Summary
Defects in myofibroblast function may cause wound healing defects in a variety of tissue types. Here we describe a simple skin-punch biopsy approach to screen mouse models for defects in wound closure that does not require extensive surgical training or expensive equipment. Experimental results may serve as an initial proof of concept to determine whether further investigation is necessary or if defects in myofibroblast function observed in other systems also result in reduced skin wound healing.
Subject areas: Cell Biology, Model Organisms, Signal Transduction
Graphical abstract
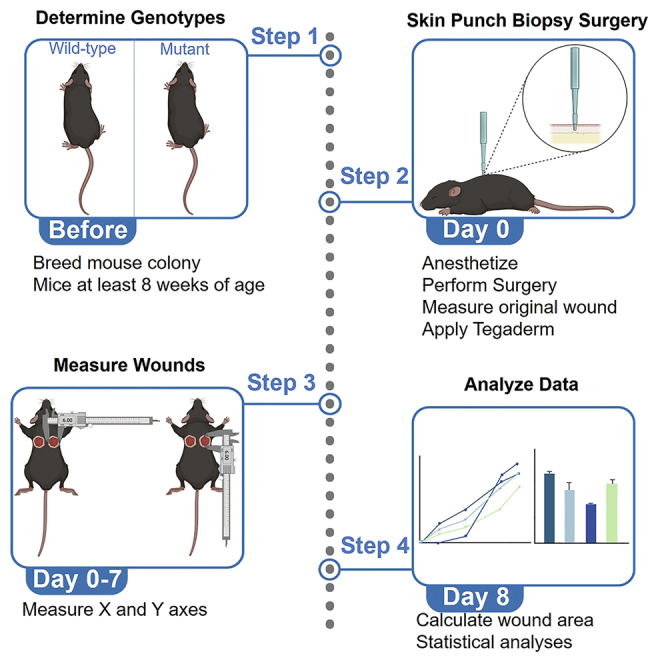
Highlights
-
•
Skin-punch biopsy protocol to investigate myofibroblast function in wound closure
-
•
Step-by-step instructions for procedure, documentation, and analysis
-
•
A simple surgical procedure as a proof-of-concept study for future experiments
-
•
Easily modified to assess topical treatments using an internal control
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Defects in myofibroblast function may cause wound healing defects in a variety of tissue types. Here we describe a simple skin-punch biopsy approach to screen mouse models for defects in wound closure that does not require extensive surgical training or expensive equipment. Experimental results may serve as an initial proof of concept to determine whether further investigation is necessary or if defects in myofibroblast function observed in other systems also result in reduced skin wound healing.
Before you begin
This protocol details the process of evaluating myofibroblast function1,2 using a skin wound healing model. Specifically, we have used this wound healing model to assess defects in untargeted gene-deleted C57BL/6J mouse models. This protocol may also be employed for cell type-specific deletion using targeted approaches such as Cre-Lox (the PostnMerCreMer line,3 for example) although this may require additional time, resources, and analyses. We have adapted this protocol to be effective for a variety of relevant loss-of-function mouse models and investigations exploring myofibroblast function. Prior to beginning the skin punch biopsies, the timing of mouse line breeding should be taken into consideration, and the surgical suite should be properly equipped.
Institutional permissions
All experiments performed using mouse models require permissions and approvals from the relevant institutions and must follow institutional and national regulations. All procedures in this protocol were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama (Protocol # 18-08-1483). Both male and female mice were used in our experiments. All animals had uninterrupted access to food and water, and all surgical techniques applied aseptic guidelines to prevent potential infection.
Prepare mouse genotypes and identify subjects
Timing: 8–12 months
-
1.Proper colony management is critical to the success of these experiments.
- a.
-
b.The breeding preparation should take into consideration the reproductive lifespan, ages of sexual maturity, and gestation period of the mouse line to be investigated, as this may vary depending on phenotype.
-
2.To maximize experimental rigor and reduce bias, investigators should incorporate blinding and randomization to all studies where possible.
-
a.Clear policies for all experimental procedures, data collection, and analysis should be generated for each study, which will increase transparency and reproducibility.
-
b.Mice of both sexes may be enrolled in the study, although we have observed sex differences in our results (see limitations section).
-
c.Sample sizes should be equal between experimental groups but must be determined empirically. We have demonstrated significant sex differences in skin wound healing using sample sizes of 15–16 mice per group (Figure 4).
-
a.
Note: Female mice picked for the experiment should not be actively breeding to remove pregnancy as a confounding variable.
Figure 4.
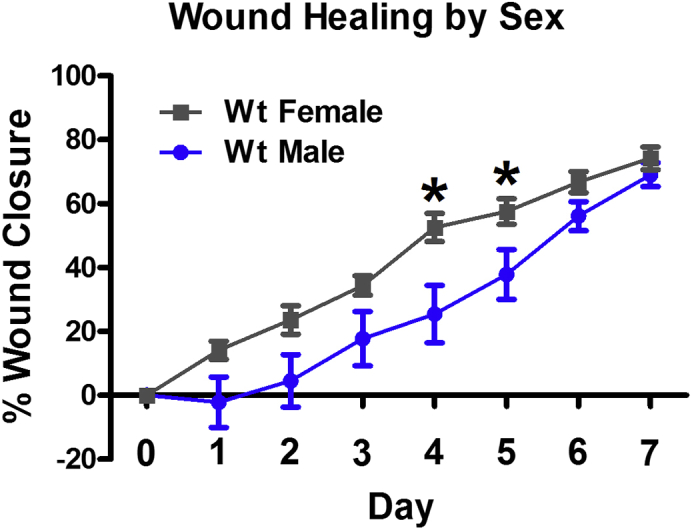
Male and female mice have different rates of wound healing
Wild-type female mice have significantly faster wound closure than wild-type male mice on post-surgery days 4 and 5. ∗p < 0.05 vs Wt Male.
Create a data sheet
Timing: 20 min
-
3.Using the mice identified as the control and experimental groups, create a data sheet (Table 1).
-
a.The data sheet should include:
-
i.Date of each day of measurements.
-
ii.Mouse identification number.
-
iii.Location to write down the X axis and Y axis measurements.
-
i.
-
a.
-
4.
Print the data sheet prior to surgery.
Table 1.
Example of raw data on Days 0 and 1 measured with calipers
| Day 0 | |||||
| 1 ♂ | WT | 2 ♂ | WT | 3 ♀ | WT |
| RX 5.5 | LX 6.0 | RX 7.8 | LX 5.2 | RX 7.1 | LX 6.1 |
| RY 5.3 | LY 6.9 | RY 6.0 | LY 5.8 | RY 6.3 | LY 5.6 |
| 4 ♀ | WT | 5 ♀ | WT | 6 ♀ | WT |
| RX 6.5 | LX 5.6 | RX 5.8 | LX 5.8 | RX 7.4 | LX 6.1 |
| RY 6.8 | LY 7.2 | RY 6.2 | LY 7.2 | RY 5.1 | LY 4.4 |
| Day 1 | |||||
| 1 ♂ | WT | 2 ♂ | WT | 3 ♀ | WT |
| RX 7.3 | LX 6.8 | RX 9.2 | LX 5.0 | RX 6.7 | LX 4.9 |
| RY 5.6 | LY 4.6 | RY 4.3 | LY 3.8 | RY 6.3 | LY 6.5 |
| 4 ♀ | WT | 5 ♀ | WT | 6 ♀ | WT |
| RX 7.4 | LX 6.7 | RX 6.5 | LX 5.6 | RX 6.1 | LX 6.5 |
| RY 5.0 | LY 5.9 | RY 5.3 | LY 4.8 | RY 3.4 | LY 2.9 |
Separate mice into individual cages
Timing: 15 min
-
5.
Prepare additional caging materials so mice may be housed individually.
Note: If the mice that have undergone a skin punch biopsy are kept in a cage with other mice, there is possibility of barbering that can aggravate the injury and influence the accuracy of the wound healing measurements.
Optional: To provide additional comfort and environmental enrichment for single-housed mice, researchers may choose to add extra nesting materials, wooden blocks, or houses to the cages.6
-
6.
Using the mouse identification numbers, separate mice chosen for each control and experimental group into their own cage.
Note: Experimenters performing surgeries and measuring wound areas should be blinded to mouse genotype to reduce bias. For this reason we recommend only including mouse identification numbers on the cage cards. This can be re-associated with genotype once all of the data have been collected.
Set up the surgical suite
Timing: 15–20 min
-
7.
Make sure all materials are positioned in a way to have easy access during the procedure.
-
8.
Place a surgical cloth on top of a warming pad on the surgical bench. Warming pad should be set to low heat only (between 37°–38°C).7
-
9.Fix the anesthesia/oxygen tube onto the surgical bench.
-
a.This ensures that the tube will not move during the procedures.
-
a.
-
10.Cut pieces of Tegaderm just large enough to cover the area of a 6 mm wound.
-
a.Always cut extra pieces to prepare for potential mistakes.
-
b.Cutting the Tegaderm beforehand makes sure that when the mice are under anesthesia, the least amount of time possible is used.
-
a.
-
11.
Pour approximately 10 mL of Betadine into a small bowl.
-
12.
Dilute 1 mL of Buprenorphine Hydrochloride with 9 mL of 0.9% Saline Solution.
-
13.Fill the syringes with the 1:10 diluted Buprenorphine Hydrochloride solution.
-
a.This ensures that time is not wasted during the surgical procedures.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Isoflurane, USP | Pivetal | 46066-755-04 |
| Betadine vet surgical scrub | Purdue Pharma | 67618-154-01 |
| Ethyl alcohol, pure, 100% | Sigma-Aldrich | 64-17-5 |
| Nair hair remover lotion with soothing aloe & lanolin | Church & Dwight Co., Inc. | N/A |
| Buprenorphine hydrochloride, injection, 0.3 mg/mL | PAR Pharmaceutical | 42023-179-05 |
| 0.9% saline solution | Teknova | S5825 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J, 8–24 weeks old, male & female | Jackson Labs | 000664 |
| Others | ||
| 0.5 mL insulin syringe with 0.36 mm (28G) permanently attached needle | BD Medical | 329461 |
| Oster Golden A5 Single Speed Clipper | Oster Pro | 78005010003 |
| Traceable carbon fiber calipers 4in. | VWR | 36934-152 |
| Cotton tipped wood applicators, sterile, 6″ | Dynarex | 4305 |
| Non-sterile woven gauze sponge, 4 IN × 4 IN, 12-ply, 100% cotton | Pivetal | 21295050 |
| Sterile disposable biopsy punch, 6 mm | Integra | 33–36 |
| Tegaderm transparent film roll | 3M | 16002 |
| Fine dressing forceps, 10 cm Long | Kent Scientific Corporation | INS650914-4 |
| Iris scissors, straight, 11 cm Long | Kent Scientific Corporation | INS750216-2 |
| Oxygen gas | Airgas | N/A |
Materials and equipment
1:10 Buprenorphine Hydrochloride Solution:
-
•
1 mL Buprenorphine Hydrochloride (0.3 mg/mL).
-
•
9 mL Saline Solution (0.9%).
Store at room temperature for up to one month.
Step-by-step method details
Anesthetize mice
Timing: 3–5 min
This section is the step-by-step procedure to monitor depth of anesthesia and ensure the mice have lost the sensation of pain before performing any surgical methods.
-
1.Place the mouse in an anesthesia induction chamber with 0.5 L per minute (LPM) of oxygen and 5% isoflurane.
-
a.Turn on the oxygen tank so that it is set to 0.5 LPM. Turn on the isoflurane vaporizer to 5%.
-
b.Confirm that the oxygen and isoflurane are flowing before you bring the mouse over to the induction chamber.
-
c.Check that the oxygen flow meter ball is floating to confirm oxygen is flowing through the tubing.
-
a.
-
2.
Once the mouse’s breathing has slowed and relaxed in the induction chamber, turn the isoflurane to 2% and move the mouse onto the surgical cloth in a prone position.
-
3.
Place its nose and mouth into the nose cone.
-
4.
Assess the depth of anesthesia using a firm toe pinch.
-
5.
Continuously monitor tail, foot, or ear for blue coloration as this could be a sign of respiratory distress.
Preparation of the wound site
Timing: 8–10 min
This is the step-by step procedure to remove all hair from and sterilize the surgical site.
-
6.
With one hand, gently pull the skin on the dorsal side of the body toward the inferior portion of the mouse.
Note: The skin tends to gather under the ears. If you do not pull the skin down, you will not remove the hair above the shoulders and the skin punch biopsy will be performed much lower than expected.
-
7.Using the clippers, shave the dorsal side of the mouse.
-
a.Ensure that the shaved area is large enough to make two skin punch biopsies.
-
b.Trim about halfway down the back and laterally toward each front appendage.
-
a.
-
8.
Add minimal depilatory cream onto a cotton swab and apply it to the area where the hair was trimmed.
-
9.After about 1 min, wipe off the depilatory cream with a piece of gauze and 70% ethanol on the shaved area.
-
a.This will help wipe off the extra depilatory cream and sterilize the area.
-
a.
Note: Do not leave the depilatory cream on the mouse too long or the skin will become damaged (see troubleshooting problem 1).
Optional: If there is still hair present, add a small amount of depilatory cream to the area and wait 30 s to one minute, and repeat the wash with 70% ethanol.
-
10.
Using a cotton swab, apply Betadine to the shaved location on the back of the mouse to sterilize the area.
-
11.
Pause for a moment to let the Betadine slightly dry so that it will be easier to perform the skin punch.
Performing the skin punch biopsy
Timing: 8–10 min
This is the step-by-step procedure to perform two skin punch biopsies on each mouse.
-
12.
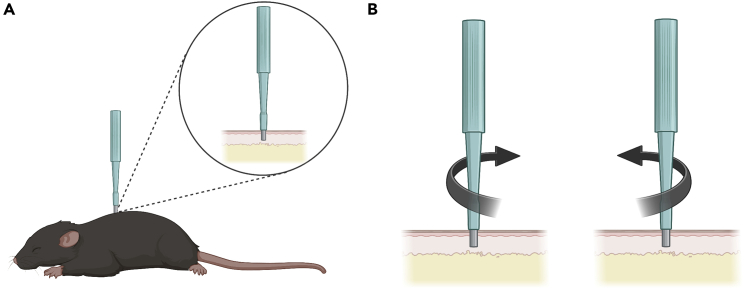
Using a sterile skin punch, apply pressure lateral to the spine on the right (Figure 1A). The pressure applied to the skin should create a full thickness cutaneous injury extending through the panniculus carnosus.8,9
-
13.Twist the skin punch biopsy clockwise and counterclockwise until you feel that the biopsy punch punctured the full thickness of the skin (Figure 1B) (see troubleshooting problem 5).
-
a.If the skin punch needs to be lifted, make sure to place it back exactly where you initially had it placed. A circular indentation should be visible on the skin from the biopsy punch.
-
b.Apply force downward until “release” is felt, which is an indication that the punch tool entered the subcutaneous area.
-
a.
-
14.
If a clean cut with the skin punch was not made, use forceps to pull the skin upward, and carefully cut a full thickness wound in the skin while following the indentation line with sterile scissors.
-
15.
Once a circular punch on the right side of the mouse is performed, follow the same steps to complete a second punch on the left side of the mouse’s back.
Figure 1.
Performing the skin punch biopsy with a sterile disposable biopsy punch
(A) Apply pressure lateral to the spine on the mouse with a sterile disposable biopsy punch.
(B) With pressure, twist the sterile disposable biopsy punch clockwise and counterclockwise until you feel that the biopsy punch has punctured the skin.
Wound measurements
Timing: 1–2 min
This is the step-by-step procedure to measure the X and Y axes of each biopsy wound.
-
16.
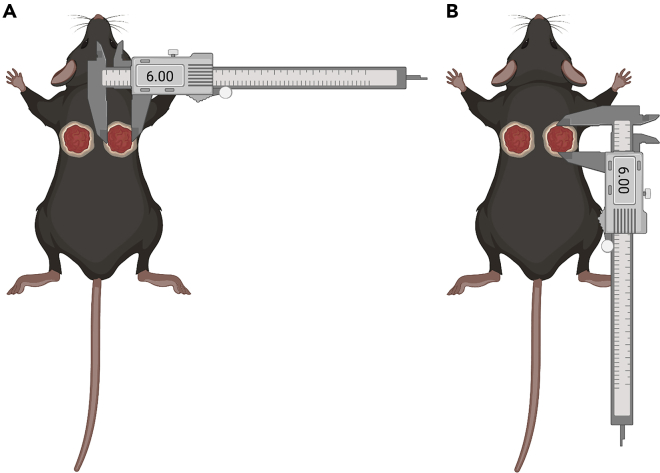
Using a caliper, measure in millimeters the X axis or the longest point spanning the length of the wound (Figure 2A).
-
17.
Record the measurement in the correct space on the data sheet according to X axis and mouse identification number (Figure 2A).
-
18.
Using a caliper, measure in millimeters the Y axis or the height of the wound (Figure 2B).
-
19.
Record the measurement in the correct space on the data sheet according to Y axis and mouse identification number (Figure 2B).
Figure 2.
Measuring biopsy wounds in mice
(A) Schematic demonstrating measurement of the longest point spanning the length of the wound using digital calipers.
(B) Schematic demonstrating measurement of the height of the wound using digital calipers.
Wound and animal care
Timing: 30 min
This is the step-by-step procedure to properly tend to the biopsy wounds.
-
20.
If Tegaderm is not already prepared, cut a piece of Tegaderm into a small square that is only big enough to cover the area of the open wound.
-
21.
Gently place the Tegaderm on the wound (see troubleshooting problem 2).
-
22.
Repeat steps 20 and 21 for the second wound.
-
23.
Using a sterile syringe with a 0.36 mm permanently attached needle, place the needle parallel to the skin and approximately 3 mm from one of the wounds. Perform a single 0.1 mL subcutaneous injection of the 1:10 Buprenorphine Hydrochloride dilution per mouse.
Note: Do not inject the Buprenorphine Hydrochloride solution too quickly or remove the syringe too quickly. Ensure that the needle is deep enough so the Buprenorphine Hydrochloride solution does not come out of the injection site. The full length of the 0.36 mm needle should be inserted underneath the skin for the injection.
-
24.
Gently take the mouse out of the nose cone and place it back into its cage.
-
25.
For the next 15–25 min, monitor the mouse for movement and quickened breathing.
Expected outcomes
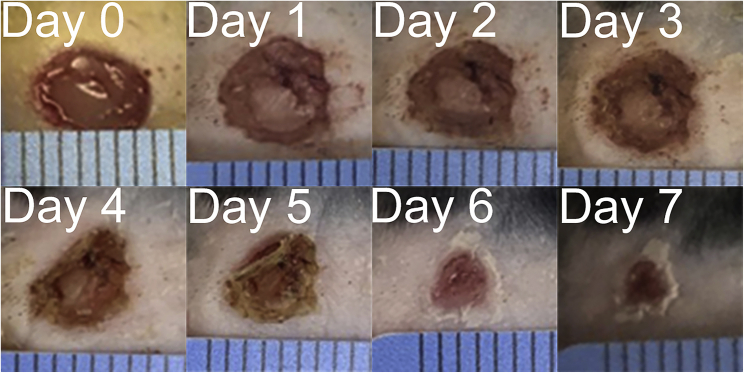
In our experience, wild-type mice will experience approximately 70–80 percent wound closure by seven days post-surgery (Figure 3). This may differ depending on genotype but should stay consistent in wild-type control mice, which will have an obvious visual improvement in wound appearance (Figure 3). Careful record-keeping will allow for qualitative and quantitative observations of wound progression.
Figure 3.
Photographs of skin wound closure in a wild-type mouse on days 0–7 post biopsy
Using a 6 mm dermal punch, wild-type mice were injured on the dorsal side. The wounds were measured daily for one week. Wound healing progression was quantified, graphed, and compared. By 7 days post-surgery, the wound is nearly healed (see troubleshooting problem 4).
We have successfully used this protocol to demonstrate differences in wound closure in gene deleted mice lacking Atf6 and Atf6b. Additionally, this protocol is similar to others previously used to determine the functions of additional genes involved in myofibroblast differentiation and wound healing.10,11,12
Quantification and statistical analysis
To quantify percent wound closure, the X axis and Y axis measurements for each individual mouse on each day were divided by two to get the semimajor and semiminor axes of each. The semimajor axis of both wounds on day zero were averaged together and the semiminor axis of both wounds on day zero were averaged together. This was repeated for seven more days of measurements. These values were then taken to find the area of an ellipse using the equation A = πab. The area of the wound for a mouse on a particular day was divided by the area of that wound on day zero. This value was subtracted by one and then multiplied by one hundred. This will result in the percent that the wound closed on that day for an individual mouse. To perform the statistical analyses and assess differences in wound closure between genotypes, Graphpad Prism 5 was used. Data should be analyzed with the appropriate statistical test (see troubleshooting problem 3). For Figure 4, a simple t-test comparing wound healing between male and female wild-type mice on each day was used.
Additionally, multilevel modeling was used to examine the rate of change post-surgery (i.e., growth curve analysis). A quadratic growth curve was used with the interactions sex∗day and sex∗day2 that enabled testing if wound closure changed differently for female relative to male mice. Given significant interactions, growth curves would be estimated separately for female and male mice. The model was estimated without an intercept because % wound closure was 0 on day 0. The random effect of day was estimated.
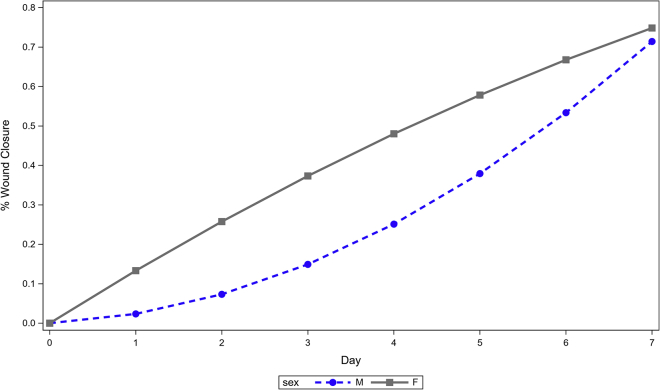
Model 1 in Table 2 presented results from the model that had the interactions. Both interactions terms were significant (p < .01), suggesting that % wound closure changed differently for female relative to male mice. Model 2 was mathematically equivalent to Model 1 except that it estimated the growth curves separately for female and male mice. The growth of % wound closure in female mice was quite linear (day, p < .01, day2, p = .059), while that for male mice had some curvature (day, p = .513, day2, p < .01) (Figure 5). It appeared that up to 4 days post-surgery female mice exhibited faster rate of change in % wound closure than male mice. After that, the rate of change was faster in male than in female mice.
Table 2.
Estimated parameters for growth curve analysis
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Female |
Male |
|||||
| Estimate | p | Estimate | p | Estimate | p | |
| Day | .010 | .513 | .138 | <.01 | .010 | .513 |
| Day∗Sex | .127 | <.01 | ||||
| Day2 | .013 | <.01 | −.004 | .059 | .013 | <.01 |
| Day2∗Sex | −.017 | <.01 | ||||
Figure 5.
Estimated growth curves for % wound closure in female and male mice
Up to 4 days post-surgery, female mice exhibited faster rate of change in % wound closure than male mice. After 4 days, the rate of change was faster in male mice than in female mice
One advantage of this protocol is that it can be modified to assess the effect of a treatment on one wound, using the other untreated wound as an internal control. For example, one dermal wound can be treated topically with an adenovirus while the other control wound is left untreated.10
Limitations
Mouse skin is composed of a superficial epidermis, dermis, subcutaneous adipose layer, and a deep panniculus carnosus muscle.13 The panniculus carnosus is largely absent in humans. However, in mice it appears to play a role in skin healing via wound closure, which underlies wound resolution to a greater extent in loose-skinned animals than in humans and primarily results from the myofibroblast activity.13,14 Because of these physiological differences causing more efficient wound closure in mouse models, previous work has largely used splinted wound circumference to simulate typical human skin repair via activity of the granulation tissue and re-epithelialization.15 Because this protocol is designed to investigate myofibroblast function specifically, the superior mouse wound contraction phenotype (largely resulting from myofibroblast activity) is a benefit, and thus these biopsy injuries do not use a splint. These inherent differences between wound healing properties of mouse and human skin must be kept in mind by investigators.
We have consistently observed accelerated wound healing in female mice consistent with reports in the literature.16 In C57BL6/J mice, we find that females heal significantly faster than male mice on days 4–5 post surgery (p < 0.05), with a strong trend towards faster wound healing on days 1–3, and on day 6 (p < 0.085) (Figure 4). For this reason, we recommend that all investigators independently assess sex differences in their model systems.
Occasionally, the Tegaderm may fall off for some mice before the 24-h mark post-surgery. Mice that have no Tegaderm tend to have slightly quicker scabbing and healing. If the Tegaderm falls off within 24 h post-surgery, it will need to be reapplied to the mice that have open wounds. Between 24–48 h post-surgery, all of the Tegaderm should be removed from the mice for the remainder of the study. This timing should be consistent for all mice in the study. It is necessary to add Tegaderm immediately following surgery to avoid substantial blood loss and infection. It may not be possible to guarantee that all mice have kept the bandage on for the exact same amount of time. This could potentially influence the resulting progression of wound healing within the first 24–48 h.
Imprecise measurement of the wound area may affect the validity of the results. Using software such as ImageJ may make the measurements and statistical analyses more reliable. If investigators choose to use an imaging software for the analysis, they must consider that photographs need to be taken from the same height, magnification, and working distance.
Troubleshooting
Problem 1
Leaving depilatory cream on too long or using too much depilatory cream may cause extra stress and additional skin damage (steps preparation of the wound site 8,9).
Potential solution
Use a minimal amount of depilatory cream necessary to remove the fur from the mouse. To avoid extra skin damage, apply the depilatory cream for a very short amount of time before removing it, and repeat this multiple times.
Problem 2
Bedding may get in the wound area even though Tegaderm was applied. Tegaderm bandage may fall off shortly after application (step wound and animal care 20, 21, 22).
Potential solution
On days 1 through 3, pay extra attention and remove bedding from the wounds with forceps. Ensure not to disturb the scab as much as possible. Reapply Tegaderm up until 24 h post-surgery to wounds where it may have fallen off.
Problem 3
Male and female mice have different rates of wound healing (Figures 4 and 5).
Potential solution
Add separate statistical analyses to compare male and female wound healing. In our studies using wild-type C57BL/6J mice, general wound healing in females happens at a quicker rate compared to males. However, any sex differences should be independently assessed in the experimenter’s lab, as this could also potentially uncover sex-specific roles for the gene of interest.
Problem 4
Depending on your mutant mouse genotype, wound closure may be delayed to the extent that the study should be continued beyond 7 days of wound measurements (expected outcomes).
Potential solution
A pilot experiment should be performed at wound closure assessed on day 7. This will provide a better perspective on the current progression of wound closure, and help researchers decide if they should expand the wound measurements beyond one week. We find that our wild-type mice will experience approximately 70–80 percent wound closure by seven days post-surgery (Figure 3).
Problem 5
It is possible for the wounds to become infected after performing this surgical procedure (step 13 under “performing the skin punch biopsy”).
Potential solution
Ensure that all proper aseptic techniques are applied while preparing the wound site. For the first 24–48 h Tegaderm should be used to protect the injury site. Single housing is used to prevent fighting and subsequent injury that could aggravate the wounds.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Robert N. Correll (rncorrell1@ua.edu).
Materials availability
This study did not generate any new or unique reagents.
Acknowledgments
This work was supported by the Department of Biological Sciences at the University of Alabama. Graphical abstract and Figures 1 and 2 were created with BioRender.com.
Author contributions
M.B.R. and R.N.C. conceived the methodology. M.B.R. performed the investigation. M.B.R. and P.E.M. wrote the original draft. C.B. performed statistical analysis and aided in interpretation of results. M.B.R., P.E.M., C.B., and R.N.C. reviewed and edited the final draft. R.N.C. supervised the work and acquired the funding and resources for this protocol.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Mary B. Rowland, Email: mbrowland@crimson.ua.edu.
Robert N. Correll, Email: rncorrell1@ua.edu.
Data and code availability
No new codes or datasets were generated by this study. However, any additional information generated by this study will be available upon request through the lead contact.
References
- 1.Davis J., Molkentin J.D. Myofibroblasts: trust your heart and let fate decide. J. Mol. Cell. Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016;64:171–177. doi: 10.1016/j.retram.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Kanisicak O., Khalil H., Ivey M.J., Karch J., Maliken B.D., Correll R.N., Brody M.J., J Lin S.C., Aronow B.J., Tallquist M.D., Molkentin J.D. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson S.J., Andrews N., Ball D., Bellantuono I., Gray J., Hachoumi L., Holmes A., Latcham J., Petrie A., Potter P., et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017;51:160–169. doi: 10.1177/0023677216653984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D.J., Mustoe T., Clark R.A.F. Cutaneous wound healing in aging small mammals: a systematic review. Wound Repair Regen. 2015;23:318–339. doi: 10.1111/wrr.12290. [DOI] [PubMed] [Google Scholar]
- 6.Zidar J., Weber E.M., Ewaldsson B., Tjader S., Lilja J., Mount J., Svensson C., Svensk E., Uden E., Tornqvist A.E. Group and single housing of male mice: collected experiences from research facilities in Sweden. Animals. 2019;9 doi: 10.3390/ani9121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolis I.N., Beale C.N., Bidot W.A., Esmail M., Perkins S.E. Performance and consistency of circulating warm water blankets for rodents. J. Am. Assoc. Lab. Anim. Sci. 2022;61:96–100. doi: 10.30802/AALAS-JAALAS-21-000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masson-Meyers D.S., Andrade T.A.M., Caetano G.F., Guimaraes F.R., Leite M.N., Leite S.N., Frade M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020;101:21–37. doi: 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong V.W., Sorkin M., Glotzbach J.P., Longaker M.T., Gurtner G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis J., Burr A.R., Davis G.F., Birnbaumer L., Molkentin J.D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J., Salomonis N., Ghearing N., Lin S.C.J., Kwong J.Q., Mohan A., Swanson M.S., Molkentin J.D. MBNL1-mediated regulation of differentiation RNAs promotes myofibroblast transformation and the fibrotic response. Nat. Commun. 2015;6:10084. doi: 10.1038/ncomms10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molkentin J.D., Bugg D., Ghearing N., Dorn L.E., Kim P., Sargent M.A., Gunaje J., Otsu K., Davis J. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation. 2017;136:549–561. doi: 10.1161/CIRCULATIONAHA.116.026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider M.R. Genetic mouse models for skin research: strategies and resources. Genesis. 2012;50:652–664. doi: 10.1002/dvg.22029. [DOI] [PubMed] [Google Scholar]
- 14.Naldaiz-Gastesi N., Bahri O.A., López de Munain A., McCullagh K.J.A., Izeta A. The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. J. Anat. 2018;233:275–288. doi: 10.1111/joa.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiano R.D., Michaels J., 5th, Dobryansky M., Levine J.P., Gurtner G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomason H.A., Williams H., Hardman M.J. In: Sex and Gender Differences in Infection and Treatments for Infectious Diseases. Klein S., Roberts C., editors. Springer; 2015. Sex and sex hormones mediate wound healing. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new codes or datasets were generated by this study. However, any additional information generated by this study will be available upon request through the lead contact.