10.1136/annrheumdis-2021-221795. [DOI] [PubMed] [Google Scholar]
- 16.Suppiah R, Robson JC, Grayson PC, Ponte C, Craven A, Khalid S, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. 2022;81:321–326. doi: 10.1136/annrheumdis-2021-221796. [DOI] [PubMed] [Google Scholar]
- 17.Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:309–314. doi: 10.1136/annrheumdis-2021-221794. [DOI] [PubMed] [Google Scholar]
- 18.Pyo JY, Ahn SS, Song JJ, Park YB, Lee SW. Reclassification of previously diagnosed GPA patients using the 2022 ACR/EULAR classification criteria. Rheumatology (Oxford) 2022 May 04; doi: 10.1093/rheumatology/keac267. [Epub]. Available at: [DOI] [PubMed] [Google Scholar]
- 19.Pyo JY, Ahn SS, Song JJ, Park YB, Lee SW. Application of the 2022 ACR/EULAR criteria for microscopic polyangiitis to patients with previously diagnosed microscopic polyangiitis. Clin Exp Rheumatol. 2022 May 19; doi: 10.55563/clinexprheumatol/vmrk76. [Epub]. Available at: [DOI] [PubMed] [Google Scholar]
- 20.Pyo JY, Ahn SS, Song JJ, Park YB, Lee SW. The reclassification of patients with previously diagnosed eosinophilic granulomatosis with polyangiitis based on the 2022 ACR/EULAR criteria for antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2022 Sep 15; doi: 10.3899/jrheum.220560. [Epub]. Available at: [DOI] [PubMed] [Google Scholar]
- 21.Kesel N, Köhler D, Herich L, Laudien M, Holl-Ulrich K, Jüngel A, et al. Cartilage destruction in granulomatosis with polyangiitis (Wegener's granulomatosis) is mediated by human fibroblasts after transplantation into immunodeficient mice. Am J Pathol. 2012;180:2144–2155. doi: 10.1016/j.ajpath.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Geetha D, Jefferson JA. ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Dis. 2020;75:124–137. doi: 10.1053/j.ajkd.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 24.Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022;400:769–786. doi: 10.1016/S0140-6736(22)01052-2. [DOI] [PubMed] [Google Scholar]
- 25.Argyropoulou OD, Goules AV, Boutzios G, Tsirogianni A, Sfontouris C, Manoussakis MN, et al. Occurrence and antigenic specificity of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) in systemic autoimmune diseases. Cells. 2021;10:2128. doi: 10.3390/cells10082128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi CB, Park YB, Lee SW. Eosinophilic granulomatosis with polyangiitis: experiences in Korean patients. Yonsei Med J. 2019;60:705–712. doi: 10.3349/ymj.2019.60.8.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaglio A, Casazza I, Grasselli C, Corradi D, Sinico RA, Buzio C. Churg-Strauss syndrome. Kidney Int. 2009;76:1006–1011. doi: 10.1038/ki.2009.210. [DOI] [PubMed] [Google Scholar]
- 28.Yoo J, Kim HJ, Ahn SS, Jung SM, Song JJ, Park YB, et al. The utility of the ACR/EULAR 2017 provisional classification criteria for granulomatosis with polyangiitis in Korean patients with antineutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Rheumatol. 2018;36 Suppl 111:S85–S87. [PubMed] [Google Scholar]
- 29.Millet A, Pederzoli-Ribeil M, Guillevin L, Witko-Sarsat V, Mouthon L. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis. 2013;72:1273–1279. doi: 10.1136/annrheumdis-2013-203255. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood SB, Ahmad H, Wu J, Haselby D, LeClaire MM, Nasr R. Anti-neutrophil cytoplasmic antibody-positive eosinophilic granulomatosis with polyangiitis: can it cause membranous nephropathy? Scand J Rheumatol. 2019;48:256–257. doi: 10.1080/03009742.2019.1580765. [DOI] [PubMed] [Google Scholar]
- 31.Pyo JY, Lee LE, Ahn SS, Song JJ, Park YB, Lee SW. The efficacy of mycophenolate mofetil in remission maintenance therapy for microscopic polyangiitis and granulomatosis with polyangiitis. Yonsei Med J. 2021;62:494–502. doi: 10.3349/ymj.2021.62.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn SS, Park YB, Lee SW. Serological biomarkers and indices for the current activity and prognosis of ANCA-associated vasculitis: experience in a single centre in Korea. Yonsei Med J. 2021;62:279–287. doi: 10.3349/ymj.2021.62.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]