Abstract
Phosphatidylinositol 3-kinase (PI3K) inhibitors are effective in chronic lymphocytic leukemia (CLL). However, the severe toxicity profile associated with the first-generation inhibitors idelalisib and duvelisib, combined with the availability of other more tolerable agents, have limited their use. CLL is still considered incurable, and relapse after treatment, development of resistance, and treatment intolerance are common. It is therefore of interest to optimize the administration of currently approved PI3K inhibitors and to develop next-generation agents to improve tolerability, so that this class of agents will be considered an effective and safe treatment option when needed. These efforts are reflected in the large number of emerging clinical trials with PI3K inhibitors in CLL. Current strategies to overcome treatment limitations include intermittent dosing, which is established for copanlisib and zandelisib and under investigation for duvelisib and parsaclisib. A second strategy is to combine the PI3K inhibitor with another novel agent, either as a continuous regimen or a fixed-duration regimen, to deepen responses. In addition to these approaches, it is of interest to identify higher-resolution actionable biomarkers that can predict treatment responses and toxicity, and inform personalized treatment decisions. Here, we discuss the current status of PI3K inhibitors in CLL, factors limiting the use of currently approved PI3K inhibitors in CLL, current strategies to overcome these limitations, and where to go next.
Introduction
Chronic lymphocytic leukemia (CLL) is a common B-cell malignancy with approximately 20,000 new cases expected in the USA in 2022.1 Targeted therapies were introduced almost a decade ago and have rapidly and remarkably improved the management of CLL. While the disease typically responds well to the novel agents, exposure to treatment will over time result in outgrowth of treatment-resistant cellular clones and most typically result in disease relapse. Multiple lines of therapy are therefore the rule in CLL management. How to sequence or combine novel therapies for optimal care and durable remission are pressing research questions.
Three classes of targeted therapies are currently approved for CLL: Bruton tyrosine kinase (BTK) inhibitors (ibrutinib and acalabrutinib), the B-cell lymphoma-2 (Bcl-2) antagonist venetoclax, and phosphatidylinositol 3-ki-nase (PI3K) inhibitors (idelalisib and duvelisib). While venetoclax and BTK inhibitors have positioned themselves as effective and well-tolerated therapies in CLL (Box 1), PI3K inhibitors have experienced some setbacks because of unexpected toxicity. However, PI3K inhibitors are effective in CLL and are needed in the CLL therapy toolbox due to the incurable nature of the disease. Optimizing their use by developing more specific next-generation inhibitors, reducing toxicity and defining the ideal sequence or combination of therapies is therefore necessary to further improve CLL outcomes. In this review article, we discuss the present status of PI3K inhibitors in CLL, factors limiting the use of currently approved PI3K inhibitors in CLL, strategies to overcome these limitations, and where to go next.
The current status of PI3K inhibitors in chronic lymphocyte leukemia
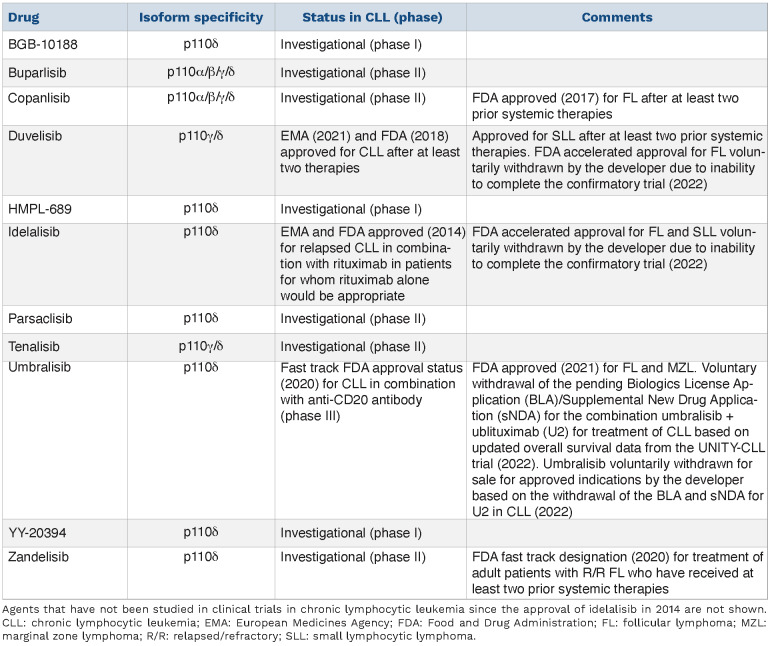
Class I PI3K consist of a regulatory unit (p85) in complex with a catalytic unit (p110). The catalytic unit exists as four isoforms; p110a, b, g, and d. p110a and p110b are ubiquitously expressed, while p110g and p110d are enriched in leuko-cytes.11 p110d is highly expressed in CLL, and has therefore in particular been the focus of drug development efforts for this disease (Table 1). In 2014, the selective p110d inhibitor idelalisib was approved for use in CLL based on a double-blind, placebo-controlled, randomized phase III study comparing idelalisib + the anti-CD20 antibody rituximab to placebo + rituximab in relapsed CLL.12 The patients enrolled on this trial had received a median of three previous drugs, which included rituximab, cyclophosphamide, fludarabine, bendamustine, and chlorambucil.12 The final follow-up of the study demonstrated a median progression-free survival (PFS) of 20.3 months in patients initially assigned to the ide-lalisib arm.13 The median overall survival (OS) was significantly improved, with 40.6 months in the idelalisib arm versus 34.6 months in the placebo arm.13 Idelalisib has been shown to inhibit B-cell receptor signaling and to induce apoptosis in CLL cells.14-17 It is administered orally at a recommended dose of 150 mg twice a day. While idelalisib is effective, serious adverse autoimmune events limit treat-ment.18,19 Of note, the Food and Drug Administration (FDA) accelerated approval of idelalisib for follicular lymphoma and small lymphocytic leukemia was voluntarily withdrawn in 2022 by the developer due to inability to complete the confirmatory trial (Table 1).
Box 1. Alternative targeted treatment options in chronic lymphocytic leukemia
BTK inhibitors and venetoclax provide excellent alternative treatment options to PI3K inhibitors in all lines of therapy. The first-in-class covalent BTK inhibitor ibrutinib is approved by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) for both previously treated and untreated CLL, as a single agent and in combination with the CD20 antibodies obinutuzumab or rituximab. The next-generation BTK inhibitor acalabrutinib has fewer of-target efects than ibrutinib,2 and was approved by the FDA for the treatment of CLL in 2019, and by the EMA as a monotherapy for previously treated CLL in 2020. Acalabrutinib and ibrutinib are being compared head-to-head in previously treated, high-risk CLL patients in the relapsed or refractory (R/R) setting (NCT02477696). At a median follow-up of 40.9 months, acalabrutinib and ibrutinib both showed a progression-free survival (PFS) of 38.4 months, while the incidence of all-grade atrial fibrillation was lower with acalabrutinib than with ibrutinib (9.4% vs. 16.0%, respect-ively).3 Discontinuation due to adverse events occurred in 14.7% and 21.3% of the patients in the acalabrutinib and ibrutinib arms, respectively.3
Zanubrutinib, another covalent and selective next-generation BTK inhibitor, was approved by the FDA in 2019 for treatment of patients with R/R mantle cell lymphoma. There are several ongoing studies with zanubrutinib in CLL, including the phase III ALPINE study, a head-to-head comparison with ibrutinib in R/R patients.4 The first interim analysis at a median follow-up of 12 months showed higher PFS (94.9% vs. 84.0%) and overall survival (OS) (97.0% vs. 92.7%) in the zanubrutinib arm. Atrial fibrillation/flutter was less frequent with zanubrutinib (2.5% vs. 10.1%), as were adverse events leading to study discontinuation (7.8% vs. 13.0%).4
A third generation of BTK inhibitors has been developed to overcome resistance to the covalent inhibitors. The most advanced non-covalent agents that inhibit both wild-type and C481S mutant BTK are pirtobrutinib5 and MK-1026.6 These agents have promising anti-tumor activity in CLL that has been treated with several lines of therapy, including with a covalent BTK inhibitor.
In addition to the expanding class of BTK inhibitors, venetoclax represents an efective therapeutic option in CLL. It was first approved by the FDA in 2016 for previously treated CLL patients with del(17p). Based on the CLL14 study,7 venetoclax is now approved by the FDA and EMA for all patients with CLL.
While studies such as the CLL17 trial (NCT04608318) compare diferent treatment options involving ibrutinib and vene-toclax, direct comparisons of PI3K inhibitors to BTK inhibitors or venetoclax in prospective clinical trials are limited. The ASCEND trial compared treatment with a BTK inhibitor to PI3K inhibitor treatment by randomizing patients with R/R CLL to acalabrutinib or investigator’s choice of idelalisib + rituximab or bendamustine + rituximab (BR).8 The PFS was significantly longer in the acalabrutinib arm than in the other arm (not reached vs. 16.5 months) at a median follow-up of 16.1 months. Grade ≥3 adverse events were significantly more frequent in the idelalisib arm, with the most common being diarrhea (22%) and neutropenia (20%). This trial thus favored acalabrutinib over idelalisib + rituximab in this setting. Similarly, the BRUIN CLL-321 study (NCT04666038) will compare pirtobrutinib to investigator’s choice of idelalisib + rituximab or BR in CLL patients who have received previous treatment with at least one BTK inhibitor. Based on available evidence, the European Society of Medical Oncology (ESMO) guidelines generally recommend PI3K inhibitors in later line therapy than BTK inhibitors or venetoclax.9 However, a PI3K inhibitor may be preferred ahead of these therapies in patients with severe renal or heart disease, or with a significant risk of tumor lysis. This practice is followed for patients outside clinical trials. A retrospective study showed that only 9% of patients (81% treated outside a clinical trial, 19% treated on a clinical trial) received idelalisib as the first B-cell receptor inhibitor.10
The next-generation PI3K inhibitor duvelisib is an oral, dual p110γ/δ inhibitor (Table 1). It received FDA approval in 2018 for CLL patients who have received at least two prior therapies. Approval by the European Medicines Agency (EMA) followed in 2021. The recommended dose is 25 mg twice a day. The indication was based on the phase III randomized DUO study of duvelisib versus the anti-CD20 antibody ofatumumab in patients with relapsed/refractory (R/R) CLL/small lymphocytic leukemia.20 One of the exclusion criteria was prior treatment with BTK or PI3κδ inhibitors.20 The median PFS was 13.3 months in the duvelisib arm and 9.9 months in the ofatumumab arm.20 The FDA accelerated approval of duvelisib for follicular lymphoma was voluntarily withdrawn in 2022 by the developer due to their inability to complete the confirmatory trial (Table 1).
The p110δ selective inhibitor umbralisib received FDA fast-track approval status for CLL in combination with the anti-CD20 antibody ublituximab in 2020, and was FDA approved for follicular lymphoma and marginal zone lym-phoma in 2021 (Table 1). It is administered orally with a recommended dose of 800 mg once a day. The phase I study of umbralisib suggested that this drug is associated with fewer autoimmune-like toxicities than idelalisib and duvelisib.21 However, head-to-head randomized trials comparing the different PI3K inhibitors would be needed to test these findings and to evaluate the relative efficacy of the different agents. Updated OS data from the randomized phase III UNITY-CLL trial showed an increasing imbalance in OS, and this led to the voluntary withdrawal of the pending Biologics License Application (BLA)/Supplemental New Drug Application (sNDA) for the combination of umbralisib + ublituximab (U2) for the treatment of CLL in 2022.22,23 Umbralisib was also voluntarily withdrawn from sale for approved indications based on these events (Table 1).
Table 1.
Approved and investigational PI3K inhibitors in chronic lymphocytic leukemia.
Factors limiting the use of currently approved PI3K inhibitors in chronic lymphocytic leukemia
Even with encouraging efficacy reports, the use of PI3K inhibitors in CLL has experienced setbacks. Barriers include the broad range of toxicities and safety warnings associated with these agents, which may deter clinicians who are unfamiliar with their management.24 Other classes of effective and more tolerable targeted therapies are available in CLL, and real-world data suggest that treatment with a PI3K inhibitor following venetoclax results in less durable remissions than when venetoclax is followed by a BTK inhibitor or cellular therapy.25 This further contributes to pushing PI3K inhibitors back in the treatment sequencing line.
Toxicity
Initial studies on the first-in-class PI3K inhibitor idelalisib showed encouraging efficacy, but serious toxicities also emerged, more frequent in frontline treatment than in the R/R setting. In a randomized, double-blind, placebo-controlled phase III study evaluating the efficacy and safety of rituximab with or without idelalisib in R/R CLL, the serious adverse event rates were similar between the idelal-isib and placebo arms (40% and 35%, respectively).12 However, the follow-up was short, and prolonged exposure to idelalisib was later reported to increase the incidence of all-grade and grade ≥3 diarrhea (46.4% and 16.4%), colitis (10.9% and 8.2%) and pneumonitis (10.0% and 6.4%).13
To evaluate idelalisib as initial therapy, a phase II study of idelalisib + rituximab in treatment-naïve older CLL patients was performed.26 The overall response rate was encouraging at 97%, and 100% in patients with TP53 aberrations. However, the frequency of adverse events was higher in this treatment-naïve population than in previously treated patients. For example, all-grade and grade ≥3 diarrhea and/or colitis occurred in 64% and 42% of the patients, respectively.26 A high frequency of serious adverse events was also seen when idelalisib was given frontline in combination with the anti-CD20 antibody ofa-tumumab.18 A follow-up outcome study showed that patients who stopped idelalisib due to toxicity (n=15) had a higher risk of subsequent disease progression than those who continued on therapy.27 However, no CLL-related deaths occurred, indicating that salvage treatment can be effective in this setting. A recent study has shown that patients who developed grade ≥3 toxicity had higher response rates and longer survival than those who did not have toxicity.28
The DUO trial, a randomized phase III study of duvelisib versus ofatumumab in R/R CLL, showed a similar toxicity profile for duvelisib as for idelalisib.20 The most common grade ≥3 adverse events in the duvelisib arm at a median overall follow-up of 22.4 months were neutropenia (30%), diarrhea (15%) and pneumonia (14%). The high incidence of adverse events associated with idelalisib and duvelisib has led to the addition of black box warnings for both agents.29,30
The broad range of toxicities associated with PI3K inhibitors does limit the use of these agents. In clinical trials with PI3K inhibitors in CLL, dose-reductions were performed for up to 58% of the patients, while drug discontinuation due to adverse events occurred in up to 39% of the cases.31 Toxicity and discontinuation rates are even higher outside clinical trials.32-35 A retrospective study of CLL patients treated with idelalisib + rituximab in the UK and Ireland (27 patients frontline, 83 R/R patients) showed that 87.3% of the patients discontinued idelalisib. The main reasons for discontinuation were adverse events (63% of frontline patients, 44.6% of R/R patients) and disease progression (3.7% of frontline patients, 20.5% of R/R patients). All-grade and grade ≥3 lower respiratory tract infection/pneumonia were reported in 34.5% and 19.1% of the patients, diarrhea in 30.9% and 6.4%, and colitis in 9.1% and 5.5% of the patients, respectively.35 Furthermore, a comparison of outcomes for CLL patients treated with idelalisib + rituximab in clinical trial participants versus Medicare beneficiaries in the USA showed that the latter group had a shorter median treatment duration (173 days vs. 473 days), higher treatment discontinuation (43.2% vs. 18%), higher mortality rate (hazard ratio 1.40), and higher fatal infection rate per 100 person-years (18.4 vs. 9.8).34 Dose reduction was more frequent in trial participants than in Medicare beneficiaries (32.6% vs. 18%). Interestingly, the number of Medicare beneficiary patients receiving idelalisib + rituximab treatment dropped by more than 60% during the first year following safety communications on risk of serious infections with idelalisib and the recommendation for prophylaxis in all idelalisib-treated pa-tients.34 This underscores the need for easily available information on how to monitor and manage PI3K inhibitor toxicities.
Opportunistic infections
Studies have shown that opportunistic infections can be caused by treatment with idelalisib combinations. Three phase III studies comparing BR or rituximab with or without idelalisib in treatment-naïve CLL or R/R indolent B-cell non-Hodgkin lymphoma were halted due to excess deaths and serious adverse events in the idelalisib arms, which were largely attributed to bacterial and opportunistic infections.36
Although an increased risk of opportunistic infections was reported for patients treated with idelalisib in combination with rituximab with or without bendamustine, these findings led to the recommendation for Pneumocystis jirovecii pneumonia (PJP) and antiviral prophylaxis as well as cyto-megalovirus monitoring in all patients treated with idelal-isib.36 The risk of cytomegalovirus reactivation appears greatest with bendamustine-based combinations.37
Resistance
Development of treatment resistance is a constant challenge for all types of cancer therapies. While resistance mechanisms to BTK inhibitors and venetoclax have been identified, revealing resistance mechanisms to PI3K inhibitors has proven more tedious.38 Initial whole-exome sequencing efforts on a PI3K inhibitor-resistant mouse model,39 as well as on samples from patients who progressed on idelalisib,40 did not identify mutations that could explain the resistance mechanisms. However, more recent studies indicate activating mutations in MAPK pathway genes and upregulation of IGF1R as resistance mechanisms in CLL.41;42 These findings suggest a role for MEK/ERK inhibitors in CLL, which were indeed shown to be effective in idelalisib-resistant CLL cells.43 In the same study, it was shown that CLL cells from idelalisib-exposed patients displayed increased sensitivity to venetoclax.43 This is in agreement with a study on follicular lymphoma which showed that treatment with idelalisib induces sensitivity to Bcl-2 inhibitors,44 and a third study showing that treatment with duvelisib sensitizes CLL cells to venetoclax in vitro.45 Combinations involving PI3K inhibitors, MEK inhibitors and Bcl-2 inhibitors would therefore be of interest to explore in CLL. Indeed, ongoing trials are investigating combinations between venetoclax and the PI3K inhibitors idelalisib (NCT03639324), duvelisib (NCT03534323), and copanlisib (NCT04685915) in CLL (Table 2). Interestingly, ex vivo testing of idelalisib-resistant tumor cells has identified signatures that stratify responders and non-re-sponders. A genetic signature was reported in follicular lymphoma,44 while a drug sensitivity profile was reported in CLL.17 Idelalisib treatment interferes with the CD40/CD40L pathway and induces downregulation of proteins involved in communication between lymphoma cells and the tumor microenvironment. This leads to reduced microenvironmental support and inhibition of tumor cell proliferation.44 High response rates to idelalisib and co-panlisib have been associated with high expression/activation of proteins in the PI3K pathway, and levels of raptor were significantly associated with response to the p110α/β/γ/δ inhibitor buparlisib.17,46-48 It will be of interest to test the predictive value of these biomarkers in prospective clinical trials.
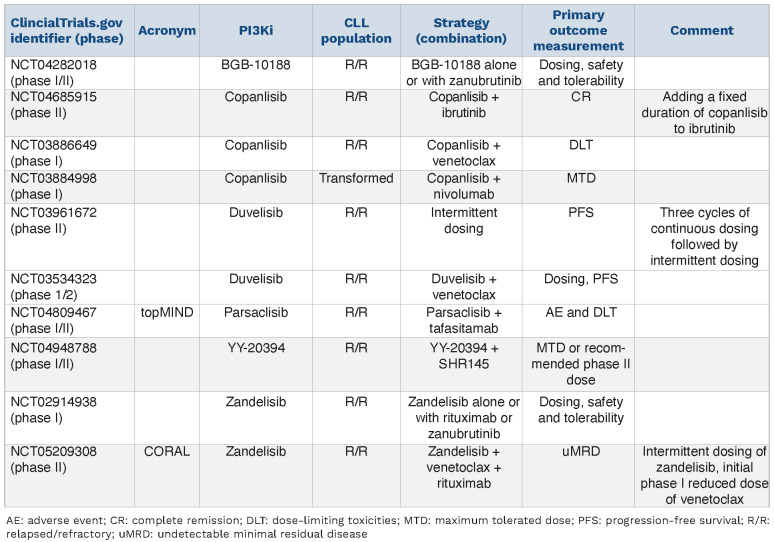
Table 2.
Select next-generation PI3K inhibitor trials in chronic lymphocytic leukemia with novel-novel combinations and/or alternative dosing schedules.
Current strategies to overcome PI3K inhibitor limitations in chronic lym-phocytic leukemia
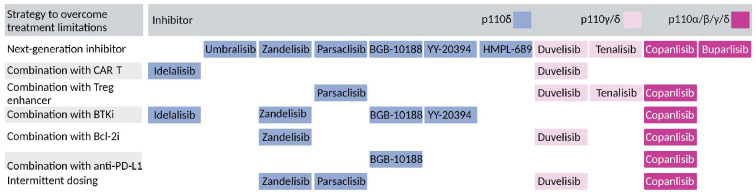
Toxicity is the main limiting factor to the use of currently approved PI3K inhibitors in CLL. Efforts are ongoing to overcome this limitation by developing more specific next-generation inhibitors (Figure 1) and to employ alternative combination or dosing regimens (Figure 2, Table 2). Below, we discuss the current strategies for PI3K inhibitors studied in clinical trials on CLL since the approval of ide-lalisib in 2014.
p110δ inhibitors
Idelalisib
Findings from a retrospective observational study of clinical outcomes of CLL patients treated with idelalisib + ri-tuximab suggested that treatment benefit extended considerably beyond the treatment duration.35 While the median treatment duration in this study was 11.9 months, the median PFS and time to next treatment were 29.6 and 29.2 months, respectively. These findings may indicate benefit from fixed-duration therapy. It would therefore be of interest to study intermittent or time-limited dosing of idelalisib in prospective clinical trials.
Umbralisib
Umbralisib shows a much higher selectivity to p110δ relative to other p110 isoforms, such as idelalisib and du-velisib, and has fewer off-targets.17,21 The phase I study in R/R CLL showed that it was well tolerated. The safety profile was distinct from that of idelalisib and duvelisib, with fewer cases of grade 1-2 and grade 3 colitis (0% and 2%), diarrhea (40% and 3%), and pneumonia (2% and 6%) at a median follow-up of 133 days.21 Toxicities associated with idelalisib and duvelisib are thought to be immune-mediated, and ex vivo or in vivo treatment with these agents affects regulatory T-cell function and numbers.49-52 Interestingly, treatment of Eμ-TCL1 CLL mice with umbralisib, as opposed to treatment with idelalisib or duvelisib, was shown to spare the regulatory T cells,53 suggesting a mechanism for the improved safety profile of umbralisib. Umbralisib in combination with ublituximab (U2) showed promising activity in a phase I trial in R/R CLL.54 Discontinuation of treatment due to adverse events occurred in 13% of the patients, and dose reduction occurred in 15% of the patients.54 These initial results led to the randomized phase III UNITY-CLL trial of U2 versus obinutuzumab + chlorambucil in treatment-naïve and R/R CLL (Table 2).55 At a median follow-up of 36.2 months, PFS was significantly longer in the U2 arm (31.9 months vs. 17.9 months). The favorable PFS was consistent in both treatment-naïve (38.5 months vs. 26.1 months) and R/R (19.5 months vs. 12.9 months) CLL patients.55 However, the choice to use obinutuzumab + chlorambucil in the control arm for R/R CLL was somewhat surprising since chemoimmunother-apy is rarely used as salvage therapy for relapsed CLL.56 Typical PI3K inhibitor toxicities were reported, but at lower rates than observed with idelalisib or duvelisib (neutrope-nia 30.6%, diarrhea 12.1%, colitis 3.4%, pneumonitis 2.9%).55 Umbralisib received fast track FDA approval status for CLL in combination with anti-CD20 antibody in 2020 (Table 1). However, the developer voluntarily withdrew the pending BLA/sNDA for the U2 combination for treatment of CLL based on updated OS data from the UNITY-CLL trial in 2022.22,23 Umbralisib was also voluntarily withdrawn from sale for approved indications by the developer based on the withdrawal of the BLA and sNDA for U2 in CLL the same year.
Figure 1.
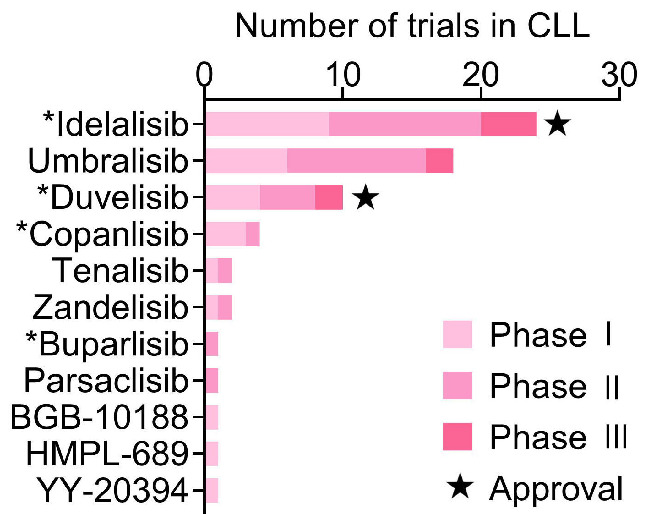
PI3K inhibitors in clinical trials in chronic lymphocytic leukemia. Overview of the number of phase I, II, and III clinical trials with the indicated PI3K inhibitors in chronic lymphocytic leukemia (CLL) registered at ClinicalTrials.gov. Agents that have not been studied in clinical trials in CLL since the approval of idelalisib in 2014 were excluded from the analysis. PI3K inhibitors with European Medicines Agency/Food and Drug Administration approval in CLL are indicated with a star. *Excluding terminated or withdrawn trials.
Figure 2.
Current strategies to overcome the limitations of PI3K inhibitors. The figure shows current strategies to overcome PI3K inhibitor limitations discussed in the main text, and indicates which strategies are employed for the different inhibitors. The color key indicates the p110 specificity of the inhibitors. CAR-T: chimeric antigen receptor T-cell; Bcl-2i: B-cell lymphoma-2 inhibitor; BTKi: Bruton tyrosine kinase inhibitor; PD-L1: programmed death ligand 1; Treg: regulatory T cell.
Zandelisib
Zandelisib is a selective p110δ inhibitor with longer p110δ occupancy than idelalisib.57,58 A phase I study in healthy volunteers identified 60 mg once a day as the optimal dose, due to approximately 90% inhibition of basophil ac-tivation.58 A phase Ib dose escalation study in follicular lymphoma and CLL/small lymphocytic leukemia showed that the most common adverse events had a delayed onset beyond cycle 2, as is typical of most PI3K immune-mediated toxicities.59 These adverse events, which included grade 3 diarrhea and rash, were reversible with drug interruption and/or corticosteroids. These findings motivated a phase I trial with intermittent dosing (7 days on/21 days off) after two continuous cycles (Figure 2).60 The rationale for the time off was based on the time it takes for regulatory T cells to repopulate (14 days), while the initial two cycles were used for tumor debulking. Preliminary results indicate a reduced rate of delayed grade 3 adverse events with maintained efficacy on the intermittent dosing schedule.60 TIDAL is a phase II study of intermittent dosing in patients with R/R follicular lymphoma (NCT03768505). Eligible patients must have received prior therapy with an anti-CD20 antibody and chemotherapy with an alkylating agent or purine analogue, and no prior PI3Kδ therapy. The trial was originally designed to compare continuous and intermittent dosing schedules,62 but has been revised to study only intermittent dosing. The phase III COASTAL study compares zandelisib + rituximab to im-munochemotherapy in patients with relapsed (≥1 prior line of therapy) indolent non-Hodgkin lymphoma.63 Eligible patients must have received an anti-CD20 antibody combined with chemotherapy or lenalidomide, and no prior PI3K inhibitor therapy. All patients in the zandelisib arm will be treated on the intermittent schedule (NCT04745832). In March 2020 the FDA granted zandelisib fast track designation for treatment of adult patients with R/R follicular lymphoma who have received at least two prior systemic therapies.
Parsaclisib
Parsaclisib is a potent and selective p110δ inhibitor.63 Intermittent dosing has been explored in a phase I trial in patients with R/R B-cell malignancies (Figure 2).64 Eligible patients had to have received one or more prior treatment regimens, excluding allogeneic hematopoietic stem cell transplant within the preceding 6 months and autologous hemato-poietic stem cell transplant within the last 3 months. Patients received 20 mg of parsaclisib once a day for the first 9 weeks followed by 20 mg once a week to decrease late-onset adverse events. This design was based on a comparative pharmacokinetic/dynamic simulation with copanlisib.64,65 Encouragingly, there were no treatment discontinuations due to adverse events in the intermittent dosing arm. Grade ≥3 adverse events were few (n=1 neutropenia, n=1 diarrhea). In the continuous treatment arm, discontinuations were observed in 13% of the patients, and grade 4 neutropenia was reported in 6% of the patients. Taken together, this suggests that intermittent treatment with par-saclisib is better tolerated than continuous treatment.
BGB-10188
BGB-10188 is a highly selective p110δ inhibitor.66 It has shown antitumor activity in B-cell lymphoma xenograft models, with an improved safety margin compared to other p110δ inhibitors.66 BGB-10188 is currently being studied as monotherapy and in combination with zanu-brutinib and the humanized IgG4 anti-PD-1 antibody tis-lelizumab in a phase I/II trial on R/R CLL (NCT04282018) (Table 2, Figure 2).
YY-20394
YY-20394 is a selective p110δ inhibitor.67 A phase I clinical trial in patients with R/R B-cell hematologic malignancies documented a median PFS of 255 days.67 Eligible patients could not have experienced disease progression on prior PI3Kδ inhibitor therapy. A durable response of approximately 36 months was observed in a CLL patient. The most frequent grade ≥3 adverse events were neutropenia (44%), pneumonia (16%), and hyperuricemia (12%). Grade 1/2 diarrhea was reported in 8% of the patients.67 Based on this study, the recommended phase II dose was set at 80 mg once daily.67 Additional phase I studies are ongoing in R/R follicular non-Hodgkin lymphoma (NCT04370405), R/R peripheral T-cell lymphoma (NCT04108325), and R/R B-cell hematologic malignancies (NCT04279405). YY-20394 is currently being studied in combination with the BTK inhibitor SHR145 in R/R B-cell non-Hodgkin lymphoma (NCT04948788) (Figure 2). Phase II trials with YY-20394 monotherapy are planned in patients with R/R follicular lymphoma (NCT04379167), R/R peripheral T/NK cell lym-phoma (NCT04705090), and R/R thymic cancer (NCT04975061). A phase I/II trial on YY-20394 combined with GEMOX (gemcitabine and oxaliplatin) is planned for patients with R/R diffuse large B-cell lymphoma (NCT04500561).
HMPL-689
HMPL-689 is a selective p110d inhibitor.68 A phase I dose escalation and expansion study with HMPL-689 in patients with R/R lymphoma is currently ongoing (NCT03786926). For eligible patients, standard-of-care treatment options should no longer exist, except for PI3Kd inhibitors. Results have not yet been reported. HMPL-689 is also being studied in patients with R/R marginal zone lymphoma and follicular lymphoma (NCT04849351). Eligible patients should not have received prior treatment with any PI3K or BTK inhibitor.
p110g/d inhibitors
Duvelisib
Intermittent dosing of duvelisib is being studied in the phase II TEMPO trial (NCT03961672) (Table 2), with the hypothesis that this may reduce toxicity (Figure 2). Duvelisib will be administered continuously for three cycles, followed by maintenance on days 1-2, 8-9, 15-16, 22-23 of each cycle in the absence of disease progression or unacceptable toxicity.
Tenalisib
Tenalisib is a highly specific, orally available p110g/d inhibitor with demonstrated anti-tumor activity in Hodgkin lymphoma cell line xenografts.69 In a phase I study in R/R hematologic malignancies, tenalisib was administered twice/thrice a day with a starting dose of 25 mg twice a day.70 Eligible patients had received at least one prior line of treatment, were unresponsive to standard therapy, and were not eligible for transplantation. Thirty-three of 35 enrolled patients completed a 28-day follow-up. The most frequently reported any-grade and grade ≥3 related adverse events were diarrhea (6% and 3%), hypertriglyceride-mia (6% and 3%), and nausea (9% and 0%).70 A phase I study of tenalisib in patients with R/R T-cell lymphoma applied a dosing scheme of 200 mg to 800 mg twice daily.71 The most frequently reported adverse events were fatigue (44.8%), aspartate/alanine aminotransferase increase (36.2%/34-5%), and diarrhea (32.8%).71 Phase II studies of tenalisib in R/R indolent non-Hodgkin lym-phoma (NCT03711578) and R/R CLL (NCT04204057) have been completed. Tenalisib was administered at the dose of 800 mg twice daily in the latter two trials. In the study on CLL, eligible patients should have received at least one prior therapy, excluding a PI3K inhibitor. Publication of results is awaited.
p110a/b/g/d inhibitors
Copanlisib
Copanlisib is a p110a/b/g/d inhibitor.72 It has been shown to be more effective at killing CLL cells than p110d selective inhibitors,17,73 and shows increased activity in tumor cells with aberrant activation of PI3K.72 It is effective both at continuous and intermittent dosing (Figure 2), and weekly dosing was shown to be sufficient to achieve sustained response in a xenograft model of non-small cell lung cancer.72 An intermittent schedule with copanlisib administered intravenously at 60 mg on days 1, 8, and 15 of a 28-day cycle was applied in the phase II CHRONOS-1 study enrolling patients with both indolent and aggressive R/R malignant lymphomas.47 For patients with indolent non-Hodgkin lymphoma, inclusion criteria included relapsed disease after prior treatment with two or more chemotherapy- or immunotherapy-based regimens, or disease refractory to two prior chemotherapy- and/or immuno-therapy-based regimens. The reported median PFS was 390 days (indolent lymphoma) and 70 days (aggressive lymphoma), and the median duration of response was 390 days and 166 days, respectively. Common adverse events were hyperglycemia (57.1% and 23.8%), hypertension (54.8% and 40.5%), and diarrhea (40.5% and 4.8%).47 The unique toxicity profile is likely associated with the inhibition of p110a. Dose delays were reported for 74% of the patients with indolent lymphoma, of which 91% were due to adverse events. Most of the delays (55%) lasted <1 week. Dose reductions occurred in 26% (to 45 mg) and 7% (30 mg) of these patients.46 Encouragingly, the 2-year follow-up of this study showed no evidence of an increased incidence or worsening of serious adverse events following longer treatment.74 Based on results from this trial, copan-lisib was approved by the FDA in 2017 for the treatment of relapsed follicular lymphoma after at least two prior systemic therapies.
The phase III CHRONOS-3 trial compares copanlisib + ri-tuximab to placebo + rituximab in patients with relapsed indolent non-Hodgkin lymphoma.75 In this study, previous exposure to a PI3K inhibitor, except copanlisib, is acceptable provided there is no resistance. Intolerance to PI3K inhibitors other than copanlisib is acceptable. At a median follow-up of 19.2 months, the PFS was 21.5 months and 13.8 months in the copanlisib arm and control arm, respectively. The most frequent grade ≥3 adverse events were hyperglycemia (56% and 8%) and hypertension (40% and 9%). This study demonstrates that copanlisib can be safely combined with rituximab.
The phase III CHRONOS-4 trial (NCT02626455) is evaluating the feasibility of combining copanlisib with standard chemoimmunotherapy (rituximab + bendamustine [BR, n=10 patients] or rituximab + cyclophosphamide, doxorubi-cin, vincristine, prednisone [R-CHOP, n=11 patients]) in R/R indolent non-Hodgkin lymphoma.76 Previous exposure to a PI3K inhibitor, except copanlisib, is acceptable provided there was no resistance. The most common all-grade and grade ≥3 adverse events in the BR arm were neutropenia (80% and 50%), nausea (70% and 0%), decreased platelet count (60% and 10%), and hyperglycemia (60% and 50%). In the R-CHOP arm they were hyperglycemia (82% and 64%), hypertension (73% and 64%), and neutropenia (64% and 64%).76 The objective response rates were 90% and 100% in the two treatment arms, respectively.76
Buparlisib
Buparlisib is an orally available p110α/β/γ/δ inhibitor able to cross the blood-brain barrier.77 It has been shown to induce cell death in CLL cells more potently than p110δ selective inhibitors.17,78 A phase II study of buparlisib in relapsed non-Hodgkin lymphoma demonstrated overall response rates between 11%-25%, depending on the indication.79 Eligible patients had received at least one prior therapy, with prior PI3K inhibitor excluded. The most common any-grade adverse events were hyperglycemia, fatigue, and nausea (all 36.1%), depression (29.2%), diarrhea (27.8%), and anxiety (25.0%). Grade ≥3 adverse events included hyperglycemia (11.1%) and neutropenia (5.6%).79 A phase II study of buparlisib in CLL reported a similar toxic-ity profile.48 Eligible patients had received at least one prior systemic treatment, excluding buparlisib. Mood alterations associated with buparlisib treatment distinguish this agent from copanlisib. There are no active clinical studies on buparlisib in CLL.
Combination therapies
As discussed above, early studies reported an increased risk of opportunistic infections among patients treated with idelalisib in combination with rituximab with or without bendamustine. Current efforts are therefore aimed at identifying combination regimens that are safe or theoretically limit toxicity.
Toxicities associated with idelalisib are thought to be im-mune-mediated.31 Idelalisib may therefore pair well with chimeric antigen receptor (CAR) T cells (Figure 2). Treatment with idelalisib during culturing of CAR T cells has been shown to increase T-cell viability and CD19-CAR expression in both healthy donors and CLL patients, overall improving the CAR T-cell products.80 Similarly, duvelisib treatment can improve the efficacy of CAR T-cells for CLL (Figure 2).81 In vitro addition of duvelisib during production of CAR T cells resulted in a CAR T product (duv-CAR) with stem-like qualities and improved metabolic fitness, which induced faster elimination of CLL cells in a xenograft mouse model.81 This finding suggests a novel place for du-velisib in CLL therapy.
Another treatment strategy could be to combine PI3K inhibitors with a drug that counteracts their effects on T cells.82 Histone deacetylase inhibitors83,84 and proteasome inhibitors85,86 have been shown to enhance regulatory T cells, and could serve as potential partners for PI3K inhibitors. The proteasome inhibitor ixazomib had demonstrated activity in an index case of relapsed CLL,87 and a phase I trial is evaluating the combination of duvelisib with either the histone deacetylase inhibitor romidepsin or the proteasome inhibitor bortezomib in R/R T-cell lymphomas (NCT02783625) (Figure 2).88 Combined treatment with ro-midepsin + duvelisib resulted in lower rates of transamini-tis compared to what was previously reported for duvelisib alone.88 Additional trials involve PI3K inhibitor + romid-epsin combinations for the same indication (NCT03770000, tenalisib; NCT04774068, parsaclisib; NCT04233697, copanlisib, withdrawn since the investigator left the institution) (Figure 2). It would be of interest to study whether similar combinations are effective and well-tolerated in CLL as well.
Idelalisib is also being studied in combination with the BTK inhibitor tirabrutinib, with or without obinutuzumab, in R/R CLL (NCT02457598, NCT02968563).89 The first report on tirabrutinib + idelalisib demonstrated promising efficacy, but with the same complete response rate (7%) as that observed with tirabrutinib monotherapy.89 All patients (100%) in the tirabrutinib + idelalisib arm (n=14) experienced grade ≥3 laboratory abnormalities, including neutropenia (43%). Two of the patients developed grade 1-2 pneumonitis and one patient died.89 Other trials are studying combined treatment with duvelisib and veneto-clax in R/R CLL (NCT03534323) (Table 2, Figure 2), the combination of zandelisib with rituximab, zanubrutinib and/or venetoclax in CLL (NCT02914938, NCT05209308) (Table 2, Figure 2), and several studies were initiated with U2-based combinations, but these were halted due to the updated OS data from UNITY-CLL.
Time-limited or intermittent dosing schedules for PI3K inhibitor combinations are also being explored as an approach to limit toxicity (Figure 2). The phase Ib/IIa topMIND study is evaluating intermittent dosing of parsaclisib combined with the anti-CD19 antibody tafasitamab in R/R CLL (NCT04809467) (Table 2), while a phase II trial (NCT04685915) is exploring a time-limited treatment with copanlisib to deepen the responses to ibrutinib. In addition, a phase I trial (NCT03886649) is studying combined treatment with copanlisib + venetoclax (Figure 2), while another trial (NCT03884998) is currently evaluating copan-lisib in combination with the PD-1 inhibitor nivolumab in transformed CLL (Table 2, Figure 2). The large number of clinical trials with copanlisib combinations in hematologic malignancies suggests that this agent is finding a home.90 Overall, it is hoped that these new combination approaches will provide more tolerable regimens compared to the initial idelalisib-based combinations.
Where do we go from here?
Although the implementation of PI3K inhibitor-based regimens in CLL management has faced various challenges, efforts remain profuse to optimize their use and identify tolerable schedules and combinations. Both patient selection and optimized patient management can reduce risks. Autoimmune toxicity is less in older patients and those who are more heavily pretreated. Infectious toxicity can be managed by prophylactic treatment of neutropenia and Pneumocystis jirovecii pneumonia, and antiviral prophylaxis. Currently, PI3K inhibitors are best used after BTK inhibitors or venetoclax in the management of CLL, as per ESMO guidelines.9 It is important to note that PI3K inhibitors can generally be used in patients with severe cardiac or renal disease, which may influence their choice in selected patients. It is also important that new clinical trial designs better reflect current recommendations by including patients who have been exposed to several lines of therapy, including targeted therapies and in some cases even a PI3K inhibitor.
For the future, intermittent dosing schedules and next-generation inhibitors hold the promise of reduced autoimmune toxicity. There is currently most activity in developing next-generation p110δ specific inhibitors, but the p110α/β/γ/δ inhibitor copanlisib is also finding its place.
With the growing number of targeted therapies available and in development for CLL, the question becomes: how to choose the right therapy for the right patient at the right time? Current treatment guidelines for CLL are based on relatively generic biomarkers such as IGVH and TP53 mutational status.9 Higher-resolution patient stratification will be possible if biomarkers identified in pre-clinical studies can be validated in biomarker-driven clinical trials and implemented in routine practice. In general, clinical trials of novel therapies should biobank samples dedicated to functional analyses such as cell signaling profiling, immune phenotyping, and ex vivo drug sensitivity testing, in addition to genetic profiling, to allow for identification of molecular signatures predictive of treatment responses, including tolerability.91 To develop truly predictive prognostic markers in this heterogeneous disease, sharing of comprehensive clinical, genetic, and functional data from both clinical trials and real-world cohorts is necessary.92 Based on these data, maximally predictive models and minimal biomarker signatures can be developed to inform personalized treatment decisions. This approach to precision medicine is exemplified by the European Union-funded CLL-CLUE consortium.93 The goal is to maximize efficacy and minimize toxicity by matching the right therapy to the right patient at the right time.
Funding Statement
Funding: SSS is supported by the Research Council of Norway under the frames of ERA PerMed (project number 322898) and Digital Life Norway (project number 294916). JRB is supported by NIH R01 CA 213442.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. [DOI] [PubMed] [Google Scholar]
- 2.Herman SEM, Montraveta A, Niemann CU, et al. The Bruton tyro-sine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Hillmen P, Ghia P, et al. First results of a head-to-head trial of acalabrutinib in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2021;39(15 Suppl):7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanu-brutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA Library. 2021;LB1900. [Google Scholar]
- 5.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892-901. [DOI] [PubMed] [Google Scholar]
- 6.Woyach J, Flinn I, Awan FT, et al. Preliminary efficacy and safety of MK-1026, a non-covalent inhibitor of wild-type and C481S mutated Bruton tyrosine kinase, in B-cell malignancies: a phase 2 dose expansion study. Blood. 2021;138(Suppl 1):392. [Google Scholar]
- 7.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzu-mab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. [DOI] [PubMed] [Google Scholar]
- 8.Ghia P, Pluda A, Wach M, et al. Acalabrutinib vs idelalisib plus ri-tuximab (IDR) or bendamustine plus rituximab (BR) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): ASCEND final results. EHA Library. 2020;294979:S159. [Google Scholar]
- 9.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23-33. [DOI] [PubMed] [Google Scholar]
- 10.Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibru-tinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050-1056. [DOI] [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Perry MWD, Brown JR, André F, Okkenhaug K. PI3K inhibitors are finally coming of age. Nat Rev Drug Discov. 2021;20(10):741-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituxi-mab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myhrvold IK, Cremaschi A, Hermansen JU, et al. Single cell profiling of phospho-protein levels in chronic lymphocytic leukemia. Oncotarget. 2018;9(10):9273-9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skånland SS, Cremaschi A, Bendiksen H, et al. An in vitro assay for biomarker discovery and dose prediction applied to ibrutinib plus venetoclax treatment of CLL. Leukemia. 2020;34(2):478-487. [DOI] [PubMed] [Google Scholar]
- 16.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, Athanasiadis P, Karlsen L, et al. Functional testing to characterize and stratify PI3K inhibitor responses in chronic lym-phocytic leukemia. Clin Cancer Res. 2022;28(20):4444-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given frontline for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idela-lisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127(20):2411-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: du-velisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burris HA, III Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19(4):486-496. [DOI] [PubMed] [Google Scholar]
- 22.TG Therapeutics. TG Therapeutics Announces Voluntary Withdrawal of the BLA/sNDA for U2 to Treat Patients with CLL and SLL. https://ir.tgtherapeutics.com/news-releases/news-release-details/tg-therapeutics-announces-voluntary-withdrawal-bla-snda-u2-treat. Accessed 15-4-2022. [Google Scholar]
- 23.Richardson NC, Kasamon Y, Pazdur R, Gormley N. The saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol. 2022;23(5):563-566. [DOI] [PubMed] [Google Scholar]
- 24.Patel K, Pagel JM. Exploring a future for PI3K inhibitors in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2019;14(4):292-301. [DOI] [PubMed] [Google Scholar]
- 25.Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of ide-lalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson PA, Stingo F, Keating MJ, et al. Outcomes of patients with chronic lymphocytic leukemia treated with first-line idela-lisib plus rituximab after cessation of treatment for toxicity. Cancer. 2016;122(16):2505-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner-Johnston ND, Sharman J, Furman RR, et al. Idelalisib immune-related toxicity is associated with improved treatment response. Leuk Lymphoma. 2021;62(12):2915-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zydelig (idelalisib) [package insert]. Gilead Science,Inc. 2018. [Google Scholar]
- 30.Copiktra (duvelisib) [package insert]. Verastem, Inc. 2018. [Google Scholar]
- 31.Hanlon A, Brander DM. Managing toxicities of phosphatidylinosi-tol-3-kinase (PI3K) inhibitors. Hematology Am Soc Hematol Educ Program. 2020;2020(1):346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199-2205. [DOI] [PubMed] [Google Scholar]
- 33.Mato AR, Samp JC, Gauthier G, Terasawa E, Brander DM. Drivers of treatment patterns in patients with chronic lymphocytic leukemia stopping ibrutinib or idelalisib therapies. Cancer Biol Ther. 2018;19(7):636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bird ST, Tian F, Flowers N, et al. Idelalisib for treatment of relapsed follicular lymphoma and chronic lymphocytic leukemia: a comparison of treatment outcomes in clinical trial participants vs medicare beneficiaries. JAMA Oncol. 2020;6(2):248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyre TA, Preston G, Kagdi H, et al. A retrospective observational study to evaluate the clinical outcomes and routine management of patients with chronic lymphocytic leukaemia treated with idelalisib and rituximab in the UK and Ireland (RETRO-idel). Br J Haematol. 2021;194(1):69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheah CY, Fowler NH. Idelalisib in the management of lym-phoma. Blood. 2016;128(3):331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skånland SS, Mato AR. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021;5(1):334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheffold A, Jebaraj BMC, Tausch E, et al. In vivo modeling of resistance to PI3Kd inhibitor treatment using EµTCL1-Tg tumor transfer model. Blood 2016;128(22):190. [Google Scholar]
- 40.Ghia P, Ljungstrom V, Tausch E, et al. Whole-exome sequencing revealed no recurrent mutations within the PI3K pathway in relapsed chronic lymphocytic leukemia patients progressing under idelalisib treatment. Blood. 2016;128(22):2770.27697770 [Google Scholar]
- 41.Murali I, Kasar S, Naeem A, et al. Activation of the MAPK pathway mediates resistance to PI3K inhibitors in chronic lymphocy-tic leukemia. Blood. 2021;138(1):44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tausch E, Ljungström V, Agathangelidis A, et al. Secondary resistance to idelalisib is characterized by upregulation of IGF1R rather than MAPK/ERK pathway mutations. Blood. 2022;139(22):3340-3344. [DOI] [PubMed] [Google Scholar]
- 43.Melvold K, Giliberto M, Karlsen L, et al. Mcl-1 and Bcl-xL levels predict responsiveness to dual MEK/Bcl-2 inhibition in B-cell malignancies. Mol Oncol. 2022;16(5):1153-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrat N, Guerrero-Hernández M, Matas-Céspedes A, et al. PI3Kdelta inhibition reshapes follicular lymphoma-immune mi-croenvironment cross talk and unleashes the activity of veneto-clax. Blood Adv. 2020;4(17):4217-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel VM, Balakrishnan K, Douglas M, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199). Leukemia. 2017;31(9):1872-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-ki-nase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898-3905. [DOI] [PubMed] [Google Scholar]
- 47.Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assouline S, Amrein L, Aloyz R, et al. IND.216: a phase II study of buparlisib and associated biomarkers, raptor and p70S6K, in patients with relapsed and refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2020;61(7):1653-1659. [DOI] [PubMed] [Google Scholar]
- 49.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the pho-sphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6598-6602. [DOI] [PubMed] [Google Scholar]
- 50.Chellappa S, Kushekhar K, Munthe LA, et al. The PI3K p110delta isoform inhibitor idelalisib preferentially inhibits human regulatory T cell function. J Immunol. 2019;202(5):1397-1405. [DOI] [PubMed] [Google Scholar]
- 51.Gadi D, Griffith A, Wang Z, et al. Idelalisib reduces regulatory T cells and activates T helper 17 cell differentiation in relapsed refractory patients with chronic lymphocytic leukaemia. Br J Hae-matol. 2022;197(2):207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gadi D, Griffith A, Tyekucheva S, et al. A T cell inflammatory phenotype is associated with autoimmune toxicity of the PI3K inhibitor duvelisib in chronic lymphocytic leukemia. Leukemia. 2022;36(3):723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maharaj K, Powers JJ, Achille A, et al. The dual PI3Kδ/γ inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020;4(13):3072-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lunning M, Vose J, Nastoupil L, et al. Ublituximab and umbrali-sib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(21):1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gribben JG, Jurczak W, Jacobs R, et al. Umbralisib plus ublituxi-mab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): results from the phase 3 Unity-CLL study [abstract]. Blood. 2020;136(Suppl 1):37-39. [Google Scholar]
- 56.Lampson BL, Brown JR. The evolving use of phosphatidylinositol 3-kinase inhibitors for the treatment of chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2021;35(4):807-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Farrell M, Ventura R, Tai A, et al. Preclinical characterization of PWT143, a novel selective and potent phosphatidylinositol 3-ki-nase delta (PI3K delta) inhibitor with ex-vivo activity in hemato-logic malignancies [abstract]. Blood. 2012;120(21):2907. [Google Scholar]
- 58.Moreno O, Butler T, Zann V, et al. Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110δ, following single ascending dose administration to healthy volunteers. Clin Ther. 2018;40(11):1855-1867. [DOI] [PubMed] [Google Scholar]
- 59.Soumerai JD, Pagel JM, Jagadeesh D, et al. Initial results of a dose escalation study of a selective and structurally differentiated PI3Kδ inhibitor, ME-401, in relapsed/refractory (R/R) follicu-lar lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) J Clin Oncol. 2018;36(15 Suppl):7519. [Google Scholar]
- 60.Zelenetz A, Soumerai JD, Jagadeesh D, et al. Preliminary safety and efficacy results with an intermittent schedule of the PI3kδ inhibitor ME-401 alone or in combination with rituximab for B-cell malignancies. Blood. 2018;132(Suppl 1):2893. [Google Scholar]
- 61.Zelenetz AD, Zinzani PL, Chan H, et al. ME-401-003 (TIDAL): a multicenter, randomized, double-blind, placebo-controlled, two-arm, phase 2 study of ME-401 investigating continuous and intermittent dosing schedules in patients with relapsed/refractory follicular lymphoma. Blood. 2019;134(Suppl 1):5244. [Google Scholar]
- 62.Jurczak W, Zinzani PL, Cunningham D, et al. Coastal: a phase 3 study of the PI3Kδ inhibitor zandelisib with rituximab (R) versus immunochemotherapy in patients with relapsed indolent non-Hodgkin's lymphoma (iNHL). Blood. 2021;138(Suppl 1):2430. [Google Scholar]
- 63.Shin N, Koblish H, Covington M, et al. INCB050465, a novel PI3Kδ inhibitor, synergizes with PIM protein kinase inhibition to cause tumor regression in a model of DLBCL. Cancer Res. 2015;75(15 Suppl):2671. [Google Scholar]
- 64.Forero-Torres A, Ramchandren R, Yacoub A, et al. Parsaclisib, a potent and highly selective PI3Kδ inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood. 2019;133(16):1742-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol. 2016;27(10):1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X, Yang X, Cui X, et al. BGB-10188, a highly selective PI3Kδ inhibitor with improved safety profile and superior anti-tumor activities in vivo. Cancer Res. 2020;80(16 Suppl):664. [Google Scholar]
- 67.Jiang B, Qi J, Song Y, et al. Phase 1 clinical trial of the PI3Kδ inhibitor YY-20394 in patients with B-cell hematological malignancies. J Hematol Oncol. 2021;14(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawrence T, Hahka-Kemppinen M, Cohen JB, Kania M. A phase 1 study of HMPL-689, a small molecule, highly selective, and potent inhibitor of phosphoinositide 3 kinase-delta, in patients with relapsed or refractory lymphoma. Blood. 2020;136(Suppl):17-18. [Google Scholar]
- 69.Locatelli SL, Careddu G, Serio S, et al. Targeting cancer cells and tumor microenvironment in preclinical and clinical models of Hodgkin lymphoma using the dual PI3Kδ/γ inhibitor RP6530. Clin Cancer Res. 2019;25(3):1098-1112. [DOI] [PubMed] [Google Scholar]
- 70.Carlo-Stella C, Delarue R, Scarfo L, et al. A first-in-human study of tenalisib (RP6530), a dual PI3Kδ/γ inhibitor, in patients with relapsed/refractory hematologic malignancies: results from the European study. Clin Lymphoma Myeloma Leuk. 2020;20(2):78-86. [DOI] [PubMed] [Google Scholar]
- 71.Huen A, Haverkos BM, Zain J, et al. Phase I/Ib study of tenalisib (RP6530), a dual PI3Kδ/γ inhibitor in patients with relapsed/refractory T-cell lymphoma. Cancers (Basel). 2020;12:2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319-2330. [DOI] [PubMed] [Google Scholar]
- 73.Göckeritz E, Kerwien S, Baumann M, et al. Efficacy of phosphatidylinositol-3 kinase inhibitors with diverse isoform selectivity profiles for inhibiting the survival of chronic lymphocytic leukemia cells. Int J Cancer 2015;137(9):2234-2242. [DOI] [PubMed] [Google Scholar]
- 74.Dreyling M, Santoro A, Mollica L, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol. 2020;95(4):362-371. [DOI] [PubMed] [Google Scholar]
- 75.Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):678-689. [DOI] [PubMed] [Google Scholar]
- 76.Matasar MJ, Dreyling M, Leppä S, et al. Feasibility of combining the phosphatidylinositol 3-kinase inhibitor copanlisib with ritu-ximab-based immunochemotherapy in patients with relapsed indolent B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(11):e886-e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Gooijer MC, Zhang P, Buil LCM, et al. Buparlisib is a brain penetrable pan-PI3K inhibitor. Sci Rep. 2018;8(1):10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amrein L, Shawi M, Grenier J, Aloyz R, Panasci L. The phosphati-dylinositol-3 kinase I inhibitor BKM120 induces cell death in B-chronic lymphocytic leukemia cells in vitro. Int J Cancer. 2013;133(1):247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes A, Salles G, Martinelli G, et al. Pan-phosphatidylinositol 3-kinase inhibition with buparlisib in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica. 2017;102(12):2104-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stock S, Übelhart R, Schubert ML, et al. Idelalisib for optimized CD19-specific chimeric antigen receptor T cells in chronic lymphocytic leukemia patients. Int J Cancer 2019;145(5):1312-1324. [DOI] [PubMed] [Google Scholar]
- 81.Funk CR, Wang S, Chen KZ, et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139(4):523-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown JR. Phosphatidylinositol 3 kinase delta inhibitors: present and future. Cancer J. 2019;25(6):394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi SW, Gatza E, Hou G, et al. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic he-matopoietic cell transplantation in humans. Blood. 2015;125(5):815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lucas JL, Mirshahpanah P, Haas-Stapleton E, et al. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257(1-2):97-104. [DOI] [PubMed] [Google Scholar]
- 85.Ramos TL, Garcia-Guerrero E, Caballero-Velazquez T, et al. Delayed administration of ixazomib modifies the immune response and prevents chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56(12):3049-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94(7):975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skånland SS, Inngjerdingen M, Bendiksen H, et al. Functional testing of relapsed chronic lymphocytic leukemia guides precision medicine and maps response and resistance mechanisms. An index case. Haematologica. 2022;107(8):1994-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horwitz SM, Nikitina A, Kotlov N, et al. The combination of duve-lisib and romidepsin (DR) is highly active against relapsed/refractory peripheral T-cell lymphoma with low rates of transaminitis: final results and biomarker analysis [abstract]. Blood. 2021;39(Suppl 2):683. [Google Scholar]
- 89.Danilov AV, Herbaux C, Walter HS, et al. Phase Ib study of tira-brutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2020;26(12):2810-2818. [DOI] [PubMed] [Google Scholar]
- 90.Le T, Jerel D, Bryan LJ. Update on the role of copanlisib in he-matologic malignancies. Ther Adv Hematol. 2021;12:20406207211006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skånland SS, Karlsen L, Taskén K. B cell signaling pathways -new targets for precision medicine in CLL. Scand J Immunol. 2020;92(5):e12931. [DOI] [PubMed] [Google Scholar]
- 92.Agius R, Parviz M, Niemann CU. Artificial intelligence models in chronic lymphocytic leukemia - recommendations toward state-of-the-art. Leuk Lymphoma. 2022;63(2):265-278. [DOI] [PubMed] [Google Scholar]
- 93.Tailoring the targeted treatment of chronic lymphocytic leukemia (CLL-CLUE). Available from: www.era-learn.eu/network-in-formation/networks/era-permed/multidisciplinary-research-projects-on-personalised-medicine-2013-pre-clinical-research-big-data-and-ict-implementation-and-user2019s-perspective/tailo-ring-the-targeted-treatment-of-chronic-lymphocytic-leukemia.ERA-LEARN. 2021. [Google Scholar]