Abstract
Neutrophils exposed to low concentrations of gram-negative lipopolysaccharide (LPS) become primed and have an increased oxidative response to a second stimulus (e.g., formyl-methionyl-leucyl-phenylalanine [fMLP]). In studies aimed at understanding newborn sepsis, we have shown that neutrophils of newborns are not primed in response to LPS. To further understand the processes involved in LPS-mediated priming of neutrophils, we explored the role of extracellular signal-related protein kinases (ERK 1 and 2) of the mitogen-activated protein kinase family. We found that LPS activated ERK 1 and 2 in cells of both adults and newborns and that activation was plasma dependent (maximal at ≥5%) through LPS-binding protein. Although fibronectin in plasma is required for LPS-mediated priming of neutrophils of adults assessed by fMLP-triggered oxidative burst, it was not required for LPS-mediated activation of ERK 1 and 2. LPS-mediated activation was dose and time dependent; maximal activation occurred with approximately 5 ng of LPS per ml and at 10 to 40 min. We used the inhibitor PD 98059 to study the role of ERK 1 and 2 in the LPS-primed fMLP-triggered oxidative burst. While Western blotting showed that 100 μM PD 98059 completely inhibited LPS-mediated ERK activation, oxidative response to fMLP by a chemiluminescence assay revealed that the same concentration inhibited the LPS-primed oxidative burst by only 40%. We conclude that in neutrophils, LPS-mediated activation of ERK 1 and 2 requires plasma and that this activation is not dependent on fibronectin. In addition, we found that the ERK pathway is not responsible for the lack of LPS priming in neutrophils of newborns but may be required for 40% of the LPS-primed fMLP-triggered oxidative burst in cells of adults.
Septicemia and shock leading to multiple organ failure remains one of the major causes of death of adults and newborn infants (21, 22). Many symptoms of septic shock, including vasodilation, myocardial dysfunction, and disseminated intravascular coagulation, are elicited by lipopolysaccharide (LPS), a membrane glycolipid from the cell wall of gram-negative bacteria. Septicemia is characterized by low levels (ng/ml) of LPS in the bloodstream (9, 29).
LPS interacts with specific cellular recognition proteins to modify cellular function (15). For example, neutrophils or polymorphonuclear leukocytes of adults exposed to LPS have increased response to bacterial oligopeptides such as formyl-methionyl-leucyl-phenylalanine (fMLP). This process is known as priming (26). It has been shown that neutrophils from newborn infants are not primed in response to LPS, in contrast to neutrophils from adults under similar in vitro conditions (2, 23, 24).
The immediate postreceptor events of LPS priming are largely unknown; however, protein tyrosine kinases have been implicated in the signaling pathways, and inhibitors of protein tyrosine kinases (e.g., genistein) block neutrophil priming (12, 13, 25). In primed neutrophils, proteins with molecular sizes in the range of approximately 40 to 46 kDa become phosphorylated on tyrosine residues (11–14, 20, 28). Thus, mitogen-activated protein kinases (MAPKs) with similar molecular sizes may be involved in LPS signaling (10, 20). This led us to question whether differences in these pathways are responsible for the biological difference between neutrophils of adults and newborns in response to LPS.
The term MAPK broadly refers to a family of serine/threonine kinases that are activated by multiple extracellular factors, respond to stress, and control cellular growth and differentiation (6, 18). The three major MAPK pathways identified in mammalian cells are p42/44 or extracellular signal-regulated kinases 1 and 2 (ERK 1 and 2), p38 MAPK, and c-Jun N-terminal kinase or stress-activated protein kinase (18). Although Nolan et al. reported that ERK 1 and 2 are activated in response to LPS, this trend was only evident with high concentrations of LPS (1 g/ml) or relatively long treatment periods (19). Others have found that in neutrophils, ERK 1 and 2 are not activated by low concentration of LPS (≤500 ng/ml) (8, 16, 17).
Here we report that in neutrophils, ERK 1 and 2 MAPK are activated by low concentrations of LPS (approximately 5 ng/ml) in a plasma- and time-dependent manner. Although plasma was required, fibronectin, a protein that plays a role in LPS priming of respiratory burst (3), was not the plasma factor responsible for LPS-mediated activation of ERK. In addition, there was no significant difference in the LPS-mediated ERK 1 and 2 activation between cells of adults and newborns. Furthermore, while we found that the ERK pathway-specific inhibitor PD 98059 completely prevented ERK activation at 100 M in cells of adults, at the same concentration it only partially inhibited the LPS-primed fMLP-triggered oxidative burst in these cells. This work indicates that LPS activation of ERK 1 and 2 in neutrophils requires extracellular factors normally found in plasma and that the ERK pathway is not solely responsible for the LPS-primed fMLP-triggered oxidative burst.
MATERIALS AND METHODS
Reagents.
Heparin was purchased from Organon Teknika (Toronto, Ontario, Canada). Dextran (6%) was bought from Abbott Laboratories Inc. (Toronto, Ontario, Canada). Ficoll-Plaque Plus was acquired from Amersham Pharmacia Biotech, Inc. (Quebec, Quebec, Canada). Hank's buffered salt solution (HBSS), 7.5% sodium bicarbonate, and 1M HEPES buffer solution were purchased from Gibco Laboratories (Burlington, Ontario, Canada). PD 98059 was obtained from Calbiochem (through Cedar Lane Laboratories, Hornby, Ontario, Canada). Radioimmunoprecipitation assay (RIPA) lysis buffer consisted of 25 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.25% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), and 1 mM EDTA (pH 7.5). Prior to lysis, the following protease and phosphatase inhibitors were added to the RIPA: 1.57 mg of benzamidine per ml, 5 mM sodium fluoride, 200 mM sodium orthovanadate, 5 μg of pepstatin A per ml, 2 mM diisopropyl fluorophosphate, 5 μg of leupeptin per ml, 10 μM phenylarsine oxide, 1 μM phenylmethylsulfonyl fluoride, and 1 mM dl-dithiothreitol; all were from Sigma Chemical (Oakville, Ontario, Canada). Purified natural human lipopolysaccharide binding protein (LBP) and mouse monoclonal antibody to human LBP (subclass immunoglobulin G1 [IgG1]) were obtained from Cedar Lane Laboratories. Lucigenin, fMLP, and human plasma fibronectin were also from Sigma Chemical. Escherichia coli LPS (serotype 0111:B4), obtained from List Biological Laboratories Inc. (Cambell, Calif.), was dissolved in HBSS at a concentration of 10 μg/ml and stored in aliquots at −70°C. Before use, LPS was thawed, diluted in HBSS to 1 μg/ml, and sonicated twice (2-s bursts). H-medium consisted of 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.8 mM CaCl2, 5 mM glucose, and 10 mM HEPES (pH 7.4). Protein A-Sepharose 4B beads were obtained from Sigma Chemical, myelin basic protein was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.), and [γ-32P]ATP was from Amersham Pharmacia Biotech Inc. All whole-cell solutions were prepared with human infusion-grade water (Abbott Laboratories Ltd.). Tris-buffered saline with Tween 20 (TBT) used for Western blotting consisted of 0.2 M Tris-base (Boehringer Mannheim), 0.17 M NaCl (BDH, Toronto, Ontario, Canada), and 0.2% Tween 20 (Bio-Rad) (pH 7.5).
Neutrophil preparation.
Venous blood from healthy adults or from umbilical cords of healthy full-term newborn infants was collected into a sterile heparinized syringe (final concentration, 10 U/ml). Approximately 1 ml of blood was used for plasma, which was obtained by centrifugation. Neutrophils were separated by dextran and Ficoll sedimentation (4). Contaminating erythrocytes were removed by hypotonic lysis with water for 20 s. Cells were suspended in H-medium (107 cells/ml for Western blotting) or HBSS without NaHCO3 and phenol red (2 × 106 cells/ml for chemiluminescence assay).
ERK activation.
Neutrophils in H-medium at a final density of 9.5 × 106 cells/ml, with the addition of the indicated concentrations of plasma and LPS, were aliquoted into Eppendorf tubes and were incubated in a 37°C water bath for 20 min. When the inhibitor PD 98059 was used, neutrophils were first incubated with plasma (5%) and inhibitor for 10 min at room temperature. After incubation, tubes were immediately placed on ice, and cold HBSS (without Ca2+, Mg2+, NaHCO3, and phenol red) was added. Cells were collected by centrifugation at 10,000 × g for 10 s and lysed, on ice, in RIPA buffer containing protease and phosphatase inhibitors (50 to 100 μl/106 cells). Cell lysate was cleared by centrifugation at 12,000 × g for 10 min at 4°C and then mixed with SDS-polyacrylamide gel electrophoresis (PAGE) reducing buffer and frozen at −70°C. ERK activity was also determined by measuring phosphorylation of the ERK-specific substrate, myelin basic protein, as outlined in an earlier report (5).
Preparation of fibronectin-depleted plasma and fibronectin.
Fibronectin-depleted human plasma was prepared according to the manufacturer's instructions by using a gelatin-Sepharose 4B affinity column (Amersham Pharmacia Biotech, Inc.). The column was prepared by washing beads with 70% ethanol followed by three washings with phosphate-buffered saline (PBS). Under sterile conditions, plasma from adults was passed through the column three times and was stored in aliquots at −70°C. The column was washed with PBS followed by a wash with 0.9% sodium chloride solution, and fibronectin was eluted from the column using 0.05 M sodium acetate and 1.0 M NaBr, pH 5.0. Fibronectin was dialyzed with PBS overnight and stored in aliquots at −70°C. Fibronectin depletion of plasma and fibronectin purification was confirmed by SDS-PAGE and Coomassie blue staining.
SDS-PAGE and Western blotting.
Protein concentration was quantified using the Bradford method, and equal amounts of protein were separated by SDS-PAGE (4% stacking gel, 12% separating gel) and transferred to nitrocellulose membranes using the Bio-Rad Mini Protean II apparatus. Nitrocellulose membranes were blocked in 5% bovine serum albumin in TBT for 60 min and were incubated with a primary antibody specific for phosphorylated ERK 1 and 2 (p-ERK E-4; Santa Cruz Biotechnology Inc.) at 4°C overnight on a shaker. Membranes were washed in TBT (three times for 5 min each) and incubated with a horseradish peroxidase-conjugated goat anti-mouse (Bio-Rad) secondary antibody for 60 min. Membranes were washed in TBT (six times for 5 min each), and antibody binding was detected with ECL reagents (Amersham Pharmacia Biotech, Inc.). Equal protein loading was confirmed by stripping membranes and reprobing with a primary antibody specific for nonphosphorylated ERK 2 (ERK 2 C-14; Santa Cruz Biotechnology Inc.) and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Cappell Research Products, Durham, N.C.). Band density was quantified by densitometric scanning of blots (NIH Image 1.6 software).
Chemiluminescence assay.
Neutrophils in HBSS at a final density of 4 × 105 cells/ml, with the addition of 5% plasma with or without LPS (5 ng/ml), were incubated in a 37°C shaker (100 rpm) for 45 min. When the inhibitor PD 98059 was used, neutrophils were incubated first with plasma (5%) and the inhibitor for 10 min at room temperature. Lucigenin (7 × 10−8 M) was added to assay tubes, and background readings were taken using a luminometer; fMLP (10−5 M) was then added to tubes, and oxidative burst was measured for 4 min.
RESULTS
LPS-mediated ERK 1 and 2 activation in neutrophils from adults and newborns.
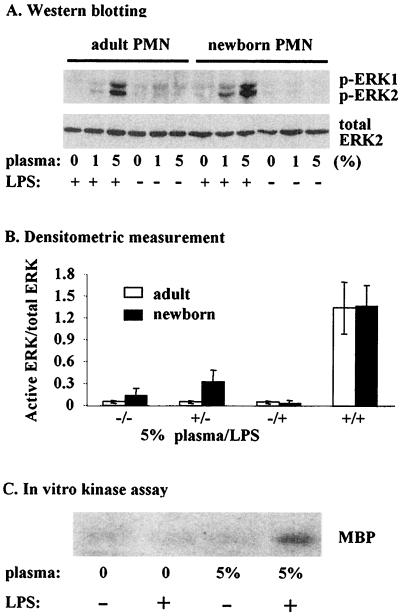
LPS is known to activate ERK in many cell types (27, 30) but results using neutrophils are controversial, with some studies indicating that ERK is not activated by LPS (8, 16, 17) and others showing activation occurs only at high concentrations (19). Using Western blotting to detect levels of ERK 1 and 2 phosphorylation, we examined the LPS-mediated activation of ERK 1 and 2 in neutrophils from both adults and newborns. We first stimulated neutrophils with 5 ng of LPS per ml for 20 min in the presence of 5% plasma, conditions that effectively prime neutrophils without activation (2). Under these conditions LPS induced dramatic activation of ERK 1 and 2 (Fig. 1). We also noted that levels of LPS-induced ERK 1 and 2 activation were similar between neutrophils from adults and newborns (Fig. 1A, upper blot). Stripping membranes and reprobing them with an antibody for nonphosphorylated ERK 2 showed that lanes had similar loading of this protein (Fig. 1A, lower blot). Data from several different individuals showed that there was no difference in the relative amount of ERK 1 and 2 activation (Fig. 1B). There was also no difference between cells from adults and newborns in the ratio of activated ERK 1 to ERK 2 (data not shown). In addition, assays of adult samples for ERK activity verified that ERK phosphorylation was indicative of ERK activity (Fig. 1C).
FIG. 1.
(A) Sample Western blots for activated ERK 1 and 2 (upper blot) and total ERK 2 (lower blot) from neutrophils of adults and newborns incubated with or without 5 ng of LPS per ml and the indicated plasma concentrations for 20 min. PMN, polymorphonuclear leukocytes. (B) Neutrophils from five adults and five newborns were incubated with or without plasma (5%) and LPS (5 ng/ml) for 20 min. The mean (± standard error of the mean) level of activated ERK 1 and 2 divided by total ERK 2 determined by densitometric scanning is indicated. (C) Autoradiograph showing phosphorylation of myelin basic protein (MBP) by ERK in the same adult samples shown in panel A.
LPS-mediated activation of ERK 1 and 2 is plasma dependent.
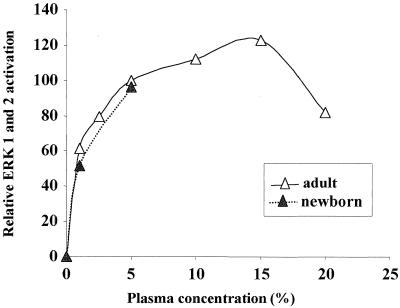
As shown in Fig. 1, LPS activated ERK 1 and 2 only when neutrophils were reconstituted with autologous plasma. In the absence of plasma, LPS failed to activate ERK 1 and 2 in cells of both adults and newborns. With the addition of 1% plasma, LPS induced a detectable increase in ERK 1 and 2 activation (Fig. 1A and 2). Increasing the concentration of plasma to 5% enhanced LPS activation of ERK 1 and 2. There was no further increase in activation with the addition of more plasma (Fig. 2). We had previously noted that plasma from newborns was not sufficient to support the LPS-mediated priming of polymorphonuclear leukocyte respiratory burst (3); however, in terms of ERK activation no difference was found between plasma from adults and newborns (Fig. 2).
FIG. 2.
ERK 1 and 2 activation by LPS in neutrophils is plasma dependent. Neutrophils from adults and newborns were incubated with LPS (5 ng/ml) and the indicated plasma concentrations for 20 min. ERK 1 and 2 activation was quantified by densitometric scanning of the bands. Values for ERK 1 and 2 were combined, and percent activation was calculated relative to values obtained for 5% plasma.
LPS-mediated activation of ERK 1 and 2 is time dependent.
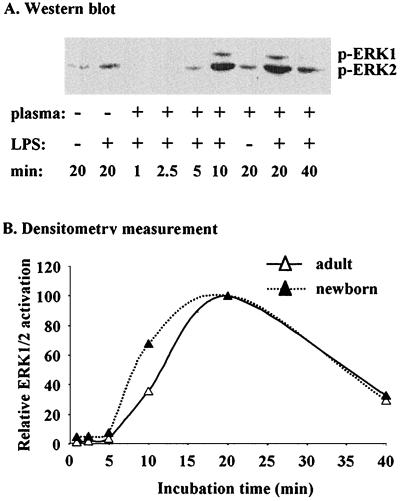
Next, we examined the time course for LPS-mediated activation of ERK 1 and 2. As shown in Fig. 3, ERK 1 and 2 activation in neutrophils increased after a lag of 5 min and was maximal after 20 min of incubation with LPS (Fig. 3A and B). When cells were incubated with LPS for longer than 20 min ERK activation rapidly decreased, though it was still higher than that in unstimulated cells (Fig. 3). No significant difference was observed in the time course for LPS-mediated activation of ERK 1 and 2 between cells of adults and newborns (Fig. 3).
FIG. 3.
(A) Sample Western blot for activated ERK 1 and 2 from neutrophils of newborns incubated with LPS for the indicated times. (B) Neutrophils from adults and newborns were incubated with LPS (5 ng/ml) and 5% plasma for the indicated times. Total ERK 1 and 2 activation was quantified by densitometric scanning of the bands (A). Results are expressed as percent activation relative to values obtained for 20 min of incubation.
LPS-mediated activation of ERK 1 and 2 is dose dependent.
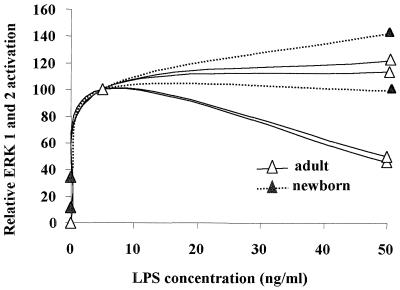
LPS has previously been shown to activate ERK 1 and 2 in human neutrophils only when used at concentrations higher than 500 ng/ml (19), but as demonstrated above, in the presence of plasma low concentrations of LPS can induce ERK 1 and 2 activation in neutrophils from adults and newborns. Therefore, we sought to measure the LPS dose response under these conditions. We found that neutrophils from different individuals responded variably to LPS stimulation when used at doses lower than 2.5 ng/ml (data not shown). Maximal ERK 1 and 2 activation generally occurred in cells of both adults and newborns when the LPS dose was approximately 5 ng/ml (Fig. 4). LPS-mediated ERK 1 and 2 activation was similar for neutrophils from adults and newborns at 5 and 50 ng/ml (Fig. 4). Increasing the LPS concentration from more than 50 ng/ml to up to 1,000 ng/ml did not cause further enhancement of ERK 1 and 2 activation (data not shown).
FIG. 4.
LPS-mediated activation of ERK 1 and 2 is dose dependent in neutrophils. Four individual neutrophil preparations of adults and two of newborns were incubated with the indicated LPS concentrations for 20 min. ERK 1 and 2 activation was quantified by densitometric scanning of the bands. Values for ERK 1 and 2 were combined, and percent activation was calculated relative to values obtained for 5 ng of LPS per ml.
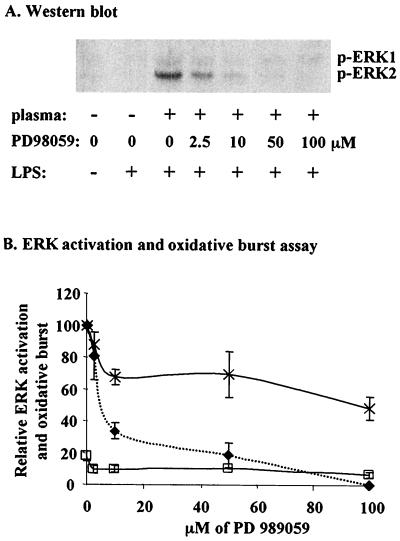
PD 98059 completely prevents LPS-mediated ERK 1 and 2 activation but only partially inhibits LPS priming of neutrophil respiratory burst.
To determine whether the ERK pathway was responsible for LPS-mediated neutrophil priming, we examined the effect of PD 98059, a MEK-specific inhibitor, on ERK activation and on fMLP-triggered oxidative burst in the presence or absence of LPS priming. We found that ERK activation was markedly inhibited (approximately 70%) at 10 M PD 98059 and completely inhibited at 100 M (Fig. 5A and B). However, in chemiluminescence assays for oxidative burst activity in neutrophils from adults primed with LPS then triggered with fMLP, only partial inhibition (approximately 40%) was seen at 100 M PD 98059 (Fig. 5B). These results indicate that pathways other than the ERK MAPK pathway are involved in the mechanism of LPS-mediated neutrophil priming.
FIG. 5.
Effects of PD 98059 on ERK activation and oxidative burst. (A) Sample Western blot for activated ERK 1 and 2 from neutrophils of adults incubated with PD 98059. (B) Neutrophils from adults were incubated with LPS (5 ng/ml) with 5% plasma and the indicated PD 98059 concentrations for 20 min. Activation of ERK 1 and 2 (⧫) was measured by Western blotting as described previously and expressed relative to values obtained for no inhibitor (plus LPS). For measurement of oxidative burst, neutrophils were incubated with (×) or without (□) LPS (5 ng/ml) with 5% plasma and a range of PD 98059 concentrations. After stimulation with fMLP, oxidative burst was measured as integrated chemiluminescence intensity for 4 min and expressed relative to values obtained for no inhibitor (plus LPS). Each data point represents the mean ± range or standard error of the mean of at least two independent measurements; oxidative burst (plus LPS) was significantly greater than ERK activity at PD 98059 concentrations of ≥10 μM (P < 0.05).
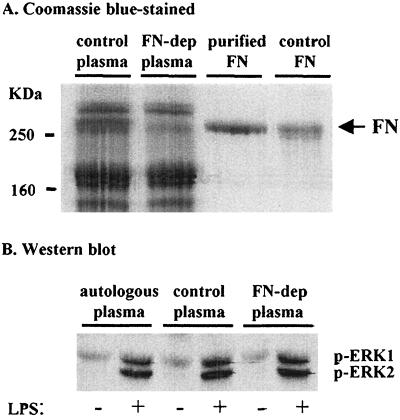
Fibronectin is not the essential plasma factor in LPS-mediated activation of ERK.
We have reported that fibronectin is required for LPS priming of the fMLP-triggered neutrophil respiratory burst (3). In this report we show that ERK activation is associated with LPS-mediated priming of neutrophils only if plasma is present. Therefore, we chose to determine if fibronectin was involved in the plasma-dependent LPS-mediated activation of ERK 1 and 2. A Sepharose gelatin 4B column was used to obtain fibronectin-depleted plasma and purified fibronectin (Fig. 6A, lanes 2 and 3). Surprisingly, depleting plasma of fibronectin did not alter LPS-mediated ERK activation (Fig. 6B). Furthermore, when plasma was fractionated by gel filtration, we found that maximal activation of ERK 1 and 2 by LPS occurred with fractions that do not contain fibronectin (data not shown). Therefore, our combined results indicate that fibronectin is not necessary for plasma-dependent LPS-mediated activation of ERK 1 and 2.
FIG. 6.
Depleting plasma of fibronectin (FN) does not affect ERK activation in neutrophils from adults. (A) Equal protein amounts of control plasma (lane 1) and fibronectin-depleted plasma (FN-dep) (lane 2) were separated by SDS-PAGE along with fibronectin purified from the control plasma (lane 3) and purchased control fibronectin (lane 4). (B) Sample Western blot for activated ERK 1 and 2 from neutrophils of adults incubated with or without 5 ng of LPS per ml and with either 5% autologous plasma, control plasma, or fibronectin-depleted plasma. The blot is representative of two experiments.
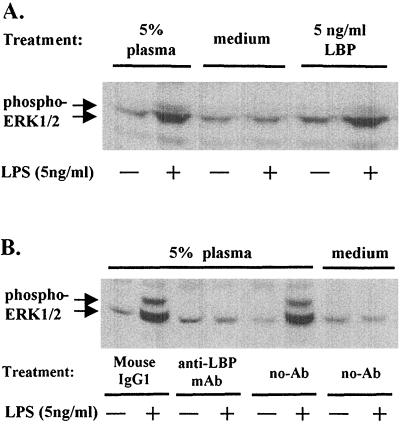
LBP is the essential plasma factor in LPS-mediated activation of ERK.
Since LBP is recognized as an important factor enhancing activation of cells at low concentrations of LPS, we determined if LBP was involved in the plasma-dependent LPS-mediated activation of ERK 1 and 2. Purified human LBP at a concentration of 5 ng/ml altered LPS-mediated ERK 1 and 2 activation to the same extent as 5% plasma (Fig. 7A). The role of LBP was confirmed by using a monoclonal antibody that neutralized functional activity of LBP. When antibody to LBP was added, we found complete inhibition of ERK 1 and 2 activation with LPS in the presence of 5% plasma (Fig. 7B).
FIG. 7.
Requirement of LBP for ERK 1 and 2 (ERK1/2) activation. Sample Western blots for activated ERK 1 and 2 from neutrophils of adults incubated with LPS (5 ng/ml) are shown. (A) Neutrophils were resuspended in media supplemented with 5% plasma, human LBP (5 ng/ml), or control media followed by stimulation with or without LPS for 20 min at 37°C before lysis. (B) Plasma was preincubated with 20 μg of mouse anti-LBP monoclonal antibody (mAb) (IgG1), control mouse IgG1, or media alone for 1 h at room temperature before being added to neutrophils to a final plasma concentration of 5%. Cells were then incubated in the presence or absence of LPS for 20 min at 37°C before lysis. phospho-ERK1/2, phosphorylated ERK1 and ERK2 proteins identified using E-4 monoclonal antibody.
DISCUSSION
We have shown that ERK 1 and 2 are activated in neutrophils exposed to low concentrations of LPS (<5 ng/ml) and that this activation is plasma dependent (Fig. 1). These results differ from earlier reports that the ERK 1 and 2 pathway is not activated by low concentrations of LPS (<500 ng/ml) in neutrophils (8, 16, 17). This discrepancy is likely due to the fact that we reconstituted neutrophils with 5% plasma prior to incubation with LPS. In contrast, earlier studies used only 0.1% bovine serum albumin (8, 16) or 0.1% heat-inactivated platelet-poor plasma (17). Our experiments indicate that plasma concentrations of less than 1% do not result in detectable activation of ERK 1 and 2 (Fig. 2). Interestingly, LPS activation of p38 occurs in the presence or absence of plasma, suggesting that LBP is not required (16, 17). In comparison to earlier reports, our findings indicate that LPS-mediated ERK activation requires either a higher concentration of plasma containing LBP or other ERK-specific plasma factors than does p38 for activation. Differences in experimental conditions (e.g., the presence of specific protease or phosphatase inhibitors in the lysis buffer) may also explain why we were able to detect ERK 1 and 2 activation by LPS.
Previously it was shown that neutrophils from newborns, in contrast to those from adults, do not exhibit an LPS-primed increase in fMLP-triggered oxidative burst (2, 24). DeLeo et al. have shown that incubating neutrophils with 100 ng of LPS per ml results in the translocation of cytosolic p47phox (a component of the NADPH oxidase) to the plasma membrane of the cell (7). Furthermore, Benna et al. have found that ERKs are involved in phosphorylation and translocation of p47phox to the plasma membrane (1). This evidence led us to ask if neutrophils from newborns fail to prime in response to LPS due to deficiencies in ERK activation. Thorough comparisons of the conditions for ERK 1 and 2 activation in cells of adults and newborns indicates that there is no significant difference in LPS-mediated activation of ERK 1 and 2 between these cells. We conclude that the inability of newborn neutrophils to prime in response to LPS is not due to deficiencies in the ERK MAPK pathway.
Although there was no detectable difference in ERK activation between cells from adults and newborns, we wondered if phosphorylated proteins downstream of ERK may be involved in the LPS-primed oxidative burst in adult cells. PD 98059, an ERK pathway-specific inhibitor, was used to assess whether ERK contributed to the LPS-primed respiratory burst in neutrophils. We found that at a concentration of 100 μM PD 98059 completely blocked LPS-mediated activation of ERK 1 and 2 in neutrophils but inhibited the LPS-primed fMLP-triggered oxidative burst by only 40% in these cells (Fig. 5). This partial inhibition of the LPS-primed fMLP-triggered oxidative burst by PD 98059 and the kinase assay results (Fig. 1) support a conclusion that ERK activation plays a minor role in LPS priming in these cells. Approximately 60% of the priming activity remained when PD 98059 was used at concentrations shown to completely inhibit ERK. Thus, although ERK 1 and 2 are involved in the LPS-primed fMLP-triggered increase in oxidative burst, other pathways also must contribute to the majority of this response, further supporting the conclusion that deficiencies in the LPS priming of neutrophils from newborns are likely due to other processes and not the ERK pathway.
Previously it was shown that plasma with the adult isoform of fibronectin is involved in LPS priming of neutrophils but plasma with the newborn isoform is not (3). As preliminary studies indicated that a plasma protein or proteins of ≥100 kDa were involved in LPS-stimulated activation of ERK 1 and 2 (data not shown), we were interested in whether fibronectin was required for plasma-dependent LPS-mediated activation of ERK 1 and 2. Use of fibronectin-depleted plasma did not significantly affect ERK activation in adult cells. Furthermore, using plasma fractionated by protein size, we found that LPS-mediated ERK 1 and 2 activation is maximal in fractions with molecular sizes of 50 to 70 kDa that do not contain fibronectin (data not shown). In contrast to the studies with fibronectin, we found LBP to be an essential factor involved in LPS-stimulated activation of ERK 1 and 2 (Fig. 7). These observations are consistent with the above findings that plasma-dependent LPS-mediated ERK activation does not differ between neutrophils from adults and newborns. We conclude that fibronectin is not one of the plasma factors necessary for LPS-mediated activation of ERKs.
Our findings demonstrate that ERK 1 and 2 activation occurs in neutrophils and contributes to some of the LPS-primed oxidative burst increase in these cells. We have shown that plasma is necessary for LPS-mediated ERK activation and that fibronectin is not involved in plasma-dependent activation of ERK. Furthermore, we have shown that there is no difference in LPS-mediated ERK 1 and 2 activation between cells from adults and newborns. This eliminates one of the major LPS-activated intracellular signaling pathways as being the source for the biological difference between neutrophils from adults and newborns in response to LPS. Future research is needed to identify the plasma factor or factors required for ERK 1 and 2 activation and other signaling pathways to explain the biological differences between neutrophils from adults and newborns.
ACKNOWLEDGMENTS
This work was supported by grant MT. 14439 from the Medical Research Council of Canada. S. R. Yan is supported by funds awarded for salary from the IWK Grace Health Center.
We acknowledge the assistance of Walla A1-Hertani in the undertaking of these experiments and Pam Kirby for laboratory and secretarial support.
REFERENCES
- 1.Benna J E, Han J, Park J-W, Schmid E, Ulevitch R J, Babior B M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 2.Bortolussi R, Howlett S, Rajaraman K, Halperin S. Deficient priming activity of newborn cord blood-derived polymorphonuclear neutrophilic granulocytes with lipopolysaccharide and tumor necrosis factor-α triggered with formyl-methionyl-leucyl-phenylalanine. Pediatr Res. 1993;34:243–248. doi: 10.1203/00006450-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bortolussi R, Rajaraman K, Qing G, Rajaraman R. Fibronectin enhances in vitro lipopolysaccharide priming of polymorphonuclear leukocytes. Blood. 1997;89:4182–4189. [PubMed] [Google Scholar]
- 4.Bortolussi R, Vandenbroucke-Grauls C M J E, Van Asbeck B S, Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987;55:3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Lin T H, Der C J, Juliano R L. Integrin-mediated activation of mitogen-activated protein (MAP) or extracellular signal-related kinase kinase (MEK) and kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- 6.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 7.DeLeo F R, Renee J, McCormick S, Nakamura M, Apicella M, Weiss J P, Nauseef W M. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Investig. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouda S I, Molski T F P, Ashour M S-E, Sha'afi R I. Effect of lipopolysaccharide on mitogen-activated protein kinases and cytosolic phospholipase A2. J Biochem. 1995;308:815–822. doi: 10.1042/bj3080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N I, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe menigococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Cambronero J, Huang C-K, Gomez-Cambronero T M, Waterman W H, Becker E L, Sha'afi R I. Granulocyte-macrophage colony-stimulating factor-induced protein tyrosine phosphoration of microtubule-associated protein kinase in human neutrophils. Proc Natl Acad Sci USA. 1992;89:7551–7555. doi: 10.1073/pnas.89.16.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Cambronero J, Wang E, Johnson G, Huang C-K, Sha'afi R I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991;266:6240–6245. [PubMed] [Google Scholar]
- 12.Lloyds D, Brindle N P J, Hallett M B. Priming of human neutrophils by tumour necrosis factor-alpha and substance P is associated with tyrosine phosphorylation. Immunology. 1995;84:220–226. [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyds D, Hallett M B. Neutrophil “priming” induced by orthovanadate: evidence of a role for tyrosine phosphorylation. Biochem Pharmacol. 1994;48:15–21. doi: 10.1016/0006-2952(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 14.McColl S R, DiPersio J F, Caon A C, Ho P, Maccache P H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991;78:1842–1852. [PubMed] [Google Scholar]
- 15.Morrison D C, Lei M-G, Kirikae T, Chen T-Y. Endotoxin receptors in mammalian cells. Immunobiology. 1993;187:212–226. doi: 10.1016/S0171-2985(11)80340-2. [DOI] [PubMed] [Google Scholar]
- 16.Nahas N, Molski T F P, Fernandez G A, Sha'afi R I. Tyrosine phosphorylation and activation of a new mitogen-activated protein (MAP)-kinase cascade in human neutrophils stimulated with various agonists. J Biochem. 1996;318:247–253. doi: 10.1042/bj3180247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nick J A, Avdi N J, Gerwins P, Johnson G L, Worthen G S. Activation of a p38 mitogen-activated protein kinase in human neutrophils by lipopolysaccharide. J Immunol. 1996;156:4867–4875. [PubMed] [Google Scholar]
- 18.Nick J A, Avdi N J, Young S K, Lehman L A, McDonald P P, Frasch S C, Billstrom M A, Henson P M, Johnson G L, Worthen G S. Selective activation and functional significance of p38α mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Investig. 1999;103:851–858. doi: 10.1172/JCI5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan B, Duffy A, Paquin L, De M, Collette H, Graziano C M, Bankey P. Mitogen-activated protein kinases signal inhibition of apoptosis in lipopolysaccharide-stimulated neutrophils. Surgery. 1999;126:406–412. [PubMed] [Google Scholar]
- 20.Ohta S, Inazu T, Taniguchi T, Nakagawara G, Yamamura H. Protein-tyrosine phosphorylations induced by concanavalin A and N-formyl-methionyl-leucyl-phenylalanine in human neutrophils. Eur J Biochem. 1992;206:895–900. doi: 10.1111/j.1432-1033.1992.tb16998.x. [DOI] [PubMed] [Google Scholar]
- 21.Perez E M, Weisman L E. Novel approaches to the prevention and therapy of neonatal bacterial sepsis. Neonatology. 1997;24:213–229. [PubMed] [Google Scholar]
- 22.Pinner R W, Teutsch S M, Simonsen L, Klug L A, Graber J M, Clarke M J, Berkelman R L. Trends in infectious diseases mortality in the United States. JAMA. 1996;275:189–193. [PubMed] [Google Scholar]
- 23.Qing G, Howlett S, Bortolussi R. Lipopolysaccharide binding proteins on polymorphonuclear leukocytes: comparison of adult and neonatal cells. Infect Immun. 1996;64:4638–4642. doi: 10.1128/iai.64.11.4638-4642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing G, Rajaraman K, Bortolussi R. Diminished priming of neonatal polymorphonuclear leukocytes by lipopolysaccharide is associated with reduced CD14 expression. Infect Immun. 1995;63:248–252. doi: 10.1128/iai.63.1.248-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafiee P, Lee J K, Leung C-C, Raffin T A. TNF-α induces tyrosine phosphorylation of mitogen-activated protein kinase in adherent human neutrophils. J Immunol. 1995;154:4785–4792. [PubMed] [Google Scholar]
- 26.Schopf R E, Keller R, Rehder M, Benes P, Kallinowski F, Vaupel P. TNF-α primes polymorphonuclear leukocytes for an enhanced respiratory burst to a similar extent as bacterial lipopolysaccharide. J Investig Dermatol. 1990;95:216S–218S. doi: 10.1111/1523-1747.ep12875802. [DOI] [PubMed] [Google Scholar]
- 27.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Tomonaga M, Kohno T, Moriuchi R, Miyamoto T, Katamine S. Tyrosine phosphorylation in neutrophils induced by G-CSF and fMLP: involvement of FGR tyrosine kinase in neutrophil activation. International Society for Experimental Hematology XXI Annual Meeting Exp Hematol. 1992;20:828. [Google Scholar]
- 29.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Young D W, Gusovsky F, Chow J C. Cellular events mediated by lipopolysaccharide-stimulated Toll-like receptor 4. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]