Abstract
This study tested the hypothesis that glycine improves intestinal barrier function through regulating oxidative stress in broilers exposed to heat stress. A total of 300 twenty-one-day-old female Arbor Acres broilers (600 ± 2.5g) was randomly allocated to 5 treatments (6 replicate of 10 birds each). The 5 treatments were as follows: the control group (CON) was kept under thermoneutral condition (24 ± 1°C) and was fed a basal diet. Broilers fed a basal diet and reared under high ambient temperature (HT) were considered as the HT group (34 ± 1°C for 8 h/d). Broilers fed a basal diet supplemented with 0.5%, 1.0%, and 2.0% glycine and exposed to HT were regarded as the HT + glycine treatments. The results exhibited that heat stress reduced growth performance, serum total antioxidant capacity (T-AOC), and glutathione (GSH) concentration (P < 0.05); increased activity of serum catalase (CAT) and the contents of hydrogen peroxide (H2O2) and malondialdehyde (MDA) (P < 0.05). HT exposure led to downregulating the mRNA expression of NAD(P)H quinone dehydrogenase 1 (NQO1), Occludin, and zonula occludens-1 (ZO-1) (P < 0.05); enhanced the mRNA levels of Kelch-like ECH-associated protein 1 (Keap1), CAT, glutathione synthetase (GSS), and glutamate-cysteine ligase modifier subunit (GCLM) (P < 0.05); impaired the intestinal morphology (P < 0.05); and altered the diversity and community of gut microbiota (P < 0.05). The final body weight (FBW), ADFI, ADG, and gain-to-feed ratio (G: F) increased linearly or quadratically, and the antioxidant capacity was improved (P < 0.05) with glycine supplementation. Glycine treatment increased the villus height (VH), and villus height to crypt depth ratio (V/C) of the duodenum linearly or quadratically, and linearly increased the VH of jejunum and ileum. The mRNA expression of Occludin, and ZO-1 were increased linearly in the ileum mucosa of broilers subjected to HT. Collectively, these results demonstrated that glycine supplementation alleviates heat stress-induced dysfunction of antioxidant status and intestinal barrier in broilers.
Key words: antioxidant status, glycine, heat stress, intestinal barrier function, Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling pathway
INTRODUCTION
The rising global temperature brings great challenges to the poultry industry. Poultry is more vulnerable to heat stress than mammals because of its high metabolic rate and lack of sweat glands. In the high-temperature environment, the surface blood flow of poultry increases but the visceral blood flow decreases compensably (Rostagno, 2020). The local ischemic environment leads to the reduction of oxygen flow to the intestinal mucosa and the production of a large number of reactive oxygen species (ROS) (Nawab et al., 2018). The resultant oxidative stress causes intestinal tight junction injury (El-Orabi et al., 2011). Oxidative stress became a main factor in reducing poultry growth performance under heat stress (Goel, 2021). Therefore, many functional nutrients with antioxidant effects are used to alleviate heat stress on poultry, which includes probiotics, amino acids, vitamins, trace elements, electrolytes, etc. (Ansari et al., 2020; Jiang et al., 2021; Calik et al., 2022; Ouyang et al., 2022).
Glycine, the smallest dispensable amino acid, can be internally synthesized by mammals such as humans, rodents, and pigs (Gibson et al., 2002). However, chicks are unable to synthesize sufficient glycine to satisfy their requirements, and it is therefore considered a conditionally indispensable amino acid (Ospina-Rojas et al., 2012). Glycine plays an important role in antioxidant (Wang et al., 2013), anti-inflammatory (Wheeler et al., 1999), cytoprotective (Zhong et al., 2003), and immunomodulatory properties (Carmans et al., 2010). Glycine inhibits the generation of ROS and reduces oxidative stress in ethanol-treated neuroblastoma cells (Amin et al., 2016), and decreases mitochondrial swelling, ROS, and lipid peroxidation (LPO) of cholestasis mice (Heidari et al., 2018). Supplementation with glycine significantly alleviates intestinal injury by inhibiting Toll-like receptor 4 (TLR4) and nucleotide-binding oligomerization domain protein (NOD) pathway of lipopolysaccharide (LPS) challenged piglets (Xu et al., 2018). Glycine is an important substrate for the synthesis of biological molecules such as porphyrin, creatine, purine, neurotransmitter, and so on (Petrat et al., 2012). Meanwhile, it is also a component of bile acid and participates in the synthesis of tissue protein and glutathione (Paolini et al., 2001). Glycine inhibits the intestinal injury caused by tumor necrosis factor alpha (TNF-α) in the chemical colitis model (McCole, 2010), and restores the glutathione (GSH) to oxidized GSH (GSSG) ratio reduced by oxidative stress (Wessner et al., 2003). Glycine's application has been widely recognized in various disease model of the medical field, such as ischemia-reperfusion injury, shock, organ transplantation, alcoholic hepatitis, liver fibrosis, arthritis, tumor, and drug toxicity (Amelio et al., 2014; Effenberger-Neidnicht et al., 2014; Al-Saeedi et al., 2019; Alves et al., 2019; Shafiekhani et al., 2019). However, there are few studies on the application of glycine under stressful conditions in poultry over the past 20 yr.
Based on the antioxidant and cytoprotective effects of glycine, we hypothesized that it has the potential to alleviate heat stress in broilers. This study was conducted to investigate the effect of glycine on the growth performance, serum and intestinal mucosa antioxidant capacity, intestinal morphology, and barrier function of broilers under heat stress.
MATERIALS AND METHODS
Ethical Approval
The experiment was conducted following the Chinese guidelines for animal welfare and ethics censorship. All experimental procedures using laboratory animals were approved by the Laboratory Animal Ethics Committee of Jiangxi Agricultural University, Nanchang, China (approval code: JXAULL-2021-036).
Animals and Treatments
A total of 400 one-day-old female Arbor Acres broiler chickens were obtained from a commercial hatchery (Changsha, Hunan province, China). The chickens were fed the basal diet during the starter periods (days 1–21) before the experiment. Three hundred broilers with similar weight (600 ± 2.5 g) were selected and completely randomized into 5 groups (6 replicate cages per treatment and 10 birds per cage) on day 22. The control group (CON) was kept in a room under thermoneutral conditions (24°C, 65%–70% RH) and the high ambient temperature (HT) groups were raised in a separate room under cycle high temperature (34°C from 9:00 to 17:00 and 24°C for the rest time, 65%–70% RH).
All broilers were kept in stainless steel cages with 24-h light and free access to feed and water. The CON was fed the basal diet for the grower period (days 22–35), whereas the HT groups were fed with basal diet and the basal diet supplemented with 0.5%, 1.0%, and 2.0% glycine (99.4% purity, Hebei Huaheng Biotechnology Co., Ltd., Hengshui, China). Basal diets (Table 1) were formulated to meet or exceed the nutrient requirement of the Feeding Standard of Chicken, China (Zhu et al., 2022). L-Alanine (99.2% purity, Hebei Huaheng Biotechnology Co., Ltd.) was used to balance nitrogen. Zeolite powder was reduced to increase the formulation space for glycine and alanine.
Table 1.
Ingredients and nutrients composition of the basal experimental diets.
| Item% | Starter phase | Grower phase |
|---|---|---|
| Ingredients | ||
| Corn | 56.00 | 58.20 |
| Soybean meal | 25.50 | 21.10 |
| Corn gluten meal | 10.00 | 10.00 |
| Soybean oil | 2.50 | 4.00 |
| Salt | 0.30 | 0.30 |
| Limestone | 1.50 | 1.40 |
| Calcium hydrogen phosphate | 1.70 | 1.40 |
| Vitamin premix1 | 0.05 | 0.05 |
| Mineral premix2 | 0.20 | 0.20 |
| L-Lysine, 79% | 0.29 | 0.36 |
| DL-Methionine, 98% | 0.15 | 0.03 |
| Choline chloride, 60% | 0.10 | 0.10 |
| Zeolite powder | 1.71 | 2.86 |
| Total | 100.00 | 100.00 |
| Nutrient level3 | ||
| Metabolizable energy, MJ/kg | 12.59 | 12.97 |
| Crude protein, % | 21.70 | 20.05 |
| Lysine, % | 1.16 | 1.10 |
| Methionine, % | 0.55 | 0.40 |
| Methionine + cysteine, % | 0.96 | 0.77 |
| Glycine, % | 0.66 | 0.35 |
| Serine, % | 0.92 | 0.93 |
| Calcium, % | 1.04 | 0.92 |
| Available phosphorous, % | 0.46 | 0.40 |
Provided per kilogram of complete diet: 10,000 IU vitamin A, 1,000 IU vitamin D3, 60 IU vitamin E, 1.5 mg vitamin K3, 2 mg thiamine, 8 mg riboflavin, 10 mg pantothenic acid, 35 mg nicotinic acid, 3.5 mg vitamin B6, 0.3 mg biotin, 1.25 mg folic acid, 0.025 mg vitamin B12.
Provided per kilogram of diet: Mn, 124 mg as MnSO4·H2O; Zn, 100 mg as ZnSO4·H2O; Cu, 14.5 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4·H2O; I, 0.7 mg as Ca (IO3) H2O; and Se, 0.3 mg as Na2SeO3.
The nutrient levels are calculated values except glycine and serine which are measured values.
Performance Measurement and Sampling
The broilers per cage were weighed in the morning at 22 and 36 d of age and feed consumption per cage was measured daily to calculate ADG, ADFI, and gain-to-feed ratio (G: F). The feed samples of the basal diet were collected and stored at −20°C.
At the end of the trial, 1 bird was randomly selected from each replicate after 12 h of fasting to collect blood (pterygoid vein). Blood samples were centrifuged at 3,000 × g for 10 min at 4°C to obtain serum and stored at −80°C for further analysis. Then the broilers were euthanized by cervical dislocation and necropsied immediately. Samples of the duodenum, jejunum, and ileum (2 cm) were isolated, washed with phosphate buffer, and fixed in 4% paraformaldehyde for morphology analysis. The mucosa samples of each intestinal segment were scraped with glass slides, snap frozen in liquid nitrogen, and then transferred to −80°C until analysis.
Serum Parameters Determination
The serum catalase (CAT), glutathione peroxidase (GPX), superoxide dismutase (SOD) activities and hydrogen peroxide (H2O2), total antioxidant capacity (T-AOC), malondialdehyde (MDA), GSH, and GSSG levels were measured with commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The serum diamine oxidase (DAO) activity, D-lactic acid (D-LA), and LPS concentration were measured with chicken ELISA Assay Kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China).
Intestinal Morphology Determination
The paraformaldehyde fixed duodenum, jejunum, and ileum were washed, dehydrated, embedded in paraffin, sectioned, patched, and stained with hematoxylin and eosin. The intestinal morphology was evaluated by villus height (VH), crypt depth (CD), and villus height: crypt depth ratio (V/C) that were measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD).
Ileal Mucosal Antioxidant Activity Determination
The ileum mucosal antioxidant enzymes (CAT, GPX, SOD) activities, and H2O2, MDA, T-AOC, GSH, and GSSG levels were measured according to the manufacturer's instructions of commercial assay kit (Nanjing Jiancheng Bioengineering Institute). The protein concentration of the supernatant was measured using G-clone BCA Protein Assay Kit (G-clone Biotechnology Co., Ltd., Beijing, China).
Quantitative Real-Time PCR
Total RNA was isolated from the ileal mucosa by using TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China). The quality and concentration of RNA were assessed using BioDrop uLite spectrophotometer (Biochrom Ltd., Cambridge, UK). gDNA removal and cDNA synthesis used EasyScript Super Mix (TransGen). The gene expression levels were quantified using a BIO-RAD CFX Connect Real-Time system (Bio-Rad Laboratories, Inc., Hercules, CA) and PerfectStart Green qPCR Super Mix (TransGen). The target gene relative expression was calculated according to the 2-∆∆Ct method and β-actin was used as the internal reference gene. The primer sequences are shown in Table 2.
Table 2.
Primer sequences for RT-PCR.
| Genes | Accession no. | Primer sequence5′-3′ | |
|---|---|---|---|
| Keap1 | MN416132.1 | Forward: | GCCCTCAACAACTGCAT |
| Reverse: | CGGGTCGTAACACTCCA | ||
| Nrf2 | MN416129.1 | Forward: | ATCACGAGCCCTGAAACCAA |
| Reverse: | GGCTGCAAAATGCTGGAAAA | ||
| Maf | XM_040707140.1 | Forward: | AGTCCTGCCGCTTCAAG |
| Reverse: | GTAGGCGTCCCTCTCCC | ||
| CAT | NM_001031215.2 | Forward: | TATCCTTCCTGGTCTTT |
| Reverse: | CATCTGTTCTACCTCCG | ||
| SOD1 | NM_205064.2 | Forward: | ATGTGACTGCAAAGGGAGGA |
| Reverse: | AGCTAAACGAGGTCCAGCAT | ||
| HO-1 | NM_205344.2 | Forward: | GAAAGCTGCCCTGGAGAAAG |
| Reverse: | CCCAGACAGGTCTCCCAAAT | ||
| NQO1 | NM_001277620.2 | Forward: | TCAATGCCGTGCTCTCA |
| Reverse: | CAGCCGCTTCAATCTTC | ||
| GSR | XM_040671422.1 | Forward: | AGTGGCTTGCTGGAGGT |
| Reverse: | GGGTCAGGAGGGCTTTG | ||
| GPX-1 | NM_001277853.3 | Forward: | GACCAACCCGCAGTACATCA |
| Reverse: | GAGGTGCGGGCTTTCCTTTA | ||
| GCLC | XM_040666478.1 | Forward: | AAATGGGACAGGCACAG |
| Reverse: | GGGATCAAACCAGGAAA | ||
| GCLM | NM_001007953.2 | Forward: | TTCGGTCATTATTGCCC |
| Reverse: | ACCTGATTGCTGCTTGG | ||
| GSS | XM_040688004.1 | Forward: | GAACCTCCTACATCCTG |
| Reverse: | CTGACATAGACACCGAA | ||
| Occludin | NM_205128.1 | Forward: | AGCCCTCAATACCAGGATGTG |
| Reverse: | CGCTTGATGTGGAAGAGCTTG | ||
| Claudin1 | NM_001013611.2 | Forward: | AGAAGATGCGGATGGCT |
| Reverse: | AACGGGTGTGAAAGGGT | ||
| Claudin2 | NM_001277622.1 | Forward: | GATACGTGTAGCAGCAGCAG |
| Reverse: | AGCTGGGATTTCTGAGCAGT | ||
| ZO-1 | XM_040680632.1 | Forward: | AAGAGGAAGCTGTGGGTAACTC |
| Reverse: | TGAAGAGTCACCGTGTGTTGT | ||
| β-actin | NM_205518.2 | Forward: | AACCCCAAAGCCAACAG |
| Reverse: | ACAGGGACAGCACAGCC | ||
Ileal Mucosal Microbiome
The HT group fed 2.0% glycine group (HTG) was selected to compare the differences between the CON and the HT groups in the ileal microbiome. Total bacterial DNA was extracted from ileal mucosal with the TGuide S96 Magnetic Soil/Stool DNA Kit (Tiangen Biotech, Beijing, China) according to manufacturer instructions. The DNA concentration of the samples was measured with the Qubit dsDNA HS Assay Kit and Qubit 4.0 Fluorometer (Invitrogen, Thermo Fisher Scientific, Eugene, OR). The 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′- GGACTACHVGGGTWTCTAAT-3′) universal primer set was used to amplify the V3-V4 region of the 16S rRNA gene from the genomic DNA. The total of PCR amplicons was purified with Agencourt AMPure XP Beads (Beckman Coulter, Indianapolis, IN) and pooled in equal amounts for the constructed library by using Illumina novaseq 6000 (Illumina, Santiago, CA) for sequencing. Sequences with similarity ≥97% were clustered into the same operational taxonomic unit (OTU) by USEARCH (v10.0) (Edgar, 2013). Taxonomy annotation of the OTUs was performed based on the Naive Bayes classifier in QIIME2 (Bolyen et al., 2019) using the SILVA database (Quast et al., 2013) (release 132) with a confidence threshold of 70%. The Alpha diversity was calculated and displayed by the QIIME2 and R software, respectively. Beta diversity was determined to evaluate the degree of similarity of microbial communities from different samples using QIIME. The linear discriminant analysis (LDA) effect size (LEfSe) (Segata et al., 2011) was used to analyze the significant taxonomic difference among groups. A logarithmic LDA score of 2.0 was set as the threshold for discriminative features.
Statistical Analysis
The data were analyzed using SPSS17.0 (SPSS Inc., Chicago, IL). Levene's test was used to test the variance homogeneity of measured data. An independent sample t test was used to compare the differences between the CON and HT. The differences between HT and HT + glycine groups were analyzed using 1-way ANOVA. Orthogonal polynomial contrasts were used to examine the linear and quadratic effects of glycine levels on the broiler under heat stress. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Performance
As expected, the growth performance of broilers exposed to high temperature decreased significantly (Table 3). The final body weight (FBW), ADFI, ADG, and the G: F ratio of the HT group were lower compared with the CON group (P < 0.05). Among the 4 high-temperature groups, the FBW, ADFI, ADG, and G: F increased linearly or quadratically as dietary glycine increased from 0.5% to 2.0% (P < 0.05).
Table 3.
Effect of glycine on the growth performance of heat-stressed broilers.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P value |
||||||||
| Items | CON | HT | 0.5%Gly | 1.0%Gly | 2.0%Gly | SEM | P value | Main effect | Linear | Quadratic |
| IBW (g) | 600.67 | 600.00 | 601.83 | 597.67 | 599.83 | 1.28 | 0.821 | 0.746 | 0.698 | 0.951 |
| FBW (g) | 1290.83 | 1104.69 | 1096.06 | 1122.41 | 1177.50 | 9.34 | <0.001 | 0.003 | 0.001 | 0.036 |
| ADFI (g) | 86.15 | 65.44 | 64.35 | 69.37 | 74.62 | 1.04 | <0.001 | <0.001 | <0.001 | 0.026 |
| ADG (g) | 49.30 | 36.05 | 35.3 | 37.48 | 41.26 | 0.66 | <0.001 | 0.001 | 0.001 | 0.029 |
| G: F | 1.72 | 1.90 | 1.86 | 1.85 | 1.79 | 0.02 | 0.001 | 0.037 | 0.007 | 0.565 |
Abbreviations: FBW, final body weight; G: F, gain: feed ratio; IBW, initial body weight.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Intestinal Morphology
As shown in Table 4, the V/C of the 3 intestinal segments and duodenal CD had a difference in the HT group compared with the CON group (P < 0.05). Dietary supplementation with glycine linearly or quadratically increased the VH and V/C ratio of duodenum (P < 0.05), and linearly alleviated villi damage of jejunum and ileum. On the contrary, the CD of duodenum decreased quadratically with increasing glycine (P > 0.05).
Table 4.
Effect of glycine on the intestinal morphology of heat-stressed broilers.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P value |
||||||||
| Items | CON | HT | 0.5%Gly | 1.0%Gly | 2.0%Gly | SEM | P value | Main effect | Linear | Quadratic |
| Duodenum | ||||||||||
| VH (μm) | 1596.93 | 1462.05 | 1699.39 | 1726.31 | 1676.52 | 17.57 | 0.024 | <0.001 | <0.001 | <0.001 |
| CD (μm) | 226.65 | 287.37 | 244.87 | 244.94 | 269.60 | 8.24 | 0.007 | 0.042 | 0.659 | 0.007 |
| V/C | 7.45 | 5.36 | 7.15 | 7.22 | 7.01 | 0.26 | 0.006 | 0.030 | 0.028 | 0.048 |
| Jejunum | ||||||||||
| VH (μm) | 1726.71 | 1483.55 | 1524.76 | 1594.36 | 1631.81 | 14.63 | <0.001 | 0.001 | <0.001 | 0.976 |
| CD (μm) | 190.83 | 215.54 | 209.68 | 196.52 | 231.93 | 6.12 | 0.210 | 0.271 | 0.509 | 0.093 |
| V/C | 9.55 | 7.25 | 6.97 | 8.03 | 7.25 | 0.24 | 0.014 | 0.703 | 0.613 | 0.599 |
| Ileum | ||||||||||
| VH (μm) | 1351.33 | 1113.69 | 1222.46 | 1219.59 | 1273.03 | 13.80 | <0.001 | <0.001 | <0.001 | 0.218 |
| CD (μm) | 165.46 | 175.44 | 176.78 | 157.71 | 164.85 | 3.93 | 0.655 | 0.242 | 0.134 | 0.735 |
| V/C | 8.12 | 6.56 | 7.47 | 7.98 | 7.27 | 0.24 | 0.010 | 0.284 | 0.213 | 0.087 |
Abbreviations: CD, crypt depth; V/C, villus height: crypt depth ratio; VH, villus height.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Antioxidant Status of Serum and Intestinal Mucosa
As shown in Table 5, the HT exhibited oxidative damages with higher serum H2O2, MDA, and CAT activity compared with the CON (P < 0.05). The change trends of T-AOC and GSH were reversed (P < 0.05). After supplementation with glycine, MDA content decreased linearly or quadratically, and H2O2 content decreased linearly (P < 0.05). The activities of SOD and GPX increased linearly, and the T-AOC activity and GSSG concentration increased quadratically with the addition of dietary glycine (P < 0.05). However, serum CAT activity, serum GSH were not affected (P > 0.05) by dietary treatments.
Table 5.
Effect of glycine on the serum antioxidant status of heat-stressed broilers.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P value |
||||||||
| Items | CON | HT | 0.5%Gly | 1.0%Gly | 2.0%Gly | SEM | P value | Main effect | Linear | Quadratic |
| H2O2 (mmol/L) | 30.94 | 43.23 | 26.97 | 28.25 | 25.40 | 2.16 | 0.023 | 0.001 | 0.001 | 0.010 |
| MDA (nmol/mL) | 3.27 | 4.26 | 3.30 | 3.29 | 2.78 | 0.21 | 0.044 | 0.043 | 0.008 | 0.567 |
| CAT (U/mL) | 3.33 | 5.10 | 5.77 | 5.34 | 4.37 | 0.22 | 0.005 | 0.342 | 0.596 | 0.092 |
| SOD (U/mL) | 130.75 | 115.33 | 200.76 | 192.55 | 235.43 | 16.98 | 0.651 | 0.046 | 0.01 | 0.498 |
| GPX (U/mL) | 834.60 | 821.89 | 902.17 | 913.44 | 1015.01 | 22.85 | 0.766 | 0.017 | 0.002 | 0.783 |
| T-AOC (U/mL) | 11.97 | 7.44c | 13.14 | 17.93 | 10.59 | 1.09 | 0.016 | 0.001 | 0.052 | <0.001 |
| GSH (μmol/L) | 29.74 | 15.69 | 18.10 | 21.16 | 18.22 | 1.19 | 0.003 | 0.479 | 0.311 | 0.310 |
| GSSG (μmol/L) | 15.35 | 15.37 | 27.15 | 21.05b | 15.52 | 1.15 | 0.991 | <0.001 | 0.302 | <0.001 |
Abbreviations: CAT, catalase; GPX, glutathione peroxidase; GSH, glutathione; GSSG, oxidized GSH; H2O2, hydrogen peroxide; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Similar trends were observed in ileal mucosa to that of serum. CAT, H2O2 concentration, and SOD, T-AOC, and GPX activities of the HT group were higher than the CON group (P < 0.05, Table 6). CAT increased significantly and GPX activity quadratically increased as the inclusion of glycine increased (P < 0.05). Meanwhile, the content of H2O2 and GSH decreased linearly and reached the lowest concentration at the level of 2.0% glycine (P < 0.05). There was no significant difference between CON, HT, and HT + glycine groups about the antioxidant index in duodenal mucosa (P > 0.05). However, the GSSG content in jejunal mucosa of HT increased significantly compared with CON (P < 0.05), and the content of MDA decreased supplemented with glycine (P < 0.05).
Table 6.
Effect of glycine on the intestinal mucosa antioxidant status of heat-stressed broilers.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P value |
||||||||
| Items | CON | HT | 0.5%Gly | 1.0%Gly | 2.0%Gly | SEM | P value | Main effect | Linear | Quadratic |
| Duodenum | ||||||||||
| CAT (U/mg prot) | 1.74 | 1.48 | 1.03 | 0.84 | 1.34 | 0.15 | 0.583 | 0.427 | 0.648 | 0.123 |
| H2O2 (mmol/mg prot) | 3.40 | 2.90 | 2.40 | 2.30 | 1.96 | 0.13 | 0.497 | 0.087 | 0.015 | 0.729 |
| MDA (nmol/mg prot) | 0.91 | 0.78 | 0.63 | 0.80 | 0.94 | 0.11 | 0.671 | 0.792 | 0.512 | 0.507 |
| SOD (U/mg prot) | 83.85 | 85.21 | 88.90 | 73.98 | 78.38 | 3.35 | 0.847 | 0.413 | 0.251 | 0.958 |
| T-AOC (U/mg prot) | 0.93 | 1.00 | 0.98 | 0.94 | 0.97 | 0.06 | 0.666 | 0.987 | 0.804 | 0.854 |
| GPX (U/mg prot) | 41.28 | 31.13 | 18.39 | 22.13 | 19.34 | 2.36 | 0.189 | 0.114 | 0.064 | 0.197 |
| T-GSH (μmol/mg prot) | 62.06 | 67.66 | 62.55 | 51.91 | 45.35 | 3.44 | 0.580 | 0.080 | 0.012 | 0.909 |
| GSH (μmol/mg prot) | 27.20 | 33.95 | 31.05 | 25.29 | 24.15 | 1.83 | 0.213 | 0.181 | 0.035 | 0.804 |
| GSSG (μmol/mg prot) | 17.43 | 16.85 | 15.75 | 13.31 | 10.60 | 1.02 | 0.874 | 0.125 | 0.021 | 0.676 |
| Jejunum | ||||||||||
| CAT (U/mg prot) | 2.37 | 2.95 | 1.99 | 3.74 | 3.03 | 0.26 | 0.545 | 0.125 | 0.381 | 0.279 |
| H2O2 (mmol/mg prot) | 4.78 | 5.68 | 4.63 | 4.89 | 3.94 | 0.30 | 0.352 | 0.264 | 0.083 | 0.949 |
| MDA (nmol/mg prot) | 2.04 | 2.52 | 1.64 | 2.16 | 1.84 | 0.11 | 0.203 | 0.016 | 0.078 | 0.144 |
| SOD (U/mg prot) | 114.86 | 126.46 | 118.26 | 145.67 | 135.04 | 4.88 | 0.381 | 0.281 | 0.243 | 0.795 |
| T-AOC (U/mg prot) | 0.96 | 1.05 | 1.04 | 1.31 | 0.99 | 0.06 | 0.575 | 0.299 | 0.734 | 0.282 |
| GPX (U/mg prot) | 69.59 | 44.37 | 42.84 | 46.09 | 38.47 | 5.539 | 0.287 | 0.972 | 0.787 | 0.799 |
| T-GSH (μmol/mg prot) | 19.92 | 30.95 | 33.78 | 29.31 | 22.06 | 2.04 | 0.049 | 0.231 | 0.103 | 0.208 |
| GSH (μmol/mg prot) | 44.01 | 64.75 | 33.67 | 44.77 | 50.50 | 4.44 | 0.115 | 0.084 | 0.393 | 0.034 |
| GSSG (μmol/mg prot) | 11.76 | 17.31 | 9.83 | 10.10 | 8.59 | 1.23 | 0.022 | 0.059 | 0.033 | 0.112 |
| Ileum | ||||||||||
| CAT (U/mg prot) | 2.33 | 4.86 | 6.94 | 4.94 | 5.09 | 0.30 | 0.003 | 0.032 | 0.585 | 0.082 |
| H2O2 (mmol/mg prot) | 2.01 | 3.62 | 3.70 | 3.07 | 2.49 | 0.16 | 0.009 | 0.007 | 0.001 | 0.188 |
| MDA (nmol/mg prot) | 0.20 | 0.26 | 0.28 | 0.32 | 0.30 | 0.03 | 0.401 | 0.946 | 0.628 | 0.802 |
| SOD (U/mg prot) | 68.95 | 136.86 | 147.49 | 137.88 | 125.15 | 5.50 | 0.004 | 0.585 | 0.384 | 0.311 |
| T-AOC (U/mg prot) | 0.28 | 0.56 | 1.13 | 1.12 | 0.94 | 0.14 | 0.036 | 0.446 | 0.366 | 0.193 |
| GPX (U/mg prot) | 24.17 | 58.38 | 76.20 | 91.07 | 32.68 | 6.19 | 0.004 | 0.001 | 0.138 | <0.001 |
| T-GSH (μmol/mg prot) | 86.93 | 86.77 | 88.3 | 68.31 | 50.56 | 5.35 | 0.991 | 0.022 | 0.004 | 0.281 |
| GSH (μmol/mg prot) | 34.97 | 46.44 | 32.17 | 31.52 | 24.60 | 2.95 | 0.102 | 0.035 | 0.007 | 0.464 |
| GSSG (μmol/mg prot) | 25.98 | 20.17 | 26.20 | 20.84 | 17.93 | 1.81 | 0.275 | 0.454 | 0.568 | 0.207 |
Abbreviations: CAT, catalase; GPX, glutathione peroxidase; GSH, glutathione; GSSG, oxidized GSH; H2O2, hydrogen peroxide; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Expression of Keap1/Nrf2 Signaling Pathway-Related Genes in Ileal Mucosa
The data of Kelch-like ECH-associated protein 1/ nuclear factor erythroid 2-related 2 (Keap1/Nrf2) signaling pathway-related genes mRNA expression in ileal mucosa were shown in Table 7. The mRNA expression of Keap1, CAT, glutathione synthetase (GSS), and glutamate-cysteine ligase modifier subunit (GCLM) in the HT group increased significantly compared with the CON group, whereas the expression of NAD(P)H quinone dehydrogenase 1 (NQO1) was on the contrary (P < 0.05). Supplementation with glycine increased the relative expression of both NQO1 and Keap1 genes (P < 0.05), with a linear trend for Keap1 (P < 0.05). The relative expression of GCLM was decreased linearly and reached the lowest point at the dosage 2.0% glycine (P < 0.05).
Table 7.
Relative mRNA expression of Keap1/Nrf2 signaling pathway genes in ileum.
| Effect of glycine under HT |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P value |
|||||||||
| Items | CON | HT | 0.5%Gly | 1%Gly | 2%Gly | SEM | P value | Main effect | Linear | Quadratic | |
| Keap1 | 1.00 | 1.88 | 2.23 | 2.52 | 2.81 | 0.27 | 0.139 | 0.703 | 0.252 | 0.965 | |
| Nrf2 | 1.00 | 1.23 | 1.31 | 1.16 | 1.23 | 0.09 | 0.105 | 0.958 | 0.863 | 0.980 | |
| Maf | 1.00 | 0.88 | 0.93 | 0.83 | 0.61 | 0.10 | 0.790 | 0.649 | 0.274 | 0.547 | |
| HO-1 | 1.00 | 0.68 | 1.02 | 0.65 | 0.58 | 0.07 | 0.246 | 0.137 | 0.296 | 0.144 | |
| NQO1 | 1.00 | 0.22 | 0.59 | 0.56 | 1.05 | 0.11 | <0.001 | 0.044 | 0.233 | 0.193 | |
| SOD1 | 1.00 | 0.72 | 0.90 | 0.61 | 0.85 | 0.15 | 0.061 | 0.685 | 0.489 | 0.618 | |
| CAT | 1.00 | 2.15 | 1.96 | 1.28 | 1.84 | 0.18 | 0.040 | 0.280 | 0.165 | 0.179 | |
| GPX | 1.00 | 0.79 | 0.97 | 0.93 | 1.25 | 0.13 | 0.493 | 0.690 | 0.289 | 0.781 | |
| GSR | 1.00 | 1.51 | 1.51 | 0.90 | 1.25 | 0.20 | 0.405 | 0.684 | 0.455 | 0.673 | |
| GSS | 1.00 | 2.10 | 2.52 | 2.25 | 1.81 | 0.22 | 0.025 | 0.747 | 0.593 | 0.361 | |
| GCLC | 1.00 | 1.80 | 1.62 | 1.10 | 1.74 | 0.21 | 0.227 | 0.733 | 0.684 | 0.451 | |
| GCLM | 1.00 | 3.21 | 2.09 | 1.27 | 1.24 | 0.27 | 0.017 | 0.022 | 0.004 | 0.236 | |
Abbreviations: CAT, catalase; GCLC, catalytic subunit of γ-glutamate cysteine ligase; GCLM, modulatory subunit of γ-glutamate cysteine ligase; GPX, glutathione peroxidase; GSR, glutathione-disulfide reductase; GSS, glutathione synthetase; HO-1, heme oxygenase-1; Keap1, Kelch-like ECH-associated protein 1; Maf, transcription factor Maf; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid 2-related factor 2; SOD1, superoxide dismutase 1.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Contents of Serum DAO, D-LA, LPS, and mRNA Expression of Tight Junction Protein-Related Genes in Ileal Mucosa
The mRNA expressions of Occludin and zonula occludens-1 (ZO-1) in ileal mucosa were decreased under HT conditions (P < 0.05, Table 8), and supplementation with glycine changed the trends linearly (P < 0.05). The concentration of serum LPS was lower in the HT + glycine groups compared with the HT group (P < 0.05, Table 9), which was the biomarker of the intestinal barrier.
Table 8.
Relative mRNA expression of tight junction protein genes in ileum.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HS |
P Value |
||||||||
| Items | CON | HT | 0.5%Gly | 1%Gly | 2%Gly | SEM | P Value | Main effect | Linear | Quadratic |
| Occludin | 1.00 | 0.64 | 0.97 | 1.23 | 1.14 | 0.080 | 0.004 | 0.030 | 0.010 | 0.126 |
| Claudin1 | 1.00 | 0.73 | 1.43 | 1.02 | 1.09 | 0.130 | 0.334 | 0.351 | 0.584 | 0.256 |
| Claudin2 | 1.00 | 1.35 | 1.37 | 1.66 | 1.05 | 0.110 | 0.330 | 0.370 | 0.465 | 0.246 |
| ZO-1 | 1.00 | 0.55 | 0.89 | 1.16 | 0.98 | 0.080 | 0.009 | 0.025 | 0.014 | 0.060 |
Abbreviations: CD, crypt depth; V/C, villus height: crypt depth ratio; VH, villus height.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Table 9.
Effect of glycine on the serum DAO, D-LA, and LPS of heat-stressed broilers.
| Effect of glycine under HT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments1 |
CON vs. HT |
P Value |
||||||||
| Items | CON | HT | 0.5%Gly | 1%Gly | 2%Gly | SEM | P Value | Main effect | Linear | Quadratic |
| DAO (ng/mL) | 2.87 | 3.06 | 3.23 | 3.19 | 2.55 | 0.137 | 0.675 | 0.263 | 0.189 | 0.142 |
| D-LA (μmol/mL) | 8.07 | 7.69 | 6.80 | 7.73 | 6.37 | 0.811 | 0.796 | 0.926 | 0.697 | 0.893 |
| LPS (EU/L) | 23.38 | 33.82 | 21.21 | 20.97 | 20.89 | 2.174 | 0.002 | 0.017 | 0.053 | 0.126 |
Abbreviations: DAO, diamine oxidase; D-LA, D-lactate; LPS, lipopolysaccharide.
CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; 0.5% Gly, 1.0% Gly, 2.0% Gly: basal diet supplemented with 0.5%, 1.0%, 2.0% glycine under cyclic high-temperature conditions (n = 6).
Ileal Microbiota
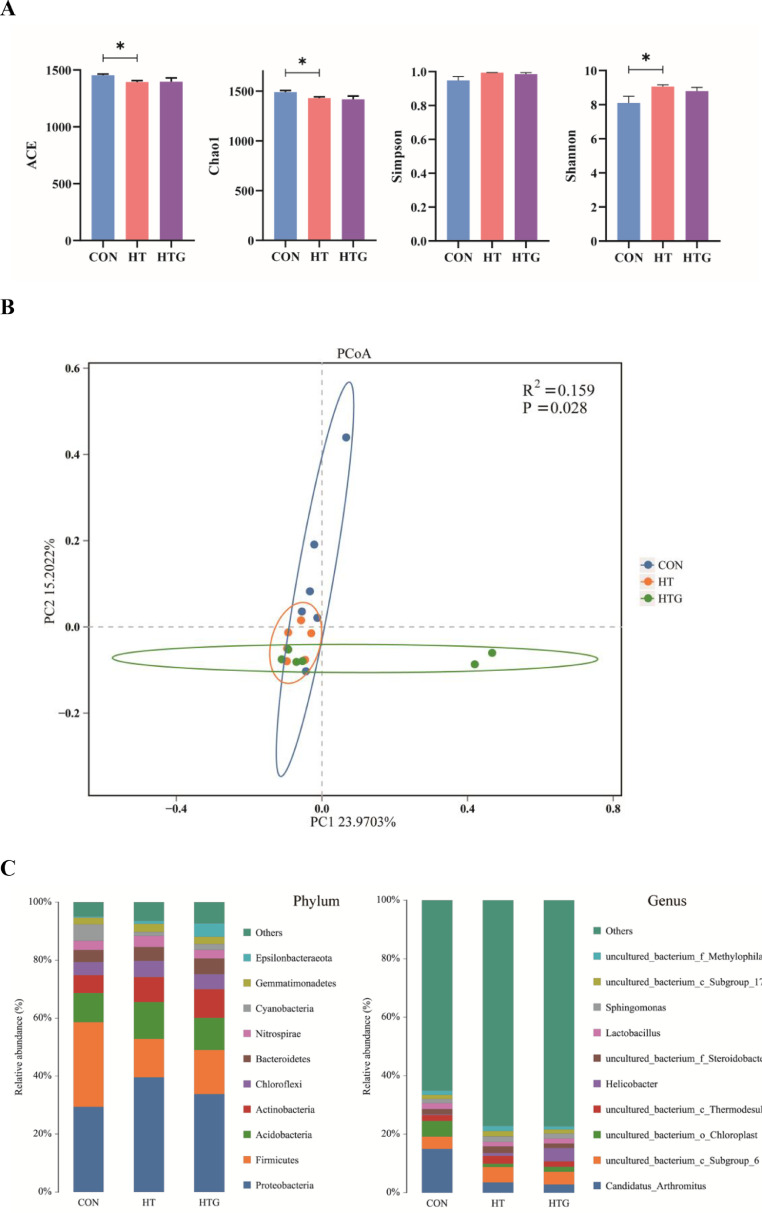
As shown in Figure 1A, alpha-diversity indexes which included ACE, Chao1, Simpson, and Shannon were investigated. The HT group decreased the species richness and diversity as shown by ACE (P < 0.05), Chao1 (P < 0.05), and Shannon (P < 0.05) compared with the CON group. However, dietary glycine supplementation had no significant effect on microbial diversity and richness (P > 0.05). The β-diversity using principal coordinates analysis (PCoA) and PERMANOVA test (R2 = 0.159, P = 0.028) showed samples were separated and divided into 3 different clusters (Figure 1B).
Figure 1.
Summary of the microbiomes in the ileal mucosa. (A) Alpha diversity indexes (ACE, Chao1, Simpson, Shannon). (B) Principal co-ordinates analysis (PCoA) and PERMANOVA test plot. (C) Relative abundance of bacterial composition at phylum and genus levels. CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; HTG: basal diet supplemented with 2.0% glycine under cyclic high-temperature conditions (n = 6). *Significant difference between CON and HT, HT and HTG by t test (P < 0.05).
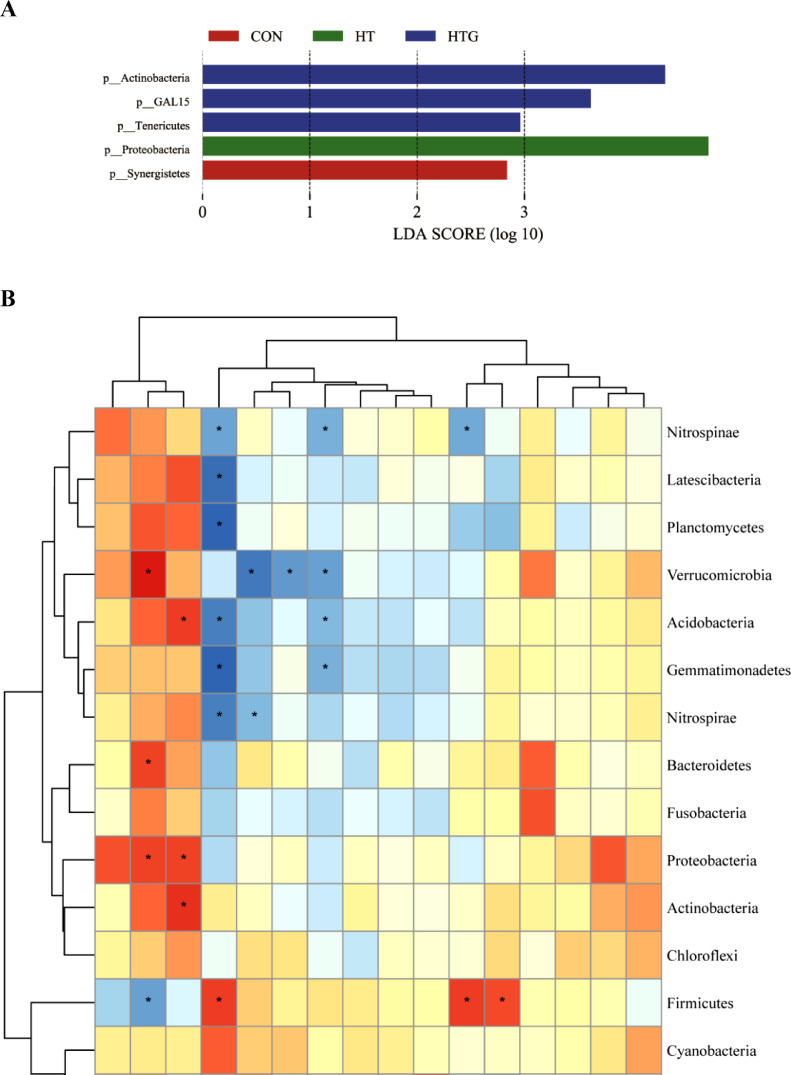
The microbiota of ileal mucosa at the phylum level was dominated by Proteobacteria, Firmicutes, Acidobacteria, and Actinobacteria in all groups (Figure 1C, Left). The percentage of Proteobacteria increased in the high-temperature group and decreased with the supplementation of glycine, whereas Firmicutes exhibited the opposite trend. At the genus level, the dominant microbiota were Candidatus_Arthromitus, uncultured_bacterium_c_Subgroup_6, uncultured_bacterium_o_Chloroplast, uncultured_bacterium_c_Thermodesulfovibrionia (Figure 1C, Right). The relative abundance of Candidatus_Arthromitus in birds decreased when exposed to high temperature. The LEfSe analysis at the phylum level revealed that the predominant microbiota of CON was Synergistetes (Figure 2A). The predominant microbiota of HT was Proteobacteria. The predominant microbiota of HTG was Actinobacteria, GAL15, and Tenericutes.
Figure 2.
Summary of the microbiomes in the ileal mucosa. (A) LEfSe analysis at phylum level (LDA score >2.0). (B) Correlation between top 15 microbiota at phylum level and mRNA expression of Keap1/Nrf2-related genes, tight junction protein. Red indicates a positive correlation, whereas blue represents a negative correlation. Significance is presented as * (P < 0.05). CON: basal diet and raised under thermoneutral conditions; HT: basal diet and raised under cyclic high-temperature conditions; HTG: basal diet supplemented with 2.0% glycine under cyclic high-temperature conditions (n = 6).
Spearman's rank correlation coefficient was used to analyze the correlation between the top 20 microbiota at phylum level and the mRNA expression of ileal mucosa genes (Figure 2B). Verrucomicrobia, Proteobacteria, and Bacteroidetes were positively correlated with Keap1, whereas Firmicutes was negatively correlated with it. Firmicutes was positively correlated with SOD1, NQO1, and glutamate-cysteine ligase catalytic subunit (GCLC), whereas Nitrospinae was negatively correlated with SOD1, ZO-1, and NQO1. Acidobacteria, Actinobacteria, and Proteobacteria were positively associated with GSS. Gemmatimonadetes, Acidobacteria, Planctomycetes, Latescibacteria, and Nitrospirae were negatively correlated with SOD1. Verrucomicrobia were negatively correlated with Clandin1, HO1, and ZO-1. Gemmatimonadetes and Acidobacteria were also negatively correlated with ZO-1.
DISCUSSION
In the present study, broilers exposed to high temperatures were injured by heat stress. Both ADFI and ADG decreased, intestinal morphology and barrier function were impaired, and microbiota was altered, which ultimately reduced growth performance.
Heat stress induces high production of ROS and disrupts the balance of antioxidant systems in the body (Li et al., 2020). ROS contains H2O2, superoxide radical (O2−), hydroxyl radical (OH−), etc. One of the main sources of ROS is the substrate end of the respiratory chain in the inner mitochondrial membrane (Zhang et al., 2022), where the electron transport chain complexes in the mitochondria transfer electrons to O2 (Yang et al., 2020). ROS is normally scavenged by antioxidative substances such as SOD, GPX, CAT, and GSH, and is in equilibrium with the antioxidant system. When the ROS produced exceeds the scavenging capacity of the antioxidant system, it will react with large molecules such as phospholipids, enzymes, and side chains of polyunsaturated fatty acids, and nucleic acids associated with cellular membranes to form LPO such as MDA and 4-hydroxynonenal (HDA), leading to alteration in the fluidity and permeability of cellular membranes, and ultimately in changes in cell structure and function (Del Rio et al., 2005). Therefore, heat stress causes damage to the intestinal epithelium of poultry by inducing oxidative stress, which disrupts the integrity and function of the intestinal barrier and reduces the production potential and reproductive performance (Huang et al., 2015; Murata et al., 2021; Vandana et al., 2021). In this study, the levels of oxidative stress markers H2O2 and MDA (Czerska et al., 2015) elevated in serum, which indicates that heat stress induces oxidative stress. The changes in H2O2 and MDA in the duodenum and jejunum were insignificant, so we focused on the functional changes of the ileum under heat stress. Previous studies have shown that glycine alleviates oxidative stress under a variety of disease conditions and reduces LPO in the liver and kidney of heat-exposed rats (Alcaraz-Contreras et al., 2011). In addition, glycine minimizes the impairment of antioxidant enzymes activity (SOD, GPX, and CAT) by inhibiting nuclear factor kappa-B (NF-κB) activation to suppress ROS formation (Zhong et al., 1999; Mauriz et al., 2001). In developing rat brains, glycine limits ethanol-induced ROS by activating the phosphatidylinositol 3 kinase (PI3K) /protein kinase B (Akt) pathway (Amin et al., 2016). Dietary glycine prevents Kupffer cell activation, ameliorates oxidative stress, and will reduce the impairment of the activities of antioxidant enzymes SOD, GPX, and CAT (Mauriz et al., 2001). Glycine is also an important component of GSH, which consists of L-glutamic acid, L-cysteine, and glycine and is an important component of the body's antioxidant system, and lack of GSH leads to oxidative stress. Whereas glycine restores the GSH/GSSG ratio reduced by oxidative stress, incubation of U937 cells with glycine for 24 h increased the concentration of GSH (Wessner et al., 2003; Perez-Torres et al., 2017). In vitro studies showed that glycine also attenuated the effects of lead acetate on oxidative stress parameters in rat liver and kidney samples and induced an increase in GSH levels (Garcia-Macedo et al., 2008). In the study, glycine increased the activities of serum SOD, GPX and T-AOC, as well as CAT and GPX activities in the ileum mucosa, and decreased serum H2O2 and MDA levels. As reported by Wang et al. (2018), glycine ameliorated the raise in urinary MDA levels and partially restored renal glutathione levels in diabetic rats. It can be concluded that glycine has the effect to alleviate oxidative stress from different triggers. However, the growth performance increased linearly or quadratically with the change in glycine levels, indicating that the optimal dosage of glycine may exceed 2.0%.
Alterations in the oxidative state of the intestine are usually accompanied by changes in intestinal morphology and permeability. Several studies have reported changes in intestinal morphology in poultry under heat stress conditions, including a decrease in VH and V/C, and an increase in CD (Varasteh et al., 2015; Nanto-Hara et al., 2020). Meanwhile, the integrity of the intestinal barrier is also disrupted, leading to increased intestinal permeability (Quinteiro-Filho et al., 2010). Glycine was found to alleviate LPS-induced intestinal mucosal damage and reduce jejunal crypt depth in pigs, attenuating Citrobacter rodentium-induced Colitis in mice (Xu et al., 2018; Zhang et al., 2021). Moreover, glycine represses endoplasmic reticulum stress-related apoptosis and intestinal barrier dysfunction of porcine intestinal epithelial cells, and increases the protein abundance of Occludin, Claudin-1, ZO-1, and zonula occludens-2 (ZO-2) by the inactivation of inositol-requiring enzyme (IRE1a)-c-Jun N-terminal kinase (JNK) signaling in a mammalian target of rapamycin complex 1 (mTORC1)-dependent manner (Yang et al., 2022). In the present study, the addition of glycine markedly increased the intestinal villus height (duodenum, jejunum, and ileum) and V/C (duodenum) in heat-stressed broilers. While the serum levels of LPS decreased and the mRNA expression of Occludin and ZO-1 in ileal mucosa linearly increased indicating that glycine could improve the intestinal barrier function and exhibit a dosage effect.
The Keap1/Nrf2 pathway is one of the most important defense mechanisms against oxidation (Jia et al., 2020), involved in regulating the expression of downstream antioxidant-related genes such as NQO1, HO-1, GPX-1, and glutathione synthesis (Jo et al., 2016; Jiang et al., 2017; Ali et al., 2022). In this study, we found that glycine increased the mRNA expression of Keap1 and NQO1 genes in the ileal mucosa. NQO1 is a flavin, an antioxidant enzyme, and an important antioxidant substance in the body. Under the catalysis of this enzyme, quinones are directly reduced to hydroquinone in vivo, reducing the oxygen radicals generated by quinone conversion, thus forming a protective mechanism against oxidative stress damage caused by the metabolism of quinones (Ross et al., 2021). γ-glutamylcysteine ligase (GCL) is composed of GCLC and GCLM subunits, which is the rate-limiting enzyme for the synthesis of GSH (Langston et al., 2011). GCLC is responsible for the formation of ATP-dependent glutamate γ-carboxyl and cysteine amino linkages, whereas GCLM increases its catalytic efficiency by interacting with GCLC (Yang et al., 2005). However, glycine reduced the mRNA expression of GCLM in the ileum, thus limiting the synthesis of GSH in the present study. Interestingly, we found similar results in the study of Jackson et al. (2004), where a decrease in erythrocyte glutathione concentration and synthesis rate after intravenous infusion of 20 μmol/L glycine was observed, which may be due to the availability of methionine limiting the synthesis of glutathione. The result may also be due to the changes in the microbiota of ileum.
The intestinal microbiota of poultry is complex and diverse, interacting closely with the host and also being affected by external factors such as heat stress along with the host. Heat stress alters the relative abundance of cecal flora in broilers and hens, such as Firmicutes, Bacteroidetes, Tenericutes, and Proteobacteria (Liu et al., 2022; Zhou et al., 2022). And the effect of heat stress on the microbial composition of ileal mucosa is more prominent than that of ileal contents (Emami et al., 2022). Ji et al., 2021 reported that dietary supplementation with 2% glycine decreased the number of pathogenic bacteria (Escherichia–Shigella, Clostridium, and Burkholderiales) and increased the number of short-chain fatty acid-producing bacteria (Blautia, Lachnospiraceae, Anaerostipes, and Prevotella) in the colon. In the present study, heat stress decreased the α-diversity and β-diversity of ileal flora in broilers, and the distribution of microorganisms was changed at the phylum and genus levels. However, glycine did not affect the richness and diversity of ileal flora. These results indicate that the microbial pathway maybe not the main mechanism by which glycine alleviates heat stress in broilers. Rom et al. (2020) also reported that the glycine-based tripeptide DT-109 did not depend on alterations in the gut microbiome to resist nonalcoholic steatohepatitis.
CONCLUSION
In summary, we have found that heat stress resulted in antioxidant capacity and intestinal function breakdown in broilers. These effects on the antioxidant state and intestinal barrier were attenuated by glycine, which is consistent with our hypothesis. The current findings suggest that glycine might be a critical nutrient in maintaining intestinal barrier function and antioxidant capacity in heat-stressed broilers. Further research to determine the optimal level of glycine to alleviate heat stress is significant, and enriches the study of functional amino acids.
AUTHOR CONTRIBUTIONS
C.D. and G.L. designed the study. C.D. and J.Z. conducted the experiments, collected and detected samples. C.D. analyzed the data and wrote the manuscript. G.L. and H.Z. directed the analyses and revised the manuscript. All authors contributed to the article and approved the final manuscript.
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (grant no. 32260851) and Jiangxi Province Postgraduate Innovation Special Fund Project (no. YC2021-B087).
DISCLOSURES
The authors declare that they have no potential conflicts of interest in the research.
REFERENCES
- Alcaraz-Contreras Y., Garza-Ocanas L., Carcano-Diaz K., Ramirez-Gomez X.S. Effect of glycine on lead mobilization, lead-induced oxidative stress, and hepatic toxicity in rats. J. Toxicol. 2011;2011 doi: 10.1155/2011/430539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I., Li C., Kuang M., Shah A.U., Shafiq M., Ahmad M.A., Abdalmegeed D., Li L., Wang G. Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022;148:54–67. doi: 10.1016/j.molimm.2022.05.121. [DOI] [PubMed] [Google Scholar]
- Al-Saeedi M., Liang R., Schultze D.P., Nickkholgh A., Herr I., Zorn M., Schemmer P. Glycine protects partial liver grafts from Kupffer cell-dependent ischemia-reperfusion injury without negative effect on regeneration. Amino Acids. 2019;51:903–911. doi: 10.1007/s00726-019-02722-5. [DOI] [PubMed] [Google Scholar]
- Alves A., Bassot A., Bulteau A.L., Pirola L., Morio B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients. 2019;11:1–28. doi: 10.3390/nu11061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelio I., Cutruzzola F., Antonov A., Agostini M., Melino G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014;39:191–198. doi: 10.1016/j.tibs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin F.U., Shah S.A., Kim M.O. Glycine inhibits ethanol-induced oxidative stress, neuroinflammation and apoptotic neurodegeneration in postnatal rat brain. Neurochem. Int. 2016;96:1–12. doi: 10.1016/j.neuint.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Ansari I., Khalaji S., Hedayati M. Potassium phosphate and potassium carbonate administration by feed or drinking water improved broiler performance, bone strength, digestive phosphatase activity and phosphorus digestibility under induced heat stress conditions. Trop. Anim. Health Prod. 2020;52:591–600. doi: 10.1007/s11250-019-02046-2. [DOI] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodriguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., 2nd, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vazquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Emami N.K., White M.B., Walsh M.C., Romero L.F., Dalloul R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part I: growth performance, body composition and intestinal nutrient transporters. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmans S., Hendriks J.J., Thewissen K., Van den Eynden J., Stinissen P., Rigo J.M., Hellings N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J. Neurosci. Res. 2010;88:2420–2430. doi: 10.1002/jnr.22395. [DOI] [PubMed] [Google Scholar]
- Czerska M., Mikolajewska K., Zielinski M., Gromadzinska J., Wasowicz W. Today's oxidative stress markers. Med. Pr. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- Del Rio D., Stewart A.J., Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Effenberger-Neidnicht K., Jagers J., Verhaegh R., de Groot H. Glycine selectively reduces intestinal injury during endotoxemia. J. Surg. Res. 2014;192:592–598. doi: 10.1016/j.jss.2014.06.016. [DOI] [PubMed] [Google Scholar]
- El-Orabi N.F., Rogers C.B., Edwards H.G., Schwartz D.D. Heat-induced inhibition of superoxide dismutase and accumulation of reactive oxygen species leads to HT-22 neuronal cell death. J. Therm. Biol. 2011;36:49–56. [Google Scholar]
- Emami N.K., Schreier L.L., Greene E., Tabler T., Orlowski S.K., Anthony N.B., Proszkowiec-Weglarz M., Dridi S. Ileal microbial composition in genetically distinct chicken lines reared under normal or high ambient temperatures. Anim. Microbiome. 2022;4:28. doi: 10.1186/s42523-022-00183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Macedo R., Sanchez-Munoz F., Almanza-Perez J.C., Duran-Reyes G., Alarcon-Aguilar F., Cruz M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur. J. Pharmacol. 2008;587:317–321. doi: 10.1016/j.ejphar.2008.03.051. [DOI] [PubMed] [Google Scholar]
- Gibson N.R., Jahoor F., Ware L., Jackson A.A. Endogenous glycine and tyrosine production is maintained in adults consuming a marginal-protein diet. Am. J. Clin. Nutr. 2002;75:511–518. doi: 10.1093/ajcn/75.3.511. [DOI] [PubMed] [Google Scholar]
- Goel A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. (Berl.). 2021;105:1136–1145. doi: 10.1111/jpn.13496. [DOI] [PubMed] [Google Scholar]
- Heidari R., Ghanbarinejad V., Mohammadi H., Ahmadi A., Ommati M.M., Abdoli N., Aghaei F., Esfandiari A., Azarpira N., Niknahad H. Mitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic mice. Biomed. Pharmacother. 2018;97:1086–1095. doi: 10.1016/j.biopha.2017.10.166. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Jackson A.A., Gibson N.R., Lu Y., Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 2004;80:101–107. doi: 10.1093/ajcn/80.1.101. [DOI] [PubMed] [Google Scholar]
- Ji Y., Fan X., Zhang Y., Li J., Dai Z., Wu Z. Glycine regulates mucosal immunity and the intestinal microbial composition in weaned piglets. Amino Acids. 2021;54:385–398. doi: 10.1007/s00726-021-02976-y. [DOI] [PubMed] [Google Scholar]
- Jia G., Yu S., Sun W., Yang J., Wang Y., Qi Y., Chen Y. Hydrogen sulfide attenuates particulate matter-induced emphysema and airway inflammation through Nrf2-dependent manner. Front. Pharmacol. 2020;11:29. doi: 10.3389/fphar.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Li H., Zhao N. Thymoquinone protects against cobalt chloride-induced neurotoxicity via Nrf2/GCL-regulated glutathione homeostasis. J. Biol. Regul. Homeost. Agents. 2017;31:843–853. [PubMed] [Google Scholar]
- Jiang S., Yan F.F., Hu J.Y., Mohammed A., Cheng H.W. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals (Basel) 2021;11:1494. doi: 10.3390/ani11061494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H.S., Kim D.S., Ahn E.H., Kim D.W., Shin M.J., Cho S.B., Park J.H., Lee C.H., Yeo E.J., Choi Y.J., Yeo H.J., Chung C.S., Cho S.W., Han K.H., Park J., Eum W.S., Choi S.Y. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016;49:617–622. doi: 10.5483/BMBRep.2016.49.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J.W., Li W., Harrison L., Aw T.Y. Activation of promoter activity of the catalytic subunit of gamma-glutamylcysteine ligase (GCL) in brain endothelial cells by insulin requires antioxidant response element 4 and altered glycemic status: implication for GCL expression and GSH synthesis. Free Radic. Biol. Med. 2011;51:1749–1757. doi: 10.1016/j.freeradbiomed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Tan H., Zou Z., Gong J., Zhou J., Peng N., Su L., Maegele M., Cai D., Gu Z. Preventing necroptosis by scavenging ROS production alleviates heat stress-induced intestinal injury. Int. J. Hyperth. 2020;37:517–530. doi: 10.1080/02656736.2020.1763483. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Pan Z.Y., Zhao Y., Guo Y., Qiu S.J., Balasubramanian B., Jha R. Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.890520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriz J.L., Matilla B., Culebras J.M., Gonzalez P., Gonzalez-Gallego J. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic. Biol. Med. 2001;31:1236–1244. doi: 10.1016/s0891-5849(01)00716-x. [DOI] [PubMed] [Google Scholar]
- McCole D.F. The epithelial glycine transporter GLYT1: protecting the gut from inflammation. J. Physiol. 2010;588:1033–1034. doi: 10.1113/jphysiol.2010.188516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Kunii H., Kusama K., Sakurai T., Bai H., Kawahara M., Takahashi M. Heat stress induces oxidative stress and activates the KEAP1-NFE2L2-ARE pathway in bovine endometrial epithelial cells. Biol. Reprod. 2021;105:1114–1125. doi: 10.1093/biolre/ioab143. [DOI] [PubMed] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2020;57:284–290. doi: 10.2141/jpsa.0190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., Zhao Y., Nawab Y., Li K., Xiao M., An L. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Eyng C., Nunes R.V., Duarte C.R., Vargas M.D. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine+serine:lysine. Poult. Sci. 2012;91:3148–3155. doi: 10.3382/ps.2012-02470. [DOI] [PubMed] [Google Scholar]
- Ouyang J., Zhou H., Li Q., Zheng J., Chen C., Guo S., You J., Li G. Tryptophan alleviates acute heat stress-induced impairment of antioxidant status and mitochondrial function in broilers. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.863156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C.L., Marconi A.M., Ronzoni S., Di Noio M., Fennessey P.V., Pardi G., Battaglia F.C. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Perez-Torres I., Zuniga-Munoz A.M., Guarner-Lans V. Beneficial effects of the amino acid glycine. Mini Rev. Med. Chem. 2017;17:15–32. doi: 10.2174/1389557516666160609081602. [DOI] [PubMed] [Google Scholar]
- Petrat F., Boengler K., Schulz R., de Groot H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br. J. Pharmacol. 2012;165:2059–2072. doi: 10.1111/j.1476-5381.2011.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rom O., Liu Y., Liu Z., Zhao Y., Wu J., Ghrayeb A., Villacorta L., Fan Y., Chang L., Wang L., Liu C., Yang D., Song J., Rech J.C., Guo Y., Wang H., Zhao G., Liang W., Koike Y., Lu H., Koike T., Hayek T., Pennathur S., Xi C., Wen B., Sun D., Garcia-Barrio M.T., Aviram M., Gottlieb E., Mor I., Liu W., Zhang J., Chen Y.E. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci Transl Med. 2020;12:eaaz2841. doi: 10.1126/scitranslmed.aaz2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D., Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020;98:skaa090. doi: 10.1093/jas/skaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiekhani M., Ommati M.M., Azarpira N., Heidari R., Salarian A.A. Glycine supplementation mitigates lead-induced renal injury in mice. J. Exp. Pharmacol. 2019;11:15–22. doi: 10.2147/JEP.S190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandana G.D., Sejian V., Lees A.M., Pragna P., Silpa M.V., Maloney S.K. Heat stress and poultry production: impact and amelioration. Int. J. Biometeorol. 2021;65:163–179. doi: 10.1007/s00484-020-02023-7. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wu Z., Dai Z., Yang Y., Wang J., Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45:463–477. doi: 10.1007/s00726-013-1493-1. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhang J., Wang L., Li W., Chen L., Li J., Zhao D., Zhang H., Guo X. Glycine mitigates renal oxidative stress by suppressing Nox4 expression in rats with streptozotocin-induced diabetes. J. Pharmacol. Sci. 2018;137:387–394. doi: 10.1016/j.jphs.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Wessner B., Strasser E.M., Spittler A., Roth E. Effect of single and combined supply of glutamine, glycine, N-acetylcysteine, and R,S-alpha-lipoic acid on glutathione content of myelomonocytic cells. Clin. Nutr. 2003;22:515–522. doi: 10.1016/s0261-5614(03)00053-0. [DOI] [PubMed] [Google Scholar]
- Wheeler M.D., Ikejema K., Enomoto N., Stacklewitz R.F., Seabra V., Zhong Z., Yin M., Schemmer P., Rose M.L., Rusyn I., Bradford B., Thurman R.G. Glycine: a new anti-inflammatory immunonutrient. Cell. Mol. Life Sci. 1999;56:843–856. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang X., Wu H., Zhu H., Liu C., Hou Y., Dai B., Liu X., Liu Y. Glycine relieves intestinal injury by maintaining mTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int. J. Mol. Sci. 2018;19:1980. doi: 10.3390/ijms19071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Magilnick N., Ou X., Lu S.C. Tumour necrosis factor alpha induces coordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1. Biochem. J. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Lian G. ROS and diseases: role in metabolism and energy supply. Mol. Cell. Biochem. 2020;467:1–12. doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Fan X., Ji Y., Li J., Dai Z., Wu Z. Glycine represses endoplasmic reticulum stress-related apoptosis and improves intestinal barrier by activating mammalian target of rapamycin complex 1 signaling. Anim. Nutr. 2022;8:1–9. doi: 10.1016/j.aninu.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang P., Tan C., Zhao Y., Zhu Y., Bai J., Xiao X. Integrated transcriptomics and metabolomics unravel the metabolic pathway variations for barley beta-glucan before and after fermentation with L. plantarum DY-1. Food Funct. 2022;13:4302–4314. doi: 10.1039/d1fo02450g. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jiang D., Jin Y., Jia H., Yang Y., Kim I.H., Dai Z., Zhang J., Ren F., Wu Z. Glycine attenuates citrobacter rodentium-induced colitis by regulating ATF6-mediated endoplasmic reticulum stress in mice. Mol. Nutr. Food Res. 2021;65 doi: 10.1002/mnfr.202001065. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Enomoto N., Connor H.D., Moss N., Mason R.P., Thurman R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12:54–62. doi: 10.1097/00024382-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wheeler M.D., Li X., Froh M., Schemmer P., Yin M., Bunzendaul H., Bradford B., Lemasters J.J. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- Zhou C., Gao X., Cao X., Tian G., Huang C., Guo L., Zhao Y., Hu G., Liu P., Guo X. Gut microbiota and serum metabolite potential interactions in growing layer hens exposed to high-ambient temperature. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.877975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Zhang X., Du P., Wang Z., Luo P., Huang Y., Liu Z., Zhang H., Chen W. Dietary herbaceous mixture supplementation reduced hepatic lipid deposition and improved hepatic health status in post-peak laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]