Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) can potentially salvage large B-cell lymphoma (LBCL) patients experiencing treatment failure after chimeric antigen receptor T-cell therapy (CAR T). Nonetheless, data on the efficacy and toxicities of alloHCT after receipt of CAR T are limited. We report a multicenter retrospective study assessing the safety, toxicities, and outcomes of alloHCT in LBCL patients following CAR T failure. Eighty-eight patients with relapsed, refractory LBCL received an alloHCT following anti-CD19 CAR T failure. The median number of lines of therapy between CAR T infusion and alloHCT was one (range, 0-7). Low intensity conditioning was used in 77% (n=68) and peripheral blood was the most common graft source (86%, n=76). The most common donor types were matched unrelated donor (39%), followed by haploidentical (30%) and matched related donor (26%). Median follow-up of survivors was 15 months (range, 1-72). One-year overall survival, progression-free survival, and graft-versus-host disease-free relapse-free survival were 59%, 45%, and 39% respectively. One-year non-relapse mortality and progression/relapse were 22% and 33% respectively. On multivariate analysis, <2 lines of intervening therapy between CAR T and alloHCT and complete response at time of alloHCT were associated with better outcomes. In conclusion, alloHCT after CAR T failure can provide durable remissions in a subset of patients.
Introduction
For patients with relapsed, refractory (R/R) large B-cell lymphoma (LBCL), chimeric antigen receptor T-cell (CAR T) therapy produces high overall response rates (ORR) ranging from 52-83%;1-7 nonetheless, the durable remission rate is much lower at 30% to 40%.4,5 Outcomes for patients with disease progression after CAR T are poor, with a median overall survival (OS) from time of progressive disease (PD) of less than 6 months.8,9
In patients with LBCL who have previously received or are ineligible for autologous hematopoietic cell transplantation (HCT), allogeneic HCT (alloHCT) has curative potential in selected patients. The 3-year progression-free survival (PFS) following alloHCT in patients with R/R LBCL is reported at 36% to 38% in more recent series.10 Despite this, there are limited data on the efficacy and toxicities of alloHCT after receipt of CAR T therapy, limited to studies of fewer than ten patients.11,12 Therefore, whether patients who fail CAR T derive similar benefit with alloHCT and to whom to offer this modality to remains unknown. In smaller reports, a low proportion of patients make it to alloHCT,9,13 which could be a consequence of an inability to attain disease control or impaired functional status after CAR T failure. Nonetheless, as we continue to expand the arsenal of effective novel therapies for R/R LBCL,14-16 we may be able to better achieve disease control and offer alloHCT to a larger proportion of patients. Given these knowledge gaps, we conducted a multicenter retrospective study to evaluate the safety, toxicities, and outcomes of alloHCT after CAR T for patients with R/R LBCL.
The primary objective of this study was to describe the 1-year OS, PFS, graft-versus-host disease (GvHD)-free relapse-free survival (GRFS), incidence of non-relapse mortality (NRM) and progression/relapse in R/R LBCL patients receiving alloHCT following anti-CD19 CAR T failure. The secondary objective was to determine patient-, disease- and treatment-related factors predicting favorable outcomes in these patients.
Methods
Study design and participants
Patients ≥18 years old with R/R LBCL treated with anti-CD19 CAR T therapy between 2013-2021 that subsequently received an alloHCT were included in this retrospective, multicenter analysis. Eighteen United States academic medical centers participated in this analysis (Online Supplementary Table S1). Patients treated with investigational CAR T therapy were included if it targeted the CD19 antigen. Patients who were in a durable complete response (CR) following CAR T therapy, who then received an alloHCT either as a consolidation or for indications other than R/R LBCL were excluded. Patients with primary central nervous system (CNS) lymphoma were excluded. Data were collected from the electronic medical record of each institution via chart review by individual investigators. Information collected included demographic and clinical characteristics, CAR T toxicities and responses, post-CAR T treatment regimens, alloHCT treatment details, neutrophil and platelet recovery, and acute and chronic GvHD.
Cytokine release syndrome (CRS) and immune effector cell neurotoxicity syndrome (ICANS) were graded according to institutional standards.17-20 Treatment responses were assessed by Lugano criteria21 by treating physicians at individual institutions without centralized review. Independent institutional review board approval was obtained at each institution. The study was conducted in accordance with the Declaration of Helsinki.
Study endpoints
The co-primary endpoints of this analysis were PFS and OS after alloHCT. PFS was defined as the time from transplant to disease progression/relapse or death. OS was defined as the time from transplant to death. Patients without PFS/OS events were censored at the time of last contact. GRFS events were defined as the first event among grade III–IV acute GvHD and aGvHD, moderate/severe chronic GvHD and cGvHD, relapse and death.10 Incidence of NRM was defined as the time from transplant to death without progression/relapse. Progression/relapse was defined as progressive lymphoma after alloHCT or lymphoma recurrence after a CR with NRM considered as a competing risk. Surviving patients without progression/relapse were censored at the date of last follow-up.
Neutrophil and platelet recovery, aGvHD and cGvHD were graded using established consensus criteria.22-24 For neutrophil and platelet recovery, death without the event was considered a competing risk. Patients with no nadir were not included in the recovery analyses.
Statistical analysis
For all time-to-event outcomes patients were followed from day 0 of the alloHCT until the event of interest, last follow-up, or the administrative truncation time (Online Supplementary Table S2). Descriptive summaries were obtained using the Kaplan-Meier method to estimate timespecific and median event time. In the presence of competing risks, the cumulative incidence was estimated using the Aalen-Johansen estimator. The effect of covariates on outcomes was assessed using Cox regression for survival outcomes, and Fine-Gray regression in the presence of competing risks. Variables considered in model building for Cox model multivariate analysis are listed in the Online Supplementary Table S2. Variables significant at the 0.2 level in the univariable analysis were included in the multivariable model. Continuous variables were assessed with Wilcoxon rank-sum testing and categorical values with Fisher’s exact test. Statistical analysis was completed in R 4.0.3 and GraphPad Prism 9.3.1. A two-sided 0.05 significance level was used.
Results
Patient characteristics
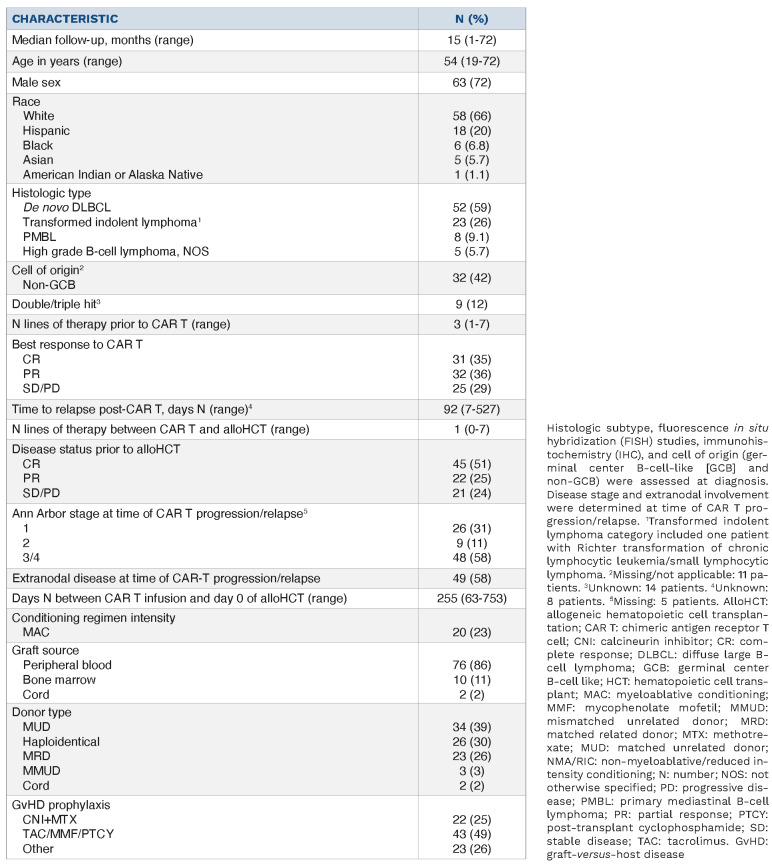
Ninety-four patients with R/R LBCL received an alloHCT following anti-CD19 CAR T. Six of these 94 patients received an alloHCT while in a durable CR following anti-CD19 CAR T and were excluded. We report findings on the remaining 88 patients with anti-CD19 CAR T failure. Demographic and clinical characteristics are described in Table 1. Median age at time of alloHCT was 54 years (range, 19-72). Most patients were male (n=63, 72%), White (n=58, 66%) and had de novo diffuse large B-cell lymphoma (n=52, 59%). Twenty-two patients (25%) had an autologous HCT prior to CAR T. The most common CAR T construct received was axicabtagene ciloleucel (n=59, 67%) followed by investigational anti-CD19 CAR T-cells (n=14, 16%), lisocabtagene maraleucel (n=11, 12%) and tisagenlecleucel (n=4, 5%). Following CAR T, rates of grade ≥III CRS were 2% (n=2) and grade ≥III ICANS were 17% (n=15). Best response to CAR T was CR in 35% (n=31), PR in 36% (n=32), and SD/PD in 28% (n=25). Median time between CAR T and alloHCT was 255 days (range, 63-753). The median number of lines of therapy between CAR T infusion and alloHCT was 1 (range, 0-7). Disease status at time of alloHCT was CR in 51% (n=45), PR in 25% (n=22) and SD/PD in 24% (n=21) of patients. RIC/NMA conditioning regimens were used in 77% (n=68) and peripheral blood was the most common graft source (86%, n=76). The most common donor types were matched unrelated donor (MUD) (39%), followed by haploidentical (30%) and MRD (26%). The most common GvHD prophylaxis regimen was tacrolimus + mycophenolate mofetil + post-transplant cyclophosphamide (49%) followed by a calcineurin inhibitor + methotrexate (25%). Median follow-up of survivors was 15 months (range, 1-72).
Table 1.
Demographic and clinical characteristics of patients with large B-cell lymphoma receiving allogeneic transplantation after CAR T therapy.
Therapies given between CAR T infusion and allogeneic hematopoietic cell transplantation
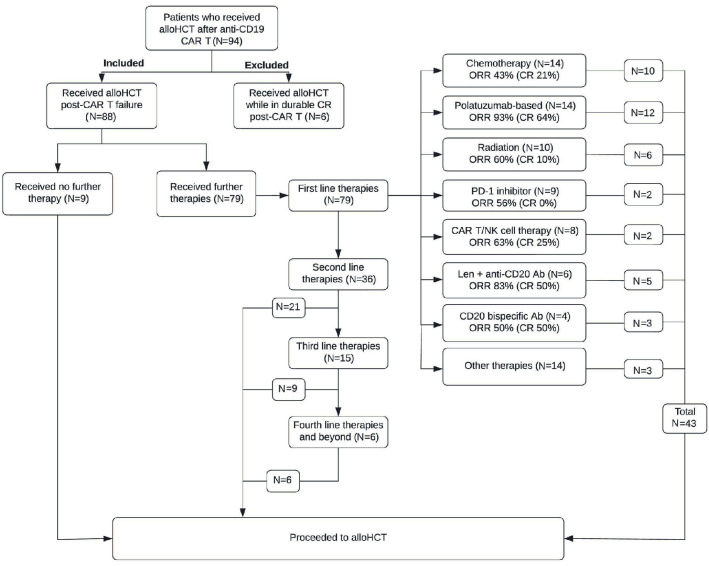
The most common first-line treatment regimens (n=79) given as salvage following CAR T failure were: chemotherapy (n=14, 18%), polatuzumab-based (n=14, 18%; n=13 received polatuzumab + bendamustine + rituximab), radiation (n=10, 13%), checkpoint inhibitors (n=9, 11%) and CAR T/NK-cell therapy (n=8, 10%). Nine patients received no therapy for their active lymphoma between CAR T and alloHCT. The therapeutic procedure for all patients between CAR T and alloHCT is depicted in Figure 1. The most common regimens given across all lines between CAR T and alloHCT (n=142 treatment regimens) were chemotherapy-based (n=31), polatuzumab-based (n=28), and radiation (n=18) (Online Supplementary Table S3). Polatuzumab-based regimens and chemotherapy-based regimens were the most common regimens to led to a CR prior to alloHCT across multiple lines (n=19 [42%] and n=10 [22%] respectively; Online Supplementary Table S4). The ORR across multiple lines between CAR T and alloHCT for chemotherapy was 60% (33% CR; n=31) and for polatuzumab + bendamustine + rituximab was 91% (71% CR; n=23).
Figure 1.
Flow chart depicting intervening lines of therapy between CAR T-cell therapy and allogeneic hematopoietic cell transplantation. Ab: antibody; alloHCT: allogeneic hematopoietic cell transplantation; CAR T: chimeric antigen receptor T-cell therapy; CR: complete remission; len: lenalidomide; n: number; NK: natural killer cell; ORR: overall response rate; PD-1: programmed cell death protein 1.
Engrafment and graf-versus-host disease
Median time to neutrophil recovery was 16 days (interquartile range [IQR], 12-18) and median time to platelet recovery was 18 days (IQR, 11-25) (Online Supplementary Figure S1). Cumulative incidence of neutrophil recovery was 94% at 28 days (95% confidence interval [CI]: 89-99). Cumulative incidence of platelet recovery at 100 days was 89% (95% CI: 83-96). There was one patient with primary graft failure. The cumulative incidence of grade II-IV and III-IV aGvHD were 34% (95% CI: 25-45) and 10% (95% CI: 5.6-19) at 100 days. The cumulative incidence of mild, moderate, and severe cGvHD at 1-year was 16% (95% CI: 9.8-28), 7.8% (95% CI: 3.6-17), and 3.8% (95% CI: 1.3-12) respectively. The cumulative incidence of grade II-IV aGvHD at 100 days was 31% (95% CI: 20-49) for patients who received PTCy-based prophylaxis versus 36% (95% CI: 24-53) for patients who did not receive PTCy (Online Supplementary Figure S2). The cumulative incidence of cGvHD at 1-year was 37% (95% CI: 24-57) for patients receiving PTCy-based prophylaxis versus 24% (95% CI: 14-41) for those who did not (Online Supplementary Figure S2).
Twenty-three patients (26%) received a PD-1 inhibitor between CAR T and alloHCT across multiple lines with last dose of the PD-1 inhibitor a median of 167 days (range, 56-527) before alloHCT. Seventeen of these patients (74%) received post-transplant cyclophosphamide (PTCy) as part of their GvHD prophylaxis. There was no significant difference in the incidence of grade II-IV aGvHD and cGvHD whether patients received an PD-1 inhibitor between CART and alloHCT (Online Supplementary Table S5).
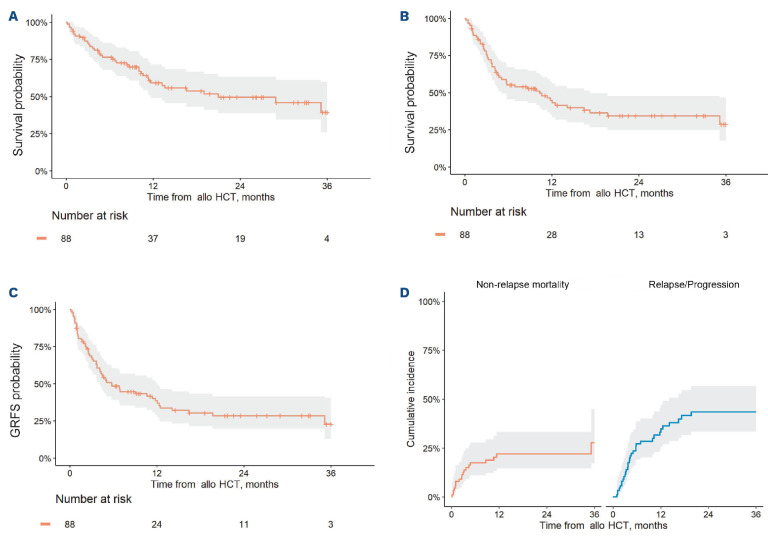
Figure 2.
Survival outcomes, progression/relapse and non-relapse mortality in patients who underwent allogeneic hematopoietic cell transplantation afer CAR T. (A) Overall survival, all patients. (B) Progression-free survival, all patients. (C) Graft-versus-host disease free, relapse-free survival (GRFS), all patients. (D) Non-relapse mortality and relapse/progression, all patients. AlloHCT: allogeneic hematopoietic cell transplantation.
Table 2.
Univariate analysis outcomes after allogeneic hematopoietic cell transplantation.1
Allogeneic hematopoietic cell transplantation outcomes
Median OS was 21 months (95% CI: 12 months – not reached [NR]), and 1-year OS was 59% (95% CI: 49-72) for all patients (Figure 2A). Median PFS was 10 months (95% CI: 5.1-17), and 1-year PFS was 45% (95% CI: 35-57) (Figure 2B). Median GRFS was 5.7 months (95% CI: 4.1–12), and 1-year GRFS was 39% (95% CI: 29-51) (Figure 2C). The incidence of NRM at 1-year was 22% (95% CI: 15-33) (Figure 2D). The incidence of progression/relapse at 1-year was 33% (95% CI: 24-45) (Figure 2D).
Univariable analysis of allogeneic hematopoietic cell transplantation outcomes
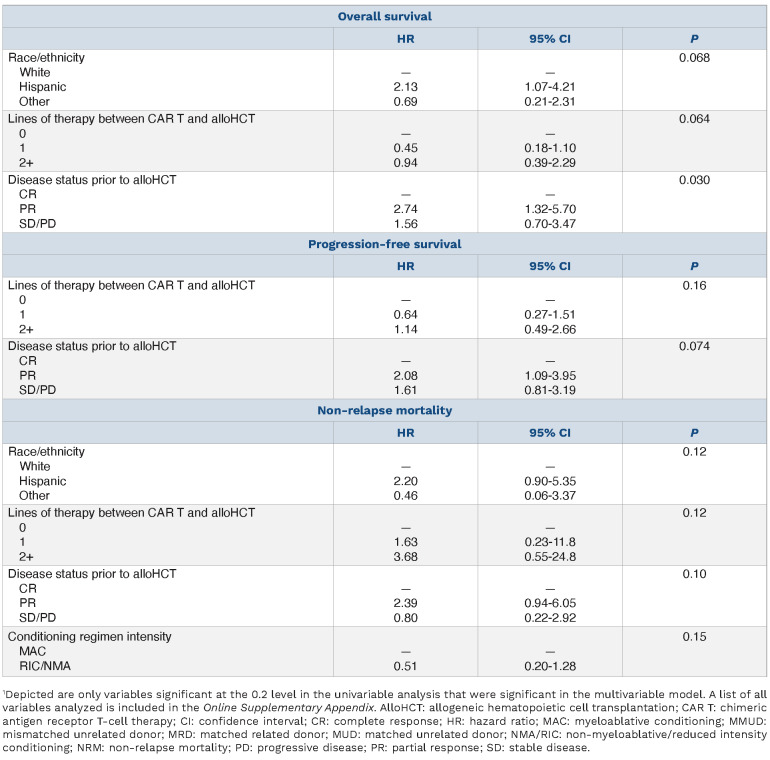
On univariable analysis, disease status prior to alloHCT was predictive of OS, with patients in CR at time of alloHCT experiencing improved OS compared to patients in PR or SD/PD (P=0.03; Table 2; Online Supplementary Table S6). There were no significant predictors of PFS, NRM, GRFS, or progression/relapse on univariate analysis (Table 2; Online Supplementary Table S6).
Multivariable analysis of allogeneic hematopoietic cell transplantation outcomes
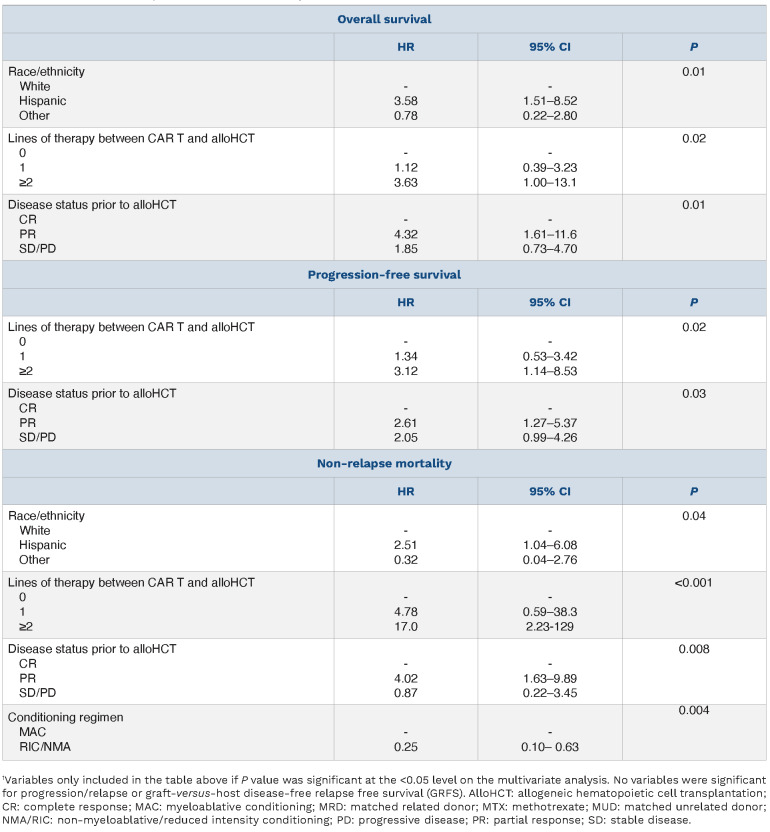
On multivariable analysis (MVA) (Table 3), predictors of inferior OS included ≥2 lines of therapy between CAR T and alloHCT (HR 3.6, 95% CI: 1.00-13.1; P=0.02 vs. 0 lines), disease status of PR at time of alloHCT (HR 4.32, 95% CI: 1.61–11.6; P=0.01 vs. CR), and Hispanic ethnicity (HR 3.6, 95% CI: 1.5-8.5; P=0.01 vs. White). Predictors of inferior PFS included disease status of PR at time of alloHCT (HR 2.6, 95% CI: 1.3-5.4; P=0.03 vs. CR) and ≥2 lines of therapy between CAR T and alloHCT (HR 3.1, 95% CI: 1.1-8.5; P=0.02 vs. 0 lines). There were no significant predictors of progression/relapse or GRFS on MVA. A higher risk of NRM was seen with patients who received ≥2 lines of therapy between CAR T and alloHCT (HR 17.0, 95% CI: 2.2-12.9; P<0.001 vs. 0 lines), those in PR at time of alloHCT (HR 4.0, 95% CI: 1.6-9.9; P=0.008 vs. CR) and those with Hispanic ethnicity (HR 2.51, 95% CI: 1.04-6.08, P=0.04 vs. White). Patients receiving RIC/NMA conditioning regimens had a lower risk of NRM (HR 0.25, 95% CI: 0.10-0.63; P=0.004) than those receiving MAC regimens.
Table 3.
Multivariate analysis outcomes after allogeneic hematopoietic cell transplantation.1
Impact of disease status at time of allogeneic hematopoietic cell transplantation
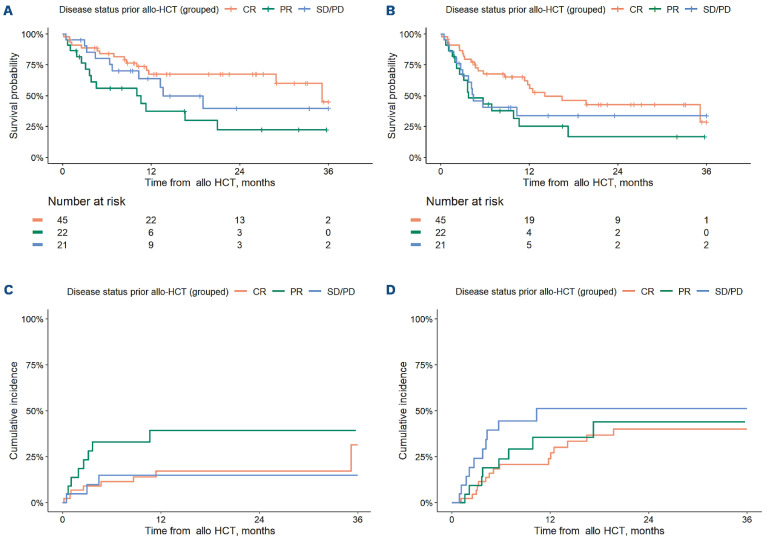
As noted above, there was a significant association between disease status at time of alloHCT and the end-points of PFS, OS, and NRM on MVA. There were no significant differences in incidence of progression/relapse or GRFS based on disease status. For patients in CR versus PR versus SD/PD at time of alloHCT, 1-year OS was estimated at 67% (95% CI: 54-84) versus 37% (95% CI: 20-68) versus 64% (95% CI: 45-90) respectively (Figure 3A), 1-year PFS was estimated at 59% (95% CI :45-76) versus 25% (95% CI: 11-56) versus 34% (95% CI: 18-64) (Figure 3B) and 1-year GRFS was estimated at 48% (95% CI: 35-66) versus 20% (95% CI: 7.8-51) versus 36% (95% CI: 20-65) (not depicted). For patients in CR versus PR versus SD/PD at time of alloHCT, the 1-year incidence of NRM was 9.0% (95% CI: 3.5-23) versus 28% (95% CI: 14-55) and 9.8% (95% CI: 2.6-37) (Figure 3C) and the 1-year incidence of progression/relapse was 24% (95% CI: 14-41) versus 36% (95% CI: 19-65) versus 51% (95% CI: 33-80) (Figure 3D). Demographic and clinical characteristics of patients in CR versus PR versus SD/PD at the time of transplant were overall similar (Online Supplementary Table S7). However, patients with SD/PD at time of alloHCT were significantly more likely to received myeloablative conditioning (MAC) versus patients in CR/PR (42.9% vs. 16.4%; P=0.02). Patients in CR versus PR/SD/PD were more likely to have received ≥2 lines of therapy between CAR-T and alloHCT (53% vs. 30%; P=0.03) (Online Supplementary Table S7). Twenty-three percent (n=5) of patients in PR at time of alloHCT and 19% (n=4) of patients with SD/PD at time of alloHCT received zero lines of therapy between CART and alloHCT (vs. 0% of those in CR at time of alloHCT).
Figure 3.
Survival outcomes, progression/relapse and non-relapse mortality based on disease status at time of allogeneic hematopoietic cell transplantation. (A) Overall survival, by disease status at allogeneic hematopoietic cell transplantation (alloHCT). (B) Progression-free survival, by disease status at alloHCT. (C) Non-relapse mortality, by disease status at alloHCT. (D) Progression/relapse, by disease status at alloHCT.
Survival and outcome curves by lines of therapy are depicted in Online Supplementary Figure S3 and survival outcomes by both disease status and number of lines of therapy in Online Supplementary Figures S4 and S5. Patients who received zero lines of intervening therapy had a relatively low incidence of NRM at 1-year (0 lines: 15%, 95% CI: 2.6-86; 1 line: 14%, 95% CI: 6.9-30%; ≥2 lines: 34%, 95% CI: 21-56), but a high incidence of progression/relapse at 1-year (0 lines: 56%, 95% CI: 31-100; 1 line: 24%, 95% CI: 14-42; ≥2 lines: 39%, 95% CI: 25-61) (Online Supplementary Figure S3). Patients in CR at time of alloHCT after ≥2 lines of therapy between CAR T and alloHCT had markedly better PFS and OS than those in PR or SD/PD after ≥2 lines (Online Supplementary Figures S4 and S5).
Cause of death
At time of last follow-up, forty patients had died. Causes of death in descending order of prevalence included: infection (n=14, 35%), progressive disease (n=12, 30%), respiratory failure (n=4, 10%) GvHD (n=3, 7.5%), hepatic sinusoidal obstruction syndrome/venoocclusive disease (SOS/VOD) (n=2; 5%), unknown (n=2, 5%), thrombotic microangiopathy (n=1, 2.5%), pulmonary embolism (n=1, 2.5%), and anaphylaxis from a local anesthetic agent (n=1, 2.5%). Three of the 14 patients who died of infection specifically died of SARS-CoV-2.
Discussion
In our study, alloHCT after CAR T therapy produced durable responses at 1-year with similar safety and outcomes to what has been observed in both modern and historical series of alloHCT for LBCL.10,25-28 Longer follow-up is needed to evaluate whether these outcomes will be durable. Engraftment, incidence of aGvHD at 100 days, and cGvHD at 1 year were similar to prior studies of alloHCT for LBCL.10,25,26 There were no unexpected or prolonged issues with hematopoiesis and no signal of increased GvHD or other unexpected complications.
Predictors of OS, PFS, and NRM on MVA included number of lines of therapy between CAR T and alloHCT and disease status at time of alloHCT. Patients receiving two or more lines of therapy between CAR T and alloHCT had significantly worse outcomes, particularly if they did not achieve a CR prior to alloHCT. The reasons for this are unknown but could be related to the cumulative toxicities of multiple therapies given the incidence of NRM at 1-year was higher in this cohort. Moreover, needing multiple lines of therapy post-CAR T to achieve a response could be reflective of a more treatment resistant disease phenotype less likely to achieve durable remission after alloHCT.
Patients not in CR at time of alloHCT also had poorer OS, PFS and NRM on MVA. Inferior PFS was primarily driven by higher NRM rather than progression/relapse. Similar to our findings, disease status prior to alloHCT in patients with LBCL has been previously shown to be predictive of OS, PFS and NRM.27,28 Since our cohort was small, these findings need validation, and this merits further study. Patients with Hispanic race/ethnicity had worse NRM and OS than their White, Black, or Asian counterparts. This is in line with prior large studies that have shown worse OS in Hispanic compared to non-Hispanic white patients in those receiving MRD alloHCT despite adjusting for clinical variables, although these studies did not consider socioeconomic or cultural variables.29,30
For this cohort of patients, polatuzumab-based regimens had the highest overall and CR rates between CAR T and alloHCT across multiple lines, although the significance of this finding is limited by an absence of data on treatment regimens received in patients with CAR T failure considered for an allograft who did not undergo transplantation (i.e., lack of a denominator). Response rates described here do not reflect the true response rates for these regimens in the post-CAR T failure setting (which are lower13) but rather, are reflective of a treatment sensitive population that is able to proceed to alloHCT. Recent data has shown higher overall response rates and longer PFS in patients receiving polaBR compared to chemotherapy in the first line following CAR T failure;13 therefore, the introduction of a novel immunotherapy may be preferable to using chemotherapy alone when trying to achieve disease control prior to alloHCT in patients with CAR T failure. Of the recently approved novel agents, no patients in this cohort received selinexor and only one patient received tafasitamab or loncastuximab tesirine between CAR T and alloHCT, likely related in part to the recent nature of these approvals (June 2020 and onward).
There were several important limitations to this analysis. Firstly, this was a multicenter retrospective analysis that focused only on patients who received an allograft. The true denominator (patients who failed CAR T and warranted consideration for an allograft) was not captured in this study and therefore the clinical interpretation of treatment practices between CAR T and alloHCT should be viewed as hypothesis-generating. Secondly, the median follow-up of this study was relatively short (15 months) and longer follow-up is needed to determine the durability of these remissions. Next, given the nature of this analysis, there was inherent heterogeneity of institutional practices, including the grading of CRS and ICANS.17-20 Lastly, we did not include correlative data such as CAR T-cell persistence (at relapse or following treatment for relapse) that might inform different treatment options such as bispecific antibodies or other immunotherapeutic approaches.
In conclusion, 1-year alloHCT outcomes following CAR T failure in patients with LBCL mirrors the outcomes of LBCL with alloHCT more generally. More long-term follow-up is needed to confirm the durable remission rate and curative potential of alloHCT after CAR T failure. Outcomes were worse in patients not in CR at time of alloHCT and in those receiving two or more lines of therapy between CAR T and alloHCT. Based on our study, transplanteligible patients who achieve a CR after CAR T failure should be considered for alloHCT.
Supplementary Material
Funding Statement
Funding: AFH was supported by the National Cancer Institute of the National Institutes of Health P50 CA107399-11A1, the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award, and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award.
References
- 1.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403-1415. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel JY, Dahiya S, Jain MD, et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood. 2021;137(13):1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreger P, Sureda A, Ahn KW, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3(3):360-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shadman M, Gauthier J, Hay KA, et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy. Blood Adv. 2019;3(20):3062-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel JY, Dahiya S, Jain MD, et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel. Blood. 2021;137(13):1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurko JC, Epperla N, Nizamuddin I, et al. Outcomes and treatment patterns in patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy. Blood. 2021;138(Suppl 1):S884. [Google Scholar]
- 14.Caimi PF, Ai WZ, Alderuccio JP, et al. Efficacy and safety of loncastuximab tesirine (ADCT-402) in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2020;136(Suppl 1):S35-37. [Google Scholar]
- 15.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. [DOI] [PubMed] [Google Scholar]
- 16.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity asociated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GvHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2021;27(8):642-649. [DOI] [PubMed] [Google Scholar]
- 25.Hamadani M, Gopal AK, Pasquini M, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6(2):486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epperla N, Ahn KW, Khanal M, et al. Impact of reduced-intensity conditioning regimens on outcomes in diffuse large B cell lymphoma undergoing allogeneic transplantation. Transplant Cell Ther. 2021;27(1):58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174(2):235-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3754-3760. [DOI] [PubMed] [Google Scholar]
- 30.Baker KS, Loberiza FR, Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23(28):7032-7042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.