Abstract
Purpose
The mean platelet volume (MPV) is regarded as a marker for thrombosis, atherosclerosis, and inflammation in various vascular diseases. However, it still remains unclear whether plasma MPV is associated with cerebral white matter hyperintensities (WMH) and cerebral microvascular pathology in the elderly population.
Materials and Methods
We examined whether MPV level is associated with the presence of cerebral WMH on brain magnetic resonance imaging from 870 non-stroke outpatient subjects. The subjects were divided into three groups according to the consecutive level of MPV (low T1, middle T2, and high T3 MPV tertile groups). To determine the association of MPV levels with the WMH, logistic regression and receiver operating characteristic curve analyses were conducted.
Results
Subjects with higher MPV level were older and more likely to have hypertension, diabetes mellitus, and low renal function. Cerebral WMH were more prevalent in subjects with higher MPV level. After adjusting for confounding factors, moderate to severe cerebral WMH were significantly associated with high MPV tertile level. This association remained significant after adjusting for other cerebral vascular pathologies. T2 [odds ratio (OR): 1.49, 95% confidence interval (CI): 1.03–2.15] and T3 MPV tertile groups (OR: 1.51, 95%CI: 1.04–2.20) had more cerebral WMH lesions compared to T1 MPV tertile group. In addition, the subjects with higher Fazekas scores showed higher MPV level (p=0.020).
Conclusion
We found that high MPV level is independently associated with cerebral WMH. This result suggests that platelet activation plays a role in the development of cerebral WMH.
Keywords: Mean platelet volume, atherosclerosis, white matter hyperintensities, platelet activation
INTRODUCTION
Cerebral white matter hyperintensities (WMH) are a common finding in brain imaging in the elderly, especially in those with cerebrovascular and neurodegenerative diseases. Cerebral WMH are initially mild and asymptomatic in the early stages, but their progression results in the development of serious neurological diseases, including neurodegenerative ones, as well as cognitive decline and mood disorder.1 Furthermore, recent evidence has suggested that cerebral WMH play a causal role in the development and progression of neurodegenerative and cerebrovascular diseases.2,3 The presence of cerebral WMH corresponds to the pathological conditions of neuronal loss, ischemic demyelination, and gliosis in the brain.4
On brain imaging, cerebral small vessel disease (CSVD) usually presents as silent lacunar infarction, cerebral microbleeds, and cerebral WMH. The underlying pathomechanism of cerebral WMH is hypothesized to be diffuse CSVD in response to chronic ischemic injury.1 Some of the underlying risk factors for cerebral WMH are increased age and arterial hypertension (HTN). In addition to vascular risk factor for atherosclerosis, endothelial dysfunction, vascular inflammation, and microthrombosis of the cerebral microvasculature may also contribute to the development of cerebral WMH.5 It has been also reported that CSVD is associated with high dietary glucose, obstructive sleep apnea, low levels of plasma omega 3-polyunsaturated fatty acids, and aortic atheroma, suggesting that various pathophysiological processes are involved in WMH.6,7,8,9 Recent studies have reported that platelet-derived thrombogenic microvesicles10,11 were increased in patients with the presence of cerebral WMH. These findings indicate the role of platelets in the development of cerebral WMH.
The mean platelet volume (MPV), defined as a machine-calculated measurement of the average size of platelets in blood, is an easily accessible parameter on blood sampling. MPV reflects platelet size and activity.12,13 Recent studies have demonstrated that high MPV is associated with HTN14 and diabetes mellitus (DM),15 both of which contribute to the development of ischemic heart disease and ischemic stroke. Furthermore, high MPV predicts poor clinical outcome of ischemic heart disease16,17,18 and ischemic stroke.19,20,21,22 MPV is also a marker of increased platelet metabolism and enzymatic activity, which has been shown to be associated with vascular injury and inflammatory processes.23,24
However, little is known about the relationship between MPV in blood and the severity of cerebral WMH due to the paucity of data to date. Although a few studies have investigated the association between MPV and brain volume, the association has not yet been clearly established.23,24,25 In the present study, we investigated the relationship between platelets in the blood and cerebral WMH in the brain in clinically non-stroke neurology outpatients.
MATERIALS AND METHODS
Study population
The study subjects were neurologically healthy volunteers, aged ≥41 years, who visited the healthcare center at CHA Bundang Medical Center, Seongnam, Korea, for routine health examination between March 2008 and August 2014. A retrospective analysis was performed for subjects who arrived for health screening. Only participants whose records contained adequate information on demographic, laboratory, and radiological data were included. We did not include patients with hematologic, renal, haptic disorder, and recent history of infection. Of the 1011 study patients extracted from our database during the study period, 141 patients were excluded for the following reasons: 1) inadequate medical information (n=5); 2) no laboratory tests performed (n=75); 3) no data on brain magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA) (n=24); 4) previous history of hematologic, hepatic and/or renal disorders (n=29); or 5) abnormal neurological findings at the time of examination (n=8). A total of 870 participants were included in the subsequent analysis. All participants and their guardians gave written informed consent before taking part in the study. This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethical Committee of the CHA Bundang Medical Center (IRB approval no. 2010-083).
Risk factor assessment
The patients’ medical records were reviewed to gather information on their medical history and laboratory data related to cardiovascular risk factors as follows: 1) HTN: a high baseline blood pressure (systolic ≥140 mm Hg or diastolic ≥90 mm Hg) or a history of antihypertensive treatment; 2) DM: a fasting plasma glucose ≥126 mg/dL or a history of hypoglycemic therapy; 3) smoking: as current smoker at the time of examination; 4) hypercholesterolemia: a fasting serum total cholesterol ≥220 mg/dL or a history of statin medication; and 5) coronary arterial occlusive disease (CAOD): a history of CAOD and percutaneous coronary interventions or coronary artery bypass grafting.
We collected plasma sample data from all study subjects within 1 month of the radiological examination. Laboratory parameters, such as fasting blood glucose, total cholesterol, triglycerides, white blood cell count (WBC), and estimated glomerular filtration rate (eGFR),26 were determined in the core laboratory of the hospital.
Blood samples were drawn from the peripheral vein and analyzed using an automated complete blood cell counter (Uni-Cel DxH 800, Beckman Coulter Inc., Miami, FL, USA). Whole blood samples for MPV were collected in EDTA, and MPV was determined in fL units.
Radiological evaluation
Brain MRI and MRA were performed using one of three 1.5-T MR systems (Sonata, Siemens Healthcare, Germany; Signa Excite, GE Healthcare, Waukesha, WI, USA; and SignaHDx, GE Healthcare). Image interpretation was performed by two neurologists, who were unaware of the patient’s clinical and laboratory data. A small cavitated lesion (3–15 mm in diameter) in an area supplied by deep perforating arteries that showed low signal intensity on T1-weighted imaging [repetition time (TR)/echo time (TE)=560/14 ms] was defined as a silent lacunar infarction.27 All series contained 16 axial images with a slice thickness of 7 mm and a 2-mm inter-slice gap. For analysis of cerebral WMH, fluid-attenuation inversion recovery (FLAIR) images (TR/TE=9000/105 ms; inversion time, 2500 ms) were used in accordance with the MR imaging protocol. The presence of cerebral WMH was evaluated on FLAIR images by two neurologists, and the severity of cerebral WMH was assessed by the use of the Fazekas score.28 Fazekas scale ranges from 0 to 3: Fazekas 0, none or a single punctate WMH lesion; Fazekas 1, multiple punctate lesions; Fazekas 2, beginning confluence of lesions (bridging); and Fazekas 3, large confluent lesions. Large artery atherosclerosis (LAA) was identified as either marked stenosis (≥50%) or total occlusion of an intracranial or extracranial cerebral artery on brain MRA (MAGNETOM Symphony, Siemens, Heidelberg, Germany).29,30 A diagnosis of LAA was made only when both investigators reached the same conclusion.
Statistical analysis
To evaluate the factors associated with MPV levels, subjects were categorized into tertile groups based on consecutive MPV level as follows; 1) low MPV tertile group (T1): less than 8.10 fL; 2) middle MPV tertile group (T2): 8.10–9.60 fL; and 3) high MPV tertile group (T3): higher than 9.60 fL. Baseline characteristics were compared between MPV tertiles. Continuous variables are reported as mean±standard deviation, whereas categorical variables are reported as frequency and percentage. Chi-square test was used to compare the categorical variables, and analysis of variance was used to compare the continuous variables. Logistic regression was used to compare the MPV levels between those with cerebral WMH and those without a corresponding lesion. Potential confounding factors, including age, sex, HTN, DM, hypercholesterolemia, smoking status, current statin medication, CAOD, WBC, eGFR, and LAA, were added to the analysis. In the logistic regression analyses, MPV was treated as a categorical variable (MPV tertiles). Analysis of variance followed by post hoc comparison was performed to assess the significance of difference according to the Fazekas scale score. Odds ratio (OR) and 95% confidence interval (CI) were calculated from the logistic regression models.
To better understand the effect of MPV level on cerebral WMH, we illustrated a smoothing spline plot of the estimated probability for the presence of cerebral WMH according to the MPV, on the basis of the generalized additive regression model.
Receiver operating characteristic (ROC) curve analysis was conducted to determine the area under the curve (AUC), sensitivity, specificity, and cut-off point for the MPV that optimally predicted WMH. ROC analysis measured using the DeLong’s test31 was used to compare the performance between with and without MPV for WMH.
Statistical analyses were conducted using SPSS (ver. 18.0; SPSS Inc., Chicago, IL, USA) and R software, ver. 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). A two-sided p<0.05 was considered statistically significant.
RESULTS
The demographic characteristics of the study participants are summarized in Table 1. The clinical characteristics of the 870 participants included in this study were analyzed according to MPV tertile. In univariate analysis, subjects in the T3 MPV tertile group tended to be older than those in the T1 and T2 tertile groups, and had higher prevalence of HTN and DM. The group with higher MPV tertile had a higher Fazekas score and higher WBC counts compared to the lower MPV tertile group. The higher MPV tertile group had lower eGFR compared to the lower MPV tertile group (Table 1).
Table 1. Clinical Characteristics of 870 Subjects according to MPV Tertile.
| All (n=870) | T1 (n=290) | T2 (n=290) | T3 (n=290) | p value | ||
|---|---|---|---|---|---|---|
| Sex, female | 516 (59.3) | 178 (61.4) | 173 (59.7) | 165 (56.9) | 0.541 | |
| Age (yr) | 63.9±9.2 | 63.5±8.8 | 62.9±9.2 | 65.1±9.4 | 0.011 | |
| Hypertension | 450 (51.7) | 127 (43.8) | 161 (55.5) | 162 (55.9) | 0.004 | |
| Diabetes mellitus | 206 (23.7) | 49 (16.9) | 69 (23.8) | 88 (30.3) | 0.001 | |
| Hypercholesterolemia | 231 (26.6) | 70 (24.1) | 71 (24.5) | 90 (31.0) | 0.106 | |
| Current smoking | 153 (17.6) | 45 (15.5) | 46 (15.5) | 63 (21.7) | 0.077 | |
| CAOD | 63 (7.2) | 25 (8.6) | 19 (6.6) | 19 (6.6) | 0.540 | |
| Statin medication | 165 (19.0) | 55 (19.0) | 52 (17.9) | 58 (20.0) | 0.817 | |
| SBP (mm Hg) | 128.7±16.4 | 128.2±15.9 | 128.5±16.7 | 129.4±16.5 | 0.649 | |
| DBP (mm Hg) | 78.5±10.8 | 78.3±10.7 | 79.3±11.3 | 77.9±10.4 | 0.259 | |
| WBC (×109/L) | 6.7±2.1 | 6.5±1.9 | 6.5±2.1 | 6.9±2.2 | 0.024 | |
| Large artery atherosclerosis | 178 (20.5) | 56 (19.3) | 51 (17.6) | 71 (24.5) | 0.101 | |
| Silent lacunar infarction | 109 (12.5) | 36 (12.4) | 35 (12.1) | 38 (13.1) | 0.929 | |
| WMH | 483 (55.5) | 140 (48.3) | 167 (57.6) | 176 (60.7) | 0.004 | |
| Fazekas score | 0.039 | |||||
| 0 | 387 (44.5) | 150 (51.7) | 123 (42.4) | 114 (39.3) | ||
| 1 | 247 (28.4) | 77 (26.6) | 77 (26.6) | 93 (32.1) | ||
| 2 | 171 (19.7) | 44 (15.2) | 68 (23.4) | 59 (20.3) | ||
| 3 | 65 (7.5) | 19 (6.6) | 22 (7.6) | 24 (8.3) | ||
| Total cholesterol (mmol/L) | 4.85±1.03 | 4.89±1.11 | 4.76±0.96 | 4.88±1.00 | 0.246 | |
| Triglyceride (mmol/L) | 1.67±1.11 | 1.73±1.29 | 1.69±1.06 | 1.60±0.96 | 0.365 | |
| eGFR (mL/min/1.73 m2) | 75.6±18.1 | 77.7±17.1 | 76.4±19.9 | 72.6±17.0 | 0.002 | |
T1, low MPV tertile; T2, middle MPV tertile; T3, high MPV tertile; MPV, mean platelet volume; CAOD, coronary arterial occlusive disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; WMH, white matter hyperintensities; eGFR, estimated glomerular filtration rate.
Data are presented as mean±standard deviation or n (%).
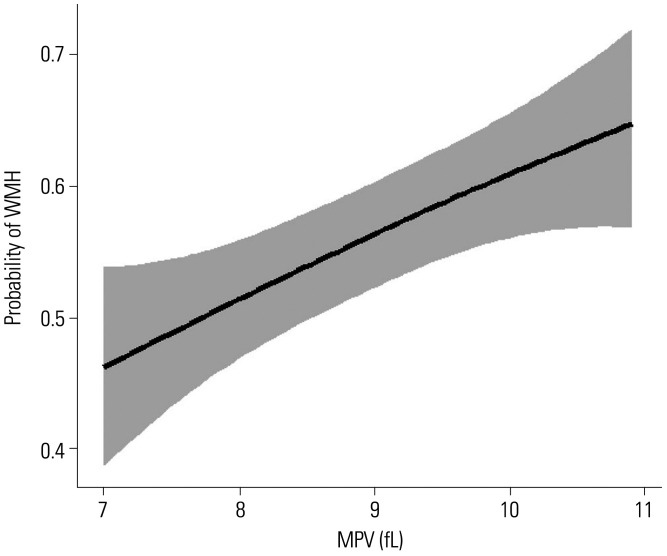
Next, we conducted logistic regression analyses between MPV tertile groups to determine whether MPV level was independently associated with the presence of cerebral WMH. Cerebral WMH were positively correlated with higher MPV tertile group in univariate analysis (Model 1 in Table 2). After adjustment for other confounding variables, the high MPV tertile group still showed statistical significance. After adjusting for confounding factors including LAA, subjects with T2 and T3 MPV tertile groups showed 49% and 51% increase in cerebral WMH lesions, respectively (Model 4 in Table 2). Furthermore, multiple-adjusted spline regressions confirmed the association between cerebral WMH and MPV levels (Fig. 1). MPV levels differed significantly with severity of cerebral WMH. There was a significant difference in MPV levels between the four groups according to the Fazekas score (p=0.020) (Fig. 2). The subjects with higher Fazekas scores showed higher MPV level.
Table 2. Multivariate Adjusted ORs for the Association between Cerebral WMH and MPV Tertile.
| Model 1* | p value | Model 2† | p value | Model 3‡ | p value | Model 4 § | p value | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CIs) | OR (95% CIs) | OR (95% CIs) | OR (95% CIs) | |||||
| T1 | Ref | - | Ref | - | Ref | - | Ref | - |
| T2 | 1.50 (1.08–2.08) | 0.016 | 1.61 (1.12–2.29) | 0.009 | 1.48 (1.03–2.14) | 0.036 | 1.49 (1.03–2.15) | 0.034 |
| T3 | 1.70 (1.22–2.36) | 0.002 | 1.64 (1.15–2.34) | 0.007 | 1.52 (1.05–2.20) | 0.029 | 1.51 (1.04–2.20) | 0.030 |
T1, low MPV tertile; T2, middle MPV tertile; T3, high MPV tertile; Ref, reference; WMH, white matter hyperintensities; MPV, mean platelet volume; OR, odds ratio; CIs, confidence intervals; WBC, white blood cell.
*Model 1: no adjustment; †Model 2: adjusted for age and sex; ‡Model 3: adjusted for sex, age, hypertension, diabetes mellitus, hypercholesterolemia, current smoking status, coronary arterial occlusive disease, current statin medication, WBC, and estimated glomerular filtration rate; §Model 4: adjusted for sex, age, hypertension, diabetes mellitus, hypercholesterolemia, current smoking status, coronary arterial occlusive disease, current statin medication, WBC, estimated glomerular filtration rate, and large artery atherosclerosis.
Fig. 1. Regression spline curve of estimated probability for cerebral WMH according to the level of MPV. The black lines and gray shadows represent the estimated probability and the 95% confidence intervals for the presence of cerebral WMH at the MPV level, respectively, based on the generalized additive model with splines. Adjustment was performed for the same covariates used in Model 4 of Table 2 (sex, age, hypertension, diabetes mellitus, hypercholesterolemia, current smoking status, coronary arterial occlusive disease, current statin medication, WBC, estimated glomerular filtration rate, and large artery atherosclerosis). The x-axis is limited from the 5th to 95th percentile of MPV level. WMH, white matter hyperintensities; MPV, mean platelet volume; WBC, white blood cell.
Fig. 2. Comparison of MPV levels according to the Fazekas score by using analysis of variance. MPV, mean platelet volume.
The ROC curves of the predictive value of MPV for WMH are shown in Fig. 3. In the ROC curves based on univariate logistic regression model with MPV, the optimal cutoff of MPV were 8.85 (specificity: 58.3%; sensitivity: 59.5%) for WMH. The AUC for WMH were 0.634 (Fig. 3A). We evaluated the AUC of multivariate logistic models for WMH by adding MPV (Fig. 3B). Addition of MPV to the models increased AUC, and it also reached statistical significance (p=0.011).
Fig. 3. ROC curves for WMH. (A) ROC curve of univariate logistic regression model for WMH with MPV. (B) Comparison of AUC for WMH in the multivariate models with and without MPV. Green indicates AUC of the model with MPV, and blue indicates AUC of the model without MPV. MPV was evaluated as a continuous variable. Adjustment was performed for the same covariates used in Model 4 of Table 2 (sex, age, hypertension, diabetes mellitus, hypercholesterolemia, current smoking status, coronary arterial occlusive disease, current statin medication, WBC, estimated glomerular filtration rate, and large artery atherosclerosis). ROC, receiver operative characteristic; WMH, white matter hyperintensities; MPV, mean platelet volume; AUC, area under curve; WBC, white blood cell.
DISCUSSION
In this study, we found that high MPV level was associated with the development of cerebral WMH. The subjects with high MPV level had increased chance of cerebral WMH in the brain after adjusting for age, sex, and classical vascular risk factors. Furthermore, this association remained statistically significant after controlling for the presence of atherosclerosis of major cerebral arteries. This finding suggested that MPV level might be an independent hematological index to identify the development of cerebral WMH in non-stroke individuals. That is, the association of MPV level and cerebral WMH indicates that platelet activation plays a role in the development of cerebral WMH, presumably via the mechanism of microthrombosis at the site of the end-zone of cerebral microvasculature.
MPV level is one of the indicators of platelet activity accounting for much platelet-derived granule secretions, thromboxane synthesis, and expression of glycoprotein IIb/IIIa receptors.12,13 Larger platelets contain more granules and lead to thrombosis at vessels in atherosclerosis. It is well known that MPV level corresponds to severity and clinical outcome in atherothrombotic diseases. MPV level also predicts restenosis after coronary and carotid angioplasty19 and long-term outcome in myocardial infarction treated with primary coronary intervention.16,17 High MPV level is positively associated with severity and a worse outcome in ischemic stroke.20,21 MPV level was an independent predictor for 90-day outcomes in stroke patients receiving thrombolysis.22 Another study showed that MPV level was higher in patients with ischemic stroke compared to the controls, and predicted poor outcomes.32,33 Although it is well known that MPV level is a useful blood parameter to predict atherosclerotic status of large cerebral arteries, it still remains unclear whether MPV level is associated with the status of cerebral microvasculature.
Given that high MPV level represents large platelets and the highly activated status of platelets, our study is comparable to previous studies which showed that activated platelets contribute to the development of cerebral WMH.10,11,34 Glycoprotein IIb/IIIa expression, numbers of platelet-derived microvesicles, and total number of thrombogenic microvesicles at baseline were associated with cerebral WMH load and progression in menopausal women.11 Another study showed a close relationship between cerebral WMH and platelet hyper-aggregability in systemic blood.34 The association between cerebral WMH and platelet activation is attributable to the anatomical and physiological property of cerebral microvasculature in white matter.34 The vascular endothelium in the cerebral microvasculature has distinctive properties, and the concentration of thrombomodulin, which regulates thrombin activity, is relatively low on the luminal surface of vessels in cerebral white matter.35 Interestingly, some recent studies found that MPV level is associated with leukoaraiosis and white matter volume.25,36 In those studies, MPV was found to be independently and positively associated with leukoaraiosis in apparently healthy elderly subjects.36 The results from our study supported the idea that platelet activation plays a role in cerebral WMH development, even in younger subjects.
Several limitations in our study should be addressed. First, the retrospective design of our study could result in selection bias. Second, although our study subjects had no neurological problems, they were visitors at an outpatient department; therefore, they would have a higher prevalence of cardiovascular risk factors compared to a healthy general population. A prospective study in the general population is required to validate our results. Third, we measured the severity of cerebral WMH using a visual rating scale, which cannot represent the volume of cerebral WMH. Although WMH severity was measured using the Fazekas score in this study, it is controversial whether WMH severity can be evaluated only with the Fazekas score, rather than quantitative WMH volume measurement. However, the visual rating method has been widely used, and it shows a high correlation with a quantitative method.37 Fourth, although we collected and adjusted for multiple risk factors and laboratory findings where possible, no data were available for a more detailed parameter regarding platelet activation and thrombogenicity. More accurate laboratory data for platelet activation and thrombogenicity should be obtained in future studies. Fifth, we did not consider the use and frequency of antithrombotic treatment. Although this study was conducted on neurologically healthy participants, the use of antiplatelets and anticoagulants is related to CSVD and WMH; therefore, future studies considering this will be required.
In the present study, we found that high MPV level was associated with the prevalence of cerebral WMH. On the basis of previous studies and our study, we suggest that platelet activation contributes to the development of cerebral WMH, and that MPV level is a useful indicator for the presence of cerebral WMH.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung-Won Choi, Kee Ook Lee, and Seung-Hun Oh.
- Data curation: Kee Ook Lee and Seung-Hun Oh.
- Formal analysis: Kee Ook Lee.
- Investigation: all authors.
- Methodology: Jung-Won Choi, Kee Ook Lee, and Seung-Hun Oh.
- Project administration: Kee Ook Lee and Seung-Hun Oh.
- Resources: Jung-Won Choi, Kee Ook Lee, and Seung-Hun Oh.
- Software: Kee Ook Lee.
- Supervision: Kee Ook Lee and Seung-Hun Oh.
- Validation: Jung-Won Choi, Kee Ook Lee, and Seung-Hun Oh.
- Visualization: Kee Ook Lee.
- Writing—original draft: Jung-Won Choi, Kee Ook Lee, and Seung-Hun Oh.
- Writing—review & editing: Kee Ook Lee.
- Approval of final manuscript: all authors.
AVAILABILITY OF DATA AND MATERIAL
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 2.Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henninger N, Khan MA, Zhang J, Moonis M, Goddeau RP., Jr Leukoaraiosis predicts cortical infarct volume after distal middle cerebral artery occlusion. Stroke. 2014;45:689–695. doi: 10.1161/STROKEAHA.113.002855. [DOI] [PubMed] [Google Scholar]
- 4.Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J. 2012;88:79–87. doi: 10.1136/postgradmedj-2011-130307. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 6.Song TJ, Chang Y, Kim AR, Kim Y, Kim YJ. High dietary glycemic load was associated with the presence and burden of cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res. 2018;51:93–101. doi: 10.1016/j.nutres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Song TJ, Chang Y, Shin MJ, Heo JH, Kim YJ. Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res. 2015;35:368–374. doi: 10.1016/j.nutres.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Song TJ, Kim YD, Yoo J, Kim J, Chang HJ, Hong GR, et al. Association between aortic atheroma and cerebral small vessel disease in patients with ischemic stroke. J Stroke. 2016;18:312–320. doi: 10.5853/jos.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song TJ, Park JH, Choi KH, Chang Y, Moon J, Kim JH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36–42. doi: 10.1016/j.sleep.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Jayachandran M, Lahr BD, Bailey KR, Miller VM, Kantarci K. Menopausal hormone therapy, blood thrombogenicity, and development of white matter hyperintensities in women of the Kronos Early Estrogen Prevention Study. Menopause. 2020;27:305–310. doi: 10.1097/GME.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles H, Smith RE, Martin JF. Platelet glycoprotein IIb-IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24:69–72. doi: 10.1111/j.1365-2362.1994.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 13.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–519. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 14.Karabacak M, Dogan A, Turkdogan AK, Kapci M, Duman A, Akpinar O. Mean platelet volume is increased in patients with hypertensive crises. Platelets. 2014;25:423–426. doi: 10.3109/09537104.2013.830181. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Salvagno GL, Nouvenne A, Meschi T, Borghi L, Targher G. The mean platelet volume is significantly associated with higher glycated hemoglobin in a large population of unselected outpatients. Prim Care Diabetes. 2015;9:226–230. doi: 10.1016/j.pcd.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 17.Wasilewski J, Desperak P, Hawranek M, Ciślak A, Osadnik T, PykaŁ, et al. Prognostic implications of mean platelet volume on short- and long-term outcomes among patients with non-ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: a single-center large observational study. Platelets. 2016;27:452–458. doi: 10.3109/09537104.2016.1143919. [DOI] [PubMed] [Google Scholar]
- 18.Yang A, Pizzulli L, Lüderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb Res. 2006;117:371–377. doi: 10.1016/j.thromres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Dai Z, Gao J, Li S, Li R, Chen Z, Liang M, et al. Mean platelet volume as a predictor for restenosis after carotid angioplasty and stenting. Stroke. 2018;49:872–876. doi: 10.1161/STROKEAHA.117.019748. [DOI] [PubMed] [Google Scholar]
- 20.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 21.Muscari A, Puddu GM, Cenni A, Silvestri MG, Giuzio R, Rosati M, et al. Mean platelet volume (MPV) increase during acute non-lacunar ischemic strokes. Thromb Res. 2009;123:587–591. doi: 10.1016/j.thromres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Xie D, Xiang W, Weng Y, Li J, Xu L, Zhang X, et al. Platelet volume indices for the prognosis of acute ischemic stroke patients with intravenous thrombolysis. Int J Neurosci. 2019;129:344–349. doi: 10.1080/00207454.2018.1536054. [DOI] [PubMed] [Google Scholar]
- 23.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–1515. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 25.Choi S, Bush AM, Borzage MT, Joshi AA, Mack WJ, Coates TD, et al. Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients. Neuroimage Clin. 2017;15:239–246. doi: 10.1016/j.nicl.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 27.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 29.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 30.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 32.Butterworth RJ, Bath PM. The relationship between mean platelet volume, stroke subtype and clinical outcome. Platelets. 1998;9:359–364. doi: 10.1080/09537109876429. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley T, Langhorne P, Elton RA, Stewart C. Platelet size in stroke patients. Stroke. 1995;26:995–999. doi: 10.1161/01.str.26.6.995. [DOI] [PubMed] [Google Scholar]
- 34.Fujita S, Kawaguchi T. Association of platelet hyper-aggregability with leukoaraiosis. Acta Neurol Scand. 2002;105:445–449. doi: 10.1034/j.1600-0404.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101:363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SJ, Park BJ, Shim JY, Lee HR, Hong JM, Lee YJ. Mean platelet volume (MPV) is associated with leukoaraiosis in the apparently healthy elderly. Arch Gerontol Geriatr. 2012;54:e118–e121. doi: 10.1016/j.archger.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Valdés Hernández Mdel C, Morris Z, Dickie DA, Royle NA, Muñoz Maniega S, Aribisala BS, et al. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology. 2013;40:13–22. doi: 10.1159/000341859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.