Asciminib is a potent first-in-class BCR::ABL1 inhibitor specifically targeting the ABL1 myristoyl pocket (STAMP). By virtue of not interacting with the ATP binding site, the drug maintains activity against chronic myeloid leukemia (CML) cells resistant to conventional tyrosine kinase inhibitors (TKI) caused by ABL1 kinase domain mutations. A phase I trial demonstrated promising results both in tolerability and treatment efficacy.1 In addition, a phase III trial (ASCEMBL) established a significant superiority of asciminib compared to bosutinib with better molecular response rates and a favorable safety profile in CML patients treated with at least two prior TKI.2 Currently, multiple phase II and III trials are ongoing, including the ASC4MORE and CMLXI trial assessing the combination of asciminib with imatinib and asciminib as first-line treatment option.3,4 In the perspective of its emergent role in the CML treatment landscape, it is relevant to provide insight in its real-world tolerability and efficacy. CML patients without further TKI treatment options are provided access to asciminib in the Early Access Programme (EAP) of Novartis in several countries including the Netherlands. We assessed real-world treatment patterns and outcomes of asciminib in the Dutch EAP patient cohort.
We approached all hematologists treating CML patients with asciminib in the Dutch EAP (i.e., not in a clinical trial setting). Patients qualified for the EAP if (i) treated with at least two prior TKI and (ii) no other registered TKI were suitable due to severe comorbidities or known ABL1 kinase domain mutations. Patients were included in our study if they consented and were older than 18 years. Deceased patients were included if they had not objected to the use of their medical information for scientific purposes in the past. Treatment failure was defined as not reaching a complete cytogenetic response or BCR::ABL1 <1%IS (CCYR/MR2.0) (= primary), losing previously achieved CCyR/MR2.0 or MMR (= secondary) or progression to advanced-phase disease (AP/BC). Event-free survival (EFS) was defined as time from start of asciminib until progression to AP/BC or death. CML-related death was defined as death preceded by progression to AP/BC in the prior 3 months. Response to treatment was assessed using the cumulative incidence competing risk (CICR) method considering the competing event of TKI discontinuation for any cause. Patients were only assessed for response if asciminib exposure was longer than 1 month. The Medical Ethics Committee of the Amsterdam University Medical Center approved this study. The study was conducted in accordance with the Declaration of Helsinki.
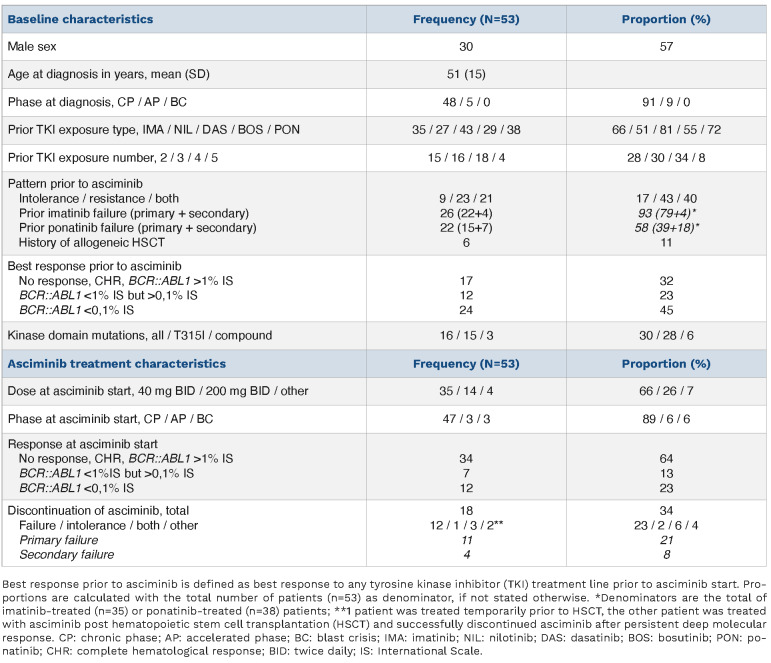
Fifty-six CML patients from 14 medical centers had received asciminib through the Dutch EAP at the time of inclusion. All hematologists agreed to participate and collected the relevant data from their patients. A total of 53 patients were included (Table 1). Patients were started on asciminib because of failure after having been on multiple TKI (43%), intolerance to multiple TKI (17%) or a combination of both (40%). Most patients (72%) had been exposed to at least three different types of TKI prior to asciminib (Online Supplementary Figure S1). Sixteen patients harbored a kinase domain mutation of whom 15 the T315I mutation and three a compound mutation. Six patients were in AP/BC when asciminib was started and six patients were treated with asciminib after an allogeneic hematopoietic stem cell transplantation (HSCT) of whom three also received asciminib prior to the HSCT. Of 38 patients previously treated with ponatinib, 15 (39%) had primary ponatinib failure and 27 (71%) discontinued ponatinib due to intolerance of whom 13 patients combined with treatment failure.
Median asciminib exposure time was 7 months (interquartile range [IQR], 3-17). Chronic phase patients started with a dose of 40 mg twice daily (BID), with the exception of patients with a T315I mutation who immediately started with a dose of 200 mg BID as currently recommended based on the asciminib phase I results.1 Patients with advanced phase disease were also recommended to start a higher dose (varying from 160 to 200 mg BID). Frequently reported adverse events (AE) were cytopenia and folliculitis, both of which were considered probably related to asciminib and were generally manageable without dose modification (Online Supplementary Table S1). Four patients definitely discontinued asciminib due to persistent anemia and/or thrombocytopenia, of whom three also because of a failing response. Two vascular events were reported: one recurrent TIA with multiple events prior to asciminib exposure, therefore classified as unlikely to be related; and one non ST-elevation myocardial infarction (NSTEMI) in a patient with a history of cardiovascular disease, but occurring at 9 days after the initiation of asciminib and therefore classified as possibly related.
Approximately one third of patients discontinued asciminib after a median exposure time of 4 months (n=18) (Table 1).
Table 1.
Baseline characteristics and asciminib treatment characteristics of included chronic myeloid leukemia patients treated with asciminib in the Dutch Early Access Programme.
The main reason for discontinuation was treatment failure. Eleven patients had primary treatment failure, of whom three also had intolerance for asciminib; four patients had secondary treatment failure. Only one patient discontinued asciminib solely based on intolerance, experiencing persistent CTC grade 3 cytopenia.
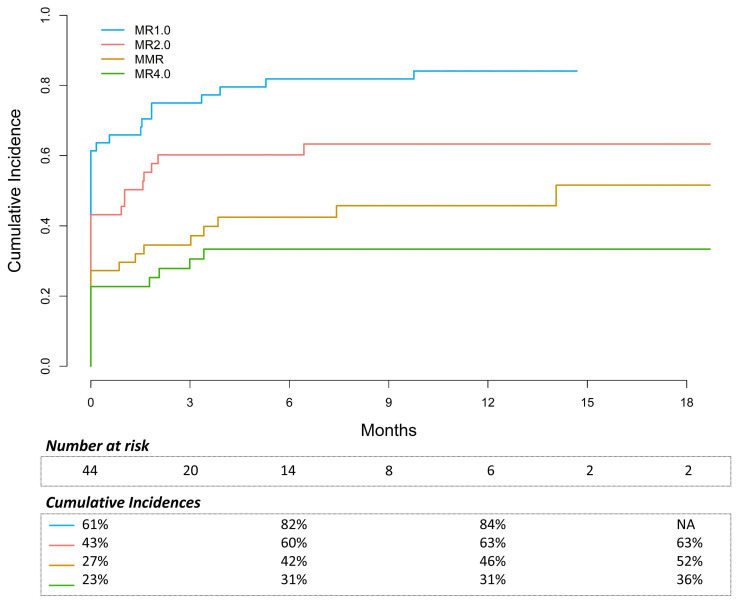
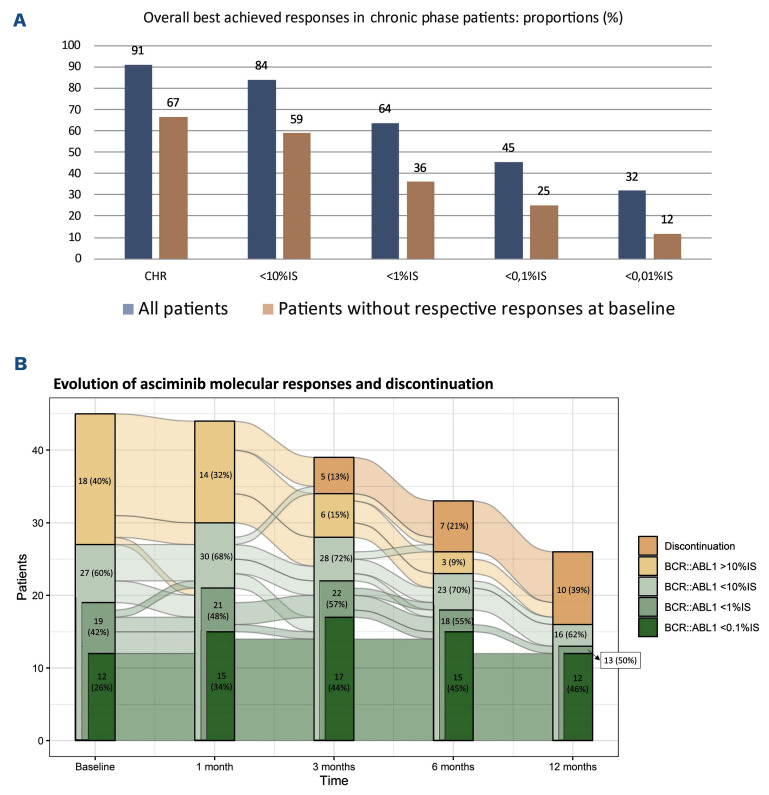
In chronic-phase (CP) patients without prior HSCT (n=44), the CI of maintaining or reaching MR2.0 and MMR were 60% and 42% by 6 months, respectively (Figure 1). In patients without MR2.0 (n=25) or MMR (n=32) at baseline, the CI of reaching these responses were 30% and 21% by 6 months, respectively. The overall best achieved response was MR2.0 and MMR in 66% and 45% of patients, respectively (Figure 2A). Figure 2B presents the evolution of molecular response and discontinuation at different points in time. Of 32 patients without MMR at baseline, 21 patients (66%) had an improvement in response, defined as a BCR::ABL1 decline of at least one-log (n=17) or a complete hematological response (CHR) if not present at baseline (n=4). The proportion of CP patients in CHR improved from 80% at baseline to 91% with asciminib. Three CP patients progressed to AP/BC, six proceeded to HSCT and three eventually died of a CML-related cause.
Of note, in CP patients with primary ponatinib failure (n=10), only one achieved MR2.0 and none of them achieved MMR. Moreover, to date six patients discontinued asciminib because of failure with a median treatment duration of 4 months. Better responses were observed in case of secondary ponatinib failure (n=5): 80% regained MR2.0 and 40% reached MMR. In case of ponatinib discontinuation due to intolerance most patients were already in MR2.0 (13/16, 81%) or MMR (10/16, 63%) at the start of asciminib and maintained this response. Three of these 16 patients deepened their response by one-log or more.
Figure 1.
Cumulative incidences of MR1.0 (BCR::ABL1 <10% IS), MR2.0 (BCR::ABL1 <1% IS), MMR (BCR::ABL1 <0,1% IS), MR4.0 (BCR::ABL1 <0,01% IS) in 44 chronic-phase chronic myeloid leukemia patients during asciminib treatment. A cumulative incidence competing risk (CICR) method was used for this response analysis, with asciminib discontinuation of any cause as competing event. IS: International Scale.
Twelve CP patients harbored the T315I or compound mutation and most of them (n=8) were already in MR4.0 at asciminib initiation. In the remaining four, only one achieved MR2.0, none achieved MMR and all eventually discontinued asciminib due to failure (Online Supplementary Figure S2A).
Of six patients in AP/BC at the start of asciminib, four had primary asciminib failure and only one patient is currently still on asciminib after 5 months of treatment without HSCT (Online Supplementary Figure S2B). Three AP/BC patients were temporarily treated with asciminib before proceeding to a HSCT; two achieved or maintained a CHR with a slight decrease in BCR::ABL1 during asciminib treatment. However, only one maintained this response until HSCT. Three AP/BC patients died, all from a CML-related cause. One CML-AP patient, known with a compound mutation (T315I and Y253H), was treated with both ponatinib and asciminib. This resulted in a BCR::ABL1 decrease from 78% to 86% IS, however accompanied with grade 3 thrombocytopenia. After 4 months, this patient discontinued the combination treatment because of an increase in BCR::ABL1 and persistent thrombocytopenia. He proceeded to HSCT in December 2021 and was in MMR at the time of analysis.
Six patients were treated with asciminib because of refractory disease post HSCT (Online Supplementary Figure S2C). One patient achieved MR2.0 at a single time point, however progressed to BC and died shortly after. The other five reachieved and maintained at least M2.0, three reached MR5.0 of whom one eventually discontinued asciminib.
In the whole cohort, OS and EFS were 87% and 81%, with a median time to event or lost-to-follow-up of 12 months. Seven patients died, all from a CML-related cause.
Our results demonstrate that asciminib is a well-tolerated novel CML treatment option with adequate response rates, even in heavily pretreated patients. Only one patient discontinued asciminib solely due to intolerance. The majority of CP patients had at least a one-log improvement in their molecular response, 60% reached or maintained MR2.0 by 6 months and 44% reached or maintained MMR by 12 months. These results are promising, especially in the context of poor responses observed in patients treated with other TKI in third line and beyond.5 Interestingly, in our cohort, only one patient (10%) with primary ponatinib resistance achieved MR2.0. This compares poorly to the remaining CP patients with an overall MR2.0 rate of 79% (P<0.001). Most of the patients with a prior ponatinib resistance were treated with an asciminib dose of 40 mg BID. As dosages up to 200 mg BID have been recommended for patients harboring the T315I mutation, it seems warranted to explore if higher dosages would also yield better results in primary ponatinib-resistant patients. Of note, limited responses were seen in CML-CP patients who were primary refractory to ponatinib (both in the presence or absence of the T315I mutation) and in patients with advanced phase CML. Presumably, BCR::ABL1-independent resistance mechanisms play an important role in this TKI refractoriness. More clinical data are needed to assess if earlier use of asciminib in CML patients that fail to respond to current kinase domain targeting TKI may prevent the development of asciminib resistance.
Figure 2.
Overall best achieved response in chronic-phase patients, presented as proportions (%) in all patients and in patients without the respective response at baseline. CHR: complete hematological response; IS: International Scale.
Our study supports the efficacy and tolerability in a real-world setting among heavily pretreated CML patients and with its favorable toxicity profile, asciminib is emerging as a valuable treatment option for CML patients with intolerance to current TKI.
Supplementary Material
References
- 1.Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rea D, Mauro MJ, Boquimpani C, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138(21):2031-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ASC4MORE trial. Available at: https://clinicaltrials.gov/ct2/show/NCT03578367 [Google Scholar]
- 4.CMLXI trial. Available at: https://clinicaltrials.gov/ct2/show/NCT03906292. [Google Scholar]
- 5.Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14(1):44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.