Abstract
To construct a prototype hybrid vaccine against Shigella and enterotoxigenic Escherichia coli (ETEC), the genes encoding the production of ETEC CS2 and CS3 fimbriae were isolated and expressed in attenuated Shigella flexneri 2a guaBA strain CVD 1204. The CS2 cotA to -D genes, isolated from ETEC strain C91F, and the CS3 cstA to -H genes, subcloned from plasmid pCS100, were cloned into ∼15-copy-number-stabilized pGA1 behind the osmotically regulated ompC promoter, resulting in high expression of both fimbriae. Under nonselective in vitro growth conditions, pGA1-CS2 and pGA1-CS3 were stable in CVD 1204, exhibiting a plasmid loss of only approximately 1% per duplication. Expression of CS2 and CS3 reduced the invasiveness of Shigella for HeLa cells and slowed the intracellular growth rate. Guinea pigs immunized intranasally with CVD 1204(pGA1-CS2) or CVD 1204(pGA1-CS3), or with a mixture of these strains, developed secretory immunoglobulin A (IgA) in tears and serum IgG antibodies against Shigella lipopolysaccharide, CS2, and CS3 antigens. Moreover, the animals were protected against keratoconjunctivitis following conjunctival challenge with virulent S. flexneri 2a strain 2457T. Animals immunized with Shigella expressing CS2 or CS3 developed serum antibodies that agglutinated Shigella as well as an ETEC strain bearing the homologous fimbriae, whereas animals immunized with combined CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) developed antibodies that agglutinated all three test strains. These observations support the feasibility of a multivalent vaccine against shigellosis and ETEC diarrhea consisting of multiple Shigella live vectors expressing relevant ETEC antigens.
Two bacterial enteric pathogens that have been identified by the World Health Organization as constituting important targets for the development of vaccines are enterotoxigenic Escherichia coli (ETEC) and Shigella (35, 38). In developing countries, ETEC is a major cause of diarrheal dehydration in infants (4), whereas Shigella is the main agent of bacillary dysentery in young children (35). Both pathogens contribute in a major way to the mortality burden attributable to enteric pathogens (4, 35). ETEC is also the most frequent etiologic agent associated with traveler's diarrhea (3, 14, 29, 51), whereas in many studies Shigella is often the second most incriminated pathogen (14, 29). Traveler's diarrhea caused by Shigella tends to be clinically more severe and debilitating than that caused by ETEC. Both ETEC and Shigella are deemed to be worthy targets for immunoprophylaxis of travelers from industrialized countries who visit developing regions of the world (45).
Among the promising candidate vaccines against Shigella are parenteral O polysaccharide-carrier protein conjugates (7, 8), intranasally administered proteosomes consisting of outer membrane protein vesicles of group B Neisseria meningitidis to which Shigella lipopolysaccharide is noncovalently bound (47, 48), and attenuated strains of Shigella used as live oral vaccines (9, 34). Within the four Shigella species (also referred to as groups), 39 main serotypes and subtypes are recognized (15, 35), and epidemiologic and experimental observations indicate that immunity is group-specific and, in many instances, serotype-specific (21, 22). Consequently, initial success with prototype vaccines will have to be followed by the development of a final vaccine formulation that incorporates a strategy for conferring broad-spectrum protection against the epidemiologically most important Shigella serotypes (35, 53).
In recent years, candidate human vaccines against ETEC have been prepared that are based on stimulating intestinal antibodies against the colonization factor fimbriae by which ETEC attaches to enterocytes and on stimulating antitoxin to neutralize heat-labile enterotoxin (LT) (1, 19, 41, 43, 61, 66, 67). Antigens to stimulate anticolonization immunity have included inactivated fimbriated ETEC whole bacteria (1, 16, 19, 60), purified ETEC fimbriae administered in native form (18, 43) or contained within polylactide-polyglycolide microspheres (67), and live oral vaccines consisting of either fimbriated nontoxigenic ETEC strains (36, 37) or of attenuated Shigella or Salmonella enterica serovars Typhi or Typhimurium live vectors expressing ETEC fimbriae and mutant LT or the LT B subunit (26, 31, 42, 54, 55). ETEC vaccines must also address the considerable antigenic heterogeneity among ETEC strains that cause human diarrheal disease (24, 39, 42). It is widely agreed that an ETEC vaccine should include colonization factor antigen I (CFA/I) and coli surface antigens 1 to 6 (CS1 to CS6) fimbrial antigens (42). The candidate ETEC vaccine that is furthest along in clinical trials consists of an oral formulation containing a mixture of inactivated, fimbriated ETEC strains that express CFA/I and CS1-6, coadministered in combination with the cholera toxin B subunit (CT-BS) (60, 61). CT and CT-BS elicit cross-reacting antibodies that can neutralize the LT variant found in ETEC strains in humans (LTh) (46, 65); CT-BS, by itself, has conferred short-term protection (for several months) against diarrhea caused by LT-producing ETEC (6, 56).
We have embarked on a long-term project to develop a multivalent hybrid vaccine to prevent both Shigella dysentery and ETEC diarrhea caused by the epidemiologically most important serotypes and antigenic types (34, 39, 53). The approach consists of engineering five attenuated Shigella strains (representing five epidemiologically and immunologically critical serotypes), each expressing two separate ETEC fimbrial antigens and an antigen to elicit antibodies against LTh (31, 39, 55). Towards this goal, we have prepared improved Shigella vaccine candidates by introducing a deletion mutation in the guaBA operon (which encodes two enzymes involved in the synthesis of guanine nucleotides) in wild-type S. flexneri 2a, resulting in vaccine strain CVD 1204 as a basis of further derivatives (34, 54). This serotype is used as a model because of its epidemiologic importance, the presence in its chromosome of a pathogenicity island that includes Shigella enterotoxin 1 (20, 52), and extensive experience with this serotype in experimental challenge studies in volunteers (11–13, 32, 33). The effect of introducing additional attenuating mutations into CVD 1204, such as deletions in virG (also referred to as icsA, encoding a protein involved with intracellular and intercellular spread of Shigella), resulting in CVD 1205 (54), and in the genes encoding Shigella enterotoxins 1 and 2 (Δset1A, Δsen), resulting in CVD 1207, have been evaluated (34).
We have previously reported cloning the genes necessary for the expression of CFA/I by CVD 1204 and the ability of that live vector to elicit both anti-S. flexneri 2a and anti-CFA/I antibodies (31). The research reported herein describes the expression of rigid CS2 fimbriae and flexible CS3 fibrillae by attenuated S. flexneri 2a strain CVD 1204; an estimation of the stability of the expression plasmids in CVD 1204; the suitability of the osmolarity-activated ompC promoter in promoting fimbrial expression; the ability of the live vector to elicit antibodies to each fimbrial antigen individually and to both fimbriae simultaneously, in addition to S. flexneri O antigen; and, finally, a demonstration that expression of ETEC fimbriae by the live vector does not diminish its ability to protect against virulent S. flexneri 2a in a challenge model.
MATERIALS AND METHODS
Strains and medium.
The following strains were used in this work: wild-type S. flexneri 2a 2457T, originally isolated from a patient in Japan (11); CVD 1204 ΔguaBA, a guanine-dependent strain derived from S. flexneri 2a strain 2457T by targeting a specific deletion that inactivates the purine metabolic pathway enzymes IMP dehydrogenase and GMP synthetase (54); S. flexneri 2a CVD 1204(pGA1), which contains the expression vector pGA1 (this work), and S. flexneri 2a CVD 1204(pGA1-CS2), expressing ETEC CS2 fimbriae (this work); S. flexneri 2a CVD 1204(pGA1-CS3) expressing ETEC CS3 fibrillae (this work); ETEC strain C91f (O6:K15:H16, biotype C), isolated from a patient with diarrhea in Ethiopia (2), was used for isolation of the CS2-encoding genes (23); and ETEC E9034A (O8:H9), a CS3-producing strain (44). E. coli DH5α was the host strain for plasmid constructions. E. coli HS (O9:H4), a nonpathogenic smooth human commensal organism, was used as a control strain in the immunization of guinea pigs (40). Shigella strains were grown on Trypticase soy agar (TSA) supplemented with 0.1% Congo red dye (Sigma Chemical Co., St. Louis, Mo.) and guanine (10 μg/ml). For expression of CS antigens, the ETEC strains were grown on CFA agar plates (17). Luria-Bertani (LB) broth and LB agar containing 50 μg of carbenicillin (Sigma Chemical Co.) per ml were used for cloning and plasmid amplification in E. coli DH5α. To induce fimbria formation in Shigella, the strains were grown in TS broth (Tryptone, 1.5%; Soytone, 0.5%) supplemented with NaCl at different concentrations.
Plasmid constructions.
Synthesis of the 7-nm-diameter rod-like CS2 fimbriae requires four contiguous chromosomal genes, cotB, cotA, cotC, and cotD, which encode the structural and assembly proteins as deduced by homology to CooD and CfaE (5, 23). CotA is the 16.5-kDa major fimbrial subunit protein (63). CotD is a minor fimbrial protein of 38.9 kDa found at the fimbrial tip. In CS1 and CFA/I fimbriae, the tip proteins encoded by cooD and cfaE, respectively, are essential for fimbria-mediated hemagglutination and for adherence of ETEC to intestinal cells (58). CotB (24.8 kDa) and CotC (94.6 kDa) proteins are responsible for the assembly of the fimbriae on the cell surface. By homology to CS1, CotB is a periplasmic protein that manifests chaperone-like activity that may prevent the misfolding and degradation of the synthesized fimbrial proteins. The CotC outer membrane protein is believed to be involved in secretion of the fimbrial proteins from the periplasm across the outer membrane (59).
CS3, which is encoded by the cstA to -H gene cluster, is a thin, flexible, wiry thread, 2 nm in diameter. CstH, the major fimbrial protein, is produced as a 17.5-kDa precursor (69). Removal of either 15 or 22 N-terminal amino acids results in two proteins of 15.5 and 14.5 kDa (44). The remaining genes, cstA to -G, encode the assembly cassette: cstA encodes a 27-kDa protein with homology to the fimbrial chaperones; cstB encodes a 104-kDa protein that is homologous to the outer membrane usher proteins (30, 49, 69).
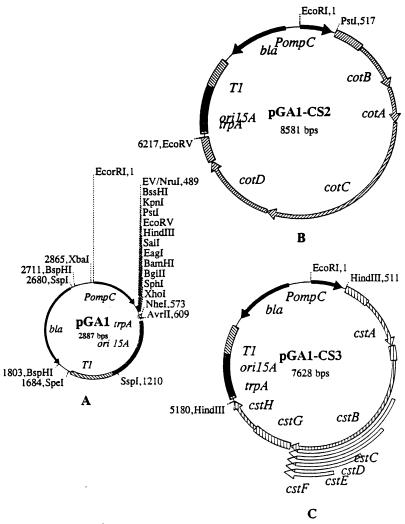
The genes encoding CS2 and CS3 were cloned in pGA1 (Fig. 1A), which was derived from pGEN91 (25) by replacing gfp with an 84-bp synthetic DNA fragment that contains multiple cloning sites (MCS) for BssHI, KpnI, PstI, EcoRV, HindIII, SalI, EagI, BamHI, BglII, SphI, XhoI, and NheI. The linker was synthesized by overlapping PCR using the following four primers: ZA3, GGGTCGCGAGCGCGCGGTACCCTGCAGGATATCAAGCTTGTCGACCGGCCGGGATCCAGATCTGCATGCC; ZA4, CCCGCTAGCCTCGAGGCATGCAGATCTGGATCCCGGCCGGTCGACAAGCTTGATATCCTGCAGGGTACCG; ZA5, GGGTCGCGAGCGCGCGGTACC; and ZA6, CCCGCTAGCCTCGAGGCATGC. The PCR fragment was cleaved with NruI and NheI enzymes and ligated to pGEN91 that was digested with EcoRV/NheI to construct plasmid pGA1.
FIG. 1.
Maps of CS2 and CS3 fimbria-expressing plasmids. (A) Map of the cloning vector pGA1, derived from pGEN91 by replacing gfp with a synthetic linker containing multiple cloning sites. (B) Map of pGA1-CS2, constructed by cloning 5,696 bp of the CS2 operon into pGA1. (C) Map of pGA1-CS3, constructed by cloning 4,746 bp of the CS3 operon into pGA1.
The plasmid contains the ori15A region (which maintains the copy number at approximately 15 per cell), an osmotically regulated ompC promoter that is located 40 bp upstream of the MCS, two transcription termination sites (trpA and T1, which are located immediately and 665 bp downstream of the MCS, respectively), and bla, which encodes β-lactamase production and carbenicillin resistance.
Cloning of CS2 operon.
The chromosomal CS2 operon consists of four genes, cotA to -D (23). Based on analysis of the DNA sequence (NCBI accession number Z47800), the total genomic DNA of strain C91f was digested with the restriction enzymes PstI (a site located 422 bp upstream of the ATG codon in CotA) and EcoRV (a site 150 bp downstream of the stop codon for CotD). DNA fragments between 5 and 8 kb were gel purified and cloned in pBluescript KS (Stratagene, La Jolla, Calif.). DH5α transformants were picked into 96-well microtiter plates, and pools of colonies were analyzed by PCR by using specific DNA primers that amplified a DNA fragment of 1,333 bp from the CS2 operon. The primers used were CS2a, 5′-CACTGTAACTGCTAGC GTTGATCCAAC-3′, and the reverse primer CS2b, 5′-ATCGGGTTAAC ATAACGGTTACTGGCGATG-3′. Individual colonies from positive pools were further analyzed by PCR. Approximately 2% of 900 screened colonies were positive in the PCR assay and were further analyzed for fimbria production by agglutination tests using rabbit antiserum against purified CS2 fimbriae. The genes encoding CS2 were further subcloned as an EcoRV/PstI fragment into pGA1 to generate the 8,587-bp CS2 fimbria-expressing plasmid pGA1-CS2 (Fig. 1B).
Cloning of CS3 operon.
The genes encoding CS3 (cstA to -H) were isolated from pCS100 as a 4,746-bp HindIII fragment (26, 55) and cloned in pGA1, resulting in the 7,628-bp pGA1-CS3 (Fig. 1C). Expression of CS3 fibrillae in DH5α transformants was confirmed by bacterial agglutination using rabbit antiserum against purified CS3 (44).
Transformation of Shigella strains.
Electroporation of competent S. flexneri 2a strain CVD 1204 was accomplished by growing the bacteria in L broth supplemented with guanine to an optical density at 600 nm (OD600) of 0.6. The cells were precipitated, washed twice with cold H2O and once with cold 10% glycerol, and resuspended to 1/100 of the original volume. A mixture containing 150 μl of bacteria plus plasmid DNA was electroporated in 0.2-cm cuvettes in a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) using 2.5 kV, 200 Ω, and 25 μF. Transformants were selected on TSA plates supplemented with carbenicillin, guanine, and Congo red.
Plasmid stability tests.
CVD 1204 strains that express ETEC CS2 or CS3 fimbriae were grown for 24 h in LB broth plus guanine. Ten-fold dilutions of the bacterial cultures were plated on LB guanine plates, and after 24 h single colonies were replica plated on LB guanine agar plates with and without carbenicillin. Colonies that failed to grow on the antibiotic-containing plates were scored for loss of the plasmid.
Detection of fimbrial synthesis.
CVD 1204 strains that expressed CS2 or CS3 were cultured in TS broth containing 0, 50, 150, or 300 mM NaCl until the logarithmic phase of growth. The bacteria were assayed for fimbria production by either dot immunoassays (DIAs) of whole bacteria or by immunoblotting of cell extracts. For the DIAs, the bacterial cultures were serially diluted in phosphate-buffered saline (PBS); 5 μl of each dilution was spotted on a 0.45-μm nitrocellulose filter (Micron Separations Inc., Westboro, Mass.) and blocked with PBS containing 2% bovine serum albumin and 0.05% Tween 20. After washing in PBS-Tween buffer, rabbit anti-CS2 or anti-CS3 was added to the blocking buffer for 60 min at room temperature. After five washings, the second antibody, goat anti-rabbit immunoglobulin G (IgG) labeled with alkaline phosphatase (Gibco BRL, Grand Island, N.Y.), was added for 30 min. After five washings, the positive dots were detected with phosphatase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) reagent. The highest dilution with a positive signal was determined. For immunoblotting experiments, the bacterial cultures were adjusted to an OD600 of 10 and boiled for 10 min in Laemmli sample buffer (Bio-Rad). The cell extract proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15%), transferred to nitrocellulose (MSI) or 0.2 μm polyvinylidene fluoride (Bio-Rad) filters, and probed with rabbit antiserum against purified CS2 or CS3 fimbriae. The protein bands on the polyvinylidene fluoride membrane were developed by adding chemiluminescent substrate (Immun-Star; Bio-Rad) and exposing the filter to X-ray film.
Invasion assays.
HeLa cells (∼2 × 105 cells/ml) in 24-well plates were inoculated with ∼107 bacteria grown on TSA-Congo red-guanine plates. The infected cells were incubated for 90 min in antibiotic-free Dulbecco's modified Eagle's medium containing guanine and 10% calf serum, washed with Hanks balanced salt solution buffer containing 100 μg of gentamicin (Gibco BRL) per ml, and incubated in Dulbecco's modified Eagle's medium containing gentamicin for 30 min (time zero) or 4 h. At 30 min or 4 h, the infected cells were washed with Hanks balanced salt buffer and lysed with PBS containing Triton X-100 (0.5%), and the free bacteria were plated on TSA plates containing Congo red, guanine, and carbenicillin.
Immunization.
Guinea pigs anesthetized subcutaneously with ketamine HCl (40 mg/kg of body weight) and xylazine (5 mg/kg) were inoculated intranasally on days 1 and 15 with ∼2 × 109 bacteria that were grown on TSA-Congo red-guanine plates and harvested in PBS. Five groups of animals were inoculated: group 1 was immunized with CVD 1204; group 2 received CVD 1204(pGA1-CS3); group 3 received CVD 1204(pGA1-CS2); group 4 received a mixture of CVD 1204(pGA1-CS3) plus CVD 1204(pGA1-CS2); and group 5, serving as a placebo control, received 2 × 1010 CFU of E. coli HS. Groups 1 to 4 contained 5 animals each, whereas group 5 had 15 guinea pigs. Sera were obtained on days 0, 14, and 30 by anterior vena cava puncture of anesthetized animals. Tears were collected on the same days by lacrimal stimulation with flakes of Capsicum bacatum, as described previously (54).
Protective efficacy.
In order to assess the protective efficacy of immunization with CVD 1204 expressing CS2 or CS3, or of immunization with a mixture of both live vector constructs, in preventing Shigella keratoconjunctivitis, the “Sereny” test was performed (62). Control animals were immunized with E. coli HS. The guinea pigs were challenged 21 days following the second dose with 10 μl containing 108 CFU of wild-type S. flexneri 2a 2457T in the conjunctival sac. The animals were examined daily for 4 days, and their inflammatory responses were graded as follows: 0 = normal eye indistinguishable from the contralateral nonchallenged eye; 1 = lacrimation or eyelid edema; 2 = 1 plus mild conjuctival hyperemia; 3 = 2 plus slight exudate; and 4 = full purulent keratoconjuctivitis (54).
Antibodies.
The sera and tears from immunized animals were assayed for antibodies by serum agglutination using Shigella and ETEC strains and by enzyme-linked immunosorbent assay using purified Shigella lipopolysaccharide (LPS) and CS2 and CS3 fimbriae as antigens. Secretory IgA (sIgA) antibodies were determined in guinea pig tears using rabbit anti-guinea pig IgA α chain-specific antibody (Bethyl Lab., Montgomery, Tex.) followed by phosphatase-conjugated goat anti-rabbit IgG antibody (Kirkegaard & Perry Laboratories). Serum IgG antibodies were determined using a goat anti-guinea pig IgG (Kirkegaard & Perry Laboratories) conjugate. The starting dilution of samples was 1:40 for tears and 1:25 for sera. Under these conditions, the preimmune sera were negative to the tested antigens. The final dilution considered positive had an OD value that was higher than two standard deviations above the mean OD values obtained from unimmunized animals.
Antigens.
S. flexneri 2a LPS was prepared from strain 2457T by the hot-water–phenol method (68). CS2 and CS3 fimbriae were purified from strains C91f and E9034A, respectively, by a method that involved shearing, differential centrifugation, gel filtration, and density-gradient ultracentrifugation (28, 44).
RESULTS
Cloning cotA to -D.
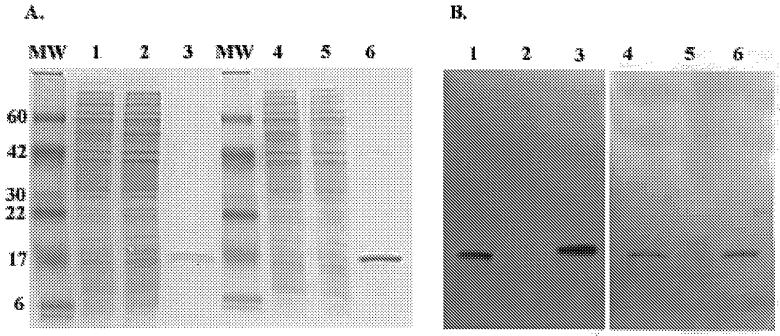
The cotA to -D cluster that encodes the CS2 fimbria was cloned from ETEC strain C91f chromosomal DNA as a 5.7-kb PstI/EcoRV DNA fragment. The entire CS2 operon was cloned into pBluescript KS and subsequently into pGA1 (Fig. 1A), downstream from the ompC promoter (Fig. 1B). Transformation of E. coli DH5α and S. flexneri 2a CVD 1204 with pKS-CS2 or pGA1-CS2 resulted in the synthesis of CS2 fimbriae. Fimbria formation was confirmed by positive colony agglutination with rabbit anti-CS2 antisera. Western immunoblotting of whole-cell lysates of CVD 1204(pGA1-CS2) probed with rabbit antiserum against purified CS2 further validated the expression of CS2. As shown in Fig. 2, a unique 16.5-kDa band, corresponding to the CS2 major fimbrial subunit, was visible in CVD 1204(pGA1-CS2) but not in CVD 1204(pGA1) containing the vector alone.
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and Western blots of CS2- and CS3-expressing CVD 1204. (A) Fast-Page BluPrint satin; (B) Western blot probed with anti-CS3 polyclonal antibody; (C) Western blot probed with anti-CS2 polyclonal antibody. Lane 1, CVD 1204(pGA1-CS3); lane 2, CVD 1204(pGA1); lane 3, purified CS3 pili; lane 4, CVD 1204(pGA1-CS2); lane 5, CVD 1204(pGA1); lane 6, purified CS2 pili.
Cloning cstA to -H.
The cstA to -H cluster, which is located on plasmids in ETEC that encode CS3, was previously cloned as a 4.7-kb HindIII DNA fragment (26, 55). The cstA to -H cluster was subcloned into vector pGA1 downstream of the ompC promoter (Fig. 1C), and CS3 fibrillae were expressed in both E. coli DH5α and S. flexneri 2a CVD 1204. CS3 expression was verified by bacterial agglutination assays, dot immunoassays (see Table 2), and Western immunoblotting assays of bacterial lysates probed with rabbit antiserum against purified CS3. The CS3 major fimbrial subunit is produced as a precursor protein; removal of either 22 or 7 amino acids from its signal peptide gives rise to two proteins of approximately 15.5 and 14.5 kDa (44). These two subunit species were seen in CVD 1204(pGA1-CS3) (Fig. 2).
TABLE 2.
DIA test for CS2 and CS3 fimbriae produced by CVD 1204 strains containing the cotA to -D and cstA to -H genes induced by NaCl
| Strain | NaCl concn (mM) | Positive end point in DIA testa |
|---|---|---|
| CVD 1204(pGA1-CS2) | 0 | 1:16 |
| 50 | 1:64 | |
| 150 | 1:256 | |
| 300 | 1:64 | |
| CVD 1204(pGA1-CS3) | 0 | 1:64 |
| 50 | 1:256 | |
| 150 | 1:256 | |
| 300 | 1:32 |
The final culture dilution considered positive in the DIA test.
Stability of cloned cotA to -D and cstA to -H genes.
The stability of pGA1, pGA1-CS2, and pGA1-CS3 in CVD 1204 was tested by growing the strains in antibiotic-free medium. The results, presented in Table 1, indicate that 86% of CVD 1204(pGA1-CS2) cells and 93% of CVD 1204(pGA1-CS3) cells maintained the plasmid during 7 and 13 duplications, respectively; 100% of CVD 1204(pGA1) cells retained the plasmid during 7 duplications.
TABLE 1.
Stability of plasmids expressing CS2 or CS3 in CVD 1204
| Strain | Relevant phenotype | No. of colonies screened | No. of Ampr colonies | % of cells that maintained the plasmid |
|---|---|---|---|---|
| CVD 1204(pGA1)a | Ampr | 324 | 324 | 100 |
| CVD 1204(pGA1-CS2)b | Ampr CS2+ | 621 | 535 | 86 |
| CVD 1204(pGA1-CS3)a | Ampr CS3+ | 620 | 577 | 93 |
Cells underwent 7 doublings.
Cells underwent 13 doublings.
Induction of fimbria formation by increased osmolarity.
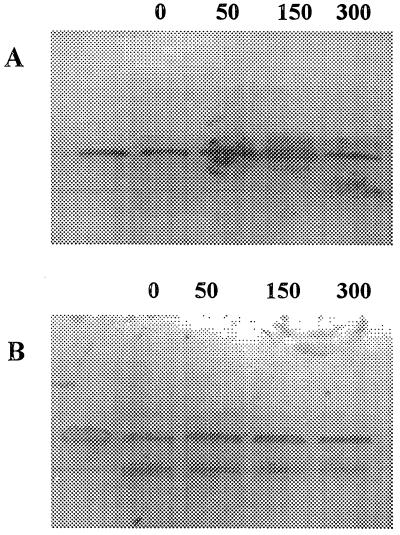
Since the ompC promoter from E. coli is osmotically regulated, the induction of fimbrial synthesis was assayed by growing CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) in medium that contained increasingly higher concentrations of NaCl (0, 50, 150, and 300 mM). Fimbrial expression was tested by DIA and immunoblotting experiments. DIA results (Table 2) indicated that growth in 150 mM NaCl led to a 16-fold induction of synthesis of CS2 fimbriae and a 4-fold induction of CS3. NaCl concentrations of 300 mM had an inhibitory effect on production of both CS2 and CS3 fimbriae. Immunoblotting of cell extracts of CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) (Fig. 3) confirmed the DIA results. Salt concentrations of up to 150 mM NaCl induced fimbrial synthesis, while higher concentrations had an inhibitory effect. No fimbriae were detected in CVD 1204(pGA1).
FIG. 3.
Induction of fimbrial expression by salt concentration. (A) Western blot probed with anti-CS2 polyclonal antibody. Lane 1, purified CS2 pili; lanes 2 to 5, CVD 1204(pGA1-CS2) grown in the indicated NaCl concentrations (millimolar). (B) Western blot probed with anti-CS3 polyclonal antibody. Lane 1, purified CS3 pili; lanes 2 to 5, CVD 1204(pGA1-CS3) grown in the indicated NaCl concentrations.
Invasion and replication in HeLa cells.
The strains were tested to ascertain whether the expression of CS2 or CS3 fimbriae in CVD 1204 interfered with the ability of the Shigella vaccine strain to invade HeLa cells and to maintain intracellular growth thereafter. CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) were more than 100-fold less invasive, and CVD 1204(pGA1) was approximately 10-fold less invasive than CVD 1204 (Table 3). Following invasion, the strains maintained their ability to grow intracellularly, albeit to a somewhat diminished degree compared to the host: CVD 1204(pGA1-CS2) demonstrated two replications and CVD 1204(pGA1-CS3) exhibited three replications, compared to the four to five replications achieved by CVD 1204 during 4 h of growth in HeLa cells.
TABLE 3.
Invasion and replication of CVD 1204 strains carrying plasmids harboring cotA to -D and cstA to -H operons and producing CS2 and CS3 fimbriae in HeLa cells
| Strain | CFU/105 HeLa cellsa at time (h):
|
|
|---|---|---|
| 0 | 4 | |
| CVD 1204 | (1.6 ± 0.9) × 104 | (3.7 ± 0.6) × 105 |
| CVD 1204(pGA1) | (1.0 ± 0.4) × 103 | (1.3 ± 0.2) × 104 |
| CVD 1204(pGA1-CS2) | 4 × 101 | (1.26 ± 1.0) × 102 |
| CVD 1204(pGA1-CS3) | (8.2 ± 1.8) × 101 | (7.7 ± 3.2) × 102 |
Arithmetic mean ± standard deviation of data points from three wells.
Immunization of guinea pigs.
The immunogenicity and protective efficacy induced by CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) were tested in guinea pigs. The animals were immunized with two intranasal administrations of live bacterial cultures. Serum, as a source of IgG, and tears, as a source of sIgA, were collected 1 day prior to the first dose and 14 days following each dose.
Bacterial agglutination assays performed with sera obtained 2 weeks after the second immunization showed that all of the animals immunized with either CVD 1204 alone, CVD 1204(pGA1-CS2), or CVD 1204(pGA1-CS3) developed antibodies capable of agglutinating wild-type S. flexneri 2a (Table 4). Animals immunized with CVD 1204 expressing CS3 or CS2 fimbriae developed antibodies which agglutinated the wild-type ETEC strain bearing the corresponding fimbriae. Moreover, animals immunized with the mixture of CVD 1204(pGA1-CS2) and CVD 1204(pGA1-CS3) produced antibodies that agglutinated both fimbriated wild-type ETEC strains.
TABLE 4.
Agglutination of Shigella, ETEC strain C91f (CS2+), and ETEC strain E9034A (CS3+) by postimmunization sera from guinea pigs immunized with various vaccines
| Vaccine straina | Agglutinationb by postimmunization sera of bacterial strain:
|
||
|---|---|---|---|
| CVD 1204 | E9034A | C91f | |
| CVD 1204 | +++ | − | − |
| CVD 1204(pGA1-CS3) | + | +++ | − |
| CVD 1204(pGA1-CS2) | + | − | +++ |
| CVD 1204(pGA1-CS3) plus CVD 1204(pGA1-CS2) | ++ | + | +++ |
| Control (preimmunization sera) | − | − | − |
The assays were performed with pooled sera from each immunized group, 34 days postimmunization with the designated strain.
+++, very strong; ++, strong; +, weak; −, none.
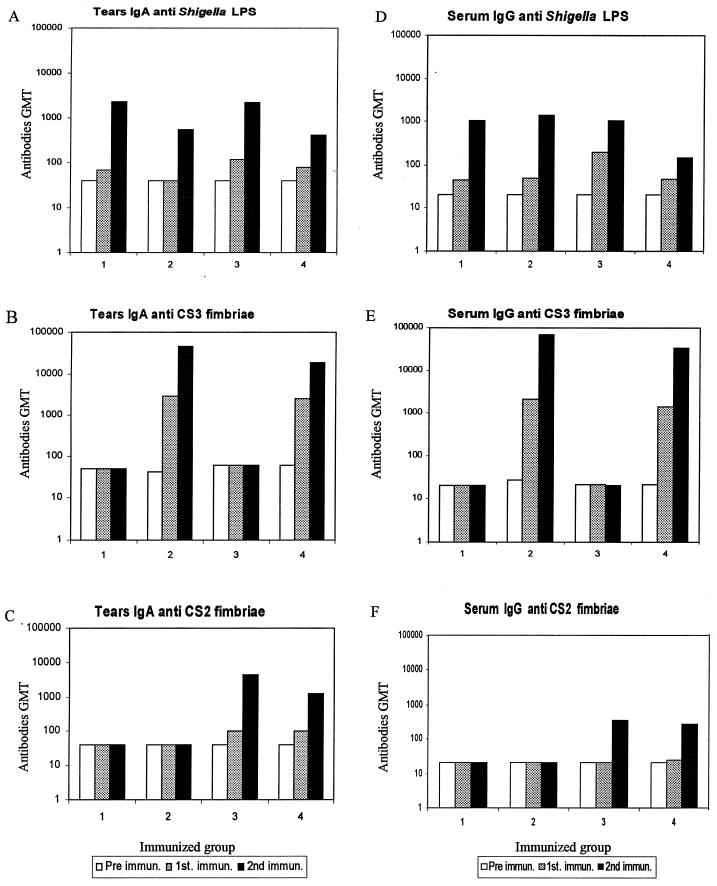
Specific immune responses to each fimbria and the Shigella vector itself were quantitated by enzyme-linked immunosorbent assay. All animals immunized with CVD 1204(pGA1-CS3) alone (group 2) or as a mixture (group 4) responded with high levels of both mucosal IgA and serum IgG anti-CS3 following a single dose (Fig. 4B and E). These titers were boosted to even higher levels following the second dose. Anti-CS3 IgG titers ranged in group 2 from 51,200 to 204,800 and in group 4 from 12,800 to 204,800.
FIG. 4.
Production of sIgA and serum IgG antigen-specific antibodies in guinea pigs immunized with Shigella strains expressing CS2 and CS3 fimbriae. The immunized groups were as follows: group 1, CVD 1204; group 2, CVD 1204(pGA1-CS3); group 3, CVD 1204(pGA1-CS2); and group 4, a mixture of CVD 1204(pGA1-CS3) and CVD 1204(pGA1-CS2). Antibody titers were determined in preimmune sera, following a single immunization (day 14) and following two immunizations (day 28). Antibodies elicited against Shigella LPS, CS2, and CS3 antigens were quantitated. The titers of tear sIgA are presented in panels A (LPS), B (CS3), and C (CS2); the titers of serum IgG are presented in panels D (LPS), E (CS3), and F (CS2).
All animals immunized with CVD 1204(pGA1-CS2) (groups 3 and 4) developed anti-CS2 mucosal IgA and serum IgG following a single dose (Fig. 4C). Two immunizations were required to elicit anti-CS2 serum IgG responses in all animals (Fig. 4F). Antifimbrial titers were comparable in groups receiving a single strain or a mixture of strains. Anti-CS2 IgG titers ranged in group 3 from 100 to 1,600 and in group 4 from 100 to 1,600.
All animals in every group responded to the vector strain itself with anti-Shigella LPS mucosal IgA and serum IgG following two doses, with comparable titers in all groups (Fig. 4A and D). Following a single dose of any CVD 1204 inoculum, half of the animals responded with anti-Shigella LPS mucosal IgA, whereas three-fourths of the animals responded with anti-Shigella LPS serum IgG. Anti-Shigella LPS IgG titers ranged in group 1 from 400 to 1,600, in group 2 from 800 to 3,200, in group 3 from 400 to 3,200, and in group 4 from 100 to 200.
Protective efficacy.
Upon Sereny test challenge with wild-type S. flexneri 2a, all 15 animals vaccinated intranasally with the placebo strain of E. coli HS developed severe keratoconjunctivitis (Table 5). In contrast, none of the animals (5 per group) immunized with either native CVD 1204 or CVD 1204 expressing ETEC fimbriae developed severe keratoconjunctivitis (P = 0.000064 for each comparison; Fisher's exact test). One animal in the group immunized with CVD 1204(pGA1-CS2) had a score of 1 on day 3. One animal in the group immunized with CVD 1204(pGA1-CS3) had a score of 2 on days 3 and 4.
TABLE 5.
Protection of immunized guinea pigs against conjunctivitis following challenge with wild-type S. flexneri 2a
| Immunization regimen | No. of guinea pigs challenged | No. of guinea pigs exhibiting severe keratoconjunctivitisa |
|---|---|---|
| CVD 1204 | 5 | 0 |
| CVD 1204(pGA1-CS3) | 5 | 0 |
| CVD 1204(pGA1-CS2) | 5 | 0 |
| CVD 1204(pGA1-CS3) plus CVD 1204(pGA1-CS2) | 5 | 0 |
| E. coli HS | 15 | 15 |
CVD 1204 versus HS, P = 0.000004; CVD 1204(pGA1-CS3) versus HS, P = 0.000064; CVD 1204(pGA1-CS2) versus HS, P = 0.000064; CVD 1204(pGA1-CS3) plus CVD 1204(pGA1-CS2) versus HS, P = 0.000064.
DISCUSSION
Shigella and ETEC are important human pathogens that cause diarrheal disease in children in developing countries and in travelers. One of the daunting obstacles that faces vaccine developers of both Shigella and ETEC vaccines is that multiple antigenic types of these pathogens cause disease in humans, and so for each a multivalent vaccine will be necessary to provide broad-spectrum protection. It is the contention of our group that a multivalent Shigella vaccine containing five serotypes could confer broad protection. The serotypes should include Shigella dysenteriae 1 (the cause of severe epidemic Shiga dysentery in the least-developed countries of the world) (35); S. flexneri 2a, S. flexneri 3a, and S. flexneri 6 (which together bear group- or type-specific antigens that are shared with the other 12 S. flexneri types and subtypes and demonstrate cross-protection against them in guinea pig challenge studies [53]); and Shigella sonnei (the main cause of traveler's shigellosis and of persisting foci of disease in endemic areas in industrialized countries) (29, 35).
Similarly, ETEC strains associated with human diarrheal disease exhibit an array of colonization fimbriae, of which the most common are CFA/I, the CFA/II family, and the CFA/IV family. CFA/I strains consist of a single antigenic moiety (37, 42). In contrast, all CFA/II strains produce CS3 but, in addition, may coexpress either CS1 or CS2. Similarly, CFA/IV strains express CS6, either alone or together with CS4 or CS5 fimbriae (24, 42, 50).
Since Shigella and ETEC are two of the most important bacterial enteric pathogens targeted for immunoprophylaxis, we have embarked on a long-term program to develop a multivalent hybrid vaccine against both pathogens that consists of attenuated Shigella strains of the above-mentioned five serotypes, each expressing different fimbrial antigens and an antigen (either mutant LTh or LTh B subunit) to stimulate LT antitoxin. The results described herein, relating further progress in the development of this complex multivalent live oral vaccine, communicate the construction of a prototype combined vaccine consisting of an attenuated ΔguaBA S. flexneri 2a strain expressing either CS2 or CS3 fimbriae. The fimbriae are expressed from a circa 15-copy-number plasmid and, as shown in Table 1, both pGA1-CS2 and pGA1-CS3 exhibited a high degree of stability upon in vitro culture in the absence of selective antibiotic. In vivo, plasmids sometimes are less stable than might be predicted by in vitro data. Therefore, future constructs that are currently in preparation will involve inserting the cloned CS2 and CS3 gene sequences reported herein onto the highly stabilized plasmid expression vectors recently described by Galen et al. (25). High-level expression of CS2 and CS3 was achieved under the direction of the osmotically activated promoter, ompC (Table 2).
Carriage of plasmids by CVD 1204 diminishes HeLa cell invasiveness 10-fold. However, the expression of CS2 or CS3 fimbriae decreased invasiveness an additional 10-fold, presumably by sterically preventing the Shigella invasion plasmid antigens from coming in contact with the surface of the eukaryotic cells. In practical terms, this diminished invasiveness would be expected to further attenuate the Shigella vaccine strain for humans. Shigella bacteria that were internalized underwent several replications (Table 3). Although the Shigella live vectors expressing CS2 or CS3 had 100-fold-diminished invasiveness for cells in tissues culture, the live vector vaccines were nevertheless highly immunogenic in eliciting both anti-Shigella and anti-ETEC fimbrial antibodies in mucosal secretions (tears) and in the blood. Particularly important is the observation that concomitant mucosal immunization with a mixture of pGA1-CS2 and pGA1-CS3 bacteria resulted in strong antibody responses to both CS2 and CS3 antigens. There was no diminution in responses to either fimbria when coadministered with the other. It is known that antifimbrial antibodies can prevent ETEC strains bearing the homologous fimbria from attaching to intestinal mucosa and thus prevent diarrhea (10, 18, 27, 37, 57, 67). Since responses against two fimbria types were accomplished with a mixture of two strains, we are highly encouraged to proceed in future studies with the administration of a mixture containing multiple Shigella live vector strains expressing different fimbrial antigens.
Another important observation made in this study is that the live vectors that elicited anti-CS antibodies in guinea pigs still conferred upon those guinea pigs protection against challenge with virulent Shigella in the Sereny test (62). These findings constitute additional encouraging preclinical data that will help to advance the project towards proof-of-principle clinical trials in humans with further improved prototype live vector constructs. Next steps will include studies with live vectors carrying further-modified stabilized plasmids carrying a kanamycin resistance gene rather than an ampicillin or carbenicillin resistance gene (which is more acceptable to regulatory agencies) and cloned CS operons with mutant LTh (e.g., K63) or the LTh B subunit.
ACKNOWLEDGMENTS
These studies were supported by grant ROI AI 29471 from NIAID and grants from Chiron Corporation and the Rockefeller Foundation (M. M. Levine, principal investigator).
We gratefully acknowledge Dave R. Maneval for the antigen production.
REFERENCES
- 1.Ahren C, Jertborn M, Svennerholm A M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect Immun. 1998;66:3311–3316. doi: 10.1128/iai.66.7.3311-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back E, Mollby R, Kaijser B, Stintzing G, Wadstrom T, Habte D. Enterotoxigenic Escherichia coli and other gram-negative bacteria of infantile diarrhea: surface antigens, hemagglutinins, colonization factor antigen, and loss of enterotoxigenicity. J Infect Dis. 1980;142:318–327. doi: 10.1093/infdis/142.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Black R E. Pathogens that cause travelers' diarrhea in Latin America and Africa. Rev Infect Dis. 1986;8:S131–S135. doi: 10.1093/clinids/8.Supplement_2.S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R E, Merson M H, Huq I, Alim A R M A, Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhoea in rural Bangladesh. Lancet. 1981;i:141–143. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- 5.Caron J, Coffield L M, Scott J R. A plasmid-encoded regulatory gene, rns, required for expression of the CS1 and CS2 adhesins of enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA. 1989;86:963–967. doi: 10.1073/pnas.86.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens J D, Sack D A, Harris J R, Chakraborty J, Neogy P K, Stanton B, Huda N, Khan M U, Kay B A, Khan M R, Ansaruzzaman M, Yunus M, Rao M R, Svennerholm A-M, Holmgren J. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988;158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 7.Cohen D, Ashkenazi S, Green M, Lerman Y, Slepon R, Robin G, Orr N, Taylor D N, Sadoff J C, Chu C, Shiloach J, Schneerson R, Robbins J B. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 9.Coster T S, Hoge C W, VanDeVerg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De la Cabada F J, Evans D G, Evans D J., Jr Immunoprotection against enterotoxigenic Escherichia coli diarrhea in rabbits by peroral administration of purified colonization factor antigen I (CFA/I) FEMS Microbiol Lett. 1981;11:303–307. [Google Scholar]
- 11.DuPont H L, Hornick R B, Dawkins A T, Snyder M J, Formal S B. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969;119:296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- 12.DuPont H L, Hornick R B, Snyder M J, Libonati J P, Formal S B, Gangarosa E J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125:12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- 13.DuPont H L, Levine M M, Hornick R B, Formal S B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 14.DuPont H L, Olarte J, Evans D G, Pickering L K, Galindo E, Evans D J. Comparative susceptibility of Latin American and United States students to enteric pathogens. N Engl J Med. 1976;285:1520–1521. doi: 10.1056/NEJM197612302952707. [DOI] [PubMed] [Google Scholar]
- 15.Edwards P, Ewing W. Identification of Enterobacteriaceae. Minneapolis, Minn: Burgess Publishing Co.; 1972. [Google Scholar]
- 16.Evans D G, Evans D J, Jr, Opekun A, Graham D Y. Non-replicating whole cell vaccine protective against enterotoxigenic Escherichia coli (ETEC) diarrhea: stimulation of anti-CFA (CFA/I) and anti-enterotoxin (anti-LT) intestinal IgA and protection against challenge with ETEC belonging to heterologous serotypes. FEMS Microbiol Lett. 1988;47:117–125. doi: 10.1111/j.1574-6968.1988.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 17.Evans D G, Evans D J, Jr, Tjoa W S, DuPont H L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978;19:727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans D G, Graham D Y, Evans D J, Jr, Opekun A. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Gastroenterology. 1984;87:934–940. [PubMed] [Google Scholar]
- 19.Evans D J, Jr, Evans D G, Opekun A, Graham D Y. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiol Lett. 1988;47:9–18. doi: 10.1111/j.1574-6968.1988.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 20.Fasano A, Noriega F R, Maneval D R, Jr, Chanasongcram S, Russell R, Guandalini S, Levine M M. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Investig. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreccio C, Prado V, Ojeda A, Cayazzo M, Abrego P, Guers L, Levine M M. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 22.Formal S B, Oaks E V, Olsen R E, Wingfield Eggleston M, Snoy P J, Cogan J P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 23.Froehlich B J, Karakashian A, Sakellaris H, Scott J R. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun. 1995;63:4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaastra W, Svennerholm A M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 25.Galen J E, Nair J, Wang J Y, Wasserman S S, Tanner M K, Sztein M B, Levine M M. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun. 1999;67:6424–6433. doi: 10.1128/iai.67.12.6424-6433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giron J A, Xu J-G, Gonzalez C R, Hone D M, Kaper J B, Levine M M. Simultaneous constitutive expression of CFA/I and CS3 colonization factors of enterotoxigenic Escherichia coli by aroC, aroD Salmonella typhi vaccine strain CVD 908. Vaccine. 1995;10:939–946. doi: 10.1016/0264-410x(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 27.Grewal H M, Valvatne H, Bhan M K, van Dijk L, Gaastra W, Sommerfelt H. A new putative fimbrial colonization factor, CS19, of human enterotoxigenic Escherichia coli. Infect Immun. 1997;65:507–513. doi: 10.1128/iai.65.2.507-513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall R H, Maneval D R, Collins J H, Theibert J L, Levine M M. Purification and analysis of colonization factor antigen I, coli surface antigen 1, and coli surface antigen 3 fimbriae from enterotoxigenic Escherichia coli. J Bacteriol. 1989;171:6372–6374. doi: 10.1128/jb.171.11.6372-6374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyams K C, Bourgeois A L, Merrell B R, Rozmahel R, Escamilla J, Thornton S A, Wasserman G M, Burke A, Echeverria P, Green K Y, Kapikian A Z, Woody J N. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 30.Jalajakumari M B, Thomas C J, Halter R, Manning P A. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989;3:1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 31.Koprowski H, II, Levine M M, Anderson R J, Losonsky G, Pizza M, Barry E M. Attenuated Shigella flexneri 2a vaccine strain CVD 1204 expressing colonization factor antigen I and mutant heat-labile enterotoxin of enterotoxigenic Escherichia coli Infect. Immun. 2000;68:4884–4892. doi: 10.1128/iai.68.9.4884-4892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotloff K L, Losonsky G A, Nataro J P, Wasserman S S, Hale T L, Taylor D N, Newland J W, Sadoff J C, Formal S B, Levine M M. Evaluation of the safety, immunogenicity and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 33.Kotloff K L, Nataro J P, Losonsky G A, Wasserman S S, Hale T L, Taylor D N, Sadoff J C, Levine M M. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 34.Kotloff K L, Noriega F R, Samandari T, Sztein M B, Losonsky G A, Nataro J P, Picking W D, Barry E M, Levine M M. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect Immun. 2000;68:1034–1039. doi: 10.1128/iai.68.3.1034-1039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotloff K L, Winickoff J P, Ivanoff B, Clemens J D, Swerdlow D L, Sansonetti P J, Adak G K, Levine M M. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull W H O. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 36.Levine M M. Travellers' diarrhoea: prospects for successful immunoprophylaxis. Scand J Gastroenterol Suppl. 1983;84:121–134. [PubMed] [Google Scholar]
- 37.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 38.Levine M M. Modern vaccines. Enteric infections. Lancet. 1990;335:958–961. doi: 10.1016/0140-6736(90)91013-z. [DOI] [PubMed] [Google Scholar]
- 39.Levine M M. Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr. 2000;31:336–355. doi: 10.1097/00005176-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 41.Levine M M, Black R E, Clements M L, Young C R, Cheney C P, Schad P, Collins H, Boedeker E C. Prevention of enterotoxigenic Escherichia coli diarrheal infection by vaccines that stimulate antiadhesion (antipili) immunity. In: Boedeker E C, editor. Attachment of organisms to the gut mucosa. Boca Raton, Fla: CRC Press; 1984. pp. 223–244. [Google Scholar]
- 42.Levine M M, Giron J A, Noriega F. Fimbrial vaccines. In: Klemm P, editor. Fimbriae: adhesion, biogenics, genetics and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 255–270. [Google Scholar]
- 43.Levine M M, Morris J G, Losonsky G, Boedeker E, Rowe B. Fimbriae (pili) adhesins as vaccines. In: Lark D L, Normark S, Uhlin B E, Wolf-Watz H, editors. Protein-carbohydrate interactions in biological systems. The molecular biology of microbial pathogenicity. London, England: Academic Press; 1986. pp. 143–145. [Google Scholar]
- 44.Levine M M, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements M L, Cheney C, Patnaik R. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984;44:409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine M M, Svennerholm A-M. Enteric vaccines: present and future. In: DuPont H L, Steffen R, editors. Textbook of travel medicine and health. B. C. New York, N.Y: Decker; 2000. pp. 252–263. [Google Scholar]
- 46.Levine M M, Young C R, Black R E, Takeda Y, Finkelstein R A. Enzyme-linked immunosorbent assay to measure antibodies to purified heat-labile enterotoxins from human and porcine strains of Escherichia coli and to cholera toxin: application in serodiagnosis and seroepidemiology. J Clin Microbiol. 1985;21:174–179. doi: 10.1128/jcm.21.2.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowell G H. Proteosomes for improved nasal, oral or injectable vaccines. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1997. pp. 193–206. [Google Scholar]
- 48.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manning P A, Timmis K N, Stevenson G. Colonization factor antigen II (CFA/II) of enterotoxigenic Escherichia coli: molecular cloning of the CS3 determinant. Mol Gen Genet. 1985;200:322–327. doi: 10.1007/BF00425443. [DOI] [PubMed] [Google Scholar]
- 50.McConnell M M, Thomas L V, Willshaw G A, Smith H R, Rowe B. Genetic control and properties of coli surface antigens of colonization factor antigen IV (PCF8775) of enterotoxigenic Escherichia coli. Infect Immun. 1988;56:1974–1980. doi: 10.1128/iai.56.8.1974-1980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merson M H, Morris G K, Sack D A, Wells J G, Feeley J C, Sack R B, Creech W B, Kapikian A Z, Gangarosa E J. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N Engl J Med. 1976;294:1299–1305. doi: 10.1056/NEJM197606102942401. [DOI] [PubMed] [Google Scholar]
- 52.Noriega F R, Liao F M, Formal S B, Fasano A, Levine M M. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J Infect Dis. 1995;172:1408–1410. doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 53.Noriega F R, Liao F M, Maneval D R, Ren S, Formal S B, Levine M M. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noriega F R, Losonsky G, Lauderbaugh C, Liao F M, Wang M S, Levine M M. Engineered guaB-A, virG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity and potential efficacy as a mucosal vaccine. Infect Immun. 1996;64:3055–3061. doi: 10.1128/iai.64.8.3055-3061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noriega F R, Losonsky G, Wang J Y, Formal S B, Levine M M. Further characterization of aroA, virG Shigella flexneri 2a strain CVD 1203 as a mucosal Shigella vaccine and as a live vector vaccine for delivering antigens of enterotoxigenic Escherichia coli. Infect Immun. 1996;64:23–27. doi: 10.1128/iai.64.1.23-27.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, Kataja M J, Cadoz M. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991;338:1285–1289. doi: 10.1016/0140-6736(91)92590-x. [DOI] [PubMed] [Google Scholar]
- 57.Rudin A, Olbe L, Svennerholm A M. Monoclonal antibodies against fimbrial subunits of colonization factor antigen I (CFA/I) inhibit binding to human enterocytes and protect against enterotoxigenic Escherichia coli expressing heterologous colonization factors. Microb Pathog. 1996;21:35–45. doi: 10.1006/mpat.1996.0040. [DOI] [PubMed] [Google Scholar]
- 58.Sakellaris H, Munson G P, Scott J R. A conserved residue in the tip proteins of CS1 and CFA/I pili of enterotoxigenic Escherichia coli that is essential for adherence. Proc Natl Acad Sci USA. 1999;96:12828–12832. doi: 10.1073/pnas.96.22.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakellaris H, Scott J R. New tools in an old trade: CS1 pilus morphogenesis. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- 60.Savarino S J, Brown F M, Hall E, Bassily S, Youssef F, Wierzba T, Peruski L, El-Masry N A, Safwat M, Rao M, Jertborn M, Svennerholm A M, Lee Y J, Clemens J D. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Egyptian adults. J Infect Dis. 1998;177:796–799. doi: 10.1086/517812. [DOI] [PubMed] [Google Scholar]
- 61.Savarino S J, Hall E R, Bassily S, Brown F M, Youssef F, Wierzba T F, Peruski L, El-Masry N A, Safwat M, Rao M, El Mohamady H, Abu-Elyazeed R, Naficy A, Svennerholm A M, Jertborn M, Lee Y J, Clemens J D. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. PRIDE Study Group. J Infect Dis. 1999;179:107–114. doi: 10.1086/314543. [DOI] [PubMed] [Google Scholar]
- 62.Sereny B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4:367–376. [PubMed] [Google Scholar]
- 63.Sjoberg P O, Lindahl M, Porath J, Wadstrom T. Purification and characterization of CS2, a sialic acid-specific haemagglutinin of enterotoxigenic Escherichia coli. Biochem J. 1988;255:105–111. doi: 10.1042/bj2550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth C J. Two mannose-resistant hemagglutinins on enterotoxigenic Escherichia coli of serotype 06:H16 or H-isolated from traveller's and infantile diarrhea. J Gen Microbiol. 1982;128:2081–2096. doi: 10.1099/00221287-128-9-2081. [DOI] [PubMed] [Google Scholar]
- 65.Svennerholm A M, Holmgren J, Black R, Levine M, Merson M. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J Infect Dis. 1983;147:514–522. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 66.Svennerholm A M, Holmgren J, Sack D A. Development of oral vaccines against enterotoxinogenic Escherichia coli diarrhoea. Vaccine. 1989;7:196–198. doi: 10.1016/0264-410x(89)90228-4. [DOI] [PubMed] [Google Scholar]
- 67.Tacket C O, Reid R H, Boedeker E C, Losonsky G, Nataro J P, Bhagat H, Edelman R. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine. 1994;12:1270–1274. doi: 10.1016/s0264-410x(94)80038-2. [DOI] [PubMed] [Google Scholar]
- 68.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further application of procedures. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 69.Yakhchali B, Manning P A. Epitope analysis of the CS3 fimbrial subunit of human enterotoxigenic Escherichia coli and the construction of novel CS3::ST and CS3::LT-B immunogens. Behring Inst Mitt. 1997;98:124–134. [PubMed] [Google Scholar]