Abstract
Despite progress in genomic characterization, no single prognostic marker that can be evaluated using an easy-to-perform and relatively inexpensive method is available for pancreatic ductal adenocarcinoma (PDAC). MicroRNAs, which are stable, tumor- and tissue-specific molecules, are potentially ideal biomarkers, and we established an inter-laboratory validated method to investigate miR-21 as a prognostic biomarker in PDAC. The study samples of PDAC patients were recruited from a test cohort of Glasgow (n = 189) and three validation cohorts of Pisa (n = 69), Sydney (n = 249), and International Cancer Genome Consortium (ICGC) (n = 249). Tissue microarrays were used for miR-21 staining by chromogenic in situ hybridization (CISH). The patients were subdivided into no/low and high miR-21 staining groups using a specific histoscore. Furthermore, miR-21 staining was evaluated against clinicopathological variables and follow-up data by Fisher/log-rank test and Cox proportional models. The prognostic variables found to be significant in univariate analysis (P value < 0.10) were included in multivariate analysis in a backward-stepwise fashion. MiR-21 expression was cytoplasmic, with more consistent staining in the malignant ductal epithelium than in the stroma. The expression of miR-21 was significantly associated with tumor size and lymph node metastasis, whereas no association was observed with other clinicopathological variables. High miR-21 staining (histoscore ≥ 45 [median score]) was an independent predictor of survival in the Glasgow test cohort (HR 2.37, 95% CI: 1.42-3.96, P < 0.0001) and three validation cohorts (Pisa, HR 2.03, 95% CI: 1.21-3.39, P = 0.007; Sydney, HR 2.58, 95% CI (1.21-3.39), P < 0.0001; and ICGC, HR 3.34, 95% CI: 2.07-5.84, P = 0.002) when adjusted for clinical variables in a multivariate model. In comparison to the patients with low miR-21, the patients with high miR-21 expression had significant increase in OS as they benefit from gemcitabine-based adjuvant chemotherapy (Glasgow 16.5 months [with chemotherapy] vs 10.5 months [without chemotherapy]); Sydney 25.0 vs 10.6; ICGC 25.2 vs 11.9. These results indicated that miR-21 is a predictor of survival, prompting prospective trials. Evaluation of miR-21 offers new opportunities for the stratification of patients with PDAC and might facilitate the implementation of clinical management and therapeutic interventions for this devastating disease.
Keywords: Pancreatic ductal adenocarcinoma, MiR-21, chromogenic in-situ hybridization, prognosis, gemcitabine adjuvant chemotherapy, overall survival, tissue microarrays
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most prevalent and aggressive type of GIT cancer with a poor survival outcomes because of its rapid progression and late diagnosis [1]. One of the most recent studies based on real-world data for PDAC has reported that the actual 5-year survival rate from the time of initial diagnosis is still less than 5% [2]. Approximately 80% of PDAC patients are not amenable to radical surgery, whereas in the remaining 15-20% of patients who undergo surgical resection, the ESPAC-4 trial has suggested the use of gemcitabine-capecitabine combination and, most recently, the PRODIGE-24/CCTG PA.6 study showed an increase in OS of 19 months for adjuvant treatment with 5-Fluorouracil, leukovorin, irinotecan and oxaliplatin (i. e., FOLFIRINOX) [3,4]. However, the Whipple procedure is a complex operation, with high rates of severe complications, and current systemic therapies, when used in an all-comer approach, are modestly effective, in a small group of undefined patients.
In the last decade, micro-RNAs have been reported as a strong regulators of oncogenic processes in different types of cancers, including PDAC [5]. These miRNAs are the evolutionarily conserved non-coding endogenous RNAs, with a length of 18-24 bases in a single-stranded form, which is capable of negatively regulating gene expression in a sequence specific fashion [6]. Recently, miRNAs have emerged as an innovative therapeutic targets, while the implementation of molecular morphology in-situ hybridization methods, providing reliable localization and quantification, has opened new opportunities to evaluate whether they can also be used as diagnostic biomarkers and/or to predict clinical outcomes [7,8]. Expression profiling of the PDAC miRNome has revealed a distinct signature playing an important role in PDAC carcinogenesis [9,10]. However, the pivotal regulatory role of each miRNA in controlling the expression of multiple gene transcripts offers a unique opportunity to identify critical miRNAs as informative biomarkers for the prognosis of tumors that result from the deregulation of multiple genes [6].
The main objective of the current study was to assess the feasibility of using a candidate miRNA as a prognostic biomarker by determining its association with OS in a test and three validation cohorts, including 686 well-characterized formalin-fixed paraffin-embedded (FFPE) samples from radically resected PDAC patients. This is the largest population ever investigated for the analysis of a miRNAs as a potential biomarker in PDAC specimens. Based on our previous PCR and microarray data, current literature, and meta-analyses, we selected miR-21 as a candidate miRNA for testing [8,11,12]. Most recent studies have found that miR-21 is overexpressed in PDAC and is responsible for increased drug resistance, particularly to gemcitabine [13-15]. Although the qPCR technique has been widely regarded as the gold standard in terms of sensitivity, the FISH technique is thought to be more dominant in terms of high specificity (99.32%) than qPCR, and even more specific than IHC [16]. Furthermore, unlike qPCR, CISH can assist in determining the localized expression of miRNAs, which is critical in understanding the pathogenesis of aggressive cancers such as PDAC. Understanding the location of miR21 is essential to understanding its function in disease because this information can be used to characterize the molecular pathways that miR-21 controls in pathological processes [17-19]. MiRNA in situ hybridization analysis is a highly sensitive technique for studying miRNA localization and expression [20]. Although several studies using simple ISH techniques yielded divergent results for miR21 cellular localization, using it with high affinity LNA-modified DNA probes along with a series of positive and negative control probes yielded significant proof of miR21 localization within tumor tissue [20]. Therefore, the most popular CISH technique is being used in this study. The majority of current, significant, and cutting-edge research studies examine miRNAs using miRNA-ISH techniques, which are enabling the most complex field of spatial transcriptomics [21]. Furthermore, the CISH-based approach is a simple and low-cost method for detecting predefined miRNA targets in any cancer sample, including PDAC, as is the case in our current study. To establish a reliable, consistent, and robust CISH assay for miR-21, we performed a validation study using repeated analyses of the test cohort in two laboratories. Here, we present a robust CISH method and guide for miR-21 quantification in PDAC specimens. By applying the validated tissue microarray to well-annotated PDAC cohorts of patients, we showed that the epithelial expression of miR-21 is an independent robust prognostic biomarker in PDAC and unravel its predictive potential for gemcitabine adjuvant chemotherapy.
Material and methods
Patients samples, ethical approval and data acquisition of study cohorts
The study samples that were recruited for testing miR-21 expression levels were retrieved from 189 consecutive patients who were diagnosed with PDAC and underwent pancreaticoduodenectomy in the West Scotland Pancreatic Unit, Glasgow Royal Infirmary, Glasgow, UK. This cohort was designated as the test cohort. In addition, three cohorts of PDAC patients were prospectively recruited from the Department of Translational Research and New Technologies in Medicine and Surgery, Hospital of Pisa, Italy, University of Pisa (n = 69), the Australian Pancreatic Cancer Genome Initiative (APGI)-associated six teaching hospitals in Sydney, Australia (n = 249), and the International Cancer Genome Consortium (ICGC) though the help of APGI (n = 179) to validate the data obtained from the test cohort (n = 189). These three validation cohorts were designated as the Pisa, Sydney, and ICGC cohorts. Ethical approval for the acquisition of data and biological material was obtained from the Human Research Ethics Committee/Ethical Review Board of each participating institution. Informed consent was obtained from each participant in the Pisa validation cohort but was not required by the human research ethics committee for retrospective patient cases in the Sydney and ICGC cohorts. The demographic and clinicopathological characteristics of the test and validation cohorts were also recorded. All samples that had technical issues with IHC processing and/or lacked complete demographic and clinicopathological data were excluded from the study.
Immunohistochemical evaluation via tissue microarrays and tumors staging
Tissue microarrays (TMAs) were constructed from formalin-fixed paraffin-embedded (FFPE) tissues. A 2.5 µm thick sections from the tissue microarray blocks were freshly cut for staining of all cohorts. TMAs were constructed using a minimum of three 0.6 mm2 cores from each area to account for intra-tumor disease heterogeneity (Beecher Scientific, Silver Spring, MD). Around 2.5 µm thick sections from each TMA block were mounted on salinized positively charged glass slides. The slides were then heated to 45°C for 1 h, cooled to room temperature, and stored at 4°C. H&E-stained sections were used to identify and mark the epithelial area of PDAC in each block. The tumors were staged according to the seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual [22].
Chromogenic in-situ hybridization (CISH) for evaluation of miR-21 expression levels
MiR-21 expression levels were evaluated in the constructed tissue microarrays (TMA), following the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines, and optimized and established a sensitive and stable one-day chromogenic in situ hybridization (CISH) method utilizing locked nucleic acid (LNA) miR-21 probes with small nuclear RNA U6 (U6) as a positive control and scrambled RNA as a negative control, as previously described [23]. Briefly, the TMA slides were deparaffinized in xylene and rehydrated with graded alcohol washes. Afterwards, the slides were incubated in Proteinase K solution (15 µg/ml) at 37°C for 8 min, washed with PBS, and then dehydrated with graded alcohol washes. Digoxigenin (DIG)-labeled mercury LNA probes (Exiqon) for miR-21, U6, and scrambled RNA were denatured for 4 min at 90°C, mixed with ISH buffer, and hybridized to slides for 2 h at 53°C. Stringency washes were then performed. The TMA slides were then incubated with an alkaline phosphatase-conjugated anti-DIG Fab fragment (Roche Diagnostics) for 2 h at room temperature. After washing and drying, slides were incubated with NBT/BCIP solution (Roche Diagnostics) at room temperature for 16 h. Finally, the slides were counterstained with nuclear fast red light and mounted using glycerol gelatin. The patient samples were subdivided into no or low/weak staining and high staining groups.
Image acquisition and quantification
In situ hybridization staining patterns (Figure 1) were scored semi-quantitatively using a weighted histoscore method (range 0-300) for each cohort [24], (Supplementary File 1). The staining intensity of miR-21 in PDAC TMA cores was categorized into the percentage of epithelial cells with negative (0), weak (1), moderate (2), and strong (3) staining. The final histoscore was calculated using the following formula: (0% negative tumor cells) + (1% weak tumor cells) + (2% moderate tumor cells) + (3% strong tumor cells) (Supplementary Table 1). The agreement between the two observers was monitored by calculating intraclass correlation coefficients. The results were reevaluated if the scores differed by > 50. The scorer was blinded to the clinical outcomes.
Figure 1.
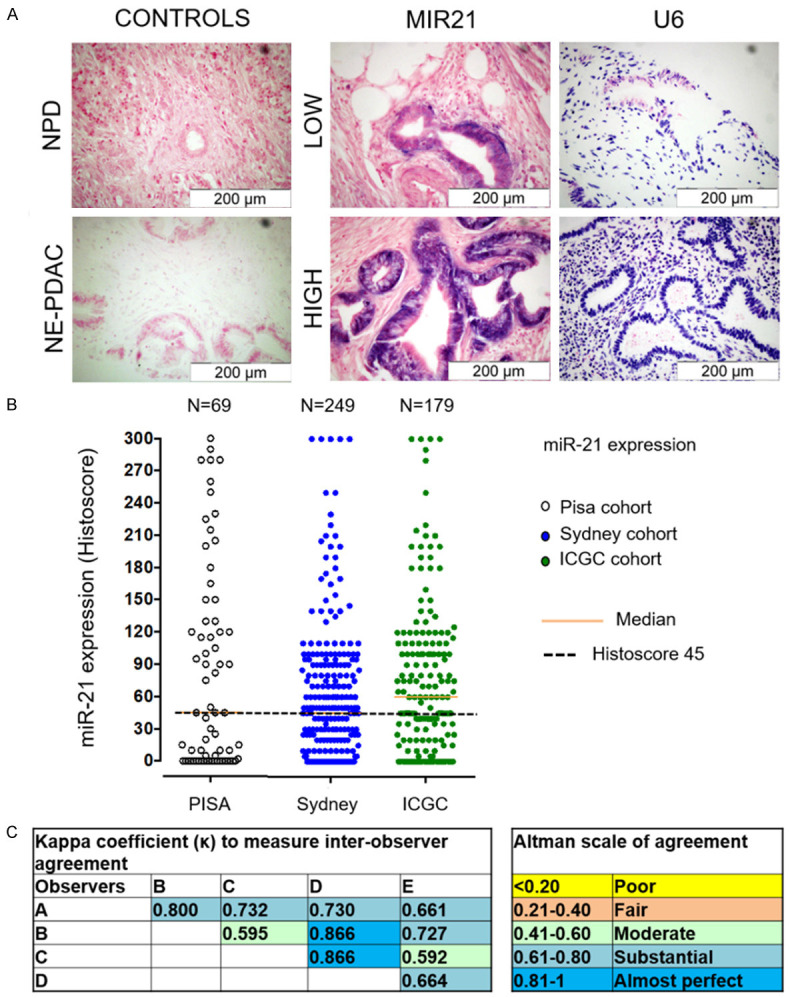
Staining patterns, histoscoring for miR-21 expression and quantifications. A. Expression of miR-21 in pancreatic ductal adenocarcinoma and normal pancreatic tissue as detected by chromogenic in-situ hybridization. B. Representation of miR-21 in the training cohorts with median range and histoscore level of 45. C. Depicted of Kappa coefficient (κ) to measure inter-observer agreement and developing an Altman scale of agreement.
Statistical analysis
The relationships between categorical variables were analyzed using the Mantel-Haenszel (χ2) test. The primary outcome measures were the length of disease-specific survival (Glasgow cohort) and OS (Pisa, Sydney, and ICGC cohorts), as measured from the time of resection with curative intent. The length of survival following surgery and cause of death were obtained from prospectively maintained databases. Kaplan-Meier survival analysis was used to analyze median survival from the time of surgery with a log-rank test performed to compare curves using SPSS Version 21 (IBM Corp., Armonk, NY). The 5-year survival rate was estimated using the life table method. Patients who were alive at the time of the follow-up were censored. A Cox proportional hazards model was used for multivariate analysis to adjust for competing risk factors, and the hazard ratio (HR) with 95% confidence intervals (CIs) was used to estimate the risk of disease-specific death. Only prognostic variables found to be significant on univariate analysis (P value < 0.10) were included in the multivariate analysis in a backward-stepwise fashion. Statistical significance was set at P ≤ 0.05. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Cohort description, demographic and clinicopathological parameters
The characteristics of all four cohorts are shown in Table 1, including age distribution, TNM staging, pathological grade, tumor size, lymph node status, metastasis, vascular invasion, lymphatic invasion, perineural invasion, resection margin status, and prognostic factor data. A total of 686 patients diagnosed with PDAC from all four cohorts were studied, with almost equal proportions of male (n = 345; 50.3%) and female patients (n = 341; 49.7%). The mean age (years) for all the study cohorts was in the range from 62-67, where the test cohort mean age was 62.2 and three validation Pisa, Sydney & ICGC cohorts were 65.1, 67.1 and 66.5 respectively. From the follow-up data of all four cohorts, it was recorded that death from PDAC was high (76.3%, 100%, 79.5%, and 47.4%, respectively). Most of the tumors were at stage III in the Glasgow (80.4%), Pisa (85.5%), and Sydney (16.4%) cohorts, while stage IV cases were only observed in the ICGC cohort (4%). A similar pattern was observed in the TNM staging system. The other clinico-pathological parameters are summarized in the sections below in association with miR-21 expressions levels. In addition, the staining patterns of miR-21 in comparison with the U6 control, the histoscore for miR-21 expression, and quantification agreement based on the Kappa coefficient (κ) are depicted in Figure 1.
Table 1.
Cohort description, demographic and clinicopathological parameters data from test and validation cohorts
| Glasgow Cohort | Pisa Cohort | Sydney Cohort | ICGC Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| n = 189 | Median OS | P value | n = 69 | Median OS | P value | n = 249 | Median OS | P value | n = 179 | Median OS | P value | |
| Variables | No. (%) | (months) | (Log rank) | No. (%) | (months) | (Log rank) | No. (%) | (months) | (Log rank) | No. (%) | (month) | (Log rank) |
| Gender | ||||||||||||
| Male | 101 (58.7) | 17.6 | 31 (44.9) | 20.9 | 125 (50.2) | 16.8 | 88 (49.2) | 16.8 | ||||
| Female | 88 (41.3) | 20.1 | 0.48 | 38 (55.1) | 17 | 0.8 | 124 (49.8) | 16 | 0.296 | 91 (50.8) | 28.7 | 0.082 |
| Age (years) | ||||||||||||
| Mean | 62.2 | 65.1 | 67.1 | 66.5 | ||||||||
| Median | 63.4 | 65 | 69 | 67.0 | ||||||||
| Range | 37.4-86.1 | 42.0-82.0 | 28.0-87.1 | 36.2-88.1 | ||||||||
| Outcome | ||||||||||||
| Follow-up (months) | 0.8-79.0 | 3.8-129.1 | 0.4-120 | 3.0-79.0 | ||||||||
| Median follow-up | 22 | 19.8 | 16.7 | 19.6 | ||||||||
| Death PDAC | 61 (76.3) | 69 (100) | 198 (79.5) | 85 (47.4) | ||||||||
| Death other | 7 (8.7) | 0 (0) | 10 (4.1) | 6 (3.5) | ||||||||
| Death unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||
| Alive | 12 (6.3) | 0 (0) | 41 (16.4) | 88 (49.1) | ||||||||
| Stage | ||||||||||||
| I | 5 (2.7) | 90.3 | 1 (1.4) | - | 19 (7.6) | 70 | 12 (6.7) | 60.1 | ||||
| II | 32 (16.9) | 22.6 | 9 (13.1) | 21.5 | 75 (30.1) | 17.5 | 160 (89.3) | 30.1 | ||||
| III | 152 (80.4) | 18.1 | 0.049 | 59 (85.5) | 19 | 0.057 | 155 (62.2) | 16.4 | 0.045 | 0.013 | ||
| IV | 7 (4.0) | 17.6 | ||||||||||
| T Stage | ||||||||||||
| T1/T2 | 15 (7.9) | 33.4 | 2 (2.8) | - | 39 (15.7) | 31 | 21 (11.7) | 61.7 | ||||
| T3/T4 | 174 (92.1) | 18.1 | 0.031 | 67 (97.2) | 19.5 | 0.032 | 210 (84.3) | 16.4 | 0.002 | 158 (88.3) | 28.6 | 0.312 |
| N Stage | ||||||||||||
| N0 | 36 (19.0) | 31 | 11 (15.9) | 24 | 94 (37.8) | 20 | 50 (27.9) | 61.8 | ||||
| N1 | 153 (81.0) | 18.5 | 0.003 | 58 (84.1) | 18 | 0.044 | 155 (62.2) | 16.7 | 0.05 | 129 (72.1) | 25.6 | 0.041 |
| Grade | ||||||||||||
| Low | 128 (67.7) | 23.1 | 39 (56.5) | 20.9 | 189 (75.9) | 16.7 | 140 (78.2) | 28.3 | ||||
| High | 61 (32.3) | 13.4 | 0.021 | 30 (43.5) | 18 | 0.079 | 60 (24.1) | 18.3 | 0.572 | 39 (21.8) | 16.3 | 0.008 |
| Tumour size | ||||||||||||
| ≤ 30 mm | 94 (49.7) | 23.1 | 30 (43.7) | 22.9 | 50 (15.7) | 36.5 | 84 (46.9) | 38.1 | ||||
| > 30 mm | 95 (50.3) | 16.1 | 0.01 | 39 (46.3) | 18 | 0.041 | 199 (84.3) | 16 | < 0.0001 | 95 (53.1) | 21.6 | < 0.0001 |
| Margins | ||||||||||||
| R0 | 49 (25.9) | 28.5 | 60 (86.9) | 19.5 | 148 (59.4) | 22.4 | 127 (71.0) | 33.4 | ||||
| R1 | 140 (74.1) | 16.4 | < 0.0001 | 9 (13.1) | 21.5 | 0.33 | 101 (40.6) | 13.2 | < 0.0001 | 52 (29.0) | 20.3 | 0.001 |
| Perineural Invasion | ||||||||||||
| Negative | 15 (7.9) | 18.2 | 0 (0) | - | 57 (22.9) | 25.6 | 32 (18.1) | 41.9 | ||||
| Positive | 174 (92.1) | 20 | 0.33 | 69 (100.0) | 19.9 | - | 184 (73.9) | 16.7 | 0.275 | 144 (81.9) | 25.6 | 0.047 |
| Venous Invasion | ||||||||||||
| Negative | 97 (51.3) | 24 | 50 (72.4) | 19.9 | 123 (49.4) | 20.7 | 73 (42.1) | 40.1 | ||||
| Positive | 92 (48.7) | 16.3 | 0.004 | 19 (27.6) | 16.8 | 0.382 | 111 (44.6) | 16.2 | 0.008 | 100 (57.9) | 23.8 | 0.013 |
| Chemotherapy | ||||||||||||
| Adjuvant | 83 (43.9) | 23.1 | 69 (100) | 19.5 | 52 (20.9) | 25.2 | 41 (23.6) | 31.4 | ||||
| No Adjuvant | 106 (56.1) | 16.3 | 0.04 | 0 (0) | - | - | 196 (79.1) | 16.3 | 0.013 | 138 (76.4) | 17.4 | 0.007 |
| miR-21 Expression (median histoscore 45) | ||||||||||||
| Low | 94 (49.7) | 26.5 | 37 (53.6) | 23.7 | 126 (50.6 | 29.3 | 85 (47.4) | 36.8 | ||||
| High | 95 (50.3) | 14.7 | < 0.0001 | 32 (46.3) | 15.5 | 0.002 | 123 (49.4) | 12.8 | < 0.0001 | 94 (52.6) | 20.3 | < 0.0001 |
| miR-21 & Lymph Node Status | ||||||||||||
| Mir21 low/LN Neg | 16 (8.5) | 90.3 | 7 (10.2) | 25.7 | 62 (6.3) | 26 | 26 (14.5) | 61.8 | ||||
| Mir21 high/LN Neg | 20 (10.6) | 13.6 | 3 (4.3) | 21.5 | 32 (10.0) | 13 | 24 (13.5) | 21.6 | ||||
| Mir21 low/LN Pos | 77 (40.7) | 24.7 | 30 (43.4) | 23.1 | 64 (43.7) | 29.6 | 59 (32.9) | 34.9 | ||||
| Mir21 High/LN Pos | 76 (40.2) | 14.7 | < 0.0001 | 29 (42.0) | 15.2 | 0.002 | 91 (40.0) | 12.6 | < 0.0001 | 70 (39.1) | 20.2 | < 0.0001 |
| miR-21 & Resection Margin Status | ||||||||||||
| Mir21 low/R0 | 16 (8.5) | 90.3 | 33 (47.8) | 23.0 | 80 (32.1) | 33.6 | 67 (37.4) | 41.9 | ||||
| Mir21 high/R0 | 20 (10.6) | 13.6 | 26 (37.6) | 15.5 | 68 (27.3) | 15.1 | 60 (33.5) | 26.5 | ||||
| Mir21 low/R1 | 77 (40.7) | 24.7 | 3 (4.3) | 23.7 | 46 (18.4) | 19.5 | 18 10.0) | 23.9 | ||||
| Mir21 High/R1 | 76 (40.2) | 14.7 | < 0.0001 | 6 (8.6) | 13.2 | 0.002 | 55 (22.1) | 10.1 | < 0.0001 | 34 (18.9) | 15.8 | 0.001 |
The expression of miR-21 was significantly associated with tumor size and lymph node metastasis, whereas no association was observed with other clinicopathological variables
In the Glasgow test cohort, a statistically significant association between the expression levels of miR-21 and tumor size was observed. In the miR-21 high group, 38/56 (68%) PDAC cases had tumors larger than 2 cm, while only 1/7 (14%) cases had tumor size less than 2 cm (P < 0.01, Fisher’s exact test). Similarly, lymph node metastasis showed a trend towards statistical significance in the group with high miR-21 expression compared to the no or low expression group (P < 0.07, Fisher’s exact test). However, no statistically significant association was observed between miR-21 expression and other clinicopathological variables, including age, T stage, grade, vascular invasion, perineural invasion, lymphatic invasion, and resection margin status. Interestingly, only high miR-21 expression was prognostic, and none of the clinicopathological variables were prognostic in the investigated cohort of 67 patients. In the validation cohort, a trend towards statistical significance between the expression levels of miR-21 and pathologic grade was observed (P < 0.09, Fisher’s exact test). However, no statistically significant association was observed between miR-21 expression and other clinicopathological variables, including age, sex, T stage, and lymphatic invasion (Table 2).
Table 2.
Multivariate cox regression analysis of test and validation cohorts
| Cohort | Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| A. Glasgow training cohort | Lymph Node Metastases (Positive) | 1.92 (1.17-3.15) | 0.01 |
| Tumour size (> 30 mm) | 1.48 (1.06-2.06) | 0.021 | |
| Tumor Grade (High) | 1.77 (1.25-2.52) | 0.001 | |
| Resection Margin Involvement (< 1 mm) | 1.79 (1.18-2.72) | 0.006 | |
| Adjuvant chemotherapy (≥ 3 cycles) | 0.54 (0.38-0.77) | 0.001 | |
| miR-21 expression (High, HS > 45) | 2.11 (1.51-2.96) | < 0.0001 | |
| B. Pisa validation cohort | Tumor Stage (T3/T4) | 4.47 (1.06-18.9) | 0.042 |
| miR-21 expression (High, HS > 45) | 2.03 (1.21-3.39) | 0.007 | |
| C. Sydney validation cohort | Tumour size (> 30 mm) | 2.10 (1.42-3.12) | < 0.0001 |
| Venous Invasion (Positive) | 1.30 (0.98-1.75) | 0.070 | |
| Resection Margin Status (Involved, < 1 mm) | 1.65 (1.23-2.20) | < 0.0001 | |
| Adjuvant chemotherapy (≥ 3 cycles) | 0.59 (0.42-0.86) | 0.006 | |
| miR-21 expression (High, HS > 45) | 2.59 (1.89-3.53) | < 0.0001 | |
| D. ICGC validation cohort | Tumour size (> 30 mm) | 2.01 (1.22-3.31) | 0.006 |
| Gender | 1.63 (1.04-2.56) | 0.032 | |
| Tumour Grade (High) | 2.40 (1.41-4.06) | 0.001 | |
| Adjuvant chemotherapy (≥ 3 cycles) | 0.41 (0.23-0.72) | 0.002 | |
| miR-21 expression (High, HS > 45) | 2.16 (1.32-3.51) | 0.002 |
Higher expression levels of miR-21 in PDAC patients have a direct prognostic impact and are associated with shorter OS
We investigated the expression levels of miR-21 in relation to survival following curative intent PDAC resection in the Glasgow test cohort. Greater than the median miR-21 tumoral expression (histoscore ≥ 45, high) was associated with shorter OS as compared to the low expression group (Histoscore < 45) i. e., (14.7 (95% CI: 12.4-17.0) vs 26.5 (95% CI: 20.4-32.6) months; P < 0.0001) (Figure 2A). High epithelial miR-21 expression was found to be independently associated with poor prognosis in a multivariate analysis (HR 2.11, 95% CI: 1.51-2.96, P < 0.0001), along with the presence of lymph node metastases, high tumor grade, tumor size > 30 mm, R1 margin status, and no adjuvant chemotherapy (Table 2).
Figure 2.
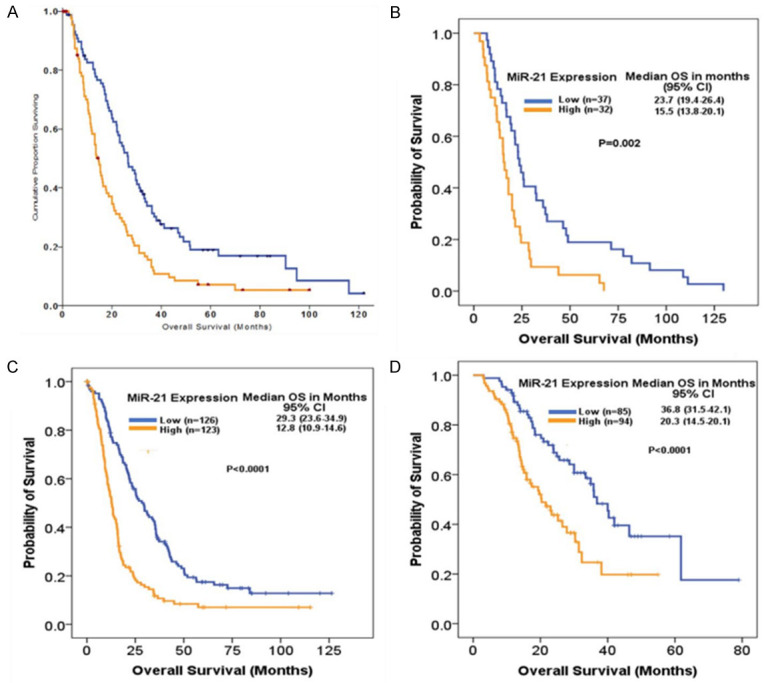
Kaplan-Meier curves of disease specific and OS in four cohorts: Kaplan-Meier curves for (A) disease specific survival and (B-D) OS of patients with low and high miR-21 expression assessed by chromogenic in-situ hybridization in tissue microarrays including (A) Glasgow training cohort (n = 189), (B) Pisa validation cohort (n = 69), (C) Sydney validation cohort (n = 249) and (D) ICGC validation cohort (n = 179).
A similar pattern was employed to analyze miR-21 prognostic impact in the validation cohorts, where miR-21 molecular phenotypes defined in the Glasgow test cohort were co-segregated with outcomes in the Pisa, Sydney, and ICGC validation cohorts. Univariate survival analysis demonstrated that when the same cut-off was applied, patients with high tumoral miR-21 expression experienced shorter OS (in months) than those in the low expression group in the Pisa cohort (15.5 95% CI (13.8-20.1) vs 23.7 95% CI (19.4-26.4); P = 0.002) (Figure 2B); Sydney cohort (12.8 95% CI (10.9-14.7) vs 29.3 95% CI (23.2-35.8); P < 0.0001) (Figure 2C); and ICGC cohort (20.3 95% CI (14.5-26.1) vs 36.8 95% CI (31.5-42.1); P < 0.0001) (Figure 2D).
On multivariate analysis, high miR-21 was an independent prognostic factor associated with shorter OS in the Pisa cohort (HR 2.03, 95% CI: 1.21-3.39, P = 0.007) along with T stage; Sydney cohort (HR 2.59, 95% CI (1.89-3.53), P < 0.0001) along with tumor size > 30 mm, R1 margin status, venous invasion, and no adjuvant chemotherapy; and ICGC cohort (HR 2.16, 95% CI (1.32-3.51); P = 0.002) along with tumor size > 30 mm, sex, high tumor grade, and no adjuvant chemotherapy (Table 2).
Patients with high miR-21 expression levels showed an association between adjuvant chemotherapy and a significant increase in OS
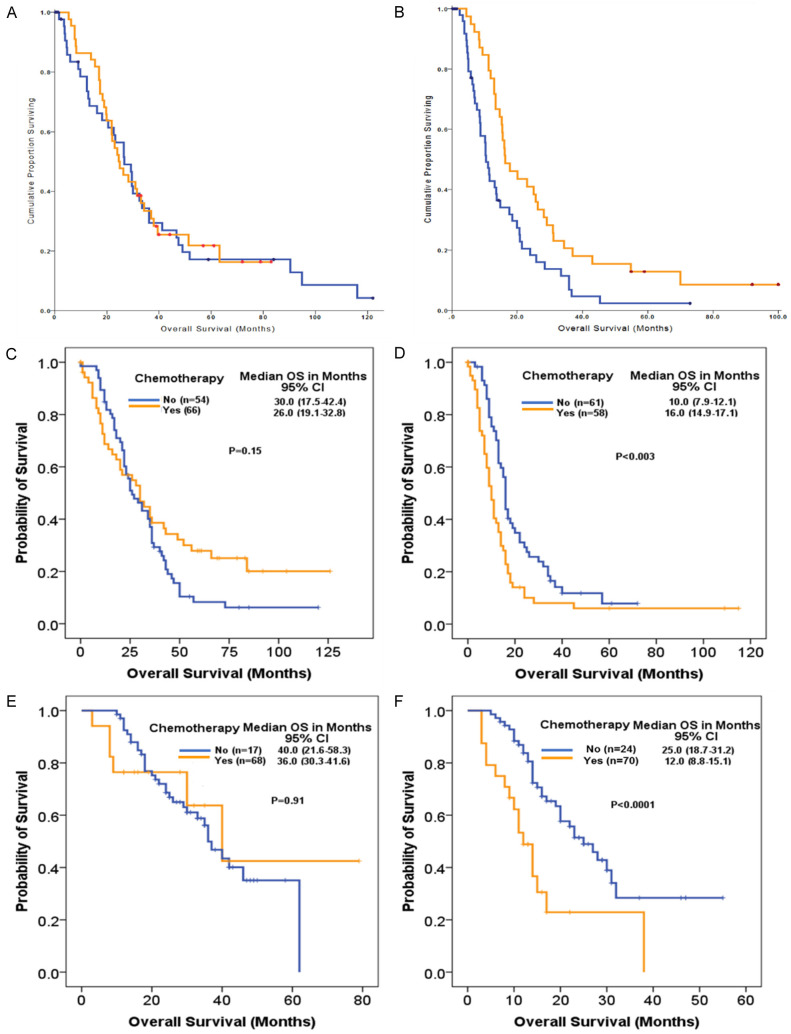
We subsequently analyzed miR-21 expression along with adjuvant chemotherapy allocation in the Glasgow test cohort as well as in the Sydney and ICGC validation cohorts. Analysis was not possible in the Pisa cohort, as all patients received adjuvant chemotherapy. Of the patients in the test cohort with low miR-21 expression, 45 received chemotherapy and 49 did not, and administration was not associated with improvement in survival; 24.7 (95% CI: 17.8-31.6) with chemotherapy vs 26.7 months (95% CI: 18.7-34.7; P = 0.827) without chemotherapy. In contrast, 40 patients with high miR-21 expression received chemotherapy, and 57 did not. Adjuvant chemotherapy was associated with a significant increase in OS, from 10.5 months (95% CI: 8.6-12.3) without chemotherapy to 16.5 months (95% CI: 10.9-21.9; P = 0.006) with chemotherapy (Figure 3A).
Figure 3.
Survival curves of disease specific and OS defined by chemotherapy: Kaplan-Meier curves for (A, B) disease specific survival and (C-F) OS of patients with PDAC. Survival curves are according to low miR-21 expression (A, C, E) and high miR-21 expression (B, D, F) subgroups of patients defined by adjuvant chemotherapy (No, Yes).
A similar pattern was also observed in the validation cohorts. The patients with tumors expressing high miR-21 levels, the adjuvant chemotherapy resulted in prolonged OS for Sydney cohort (25.0 vs 10.6 months; P < 0.0001) and ICGC cohort (25.2 vs 11.9 months; P < 0.0001). In contrast, for patients with tumors with low miR-21 expression, no survival advantage could be shown, with chemotherapy failing to significantly prolong OS following resection in Sydney cohort (25.2 vs 29.6 months; P = 0.883) and ICGC cohorts (40.0 vs 36.0 months; P = 0.945) (Figure 3B and 3C).
The predictive utility of miR-21 expression revealed that patients with high miR-21 expressing tumors receive more benefit from gemcitabine adjuvant chemotherapy
To assess miR-21 expression as a true predictive biomarker of adjuvant chemotherapeutic responsiveness, a test of interaction using a Cox regression model was performed. After adjusting for the prognostic effect of miR-21 expression and adjuvant chemotherapy, the interaction variable (miR-21 × chemotherapy [≥ 3 cycles]) remained statistically significant in the Glasgow test cohort (HR = 0.51, 95% CI: 0.33-0.80, P = 0.004), the Sydney validation cohorts (HR = 0.33, 95% CI: 0.16-0.66, P = 0.002), and ICGC validation cohort (HR = 0.30, 95% CI: 0.10-0.87, P = 0.027) (Supplementary File 1).
Therefore, we can conclude that the influence of adjuvant chemotherapy on survival was correlated with miR-21 status (interaction variable: miR-21 × chemotherapy) and that patients with high miR-21 expressing tumors receive more benefit from adjuvant chemotherapy.
Discussion
Pancreatic ductal adenocarcinoma (PDAC) still remains the most aggressive cancer with dismissal prognostic outcomes, and the genomics and proteomics studies have reported its underlying molecular heterogeneity [25,26]. We believe that clinical progress in patients with PDAC would depend on the development of novel and effective therapies and the parallel establishment of novel prognostic and predictive biomarkers [27]. Accumulating evidence has revealed that aberrant overexpression of numerous micro-RNAs, including miR-21, is associated with different types of cancers, including PDAC [28]. However, the potential association between miR-21 and PDAC in multiple international cohorts is not well established. In the current study, we demonstrated that miR-21 is significantly overexpressed in epithelial PDAC specimens from multinational cohorts of 686 patients and has a prognostic impact, indicating that miR-21 may function as an oncogene in the pathogenesis of PDAC.
It has been widely reported that miR-21 is an important player in carcinogenesis and has been correlated with survival and clinical outcome in various cancers [27-31]. Previous studies have demonstrated that miR-21 plays a vital role not only in cancer proliferation, but also in invasion and metastasis by regulating multiple oncogenes and tumor suppressor genes. miR-21 acts as an oncomir that leads to the inhibition of negative regulators of the RAS pathway, pro-apoptotic genes, and other key genes in PDAC tumorigenesis and aggressive behaviors, such as SPRY2, PDCD4, PTEN, TPM1, Maspin, NFIB, RhoB, Apaf1, Bcl2, and TIMP3 [32-34]. Most of these key signaling molecules are well known for their role in pancreatic cancer cell proliferation, prevention of apoptosis of cancerous cells, enhancement of angiogenesis, and promotion of metastasis [28,35-38], as depicted in the Figure 4 below.
Figure 4.
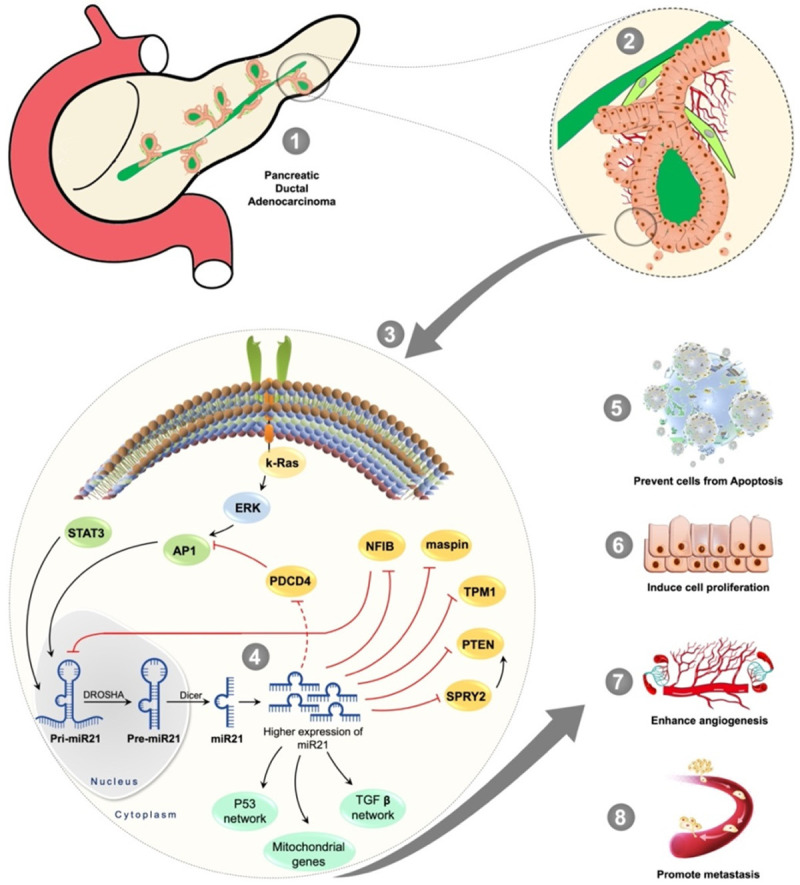
Depiction of miR21 potential role in pancreatic cancer cell signaling: The miR21 plays a major role in the k-Ras signaling that has been upregulated in majority of cancers including the PDAC. The upregulated AP1 induces the transcription of pri-miR21 inside a nucleus where the DROSHA converts the pri-miR21 into pre-miR21 and Dicer makes it a mature functional miR21 following its translocation into the cytoplasm where the pancreatic cancer cells overexpress the miR21 levels. The higher expression of miR21 negatively regulates key signaling molecules and influences the mitochondrial apoptotic pathway genes, as well as the P53 network and TGF-β network. This results in the pancreatic cancer cells’ proliferation, preventing the PDAC cells from apoptosis, enhancing an angiogenesis, and promoting metastasis. The pathway is produced based on the references from [28,35-38].
In the current study, we demonstrated that the overexpression of miR-21 was significantly associated with tumor size and lymph node metastasis, as previous studies have reported the similar outcomes in different types of cancers, including the PDAC [39-43]. Moreover, the overexpression of miR-21 was an independent poor prognostic factor in four cohorts of patients with resected PDAC. Our findings support the evidence that miR-21 is a prognostic biomarker, as previously described in a cohort of 31 and 72 patients with resected PDAC [6]. The prognostic significance of miR-21 in PDAC has also been reviewed and quantified in meta-analyses [44-46].
In addition, we also demonstrated that patients with high miR-21 expression levels showed an association between adjuvant chemotherapy and a significant increase in overall survival. Epithelial overexpression of miR-21 is predictive of gemcitabine-based adjuvant chemotherapy. Patients with high miR-21 levels benefited from gemcitabine-based adjuvant chemotherapy with a survival advantage of 6.0 months, 14.4 months and 13.3 months in Glasgow, Sydney and ICGC cohorts. In contrast, for patients with low tumor miR-21 expression, no survival advantage was observed, and chemotherapy failed to significantly prolong survival following resection. This stratification based on miR-21 expression level could significantly improve the current management algorithms for PDAC, with the hope that miR-21 could potentially be used as a predictive biomarker for gemcitabine-based therapies. Previous research studies have also reported that the overexpression miR-21 has association with gemcitabine resistance in PDAC [23,47-49]. Nonetheless, these studies stratified patients who received gemcitabine into low miR-21 and high miR-21 groups and showed a differential survival pattern with a longer survival in patients with low miR-21. Our results are consistent with these findings. We further stratified patients with high miR-21 levels into those who received adjuvant chemotherapy and those who did not. Longer survival was observed in patients who received chemotherapy, and this finding was consistent across all the study cohorts. The predictive effect in published studies could potentially be due to the prognostic effect of miR-21 or chemotherapy itself. We adjusted for the prognostic effect of miR-21 and chemotherapy and found that the interactive variable miR-21 × chemotherapy was still a true predictive biomarker. Functional studies have shown that gemcitabine exposure downregulates miR-21 expression and upregulates FasL, which is a direct target of miR-21. Upregulated FasL subsequently induces apoptosis in cancer cell apoptosis [49]. This could potentially be the mechanism by which patients with high miR-21 expression benefit from gemcitabine-based chemotherapy. Further mechanistic studies are required to elucidate how gemcitabine exposure leads to better survival in the group with high miR-21 expression. There is an ongoing effort to identify a suitable predictive biomarker for the current standard of care drug, gemcitabine. Predictive biomarkers investigated for gemcitabine response include hENT1, ERCC1, RRM1, HuR and S100A2 [50,51]. In contrast, the predictive ability of miR-21 is unique for these predictive biomarkers, as discussed earlier.
Earlier research has either used miRNA microarray technology or quantitative polymerase chain reaction (qPCR) to investigate the levels of miR-21 expression in PDAC [6,23,47]. In contrast, CISH confers the ability to localize miRNAs in tissue and cellular compartments and provides clues to the interaction between the epithelium and stroma. It is important to reiterate here that in our study, it was the epithelial and not the stromal overexpression of miR-21, which predicts adverse clinical outcomes. Theoretically, this might have an important clinical implication where samples obtained could contain stromal elements possibly confounding accurate assessment of “prognostic miR-21 expression” by PCR but not by CISH. Of note, our CISH can be used for both histological and cytological samples and can provide a clear clinical advantage in the preoperative setting. Furthermore, the preoperative clinical advantage of these findings is the identification of poor or better prognostic groups in cytology samples through knowledge of miR-21 expression levels, thereby potentially discussing the surgical outcomes of patients. Surgical resection is currently the only curative option that can increase long-term survival in pancreatic cancer; however, it carries significant mortality and morbidity, and not all patients benefit from surgery [52,53]. Most clinically significant variables, including resection margin status, lymph node status, and tumor differentiation, are unknown until surgical resection. Thus, there is a need for better identification, ideally preoperatively, of patients who will not benefit from surgery and those who might require aggressive therapeutic strategies. MiR-21 is therefore worth investigating in pancreatic cytological samples.
Several recent reports have demonstrated that large-scale prognostic studies of pancreatic cancer from multinational cohorts are limited [54-57]. From a total of 89 articles reporting 103 potential prognostic biomarkers included in one systematic review [54], the median sample size was 73 (range, 48-300). Only six out of 89 studies had a sample size of more than 200 cases. Our study was sufficiently powered, with a total sample size of 686 patients from four multinational cohorts. However, it is important to mention that despite these results, the current study is limited by the small sample size in each cohort, and most importantly, the utility as a predictive marker should be tested in an adequately powered, randomized prospective trial.
Conclusion
This study reported the independent prognostic and predictive utility of miR-21 expression in multinational cohorts. miR-21 expression is predictive of gemcitabine-based adjuvant chemotherapy, and patients with high miR-21 expression levels benefit from adjuvant chemotherapy. This stratification based on miR-21 expression level could significantly improve the current management algorithms for PDAC, with the hope that miR-21 could potentially be used as a prognostic and predictive biomarker for gemcitabine-based therapies.
Acknowledgements
Bio-specimens and clinical data for the Sydney cohort were provided by the Australian Pancreatic Cancer Genome Initiative (APGI, https://www.pancreaticcancer.net.au/), which is supported by an Avner Pancreatic Cancer Foundation Grant (www.avnersfoundation.org.au).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Mortoglou M, Miralles F, Arisan ED, Dart A, Jurcevic S, Lange S, Uysal-Onganer P. microRNA-21 regulates stemness in pancreatic ductal adenocarcinoma cells. Int J Mol Sci. 2022;23:1275. doi: 10.3390/ijms23031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. doi: 10.1038/s41598-020-73525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, Engebretson A, Herman JM, Strickler JH, Benson AB 3rd, Urba S, Yee NS. Locally advanced, unresectable pancreatic cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016;34:2654–2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 4.Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, Ruggiero JT, Copur MS, Lau M, Urba S, Laheru D. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daoud AZ, Mulholland EJ, Cole G, McCarthy HO. MicroRNAs in pancreatic cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer. 2019;19:1130. doi: 10.1186/s12885-019-6284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson NB, Morran DC, Morton JP, Ali A, Dickson EJ, Carter CR, Sansom OJ, Evans TJ, McKay CJ, Oien KA. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clin Cancer Res. 2012;18:534–545. doi: 10.1158/1078-0432.CCR-11-0679. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O’Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 9.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 10.Steele CW, Oien KA, McKay CJ, Jamieson NB. Clinical potential of microRNAs in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1165–1171. doi: 10.1097/MPA.0b013e3182218ffb. [DOI] [PubMed] [Google Scholar]
- 11.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, Goel R, Mathivanan S, Marimuthu A, Kashyap M, Vizza RF, Mayer RJ, Decaprio JA, Srivastava S, Hanash SM, Hruban RH, Pandey A. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo-or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 13.Mortoglou M, Miralles F, Arisan ED, Dart A, Jurcevic S, Lange S, Uysal-Onganer P. microRNA-21 regulates stemness in pancreatic ductal adenocarcinoma cells. Int J Mol Sci. 2022;23:1275. doi: 10.3390/ijms23031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciernikova S, Earl J, Garcia Bermejo ML, Stevurkova V, Carrato A, Smolkova B. Epigenetic landscape in pancreatic ductal adenocarcinoma: on the way to overcoming drug resistance? Int J Mol Sci. 2020;21:4091. doi: 10.3390/ijms21114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Yao J, Li W, Zhang C. Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol Res. 2018;26:827–835. doi: 10.3727/096504017X14934840662335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YC, Chang IC, Wang CL, Chen TD, Chen YT, Liu HP, Chu Y, Chiu YT, Wu TH, Chou LH, Chen YR, Huang SF. Comparison of IHC, FISH and RT-PCR methods for detection of ALK rearrangements in 312 non-small cell lung cancer patients in Taiwan. PLoS One. 2013;8:e70839. doi: 10.1371/journal.pone.0070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, Tian L. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31:148. doi: 10.1007/s12032-014-0148-8. [DOI] [PubMed] [Google Scholar]
- 19.Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen BS, Jørgensen S, Fog JU, Søkilde R, Christensen IJ, Hansen U, Brünner N, Baker A, Møller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 2011;28:27–38. doi: 10.1007/s10585-010-9355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckstein M, Sailer V, Nielsen BS, Wittenberg T, Wiesmann V, Lieb V, Nolte E, Hartmann A, Kristiansen G, Wernert N, Wullich B, Taubert H, Wach S German Prostate Cancer Consortium (DPKK) Co-staining of microRNAs and their target proteins by miRNA in situ hybridization and immunohistofluorescence on prostate cancer tissue microarrays. Lab Invest. 2019;99:1527–1534. doi: 10.1038/s41374-019-0251-8. [DOI] [PubMed] [Google Scholar]
- 22.Rice TW, Rusch VW, Ishwaran H, Blackstone EH Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American joint committee on cancer/international union against cancer cancer staging manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 23.Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A, Danesi R, Campani D, Verheul HM, Boggi U. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70:4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 24.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13:340–351. doi: 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandana S, Babiker HM, Mahadevan D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC) Expert Opin Investig Drugs. 2019;28:161–177. doi: 10.1080/13543784.2019.1557145. [DOI] [PubMed] [Google Scholar]
- 26.Panchal K, Sahoo RK, Gupta U, Chaurasiya A. Role of targeted immunotherapy for pancreatic ductal adenocarcinoma (PDAC) treatment: an overview. Int Immunopharmacol. 2021;95:107508. doi: 10.1016/j.intimp.2021.107508. [DOI] [PubMed] [Google Scholar]
- 27.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22. doi: 10.1002/jso.23192. [DOI] [PubMed] [Google Scholar]
- 28.Smolarz B, Durczyński A, Romanowicz H, Hogendorf P. The role of microRNA in pancreatic cancer. Biomedicines. 2021;9:1322. doi: 10.3390/biomedicines9101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer. 2012;107:1354–1360. doi: 10.1038/bjc.2012.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frampton AE, Gall TM, Castellano L, Stebbing J, Jiao LR, Krell J. Towards a clinical use of miRNAs in pancreatic cancer biopsies. Expert Rev Mol Diagn. 2013;13:31–34. doi: 10.1586/erm.12.136. [DOI] [PubMed] [Google Scholar]
- 31.Giovannetti E, van der Velde A, Funel N, Vasile E, Perrone V, Leon LG, De Lio N, Avan A, Caponi S, Pollina LE, Gallá V, Sudo H, Falcone A, Campani D, Boggi U, Peters GJ. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS One. 2012;7:e49145. doi: 10.1371/journal.pone.0049145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 33.Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. 2011;30:371–80. doi: 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Q, Hou W. Regulation of angiogenesis by microRNAs in cancer. Mol Med Rep. 2021;24:583. doi: 10.3892/mmr.2021.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammouz RY, Kołat D, Kałuzińska Ż, Płuciennik E, Bednarek AK. MicroRNAs: their role in metastasis, angiogenesis, and the potential for biomarker utility in bladder carcinomas. Cancers (Basel) 2021;13:891. doi: 10.3390/cancers13040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Q, Ye A, Ye W, Liao X, Qin G, Xu Y, Yin Y, Luo H, Yi M, Xian L, Zhang S, Qin X, Zhu W, Li Y. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021;12:576. doi: 10.1038/s41419-021-03803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortoglou M, Wallace D, Buha Djordjevic A, Djordjevic V, Arisan ED, Uysal-Onganer PJS. MicroRNA-regulated signaling pathways: potential biomarkers for pancreatic ductal adenocarcinoma. Stresses. 2021;1:30–47. [Google Scholar]
- 39.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavano F, Di Mola FF, Piepoli A, Panza A, Copetti M, Burbaci FP, Latiano T, Pellegrini F, Maiello E, Andriulli A, di Sebastiano P. Changes in miR-143 and miR-21 expression and clinicopathological correlations in pancreatic cancers. Pancreas. 2012;41:1280–1284. doi: 10.1097/MPA.0b013e31824c11f4. [DOI] [PubMed] [Google Scholar]
- 41.Kadera BE, Li L, Toste PA, Wu N, Adams C, Dawson DW, Donahue TR. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS One. 2013;8:e71978. doi: 10.1371/journal.pone.0071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Sun J, Xu J, Li Q, Guo Y, Zhang Q. miR-21 is a promising novel biomarker for lymph node metastasis in patients with gastric cancer. Gastroenterol Res Pract. 2012;2012:640168. doi: 10.1155/2012/640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunetti O, Russo A, Scarpa A, Santini D, Reni M, Bittoni A, Azzariti A, Aprile G, Delcuratolo S, Signorile M, Gnoni A, Palermo L, Lorusso V, Cascinu S, Silvestris N. MicroRNA in pancreatic adenocarcinoma: predictive/prognostic biomarkers or therapeutic targets? Oncotarget. 2015;6:23323–41. doi: 10.18632/oncotarget.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu GY, Tao F, Wang W, Ji KW. Prognostic value of microRNA-21 in pancreatic ductal adenocarcinoma: a meta-analysis. World J Surg Oncol. 2016;14:82. doi: 10.1186/s12957-016-0842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Wang X, Huang Z, Wang J, Zhu W, Shu Y, Liu P. Prognostic value of miR-21 in various cancers: an updating meta-analysis. PLoS One. 2014;9:e102413. doi: 10.1371/journal.pone.0102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH, Kim SW, Del Chiaro M, Peters GJ, Giaccone G. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5:e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morinaga S, Nakamura Y, Atsumi Y, Murakawa M, Yamaoku K, Aoyama T, Kobayashi S, Ueno M, Morimoto M, Yokose T, Miyagi Y. Locked nucleic acid in situ hybridization analysis of microRNA-21 predicts clinical outcome in patients after resection for pancreatic cancer treated with adjuvant gemcitabine monotherapy. Anticancer Res. 2016;36:1083–1088. [PubMed] [Google Scholar]
- 49.Wang P, Zhuang L, Zhang J, Fan J, Luo J, Chen H, Wang K, Liu L, Chen Z, Meng Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol Oncol. 2013;7:334–345. doi: 10.1016/j.molonc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, Dicker AP, Mackey JR. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 51.Bachet JB, Maréchal R, Demetter P, Bonnetain F, Cros J, Svrcek M, Bardier-Dupas A, Hammel P, Sauvanet A, Louvet C, Paye F, Vaillant JC, André T, Closset J, Salmon I, Emile JF, Van Laethem JL. S100A2 is a predictive biomarker of adjuvant therapy benefit in pancreatic adenocarcinoma. Eur J Cancer. 2013;49:2643–2653. doi: 10.1016/j.ejca.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Schniewind B, Bestmann B, Henne-Bruns D, Faendrich F, Kremer B, Kuechler T. Quality of life after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head. Br J Surg. 2006;93:1099–1107. doi: 10.1002/bjs.5371. [DOI] [PubMed] [Google Scholar]
- 53.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY, Deshpande V, Mino-Kenudson M, Fernández-del Castillo C, Lillemoe KD, Warshaw AL. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152(Suppl 1):S43–S49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamieson NB, Carter CR, McKay CJ, Oien KA. Tissue biomarkers for prognosis in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Clin Cancer Res. 2011;17:3316–3331. doi: 10.1158/1078-0432.CCR-10-3284. [DOI] [PubMed] [Google Scholar]
- 55.Le N, Sund M, Vinci A GEMS collaborating group of Pancreas 2000. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2016;48:223–230. doi: 10.1016/j.dld.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Dhara S, Chhangawala S, Chintalapudi H, Askan G, Aveson V, Massa AL, Zhang L, Torres D, Makohon-Moore AP, Lecomte N, Melchor JP, Bermeo J, Cardenas A 3rd, Sinha S, Glassman D, Nicolle R, Moffitt R, Yu KH, Leppanen S, Laderman S, Curry B, Gui J, Balachandran VP, Iacobuzio-Donahue C, Chandwani R, Leslie CS, Leach SD. Pancreatic cancer prognosis is predicted by an ATAC-array technology for assessing chromatin accessibility. Nat Commun. 2021;12:3044. doi: 10.1038/s41467-021-23237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pietrasz D, Wang-Renault S, Taieb J, Dahan L, Postel M, Durand-Labrunie J, Le Malicot K, Mulot C, Rinaldi Y, Phelip JM, Doat S, Blons H, de Reynies A, Bachet JB, Taly V, Laurent-Puig P. Prognostic value of circulating tumour DNA in metastatic pancreatic cancer patients: post-hoc analyses of two clinical trials. Br J Cancer. 2022;126:440–448. doi: 10.1038/s41416-021-01624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.