Abstract
Omalizumab, which downregulates the immunoglobulin E (IgE) receptor site on plasmacytoid dendritic cells and thereby increases interferon-α (INF-α) production, may shorten the duration of viral infections by enhancing the antiviral immunity.
A systematic review was conducted to investigate whether previous anti-IgE treatment with omalizumab could protect against SARS-CoV-2 disease (“COVID-19”) (infection, disease duration, and severity), and whether IFN-α upregulation could be involved. The research included articles published from March 2020 to January 2022. An accurate search was performed on bibliographic biomedical database (MEDLINE – Pubmed, SCOPUS, EMBASE, BIOMED CENTRAL, Google scholar, COCHRANE LIBRARY, ClinicalTrial.gov) including cohorts, case reports and reviews. Different methods were used, based on the study design, to assess the quality of eligible studies.
Several authors link omalizumab to a possible protection against viruses, but they often refer to studies carried out before the pandemic and with viruses other than SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) (eg, rhinoviruses -RV). Few cases of COVID-19 patients treated with omalizumab have been recorded, and, in most of them, no increased susceptibility to severe disease was observed. According to these data, the current indication is to continue omalizumab therapy during the pandemic.
Moreover, although omalizumab may enhance the antiviral immune response even for SARS-CoV-2, further studies are needed to confirm this hypothesis. It would be helpful to establish a registry of omalizumab-treated (or in treatment) patients who have developed COVID-19. Finally, randomized controlled trials could be able to demonstrate the effect of omalizumab in protecting against severe SARS-CoV-2, through IFN-α upregulation or other immunological pathways.
Keywords: COVID-19, Interferon, IFN-Alpha, Omalizumab, SARS-CoV-2
Introduction
COVID-19 spread rapidly around the world causing numerous deaths. The immune response against SARS-CoV-2 follow several stages. During the early stages of the disease, type I interferon (including IFN-α) and adaptive immunity preclude the disease progression.1, 2, 3, 4, 5, 6, 7 When this immune response is impaired, high virus loads lead to pathological inflammation, massive organ dysfunction, and acute respiratory distress syndrome (ARDS).9 This hyperinflammatory immune response, evoked by immune cells and various cytokines,6 causes an exaggerated immune response (called “cytokine storm”) with multi-organ damage, leading to high mortality.1,10
Type I interferon is a family of potent antiviral cytokines that restricts viral replication and improves antiviral immunity.3 Each cell can produce IFN (type I), but only plasmacytoid cells can produce it rapidly in high quantities.4 In humans, the IFN type I multigene cytokine family includes 13 subtypes of IFN-α, only one IFN-β, a single (poorly defined) IFN-ε, IFN-κ, and IFN-ω subtypes.11 IFN-α acts at cellular and intercellular level with autocrine and paracrine activities, hindering the spread of the virus and above all eliminating virus-infected cells.12
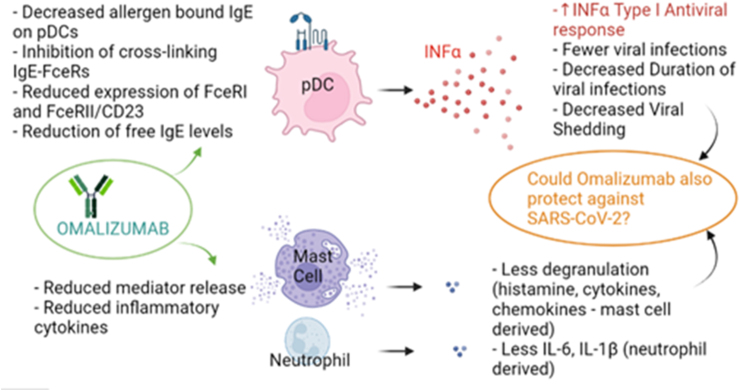
Omalizumab is actually approved to treat severe asthma and also chronic spontaneous urticaria.13 IgEs are a very important part of hypersensitivity; they also play a role in susceptibility to respiratory infections and they can activate mast cells or inhibit inflammatory cells such as neutrophils.2 Furthermore, after omalizumab treatment, interleukin 33 levels decrease. It's important to notice that IL-33 is involved in production of inflammatory cytokines (such as IL-6, IL-1 β, tumor necrosis factor and prostaglandin D2).14 Some studies showed a synergistic effect between omalizumab and immunotherapy to induce the development of regulatory T cells.15 Anti-IgE antibody seems also able to upregulate the production of IFNs (with a complex mechanism that prevent IgE-FcεRI cross-linking on dendritic cells).2 Between IgE levels and IFN-α levels a negative correlation has been demonstrated.2,16 Toll-like receptor-9 expression and TLR-7 signaling usually enhance interferon production from pDCs, but they are reduced by IgE cross-linking (Fig. 1).
Fig. 1.
Multiple effects of omalizumab. (IgE immunoglobulin E; pDCs plasmacytoid dendritic cells; FceRs Fc epsilon receptor, type I and II; INF α interferon alpha; IL-6 interleukin 6; IL1 beta interleukin 1 beta)
Some authors noticed that omalizumab could stabilize effector cells and make innate immunity much more efficient at fighting some respiratory viral infections.2,3 It appears to improve antiviral immunity mainly by downregulating IgE receptor on plasmacytoid dendritic cells.17
No increased susceptibility for severe COVID-19 was observed in some cohorts of omalizumab-treated patients who contracted the disease.17, 18, 19 The PROSE study showed that anti-IgE antibody (omalizumab) reduces respiratory virus-associated asthma exacerbations in patients with severe asthma.20 We can suppose a potential omalizumab-induced effect on antiviral responses also against SARS-CoV-2.
To investigate whether omalizumab treatment could protect against severe COVID-19 and whether type I IFN (especially IFN-α) upregulation could be involved a systematic review was performed. We analyzed available evidence to understand whether concurrent or previous use of omalizumab could have a protective effects on SARS-CoV-2 infection and COVID-19 severity and/or duration.
Materials and methods
An extensive literature search using validated keyword and filters to select articles related to omalizumab as a potential IFN I inducer to prevent and/or protect against SARS-CoV-2 infection was conducted.
We considered articles published from March 2020 to January 2022, a selective search was performed on bibliographic biomedical databases (MEDLINE (PubMed): https://pubmed.ncbi.nlm.nih.gov/, EMBASE: https://www.embase.com/#search/, SCOPUS: https://www.scopus.com/search/form.uri?display=basic#basic - accessed on 20 January 2021, Google Scholar: https://scholar.google.com/, BIOMED CENTRAL https://www.biomedcentral.com/, COCHRANE LIBRARY https://www.cochranelibrary.com/, ClinicalTrial.gov).
Following the PICO process we developed a literature search strategies including patients treated (or in treatment) with omalizumab who had COVID-19 (P –population), investigating a possible positive effect of omalizumab mediated by increase virus-induced IFN-α responses (I- Intervention), comparing with COVID-19 patients without omalizumab treatment (C – Comparison), in order to clarify if a protective effects on COVID-19 could be present, or an effect on his duration or severity (O – Outcome). We wrote a protocol including review questions, research strategy, inclusion/exclusion criteria, and eventual study bias.
At first, titles and abstracts were screened to choose eligible studies, then full texts were selected for the final evaluation. Systematic reviews, research papers, letters to the editors, abstracts, case reports and series were included, considering the limited time window of pandemic.
Inclusion criteria were:
-
1)
full text availability
-
2)
English language
-
3)
presence of the keywords “omalizumab”AND“COVID-19″AND“interferon”. The following Mesh term were used: “omalizumab”; “COVID-19”; “interferon”.
Exclusion criteria were:
-
•
articles with themes or topics not related to the present review
-
•
duplicate articles (when one article appeared in different literature sources, we only counted it one time)
-
•
outcomes far away from our purposes
Primary outcome
-
1)
to evaluate if omalizumab is a potential IFN I inducer, thus preventing COVID-19 and/or limiting COVID-19 severity.
Secondary outcomes
-
1)
to analyze omalizumab adverse events in patients with previous or concomitant SARS CoV-2 infection
-
2)
to evaluate the continuation of biological therapy with omalizumab during the illness
-
3)
to dissect possible bias and assess the quality of evidence
Most authoritative documents were considered on the basis of chronological parameters, in particular date of publication, to set up a definite research path and a coherent development of the argument supporting conclusions.
Two reviewers independently agreed on the selection of studies. The characteristics of included studies were clarified and unified in a graphical way in Table 1.
Table 1.
Results of the literature review. (aICU: intensive care unit)
| N | Study | Year | Type of study | Scale/score | Population/comparator | “Intervention" | Outcome | Founding |
|---|---|---|---|---|---|---|---|---|
| Omalizumab for a better antiviral response (increasing IFN-α production) (e.g. against rhinovirus, influenzavirus, etc.) | ||||||||
| 1 | Farmani A.R. | 2021 | Review | AMSTAR low | / | / | / | no |
| 2 | Menzella F. | 2021 | Review | AMSTAR low | / | / | / | no |
| 3 | Costanzo G. | 2021 | Review | AMSTAR low | / | / | / | no |
| 4 | Greene T.T. | 2021 | Review | AMSTAR low | / | / | / | YES, NIH grants AI145314, AI081923, AI113923 and AI132122 |
| 5 | Padayachee Y. | 2021 | Narrative Review | SANRA 1-0-0-2-1-1 | / | / | / | no |
| 6 | Bencze D. | 2021 | Review | AMSTAR low | / | / | / | YES, NKFIH FK 128294 to K.P., NKFIH PD 135193;, also supported by GINOP-2.3.2-15-2016-00050 project |
| 7 | Galati D. | 2021 | Narrative Review | SANRA 2-2-0-2-2-2 | / | / | / | no |
| 8 | Kumar K. | 2020 | Narrative Review | SANRA 2-2-0-2-2-2 | / | / | / | YES, Novartis |
| 9 | Criado P.R. | 2020 | Review | SANRA 2-2-0-2-2-1 | / | / | / | YES, authors are speaker of Novartis |
| 10 | Novak N. | 2020 | Narrative Review | SANRA 2-2-1-2-2-2 | / | / | / | YES, grant number: 2019-T1/BIO-12690; PI20/00351 |
| 11 | Visentin A.H. | 2020 | Review | AMSTAR low | / | / | / | no |
| 12 | Ding M. | 2021 | Narrative Review | SANRA 2-2-0-2-2-1 | / | / | / | no |
| 13 | Sarioglu N. | 2020 | Narrative Review | SANRA 1-2-0-2-1-2 | / | / | / | no |
| 14 | Assaf S.M. | 2020 | Narrative Review | SANRA 1-2-0-2-2-2 | / | / | / | no |
| 15 | Liu S. | 2020 | Narrative Review | SANRA 2-2-1-2-2-2 | / | / | / | no |
| 16 | Morais-Almeida M. | 2020 | Narrative Review | SANRA 2-2-0-2-2-2 | / | / | / | no |
| 17 | Wang C.-J. | 2021 | Review | AMSTAR low | / | / | / | no |
| 18 | Ramakrishnan, R.K. | 2021 | Narrative Review | SANRA 1-0-0-1-1-2 | / | / | / | no |
| 19 | Ayobami T. | 2020 | Narrative Review | SANRA 1-0-0-2-1-1 | / | / | / | no |
| 20 | Lin Y. | 2021 | Narrative Review | SANRA 2-1-0-2-1-2 | / | / | / | no |
| 21 | Klimek L. | 2020 | Position Paper | AMSTAR low | / | / | / | YES |
| 22 | Pfaar O. | 2021 | Position Paper | AMSTAR low | / | / | / | YES |
| 23 | Vultaggio A. | 2020 | Position Paper | AMSTAR low | / | / | / | YES |
| 24 | Paladini E. | 2021 | Pediatric case report | JBI-case report high | 1 patient in Omalizumab | COVID-19 | mild disease | YES, Novartis |
| No increased risk of COVID-19 (and severe COVID-19) in patients treated with Omalizumab | ||||||||
| 2 | Menzella F. | 2021 | Review | AMSTAR low | / | / | / | no |
| 3 | Costanzo G. | 2021 | Review | AMSTAR low | / | / | / | no |
| 9 | Criado P.R. | 2020 | Review | SANRA 2-2-0-2-2-1 | / | / | / | YES, authors are speaker of Novartis |
| 12 | Ding M. | 2021 | Narrative Review | SANRA 2-2-0-2-2-1 | / | / | / | no |
| 13 | Sarioglu N. | 2020 | Narrative Review | SANRA 1-2-0-2-1-2 | / | / | / | no |
| 14 | Assaf S.M. | 2020 | Narrative Review | SANRA 1-2-0-2-2-2 | / | / | / | no |
| 23 | Vultaggio A. | 2020 | Position Paper | AMSTAR low | / | / | / | YES |
| 25 | Lombardi C. | 2021 | Narrative Review | SANRA 2-2-0-2-2-1 | / | / | / | no |
| 26 | Ortega J. | 2020 | Observational cohort study | STROBE low | 46 patients in Omalizumab | 5 COVID-19 | 0 ICUa (intensive care unit) | no |
| 27 | Izquierdo J.L. | 2021 | Observational cohort study | STROBE high | 641 patients in Omalizumab | 9 COVID-19 | 0 ICUa | YES |
| 28 | Adir Y. | 2021 | Observational cohort study | STROBE high | 8242 asthmatic COVID-19 vs 72.360 asthmatic without COVID-19 | 24 in Omalizumab vs 200 in Omalizumab | 0 ICUa | YES |
| 29 | Rial M.J. | 2020 | Observational cohort study | STROBE low | 263 patients in Omalizumab | 14 COVID-19 | 1 ICUa | YES |
| 30 | Matucci A. | 2021 | Observational cohort study | STROBE low | 145 patients in Omalizumab | 2 COVID-19 | 1 ICUa | no |
| 31 | Hanon S. | 2021 | Observational cohort study | STROBE low | 129 patients in Omalizumab | 4 igG COVID-19 | 0 admitted in Hospital/0 ICUa | YES |
| 32 | Antonicelli L. | 2021 | Observational cohort study | STROBE low | 558 asthmatic patients (NOT KNOWN how many in omalizumab) | 3 COVID-19 | 1 admitted in Hospital/0 ICUa | no |
| 33 | Garcıa-Menaya G.M. | 2020 | Observational cohort study | STROBE medium | 112 COVID-19 patients | 1 Omalizumab treatment | good outcome | YES, Grants RETICS RD16/0006/0004 (ARADyAL), PI15/00303, and PI18/00540 |
| 34 | Nassim D. | 2020 | Letter to the editor | SANRA 1-1-0-1-1-1 | / | / | / | no |
| 35 | Licari A. | 2020 | Observational cohort study | STROBE medium | 308 patients in biologics/286 in omalizumab | 3 patient had COVID-19 in omalizumab | paucisymptomatic disease | no |
| Omalizumab may enhance an antiviral immune response against SARS-CoV-2 (throught interferon) | ||||||||
| 1 | Farmani A.R. | 2021 | Review | AMSTAR low | / | / | / | no |
| 17 | Wang C.-J. | 2021 | Review | AMSTAR low | / | / | / | no |
| 18 | Ramakrishnan, R.K. | 2021 | Narrative Review | SANRA 1-0-0-1-1-2 | / | / | / | no |
| 27 | Izquierdo J.L. | 2021 | Observational cohort study | STROBE high | 641 patients in Omalizumab | 9 COVID-19 | 0 ICUa | YES |
| 36 | Yalcin A.D. | 2021 | Narrative Review | SANRA 2-2-0-2-2-2 | / | / | / | no |
| 37 | Wisnu Wardana V. A. | 2021 | Review | AMSTAR low | / | / | / | no |
| 38 | Tan C. | 2021 | Observational cohort study | STROBE high | / | / | / | no |
| 39 | Manti S. | 2020 | Narrative Review | SANRA 1-1-0-2-2-2 | / | / | / | no |
| 40 | Marques J.G. | 2022 | Narrative Review | SANRA 1-2-2-2-1-2 | / | / | / | no |
| 41 | Abdelmaksoud A. | 2020 | Letter to the editor | JBI-case report low | / | / | / | no |
| 42 | Wang J-Y. | 2021 | Letter to the editor | JBI-case report low | / | / | / | no |
| 43 | Lommatzsch M. | 2020 | Case report (1 pt) | JBI-case report high | 1 allergic asmatic patient with COVID-19 | 1 patient continued Omalizumab | good outcome | no |
| 44 | Lanoue D. | 2021 | Case report (1 pt) | JBI-case report very low | 1 chronic spontaneous urticaria (CSU) patient with COVID-19 | 2 patient continued Omalizumab | good outcome | no |
| 50 | Mantlo E. | 2021 | Experimental study | JADAD scale low | Vero cells infected by SARS-CoV-2 | tested the antiviral efficacy of IFN-α and IFN-β | significant reduction of viral titer | no |
| No sufficient data or clinical evidence about Omalizumab and reduced risk of SARS-CoV-2 infection/duration/severity | ||||||||
| 3 | Costanzo G. | 2021 | Review | AMSTAR low | / | / | / | no |
| 14 | Assaf S.M. | 2020 | Narrative Review | SANRA 1-2-0-2-2-2 | / | / | / | no |
| 15 | Liu S. | 2020 | Narrative Review | SANRA 2-2-1-2-2-2 | / | / | / | no |
| 16 | Morais-Almeida M. | 2020 | Narrative Review | SANRA 2-2-0-2-2-2 | / | / | / | no |
| 22 | Pfaar O. | 2021 | Position Paper | AMSTAR low | / | / | / | YES |
| 24 | Paladini E. | 2021 | Pediatric case report | JBI-case report high | 16 years old female in Omalizumab | COVID-19 | mild disease | YES, Novartis |
| 25 | Lombardi C. | 2021 | Narrative Review | SANRA 2-2-0-2-2-1 | / | / | / | no |
| 28 | Adir Y. | 2021 | Observational cohort study | STROBE high | 8242 asthmatic COVID-19 vs 72.360 asthmatic without COVID-19 | 24 in Omalizumab vs 200 in Omalizumab | 0 ICUa | YES |
| 45 | Schön M.P. | 2020 | Narrative Review | SANRA 2-1-0-2-1-2 | / | / | / | no |
| 46 | Chhiba K. D. | 2020 | Observational cohort study | STROBE low | 220 patients in Omalizumab | 1 COVID-19 | 1 ICUa | no |
| 48 | Pfaar O. | 2020 | Review | AMSTAR low | / | / | / | no |
| 49 | Humbert M. | 2021 | Review | AMSTAR medium | / | / | / | no |
| Biologic therapy for asthma linked to a more severe course of COVID-19 | ||||||||
| 47 | Eger K. | 2021 | Retrospective cohort study: 120 in Omalizumab/2 COVID-19/2 ICU | 120 patients in Omalizumab | 2 COVID-19 | 2 ICUa | no | |
Different methods were used, based on the study design, to assess the quality of eligible studies. The Amstar Checklist for Systematic Reviews, The SANRA scale for Narrative Reviews, The Jadad score for Randomized Clinical Trials (RCT), The Strobe Checklist for the Observational Studies, Joanna Briggs Institute Critical Appraisal Checklist for Case Reports and Joanna Briggs Institute Critical Appraisal Checklist for Case Series. Information regarding study design, date of publication, setting, characteristics of the population sample, objective of the study and outcome measure were considered.
The source of funding for each article included was reported in Table 1. The authors of the present review had no competing interests.
Results
According to inclusion and exclusion criteria 155 studies were retrieved.
The overall results of literature search were displayed in Fig. 2.
Fig. 2.
The studies checked according to PRISMA 2020 for systematic reviews
The studies included were 50 from different databases and other sources: PUBMED (N = 4); EMBASE (N = 9); SCOPUS (N = 19), Google Scholar (n = 3), Citation searching (n = 14), Congress Abstract (n = 1).
Studies can be divided into 5 groups (Table 1):
-
1)
studies suggesting a link between omalizumab and a better antiviral response (increasing IFN-α production), but referring to other viruses besides SARS-CoV-2 (eg, rhinovirus, influenza virus);
-
2)
studies that do not identify an increased risk of COVID-19 and severe disease in omalizumab-treated patients;
-
3)
studies hypothesizing that omalizumab may potentiate antiviral responses also against SARS-CoV-2:
-
4)
studies highlighting the lack of sufficient data or clinical evidence that omalizumab may be an advantage in COVID-19;
-
5)
a single study suggesting that omalizumab therapy for asthma may be the cause of a more severe COVID-19 (course and duration).
We reported the first author, publication year, and type of study and assessed their quality, which was not as high as scoring scale shows (see Table 1).
The population treated (or in treatment) with omalizumab was recorded with precise numbers (that were relatively high), investigating possible consequences of SARS-CoV-2 infections (comparing with general population) in order to clarify outcomes. A possible protective effect against SARS-CoV-2 duration and severity were supposed by some authors because of the low rate of infection and, above all, the low rate of patients in intensive care units.
We also reported eventual funding above all by Novartis (when explicated) to clarify the eventual financial bound that could have conditionate the results (see Table 1).
Discussion
Evidence supporting the effect of omalizumab in enhancing the antiviral response to viruses other than SARS-CoV-2
Several studies link omalizumab to an enhanced antiviral response (via dendritic cells and IFN-α). However, the studies were carried out before the pandemic and with viruses other than SARS-CoV-2.21 During immune responses to viruses the activation of innate immunity and the production of type I and II are essential to control viral spread.3
Atopic patients often show a reduction in IFN production by pDCs and epithelial cells with delayed and inefficient antiviral defenses.2,22 Serum IgE levels are inversely related to the intensity of INF-α response: antiviral responses of pDCs can be suppressed in atopic patients.3 The great susceptibility to respiratory viral infections in asthmatics often results in possible triggers for exacerbations.
Omalizumab seems to reduce virus-induced seasonal exacerbations in both adults and children.3,23 The most common causes of viral asthma exacerbations are human rhinoviruses (RV), respiratory syncytial virus (RSV), influenza viruses, parainfluenza viruses, coronaviruses, adenoviruses, and human metapneumovirus.24 Omalizumab reduced the duration of several infections: RSV,2 flu virus,3 RV (above all RV16).3,12,20,21,25,26 In fact, viral shedding and risk of virus-related disease are lower compared with guideline-driven care alone. Omalizumab decreases the expression of the pDC FcεRIα protein and in this way seems to increase IFN-α responses to RV and influenza virus.27, 28, 29 Type I IFNs other than IFN-α such as IFN-β1 or IFN-γ (usually low in allergic asthmatic patients) could also be upregulated and responsible for the increased viral clearance seen in patients treated with omalizumab as Cardet and Casale hypothesized.30 Of note, all these studies analyzing the cellular and molecular mechanisms that link omalizumab therapy and IFN-α production refer to viruses different than SARS-CoV-2 (Table 2). Moreover, restoration of IFN-α during omalizumab treatment involved ex vivo experiments on peripheral blood and not interferon generation by airway cells. Consequently, the association between blood cell IFN-α production and clinical improvement may be only considered surrogate markers for in vivo events in the lung. Regarding the IFN production in the lung, some authors demonstrated that type III IFN (IFN-λ1-4) play a crucial role in limiting SARS-CoV-2 infection in the airways during the early phase of the disease. Overall, these data highlight the complexity of the IFN biology and the importance of assessing the IFN effect in the different anatomical sites.
Table 2.
The most cited studies about Omalizumab and a better antiviral response (increasing IFN- α production)
| STUDY | VIRUS | OUTCOME |
|---|---|---|
| Busse (2011) | influenza virus, rhinovirus | “Omalizumab significantly reduced the proportion of participants who had one or more exacerbations” |
| Durrani SR (2012) | rhinovirus, RV-16 | “Innate immune responses reduced by the high-affinity IgE receptor in allergic asthmatic children; after FcεRI cross-linking, allergic asthmatic children had significantly lower HRV-induced IFN responses” |
| Teach SJ (2015) | rhinovirus RV-16 | “Omalizumab improved IFN-α responses to rhinovirus, and within the omalizumab group, greater IFN-α increases were associated with fewer exacerbations” (PROSE STUDY) |
| Saini SS (2015) | upper airway infections | “Targeting IgE with Omalizumab in asthma or chronic spontaneous urticaria patients did not increase upper airway infections” |
| Kantor DB (2016) | rhinovirus | “Omalizumab reduces acute severity of rhinovirus-triggered asthma exacerbation” |
| Esquivel A (2017) | rhinovirus | “Direct evidence that blocking IgE decreases susceptibility to RV infections and related-illness” |
| Edwards MR (2017) | viral infections | “Anti-T2 biologics are particularly successful in controlling asthma exacerbations” |
| Gill MA (2018) | rhinovirus- and influenza | “Omalizumab enhanced plasmacytoid dendritic cell antiviral responses” |
| Jartti T (2019) | rhinovirus | “Pre-seasonal treatment with Omalizumab has been shown to eliminate the seasonal peaks in asthma exacerbations, most of which are associated with RV infection” |
| Heymann PW (2020) | rhinovirus, RV-16 | “The effect of administering Omalizumab on the response to rhinovirus was most pronounced during the early/innate phase of the infection” |
| Altman MC (2020) | viral infection | “There are emerging therapeutic options to decrease severity of wheezing exacerbations caused by respiratory viral infections” |
Is it logical to think that IgE blockade can reduce susceptibility also to COVID-19 infection? We must notice that there are remarkable (or evident or striking) differences in the cell receptor for rhinovirus and coronavirus, can the observations on rhinovirus be extended to SARS-CoV-2? Rhinovirus and influenza virus are among the most common viruses involved in the development of asthma exacerbations, but also coronavirus are involved, in a weaker way.23,24,35
The cell receptors are indeed very different between three viruses: receptor of major group serotype of rhinovirus is ICAM 1 (intercellular adhesion molecule 1), sialic acid is the one for influenza virus and ACE-2 the one for SARS-CoV-2.24 But as some valid observation for rhinovirus have been proven and then extended to the influenza virus, we can hypothesize that it could also happen for SARS-CoV-2. All are single-stranded RNA viruses, both influenza virus and coronaviruses have the envelope. However, the beneficial effect of omalizumab in treating virus-induced exacerbations may be due to its effect on IFN-α production (and not about the receptor involved).38 In adults and children omalizumab treatment (for 6 months) increased IFN production against influenza virus A (in a statistically significant way), also a positive trend versus RV and an improvement in control of asthma symptoms were observed.3,21 It has also been supposed for SARS-CoV-2 (even if there are not solid studies about this hypothesis).
Evidence showing no increased risk of COVID-19 (and severe COVID-19) in patients treated with omalizumab
In the first period of pandemic, one of allergists' first questions was whether patients they were treating with biologics would be at increased risk of infection. Patients with severe asthma treated with biologics don't have an increased risk of SARS-CoV-2 infection or progression to severe forms than the age- and geography-matched non-asthmatic population.3,17
It is recommended to continue biologics in allergic patients.3,10,31, 32, 33, 34, 35, 36, 37 Discontinuation of biologics may lead to an increased risk of asthma exacerbations, oral corticosteroid use and a greater likelihood of access to the emergency room and hospitalization which represent risk factors for SARS-CoV-2 infection.
Biological treatment for asthma or dermatologic allergic disease do not seem to increase the risk of COVID-19 as several cohorts show.38,39 In Belgian Severe Asthma Registry and Italian Severe Asthma Network biologic therapy was not associated with increased risk of infection of disease severity. 38,40, 41, 42 In a multivariate analyze, Adir et al demonstrated that biologic use was not associated with increased risk of COVID-19 severity or mortality (adjusted odds ratio 0.99; 95% CI 0.73–1.33).38,41 Extrapolating data from a large Spanish database Izquierdo et al reported that COVID-19 related hospital admission and mortality was very low among asthmatic patients treated with biologics. 38,41,43 No increased risk for COVID-19 was found also by Matucci after a cross-sectional telephone-based survey including 473 patients on biological therapy.44 In Ortega's cohort, 5 patients out of 46 in omalizumab treatment had COVID-19 infection, and none was admitted to the hospital: the use of biologics for severe asthma seemed not have an impact on poorer clinical outcomes due to SARS-CoV-2 respiratory infection as the authors suggested.45 The same results (no increased risk of infection or disease severity or mortality) were found in another Spanish study that included 545 asthmatic patients treated with biologics.46 A Turkiye study enrolled 75 asthmatic patients treated with omalizumab, comparing patient who continued biologic treatment and patients who interrupted it. Only 12 patients interrupted the treatment (because they were afraid of pandemic and refused to return to the hospital): the risk of infection and severe disease was higher in these second group (relative risk of 2.71; 95% CI, 1.21–6.06).1,47 Between 13 Turkish patients with severe asthma (treated with omalizumab or mepolizumab) that contracted SARS-CoV-2, five (38.5%) had mild COVID-19, eight (61.5%) had moderate disease, none required mechanical ventilation or intensive care, all patients fully recovered.1,47 In the Garcıa-Menaya cohort, only 1 patient of 113 with COVID-19 had taken omalizumab before hospital admission.48
Evidence supporting that omalizumab may enhance the antiviral immune response even against SARS-CoV-2
Several authors suggest that omalizumab treatment could protect against severe forms of COVID-19. Might misfortune (allergic disease and biologic treatment) be a blessing in disguise (pandemic)?1,48
The antiviral and anti-inflammatory effects against virus-exacerbated asthma that omalizumab showed in previous studies may be relevant to the treatment of COVID-19.2 Anti-IgE drugs could reduce the severity and duration of COVID-19, the risks of severe outcomes, and perhaps pneumonia during SARS-CoV-2 infection.3,17 A letter published in the journal Allergy details an asthma patient who developed a COVID-19 infection while receiving ongoing treatment with omalizumab.17 Because the patient's COVID 19 illness did not progress into a more severe illness, the authors of this paper raised the possibility that omalizumab may have attenuated the seriousness of the infection. This interpretation extends beyond the available data and does not necessarily support the hypothesis under review, however, numerous cohorts show relevant data about the low severity of COVID-19 in omalizumab-treated patients compared to the incidence data of the general population (Table I). When acute respiratory distress syndrome (ARDS) develops in the severe phase of COVID-19 omalizumab perhaps could contribute to a better respiratory function.8 A patient with chronic spontaneous urticaria succeeded in relieve symptom of exacerbated urticaria and angioedema due to COVID-19 with omalizumab.18 Criado described the first favorable outcome of omalizumab in Chronic Spontaneous Urticaria in a patient with COVID-19, without deterioration of clinical status and excellent response.19 Thus, the IgE blockade (enhancing IFN-α) could act as immunotherapy in case of SARS-Cov-2 infection.49 In fact, omalizumab has also been included in the list of the possible drugs useful in treating SARS-CoV-2 infection.50,51 The same IFN-alpha was considered as a potential therapeutic strategy to treat COVID-19 in that early phase in which the immune system produces cytokine as first line defense against viral infection.4,12 The key of the process seems to be that pDC-derived IFN-I crucial for the host to control SARS-CoV-2 infection.52 This early activation of the innate immunity is intact in adult patients with mild disease and is enhanced in children that usually experience a benign disease course.53,54 The same protective effect was also hypothesized in pediatric patients treated with omalizumab.55 Among 308 Italian children and adolescents (mean age 12.8 years) treated with biologics, only 3 subjects (1%) who were treated with omalizumab experienced pauci-symptomatic COVID-19. As Licari et al showed their symptoms promptly resolved.56
But could allergic phenotype (and not omalizumab) exert a protective effect against SARS CoV-2?17,47,51,57, 58, 59
Asthmatic phenotype was not included between risk factors for an increased severe SARS-CoV-2 infection.60
In these patients, the low expression of angiotensin-converting enzyme 2 (ACE2) in bronchial epithelial cells seemed to be a protection from COVID-19.18,61 Asthma and allergic disorders are associated with a significant reduction of ACE-2 expression in nasal and bronchial cells, both in adults and children.21,61 ACE-2 expression appears to be inversely related to type 2 cytokine levels,17,33 and, as it has been well demonstrated, to be related to patient's age. The production of pro inflammatory cytokines can be inhibited by type 2 cytokines.3,62
The epithelium with his mucus lining is the first physical barrier that blocks the invasion of the virus. Then activated eosinophils with their ribonucleases are another defense promoting viral clearance. Early airway inflammation caused by virus infection is attenuated also by low-dose inhaled corticosteroids (part of maintenance therapy). Allergen immunotherapy induces regulatory T cells (Tregs) with consequent suppression of inflammation. Tregs also prevent tissue damage: in fact they downregulate the induction and proliferation of other T-cell subsets.15
A various prevalence of asthma was found among COVID-19 patients in different countries.
Some authors observed that the link between a history of asthma and COVID infection or mortality was not statistically significant.21 In one of the first China-nationwide analysis of comorbidities in COVID-19 patients, 1590 patients were screened and none of them reported a diagnosis of asthma.61, 63 A very low prevalence of asthmatic patient was also found in the cohort of Li and Zhang compared to the general population.64,65
It seems true also in pediatric allergic patients. Only one of 52 young patient hospitalized for COVID-19 had a diagnosis of asthma in Ciprandi's study (including patients from 2 big Italian hospitals of Liguria and Lombardy).58 Other worldwide studies suggest a different interpretation. Some authors suppose that there was an underreporting of chronic respiratory disease. Others suggested that there is an epidemiologically relevant difference in the prevalence of comorbidities (such asthma) between countries and that this was the reason of low prevalence of asthma in COVID-19 patients in early Asian studies.66
In children the type-2 skewed immunity drives a less pathogen associated molecular patterns (PAMPs) activation resulting in less inflammation. This mechanism decreases host's protection against viruses, but also determine a hypo-inflammatory state.
The key role of pDC in anti-viral immunity is underlined also by some interesting considerations about multisystem inflammatory syndrome in children (MIS-C). Circulating pDCs were severely decreased in MISC-C (comparing this condition to COVID-19 children). IFN- α plasma levels and expression of type I IFNs inducible genes were higher in COVID-19 children than in MIS-C patients.21
Most studies emphasize the lack of sufficient data or clinical evidence that omalizumab may be an advantage in SARS-CoV-2 infection
Most studies suggest insufficient data about COVID-19 incidence of severity in omalizumab-treated patients. It is not known if omalizumab reduces (or increase) SARS-CoV2 risk.41,57 We agree that the evidence is too few because there are not double-blind placebo-controlled, randomized controlled trials, or open controlled trials to strongly link omalizumab to protection against severe SARS-CoV-2 (through IFN alpha or other ways).15
The number of omalizumab-treated patients with severe COVID-19 remains low, but the right sample size or study design to affirm causal relationships is lacking.67,68
There are ethical and temporal reasons why we do not have controlled trials. Further investigations and studies are actually needed to prove that the protective role of anti-IgE on rhinovirus clearance can be extrapolated and generalized to SARS-CoV-2.15,23 If omalizumab effectively reduces the risk of COVID-19 infections, the benefit may occur because interferon production generation is enhanced, but no data in the literature, either from the airway or peripheral blood cells, have indicated or assessed this possibility with SARS-CoV-2. This idea is almost like a syllogism. pDCs are involved in antiviral response in asthmatic patients treated with omalizumab, because they target the immune response with rapid and massive IFN I type production, enhanced by TLRs.3,24 IFN- α and IFN- β inhibit virus infection in a dose-dependent way. IFN- α in a concentration as low as 5 IU/mL already has an anti-SARS-CoV-2 activity: viral titer result in a reduction of 1 log (p < 0.01). Increasing progressively IFN- α concentrations virus titers decrease in a proportional way.4 Is it possible to conclude that omalizumab enhancing INF decreases SARS-CoV-2 activity?
Only 1 study suggests that biological therapy for asthma may be associated with a poorer COVID-19 outcome
Only 2 patients out of 120 omalizumab-treated developed COVID-19 and were both hospitalized in a retrospective study.69 However, some confounding factors are present in the study: for example higher prevalence of comorbidities such as obesity in omalizumab-treated population. COVID-19 severity can be explained by this comorbidities independently from biological therapy.
A possible “IgE mediated protection” is suggested by a lower risk of COVID-19 in allergic patients with higher IgE values. Therefore, can we suppose that reducing IgE with omalizumab may enhance the risks of infection and be a complication from the same therapy? Probably this hypothesis is not correct. In fact the apparent protective effects is not due to the higher IgE expression, but it is a marker of an higher activity of the higher humoral immune system, as Farmani showed in some asthmatic patients.2 Moreover, omalizumab reduces only IgE and not also the other immunoglobulins. The allergic patient has other numerous protective pathway that does not involve “only” IgE mediated effect. Larsson in 2021 demonstrated a positive association between genetic predisposition to any allergic disease and lower risk of SARS-CoV-2 infection (with a mendelian randomization analysis of 136 uncorrelated single nucleotide polymorphisms related with a broad allergic disease phenotype). We must also notice that patients with more elevated IgE levels seems to be more prone to virus-induced asthma exacerbations: between IgE and IFN-α seems to be is a negative correlation. IL-4 and IL-13 can downregulate ACE2 expression and are not very influenced by omalizumab.
ACE2 also circle in a soluble form that is upregulated in asthma. It can limit the ability of SARS-CoV-2 to bind to airway cell membranes acting as a competitive soluble interceptor. The increase of eosinophils number in asthmatic patients could be another protective factor against excessive inflammatory responses in severe COVID-19. Omalizumab decreases their lung recruitment but not the total number, and a reduction in recruitment could also be linked with a minor risk of cytokine storm. Omalizumab has pleiotropic effects for example it not only reduces IgE, but also IL-33 and as a consequence, other inflammatory cytokines such as IL-6, TNF- α, IL-1 β and prostaglandin.70 In this way it seems able to protect from severe COVID-19.
Levels of specific IgE are associated with COVID-19 progression and severity at admission in the hospital. Tan and al. in 2021 showed that anti SARS-CoV-2 spike S1-specific IgE (SP-IgE) and anti-SARS-CoV-2 nucleocapsid specific IgE (NP-IgE) were higher in worst cases. The total lung severity score (TLSS) was correlated with IgE level with a positive correlation, the PoO2/FiO2 ratio with a negative one.71 Omalizumab reducing IgE response could be protective in these severe COVID-19 cases.
What are the limitations of the present review?
We performed a broad literature search and we found that there is little convincing evidence to indicate that omalizumab affects the course of a COVID-19 illness. Most of the evidence referred to is circumstantial or based on observational studies.
Some data show that a reduction of cell surface IgE enhances viral defense. Little data are presented to suggest that a reduction of cell surface bound IgE is beneficial with a COVID-19 illness. A counterregulatory mechanism was revealed between FcεRI (Fc epsilon RI - high affinity receptor of IgE) and TLR 7.20,28 Activation of TLR-7 or a component of the TLR-7 pathway may inhibit the expression of FcεRI.20,28 It is not very clear also the relative effect of bound IgE and free serum IgE.
In the different cohort it is not always well explained if omalizumab was administered before or concomitant COVID-19 and how many weeks before the infection in the first case. In fact, the modulation could be evanescent in time and to establish a correlation it is important to clarify temporal correlation.
Conclusions
Omalizumab is an anti-IgE biologic therapy with proven antiviral activity. It reduces incidence and severity of upper respiratory tract infections and asthma exacerbations.69 This is a very interesting quality in pandemic time: the hypothesis of a possible protection against severe COVID-19 is very fascinating, although currently there are not sufficient data on a possible positive effect of anti-IgE therapy on COVID-19.
An EAACI consensus paper states that “patients with SARS-CoV-2 infection, irrespective of the severity of the infection, should withhold the application of biologicals until recovered”, while treatment of patients on biologicals should be maintained in noninfected individuals.10
An international registry of omalizumab-treated COVID-19 cases could be very useful to clarify the outstanding issues in this area. Moreover, randomized controlled trials are necessary to clearly demonstrate the effect of omalizumab in protecting against severe SARS-CoV-2, through IFN-α upregulation or other immunological pathways.
Abbreviations
IgE, Immunoglobulin E; INF-α, interferon-α; COVID-9, Corona Virus Disease 19; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; RV, rhinoviruses; ARDS, Acute Respiratory Distress Syndrome; IL, interleukin; TNF, Tumor Necrosis Factor; pDCs, plasmacytoid dendritic cells; FceRs, Fc epsilon receptor; RCT, Randomized Clinical Trials; JBI, Joanna Briggs Institute; ICU, intensive care unit; ICAM1, intercellular adhesion molecule 1; ACE2, angiotensin-converting enzyme 2; Tregs, regulatory T cells; PAMPs, pathogen-associated molecular patterns; MIS-C, multisystem inflammatory syndrome in children; SP-IgE, protein-specific IgE; NP-IgE, nucleocapsid protein-specific IgE.
Funding
No found were used for the present study.
Availability of data and materials
The source of data and materials are mentioned in the manuscript, in support of the findings. The authors are available (upon request) to send all the articles used for the review and the complete list of excluded articles.
Authorship
Ghiglioni Daniele Giovanni has made substantial contributions to conception and interpretation of data.
Cozzi Laura has made substantial contributions to conception and design, or acquisition of data, analysis and interpretation of data.
Castagnoli Riccardo has been involved in drafting the manuscript or revising it critically for important intellectual content.
Maffeis Laura contributed with data collection.
Bruschi Gaia contributed with data collection.
Marchisio Paola Giovanna has been involved in drafting the manuscript or revising it critically for important intellectual content.
Marseglia Gian Luigi has been involved in drafting the manuscript or revising it critically for important intellectual content.
Licari Amelia has been involved in drafting the manuscript or revising it critically for important intellectual content.
All the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval
Not applicable.
Authors’ consent for publication
All authors’ gave their permission to publish research findings.
Potential competing interests
The authors report no competing interests.
To note
Ghiglioni Daniele Giovanni and Cozzi Laura should be considered joint first author.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Wang C.J., Cheng S.L., Kuo S.H. Asthma and COVID-19 associations: focus on IgE-related immune pathology. Life (Basel) 2022;12(2):153. doi: 10.3390/life12020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farmani A.R., Mahdavinezhad F., Moslemi R., et al. Anti-IgE monoclonal antibodies as potential treatment in COVID-19. Immunopharmacol Immunotoxicol. 2021;43(3):259–264. doi: 10.1080/08923973.2021.1925906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menzella F., Ghidoni G., Galeone C., Capobelli S., Scelfo C., Facciolongo N.C. Immunological aspects related to viral infections in severe asthma and the role of omalizumab. Biomedicines. 2021;9(4):348. doi: 10.3390/biomedicines9040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastard P., Gervais A., Le Voyer T., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6(62) doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abers M.S., Rosen L.B., Delmonte O.M., et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol Cell Biol. 2021;99(9):917–921. doi: 10.1111/imcb.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yalcin A.D., Yalcin A.N. Future perspective: biologic agents in patients with severe COVID-19. Immunopharmacol Immunotoxicol. 2021;43(1):1–7. doi: 10.1080/08923973.2020.1818770. [DOI] [PubMed] [Google Scholar]
- 9.Abers M.S., Delmonte O.M., Ricotta E.E., et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI insight. 2021;6(1) doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vultaggio A., Agache I., Akdis C.A., et al. Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: an EAACI statement. Allergy. 2020;75(11):2764–2774. doi: 10.1111/all.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbert K., Schlaak J.F., Yang D., Dittmer U. IFN-α subtypes: distinct biological activities in anti-viral therapy. Br J Pharmacol. 2013;168(5):1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bencze D., Fekete T., Pázmándi K. Type I interferon production of plasmacytoid dendritic cells under control. Int J Mol Sci. 2021;22(8):4190. doi: 10.3390/ijms22084190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okayama Y., Matsumoto H., Odajima H., Takahagi S., Hide M., Okubo K. Roles of omalizumab in various allergic diseases. Allergol Int. 2020;69(2):167–177. doi: 10.1016/j.alit.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan R.K., Al Heialy S., Hamid Q. Implications of preexisting asthma on COVID-19 pathogenesis. Am J Physiol Lung Cell Mol Physiol. 2021;320(5):L880–L891. doi: 10.1152/ajplung.00547.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID-19. Allergy. 2021;76(3):866–868. doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lommatzsch M., Stoll P., Virchow J.C. COVID-19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75(10):2705–2708. doi: 10.1111/all.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanoue D., Pham H., Valois J., Yang W., Olynych T. Mild COVID-19 respiratory infection associated with moderate fare of chronic spontaneous urticaria in a 43-year old female long-term care worker on omalizumab. Allergy Asthma Clin Immunol. 2021;17(1):68. [Google Scholar]
- 19.Criado P.R., Criado R.F.J., Pincelli T.P., Yoshimoto T.A., Naufal G.G.A., Abdalla B.M.Z. Chronic spontaneous urticaria exacerbation in a patient with COVID-19: rapid and excellent response to omalizumab. Int J Dermatol. 2020;59(10):1294–1295. doi: 10.1111/ijd.15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teach S.J., Gill M.A., Togias A., Sorkness C.A., Arbes S.J., Jr., Calatroni A. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galati D., Zanotta S., Capitelli L., Bocchino M. A bird's eye view on the role of dendritic cells in SARS-CoV-2 infection: perspectives for immune-based vaccines. Allergy. 2022;77(1):100–110. doi: 10.1111/all.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padayachee Y., Faiez T.S., Singanayagam A., Mallia P., Johnston S.L. Asthma and viruses: a focus on rhinoviruses and SARS-CoV-2. J Allergy Clin Immunol. 2021;147(5):1648–1651. doi: 10.1016/j.jaci.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaar O., Creticos P.S., Kleine-Tebbe J., Canonica G.W., Palomares O., Schülke S. One hundred ten years of allergen immunotherapy: a broad look into the future. J Allergy Clin Immunol Pract. 2021;9(5):1791–1803. doi: 10.1016/j.jaip.2020.12.067. [DOI] [PubMed] [Google Scholar]
- 24.Kumar K., Singanayagam A., Johnston S.L. Respiratory virus infections in asthma: research developments and therapeutic advances. Acta Med Acad. 2020;49(2):130–143. doi: 10.5644/ama2006-124.292. [DOI] [PubMed] [Google Scholar]
- 25.Esquivel A., Busse W.W., Calatroni A., et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med. 2017;196:985–992. doi: 10.1164/rccm.201701-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrani S.R., Montville D.J., Pratt A.S., et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130(2):489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Criado P.R., Pagliari C., Criado R.F.J., Marques G.F., Belda W., Jr. What the physicians should know about mast cells, dendritic cells, urticaria, and omalizumab during COVID-19 or asymptomatic infections due to SARS-CoV-2? Dermatol Ther. 2020;33(6) doi: 10.1111/dth.14068. [DOI] [PubMed] [Google Scholar]
- 28.Gill M.A., Liu A.H., Calatroni A., et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141(5):1735–1743.e9. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse W.W., Morgan W.J., Gergen P.J., et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardet J.C., Casale T.B. New insights into the utility of omalizumab. J Allergy Clin Immunol. 2019;143(3):923–926.e1. doi: 10.1016/j.jaci.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes-Visentin A., Paul A.B.M. Asthma and COVID-19: what do we know now. Clin Med Insights Circulatory, Respir Pulm Med. 2020;14 doi: 10.1177/1179548420966242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding M., Dong X., Sun Y.L., et al. Recent advances and developments in COVID-19 in the context of allergic diseases. Clin Transl Allergy. 2021;11(7) doi: 10.1002/clt2.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarıoğlu N. Asthma and COVID-19: what do we know? Tuberk Toraks. 2020;68(2):141–147. doi: 10.5578/tt.69775. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi C., Gani F., Berti A., Comberiati P., Peroni D., Cottini M. Asthma and COVID-19: a dangerous liaison? Asthma Res Pract. 2021;7(1):9. doi: 10.1186/s40733-021-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaar O., Klimek L., Jutel M., et al. COVID-19 pandemic: practical considerations on the organization of an allergy clinic-An EAACI/ARIA Position Paper. Allergy. 2021;76(3):648–676. doi: 10.1111/all.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaker M.S., Oppenheimer J., Grayson M., et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol. 2020;8(5):1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowalski M.L., Bartuzi Z., Bręborowicz A., et al. Position statement of expert panel of the Polish Allergology Society on the management of patients with bronchial asthma and allergic diseases during SARS-Cov-2 pandemics. Alergologia Polska – Polish Journal of Allergology. 2020;7(3):117–130. [Google Scholar]
- 38.Adir Y., Humbert M., Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol. 2021;148(2):361–367.e13. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nassim D., Jfri A., Litvinov I.V., Netchiporouk E. Preliminary data suggests that biologics in dermatology are not associated with adverse COVID-19 outcomes. J Cutan Med Surg. 2020;24(4):420–421. doi: 10.1177/1203475420929250. [DOI] [PubMed] [Google Scholar]
- 40.Hanon S., Brusselle G., Deschampheleire M., et al. COVID-19 and biologics in severe asthma: data from the Belgian Severe Asthma Registry. Eur Respir J. 2020;56(6) doi: 10.1183/13993003.02857-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adir Y., Saliba W., Beurnier A., Humbert M. Asthma and COVID-19: an update. Eur Respir Rev. 2021;30(162) doi: 10.1183/16000617.0152-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonicelli L., Tontini C., Manzotti G., et al. Severe asthma in adults does not significantly affect the outcome of COVID-19 disease: results from the Italian Severe Asthma Registry. Allergy. 2021;76(3):902–905. doi: 10.1111/all.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izquierdo J.L., Almonacid C., González Y., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57(3) doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matucci A., Caminati M., Vivarelli E., et al. COVID-19 in severe asthmatic patients during ongoing treatment with biologicals targeting type 2 inflammation: results from a multicenter Italian survey. Allergy. 2021;76(3):871–874. doi: 10.1111/all.14516. [DOI] [PubMed] [Google Scholar]
- 45.Domínguez-Ortega J., López-Carrasco V., Barranco P., et al. Early experiences of SARS-CoV-2 infection in severe asthmatics receiving biologic therapy. J Allergy Clin Immunol Pract. 2020;8(8):2784–2786. doi: 10.1016/j.jaip.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rial M.J., Valverde M., Del Pozo V., et al. Clinical characteristics in 545 patients with severe asthma on biological treatment during the COVID-19 outbreak. J Allergy Clin Immunol Pract. 2021;9(1):487–489. doi: 10.1016/j.jaip.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J.Y., Pawankar R., Tsai H.J., Wu L.S., Kuo W.S. COVID-19 and asthma, the good or the bad? Allergy. 2021;76(2):565–567. doi: 10.1111/all.14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.García-Menaya J.M., Cordobés-Durán C., Rangel-Mayoral J.F., García-Martín E., Agúndez J.A.G. Outcomes and laboratory and clinical findings of asthma and allergic patients admitted with covid-19 in a Spanish university hospital. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.570721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pashaei M., Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expet Opin Biol Ther. 2020;20(10):1111–1116. doi: 10.1080/14712598.2020.1807933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdelmaksoud A., Goldust M., Vestita M. Comment on "Chronic spontaneous urticaria exacerbation in a patient with COVID-19: rapid and excellent response to omalizumab. Int J Dermatol. 2020;59(11):1417–1418. doi: 10.1111/ijd.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manti S., Licari A., Montagna L., et al. SARS-CoV-2 infection in pediatric population. Acta Biomed. 2020;91(11-S) doi: 10.23750/abm.v91i11-S.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q., Bastard P. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature. 2022;603(7902):587–598. doi: 10.1038/s41586-022-04447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greene T.T., Zuniga E.I. Type I interferon induction and exhaustion during viral infection: plasmacytoid dendritic cells and emerging COVID-19 findings. Viruses. 2021;13(9):1839. doi: 10.3390/v13091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 55.Yu L., Zhang H., Pan J., Ye L. Pediatric usage of Omalizumab: a promising one. World Allergy Organ J. 2021;14(12) doi: 10.1016/j.waojou.2021.100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Licari A., Castagnoli R., Votto M., Brambilla I., Ciprandi G., Marseglia G.L. Biologic use in allergic and asthmatic children and adolescents during the COVID-19 pandemic. Pediatr Allergy Immunol Pulmonol. 2020;33(3):155–158. doi: 10.1089/ped.2020.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schön M.P., Berking C., Biedermann T., et al. COVID-19 and immunological regulations - from basic and translational aspects to clinical implications. J Dtsch Dermatol Ges. 2020;18(8):795–807. doi: 10.1111/ddg.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciprandi G., Licari A., Filippelli G., Tosca M.A., Marseglia G.L. Children and adolescents with allergy and/or asthma seem to be protected from coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125:361–362. doi: 10.1016/j.anai.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Filippo M., Votto M., Brambilla I., et al. Allergy and COVID-19. Acta Biomed. 2021;92(S7) doi: 10.23750/abm.v92iS7.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novak N., Cabanillas B. Viruses and asthma: the role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology. 2020;161(2):83–93. doi: 10.1111/imm.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson D.J., Busse W.W., Bacharier L.B., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cameron M.J., Ran L., Xu L., et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X., Xu S., Yu M., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J.J., Cao Y.Y., Dong X., et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy. 2020;75:1809–1812. doi: 10.1111/all.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chhiba K.D., Patel G.B., Vu T.H.T., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morais-Almeida M., Aguiar R., Martin B., et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5) doi: 10.1016/j.waojou.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eger K., Hashimoto S., Braunstahl G.J., et al. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med. 2020;177 doi: 10.1016/j.rmed.2020.106287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Licari A., Marseglia A., Caimmi S., et al. Omalizumab in children. Paediatr Drugs. 2014;16(6):491–502. doi: 10.1007/s40272-014-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan C., Zheng X., Sun F., et al. Hypersensitivity may be involved in severe COVID-19. Clin Exp Allergy. 2022;52(2):324–333. doi: 10.1111/cea.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source of data and materials are mentioned in the manuscript, in support of the findings. The authors are available (upon request) to send all the articles used for the review and the complete list of excluded articles.